Abstract
Background
Gastro‐oesophageal reflux (GOR) is characterised by the regurgitation of gastric contents into the oesophagus. GOR is a common presentation in infancy, both in primary and secondary care, affecting approximately 50% of infants under three months old. The natural history of GOR in infancy is generally of a self‐limiting condition that improves with age, but older children and children with co‐existing medical conditions can have more protracted symptoms. The distinction between gastro‐oesophageal reflux disease (GORD) and GOR is debated. Current National Institute of Health and Care Excellence (NICE) guidelines define GORD as GOR causing symptoms severe enough to merit treatment. This is an update of a review first published in 2014.
Objectives
To assess the effects of pharmacological treatments for GOR in infants and children.
Search methods
For this update, we searched CENTRAL, MEDLINE, Embase, and Web of Science up to 17 September 2022. We also searched for ongoing trials in clinical trials registries, contacted experts in the field, and searched the reference lists of trials and reviews for any additional trials.
Selection criteria
We included randomised controlled trials (RCTs) that compared any currently‐available pharmacological treatment for GOR in children with placebo or another medication. We excluded studies assessing dietary management of GORD and studies of thickened feeds. We included studies in infants and children up to 16 years old.
Data collection and analysis
We used standard methodology expected by Cochrane.
Main results
We included 36 RCTs involving 2251 children and infants. We were able to extract summary data from 14 RCTs; the remaining trials had insufficient data for extraction. We were unable to pool results in a meta‐analysis due to methodological differences in the included studies (including heterogeneous outcomes, study populations, and study design).
We present the results in two groups by age: infants up to 12 months old, and children aged 12 months to 16 years old.
Infants
Omeprazole versus placebo: there is no clear effect on symptoms from omeprazole. One study (30 infants; very low‐certainty evidence) showed cry/fuss time in infants aged three to 12 months had altered from 246 ± 105 minutes/day at baseline (mean +/‐ standard deviation (SD)) to 191 ± 120 minutes/day in the omeprazole group and from 287 ± 132 minutes/day to 201 ± 100 minutes/day in the placebo group (mean difference (MD) 10 minutes/day lower (95% confidence interval (CI) ‐89.1 to 69.1)). The reflux index changed in the omeprazole group from 9.9 ± 5.8% in 24 hours to 1.0 ± 1.3% and in the placebo group from 7.2 ± 6.0% to 5.3 ± 4.9% in 24 hours (MD 7% lower, 95% CI ‐4.7 to ‐9.3).
Omeprazole versus ranitidine: one study (76 infants; very low‐certainty evidence) showed omeprazole may or may not provide symptomatic benefit equivalent to ranitidine. Symptom scores in the omeprazole group changed from 51.9 ± 5.4 to 2.4 ± 1.2, and in the ranitidine group from 47 ± 5.6 to 2.5 ± 0.6 after two weeks: MD ‐4.97 (95% CI ‐7.33 to ‐2.61).
Esomeprazole versus placebo: esomeprazole appeared to show no additional reduction in the number of GORD symptoms compared to placebo (1 study, 52 neonates; very low‐certainty evidence): both the esomeprazole group (184.7 ± 78.5 to 156.7 ± 75.1) and placebo group (183.1 ± 77.5 to 158.3 ± 75.9) improved: MD ‐3.2 (95% CI ‐4.6 to ‐1.8).
Children
Proton pump inhibitors (PPIs) at different doses may provide little to no symptomatic and endoscopic benefit.
Rabeprazole given at different doses (0.5 mg/kg and 1 mg/kg) may provide similar symptom improvement (127 children in total; very low‐certainty evidence). In the lower‐dose group (0.5 mg/kg), symptom scores improved in both a low‐weight group of children (< 15 kg) (mean ‐10.6 ± SD 11.13) and a high‐weight group of children (> 15 kg) (mean ‐13.6 ± 13.1). In the higher‐dose groups (1 mg/kg), scores improved in the low‐weight (‐9 ± 11.2) and higher‐weight groups (‐8.3 ± 9.2). For the higher‐weight group, symptom score mean difference between the two different dosing regimens was 2.3 (95% CI ‐2 to 6.6), and for the lower‐weight group, symptom score MD was 4.6 (95% CI ‐2.9 to 12).
Pantoprazole: pantoprazole may or may not improve symptom scores at 0.3 mg/kg, 0.6 mg/kg, and 1.2 mg/kg pantoprazole in children aged one to five years by week eight, with no difference between 0.3 mg/kg and 1.2 mg/kg dosing (0.3 mg/kg mean −2.4 ± 1.7; 1.2 mg/kg −1.7 ± 1.2: MD 0.7 (95% CI ‐0.4 to 1.8)) (one study, 60 children; very low‐certainty evidence).
There were insufficient summary data to assess other medications.
Authors' conclusions
There is very low‐certainty evidence about symptom improvements and changes in pH indices for infants. There are no summary data for endoscopic changes. Medications may or may not provide a benefit (based on very low‐certainty evidence) for infants whose symptoms remain bothersome, despite nonmedical interventions or parental reassurance. If a medication is required, there is no clear evidence based on summary data for omeprazole, esomeprazole (in neonates), H₂antagonists, and alginates for symptom improvements (very low‐certainty evidence). Further studies with longer follow‐up are needed.
In older children with GORD, in studies with summary data extracted, there is very low‐certainty evidence that PPIs (rabeprazole and pantoprazole) may or may not improve GORD outcomes. No robust data exist for other medications.
Further RCT evidence is required in all areas, including subgroups (preterm babies and children with neurodisabilities).
Keywords: Adolescent; Child; Humans; Infant; Infant, Newborn; Esomeprazole; Gastroesophageal Reflux; Gastroesophageal Reflux/complications; Gastroesophageal Reflux/drug therapy; Omeprazole; Pantoprazole; Proton Pump Inhibitors; Proton Pump Inhibitors/therapeutic use; Rabeprazole; Ranitidine
Plain language summary
Medicines for children with reflux
Review question
What is the best and safest treatment for babies and children with gastro‐oesophageal reflux?
Key messages:
‐ the evidence for medications for babies with gastro‐oesophageal reflux/reflux disease is very uncertain;
‐ for children with gastro‐oesophageal reflux disease, the evidence is very uncertain regarding the effects of proton pump inhibitors. There was no adequate evidence to draw conclusions regarding other medications.
What is gastro‐oesophageal reflux?
Gastro‐oesophageal reflux happens when stomach contents come back up into the oesophagus (food pipe). Most babies (under 1 year) grow out of reflux symptoms, but does medicine help? Children (older than 1 year) can have reflux just like adults. Reflux can be normal ('physiological reflux'), but in babies and children, it can cause symptoms, including pain or weight loss, as the oesophagus becomes inflamed (oesophagitis). Bothersome symptoms of reflux are called 'gastro‐oesophageal reflux disease' (GORD).
How is gastro‐oesophageal reflux treated?
Medicines can thicken the stomach contents (alginates), neutralise stomach acid (ranitidine, omeprazole, lansoprazole), or help the stomach to empty faster (domperidone, erythromycin).
What did we want to find out?
We wanted to learn the best way to reduce reflux in babies and children. We wanted to see if medicines help infants and children to feel better (symptom scores), heal the oesophagus (which is checked by using endoscopy, where a tiny camera is put down the oesophagus), or lower the time the oesophagus is exposed to stomach acid. We also investigated whether the medicines were safe by considering the harmful or unwanted effects reported in the studies.
What did we do?
We searched for studies testing gastro‐oesophageal reflux medicines in babies and children. We included all studies comparing these medicines, or comparing them to an inactive medicine (placebo). We assessed results which are important to doctors, nurses, and parents, and performed our own analysis of the results. We rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 36 suitable studies (involving 2251 babies and children), conducted worldwide, with most in the USA. The largest study recruited 268 babies, the smallest, 16 children. Fifteen studies compared an active medicine to placebo; 8 compared one active medication to another; and 11 studies gave the same medication at different doses. We found useable outcome information in 14 of the 36 studies. The remaining studies either did not report outcomes we were interested in or did not report them in a way we could analyse. We could not combine the results of any studies because they were too different (in terms of how long they followed participants up and the outcomes they investigated) to use in a meaningful way.
Key results
Babies. There is no clear effect on symptoms or measured acidity (one measure is reflux index, which is the percentage of time in 24 hours the oesophagus is exposed to stomach acid) between babies given omeprazole or placebo. One study (30 babies) showed cry/fuss time went down from 287 to 201 minutes/day in the placebo group and 246 to 191 minutes/day in the omeprazole group. Reflux index changed in the omeprazole group from 9.9% to 1.0% in 24 hours, and in the placebo group from 7.2% to 5.3%. One study (76 babies) showed that omeprazole and ranitidine may have a similar benefit for symptoms after 2 weeks: symptom scores (higher scores mean worse symptoms) in the omeprazole group dropped from 51.9 to 2.4, and in the ranitidine group, from 47 to 2.5. In one study of 52 newborn babies, esomeprazole appeared to show no reduction in the number of symptoms (184.7 to 156.7) compared to placebo (183.1 to 158.3). None of the studies reported harmful events or results about changes to babies' oesophaguses.
Children. In children older than 1 year of age, no studies assessed medical treatment versus placebo. Proton pump inhibitors (PPIs), which block stomach acid production, at different doses may provide little to no improvements in symptoms or oesophagus healing. In one study (127 children), both lower‐weight and higher‐weight children given rabeprazole at lower and higher doses had both minimal – probably unimportant – changes in symptom scores and endoscopic scores (which indicate whether healing of the oesophagus has occurred). Pantoprazole may or may not improve symptom scores in children aged 1 to 5 years by week 8: there was no difference between lower and higher dosing in one study (60 children). Studies investigating other medications did not report enough information for us to assess their results properly.
Quality of the evidence
We are not confident in the evidence, which was mainly based on single studies with few babies and children. Several studies had pharmaceutical company help with manuscript writing. The question of how best to treat children with disabilities, and whether any PPIs are better than other medicines remain. The evidence is current to 17 September 2022.
Summary of findings
Summary of findings 1. Omeprazole compared to placebo for GORD in infants.
| Omeprazole compared to placebo for GORD in infants | ||||||
| Patient or population: infants with GORD Setting: outpatients Intervention: omeprazole Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with omeprazole | |||||
| Improvement in symptoms in infants assessed with: cry/fuss diary (minutes/day) Follow‐up: mean 2 weeks | The mean improvement in symptoms in infants was ‐66 minutes/day | MD 10 minutes/day lower (89.1 lower to 69.1 higher) | ‐ | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Cry/fuss time in infants between 3 and 12 months of age (mean 5.4 months) improved from 246 ± 105 minutes/day at baseline (mean +/‐ SD) to 191 ± 120 minutes/day in the omeprazole group and from 287 ± 132 minutes/day to 201 ± 100 minutes/day in the placebo group (mean difference (MD) 10 minutes/day lower (95% confidence interval (CI) ‐89.1 to 69.1)) |
| Adverse events ‐ not reported | There were no reports of adverse events in either the omeprazole or placebo group | ‐ | ‐ | |||
| Improvement in pH metrics in infants assessed with: reflux index Follow‐up: mean 2 weeks | The mean improvement in pH metrics in infants was 1.9 % of time in 24 hours | MD 7% of time in 24 hours lower (4.66 lower to 9.34 lower) | ‐ | 30 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | In the omeprazole group, the reflux index improved from 9.9 ± 5.8% in 24 hours to 1.0 ± 1.3% in 24 hours. In the placebo group, the reflux index improved from 7.2 ± 6.0% in 24 hours to 5.3 ± 4.9% in 24 hours. |
| Endoscopic metrics ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | There were no data to assess this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429021146969853213. | ||||||
aRisk of bias: outcomes were assessed with behaviour diary (potential for recall bias) and visual analogue score (potential for parental observer bias). There were concerns that some of these infants may not have had significant endoscopic or reflux index changes at inclusion. North American Society of Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) guidance in place at the time considered reflux index > 10% to be pathological in infants, and no evidence of reflux oesophagitis was seen (erosions or ulcers) at entry endoscopy. The inclusion criteria considered loss of vascular pattern or friability enough for inclusion. Only seven infants had both endoscopic changes and reflux index > 5%. With these concerns, we have downgraded the evidence by one step. bImprecision: for Moore 2003, there was a wide confidence interval crossing the clinical decision threshold and only 15 infants in each group so we have downgraded the evidence by two steps. cRisk of bias: there were concerns that some of these infants may not have had significant endoscopic or reflux index changes at inclusion. NASPGHAN guidance in place at the time considered reflux index > 10% to be pathological in infants, and no evidence of reflux oesophagitis was seen (erosions or ulcers) at entry endoscopy. The inclusion criteria considered loss of vascular pattern or friability enough for inclusion. Only seven infants had both endoscopic changes and reflux index.
Summary of findings 2. Omeprazole compared to ranitidine for GORD in infants.
| Omeprazole compared to ranitidine for GORD in infants | ||||||
| Patient or population: GORD in infants Setting: outpatients Intervention: omeprazole Comparison: ranitidine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with ranitidine | Risk with omeprazole | |||||
| Improvement in symptoms in infants assessed with: weekly gastro‐oesophageal reflux score (WGSS) Follow‐up: mean 2 weeks | The mean improvement in symptoms in infants was ‐44.5 points | MD 4.97 points lower (2.47 lower to 7.33 lower) | ‐ | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Omeprazole (0.5 mg/kg/day) appears to provide some symptomatic benefit in infants between 2 and 12 months old, with improved scores after 2 weeks (51.93 ± 5.42 to 2.43 ± 1.15) equivalent to ranitidine (2 to 4 mg/kg/day) with scores improving (47 ± 5.6 to 2.47 ± 0.58): no differences between omeprazole and ranitidine were noted: MD ‐4.97 (95% CI ‐2.47 to ‐7.33). |
| Adverse events in infants ‐ not measured | ‐ | ‐ | No data were available for this outcome | |||
| Improvement in pH metrics in infants ‐ not measured | ‐ | ‐ | No data were available for this outcome | |||
| Improvement in endoscopic findings in infants ‐ not measured | ‐ | ‐ | No data were available for this outcome | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429020868699799221. | ||||||
aRisk of bias: the certainty of evidence was downgraded by two steps due to issues with blinding (performance bias) as omeprazole was delivered as a capsule and ranitidine as a syrup so parents would be aware which medication was being offered. In addition, 16 infants were lost to follow‐up (attrition bias), severe pneumonia, premature discontinued drugs, and parental issues with the questionnaire bImprecision: as the confidence intervals do not overlap the clinical decision threshold between recommending and not recommending treatment, and the study had very small numbers, we downgraded the certainty of evidence by two steps (very serious), but the certainty of evidence was already very low.
Summary of findings 3. Esomeprazole compared to placebo for GORD in infants.
| Esomeprazole compared to placebo for GORD in infants | ||||||
| Patient or population: GORD in infants Setting: inpatients in 3 neonatal intensive care units Intervention: esomeprazole Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with esomeprazole | |||||
| Improvement in symptoms and signs in infants assessed with: total number of gastro‐oesophageal reflux disease (GORD) symptoms Follow‐up: mean 2 weeks | The mean improvement in symptoms and signs in infants was ‐24.5 episodes | MD 3.2 episodes fewer (4.6 fewer to 1.8 fewer) | ‐ | 52 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,d | Included data from premature babies to 1 m corrected gestational age. No data in older infants. For total number of GORD symptoms (from video monitoring) and GORD‐related signs (from cardiorespiratory monitoring), the esomeprazole group improved from baseline 184.7 (78.5) to 156.7 (75.1) and placebo group improved from 183.1 (77.5) to 158.3 (75.9). |
| Adverse events ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | It was not possible to extract summary data, although there were no reported differences between the placebo and esomeprazole groups. |
| pH indices ‐ not measured | ‐ | ‐ | No data were available for this outcome | |||
| Endoscopic metrics ‐ not measured | ‐ | ‐ | No data were available for this outcome | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429020489057615413. | ||||||
aRisk of bias: the certainty of evidence was downgraded by one step as the study was terminated early due to poor recruitment (the power calculation estimated needing 38 neonates in each group). bIndirectness: the certainty of evidence was downgraded by one step as the population studied (neonates) is only a part of the population under assessment (infants). cImprecision: the certainty of evidence was downgraded by two steps due to small numbers and wide confidence intervals crossing the clinical decision threshold. dPublication bias: this single study was industry‐funded, with support for manuscript‐writing, but the certainty of evidence was not downgraded by one step, as already at 'very low'
Summary of findings 4. Rabeprazole at higher doses (1 mg/kg) compared to rabeprazole at lower doses (0.5 mg/kg) for GORD in children over 1 year of age.
| Rabeprazole at higher doses (1 mg/kg) compared to rabeprazole at lower doses (0.5 mg/kg) for GORD in children over 1 year of age | ||||||
| Patient or population: GORD in children over 1 year of age Setting: outpatients Intervention: rabeprazole at higher doses (1 mg/kg) Comparison: rabeprazole at lower doses (0.5 mg/kg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with rabeprazole at lower doses (0.5 mg/kg) | Risk with rabeprazole at higher doses (1 mg/kg) | |||||
| Improvement in symptoms assessed with: 'Total GORD Symptoms and Severity' score Follow‐up: mean 12 weeks | The mean improvement in symptoms was ‐9.9 points | MD 2.3 points higher (2 lower to 6.6 higher) | ‐ | 127 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Rabeprazole at 0.5 mg/kg and 1 mg/kg may provide similar symptom improvement: in the 0.5 mg/kg group, symptom score improved in both the low‐weight (< 15 kg) (n = 21 mean ‐10.6 ± SD 11.13)) and high‐weight (> 15 kg) groups (n = 44 mean ‐13.6 ± 13.1). In the 1 mg/kg group, scores improved in the low‐weight (n = 19, ‐9 ± 11.2)) and higher‐weight groups (n = 43, ‐8.3 ± 9.2). For the higher‐weight group, MD 2.3 (95% CI ‐2 to 6.6), and low‐weight group: 0.5 mg/kg vs 1 mg/kg: MD 4.6 (95% CI ‐2.9 to 12). |
| Adverse events assessed with: parent‐reported events | Rabeprazole at 0.5 mg/kg and 1 mg/kg may lead to some adverse events: 95 (84%) children had adverse events, including abdominal pain, nausea, vomiting, bronchopneumonia, gastroenteritis, cough, and choking. | 127 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | There was no difference between the groups. | ||
| Improvement in endoscopic appearances assessed with: Hetzel‐Dent score Follow‐up: mean 12 weeks | The mean improvement in endoscopic appearances was ‐1.4 points | MD 0.1 points higher (0.23 lower to 0.43 higher) | ‐ | 127 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | In the 0.5 mg/kg group, endoscopic appearances improved in both the low‐weight (‐1.4 ± 1.06) and higher‐weight groups (‐1.2 ± 0.75). In the 1 mg/kg group, endoscopic appearances also improved in the low‐weight (‐1.1 ± 0.72) and high‐weight groups (‐1.0 ± 0.85). In the low‐weight group: 0.5 mg/kg vs 1 mg/kg: MD 0.30 (95% CI ‐0.27 to 0.87) and in the higher‐weight group MD 0.1 (95% CI ‐0.23 to 0.43). |
| pH indices ‐ not measured | ‐ | ‐ | No data were available for this outcome. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429021824944230742. | ||||||
aRisk of bias: the certainty of evidence was downgraded by one step for selection bias: 30% of children had already received proton pump inhibitors, 15% H2 antagonists, and 2% prokinetics. 15% of participants had also withdrawn. bImprecision: the certainty of evidence was downgraded by one step as the wide confidence intervals crossed the clinical decision threshold. cPublication bias: the certainty of evidence was downgraded by one step as this was a single study and was industry‐funded, with assistance in manuscript preparation, and authors were employed by a pharmaceutical company. The study design involved the same medication at different doses which is less clinically useful than comparison to placebo or an alternative medication. We do not have other studies to assess whether this would have had a material impact.
Summary of findings 5. Pantoprazole in higher doses (1.2 mg/kg) compared to pantoprazole at lower doses (0.3 mg/kg) for GORD in children over 1 year of age.
| Pantoprazole in higher doses (1.2 mg/kg) compared to pantoprazole at lower doses (0.3 mg/kg) for GORD in children over 1 year of age | ||||||
| Patient or population: GORD in children over 1 year of age Setting: outpatients Intervention: pantoprazole in higher doses (1.2 mg/kg) Comparison: pantoprazole at lower doses (0.3 mg/kg) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with pantoprazole at lower doses (0.3 mg/kg) | Risk with Pantoprazole in higher doses (1.2 mg/kg) | |||||
| Improvement in symptoms assessed with: weekly gastro‐oesophageal reflux score (WGSS) Follow‐up: mean 8 weeks | The mean improvement in symptoms was ‐2.37 points | MD 0.7 points higher (0.4 lower to 1.8 higher) | ‐ | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | Pantoprazole appears to improve symptoms at 0.3 mg/kg, 0.6 mg/kg, and 1.2 mg/kg pantoprazole in 60 children aged 1 to 5 years. Symptom scores improved from baseline to week 8 (0.3 mg/kg MD ‐2.4, 95% CI ‐3.2 to ‐1.5; 1.2 mg/kg ‐1.7, 95% CI ‐2.9 to ‐0.39). There was no difference between 0.3 mg/kg and 1.2 mg/kg dosing: MD 0.7 (95% CI ‐0.4 to 1.8). Individual symptoms (abdominal pain, burping, heartburn, pain after eating and difficulty swallowing) improved in all groups after 8 weeks. |
| Adverse events assessed with: individual symptom reporting Follow‐up: 8 weeks | Pantoprazole at all doses investigated may lead to adverse events: in the 0.3 mg/kg group, 1 child developed diarrhoea and nappy rash; in the 0.6 mg/kg group, 1 child had sleep disturbance and 1 developed abdominal pain; and in the 1.2 mg/kg group, 1 child had rectal bleeding. | 60 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c | There was no difference between the groups. | ||
| Improvement in pH indices ‐ not measured | ‐ | ‐ | No data were available for this outcome. | |||
| Improvement in endoscopic metrics ‐ not measured | ‐ | ‐ | No data were available for this outcome. | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_429032441167302611. | ||||||
aRisk of bias: we downgraded the certainty of evidence by one step as no comment was made about blinding and randomisation technique. bImprecision: we downgraded the certainty of evidence due to the small sample size, which would not meet the optimal information size, and confidence intervals that cross the decision‐making threshold. We would have downgraded by two steps but the certainty of evidence was already 'very low'. cPublication bias: we downgraded the certainty of evidence by one step as this study was industry‐funded with support with manuscript writing. The study design involved the same medication at different doses which is less clinically useful than comparison to placebo or an alternative medication. It is difficult to estimate the degree of effect given other studies were not available to compare.
Background
Description of the condition
Gastro‐oesophageal reflux (GOR) occurs when gastric contents come back up into the oesophagus (NASPGHAN‐ESPGHAN guidelines 2018). GOR is a very common presentation, both in primary and secondary care settings. Symptoms of GOR can affect approximately 50% of infants aged one to three months old (Miyazawa 2002; Nelson 1997). The natural history of GOR is generally of improvement with age, with less than 5% to 10% of children with vomiting or regurgitation in infancy continuing to have symptoms after the age of 12 to 14 months (Campanozzi 2009; Martin 2002). This is due to a combination of growth in length of the oesophagus, a more upright posture, increased tone of the lower oesophageal sphincter, and a more solid diet.
Gastro‐oesophageal reflux disease (GORD) is defined as "GOR associated with bothersome symptoms or complications" (NASPGHAN‐ESPGHAN guidelines 2018; Sherman 2009). Sherman and colleagues caution that this definition is complicated by unreliable reporting of symptoms in children under eight years of age (Sherman 2009). Gastrointestinal sequelae include oesophagitis, haematemesis, oesophageal stricture formation, and Barrett's oesophagitis. Extraintestinal sequelae can include acute life‐threatening events, apnoea, chronic otitis media, sinusitis, secondary anaemia, and chronic respiratory disease (chronic wheezing/coughing or aspiration), as well as failure to thrive. The presence of severe oesophagitis has historically been shown to predict the need for surgical reconstruction (Hyams 1988).
GOR is distinguished from vomiting physiologically by the absence of (1) a central nervous system emetic reflex, (2) retrograde upper intestinal contractions, (3) nausea, and (4) retching. GOR is generally characterised as effortless and non‐projectile, although it may be forceful in infants (Sherman 2009). Other conditions, such as rumination syndrome, are distinguished by the absence of nighttime symptoms, and features such as early satiety and bloating may point to functional dyspepsia (Hyams 2016).
Children with certain predisposing conditions are more prone to severe GORD. These conditions include neurological impairment (e.g. cerebral palsy), repaired oesophageal atresia or congenital diaphragmatic hernia, and chronic lung disease.
Diagnosis of physiological or functional GOR (i.e. reflux symptoms that are likely to improve with gut maturation) in infants is usually made based on the symptoms alone, avoiding the need for expensive and possibly harmful investigations. Investigations to assess the severity of GORD, or in cases where GOR cannot be diagnosed on clinical grounds, include 24‐hour oesophageal pH monitoring, which can be combined with impedance monitoring, upper gastrointestinal endoscopy, scintigraphy, or oesophageal manometry. All have been shown to correlate poorly with symptomatology, and may not accurately predict the degree of improvement with treatment (Augood 2003; NICE 2019).
Clinical symptoms are commonly scored and reported individually. These symptoms include:
number of vomiting episodes, back arching, regurgitation, failure to thrive, feeding difficulties, and abdominal pain in infants;
heartburn, epigastric pain, and regurgitation symptoms in older children.
Common scoring systems include the Paediatric Gastro‐oesophageal Symptom Questionnaire (PGSQ) for older children (Kleinman 2011), the GORD Assessment of Symptoms in Pediatrics Questionnaire (GASP‐Q) for younger children (Fitzgerald 2003), and the Infant Gastro‐oesophageal Reflux Questionnaire Revised (I‐GERQ‐R) for infants (Orenstein 2010).
Normal gastric juices are acidic in nature, with a pH of approximately 1 to 3. The pH scale goes from 1 (strongly acidic) through 7 (neutral), to 14 (strongly alkaline).
Investigations to assess disease severity include:
pH‐impedance indices over 24 hours, including: reflux index on pH probe (percentage of time that oesophageal pH < 4 in 24 hours); number of acid reflux/impedance episodes; and time length of reflux episodes where oesophageal pH is less than 4;
endoscopic findings, including macroscopic appearance of oesophagus on endoscopy, and histological appearances.
Consensus exists that there are insufficient data to recommend histology as a tool to diagnose or exclude GORD in children, but that histology is useful in confirming the presence of oesophagitis and ruling out other conditions, such as eosinophilic oesophagitis, Barrett's oesophagus, Crohn's disease, infection, and graft‐versus‐host disease (NICE 2019). Histological scoring scales (e.g. the Hetzel‐Dent classification) are also commonly utilised to help assess improvement (NASPGHAN‐ESPGHAN guidelines 2018).
Description of the intervention
Proton pump inhibitors (PPIs)
PPIs, such as omeprazole and lansoprazole, are a group of drugs that irreversibly inactivate H+/K+ ATPase, in the parietal cells of the stomach. There are five PPIs approved by the US Food and Drug Administration (FDA) in adults: omeprazole (since 1988), lansoprazole, pantoprazole, rabeprazole, and esomeprazole (the pure S‐isomer of omeprazole). The current National Institute for Health and Clinical Excellence (NICE) guidelines recommend only a two‐week trial of a PPI or a histamine receptor antagonist (H₂RA) for infants whose symptoms fail to improve with nonmedical interventions (NICE 2019). Omeprazole is licensed for use in children over one year of age in the UK, with a half‐life of one hour, but due to the permanent receptor block, the effect can last for five to seven days. The dose range is 5 mg to 10 mg daily in infants, 10 mg to 20 mg daily in young children, and 20 mg to 40 mg daily in older children and adolescents. Lansoprazole is only recommended by the British National Formulary for children when treatment with the available formulations of omeprazole is unsuitable (BNFc 2021). It is used in doses of 7.5 mg to 15 mg in young children, and 15 mg to 30 mg in older children. The average elimination half‐life is 1.5 hours in infants and young children. The inhibition of acid secretion is about 50% of maximum at 24 hours and the duration of action is approximately 72 hours (Ward 2013). Esomeprazole is also licensed for GORD: for children aged one to 11 years with a body‐weight of 10 kg to 19 kg, 10 mg once daily; for children aged one to 11 years with a body‐weight of 20 kg and above, 10 mg to 20 mg once daily; for children aged 12 to 17 years, 20 mg to 40 mg once daily, and a maintenance dose of generally 20 mg daily. Pantoprazole and rabeprazole are not currently licensed for use in children.
Gastric pH provides some protection against infection in children. Thus, there is evidence that potentiating the hypochlorhydria (low levels of stomach acid) in neonates further with PPIs can result in bacterial overgrowth (De Bruyne 2018). Increases in respiratory infections in critically‐ill inpatients have been identified, but in infants and children who are otherwise well, no clear ill effects have been demonstrated from this overgrowth. A Medicines and Healthcare products Regulatory Agency (MHRA) alert in 2012 highlighted that PPIs used for longer than three months may be associated with hypomagnesaemia (especially in those on therapy lasting for more than five years), and a possible increased risk of fractures (Fleishmann 2021; MHRA 2012). Since then, concerns have been raised about hypergastrinaemia (but the risk of cancer is not thought to be increased), Clostridioides difficile colitis, vitamin B12 deficiency (due to atrophic gastritis and hypochlorhydria, which produce bacterial overgrowth promoting increased digestion of cobalamin), and acute interstitial nephritis (a hypersensitivity reaction that can occur within days to 18 months of starting treatment and resolves on discontinuing the PPI) (BNFc 2021; NICE 2019). There have been a handful of cases reported of PPI‐induced systemic cutaneous lupus erythematosus, and significant drug interactions (itraconazole, ketoconazole, isoniazid, oral iron supplements) (Schoenfeld 2016). PPIs are metabolised by the cytochrome P450 system in the liver and interactions include those medications that inhibit or enhance cytochrome P450 metabolism (listed in BNFc 2021).
Histamine (H₂) receptor antagonists (H2RAs)
The most commonly used H2RA is ranitidine, which competitively blocks selective histamine receptors. Ranitidine is metabolised in the liver and renally excreted with a half‐life of two to four hours and length of action of 12 to 24 hours. Ranitidine is well‐tolerated and has a low incidence of side effects; these commonly include fatigue, dizziness, and diarrhoea (Tighe 2009). It also affects metabolism of other drugs by the cytochrome P450 system (BNFc 2021). Ranitidine has been withdrawn worldwide due to concerns regarding a low level of impurity of N‐nitrosodimethylamine (NDMA) (MHRA 2019). Cimetidine is rarely used clinically because of concerns about its greater effects on the cytochrome P450, which cause multiple drug interactions, as well as its interference with vitamin D metabolism and endocrine function. Famotidine is a recently‐developed H₂ antagonist not commonly used in children but with similar pharmacodynamics to ranitidine. Tachyphylaxis from H₂ antagonists has been reported (McRorie 2014).
Magnesium hydroxide and aluminium hydroxide (MHAH)
Magnesium hydroxide and aluminium hydroxide reduce gastric pH and are commercially available as Maalox. Aluminium should be avoided in chronic use, especially in infants and children with chronic renal failure, due to the risk of aluminium accumulation.
Prokinetics
Domperidone is a dopamine‐receptor (D‐2) blocker that has relatively few side effects, but case reports of extrapyramidal side effects exist (Franckx 1984; Shafrir 1985), and there is concern about the risk of cardiac side effects (EMA 2014b). Its use has declined except in specialist indications, since the publication of NICE guidance (NICE 2019). Current advice is to not use it in children with co‐existing cardiac disease or in those taking CYP3A4 inhibitors, and not to exceed a daily dose of 30 mg/day in children over 12 years old and 250 micrograms/kg three times a day in younger children (EMA 2014b). Domperidone is no longer marketed in the USA (Bashashati 2016), but can be used as an investigational new drug and should not be used for nausea and vomiting for more than one week.
Erythromycin is a macrolide antibiotic; its use as a prokinetic is as an unlicensed indication (BNFc 2021).
Metoclopramide has been the subject of an FDA 'black box' warning (FDA 2009). In August 2013, the European Medicines Agency released a statement that the risk of neurological adverse events (such as short‐term extrapyramidal disorders and tardive dyskinesia) with metoclopramide outweighed the benefit, when taken for a prolonged period at a high dose (EMA 2014a). Metoclopramide has also been assessed in a separate Cochrane Review (Craig 2004), so we did not review the associated literature for metoclopramide as it is not used to treat reflux in children, given the adverse event profile and NICE guidance (NICE 2019).
At its peak use, cisapride was prescribed to over 36 million children worldwide for GOR (Vandenplas 1999). However, concerns about the effect of cisapride in prolonging the QT interval led to its removal from general paediatric use (Com Safety Med 2000). A Cochrane Review found that there was no clear evidence that cisapride reduces symptoms of GOR, and found evidence of substantial publication bias favouring studies showing a positive effect of cisapride (Augood 2003). Given the known risks of toxicity and its suspension of manufacture, further trials of cisapride are unlikely.
Quince syrup (heated extract of Cydonia oblonga Mill.) belongs to the rose family (Rosacea) as a traditional Persian medicine to treat GORD (Zohalinezhad 2015). It is unlicensed in the UK.
Alginates
Compound alginate preparations differ from other alginate preparations, which can also contain sodium bicarbonate or potassium bicarbonate (BNFc 2021).
Caution should be used with alginates that contain aluminium (see below), and in children with vomiting or diarrhoea, or children at risk of intestinal obstruction (Gaviscon Product Information 2021). In children whose feeds are already thickened (e.g. Enfamil AR/SMA Staydown), coexistent Gaviscon Infant could potentially cause intestinal obstruction (Keady 2007). Some alginate preparations contain sodium: for example, Gaviscon Infant contains 0.92 mmol Na+/dose, which should be considered if a child’s sodium intake needs to be monitored with caution (e.g. renal impairment, congestive cardiac failure, preterm infants, or children with diarrhoea and vomiting) (BNFc 2021). Gaviscon Infant has changed to become aluminium‐free, with different proportions of alginate, and other forms are now available (Gastrotuss and Refluxsan Nipio). Alginates for infants are generally prescribed at up to six doses per day with half a dual sachet in formula bottles of less than 210 mL and one dual sachet in formula bottles of more than 210 mL of milk. Breastfed babies have a dose mixed with expressed breast milk and given before or with a breastfeed by syringe.
Two other Cochrane Reviews have assessed thickened feeds (Craig 2004; Kwok 2017).
Antispasmodics
Baclofen is primarily an antispasmodic acting on gamma‐aminobutyric acid (GABA) receptors, commonly used in children with neurodisability, such as cerebral palsy (Omari 2006). It is not licensed for children with GORD (BNFc 2021).
Conservative options
These include reassurance of parents, and positioning of the baby to reduce gastro‐oesophageal reflux, through the effect of gravity on gastric contents. This can include elevating the head of the cot or basket in which the baby is placed to sleep, or keeping the baby upright after a feed.
Altering the feed's consistency can be achieved with feed thickeners (e.g. with rice starch/carob bean gum) and may reduce the reflux of gastric contents with increased viscosity. Some feeds are manufactured with a thickening agent added. Weaning also has a similar effect by increasing the viscosity of gastric contents, and gastro‐oesophageal reflux is known to improve with weaning. We have considered compound alginates in this review, but not other feed‐thickeners, which are assessed elsewhere (Craig 2004; Kwok 2017).
Changes in feeding can also improve GOR. For breastfed babies, a breastfeeding assessment by health professionals experienced in breastfeeding is recommended initially, then elimination of cow's milk from the maternal diet can be trialled. For formula‐fed infants, after assessing for and correcting overfeeding, clinicians can consider recommendations supporting two to four weeks of a protein hydrolysate or amino acid‐based formula (NASPGHAN‐ESPGHAN guidelines 2018; NICE 2019).
Surgical options
Surgery is used to limit GORD. The most common strategy is a Nissen's fundoplication involving a 360º wrap (Hassall 2005). This aims to combine antireflux factors, including creation of a high pressure zone at the distal oesophagus and recreation of the diaphragmatic crural mechanism. However, underlying dysmotility may persist and retching may continue as a prominent feature. Comparisons of these techniques are considered elsewhere (NICE 2019). We have not assessed conservative and surgical strategies in this Cochrane Review, which seeks to assess medical treatments, to better inform medical practitioners (GPs/paediatricians). Surgery relates to a small minority of children with gastro‐oesophageal reflux and is beyond the scope of this review.
How the intervention might work
Pharmacological treatments work by altering the gastric pH (e.g. PPIs, H₂ antagonists) and reducing the acidity of refluxate, by promoting gut motility (prokinetics), or by altering the viscosity of refluxate (alginates). Pharmacological treatments are considered if nonmedical measures have been ineffective. Dosing, metabolism interactions, and associated adverse events are described above.
Proton pump inhibitors (PPIs)
PPIs irreversibly inactivate H+/K+ ATPase, at the level of the parietal cell membrane transporter. This increases the pH of gastric contents and decreases the total volume of gastric secretion. Of the five PPIs approved by the FDA, three are licensed in the UK for children: omeprazole, lansoprazole, and esomeprazole. PPIs were the subject of a 'Pediatric Written Request' (PWR) made by the FDA to improve our knowledge of PPIs in children and infants. There is good clinical experience with PPIs in children, and an excellent evidence‐base of efficacy in adults (NICE 2019).
H₂ receptor antagonists (H2RAs)
H₂ antagonists also aim to increase the pH of gastric contents in children, and there is good clinical experience with H₂ antagonists in infants, children, and adults (NICE 2019).
Magnesium hydroxide and aluminium hydroxide (MHAH)
MHAH is designed to reduce gastric acid, and forms water as a by‐product. Its use in children is unlicensed.
Prokinetics
Prokinetics are considered when GOR fails to improve with conservative measures. There are several classes of drugs designed to increase gastrointestinal motility.
Domperidone acts to increase motility and gastric emptying through acting on dopamine receptors and decreases post‐prandial reflux time (Franckx 1984; Shafrir 1985). Domperidone had been commonly used in clinical practice, either as part of empirical medical therapy of gastro‐oesophageal reflux disease or if delayed gastric emptying has been demonstrated on a barium swallow or milk scan.
Erythromycin binds to motilin receptors to promote peristalsis and gastric emptying, to decrease post‐prandial reflux time. Its use as a prokinetic is unlicensed.
Metoclopramide has also been assessed in a separate Cochrane Review (Craig 2004), so we did not review the associated literature for metoclopramide as it is not used to treat reflux in children, given the adverse event profile and NICE guidance (NICE 2019).
Cisapride is a gastro‐oesophageal prokinetic agent which stimulates motility in the gastrointestinal tract by increasing acetylcholine release in the myenteric plexus, controlling smooth muscle. As cisapride has been the subject of a separate Cochrane Review (Augood 2003), and is now no longer manufactured, we have not reviewed the literature for this drug.
Quince syrup has ulcer‐healing properties and is thought to increase the lower oesophageal sphincter tone (Zohalinezhad 2015).
Alginates
Compound alginate preparations prevent reflux in infants by increasing the viscosity of gastric contents (BNFc 2021). This contrasts with other Gaviscon preparations, which can also contain sodium bicarbonate/potassium bicarbonate that – in the presence of gastric acid – forms a gel in which carbon dioxide (derived from the breakdown of bicarbonate) is trapped. This 'foam raft' floats on top of the gastric contents and is designed to neutralise gastric acid (providing symptomatic relief), to thicken the feed (to reduce reflux), and to reduce oesophageal irritation (Mandel 2000).
Sodium and magnesium alginate (Gaviscon Infant) is a thickener, and other forms are now available (Gastrotuss and Refluxsan Nipio).
Other thickening agents, such as carob bean gum (Carobel), have been assessed separately (Craig 2004; Kwok 2017). Current NICE guidance recommends discontinuing pre‐thickened formulas if alginates are trialled (NICE 2019).
Antispasmodics
Baclofen has been used to treat co‐existing reflux by aiming to improve the incoordination of the lower oesophageal sphincter, reducing the number of transient lower oesophageal sphincter relaxations (TLESRs) (Omari 2006). It is not part of clinical GORD consensus guidelines (NASPGHAN‐ESPGHAN guidelines 2018).
Why it is important to do this review
Gastro‐oesophageal reflux in children is a common condition. Healthcare professionals frequently use pharmacological treatment of this condition for symptom relief. New studies have been published since the original version of this review (Tighe 2014), and new medicines to treat gastro‐oesophageal reflux are available. Thus, an up‐to‐date synthesis of the evidence, including the current balance of benefits and harms of these treatments, is required.
Objectives
To assess the effects of pharmacological treatments for GOR in infants and children.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised controlled trials (RCTs) for inclusion.
Types of participants
We included all children (aged 0 to 16 years) with "GOR associated with bothersome symptoms or complications" (NASPGHAN‐ESPGHAN guidelines 2018; see also Sherman 2009).
We predefined two groups organised by age: infants up to 12 months old, and children aged 12 months to 16 years old. We included studies assessing preterm neonates and children with a neurodisability.
Types of interventions
We included all currently available medical treatments for gastro‐oesophageal reflux in children.
We considered all RCTs that compared a medication for GOR with a placebo or another medication. We imposed no restrictions on dosage, frequency, or duration of pharmacological treatment.
We attempted comparisons of all active treatments versus placebo, by treatment class:
proton pump inhibitors (PPIs: omeprazole, lansoprazole, pantoprazole, rabeprazole, and esomeprazole) versus placebo;
H₂ antagonists (ranitidine, famotidine, cimetidine) versus placebo;
prokinetics (domperidone, erythromycin, bethanechol) versus placebo;
compound alginate preparations versus placebo
sucralfate versus placebo.
We included studies assessing quince syrup, a traditional Persian medicine to treat GOR. We outline the evidence base, but note that quince syrup is not currently a prescribable medicine in many countries, including the United Kingdom.
We excluded studies assessing metoclopramide, thickened feeds, or using thickened feeds as a comparator. (In a 2004 Cochrane Review, Craig and colleagues assessed metoclopramide and thickened feeds for GOR in children under two years of age (Craig 2004); this review has since been withdrawn.) We excluded studies employing conservative treatment and surgical techniques for GOR, as well as studies assessing dietary management of GORD. We excluded studies assessing pharmacological treatments for GORD in people with coexistent conditions, such as tracheo‐oesophageal fistula (TOF) or asthma, that predispose them to GORD, to avoid heterogeneity between participants.
Types of outcome measures
To make this update as robust as possible, and to assist the potential for meta‐analysis, we selected the same outcome measures in this updated review as in the previous version (Tighe 2014). We included all reported outcomes that are likely to be meaningful to clinicians making medical decisions about treating gastro‐oesophageal reflux. We included all time points for assessments. We identified studies with very short follow‐up periods (fewer than two weeks) as a potential source of bias.
We did not exclude studies based on outcomes measured. However, we excluded studies assessing purely pharmacokinetic outcomes or taste, as these were not considered as primary or secondary outcome measures of interest. Nevertheless, to exclude outcome bias, we contacted corresponding authors of such trials to establish if there were any relevant data that had not been published. In cases of uncertainty, we contacted corresponding authors for clarification.
Primary outcomes
Our primary outcome was improvement in clinical symptoms, which was usually assessed through questionnaires completed by parents and childcare providers. The symptoms monitored included:
number of vomiting episodes (continuous data);
episodes of back arching (continuous data);
number of regurgitation episodes (continuous data);
failure to thrive (binary outcome);
feeding difficulties (binary outcome);
abdominal pain in infants (continuous data).
In older children, the number of episodes of heartburn, epigastric pain, or regurgitation (continuous data) were again assessed through questionnaires completed by participants, parents, and health professionals. These included, for example, the Paediatric Gastro‐oesophageal Symptom Questionnaire (PGSQ) and the Infant Gastro‐oesophageal Reflux Questionnaire–Revised (I‐GER‐Q), which were completed daily by parents and health professionals to provide quantitative data through validated symptom scores.
Secondary outcomes
Our secondary outcomes were: adverse effects, 24‐hour pH‐impedance indices, and endoscopic metrics.
Adverse effects
We explored all studies for any adverse effects, as defined by the Medicines Health Regulation Authority (MHRA 2012). In cases of uncertainty, we contacted corresponding authors for clarification. This exploratory approach aimed to identify unanticipated and rare adverse effects of an intervention and to look for data on possible associations between an intervention and a list of observed adverse events, to add to existing safety profiles. We assessed and reported adverse effects, and studies reporting the absence of adverse effects without separate data extraction, in line with Cochrane guidance (Higgins 2022).
24‐hour pH‐impedance indices
Reflux monitoring measures the amount of reflux in the oesophagus during a 24‐hour period. The test is carried out by placing a catheter in the oesophagus. These indices assess:
improvement in the reflux index (continuous data);
number and duration of reflux episodes on a 24‐hour pH‐impedance probe (continuous data);
results of non‐acid impedance studies (continuous data).
Endoscopic metrics
Improvement of oesophagitis on endoscopy (visual appearance – this can be a binary outcome or continuous data if scored (e.g. Hetzel‐Dent classification));
Histology (continuous data).
Different grading scales currently exist for classifying macroscopic appearances of the oesophagus, but no one grading scale has been demonstrated to show superior validity to the alternatives. We considered the description of histological changes, and histological scoring scales, and where relevant to help clinicians, we describe useful findings below. However, we did not include histological data in the summary of findings tables.
The number of children within a study population who failed to improve and required fundoplication was considered a potential secondary outcome (binary outcome).
Search methods for identification of studies
Electronic searches
We searched the following databases on 17 September 2022:
Cochrane Central Register of Controlled Trials (CENTRAL) via Ovid Evidence‐Based Medicine Reviews Database (EBMR) (from inception to 2022) (Appendix 1);
MEDLINE via Ovid (from 1946 to 17 September 2022) (Appendix 2);
Embase via Ovid (from 1974 to 17 September 2022) (Appendix 3);
Science Citation Index via Web of Science (from inception to 17 September 2022) (Appendix 4).
We searched the World Health Organization International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/) and ClinicalTrials.gov (www.ClinicalTrials.gov).
We developed this search strategy with assistance from the Information Specialist of the Cochrane Gut Group.
Searching other resources
We checked the reference lists of all eligible studies and relevant reviews identified by the search and published within the past five years for possible references to RCTs. We also contacted experts in the field for any additional trials.
Adverse outcomes
We did not conduct a separate search for adverse events.
Language
We did not restrict our searches by language, and translated papers as necessary.
Data collection and analysis
We used Review Manager 5.4 and RevMan Web for data collection and analysis (RevMan 2019; RevMan Web 2022).
Selection of studies
Two review authors (MT, IL) downloaded all titles and abstracts retrieved by electronic searching to a reference management database and removed duplicates. Four review authors (MT, IL, EA, RMB) independently screened titles and abstracts for inclusion. We retrieved the full‐text reports/publications and independently applied the eligibility criteria to the full texts, identified studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. We resolved any disagreement through discussion or, when required, through consulting a fifth review author (NAA).
We listed studies that initially appeared to meet the inclusion criteria but that we later excluded in the Characteristics of excluded studies table, with the reasons for their exclusion. We collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We also provided any information we could obtain about ongoing studies. We entered studies that were only in abstract form, or were only identified in the ISRCTN register into the Characteristics of studies awaiting classification table. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Liberati 2009).
Data extraction and management
Three review authors (MT, IL, EA) independently extracted the data using a robust data extraction form (utilised in the first review), checked and entered the data into RevMan 5.4/RevMan Web, analysed the data, and highlighted any discrepancies, with statistician supervision (AH). RMB supervised data collection and acted as arbiter for any disagreements. If studies had insufficient data, we did not extract summary data. We collected and archived data in a format to facilitate future access and data sharing. Where statistical analyses were not possible (or were inappropriate), we provided a descriptive summary. We looked at all studies, performing further analysis of those employing an intention‐to‐treat (ITT) analysis where such information existed, and have included single forest plots of studies with summary data extracted.
Assessment of risk of bias in included studies
As in the original review, we have described each study in a risk of bias table, and addressed the following issues, which may be associated with biased estimates of treatment effect: recruitment strategy, random sequence generation, allocation sequence concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias (Higgins 2011). We commented specifically on:
the method of generation of the randomisation sequence;
the method of allocation concealment – it is considered 'adequate' if the assignment could not be foreseen, and should be independent of and remote from the investigators;
who was blinded and not blinded (participants, clinicians, outcome assessors) if this was appropriate (up to and after the point of treatment allocation);
how many participants were lost to follow‐up in each arm, and whether reasons for losses were adequately reported;
whether all participants were analysed in the groups to which they were originally randomised (intention‐to‐treat principle).
We also reported on:
the baseline assessment of the participants for age, sex, and duration of symptoms, if suggestive of bias between the groups;
whether outcome measures were described and their assessment was standardised;
the use and appropriateness of statistical analyses, where we could not extract tabulated data from the original publication.
Measures of treatment effect
The outcomes described above yielded both continuous and dichotomous data.
Clinical symptoms produced continuous data (e.g. number of vomiting episodes), yielding outcomes described as the mean difference (MD) and standardised mean difference (SMD). We extracted continuous data (e.g. reflux index) for summary data: we used means and standard deviations (SDs) to derive a mean difference (MD) with a 95% confidence interval (95% CI) using a fixed‐effect model.
The latest guidelines of the North American Society of Paediatric Gastroenterology Hepatology and Nutrition (NASPGHAN) and the European Society of Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) do not define normal values for pH‐metry and pH‐impedance (NASPGHAN‐ESPGHAN guidelines 2018). We therefore continued to treat reflux index as continuous data but removed consideration of whether baseline values were normal or abnormal (which had been discussed in the previous version of this review), and included any improvement/non‐improvement in values compared to the other agent or dose being tested, expressed as MD ± 95% CI.
Dichotomous data, such as improvement/non‐improvement in endoscopic appearance, produced outcome data we presented as risk ratios. For studies of a single pharmacological agent (e.g. omeprazole) versus either placebo or a different drug, if sufficient trials were available and participant characteristics were clinically similar, we planned to conduct meta‐analyses of primary and secondary outcomes.
Unit of analysis issues
We considered unit of analysis issues for any included trials with multiple treatment groups and cluster‐randomised designs. We considered cross‐over trials for inclusion and assessed only the first stage of therapy prior to cross‐over, but commented on results obtained after cross‐over if clinically relevant. We also considered issues arising from multiple observations for the same outcome (e.g. repeated pH‐impedance measurements), and planned to consult the Cochrane Gut group if clarification was required. For multi‐arm studies, we analysed multiple intervention groups appropriately to prevent arbitrary omission of relevant groups or double‐counting of participants.
Dealing with missing data
Where we were uncertain about the specifics of a trial pertinent to analysis, we contacted trial authors or sponsors of studies published from 2014 to 2022 to request missing data or clarification. We detailed authors' and sponsors' contribution in Characteristics of included studies.
Assessment of heterogeneity
We screened studies to assess clinical heterogeneity and planned subgroup analyses if appropriate, reporting on the extent of any heterogeneity using the I2 statistic (Higgins 2003). Where we found evidence of significant heterogeneity (I2 > 50%) in summary data extraction, we downgraded the evidence certainty.
Assessment of reporting biases
We assessed selective reporting of results by comparing (where available) the outcomes listed in trials' original protocols to those reported in the final papers. We also searched clinical trials registries for details of the included trials. We contacted the primary investigator(s) of included trials to determine whether they were aware of any relevant unpublished data. We aimed to identify publication bias with the construction of funnel plots (Page 2020). However, insufficient trials were eligible for inclusion in the current version of the review. We plan to undertake this analysis in future if we can include more trials.
Data synthesis
We were unable to combine studies meaningfully due to the heterogeneity of studies in terms of outcomes, comparisons, and populations. For continuous measurements, we had planned to use weighted mean differences to pool results from studies using a common measurement scale. Where studies used different measurement scales, we planned to pool standardised mean differences. Instead, we have presented difference in means and 95% confidence intervals for individual studies and summary effects, using the following order: Population > Comparison > Outcome. We assessed all individual treatments separately, given the individual study differences and heterogeneity in study design. We considered combining data – for example, on high‐dose versus low‐dose proton pump inhibitors (please see Effects of interventions) – to attempt to increase the population size on which conclusions were based, only where similar outcomes in similar participants were assessed. However, we were unable to undertake this method due to the heterogeneity of study methodology. Due to the number of summary of finding tables, we limited our assessment of quince syrup, as it is not a prescribable medicine. We have not included quince syrup in the summary of findings tables, nor assessed the certainty of evidence for this intervention.
Subgroup analysis and investigation of heterogeneity
We addressed subgroup analysis in two ways. First, we have distinguished between infants (up to 12 months in age) and children (one to 16 years in age) throughout the review. These subgroups have different GOR characteristics. For example, infants with symptomatic gastro‐oesophageal reflux have different symptoms from older children (who are generally on a more solid diet, and are upright). Some treatments, such as alginates, are mainly used in the infant cohort.
Secondly, we looked for studies evaluating specific subgroups: (1) preterm infants, as this group of babies can be problematic to assess and often have empirical treatment for common symptoms (e.g. apnoeas and bradycardias) that can be caused by GORD, but are more commonly caused by other issues associated with prematurity; and (2) children with neurodisability, who often have considerable gut dysmotility, and are often on long‐term antireflux therapy. The results are outlined within Effects of interventions.
Where we found substantial heterogeneity (I2 > 50%) between studies for the primary outcome, we explored the reasons for heterogeneity (including severity of reflux, demographic differences (age and comorbidity) within the age subgroups, having considered varying outcomes, different comparison agents (same drug, different dosing)) and downgraded the evidence certainty. As it was inappropriate to pool the data because of clinical or statistical heterogeneity, which we discuss in Overall completeness and applicability of evidence, we did not conduct meta‐analysis. There were insufficient studies within other specific subgroups (preterm infants and children within neurodisability) to consider heterogeneity.
Sensitivity analysis
In this review update, we could not undertake meta‐analysis due to the heterogeneity of the included studies' populations, comparisons, and outcomes. Thus, sensitivity analysis was not required. In future updates of the review, if meta‐analysis is possible, we plan to undertake sensitivity analysis to explore whether a 12‐month age threshold for subgroups influences meta‐analytic robustness. We plan to integrate these findings into the results and conclusions. Additionally, if there are sufficient data in future updates of the review, we plan to explore whether endoscopic metrics, pH indices, and symptomatic outcomes are affected by either endoscopic descriptors (such as erosive or non‐erosive oesophagitis) or severity markers on 24‐hour pH‐impedance monitoring (such as reflux index). Other possible sensitivity analyses will depend on the type of meta‐analysis possible.
Summary of findings and assessment of the certainty of the evidence
Two authors (MT, EA) independently used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome, and to draw conclusions about the certainty of evidence within the text of the review. We resolved any disagreements through discussion, involving all review authors if a disagreement could not be resolved.
Our summary of findings tables prioritise comparisons and outcomes that will be of use to decision‐makers. We deferred the creation of summary of findings tables for treatments that are not currently available by prescription to future review updates. All review author reviewed the GRADE considerations in assessing the certainty of evidence (Schünemann 2013), and integrated judgements into the summary of findings tables.
All review authors agreed prior to data collection that the summary of findings tables should distinguish results by age (infants: 0 to 12 months; and children: aged 1 to 16 years old). The tables present these outcomes: symptoms, adverse events, pH impedance indices, and endoscopic metrics. We provide clear rationales where we downgraded evidence according to GRADE criteria.
Results
Description of studies
Results of the search
The first version of this review included 24 studies (Tighe 2014). In the September 2022 update searches, we identified 1427 records through electronic database searches and supplemental search methods. After the removal of duplicates, 1034 records remained. At this stage, we discarded 978 records as clearly irrelevant. We screened the full‐text publications of 54 studies (56 records). We excluded 40 studies (42 records) and listed two studies as 'awaiting classification.' We identified 12 new studies for inclusion. Thus, in this updated version, we have included a total of 36 RCTs assessing 2251 participants. We identified no ongoing studies (see Figure 1).
1.
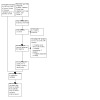
PRISMA study flow diagram
Included studies
We present the main characteristics of the included studies in the Characteristics of included studies table.
Study design
Of the 36 RCTs, most (31) were of parallel‐group design (Azizollahi 2016; Baker 2010; Ballengee 2018; Bines 1992; Borrelli 2002; Buts 1987; Carroccio 1994; Cresi 2008; Cucchiara 1984; Cucchiara 1993; Davidson 2013; Famouri 2017; Forbes 1986; Gilger 2006; Gunesekaran 2003; Haddad 2013; Kierkus 2011; Loots 2014; Miller 1999; Naeimi 2019; Omari 2006; Omari 2007; Orenstein 2008; Pfefferkorn 2006; Simeone 1997; Tolia 2006; Tolia 2010a; Tolia 2010b; Tsou 2006; Ummarino 2015; Zohalinezhad 2015); two were cross‐over studies (Baldassarre 2020; Moore 2003), two were withdrawal studies (Hussain 2014; Orenstein 2002), and one had a more complex design (Del Buono 2005). Twenty‐two studies (61%) enroled more than 40 participants (Azizollahi 2016; Baker 2010; Baldassarre 2020; Carroccio 1994; Cucchiara 1984; Davidson 2013; Famouri 2017; Gilger 2006; Gunesekaran 2003; Haddad 2013; Hussain 2014; Loots 2014; Miller 1999; Naeimi 2019; Omari 2007; Orenstein 2008; Tolia 2006; Tolia 2010a; Tolia 2010b; Tsou 2006; Ummarino 2015; Zohalinezhad 2015), and 14 studies enroled fewer than 40 participants (Ballengee 2018; Bines 1992; Borrelli 2002; Buts 1987; Cresi 2008; Cucchiara 1993; Del Buono 2005; Forbes 1986; Kierkus 2011; Moore 2003; Omari 2006; World Bank 2022; Pfefferkorn 2006; Simeone 1997). The largest study enroled 268 participants (Hussain 2014).
Fifteen studies were multicentre (Baker 2010; Baldassarre 2020; Davidson 2013; Gilger 2006; Gunesekaran 2003; Haddad 2013; Hussain 2014; Loots 2014; Miller 1999; Orenstein 2002; Orenstein 2008; Tolia 2006; Tolia 2010a; Tolia 2010b; Tsou 2006) and 21 were single‐centre (Azizollahi 2016; Ballengee 2018; Bines 1992; Borrelli 2002; Buts 1987; Carroccio 1994; Cresi 2008; Cucchiara 1984; Cucchiara 1993; Del Buono 2005; Famouri 2017; Forbes 1986; Kierkus 2011; Moore 2003; Naeimi 2019; Omari 2006; Omari 2007; Pfefferkorn 2006; Simeone 1997; Ummarino 2015; Zohalinezhad 2015). Seventeen studies had a placebo‐controlled arm (Ballengee 2018; Bines 1992; Buts 1987; Carroccio 1994; Cresi 2008; Davidson 2013; Del Buono 2005; Famouri 2017; Forbes 1986; Hussain 2014; Loots 2014; Miller 1999; Moore 2003; Omari 2006; Orenstein 2002; Orenstein 2008; Simeone 1997). Ten studies compared the active medication to a comparator medication (Azizollahi 2016; Baldassarre 2020; Borrelli 2002; Carroccio 1994; Cucchiara 1984; Cucchiara 1993; Naeimi 2019; Pfefferkorn 2006; Ummarino 2015; Zohalinezhad 2015), and 10 studies used the same medication at different doses (Baker 2010; Gilger 2006; Tolia 2010b; Gunesekaran 2003; Haddad 2013; Kierkus 2011; Tolia 2006; Tolia 2010a; Tolia 2010b; Tsou 2006). One study compared improvements on the active medication to baseline (Omari 2006). All the studies were conducted on outpatients except Cresi 2008 and Davidson 2013 which were conducted on inpatients in neonatal intensive care units (NICUs). All bar four of the studies were conducted in high‐income countries: Azizollahi 2016, Famouri 2017, Naeimi 2019, and Zohalinezhad 2015 were conducted in Iran, a lower‐middle income country (World Bank 2022).
Participants
Nineteen studies assessed infants only, six studies assessed infants and children, and 11 assessed children aged one year or older. Of the studies that assessed infants only, 14 included infants with symptomatic GORD (Azizollahi 2016; Baldassarre 2020; Bines 1992; Cresi 2008; Davidson 2013; Del Buono 2005; Famouri 2017; Forbes 1986; Hussain 2014; Loots 2014; Miller 1999; Orenstein 2002; Orenstein 2008; Ummarino 2015), four studies included infants with symptoms and signs of GORD on 24‐hour pH/impedance monitoring (Ballengee 2018; Kierkus 2011; Moore 2003; Omari 2007); one study included infants with endoscopic changes (Pfefferkorn 2006); and one study included infants with either significant pH indices or endoscopic changes (Moore 2003). Of those studies in both infants and children, one study included participants with symptomatic GORD (Zohalinezhad 2015), and six studies undertook corroborative investigations (pH/impedance and endoscopy) (Buts 1987; Carroccio 1994; Cucchiara 1984; Cucchiara 1993; Kierkus 2011; Simeone 1997). Of the studies in children aged one year or older, one study included children with symptomatic GORD (Naeimi 2019), and 10 studies undertook corroborative investigations (endoscopy, pH/impedance studies, or both) (Baker 2010; Borrelli 2002; Gilger 2006; Gunesekaran 2003; Haddad 2013; Omari 2006; Tolia 2006; Tolia 2010a; Tolia 2010b; Tsou 2006). Fourteen studies contained suitable summary data for extraction (described below in 'Interventions and comparisons'). Of those 14 studies, two studies had data on both infants and children (Cucchiara 1984; Zohalinezhad 2015); we discuss these in Included studies and Effects of interventions but do not present them in the summary of findings tables.
Interventions and comparisons
Studies in infants
Two studies with summary data assessed proton pump inhibitors (PPIs) versus placebo (Moore 2003 assessed omeprazole, Davidson 2013 assessed esomeprazole); one study with summary data compared a PPI (omeprazole) with another medication (ranitidine) (Azizollahi 2016); and two studies with summary data assessed a PPI given in different doses (Kierkus 2011 assessed pantoprazole, Hussain 2014 assessed rabeprazole). For H₂ antagonists, Azizollahi 2016 compared ranitidine with another medication (omeprazole). There were no studies with summary data that assessed prokinetics or magnesium alginate.
Studies in children
Six studies with summary data assessed a PPI. Two studies compared a PPI with another medication: Pfefferkorn 2006 compared omeprazole to additional ranitidine, and Zohalinezhad 2015 compared omeprazole to quince syrup. Three studies compared different doses of a PPI: Baker 2010 and Tolia 2006 assessed pantoprazole, Haddad 2013 assessed rabeprazole. For H₂ antagonists, as noted above, Azizollahi 2016 compared ranitidine to omeprazole. Two studies assessed quince syrup: as noted above, Zohalinezhad 2015 compared quince syrup to omeprazole, and Naeimi 2019 compared ranitidine plus quince syrup to ranitidine alone.
Outcomes
Studies in infants
Of studies which compared a PPI to placebo, one study with summary data provided data on clinical symptoms (Moore 2003), and three studies provided data on pH/impedance outcomes: Moore 2003 assessed omeprazole, Davidson 2013 assessed esomeprazole, and Kierkus 2011 assessed pantoprazole. One study on a PPI versus another medication (ranitidine) provided summary symptomatic data (Azizollahi 2016). One study on pantoprazole at different doses provided 24‐hour pH/impedance outcome data (Kierkus 2011). One study with summary data assessed a PPI given in different doses (rabeprazole) (Hussain 2014). For H₂ antagonists, one study with symptomatic summary data compared ranitidine to another medication (omeprazole: Azizollahi 2016). There were no studies with summary data assessing prokinetics or alginates.
Studies in children
We included six studies assessing a PPI from which we were able to extract summary data, as follows. Pfefferkorn 2006 assessed omeprazole versus additional ranitidine and provided symptoms scores and reflux index data. Zohalinezhad 2015 provided symptom scores in a comparison of omeprazole to quince syrup. Three studies of pantoprazole provided extracted summary data: Baker 2010 and Tolia 2006 provided symptom scores, and endoscopic and histological scores, and Tsou 2006 provided symptom scores. Haddad 2013 studied rabeprazole and provided symptom scores and endoscopic and histological scores.
As stated above, Azizollahi 2016 assessed an H₂ antagonist (additional ranitidine with omeprazole) and provided summary symptomatic data. Two studies provided symptomatic summary data only on quince syrup (Zohalinezhad 2015 compared quince syrup to omeprazole, and Naeimi 2019 compared ranitidine plus quince syrup to ranitidine alone).
Included studies adopted different definitions of adverse events. Studies also varied in terms of reporting of adverse events and patient monitoring, and potential incomplete reporting. We have presented all reported adverse events.
No studies provided long‐term data on the number of infants or children failing to respond to medication and requiring fundoplication.
Excluded studies
We excluded 40 studies (42 records) at the full‐text screening stage. The primary reasons for exclusion were: ineligible study design (36 studies); ineligible intervention (three studies); ineligible population (one study). Please see Characteristics of excluded studies for further details.
Risk of bias in included studies
We detail our risk of bias assessments by study in Figure 2 and Figure 3. With many of the older studies, it was difficult to clarify methodological issues from the published protocol.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.

Risk of bias summary: review authors' judgements presented as percentages across all included studies
Random sequence generation (selection bias)
Fifteen studies clearly described an adequate method of random sequence generation, such as blocked randomisation; we assessed these as having a low risk of bias (Azizollahi 2016; Baldassarre 2020; Ballengee 2018; Borrelli 2002; Carroccio 1994; Cresi 2008; Davidson 2013; Gunesekaran 2003; Kierkus 2011; Loots 2014; Moore 2003; Naeimi 2019; Orenstein 2008; Pfefferkorn 2006; Zohalinezhad 2015). Nineteen studies made no reference to or incompletely outlined the method of randomisation used in their trial; we assessed these as having an unclear risk of bias (Baker 2010; Bines 1992; Buts 1987; Cucchiara 1984; Cucchiara 1993; Del Buono 2005; Forbes 1986; Famouri 2017; Gilger 2006; Haddad 2013; Hussain 2014; Miller 1999; Omari 2006; Omari 2007; Orenstein 2002; Simeone 1997; Tolia 2006; Tsou 2006; Ummarino 2015). We judged the remaining two studies to be at high risk of selection bias (Tolia 2010a due to the nature of a post hoc analysis and the risk of bias posed by the selection of participants with oesophagitis who have not responded satisfactorily to other approved therapy; Tolia 2010b due to the risk of bias posed by the selection of participants for initial endoscopy and then enrolment being performed at the discretion of the investigator).
Allocation
Nine studies specified the method of allocation, such as randomised computer‐generated allocation; we assessed these studies as having a low risk of bias in this domain (Azizollahi 2016; Baldassarre 2020; Carroccio 1994; Cresi 2008; Davidson 2013; Gunesekaran 2003; Haddad 2013; Moore 2003; Naeimi 2019). Twenty‐four studies made no reference to or incompletely outlined the method of allocation used in their trial; we assessed these as having an unclear risk of bias (Baker 2010; Ballengee 2018; Bines 1992; Borrelli 2002; Buts 1987; Cucchiara 1984; Cucchiara 1993; Del Buono 2005; Forbes 1986; Famouri 2017; Gilger 2006; Hussain 2014; Kierkus 2011; Loots 2014; Miller 1999; Omari 2006; Omari 2007; Orenstein 2002; Orenstein 2008, Pfefferkorn 2006; Simeone 1997; Tolia 2006; Tsou 2006; Ummarino 2015). We judged the remaining three studies to be at high risk of selection bias: Tolia 2010a due to the nature of a post hoc analysis and the risk posed by the enrolment of participants who have not responded satisfactorily to other approved therapy; Tolia 2010b due to the risk of bias of enrolment based on initial endoscopy being performed at the discretion of the investigator; Zohalinezhad 2015 due to the marked difference in baseline symptom score in the omeprazole group, affecting outcomes such as refusal to feed and weight gain.
Blinding
Performance bias
We assessed 13 studies as low risk, with additional detail outlining methodological strategies to ensure equal care between groups and blinding of parents and participants (Ballengee 2018; Cucchiara 1984; Del Buono 2005; Gilger 2006; Haddad 2013; Moore 2003; Naeimi 2019; Omari 2006; Orenstein 2008; Pfefferkorn 2006; Tolia 2010a; Tolia 2010b; Zohalinezhad 2015). Eleven studies had an unclear risk of performance bias, where the blinding between groups was not explained in sufficient detail (Baker 2010; Borrelli 2002; Buts 1987; Carroccio 1994; Davidson 2013; Gunesekaran 2003; Hussain 2014; Miller 1999; Omari 2006; Simeone 1997; Tolia 2006). Hussain 2014 did not specify blinding technique but did use identical placebo and active preparations, we assessed this study as having an unclear risk of bias in this domain. Twelve studies were at high risk of performance bias (Azizollahi 2016; Baldassarre 2020; Bines 1992; Cucchiara 1993; Cresi 2008; Famouri 2017; Forbes 1986; Kierkus 2011; Loots 2014; Orenstein 2002; Tsou 2006; Ummarino 2015). The Azizollahi 2016 study used different preparations (omeprazole capsule versus ranitidine syrup), increasing the risk of bias. Baldassarre 2020, Cresi 2008, Famouri 2017, Kierkus 2011, and Tsou 2006 were open‐label, as was the second part of the Bines 1992 study and the first part of the Orenstein 2002 study. There was no detail regarding blinding for Cucchiara 1993, but the participants were on ranitidine twice daily before the trial, and the ranitidine group continued receiving twice‐daily dosing, while the omeprazole group received once‐daily dosing. Forbes 1986 and Ummarino 2015 had clinician blinding but not parental blinding. In Loots 2014, the medications were double‐blind but the children's body positioning was single‐blind and parents were aware. This could affect reported symptom control outcomes, which rely heavily on parental reporting and diaries.
Detection bias
We assessed 10 studies as low risk with additional detailing of blinding assessment methods for assessors (Ballengee 2018; Davidson 2013; Del Buono 2005; Forbes 1986; Haddad 2013; Loots 2014; Naeimi 2019; Tolia 2010b; Ummarino 2015; Zohalinezhad 2015). Twenty studies had unclear risk, as they provided insufficient details about assessor blinding to determine risk of bias (Azizollahi 2016; Baker 2010; Bines 1992; Borrelli 2002; Buts 1987; Carroccio 1994; Cucchiara 1984; Cucchiara 1993; Famouri 2017; Gilger 2006; Gunesekaran 2003; Hussain 2014; Miller 1999; Omari 2006; Omari 2007; Orenstein 2008; Pfefferkorn 2006; Simeone 1997; Tolia 2010a; Tsou 2006). We assessed the six remaining studies as high risk (Baldassarre 2020; Cresi 2008; Kierkus 2011; Moore 2003; Orenstein 2002; Tolia 2006). Assessors were aware of the outcome for Baldassarre 2020, Cresi 2008, and Kierkus 2011. Parental diaries and visual analogue scores in Moore 2003 were open to recall bias. In Orenstein 2002, the parents were unblinded to the interventions in part one, affecting the risk of bias for the parental assessment. Tolia 2006 was assessed as high risk as the endoscopic outcomes were not assessed in one group.
Incomplete outcome data
Studies with good completion of outcome data were: Baker 2010; Baldassarre 2020; Ballengee 2018; Carroccio 1994; Cucchiara 1984; Davidson 2013; Gilger 2006; Loots 2014; Moore 2003; Naeimi 2019; Orenstein 2002; Tolia 2006; Ummarino 2015; and Zohalinezhad 2015. We rated these fourteen studies as low risk of bias in this domain. In both Zohalinezhad 2015 and Gilger 2006, only one participant was lost. Loots 2014 lost six of 51 participants. Ballengee 2018 lost two participants (one from each arm of the study) out of 31 and clearly stated the reason for loss (one died and the other developed sepsis). Baldassarre 2020 had no loss of participants after randomisation. In Naeimi 2019, four participants withdrew from the control (ranitidine) arm and one withdrew from the treatment arm (ranitidine and quince syrup).
Eighteen studies did not have enough evidence to determine risk of attrition bias: Bines 1992; Borrelli 2002; Buts 1987; Cresi 2008; Cucchiara 1993; Del Buono 2005; Famouri 2017; Forbes 1986; Gunesekaran 2003; Haddad 2013; Hussain 2014; Kierkus 2011; Miller 1999; Omari 2006; Omari 2007; Orenstein 2008; Pfefferkorn 2006; Simeone 1997. We assessed these as having an unclear risk of bias in this domain.
Four studies showed evidence of significant incomplete outcome data, particularly Azizollahi 2016, Tolia 2010a, Tolia 2010b, and Tsou 2006, sufficient to be considered at high risk of bias in this domain. We obtained further data for Tolia 2010a after direct communication with the authors.
Selective reporting
The risk of bias was low in Baldassarre 2020, Davidson 2013, Omari 2006, Ummarino 2015, and Zohalinezhad 2015.
Twenty‐five studies did not have enough evidence to determine risk of reporting bias (Azizollahi 2016; Baker 2010; Ballengee 2018; Buts 1987; Carroccio 1994; Cresi 2008; Cucchiara 1984; Cucchiara 1993; Del Buono 2005; Famouri 2017; Forbes 1986; Gilger 2006; Gunesekaran 2003; Haddad 2013; Kierkus 2011; Loots 2014; Miller 1999; Moore 2003; Naeimi 2019; Omari 2007; Orenstein 2002; Orenstein 2008; Pfefferkorn 2006; Simeone 1997; Tolia 2006).
Reporting bias was evident in: Bines 1992; Borrelli 2002 (excluded severe oesophagitis); Hussain 2014 due to the post hoc analyses; Tolia 2010a; Tsou 2006; Tolia 2010b. We rated these as high risk.
Other potential sources of bias
We identified other sources of bias leading to a judgement of 'high risk' in 23 studies. These included studies with a follow‐up of less than two weeks (Baldassarre 2020; Buts 1987; Cresi 2008; Davidson 2013; Del Buono 2005; Gunesekaran 2003; Kierkus 2011; Omari 2006; Omari 2007). In Bines 1992, participants agreeing to an open‐label trial may be biased towards those who believed they had an initial benefit from treatment. In Baldassarre 2020, the comparator was a thickened formula. In Carroccio 1994, children received frequent short feeds, positioning advice, and all formula milk was thickened with Medigel 1%. Similarly, in Cucchiara 1984, all infants had thickener added (Nestargel 1%). Davidson 2013 was discontinued prematurely because of poor enrolment: the study estimated needing to recruit 90 participants to achieve 38 neonates in each group to achieve higher than 80% power at the 2‐sided alpha level of 0.05 to detect a difference between esomeprazole and placebo in the change in number of symptoms from baseline. In Moore 2003, there was no evidence of reflux oesophagitis seen (erosions or ulcers) at entry endoscopy; loss of vascular pattern or friability was sufficient for inclusion. Moore 2003 included infants with reflux index higher than 5% in 24 hours, only seven infants had endoscopic changes and reflux index higher than 5% in 24 hours, so some of these infants may have had functional reflux.
We assessed the following studies as high risk for other bias as they either had pharmaceutical company support for manuscript‐writing, an author was employed by a pharmaceutical company, or both (Baker 2010, Baldassarre 2020; Cucchiara 1993; Davidson 2013; Gilger 2006; Haddad 2013; Hussain 2014; Kierkus 2011; Omari 2007; Tolia 2006; Tolia 2010a; Tolia 2010b; Tsou 2006).
Other sources of bias are diverse, and are discussed in Included studies but were not assessed as providing a significant additional risk of bias.
We considered three studies to be of lower risk due to identified independent funding (Naeimi 2019; Pfefferkorn 2006; Zohalinezhad 2015).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
We present results below ordered by population (first infants (aged zero to 12 months) then children (aged one to 16 years)), treatment class, comparisons, and outcomes (improvement in clinical symptoms; adverse events; pH‐impedance indices; endoscopic findings). Most of the included studies offered an appraisal of improvement in clinical symptoms. However, there was considerable heterogeneity in symptom assessment, such as the use of composite scores as well as individual symptom assessment. Studies in infants commonly assessed number of vomiting episodes, back arching, regurgitation, failure to thrive, feeding difficulties, or abdominal pain/colic. Studies in older children commonly assessed heartburn, epigastric pain, or regurgitation symptoms. Several studies used 24‐hour pH/impedance studies, with reflux index and number of reflux episodes the most commonly‐used outcomes. The macroscopic appearance of the oesophagus on endoscopy and histological improvement were the most common endoscopic improvement metrics. Most studies described adverse events, and we have summarised these below. We attempted to extract summary statistics from all studies, and where available, we expressed these as the difference in means (i.e. the mean difference, MD) with a 95% confidence interval (CI).
I. Infants
Proton pump inhibitors (PPIs)
Omeprazole
Omeprazole versus placebo
See Table 1.
Improvement in clinical symptoms
Based on the results of one study (Moore 2003), the evidence is very uncertain about the effect of omeprazole on cry/fuss time compared to placebo (MD ‐10.0 minutes (min)/24 hours, 95% CI ‐89.1 to 69.1; 30 infants; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Omeprazole compared to placebo for infants with GORD, Outcome 1: Improvement in cry/fuss time
Adverse events
Although Moore 2003 reported no adverse events in either the omeprazole or placebo groups (30 infants), there was insufficient evidence to extract summary data on adverse events.
pH‐impedance indices
The evidence is very uncertain about the effect of omeprazole on reflux index compared to placebo, based on one study assessing reflux index (Moore 2003) (MD ‐7.0% in 24 hours, 95% CI ‐4.66 to ‐9.34; 30 infants; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Omeprazole compared to placebo for infants with GORD, Outcome 2: Improvement in reflux index
Endoscopic findings
No studies were available for this outcome.
Omeprazole versus other treatments: ranitidine
See Table 2.
Improvement in clinical symptoms
Omeprazole and ranitidine may result in similar symptomatic improvement. Based on evidence from a single study (Azizollahi 2016), omeprazole (0.5 mg/kg/day) provided symptomatic benefit equivalent to ranitidine (2 to 4 mg/kg/day) in 76 infants with troublesome symptoms of GORD. Symptom scores in the omeprazole group improved from 51.93 ± 5.42 to 2.43 ± 1.15, and in the ranitidine group, from 47 ± 5.6 to 2.47 ± 0.58 after two weeks (MD ‐4.97, 95% CI ‐2.47 to ‐7.33; 1 study, 76 infants; very low‐certainty evidence; Analysis 2.1). Another study noted improvements in symptom scores in participants treated with omeprazole (Cucchiara 1993). However, Cucchiara 1993 did not report the outcome in sufficient detail to allow extraction of summary statistics and did not differentiate between infants and children, so we did not include this study in the assessment of evidence certainty.
2.1. Analysis.

Comparison 2: Omeprazole compared to ranitidine for infants with GORD, Outcome 1: Improvement in symptom scores (WGSS)
Adverse events
Cucchiara 1993 noted that, in those randomised to eight weeks of either standard doses of omeprazole (40 mg/day/1.73 m2 surface area) or higher doses of ranitidine (20 mg/kg/day), one participant sustained a fever and a respiratory infection and was withdrawn. The study did not specify the participant's age or treatment group, so we did not include this result in the assessment of evidence certainty, and there were insufficient data for summary data extraction. Azizollahi 2016 found that, overall, 16 participants withdrew, due to being lost to follow‐up, prematurely discontinuing medication, having severe pneumonia, and the mother being unable to complete questionnaires. Azizollahi 2016 did not describe the number of participants with severe pneumonia, so this result was not suitable for inclusion in the summary of findings table.
pH‐impedance indices
Although the Cucchiara 1993 study assessed this outcome, trialists did not distinguish between infants and children. We were therefore unable to extract suitable data and did not include this study in our assessment of the evidence certainty.
Endoscopic findings
Although the Cucchiara 1993 study assessed this outcome, trialists did not distinguish between infants and children. We were therefore unable to extract suitable data and did not include this study in our assessment of the evidence certainty.
Omeprazole versus other treatments: quince syrup
Zohalinezhad 2015 used omeprazole as a comparator to assess the efficacy of quince syrup for gastro‐oesophageal reflux.
Improvement in clinical symptoms
Although the Zohalinezhad 2015 study assessed this outcome, trialists did not distinguish between infants and children. We were therefore unable to extract suitable data and did not include this study in our assessment of the evidence certainty.
Adverse events
Although the Zohalinezhad 2015 study assessed this outcome, trialists did not distinguish between infants and children. We were therefore unable to extract suitable data and did not include this study in our assessment of the evidence certainty. Zohalinezhad 2015 reported no adverse events in 37 infants and young children (under 60 months of age) in either the quince syrup or omeprazole groups.
pH‐impedance indices
No studies were available for this outcome.
Endoscopic findings
Zohalinezhad 2015 did not identify how many participants underwent endoscopy, so we did not include this result in the summary of findings tables and did not assess evidence certainty.
Omeprazole at different doses
No studies were available for this comparison.
Lansoprazole
Lansoprazole versus placebo
Improvement in clinical symptoms
A single study assessing 162 infants (age range: four to 51 weeks) showed no improvement in the lansoprazole group compared to placebo, based on observer assessments and symptom diaries (Orenstein 2008). For participants who went on to take lansoprazole open‐label (n = 55), there was no improvement in symptoms, but the trial did not report the outcome in sufficient detail to allow extraction of summary statistics. There was no investigation to confirm GORD in participants, and many of the participants enroled may have had functional reflux. Thus, we were unable to extract summary statistics with which to judge the efficacy of lansoprazole on symptomatic improvement compared to placebo.
Adverse events
The Orenstein 2008 study noted that adverse events were more common in the lansoprazole group (10 participants versus two placebo participants from a total of 162 participants). These included lower respiratory‐tract infections (five participants versus one on placebo), diarrhoea (two participants), ileus (one participant), and dehydration (one participant). The trial did not report the outcome in sufficient detail to allow extraction of summary statistics or to estimate the certainty of the evidence. Thus, we were unable to extract summary statistics with which to judge the risk of adverse events.
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Lansoprazole versus other treatments or at different doses
No studies were available for these comparisons.
Esomeprazole
Esomeprazole versus placebo
See Table 3.
Improvement in clinical symptoms
Esomeprazole may provide no symptomatic improvement compared to placebo, based on evidence from a single study. Davidson 2013 compared 52 neonates (premature to one month corrected age) given 0.5 mg/kg esomeprazole or placebo, and noted no improvement compared to placebo in the total number of GORD symptoms (from video monitoring) and GORD‐related signs (from cardiorespiratory monitoring) (MD 3.2 episodes fewer, 95% CI 4.6 fewer to 1.8 fewer; 1 study, 52 neonates; very low‐certainty evidence; Analysis 3.1).
3.1. Analysis.

Comparison 3: Esomeprazole compared to placebo for infants with GORD, Outcome 1: Improvement in number of GORD‐related symptoms and signs
Adverse events
In the single study comparing esomeprazole to placebo (Davidson 2013), the study did not report the outcome in sufficient detail to allow extraction of summary statistics. We were therefore unable to include this study in our assessment of the evidence certainty.
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Esomeprazole versus other treatments: magnesium hydroxide
Improvement in clinical symptoms
A single study was available for this comparison: Loots 2014 compared esomeprazole and antacid (aluminium hydroxide and magnesium hydroxide (Mylanta)) therapy to left lateral positioning (LLP). In this study, 51 infants (aged two weeks to 26 weeks) with symptoms of GOR were randomised to one of four groups: (1) esomeprazole plus LLP; (2) esomeprazole plus head‐of‐cot elevation; (3) antacid plus LLP; or (4) antacid plus head‐of‐cot elevation. After two weeks, the esomeprazole groups' symptoms were reported to have improved more than those of the antacid groups, but the trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Adverse events
Loots 2014 reported five adverse events. Three were not considered serious: urinary tract infection, constipation, and diarrhoea and vomiting (following immunisation). Two of the adverse events were deemed serious: one participant (randomised to esomeprazole plus head elevation) was admitted with rotavirus infection; another (randomised to esomeprazole plus head elevation) was admitted because of reduced oral intake and weight loss. None were thought to be treatment‐related. However, the study did not report adverse events in sufficient detail to allow extraction of summary statistics or to assess evidence certainty.
pH‐impedance indices
Loots 2014 reported improved reflux index for the esomeprazole groups compared with the antacid groups (although reflux index < 10% in 24 hours in infants is not considered pathological by the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN)). This study did not report the outcome in sufficient detail to allow extraction of summary statistics or to judge evidence certainty.
Endoscopic findings
No studies were available for this outcome.
Esomeprazole at different doses
Improvement in clinical symptoms
Omari 2007 reported on 50 infants with symptoms of GORD and a reflux index suggestive of acid GOR (> 4% in 24 hours) who were given oral esomeprazole 0.25 mg/kg or 1 mg/kg for eight days. They noted greater symptom improvement in the lower‐dose group. However, the trial did not report the outcome in sufficient detail to allow extraction of summary statistics for judging the efficacy of different doses of esomeprazole on symptomatic improvement.
Adverse events
Omari 2007 reported that only one infant with pre‐existing colic developed excessive irritability and was withdrawn. However, this study did not report the outcome in sufficient detail to allow extraction of summary statistics for judging the risk of adverse events at different doses.
pH‐impedance indices
Omari 2007 reported that reflux index improved in both groups (oral esomeprazole 0.25 mg/kg or 1 mg/kg), with greater improvement seen in the lower‐dose group. However, this study did not report the outcome in sufficient detail to allow extraction of summary statistics for assessing the efficacy of esomeprazole on pH‐impedance indices.
Endoscopic findings
No studies were available for this outcome.
Rabeprazole
Rabeprazole versus placebo or other treatments
No studies were available for these comparisons.
Rabeprazole at different doses
Improvement in clinical symptoms
The Hussain 2014 study was a five‐week, double‐blind withdrawal study (following a one‐ to three‐week open‐label phase) that compared rabeprazole 5 mg and 10 mg groups to placebo in 268 infants (aged one to 11 months). Only those infants who had improved went on to the double‐blind withdrawal phase. Of the 268 randomised infants (placebo: 90; rabeprazole 5 mg: 90; rabeprazole 10 mg: 88), 231 (86%) completed the study. Efficacy endpoints were similarly improved during the open‐label phase in all groups, and continued improving during the double‐blind withdrawal phase with no difference between the placebo and combined rabeprazole groups. No difference in primary endpoints (I‐GERQ scores) were noted. On post hoc analysis outcomes, a reduction in mean regurgitation frequency was seen (−0.79 versus −1.20 times a day), in I‐GERQ‐Revised scores (−3.6 (−25%) versus −3.9 points (−27%); MD 0.5, 95% CI −1.4 to 2.4), in I‐GERQ‐Daily Diary scores (−1.87 (−19%) versus −1.85 (−19%); MD 0.5, 95% CI −1.12 to 2.12), indicating equal improvement between the groups. However, given the serious risk of bias inherent in the post hoc nature of the study and given that only infants who had improved went on to the withdrawal phase, we did not include these data in the summary of findings tables.
Adverse events
In the double‐blind phase of the study, Hussain 2014 noted equal rates (47%: n = 42) of adverse events between placebo and combined rabeprazole groups (diarrhoea, constipation, flatulence, crying, and rash). Vomiting and worsened GORD was reportedly more common in the placebo group, and eight participants in the rabeprazole groups had elevated gastrin levels noted. However, the study did not report the outcome in sufficient detail to allow extraction of summary adverse events statistics at different doses.
pH‐impedance indices
No studies were available for this outcome.
Endoscopic findings
Hussain 2014 performed 12 endoscopies at baseline, but did not repeat them, so we were therefore unable to extract summary data and did not include this study in our assessment of the evidence certainty.
Pantoprazole
Pantoprazole versus placebo or other treatments
No studies were available for these comparisons.
Pantoprazole at different doses
Improvement in clinical symptoms
No studies were available for this outcome.
Adverse events
Kierkus 2011 assessed high‐dose (1.2 mg/kg) versus low‐dose pantoprazole (0.6 mg/kg) with a 24‐hour pH probe performed at baseline and then at day five. One participant developed excessive vomiting (trialists did not explicitly state which group this infant was in), and the participant was withdrawn from the study during the open‐label phase (24 infants). There was not enough evidence for pantoprazole at different doses to assess adverse events appropriately.
pH‐impedance indices
The evidence is very uncertain about the effect of different doses of pantoprazole on pH‐impedance indices, based on the Kierkus 2011 study. Trialists noted improvements in reflux index from baseline in both groups (MD −0.4% in 24 hours (95% CI −0.99 to 0.19)), number of episodes (103.0 (95% CI −3.8 to 209.8)), number of reflux episodes lasting more than five minutes (2.0 (95% CI −0.38 to 4.38)), and duration of the longest reflux episodes (9.0 minutes (95% CI 1.46 to 16.54)). In each group, 50% to 70% of infants had normal reflux indices on enrolment (reflux index < 5% in 24 hours; defined by the authors), but there was no difference between the low‐dose and high‐dose groups (1 study, 24 infants; very low‐certainty evidence). However, the insufficient data at baseline affected our ability to extract summary data, so we have not included this study in the summary of findings tables.
Endoscopic findings
No studies were available for this outcome.
H₂ antagonists
Ranitidine
Ranitidine versus placebo
No studies were available for this comparison
Ranitidine versus other treatments: omeprazole
Improvement in clinical symptoms
Evidence from Azizollahi 2016 indicated that ranitidine was likely to improve clinical symptoms but was not superior to omeprazole. Azizollahi 2016 performed a randomised double‐blind trial in 76 infants with troublesome symptoms (diagnosed as GORD) aged between two months and 12 months. Participants had two weeks of standard treatment (smaller, more frequent feeds, hypoallergenic thickened formula), and were then randomised for two weeks to ranitidine (2 to 4 mg/kg/day) or omeprazole (0.5 mg/kg/day). Symptom scores (one of five GORD symptoms (vomiting/regurgitation, irritability/fussing, choking/gagging, arching back, refusal to feed) were assessed weekly for two weeks. In the ranitidine group, symptom scores improved from 47 ± 5.6 to 2.47 ± 0.58 (mean ± SD) compared to the omeprazole group, which improved from a higher mean baseline of 51.93 ± 5.42 to 2.43 ± 1.15 (MD of symptom score change ‐4.97 (95% CI ‐2.47 to ‐7.33). No difference between ranitidine and omeprazole was seen (1 study, 76 infants; very low‐certainty evidence). Please see Table 2.
Adverse events
Azizollahi 2016 found no adverse events (76 infants) but provided insufficient detail. We were therefore unable to extract summary data and did not include this study in our assessment of the evidence certainty regarding adverse events.
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Ranitidine versus other treatments: hypoallergenic diet or formula
Improvement in clinical symptoms
There were no suitable summary statistics to assess outcomes. A single study assessed ranitidine (6 mg/kg daily in two divided doses) against a hypoallergenic diet (mothers of breastfed infants were advised to consume only a hypoallergenic diet; formula‐fed infants were to feed on hydrolysed protein or amino acid‐based formula) in 50 infants aged less than one year with I‐GERQ‐R scores of more than 7 (Famouri 2017). The study reported that an improvement in vomiting and respiratory symptoms was noted, but improvement in irritability, arching, or feed refusal was not observed. The study did not report the outcome in sufficient detail to allow extraction of summary statistics.
Adverse events
Although a single study noted no adverse events (50 infants) (Famouri 2017), we were unable to extract suitable data and did not include this study in our assessment of the evidence certainty.
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Ranitidine at different doses
No studies were available for any outcome.
Cimetidine
Cimetidine versus placebo
No studies were available for any outcome.
Cimetidine versus other treatment: Maalox
Improvement in clinical symptoms
One study compared cimetidine to Maalox over 12 weeks in 33 infants and children (aged two months to 42 months) with a diagnosis of GORD based on symptoms, oesophagitis on endoscopy, and acid reflux on pH probe (Cucchiara 1984). They found that cimetidine and Maalox both provided symptomatic relief (MD 0.29 (95% CI ‐0.27 to 3.24). However, this study did not report the outcome in sufficient detail to differentiate between infants and children and was not included in the assessment of evidence certainty.
Adverse events
Cucchiara 1984 reported only that two participants on cimetidine had diarrhoea compared to placebo. However, this study did not differentiate between infants and children, so we did not include these data in the assessment of evidence certainty.
pH‐impedance indices
Cucchiara 1984 observed that the reflux index was improved in both groups compared to baseline; neither group was superior (MD 0.31% in 24 hours (95% CI −1.45 to 2.1)). However, this study did not report the outcome in sufficient detail to differentiate between infants and children, so we did not include these data in the assessment of evidence certainty.
Endoscopic findings
Cucchiara 1984 found that endoscopic appearances were improved (MD 0.19 (95% CI ‐2.47 to 2.85)) but the trial did not report the outcome in sufficient detail to differentiate between infants and children and was not included in the assessment of evidence certainty.
Cimetidine at different doses
No studies were available for any outcome.
Famotidine
Famotidine versus placebo
Improvement in clinical symptoms
The single RCT for this comparison, Orenstein 2002, assessed 35 infants (age range 1.3 to 10.5 months) with symptomatic GORD who were given four weeks of famotidine 0.5 mg/kg versus famotidine 1 mg/kg (Phase 1: discussed in 'Famotidine at different doses' comparison below) and then four weeks' double‐blind withdrawal comparison of each dose with placebo (Phase 2). Only 8 of 35 participants completed phase 2, giving insufficient data for meaningful comparison. There were no suitable summary statistics to assess improvement outcomes.
Adverse events
Orenstein 2002 noted that only eight of 35 participants completed phase 2 of the study, giving insufficient data for meaningful comparison, so we were therefore unable to extract summary data and did not include this study in our assessment of the evidence certainty. However, in addition to the adverse events reported below, the study report noted that four participants in the famotidine group and four in the placebo group experienced asymptomatic neutropenia which resolved.
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Famotidine versus other treatments
No studies were available for any outcome.
Famotidine at different doses
Improvement in clinical symptoms
As described above, a single RCT for this comparison, Orenstein 2002, assessed 35 infants (age range 1.3 to 10.5 months) with symptomatic GORD who were given four weeks of famotidine 0.5 mg/kg versus famotidine 1 mg/kg (Phase 1), followed by four weeks' double‐blind withdrawal comparison of each dose with placebo (Phase 2). Twenty‐seven participants completed phase 1. In terms of symptom outcomes, a slight improvement in regurgitation frequency/volume and crying time in those infants receiving the reduced dose of famotidine was noted, as well as improved global assessments by parents and physicians. However, the data were not expressed in enough detail to allow extraction of summary symptom or observer statistics. There were no suitable summary statistics to assess outcomes.
Adverse events
Orenstein 2002 observed that 72% of infants receiving famotidine 0.5 mg/kg experienced adverse events and all infants receiving famotidine 1 mg/kg experienced adverse events. However, the data provided did not distinguish between the two groups, so we were unable to extract summary data to assess the certainty of evidence for this outcome. Trialists noted six participants with agitation or irritability (manifested as head‐rubbing in two), three participants with somnolence, two participants with anorexia, two with headache, one participant with vomiting, one participant with hiccups, and one participant with candidiasis. Six infants on famotidine experienced new agitation/irritability; two had accompanying head rubbing and all resolved within days of ending therapy (35 infants).
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Nizatidine
No studies were available for any comparison.
Prokinetics
Domperidone
Domperidone versus placebo
Improvement in clinical symptoms
Carroccio 1994 assessed symptoms, and noted no difference between domperidone and placebo. The trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and did not differentiate between infants and children over one year of age, so we did not consider this study in assessing the certainty of evidence.
Adverse events
There was insufficient evidence to assess adverse events appropriately for domperidone compared to placebo, based on one study (Cresi 2008). No adverse events were seen (26 neonates).
pH‐impedance indices
Two studies were considered. Cresi 2008 randomised 26 neonates to domperidone 0.3 mg/kg or placebo over 24 hours with assessment through a 24‐hour oesophageal pH study. Reflux frequency was increased (difference in means between domperidone epochs and placebo: MD ‐1.26 episodes/hour (95% CI ‐2.31 to ‐0.21), but duration was improved in this brief study (MD 3.5 seconds (95% CI ‐1.67 to 8.67)). Reflux height (MD ‐0.03 (95% CI ‐0.63 to 0.57)) and mean reflux pH (‐0.12 (95% CI ‐0.90 to 0.66)) were no different. As this study only assessed short epochs (8 hours) of data, we have not assessed the certainty of the evidence nor included it in our summary of findings table. Carroccio 1994 also assessed pH indices, and noted no difference between domperidone and placebo. The trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and did not differentiate between infants and children over one year of age, so we did not consider this study in assessing the certainty of the evidence.
Endoscopic findings
No studies were available for this outcome.
Domperidone compared to other treatments
Improvement in clinical symptoms
Carroccio 1994 performed an RCT in 80 participants (one month to 18 months old, with symptoms of reflux) in four groups (domperidone with alginate, domperidone alone, placebo, and domperidone with Maalox, with 20 participants in each group). Trialists assessed symptoms and 24‐hour oesophageal pH indices. There were suggested improvement in symptoms in all four groups: it was reported that symptoms were resolved in 16 of 20 participants in the domperidone with Maalox group; in eight of 20 participants in the domperidone with alginate group; in nine of 20 participants in the domperidone alone group; and an improvement in symptoms in seven of 20 participants in the placebo group. However, the trial did not report the outcome in sufficient detail to allow extraction of summary statistics and did not differentiate between infants and children over one year of age, so we did not consider this study in assessing the certainty of the evidence.
Adverse events
No studies were available for this outcome.
pH‐impedance indices
Carroccio 1994 assessed 24‐hour oesophageal pH indices but did not report the outcome in sufficient detail to allow extraction of summary statistics and did not differentiate between infants and children over one year of age, so we did not consider this study in assessing the certainty of the evidence.
Endoscopic findings
No studies were available for this outcome.
Domperidone at different doses
No studies were available for this comparison.
Erythromycin
Erythromycin versus placebo
Improvement in clinical symptoms
Ballengee 2018 compared erythromycin (50 mg/kg/day) to a visually‐indistinguishable placebo in 33 preterm infants with reflux events on pH‐impedance monitoring (mean gestational age 27 weeks), and noted no improvement in nurse‐reported apnoea/bradycardia and desaturations compared to placebo. However, the trial did not report the outcome in sufficient detail to allow extraction of summary statistics or assessment of evidence certainty.
Adverse events
Whilst Ballengee 2018 reported no increase in apnoeas, bradycardias, and desaturations in the erythromycin group compared to placebo, there were insufficient data for extraction of summary statistics (33 infants) and for judging evidence certainty.
pH‐impedance indices
For Ballengee 2018, the primary outcome measure was the total number of reflux events on 24‐hour pH‐impedance monitoring after one week on erythromycin or placebo. The study found that erythromycin may be inferior to placebo in reducing reflux events, but the trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Endoscopic findings
No studies were available for this outcome.
Erythromycin versus other treatments or at different doses
No studies were available for these comparisons.
Compound alginate preparations
Sodium alginate plus magnesium alginate versus placebo
Improvement in clinical symptoms
Five studies evaluated sodium alginate plus magnesium alginate (sodium+magnesium alginate; i.e. Gaviscon Infant) (Buts 1987; Carroccio 1994; Del Buono 2005; Forbes 1986; Miller 1999). Miller 1999 and Buts 1987 found symptomatic improvement, but were limited by short follow‐up and did not have enough data for extraction of summary statistics. Buts 1987 noted that the number of episodes of regurgitations per day reported by parents was reduced during the trial (20 participants), although summary statistics could not be extracted and the authors did not differentiate between infants and children over one year of age. We did not include these data in the summary of findings tables.
Adverse events
Four studies in sodium+magnesium alginate evaluated adverse events (Buts 1987; Carroccio 1994; Forbes 1986; Miller 1999). Buts 1987 found no adverse events in 20 children but had insufficient data for extraction of summary statistics. Trialists did not differentiate infants from children over one year of age, so we did not assess the certainty of the evidence or include the data in the summary of findings tables. Forbes 1986 noted no adverse events, but these authors also did not differentiate between infants and children, so we did not include the data in the summary of findings tables or assess the certainty of the evidence. Carroccio 1994 also did not report adverse events in sufficient detail to allow extraction of summary statistics and did not differentiate between infants and children, so we did not consider these data in assessing the certainty of the evidence. Miller 1999 noted that 13 participants withdrew due to adverse effects, including diarrhoea and constipation, but with no difference between groups. The trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and we did not consider these data in assessing the certainty of the evidence.
pH‐impedance indices
Two studies assessed this outcome: Del Buono 2005 only noted an improvement in reflux height on manometry, with no other differences compared to placebo, but the trial did not report the outcome in sufficient detail to allow extraction of summary pH statistics. An older formulation of sodium+magnesium alginate (Gaviscon Infant) was evaluated by Forbes 1986, showing no difference in pH indices after 24 hours of treatment, but conclusions were limited by the short‐term nature of this study (24 hours). The total number of reflux events per hour were similar between groups (MD −32.0 (95% CI −85.5 to 21.5)) as well as total duration of reflux episodes (MD 22.0 seconds (95% CI −63.6 to 107.1)). As the authors did not differentiate between infants and children over one year of age, we did not include the data in the summary of findings tables or judge the certainty of the evidence.
Endoscopic findings
No data were available for this outcome.
Sodium+magnesium alginate versus other treatments
Improvement in clinical symptoms
No data were available for this outcome. As discussed above, Carroccio 1994 demonstrated no symptomatic benefit in the domperidone and sodium+magnesium alginate group (20 children), compared to placebo or to domperidone, but the trial did not report the outcome in sufficient detail to allow extraction of summary statistics. However, a confounding factor may have been the thickening of all feeds in all groups by Medigel 1%.
Adverse events
As discussed above, Carroccio 1994 did not comment on adverse events, so we did not consider this study in assessing the certainty of the evidence.
pH‐impedance indices
Carroccio 1994 performed an RCT in 80 participants (infants aged one month to 18 months old with symptoms of reflux) in four groups, and assessed 24‐hour oesophageal pH indices. However, the trial did not report the outcome in sufficient detail to allow extraction of summary statistics and did not differentiate between infants and children over one year of age, so we did not consider this study in assessing the certainty of the evidence.
Endoscopic findings
No studies were available for this outcome.
Sodium+magnesium alginate at different doses
No studies were available for this comparison.
Magnesium (Mg) alginate
Magnesium alginate versus placebo
No studies were available for this comparison
Magnesium alginate versus other treatments
Improvement in clinical symptoms
We identified two studies. Baldassarre 2020 assessed 53 formula‐fed infants and 19 breastfed infants (aged three weeks to four months) with IGERQ‐R scores above 16, and noted that in formula‐fed infants, while both groups improved, no differences in score reduction between magnesium alginate and thickened formula was seen. The breastfed arm did not have a control arm so was not considered. However, the trial included a thickener as a comparator arm, so we have not considered these data in assessing the certainty of evidence. Ummarino 2015 was not included as the trial did not report the outcome in sufficient detail to allow extraction of summary statistics. This study noted magnesium alginate plus simethicone showed improved symptoms compared to thickened formula and lifestyle advice.
Adverse events
We identified two studies. One study noted no adverse events in the magnesium alginate group (75 infants) (Ummarino 2015). Baldassarre 2020 monitored for adverse events and reported that no participants withdrew due to adverse events. However, trialists did not report the presence or absence of adverse events, and so we did not consider this study in assessing evidence certainty.
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Magnesium alginate at different doses
No studies were available for this comparison.
II. Children older than one year
Proton pump inhibitors
Omeprazole
Omeprazole versus placebo
No studies were available for this comparison
Omeprazole versus ranitidine
Improvement in clinical symptoms
We assessed two studies (Cucchiara 1993; Pfefferkorn 2006). Pfefferkorn 2006 assessed nocturnal acid breakthrough symptoms after three weeks in 16 participants (aged one to 13 years) with symptomatic GORD with endoscopic/histological changes treated with omeprazole. In the omeprazole group, symptom scores improved from 2.0 ± 0 at baseline, to 0.6 ± 0.4 at week three, to 0.4 ± 0.45 at week 9, to 0.4 ± 0.5 at week 17; difference in means from baseline to week 17: MD ‐1.6 (95% CI ‐1.9 to ‐1.2). The study also reported no additional benefit from adding ranitidine in those with breakthrough symptoms. There was insufficient detail to compare omeprazole plus ranitidine to omeprazole plus placebo for inclusion in a summary of findings table or to assess the certainty of evidence. As discussed above, Cucchiara 1993 noted similar improvements in symptoms in both those randomised to eight weeks of standard doses of omeprazole (40 mg/day/1.73m2 surface area) and higher doses of ranitidine (20 mg/kg/day). However, we did not consider this evidence further as the trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and did not differentiate between infants and children so was not considered in the certainty of evidence.
Adverse events
We assessed two studies (Cucchiara 1993; Pfefferkorn 2006). One study noted no adverse events (16 participants) (Pfefferkorn 2006). Cucchiara 1993 noted one participant was withdrawn due to a temperature and a respiratory infection, but it was uncertain which treatment group this participant was from (omeprazole or high‐dose ranitidine). We did not consider this evidence further as the trial did not report the outcome in sufficient detail to allow extraction of summary statistics, or differentiate between infants and children so was not considered in the certainty of evidence.
pH‐impedance indices
Two studies assessed the impact of omeprazole versus ranitidine on pH‐impedance indices. Additional ranitidine may not provide additional benefit. Pfefferkorn 2006 assessed nocturnal acid breakthrough symptoms after three weeks in 16 participants with symptomatic GORD with endoscopic/histological changes (aged one to 13 years) treated with omeprazole. Oesophageal pH studies were performed at baseline, week three, and week 17 on omeprazole. Reflux index improved following initiation of therapy from 14.3 ± 11.5% in 24 hours at baseline to 2.0 ± 2.9% in 24 hours at week three (MD ‐12.3 (95% CI‐18.4 to ‐6.4)). The reflux index did not change from week three (2.0 ± 2.9% in 24 hours) to week 17 after initiation of ranitidine (5.1 ± 5.1% in 24 hours) (MD 3.1 (95% CI‐1.0 to 7.2)). However, as both arms contained omeprazole, we have not included this result in a summary of findings table or assessed the certainty of evidence. Cucchiara 1993 noted improvements in pH indices but was not further considered as the trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and did not differentiate between infants and children so was not considered in the certainty of evidence.
Endoscopic findings
Two studies assessed the impact of omeprazole versus ranitidine on endoscopic findings. Additional ranitidine may not provide additional benefit. Pfefferkorn 2006 assessed endoscopy appearances at baseline and week 17 using Hetzel‐Dent scoring (grade 0 to 4). Improvement in endoscopic grade from 3.1 ± 1.4 to 1.6 ± 1.8 occurred in those children receiving omeprazole: MD ‐1.5 (95% CI‐3.1 to 0.1). Improvement in mean histology scores of all participants from baseline (1.8 ± 0.7) to week 17 (0.8 ± 0.9): MD ‐1.0 (95% CI ‐1.8 to ‐0.2) was also seen. However, as both arms contained omeprazole, we have not included this result in a summary of findings table nor assessed the certainty of evidence. Further detail on the effect of ranitidine is discussed below. As discussed above, Cucchiara 1993 noted similar improvements in endoscopic appearances in those randomised to eight weeks of standard doses of omeprazole (40 mg/day/1.73m2 surface area) or higher doses of ranitidine (20 mg/kg/day). This result was not further considered as the trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and did not differentiate between infants and children so was not considered in the certainty of evidence.
Omeprazole versus quince syrup
Zohalinezhad 2015 was the only included study which investigated this comparison, using omeprazole as a comparator to assess the efficacy of quince syrup.
Improvement in clinical symptoms
Omeprazole may provide symptomatic relief but was not superior to quince syrup, based on one study in 80 children (aged 0 to 18 years) with GORD (Zohalinezhad 2015). Composite Symptom Scores (CSS) improved in both groups (quince syrup versus omeprazole (2 mg/kg/day)). This was seen in infants and young children (aged less than 60 months: discussed above) and 42 children (aged more than 60 months to 18 years) at four and seven weeks compared to baseline. In children aged over five years, at week 7, CSS scores were 37 in the quince group and 43 in the omeprazole group. However, as quince syrup is not clinically prescribable, we have not assessed the certainty of evidence in the summary of findings tables. Please see 'Quince syrup versus other treatments' comparison below for a mean difference (MD).
Adverse events
Zohalinezhad 2015 identified no adverse events in either group (42 children), but the trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
pH‐impedance indices
No studies were available for this outcome.
Endoscopic findings
One study was unclear about how many children had had endoscopy (Zohalinezhad 2015), so there were no suitable data for this outcome, and we did not assess evidence certainty.
Omeprazole at different doses
No studies were available for this comparison.
Lansoprazole
Lansoprazole versus placebo
No studies were available for this comparison.
Lansoprazole versus other treatments: alginate
Improvement in clinical symptoms
One study compared lansoprazole plus alginate versus lansoprazole alone in 36 children (age range: 12 months to 12 years) with GORD (based on symptoms, 24‐hour pH probe and endoscopy), and reported that symptom scores improved in all groups by week 8 (Borrelli 2002). The trial did not report the outcome in sufficient detail to allow extraction of summary outcome statistics.
Adverse events
One study noted the absence of adverse events (36 children) (Borrelli 2002), but the study did not report the outcome in sufficient detail to allow extraction of summary statistics
pH‐impedance indices
Borrelli 2002 compared lansoprazole plus alginate versus lansoprazole alone in 36 children (age range: 12 months to 12 years) with GORD (based on symptoms, 24‐hour pH probe and endoscopy). Reflux index improved in all groups by week 8. The trial did not report the outcome in sufficient detail to allow extraction of summary outcome statistics.
Endoscopic findings
Borrelli 2002 compared lansoprazole plus alginate versus lansoprazole alone in 36 children (range 12 months to 12 years) with GORD (based on symptoms, 24‐hour pH probe and endoscopy). Endoscopic appearances improved in both groups by week 8. The trial did not report the outcome in sufficient detail to allow extraction of summary outcome statistics.
Lansoprazole at different doses
Improvement in clinical symptoms
One study assessed 63 adolescents (age range: 12 to 17 years) with symptomatic/endoscopic GORD, randomised to lansoprazole 30 mg versus 15 mg (Gunesekaran 2003). After five days of treatment, the symptom diaries and physician assessments in both groups noted improvements in the frequency and severity of heartburn and other symptoms, but the trial did not report the outcomes in sufficient detail to allow extraction of summary statistics.
Adverse events
Gunesekaran 2003 noted that pharyngitis 6% (2/32 in lansoprazole 15 mg) and headache (16% 4/31) were the most commonly‐reported adverse events (63 children), but the study did not report adverse events in sufficient detail to allow extraction of summary statistics.
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Esomeprazole
Esomeprazole versus placebo or other treatments
No studies were available for these comparisons.
Esomeprazole at different doses
Two studies were considered. Tolia 2010b demonstrated resolution of endoscopically‐proven erosive oesophagitis after eight weeks of esomeprazole in 45/109 children aged one to 11 years. Symptoms and safety data were published by the group separately (Gilger 2006), and then a post hoc analysis of some of these children with endoscopically‐confirmed GORD (aged 12 months to 36 months old) compared esomeprazole 5 mg or 10 mg daily for eight weeks (Tolia 2010a). The trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Improvement in clinical symptoms
Two studies were considered. Tolia 2010b reported improvement in reflux symptoms as assessed by physician's global assessment (PGA) and parental diaries, but the trial did not report the outcome in sufficient detail to allow extraction of summary statistics. Subsequently, a post hoc analysis of some of these children with endoscopically‐confirmed GORD (aged 12 months to 36 months old) compared esomeprazole 5 mg or 10 mg daily for eight weeks (Gilger 2006). They noted 84.2% had improved symptom scores by the final visit, and no difference was reported between low‐dose and high‐dose groups. However, the trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Adverse events
Two studies were considered. Gilger 2006 noted improvement in reflux symptoms as assessed by PGA and parental diaries, but the study did not report the outcome in sufficient detail to allow extraction of summary statistics. Only 9.3% of participants reported 13 adverse events; the most common were diarrhoea (2.8% (3/108)), headache (1.9% (2/108)), and somnolence (1.9% (2/108)). Vomiting in two participants was not judged to be related to treatment. Tolia 2010b did not note any additional adverse events. Again, the study did not report the outcome in sufficient detail to allow extraction of summary statistics
pH‐impedance indices
No studies were available for this outcome.
Endoscopic findings
The two available trials did not report the outcome in sufficient detail to allow extraction of summary statistics.
Rabeprazole
Rabeprazole versus placebo or other treatments
No studies were available for these comparisons.
Rabeprazole at different doses
Improvement in clinical symptoms
Rabeprazole at different doses (0.5 mg/kg and 1 mg/kg) may provide similar symptomatic improvement, based on a single study: Haddad 2013 noted a decrease in the mean 'Total GERD Symptoms and Severity' score. The symptom score improved for 0.5 mg/kg and 1 mg/kg dosing in both the low‐weight group (< 15 kg) (mean ‐13.6 ± 13.1) and (‐9 ± 11.2): MD 4.6 (95% CI ‐2.9 to 12.1), and the high‐weight group (> 15 kg) (‐10.6 ± 11.1) and (‐8.3 ± 9.2): MD 2.3 (95% CI ‐2 to 6.6) by week 12 (1 study, 127 children; very low‐certainty evidence; Analysis 4.1). Please see Table 4.
4.1. Analysis.

Comparison 4: Rabeprazole at higher doses (1 mg/kg) compared to rabeprazole at lower doses (0.5 mg/kg) for GORD in children older than 1 year, Outcome 1: Improvement in symptom score ('Total GERD Symptoms and Severity' score)
Adverse events
Rabeprazole at 0.5 mg/kg and 1 mg/kg is likely to give some adverse events, based on a single study: Haddad 2013 noted 95 (84%) children had adverse events, including abdominal pain, nausea, vomiting, bronchopneumonia, gastroenteritis, cough, and choking (1 study, 127 children; very low‐certainty evidence).
pH‐impedance indices
No studies were available for this outcome.
Endoscopic findings
Rabeprazole at different doses may provide endoscopic and histological improvement, based on a single study: Haddad 2013 found that endoscopic scores in both the low‐weight group (0.5 mg/kg mean ‐1.4 ± 1.1) and 1 mg/kg ‐1.1 ± 0.7): MD 0.3 (95% CI ‐0.3 to 0.9) and high‐weight group (0.5 mg/kg ‐1.1 ± 0.7) and (1 mg/kg ‐1 ± 0.9): MD 0.1 (95% CI ‐0.2 to 0.4) improved at week 12 using Hetzel‐Dent criteria (1 study, 127 children; very low‐certainty evidence; Analysis 4.2). Please see Table 4. Histological scores (Grades 1 to 5) also improved: MD ‐0.8 (95% CI ‐1.06 to ‐0.53) but this is not reported in the summary of findings table, as discussed in Types of outcome measures.
4.2. Analysis.

Comparison 4: Rabeprazole at higher doses (1 mg/kg) compared to rabeprazole at lower doses (0.5 mg/kg) for GORD in children older than 1 year, Outcome 2: Improvement in endoscopic scores (Hetzel Dent scores)
Pantoprazole
Pantoprazole versus placebo or other treatments
No studies were available for these comparisons.
Pantoprazole at different doses
Pantoprazole may or may not improve symptom scores at 0.3 mg/kg, 0.6 mg/kg, and 1.2 mg/kg pantoprazole in children aged one to five years by week 8 with no difference between 0.3 mg/kg and 1.2 mg/kg dosing (0.3 mg/kg mean −2.4 ± 1.7); 1.2 mg/kg −1.7 ± 1.2): MD 0.7 (95% CI ‐0.4 to 1.8; 1 study, 60 children; very low‐certainty evidence) and may confer some to no increase in the risk of adverse events (very low‐certainty evidence).
Improvement in clinical symptoms
Pantoprazole appears to improve symptoms at the different dose‐regimens, based on a single study. Baker 2010 looked at 0.3 mg/kg, 0.6 mg/kg, and 1.2 mg/kg pantoprazole in 60 children (aged one to five years) with symptoms of GORD and endoscopic or histological signs of GORD over eight weeks. Symptom scores improved in all dose‐regimens from baseline to week 8 (0.3 mg/kg MD −2.4, 95% CI −3.2 to −1.5; 0.6 mg/kg MD −0.6, 95% CI −1.7 to 0.5; 1.2 mg/kg MD −1.7, 95% CI −2.9 to −0.39). Symptom scores improved from baseline to week 8 (0.3 mg/kg mean −2.4 ± 1.7); 1.2 mg/kg −1.7 ± 1.2). There was no difference between 0.3 mg/kg and 1.2 mg/kg dosing: MD 0.7 (95% CI ‐0.4 to 1.8). Please see Table 5 and Analysis 5.1. Individual symptoms (abdominal pain, burping, heartburn, pain after eating, difficulty swallowing) improved in all groups after eight weeks (1 study, 60 children; very low‐certainty evidence). Two other studies were considered: Tsou 2006 assessed 136 children (aged 12 to 16 years) with symptoms of GORD given either pantoprazole 40 mg (n = 68) or pantoprazole 20 mg (n = 68) over eight weeks. In both groups, composite symptom scores reportedly improved from baseline to end of trial from 177 and 174 by at least 100 points, with improvements in the number of vomiting episodes per day, heartburn symptom score, and epigastric pain score, although the trial did not report the results in sufficient detail to allow independent extraction of summary statistics. Tolia 2006 compared 10 mg, 20 mg, and 40 mg pantoprazole over eight weeks in 53 children (five to 11 years) with symptomatic GORD, and noted symptomatic improvements in all groups treated with pantoprazole, although there was not enough detail to extract summary statistics.
5.1. Analysis.

Comparison 5: Pantoprazole in higher doses (1.2 mg/kg) compared to pantoprazole at lower doses (0.3 mg/kg) for GORD in children older than 1 year, Outcome 1: Improvement in symptom scores (WGSS)
Adverse events
Three studies reported data on adverse events. Tsou 2006 found 82% of 136 children reported an adverse event, mainly headache, and in the high‐dose group (40 mg) reported diarrhoea. Five participants had minor derangement of their liver function tests. Tolia 2006, assessing 53 children, noted in the pantoprazole 10 mg group the following: headache (seven participants; 36.8%), rhinitis (five participants; 26.3%), and nausea (three participants; 15.8%); in the pantoprazole 20 mg group, the following: headache (five participants; 27.8%) and rhinitis (three participants each; 16.7%); in the 40 mg group, the following: headache (four participants; 25%). Baker 2010, assessing 60 children aged one to five years, observed no difference between the groups. In the low‐dose group, one participant had diarrhoea and nappy rash; in the medium‐dose group, one participant had sleep disturbance, one had abdominal pain; in the high‐dose group, one participant had rectal bleeding (1 study, 60 participants, very low‐certainty evidence). Unfortunately, due to the multiple different dose‐regimes, it was only possible to include information for Baker 2010 in the summary of findings table in narrative format.
pH‐impedance indices
No studies were available for this outcome.
Endoscopic findings
Two studies reported endoscopy outcomes: there was not enough detail to extract summary statistics. Tolia 2006 had observed that endoscopy appearances showed no improvements in any group, and histologically, in the 10 mg pantoprazole group. In those with non‐erosive GORD, 36% improved and no participants with erosive disease were treated within this group. In those receiving pantoprazole 20 mg, of those with non‐erosive GORD, 50% of participants improved (n = 9) with 44% unchanged (n = 8). In those with erosive disease, all three were healed at eight weeks. In those treated with pantoprazole 40 mg, of those with non‐erosive disease, 68% of participants improved (n = 11) with 25% unchanged (n = 4); 6.2% worsened (n = 1). There was no correlation between composite symptom score changes and endoscopy/biopsy changes. In younger children, endoscopy was performed in four participants with erosive changes (Baker 2010); all four healed, but the trial did not report the outcome in sufficient detail to allow extraction of summary endoscopic statistics. For histology appearances, no scope was performed after treatment in those participants with histological changes only.
H₂ antagonists
Ranitidine
Ranitidine versus placebo
No studies were available for this comparison.
Ranitidine versus other treatments
Improvement in clinical symptoms
Two studies were considered: Cucchiara 1993 (see above), who found similar improvements in symptoms in those randomised to eight weeks of either higher doses of ranitidine (20 mg/kg/day) or standard doses of omeprazole (40 mg/day/1.73 m2 surface area). The trial did not report the outcome in sufficient detail to allow extraction of summary statistics. Pfefferkorn 2006 noted an improvement in nocturnal acid breakthrough symptoms after three weeks in 18 participants with symptomatic GORD with endoscopic/histological changes (aged one to 13 years) treated with omeprazole, but no additional benefit from additional ranitidine in those with breakthrough symptoms was seen. Symptom scores in both groups reportedly improved from baseline (see omeprazole above), but the trial did not report the outcome in sufficient detail to allow extraction of summary statistics comparing the two groups (symptoms and pH indices) so this study is not reported in the summary of findings table.
Adverse events
Two studies were considered, although one study, Pfefferkorn 2006, noted no adverse events (16 participants). Cucchiara 1993 noted one participant was withdrawn due to a temperature and a respiratory infection, but it was uncertain which treatment group this participant was from (omeprazole or high‐dose ranitidine). The trial did not report the outcome in sufficient detail to allow extraction of summary statistics, or differentiate between infants and children, so these data were not considered in the certainty of evidence.
pH‐impedance indices
Two studies were considered: Pfefferkorn 2006 assessed nocturnal acid breakthrough symptoms after three weeks in 16 participants with symptomatic GORD with endoscopic/histological changes (aged one to 13 years) treated with omeprazole. The reflux index did not change from week three (2.0 ± 2.9% in 24 hours) to week 17 after initiation of additional ranitidine (5.1 ± 5.1% in 24 hours): MD 3.1 (95% CI‐1.0 to 7.2), but direct comparison between omeprazole and ranitidine was not possible. Cucchiara 1993 noted improvements in pH indices but was not further considered as the trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and did not differentiate between infants and children so was not considered in the certainty of evidence.
Endoscopic findings
Two studies were considered: Pfefferkorn 2006 assessed endoscopy appearances at baseline and week 17 using the Hetzel‐Dent score (grade 0 to 4), and saw an improvement in grade from 3.1 ± 1.4 to 1.6 ± 1.8: MD ‐1.5 (95% CI‐3.1 to 0.1). An improvement in mean histology scores of all participants from baseline (1.8 ± 0.7) to week 17 (0.8 ± 0.9): MD ‐1.0 (95% CI ‐1.8 to ‐0.2) was also seen, but a direct comparison between omeprazole and ranitidine was not possible as ranitidine was introduced at week 3. As discussed above, Cucchiara 1993 noted similar improvements in endoscopic appearances in both the group randomised to eight weeks of higher doses of ranitidine (20 mg/kg/day) and the group receiving standard doses of omeprazole (40 mg/day/1.73m2 surface area). This result was not further considered as the trial did not report the outcome in sufficient detail to allow extraction of summary statistics, and the trial did not differentiate between infants and children so was not considered in the certainty of evidence.
Quince syrup and ranitidine versus ranitidine alone
Improvement in clinical symptoms
Ranitidine may provide symptomatic relief but was not superior to quince syrup and ranitidine, based on one study: Naeimi 2019 undertook an outpatient double‐blind RCT of ranitidine 8 mg/kg/day plus quince syrup (0.5 mL/kg/day) versus ranitidine alone (8 mg/kg/day) in 96 children aged between one and four years with GORD (diagnosed on clinical symptoms: two of five symptoms (regurgitation or vomiting, poor weight gain for one month, respiratory distress after feeding, feed refusal, and restlessness after feeding)). These symptoms were assessed at two, four, and six weeks with the Global Severity Questionnaire (GSQ‐YC). Comparing the total symptom scores showed that ranitidine was effective but that ranitidine plus quince syrup was superior to ranitidine alone: at week 2 (mean ± SD 17.8 ± 2.6 versus 23.4 ± 4.0), week 4 (11.5 ± 2.3 versus 18.8 ± 3.6), and week 6 (12.2 ± 2.3 versus 21.1 ± 4.1): MD at week 6 was 8.9 (95% CI 7.6 to 10.2). In terms of individual symptoms at six weeks, all symptoms were improved in both groups, but there was greater improvement in vomiting (MD 1.3 (95% CI 0.9 to 1.7)), feed refusal (MD 1.9 (95% CI 1.5 to 2.3)), burping/belching (MD 1.8 (95% CI 1.3 to 2.3)), and abdominal pain (MD 1.0 (95% CI 0.5 to 1.5)) in the ranitidine with quince syrup group. No differences in swallowing difficulties (MD 1.7 (95% CI 1.2 to 2.2)) or choking during eating (MD 0.8 (95% CI 0.3 to 1.3)) were seen between the groups. These differences emerged between two and four weeks after starting treatment. As both groups contained ranitidine, we included the certainty of evidence in the quince syrup section.
Adverse events
There was not enough evidence to assess adverse events appropriately for this comparison. However, one study identified no adverse events in either group (96 children) (Naeimi 2019).
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Ranitidine at different doses
No studies were available for this comparison.
Cimetidine and Famotidine
No studies were available for any comparisons.
Nizatidine
Nizatidine versus placebo
Improvement in clinical symptoms
Simeone 1997 assessed 26 participants (age range: six months to eight years) with histologic evidence of oesophagitis, randomised to either nizatidine 10 mg/kg twice a day or placebo for eight weeks. Improvement of symptoms was only seen in the nizatidine group, with reductions in abdominal pain (MD 1.5 (95% CI −0.6 to 2.4)), chest pain (MD 0.6 (95% CI −0.50 to 1.70)), regurgitation (MD 1.4 (95% CI 0.5 to 2.3)), and vomiting (MD 1.2 (95% CI 0.15 to 2.3)). However, as the trial included some infants under one year of age, we did not include these data in the summary of findings tables.
Adverse events
Simeone 1997 assessed 26 participants (age range: six months to eight years) randomised to either nizatidine 10 mg/kg twice a day or placebo for eight weeks. A single participant on nizatidine had an urticarial rash, and one participant on placebo was withdrawn due to worsened clinical symptoms. Study authors did not mention the adverse event severity or the ages of the affected participants, so we did not assess the certainty of evidence. No other adverse events were noted.
pH‐impedance indices
Simeone 1997 included some infants under one year of age, so we did not include these data in the summary of findings tables. Post‐treatment pH measurement showed improved event rates (reflux index, number of episodes pH < 4, number of episodes > 5 minutes, duration of episodes of pH < 4) in the nizatidine group versus placebo, but the trial did not report the outcome in sufficient detail to allow extraction of summary pH statistics.
Endoscopic findings
Simeone 1997 included some infants under one year of age, so we did not include these data in the summary of findings tables. Endoscopy findings included better healing in 69% of participants in the nizatidine group, but the trial did not report the outcome in sufficient detail to allow extraction of summary endoscopy statistics.
Nizatidine versus other treatments or at different doses
No studies were available for these comparisons.
Prokinetics
Domperidone
Domperidone versus placebo
Improvement in clinical symptoms
Bines 1992 assessed the impact of domperidone over four weeks (double‐blind) then a further four weeks (open‐label) versus placebo in 17 participants (aged five months to 11.3 years). No individual symptom was improved by four weeks. After eight weeks of therapy, 33% of participants treated with domperidone noted an improvement in symptoms. Some improvements were reported after four weeks of little symptom improvement, but as the study included some infants under one year of age, we did not include these data in the summary of findings tables and the trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Adverse events
Bines 1992 noted no serious adverse events, but six participants had self‐limiting diarrhoea (four participants on domperidone, two on placebo). As the study included some infants under one year of age, we did not include these data in the summary of findings tables and the trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
pH‐impedance indices
On pH monitoring, Bines 1992 observed there was only an improvement reported in total reflux episodes, with other metrics unchanged. The low number of participants (with some infants under one year of age) and lack of full (24‐hour) pH probes limited the applicability of this study. The trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Endoscopic findings
No studies were available for this outcome.
Domperidone versus other treatments or at different doses
No studies were available for these comparisons.
Quince syrup
Quince syrup versus placebo
No studies were available for this comparison.
Quince syrup versus other treatments: omeprazole
Improvement in clinical symptoms
Quince syrup was as good or better than omeprazole in improving symptoms based on one study (Zohalinezhad 2015). In this study in 80 children (aged 0 to 18 years) with GORD, Composite Symptom Scores (CSS) improved in both the quince syrup and omeprazole (2 mg/kg/day) groups. This was seen in infants and young children (aged less than 60 months: discussed above) and 42 children (aged more than 60 months to 18 years) at four and seven weeks compared to baseline. In children aged over five years, at week 7, CSS scores were 43 in the omeprazole group and 37 in the quince group (MD ‐6 (95% CI‐39.9 to 27)). However, as quince syrup is not a prescribable medicine, we did not include this in the summary of findings tables nor assess the certainty of evidence (please see Methods).
Adverse events
There was not enough evidence to assess adverse events appropriately for quince syrup compared to omeprazole. However, Zohalinezhad 2015 identified no adverse events in either group (42 children).
pH‐impedance indices
No studies were available for this outcome.
Endoscopic findings
In Zohalinezhad 2015, it was unclear how many children had had endoscopy, so we did not consider this result in the certainty of evidence.
Quince syrup versus ranitidine
Improvement in clinical symptoms
Quince syrup may provide symptomatic relief in addition to ranitidine, based on one study: Naeimi 2019 undertook an outpatient double‐blind RCT of ranitidine (8 mg/kg/day) versus ranitidine 8 mg/kg/day plus quince syrup (0.5 mL/kg/day) in 96 children aged between one and four years with GORD diagnosed on clinical symptoms (two of five symptoms (regurgitation or vomiting, poor weight gain for one month, respiratory distress after feeding, feed refusal, and restlessness after feeding)). These were assessed at two, four, and six weeks with the Global Severity Questionnaire (GSQ‐YC). Comparing the total symptom scores showed that ranitidine plus quince syrup was superior to ranitidine alone: at week 2 (mean ± SD 17.8 ± 2.6 versus 23.4 ± 4.0), week 4 (11.5 ± 2.3 versus 18.8 ± 3.6), and week 6 (12.2 ± 2.3 versus 21.1 ± 4.1): MD at week 6 8.9 (95% CI 7.6 to 10.2). In terms of individual symptoms at six weeks, all symptoms were improved in both groups, but there was greater improvement in vomiting (MD 1.3 (95% CI 0.9 to 1.7)), feed refusal (1.9 (95% CI 1.5 to 2.3)), burping/belching (1.8 (95% CI 1.3 to 2.3)), and abdominal pain (1.0 (95% CI 0.5 to 1.5)) in the ranitidine with quince syrup group. No differences in swallowing difficulties (MD 1.7 (95% CI 1.2 to 2.2)) or choking during eating (0.8 (95% CI 0.3 to 1.3)) were seen between the groups. These differences emerged between two and four weeks after starting treatment. However, as quince syrup is not a prescribable medicine, we did not include this result in the summary of findings tables nor assess the certainty of evidence (see above).
Adverse events
There was not enough evidence to assess adverse events appropriately for quince syrup compared to ranitidine alone. However, Naeimi 2019 identified no adverse events in either group (96 children).
pH‐impedance indices and endoscopic findings
No studies were available for these outcomes.
Quince syrup at different doses
No studies were available for this comparison.
Compound alginate preparations
Alginate versus placebo
No studies were available for this comparison.
Alginate versus other treatments: lansoprazole
Improvement in clinical symptoms
No suitable data were available for this outcome; one study was considered. Gaviscon liquid was assessed by Borrelli 2002, who, as discussed above, noted improvements in symptoms in children (aged 12 months to 12 years) with erosive oesophagitis given alginate alone, and noted that the greatest improvement in symptoms was seen in children treated with alginate and lansoprazole. The trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Adverse events
There was not enough evidence to assess adverse events appropriately for alginates compared to lansoprazole. Borrelli 2002 noted no adverse events.
pH‐impedance indices
Borrelli 2002 noted improvements in pH indices in children (aged 12 months to 12 years) with erosive oesophagitis given alginate alone, and noted that the greatest improvement was seen in children treated with alginate and lansoprazole. The trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Endoscopic findings
Borrelli 2002 noted improvements in endoscopic indices in children (aged 12 months to 12 years) with erosive oesophagitis given alginate alone, and noted that the greatest improvement was seen in children treated with alginate and lansoprazole. The trial did not report the outcome in sufficient detail to allow extraction of summary statistics.
Alginate at different doses
No studies were available for this comparison.
Antispasmodics
Baclofen
Baclofen versus placebo
Improvement in clinical symptoms
No studies were available for this outcome.
Adverse events
There was not enough evidence to assess adverse events appropriately for baclofen. Omari 2006 noted no adverse events in the baclofen group in the 48 hours following the trial.
pH‐impedance indices
A single study compared baclofen to placebo in a double‐blinded RCT in 30 children with resistant GORD (mean age 10.0 ± 0.8 years) (Omari 2006). Children were assessed with manometry/pH for two hours after they were given 0.5 mg/kg baclofen or placebo. The primary outcome (measurement of the incidence of transient lower oesophageal sphincter relaxations (TLESR)) was not a prespecified outcome of this review. The Omari 2006 study assessed pH indices, but did not report the outcome in sufficient detail to allow extraction of summary pH statistics.
Endoscopic findings
No studies were available for this outcome.
Baclofen versus other treatments or at different doses
No studies were available for these comparisons.
III. Other subgroups
We identified no studies assessing the efficacy of drug treatment in children with neurodisability and GORD.
Discussion
We included a total of 36 trials assessing 2251 participants in this review. We were able to extract summary data from 14 RCTs, with the remaining studies having insufficient data for extraction.
Summary of main results
In this review, we cover a wide range of potential treatments for GOR/GORD in a range of population groups. We place the extracted summary data into context with other studies, where available. The Cochrane Gut Group ran the review searches independently to ensure reproducibility.
Overall, the evidence evaluating the role of medications in GORD is of very low certainty. There are several reasons for this, including the heterogeneity of the population, the lack of head‐to‐head trials, variation in outcome measures, and variability in how well outcome measures (e.g. symptom scores/reflux index/endoscopic appearances) correlate in estimating the severity of GORD. There is also a group of infants and children who have physiological reflux that is problematic but not a pathological disease.
Below, we present outcomes (symptom scores, pH‐impedance indices, endoscopic/histological appearances, and adverse events) structured by population (first infants, then children), then by treatment class. We discuss evidence certainty if we were able to extract summary data, and outline our findings below.
I. Infants
Proton pump inhibitors
Please see Table 1; Table 2; Table 3.
For proton pump inhibitor studies with extracted summary data, there is very low‐certainty evidence based on single studies that infants with GOR and GORD may or may not benefit from omeprazole and esomeprazole. There is very low‐certainty evidence that PPIs improve reflux index and other pH probe markers of GORD, and the correlation between pH probe results and direct symptomatic benefit was very weak in infants. There were no suitable data regarding adverse events or endoscopic metrics in any study in infants. Other studies – from which we could not extract summary statistics – showed mixed efficacy.
Omeprazole
One study (with summary data extracted) compared omeprazole to placebo in infants only. This study noted that crying occurred in both omeprazole‐treated and untreated irritable infants, concluding that the cry/fuss time decreased spontaneously with time, and that empirical acid suppression was not indicated in this group. One study (with summary data extracted) comparing omeprazole and ranitidine noted similarly improved GORD symptom scores in infants.
Lansoprazole
No studies provided suitable summary data. Of the studies without summary data, one found no symptomatic difference between lansoprazole and placebo, and of those who went on to take lansoprazole open‐label, there was no improvement in symptoms. Many participants may have had physiological reflux, and a risk of adverse events was noted, including lower‐respiratory tract infections in infants treated with lansoprazole.
Esomeprazole
One study of neonates showed very low‐certainty evidence that esomeprazole was no better than placebo in treating the total number of GORD symptoms (from video monitoring) and GORD‐related signs (from cardiorespiratory monitoring). One other study of older infants without summary data reported that infants treated with esomeprazole had improved symptoms and improved reflux index compared to those treated with antacid. For different doses of esomeprazole, one other study without summary data noted improved clinical symptomsin the low‐dose group. In terms of adverse events in the same study, one infant (with pre‐existing colic) withdrew with irritability. Reflux index was improved in both groups, with greater improvement seen in the lower‐dose group.
Pantoprazole
There were no studies assessing symptomatic improvements in infants treated with pantoprazole. However, in terms of pH indices, one study (with summary data extracted) assessing infants only noted improvements in reflux index from baseline in both groups, as well as in number of episodes, number of reflux episodes lasting more than five minutes, and duration of the longest reflux episodes (very low‐certainty evidence).
Rabeprazole
There were no studies comparing rabeprazole to placebo or to other drugs. For rabeprazole at different doses, we were unable to extract any summary data. However, a withdrawal study noted no difference in primary symptom scores, and equally high rates of adverse events (diarrhoea, constipation, flatulence, crying, and rash) between groups.
H₂ antagonists
The evidence is very uncertain about the effect of H₂ antagonists on outcome. For H₂ antagonists, based on extracted summary data, there is very low‐certainty evidence based on a single study that infants with GORD may or may not have symptomatic benefit from ranitidine. Other studies – from which we could not extract summary statistics – showed mixed efficacy for ranitidine, famotidine, cimetidine, and nizatidine.
Ranitidine
In terms of studies involving infants only, one study with summary data comparing ranitidine to omeprazole showed that the two agents were equivalent in improving symptom scores for infants with symptoms of GORD. For studies without summary data, one study without a control arm reported improved vomiting and respiratory symptoms, but no change in irritability, arching, or feed refusal over two weeks. For adverse events, no summary data were available, but two other studies found no adverse events. One other study assessing both infants and children found similar improvements in symptoms, 24‐hour pH and endoscopic indices in those given either standard doses of omeprazole or high doses of ranitidine (20 mg/kg/day) in children refractory to standard‐dose ranitidine.
Cimetidine
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made. One RCT without summary data compared cimetidine to Maalox in infants and young children with GORD, based on symptoms, oesophagitis on endoscopy, and acid reflux on pH probe. This study reported that cimetidine and Maalox provided symptomatic relief, and reflux index and endoscopic appearances were also improved in both groups.
Famotidine
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made. In terms of studies without summary data,one study noted slight improvement in regurgitation frequency/volume and crying time and improved global assessments by parents and physicians. However, only eight of 35 participants completed phase 2 (double‐blind withdrawal versus placebo), giving insufficient data. A high proportion of infants experienced adverse events, including agitation or irritability, somnolence, weight loss, headache, vomiting, hiccups, candidiasis, and asymptomatic neutropenia which resolved.There were no data regarding pH or endoscopic indices.
Nizatidine
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy can be made. However, one study of children and infants reported improved symptoms in the nizatidine group and only one participant had an adverse event (urticaria). Post‐treatment pH‐indices showed improved reflux index, number of episodes of pH less than 4, number of episodes of more than five minutes, and duration of episodes of pH less than 4 in the nizatidine group compared to those given placebos. Endoscopy findings included better healing in a high proportion of participants in the nizatidine group.
Prokinetics
As discussed above, we did not assess metoclopramide here, and there were no studies of prokinetics with suitable summary data, so no robust judgement about the certainty of evidence of efficacy can be made.
Domperidone
No studies provided suitable summary data for assessment. In terms of symptom improvements, there were no studies of infants alone, but two studies involved infants and children older than one year. One found no improvement in symptoms between domperidone and placebo; the other study noted individual symptoms improved after four weeks (double‐blind); after a further four weeks (open‐label), only a few participants given domperidone noted further symptom improvement. In terms of adverse events, one short study reported no adverse events. Three studies measured pH indices. One very short‐term study (8‐hour epochs) noted reflux frequency increased in the domperidone group, but duration was improved. In another study, no difference between domperidone and placebo was seen, but the placebo included an added thickener (Medigel 1%). The final study found no difference in reflux index and only improvement in total reflux episodes.
Erythromycin
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made. One study compared erythromycin in preterm infants over one week to placebo, and found no difference between the two groups in either number of apnoeas/bradycardias and desaturations or reflux events.
Quince syrup
Quince syrup is a traditional Persian medicine. One study compared it to omeprazole, which demonstrated improvement in symptom scores for both the infant groups, and older children from baseline. However, no difference between the treatment groups (quince syrup or omeprazole) was reported. The improvement in symptoms was present at four weeks after initiation of therapy and sustained at the 7‐week follow‐up. Quince syrup was also compared in combination with ranitidine to ranitidine alone in another study in children. Trialists noted improved global symptom scores, with improvements in vomiting, refusal of eating, burping/belching, and abdominal pain. The evidence for use of quince is limited, with only two studies with relatively small numbers and short follow‐up. We did not assess the certainty of the evidence as this medicine is not clinically available.
Compound alginate preparations
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made.
Sodium+magnesium alginate
There were no summary data available to assess the certainty of evidence for sodium+magnesium alginate (Gaviscon Infant). We identified five studies without summary data; two of these evaluated the current formulation. Both studies noted symptomatic improvement, limited by short follow‐up. In terms of pH indices, one study only noted an improvement in reflux height on manometry, with no other differences when compared to placebo in a short‐term study. Assessing older preparations of Gaviscon Infant, three studies showed some to no difference in pH indices after 24 hours of treatment with sodium+magnesium alginate.
Magnesium alginate
There were no summary data available to assess the certainty of evidence for magnesium alginate. Of the remaining studies without summary data, one study compared magnesium alginate to thickener. Both groups containing formula‐fed infants improved, with no differences in symptom score reduction between magnesium alginate and thickened formula. There was a breastfed study group which was not placebo‐controlled. One open‐label RCT noted symptom scores were reportedly reduced in the magnesium alginate‐plus‐simethicone group compared with thickened formula or reassurance groups, although there was some improvement in all groups, with the study limited by relatively small numbers and 8‐week follow‐up. Whilst there were no summary data, no adverse events were seen in the magnesium alginate group.No studies provided suitable summary data on pH or endoscopic indices for assessment.
Antispasmodics
Baclofen
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made.
II. Children older than one year of age
Proton pump inhibitors
In children over one year of age, very low‐certainty evidence indicates that PPIs may or may not help GORD outcomes. Single studies with summary data extracted showed PPIs may or may not improve symptom scores, or erosive changes on endoscopy due to GORD, particularly in older children. There were very low‐certainty summary data assessing frequency of adverse events, and no robust summary data regarding pH indices in older children. Other studies – from which we could not extract summary statistics – showed efficacy.
Omeprazole
No studies had suitable summary data. Of three other studies, one study comparing omeprazole with high‐dose ranitidine noted similar improvements in symptoms, endoscopic findings, and reflux index. No significant adverse events were noted. A second study compared omeprazole to quince syrup in both infants and older children, and found that both treatments provided similar improvements in symptom scores from baseline. We extracted summary data for older children (> 60 months of age) but did not present these in a summary of findings table (very low‐certainty evidence). There are insufficient data from RCTs about the long‐term safety of omeprazole.
Lansoprazole
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made. Of the studies without summary data, one study in children aged one to 12 years, reported symptom scores improving in all groups, with the lansoprazole and alginate group superior to the other two groups. A 24‐hour pH study also showed improved reflux index, with the lansoprazole and alginate group superior to the other two groups. Endoscopy appearances were much improved in all three groups. No adverse events were reported. In older children (12‐ to 17‐year‐old children), one study of lansoprazole at different doses noted after five days that reflux symptoms were better in both groups. There are insufficient data from RCTs about the long‐term safety of lansoprazole.
Esomeprazole
No studies provided suitable summary data for assessment in children, and only studies of esomeprazole at different doses were available. It was not possible to ascertain the certainty of evidence. Of the linked studies without summary data, one studynoted improvement in reflux symptoms, as assessed by physician's global assessment and parental diaries. Reported adverse events included diarrhoea, headache, and somnolence. One other study did not note any additional adverse events. There were no data presented on pH indices. One study noted endoscopic improvement in all groups, with a post hoc analysis of some participants with endoscopically‐confirmed GORD (aged 12 months to 36 months) showing improved symptoms but no difference between low‐dose and high‐dose groups. Repeat endoscopy showed healing in all, confirmed on histology.
Rabeprazole
Please see Table 4, Analysis 4.1, and Analysis 4.2. One study provided suitable summary data for assessment in children older than one year of age, but only data regarding rabeprazole at different doses were available. Rabeprazole at different doses (0.5 mg/kg and 1 mg/kg) may provide similar symptomatic improvement (very low‐certainty evidence). There was an equivalent adverse event profile in both the low‐ and higher‐dose rabeprazole groups. There were no data for pH indices. Rabeprazole at different doses is likely to provide endoscopic and histological improvement,with a minimal clinically useful difference between doses in low‐weight and high‐weight groups (very low‐certainty evidence).
Pantoprazole
Please see Table 5 and Analysis 5.1. One study of pantoprazole at different doses provided suitable summary data of clinical symptoms for assessment. Pantoprazole appears to improve symptoms at the different dose regimens in one study with summary data (very low‐certainty evidence). Two other studies without summary data noted symptomatic improvements in all groups. One study had summary data for adverse events. It noted diarrhoea, nappy (i.e. diaper) rash, sleep disturbance, abdominal pain, and rectal bleeding (one participant each) (very low‐certainty evidence). The remaining two studies noted high rates of adverse events (mainly headache). There were no summary data available for pH indices or endoscopic outcomes. Two other studies noted endoscopy outcomes, finding no correlation between symptom scores and endoscopy/biopsy changes.
H₂ antagonists
The evidence is very uncertain about the effect of H₂ antagonists on outcome, due to the absence of summary data. In those studies from which we could not extract summary statistics, the H₂ antagonists ranitidine, cimetidine, and nizatidine showed efficacy in terms of symptom score, pH indices, and endoscopic appearances.
Ranitidine
No studies provided suitable summary data for assessment in children. One study found similar improvements in symptoms, 24‐hour pH probe data indices, and endoscopy appearances in those receiving eight weeks of either standard doses of omeprazole or high doses of ranitidine in children who had not responded to standard‐dose ranitidine. One other study looked at the addition of ranitidine or placebo, to reduce nocturnal acid breakthrough, in children who had recently started on omeprazole. In this study, symptom scores, pH indices, and endoscopic appearances reportedly improved over 17 weeks, with no difference between ranitidine and placebo groups. There was therefore no additional benefit seen from supplementation of PPI therapy with ranitidine.
Cimetidine
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made. The only RCT compared cimetidine to Maalox over 12 weeks in infants and young children with a diagnosis of GORD, based on symptoms, oesophagitis on endoscopy, and acid reflux on pH probe. This study reported that cimetidine and Maalox provided symptomatic relief, and that reflux index and endoscopic appearances were also improved in both groups.
Famotidine
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made.
Nizatidine
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy can be made. However, one study reported improved symptoms in the nizatidine group. No summary data could be extracted regarding adverse events, but the study reported only one participant with urticaria. Post‐treatment pH indices showed improved reflux index, number of episodes of pH less than 4, number of episodes of more than five minutes, and duration of episodes of pH less than 4 in the nizatidine group compared to placebo. Endoscopy findings included better healing in the majority of participants in the nizatidine group.
Prokinetics
Domperidone
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made. RCTs evaluating the use of domperidone included two studies involving infants and children older than one year. One study found no difference in improvement in symptoms between domperidone and placebo, but that the thickened feeds (Medigel 1%) could account for improvements in pH outcomes in the placebo group. Symptom improvement remained through six months of follow‐up. One shorter‐term study noted individual symptoms were improved after four weeks (double‐blind). After a further four weeks of domperidone(open‐label) versus placebo, only a low proportion of participants treated with domperidone noted an improvement in symptoms. On pH probe, there was only improvement in total reflux episodes, and some improvement in growth metrics was seen.
Erythromycin
No studies provided suitable summary data for assessment, so no robust judgement about the certainty of evidence of efficacy or risk of adverse events can be made.
Quince syrup
Two studies provided summary symptom data in children older than one year. However, as discussed in the Methods and Results, we have not created a summary of findings table or assessed evidence certainty. One study comparing quince syrup with omeprazole demonstrated improved symptom scores for older children from baseline, with no difference between groups. The improvement in symptoms was present at four weeks after initiation of therapy and sustained at the 7‐week follow‐up. Quince syrup with ranitidine compared to ranitidine alone showed improved global symptom scores in both groups, with ranitidine and quince syrup superior to ranitidine alone for individual symptoms. There were not enough summary data to appropriately assess evidence certainty for adverse events. However, both studies identified no adverse events.
Antispasmodics
Baclofen
A single study showed improvement in acid reflux and transient lower‐oesophageal sphincter relaxations in children treated with baclofen, but this was a short‐duration (2‐hour) trial (Omari 2006), with no other studies available in this group.
Overall completeness and applicability of evidence
We consider the completeness and applicability of evidence for each class of medication in turn, in infants and children with GORD. Overall, all but two studies were conducted on outpatients across primary and secondary care, where the vast majority of infants and children present with symptoms. The very low‐certainty evidence is likely to be applicable to inpatient infants and children diagnosed with GOR and GORD and augment the two neonatal studies, though further studies would confirm this. There were three studies in lower‐middle‐income settings and 33 in high‐income settings, but no studies of infants or children with GORD in low‐income settings (see World Bank 2022 for definitions). In terms of outcomes, symptom scores summary data were often available, but adverse events summary data were often lacking. There was evidence of incomplete reporting regarding endoscopic findings and pH indices in three studies. These procedures may discourage recruitment, and are generally only available in tertiary centres.
Proton pump inhibitors
There is incomplete evidence for commonly used PPIs, as the results are based on single studies, with heterogeneity of case definition in infants. There were insufficient data (one study of esomeprazole) involving premature and term neonates and no data on children with neurodisability. There were also insufficient data about the most effective PPI in infants and children, though we presented data on adverse events profiles where available, and included overall messages suggested by studies without summary data for context. Long‐term safety needs to be evaluated, and consistency between studies regarding symptom scoring would help meta‐analysis.
H₂ antagonists (ranitidine and nizatidine)
With so few RCTs in infants or children, and no appropriate head‐to‐head comparisons against PPIs, meta‐analysis to further investigate the effect of treatment was not possible. Our interpretation of summary data was limited to single studies. No RCTs evaluated the use of H₂ antagonists in physiological reflux. Subgroups of particular importance in which evidence is lacking include neonates and premature babies, as well as children with neurodisability. Evidence of efficacy in resource‐limited settings would also be useful to consider.
Prokinetics
For domperidone and erythromycin, there were no studies based on summary data in infants or children. Further studies will substantially alter our understanding of the effect size, either compared to placebo or the other prokinetic agent. There are major limitations in published study design and length of follow‐up. Subgroups of particular importance include neonates and children with neurodisability, for which there are no studies in domperidone or erythromycin.
Other agents
The utility of quince syrup in settings outside the single country in which it has been studied needs to be evaluated, as there may be other confounding factors that may affect efficacy in other settings. As quince syrup is not clinically available for prescription, it is difficult to assess how applicable the summary data are.
There were no studies in alginates based on summary data in infants or children over one year of age. Further studies in infants will substantially alter our understanding of the effect size, particularly as the available formulations have evolved over the past three decades.
Further studies to assess whether baclofen has a role in improving GORD in children with neurodisability, who are often prescribed baclofen for concomitant spasticity, would be useful. We have not found enough additional trials in this update to change our assessment of the evidence.
No studies assessed whether children who did not improve with treatment went on to need fundoplication.
Certainty of the evidence
We have included decisions about the certainty of the evidence within the summary of findings tables and considered whether risk of bias affected the certainty of the results enough to merit downgrading the evidence certainty. We appraised the general certainty of all the studies using GRADE criteria. There were insufficient trials for meta‐analysis or a funnel plot to investigate reporting (publication) bias. We would have conducted a sensitivity analysis if the exclusion of studies with a high risk of bias was required.
As discussed above, the overall GRADE ratings for the certainty of evidence were all very low as the evidence was mainly based on single studies, with significant methodological concerns about several studies (as summarised in Risk of bias in included studies, Figure 2, and Figure 3). Only nine studies specified the method of allocation, and 13 studies specified the blinding technique. Fifteen studies had a low risk of attrition bias, but only five studies were found to be at low risk for selective reporting bias. Only three studies had independent funding; pharmaceutical companies provided support for manuscript‐writing and funding in several studies. None of the studies assessed in this update has improved the certainty of the evidence, meaning that further studies would add substantially to the evidence base. There was considerable heterogeneity, such as: outcomes analysed by different symptom scores; different participant groups (infants versus children, GOR versus GORD); PPIs in different dosing comparisons, rather than comparing different agents; and different indices (for example, on 24‐hour pH/impedance monitoring). Further comparative studies in both infants and children are likely to improve the certainty of evidence.
Potential biases in the review process
The strengths of this review include the incorporation of further papers to reinforce the evidence base and the use of a similar systematic literature search (including handsearching) of multiple databases and relevant reviews, using wide search terms. The last update to the search was on 17 September 2022. Three review authors appraised each study, and a statistician verified the statistical analysis. Questions about newer studies (published from 2018 onward) were addressed by writing to the study authors, but we received no replies. Additional author data would help the robustness of evidence appraisal. There are no conflicts of interest to declare. Potential weaknesses of the review include the absence of sufficient summary data to perform a meta‐analysis (and inability to perform a sensitivity analysis to test robustness), the inclusion of studies with few children, the short study duration of most studies, and the degree of outcome heterogeneity affecting the accuracy of conclusions.
Agreements and disagreements with other studies or reviews
This review is broadly consistent with NASPGHAN‐ESPGHAN guidelines 2018, and National Institute for Health and Care Excellence (NICE) guidelines (NICE 2019). Other reviews, which include other study designs (such as case‐control and cohort studies), draw similar conclusions about the paucity of evidence, and call for further research, particularly into the subgroups discussed above (NICE 2019; Tighe 2009).
We identified no data regarding alerts of concern for specific medications, as discussed in the Description of the intervention section. For example, for ranitidine, we identified no patients experiencing tachyphylaxis in the studies assessed, but this has been identified elsewhere as a concern (Hyman 1985). Additionally, a multicentre observational study noted a nearly seven‐fold increased relative risk of necrotising enterocolitis (95% CI 1.7 to 25.0) in ranitidine‐treated very low birth weight infants (Terrin 2012), but no studies we analysed identified this complication. Domperidone does not have a Food and Drug Administration (FDA) licence for marketing in the USA, and NICE guidance advises 'do not use' except in specialist paediatric settings (NICE 2019), but no studies we analysed identified this complication. There is a known association between erythromycin and development of pyloric stenosis (Cooper 2002), as well as potential side effects affecting the neonatal microbiome and antimicrobial resistance. NICE guidance advises 'do not use' except in specialist paediatric settings (NICE 2019), but no studies we analysed identified this complication.
Authors' conclusions
Implications for practice.
Based on studies with summary data, there was no evidence to draw conclusions about the efficacy of medications (proton pump inhibitors (PPIs), H₂ antagonists, alginates, and prokinetics) compared to placebo for infants with gastro‐oesophageal reflux (GOR).
For infants with gastro‐oesophageal reflux disease (GORD), there was very low‐certainty evidence that PPIs (omeprazole and esomeprazole) were effective in improving symptoms, and very low‐certainty evidence of absence of symptomatic benefit from PPIs in preterm infants. There were no studies with summary data for prokinetics (such as domperidone), which remain of use only in specialist situations. There were no studies with summary data for alginates. As quince syrup is not clinically available, we did not evaluate the certainty of evidence for this treatment.
In older children with GORD, there was very low‐certainty evidence that PPIs (pantoprazole and rabeprazole) may or may not help GORD outcomes, including symptoms and endoscopic/histological metrics. There were no data on pH indices. There was no clear evidence of a possible risk of increased adverse events based on very low‐certainty evidence with rabeprazole. There were no studies assessing the use of PPIs in physiological reflux. There was insufficient evidence based on summary data to assess the benefit from H₂ antagonists in providing symptomatic relief. There was no evidence for prokinetics (such as domperidone or erythromycin). As quince syrup is not clinically available, we did not evaluate the certainty of evidence for this treatment. Understanding the applicability to children with GOR of the single low‐quality study of baclofen is difficult. It should be noted that the current main clinical use of baclofen is in children with neurodisability receiving baclofen for hypertonia. We identified no studies in children with neurodisability (please see Implications for research).
Implications for research.
The burden on primary and secondary care of physiological reflux and GORD is large, and further research is essential to clarify the role of medications in treating GORD. Despite the Pediatric Written Request made by the Food and Drug Administration (FDA) in the USA to improve our knowledge of a class of medications (PPIs) that are widely prescribed, the summary data available remain scarce. Further studies are needed to confirm whether PPIs or H₂ antagonists are superior, and whether individual drugs offer superior efficacy. Our review confirms that the quality and certainty of the evidence would benefit from further studies, especially assessing common medications used to treat GOR (e.g. H₂ antagonists, sodium+magnesium alginate). We would also call for comparisons that include a placebo or a different drug arm, in addition to the current comparisons between same‐drug different‐dosing. It was also evident that some studies gave participants confounding interventions (e.g. thickened or hydrolysed feeds to infants) that may provide improvements as interventions in their own right. Agreeing on consistent outcome measures and normative values for pH‐impedance monitoring and endoscopy would help research progress in the field. Further studies with longer follow‐up periods are needed. Further studies of prokinetics, including quince syrup, are recommended.
We would also highlight the need for randomised controlled trials (RCTs) specifically in children with underlying oesophageal dysmotility (e.g. children with cerebral palsy), who often have difficult and protracted reflux. Most of the trials included in this review excluded this subgroup. These children are often on maximal medical therapies, including prokinetics, for prolonged time periods. Treatment regimens for this group are often extrapolated from other groups of children. Premature babies are often also treated empirically for gastro‐oesophageal reflux; for example, as part of managing apnoea. Further RCTs in this age group are also recommended, using consistent outcomes.
Separating industry funding for trials from involvement in manuscript preparation would improve the strength of evidence according to GRADE criteria, when considering future trial design.
Evidence of medication efficacy in low‐income countries and over a longer time period would also be useful to consider.
What's new
| Date | Event | Description |
|---|---|---|
| 22 August 2023 | New citation required and conclusions have changed | Conclusions have been updated regarding the certainty of the evidence |
| 22 August 2023 | New search has been performed | Review updated with latest literature search. |
History
Protocol first published: Issue 6, 2010 Review first published: Issue 11, 2014
| Date | Event | Description |
|---|---|---|
| 1 December 2020 | New citation required and conclusions have changed | 36 (12 new) RCTs are included in this review. Summary data extracted from 14 studies and conclusions updated. |
Acknowledgements
Current version:
We would like to thank the following editors and peer referees who provided comments to improve the review: Sarah Rhodes (Statistical Editor), Yvan Vandenplas and Kornilia Nikaki (Peer Reviewers), Alfretta Vanderheyden (Consumer Reviewer), Yuhong Yuan and Teo Quay (Managing Editors) and Grigoris Leontiadis (Co‐ordinating Editor). The updated search strategies for CENTRAL, MEDLINE and Embase were designed by Yuhong (Cathy) Yuan (Information Specialist, Cochrane Gut). We would like to acknowledge the very kind work of University Hospitals Dorset Library, in accessing articles.
Previous version: We would like to again acknowledge the work that Amanda Bevan, Andrew Hayen and Alasdair Munro provided in the first review, as well as Bernie Higgins for his initial work in drafting the data collection form and the protocol. We would like to acknowledge the very kind work of Poole Hospital Library, in accessing articles.
We would also like to acknowledge the support of the Cochrane Gut Group, particularly Yuhong Yuan in performing the search.
Cochrane Gut supported the authors in the development of this Review Update.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Bob Boyle, Imperial College London, UK
Managing Editor (selected peer reviewers, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Colleen Ovelman and Anne‐Marie Stephani, Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Lisa Wydrzynski, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Faith Armitage, Cochrane Central Production Service
Peer‐reviewers (provided comments and recommended an editorial decision): Yvan Vandenplas, Vrije Universiteit Brussel, UZ Brussel, KidZ Health Castle (clinical/content review), Kornilia Nikaki, Paediatric Gastroenterology Consultant, Great Ormond Street Hospital, UK (clinical/content review), Nuala Livingstone, Cochrane Evidence Production and Methods Directorate (methods review), Robin Featherstone, Cochrane Central Editorial Service (search review).
Appendices
Appendix 1. CENTRAL search strategy (via Ovid Evidence‐Based Medicine Reviews Database (EBMR))
exp Gastroesophageal Reflux/
((gastroesophag* or gastro‐esophag* or gastro‐oesophag* or gastric or esophag* or oesophag*) adj3 reflux).tw,kw.
(GERD or GORD or NERD or NORD or GER or GOR).tw,kw.
(acid adj2 reflux).tw,kw.
exp Duodenogastric Reflux/
((duodenogastric or duodeno‐gastric or duodenal) adj3 reflux).tw,kw.
exp Bile Reflux/
(bile adj2 reflux).tw,kw.
((laryngopharyngeal or supraesophag*) adj3 reflux).tw,kw.
gastric regurgitation.tw,kw.
exp ESOPHAGITIS/
(esophagitis or oesophagitis or non‐erorisve reflux disease or nonerosive reflux disease).tw,kw.
or/1‐12
exp Proton Pump Inhibitors/
proton pump inhibitor*.mp.
(PPI or PPIs).tw,kw.
(omeprazole or h 16868 or losec or prilosec or rapinex or zegerid).mp.
(lansoprazole or lanzoprazole or ag 1749 or agopton or bamalite or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).mp.
(pantoprazole or by 1023 or protium or protonix or skf‐96022).mp.
(esomeprazole or nexium).mp.
(rabeprazole or aciphex or dexrabeprazole or e 3810 or ly‐307640 or pariet).mp.
(dexlansoprazole or Kapidex or Dexilant or AGN 20194* or AGN20194* or dexrabeprazole).mp.
(tenatoprazole or CAS 113712‐98‐4 or STU‐Na or TAK‐390* or TAK390* or TAK‐438 or TAK438 or AZD0865 or "AZD 0865").mp.
exp Histamine H2 Antagonists/
((histamine or H2 or H‐2 or H2R or H 2 R) adj3 (antagonist* or blocker* or blockage* or blockader*)).tw,kw.
(H2RA or H2RAs or H2‐RA or H2RAs).tw,kw.
(antihistaminic* adj2 (H2 or H‐2)).tw,kw.
(Cimetidine or Tagamet or altramet or biomet or biomet400 or eureceptor or histodil or skf 92334 or skf92334).tw,kw.
(ranitidine or zantac or ah 19065 or ah19065 or biotidin or ranisen or ranitidine or sostril or zantic).tw,kw.
(Famotidine or Pepcid or mk 208 or mk208 or ym 11170 or ym11170).tw,kw.
(Nizatidine or Axid or axid or ly 139037 or ly139037).tw,kw.
(Roxatidine or Rotane or Zorpe).tw,kw.
(prokinetic* or gastroprokinetic* or gastrokinetic* or gastro‐kinetic*).tw,kw.
(antiemetic* or anti‐emetic).tw,kw.
exp Benzamides/
(Benzoic Acid Amide or Amides or Phenyl Carboxyamide or Benzamide* or Benzoylamide or benzoates).tw,kw.
(Phenylcarboxyamide or Phenylcarboxamide or Benzenecarboxamide or Amid kyseliny benzoove).tw,kw.
exp Domperidone/
(domperidon* or domidon or Domperi or Domstal or evoxin or gastrocure or motilium or motilium).tw,kw.
(motis or nauzelin or Motinorm Costi or Nomit or Brulium or Molax).tw,kw.
exp Antiemetics/
exp Metoclopramide/
(Metoclopramide or cerucal or clopra or gastrese or gastrobid or gastroflux or gastromax or maxolon).tw,kw.
(metaclopramide or metozolv or metramid or migravess or mygdalon or octamide or parmid).tw,kw.
(primperan or reglan or reliveran or rimetin or Degan or Maxeran or Pylomid or Pramin).tw,kw.
exp Cisapride/
(Cisapride or alimix or Prepulsid or Propulsid).tw,kw.
exp Cholinesterase Inhibitors/
(Itopride or ganaton).tw,kw.
Mosapride.tw,kw.
exp Erythromycin/
(erythromycin or aknemycin or emcin or emgel or emycin or eryderm or erygel or erymax).tw,kw.
(erymin or eryped or gallimycin or ilosene or ilosone or ilotycin or lauromicina or maracyn).tw,kw.
(monomycin or ornacyn or retcin or rommix or romycin or roymicin or staticin or stiemycin or theramycin or tiloryth or wyamycin).tw,kw.
(Motilin adj3 (receptor* or agonist*)).tw,kw.
((5HT3 or 5HT 3 or 5‐HT3 or 5‐HT 3) adj3 antagonist*).tw,kw.
((5HT or 5‐HT or 5‐hydroxytryptamine*) adj3 (agonist* or antagonist*)).tw,kw.
((5‐HT1A or 5HT1A or 5‐HT 1A or 5HT 1A) adj3 agonist*).tw,kw.
exp Serotonin Antagonists/
exp Serotonin 5‐HT3 Receptor Antagonists/
exp Serotonin 5‐HT4 Receptor Agonists/
exp Serotonin 5‐HT1 Receptor Agonists/
(serotonin adj3 receptor adj3 (agonist* or antagonist* or block*)).tw,kw.
(tegaserod or Zelnorm or Zelmac).tw,kw.
ABT‐229.tw,kw.
(Tandospirone or Sediel or metanopirone or buspirone).tw,kw.
(alosetron or Lotronex).tw,kw.
(Acotiamide or YM‐443 or Z‐338D).tw,kw.
(acetylcholinesterase inhibitor* or cholinesterase Inhibitor* or anti‐cholinesterase* or anticholinesterase*).tw,kw.
((5HT‐4 or 5HT4 or 5‐HT 4 or 5‐HT4) adj3 agonist*).tw,kw.
exp Alginates/
(Alginates or alginic acid).tw,kw.
exp Antacids/
(antacid* or alkalinizing agent* or antigastralgic agent*).tw,kw.
(aluminum or aldrox or algeldrate or alhydrogel or alugel or amphojel or basalgel or brasivil or dialume or nephrox or pepsamer or rocgel).tw,kw.
(calcium carbonate or aragonite or calcite or calcium milk or Chalk or limestone or marble or vaterite).tw,kw.
(magnesium or brucite or magnesia).tw,kw.
(alexitol sodium or algicon or Almagate or almagel or alubifar or alugastrin or andursil or attapulgite or bicarbonate or carbex or dihydroxyaluminum sodium carbonate or gaviscon or hydrotalcite or magaldrate or Mylanta or novaluzid or rennie or solugastril or titralac or vangatalcite).tw,kw.
((gastro* or gastric or stomach) and mucosa* and protect* and (agent* or drug* or medicine* or medication*)).tw,kw.
(sucralfate or sulfate or antepsin or carafate or ulcerban or ulcogant or ulsanic).tw,kw.
(adopilon or alsucral or sulphate or alusac or andapsin or bisma or dolisec or exinol or hexagastron or inpepsa or iselpin or keal or melicide or musin or neciblok or peptonorm or succosa or sucrabest or sucralbene or sucralfin or sucramal or sulcran or sulcrate or treceptan or ufarene or ulcar or ulcekon or ulcerimin or ulcerlmin or ulcertec orulcogant or ulcyte or ulsaheal or ulsanic or ulsicral or ulsidex forte or unival or urbal or venter).tw,kw.
exp bismuth/
bismuth*.tw,kw.
exp Baclofen/
(Baclofen or Antispasmodic*).tw,kw.
or/14‐85
13 and 86
exp Adolescent/
exp Child/
exp Infant/
exp Minors/
exp Pediatrics/
exp Puberty/
exp Schools/
(baby or babies or child or children or neonatal or pediatric* or paediatric* or peadiatric* or infant* or infancy or neonat* or newborn* or new born* or kid or kids or adolescen* or preschool or pre‐school or toddler*).tw,kw.
(postmatur* or prematur* or preterm* or perinat* or boy* or girl* or teen* or minors* or prepubescen* or prepuberty* or pubescen* or puber*).tw,kw.
(elementary school* or high school* or highschool* or kindergar* or nursery school* or primary school* or secondary school* or youth* or young or student* or juvenil* or underage* or (under* adj age*) or under 16).tw,kw.
or/88‐97
87 and 98
Appendix 2. MEDLINE search strategy (via Ovid)
exp Gastroesophageal Reflux/
((gastroesophag* or gastro‐esophag* or gastro‐oesophag* or gastric or esophag* or oesophag*) adj3 reflux).tw,kw.
(GERD or GORD or NERD or NORD or GER or GOR).tw,kw.
(acid adj2 reflux).tw,kw.
exp Duodenogastric Reflux/
((duodenogastric or duodeno‐gastric or duodenal) adj3 reflux).tw,kw.
exp Bile Reflux/
(bile adj2 reflux).tw,kw.
((laryngopharyngeal or supraesophag*) adj3 reflux).tw,kw.
gastric regurgitation.tw,kw.
exp ESOPHAGITIS/
(esophagitis or oesophagitis or non‐erorisve reflux disease or nonerosive reflux disease).tw,kw.
or/1‐12
exp Proton Pump Inhibitors/
proton pump inhibitor*.mp.
(PPI or PPIs).tw,kw.
(omeprazole or h 16868 or losec or prilosec or rapinex or zegerid).mp.
(lansoprazole or lanzoprazole or ag 1749 or agopton or bamalite or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).mp.
(pantoprazole or by 1023 or protium or protonix or skf‐96022).mp.
(esomeprazole or nexium).mp.
(rabeprazole or aciphex or dexrabeprazole or e 3810 or ly‐307640 or pariet).mp.
(dexlansoprazole or Kapidex or Dexilant or AGN 20194* or AGN20194* or dexrabeprazole).mp.
(tenatoprazole or CAS 113712‐98‐4 or STU‐Na or TAK‐390* or TAK390* or TAK‐438 or TAK438 or AZD0865 or "AZD 0865").mp.
exp Histamine H2 Antagonists/
((histamine or H2 or H‐2 or H2R or H 2 R) adj3 (antagonist* or blocker* or blockage* or blockader*)).tw,kw.
(H2RA or H2RAs or H2‐RA or H2RAs).tw,kw.
(antihistaminic* adj2 (H2 or H‐2)).tw,kw.
(Cimetidine or Tagamet or altramet or biomet or biomet400 or eureceptor or histodil or skf 92334 or skf92334).tw,kw.
(ranitidine or zantac or ah 19065 or ah19065 or biotidin or ranisen or ranitidine or sostril or zantic).tw,kw.
(Famotidine or Pepcid or mk 208 or mk208 or ym 11170 or ym11170).tw,kw.
(Nizatidine or Axid or axid or ly 139037 or ly139037).tw,kw.
(Roxatidine or Rotane or Zorpe).tw,kw.
(prokinetic* or gastroprokinetic* or gastrokinetic* or gastro‐kinetic*).tw,kw.
(antiemetic* or anti‐emetic).tw,kw.
exp Benzamides/
(Benzoic Acid Amide or Amides or Phenyl Carboxyamide or Benzamide* or Benzoylamide or benzoates).tw,kw.
(Phenylcarboxyamide or Phenylcarboxamide or Benzenecarboxamide or Amid kyseliny benzoove).tw,kw.
exp Domperidone/
(domperidon* or domidon or Domperi or Domstal or evoxin or gastrocure or motilium or motilium).tw,kw.
(motis or nauzelin or Motinorm Costi or Nomit or Brulium or Molax).tw,kw.
exp Antiemetics/
exp Metoclopramide/
(Metoclopramide or cerucal or clopra or gastrese or gastrobid or gastroflux or gastromax or maxolon).tw,kw.
(metaclopramide or metozolv or metramid or migravess or mygdalon or octamide or parmid).tw,kw.
(primperan or reglan or reliveran or rimetin or Degan or Maxeran or Pylomid or Pramin).tw,kw.
exp Cisapride/
(Cisapride or alimix or Prepulsid or Propulsid).tw,kw.
exp Cholinesterase Inhibitors/
(Itopride or ganaton).tw,kw.
Mosapride.tw,kw.
exp Erythromycin/
(erythromycin or aknemycin or emcin or emgel or emycin or eryderm or erygel or erymax).tw,kw.
(erymin or eryped or gallimycin or ilosene or ilosone or ilotycin or lauromicina or maracyn).tw,kw.
(monomycin or ornacyn or retcin or rommix or romycin or roymicin or staticin or stiemycin or theramycin or tiloryth or wyamycin).tw,kw.
(Motilin adj3 (receptor* or agonist*)).tw,kw.
((5HT3 or 5HT 3 or 5‐HT3 or 5‐HT 3) adj3 antagonist*).tw,kw.
((5HT or 5‐HT or 5‐hydroxytryptamine*) adj3 (agonist* or antagonist*)).tw,kw.
((5‐HT1A or 5HT1A or 5‐HT 1A or 5HT 1A) adj3 agonist*).tw,kw.
exp Serotonin Antagonists/
exp Serotonin 5‐HT3 Receptor Antagonists/
exp Serotonin 5‐HT4 Receptor Agonists/
exp Serotonin 5‐HT1 Receptor Agonists/
(serotonin adj3 receptor adj3 (agonist* or antagonist* or block*)).tw,kw.
(tegaserod or Zelnorm or Zelmac).tw,kw.
ABT‐229.tw,kw.
(Tandospirone or Sediel or metanopirone or buspirone).tw,kw.
(alosetron or Lotronex).tw,kw.
(Acotiamide or YM‐443 or Z‐338D).tw,kw.
(acetylcholinesterase inhibitor* or cholinesterase Inhibitor* or anti‐cholinesterase* or anticholinesterase*).tw,kw.
((5HT‐4 or 5HT4 or 5‐HT 4 or 5‐HT4) adj3 agonist*).tw,kw.
exp Alginates/
(Alginates or alginic acid).tw,kw.
exp Antacids/
(antacid* or alkalinizing agent* or antigastralgic agent*).tw,kw.
(aluminum or aldrox or algeldrate or alhydrogel or alugel or amphojel or basalgel or brasivil or dialume or nephrox or pepsamer or rocgel).tw,kw.
(calcium carbonate or aragonite or calcite or calcium milk or Chalk or limestone or marble or vaterite).tw,kw.
(magnesium or brucite or magnesia).tw,kw.
(alexitol sodium or algicon or Almagate or almagel or alubifar or alugastrin or andursil or attapulgite or bicarbonate or carbex or dihydroxyaluminum sodium carbonate or gaviscon or hydrotalcite or magaldrate or Mylanta or novaluzid or rennie or solugastril or titralac or vangatalcite).tw,kw.
((gastro* or gastric or stomach) and mucosa* and protect* and (agent* or drug* or medicine* or medication*)).tw,kw.
(sucralfate or sulfate or antepsin or carafate or ulcerban or ulcogant or ulsanic).tw,kw.
(adopilon or alsucral or sulphate or alusac or andapsin or bisma or dolisec or exinol or hexagastron or inpepsa or iselpin or keal or melicide or musin or neciblok or peptonorm or succosa or sucrabest or sucralbene or sucralfin or sucramal or sulcran or sulcrate or treceptan or ufarene or ulcar or ulcekon or ulcerimin or ulcerlmin or ulcertec orulcogant or ulcyte or ulsaheal or ulsanic or ulsicral or ulsidex forte or unival or urbal or venter).tw,kw.
exp bismuth/
bismuth*.tw,kw.
exp Baclofen/
(Baclofen or Antispasmodic*).tw,kw.
or/14‐85
13 and 86
exp Adolescent/
exp Child/
exp Infant/
exp Minors/
exp Pediatrics/
exp Puberty/
exp Schools/
(baby or babies or child or children or neonatal or pediatric* or paediatric* or peadiatric* or infant* or infancy or neonat* or newborn* or new born* or kid or kids or adolescen* or preschool or pre‐school or toddler*).tw,kw.
(postmatur* or prematur* or preterm* or perinat* or boy* or girl* or teen* or minors* or prepubescen* or prepuberty* or pubescen* or puber*).tw,kw.
(elementary school* or high school* or highschool* or kindergar* or nursery school* or primary school* or secondary school* or youth* or young or student* or juvenil* or underage* or (under* adj age*) or under 16).tw,kw.
or/88‐97
87 and 98
randomized controlled trial.pt.
controlled clinical trial.pt.
random*.mp.
placebo.ab.
drug therapy.fs.
trial.ab.
groups.ab.
or/100‐106
exp animals/ not humans.sh.
107 not 108
99 and 109
Note: Lines 100‐109, Cochrane handbood RCT filter: “Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format”. We made the following minor revision: we used “random*” instead of “randomized.ab” or “randomly.ab.” to capture word variations such as “randomised, randomization, random”.
Appendix 3. Embase search strategy (via Ovid)
exp gastroesophageal reflux/
((gastroesophag* or gastro‐esophag* or gastro‐oesophag* or gastric or esophag* or oesophag*) adj3 reflux).tw,kw.
(GERD or GORD or NERD or NORD or GER or GOR).tw,kw.
(acid adj2 reflux).tw,kw.
exp duodenogastric reflux/
((duodenogastric or duodeno‐gastric or duodenal) adj3 reflux).tw,kw.
exp bile reflux/
(bile adj2 reflux).tw,kw.
((laryngopharyngeal or supraesophag*) adj3 reflux).tw,kw.
gastric regurgitation.tw,kw.
exp esophagitis/
(esophagitis or oesophagitis or non‐erorisve reflux disease or nonerosive reflux disease).tw,kw.
or/1‐12
exp proton pump inhibitor/
proton pump inhibitor*.mp.
(PPI or PPIs).tw,kw.
(omeprazole or h 16868 or losec or prilosec or rapinex or zegerid).mp.
(lansoprazole or lanzoprazole or ag 1749 or agopton or bamalite or lanzor or monolitum or ogast or ogastro or opiren or prevacid or prezal or pro ulco or promeco or takepron or ulpax or zoton).mp.
(pantoprazole or by 1023 or protium or protonix or skf‐96022).mp.
(esomeprazole or nexium).mp.
(rabeprazole or aciphex or dexrabeprazole or e 3810 or ly‐307640 or pariet).mp.
(dexlansoprazole or Kapidex or Dexilant or AGN 20194* or AGN20194* or dexrabeprazole).mp.
(tenatoprazole or CAS 113712‐98‐4 or STU‐Na or TAK‐390* or TAK390* or TAK‐438 or TAK438 or AZD0865 or "AZD 0865").mp.
exp histamine H2 receptor antagonist/
((histamine or H2 or H‐2 or H2R or H 2 R) adj3 (antagonist* or blocker* or blockage* or blockader*)).tw,kw.
(H2RA or H2RAs or H2‐RA or H2RAs).tw,kw.
(antihistaminic* adj2 (H2 or H‐2)).tw,kw.
(Cimetidine or Tagamet or altramet or biomet or biomet400 or eureceptor or histodil or skf 92334 or skf92334).tw,kw.
(ranitidine or zantac or ah 19065 or ah19065 or biotidin or ranisen or ranitidine or sostril or zantic).tw,kw.
(Famotidine or Pepcid or mk 208 or mk208 or ym 11170 or ym11170).tw,kw.
(Nizatidine or Axid or axid or ly 139037 or ly139037).tw,kw.
(Roxatidine or Rotane or Zorpe).tw,kw.
(prokinetic* or gastroprokinetic* or gastrokinetic* or gastro‐kinetic*).tw,kw.
(antiemetic* or anti‐emetic).tw,kw.
exp benzamide derivative/
(Benzoic Acid Amide or Amides or Phenyl Carboxyamide or Benzamide* or Benzoylamide or benzoates).tw,kw.
(Phenylcarboxyamide or Phenylcarboxamide or Benzenecarboxamide or Amid kyseliny benzoove).tw,kw.
exp domperidone/
(domperidon* or domidon or Domperi or Domstal or evoxin or gastrocure or motilium or motilium).tw,kw.
(motis or nauzelin or Motinorm Costi or Nomit or Brulium or Molax).tw,kw.
exp antiemetic agent/
exp metoclopramide/
(Metoclopramide or cerucal or clopra or gastrese or gastrobid or gastroflux or gastromax or maxolon).tw,kw.
(metaclopramide or metozolv or metramid or migravess or mygdalon or octamide or parmid).tw,kw.
(primperan or reglan or reliveran or rimetin or Degan or Maxeran or Pylomid or Pramin).tw,kw.
exp cisapride/
(Cisapride or alimix or Prepulsid or Propulsid).tw,kw.
exp cholinesterase inhibitor/
(Itopride or ganaton).tw,kw.
Mosapride.tw,kw.
exp erythromycin/
(erythromycin or aknemycin or emcin or emgel or emycin or eryderm or erygel or erymax).tw,kw.
(erymin or eryped or gallimycin or ilosene or ilosone or ilotycin or lauromicina or maracyn).tw,kw.
(monomycin or ornacyn or retcin or rommix or romycin or roymicin or staticin or stiemycin or theramycin or tiloryth or wyamycin).tw,kw.
(Motilin adj3 (receptor* or agonist*)).tw,kw.
exp motilin receptor agonist/
((5HT3 or 5HT 3 or 5‐HT3 or 5‐HT 3) adj3 antagonist*).tw,kw.
((5HT or 5‐HT or 5‐hydroxytryptamine*) adj3 (agonist* or antagonist*)).tw,kw.
((5‐HT1A or 5HT1A or 5‐HT 1A or 5HT 1A) adj3 agonist*).tw,kw.
exp serotonin antagonist/
exp serotonin 3 antagonist/
exp serotonin 4 agonist/
exp serotonin 1 agonist/
exp Serotonin 5‐HT3 Receptor Antagonists/
exp Serotonin 5‐HT4 Receptor Agonists/
exp Serotonin 5‐HT1 Receptor Agonists/
(serotonin adj3 receptor adj3 (agonist* or antagonist* or block*)).tw,kw.
(tegaserod or Zelnorm or Zelmac).tw,kw.
exp tegaserod/
ABT‐229.tw,kw.
exp tandospirone/
(Tandospirone or Sediel or metanopirone or buspirone).tw,kw.
exp alosetron/
(alosetron or Lotronex).tw,kw.
exp acotiamide/
(Acotiamide or YM‐443 or Z‐338D).tw,kw.
(acetylcholinesterase inhibitor* or cholinesterase Inhibitor* or anti‐cholinesterase* or anticholinesterase*).tw,kw.
((5HT‐4 or 5HT4 or 5‐HT 4 or 5‐HT4) adj3 agonist*).tw,kw.
alginic acid/
(Alginates or alginic acid).tw,kw.
exp antacid agent/
(antacid* or alkalinizing agent* or antigastralgic agent*).tw,kw.
(aluminum or aldrox or algeldrate or alhydrogel or alugel or amphojel or basalgel or brasivil or dialume or nephrox or pepsamer or rocgel).tw,kw.
(calcium carbonate or aragonite or calcite or calcium milk or Chalk or limestone or marble or vaterite).tw,kw.
(magnesium or brucite or magnesia).tw,kw.
(alexitol sodium or algicon or Almagate or almagel or alubifar or alugastrin or andursil or attapulgite or bicarbonate or carbex or dihydroxyaluminum sodium carbonate or gaviscon or hydrotalcite or magaldrate or Mylanta or novaluzid or rennie or solugastril or titralac or vangatalcite).tw,kw.
exp gastrointestinal mucosa protective agent/
((gastro* or gastric or stomach) and mucosa* and protect* and (agent* or drug* or medicine* or medication*)).tw,kw.
(sucralfate or sulfate or antepsin or carafate or ulcerban or ulcogant or ulsanic).tw,kw.
(adopilon or alsucral or sulphate or alusac or andapsin or bisma or dolisec or exinol or hexagastron or inpepsa or iselpin or keal or melicide or musin or neciblok or peptonorm or succosa or sucrabest or sucralbene or sucralfin or sucramal or sulcran or sulcrate or treceptan or ufarene or ulcar or ulcekon or ulcerimin or ulcerlmin or ulcertec orulcogant or ulcyte or ulsaheal or ulsanic or ulsicral or ulsidex forte or unival or urbal or venter).tw,kw.
exp bismuth/
bismuth*.tw,kw.
exp baclofen/
(Baclofen or Antispasmodic*).tw,kw.
or/14‐94
13 and 95
exp adolescence/
exp adolescent/
exp child/
exp high school/
exp kindergarten/
exp middle school/
exp newborn/
exp nursery school/
exp pediatrics/
exp primary school/
exp puberty/
exp school/
exp newborn/ or exp pediatrics/
(baby or babies or child or children or neonatal or pediatric* or paediatric* or peadiatric* or infant* or infancy or neonat* or newborn* or new born* or kid or kids or adolescen* or preschool or pre‐school or toddler*).tw,kw.
(postmatur* or prematur* or preterm* or perinat* or boy* or girl* or teen* or minors* or prepubescen* or prepuberty* or pubescen* or puber*).tw,kw.
(elementary school* or high school* or highschool* or kindergar* or nursery school* or primary school* or secondary school* or youth* or young or student* or juvenil* or underage* or (under* adj age*) or under 16).tw,kw.
or/97‐112
96 and 113
random:.tw.
placebo:.mp.
double‐blind:.tw.
or/115‐117
exp animal/ not human/
118 not 119
114 and 120
Note: Lines #115‐117, Hedge Best balance of sensitivity and specificity filter for identifying "therapy studies"in Embase. https://hiru.mcmaster.ca/hiru/HIRU_Hedges_EMBASE_Strategies.aspx
Appendix 4. Web of Science search strategy
| # 16 | #15 AND #14 Databases=SCI‐EXPANDED Timespan=All Years |
| # 15 | Topic=(single blind*) OR Topic=(double blind*) OR Topic=(clinical trial*) OR Topic=(placebo*) OR Topic=(random*) OR Topic=(controlled clinical trial) OR Topic=(research design) OR Topic=(comparative stud*) OR Topic=(controlled trial) OR Topic=(follow up stud*) OR Topic=(prospective stud*) Databases=SCI‐EXPANDED Timespan=All Years |
| # 14 | #13 NOT #11 Databases=SCI‐EXPANDED Timespan=All Years |
| # 13 | #12 AND #1 Databases=SCI‐EXPANDED Timespan=All Years |
| # 12 | #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 Databases=SCI‐EXPANDED Timespan=All Years |
| # 11 | Topic=(Adult* or Elderly or Middle Aged or Aged) NOT Topic=(infant* or Newborn* or Pediatric* or child* or baby or babies or babe or Adolescent) Databases=SCI‐EXPANDED Timespan=All Years |
| # 10 | Topic=(Rabeprazole or Esomeprazole or metoclopramide or domperidon* or bethanechol) OR Topic=(Sucralfate) Databases=SCI‐EXPANDED Timespan=All Years |
| # 9 | Topic=(lansoprazol* or Pantoprazole or omeprazole) Databases=SCI‐EXPANDED Timespan=All Years |
| # 8 | Topic=(Proton Pump Inhibitor* OR PPI) Databases=SCI‐EXPANDED Timespan=All Years |
| # 7 | Topic=(Ranitidin*) OR Topic=(Cimetidine) OR Topic=(Famotidine) Databases=SCI‐EXPANDED Timespan=All Years |
| # 6 | Topic=(H2 antagonist*) Databases=SCI‐EXPANDED Timespan=All Years |
| # 5 | Topic=(Maalox*) Databases=SCI‐EXPANDED Timespan=All Years |
| # 4 | Topic=(antacid*) Databases=SCI‐EXPANDED Timespan=All Years |
| # 3 | Topic=(Gaviscon) Databases=SCI‐EXPANDED Timespan=All Years |
| # 2 | Topic=(Alginate*) Databases=SCI‐EXPANDED Timespan=All Years |
| # 1 | Topic=(Gastroesophageal Reflux) OR Topic=(GER or GOR) OR Topic=(GERD or GORD) Databases=SCI‐EXPANDED Timespan=All Years |
Data and analyses
Comparison 1. Omeprazole compared to placebo for infants with GORD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Improvement in cry/fuss time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.2 Improvement in reflux index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Omeprazole compared to ranitidine for infants with GORD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Improvement in symptom scores (WGSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Esomeprazole compared to placebo for infants with GORD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Improvement in number of GORD‐related symptoms and signs | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Rabeprazole at higher doses (1 mg/kg) compared to rabeprazole at lower doses (0.5 mg/kg) for GORD in children older than 1 year.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Improvement in symptom score ('Total GERD Symptoms and Severity' score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.1 Children > 15 kg | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1.2 Children < 15 kg | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2 Improvement in endoscopic scores (Hetzel Dent scores) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.1 Children > 15 kg | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.2.2 Children < 15 kg | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Pantoprazole in higher doses (1.2 mg/kg) compared to pantoprazole at lower doses (0.3 mg/kg) for GORD in children older than 1 year.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Improvement in symptom scores (WGSS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Azizollahi 2016.
| Study characteristics | ||
| Methods | Double‐blind, single‐centre, parallel design outpatient RCT | |
| Participants | 76 infants aged between 2 and 12 months with symptoms after standard treatment (smaller, more frequent feeds, hypoallergenic thickened formula) for 2 weeks | |
| Interventions | Omeprazole (0.5 mg/kg/day) versus ranitidine (2 to 4 mg/kg/day) | |
| Outcomes | Symptom scores (1 of 5 GORD symptoms (vomiting/regurgitation, irritability/fussing, choking/gagging, arching back, refusal to feed) assessed weekly for 2 weeks | |
| Notes | No funding declaration given Location: Iran; single centre No potential conflict of interest was reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked random number generation |
| Allocation concealment (selection bias) | Unclear risk | Patients randomly assigned using randomisation software as above with similar characteristics. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No comment on technique, but ranitidine delivered by syrup and omeprazole by capsule |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The report mentions double‐blinding but no evidence to judge blinding of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 participants excluded: lost to follow‐up, pneumonia, prematurely discontinued medications, and mother's inability to complete questionnaire. Remainder (60) completed study. |
| Selective reporting (reporting bias) | Unclear risk | No evidence to judge risk of selective reporting |
| Other bias | Unclear risk | Funding source not declared. |
Baker 2010.
| Study characteristics | ||
| Methods | Randomised, double‐blind, parallel design outpatient study | |
| Participants | 60 children (aged 1 to 5 years) with symptoms of GORD and endoscopic or histological signs of GORD at recruitment | |
| Interventions | 3 groups: (1) pantoprazole 0.3 mg/kg daily; (2) pantoprazole 0.6 mg/kg daily; (3) pantoprazole 1.2 mg/kg daily delayed‐release | |
| Outcomes | Outcomes were assessed in terms of symptoms, endoscopy (in those with erosive changes and side effects). Symptoms were recorded as a validated GOR symptom score (weekly GOR frequency scores: Weekly Gastro‐oesophageal Severity Score (WGSS) at baseline and week 8, and individual symptoms (abdominal pain, burping, heartburn, pain after eating, difficulty swallowing) were recorded by parents daily using an eDiary, and endoscopy was performed at week 8 again only in those with erosive changes. | |
| Notes | Followed a Paediatric Written Request (PWR) template, after widespread call from FDA for manufacturers of PPIs for children to carry out RCTs in children. Exclusions: recent acute life‐threatening event, eosinophilic oesophagitis, cystic fibrosis, cow's milk protein allergy, Helicobacter pylori infection. Location: multicentre, with sites across the USA No potential conflict of interest was reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment made |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No comment made re: blinding. Parents recorded symptoms daily on an eDiary |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not discussed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data included on symptom score and those participants with erosive oesophagitis who were re‐scoped. All participants accounted for, including analysis of those not enroled. Of the 41 participants not included, 17 had normal biopsy. Eosinophilic oesophagitis was noted in 8 participants and withdrawal of consent in 5, H pylori positive in 4 and use of prohibited treatments in 3 children. Of those who withdrew: 1 in low‐dose group, 4 in medium‐dose, 3 in high‐dose group. |
| Selective reporting (reporting bias) | Unclear risk | No comment made |
| Other bias | High risk | Writing support (Wyeth). Institutional support from drug companies |
Baldassarre 2020.
| Study characteristics | ||
| Methods | Multicentre, randomised, cross‐over outpatient study | |
| Participants | 53 formula‐fed infants and 19 breast‐fed infants (aged 3 weeks to 4 months) with persisting regurgitation (I‐GERQ‐R > 16). 72 infants completed the study. | |
| Interventions | Infants with symptoms of reflux (at least 2 episodes of reflux a day and I‐GERQ‐R scores > 16 at enrolment) had 1 week of behaviour and nutrition advice (e.g. avoid overfeeding, and passive smoking), and feeding support (e.g. on positioning). If symptoms of reflux persisted, formula‐fed infants randomly assigned to receive 2 weeks of a magnesium‐alginate‐based formulation followed by 2 weeks of thickened formula (chosen by parents), or vice‐versa, with 1 week washout between groups. Exclusively breastfed infants were followed up for 2 weeks while receiving magnesium alginate as a cohort and are considered separately as not randomised. | |
| Outcomes | GOR symptoms were evaluated through the Infant Gastroesophageal Reflux Questionnaire Revised (I‐GERQ‐R). Direct cost of treatments was also calculated. | |
| Notes | No funding declaration given Location: Italy Pharmaceutical support (Aurora Biofarma) in providing the study medicine; employed 1 author. The company had no input in the: design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated two‐treatment allocation sequence nQuery Advisor (v.7.0 software, Statistical Solutions Ltd., Cork, Ireland). Randomisation scheme was performed in blocks of 4 participants |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not undertaken, but participants not aware that I‐GERQ‐R to be performed at follow‐up visits |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors aware of outcome but participants unaware of primary outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up after randomisation, but 16 participants had improved with lifestyle advice and feeding support prior to randomisation. |
| Selective reporting (reporting bias) | Low risk | No children lost to follow‐up |
| Other bias | High risk | Very short‐term follow‐up of 2 weeks. Each participant served as their own control. Comparator was thickened formula (choice of the commercial infant thickened formula was left to the parents) |
Ballengee 2018.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled, parallel, single‐centre outpatient trial | |
| Participants | 46 preterm infants under 35 weeks gestation (mean gestational age 27 weeks) with clinical signs of GORD, including only those who had undergone a 24‐hour pH‐multichannel intraluminal impedence monitoring. 33 infants were randomised after meeting the inclusion criteria. | |
| Interventions | Erythromycin 50 mg/kg/day in divided doses or visually identical 5% dextrose water preparation placebo for 1 week duration. After the 7‐day study period, repeat pH‐multichannel intraluminal impedence monitoring was performed. | |
| Outcomes | Outcomes assessed as symptoms and reflux events on 24‐hour pH study. The primary outcome was changes in the total number of reflux events, with secondary outcomes including changes in the number of acidic and non‐acidic events, proximal reflux events, duration of reflux events and nurse‐reported apnoea/bradycardia/desaturation. |
|
| Notes | The erythromycin dose is standard, as the BNFc 2021 dose is 12.5 mg/kg four times a day. Funding declaration: "All phases of this study were supported through grants obtained from The Gerber Foundation and Thrasher Research Fund." Location: USA No potential conflict of interest was reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Permuted block design with stratification by gestational age at birth |
| Allocation concealment (selection bias) | Unclear risk | No evidence to judge selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Visually indistinguishable placebo; staff blinded to randomisation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome data initially reported by pH probe software independent of assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition and all data reported |
| Selective reporting (reporting bias) | Unclear risk | No evidence to judge risk of selective reporting |
| Other bias | Unclear risk | There is little normative premature neonate impedance data on which to judge impact. Funding from the Gerber Foundation and Thrasher Research Fund. |
Bines 1992.
| Study characteristics | ||
| Methods | Double‐blind, placebo‐controlled outpatient trial | |
| Participants | 17 participants aged 5 months to 11.3 years with pH‐probe‐confirmed gastro‐oesophageal reflux, rated moderate to severe based on symptoms | |
| Interventions | Domperidone (0.6 mg/kg) 30 minutes before meal time or placebo over 4 weeks (double‐blind) then a further 4 weeks (open‐label) | |
| Outcomes | Outcomes were assessed by symptomatic change (a detailed symptom analysis was not given); 8 to 12 hours oesophageal pH probe (number of episodes pH < 4; longest episode pH < 4 (in minutes)); and adverse events. Growth (weight and height Z scores)and gastric emptying time were reported but were not pre‐specified outcomes so are not reported here. | |
| Notes | No funding declaration given Location: USA; single site No potential conflict of interest statement was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Part 2 of the trial was open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described by authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some data not included |
| Selective reporting (reporting bias) | High risk | Numerous data from outcomes not presented |
| Other bias | High risk | Participants agreeing to open‐label trial likely to be biased towards those who believed they had an initial benefit from treatment. Pharmaceutical support with funding also noted (Janssen) |
Borrelli 2002.
| Study characteristics | ||
| Methods | Open‐label, parallel‐design, single‐centre outpatient RCT | |
| Participants | 36 participants, median age 5.6 years (12 months to 12 years) with diagnosis of GORD based on symptoms, 24‐hour pH probe and endoscopy | |
| Interventions | Group A: alginate alone (2 mL/kg/day in divided doses) Group B: lansoprazole 1.5 mg/kg twice daily before meals Group C: lansoprazole and alginate over 8 weeks |
|
| Outcomes | Symptoms: reported as symptom score (regurgitation/vomiting, chest pain/irritability, epigastric pain/bloating, nocturnal cough/post‐feeding cough) median (range) at baseline, week 4 and week 8. Adverse events were reported. 24‐hour pH study (at baseline then week 1) reported using reflux index (% of time oesophageal pH < 4 in 24 hours). Endoscopy appearances: (performed at baseline then week 8) scored using Hetzel‐Dent scoring: Grade 0 to 4. Children with grade 3 to 4 oesophagitis on endoscopy were not enroled but given high‐dose lansoprazole. | |
| Notes | 4 participants lost to follow‐up: 1 had upper respiratory tract infection with fever, 2 had poor drug compliance. No list of participants excluded: but infectious diseases, cow's milk protein allergy, neurometabolic conditions, and structural gut abnormalities excluded on investigations as part of workup. No funding declaration given Location: Italy; single site No conflict of interest statement was present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation sequence |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No comment made |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No comment made |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No comment made |
| Selective reporting (reporting bias) | High risk | Children with severe erosive oesophagitis excluded from trial |
| Other bias | Unclear risk | No comment about funding |
Buts 1987.
| Study characteristics | ||
| Methods | Blinded, single‐centre, outpatient RCT | |
| Participants | 20 infants and children with characteristic symptoms of GOR (vomiting, acid regurgitation related to meals and posture, heartburn, recurrent respiratory tract disorders) | |
| Interventions | Either Gaviscon (10 participants, mean age: 21 months) or a placebo (lactose sachet, 10 participants, mean age: 35 months). 24‐hour pH probe at baseline and Day 8; symptom assessment (vomiting and number of episodes of regurgitation within 24 hours) during the time of the recordings were observed by staff. | |
| Outcomes | Symptoms: were recorded as number of episodes of regurgitations per day, and vomiting frequency and volume. No further evaluation of symptoms given 24‐hour pH probe was assessed at baseline and day 8. Symptoms including vomiting and number of episodes of regurgitation within 24 hours during the time of the recordings were observed by staff. |
|
| Notes | No oesophagitis seen on endoscopy of 14 participants (6 treated with Gaviscon, 8 with placebo). As the study was underpowered (only 10 participants in each group), and data presented as mean (standard error), independent data extraction was not undertaken. No funding declaration given Location: Belgium No conflict of interest statement was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment made |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind: but no methodological comment made as to blinding technique and who was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No comment made |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 14 participants endoscoped; none had oesophagitis. Further details on symptom evaluation required |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | High risk | No funding/competing interests declaration made. Very short‐term follow‐up |
Carroccio 1994.
| Study characteristics | ||
| Methods | Double‐blind, parallel‐group, placebo‐controlled, single‐centre outpatient RCT | |
| Participants | 80 participants (45 male, 35 female: aged 1 to 18 months – median 4.5 months) with symptoms of GORD: 50 had vomiting and slowed growth, 20 had weight loss, 4 had recurrent bronchopneumonia, 5 infants had prolonged crying worse after feeding, 1 had apnoeas | |
| Interventions | Group A: domperidone (0.3 mg/kg/dose) ‐ Gaviscon (0.7 mL/kg/dose) Group B: domperidone (0.3 mg/kg/dose) ‐ Maalox (41 g/1.73m2/day) Group C: domperidone (0.3 mg/kg/dose) Group D: placebo |
|
| Outcomes | Outcomes were measured by symptoms, and 24‐hour pH indices (number of episodes pH < 4, duration of episodes of pH < 4, and number of reflux episodes > 5 minutes). Symptom improvement was confirmed on monthly follow‐up for 6 months, but a detailed symptom analysis was not given. | |
| Notes | No child had erosions/ulcers on endoscopy prior to treatment. 80 participants divided into small groups limiting power of study. Participants were stratified by age (< 12 months, > 12 months) and reflux index (< 10% in 24 hours, > 10% in 24 hours). All children had their feeds thickened with Medigel 1%, potentially reducing the impact of alginate, and explaining the significant improvement in pH outcomes in placebo group. All participants who were not cured (40 participants) were treated with cisapride/ranitidine (36 participants responded). No funding declaration given Location: Italy No conflict of interest statement was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratification and successive block randomisation |
| Allocation concealment (selection bias) | Low risk | Strata 1: age < 12 months or > 12 months then dependent on results of baseline pH probe (reflux index < 10% in 24 hours or > 10% in 24 hours) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reportedly double‐blind (participants, parents, observers) but no comment made as to how parents were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No comment made as to blinding method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants also reviewed at 6 months; all those who were cured at 8 weeks remained well. 40 participants with persistent symptoms required cisapride and ranitidine: 36 improved but 4 participants went on to require surgery. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | High risk | All children received frequent short feeds, positioning advice, and formula milk was thickened with Medigel 1%. Funding not declared |
Cresi 2008.
| Study characteristics | ||
| Methods | Single‐centre, parallel‐design RCT over 24 hours in inpatient neonates | |
| Participants | 26 neonates. Mean age (SD): control group 29.5 days (7.4) versus treatment group 24.7 days (13.7) | |
| Interventions | In treatment group: domperidone 0.3 mg/kg two doses in 24 hours. First epoch: P0 = 8h baseline. Time from 1st dose to 2nd dose (8h) = Second epoch P1. Time from second dose to end of study (8h) = third epoch P2 then compared to control group over 24 hours | |
| Outcomes | 24‐hour pH probe and impedance assessing reflux frequency P1 + P2 versus P0 (Mean (SD)); Reflux duration; Reflux height; and Reflux pH. | |
| Notes | No placebo. No blinding evident. Very short follow‐up (24 hours only) No funding declaration given Location: Italy, single centre The authors declared no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consecutive recruitment |
| Allocation concealment (selection bias) | Low risk | Random allocation from odds‐on pair from random‐number table. Pairing occurred after treatment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, for participants/parents, operator/analyser or authors |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant's pH/impedance recording was stopped early: that period was discarded in the analysis. 8% data within pH probes also discarded due to interruptions |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | High risk | Very short‐term follow‐up. No funding issues/conflicts of interest |
Cucchiara 1984.
| Study characteristics | ||
| Methods | Single‐centre, single‐blinded, parallel‐design outpatient RCT | |
| Participants | 46 children (29 boys and 17 girls) aged 2 to 58 months (mean 10.3 months) with symptoms of GORD were assessed. Of these, 33 children (20 boys and 13 girls aged 2 to 42 months of age (mean 9 months)) met the criteria for gastro‐oesophageal reflux with oesophagitis: with symptoms, oesophagitis on endoscopy, and acid reflux on pH probe. | |
| Interventions | Randomised to either cimetidine 20 mg/kg/day or Maalox 700 mmol/1.73m2/day, 7 doses a day | |
| Outcomes | Outcomes assessed included symptoms (composite score in brackets): individual symptoms included vomiting/regurgitation (number of episodes a week), anorexia (absent to severe, 0 to 4 points), pneumonia/apnoea (number of episodes in 3 months) > 1:15 points; anaemia (haemoglobin < 7 g/dL = 9 points), weight:height ratio (centiles) < 5th 6 points. 24‐hour pH study (reflux index: mean (SD) and number of episodes of gastro‐oesophageal reflux), and endoscopy appearances: graded as healed, improved, unchanged/worsened: number (%) at baseline and at 12 weeks | |
| Notes | Exclusions: 13 had an alternative diagnosis including GOR without oesophagitis (5), cows' milk protein intolerance (3), coeliac disease (2), intestinal malrotation (1), and urinary tract infections (2). Of those included, 4 children didn't complete the study: 2 participants in the cimetidine group were excluded (poor drug compliance), and 2 children in the antacid group were excluded (diarrhoea and subsequent reduced antacid intake). No funding declaration given Location: Italy No conflict of interest statement was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation technique or allocation not stated |
| Allocation concealment (selection bias) | Unclear risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Observers of pH probe, endoscopy and manometry blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | High risk | All children had positioning advice, and infants had thickener added (Nestargel 1%). Respiratory complications (e.g. recurrent pneumonia or apnoea) present in 18% of the children studied. |
Cucchiara 1993.
| Study characteristics | ||
| Methods | Single‐centre, parallel‐design, outpatient RCT | |
| Participants | 32 children (aged 6 months to 13.4 years) with GOR based on symptomatology, pH probe and endoscopic findings. All had been unresponsive to an antireflux treatment including combined administration of ranitidine (8 mg/kg/day, given in 2 doses) and cisapride (0 to 8 mg/kg/day, given in 3 doses) for 8 weeks. (Unresponsiveness defined as persistent symptoms and absence of resolution on endoscopy.) | |
| Interventions | 8 weeks of either standard doses of omeprazole (40 mg/day/1.73 m2 surface area) or high doses of ranitidine (20 mg/kg/day) | |
| Outcomes | Improvement was assessed using symptoms, adverse events, 24‐hour pH probe data (reflux index: % time oesophageal pH < 4 in 24 hours), and endoscopy. Reflux symptoms were recorded at baseline by parents through a diary card, then weekly through the study. The scoring system was out of 45: vomiting or regurgitation or both (0 to 9 points: 9 if vomiting for more than 5 days in the week); recurrent pneumonia or asthma or both (number of episodes in 6 months: 6 points per episode: maximum of 18 points); anorexia or early satiety (% reduction compared to daily calorie requirement: maximum of 9 points if intake is less than 25% of that expected); pyrosis/chest pain/irritability (number of days/week: maximum of 9 points if affected 7 days a week). pH probe assessment was undertaken at baseline and 8 weeks. Repeat endoscopies were performed within 48 hours of completing the 8‐week trial. In terms of histological improvements, healing of oesophagitis was assessed (defined as return to grade 0 or grade 2 of histological score). | |
| Notes | Exclusions were: oesophageal strictures, neurological pathology, and systemic extraintestinal diseases No funding declaration given Location: Italy; single centre No conflict of interest statement was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment made |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No comment made, but the dosing regimes were different between the omeprazole and high‐dose ranitidine |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough evidence to draw conclusions. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7 withdrew: 3 on ranitidine and 4 on omeprazole. 4 of these participants were excluded as a result of noncompliance with the protocol, 2 were lost to follow‐up, and 1 was withdrawn because of prolonged fever and upper respiratory infections. |
| Selective reporting (reporting bias) | Unclear risk | Not enough evidence to comment. |
| Other bias | High risk | No funding disclosures were made; one author worked for Schering‐Plough |
Davidson 2013.
| Study characteristics | ||
| Methods | Multicentre, double‐blinded, parallel‐design, RCT in neonates in neonatal intensive care units | |
| Participants | 52 neonates (premature to 1 month corrected age), with signs and symptoms of GORD | |
| Interventions | 0.5 mg/kg esomeprazole once daily for up to 14 days versus placebo | |
| Outcomes | Change from baseline in the total number of GORD symptoms (from video monitoring) and GORD‐related signs (from cardiorespiratory monitoring) assessed with simultaneous oesophageal pH/impedance at baseline and 14 days: with cardiorespiratory, and 8‐hour video monitoring. Adverse events were recorded. Data readers blinded | |
| Notes | Location: Australia, Germany, UK The study was sponsored by AstraZeneca; it was involved in manuscript‐writing, and employed one of the authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation used |
| Allocation concealment (selection bias) | Low risk | Evidence for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Data readers blinded but unclear if nursing staff were blinded. Identical placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data readers recording outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant in the esomeprazole group was excluded from the modified ITT analysis because of invalid efficacy measurements, but was included in the safety analysis. 1 participant in the placebo group completed the study, but was lost to follow‐up between study completion and the safety follow‐up visit. |
| Selective reporting (reporting bias) | Low risk | No evidence of reporting bias |
| Other bias | High risk | Very short‐term study: the study was discontinued prematurely because of poor enrolment: the study estimated needing to recruit 90 participants to achieve 38 participants in each group to achieve > 80% power at the 2‐sided alpha level of 0.05 to detect a difference between esomeprazole and placebo in the change in number of symptoms from baseline. The study was funded by AstraZeneca with support with manuscript writing. |
Del Buono 2005.
| Study characteristics | ||
| Methods | Double‐blind, single‐centre outpatient RCT | |
| Participants | 20 infants (mean age 163.5 days, range 34 to 319 days) exclusively bottle‐fed (formula milk or expressed breast milk), with symptoms of GOR (regurgitation > 3 x day any amount or > once/day half the feed); weighing > 2 kg in weight and no signs of infection | |
| Interventions | 6 random administrations (3+3) of Gaviscon Infant (625 mg in 225 mL milk) or placebo (mannitol and Solvito N, 625 mg in 225 mL milk) were given (double‐blind) | |
| Outcomes | 24‐hour studies of impedance and dual‐channel pH monitoring. pH indices: median number of reflux events/hour, acid reflux events/hour, minimum distal or proximal pH, total acid clearance time per hour (time with pH below 4), and total reflux duration per hour were assessed. No comment on symptoms or adverse events | |
| Notes | Very short‐term study. Funding was declared from Reckitt Benckiser Location: UK Conflict of interest declared: Reckitt Benckiser Healthcare (UK) Ltd, the producers of Gaviscon Infant, funded one of the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment made |
| Allocation concealment (selection bias) | Unclear risk | Identical preparations given to infants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants/parents reportedly blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded observer interpreted pH data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No evidence of this |
| Selective reporting (reporting bias) | Unclear risk | No evidence of this |
| Other bias | High risk | Very short‐term study. Reckitt Benckiser Healthcare (UK) Ltd, the producers of Gaviscon Infant, funded one of the authors (Dr R Del Buono). However, there was no discernable impact on study design. |
Famouri 2017.
| Study characteristics | ||
| Methods | Unblinded, parallel‐design, randomised clinical outpatient trial | |
| Participants | 50 infants (0 to 12 months old). Mean age of ranitidine group 2.8 months; mean age of hypoallergenic diet group 3.4 months with I‐GERQ‐R score of > 7 | |
| Interventions | Ranitidine 6 mg/kg daily in 2 divided doses versus hypoallergenic diet (in breastfed infants, mothers were advised to eat only hypoallergenic diet and in formula‐fed infants, hydrolysed protein or amino‐acid based formula) | |
| Outcomes | Symptoms: parental reporting of symptoms of irritability, vomiting, respiratory symptoms, arching and refusal of feeds. I‐GERQ at baseline and 2 weeks post intervention. No comment on adverse events | |
| Notes | The authors do not include detailed information regarding attrition in this study. No funding declaration given Location: Iran; single centre. No conflict of interest statement present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors state only that "participants were randomly allocated" |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Parents were not blinded, and no comment made regarding the blinding of personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence of this |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | This is not made clear by the authors |
| Selective reporting (reporting bias) | Unclear risk | No evidence of this |
| Other bias | Unclear risk | There is no disclosure of interest statement available for this study |
Forbes 1986.
| Study characteristics | ||
| Methods | Single‐centre, observer‐blinded, parallel‐design outpatient RCT | |
| Participants | 10 children (mean age 68 months: range 6 to 168 months). All had symptoms of vomiting and water brash at enrolment | |
| Interventions | Gaviscon Infant liquid (antacid plus alginate) 10 mL every 6 hours (for infants) or 20 mL every 6 hours for older children versus placebo 3 times a day. 24‐hour pH probe at baseline then consecutively after 24 hours of treatment, so 2 24‐hour pH recordings were made. | |
| Outcomes | pH indices: number of reflux episodes, total duration of reflux episodes recorded. No adverse events were reported. | |
| Notes | Observer interpreting pH results was blinded. We did not consider the metoclopramide group (also 10 children): please see Methods and Differences between protocol and review. No standard nursing positions were adopted, and children could move around the bed. No funding declaration given Location: Australia No conflict of interest statement present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment made |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and parents not blinded as placebo administered 3 times daily and Gaviscon liquid 4 times daily for infants and children |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interpretation of pH data made by blinded observer |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No subgroup analysis of those with endoscopic evidence of oesophagitis |
| Selective reporting (reporting bias) | Unclear risk | No evidence of this |
| Other bias | High risk | Very short‐term study. No funding declarations |
Gilger 2006.
| Study characteristics | ||
| Methods | Multicentre, randomised, double‐blinded (for dose), uncontrolled, parallel‐group outpatient study | |
| Participants | 109 children aged 1 to 11 years with endoscopically/histologically‐confirmed erosive oesophagitis | |
| Interventions | Doses of 5 mg or 10 mg of esomeprazole (8 kg to 20 kg children), 10 mg or 20 mg esomeprazole (> 20 kg children) for 8 weeks | |
| Outcomes | Symptom improvement (assessed by physician's global assessment (PGA) and parental daily diaries at baseline then fortnightly). Adverse events reported regardless of causality | |
| Notes | Endoscopic outcomes published separately (Tolia 2010b) Funding: AstraZeneca Location: USA, Belgium, France, Italy Conflict of interest: study was funded by AstraZeneca with leadership, project management, and editorial assistance |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents reported outcomes but blinded to dose |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not described by authors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient safety data not supplied and excluded; otherwise all participants fully accounted for |
| Selective reporting (reporting bias) | Unclear risk | No evidence of reporting bias |
| Other bias | High risk | Study funded by AstraZeneca with pharmaceutical writing support noted |
Gunesekaran 2003.
| Study characteristics | ||
| Methods | Phase I, multicentre, parallel‐design, double‐blind, outpatient RCT | |
| Participants | 63 adolescents with symptomatic/endoscopic GORD, or histological changes. Mean age 14.1 years (12 to 17 years) | |
| Interventions | Randomised to 2 arms: 7‐day pre‐treatment then 5 days of treatment with lansoprazole 15 mg versus 30 mg | |
| Outcomes | In the pre‐treatment phase, a physician assessment was followed by 24‐hour intragastric pH probe, endoscopy and biopsy, H pylori testing, and a symptom diary for one week. After 5 days of treatment, participants underwent physician assessment and analysis of symptom diaries. The pharmacokinetics and intragastric pH monitoring are not considered here, as intragastric pH not an outcome relevant in oesophagitis, and pharmacokinetics are not clinical outcomes being considered within the remits of this review. Severity scores were graded 0 (none) to 3 (severe) for each item. Adverse events recorded: pharyngitis 6% (2/32 in lansoprazole 15 mg) and headache 16% (4/31 in lansoprazole 30 mg) were the most commonly reported adverse events amongst adolescents treated with lansoprazole 15 mg and 30 mg, respectively. 5 participants experienced adverse events considered to be possibly treatment‐related. 1 participant with a history of environmental allergies experienced a mild allergic reaction after 3 days of treatment with lansoprazole 15 mg. Amongst those treated with lansoprazole 30 mg, 4 participants each reported 1 occurrence of pain (toothache), diarrhoea, dizziness, and rash. | |
| Notes | Exclusions: systemic disease (e.g. scleroderma)/infection of oesophagus/chronic use of ulcerogenic drugs/use of PPIs. Funding declaration: study supported by a grant from TAP Pharmaceutical Products Inc. Location: USA No conflict of interest statement present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised in 1:1 fashion to each group |
| Allocation concealment (selection bias) | Low risk | Difference between treatments concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants/carers blinded. Pathologist examining histological specimens blinded (but not an outcome measure). No discussion of blinding of clinical observers |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No evidence of this |
| Selective reporting (reporting bias) | Unclear risk | No oesophageal data on pH probe reported |
| Other bias | High risk | Very short‐term follow‐up study. However, participants who demonstrated a positive response were offered 3 months of treatment with lansoprazole. Study supported by a grant from TAP Pharmaceuticals. |
Haddad 2013.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, parallel‐design, outpatient RCT | |
| Participants | 127 children aged 1 to 11 years | |
| Interventions | Randomised to rabeprazole 0.5 mg/kg or 1 mg/kg. Children 6 kg to 14 kg received 5 mg if in 0.5 mg/kg group and 10 mg if in 1 mg/kg group. Children > 15 kg received 10 mg if in 0.5 mg/kg group and 20 mg if in 1 mg/kg group. Medications given for 12 weeks | |
| Outcomes | Symptom score: mean 'Total GERD Symptoms and Severity' score, Global Treatment Satisfaction scale by the investigator and Clinical Global Impressions‐Improvement scale by the parent/caregiver. Endoscopy/histological healing at baseline and week 12: histological scores (Grades 1‐5). Adverse events were reported (as treatment‐emergent adverse events). | |
| Notes | At recruitment, 30% of children had already received PPIs: 15% H₂ antagonists, and 2% prokinetics. Funding declared from Janssen Research & Eisai Medical Research Inc. Location: USA, Belgium, Denmark, France, Italy, Poland, Israel, South Africa, and India No conflict of interest statement present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment on randomisation technique |
| Allocation concealment (selection bias) | Low risk | No difference in baseline characteristics |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Everyone, including the investigator, the contract research organisation, and in‐house study personnel, was blinded in the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Everyone, including the investigator, the contract research organisation, and in‐house study personnel, was blinded in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15% withdrew during study: reasons not given |
| Selective reporting (reporting bias) | Unclear risk | No evidence to judge risk of selective reporting bias |
| Other bias | High risk | Janssen provided funding and reviewed the manuscript prior to submission. 4 authors are employees of Janssen Research & Development. This may have affected study design: i.e. same medication, different dose design. |
Hussain 2014.
| Study characteristics | ||
| Methods | Double‐blind, multicentre, withdrawal outpatient RCT (following 1‐ to 3‐week open‐label phase) | |
| Participants | 268 infants (aged 0 to 11 months) | |
| Interventions | Placebo versus rabeprazole 5 mg and 10 mg groups for 5 weeks. Only those children who had improved went on to the double‐blind withdrawal phase. | |
| Outcomes | Outcomes were measured by symptom scores (I‐GERQ) assessed based on daily diary; endoscopic appearances; and side effects. Weight for height Z‐scores also assessed but not reported as not a prespecified outcome. 231 completed first part of study. 108 improved children went into the double‐blind withdrawal phase. Post hoc analysis was performed after unblinding the data, based on age subgroups (1 to 4 months; 4 to 8 months; 8 to 12 months): previous acid suppressive treatment–exposed versus treatment‐naïve infants; initial I‐GERQ‐R scores over 23 versus under 23 at entry into the open‐label phase; and improvement of the I‐GERQ‐R score by 10 points versus more than 10 points during the open‐label phase. Post hoc analyses were also based on 3 individual questions scored on the I‐GERQ‐R at entry into the open‐label phase. These included frequency of regurgitation more than 3 times a day versus 3 times a day; crying for 1 hour/day versus less than 1 hour a day; and crying during or within 1 hour of feeding always or often versus sometimes, rarely, or never. Endoscopy: only 12 of 268 underwent endoscopy, but the endoscopy was not repeated. |
|
| Notes | The study was financially supported by Janssen Research & Development. Multiple authors were employees of Janssen Research and one a consultant to Janssen Research. Location: USA, the Netherlands, South Africa, Belgium, Hungary, Israel, Bulgaria, Italy, Poland Endoscopy: of the 12 of 268 who underwent endoscopy, 10 had signs of GORD on scope Adverse events were recorded: equal percentages (47%) reported adverse events in placebo and combined rabeprazole groups (diarrhoea, constipation, flatulence, crying and rash). 8 participants in rabeprazole groups had elevated gastrin levels. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation technique not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding technique not specified but identical placebo and rabeprazole preparations used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding technique not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 37 participants didn't complete the study |
| Selective reporting (reporting bias) | High risk | Post hoc analysis based on age‐bands (1 to 4 months, 4 to 8 months, 8 to 12 months), those who had previously had treatment with PPI versus those who were PPI‐naïve, those with high I‐GERQ scores (< 23) versus lower I‐GERQ scores, and analyses of certain I‐GERQ questions (crying, frequency of regurgitation, and crying within 1 hour of feeding). |
| Other bias | High risk | The aim of the lead‐in period was to identify those participants who were PPI‐responsive: these infants were then more likely to show signs on withdrawal. Pharmaceutical help with funding the study and manuscript preparation (Janssen) was noted, which may have affected study design (post hoc analysis). |
Kierkus 2011.
| Study characteristics | ||
| Methods | Unblinded, single‐centre, parallel‐design, outpatient RCT | |
| Participants | Study 1: neonates/preterm infants pantoprazole 2.5 mg (approximately 1.2 mg/kg once daily). This study not analysed as not randomised Study 2: 24 participants. Mean age 6.9 months (range 1.3 to 11 months; 1 extremely premature baby) in low‐dose treatment group. Mean age 3.6 months (1.1 to 12.1 months; 2 extremely premature babies) in high‐dose treatment group. |
|
| Interventions | Study 2: randomised to high‐dose (1.2 mg/kg) or low‐dose pantoprazole (0.6 mg/kg). Mainly pharmacokinetic data but 24‐hour pH probe at baseline then day 5. Treatment for 6 weeks | |
| Outcomes | Baseline and steady‐state (day 5): pH indices: reflux index (mean ± SD); number of episodes pH < 4; number of episodes lasting more than five minutes; duration of episodes of pH < 4. Related and unrelated adverse events were reported: no serious adverse events after 6 weeks of treatment, although 58% of the 24 participants reported at least 1 adverse event (unrelated). |
|
| Notes | Funded by Wyeth, including funding for writing assistance Location: USA, Europe, Australia Conflict of interests disclosed: Wyeth was acquired by Pfizer Inc in October 2009. Multiple authors were employees of Wyeth Research and may have held Wyeth stock. Other investigators or their institutions received compensation from Wyeth Research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of randomised numbers in strict ascending sequential order |
| Allocation concealment (selection bias) | Unclear risk | At end of trial, infants could continue on same dose or higher dose for 6 weeks |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant excluded in low‐dose treatment group: error in pH probe. 2 excluded in high‐dose group: 1 pH probe error; 1 at investigator request |
| Selective reporting (reporting bias) | Unclear risk | No evidence found, although no symptom change reported |
| Other bias | High risk | Very short‐term follow‐up. Funded by Wyeth, including funding for writing assistance |
Loots 2014.
| Study characteristics | ||
| Methods | Multicentre, parallel‐design, double‐blind outpatient RCT | |
| Participants | 51 infants aged 2 to 26 weeks with symptomatic GORD | |
| Interventions | Mainly assessing the role of left lateral positioning (LLP), but esomeprazole (PPI) and antacid therapy included. Infants demonstrating a positive GOR symptom association were randomised to 1 of 4 groups:
Cot elevation and antacid were considered “sham” therapies. |
|
| Outcomes | Nurse‐led symptom observation; using cardiorespiratory and video monitoring, and I‐GERQ‐R. Also 8‐hour pH‐impedance (reflux index) at baseline and after 2 weeks. | |
| Notes | Efficacy of positioning not considered in this review. However, the antacid groups did have different pH indices at baseline, and in this age‐group, a reflux index of less than 10% in 24 hours was not considered pathological. The study was supported by grants from the National Health and Medical Research Council, the Financial Markets Foundation for Children, the Dutch Digestive Disease Foundation, the Channel 7 Children’s Research Foundation, and the Women’s & Children’s Hospital Foundation. Part of the equipment was provided by AstraZeneca. Location: Australia, the Netherlands The authors reported no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation independently generated by monitor |
| Allocation concealment (selection bias) | Unclear risk | No evidence to judge risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Medications were double‐blinded. Body positioning was single‐blind as parents aware |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 infants withdrew: 3 before starting treatment; 1 poorly compliant; 1 withdrawn by parents at day 9 (did not wish to proceed to head of cot (bed) elevation and antacid) and 1 infant admitted due to poor intake (from 'PPI plus head of cot elevated' group). |
| Selective reporting (reporting bias) | Unclear risk | No evidence to judge risk of reporting bias |
| Other bias | Unclear risk | Some equipment provided by AstraZeneca but no influence on study design, or manuscript writing. Additional Sudden Infant Death Syndrome (SIDS) precautions taken, including continuous O2 saturations monitoring. |
Miller 1999.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, placebo‐controlled, outpatient RCT | |
| Participants | 90 infants with symptoms of GOR at least twice a day for 2 days prior to start of study | |
| Interventions | Sodium alginate (aluminium‐free Infant Gaviscon) 312.5 mg/sachet, one to two sachets per feed versus placebo | |
| Outcomes | Improvement in symptoms noted by parents (daily diary) and investigators, at baseline, day 7, and day 14. Symptoms assessed: number of vomiting episodes expressed as median (range) as primary outcome; and assessment of vomiting severity and parental global assessment of improvement at day 14 as secondary outcomes. Infants received up to 4 days additional treatment after day 14. No difference in adverse events reported between the 2 groups, but no further details given. |
|
| Notes | Exclusions: infants with oesophageal, neuro, cardiac, respiratory, metabolic, hepatic, or renal disease; below 2.5 kg in weight; below 37 weeks' gestation The authors received funding from Parexel International Location: United Kingdom across 25 centres No conflict of interest statement was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment made |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reportedly double‐blind but technique not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Technique not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | From 90 participants: 2 infants in placebo group did not receive treatment = ITT population 88. During study: 20 withdrawals (7 from alginate group; 13 from placebo group; P > 0.2) due to adverse events (alginate: 4; placebo: 7) and lack of efficacy (alginate: 2; placebo: 3). ITT analysis included withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | No evidence found, but data for Day 7 investigator assessment were not presented |
| Other bias | Unclear risk | Funded by Reckitt & Colman and Parexel International |
Moore 2003.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, cross‐over outpatient trial | |
| Participants | 30 infants with symptomatic GORD between 3 and 12 months of age (mean 5.4 months) who had previous empirical gastroesophageal reflux treatment, excluding PPI therapy, with either reflux index over 5% in 24 hours OR biopsy evidence of oesophagitis | |
| Interventions | 4‐week study: omeprazole (2 weeks) then placebo (2 weeks) or vice versa. Infants from 5 kg to 10 kg were given 10 mg daily and > 10 kg were given 10 mg twice daily omeprazole or identical placebo. | |
| Outcomes | Symptoms assessed as crying/fuss time: mean (SD) by Symptom Diary and Visual Analogue Score; sliding scale from 0 to 10 assessing irritability reported by parent over 4 weeks. pH indices assessed as change in reflux index; mean (SD) ‐ % of time spent with oesophageal pH < 4 in 24 hours. Authors reported PPI improved reflux index with no effect on crying/fussing compared to placebo. Of note, there was significant reduction in both groups over the 4‐week study period compared to baseline. Adverse events were recorded (none noted). |
|
| Notes | The study was jointly funded by the J.H. and J.D. Gunn Medical Research Foundation and the Channel 7 Children’s Research Foundation. The omeprazole and placebo capsules were supplied free of charge by AstraZeneca. Location: Australia; single‐centre study No potential conflict of interest statement was present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described by authors but randomisation code used. |
| Allocation concealment (selection bias) | Low risk | Not described by authors but code broken at end of study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded: parents/infants and observers, code broken at end of study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes expressed as behaviour diary (potential for recall bias) and visual analogue score (potential for parental observer bias). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No table of baseline characteristics. 4 infants dropped out due to significant crying. |
| Selective reporting (reporting bias) | Unclear risk | No comment made. Only 7 infants had both endoscopic changes and reflux index > 5% in 24 hours. |
| Other bias | High risk | 64 infants evaluated for inclusion. Note NASPGHAN guidance in place at the time considers reflux index > 10% in 24 hours to be pathological in infants. No evidence of reflux oesophagitis seen (erosions or ulcers) at entry endoscopy: loss of vascular pattern or friability enough for inclusion. Some of these infants may have had functional reflux. Independent funding: AstraZeneca only provided the placebo and omeprazole free of charge. |
Naeimi 2019.
| Study characteristics | ||
| Methods | Double‐blind, parallel‐design, single‐centre, outpatient RCT | |
| Participants | 96 children between 1 and 4 years old with GORD, diagnosed on clinical symptoms: needed 2 of 5 of the following symptoms: (1) regurgitation (or vomiting immediately after feeding); (2) poor weight gain for one month; (3) respiratory distress immediately after feeding; (4) feed refusal; and (5) restlessness up to 3 hours after eating over the preceding month. | |
| Interventions | Two groups: ranitidine 8 mg/kg/day versus ranitidine 8 mg/kg/day plus quince syrup (0.5 mL/kg/day). Assessed at 2, 4, and 6 weeks. | |
| Outcomes | Global Severity Questionnaire (GSQ‐YC): assessing severity and frequency of vomiting, refusal to eat, difficulty in swallowing, choking during eating, burping/belching, and abdominal or belly pain. Adverse effects of ranitidine or quince syrup were recorded (none noted). | |
| Notes | Significant differences emerged between 2 and 4 weeks after starting the trial. Small sample size and short study duration were other limitations noted, and the authors recommend a larger study to explore the effect of different doses and improve the reliability of the results. The study was funded by a university grant. Location: Iran No conflict of interest statement was present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation software was used to prepare the randomised list but software not specified. |
| Allocation concealment (selection bias) | Low risk | The participants in this study were selected randomly and divided into 2 parallel groups. Detailed analyses for differences between groups were undertaken regarding symptom characteristics, age, weight, height, number of siblings, maternal occupation and education, co‐existing conditions, atopy, method of birth, and type of feeding (as well as age of weaning). No significant differences identified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants were blinded regarding drug allocation. The research team (a paediatrician and a physician) were also unaware of the participants' allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research team (a paediatrician and a physician) were unaware of the participants' allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants in the ranitidine group (n = 47) and 1 in the quince syrup plus ranitidine group (n = 49) were excluded due to worsened symptoms or poor compliance. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of post hoc analyses or changes in end points |
| Other bias | Low risk | No conflict of interest statement. Funded by a university grant (from Babol University of Medical Sciences, Iran). |
Omari 2006.
| Study characteristics | ||
| Methods | Randomised, double‐blinded, single‐centre, outpatient, placebo‐controlled trial | |
| Participants | 30 children with resistant GORD. Mean age 10 ± 0.8 years. All children had failed standard therapy (positioning, reassurance, feed thickener, antacids, PPI and H₂ antagonist) | |
| Interventions | Assessed with manometry/pH at baseline for 2 hours after 250 mL of cow's milk and dose of baclofen to ensure tolerability (control period). 0.5 mg/kg baclofen or placebo was then administered. One hour later, 250 mL of milk was given and measurements performed for another 2 hours (test period). | |
| Outcomes | Impedance: transient lower oesophageal sphincter relaxations (TLESR) (median ± CI) versus placebo: during the 2‐hour test period compared with the control period. pH: number of acid reflux episodes (pH < 4) detected. Adverse events assessed (1 causing early withdrawal but thought to be unrelated): up to 48 hours following trial. | |
| Notes | Exclusions: previous gastrointestinal surgery, neurological disease, cardiac/respiratory disease, peptic ulcers or cow's milk protein intolerance (CMPI)/lactose intolerance. Significantly higher number of acid reflux episodes and TLESRs at baseline in control group. Very short trial period. Gastric emptying was not evaluated in this review as it was not a prespecified outcome. The study was supported by the Women & Children's Hospital Research Foundation, the J.H. & J.D. Gunn Medical Research Foundation, the Netherlands Organization for Scientific Research and AstraZeneca Location: Australia No conflict of interest statement was available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No evidence provided |
| Allocation concealment (selection bias) | Unclear risk | No evidence provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents and staff remained blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No evidence provided |
| Selective reporting (reporting bias) | Low risk | All participants had initially received a test dose to assess tolerability; no data for children who had not tolerated the initial test dose. |
| Other bias | High risk | Very short‐term follow‐up. Funded by Women and Children's Research Foundation, the JH & JD Gunn Medical Research Foundation and AstraZeneca R&D. |
Omari 2007.
| Study characteristics | ||
| Methods | Single‐centre, randomised, single‐blind outpatient study (SH‐NEC‐0001) | |
| Participants | 50 infants with symptoms of GORD (irritability/crying, vomiting, choking/gagging) and reflux index on 24‐hour pH probe suggestive of acid GOR (> 4% in 24 hours) | |
| Interventions | Oral esomeprazole 0.25 mg/kg or 1 mg/kg for 8 days | |
| Outcomes | Symptoms were recorded on a symptom chart at baseline and at day 7, based on the I‐GERQ. Severity scores were graded 0 (none) to 3 (severe) for each item. A 24‐hour pH probe (assessing reflux index) was performed at baseline and on day 7. Adverse events were also monitored through physician assessment. | |
| Notes | Exclusions included a history of upper gastro‐intestinal surgery, and congenital drug addiction. Use of any pharmacological antireflux therapy up to 24 hours before, or any PPI up to 72 hours before the first dose of study medication was not permitted. Contemporaneous treatment with medications known to interact with esomeprazole, or to improve symptoms of reflux (e.g. H₂ antagonists) was not permitted. Also republished in full in 2015: other exclusion criteria listed there were: any current/previous clinically significant illness that may interfere with study procedures or with the metabolism of esomeprazole, or that may jeopardise infant safety; any experimental drug or device in the 8‐week period before screening; history of surgery of the oesophagus, stomach, duodenum, or jejunum; and congenital drug addiction. Use of any pharmacological antireflux therapy up to 24 hours before, or any PPI up to 72 hours before, the first dose of study medication was not permitted. Treatment with anticholinergics, antineoplastic agents, H₂‐receptor antagonists, sucralfate, bismuth‐containing compounds, methylxanthines, promotility drugs, macrolide antibiotics, or barbiturates was not permitted. Known hypersensitivity to esomeprazole, substituted benzimidazoles, or any constituents of the esomeprazole formulation also precluded infants from the study. No funding declaration given. Medical writing support was funded by AstraZeneca Location: Australia No conflict of interest statement was present. Further details were supplied by author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No evidence provided |
| Allocation concealment (selection bias) | Unclear risk | No evidence provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Staff became aware of which treatment a participant was on based on the weight. Parents remained blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No evidence provided |
| Selective reporting (reporting bias) | Unclear risk | No evidence |
| Other bias | High risk | Very short‐term study. No funding statement but medical writing support by AstraZeneca which may have influenced study design (same medication: different dose comparison) which has less clinical utility. |
Orenstein 2002.
| Study characteristics | ||
| Methods | Eight‐week, multicentre, placebo‐controlled, two‐phase outpatient RCT | |
| Participants | 35 infants, mean age 5.5 months (range 1.3 to 10.5 months), male:female 12:14, previous H₂ antagonist therapy in 57%, previous prokinetic use in 37%. All had a clinical diagnosis of GORD. | |
| Interventions | First 4 weeks: observer blind trial of famotidine; second 4 weeks: double‐blind withdrawal comparison of each dose with placebo. Phase 1 ‐ famotidine 0.5 mg/kg dose versus famotidine 1 mg/kg dose Phase 2 ‐ each dose category split to continue on dose or receive placebo |
|
| Outcomes | Symptoms assessed in terms of improvement in regurgitation frequency, improvement in regurgitation volume, improvement in crying time, and global assessments by parents and physicians. Adverse events were monitored: 6 infants on famotidine experienced new agitation/irritability; 2 of these had accompanying head rubbing. All resolved within days of ending therapy. No breakdown as to which group. | |
| Notes | Exclusion criteria: respiratory complication, previous gastrointestinal surgery, cardiovascular, renal, hepatic, neoplastic or diabetic disease, inability to discontinue previous proton pump inhibitor therapy, sensitivity to famotidine or H₂ antagonists. Study supported by a grant by Merck & Co. Inc. to each of the 3 sites. Location: USA No conflict of interest statement was present |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described by authors |
| Allocation concealment (selection bias) | Unclear risk | Not described by authors. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Parents unblinded to intervention in part one |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Parents unblinded to intervention in part one, with parental assessment a key outcome measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All infants accounted for, all outcomes clearly defined and reported |
| Selective reporting (reporting bias) | Unclear risk | No evidence of this, although infants with previous sensitivity to famotidine were excluded. |
| Other bias | Unclear risk | In selection, infants with previously failed GORD treatment far more likely to be enroled. Study supported by a grant by Merck & Co. Inc. to each of the 3 sites. |
Orenstein 2008.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, placebo‐controlled outpatient RCT | |
| Participants | 162 infants (mean age 16 weeks, range 4 to 51 weeks) with symptoms of GORD – 'crying, fussing or irritability' – within 1 hour after feeding (specifically daily crying noted in diary in > 25% of feeds over 4 days), after 1 week of non‐pharmacological treatment. | |
| Interventions | The trial occurred in 3 phases. In the pretreatment phase: small frequent feeds were recommended, as was reduction in smoking, hypoallergenic feeds (or if breastfed, mothers started dairy‐free diet), and positioning advice. The treatment phase lasted 4 weeks and infants were randomised to lansoprazole 1:1 (0.2 to 0.3 mg/kg/day in those < 10 weeks old, 1 to 1.5 mg/kg/day in those > 10 weeks old) versus placebo. In the post‐treatment phase, investigators could choose to put children on lansoprazole. | |
| Outcomes | Symptom assessment for 30 days following the study was performed. Parent diaries were assessed for symptom scores and individual symptoms (crying/regurgitation/back arching/hoarseness/feed refusal or early stopping/cough or wheeze). | |
| Notes | Takeda Global Research and Development sponsored the clinical trial and data analysis. Location: USA and Poland. 16 centres participated. No potential conflict of interest statement was present. Infants were excluded if PPI taken in previous 30 days or H₂ receptor antagonists within 7 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation 1:1 lansoprazole:placebo |
| Allocation concealment (selection bias) | Unclear risk | No evidence of this |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding reported: randomisation blinded and parents blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators able to find out after 4 weeks who was taking which treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One participant in lansoprazole group: data missing |
| Selective reporting (reporting bias) | Unclear risk | No evidence of this |
| Other bias | Unclear risk | Takeda funded the trial and data analysis but took no part in manuscript preparation |
Pfefferkorn 2006.
| Study characteristics | ||
| Methods | Double‐blind, parallel‐design, single‐centre, outpatient RCT | |
| Participants | 18 participants, ages 1 to 13 years (mean 10.3 years) with symptomatic GORD with endoscopic/histological changes | |
| Interventions | Of the 18 participants who received omeprazole (1.4 mg/kg once daily, maximum 60 mg) for the first 3 weeks, 16 (89%) had nocturnal acid breakthrough on pH monitoring and were randomised to ranitidine 4 mg/kg or placebo, whilst continuing omeprazole. | |
| Outcomes | Participants were evaluated for symptoms and adverse events during follow‐up at 3, 9 and 17 weeks. Symptoms (heartburn, abdominal pain, vomiting, dysphagia, and ‘‘others’’) were recorded (none, same, better, worse) at follow‐up. Details of the symptom scoring were not given. At week 17, all participants had repeat 24‐hour pH monitoring (reflux index) and endoscopy/histology evaluation using Hetzel‐Dent score (grade 0 to 4). Adverse events were monitored: none were seen. | |
| Notes | One participant received esomeprazole 40 mg twice daily; 2 participants in ranitidine group withdrew; 1 participant was lost to follow‐up. Location: USA No potential conflict of interest statement was present: funding from Grant‐in‐Aid from the Riley Childrens' Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician provided a randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not clear whether there was block allocation, or how participants were randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Ranges not included on some data. Two participants in ranitidine group withdrew; 1 was lost to follow‐up; 1 participant received esomeprazole 40 mg twice daily. |
| Selective reporting (reporting bias) | Unclear risk | None |
| Other bias | Low risk | Funded by a Grant‐in‐Aid from the Riley Childrens' Foundation. |
Simeone 1997.
| Study characteristics | ||
| Methods | Double‐blind, single‐centre, parallel‐design outpatient RCT | |
| Participants | 26 infants and children with histological features of oesophagitis (mild‐moderate). 17 boys and 9 girls (median age 1.66 years; range 6 months to 8 years) were recruited. | |
| Interventions | Nizatidine 10 mg/kg twice daily versus placebo for 8 weeks. All participants received positional therapy and dietary manipulation with thickened feeds (dry rice cereal). | |
| Outcomes | Outcomes were assessed in terms of symptoms (symptomatic score assessment by daily diary card kept by parents to record the frequency/severity of GOR symptoms (abdominal pain, chest pain, regurgitation, and vomiting), and physical and symptomatologic physician assessment was performed at baseline and after 4 weeks of therapy); 24‐hour pH scores (reflux index, number of episodes pH < 4, no of episodes > 5 minutes, duration of episodes of pH < 4), and endoscopy/histology appearances (healed/improved/unchanged/worse) 48 hours before the end of the therapy at 8 weeks. Adverse events were monitored: 1 participant developed urticaria. | |
| Notes | Children receiving ulcerogenic drugs or with an antireflux agent were excluded from the study. Also excluded were participants with systemic extraintestinal diseases, neurological disorders, or a history of previous surgery. Post‐treatment pH‐metry was repeated in only 10 participants of the nizatidine group (83.3%) and 9 of the placebo group (75%). No funding declaration given. Location: Italy No potential conflict of interest statement was present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment made |
| Allocation concealment (selection bias) | Unclear risk | No comment made |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No comment made |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No comment made |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | pH‐metry was repeated in 10 participants of the nizatidine group (83.3%) and 9 of the placebo group (75%). Five parents refused re‐evaluation. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of this |
| Other bias | Unclear risk | No comment made. Funding not stated. |
Tolia 2006.
| Study characteristics | ||
| Methods | Multicentre, double‐blind, parallel‐design, outpatient RCT | |
| Participants | 53 children (5 to 11 years) with symptomatic GORD | |
| Interventions | Comparison of 10, 20, and 40 mg pantoprazole for 8 weeks | |
| Outcomes | Symptom score, endoscopic appearance and histological assessment, adverse events. Overall symptom score assessed using GASP‐Q to produce a composite symptom score (CSS). Individual symptoms also assessed (number of vomiting episodes, heartburn, epigastric pain) at week 0 then week 1 then 8. Endoscopy appearances were assessed using Hetzel‐Dent scoring. Adverse events were recorded. |
|
| Notes | There was no correlation between composite symptom score changes and endoscopy/biopsy changes. Statistically significant increases from baseline were noted in mean values for weight and height at week 8 in the pantoprazole 10 mg and 40 mg dose groups (P < 0.04). The participants in the 20 mg group had a significant mean increase in weight at week 8 (P = 0.023). Antacid use was reduced in the 20 mg and 40 mg groups at end of treatment. Adverse events noted: pantoprazole 10 mg group: headache (7 participants: 36.8%), rhinitis (5 participants; 26.3%), and nausea (3 participants; 15.8%). Pantoprazole 20 mg group: headache (5 participants; 27.8%), rhinitis (3 participants; 16.7%). Pantoprazole 40 mg group: headache (4 participants; 25%), abdominal pain, asthma, and pharyngitis (3 participants each; 18.8%). No funding declaration given. Location: USA No potential conflict of interest statement was present. Wyeth Research assisted in the preparation of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No comment on randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | No comment on allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blinded but no comment as to technique. Physician not blinded, but endoscopic findings read by blinded observer. No comment as to how participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No analysis of endoscopy appearances after treatment given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All enroled participants accounted for. No evidence of consecutive enrolment, or discussion of those children who refused consent or who were excluded. |
| Selective reporting (reporting bias) | Unclear risk | No evidence of selective reporting |
| Other bias | High risk | Wyeth Research involved in the preparation of the manuscript which may have affected study design (same medication, different dosing comparison). |
Tolia 2010a.
| Study characteristics | ||
| Methods | Post hoc analysis of subgroup of multicentre, parallel‐design RCT | |
| Participants | Subgroup of 109 participants weighing 8 kg to < 20 kg were randomised 1:1 to receive esomeprazole 5 mg or 10 mg daily. | |
| Interventions | Esomeprazole 10 mg once daily for 8 weeks versus esomeprazole 5 mg once daily | |
| Outcomes | Symptoms were graded as none/mild/moderate/severe (PGA ‐ Physician's Global Assessment symptom score) and by parents telephoning daily to report on the preceding 24 hours' symptoms. Also, the number of vomiting episodes and the use of antacids were assessed. Adverse events were monitored. Endoscopic findings were graded using the Los Angeles (LA) classification for erosive oesophagitis: Grade A is > 1 mucosal break < 5 mm that does not extend between the tops of 2 mucosal folds Grade B is > 1 mucosal break > 5 mm that does not extend between the tops of 2 mucosal folds Grade C is > 1 mucosal break that is continuous between the tops of > 2 mucosal folds but involves < 75% of the circumference of the oesophagus Grade D is > 1 mucosal break that involves > 75% of the circumference of the oesophagus Histology appearances were graded as healed/improved/unchanged. |
|
| Notes | Study supported by AstraZeneca LP. Medical writing services provided by Scientific Connexions, Newtown, PA, on behalf of AstraZeneca LP. Multiple authors received grant/research support from, and or were employees of, and or speakers for and or were consultants to AstraZenaca, Wyeth, Johnson and Johnson, TAP, Nutricia, Nestle and GlaxoSmithKline. Location: USA, France, Belgium, and Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | See Tolia 2010b: no comment made: higher risk as post hoc analysis and authors also note the potential for selection bias due to enrollment of patients who have not responded satisfactorily to other approved therapy |
| Allocation concealment (selection bias) | High risk | See Tolia 2010b: no comment made: higher risk as post hoc analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind by dose strata |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No comment made |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher risk as post hoc analysis |
| Selective reporting (reporting bias) | High risk | ITT analysis of all those participants with oesophagitis. Authors wondered about selection bias of those children with oesophagitis (sicker children); 2 children with erosive oesophagitis didn't have follow‐up endoscopy |
| Other bias | High risk | See funding comments: likely influence on study design (same medication: different dose comparison). |
Tolia 2010b.
| Study characteristics | ||
| Methods | Multicentre, double‐blinded (for dose), parallel‐group, outpatient RCT | |
| Participants | 109 children aged 1 to 11 years across Europe and the USA with endoscopically/histologically confirmed erosive oesophagitis | |
| Interventions | Doses of 5 mg or 10 mg of esomeprazole (8 kg to 20 kg children), 10 mg or 20 mg esomeprazole (> 20 kg children) for 8 weeks | |
| Outcomes | Children with erosive oesophagitis underwent an endoscopy after 8 weeks to assess healing of erosions. Outcomes assessed included resolution on endoscopy and side effects. Safety data (adverse events) and symptoms were published by the group separately (Gilger 2006). Endoscopy appearance ‐ presence/absence of erosive oesophagitis | |
| Notes | Baseline symptom characteristics were recorded and mention of record at follow‐up, but no follow‐up data available, and the trial did not report the outcome in sufficient detail to allow extraction of summary statistics. Baseline histologic appearance recorded and mention of record at follow‐up but no follow‐up data available. 49 children were excluded: 4 had eosinophilic oesophagitis, 29 had no evidence of reflux oesophagitis on endoscopy, and 16 were excluded for reasons 'not related to endoscopy'. Study funded by AstraZeneca. Location: USA, Belgium, France, Italy Competing interests declared: multiple authors had received grant/research support from AstraZeneca. An author had served as a speaker and a consultant for AP and AstraZeneca and has served as a speaker for Nestle. Another author has received research grants from Wyeth, Johnson & Johnson, and GlaxoSmithKline and has served as a speaker for Takeda and SHS Nutritionals. Three authors were employees of AstraZeneca LP. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not described by authors, and initial endoscopy and then enrolment performed at the discretion of the investigator. |
| Allocation concealment (selection bias) | High risk | Not described by authors |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Parents reported outcomes but blinded to dose |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Endoscopy performed by blinded examiners |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A high number of participants did not undergo follow‐up endoscopic examination (> 50%) |
| Selective reporting (reporting bias) | High risk | Of 3 potential outcome measures (endoscopic appearance, histologic appearance, and symptoms) only 1 had any follow‐up data recorded despite all 3 being recorded at baseline and follow‐up measurement described by the authors. |
| Other bias | High risk | Study funded by AstraZeneca, with pharmaceutical writing support also noted, which may have affected study design (same medication, different dosing comparison). |
Tsou 2006.
| Study characteristics | ||
| Methods | Outpatient, multicentre, randomised, double‐blind, multidose, parallel‐group study | |
| Participants | 112 children aged 12 to 16 years with symptomatic GORD | |
| Interventions | Pantoprazole 40 mg (n = 68) versus pantoprazole 20 mg (n = 68) | |
| Outcomes | Improvements were assessed using the GORD Assessment of Symptoms‐Pediatric questionnaire (GASP‐Q): outcomes expressed as composite symptom score and individual symptom score (vomiting episodes per day, heartburn symptom score, and epigastric pain score), through patient/parent records and physician assessment at baseline and week 8 (Likert score). Side effects also reported. | |
| Notes | In terms of adverse events (expressed as 'treatment‐associated adverse events'), a total of 112 participants (82.4%) had a treatment‐associated adverse event (AE), as follows: 1 or more treatment‐associated AEs = 59 participants (86.8%) in 20 mg group, 53 participants (77.9%) in 40 mg group. No serious AEs/deaths occurred. Commonest treatment‐associated AE was headache: 25 participants in 20 mg group; 22 participants in the 40 mg group. The majority were mild. Headache led to early withdrawal of 3 participants in the 40 mg group. 1 participant in the 20 mg group and 7 participants in the 40 mg group reported diarrhoea. Liver function fluctuation in 5 children was noted, and mild uric acid rise in 15 children. The study was supported by Wyeth Research. Wyeth Research were involved in the preparation of the manuscript. Location: USA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No evidence provided |
| Allocation concealment (selection bias) | Unclear risk | No evidence provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence provided as to method of blinding; no true control arm |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No evidence provided as to blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 159 children screened, and only 139 children entered the study: reasons for the other 20 not given. Otherwise, results analysed on intention‐to‐treat basis. Good assessment of compliance in teenagers |
| Selective reporting (reporting bias) | High risk | Children may not have been seen at trial entry by physician, potentially causing recall bias |
| Other bias | High risk | Final author employed by Wyeth, who funded the research, which may have affected study design (same medication, different dosing comparison). |
Ummarino 2015.
| Study characteristics | ||
| Methods | Single‐centre, outpatient, single‐blinded, parallel design RCT | |
| Participants | 75 infants younger than 1 year old (mean age 5 months, range 1 to 10 months), affected by symptoms of GOR (score > 7/35 on I‐GERQ) | |
| Interventions | 8 weeks' treatment with magnesium alginate and reassurance, thickened formula feeding (rice‐starch) and reassurance, or reassurance (lifestyle changes and reassurance on the condition). Evaluation after 1 (T1) and 2 months (T2). | |
| Outcomes | Parent‐reported symptom score (I‐GERQ) and individual symptoms (regurgitation, vomiting, and vomiting causing pain). Adverse events were monitored: 1 infant treated with magnesium alginate and simeticone developed constipation. | |
| Notes | This study assessed magnesium alginate and simeticone [Gastrotuss] over sodium alginate, given the theoretical advantages of a higher viscosity and lower sodium exposure. Location: Italy, from September 2012 to September 2013 The authors reported no conflicts of interest. There was no funding declaration present. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation confirmed but technique unclear |
| Allocation concealment (selection bias) | Unclear risk | No information given regarding allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician blinding but no patient blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Clinician evaluating questionnaire results and follow‐ups was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate of 15% was noted: 2 infants started PPI, 1 infant started Gastrotuss baby therapy, and 5 infants saw a different paediatrician due to persistence of symptoms |
| Selective reporting (reporting bias) | Low risk | Complete data in enroled infants |
| Other bias | Unclear risk | No manufacturer support identified |
Zohalinezhad 2015.
| Study characteristics | ||
| Methods | Outpatient, single‐centre, double‐blind, parallel‐group RCT | |
| Participants | 80 children (0 to 18 years) with GORD | |
| Interventions | Quince syrup versus omeprazole (2 mg/kg/day) | |
| Outcomes | Symptomatic improvement (composite symptom score) based on parental reports assessed at weeks 4 and 7; and adverse events. | |
| Notes | Adequately powered to show a 1‐sided significance of 0.05 (80% power) with 32 participants in each group. Unclear how many participants had had endoscopy. Diagnosis of GORD based on 1 month of 2 of 5 symptoms refractory to 'routine' treatments: vomiting immediately after eating, restlessness 1 to 3 hours after feeding, apnoea and respiratory distress after feeding, poor weight gain or refusal to eat. 9 participants declined to participate as they were already on PPIs. No adverse events were noted. Location: Iran The study was financially supported by Shiraz University of Medical Sciences grants. No potential conflict of interest statement was available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation software used |
| Allocation concealment (selection bias) | High risk | More children in omeprazole‐only group were refusing to eat at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study team (paediatricians, physician administering medications, and statisticians) and participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No evidence of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant lost to follow‐up or excluded from analysis (due to car accident) |
| Selective reporting (reporting bias) | High risk | No evidence to judge risk of reporting bias: no conflict of interest statement |
| Other bias | Low risk | Financial support from Shiraz University |
FDA: Food and Drug Administration; GASP‐Q: GORD Assessment of Symptoms–Pediatric; GOR: gastro‐oesophageal reflux; GORD: gastro‐oesophageal reflux disease; I‐GERQ‐R: Infant Gastroesophageal Reflux Questionnaire–Revised; ITT: intention‐to‐treat; PPI: proton pump inhibitor; RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Biltagi 2012 | Ineligible study design |
| Ameen 2006 | Ineligible study design |
| Bestebreurtje 2020 | Ineligible study design |
| Bestebreurtje 2017 | Ineligible study design |
| Corvaglia 2010 | Ineligible study design |
| Dhillon 2004 | Ineligible study design |
| Fiedorek 2005 | Ineligible study design |
| Franco 2000 | Ineligible study design |
| Gunesekaran 1993 | Ineligible study design |
| Haddad 2014 | Ineligible study design |
| Hassall 2000 | Ineligible study design |
| Hassall 2012 | Ineligible study design |
| James 2007 | Ineligible study design |
| Kaguelidou 2016 | Ineligible study design |
| Kukulka 2012 | Ineligible study design |
| Kushki 2020 | Ineligible study design |
| Li 2006 | Ineligible study design |
| Loots 2011 | Ineligible intervention |
| Madrazo‐de la Garza 2003 | Ineligible study design |
| Martin 2006 | Ineligible study design |
| Nielsen 2004 | Ineligible intervention |
| Omari 2009 | Ineligible study design |
| Orenstein 2005 | Ineligible study design |
| Orsi 2011 | Ineligible study design |
| Pfizer 2021 | Ineligible study design |
| Rabie 2016 | Ineligible intervention |
| Sabahi 2020 | Ineligible study design |
| Salvatore 2006 | Ineligible study design |
| Salvatore 2018 | Ineligible study design |
| Størdal 2005 | Ineligible population |
| Tammara 2011 | Ineligible study design |
| Terrin 2012 | Ineligible study design |
| Tolia 2002 | Ineligible study design |
| Tran 2002 | Ineligible study design |
| Treepongkaruna 2011 | Ineligible study design |
| Ward 2011 | Ineligible study design |
| Winter 2010 | Ineligible study design |
| Winter 2012 | Ineligible study design |
| Zannikos 2011 | Ineligible study design |
| Zhao 2006 | Ineligible study design |
Characteristics of studies awaiting classification [ordered by study ID]
Paknejad 2021.
| Methods | Double‐blind, single‐centre, randomised controlled trial |
| Participants | Children aged 1 to 7 years old, diagnosed with gastroesophageal reflux disease (GERD) |
| Interventions | Omeprazole and “myrtle fruit syrup” (syrup made from Myrtus communis L. fruit) versus control group (omeprazole and placebo syrup) for 8 weeks |
| Outcomes | GERD symptom questionnaire for young children (GSQ‐YC) at baseline, eighth week, and twelfth week (4 weeks after cessation of intervention). |
| Notes | Awaiting classification |
Shahmirzadi 2020.
| Methods | Single‐blinded randomised controlled trial |
| Participants | Children 6 months to 12 years old with symptomatic gastro‐oesophageal reflux disease |
| Interventions | Control group: omeprazole 1 mg/kg treatment Intervention group: omeprazole plus baclofen 0.25 mg/kg 2 times per day |
| Outcomes | 62 participants in each group: 46 (85.2%) cases in the baclofen treatment group and 32 cases (55.2%) in the non‐baclofen treatment group improved (moderate or full remission) at 1 month based on parental reporting. |
| Notes | No symptom scores. 8 cases in the treatment group with baclofen and 4 cases in the control group were excluded due to lack of follow‐up, lack of medication, and incomplete records of participants. No adverse events reported in baclofen group (control group not reported). |
Differences between protocol and review
Differences between the protocol and the present update:
Data collection and analysis: we used Review Manager 5.4 and RevMan Web for data collection and analysis, updated from RevMan 5.1.
Selection of studies: we added reprints of articles to the reference lists of included studies but did not consider them separately if they contained no new data. In the 2014 review, we discarded article reprints. We listed studies that were available only in abstract form, or were only identified in the ISRCTN register, as studies 'awaiting classification'.
Outcomes: we redesignated the outcome of 'pH/impedance studies' to 'pH/impedance indices' to account for the range of pH measurements described in the available literature.
Data extraction and management: three review authors (MT, IL, EA) independently extracted study data using a robust data extraction form and checked and entered the data into RevMan 5.4/RevMan Web, with MT, EA, and IL analysing the data and highlighting any discrepancies. In the 2014 review, two review authors extracted and entered study data into RevMan 5.1.
Measures of treatment effect: we extracted continuous data (e.g. reflux index) for summary data: we used means and standard deviations to derive a standardised mean difference (SMD) with a 95% confidence interval using a fixed‐effect model. The latest NASPGHAN/ESPGHAN guidelines do not define normal values for pH‐metry and pH‐impedance (NASPGHAN‐ESPGHAN guidelines 2018). The values of reflux index mentioned in the 2014 review (> 10% in 24 hours in infants and > 4% in 24 hours in children > 12 months) have been modified here with a judgement regarding improvement/non‐improvement. Dichotomous data, such as improvement/non‐improvement in endoscopic appearance, produced outcome data we presented as risk ratios. In the 2014 review, we used reported data rather than extracting summary data.
Unit of analysis issues: we considered issues related to multiple observations for the same outcome (e.g. repeated pH‐impedance measurements), and consulted the Cochrane Gut group if clarification was required. If we included multi‐arm studies, we would analyse multiple intervention groups to prevent arbitrary omission of relevant groups or double‐counting of participants. In the 2014 review, there was some overlap in reported data (e.g. according to age criteria); this was corrected in this review.
Dealing with missing data: we contacted trial authors or sponsors of studies published from 2014 to 2022 to provide missing data, or to seek clarification when we were uncertain about the specifics of a trial pertinent to analysis. In the 2014 review, we contacted study authors of studies published within the previous 10 years (e.g. to 2004).
Data synthesis: were unable to combine studies meaningfully due to the heterogeneity of studies in terms of outcomes, comparisons, and populations. For continuous measurements, we had planned to use weighted mean differences to pool results from studies using a common measurement scale. Where studies used different measurement scales, we planned to pool standardised mean differences. Instead, we have presented difference in means and 95% confidence intervals for individual studies and summary effects, using the following order: Population > Comparison > Outcome, following updated guidance. Given the individual study differences and heterogeneity in study design, we provided guidance based on individual treatments to give better focus for decision‐makers. This differs from the 2014 review.
Sensitivity analysis: if meta‐analysis had been possible, we intended to undertake sensitivity analysis using RevMan Web, to ascertain whether any decisions regarding thresholds influenced result reporting (e.g. choosing age thresholds at 12 months influencing meta‐analytic robustness). We planned to integrate the findings into the results and conclusions. This was not considered in the 2014 review. However, a meta‐analysis was not possible and sensitivity analysis not required.
Summary of findings and assessment of the certainty of the evidence: working independently, two authors used the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome, and to draw conclusions about evidence certainty within the text of the review. We resolved disagreements through discussion, and involved all review authors involved if the initial two authors could not reach agreement. All authors then reviewed the GRADE considerations in assessing the certainty of evidence and integrated this into the summary of findings tables. The summary of findings tables distinguish results by age (infants, and children aged one to 16 years), then comparison, and the evidence is presented by outcome (symptoms, adverse events, pH‐impedance indices, and endoscopic findings), with clear rationales given where evidence was downgraded or upgraded according to GRADE criteria, including if the risk of bias was so great the evidence needed downgrading by two steps.
Literature search in this update version: we did not search the Cochrane Review Group Specialised Register as it was not updated since the 2014 version and the included RCTs are included in Cochrane CENTRAL, which we did search. We did not search the Centralised Information Service for Complementary Medicine (CISCOM). This database did not yield additional eligible studies for our review in the 2014 version, and it was not available to us for this update. In the 2014 version, we handsearched published abstracts from conference proceedings. For this update, we did not handsearch proceedings from conferences that took place after 2014 because Embase now includes proceedings from these conferences (2000 onwards); these abstracts were searched electronically through our main electronic search. In the 2014 version, we searched the clinical trials register mRCT. In this updated version, we searched the World Health Organization's clinical trials register (ICTPR) and ClinicalTrials.gov (https://clinicaltrials.gov/). We also revisited the search strategies, and added some new terms to reflect the current practice of treatment in the updated search.
We have used the terms GOR and GORD throughout the review, following NASPGHAN‐ESPGHAN guidelines 2018 and NICE 2019 definitions, and we acknowledge that different groups may have used different definitions for these terms in their studies. We have included some narrative where relevant and encourage readers to review the original articles if they wish to ascertain in more detail how other authors distinguish between GOR and GORD.
Contributions of authors
Roles and responsibilities
Agree the protocol is still appropriate: Mark Tighe, Mark Beattie, Confirm the search strategy: Mark Tighe, Mark Beattie Search for new trials (2 people): Mark Tighe, Iona Liddicoat, Obtain copies of new trials: Mark Tighe, Iona Liddicoat Select which trials to include (2 + 1 arbiter) Mark Tighe, Iona Liddicoat +Mark Beattie. Extract data from trials (2 people) Mark Tighe, Iona Liddicoat Enter data into RevMan: Mark Tighe, Iona Liddicoat Carry out the analysis: Mark Tighe, Iona Liddicoat, Edward Andrews, Mark Beattie. Interpret the analysis Mark Tighe, Nadeem Afzal, Mark Beattie, Iona Liddicoat, Edward Andrews, Andrew Hayen Update the review Mark Tighe, Iona Liddicoat, Edward Andrews, Nadeem Afzal, Mark Beattie, Andrew Hayen.
Sources of support
Internal sources
-
Library, University Hospitals Dorset NHS Foundation Trust, UK
Obtaining manuscripts
External sources
-
Cochrane Gut Group, Canada
We would also like to thank the following editors and peer referees who provided comments to improve the review: Sarah Rhodes (editor), Yvan Vandenplas and Kornilia Nikaki (peer reviewers), and Alfretta Vanderheyden (consumer reviewer), Yuhong Yuan (managing editor) and to Grigoris Leontiadis (co‐ordinating editor). The update search strategies of Cochrane, MEDLINE and Embase were designed by Yuhong (Cathy) Yuan (Information Specialist at the Cochrane Gut group).
Declarations of interest
There are no authors' conflicts of interest.
A previous review of the medical treatment of gastro‐oesophageal reflux was completed for 'Pediatric Drugs' (publishers: 'Adis') and published in early 2009. However that article is substantially different from the Cochrane review. The Pediatric Drugs article was not funded.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Azizollahi 2016 {published data only}
- Azizollahi HR, Rafeey M. Efficacy of proton pump inhibitors and H2 blocker in the treatment of symptomatic gastroesophageal reflux disease in infants. Korean Journal of Pediatrics 2016;59(5):226-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2010 {published data only}
- Baker R, Tsou VM, Tung J, Baker SS, Li H, Wang W, et al. Clinical results from a randomised, double-blind, dose-ranging study of pantoprazole in children aged 1 through 5 years with symptomatic histologic or erosive esophagitis. Clinical Pediatrics 2010;49(9):852-65. [DOI] [PubMed] [Google Scholar]
Baldassarre 2020 {published data only}
- Baldassarre ME, Di Mauro A, Pignatelli MC, Fanelli M, Salvatore S, Di Nardo G, et al. Magnesium alginate in gastro-esophageal reflux: a randomized multicenter cross-over study in infants. International Journal of Environmental Research and Public Health 2020;17(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballengee 2018 {published data only}
- Ballengee CR, Davalian F, Conaway MR, Sauer CG, Kaufman DA. Erythromycin and reflux events in premature neonates: a randomized clinical trial. Journal of Pediatric Gastroenterology and Nutrition 2018;67(6):720-25. [DOI] [PubMed] [Google Scholar]
Bines 1992 {published data only}
- Bines JE, Quinlan JE, Treves S, Kleinman RE, Winter HS. Efficacy of domperidone in infants and children with gastroesophageal reflux. Journal of Pediatric Gastroenterology and Nutrition 1992;14(4):400-5. [DOI] [PubMed] [Google Scholar]
Borrelli 2002 {published data only}
- Borrelli O, Rea P, Bueno De Mesquita M, Ambrosini A, Mancini V, Di Nardo G, et al. Efficacy of combined administration of an alginate formulation (Gaviscon) and Lansoprazole for children with gastroesophageal reflux. Italian Journal of Pediatrics 2002;28(4):304-9. [Google Scholar]
Buts 1987 {published data only}
- Buts JP, Barudi C, Otte JB. Double-blind controlled study on the efficacy of sodium alginate (Gaviscon) in reducing gastroesophageal reflux assessed by 24 h continuous pH monitoring in infants and children. European Journal of Pediatrics 1987;146(2):156-8. [DOI] [PubMed] [Google Scholar]
Carroccio 1994 {published data only}
- Carroccio A, Iacono, Montalto G, Cavataio F, Soresi M, Notarbartolo A. Domperidone plus magnesium hydroxide and aluminium hydroxide: a valid therapy in children with gastroesophageal reflux. Scandinavian Journal of Gastroenterology 1994;29(4):300-4. [DOI] [PubMed] [Google Scholar]
Cresi 2008 {published data only}
- Cresi F, Marinaccio C, Russo MC, Miniero R, Silvestro L. Short-term effect of domperidone on gastroesophageal reflux in newborns assessed by combined intraluminal impedance and pH monitoring. Journal of Perinatology 2008;28:766-70. [DOI] [PubMed] [Google Scholar]
Cucchiara 1984 {published data only}
- Cucchiara S, Staiano A, Romaniello G, Capobianco S, Aurcchio S. Antacids and cimetidine treatment for gastrooesophageal reflux and peptic oesophagitis. Archives of Disease in Childhood 1984;59:842-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cucchiara 1993 {published data only}
- Cucchiara S, Minella R, Iervolino C, Franco MT, Campanozzi A, Franceschi M, et al. Omeprazole and high dose ranitidine in the treatment of refractory reflux oesophagitis. Archives of Disease in Childhood 1993;69:655-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 2013 {published data only}
- Davidson G, Wenzl TG, Thomson M, Omari T, Barker P, Lundborg P, et al. Efficacy and safety of once-daily esomeprazole for the treatment of gastroesophageal reflux disease in neonatal patients. Journal of Pediatrics 2013;163(3):692-8. [DOI] [PubMed] [Google Scholar]
Del Buono 2005 {published data only}
- Del Buono R, Wenzl TG, Ball G, Keady S, Thomson M. Effect of Gaviscon Infant on gastro-oesophageal reflux in infants assessed by combined intraluminal impedance/pH. Archives of Disease in Childhood 2005;90:460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Famouri 2017 {published data only}
- Famouri F, Zibanejad N, Kabiri P, Kelishad R. Comparison of hypoallergenic diet vs. ranitidine in treatment of gastroesophageal reflux disease of infants: a randomized clinical trial. Iranian Journal of Pediatrics 2017;27(4):e5343. [Google Scholar]
Forbes 1986 {published data only}
- Forbes D, Hodgson M, Hill R. The effects of gaviscon and metoclopramide in gastroesophageal reflux in children. Journal of Pediatric Gastroenterology and Nutrition 1986;5(4):556-9. [DOI] [PubMed] [Google Scholar]
Gilger 2006 {published data only}
- Gilger M, Tolia V, Vandenplas Y, Youssef N, Traxler B, Illueca M. Safety and tolerability of esomeprazole in children with gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2006;43(4):E20-21. [DOI] [PubMed] [Google Scholar]
- Gilger MA, Tolia V, Vandenplas Y, Youssef NN, Traxler B, Illueca M. Safety and tolerability of esomeprazole in children with gastro-esophageal reflux disease. Gastroenterology 2015;46(5):S16-23. [DOI] [PubMed] [Google Scholar]
Gunesekaran 2003 {published data only}
- Gunasekaran T, Gupta S, Gremse D, Karol M, Pan W-J, Chin Y-L, et al. Lansoprazole in adolescents with gastroesophageal reflux disease: pharmacokinetics, pharmacodynamics, symptom relief efficacy, and tolerability. Journal of Pediatric Gastroenterology and Nutrition 2002;35(4):S327-35. [DOI] [PubMed] [Google Scholar]
Haddad 2013 {published data only}
- Haddad I, Kierkus J, Tron E, Ulmer A, Hu P, Sloan S, et al. Efficacy and safety of rabeprazole in children (1-11 years) with gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2013;57(6):798-807. [DOI] [PubMed] [Google Scholar]
Hussain 2014 {published data only}
- Hussain S, Kierkus J, Hu P, Hoffman D, Lekich R, Sloan S, et al. Safety and efficacy of delayed release rabeprazole in 1- to 11-month-old infants with symptomatic GERD. Journal of Pediatric Gastroenterology and Nutrition 2014;58(2):226-36. [DOI] [PubMed] [Google Scholar]
Kierkus 2011 {published data only}
- Kierkus J, Furmaga-Jablonska W, Sullivan JE, David ES, Stewart DL, Rath N, et al. Pharmacodynamics and safety of pantoprazole in neonates, preterm infants, and infants aged 1-11 months with a clinical diagnosis of gastroesophageal reflux disease. Digestive Diseases and Sciences 2011;56:425-34. [DOI] [PubMed] [Google Scholar]
Loots 2014 {published data only}
- Loots C, Kritas S, Wijk M, McCall L, Peeters L, Lewindon P, et al. Body positioning and medical therapy for infantile gastroesophageal reflux symptoms. Journal of Pediatric Gastroenterology and Nutrition 2014;59(2):237-43. [DOI] [PubMed] [Google Scholar]
Miller 1999 {published data only}
- Miller S. Comparison of the efficacy and safety of a new aluminum-free paediatric alginate preparation and placebo in infants with recurrent gastro-oesophageal reflux. Current Medical Research and Opinion 1999;15(3):160-8. [DOI] [PubMed] [Google Scholar]
Moore 2003 {published data only}
- Moore DJ, Tao BS, Lines DR, Hirte C, Heddle ML, Davidson GP. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. Journal of Pediatrics 2003;143(2):219-23. [DOI] [PubMed] [Google Scholar]
Naeimi 2019 {published data only}
- Naeimi M, Kianifar H, Memariani Z, Kamalinejad M, Bijani A, Saghebi R, et al. Comparison of the efficacy of ranitidine and quince syrup on gastroesophageal reflux disease in children. Complementary Therapies in Medicine 2019;45:215-21. [DOI] [PubMed] [Google Scholar]
Omari 2006 {published data only}
- Omari TI, Benninga MA, Sansom L, Butler RN, Dent J, Davidson GP. Effect of baclofen on esophagogastric motility and gastroesophageal reflux in children with gastroesophageal reflux disease: a randomized controlled trial. Journal of Pediatrics 2006;149(4):468-74. [DOI] [PubMed] [Google Scholar]
Omari 2007 {published data only}
- Omari T, Davidson G, Bondarov P, Naucler E, Nilsson C, Lundborg P. Pharmacokinetics and acid-suppressive effects of esomeprazole in infants 1-24 months old with symptoms of gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2007;45(5):530-7. [MEDLINE: Published in abstract form in JPGN:2006: 42: E1-110] [DOI] [PubMed] [Google Scholar]
- Omari T, Davidson G, Bondarov P, Naucler E, Nilsson C, Lundborg P. Pharmacokinetics and acid-suppressive effects of esomeprazole in infants 1-24 months old with symptoms of gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2015;60:S2-S8. [DOI] [PubMed] [Google Scholar]
Orenstein 2002 {published data only}
- Orenstein SR, Shalaby TM, Devandry SN, Liacouras CA, Czinn SJ, Dice JE, et al. Famotidine for infant gastro-oesophageal reflux: a multi-centre, randomized, placebo-controlled, withdrawal trial. Alimentary Pharmacology and Therapeutics 2003;17(9):1097-107. [DOI] [PubMed] [Google Scholar]
Orenstein 2008 {published data only}
- Orenstein SR, Hassall E, Furmaga-Jablonska W, Atkinson S, Raanan M. Multicenter, double-blind, randomized, placebo-controlled trial assessing the efficacy and safety of proton pump inhibitor lansoprazole in infants with symptoms of gastroesophageal reflux disease. Journal of Pediatrics 2009;154(4):514-20. [DOI] [PubMed] [Google Scholar]
Pfefferkorn 2006 {published data only}
- Pfefferkorn MD, Croffie JM, Gupta SK, Molleston JP, Eckert GJ, Corkins MR, et al. Nocturnal acid breakthrough in children with reflux esophagitis taking proton pump inhibitors. Journal of Pediatric Gastroenterology and Nutrition 2006;42(2):160-5. [DOI] [PubMed] [Google Scholar]
Simeone 1997 {published data only}
- Simeone D, Caria MC, Miele E, Staiano A. Treatment of childhood peptic esophagitis: a double-blind placebo-controlled trial of nizatidine. Journal of Pediatric Gastroenterology and Nutrition 1997;25(1):51-5. [DOI] [PubMed] [Google Scholar]
Tolia 2006 {published data only}
- Tolia V, Bishop PR, Tsou VM, Gremse D, Soffer EF, Comer GM, et al. Multicenter, randomized, double-blind study comparing 10, 20 and 40 mg pantoprazole in children (5-11 years) with symptomatic gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2006;42(4):384-91. [DOI] [PubMed] [Google Scholar]
Tolia 2010a {published data only}
- Tolia V, Gilger MA, Barker PN, Illueca M. Healing of erosive esophagitis and improvement of symptoms of gastroesophageal reflux disease after esomeprazole treatment in children 12 to 36 months old. Journal of Pediatric Gastroenterology and Nutrition 2010;51(5):593-8. [DOI] [PubMed] [Google Scholar]
Tolia 2010b {published data only (unpublished sought but not used)}
- Tolia V, Youssef NN, Gilger MA, Traxler B, Illueca M. Esomeprazole for the treatment of erosive esophagitis in children: an international, multicenter, randomized, parallel-group, double-blind (for dose) study. BMC Pediatrics 2010;10:41-50. [DOI: 10.1186/1471-2431-10-41] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolia V, Youssef NN, Gilger MA, Traxler B, Illueca M. Esomeprazole for the treatment of erosive esophagitis in children: an international, multicenter, randomized, parallel-group, double-blind (for dose) study. Journal of Pediatric Gastroenterology and Nutrition 2015;10(1):S24-30. [DOI] [PubMed] [Google Scholar]
Tsou 2006 {published data only}
- Tsou VM, Baker R, Book L, Hammo AH, Soffer EF, Wang W, et al. Multicenter, randomized, double-blind study comparing 20 and 40 mg of pantoprazole for symptom relief in adolescents (12 to 16 years of age) with gastroesophageal reflux disease (GERD). Clinical Pediatrics 2006;45(8):741-9. [DOI] [PubMed] [Google Scholar]
Ummarino 2015 {published data only}
- Ummarino D, Miele E, Martinelli M, Scarpato E, Crocetto F, Sciorio E, et al. Effect of magnesium alginate plus simethicone on gastroesophageal reflux in infants. Journal of Pediatric Gastroenterology and Nutrition 2015;60(2):230-5. [DOI] [PubMed] [Google Scholar]
Zohalinezhad 2015 {published data only}
- Zohalinezhad ME, Imanieh MH, Samani SM, Mohagheghzadeh A, Dehghani SM, Haghighat M, et al. Effects of quince syrup on clinical symptoms of children with symptomatic gastroesophageal reflux disease: a double-blind randomized controlled clinical trial. Complementary Therapies in Clinical Practice 2015;21(4):268-76. [DOI] [PubMed] [Google Scholar]
- Zohalinezhad ME, Zohalinezhad IS, Samani A, Mohagheghzadeh F, Dehghani H. Efficacy of quince (Cydonia oblonga M) syrup in symptomatic gastroesophageal reflux disease: a double-blind randomized controlled clinical trial. Planta Medica 2016;82(1):1439. [Google Scholar]
References to studies excluded from this review
Al‐Biltagi 2012 {published data only}
- Al-Biltagi M, Bediwy AS, Deraz S, Amer HG, Saeed NK. Esomeprazole, versus esomeprazole and domperidone in treatment of gastroesophageal reflux in children with difficult-to-treat asthma. European Respiratory Journal 2013;42(Suppl 57):1138. [NCT ID: NCT04821310] [Google Scholar]
Ameen 2006 {published data only}
- Ameen VZ, Pobiner BF, Giguere GC, Carter EG. Ranitidine (Zantac) syrup vs ranitidine effervescent tablets (Zantac EFFERdose) in children, a single-center taste preference study. Pediatric Drugs 2006;8(4):265-70. [DOI] [PubMed] [Google Scholar]
Bestebreurtje 2017 {published data only}
- Bestebreurtje P, De Koning B, Knibbe CA, Tibboel D, De Wildt SN. Rectal omeprazole in young infants: the magic bullet. Clinical Pharmacology and Therapeutics 2017;101(S1):S22. [Google Scholar]
Bestebreurtje 2020 {published data only}
- Bestebreurtje P, Koning B, Roeleveld N, Knibbe CA, Tibboel D, Groen B, et al. Rectal omeprazole in infants with gastroesophageal reflux disease: a randomized pilot trial. European Journal of Drug Metabalism and Pharmacokinetics 2020;45(5):635-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corvaglia 2010 {published data only}
- Corvaglia L, Aceti A, Mariani E, De Giorgi M, Capretti MG, Faldella G. The efficacy of sodium alginate (Gaviscon) for the treatment of gastro-oesophageal reflux in preterm infants. Alimentary Pharmacology and Therapeutics 2011;33:466-70. [DOI] [PubMed] [Google Scholar]
Dhillon 2004 {published data only}
- Dhillon AS, Ewer AK. Diagnosis and management of gastro-oesophageal reflux in preterm infants in neonatal intensive care units. Acta Paediatrica 2004;93:88-93. [PubMed] [Google Scholar]
Fiedorek 2005 {published data only}
- Fiedorek S, Tolia V, Gold BD, Huang B, Stolle J, Lee C, et al. Efficacy and safety of lansoprazole in adolescents with symptomatic erosive and non-erosive gastro-oesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2005;40(3):319-27. [DOI] [PubMed] [Google Scholar]
Franco 2000 {published data only}
- Franco MT, Salvia G, Terrin G, Spadaro R, De Rosa I, Iula VD, et al. Lansoprazole in the treatment of gastro-oesophageal reflux disease in childhood. Digestive and Liver Disease 2000;32(8):660-6. [DOI] [PubMed] [Google Scholar]
Gunesekaran 1993 {published data only}
- Gunasekaran TS, Hassall EG. Efficacy and safety of omeprazole for severe gastroesophageal reflux in children. Journal of Pediatrics 1994;124(2):332-4. [DOI] [PubMed] [Google Scholar]
Haddad 2014 {published data only}
- Haddad I, Kierkus J, Tron E, Ulmer A, Hu P, Sloan S, et al. Maintenance of efficacy and safety of rabeprazole in children with endoscopically proven GERD. Journal of Pediatric Gastroenterology and Nutrition 2014;58(4):510-7. [DOI] [PubMed] [Google Scholar]
Hassall 2000 {published data only}
- Hassall E, Israel D, Shepherd R, Radke M, Dalväg A, Sköld B, et al. Omeprazole for treatment of chronic erosive esophagitis in children: a multicenter study of efficacy safety, tolerability and dose requirements. Journal of Pediatrics 2000;137(6):800-7. [DOI] [PubMed] [Google Scholar]
Hassall 2012 {published data only}
- Hassall E, Shepherd R, Koletzko S, Radke M, Henderson C, Lundborg P. Long-term maintenance treatment with omeprazole in children with healed erosive oesophagitis: a prospective study. Alimentary Pharmacology and Therapeutics 2012;35:368-79. [DOI] [PubMed] [Google Scholar]
James 2007 {published data only}
- James L, Walson P, Lomax K, Kao R, Varughese S, Reyes J. Pharmacokinetics and tolerability of rabeprazole sodium in subjects aged 12 to 16 years with gastroesophageal reflux disease: an open-label, single- and multiple-dose study. Clinical Therapeutics 2007;29(9):2082-92. [DOI] [PubMed] [Google Scholar]
Kaguelidou 2016 {published data only}
- Kaguelidou F, Alberti C, Biran V, Bourdon O, Farnoux C, Zohar S, et al. Dose-finding study of omeprazole on gastric pH in neonates with gastro-esophageal acid reflux using a Bayesian sequential approach. PloS One 2016;11(12):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kukulka 2012 {published data only}
- Kukulka M, Wu J, Perez MC. Pharmacokinetics and safety of dexlansoprazole MR in adolescents with symptomatic GERD. Journal of Pediatric Gastroenterology and Nutrition 2012;54(1):41-7. [DOI] [PubMed] [Google Scholar]
Kushki 2020 {unpublished data only}
- Kushki AM. Study of the effect of Esomeprazole and combination of Esomeprazole plus Baclofen in the treatment of clinical signs of infants reflux [sic]. Iranian Registry of Clinical Trials 2021.
Li 2006 {published data only}
- Li J, Zhao J, Hamer-Maansson JE, Andersson T, Fulmer R, Illueca M, et al. Pharmacokinetic properties of esomeprazole in children aged 1 to 11 years with symptoms of gastroesophageal reflux disease: a randomized, open-label study. Clinical Therapeutics 2006;28(11):1868-76. [DOI] [PubMed] [Google Scholar]
Loots 2011 {published data only}
- Loots CM, Smits MJ, Wijnakker R, Wijk MP, Davidson G, Benninga MA, et al. Esophageal impedance baselines in infants before and after placebo, antacid and proton pump inhibitor therapy. Journal of Pediatric Gastroenterology and Nutrition 2011;53(2):S68. [Google Scholar]
Madrazo‐de la Garza 2003 {published data only}
- Madrazo-de la Garza A, Dibildox M, Vargas A, Delgado J, Gonzalez J, Yañez P. Efficacy and safety of oral pantoprazole 20 mg given once daily for reflux esophagitis in children. Journal of Pediatric Gastroenterology and Nutrition 2003;36(2):261-5. [DOI] [PubMed] [Google Scholar]
Martin 2006 {published data only}
- Martin PB, Imong SM, Krischer J, Noblett HR, Sandhu BK. The use of omeprazole for resistant oesophagitis in children. European Journal of Pediatric Surgery 1996;6(4):195-7. [DOI] [PubMed] [Google Scholar]
Nielsen 2004 {published data only}
- Nielsen RG, Bindslev-Jensen C, Kruse-Andersen S, Husby S. Severe gastroesophageal reflux disease and cow milk hypersensitivity in infants and children: disease association and evaluation of a new challenge procedure. Journal of Pediatric Gastroenterology and Nutrition 2004;39(4):383-91. [DOI] [PubMed] [Google Scholar]
Omari 2009 {published data only}
- Omari T, Lundborg P, Sandstrom M, Bondarov P, Fjellman M, Haslam R, et al. Pharmacodynamics and systemic exposure of esomeprazole in preterm infants and term neonates with gastroesophageal reflux disease. Journal of Pediatrics 2009;155(2):222-8. [DOI] [PubMed] [Google Scholar]
Orenstein 2005 {published data only}
- Orenstein SR, Gremse DA, Pantaleon CD, Kling DF, Rotenberg KS. Nizatidine for the treatment of pediatric gastroesophageal reflux symptoms: an open-label, multiple-dose, randomized, multicenter clinical trial in 210 children. Clinical Therapeutics 2005;27(4):472-83. [DOI] [PubMed] [Google Scholar]
Orsi 2011 {published data only}
- Orsi M, Donato G, Busoni V, Naisberg G, Caruso N. Gastric acid suppression of a new oral powder omeprazole suspension for infants with gastroesophageal reflux disease. A pilot study [Eficacia acidosupresora del omeprazol en polvo en lactantes con reflujo gastroesofagico. Estudio piloto]. Acta Gastroenterologica Latinoamericana 2011;41(2):111-8. [PubMed] [Google Scholar]
Pfizer 2021 {unpublished data only}
- Pfizer. Protonix (Pantoprazole) treatment of maintenance of healing in pediatric participants aged 1-11 years and 12-17 years. clinicaltrials.gov/ct2/show/NCT04821310 (first posted 29 March 2021).
Rabie 2016 {published and unpublished data}
- Rabie DE, Elshimi MS, Eltahery AM, Abusaif IS, Abushady NM, Ismail RI. G401 (P) Evaluation of gastroesophageal reflux disease in neonates pre and post proton pump inhibitors therapy using oesophageal multichannel intraluminal impedance and PH monitoring. Archives of Disease in Childhood 2016;101 (Suppl):A234-5. [Google Scholar]
Sabahi 2020 {unpublished data only}
- IRCT20191027045256N1. Effect of omeprazole and es-omeprazole in reflux disease [sic]. trialsearch.who.int/Trial2.aspx?TrialID=IRCT20191027045256N1 (first registered 09 March 2020).
Salvatore 2006 {published data only}
- Salvatore S, Hauser B, Salvatoni A, Vandenplas Y. Oral ranitidine and duration of gastric pH <4.0 in infants with persisting reflux symptoms. Acta Paediatrica 2006;95(2):176-81. [DOI] [PubMed] [Google Scholar]
Salvatore 2018 {published data only}
- Salvatore S, Ripepi A, Huysentruyt K, de Maele K, Nosetti L, Agosti M, Salvatoni A, Vandenplas Y. The effect of alginate in gastroesophageal reflux in infants. Paediatric Drugs 2018;20(6):575-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Størdal 2005 {published data only}
- Størdal K, Johannesdottir GB, Bentsen BS, Knudsen PK, Carlsen KC, Closs O, et al. Acid suppression does not change respiratory symptoms in children with asthma and gastro-oesophageal reflux disease. Archives of Disease in Childhood 2005;90(9):956-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tammara 2011 {published data only}
- Tammara BK, Sullivan JE, Adcock KG, Kierkus J, Giblin J, Rath N, et al. Randomized, open-label, multicentre pharmacokinetic studies of two dose levels of pantoprazole granules in infants and children aged 1 month through < 6 years with gastro-oesophageal reflux disease. Clinical Pharmacokinetics 2011;50(8):541-50. [DOI] [PubMed] [Google Scholar]
Terrin 2012 {published data only}
- Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, et al. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics 2012;129:e40. [DOI] [PubMed] [Google Scholar]
Tolia 2002 {published data only}
- Tolia V, Ferry G, Gunesekaran T, Huang B, Keith R, Book L. Efficacy of lansoprazole in the treatment of gastrooesophageal reflux in children. Journal of Pediatric Gastroenterology and Nutrition 2002;35:S308-18. [DOI] [PubMed] [Google Scholar]
Tran 2002 {published data only}
- Tran A, Rey E, Pons G, Pariente-Khayat A, D'Athis P, Sallerin V, et al. Pharmacokinetic-pharmacodynamic study of oral lansoprazole in children. Clinical Pharmacology and Therapeutics 2002;71(5):359-67. [DOI] [PubMed] [Google Scholar]
Treepongkaruna 2011 {published data only}
- Treepongkaruna S, Ngoenmak T, Petchsrikul K, Aroonwan Preutthipan RN. Effect of lansoprazole plus domperidone for treatment of gastroesophageal reflux disease in infants. Journal of Pediatric Gastroenterology and Nutrition 2011;53(2):S69. [Google Scholar]
Ward 2011 {published data only}
- Ward RM, Kearns GL, Tammara B, Bishop P, O’Gorman MA, James LP, et al. A multicenter, randomized, open-label, pharmacokinetics and safety study of pantoprazole tablets in children and adolescents aged 6 through 16 years with gastroesophageal reflux disease. Journal of Clinical Pharmacology 2011;51(6):876-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winter 2010 {published data only}
- Winter H, Kum-Nji P, Mahomedy SH, Kierkus J, Hinz M, Li H, et al. Efficacy and safety of pantoprazole delayed-release granules for oral suspension in a placebo-controlled treatment-withdrawal study in infants 1-11 months old with symptomatic GERD. Journal of Pediatric Gastroenterology and Nutrition 2010;50(6):609-18. [DOI] [PubMed] [Google Scholar]
Winter 2012 {published data only}
- Winter H, Gunasekaran T, Tolia V, Gottrand F, Barker PN, Illueca M. Esomeprazole for the treatment of GERD in infants ages 1-11 months. Journal of Pediatric Gastroenterology and Nutrition 2012;55(1):14-20. [DOI] [PubMed] [Google Scholar]
- Winter H, Gunasekaran T, Tolia V, Gottrand F, Barker PN, Illueca M. Esomeprazole for the treatment of GERD in infants ages 1-11 months. Journal of Pediatric Gastroenterology and Nutrition 2015;60:S9-15. [DOI] [PubMed] [Google Scholar]
Zannikos 2011 {published data only}
- Zannikos PN, Doose DR, Leitz GJ, Rusch S, Gonzalez MD, Solanki B, et al. Pharmacokinetics and tolerability of rabeprazole in children 1 to 11 years old with gastroesophageal reflux disease. Journal of Pediatric Gastroenterology and Nutrition 2011;52(6):691-702. [DOI] [PubMed] [Google Scholar]
Zhao 2006 {published data only}
- Zhao J, Li J, Hamer-Maansson JE, Andersson T, Fulmer R, Illueca M, et al. Pharmacokinetic properties of esomeprazole in children aged 1 to 11 years with symptoms of gastroesophageal reflux disease: a randomized, open-label study. Clinical Therapeutics 2006;28(11):1868-76. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Paknejad 2021 {published data only}
- Paknejad MS, Eftekhari K, Rahimi R, Vigeh M, Naghizadeh A, Karimi M. Myrtle (Myrtus communis L.) fruit syrup for gastroesophageal reflux disease in children: a double-blind randomized clinical trial. Phytotherapy Research 2021;35(11):6369-76. [DOI] [PubMed] [Google Scholar]
Shahmirzadi 2020 {published data only}
- Sobhani Shahmirzadi M, Barati L, Ebraimi M, Shiroodbakhshi K. The efficacy of baclofan to treat gastroesophageal reflux disease in children aged 6 months to 12 years: a clinical trial study. International Journal of Pediatrics 2020;8(5):11287-96. [Google Scholar]
Additional references
Augood 2003
- Augood C, MacLennon S, Gilbert R, Logan S. Cisapride treatment for gastrooesophageal reflux in children. Cochrane Database of Systematic Reviews 2003, Issue 3. Art. No: CD002300. [DOI: 10.1002/14651858.CD002300] [DOI] [PubMed] [Google Scholar]
Bashashati 2016
- Bashashati M, Sarosiek I, Siddiqui T, McCallum R. Adverse effects of domperidone: prolonged quest for knowledge? Digestive Diseases and Sciences 2016;61:3384-6. [DOI] [PubMed] [Google Scholar]
BNFc 2021
- British Medical Association, Royal Pharmaceutical Society of Great Britain, Royal College of Paediatrics and Child Health, Neonatal and Paediatric Pharmacists Group. British National Formulary for Children. London, UK: BMJ Group, Pharmaceutical Press, RCPCH Publications, 2021. [Google Scholar]
Campanozzi 2009
- Campanozzi A, Boccia G, Pensabene L, Panetta F, Marseglia A, Strisciuglio P, et al. Prevalence and natural history of gastroesophageal reflux: pediatric prospective survey. Pediatrics 2009;123(3):779-83. [DOI] [PubMed] [Google Scholar]
Com Safety Med 2000
- Committee on Safety of Medicines and Medicines Control Agency. Cisapride (Propulsid) withdrawn. Curr Prob Pharmacovigil 2000;26:9-14. [Google Scholar]
Cooper 2002
- Cooper WO, Griffin MR, Arbogast P, et al. Very early exposure to erythromycin andinfantile hypertrophic pyloric stenosis. Archives of Pediatrics & Adolescent Medicine 2002;156:647-50. [DOI] [PubMed] [Google Scholar]
Craig 2004
- Craig WR, Hanlon-Dearman A, Sinclair C, Taback S, Moffatt M. Metoclopramide, thickened feedings, and positioning for gastro-oesophageal reflux in children under two years. Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No: CD003502. [DOI: 10.1002/14651858.CD003502.pub3] [DOI] [PubMed] [Google Scholar]
De Bruyne 2018
- De Bruyne P, Ito S. Toxicity of long-term use of proton pump inhibitors in children. Archives of Disease in Childhood 2018;103(1):78-82. [DOI] [PubMed] [Google Scholar]
EMA 2014a
- European Medicine Agency Committee on Medicinal Products for Human Use. European Medicines Agency recommends changes to the use of metoclopramide. www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/07/news_detail_001854.jsp&mid=WC0b01ac058004d5c1 Accessed prior to 20 May 2023.
EMA 2014b
- European Medicines Agency. PRAC recommends restricting use of domperidone; March 2014. www.ema.europa.eu/en/news/prac-recommends-restricting-use-domperidone\ Accessed prior to 20 May 2023. [EMA: EMA/129231/2014]
FDA 2009
- Food and Drug Administration (FDA). FDA requires boxed warning and risk mitigation strategy for metoclopramide-containing drugs [press release]. Food and Drug Administration; Rockville, MD 2009.
Fitzgerald 2003
- Fitzgerald JF, Vasundhara T, Fraga P, Peters S, Donnelly C, Mack M, et al. Age-specific questionaires distinguish symptom frequency and severity in young children with and without gastroesophageal reflux disease. In: Sixteenth Annual Meeting of NASPGHAN Poster, Montreal, Quebec. 2003.
Fleishmann 2021
- Fleishman N, Richardson T, Attard T. The clinical characteristics of fractures in pediatric patients exposed to proton pump inhibitors. Journal of Pediatric Gastroenterology and Nutrition 2020;70(6):815-9. [DOI] [PubMed] [Google Scholar]
Franckx 1984
- Franckx J, Noel P. Acute extrapyramidal dysfunction after domperidone administration: Report of a case. Helvetica Paediatrica Acta 1984;39(3):285-8. [PubMed] [Google Scholar]
Gaviscon Product Information 2021
- Reckitt Benckiser Healthcare (UK) Limited. Gaviscon Product Information. Reckitt Benckiser Healthcare (UK) Limited, Dansom Lane, Hull.
Hassall 2005
- Hassall E. Outcomes of fundoplication: causes for concern, newer options. Archives of Disease in Childhood 2005;90(10):1047-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3. Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Hyams 1988
- Hyams JS, Ricci A Jr, Leichtner AM. Clinical and laboratory correlates of esophagitis. Journal of Pediatric Gastroenterology and Nutrition 1988;7(1):52-6. [DOI] [PubMed] [Google Scholar]
Hyams 2016
- Hyams JS, Di Lorenzo C, Saps M, Shulman RJ, Staiano A, Tilburg M. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology 2016;150:1456–68. [Google Scholar]
Hyman 1985
- Hyman PE, Abrams C, Garvey TO. Ranitidine tachyphylaxis. Gastroenterology 1985;88:1426. [Google Scholar]
Keady 2007
- Keady S. Update on drugs for gastro-oesophageal reflux disease. Archives of Disease in Childhood 2007;92:ep114-ep118. [DOI] [PubMed] [Google Scholar]
Kleinman 2011
- Kleinman L, Nelson S, Kothari-Talwar S, Roberts L, Orenstein SR, Mody RR, et al. Development and psychometric evaluation of 2 age-stratified versions of the Pediatric GERD Symptom and Quality of Life Questionnaire. Journal of Pediatric Gastroenterology and Nutrition 2011;52(5):514-22. [DOI] [PubMed] [Google Scholar]
Kwok 2017
- Kwok TC, Ojha S, Dorling J. Feed thickener for infants up to six months of age with gastro-oesophageal reflux. Cochrane Database of Systematic Reviews 2017;12:CD003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1-34. [DOI] [PubMed] [Google Scholar]
Mandel 2000
- Mandel KG, Daggy BP, Brodie DA, Jacoby HI. Review article: alginate-raft formulations in the treatment of heartburn and acid reflux. Alimentary Pharmacology Therapeutics 2000;14(6):669-90. [DOI] [PubMed] [Google Scholar]
Martin 2002
- Martin AJ, Pratt N, Kennedy JD, Ryan P, Ruffin RE, Miles H, et al. Natural history and familial relationships of infant spilling to 9 years of age. Pediatrics 2002;109(6):1061-7. [DOI] [PubMed] [Google Scholar]
McRorie 2014
- McRorie JW, Kirby JA, Miner PB. Histamine2-receptor antagonists: rapid development of tachyphylaxis with repeat dosing. World Journal of Gastrointestinal Pharmacology and Therapeutics 2014;5(2):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
MHRA 2012
- Medicines Health Regulatory Authority. Proton pump inhibitors in long-term use: reports of hypomagnesaemia. Drug Safety Update 2012;5(9):1. [Google Scholar]
MHRA 2019
- Medicines and Healthcare products Regulatory Agency. Ranitidine – MHRA drug alert issued for Teva UK recall, 2019. Available at: https://www.gov.uk/government/news/ ranitidine-mhra-drug-alert-issued-for-teva-uk-recall.
Miyazawa 2002
- Miyazawa R, Tomomasa T, Kaneko H, Tachibana A, Ogawa T, Morikawa A. Prevalence of gastro-esophageal reflux-related symptoms in Japanese infants. Pediatrics International 2002;44(5):513-6. [DOI] [PubMed] [Google Scholar]
NASPGHAN‐ESPGHAN guidelines 2018
- Rosen R, Vandenplas Y, Singendonk M, Cabana M, DiLorenzo C, Gottrand F, et al. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2018;66(3):516-54. [DOI: 10.1097/MPG.0b013e3181b7f563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 1997
- Nelson SP, Chen EH, Syniar GM, Christoffel K. Prevalence of symptoms of gastro-oesophageal reflux during infancy: a paediatric practice-based survey. Archives of Pediatrics and Adolescent Medicine 1997;151:569-72. [DOI] [PubMed] [Google Scholar]
NICE 2019
- National Institute for Health and Care Excellence. Gastro-oesophageal reflux disease in children and young people: diagnosis and management (NG1); October 2019. www.nice.org.uk/guidance/ng1. [PubMed]
Orenstein 2010
- Orenstein SR. Symptoms and reflux in infants: Infant Gastroesophageal Reflux Questionnaire Revised (I-GERQ-R): utility for symptom tracking and diagnosis. Curr Gastroenterol Rep. 2010;12(6):431–6. [DOI] [PubMed] [Google Scholar]
Page 2020
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. www.training.cochrane.org/handbook.
RevMan 2019 [Computer program]
- Review Manager (RevMan). Version 5.4. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2019.
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Schoenfeld 2016
- Schoenfeld AJ, Grady D. Adverse effects associated with proton pump inhibitors. JAMA Intern Med. 2016;176(2):172-4. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013 Available from guidelinedevelopment.org/handbook. Available from guidelinedevelopment.org/handbook.
Shafrir 1985
- Shafrir Y, Levy Y, Ben-Amitai D, Nitzan M, Steinherz R. Oculogyric crisis due to domperidone therapy. Helvetica Paediatrica Acta 1985;40(1):95. [PubMed] [Google Scholar]
Sherman 2009
- Sherman PM, Hassall E, Fagundes-Neto U, Gold BD, Kato S, Koletzko S, et al. A global, evidence-based consensus on the definition of gastroesophageal reflux disease in the pediatric population. American Journal of Gastroenterology 2009;104(5):1278-95. [DOI] [PubMed] [Google Scholar]
Tighe 2009
- Tighe MP, Afzal NA, Bevan A, Beattie RM. Current pharmacological management of gastro-esophageal reflux in children. Pediatric Drugs 2009;11(3):185-202. [DOI] [PubMed] [Google Scholar]
Vandenplas 1999
- Vandenplas Y. Diagnosis and treatment of gastroesophageal reflux disease in infants and children. World J Gastroenterol 1999 Oct;5(5):375-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2013
- Ward RM, Kearns GL. Proton pump inhibitors in pediatrics: mechanism of action, pharmacokinetics, pharmacogenetics, and pharmacodynamics. Paediatr Drugs 2013 Apr;15(2):119-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
World Bank 2022
- World Bank. Country and Lending Groups classification of countries by income. datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html Accessed prior to 20 May 2023.
References to other published versions of this review
Tighe 2014
- Tighe M, Afzal NA, Bevan A, Hayen A, Munro A, Beattie RM. Pharmacological treatment of children with gastro-oesophageal reflux. Cochrane Database of Systematic Reviews 2014, Issue 11. Art. No: CD008550. [DOI: 10.1002/14651858.CD008550.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


