Abstract
Hepadnavirus polymerases initiate reverse transcription in a protein-primed reaction. We previously described a complementation assay for analysis of the roles of the TP and RT domains of HBV reverse transcriptase (pol) in the priming reaction. Independently expressed TP and RT domains form a complex functional for in vitro priming reactions. To map the minimal functional TP and RT domains, we prepared baculoviruses expressing amino- and carboxyl-terminal deletions of both the TP and RT domains and analyzed the proteins for the ability to participate in transcomplementation for the priming reaction. The minimal TP domain spanned amino acids 20 to 175; however, very little activity was observed without a TP domain spanning amino acids 1 to 199. The minimal RT domain spanned amino acids 300 to 775; however, little activity was observed unless the carboxyl end of the RT domain extended to amino acid 800. Thus, most of the RNase H domain was required. In previous studies, we observed a TP inhibitory domain between amino acids 199 and 344. The current analysis narrowed this domain to residues 300 to 334, which is a portion of the minimal RT domain. In addition, the ability of TP and RT deletion mutants to form stable TP-RT complexes was examined in coimmunoprecipitation assays. The minimal TP and RT domains capable of protein-protein interaction were considerably smaller than the domains required for functional interaction in the transcomplementation assays, and unlike priming activity, TP-RT interaction did not require the epsilon RNA stem-loop. These studies help to further define the complex protein-protein interactions required in HBV genome replication.
Hepadnaviruses replicate their nucleic acids through a reverse transcription step (12, 27, 41, 45). The hepatitis B virus (HBV) reverse transcriptase, designated pol, is composed of four domains. From the amino terminus, the domains are (i) the TP domain, which becomes covalently linked to negative-strand DNA by virtue of the protein-primed initiation of reverse transcription; (ii) the spacer domain, which is tolerant of mutations; (iii) the RT domain, which contains the YMDD consensus motif for reverse transcriptases; and (iv) the RNase H domain (3, 35). Hepadnavirus genome replication has been defined by a variety of methods. The initial steps appear to require the cotranslational recognition by pol of the 5′ epsilon sequence on pregenomic RNA (2, 13, 14, 17, 18, 33). Neither pol nor pregenomic RNA is packaged in the absence of the other (2, 4, 13).
Initiation of replication occurs via a priming reaction in which a nucleotide becomes covalently linked to a tyrosine residue within the TP domain of pol (3, 6, 24, 30, 47, 50, 53, 56). The addition of the first four nucleotides is templated by a sequence in a bulge in the 5′ copy of epsilon (49, 51). Whether priming occurs prior to or immediately following encapsidation has not been experimentally determined; however, pol expressed in the absence of core is functional in priming reactions (23, 43, 47, 50). Following the priming reaction, pol is translocated to a complementary sequence in the 3′ copy of DR1 (direct repeat 1), where the synthesis of minus-strand DNA resumes (10, 26, 31, 37, 39, 40, 49, 51, 54).
Minus-strand DNA terminates at the 5′ end of pregenomic RNA (37, 54); a short oligoribonucleotide remnant of the pregenomic RNA is translocated, in the second strand jump, to a homologous site, DR2, on minus-strand DNA, where it serves as the primer for plus-strand DNA (25, 28, 38, 44). A third and final strand translocation occurs once plus-strand DNA synthesis reaches the 5′ terminus of minus-strand DNA. The translocation from the 5′ to the 3′ end of minus-strand DNA results in the formation of a noncovalently closed, circular DNA molecule. Plus-strand DNA is only partially completed in mature virions, yielding the gapped, double-stranded, circular DNA characteristic of mature virions.
Several systems which permit the direct analysis of pol function in the absence of viral replication and other viral proteins have been described (23, 43, 47, 50). A functional duck hepatitis B virus (DHBV) pol has been expressed by in vitro translation (50) and as an active fusion protein of DHBV Pol in a virus-like particle from the yeast retrotransposon Ty1 (47). Both systems yield pol that possesses accurate protein-primed, reverse transcriptase activity that synthesizes minus-strand DNA originating at epsilon and DR1 (49, 51); however, for reasons not understood, these systems have not been applicable to human HBV. Functional human HBV pol has been expressed via the baculovirus-insect cell expression system (23). The purified HBV pol is active for in vitro protein-priming and reverse transcriptase reactions. This system has also been used to develop a complementation system in which the independent expression of the HBV pol TP and RT domains results in the formation of a stable complex with epsilon RNA that upon purification is active for nucleotide priming and reverse transcription (24). In addition, coexpression of HBV pol and core proteins in insect cells results in the encapsidation of functional pol (42).
In this study, we used the transcomplementation assay to map the minimal domains of TP and RT capable of a functional interaction in protein priming and reverse transcription, as well as the minimal domains capable of a stable protein-protein interaction. The minimal functional TP domain closely adheres to boundaries previously defined by analysis of pol mutants in HBV replication, while the minimal RT domain for transcomplementation extends well into the RNase H domain. Much smaller TP and RT polypeptides were required for the formation of stable TP-RT complexes, suggesting that for most constructs the lack of functional activity in the transcomplementation assay could not be explained by a failure of TP and RT to interact.
MATERIALS AND METHODS
Cells and viruses.
The Sf9 cell line was cultivated in spinner culture as previously described (22). The cultivation medium was TNMFH supplemented with 5% fetal bovine serum and 0.1% pluronic F68 prior to infection and was changed to Grace’s medium supplemented with 2% fetal bovine serum and 0.1% pluronic F68 after infection. The same conditions were used for adherent cultures except that pluronic F68 was omitted. The methods for growth, isolation, and assay of recombinant baculoviruses were as previously described (46) except that viruses were generated by the Bac to Bac system (Gibco BRL, Gaithersburg, Md.), in which transposition in bacteria creates the recombinant baculovirus genome rather than homologous recombination in insect cells (29).
Plasmid constructs.
HBV sequences of the ayw subtype are numbered as designated by Galibert and coworkers (11). The FLAG–pol–stem-loop (FPL-pol) construct was previously described (23). The amino terminus of the pol open reading frame (ORF) was fused in frame with the FLAG epitope (International Biotechnologies Inc., New Haven, Conn.) such that the sequence Met Asp Tyr Lys Asp Asp Asp Asp Lys Leu preceded the polymerase AUG codon. Following the pol ORF, the transcript includes 3′ copies of DR1 and epsilon. The TP199 construct, described previously (1, 24), contains the first 199 amino acids of pol but lacks a FLAG epitope and all HBV sequences downstream of pol amino acid 199. FTP199 was similar to TP199 except that it contained the FLAG epitope, and FTP334 was similar to FTP199 except that it terminated at amino acid 334 (24). FΔTPL was constructed from FPL-pol by an in-frame deletion that removed pol amino acids 8 to 175, thus removing the TP domain (24). Pol 177-832, described previously (1, 24), contained pol amino acids 177 to 832 fused to an amino-terminal polyhedrin leader of four amino acids and thus lacked the FLAG epitope and the HBV sequences downstream of the pol ORF.
The deletion mutants of the TP and RT domains were created by PCR mutagenesis. In each case, a small fragment was amplified with the required changes and then used for fragment replacement into one of the vectors described above. For amino-terminal deletions of both TP and RT, the forward primer fused the FLAG epitope to the HBV sequence starting at the first amino acid number designated in the construct name (e.g., FRTn250 for FLAG-RT construct with N terminus beginning at pol amino acid 250). For carboxyl-terminal deletions of TP and RT, the reverse primer contained, 5′ to 3′, a PstI restriction site, a termination codon, and the HBV sequence starting at the terminal amino acid number designated in the construct name (e.g., FTPc300).
SDS-PAGE and immunoblot analysis.
Insect cell lysates and immunoprecipitated Pol polypeptides were disrupted in electrophoresis sample buffer containing 2% sodium dodecyl sulfate (SDS) and 2% 2-mercaptoethanol and were heated to 100°C for 5 min. Proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) as previously described (19, 20). Gels from in vitro assays for Pol function were stained with Coomassie blue, dried, and autoradiographed. For immunoblot analysis, proteins were electrophoretically transferred to a Flurotrans polyvinylidene difluoride blotting membrane (Pall Biosupport, Glen Cove, N.Y.), and membranes were processed as previously described (21). Membranes were blotted with a rabbit polyclonal antibody to full-length Pol (23) followed by 125I-protein A (NEN, Boston, Mass.). The rabbit antibody to Pol detects both TP and RT polypeptides but reacts better with the TP polypeptide. For the TP-RT binding studies, visualization of RT was enhanced by blotting with 125I-labeled 9-14 (a monoclonal antibody that recognizes the carboxyl terminus of pol [57]).
Polymerase assays.
The polymerase assays were conducted with immunoprecipitated pol polypeptides still bound to the anti-FLAG affinity beads (M2 monoclonal antibody beads; Sigma). Insect cells were infected or coinfected with baculoviruses at a multiplicity of 5 to 10. Cultures were harvested at 48 h postinfection by washing three times in phosphate-buffered saline (PBS) and extracted with PEB (PBS containing 10% glycerol, 0.5% Nonidet P-40, and protease/RNase inhibitors) as previously described (23, 24). Pol polypeptides were immunoprecipitated for 2 h at 4°C with anti-FLAG affinity beads. The beads were washed one time with TNG (100 mM Tris HCl [pH 7.5], 30 mM NaCl, 10% glycerol), one time with TNG containing 1 M NaCl, and a final time with TNM (100 mM Tris HCl [pH 7.5], 30 mM NaCl, 10 mM MgCl2). Following the final wash, the beads were suspended in TNM containing 100 μM unlabeled deoxyribonucleoside triphosphates (dATP, dGTP, and dCTP) and 5 μCi of [α-32P]TTP (3,000Ci/mmol; NEN). Assays were routinely performed at 30°C for 30 min unless stated otherwise. Following the reaction, the beads were washed a final time in PBS to remove excess labeled TTP, and pol polypeptides were eluted in SDS-gel sample buffer.
Coimmunoprecipitation assays.
Sf9 cells were infected and harvested as described above for polymerase assays, and pol polypeptides were immunoprecipitated with anti-FLAG antibodies under the same conditions except that the washes consisted of one time in PEB, one time in PEB containing 1 or 2 M NaCl (as specified) and one time in PEB. Pol polypeptides were eluted in SDS-gel sample buffer and processed for immunoblotting as described above.
RESULTS
Analysis of carboxyl-terminal deletions of TP for nucleotide priming activity in transcomplementation assays.
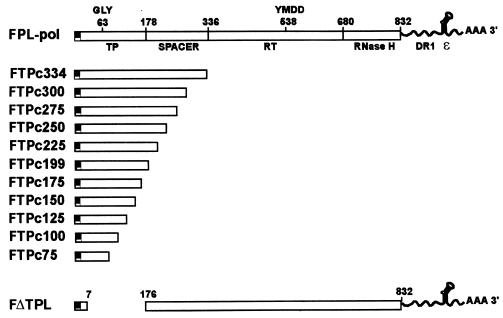
To define the minimal domain of the TP polypeptide capable of interacting with and complementing the RT domain in a transcomplementation assay for nucleotide priming and reverse transcription, we constructed a series of carboxyl-terminal deletions of the TP domain and prepared recombinant baculoviruses from each construct. We previously demonstrated that a TP polypeptide consisting of amino acids 1 to 199 was functional in this assay, while a TP polypeptide extending to amino acid 344 was inactive (24). The domain between amino acids 199 and 344 was hypothesized to contain an inhibitory region; thus, deletions were prepared to span this domain as well as much of the TP polypeptide. Each construct contained an amino-terminal FLAG epitope, followed by a TP domain which terminated between amino acids 75 and 300, progressively deleting 25 amino acids in each subsequent construct. A construct terminating at amino acid 200 was not prepared, since a TP construct terminating at 199 had been prepared previously. The constructs were designated FTPc75 through FTPc344 to indicate FLAG-TP construct with C terminus at amino acids 75 to 344 (Fig. 1).
FIG. 1.
Carboxyl-terminal deletions of the TP domain. The structures of carboxyl-terminal deletions of the TP domain are diagramed in context of the full-length pol construct, FPL-pol. Previously estimated boundaries of the TP, spacer, RT, and RNase H domains are shown for FPL-pol. A FLAG epitope is present at the amino terminus of each TP construct. The deletions remove 25 carboxyl-terminal amino acids of the TP domain at a time, from amino acids 300 to 75. The defective FTPc334 construct is shown as well. A deletion at amino acid 200 was not created, since a deletion terminating at amino acid 199 was available from previous studies. The structure of FΔTPL, which serves as the RT partner for TP constructs in transcomplementation assays, is shown at the bottom.
The constructs were tested in transcomplementation assays by coinfection of Sf9 cells with a TP mutant and the RT construct FΔTPL, which has a deletion of the TP domain and contains DR1 and epsilon at the 3′ noncoding portion of the transcript (Fig. 1). Pol polypeptides were immunoprecipitated with anti-FLAG affinity beads, nucleotide priming-reverse transcriptase assays were conducted with the polypeptides still bound to the beads, and the products (TP polypeptides with covalently attached DNA) were analyzed by SDS-PAGE and autoradiography. Although the transcomplementation assay is primarily qualitative in nature due to some variation between assays in the exact percent activity of one construct in comparison to another, where appropriate numerical comparisons have been made, and in each case, the negative results for constructs defining the functional boundaries for TP and RT were not due to decreased expression of the corresponding polypeptides, since the inactive polypeptides were visible in the Coomassie blue-stained gels from the assays (data not shown).
As previously observed (24), FTPc334 was negative in the priming assay; however, constructs terminating between amino acids 300 (FTPc300) and 199 (FTPc199) were positive in the assay (Fig. 2). FTPc175 (not clearly visible in Fig. 2) exhibited 1.3% of the priming activity observed with FTPc199. FTPc199 routinely yielded greater priming activity than the larger constructs. In this assay, FTPc300, FTPc275, FTPc250, and FTPc225 were reduced in priming activity by 80, 74, 92, and 67%, respectively, in comparison to FTP199. These data indicate that the inhibitory domain lies between amino acids 334 and 300 and that the carboxyl-terminal boundary for a functional TP polypeptide is at amino acid 175 for minimal activity and amino acid 199 for maximum activity.
FIG. 2.
Transcomplementation assays with TP carboxyl-terminal deletions. Sf9 insect cells were coinfected with baculoviruses expressing FΔTPL and the carboxyl-terminal TP deletion constructs (FTPc334 to FTPc125). Pol polypeptides were immunoprecipitated with anti-FLAG affinity beads, and nucleotide priming-reverse transcriptase reactions were conducted with the pol polypeptides still bound to the beads as described in Materials and Methods. The products (TP polypeptides with covalently attached DNA) were analyzed by SDS-PAGE and autoradiography. A single infection with FPL-pol was conducted as a positive control. Sizes are indicated in kilodaltons.
Analysis of amino-terminal deletions of TP for nucleotide priming activity in transcomplementation assays.
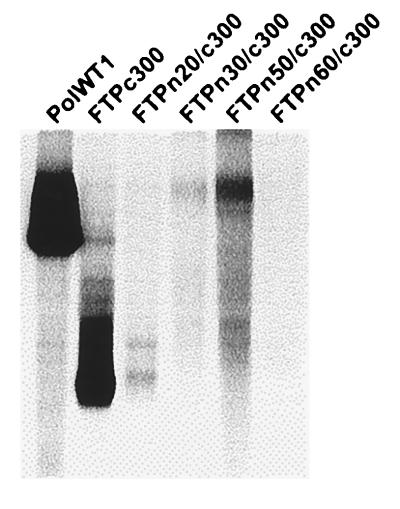
The amino-terminal boundary for TP function was determined by preparing a series of deletions that removed 20, 30, 50, and 60 amino acids from the amino terminus of the TP domain. Deletions were reconstructed into the TP construct extending to amino acid 300, FTPc300, and were designated FTPn20/c300 through FTPn60/c300 (Fig. 3). Coinfections were performed with the TP deletions and FΔTPL, and the immunoprecipitated pol polypeptides were examined in the priming assay. Deletion of 20 amino-terminal amino acids resulted in minimal nucleotide priming activity for FTPn20/300 (8% in comparison to FTPc300), and all constructs with larger deletions were negative (Fig. 4). The amino-terminal deletions were also reconstructed into full-length FPL-pol with essentially the same result: deletion of 20 amino acids resulted in minimal activity (data not shown).
FIG. 3.
Amino-terminal deletions of the TP domain. The structures of amino-terminal deletions of the TP domain are shown in context of the full-length pol construct, FPL-pol. The amino-terminal deletions were constructed in the FTPc300 construct (FTPn20/c300 through FTPn60/c300). The structure of FΔTPL, which serves as the RT partner for TP constructs in transcomplementation assays, is shown at the bottom.
FIG. 4.
Transcomplementation assays with TP amino-terminal deletions. Sf9 insect cells were coinfected with baculoviruses expressing FΔTPL and the amino-terminal TP deletion constructs (FTPn20/c300 to FTPn60/c300). Priming assays were conducted as described in the legend to Fig. 2 and Materials and Methods. FPL-pol (PolWT1) was included as a positive control.
Analysis of amino-terminal deletions of RT for nucleotide priming activity in transcomplementation assays.
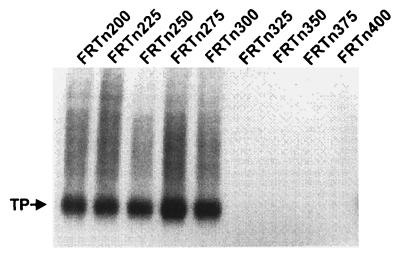
Mapping of the amino- and carboxyl-terminal limits of the RT domain was accomplished in a similar manner as for the TP domain. To map the amino-terminal boundary of the RT domain, we prepared a series of deletion mutants that fused the FLAG epitope to various locations in the RT domain, creating constructs that began at 25-amino-acid intervals within the spacer and RT domains from amino acids 200 to 400. The constructs were similar to FΔTPL in that they contained DR1 and epsilon in the 3′ noncoding region of the transcript. Recombinant baculoviruses created for the constructs were designated FRTn200L through FRTn400L (Fig. 5). Transcomplementation assays were performed by coinfection with the RT deletion constructs and FTPc199. All deletions from FRTn200 to FRTn300 exhibited similar activities in the priming assay, while deletions beyond amino acid 300 were negative (Fig. 6). These data indicate that the amino-terminal boundary of the RT domain in transcomplementation assays extends into the spacer domain and contains the inhibitory region observed in the analysis of the TP constructs.
FIG. 5.
Amino-terminal deletions of the RT domain. The structures of amino-terminal deletions of the RT domain are diagramed in context of the full-length pol construct, FPL-pol. A FLAG epitope is present at the amino terminus of each RT construct, and epsilon (ɛ) and DR1 sequences are present in the 3′ noncoding region of the transcripts. The structure of FTPc199, which serves as the TP partner for RT constructs in transcomplementation assays, is shown at the bottom.
FIG. 6.
Transcomplementation assays with RT amino-terminal deletions. Sf9 insect cells were coinfected with baculoviruses expressing FTPc199 and the amino-terminal RT deletion constructs (FRTn200 through FRTn400). Priming assays were conducted as described in the legend to Fig. 2 and Materials and Methods.
Analysis of carboxyl-terminal deletions of RT for nucleotide priming activity in transcomplementation assays.
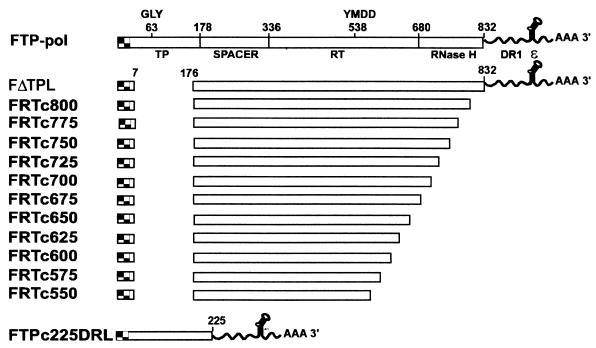
To map the carboxyl-terminal boundary of the RT domain, we prepared a series of deletion mutants in which we progressively removed the terminus of the FΔTPL construct, 25 amino acids per deletion, from amino acids 800 to 550 (FRTc800 to FRTc550 [Fig. 7]). Since the YMDD consensus motif for reverse transcriptases maps to amino acid 538, deletion beyond 550 was not deemed necessary. A new TP construct was made for the transcomplementation assays with this deletion series, since deletion of DR1 and epsilon from the end of the FΔTPL construct necessitated the addition of these elements to the TP partner. We have previously demonstrated the requirement for epsilon in the transcomplementation assay and the ability to provide epsilon on either the TP construct or in trans from a third baculovirus (24). FTPc225 was altered by the addition of DR1 and epsilon to the 3′ noncoding region of the transcript to create FTPc225DRL. In transcomplementation assays, removal of the terminal 32 amino acids of pol (FRTc800) resulted in a 25% decrease in priming activity in comparison to FΔTPL, while deletion to amino acid 775 resulted in a 66% decrease in priming activity. Deletion to amino acid 750 completely abolished priming activity (Fig. 8). Although not shown in Fig. 8, deletions from amino acids 700 to 550 were also negative in this assay. These results indicate that the majority of the RNase H domain is required as a component of the RT construct for priming activity in the transcomplementation assay.
FIG. 7.
Carboxyl-terminal deletions of the RT domain. The structures of carboxyl-terminal deletions of the RT domain are diagramed in context of the full-length pol construct, FPL-pol. The carboxyl-terminal deletions were constructed into the FΔTPL vector and thus have a deletion in the TP domain. A FLAG epitope is present at the amino terminus of each RT construct. The structure of FTPc225DRL, which serves as the TP partner for RT constructs in transcomplementation assays, is shown at the bottom. This TP construct contains epsilon (ɛ) and DR1 sequences in the 3′ noncoding region of the transcript, since these elements were deleted from the carboxyl-terminal RT deletions.
FIG. 8.
Transcomplementation assays with RT carboxyl-terminal deletions. Sf9 insect cells were coinfected with baculoviruses expressing FTPc199 and the carboxyl-terminal RT deletion constructs (FRTc800 through FRTc725). Priming reactions were conducted as described in the legend to Fig. 2 and Materials and Methods. A single infection with FPL-pol (PolWT1) and a transcomplementation with FTPc199 and FΔTPL were included as positive controls.
Mapping of the minimal TP domain capable of protein-protein interaction with RT.
The analysis of TP and RT deletion mutants in transcomplementation assays provided information with regard to the minimal boundaries for a functional interaction between these domains in nucleotide priming and reverse transcriptase activities. The failure to form a functional complex in a transcomplementation assay could be due to a number of factors, including lack of association with epsilon or essential host factors, lack of interaction between TP and RT, or deletion of an essential portion of the polypeptides for a correct conformational interaction. To determine whether functionally inactive polypeptides were still capable of forming a stable TP-RT complex, coimmunoprecipitation assays between TP and RT were conducted. These analyses were performed by coinfection of Sf9 cells with TP and RT deletion mutants, followed by immunoprecipitation with anti-FLAG antibodies and Western blot analysis of the immunoprecipitates for TP and RT polypeptides. Since immunoprecipitation was performed with anti-FLAG antibodies, constructs were chosen such that one partner in the assay, TP or RT, lacked the FLAG epitope.
The binding of RT to TP deletion mutants used the Pol 177-832 construct (1, 24), which lacks the FLAG epitope and DR1 and epsilon sequences downstream of the pol ORF. To reduce nonspecific precipitation of RT with the anti-FLAG affinity beads, the second of three washes contained 2 M NaCl; thus, only strong interactions between TP and RT could be detected in this assay. Under these conditions, Pol 177-832 specifically coprecipitated with FTPc300 through FTPc175 (Fig. 9). The level of RT coprecipitation with FTPc175 was 45% of the level observed with FTPc300, and no binding of RT to deletions beyond amino acid 175 could be detected (data not shown). These results are similar to what was observed in the transcomplementation analysis, suggesting that the inactivity of the TP polypeptides deleted beyond amino acid 175 was due to a failure to associate with RT; however, a weak interaction between RT and these polypeptides cannot be ruled out.
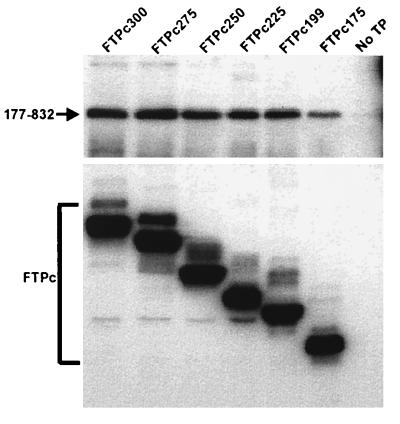
FIG. 9.
Coimmunoprecipitation of RT with carboxyl-terminal deletions of TP. Sf9 insect cells were coinfected with baculoviruses expressing an RT construct lacking a FLAG epitope, Pol 177–832, and the carboxyl-terminal TP deletion constructs (FTPc300 to FTPc175). TP polypeptides were immunoprecipitated with anti-FLAG affinity beads and analyzed for the coimmunoprecipitation of Pol 177–832 by SDS-PAGE and Western blotting. A single infection with Pol 177–832 (no TP) served as a negative control for nonspecific precipitation of RT. Western blotting was performed for the detection of both TP and RT polypeptides as described in Materials and Methods.
Each of the amino-terminal TP deletions specifically coprecipitated Pol 177–832 (Fig. 10). The level of RT coprecipitated by the amino-terminal deletions in comparison to FTPc300 progressively decreased; 65% (FTPn20), 45% (FTPn30), 45% (FTPn50), and 7.5% (FTPn60). The migration of FTPn20/c300 in the SDS-gels was anomalous in comparison to the migration of FTPc300 for unknown reasons, but sequence analysis confirmed that the construct was accurate. The anomalous migration could be due to differences in phosphorylation. Phosphorylation of the TP domain of pol has been previously described (1). These results indicate that the inactivity of the amino-terminal deletions of TP in transcomplementation assays was not due to an inability to associate with RT.
FIG. 10.
Coimmunoprecipitation of RT with amino-terminal deletions of TP. Sf9 insect cells were coinfected with baculoviruses expressing an RT construct lacking a FLAG epitope, pol 177–832, and the amino-terminal TP deletion constructs (FTPn20/c300 to FTPn60/c300). Undeleted FTPc300 and FTPc199 served as positive controls. TP polypeptides were immunoprecipitated with anti-FLAG affinity beads and analyzed for the coimmunoprecipitation of Pol 177–832 by SDS-PAGE and Western blotting. A single infection with Pol 177–832 (no TP) served as a negative control for nonspecific precipitation of RT. Western blotting was performed for the detection of both TP and RT polypeptides as described in Materials and Methods.
Mapping of the minimal RT domain capable of protein-protein interaction with TP.
Analysis of the RT deletion mutants employed coinfection with TP199, which lacks a FLAG epitope. To reduce nonspecific precipitation of TP with the anti-FLAG affinity beads, immunoprecipitates were washed with 1 M NaCl. Each of the RT carboxyl-terminal deletions specifically precipitated TP199 (Fig. 11). A decrease in the level of TP199 precipitated by the RT carboxyl-terminal deletion mutants beyond amino acid 700 was observed with the progressive deletions, ranging from 56% (FRTc675) to 14% (FRTc600) of the level of FRTc700. However, in a separate experiment, TP199 still clearly coprecipitated with the smallest RT polypeptide, FRTc550. Each of the amino-terminal deletions of RT specifically precipitated TP199 as well, although the level of TP199 coprecipitated with progressive deletions was reduced, with FRTn400L precipitating only 15% the level of TP precipitated by FRTc300L (Fig. 12). These results indicate that the failure of RT amino-terminal deletions beyond amino acid 300 and RT carboxyl-terminal deletions beyond amino acid 775 to function with TP in transcomplementation assays was not due to an inability to form a stable complex with TP.
FIG. 11.
Coimmunoprecipitation of TP with carboxyl-terminal deletions of RT. Sf9 insect cells were coinfected with baculoviruses expressing a TP construct lacking a FLAG epitope, TP199, and the carboxyl-terminal RT deletion constructs (FRTc775 to FRTc550). Undeleted FΔTPL served as a positive control. RT polypeptides were immunoprecipitated with anti-FLAG affinity beads and analyzed for the coimmunoprecipitation of TP199 by SDS-PAGE and Western blotting. A single infection with TP199 (no RT) served as a negative control for nonspecific precipitation of TP. Western blotting was performed for the detection of both TP and RT polypeptides as described in Materials and Methods.
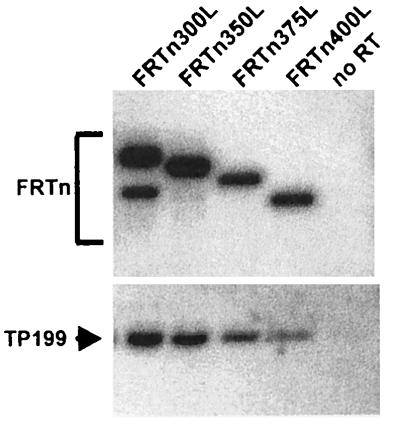
FIG. 12.
Coimmunoprecipitation of TP with amino-terminal deletions of RT. Sf9 insect cells were coinfected with baculoviruses expressing a TP construct lacking a FLAG epitope, TP199, and the amino-terminal RT deletion constructs (FRTn225L to FRTn400L). RT polypeptides were immunoprecipitated with anti-FLAG affinity beads and analyzed for the coimmunoprecipitation of TP199 by SDS-PAGE and Western blotting. A single infection with TP199 (no RT) served as a negative control for nonspecific precipitation of TP. Western blotting was performed for the detection of both TP and RT polypeptides as described in Materials and Methods.
DISCUSSION
Many viruses have proteins covalently bound to the 5′ ends of their RNA or DNA genomes. In most instances, these terminal proteins are involved in the protein priming of genome replication (reviewed in reference 36). The terminal protein provides a 3′ OH from a serine, threonine, or tyrosine to replace the requirement for a 3′ OH from an RNA primer to initiate nucleic acid synthesis. The TP function of hepadnaviruses is unique in that it is part of the same polypeptide as the polymerase, which in this case is a reverse transcriptase. Such an organizational scheme dictates that the polypeptide fold in a manner that achieves intimate contact between the TP and RT domains in order to introduce the OH group of a tyrosine into the catalytic pocket of the reverse transcriptase.
Previously, we observed that full-length HBV pol proteins with mutations in the TP and RT domains are unable to complement each other in nucleotide priming reactions (24). This observation may have been predicted, since intramolecular interaction of TP and RT would be favored over intermolecular interaction between two different pol polypeptides, but it demonstrated that the TP and RT domains had specific, stable interactions. In fact, very strong interactions between TP and RT were observed when these proteins were expressed independently, and their interaction resulted in the reconstitution of a functional TP-RT complex that also required epsilon for in vitro nucleotide priming activity (24). In this study, we have exploited this complementation system to map the boundaries of the TP and RT domains required for the formation of a functional complex, as well as for protein-protein interactions. Our analyses relied on the construction of multiple baculoviruses expressing truncated TP and RT polypeptides. A total of 46 viruses were used in the analysis, making it one of the most extensive mutagenesis studies performed with the baculovirus system.
Some of the results from transcomplementation analysis were consistent with previous designations of the TP and RT domains based on conservation of sequence among hepadnaviruses, homology to retroviruses and analysis of pol mutants in HBV replication (35). However, the previous studies could not estimate the actual requirement of these domains in priming activity. The proposed TP domain was assigned to the entire sequence amino terminal of the spacer domain, since it was covalently linked to the minus strand of DNA. In our analysis, which specifically examined TP function, the minimal TP domain was mapped to amino acids 20 through 175 (Fig. 13); however, maximum activity required amino acids 1 to 199. Since the known function of TP is to provide a primer for the catalytic domain, a small peptide could likely have provided this function. The fact that 200 amino acids were required for this function suggests that the TP domain may function in other aspects required for priming activity such as template recognition or interaction with required cellular factors.
FIG. 13.
Minimal TP and RT domains of HBV polymerase. The functional domains of pol are shown in context of full-length HBV polymerase. In addition, the boundaries are shown for the minimal TP and RT domains functional in a transcomplementation assay for protein-primed reverse transcription (Priming) and the minimal TP and RT domains capable of protein-protein interaction in a binding assay (Binding). The inhibitory domain that inhibits transcomplementation when part of the TP domain is shown as a black box above the minimal RT domains.
The minimal RT domain spanned residues 300 to 775 (Fig. 13). Thus, a portion of the spacer domain was required for both TP and RT function. These results are consistent with the defect in HBV replication noted in mutants with an insertion at amino acid 178 and a deletion between amino acids 293 and 335 (35), although the defect in these mutants could not be ascribed directly to a loss in priming function. The more important finding in the analysis of the RT domain was the requirement for the RNase H domain. It is unlikely that the RNase H function is directly involved in priming activity, but the RNase H domain may aid in stabilizing the TP-RT interaction or interactions with the template or cellular proteins. Point mutations in the RNase H domain disrupt the packaging of pol with pregenomic RNA (2, 8, 9, 13) and efficient elongation of minus-strand DNA synthesis (7), suggesting that the RNase H domain may be a participant in several steps of genomic replication.
Differences were noted between the results of this study and those obtained with DHBV pol in priming assays following in vitro translation. The most notable difference was in the requirement for the RNase H domain for transcomplementation. DHBV pol could be deleted to amino acid 568 without loss of priming function (34), while deletion of the HBV RT domain to amino acid 750 resulted in complete loss of activity. Although the amino acid sequences of HBV and DHBV pol are not highly conserved, the sequences can be partially aligned by making a number of deletions and insertions (35). The alignment suggests that HBV pol should tolerate a COOH-terminal deletion to amino acid 607 and still retain priming activity. These differences may underscore a fundamental divergence between the avian and human viruses; however, unlike the DHBV studies that employed truncations of full-length pol polypeptides, transcomplementation requires the formation of a stable complex between TP and RT. Deletions at the amino terminus of DHBV pol and the HBV TP domain exhibited differences as well. While DHBV pol could be deleted to amino acid 74 without deleterious effects on priming activity (52), deletion of HBV TP to amino acid 20 almost completely abolished priming activity. In this case, the differences cannot be attributed to the additional constraints of the transcomplementation assay, since amino-terminal deletion of full-length HBV pol to amino acid 20 also resulted in loss of priming activity. Alignment of DHBV and HBV pol sequences suggests that HBV pol should tolerate an NH2-terminal deletion of 41 amino acids, indicating that fundamental differences do exist in the manner in which the TP and RT domains of these two viruses interact.
Analysis of TP truncations also provided more concise mapping of the inhibitory domain previously noted with TP proteins extending to amino acid 334. In this study, the inhibitory domain was mapped to amino acids 300 through 334, but this provided no additional information with regard to the nature of the inhibition. Gross misfolding of the FTPc334 polypeptide is unlikely, because it was still capable of binding to RT. This domain is within the minimal RT polypeptide functional in transcomplementation with TP (Fig. 13). One possibility is that this domain contains sites in which RT normally binds to TP and that when present on TP, they result in intradomain interactions that prevent correct alignment between TP and RT without completely eliminating binding.
In addition to mapping the minimal TP and RT domains required for a functional interaction, this approach allowed an evaluation of the minimal domains required for protein-protein interactions between TP and RT. Although large domains were required for priming activity, the minimal binding domains could be reduced to 115 and 150 amino acids for TP and RT, respectively. Even smaller domains may be competent for binding, since in most cases even the most severely truncated polypeptides were capable of interaction. The COOH terminus of TP was the only domain for which deletions extended beyond the last polypeptide positive in the coimmunoprecipitation assay. Nonetheless, the data from four series of deletion mutants suggest that the binding domains can be reduced to amino acids 60 to 175 of TP and amino acids 400 to 550 of RT (Fig. 13). Binding within these domains was not unexpected, since the tyrosine at amino acid 63 must interact with the RT catalytic site at amino acids 538 to 541 (YMDD). These studies do not exclude the possibility of additional contact sites outside of these boundaries and/or multiple contact sites within these domains. Fine mapping of the contact sites between TP and RT will require additional studies.
The differences between the minimal functional and the minimal binding domains of TP and RT suggest that the surrounding sequences may be involved in other interactions required for priming activity. Although required for priming, epsilon was not required for TP-RT binding. The domain of pol involved in epsilon recognition is still undefined and is likely to be a complex interaction, since studies with DHBV pol suggest that Hsp90 is involved in epsilon binding (15) and packaging of the pol-RNA complex (16). The binding of epsilon to pol is accompanied by a conformational change in pol that is associated with the acquisition of polymerase activity (48). Additional interactions of pol with the core protein are presumably required for packaging, and mutagenesis of the core ORF suggests that interactions with core may be required for multiple steps in genome replication (5, 32, 55). During replication, pol must proceed through a series of interactions from a packaging reaction, to a priming complex, to recognition of DR1, and eventually to primer translocation to DR2. These events no doubt require multiple changes in conformation, posttranslational modifications, and/or interaction with viral and host proteins. Further exploitation of the transcomplementation assay between TP and RT should be useful in delineating the numerous interactions required of pol during genome replication.
ACKNOWLEDGMENT
This work was supported by grant CA53246 from the National Institutes of Health.
REFERENCES
- 1.Ayola B, Kanda P, Lanford R E. High level expression and phosphorylation of hepatitis B virus polymerase in insect cells with recombinant baculoviruses. Virology. 1993;194:370–373. doi: 10.1006/viro.1993.1270. [DOI] [PubMed] [Google Scholar]
- 2.Bartenschlager R, Junker-Niepmann M, Schaller H. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J Virol. 1990;64:5324–5332. doi: 10.1128/jvi.64.11.5324-5332.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartenschlager R, Schaller H. The amino-terminal domain of the hepadnaviral P-gene encodes the terminal protein (genome-linked protein) believed to prime reverse transcription. EMBO J. 1988;7:4185–4192. doi: 10.1002/j.1460-2075.1988.tb03315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartenschlager R, Schaller H. Hepadnaviral assembly is initiated by polymerase binding to the encapsidation signal in the viral RNA genome. EMBO J. 1992;11:3413–3420. doi: 10.1002/j.1460-2075.1992.tb05420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beames B, Lanford R E. Carboxy-terminal truncations of the HBV core protein affect capsid formation and size of the encapsidated HBV RNA. Virology. 1993;194:597–607. doi: 10.1006/viro.1993.1299. [DOI] [PubMed] [Google Scholar]
- 6.Bosch V, Bartenschlager R, Radziwill G, Schaller H. The duck hepatitis B virus P-gene codes for protein strongly associated with the 5′-end of the viral DNA minus strand. Virology. 1988;166:475–485. doi: 10.1016/0042-6822(88)90518-1. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Marion P L. Amino acids essential for RNase H activity of hepadnaviruses are also required for efficient elongation of minus-strand viral DNA. J Virol. 1996;70:6151–6156. doi: 10.1128/jvi.70.9.6151-6156.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Robinson W S, Marion P L. Naturally occurring point mutation in the C terminus of the polymerase gene prevents duck hepatitis B virus RNA packaging. J Virol. 1992;66:1282–1287. doi: 10.1128/jvi.66.2.1282-1287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Robinson W S, Marion P L. Selected mutations of the duck hepatitis B virus P gene RNase H domain affect both RNA packaging and priming of minus-strand DNA synthesis. J Virol. 1994;68:5232–5238. doi: 10.1128/jvi.68.8.5232-5238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Condreay L D, Wu T-T, Aldrich C E, Delaney M A, Summers J, Seeger C, Mason W S. Replication of DHBV genomes with mutations at the sites of initiation of minus- and plus-strand DNA synthesis. Virology. 1992;188:208–216. doi: 10.1016/0042-6822(92)90751-a. [DOI] [PubMed] [Google Scholar]
- 11.Galibert F, Mandart E, Fitoussi F, Tiollais P, Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979;281:646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- 12.Ganem D, Varmus H E. The molecular biology of the hepatitis B viruses. Annu Rev Biochem. 1987;56:651–693. doi: 10.1146/annurev.bi.56.070187.003251. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch R C, Lavine J E, Chang L-J, Varmus H E, Ganem D. Polymerase gene products of hepatitis B viruses are required for genomic RNA packaging as well as for reverse transcription. Nature. 1990;344:552–555. doi: 10.1038/344552a0. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch R C, Loeb D D, Pollack J R, Ganem D. cis-acting sequences required for encapsidation of duck hepatitis B virus pregenomic RNA. J Virol. 1991;65:3309–3316. doi: 10.1128/jvi.65.6.3309-3316.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu J M, Seeger C. Hsp90 is required for the activity of a hepatitis B virus reverse transcriptase. Proc Natl Acad Sci USA. 1996;93:1060–1064. doi: 10.1073/pnas.93.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu J M, Toft D O, Seeger C. Hepadnavirus assembly and reverse transcription require a multi-component chaperone complex which is incorporated into nucleocapsids. EMBO J. 1997;16:59–68. doi: 10.1093/emboj/16.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Junker-Niepmann M, Bartenschlager R, Schaller H. A short cis-acting sequence is required for hepatitis B virus pregenome encapsidation and sufficient for packaging of foreign RNA. EMBO J. 1990;9:3389–3396. doi: 10.1002/j.1460-2075.1990.tb07540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knaus T, Nassal M. The encapsidation signal on the hepatitis B virus RNA pregenome forms a stem-loop structure that is critical for its function. Nucleic Acids Res. 1993;21:3967–3975. doi: 10.1093/nar/21.17.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lanford R E, Butel J S. Antigenic relationship of SV40 early proteins to purified large T polypeptide. Virology. 1979;97:295–306. doi: 10.1016/0042-6822(79)90341-6. [DOI] [PubMed] [Google Scholar]
- 21.Lanford R E, Carey K D, Estlack L E, Smith G C, Hay R V. Analysis of plasma protein and lipoprotein synthesis in long-term primary cultures of baboon hepatocytes maintained in serum-free medium. In Vitro Cell Dev Biol. 1989;25:174–182. doi: 10.1007/BF02626175. [DOI] [PubMed] [Google Scholar]
- 22.Lanford R E, Notvall L. Expression of hepatitis B virus core and precore antigens in insect cells and characterization of a core-associated kinase activity. Virology. 1990;176:222–233. doi: 10.1016/0042-6822(90)90247-o. [DOI] [PubMed] [Google Scholar]
- 23.Lanford R E, Notvall L, Beames B. Nucleotide priming and reverse transcriptase activity of hepatitis B virus polymerase expressed in insect cells. J Virol. 1995;69:4431–4439. doi: 10.1128/jvi.69.7.4431-4439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lanford R E, Notvall L, Lee H, Beames B. Transcomplementation of nucleotide priming and reverse transcription between independently expressed TP and RT domains of the hepatitis B virus reverse transcriptase. J Virol. 1997;71:2996–3004. doi: 10.1128/jvi.71.4.2996-3004.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien J-M, Aldrich C E, Mason W S. Evidence that a capped oligoribonucleotide is the primer for duck hepatitis B virus plus-strand DNA synthesis. J Virol. 1986;57:229–236. doi: 10.1128/jvi.57.1.229-236.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lien J, Petcu D J, Aldrich C E, Mason W S. Initiation and termination of duck hepatitis B virus DNA synthesis during virus maturation. J Virol. 1987;61:3832–3840. doi: 10.1128/jvi.61.12.3832-3840.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loeb D D, Ganem D. Reverse transcription pathway of the hepatitis B viruses. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 329–355. [Google Scholar]
- 28.Loeb D D, Hirsch R C, Ganem D. Sequence-independent RNA cleavages generate the primers for plus strand DNA synthesis in hepatitis B viruses: implications for other reverse transcribing elements. EMBO J. 1991;10:3533–3540. doi: 10.1002/j.1460-2075.1991.tb04917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luckow V A, Lee S C, Barry G F, Olins P O. Efficient generation of infectious recombinant baculoviruses by site-specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67:4566–4579. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molnar-Kimber K L, Summers J, Taylor J M, Mason W S. Protein covalently bound to minus-strand DNA intermediates of duck hepatitis B virus. J Virol. 1983;45:165–172. doi: 10.1128/jvi.45.1.165-172.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Molnar-Kimber K L, Summers J W, Mason W S. Mapping of the cohesive overlap of duck hepatitis B virus DNA and of the site of initiation of reverse transcription. J Virol. 1984;51:181–191. doi: 10.1128/jvi.51.1.181-191.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nassal M. The arginine-rich domain of the hepatitis B virus core protein is required for pregenome encapsidation and productive viral positive-strand DNA synthesis but not for virus assembly. J Virol. 1992;66:4107–4116. doi: 10.1128/jvi.66.7.4107-4116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollack J R, Ganem D. An RNA stem-loop structure directs hepatitis B virus genomic RNA encapsidation. J Virol. 1993;67:3254–3263. doi: 10.1128/jvi.67.6.3254-3263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollack J R, Ganem D. Site-specific RNA binding by a hepatitis B virus reverse transcriptase initiates two distinct reactions: RNA packaging and DNA synthesis. J Virol. 1994;68:5579–5587. doi: 10.1128/jvi.68.9.5579-5587.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radziwill G, Tucker W, Schaller H. Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol. 1990;64:613–620. doi: 10.1128/jvi.64.2.613-620.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salas M. Protein-priming of DNA replication. Annu Rev Biochem. 1991;60:39–71. doi: 10.1146/annurev.bi.60.070191.000351. [DOI] [PubMed] [Google Scholar]
- 37.Seeger C, Ganem D, Varmus H E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986;232:477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- 38.Seeger C, Maragos J. Molecular analysis of the function of direct repeats and a polypurine tract for plus-strand DNA priming in woodchuck hepatitis virus. J Virol. 1989;63:1907–1915. doi: 10.1128/jvi.63.5.1907-1915.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seeger C, Maragos J. Identification and characterization of the woodchuck hepatitis virus origin of DNA replication. J Virol. 1990;64:16–23. doi: 10.1128/jvi.64.1.16-23.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seeger C, Maragos J. Identification of a signal necessary for initiation of reverse transcription of the hepadnavirus genome. J Virol. 1991;65:5190–5195. doi: 10.1128/jvi.65.10.5190-5195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seeger C, Summers J, Mason W S. Viral DNA synthesis. Curr Top Microbiol Immunol. 1991;168:41–60. doi: 10.1007/978-3-642-76015-0_3. [DOI] [PubMed] [Google Scholar]
- 42.Seifer M, Hamatake R, Bifano M, Standring D N. Generation of replication-competent hepatitis B virus nucleocapsids in insect cells. J Virol. 1998;72:2765–2776. doi: 10.1128/jvi.72.4.2765-2776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seifer M, Standring D N. Recombinant human hepatitis B virus reverse transcriptase is active in the absence of the nucleocapsid or the viral replication origin, DR1. J Virol. 1993;67:4513–4520. doi: 10.1128/jvi.67.8.4513-4520.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staprans S, Loeb D D, Ganem D. Mutations affecting hepadnavirus plus-strand DNA synthesis dissociate primer cleavage from translocation and reveal the origin of linear viral DNA. J Virol. 1991;65:1255–1262. doi: 10.1128/jvi.65.3.1255-1262.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summers J, Mason W S. Replication of the genome of a hepatitis B-like virus by reverse transcription of an RNA intermediate. Cell. 1982;29:403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- 46.Summers M D, Smith G E. A manual of methods for baculovirus vectors and insect cell culture procedures. Tex Agric Exp Stn Bull. 1987;1555:1–48. , plus appendix. [Google Scholar]
- 47.Tavis J E, Ganem D. Expression of functional hepatitis B virus polymerase in yeast reveals it to be the sole viral protein required for correct initiation of reverse transcription. Proc Natl Acad Sci USA. 1993;90:4107–4111. doi: 10.1073/pnas.90.9.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tavis J E, Massey B, Gong Y H. The duck hepatitis B virus polymerase is activated by its RNA packaging signal, ɛ. J Virol. 1998;72:5789–5796. doi: 10.1128/jvi.72.7.5789-5796.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tavis J E, Perri S, Ganem D. Hepadnavirus reverse transcription initiates within the stem-loop of the RNA packaging signal and employs a novel strand transfer. J Virol. 1994;68:3536–3543. doi: 10.1128/jvi.68.6.3536-3543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang G-H, Seeger C. The reverse transcriptase of hepatitis B virus acts as a protein primer for viral DNA synthesis. Cell. 1992;71:663–670. doi: 10.1016/0092-8674(92)90599-8. [DOI] [PubMed] [Google Scholar]
- 51.Wang G-H, Seeger C. Novel mechanism for reverse transcription in hepatitis B viruses. J Virol. 1993;67:6507–6512. doi: 10.1128/jvi.67.11.6507-6512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang G-H, Zoulim F, Leber E H, Kitson J, Seeger C. Role of RNA in enzymatic activity of the reverse transcriptase of hepatitis B viruses. J Virol. 1994;68:8437–8442. doi: 10.1128/jvi.68.12.8437-8442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber M, Bronsema V, Bartos H, Bosserhoff A, Bartenschlager R, Schaller H. Hepadnavirus P protein utilizes a tyrosine residue in the TP domain to prime reverse transcription. J Virol. 1994;68:2994–2999. doi: 10.1128/jvi.68.5.2994-2999.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Will H, Reiser W, Weimer T, Pfaff E, Büscher M, Sprengel R, Cattaneo R, Schaller H. Replication strategy of human hepatitis B virus. J Virol. 1987;61:904–911. doi: 10.1128/jvi.61.3.904-911.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu M, Summers J. A domain of the hepadnavirus capsid protein is specifically required for DNA maturation and virus assembly. J Virol. 1991;65:2511–2517. doi: 10.1128/jvi.65.5.2511-2517.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zoulim F, Seeger C. Reverse transcription in hepatitis B viruses is primed by a tyrosine residue of the polymerase. J Virol. 1994;68:6–13. doi: 10.1128/jvi.68.1.6-13.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zu Putlitz, J., and R. E. Lanford. Unpublished data.