Abstract
Background ABP 980 is a biosimilar antibody to reference trastuzumab (RTZ). Aim of the following study is to confirm the similarity of ABP 980 and RTZ in terms of clinical efficacy and safety in patients with HER2-positive early breast cancer (EBC) undergoing neoadjuvant trastuzumab-containing chemotherapy in a clinical real-world situation that also includes patients receiving pertuzumab.
Methods Patients with HER2-positive EBC, who were treated from 12/2010 to 03/2020 at the Department of Women’s Health at Tuebingen University Hospital, Germany, with at least four cycles of neoadjuvant chemotherapy (+/− pertuzumab) in combination with ABP 980 or RTZ were included in a retrospective analysis. For efficacy analysis patients achieving a pathologic complete remission (pCR = no invasive tumor in breast and lymph nodes) were compared. Safety was evaluated by comparing the number of patients with a decrease in left ventricular function (LVEF) of > 10%.
Results 124 patients were included of whom 46 (37.1%) have received ABP 980 and 77 (62.9%) were treated with RTZ. A pCR was found in 77 patients (62.1%). For patients treated with ABP 980 as compared to RTZ, there was no significant difference regarding efficacy (pCR-rates of 60.9% versus 62.8%, p = 0.829) or cardiac safety (LVEF decline in 6.5% versus 2.6%, p = 0.274).
Conclusion Similarity of ABP 980 as compared to RTZ was confirmed in a real-world situation, including a large proportion of patients that have also received pertuzumab treatment.
Keywords: trastuzumab, biosimilar, primary systemic therapy, pathological complete response
Zusammenfassung
Hintergrund ABP 980 ist ein Biosimilar-Antikörper von Referenz-Trastuzumab (RTZ). Ziel dieser Studie war es, die Ähnlichkeit von ABP 980 mit RTZ zu bestätigen in Bezug auf die klinische Wirksamkeit und Sicherheit in Patientinnen mit HER2-positivem Brustkrebs im Frühstadium, die sich einer neoadjuvanten Chemotherapie mit Trastuzumab unterziehen. Die Studie sollte reale klinische Bedingungen abbilden und schloss somit auch Patientinnen ein, die Pertuzumab erhielten.
Methoden Patientinnen mit HER2-positivem Brustkrebs im Frühstadium, die zwischen 12/2010 und 03/2020 in der Frauenklinik des Universitätsklinikums Tübingen, Deutschland, mindestens 4 Zyklen einer neoadjuvanten Chemotherapie (+/− Pertuzumab) in Kombination mit ABP 980 oder RTZ erhielten, wurden in diese retrospektive Analyse aufgenommen. Für die Wirksamkeitsanalyse wurden Patientinnen mit pathologischer Komplettremission (pCR = kein invasiver Tumor in der Brust und negativer Lymphknotenstatus) verglichen. Zur Beurteilung der Sicherheit von ABP 980 wurde die Anzahl an Patientinnen, die eine Minderung ihrer linksventrikulären Funktion (LVEF) von mehr als 10% aufwiesen, verglichen.
Ergebnisse Insgesamt wurden 124 Patientinnen in die Studie aufgenommen. Davon erhielten 46 (37,1%) Frauen ABP 980, und 77 (62,9%) Patientinnen wurden mit RTZ behandelt. Eine pCR stellte sich bei 77 (62,1%) Patientinnen ein. Es gab keinen signifikanten Unterschied bezüglich der klinischen Wirksamkeit zwischen mit ABP 980 behandelten Patientinnen und den mit RTZ behandelten Patientinnen (die jeweiligen pCR-Raten betrugen 60,9% bzw. 62,8%, p = 0,829), auch nicht in Bezug auf die kardiologische Sicherheit (eine LVEF-Minderung wurde bei 6,5% bzw. 2,6% der Fälle vermerkt, p = 0,274).
Schlussfolgerung Die Ähnlichkeit von ABP 980 und RTZ in einer realen klinischen Situation, die auch eine große Anzahl an mit Pertuzumab behandelten Patientinnen einschloss, konnte bestätigt werden.
Schlüsselwörter: Trastuzumab, Biosimilar, primäre systemische Therapie, pathologische Komplettremission
Introduction
Overexpression of the human epidermal growth factor receptor 2 (HER2) occurs in about 15–20 % of breast cancer and is associated with high risk of recurrence and metastasis 1 2 . Trastuzumab is a humanized antibody against HER2, its addition to chemotherapy significantly improves outcome in metastatic and early breast cancer (EBC) 3 4 5 6 7 8 and the use is inherent part of daily clinical practise 9 10 11 . When given in combination with chemotherapy prior to surgery (neoadjuvant therapy, NAT), the proportion of pathological complete responses (pCR) merely doubles, as compared to chemotherapy alone 12 13 . Since pCR is a strong predictor of prognosis, especially in the subgroup of HER2-positive patients, it is commonly used by clinicians to guide treatment decisions and as an established endpoint in clinical trials 14 15 . Moreover, it was recently shown that the addition of a second HER2-directed antibody, pertuzumab, further enhances the efficacy of anti-HER2 therapy in terms of outcome and achievement of pCR 16 17 18 19 .
Biosimilars are an emerging group of substances, which have high potential to facilitate access to targeted oncological therapy by lowering the financial burden on healthcare budgets. The exact definition by the European Medicines Agency reads as follows: “a biological medicinal product, that contains a version of the active substance of an already authorised original biological medicinal product in the European Economic Area.” 20 .
Given the impossibility to produce an absolutely identical product compared to the originator, which is due to complex manufacturing processes of macromolecules, an abbreviated approval pathway has been generated to accelerate the approval of biosimilars 21 22 . At the end of this pathway a comparative clinical efficacy study is performed to confirm the clinical equivalence between the biosimilar and its reference product. These studies should be conducted in a sensitive population to subsequently allow extrapolation to other indications of the reference product 23 24 .
ABP 980 is a biosimilar antibody to reference trastuzumab (RTZ) that showed no differences in terms of structure, function and pharmacokinetic profile 25 26 . In the LILAC study, patients were randomized to receive NAT that contained four cycles of either ABP 980 or RTZ in combination with standard anthracycline and taxane containing chemotherapy 27 . After surgery, adjuvant treatment with ABP 980 or RTZ was continued for up to 1 year. Similar rates of pCR were observed indicating similar efficacy, while the safety outcome in both, the neoadjuvant and the adjuvant phases, was also comparable between treatment arms.
Due to the abbreviated approval steps continuous clinical monitoring of biosimilars in everyday clinical practice and an appropriate introduction of the substance is important, especially in treatment situations that have not been investigated in the registration studies (e.g. other therapy lines or other therapy combinations) 28 . We therefore investigated the pCR rate of NAT containing RTZ versus ABP 980 in the clinical routine of a large German breast cancer center that also includes the combination of RTZ and ABP 980, respectively, with pertuzumab. Additionally, safety was investigated in the context of cardiac events and impaired left ventricular ejection fraction (LVEF).
Patients and Methods
Study Population
Patients with early HER2-positive breast cancer, who received NAT containing RTZ or ABP 980 between 12/2010 and 03/2020 at the Department of Women’s Health at Tuebingen University Hospital, Germany, were included in a retrospective analysis. From 12/2010 to 05/2018 patients were treated with RTZ and from 06/2018 on ABP 980 was applied. For inclusion the following criteria need to be met: tumor stage cT1–cT4, cN0–cN3, early stage breast cancer with no evidence of metastatic disease, at least 4 cycles of anthracycline and/or taxane containing NAT, at least 4 cycles of neoadjuvant ABP 980 or RTZ and normal LVEF at baseline. Combination with pertuzumab was permitted. Surgery was performed at the Department of Women’s Health, Tuebingen University Hospital, Germany and the tissue was examined by the Institute of Pathology, Tuebingen University Hospital, Germany. The study was approved by the local ethics committee (636/2021B02).
HER2 diagnostics
The HER2-status was assessed to local standards by using the HERCEPT test (DAKO, Glostrup, Denmark). Expression of HER2 was scored on a 0 to +3 scale. Tumors with a score of +3 were considered HER2-positive. In case of a score of +2, HER2 amplification was determined by fluorescence in-situ hybridization using the Pathvysion Kit (Vysis, Downers Grove, IL).
Therapeutic setting
RTZ/ABP 980 was administered intravenously every three weeks with an initial loading dose of 8 mg/kg and maintenance doses of 6 mg/kg. If a treatment delay of more than 7 days occurred, the initial loading dose was given again. NAT was administered according to local clinical practice and consisted of at least 4 cycles of an anthracycline and/or taxan containing standard regime. Due to potential risks of cumulative cardiac toxicity, RTZ/ABP 980 was not administered simultaneously with anthracyclines but in sequence (i.e. in combination with taxanes). After completion of breast surgery, pathological response was determined. To investigate clinical efficacy of RTZ and ABP 980, rates of pathological complete remission (pCR) in breast and axillary nodes (ypT0/is, ypN0) were compared. The frequency of cardiac adverse events was assessed as per local practice and in accordance with national guidelines, which recommends LVEF assessments to be performed at the time of treatment initiation and every 3 months during treatment and as clinically indicated. An LVEF decrease of more than 10 % from the baseline value was defined as a relevant decline in cardiac function 29 .
Statistical analysis
The primary objective was to compare the association of RTZ versus ABP 980 with pCR. For this purpose, predictors of a pCR were identified by a multivariate logistic regression analysis. The following factors were included in the first block: clinical tumor size (ordinal; T1, T2, T3/4), lymph node involvement before NAT (categorical; no, yes) grading (categorical, G2, G3), hormone receptor status (categorical; positive, negative), HER2-Score (categorical; 2+, 3+), anthracycline containing NAT (categorical; no, yes), dual HER2-blockade with pertuzumab (categorical; no, yes). The type of trastuzumab used (categorical; RTZ, ABP 980) was entered in a second block to evaluate whether this would significantly improve the first model. The performance of the logistic regression model was measured using the area under the receiver operating characteristic curve (AUC) and the Hosmer–Lemeshow test. Odds Ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. For statistical analyses SPSS software version 27 was used. All the tests were two-sided, and a P value of < 0.05 was regarded as statistically significant.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval was waived by the local Ethics Committee of University Tuebingen (No. 636/2021B02) in view of the retrospective nature of the study and all procedures being performed were part of routine care.
Results
Characteristics of the study group
124 patients were included, 46 patients (37.1%) were treated with ABP 980, while 78 patients (62.9%) received RTZ. Baseline patients’ characteristics were balanced between groups regarding tumor size, nodal involvement, hormone receptor status and HER2 status as described in Table 1 . However, patients treated with ABP 980 were more frequently node negative, of higher grade, hormonal receptor positive and more often treated with pertuzumab. In both groups, all patients received taxane-based NAT while anthracyclines were administered in approximately 90% of the patients.
Table 1 Patient characteristics.
| ABP 980 | RTZ | total | P value* | ||
| * chi-squared-test; ER = estrogen receptor; PR = progesterone receptor; RTZ = reference trastuzumab | |||||
| All patients, N | 46 | 78 | 124 | ||
| Mean age, years (SD) | 51.3 (9.0) | 53.7 (10.0) | 52.2 (9.4) | 0.176 | |
| Tumor size, N (%) | 0.436 | ||||
|
9 (19.6) | 16 (20.5) | 25 (20.2) | ||
|
32 (69.6) | 47 (60.3) | 79 (63.7) | ||
|
5 (10.9) | 15 (19.2) | 20 (16.1) | ||
| Nodal involvement, N (%) | 0.063 | ||||
|
25 (54.3) | 29 (37.2) | 54 (43.5) | ||
|
21 (45.7) | 49 (62.8) | 70 (56.5) | ||
| Grading, N (%) | 0.024 | ||||
|
9 (20.0) | 31 (39.7) | 40 (32.5) | ||
|
36 (80.0) | 47 (60.3) | 83 (67.5) | ||
| ER status, N (%) | 0.256 | ||||
|
17 (37.0) | 37 (47.4) | 54 (43.5) | ||
|
29 (63.0) | 41 (52.6) | 70 (56.5) | ||
| PR status, N (%) | 0.067 | ||||
|
23 (50.0) | 52 (66.7) | 75 (60.5) | ||
|
23 (50.0) | 26 (33.3) | 49 (39.5) | ||
| HER2 status, N (%) | 0.695 | ||||
|
7 (15.2) | 14 (17.9) | 21 (16.9) | ||
|
39 (84.8) | 64 (82.1) | 103 (83.1) | ||
| Neoadjuvant chemotherapy, N (%) | |||||
|
yes | 46 (100.0) | 78 (100.0) | 122 (100.0) | 1.000 |
|
yes | 40 (88.9) | 72 (92.3) | 112 (91.1) | 0.522 |
| no | 5 (11.1) | 6 (7.7) | 11 (8.9) | ||
|
yes | 44 (95.7) | 53 (67.9) | 97 (78.2) | < 0.001 |
| no | 2 (4.3) | 25 (32.1) | 27 (21.8) | ||
Comparison of pCR rates under treatment with RTZ or ABP 980
In total, 77 patients (62.1%) experienced a pCR in breast and axillary lymph nodes (ypT0/is, ypN0). Univariate analysis revealed no significant difference in patients treated with ABP 980 as compared to RTZ (p = 0.829). As displayed in Fig. 1 the rate of pCR was 60.9% (95% confidence interval: 46.6%–74.0%) in patients treated with ABP 980 as compared to 62.8% (95% confidence interval: 51.8%–72.9%) in the RTZ group. Associations between established predictors of pCR and pCR rates are shown in Table 2 . The pCR rates decreased with increasing tumor size, whereas negative hormonal receptor status, HER2 score of 3+ and higher grading were associated with higher pCR rates. Treatment with pertuzumab numerically increased the rate of pCR, however this observation was not statistically significant.
Fig. 1.
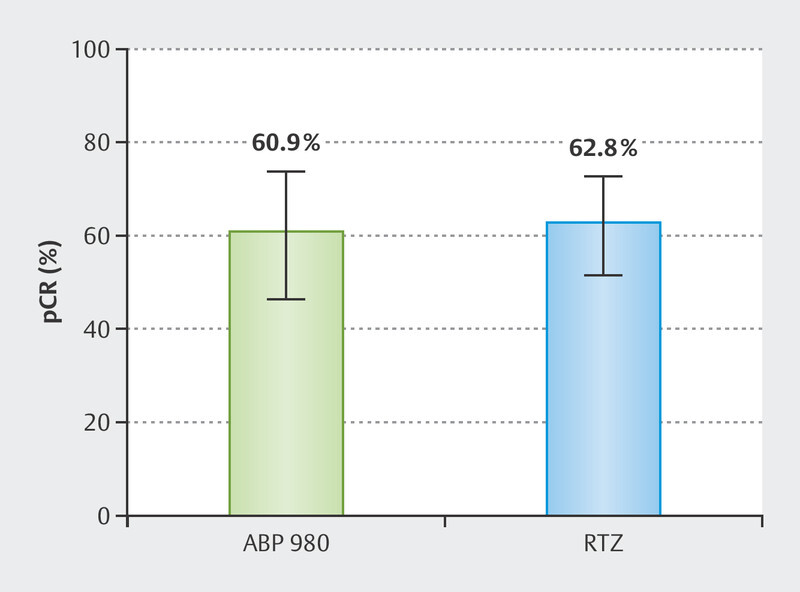
Univariate analysis of pathological complete response (pCR) rates after neoadjuvant therapy (NAT) in patients treated with ABP 980 versus reference trastuzumab (RTZ). Error bars represent 95% confidence intervals. p = 0.829 (chi-squared-test).
Table 2 Univariate analysis of pathologic complete response (pCR) rates according to patient characteristics.
| pCR | no pCR | P value | |
| * chi-squared-test; pCR = pathologic complete response; ER = estrogen receptor; PR = progesterone receptor | |||
| All Patients, N | 77 (62.1) | 47 (37.9) | |
| Mean age, years (SD) | 51.8 (9.7) | 52.9 (8.8) | 0.554 |
| Tumor size, N (%) | 0.051 | ||
|
20 (80.0) | 5 (20.0) | |
|
48 (60.8) | 31 (39.2) | |
|
9 (45.0) | 11 (55.0) | |
| Nodal involvement, N (%) | 0.195 | ||
|
37 (68.5) | 17 (31.5) | |
|
40 (57.1) | 30 (42.9) | |
| Grading, N (%) | 0.611 | ||
|
26 (65.0) | 14 (35.0) | |
|
50 (60.2) | 33 (39.8) | |
| ER status, N (%) | 0.005 | ||
|
41 (75.9) | 13 (24.1) | |
|
36 (51.4) | 34 (48.6) | |
| PR status, N (%) | 0.005 | ||
|
54 (72.0) | 21 (28.0) | |
|
23 (46.9) | 26 (53.1) | |
| HR status, N (%) | 0.002 | ||
|
41 (77.4) | 12 (22.6) | |
|
36 (50.7) | 35 (49.3) | |
| HER2 status, N (%) | 0.046 | ||
|
9 (42.9) | 12 (57.1) | |
|
68 (66.0) | 35 (34.0) | |
| Anthracyclines, N (%) | 0.467 | ||
|
8 (72.7) | 3 (27.3) | |
|
69 (61.6) | 43 (38.4) | |
| Dual HER2 blockade, N (%) | 0.215 | ||
|
14 (51.9) | 13 (48.1) | |
|
63 (64.9) | 34 (35.1) | |
Analysis of cardial side effects
As displayed in Table 3 , a drop in LVEF of more than 10 % was observed in 5 patients (4.0%). Three of these (6.5%) were treated with ABP 980 as compared to two patients (2.6 %) that were under treatment with RTZ (p = 0.274). All patients developed their drop of LVEF during neoadjuvant therapy, consisting of chemotherapy and antibody treatment. Four of these patients were without clinical impairment (two were treated with ABP 980, two with RTZ), their adjuvant therapy with trastuzumab could be completed as planned. During adjuvant treatment cardial function remained stable or even improved.
Table 3 Decrease of left ventricular ejection fraction under therapy with ABP 980 compared with reference trastuzumab (RTZ).
| ABP 980 | RTZ | total | P value* | |
| * chi-squared-test; LVEF = left ventricular ejection fraction; RTZ = reference trastuzumab | ||||
| LVEF | 0.279 | |||
|
43 (93.5) | 76 (97.4) | 119 (95.9) | |
|
5 (6.5) | 2 (2.6) | 5 (4.1) | |
One patient in the ABP 980 group developed a dilatative cardiomyopathy, an adjuvant treatment with ABP 980 was not initiated. Under pharmaceutical treatment the clinical condition was stable.
Logistic regression model analysis to identify predictors of pCR
A logistic regression model was used to examine whether the use of ABP 980 impacts pCR. The first model that did not include the type of trastuzumab treatment, was a significant improvement over the constant-only model [χ 2 (7) = 23.496, p = 0.001], explained 23.9% (Nagelkerke R 2 ) of the variance and correctly classified 68.0% of cases. The difference between actual and predicted events was low (p = 0.892, Hosmer–Lemeshow test). Addition of trastuzumab treatment (ABP 980 versus RTZ) did not significantly improve the model χ 2 (1) = 0.343, p = 0.558. The area under the ROC curve was 0.748 (95% CI: 0.660 to .838), indicating an excellent level of discrimination 30 . As displayed in Table 4 , significant predictors of pCR were tumor size before NAT and hormonal receptor status.
Table 4 Multivariate regression analysis of predictors of pathological complete response (pCR) after primary systemic therapy.
| Predictor | Coefficient (SE) | Odds Ratio (95% CI) | P value |
| * stage cT3 and cT4 were combined in one category; CI = confidence interval; RZT = reference trastuzumab; SE = standard error | |||
| Tumor size before NAT (per Stage*) | −0.75 (0.36) | 0.48 (0.24–0.96) | 0.038 |
| Nodal involvement before NAT | |||
|
0 | 1 | |
|
−0.81 (0.46) | 0.45 (0.18–1.09) | 0.077 |
| Grading | |||
|
0 | 1 | |
|
−0.53 (0.48) | 0.59 (0.23–1.52) | 0.273 |
| Hormonal receptor status | |||
|
0 | 1 | |
|
1.48 (0.48) | 4.37 (1.72–11.13) | 0.002 |
| HER2 Status | |||
|
0 | 1 | |
|
0.82 (0.54) | 2.27 (0.79–6.53) | 0.130 |
| Anthracycline treatment | |||
|
0 | 1 | |
|
−0.66 (0.82) | 0.52 (0.10–2.56) | 0.419 |
| Pertuzumab treatment | |||
|
0 | 1 | |
|
1.00 (0.55) | 2.71 (0.93–7.96) | 0.069 |
| Type of trastuzumab | |||
|
0 | 1 | |
|
−0.28 (0.48) | 0.76 (0.30–1.92) | 0.558 |
Discussion
ABP 980 is a biosimilar of trastuzumab that is, among other indications, approved for the treatment of early HER2-positive breast cancer. Results of comparative physicochemical characterization confirmed the structural and functional similarity between ABP 980 and RTZ while pharmacokinetic similarity was demonstrated in a phase I study 31 . The randomized double-blind phase III LILAC trial found similar efficacy in terms of pCR-rates when ABP 980 versus RTZ was administered in combination with an anthracycline and taxane containing NAT to treat early HER2-positive breast cancer 27 . Safety was assessed in the neoadjuvant as well as in a subsequent adjuvant phase, and again no differences were seen. In the current study, we compared ABP 980 and trastuzumab in a clinical real-world setting and confirmed the results of LILAC by showing equivalence in pCR rates and safety.
In LILAC, pCR rates of 48% and 41% were found for NAT containing ABR 980 and RZT, respectively. The higher pCR rates of 61% and 63% we found can be explained by differences in patient characteristics and treatment. Regarding the former a greater proportion of patients were ER/PR negative, fewer patients were node positive, and more patients had high-grade (G3) tumors in our analysis as compared to LILAC. Regarding the latter, a large proportion of our patients received dual anti-HER2 blockade with pertuzumab. Although we failed to show a significant difference between pCR rates in patients that were treated with or without pertuzumab our results are numerically in line with data from earlier trials and real-world registries 14 18 32 33 . A decline in LVEF of > 10% was observed in 4% of our patients, with no differences between treatment groups. This is in line with other real-world data but higher than in the LILAC study, in which 0.3–0.9% of patients suffered a decrease in LVEF 27 34 .
Randomized clinical trials are designed to control variability and to ensure the quality of the data they generate 35 . However, because populations may differ from those in clinical practice in terms of, for example, tumor characteristics, comorbidities, and prior treatment, the data generated in a traditional clinical trial may not reflect the real-world situation. Particularly, registration studies of biosimilars only examine distinct clinical situations and then transfer the results to additional indications 24 . Other biosimilars of trastuzumab that have shown similar pCR rates as compared to RZT in neoadjuvant treatment of early HER2-positive breast cancer are SB3, CT-P6 and PF-05280014 36 37 38 . MYL-1401O and PF-05280014 were also assessed in metastatic breast cancer, showing similar objective-response-rates as compared to RZT 39 40 . Of note, neither the neoadjuvant nor the metastatic trials combined trastuzumab treatment with pertuzumab.
Due to the non-randomized use of ABP 980 und RTZ, our study has several limitations. Both substances were used in a time-dependent manner, and since RZT was used first and ABP 980 later, the patient populations of the two comparison groups were not homogeneous. Additionally, pertuzumab is increasingly used over the last years and therefore more patients in the ABP 980 group were additionally treated with pertuzumab. To address these limitations, we performed a multivariate regression analysis of predictors of pCR. After adjusting for potential confounders we still found no difference regarding pCR in patients treated with ABP 980 as compared to RTZ. Although a nonsignificant result does not rule out existing differences, the pCR rates we observed are in line with those of recent data 18 32 . In addition to the existing data from LILAC, this suggests that even with a larger sample size, it would have been unlikely to show a significant effect of ABP 980 over RTZ on pCR 27 . Furthermore, the analysis of safety may be biased by the fact that cardiac adverse events leading to discontinuation of NAT during the first three cycles were not assessed, as only patients who had received at least four cycles of NAT were included in our retrospective analysis.
In conclusion we confirmed similarity of ABP 980 as compared to RZT regarding efficacy and safety. The data supports findings from previous equivalence studies in a real-world situation where a large proportion of patients have also received pertuzumab treatment. The admission of biosimilars increases the accessibility to highly effective biologicals and offers additional therapeutical options. Competition between different providers lower the costs of the applied therapy for health care systems. Consequently, a larger number of patients could benefit from a well-established, effective therapy. Real-world data from routine clinical practice should be collected and evaluated continuously to answer questions that have not yet been addressed or cannot be addressed in clinical trials.
Compliance with Ethical Standards
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Previous presentation: The data was already presented online on a poster on the DGGG Congress, German Society for Gynecology and Obstetrics, October 7 th –10th 2020 in Munich.
Contributorsʼ Statement
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by S.M., H.M. and A.D.H. The first draft of the manuscript was written by S.M. and A.D.H. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Footnotes
Conflict of Interest S.M. has received honoraria from Roche. T.E. declares no conflicts of interest. H.M. declares no conflicts of interest. L.V. has received honoraria and support for attending meetings from Roche. E.-M.G. declares no conflicts of interest. A.S. declares no conflicts of interest. M.H. declares no conflicts of interest. S.Y.B. has received honoraria/travel support from Roche, Novartis, Pfizer, AstraZeneca, Storz and Sanofi Aventis. A.D.H. has received honoraria, consulting fees and support for attending meetings from Roche, Pfizer, MSD, Hexal, Amgen.
References
- 1.Slamon D, Clark G, Wong S et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Press MF, Bernstein L, Thomas PA et al. HER-2/neu gene amplification characterized by fluorescence in situ hybridization: Poor prognosis in node-negative breast carcinomas. J Clin Oncol. 1997;15:2894–2904. doi: 10.1200/JCO.1997.15.8.2894. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Romond EH, Perez EA, Bryant J et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 5.Slamon D, Eiermann W, Robert N et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S et al. Use of chemotherapy plus a monoclonal antibody against her2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Marty M, Cognetti F, Maraninchi D et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: The M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 8.Untch M, Fasching PA, Konecny GE et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: Results from the TECHNO Trial of the AGO and GBG study groups. J Clin Oncol. 2011;29:3351–3357. doi: 10.1200/JCO.2010.31.4930. [DOI] [PubMed] [Google Scholar]
- 9.Schneeweiss A, Fasching PA, Fehm T et al. AGO Algorithms for the Treatment of Breast Cancer: Update 2021. Geburtshilfe Frauenheilkd. 2021;81:1101–1111. doi: 10.1055/A-1519-7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomssen C, Fehm TN, Stickeler E et al. Update Breast Cancer 2021 Part 4 – Prevention and Early Stages. Geburtshilfe Frauenheilkd. 2022;82:206–214. doi: 10.1055/A-1724-9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tesch H, Müller V, Wöckel A et al. Update Breast Cancer 2020 Part 4 – Advanced Breast Cancer. Geburtshilfe Frauenheilkd. 2020;80:1115–1122. doi: 10.1055/A-1270-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzdar AU, Ibrahim NK, Francis D et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Eiermann W, Semiglazov V et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 14.Cortazar P, Zhang L, Untch M et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 15.von Minckwitz G, Huang CS, Mano MS et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/nejmoa1814017. [DOI] [PubMed] [Google Scholar]
- 16.Swain SM, Miles D, Kim SB et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:519–530. doi: 10.1016/S1470-2045(19)30863-0. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Procter M, de Azambuja E et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N Engl J Med. 2017;377:122–131. doi: 10.1056/nejmoa1703643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneeweiss A, Chia S, Hickish T et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA) Ann Oncol. 2013;24:2278–2284. doi: 10.1093/annonc/mdt182. [DOI] [PubMed] [Google Scholar]
- 19.Gianni L, Pienkowski T, Im YH et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 20.European Medicines Agency . Similar biological medicinal products. https://www.ema.europa.eu/en/similar-biological-medicinal-products https://www.ema.europa.eu/en/similar-biological-medicinal-products
- 21.Declerck P, Farouk Rezk M. The road from development to approval: evaluating the body of evidence to confirm biosimilarity. Rheumatology (Oxford) 2017;56:iv4–iv13. doi: 10.1093/rheumatology/kex279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weise M, Bielsky MC, De Smet K et al. Biosimilars: what clinicians should know. Blood. 2012;120:5111–5117. doi: 10.1182/BLOOD-2012-04-425744. [DOI] [PubMed] [Google Scholar]
- 23.Barbier L, Declerck P, Simoens S et al. The arrival of biosimilar monoclonal antibodies in oncology: clinical studies for trastuzumab biosimilars. Br J Cancer. 2019;121:199–210. doi: 10.1038/s41416-019-0480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stebbing J, Mainwaring PN, Curigliano G et al. Understanding the Role of Comparative Clinical Studies in the Development of Oncology Biosimilars. J Clin Oncol. 2020;38:1070–1080. doi: 10.1200/JCO.19.02953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanes V, Chow V, Zhang N et al. A randomized, single-blind, single-dose study evaluating the pharmacokinetic equivalence of proposed biosimilar ABP 980 and trastuzumab in healthy male subjects. Cancer Chemother Pharmacol. 2017;79:881–888. doi: 10.1007/s00280-017-3286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolberg HC, Colleoni M, Santi P et al. Totality of Scientific Evidence in the Development of ABP 980, a Biosimilar to Trastuzumab. Target Oncol. 2019;14:647–656. doi: 10.1007/S11523-019-00675-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Minckwitz G, Colleoni M, Kolberg HC et al. Efficacy and safety of ABP 980 compared with reference trastuzumab in women with HER2-positive early breast cancer (LILAC study): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2018;19:987–998. doi: 10.1016/S1470-2045(18)30241-9. [DOI] [PubMed] [Google Scholar]
- 28.Hester A, Gaß P, Fasching PA et al. Trastuzumab Biosimilars in the Therapy of Breast Cancer – “Real World” Experiences from four Bavarian University Breast Centres. Geburtshilfe Frauenheilkd. 2020;80:924–931. doi: 10.1055/A-1226-6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AGO Breast Committee . Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer. Recommendations 2021. https://www.ago-online.de/leitlinien-empfehlungen/leitlinien-empfehlungen/kommission-mamma https://www.ago-online.de/leitlinien-empfehlungen/leitlinien-empfehlungen/kommission-mamma
- 30.Hosmer DW, Lemeshow S, Sturdivant RX. Hoboken: Wiley; 2013. Applied Logistic Regression. [Google Scholar]
- 31.Hanes V, Chow V, Zhang N et al. A randomized, single-blind, single-dose study evaluating the pharmacokinetic equivalence of proposed biosimilar ABP 980 and trastuzumab in healthy male subjects. Cancer Chemother Pharmacol. 2017;79:881–888. doi: 10.1007/s00280-017-3286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain SM, Ewer MS, Viale G et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): a phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol. 2018;29:646–653. doi: 10.1093/annonc/mdx773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fasching PA, Hartkopf AD, Gass P et al. Efficacy of neoadjuvant pertuzumab in addition to chemotherapy and trastuzumab in routine clinical treatment of patients with primary breast cancer: a multicentric analysis. Breast Cancer Res Treat. 2019;173:319–328. doi: 10.1007/s10549-018-5008-3. [DOI] [PubMed] [Google Scholar]
- 34.Lidbrink E, Chmielowska E, Otremba B et al. A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Cancer Res Treat. 2019;174:187–196. doi: 10.1007/s10549-018-5058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherman RE, Anderson SA, Dal Pan GJ et al. Real-World Evidence – What Is It and What Can It Tell Us? N Engl J Med. 2016;375:2293–2297. doi: 10.1056/NEJMsb1609216. [DOI] [PubMed] [Google Scholar]
- 36.Pivot X, Bondarenko I, Nowecki Z et al. Phase III, Randomized, Double-Blind Study Comparing the Efficacy, Safety, and Immunogenicity of SB3 (Trastuzumab Biosimilar) and Reference Trastuzumab in Patients Treated With Neoadjuvant Therapy for Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer. J Clin Oncol. 2018;36:968–974. doi: 10.1200/JCO.2017.74.0126. [DOI] [PubMed] [Google Scholar]
- 37.Stebbing J, Baranau Y, Baryash V et al. CT-P6 compared with reference trastuzumab for HER2-positive breast cancer: a randomised, double-blind, active-controlled, phase 3 equivalence trial. Lancet Oncol. 2017;18:917–928. doi: 10.1016/S1470-2045(17)30434-5. [DOI] [PubMed] [Google Scholar]
- 38.Lammers PE, Dank M, Masetti R et al. Neoadjuvant PF-05280014 (a potential trastuzumab biosimilar) versus trastuzumab for operable HER2+ breast cancer. Br J Cancer. 2018;119:266–273. doi: 10.1038/s41416-018-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pegram MD, Bondarenko I, Zorzetto MMC et al. PF-05280014 (a trastuzumab biosimilar) plus paclitaxel compared with reference trastuzumab plus paclitaxel for HER2-positive metastatic breast cancer: a randomised, double-blind study. Br J Cancer. 2019;120:172–182. doi: 10.1038/s41416-018-0340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rugo H, Barve A, Waller CF et al. Heritage, a phase III safety and efficacy trial of the proposed trastuzumab biosimilar, Myl-1401O vs trastuzumab. Ann Oncol. 2016;27:vi555. doi: 10.1093/ANNONC/MDW435.06. [DOI] [Google Scholar]


