Abstract
In patients with existing ovarian function, there are some special aspects to adjuvant endocrine therapy in premenopausal patients with hormone receptor-positive, HER2-negative (HR pos./HER2 neg.) breast cancer. Treatment options include tamoxifen with or without a GnRH analog, and aromatase inhibitors with a GnRH analog. Furthermore, ovarian function is affected by previous chemotherapy. Both aromatase inhibitors (+GnRH analogs) and GnRH analogs in combination with tamoxifen are supposed to be indicated for patients at increased risk of recurrence.
However, national and international guidelines and therapy recommendations do not provide a clear definition of intermediate or high risk; as a result, therapy decisions are often made for each patient on an individual basis. This is also reflected in the considerable variability at national and international levels, e.g., with regard to the use of aromatase inhibitors + GnRH analogs.
This review summarizes the data on completed studies (e.g., SOFT, TEXT, EBCTCG meta-analyses) and the current multigene testing studies (TailorX, RxPonder, ADAPT), discusses the rationale for current studies (e.g., CLEAR-B), and looks ahead to future questions.
Keywords: breast cancer, premenopausal therapy, aromatase inhibitor, tamoxifen, GnRH
Introduction
Patients who develop breast cancer at a young age often have disease characteristics that are associated with a poorer prognosis. For example, they develop triple-negative (TNBC) or HER2-positive (HER2 pos.) breast cancer more frequently, and the disease takes a more aggressive course. Moreover, their response to systemic therapies is quite different to that of postmenopausal patients 1 2 .
Risk factors for breast cancer in young patients include genetic factors, environmental factors, and reproductive behavior. Patients who develop the disease at a younger age are more likely to have germline mutations in the BRCA1 or BRCA2 genes 3 4 . Late first pregnancy has been associated with a transiently increased risk of breast cancer 5 . It is interesting to note the different effect of body weight on breast cancer risk in premenopausal and postmenopausal patients: while being overweight is associated with an increased risk of postmenopausal breast cancer, this could not be demonstrated in patients with premenopausal breast cancer 6 . Fig. 1 shows the different mechanisms of estrogen production in premenopausal and postmenopausal patients in the context of the pathogenesis of breast cancer. While in premenopausal patients estrogen is mainly synthesized in the ovaries, in postmenopausal women, fatty tissue is predominantly responsible for estrogen production.
Fig. 1.
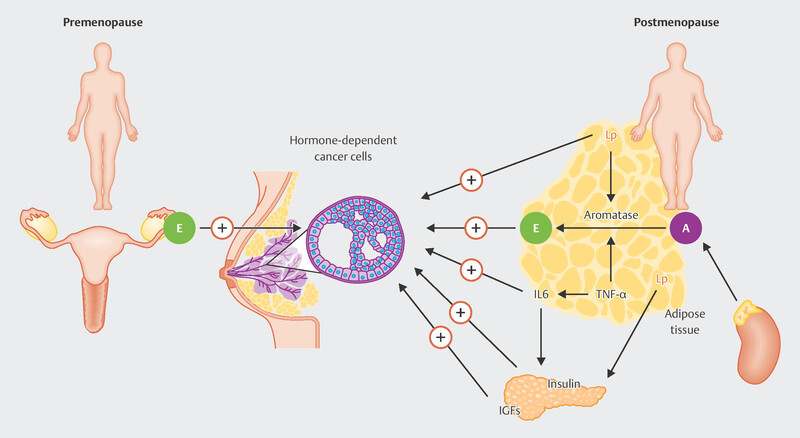
Different origins of estrogen (E) in premenopausal and postmenopausal breast cancer patients. While in premenopausal patients the ovaries are the main site of estrogen production, in postmenopausal patients this mainly occurs in the fatty tissue, where aromatase (A) is the key enzyme in the production of estradiol. Due to the formation of estrogen in fatty tissue, its production is closely linked to other metabolic regulatory mechanisms, which include leptin (Lp), interleukin 6 (IL6), tumor necrosis factor alpha (TNFα), insulin, and insulin growth factors (IGF) (Source: Maccio A, Madeddu C. Obesity, inflammation, and postmenopausal breast cancer: therapeutic implications. ScientificWorldJournal 2011; 11: 2020–2036. doi:10.1100/2011/806787. Creative Commons Attribution License (CC BY), https://creativecommons.org/licenses/by/3.0/
Due to the different mechanisms of production, the regulatory circuits for estrogen production in premenopausal and postmenopausal patients are fundamentally different. In premenopausal women, estrogen production is subject to a feedback mechanism involving the hypothalamus (in which luteinizing hormone-releasing hormones (LHRH) are produced physiologically), the pituitary gland, and the ovaries ( Fig. 2 ). Estrogen production in postmenopausal women largely takes place without such strong regulatory mechanisms, but is linked to the production of cortisol and testosterone, from which estrogen is made. The enzyme that catalyzes the conversion of androgens to estrogens is aromatase (CYP19A1) 7 . Compared to the premenopausal stage, estrogen levels in the postmenopausal stage remain relatively constant.
Fig. 2.

Different regulatory mechanisms and antiestrogen therapy options in patients with premenopausal and postmenopausal breast cancer (data from 8 , Creative Commons Attribution License (CC BY), https://creativecommons.org/licenses/by/4.0/ ).
The different production mechanisms result in direct therapeutic consequences for antihormone therapy. In principle, all antiestrogen drugs can be used in both premenopausal and postmenopausal patients. However, when aromatase inhibitors are used in premenopausal patients, they must be used exclusively in combination with GnRH analogs, as a reduction in estrogen production via the feedback mechanism would otherwise lead to a secondary increase in estrogen production 8 . With tamoxifen, the addition of a GnRH analog is optional.
Systemic Therapies in the Premenopausal Patients
Chemotherapy
Young age or premenopausal status are not in themselves an indication for chemotherapy. However, in addition to other unfavorable prognostic factors, young age is an indicator of poorer recurrence-free survival and poorer overall survival. In light of the poorer response to chemotherapy in patients with HR pos./HER2 neg. breast cancer and thus the limited benefit from chemotherapy in HR pos./HER2 neg. tumors 9 10 , in recent years efforts have been made to de-escalate the treatment of patients with this tumor type. In particular, studies that integrated multigene testing into the decision-making process for or against chemotherapy have presented separate evaluations for the benefits of chemotherapy in premenopausal patients (MINDACT, TailorX, RxPONDER, ADAPT). In principle, these studies were designed so that patients with an intermediate risk of recurrence according to a multigene test, i.e., patients for whom the indication for chemotherapy was uncertain, were randomized to treatment arms either with chemotherapy followed by endocrine therapy or with endocrine therapy alone. The study showed that in young patients aged < 50 or in premenopausal patients, chemotherapy leads to an improvement in prognosis. In postmenopausal patients, no benefit could be demonstrated for chemotherapy in addition to endocrine therapy 11 12 13 . Thus, chemotherapy is important in the treatment of premenopausal patients at moderate or significantly increased risk of recurrence. However, the ADAPT study showed that for patients aged under 50 with up to three affected lymph nodes, a recurrence score of 12–25, and a drop in Ki-67 to 10% or less after short-term endocrine therapy over three weeks, omitting chemotherapy is an option 14 .
Endocrine therapy options in the premenopausal patients
While chemotherapy is clearly only indicated for patients at increased risk of recurrence, endocrine therapy should be offered to all patients with HR pos./HER2 neg. breast cancer 15 .
Tamoxifen
Tamoxifen, a selective estrogen receptor modulator (SERM), was developed in 1963 and approved in the United States in 1977 for the treatment of patients with advanced breast cancer 16 . The effect of tamoxifen in the adjuvant therapy setting was estimated in a meta-analysis from 1998 to confer an absolute 10-year survival difference of over 5% in node-negative and over 10% in node-positive patients. This effect was largely independent of patient and tumor characteristics. Since then, tamoxifen has been one of the standards in the treatment of premenopausal patients with early stage breast cancer.
SERDs
Selective estrogen receptor degraders (SERDs) result in degradation and thus elimination of the estrogen receptor. Most of the available data relate to the SERD fulvestrant used in postmenopausal patients 17 18 . Fulvestrant has not been developed for use in early stage breast cancer, and is therefore not available for the treatment of premenopausal or early postmenopausal patients with early stage breast cancer. An adjuvant study in postmenopausal patients was started but discontinued before completion 19 . In patients with metastases, there have been studies in which treatment success has been achieved with fulvestrant in combination with GnRH analogs in patients with advanced breast cancer 20 . The combination of CDK4/6 inhibitors, fulvestrant, and GnRH analogs has also been described as effective 21 .
Aromatase inhibitors
Blocking the aromatase enzyme (CYP19A1) serves to suppress the conversion of testosterone to estradiol 22 , thus limiting the overall production of estrogen. In patients with postmenopausal breast cancer, three drugs, anastrozole, letrozole, and exemestane, were introduced almost 20 years ago to treat patients with early stage breast cancer 23 24 25 . A meta-analysis by the EBCTCG (Early Breast Cancer Trialists Collaborative Group) showed that recurrence was reduced by approximately 30%. Similarly, deaths after 10 years were reduced relatively by approximately 15%, even though most deaths were not related to breast cancer 26 .
GnRH analogs
Estrogen production in premenopausal women is controlled by the hypothalamus-pituitary-ovarian axis. Estrogen production can be largely suppressed by switching off this signal axis 7 ; this mechanism is used not only in the treatment of breast cancer, but also in other hormone-dependent diseases that affect women, such as endometriosis or myoma 27 . The continuous action of GnRH analogs in the body leads to downregulation of the pituitary GnRH receptors, which stops the release of follicle-stimulating hormones (FSH) and luteinizing hormones (LH), and stops estrogen production in the ovaries over a period of 2–3 weeks after the start of therapy. Thus, the GnRH analogs are an effective class of drugs available for OFS 7 .
Comparisons between therapies are quite complex. In premenopausal women, a GnRH analog must also be added during therapy with an aromatase inhibitor. Tamoxifen, on the other hand, can be administered with or without ovarian function suppression (OFS) 7 . As a rule, patients at higher risk of recurrence receive OFS as well 15 . Previous chemotherapy also plays a role in this context because it can compromise ovarian function in some patients 7 28 29 30 31 32 33 34 35 36 37 38 39 40 . The history of studies investigating the treatment of premenopausal patients is set out below.
Development of Endocrine Therapy Options in Premenopausal Patients
Discovery of the correlation between ovarian function and breast cancer
Over time, the recommendations regarding adjuvant endocrine therapy in premenopausal patients have changed several times in different ways. To understand these trends, it is helpful to take a look at the history of endocrine treatment in premenopausal patients.
In 1896, a surgeon named George Beatson reported on the positive clinical effect of surgical oophorectomy in premenopausal patients with advanced breast cancer 41 . Not only surgical removal but also radiation therapy of the ovaries has been associated with a therapeutic effect on breast cancer in premenopausal breast cancer patients 42 .
Introduction of GnRH analogs
Almost a century later, after the introduction of GnRH analogs, the effect of OFS on the prognosis for patients with early stage breast cancer has been systematically researched. In one of the first large-scale EBCTCG studies investigating whether early stage patients benefited from the addition of a GnRH analog in terms of their prognosis, it was concluded that this could improve both recurrence-free survival and overall survival 43 .
Initial indications of GnRH analogs having a weaker effect in patients after chemotherapy
However, most patients in this analysis had not received adjuvant chemotherapy, which only became widespread in the early 1980s 44 . This is of particular interest because in a later meta-analysis by the EBCTCG, no benefit from treatment with a GnRH analog could be demonstrated for the group of patients who had previously undergone adjuvant chemotherapy 45 . The most likely explanation for this was the effect of adjuvant chemotherapy on ovarian function. It should also be noted that in the initial studies, a significant proportion of patients had not been treated on the basis of hormone receptor positivity. In most cases, patients with unknown hormone receptor status were also able to participate in the studies. Only in later studies was inclusion strictly limited to hormone receptor-positive patients. Over the years this has led to differing therapy recommendations as a consequence of inconsistent data. Initially, OFS was generally recommended. However, this recommendation was later partially revoked. Currently, the recommendation for OFS therapy is risk-adapted 46 47 48 49 50 51 52 53 54 .
One of the studies conducted in this context was the ZEBRA study 55 . In this randomized trial, patients were treated either with the then standard chemotherapy CMF (cyclophosphamide/methotrexate/5-fluorouracil) or with OFS alone using a GnRH analog (goserelin), over a period of two years. During this period, ovarian function was suppressed in almost 100% of patients, while after the end of GnRH analog therapy, the amenorrhea rate in the chemotherapy arm was even higher than in the GnRH analog arm ( Fig. 3 ). In the group of ZEBRA patients with positive estrogen receptor status, a comparison of the two study arms showed no difference in terms of recurrence-free survival 55 .
Fig. 3.

Course of amenorrhea rate after treatment with either goserelin or CMF chemotherapy in the ZEBRA study 55 .
Chemotherapy, amenorrhea, and ovarian function
Some observations from previous studies are relevant for the interpretation of current findings 7 :
Patients who develop amenorrhea after adjuvant chemotherapy have a better prognosis 7 28 29 .
The effects of amenorrhea after chemotherapy were observed in patients with hormone receptor-positive breast cancer, but not in patients with hormone receptor-negative breast cancer 7 30 31 .
The positive effect of chemotherapy-induced amenorrhea was observed in both patients receiving tamoxifen and those without tamoxifen 7 32 .
Patients aged under 35 and on adjuvant chemotherapy had a worse prognosis than older premenopausal patients 7 33 34 35 .
Patients aged under 35 were less likely to develop chemotherapy-induced amenorrhea 7 36 .
Amenorrhea can be reversible. This is more common in younger patients 7 36 37 .
Treatment with an aromatase inhibitor can restimulate ovarian function even after chemotherapy-induced amenorrhea 7 38 39 40 .
Patients who have undergone chemotherapy have reduced ovarian function and may reach menopause earlier 7 .
Based on this knowledge, the following studies were conducted almost 20 years ago:
SOFT (Suppression of Ovarian Function Trial) 56 ,
TEXT (Tamoxifen and Exemestane 57 Trial), and
PERCHE (Premenopausal Endocrine Responsive Chemotherapy) 58
A summary of the study designs is shown in Fig. 4 . The data is further supplemented by the ABCSG-12 and HOBOE studies, which, in addition to a question around bisphosphonate, also included a comparison of tamoxifen vs. aromatase inhibitors in the study design ( Fig. 4 ) 59 60 . It should be noted that all studies except PERCHE were successfully completed. The PERCHE study had to be discontinued in 2006 due to a lack of recruitment.
Fig. 4.

Design of the SOFT, TEXT, PERCHE, HOBOE, and ABCSG-12 studies 56 57 58 59 60 .
Aromatase inhibitors versus tamoxifen with and without ovarian function suppression in the adjuvant therapy setting in premenopausal patients
In an EBCTCG meta-analysis comparing tamoxifen vs. aromatase inhibitors, the difference in efficacy was investigated in detail 61 . This analysis included all patients from studies who were treated with OFS and randomized to treatment arms with either tamoxifen or an aromatase inhibitor 61 62 . A total of 7030 patients were enrolled in the following studies: ABCSG-12, SOFT, TEXT, HOBOE (see Fig. 4 ). The median follow-up period for this analysis was 8.0 years. It was shown that the recurrence rate with OFS and aromatase inhibitors was reduced from 17.5% to 14.7% after 10 years, compared to OFS and tamoxifen (RR = 0.79; 95% CI: 0.69–0.90). Although distant metastasis-free survival had a relative risk of 0.83 (95% CI: 0.71–0.97), there was no improvement in terms of overall survival. The death rate at 10 years was 7.2% with tamoxifen plus OFS and 6.8% with aromatase inhibitors plus OFS (RR = 1.01; 95% CI: 0.82–1.24). Interestingly, the effect was only observed in years 2–4 after surgery and in patients with up to 3 affected lymph nodes. The benefit was no longer detectable in patients with more than four affected lymph nodes (RR = 1.03; 95% CI: 0.73–1.46) 61 62 .
Latest data on the addition of GnRH analogs from the SOFT and TEXT studies
The most recent analysis of the SOFT and TEXT studies with regard to the question of additional ovarian function suppression was conducted with a median follow-up period of 12 years (SOFT study) and 13 years (TEXT study) 63 . In this analysis, it was shown that overall survival could be improved by the addition of OFS. In the subgroup analyses, this effect was greatest in the cohort at increased risk of recurrence, e.g., in patients who had undergone (neo)adjuvant chemotherapy, were aged < 35, and had more than 3 affected lymph nodes and a tumor grade of 3 63 . No differences were shown in patients at low risk of recurrence. It should be noted that some of these subgroup analyses are based on very small case numbers. In the groups with tamoxifen alone, tamoxifen plus OFS, and exemestane plus OFS, there were only 103, 103, and 126 deaths respectively 63 . Accordingly, the statistical power for comparisons was low.
Side effects of endocrine therapy in premenopausal patients
Most patients report side effects that affect them to varying degrees during endocrine therapy. Musculoskeletal complaints, vasomotor symptoms, sexual dysfunction, fatigue, insomnia, weight gain, and cognitive problems are the most common side effects of tamoxifen and aromatase inhibitors 64 . GnRH analogs also increase vasomotor complaints, sexual dysfunction, vaginal dryness, and insomnia 64 . When comparing patients with and without adjuvant endocrine therapy, these side effects have a measurable impact on the quality of life of patients in follow-up care. A deterioration in quality of life two years after diagnosis is caused for the most part by endocrine therapy, and not by adjuvant chemotherapy where this is administered 65 . In the SOFT and TEXT studies, no significant difference in quality of life was found when comparing therapy with aromatase inhibitors + OFS vs. tamoxifen + OFS. The addition of OFS to treatment with tamoxifen showed a deterioration in quality of life, especially in the group of patients without prior chemotherapy 66 67 68 .
The large-scale adjuvant studies 25 26 69 showed that aromatase inhibitors lead to reduced bone density and increased risk of fracture compared to tamoxifen 70 . Aromatase inhibitors also have a significant influence on bone structure in premenopausal patients 71 . Against this background, the ABCSG-12 study ( Fig. 4 ) investigated how the addition of zoledronic acid to an endocrine therapy affected bone health 72 . After three years of treatment, reduced bone density was observed, especially in the group of patients treated with an aromatase inhibitor. Treatment with zoledronic acid was able to prevent this loss of bone density 72 .
Even though the benefits of adjuvant endocrine therapy have been consistently described, a significant proportion of patients choose to discontinue the therapy before completion. In addition to patient and tumor characteristics, the occurrence of side effects is one of the most important predictors for early discontinuation of a therapy 73 74 75 .
Current therapy recommendations
Current therapy recommendations are based on the individual patient’s risk of recurrence ( Fig. 5 ) 46 . Accordingly, patients at low risk of recurrence should be treated with tamoxifen therapy alone, while patients at increased risk of recurrence should be treated with either tamoxifen + OFS or with aromatase inhibitors + OFS. Prognostic assessment is not described in more detail in the guidelines and treatment recommendations 15 .
Fig. 5.
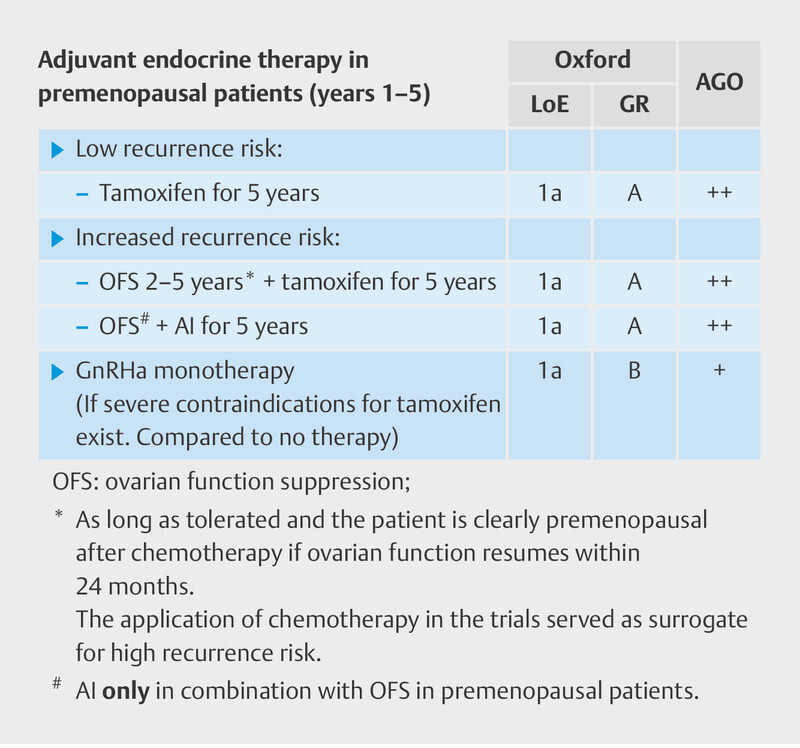
Current therapy recommendations from the Breast Committee of the Gynecological Oncology Working Group (AGO) for the adjuvant endocrine treatment of premenopausal patients.
Determining the Prognosis
Assessing the prognosis in HR pos./HER2 neg. premenopausal patients is complex. As shown in Fig. 6 , the choice of therapies is determined by the prognostic parameters. These have an influence on ovarian function, which in turn has an influence on prognosis. Against this background, therapy studies that investigate prognosis and choice of therapy are of particular importance, especially for premenopausal patients.
Fig. 6.

Interrelationship between prognosis, chemotherapy, antihormone therapy, and ovarian function with regard to the prognosis for premenopausal patients with early stage breast cancer.
Therapy studies conducted over the past 15 years have been characterized by the attempt to administer adjuvant chemotherapy to as few patients as possible when no clear benefit can be expected. While chemotherapy in TNBC and HER2-positive patients forms an integral part of the adjuvant therapy 15 , it must be assumed that overtreatment occurs in a large proportion of HR pos./HER2 neg. patients. An initial evaluation of the neoadjuvant studies showed that pCR can be achieved in less than 10% of cases in HR pos./HER2 neg. patients compared to over 30% in TNBC and over 40% in HER2-positive tumors 76 .
New insights regarding premenopausal patients with HR pos./HER2 neg. breast cancer were expected in the context of studies with a design that integrated multigene testing to determine prognosis when deciding for or against chemotherapy 11 13 77 78 79 . In these studies, adjuvant endocrine therapy was performed as standard in all women. Whether or not they received adjuvant chemotherapy depended on which study arm they were randomized to. While in postmenopausal randomized patients, these studies were able to conclude that chemotherapy can be omitted, in premenopausal randomized patients, there was a significant benefit to the addition of chemotherapy. However, this must be considered in the context of the endocrine therapy used.
The latest analysis of the RXponder study (node-positive population) has been presented, with a median follow-up period of 6.1 years. It confirmed the previously identified benefits of chemotherapy for premenopausal HR pos./HER2 neg. patients. Both recurrence-free survival (HR = 0.64; 95% CI: 0.47–0.87) and distant metastasis-free survival (HR = 0.66; 95% CI: 0.45–0.97) were improved by chemotherapy 80 . In the chemotherapy arm, approximately 75% of women no longer had menstrual bleeding in the first 6 months after randomization (GnRH therapy performed in 3–6% of patients). In the study arm with endocrine therapy alone, the amenorrhea rate was 50% (GnRH therapy performed in 14–16% of patients). Although this aspect was not formally analyzed, there appears to be no difference between patients with endocrine therapy alone vs. treatment with chemotherapy, especially in patients in whom menstrual bleeding continued. In patients whose menstrual bleeding had stopped, there appeared to be a slight benefit in the group of patients receiving chemotherapy ( Fig. 7 ).
Fig. 7.

Invasive disease-free survival in the RxPONDER study in the group of premenopausal patients; ET: endocrine therapy, CT → ET: chemotherapy and endocrine therapy (reconstructed using 81 according to 80 ). Source: Welslau M, Muller V, Luftner D et al. Update Breast Cancer 2022 Part 1 – Early Stage Breast Cancer. Geburtshilfe Frauenheilkd 2022; 82: 580–589. doi: 10.1055/a-1811-6106 ; Creative Commons Attribution-NonDerivative-NonCommercial-License.
The long-term survival analysis of the TailorX study (node-negative population; median follow-up of 11 years) also showed that chemotherapy was beneficial in terms of invasive disease-free survival, especially in patients aged under 50 with a recurrence score of 21–25. The absolute improvement in the 12-year invasive disease-free survival (iDFS) rate was 7.4 percent (improvement from 75% to 82.4%) 82 . This difference was greater if, in addition to a recurrence score of 21–25, there was also a clinically high risk of recurrence (absolute improvement in iDFS of 11.7%). In the patients with a low clinical risk of recurrence and a recurrence score of 21–25, there was still a 5.9% benefit from chemotherapy 82 . The reason for this observation is unclear. However, if this effect is due to the effect of chemotherapy on ovarian function in the form of primary ovarian insufficiency (see Fig. 6 ), it is necessary to investigate whether OFS can be performed in premenopausal patients in order to spare them chemotherapy.
How to identify the group of premenopausal patients in whom chemotherapy can be omitted without worsening the prognosis remains a question for future studies. Until then, chemotherapy must be indicated in this group depending on the patient and disease characteristics. In this context it may be helpful to determine the patient’s response to endocrine therapy by carrying out a short preoperative course of this therapy, analogous to the procedure in the ADAPT studies. All future studies should record menopausal status over time and in detail in order to better investigate the effect of chemotherapy on ovarian function and prognosis.
Therapeutic Preferences – Variability in Practice
As already discussed, in terms of adjuvant endocrine therapy there are no clear data regarding the level of recurrence risk at which premenopausal patients should be given a GnRH analog or therapy with aromatase inhibitors (+GnRH analog). In this context, the treatment choices that are made have been the subject of international studies.
International comparison of therapeutic preferences
An illuminating example is the monarchE study, which primarily investigated the addition of abemaciclib to a standard endocrine therapy for HR pos./HER2 neg. tumors with a high risk of recurrence. The study included breast cancer patients with more than 3 affected lymph nodes, or with 1–3 affected lymph nodes plus additional risk factors. For cases with 1–3 affected lymph nodes, a tumor grade of 3 or a tumor of at least 5 cm in size was also required 83 . The monarchE study is interesting, in part because the standard endocrine therapy could be freely chosen. The guidelines and therapy recommendations on the use of aromatase inhibitors or the addition of GnRH analogs are based on the risk of recurrence 46 47 . However, the level of increased risk that would require therapy with GnRH or with aromatase inhibitors is not defined in the guidelines. Thus, in the monarchE study, the frequency with which the different therapies are selected sheds light on how the risk is assessed across different countries ( Fig. 8 ) 84 . While in countries such as China, Italy, the USA, Australia, and Mexico the majority of premenopausal patients are treated with aromatase inhibitors (> 50–90%), in countries such as Germany, France, Taiwan, Korea, Japan, and Denmark, the rate of aromatase inhibitor use, at approximately 20%, is much lower in this patient population at increased risk of recurrence.
Fig. 8.

Distribution of premenopausal therapy preferences in the high-risk patient population of the MonarchE study sorted by frequency of aromatase inhibitor use. An international comparison shows that German physicians tend to be more cautious in the use of aromatase inhibitors in premenopausal patients (Data extracted from 84 using 81 ; * In these countries, less than 50 patients were included in the evaluation).
CLEAR-B
Accordingly, the evidence presented to date for the use of endocrine therapies in premenopausal patients is not sufficient. Gaining a better understanding with regard to the prognostic parameters that should be considered, the indication for chemotherapy, and the choice of antihormone therapy is of great clinical relevance. There are gaps in healthcare research data on which parameters influence the choice of therapy. In addition, it is possible that in different risk constellations (see Fig. 6 ), various therapy decisions have a different effect on prognosis. There are hardly any data available on this question either. Especially in view of the introduction of CDK 4/6 inhibitors in the adjuvant therapy setting 85 86 , knowing the correct prognosis is particularly important. The CLEAR-B study (https://clear-b.de/; AGO-B-059, Fig. 9 ) was set up in order to generate extensive data on this question. In this project, the treatment of approximately 3000 premenopausal patients will be documented in 75 certified breast cancer centers in order to investigate their care and its prognostic effects. The results will provide a better understanding of therapeutic practice in Germany.
Fig. 9.

Design of the CLEAR-B study.
Outlook
In recent years, the introduction of prognostic models, a better understanding of known biomarkers, the use of endocrine induction therapy, and the implementation of multigene testing have made it easier to assess the risk of recurrence in a more differentiated manner and adapt therapies to individual patients. Especially in premenopausal patients, the various therapeutic options (chemotherapy vs. no chemotherapy, aromatase inhibitors vs. tamoxifen, GnRH analog vs. no GnRH analog) have long-term effects on quality of life. Also, the effects of widespread routine use of abemaciclib on long-term quality of life in the adjuvant therapy setting have not yet been assessed. One of the most important tasks in the coming years will be to achieve a better assignment of risk level to the best possible therapy combination. Adjuvant endocrine treatment of hormone receptor-positive patients is associated with high requirements in terms of patient care and support. It is especially important to achieve the right balance between side effects and therapeutic benefit, because a significant number of these patients discontinue their therapy before completion. A better knowledge of the treatment situation could help to optimize the support currently available to patients. Current studies such as CLEAR-B will provide useful information in this regard.
Acknowledgement
This work is in part the result of funding from Novartis and ClinSol. The authors are solely responsible for the content of the manuscript.
Danksagung
Diese Arbeit entstand teilweise in Folge von Förderungen der Firmen Novartis und ClinSol. Für den Inhalt des Manuskriptes sind allein die Autoren verantwortlich.
Footnotes
Conflict of Interest B. A. received honoria and travel grants from AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo and Pfizer. M. B.-P. received honoraria for lectures and advisory role from Roche, Novartis, Pfizer, pfm, Eli Lilly, Onkowissen, Seagen, AstraZeneca, Eisai, Amgen, Samsung, MSD, GSK, Daiichi Sankyo, Gilead, Sirius Pintuition, Pierre Fabre, and study support from Mammotome, Endomag and Merit Medical. E. B. received honoraria from Gilead, Ipsen, Sanofi, Sandoz, SunPharma, AstraZeneca, Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, BBraun and onkowissen.de for clinical research management and/or medical education activities. N. D. has received honoraria from MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen, Gilead, MCI Healthcare. P. A. F. reports personal fees from Novartis, grants from BioNTech, personal fees from Pfizer, personal fees from Daiichi Sankyo, personal fees from AstraZeneca, personal fees from Eisai, personal fees from Merck Sharp & Dohme, grants from Cepheid, personal fees from Lilly, personal fees from Pierre Fabre, personal fees from SeaGen, personal fees from Roche, personal fees from Hexal, personal fees from Agendia, personal fees from Gilead. T. N. F. has participated on advisory boards for Amgen, Daiichi Sankyo, Novartis, Pfizer, and Roche and has received honoraria for lectures from Amgen, Celgene, Daiichi Sankyo, Roche, Novartis and Pfizer. A. D. H. received speaker and consultancy honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo, Hexal and Pfizer. N. H. received honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi Sankyo, Exact Sciences, Gilead, Lilly, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz and Seagen. W. J. has received research grants and/or honoraria from Sanofi-Aventis, Daiichi Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene and Johnson & Johnson. I. J.-B. declares no conflict of interest. H.-C. K. has received honoraria from Pfizer, Seagen, Novartis, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lilly, SurgVision, Onkowissen, Gilead, Daiichi Sankyo and MSD, travel support from Carl Zeiss, Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro and owns stock of Theraclion SA and Phaon Scientific GmbH. J. K. is an employee of Novartis. T. K. has received speaker honoraria from AstraZeneca, Daiichi Sankyo, Pfizer, MSD, Gilead, Seagen, Endomag, Merit Medical, Hologic. V. M. received speaker honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead. Consultancy honoraria from Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead. Institutional research support from Novartis, Roche, Seagen, Genentech. Travel grants: Roche, Pfizer, Daiichi Sankyo. N. N. is an employee of Novartis. E. S. received honoraria from Roche, Celgene, AstraZeneca, Novartis, Pfizer, Tesaro, Aurikamed GmbH, Seagen, Pierre Fabre, MCI Deutschland GmbH, bsh medical communications GmbH, Onkowissen TV. M. S. reports personal fees from AstraZeneca, BioNTech, Daiichi Sankyo, Eisai, Lilly, MSD, Novartis, Pantarhei Bioscience, Pfizer, Roche and Seagen. M. T. has participated on advisory boards for AstraZeneca, Clovis, Daiichi Sankyo, Eisai, Gilead Science, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Pierre Fabre, Seagen and Roche and has received honoraria for lectures from Amgen, Clovis, Daiichi Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, Vifor and AstraZeneca and has received trial funding by Exact Sciences and Endomag. Manuscript support was done by Amgen, ClearCut, pfm medical, Roche, Servier, Vifor. M. U.: All honoraria went to the institution/employer: Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Myriad Genetics, Pfizer, Roche, Sanofi-Aventis, Novartis, Pierre Fabre, Seagen; Gilead.
References/Literatur
- 1.Lambertini M, Pinto AC, Ameye L et al. The prognostic performance of Adjuvant! Online and Nottingham Prognostic Index in young breast cancer patients. Br J Cancer. 2016;115:1471–1478. doi: 10.1038/bjc.2016.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins LC, Marotti JD, Gelber S et al. Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat. 2012;131:1061–1066. doi: 10.1007/s10549-011-1872-9. [DOI] [PubMed] [Google Scholar]
- 3.Engel C, Rhiem K, Hahnen E et al. Prevalence of pathogenic BRCA1/2 germline mutations among 802 women with unilateral triple-negative breast cancer without family cancer history. BMC Cancer. 2018;18:265. doi: 10.1186/s12885-018-4029-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasching PA, Yadav S, Hu C et al. Mutations in BRCA1/2 and Other Panel Genes in Patients With Metastatic Breast Cancer -Association With Patient and Disease Characteristics and Effect on Prognosis. J Clin Oncol. 2021;39:1619–1630. doi: 10.1200/JCO.20.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambe M, Hsieh C, Trichopoulos D et al. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 6.van den Brandt PA, Spiegelman D, Yaun SS et al. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 7.Francis PA. Role of Ovarian Suppression in Early Premenopausal Breast Cancer. Hematol Oncol Clin North Am. 2023;37:79–88. doi: 10.1016/j.hoc.2022.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Lu YS, Wong A, Kim HJ. Ovarian Function Suppression With Luteinizing Hormone-Releasing Hormone Agonists for the Treatment of Hormone Receptor-Positive Early Breast Cancer in Premenopausal Women. Front Oncol. 2021;11:700722. doi: 10.3389/fonc.2021.700722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Untch M, von Minckwitz G, Konecny GE et al. PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel, and CMF versus a standard-dosed epirubicin-cyclophosphamide followed by paclitaxel with or without darbepoetin alfa in primary breast cancer--outcome on prognosis. Ann Oncol. 2011;22:1999–2006. doi: 10.1093/annonc/mdq713. [DOI] [PubMed] [Google Scholar]
- 10.Cortazar P, Zhang L, Untch M et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 11.Kalinsky K, Barlow WE, Gralow JR et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N Engl J Med. 2021;385:2336–2347. doi: 10.1056/NEJMoa2108873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piccart M, van ’t Veer LJ, Poncet C et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22:476–488. doi: 10.1016/S1470-2045(21)00007-3. [DOI] [PubMed] [Google Scholar]
- 13.Sparano JA, Gray RJ, Makower DF et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2018;379:111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitz UA, Gluz O, Kummel S et al. Endocrine Therapy Response and 21-Gene Expression Assay for Therapy Guidance in HR+/HER2- Early Breast Cancer. J Clin Oncol. 2022;40:2557–2567. doi: 10.1200/JCO.21.02759. [DOI] [PubMed] [Google Scholar]
- 15.Ditsch N, Kolberg-Liedtke C, Friedrich M et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2021. Breast Care (Basel) 2021;16:214–227. doi: 10.1159/000516419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemons M, Danson S, Howell A. Tamoxifen (“Nolvadex”): a review. Cancer Treat Rev. 2002;28:165–180. doi: 10.1016/s0305-7372(02)00036-1. [DOI] [PubMed] [Google Scholar]
- 17.ShaguftaAhmad I, Mathew S et al. Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer. RSC Med Chem. 2020;11:438–454. doi: 10.1039/c9md00570f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernando C, Ortega-Morillo B, Tapia M et al. Oral Selective Estrogen Receptor Degraders (SERDs) as a Novel Breast Cancer Therapy: Present and Future from a Clinical Perspective. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22157812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Borrego M, Guerrero-Zotano A, Bermejo B et al. Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2-) early breast cancer (EBC): results from the GEICAM/2006–10 study. Breast Cancer Res Treat. 2019;177:115–125. doi: 10.1007/s10549-019-05296-8. [DOI] [PubMed] [Google Scholar]
- 20.Bartsch R, Bago-Horvath Z, Berghoff A et al. Ovarian function suppression and fulvestrant as endocrine therapy in premenopausal women with metastatic breast cancer. Eur J Cancer. 2012;48:1932–1938. doi: 10.1016/j.ejca.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Loibl S, Turner NC, Ro J et al. Palbociclib Combined with Fulvestrant in Premenopausal Women with Advanced Breast Cancer and Prior Progression on Endocrine Therapy: PALOMA-3 Results. Oncologist. 2017;22:1028–1038. doi: 10.1634/theoncologist.2017-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santen RJ. Recent progress in development of aromatase inhibitors. J Steroid Biochem Mol Biol. 1990;37:1029–1035. doi: 10.1016/0960-0760(90)90461-s. [DOI] [PubMed] [Google Scholar]
- 23.Breast International Group (BIG) 1–98 Collaborative Group . Thurlimann B, Keshaviah A, Coates AS et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 24.Coombes RC, Hall E, Gibson LJ et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 25.Baum M, Budzar AU, Cuzick J et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 26.Early Breast Cancer Trialists’ Collaborative Group . Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet. 2015;386:1341–1352. doi: 10.1016/S0140-6736(15)61074-1. [DOI] [PubMed] [Google Scholar]
- 27.Capezzuoli T, Rossi M, La Torre F et al. Hormonal drugs for the treatment of endometriosis. Curr Opin Pharmacol. 2022;67:102311. doi: 10.1016/j.coph.2022.102311. [DOI] [PubMed] [Google Scholar]
- 28.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–5779. doi: 10.1200/JCO.2006.07.2793. [DOI] [PubMed] [Google Scholar]
- 29.Pagani O, O’Neill A, Castiglione M et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the International Breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34:632–640. doi: 10.1016/s0959-8049(97)10036-3. [DOI] [PubMed] [Google Scholar]
- 30.Swain SM, Jeong JH, Geyer CE, jr. et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–2065. doi: 10.1056/NEJMoa0909638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swain SM, Jeong JH, Wolmark N. Amenorrhea from breast cancer therapy--not a matter of dose. N Engl J Med. 2010;363:2268–2270. doi: 10.1056/NEJMc1009616. [DOI] [PubMed] [Google Scholar]
- 32.International Breast Cancer Study Group . Colleoni M, Gelber S, Goldhirsch A et al. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: International Breast Cancer Study Group Trial 13–93. J Clin Oncol. 2006;24:1332–1341. doi: 10.1200/JCO.2005.03.0783. [DOI] [PubMed] [Google Scholar]
- 33.Ahn SH, Son BH, Kim SW et al. Poor outcome of hormone receptor-positive breast cancer at very young age is due to tamoxifen resistance: nationwide survival data in Korea--a report from the Korean Breast Cancer Society. J Clin Oncol. 2007;25:2360–2368. doi: 10.1200/JCO.2006.10.3754. [DOI] [PubMed] [Google Scholar]
- 34.Goldhirsch A, Gelber RD, Yothers G et al. Adjuvant therapy for very young women with breast cancer: need for tailored treatments. J Natl Cancer Inst Monogr. 2001:44–51. doi: 10.1093/oxfordjournals.jncimonographs.a003459. [DOI] [PubMed] [Google Scholar]
- 35.Aebi S, Gelber S, Castiglione-Gertsch M et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355:1869–1874. doi: 10.1016/s0140-6736(00)02292-3. [DOI] [PubMed] [Google Scholar]
- 36.Petrek JA, Naughton MJ, Case LD et al. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24:1045–1051. doi: 10.1200/JCO.2005.03.3969. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin PJ, Ennis M, Pritchard KI et al. Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol. 1999;17:2365–2370. doi: 10.1200/JCO.1999.17.8.2365. [DOI] [PubMed] [Google Scholar]
- 38.Krekow LK, Hellerstedt BA, Collea RP et al. Incidence and Predictive Factors for Recovery of Ovarian Function in Amenorrheic Women in Their 40 s Treated With Letrozole. J Clin Oncol. 2016;34:1594–1600. doi: 10.1200/JCO.2015.62.2985. [DOI] [PubMed] [Google Scholar]
- 39.Guerrero A, Gavila J, Folkerd E et al. Incidence and predictors of ovarian function recovery (OFR) in breast cancer (BC) patients with chemotherapy-induced amenorrhea (CIA) who switched from tamoxifen to exemestane. Ann Oncol. 2013;24:674–679. doi: 10.1093/annonc/mds464. [DOI] [PubMed] [Google Scholar]
- 40.Smith IE, Dowsett M, Yap YS et al. Adjuvant aromatase inhibitors for early breast cancer after chemotherapy-induced amenorrhoea: caution and suggested guidelines. J Clin Oncol. 2006;24:2444–2447. doi: 10.1200/JCO.2005.05.3694. [DOI] [PubMed] [Google Scholar]
- 41.Beatson GT. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;ii:104–117. [PMC free article] [PubMed] [Google Scholar]
- 42.De Courmelles FV. La radiothérapie indirecte, ou dirigée par les corrélations organiques. Arch Elect Med. 1922;32:264. [Google Scholar]
- 43.Early Breast Cancer Trialists’ Collaborative Group . Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1992;339:71–85. [PubMed] [Google Scholar]
- 44.Bonadonna G, Brusamolino E, Valagussa P et al. Combination chemotherapy as an adjuvant treatment in operable breast cancer. N Engl J Med. 1976;294:405–410. doi: 10.1056/NEJM197602192940801. [DOI] [PubMed] [Google Scholar]
- 45.Early Breast Cancer Trialists’ Collaborative Group . Ovarian ablation in early breast cancer: overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1996;348:1189–1196. [PubMed] [Google Scholar]
- 46.Ditsch N, Wöcke A, Untch M et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2022. Breast Care. 2022 doi: 10.1159/000524879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft und Deutsche Krebshilfe und AWMF) . S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Version 4.0, AWMF Registernummer:032–045OL. 2017. http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/ http://www.leitlinienprogramm-onkologie.de/leitlinien/mammakarzinom/
- 48.Goldhirsch A, Winer EP, Coates AS et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldhirsch A, Wood WC, Coates AS et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goldhirsch A, Ingle JN, Gelber RD et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldhirsch A, Wood WC, Gelber RD et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 52.Goldhirsch A, Glick JH, Gelber RD et al. Meeting highlights: international expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 53.Goldhirsch A, Wood WC, Gelber RD et al. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365. doi: 10.1200/jco.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 54.Griggs JJ, Somerfield MR, Anderson H et al. American Society of Clinical Oncology endorsement of the cancer care Ontario practice guideline on adjuvant ovarian ablation in the treatment of premenopausal women with early-stage invasive breast cancer. J Clin Oncol. 2011;29:3939–3942. doi: 10.1200/JCO.2011.36.4950. [DOI] [PubMed] [Google Scholar]
- 55.Jonat W, Kaufmann M, Sauerbrei W et al. Goserelin versus cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association Study. J Clin Oncol. 2002;20:4628–4635. doi: 10.1200/JCO.2002.05.042. [DOI] [PubMed] [Google Scholar]
- 56.clinicaltrials.gov . NIH US National Library of Medicine; 2003. NCT00066690. Suppression of Ovarian Function With Either Tamoxifen or Exemestane Compared With Tamoxifen Alone in Treating Premenopausal Women With Hormone-Responsive Breast Cancer (SOFT) [Google Scholar]
- 57.clinicaltrials.gov . NIH US National Library of Medicine; 2003. NCT00066703. Triptorelin With Either Exemestane or Tamoxifen in Treating Premenopausal Women With Hormone-Responsive Breast Cancer (TEXT) [Google Scholar]
- 58.clinicaltrials.gov . NIH US National Library of Medicine; 2003. NCT00066807. Premenopausal Endocrine Responsive Chemotherapy Trial (PERCHE) [Google Scholar]
- 59.clinicaltrials.gov . NIH US National Library of Medicine; 2006. NCT00295646. Tamoxifen Versus Anastrozole, Alone or in Combination With Zoledronic Acid. [Google Scholar]
- 60.clinicaltrials.gov ; https://clinicaltrials.gov/ct2/show/ NCT00412022 . NIH US National Library of Medicine; 2006. NCT00412022. HOBOE: A Phase 3 Study of Adjuvant Triptorelin and Tamoxifen, Letrozole, or Letrozole and Zoledronic Acid in Premenopausal Patients With Breast Cancer. (HOBOE) [Google Scholar]
- 61.Early Breast Cancer Trialists’ Collaborative Group . Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022;23:382–392. doi: 10.1016/S1470-2045(21)00758-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bradley R, Braybrooke J, Gray R . 2021. Aromatase inhibitors versus tamoxifen in pre-menopausal women with estrogen receptor positive early stage breast cancer treated with ovarian suppression: A patient level meta-analysis of 7,030 women in four randomised trials; p. GS2–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Regan MM, Walley BA, Fleming GF . 2022. Randomized comparison of adjuvant aromatase inhibitor exemestane (E) plus ovarian function suppression (OFS) vs tamoxifen (T) plus OFS in premenopausal women with hormone receptor-positive (HR+) early breast cancer (BC): update of the combined TEXT and SOFT trials; p. GS2–05. [Google Scholar]
- 64.Vaz-Luis I, Francis PA, Di Meglio A et al. Challenges in Adjuvant Therapy for Premenopausal Women Diagnosed With Luminal Breast Cancers. Am Soc Clin Oncol Educ Book. 2021;41:1–15. doi: 10.1200/EDBK_320595. [DOI] [PubMed] [Google Scholar]
- 65.Ferreira AR, Di Meglio A, Pistilli B et al. Differential impact of endocrine therapy and chemotherapy on quality of life of breast cancer survivors: a prospective patient-reported outcomes analysis. Ann Oncol. 2019;30:1784–1795. doi: 10.1093/annonc/mdz298. [DOI] [PubMed] [Google Scholar]
- 66.Bernhard J, Luo W, Ribi K et al. Patient-reported outcomes with adjuvant exemestane versus tamoxifen in premenopausal women with early breast cancer undergoing ovarian suppression (TEXT and SOFT): a combined analysis of two phase 3 randomised trials. Lancet Oncol. 2015;16:848–858. doi: 10.1016/S1470-2045(15)00049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ribi K, Luo W, Bernhard J et al. Adjuvant Tamoxifen Plus Ovarian Function Suppression Versus Tamoxifen Alone in Premenopausal Women With Early Breast Cancer: Patient-Reported Outcomes in the Suppression of Ovarian Function Trial. J Clin Oncol. 2016;34:1601–1610. doi: 10.1200/JCO.2015.64.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saha P, Regan MM, Pagani O et al. Treatment Efficacy, Adherence, and Quality of Life Among Women Younger Than 35 Years in the International Breast Cancer Study Group TEXT and SOFT Adjuvant Endocrine Therapy Trials. J Clin Oncol. 2017;35:3113–3122. doi: 10.1200/JCO.2016.72.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnston SRD, Harbeck N, Hegg R et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE) J Clin Oncol. 2020;38:3987–3998. doi: 10.1200/JCO.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rachner TD, Gobel A, Jaschke NP et al. Challenges in Preventing Bone Loss Induced by Aromatase Inhibitors. J Clin Endocrinol Metab. 2020;105:dgaa463. doi: 10.1210/clinem/dgaa463. [DOI] [PubMed] [Google Scholar]
- 71.Ramchand SK, Seeman E, Wang XF et al. Premenopausal women with early breast cancer treated with estradiol suppression have severely deteriorated bone microstructure. Bone. 2017;103:131–135. doi: 10.1016/j.bone.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 72.Gnant M, Mlineritsch B, Luschin-Ebengreuth G et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9:840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 73.Nabieva N, Fehm T, Haberle L et al. Influence of side-effects on early therapy persistence with letrozole in post-menopausal patients with early breast cancer: Results of the prospective EvAluate-TM study. Eur J Cancer. 2018;96:82–90. doi: 10.1016/j.ejca.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 74.Nabieva N, Haberle L, Brucker SY et al. Preexisting musculoskeletal burden and its development under letrozole treatment in early breast cancer patients. Int J Cancer. 2019;145:2114–2121. doi: 10.1002/ijc.32294. [DOI] [PubMed] [Google Scholar]
- 75.Nabieva N, Kellner S, Fehm T et al. Influence of patient and tumor characteristics on early therapy persistence with letrozole in postmenopausal women with early breast cancer: results of the prospective Evaluate-TM study with 3941 patients. Ann Oncol. 2018;29:186–192. doi: 10.1093/annonc/mdx630. [DOI] [PubMed] [Google Scholar]
- 76.von Minckwitz G, Untch M, Nuesch E et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125:145–156. doi: 10.1007/s10549-010-1228-x. [DOI] [PubMed] [Google Scholar]
- 77.Sparano JA, Gray RJ, Makower DF et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373:2005–2014. doi: 10.1056/NEJMoa1510764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cardoso F, van’t Veer LJ, Bogaerts J et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375:717–729. doi: 10.1056/NEJMoa1602253. [DOI] [PubMed] [Google Scholar]
- 79.Sparano JA, Gray RJ, Makower DF et al. Impact of clinical risk category on prognosis and prediction of chemotherapy benefit in early breast cancer (EBC) by age and the 21-gene recurrence score (RS) in TAILORx. J Clin Oncol. 2019;37 15:503. doi: 10.1200/JCO.2019.37.15_suppl.503. [DOI] [Google Scholar]
- 80.Kalinsky KM, Barlow WE, Gralow JR . 2021. Distant-disease free interval in participants (pts) with 1–3 positive lymph nodes (LN), hormone receptor-positive (HR+) and her2-negative (HER2-) breast cancer (BC) with recurrence score (RS) < or = 25 randomized to endocrine therapy (ET) +/- chemotherapy (CT): SWOG s1007 (RxPONDER) p. GS2–07. [Google Scholar]
- 81.Rohatgi A. WebPlotDigitizer Version: 4.6. https://automeris.io/WebPlotDigitizer https://automeris.io/WebPlotDigitizer
- 82.Sparano J, Gray RJ, Makower D . 2022. Trial Assigning Individualized Options for Treatment (TAILORx): An update including 12-year event rates; p. GS1–05. [Google Scholar]
- 83.Harbeck N, Rastogi P, Martin M et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Paluch-Shimon S, Lueck H, Beith J et al. Adjuvant endocrine therapy combined with abemaciclib in monarchE patients with high-risk early breast cancer: Disease characteristics and endocrine therapy choice by menopausal status. Ann Oncol. 2021;32 05:S407–S446. [Google Scholar]
- 85.clinicaltrials.gov . 2018. NCT03701334. A Trial to Evaluate Efficacy and Safety of Ribociclib With Endocrine Therapy as Adjuvant Treatment in Patients With HR+/HER2- Early Breast Cancer (NATALEE) [Google Scholar]
- 86.Slamon DJ, Fasching PA, Patel R et al. NATALEE: Phase III study of ribociclib (RIBO) + endocrine therapy (ET) as adjuvant treatment in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) early breast cancer (EBC) J Clin Oncol. 2019;37:TPS597. doi: 10.1200/JCO.2019.37.15_suppl.TPS597. [DOI] [Google Scholar]
- 87.Maccio A, Madeddu C. Obesity, inflammation, and postmenopausal breast cancer: therapeutic implications. ScientificWorldJournal. 2011;11:2020–2036. doi: 10.1100/2011/806787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Welslau M, Muller V, Luftner D et al. Update Breast Cancer 2022 Part 1 – Early Stage Breast Cancer. Geburtshilfe Frauenheilkd. 2022;82:580–589. doi: 10.1055/a-1811-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]











