Abstract

A general strategy for the synthesis of 2N,4N′-disubstituted glycoluril enantiomers on a multigram scale using orthogonal protection is reported. The use of these glycolurils is demonstrated in the synthesis of enantiomerically pure bambus[6]uril macrocycles. Moreover, the deprotection of (S)-1-phenylethyl substituents on the macrocycle was achieved, opening access to various chiral bambus[6]urils via post-macrocyclization modification strategy.
Introduction
Chiral macrocycles play an important role among supramolecular host molecules because of their ability to specifically interact with chiral guest molecules. Selective and strong host–guest complex formation is due to a well-confined binding site of the macrocycle that offers a multivalent interaction with the guest. Thus, chiral macrocycles are often used for the differentiation, separation, and sensing of chiral compounds with great potential in pharmaceutical, material chemistry, and biology.1,2
Glycoluril and its derivatives are rigid heterocycles with a curved backbone that have been used as building blocks for supramolecular hosts3−5 such as molecular clips,6 tweezers,7 baskets,8 and three-dimensional capsules.9,10 The most widely used glycoluril-based hosts are barrel-shaped macrocyclic molecules: cucurbit[n]urils4,11−13 and bambus[n]urils.14,15 Preparation of chiral cucurbituril derivatives as racemates was only reported without further isolation of the corresponding enantiomers.16−21 Other closely related chiral macrocycles such as biotin[6]uril22 and cyclohexylhemicucurbiturils23−26 were isolated as pure enantiomers, but their enantioselective binding of chiral carboxylates was demonstrated only in one case.24
Bambus[n]urils are macrocyclic molecules consisting of n alternating 2N,4N′-disubstituted glycoluril units connected by one row of methylene bridges. Six-membered bambusurils, bambus[6]urils, form stable inclusion complexes with various inorganic anions in which a single anion is usually positioned in their electron-positive cavity, further stabilized by hydrogen bonds with hydrogen methine atoms of glycoluril constitutional units. Bambus[6]urils show high association constants ranging up to 1011 M–1 in organic solvents.27 They function as efficient anion transporters,27 supramolecular hydrogels,28 as rotaxane constituents,29 and in the selective recognition of diacyanoaurate(I).30
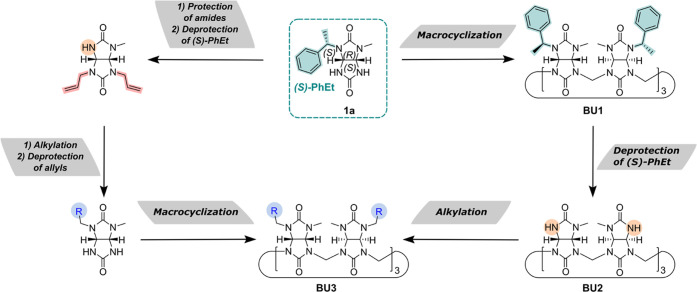
Majority of bambusurils are achiral.15 However, the synthesis of the first chiral bambusurils was recently reported, and their ability to bind enantiomers of biologically relevant compounds with selectivity exceeding 3 was demonstrated.31 The starting monomers for the synthesis of enantiomerically pure bambusurils are 2N,4N′-disubstituted glycolurils, bearing two different substituents, which are produced as a mixture of two stereoisomers. When a racemic mixture of glycolurils was used for the bambus[4]urils synthesis, a mixture of macrocycle stereoisomers was obtained, from which chiral macrocycles were separated by time-consuming and expensive high-performance liquid chromatography with chiral stationary phase.32 On the other hand, the use of a single stereoisomer in the macrocyclization reaction resulted in the selective preparation of enantiomerically pure bambusuril.33 Thus, isolation of a single glycoluril stereoisomer from the mixture is highly beneficial prior to the macrocyclization. When both substituents of 2N,4N′-disubstituted glycolurils are achiral, the glycolurils are produced as a racemic mixture from which isolation of a pure enantiomer can be challenging. This is why our previously reported approach was based on the preparation of 2N,4N′-disubstituted glycoluril 1 bearing (S)-phenylethyl and methyl substituents.31 As a consequence, glycoluril 1 was prepared as a mixture of diastereomers 1a and 1b. A single stereoisomer 1a (for structure, see Scheme 1) was separated on a multigram scale from the mixture based on its different solubility in methanol and isopropanol. Simple preparation and isolation lacking chromatography purification and the use of relatively inexpensive starting material, (S)-1-phenylethylamine, make glycoluril 1a ideal for the preparation of chiral bambus[6]uril BU1 on gram scale. (S/R)-1-Phenylethylamine is used in diastereoselective additions of nucleophiles34−36 mainly as chiral auxiliary,37 which can be removed by hydrogenolysis36 or by using organic acids.38−40 If such a deprotection is possible on glycolurils, we may use it in conjunction with 1a in the synthesis of a wide variety of chiral glycolurils and, consequently, the synthesis of chiral bambus[6]urils (BU3, Scheme 1). Deprotection of the (S/R)-1-phenylethyl group on bambus[6]uril BU1 represents the alternative pathway to chiral bambus[6]urils (BU3). Here, we report our results on this line.
Scheme 1. Schematic Overview of the Preparation of Enantiomerically Pure Bambus[6]uril BU3via Orthogonal Protective Steps of 1a and Post-Macrocyclization Modification of BU1.
Results and Discussion
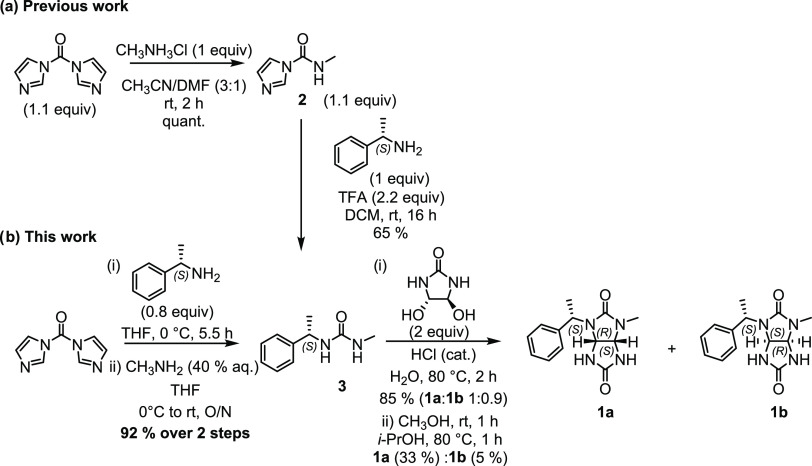
The previously described synthesis of glycoluril 1a (Scheme 2a) had several drawbacks.31 The synthesis of chiral urea 3 starting from 1,1-carbonyldiimidazole (CDI) was time-consuming, as the intermediate N-methyl carbamoylimidazole 2 was obtained only after column chromatography purification.41 Furthermore, the reaction of 2 and (S)-1-phenylethylamine yielded urea 3 only in a moderate yield of 65%.31 Thus, we searched for an alternative approach. Inspired by a procedure reported by Padiya et al.,42 we prepared 3 in one pot by a two-step reaction employing CDI (Scheme 2b and Table S1). The reaction of CDI with (S)-1-phenylethylamine in THF resulted in 3 (65%), which was contaminated by undesired N,N′-bis((S)-1-phenylethyl)urea (35%). The formation of the side product was suppressed by slow addition of a dilute solution of (S)-1-phenylethylamine in THF using a syringe pump at 0 °C. In contrast to the slow addition of (S)-1-phenylethylamine, aqueous methylamine was added in one portion, followed by overnight stirring at room temperature. The resulting urea 3 was obtained with an overall yield of 92%.
Scheme 2. Synthesis of Glycolurils 1a and 1b.
(a) Previously reported synthesis route.31,41 (b) This work.
Following a modified literature procedure,31 condensation of urea 3 with trans-4,5-dihydroxyimidazolidin-2-one afforded a mixture of diastereomers 1a and 1b (Scheme 2). For the following steps, we decided to use less soluble glycoluril 1a, which was isolated from the glycoluril mixture by washing it with methanol and isopropanol.
Glycoluril 1a was alkylated with allyl bromide in the presence of a base (Scheme 3, Table S2). We first tested sodium hydroxide to obtain glycoluril 4 in a yield of 58 and 68%. Later, we found that the reaction gives a better yield (96%) of 4 in the presence of cesium carbonate (Scheme 3). Two main reasons for the high yield of 4 were identified: (a) the cesium salt is a mild base and does not cause decomposition of both the starting material and the product, and (b) cesium salts can be removed from the reaction mixture by simple filtration.
Scheme 3. Protection of Nitrogen Atoms of Glycoluril 1a with Allyl Bromide.
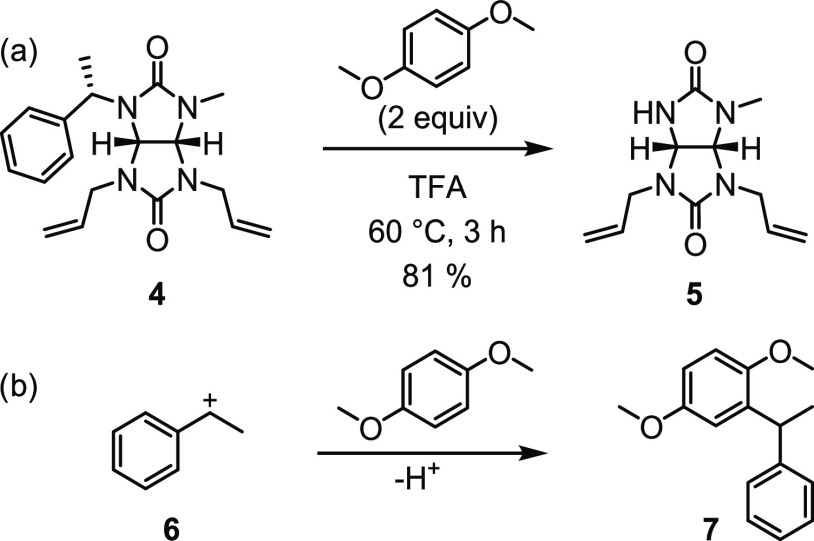
The allyl group was chosen as a suitable orthogonal protective group for the two NH positions of glycoluril 1a, since it can withstand acidic conditions required for the deprotection of (S)-1-phenylethyl group. The removal of the (S)-1-phenylethyl group from glycoluril 4 was first tested by formic acid (Table S3).38 However, the long reaction time resulted in the decomposition of the starting material and the product. Better results were achieved using neat trifluoroacetic acid (TFA) at 60 °C, which yielded the desired product 5 (Scheme 4a, Table S3). However, TLC analysis of the reaction mixture showed the presence of multiple products. We hypothesized that it could be caused by an undesired reaction of cleaved 1-phenylethan-1-ylium cation 6.43 Thus, we included a cation scavenger, 1,4-dimethoxybenzene, in the reaction, which resulted in a less complex reaction mixture and in an improvement of the yield of 5 from 70 to 81% (Scheme 4a). We were also able to isolate the byproduct 7 formed by the reaction of phenylethyl cation 6 and 1,4-dimethoxybenzene (Scheme 4b).44
Scheme 4. (a) Cleavage of the (S)-1-Phenylethyl Group and (b) Reaction of 1-Phenylethan-1-ylium Cation 6 with 1,4-Dimethoxybenzene.
Our next step was to alkylate the NH position of enantiomerically pure glycoluril 5 with benzyl derivatives bearing various substituents (H, NO2, CF3, COOCH3, Scheme 5). The reactions were carried out in CH3CN at 60 °C in the presence of cesium carbonate, yielding 8a–8d in yields of 72–93%.45
Scheme 5. Alkylation of Glycoluril 5 followed by Cleavage of the Allyl Groups of 8a–8d.
The final step in the synthesis of 2N,4N′-disubstituted chiral glycolurils 9a–9d was the deprotection of allyl groups on 8a–8d (Scheme 5). Three different reaction conditions were tested using 8c as a model compound (Table S4). The first attempt was inspired by Zacuto and Xu.46 RhCl3 and glycoluril 8c were refluxed in anhydrous n-propanol for 16 h, yielding the desired glycoluril 9c in a yield of 46% (Table S4). Next, we followed a modified procedure reported by Cadierno.47 The starting materials, ruthenium catalyst dichloro-[(2,6,10-dodecatriene)-1,12-diyl]ruthenium(IV) and KIO4, were heated at 80 °C in H2O/CH3CN mixture for 3 days, but the desired product was not detected. Lastly, we tested the conditions published by Ohmura.48 Glycoluril 8c, palladium(II) trifluoroacetate, and 1,3-bis(diphenylphosphino)propane (dppp) were heated in H2O/CH3CN mixture at 60 °C (Scheme 5, Table S4). The reaction took 6 days to complete with an 85% yield of 9c. We were able to reduce the reaction time to 30 min by performing the reaction in a closed vessel using a microwave reactor at 120 °C, obtaining 9c in 89% yield. The latter procedure was used to convert glycolurils 8a–8d into 9a–9d with high yields of 84–89%. To demonstrate the potential of prepared chiral glycolurils, we selected glycoluril 9a and used it in the synthesis of bambusuril BU3a. The reaction was performed in dry dioxane in the presence of paraformaldehyde and a catalytic amount of sulfuric acid. The compound was isolated as HSO4–@BU3a in 46% yield.
The successful deprotection of the (S)-1-phenylethyl group on glycoluril inspired us to investigate the deprotection of the same group in the case of bambusuril BU1 (Scheme 6). Anion free macrocycle BU1 was previously prepared in our group in 14% yield.31 However, in this work, we were able to increase its yield to 61% by improving its isolation and purification. Deprotection of BU1 was performed in TFA/DCM (1:1) in the presence of 1,4-dimethoxybenzene at 45 °C for 2.5 h (Scheme 6). BU2 was isolated in 83% yield.
Scheme 6. Cleavage of the (S)-1-Phenylethyl Group from BU1 Resulting in BU2 and Subsequent Alkylation of BU2 into BU3a–BU3e.
To demonstrate that BU2 can be further modified and used for the synthesis of various enantiomerically pure bambus[6]uril derivatives, BU2 was alkylated with benzyl bromide derivatives or propargyl bromide(Scheme 6). The alkylation was carried out in dry DMF or DMSO in the presence of Cs2CO3, NaH, or LiH under an argon atmosphere overnight. Enantiomerically pure bambusurils BU3a–BU3e were isolated anion free or as bromide complexes in yields of 53–94%.
Conclusions
Two general synthetic strategies to obtain enantiomerically pure bambus[6]urils were described (Scheme 1). Both routes utilized deprotection of (S)-1-phenylethyl group attached to glycoluril. The first route, derived from diastereomerically pure glycoluril 1a using orthogonal protection and deprotection cycles of allyl and (S)-1-phenylethyl groups, resulted in several enantiomerically pure glycolurils 9a–9d. p-Methoxybenzyl,49,50tert-butyloxycarbonyl,49 benzyl,51 and acetyl51 groups have been used in the protection of glycoluril’s nitrogen atoms. However, to the best of our knowledge, orthogonal (de)protection of glycolurils has not been reported to date. Glycoluril 9a was further macrocyclized into enantiomerically pure bambus[6]uril BU3a to demonstrate the potential of these glycoluril derivatives. The second route leading to enantiomerically pure bambus[6]urils BU3a–BU3e was based on the deprotection of (S)-1-phenylethyl groups of BU1 and subsequent alkylation of BU2. Both synthetic strategies allow straightforward access to a large library of enantiomerically pure bambus[6]uril macrocycles. However, the second route based on the deprotection of BU1 is preferable over the first route for the bambus[6]uril synthesis as it comprises less synthetic steps and affords BU3 in a slightly higher yield. The presence of functional groups such as bromobenzyl (BU3b) and propargyl (BU3e) allows further modification on the macrocycles by using, for example, cross-coupling and azide-alkyne Huisgen cycloaddition reactions.
Experimental Section
General
All reagents and solvents used were purchased from commercial suppliers and used without further purification. trans-4,5-Dihydroxyimidazolidin-2-one was synthesized based on a reported procedure.52 Reaction mixtures were heated on DrySyn heating blocks, and the reaction temperatures stated refer to the settings of the magnetic stirrer. Microwave syntheses were performed in pressurized sealed Discovered SP vessels closed with Activent caps. MW material was purchased from CEM. The Dynamic Control method was used for all microwave reactions, where the temperature and the pressure were set (P = 150 W max, T = 120 °C, 300 PSI max). Reactions were monitored by thin-layer chromatography (TLC) using aluminum plates precoated with silica gel (60 F254, Merck) impregnated with a fluorescent indicator. TLC plates were visualized with ultraviolet light (λ = 254 nm) and by staining with aqueous potassium permanganate (KMnO4) or ceric ammonium molybdate (CAM), followed by heating. Flash column chromatography was performed using silica gel (60 Å, 40–63 μm, Fluorochem) or CombiFlash NextGen 300 from Teledyne ISCO. NMR spectra were recorded on a Bruker Avance III HD 500 and Avance III 300 MHz spectrometer equipped with a BBFO probe with working frequency 500 MHz or 300 MHz for 1H, 126 MHz for 13C{1H}, and 471 or 282 MHz for 19F{1H}. All experiments were recorded at 303.15 K. NMR chemical shifts (δ) are reported in parts per million (ppm) using a residual solvent signal as a reference for the measured spectra in DMSO-d6 (1H = 2.50, 13C = 39.52) and CD3CN (1H = 1.94, 13C = 1.32). 19F NMR spectra were not referenced. Multiplicities are reported as singlet (s), doublet (d), doublet of doublets (dd), doublet of doublet of triplets (ddt), doublet of quartets (dq), triplet (t), quartet (q), multiplet (m), and broad (br). Signals were assigned with the aid of 1H-1H COSY, 1H-13C HSQC, and 1H-13C HMBC experiments. High-resolution mass spectra (HRMS) were obtained on Agilent 6224 accurate-mass time-of-flight (TOF) mass spectrometer. Samples were ionized by electrospray ionization (ESI) or atmospheric pressure chemical ionization (APCI). Matrix-assisted laser desorption ionization with detection of time-of-flight (MALDI-TOF) mass spectra were measured on the MALDI-TOF MS UltrafleXtreme (Bruker Daltonics). Samples were ionized by Nd-YAG laser (355 nm) from 2,5-dihydroxybenzoic acid (DHB) matrix. Melting points were measured on a Stuart SMP40 melting point apparatus.
Glycolurils 1a and 1b
The reaction procedure for separation of diastereomers was modified from a previously published procedure.31 Urea 1 (7.68 g, 43.09 mmol, 1.0 equiv) and trans-4,5-dihydroxyimidazolidin-2-one (10.15 g, 85.95 mmol, 2.0 equiv) were weighed into a 250 mL round-bottom flask. Water (70 mL) was added, and the mixture was heated to 80 °C. After 15 min, HCl (10%; 2 mL) was added, and heating was continued at 80 °C. The solids gradually dissolved, and a white precipitate emerged. After 2 h, the reaction mixture was cooled to 0 °C, and the resulting solid was isolated by filtration, washed with water (2 × 25 mL), and dried in vacuo, yielding a mixture of diastereomers (9.50 g; 85%; 1a/1b 1:0.9). Rf = 0.33 (DCM/CH3OH 9:1; UV, KMnO4). The mixture of diastereomers (9.50 g) was suspended in CH3OH (60 mL), and the mixture was stirred at room temperature for 1 h. Solid was isolated by filtration and dried in vacuo. The solid was then suspended in i-PrOH (30 mL) and stirred at 80 °C for 1 h, and the resulting solid was isolated by filtration. The filtrate was left to stand at room temperature, and more precipitate was collected and dried in vacuo. The solids were combined, yielding the less soluble diastereomer 1a as a white solid (3.70 g; 33%). The methanolic filtrate was evaporated under reduced pressure to give a white solid, which was then recrystallized from boiling water (190 mL). Crystals were collected by filtration and dried in vacuo to give a mixture of diastereomers, which could be separated again. The aqueous filtrate was evaporated under reduced pressure to give a white solid, which was recrystallized from boiling i-PrOH (25 mL) to give the more soluble diastereomer 1b (561 mg; 5%). The spectroscopic data correspond to the literature.31
Urea 3
1,1′-Carbonyldiimidazole (10.50 g, 64.75 mmol, 1.0 equiv) was weighed into a 250 mL round-bottom flask equipped with a stir bar and septum. The flask was flushed with argon, and THF (80 mL, precooled) was added by a syringe. The resulting white suspension was cooled to 0 °C while stirring the mixture vigorously. Solution of (S)-1-phenylethylamine (6.03 g, 49.76 mmol, 0.8 equiv) in THF (80 mL) was added to the reaction mixture by a syringe pump (20 mm diameter syringe with a volume of 20 mL, addition rate 300 μL min–1). The reaction mixture was cooled the whole time. The reaction mixture gradually dissolved to give a yellow transparent solution. After the addition was finished (5 h), the reaction was stirred for another 30 min to complete the transformation to intermediate. Methylamine (40% aq.; 6.00 g, 77.27 mmol, 1.2 equiv) was added to the reaction mixture in one portion at 0 °C, and the resulting yellow solution was stirred overnight, allowing the temperature to grow gradually to room temperature. The reaction mixture was evaporated under reduced pressure to give a yellow oily liquid, which solidified upon standing. The crude was diluted with HCl (10%; 50 mL), and a sticky white precipitate emerged. The mixture was extracted with DCM (3 × 50 mL); the combined organic layers were washed with HCl (10%; 25 mL) and brine (50 mL) and dried over anhydrous magnesium sulfate. The drying agent was filtered off, and the filtrate was evaporated under reduced pressure to give a white solid (8.18 g, 92%). The analytical sample was obtained by recrystallization from water. The spectroscopic data correspond to the literature.31Mp: 104–106 °C; Rf = 0.27 (DCM/CH3OH 19:1; UV, KMnO4). 1H NMR (500 MHz, DMSO-d6): δ 7.33–7.25 (m, 4H), 7.23–7.16 (m, 1H), 6.30 (d, J = 8.2 Hz, 1H), 5.63 (q, J = 4.8 Hz, 1H), 4.77–4.68 (m, 1H), 2.53 (d, J = 4.6 Hz, 3H), 1.30 (d, J = 6.9 Hz, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 157.8, 145.8, 128.1, 126.3, 125.7, 48.6, 26.2, 23.3. HRMS (APCI+) m/z: [M + H]+ Calcd for C10H15N2O 179.1179; found: 179.1181.
Glycoluril 4
Glycoluril 1a (2.59 g, 9.95 mmol, 1.0 equiv) and Cs2CO3 (9.82 g, 30.14 mmol, 3.0 equiv) were weighed into a 100 mL round-bottom flask equipped with a stir bar and septum. The flask was flushed with argon. Dry DMF (40 mL) was added, and the resulting white suspension was heated at 60 °C for 1 h. Allyl bromide (3.65 g, 30.17 mmol, 3.0 equiv) was added dropwise over 30 min. The resulting off-white suspension was stirred at 60 °C. TLC analysis after 4 h indicated the disappearance of the starting material. The reaction mixture was filtered through a Celite pad, and the pad was washed with additional DMF (20 mL). The clear yellow filtrate was evaporated under reduced pressure to give a dark orange oily liquid. The crude product was purified by column chromatography (SiO2, DCM/CH3OH 40:1) to give a white solid (3.25 g, 96%). Mp: 89–91 °C; Rf = 0.72 (DCM/CH3OH 9:1; UV, KMnO4). 1H NMR (500 MHz, DMSO-d6): δ 7.37–7.26 (m, 5H), 5.78 (m, 1H), 5.52 (m, 1H), 5.23–5.16 (m, 2H), 5.16 (d, J = 8.5 Hz, 1H), 5.12 (d, J = 8.5 Hz, 1H), 5.00 (dq, J = 10.4, 1.4 Hz, 1H), 4.87–4.78 (m, 2H), 3.98 (m, 2H), 3.81 (dd, J = 16.4, 6.2 Hz, 1H), 3.26 (dd, J = 16.2, 7.2 Hz, 1H), 2.80 (s, 3H), 1.58 (d, J = 7.0 Hz, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 158.1, 141.0, 133.8, 132.8, 128.3, 126.9, 126.7, 117.46, 116.92, 70.26, 67.70, 52.82, 45.10, 44.53, 29.90, 18.84. HRMS (APCI+) m/z: [M + H]+ Calcd for C19H25N4O2: 341.1972; found: 341.1975. Optical rotation: [α]58923 = 17.3° (c = 1.25 g/100 mL, MeOH)
Glycoluril 5
Glycoluril 4 (5.24 g, 15.39 mmol, 1.0 equiv) and 1,4-dimethoxybenzene (4.26 g, 30.83 mmol, 2.0 equiv) were weighed into a 100 mL round-bottom flask. TFA (15 mL) was added, and the resulting clear brown solution was stirred at 60 °C. TLC analysis (EtOAc) after 3 h indicated the disappearance of the starting material. The reaction mixture was cooled to 0 °C, and the reaction mixture was basified with saturated sodium carbonate solution (55 mL), strong gas evolution was observed, and the pH level reached 8–9. The resulting mixture was extracted with DCM (3 × 50 mL); the combined organic layers were washed with brine and dried over anhydrous magnesium sulfate. The drying agent was filtered off, and the filtrate was evaporated under reduced pressure to give a dark orange oil, which solidified upon standing. The crude product was purified by column chromatography (SiO2, DCM/CH3OH 20:1) to give an off-white solid (2.96 g, 81%). Mp: 90–93 °C; Rf = 0.15 (EtOAc; KMnO4). 1H NMR (500 MHz, DMSO-d6): δ 7.61 (s, 1H), 5.83–5.68 (m, 2H), 5.23– 5.12 (m, 6H), 3.98 (dd, J = 16.4, 5.0 Hz, 1H), 3.93 (dd, J = 15.9, 4.8 Hz, 1H), 3.77 (dd, J = 16.4, 6.2 Hz, 1H), 3.54 (dd, J = 16.0, 6.8 Hz, 1H), 2.74 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 159.6, 157.2, 134.1, 133.1, 117.3, 116.9, 71.4, 63.5, 45.0, 43.1, 29.4. HRMS (APCI+) m/z: [M + H]+ Calcd for C11H17N4O2 237.1346; found: 237.1349. Optical rotation: [α]58923 = 17.4° (c = 1.22 g/100 mL, MeOH)
General Procedure for the Alkylation of Glycoluril 5
Glycoluril 5 (4.32 mmol, 1.0 equiv, 0.43 M) and Cs2CO3 (8.62 mmol, 2.0 equiv, 0.86 M) were weighed into a 50 mL round-bottom flask equipped with a stir bar and septum. The flask was flushed with argon, and CH3CN (10 mL) was added. The resulting off-white suspension was stirred at 60 °C for 1 h under an argon atmosphere. Benzyl bromide derivative (6.48 mmol, 1.5 equiv, 1.3 M) in CH3CN (5 mL) was added to the reaction mixture dropwise over 40 min. The resulting mixture was stirred at 60 °C. TLC analysis (DCM/CH3OH) indicated the disappearance of the starting material. The reaction mixture was filtered through a Celite pad and was washed with additional CH3CN (10 mL). The filtrate was evaporated under reduced pressure, and the crude was purified by column chromatography.
Glycoluril 8a
Alkylation was performed based on the general procedure (reaction time: overnight). White solid; 150 mg (72%, calculated yield) from 150 mg (0.63 mmol) of 5 in CH3CN (2 mL); SiO2, gradient from DCM to DCM/CH3OH 99:1 as eluent. 1H NMR (500 MHz, DMSO-d6): δ 7.38–7.19 (m, 5H), 5.84–5.73 (m, 1H), 5.69–5.57 (m, 1H), 5.23–5.19 (m, 3H), 5.11–5.05 (m, 2H), 5.01 (dq, J = 17.1, 1.7 Hz, 1H), 4.54 (d, J = 16.3 Hz, 1H), 4.32 (d, J = 16.2 Hz, 1H), 4.06–3.80 (m, 3H), 3.40 (ddt, J = 16.2, 6.7, 1.3 Hz, 1H), 2.84 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 158.6, 157.8, 137.5, 133.9, 133.4, 128.5, 127.2, 127.1, 117.2, 116.9, 70.1, 67.7, 46.1, 45.2, 44.9, 30.1. HRMS (APCI+) m/z: [M + H]+ Calcd for C18H22N4O2: 327.1816; found: 327.1815. [α]58923 = −18.4° (c = 0.60 g/100 mL, MeOH).
Glycoluril 8b
Alkylation was performed based on the general procedure (reaction time: 4 h). Orange solid; yield 1.28 g (80%) from 1.02 g (4.32 mmol) of 5 in CH3CN (15 mL); SiO2, DCM/CH3OH 45:1 as eluent; Mp: 77–78 °C (decomp.); Rf = 0.51 (DCM/CH3OH 19:1; UV, KMnO4). 1H NMR (500 MHz, DMSO-d6): δ 8.21 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 8.4 Hz, 2H), 5.85–5.74 (m, 1H), 5.68–5.57 (m, 1H), 5.26–5.16 (m, 4H), 5.11–4.98 (m, 2H), 4.58 (d, J = 17.1 Hz, 1H), 4.53 (d, J = 17.1 Hz, 1H), 4.04–3.96 (m, 1H), 3.94–3.80 (m, 2H), 3.38 (dd, J = 16.3, 6.7 Hz, 1H), 2.85 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 158.6, 157.7, 146.7, 146.0, 133.8, 133.3, 128.1, 123.6, 117.3, 117.0, 70.3, 68.3, 46.0, 45.2, 45.0, 30.2. HRMS (APCI+) m/z: [M + H]+ Calcd for C18H22N5O4: 372.1666; found: 372.1666. [α]58923 = −13.9° (c = 1.19 g/100 mL, MeOH)
Glycoluril 8c
Alkylation was performed based on the general procedure (reaction time: 2 h). White solid; yield 4.11 g (93%) from 2.26 g (9.57 mmol) of 5 in CH3CN (25 mL); SiO2, DCM/CH3OH 60:1 as eluent; Mp: 114–117 °C; Rf = 0.54 (DCM/CH3OH 19:1; UV, KMnO4). 1H NMR (500 MHz, DMSO-d6): δ 8.02 (s, 1H), 7.93 (s, 2H), 5.79 (m, 1H), 5.57 (m, 1H), 5.26–5.14 (m, 4H), 5.03–4.91 (m, 2H), 4.61 (s, 2H), 3.99 (ddt, J = 16.5, 4.8, 1.6 Hz, 1H), 3.93–3.80 (m, 2H), 3.43 (ddt, J = 16.3, 6.6, 1.4 Hz, 1H), 2.84 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 158.7, 157.8, 141.8, 133.8, 133.4, 130.3 (q, J = 32.8 Hz), 127.9, 122.8 (q, J = 272.7 Hz), 120.9, 116.9, 116.8, 70.4, 68.6, 45.8, 45.1, 45.0, 30.1. 19F{1H} NMR (471 MHz, DMSO-d6): δ −61.35. HRMS (APCI+) m/z: [M + H]+ Calcd for C20H21F6N4O2: 463.1563; found: 463.1566. [α]58923 = 7.6° (c = 1.25 g/100 mL, MeOH)
Glycoluril 8d
Alkylation was performed based on the general procedure (reaction time: 3 h). Yellow wax; yield 1.22 g (85%) from 876 mg (3.71 mmol) of 5 in CH3CN (20 mL); SiO2, DCM/CH3OH 50:1 as eluent; Rf = 0.47 (DCM/CH3OH 19:1; UV, KMnO4). 1H NMR (500 MHz, DMSO-d6): δ 7.94 (d, J = 8.3 Hz, 2H), 7.37 (d, J = 8.3 Hz, 2H), 5.82–5.76 (m, 1H), 5.62-5.57 (m, 1H), 5.24–5.17 (m, 2H), 5.22 (d, J = 8.5 Hz, 1H), 5.15 (d, J = 8.5 Hz, 1H), 5.06 (dd, J = 10.3, 1.5 Hz, 1H), 4.99 (dd, J = 17.2, 1.7 Hz, 2H), 4.55 (d, J = 16.8 Hz, 1H), 4.45 (d, J = 16.8 Hz, 1H), 3.99 (dd, J = 16.5, 4.9 Hz, 1H), 3.89–3.84 (m, 2H), 3.85 (s, 3H), 3.35 (dd, J = 16.2, 6.7, 1.4 Hz, 1H), 2.85 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 166.0, 158.6, 157.7, 143.4, 133.9, 133.3, 129.4, 128.5, 127.3, 117.3, 116.9, 70.2, 68.0, 52.0, 46.1, 45.2, 44.9, 30.2. HRMS (APCI+) m/z: [M + H]+ Calcd for C20H24N4O4: 385.1870; found: 385.1868; [α]58923 = 19.1° (c = 1.48 g/100 mL, MeOH).
General Procedure for Deprotection of Allyl Groups
Pd(CF3COO)2 (0.1 equiv) and dppp (0.1 equiv) were added to a microwave (MW) vial with a stir bar, and the solids were flushed with argon. CH3CN (2.0 mL) and water (1.6 mL) were added to the solids, and the resulting solution was stirred for 10 min at room temperature. Allyl-protected glycoluril (8a–8d) (3.0 mmol, 1 equiv) in CH3CN (5.0 mL) was then added to the MW vial. The resulting solution was irradiated for 0.5–3 h. TLC analysis (DCM/CH3OH) indicated the disappearance of the starting material. The reaction mixture was filtered through a cotton wool, which was further washed with CH3CN (15 mL). The clear filtrate was evaporated under reduced pressure and further purified by column chromatography.
Glycoluril 9a
Deprotection of allyl groups was performed based on the general procedure. Irradiation parameters: 120 °C, 150 W max, 300 PSI max, medium stirring, 0.5 h. White solid; yield 100 mg (89%) from 150 mg (0.46 mmol) of 8a in CH3CN (3 mL) and H2O (330 μL); SiO2, DCM/CH3OH 50:1 as eluent. This compound was previously reported as a part of a racemic mixture with second enantiomer.321H NMR (500 MHz, DMSO-d6): δ 7.56 (m, 1H), 7.37–7.22 (m, 5H), 5.15 (dd, J = 8.0, 1.8 Hz, 1H), 5.02 (dd, J = 8.0, 1.8 Hz, 1H), 4.58 (d, J = 15.6 Hz, 1H), 4.00 (d, J = 15.6 Hz, 1H), 2.69 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 161.0, 157.4, 137.4, 128.4, 127.7, 127.1, 67.2, 65.0, 43.9, 27.7. HRMS (APCI+) m/z: [M + H]+ Calcd for C12H14N4O2: 247.1190; found: 247.1192. [α]58923 = −24.0° (c = 0.78 g/100 mL, MeOH).
Glycoluril 9b
Deprotection of allyl groups was performed based on the general procedure. Irradiation parameters: 120 °C, 150 W max, 300 PSI max, medium stirring, 0.5 h. Off-white foamy solid; yield 431 mg (86%) from 639 mg (1.72 mmol) of 8b in CH3CN (4 mL) and H2O (1.2 mL); SiO2, DCM/CH3OH 10:1 as eluent; Mp: 110 °C (decomp.); Rf = 0.23 (DCM/CH3OH 9:1; UV, CAM). 1H NMR (500 MHz, DMSO-d6): δ 8.21 (d, J = 8.6 Hz, 2H), 7.59 (s, 1H), 7.52 (d, J = 8.7 Hz, 2H), 7.50 (s, 1H), 5.19 (dd, J = 8.0, 1.7 Hz, 1H), 5.13 (dd, J = 8.1, 1.9 Hz, 1H), 4.61 (d, J = 16.4 Hz, 1H), 4.24 (d, J = 16.3 Hz, 1H), 2.70 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.8, 157.5, 146.7, 145.9, 128.6, 123.5, 67.4, 65.5, 43.8, 27.7. HRMS (APCI+) m/z: [M + H]+ Calcd for C12H14N5O4: 292.1040; found: 292.1038. [α]58923 = −46.5° (c = 1.20 g/100 mL, MeOH)
Glycoluril 9c
Deprotection of allyl groups was performed based on the general procedure. Irradiation parameters: 120 °C, 150 W max, 300 PSI max, medium stirring, 0.5 h. Off-white foamy solid; yield 144 mg (89%) from 194 mg (0.42 mmol) of 8c in CD3CN (1 mL) and D2O (0.3 mL); SiO2, DCM/CH3OH 10:1 as eluent; Mp: 99–101 °C (decomp.); Rf = 0.07 (DCM/CH3OH 19:1; UV, CAM). 1H NMR (500 MHz, DMSO-d6): δ 8.00 (s, 1H), 7.94 (s, 2H), 7.60 (s, 1H), 7.48 (s, 1H), 5.23–5.15 (m, 2H), 4.60 (d, J = 16.4 Hz, 1H), 4.33 (d, J = 16.4 Hz, 1H), 2.71 (d, J = 1.2 Hz, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 160.8, 157.6, 141.6, 130.2 (q, J = 32.7 Hz), 128.4 (d, J = 4.0 Hz), 122.8 (q, J = 273.4 Hz), 120.9, 67.52, 65.7, 43.9, 27.8. 19F{1H} NMR (471 MHz, DMSO-d6): δ −61.21. HRMS (APCI+) m/z: [M + H]+ Calcd for C14H13F6N4O2: 383.0937; found: 383.0935. [α]58923 = 16.5° (c = 1.04 g/100 mL, MeOH)
Glycoluril 9d
Deprotection of allyl groups was performed based on the general procedure. Irradiation parameters: 140 °C, 200 W max, 300 PSI max, medium stirring, 3 h. Off-white foamy solid; yield 753 mg (84%) from 1.14 g (2.95 mmol) of 8d in CD3CN (7 mL) and H2O (1.6 mL); SiO2, DCM/CH3OH 10:1 as eluent; Mp: 88–90 °C; Rf = 0.26 (DCM/CH3OH 9:1; UV, CAM). 1H NMR (500 MHz, DMSO-d6): δ 7.93 (d, J = 8.3 Hz, 2H), 7.57 (s, 1H), 7.53 (s, 1H), 7.39 (d, J = 8.3 Hz, 2H), 5.18 (dd, J = 8.1, 1.8 Hz, 1H), 5.08 (dd, J = 8.0, 1.8 Hz, 1H), 4.60 (d, J = 16.0 Hz, 1H), 4.14 (d, J = 16.1 Hz, 1H), 3.84 (s, 3H), 2.70 (s, 3H). 13C{1H} NMR (126 MHz, DMSO-d6): δ 166.0, 160.9, 157.5, 143.3, 129.3, 128.5, 127.8, 67.3, 65.3, 52.0, 43.8, 27.7. HRMS (APCI+) m/z: [M + H]+ Calcd for C14H16N4O4: 305.1244; found: 305.1242; [α]23589 = 68.2° (c = 1.45 g/100 mL, MeOH).
Macrocyclization
Bambus[6]uril HSO4–@BU3a
Glycoluril 9a (100 mg, 0.41 mmol, 1 equiv) and paraformaldehyde (15 mg, 0.50 mmol, 1.2 equiv) were suspended in dry dioxane (2 mL) and heated to 80 °C. H2SO4 (60 μL; 2.9% v/v) was added to the hot solution, and the clear solution was stirred at 80 °C for 2 h. The mixture was cooled to room temperature, and Et2O was added to precipitate the crude. The filtrated solid was further sonicated in CHCl3, filtrated, and dried in vacuo to yield HSO4–@BU3a as a white solid (51 mg, 46%). 1H NMR (500 MHz, DMSO-d6) δ 7.33–6.97 (m, 30H), 5.61 (dd, J = 8.5 Hz, 6H), 5.43 (dd, J = 8.5 Hz, 6H), 5.08–4.66 (m, 12H), 4.57 (d, J = 16.2 Hz, 6H), 4.25 (s, 6H), 2.98 (s, 18H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 159.1, 158.7, 139.2, 128.0, 126.6, 68.9, 68.6, 48.0, 46.9, 46.7, 30.6. HRMS (ESI+) m/z: [M + H]+ Calcd for C78H85N24O12: 1549.6773; found: 1549.6755. [α]58923 = −15.3° (c = 0.57 g/100 mL, MeOH)
Bambus[6]uril BU1
The reaction procedure was slightly modified from previously published procedure.31 Glycoluril 1a (2.50 g, 9.6 mmol, 1 equiv) and paraformaldehyde (325 mg, 10.8 mmol, 1.1 equiv) were dissolved in a mixture of dry dioxane (50 mL) and H2SO4 (1.4 mL; 2.7% v/v). The mixture was stirred at 80 °C for 80 min, after which the reaction mixture was allowed to cool to room temperature. Solid was collected by filtration, washed with dioxane (5 mL) and Et2O (2 × 25 mL), and dried in vacuo. Dried solid crude was dissolved in methanol (20 mL). Water (30 mL) and 25% ammonia (10 mL) were added, which resulted in precipitation of a white solid. The suspension was stirred at 80 °C overnight, after which it was cooled to room temperature and filtered. The collected solid was dissolved in DCM (25 mL) and sonicated for 10 min. The mixture was filtered through filter paper with fine pores to remove insoluble impurities. The filtrate was evaporated under reduced pressure to half of its volume when methanol (10 mL) was added. Evaporation under reduced pressure was continued to give BU1 as a white solid (1.60 g, 61%). All data correspond to those in the literature.31
Bambus[6]uril BU2
Bambus[6]uril BU1 (1.0 g, 0.61 mmol, 1 equiv) and 1,4-dimethoxybenzene (761 mg, 5.51 mmol, 9 equiv) were flushed with argon and dissolved in dry DCM (5 mL). TFA (5 mL) was added, and the solution was stirred at 45 °C for 2.5 h under an argon atmosphere. The solution was evaporated under reduced pressure and additionally co-evaporated with dichloromethane and methanol to dryness. The residue was suspended in ethanol (25 mL) and sonicated for 10 min, after which the suspension was centrifuged. The solid was washed with ethanol (2 × 4 mL) and Et2O (3 × 10 mL). The suspension was centrifuged after each wash to obtain BU2 as a white solid (513 mg, 83%). Mp: 356 °C (decomp.); 1H NMR (500 MHz, DMSO-d6) δ 7.82 (s, 6H), 5.22 (d, J = 8.2 Hz, 6H), 5.17 (d, J = 8.2 Hz, 6H), 4.82 (s, 6H), 4.66 (s, 6H), 2.96 (s, 18H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 159.5, 158.0, 70.3, 63.6, 48.3, 46.4, 30.0. HRMS (ESI−) m/z: [M + I]− Calcd for C36H48N24O12I: 1135.2934; found: 1135.2914. [α]58923 = −17.6° (c = 0.50 g/100 mL, MeOH)
Alkylation of Bambus[6]uril BU2
Bambus[6]uril BU3a
Bambus[6]uril BU2 (200 mg, 0.20 mmol, 1 equiv) and Cs2CO3 (600 mg, 1.84 mmol, 9.2 equiv) were dispersed in dry DMF (3.5 mL) under an argon atmosphere. Benzyl bromide (160 μL, 230 mg, 1.34 mmol, 6.7 equiv) was added, and the reaction mixture was stirred at room temperature for 18 h. The mixture was then filtered, and the reaction flask and the solids were washed with DMF (2 × 1 mL). DMF filtrate was evaporated to dryness, and the resulting solids were dispersed in Et2O (10 mL), collected by vacuum filtration, washed with Et2O (10 mL), and left to dry on the frit. The crude was treated with chloroform (10 mL) and sonicated for 5 min. The resulting suspension was filtered, and the solid was washed with chloroform (2 × 5 mL) and dried in vacuo, yielding Br–@BU3a (250 mg). The solid was dissolved in a mixture of CH3OH (4 mL) and DCM (2 mL). To the solution was added AgSbF6 (72 mg, 0.21 mmol) in CH3OH (3 mL), and the resulting suspension was stirred for 30 min. The solid was removed by filtration. The filtrate was concentrated in vacuo to approximately 3 mL, resulting in precipitation of a solid. The suspension was diluted with water (10 mL), and the solid was collected by filtration, washed with water (2 × 10 mL), and dried in vacuo yielding BU3a as a white solid (194 mg, 63%). 1H NMR (500 MHz, DMSO-d6) δ 7.27–7.14 (m, 30H), 5.23 (d, J = 8.3 Hz, 6H), 5.10 (d, J = 8.3 Hz, 6H), 4.84 (s, 6H), 4.64 (d, J = 16.3 Hz, 6H), 4.53 (d, J = 16.3 Hz, 6H), 4.16 (s, 6H), 3.05 (s, 18H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 159.1, 158.2, 138.1, 128.3, 127.0, 126.8, 69.7, 68.8, 48.3, 47.7, 47.0, 31.0. HRMS (ESI+) m/z: [M + H]+ Calcd for C78H85N24O12: 1549.6773; found: 1549.6718. [α]58923 = −176.0° (c = 0.50 g/100 mL, MeOH).
Bambus[6]uril Br–@BU3b
NaH (60% dispersion in oil; 182 mg, 7.93 mmol, 24 equiv) was weighed into a Schlenk flask, and argon was flushed through for 5 min. Dry DMF (4.8 mL) was added at 0 °C. Subsequently, BU2 (200 mg, 0.20 mmol, 1 equiv) was added in portions as a solid under an argon flow. The suspension was stirred at 0 °C for 1 h. 4-Bromobenzyl bromide (891 mg, 3.57 mmol, 18 equiv) was added in portions as a solid under an argon flow. The white mixture was stirred at 0 °C for 10 min, the cooling bath was removed, and the suspension was stirred at room temperature for 24 h. The reaction mixture was quenched with H2O (milli-Q; 1.5 mL) at 0 °C and stirred for another 10 min. EtOAc was added and transferred to a separating funnel. The organic layer was extracted with brine (5 × 10 mL) and dried over anhydrous Na2SO4. The solvent was removed under reduced pressure. The crude was dissolved in CH3OH/EtOAc (4:1) assisted by heating, and an excess of Et2O was added to precipitate the product Br–@BU3b as a white solid (300 mg, 72%). 1H NMR (500 MHz, CD3CN) δ 7.35 (d, J = 8.4 Hz, 12H), 7.18 (d, J = 8.1 Hz, 12H), 5.65 (d, J = 7.9 Hz, 6H), 5.46 (d, J = 8.5 Hz, 6H), 5.10 (s, 6H), 4.79 (d, J = 16.7 Hz, 6H), 4.53 (d, J = 16.7 Hz, 6H), 4.39 (s, 6H), 3.00 (s, 18H). 13C{1H} NMR (126 MHz, CD3CN) δ 160.0, 159.7, 140.0, 132.3, 129.5, 121.1, 69.4, 69.2, 49.9, 48.5, 47.9, 31.3. HRMS (ESI+) m/z: [M + Na]+ Calcd for C78H78Br6N24O12Na: 2045.1183; found: 2045.1125. [α]58923 = −14.6° (c = 0.54 g/100 mL, MeOH).
Bambus[6]uril Br–@BU3c
NaH (60% dispersion in oil; 48 mg, 1.2 mmol, 24 equiv) was weighed into a Schlenk flask, and argon was flushed through for 5 min. Dry DMF (1 mL) was added at 0 °C. Subsequently, BU2 (50 mg, 0.05 mmol, 1 equiv) was added in portions as a solid under an argon flow. The suspension was stirred at 0 °C for 1 h. Then, a solution of 3,5-bis(trifluoromethyl)benzyl bromide (368 mg, 1.2 mmol, 24 equiv) in dry DMF (1 mL) was added dropwise over 0.5 h. The white mixture was stirred at 0 °C for 10 min, the cooling bath was removed, and the suspension was stirred overnight at room temperature. The reaction mixture was quenched with H2O (milli-Q; 5 mL) at 0 °C and stirred for another 10 min. The precipitate was filtered and washed with H2O (milli-Q). The crude was purified by flash column chromatography to yield Br–@BU3c as a yellow solid (89 mg, 73%). 1H NMR (500 MHz, CD3CN) δ 7.81 (m, 18H), 5.66 (d, J = 8.5 Hz, 6H), 5.56 (d, J = 8.5 Hz, 6H), 5.12 (s, 6H), 4.99 (d, J = 17.4 Hz, 6H), 4.68 (d, J = 17.1 Hz, 6H), 4.14 (s, 6H), 3.05 (s, 18H). 13C{1H} NMR (126 MHz, CD3CN) δ 160.2, 159.8, 144.1, 132.1 (q, J = 33.6 Hz), 128.0 (br), 124.5 (q, J = 272.0 Hz), 121.8(br), 69.8, 69.6, 49.9, 48.3, 48.1, 31.3. 19F{1H} NMR (282 MHz, CD3CN): δ −63.33. MALDI-TOF(+)MS m/z: [M + Na]+ Calcd for C90H72F36N24O12Na: 2387.508; found: 2387.568. [α]58923 = −23.4° (c = 0.55 g/100 mL, MeOH).
Bambus[6]uril Br–@BU3d
Bambus[6]uril BU2 (100 mg, 0.10 mmol, 1 equiv) and Cs2CO3 (297 mg, 0.91 mmol, 9.1 equiv) were dispersed in dry DMF (2 mL) under an argon atmosphere. Methyl 4-(bromomethyl) benzoate (210 mg, 0.87 mmol, 8.7 equiv) was added, and the reaction mixture was stirred at room temperature for 24 h. The mixture was then filtered, and the reaction flask and the solid were washed with DMF (2 × 1 mL). Isolated solid was washed with Et2O (2 × 10 mL) and dried in a stream of air. The solid was poured into a mixture of water (10 mL) and acetic acid (1 mL) and sonicated for 5 min. The resulting suspension was filtered, and the isolated solid was washed with water (2 × 10 mL) and dried in vacuo. The compound was again washed with DMF (2 × 2 mL) and water (2 × 10 mL) and dried in vacuo yielding Br–@BU3d (120 mg, 53%). 1H NMR (300 MHz, DMSO-d6) δ 7.77 (d, J = 8.4 Hz, 12H), 7.28 (d, J = 8.3 Hz, 12H), 5.64 (d, J = 8.7 Hz, 6H), 5.47 (d, J = 8.5 Hz, 6H), 5.06 (s, 6H), 4.80 (d, J = 17.3 Hz, 6H), 4.59 (d, J = 17.2 Hz, 6H), 4.21 (s, 6H), 3.81 (s, 18H), 2.96 (s, 18H). All data correspond to those in the literature.33
Bambus[6]uril Br–@BU3e
BU2 (50 mg, 0.05 mmol, 1 equiv) and LiH (9.6 mg, 1.2 mmol, 24 equiv) were weighed into a vial and flushed with argon. The vial was sealed with a septum and attached with an argon balloon. Dry DMSO-d6 was added (750 μL), and the reaction mixture was stirred at 40 °C for 1 h. Propargyl bromide solution (80% in toluene; 99 μL, 0.75 mmol, 15 equiv) was added dropwise to the mixture, and the solution was stirred at 40 °C for 24 h. The reaction mixture was quenched with H2O (milli-Q; 5 mL). The precipitate was filtered, washed with H2O (milli-Q), and dried in vacuo to yield Br–@BU3e as a brown solid (62 mg, 94%). Mp: 310 °C (decomp.); 1H NMR (500 MHz, DMSO-d6) δ 5.75 (d, J = 8.6 Hz, 6H), 5.37 (d, J = 8.7 Hz, 6H), 5.05 (s, 6H), 5.00 (s, 6H), 4.43 (dd, J = 17.9, 2.4 Hz, 6H), 4.24 (dd, J = 17.9, 2.4 Hz, 6H), 3.02 (s, 18H), 2.66 (t, J = 2.4 Hz, 6H). 13C{1H} NMR (126 MHz, DMSO-d6) δ 158.3, 158.2, 80.3, 73.0, 68.5, 66.4, 48.6, 46.7, 33.7, 30.0. MALDI-TOF(-)MS m/z: [M + Br]- Calcd for C54H60N24O12Br: 1315.401; found: 1315.407. [α]58923 = 14.4° (c = 0.69 g/100 mL, MeOH).
Acknowledgments
This work was supported by the Czech Science Foundation (No. 23-05271S). The authors thank the RECETOX Research Infrastructure (No. LM2018121) financed by the Ministry of Education, Youth and Sports, and the Operational Programme Research, Development and Education (the CETOCOEN EXCELLENCE project No. CZ.02.1.01/ 0.0/0.0/17_043/0009632) for supportive background. This project was supported by the European Union’s Horizon 2020 Research and Innovation Programme under grant agreement No. 857560. This publication reflects only the author‘s view, and the European Commission is not responsible for any use that may be made of the information it contains. The authors acknowledge Proteomic Core Facility of CIISB, Instruct-CZ Centre, supported by MEYS CR (LM2018127).
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.3c00667.
Screening of reaction conditions of glycolurils (Tables) and NMR and MS spectra for all new compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Liang X.; Liang W.; Jin P.; Wang H.; Wu W.; Yang C. Advances in Chirality Sensing with Macrocyclic Molecules. Chemosensors 2021, 9, 279. 10.3390/chemosensors9100279. [DOI] [Google Scholar]
- Wang Y.; Wu H.; Stoddart J. F. Molecular Triangles: A New Class of Macrocycles. Acc. Chem. Res. 2021, 54, 2027–2039. 10.1021/acs.accounts.1c00108. [DOI] [PubMed] [Google Scholar]
- Rowan A. E.; Elemans J. A. A. W.; Nolte R. J. M. Molecular and Supramolecular Objects from Glycoluril. Acc. Chem. Res. 1999, 32, 995–1006. 10.1021/ar9702684. [DOI] [Google Scholar]
- Kim K.; Selvapalam N.; Ko Y. H.; Park K. M.; Kim D.; Kim J. Functionalized Cucurbiturils and Their Applications. Chem. Soc. Rev. 2007, 36, 267–279. 10.1039/B603088M. [DOI] [PubMed] [Google Scholar]
- Hardouin–Lerouge M.; Hudhomme P.; Sallé M. Molecular Clips and Tweezers Hosting Neutral Guests. Chem. Soc. Rev. 2011, 40, 30–43. 10.1039/B915145C. [DOI] [PubMed] [Google Scholar]
- Smeets J. W. H.; Sijbesma R. P.; Niele F. G. M.; Spek A. L.; Smeets W. J. J.; Nolte R. J. M. Novel Concave Building Block for the Synthesis of Organic Hosts. J. Am. Chem. Soc. 1987, 109, 928–929. 10.1021/ja00237a064. [DOI] [Google Scholar]
- Burnett C. A.; Witt D.; Fettinger J. C.; Isaacs L. Acyclic Congener of Cucurbituril: Synthesis and Recognition Properties. J. Org. Chem. 2003, 68, 6184–6191. 10.1021/jo034399w. [DOI] [PubMed] [Google Scholar]
- Smeets J. W. H.; Sijbesma R. P.; Van Dalen L.; Spek A. L.; Smeets W. J. J.; Nolte R. J. M. Synthesis and Binding Properties of Basket-Shaped Hosts. J. Org. Chem. 1989, 54, 3710–3717. 10.1021/jo00276a037. [DOI] [Google Scholar]
- Wyler R.; de Mendoza J.; Rebek J. Jr. A Synthetic Cavity Assembles Through Self-Complementary Hydrogen Bonds. Angew. Chem., Int. Ed. 1993, 32, 1699–1701. 10.1002/anie.199316991. [DOI] [Google Scholar]
- Meissner R. S.; Rebek J.; de Mendoza J. Autoencapsulation Through Intermolecular Forces: A Synthetic Self-Assembling Spherical Complex. Science 1995, 270, 1485–1488. 10.1126/science.270.5241.1485. [DOI] [PubMed] [Google Scholar]
- Freeman W. A.; Mock W. L.; Shih N. Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. 10.1021/ja00414a070. [DOI] [Google Scholar]
- Assaf K. I.; Nau W. M. Cucurbiturils: From Synthesis to High-Affinity Binding and Catalysis. Chem. Soc. Rev. 2015, 44, 394–418. 10.1039/C4CS00273C. [DOI] [PubMed] [Google Scholar]
- Barrow S. J.; Kasera S.; Rowland M. J.; del Barrio J.; Scherman O. A. Cucurbituril-Based Molecular Recognition. Chem. Rev. 2015, 115, 12320–12406. 10.1021/acs.chemrev.5b00341. [DOI] [PubMed] [Google Scholar]
- Svec J.; Necas M.; Sindelar V. Bambus[6]Uril. Angew. Chem., Int. Ed. 2010, 49, 2378–2381. 10.1002/anie.201000420. [DOI] [PubMed] [Google Scholar]
- Lizal T.; Sindelar V. Bambusuril Anion Receptors. Isr. J. Chem. 2018, 58, 326–333. 10.1002/ijch.201700111. [DOI] [Google Scholar]
- Lagona J.; Fettinger J. C.; Isaacs L. Cucurbit[n]Uril Analogues. Org. Lett. 2003, 5, 3745–3747. 10.1021/ol035468w. [DOI] [PubMed] [Google Scholar]
- Huang W.-H.; Zavalij P. Y.; Isaacs L. Chiral Recognition inside a Chiral Cucurbituril. Angew. Chem., Int. Ed. 2007, 46, 7425–7427. 10.1002/anie.200702189. [DOI] [PubMed] [Google Scholar]
- Huang W.-H.; Zavalij P. Y.; Isaacs L. Metal-Ion-Induced Folding and Dimerization of a Glycoluril Decamer in Water. Org. Lett. 2009, 11, 3918–3921. 10.1021/ol901539q. [DOI] [PubMed] [Google Scholar]
- Lucas D.; Minami T.; Iannuzzi G.; Cao L.; Wittenberg J. B.; Anzenbacher P. Jr; Isaacs L. Templated Synthesis of Glycoluril Hexamer and Monofunctionalized Cucurbit[6]Uril Derivatives. J. Am. Chem. Soc. 2011, 133, 17966–17976. 10.1021/ja208229d. [DOI] [PubMed] [Google Scholar]
- Cheng X.-J.; Liang L.-L.; Chen K.; Ji N.-N.; Xiao X.; Zhang J.-X.; Zhang Y.-Q.; Xue S.-F.; Zhu Q.-J.; Ni X.-L.; Tao Z. Twisted Cucurbit[14]Uril. Angew. Chem., Int. Ed. 2013, 52, 7252–7255. 10.1002/anie.201210267. [DOI] [PubMed] [Google Scholar]
- Li Q.; Qiu S.-C.; Zhang J.; Chen K.; Huang Y.; Xiao X.; Zhang Y.; Li F.; Zhang Y.-Q.; Xue S.-F.; Zhu Q.-J.; Tao Z.; Lindoy L. F.; Wei G. Twisted Cucurbit[n]Urils. Org. Lett. 2016, 18, 4020–4023. 10.1021/acs.orglett.6b01842. [DOI] [PubMed] [Google Scholar]
- Lisbjerg M.; Jessen B. M.; Rasmussen B.; Nielsen B. E.; Madsen A. Ø.; Pittelkow M. Discovery of a Cyclic 6 + 6 Hexamer of D-Biotin and Formaldehyde. Chem. Sci. 2014, 5, 2647–2650. 10.1039/C4SC00990H. [DOI] [Google Scholar]
- Aav R.; Shmatova E.; Reile I.; Borissova M.; Topić F.; Rissanen K. New Chiral Cyclohexylhemicucurbit[6]Uril. Org. Lett. 2013, 15, 3786–3789. 10.1021/ol401766a. [DOI] [PubMed] [Google Scholar]
- Prigorchenko E.; Öeren M.; Kaabel S.; Fomitšenko M.; Reile I.; Järving I.; Tamm T.; Topić F.; Rissanen K.; Aav R. Template-Controlled Synthesis of Chiral Cyclohexylhemicucurbit[8]Uril. Chem. Commun. 2015, 51, 10921–10924. 10.1039/C5CC04101E. [DOI] [PubMed] [Google Scholar]
- Mishra K. A.; Adamson J.; Öeren M.; Kaabel S.; Fomitšenko M.; Aav R. Dynamic Chiral Cyclohexanohemicucurbit[12]Uril. Chem. Commun. 2020, 56, 14645–14648. 10.1039/D0CC06817A. [DOI] [PubMed] [Google Scholar]
- Aav R.; Mishra K. A. The Breaking of Symmetry Leads to Chirality in Cucurbituril-Type Hosts. Symmetry 2018, 10, 98. 10.3390/sym10040098. [DOI] [Google Scholar]
- Valkenier H.; Akrawi O.; Jurček P.; Sleziaková K.; Lízal T.; Bartik K.; Šindelář V. Fluorinated Bambusurils as Highly Effective and Selective Transmembrane Cl–/HCO3– Antiporters. Chem 2019, 5, 429–444. 10.1016/j.chempr.2018.11.008. [DOI] [Google Scholar]
- Kokan Z.; Dušková-Smrčková M.; Šindelář V. Supramolecular Hydrogelation via Host-Guest Anion Recognition: Lamellar Hydrogel Materials for the Release of Cationic Cargo. Chem 2021, 7, 2473–2490. 10.1016/j.chempr.2021.06.024. [DOI] [Google Scholar]
- Kandrnálová M.; Kokan Z.; Havel V.; Nečas M.; Šindelář V. Hypervalent Iodine Based Reversible Covalent Bond in Rotaxane Synthesis. Angew. Chem., Int. Ed. 2019, 58, 18182–18185. 10.1002/anie.201908953. [DOI] [PubMed] [Google Scholar]
- Rando C.; Vázquez J.; Sokolov J.; Kokan Z.; Nečas M.; Šindelář V. Highly Efficient and Selective Recognition of Dicyanoaurate(I) by a Bambusuril Macrocycle in Water. Angew. Chem., Int. Ed. 2022, 61, e202210184 10.1002/anie.202210184. [DOI] [PubMed] [Google Scholar]
- Sokolov J.; Šindelář V. Chiral Bambusurils for Enantioselective Recognition of Carboxylate Anion Guests. Chem. - Eur. J. 2018, 24, 15482–15485. 10.1002/chem.201802748. [DOI] [PubMed] [Google Scholar]
- Mohite A. R.; Reany O. Inherently Chiral Bambus[4]Urils. J. Org. Chem. 2020, 85, 9190–9200. 10.1021/acs.joc.0c01174. [DOI] [PubMed] [Google Scholar]
- Sokolov J.; Štefek A.; Šindelář V. Functionalized Chiral Bambusurils: Synthesis and Host-Guest Interactions with Chiral Carboxylates. ChemPlusChem 2020, 85, 1307–1314. 10.1002/cplu.202000261. [DOI] [PubMed] [Google Scholar]
- Yamashita A.; Norton E. B.; Williamson R. T.; Ho D. M.; Sinishtaj S.; Mansour T. S. Use of Bis-(Chiral α-Methylbenzyl)Glycine Esters for Synthesis of Enantiopure β-Hydroxyamino Esters. Org. Lett. 2003, 5, 3305–3308. 10.1021/ol030085j. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y.; Maruyama K.; Komatsu T.; Ito W. Very High 1,2- and 1,3-Asymmetric Induction in the Reactions of Allylic Boron Compounds with Chiral Imines. J. Am. Chem. Soc. 1986, 108, 7778–7786. 10.1021/ja00284a048. [DOI] [PubMed] [Google Scholar]
- Nugent T. C.; Ghosh A. K.; Wakchaure V. N.; Mohanty R. R. Asymmetric Reductive Amination: Convenient Access to Enantioenriched Alkyl-Alkyl or Aryl-Alkyl Substituted α-Chiral Primary Amines. Adv. Synth. Catal. 2006, 348, 1289–1299. 10.1002/adsc.200606073. [DOI] [Google Scholar]
- Juaristi E.; Escalante J.; León-Romo J. L.; Reyes A. Recent Applications of α-Phenylethylamine (α-PEA) in the Preparation of Enantiopure Compounds. Part 1: Incorporation in Chiral Catalysts. Part 2: α-PEA and Derivatives as Resolving Agents. Tetrahedron: Asymmetry 1998, 9, 715–740. 10.1016/S0957-4166(98)00058-5. [DOI] [Google Scholar]
- Joullie M. M.; Wang P. C.; Semple J. E. Total Synthesis and Revised Structural Assignment of (+)-Furanomycin. J. Am. Chem. Soc. 1980, 102, 887–889. 10.1021/ja00522a095. [DOI] [Google Scholar]
- Paik S.; Lee J. Y. Simple and Efficient Cleavage of the N-(1-Phenylethyl) Unit of Carboxamides with Methanesulfonic Acid. Tetrahedron Lett. 2006, 47, 1813–1815. 10.1016/j.tetlet.2006.01.019. [DOI] [Google Scholar]
- Lorenc C.; Reeves J. T.; Busacca C. A.; Senanayake C. H. Acid Mediated Deprotection of N-Isopropyl Tertiary Amides. Tetrahedron Lett. 2015, 56, 1280–1282. 10.1016/j.tetlet.2015.01.161. [DOI] [Google Scholar]
- Duspara P. A.; Islam M. S.; Lough A. J.; Batey R. A. Synthesis and Reactivity of N-Alkyl Carbamoylimidazoles: Development of N-Methyl Carbamoylimidazole as a Methyl Isocyanate Equivalent. J. Org. Chem. 2012, 77, 10362–10368. 10.1021/jo302084a. [DOI] [PubMed] [Google Scholar]
- Padiya K. J.; Gavade S.; Kardile B.; Tiwari M.; Bajare S.; Mane M.; Gaware V.; Varghese S.; Harel D.; Kurhade S. Unprecedented “In Water” Imidazole Carbonylation: Paradigm Shift for Preparation of Urea and Carbamate. Org. Lett. 2012, 14, 2814–2817. 10.1021/ol301009d. [DOI] [PubMed] [Google Scholar]
- Wuts P. G. M.Greene’s Protective Groups in Organic Synthesis, 5th ed.; John Wiley & Sons: Hoboken, New Jersey, 2014. [Google Scholar]
- Jung M. E.; Koch P. Mild, Selective Deprotection of PMB Ethers with Triflic Acid/1,3-Dimethoxybenzene. Tetrahedron Lett. 2011, 52, 6051–6054. 10.1016/j.tetlet.2011.08.102. [DOI] [Google Scholar]
- De Simone N. A.; Chvojka M.; Lapešová J.; Martínez-Crespo L.; Slávik P.; Sokolov J.; Butler S. J.; Valkenier H.; Šindelář V. Monofunctionalized Fluorinated Bambusurils and Their Conjugates for Anion Transport and Extraction. J. Org. Chem. 2022, 87, 9829–9838. 10.1021/acs.joc.2c00870. [DOI] [PubMed] [Google Scholar]
- Zacuto M. J.; Xu F. One-Step RhCl3-Catalyzed Deprotection of Acyclic N-Allyl Amides. J. Org. Chem. 2007, 72, 6298–6300. 10.1021/jo070553t. [DOI] [PubMed] [Google Scholar]
- Cadierno V.; Gimeno J.; Nebra N. Efficient Tandem Process for the Catalytic Deprotection of N-Allyl Amides and Lactams in Aqueous Media: A Novel Application of the Bis(Allyl)–Ruthenium(IV) Catalysts [Ru(H3:H2:H3-C12H18)Cl2] and [Ru(H3:H3-C10H16)(μ-Cl)Cl2]. Chem. - Eur. J. 2007, 13, 6590–6594. 10.1002/chem.200700477. [DOI] [PubMed] [Google Scholar]
- Ohmura N.; Nakamura A.; Hamasaki A.; Tokunaga M. Hydrolytic Deallylation of N-Allyl Amides Catalyzed by PdII Complexes. Eur. J. Org. Chem. 2008, 2008, 5042–5045. 10.1002/ejoc.200800771. [DOI] [Google Scholar]
- Rudkevich D. M.; Rebek J. Jr. Chemical Selection and Self-Assembly in a Cyclization Reaction. Angew. Chem., Int. Ed. 1997, 36, 846–848. 10.1002/anie.199708461. [DOI] [Google Scholar]
- Rivera J. M.; Martín T.; Rebek J. Structural Rules Governing Self-Assembly Emerge from New Molecular Capsules. J. Am. Chem. Soc. 1998, 120, 819–820. 10.1021/ja973341u. [DOI] [Google Scholar]
- Stancl M.; Khan M. S. A.; Sindelar V. 1,6-Dibenzylglycoluril for Synthesis of Deprotected Glycoluril Dimer. Tetrahedron 2011, 67, 8937–8941. 10.1016/j.tet.2011.08.097. [DOI] [Google Scholar]
- Fiala T.; Sleziakova K.; Marsalek K.; Salvadori K.; Sindelar V. Thermodynamics of Halide Binding to a Neutral Bambusuril in Water and Organic Solvents. J. Org. Chem. 2018, 83, 1903–1912. 10.1021/acs.joc.7b02846. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.