Abstract
The human immunodeficiency virus type 1 (HIV-1) Pr55gag gene product directs the assembly of virions at the inner surface of the cell plasma membrane. The specificity of plasma membrane binding by Pr55gag is conferred by a combination of an N-terminal myristoyl moiety and a basic residue-rich domain. Although the myristate plus basic domain is also present in the p17MA proteolytic product formed upon Pr55gag maturation, the ability of p17MA to bind to membranes is significantly reduced. It was previously reported that the reduced membrane binding of p17MA was due to sequestration of the myristate moiety by a myristoyl switch (W. Zhou and M. D. Resh, J. Virol. 70:8540–8548, 1996). Here we demonstrate directly that treatment of membrane-bound Pr55gag in situ with HIV-1 protease generates p17MA, which is then released from the membrane. Pr55gag was synthesized in reticulocyte lysates, bound to membranes, and incubated with purified HIV-1 protease. The p17MA product in the membrane-bound and soluble fractions was analyzed following proteolysis. Newly generated p17MA initially was membrane bound but then displayed a slow, time-dependent dissociation resulting in 65% solubilization. Residual p17MA could be extracted from the membranes with either high pH or high salt. Treatment of membranes from transfected COS-1 cells with protease revealed that Pr55gag was present within sealed membrane vesicles and that the release of p17MA occurred only when detergent and salt were added. We present a model proposing that the HIV-1 protease is the “trigger” for a myristoyl switch mechanism that modulates the membrane associations of Pr55gag and p17MA in virions and membranes.
The assembly of lentiviruses and type C retroviruses occurs at the plasma membrane and is directed by the viral gag gene product (34). In human immunodeficiency virus type 1 (HIV-1), Pr55gag contains an N-terminal myristoyl group and a cluster of positively charged residues that act synergistically to promote plasma membrane targeting (37, 38). A combination of a myristate and a basic residue cluster also functions in the Src tyrosine kinase and in the MARCKS protein as a plasma membrane binding motif (5, 29). In addition to plasma membrane binding, the functional properties of Pr55gag responsible for the production of an infectious virion include recruitment of the HIV-1 genome and specific viral proteins into budding virions and processing by HIV-1 protease to generate the structural proteins of the mature virus (8, 17, 18, 21, 22, 36).
The process of viral maturation occurs during or immediately following retroviral assembly (19) and involves proteolysis of the Gag protein by the virus-encoded protease. In HIV-1, processing of Pr55gag by protease generates the following viral structural proteins (from N to C terminus): matrix (p17MA), capsid (p24CA), nucleocapsid (p7NC), and p6 (14). Two small peptides, p2, located between p24CA and p7NC, and p1, located between p7NC and p6, have also been described (1). Additionally, maturation involves processing of Pr160gag-pol, a polypeptide synthesized as a result of ribosomal frameshifting and produced at about 5% the level of Pr55gag. Maturation of Gag-Pol generates the retroviral enzymes protease, reverse transcriptase, and integrase.
The localization of each of the products of Pr55gag within the virion has been determined (13). The matrix protein p17MA forms a shell immediately underneath the viral membrane envelope. The capsid protein polymerizes to give rise to the conical structure inside mature viral particles. The nucleocapsid is found associated with the viral genome within the mature viral core, which carries two copies of RNA per viral particle. The function of the p2 spacer peptide is not well understood, but it appears to be essential for the correct morphology of the viral core (1, 26). p6 appears to be essential for the release of the viral particle from the infected cell (21).
The matrix protein p17MA also plays a role during the early stages of the HIV-1 viral life cycle. After viral infection and fusion with the cell plasma membrane, a portion of p17MA dissociates from the membrane. A subpopulation is phosphorylated and transported into the nucleus as a component of the preintegration complex (32). Thus, the matrix region of Gag displays different subcellular localizations, depending on the stage of viral infection.
In a previous report from our laboratory, the molecular basis for the differential membrane binding of Pr55gag and p17MA was examined (39). The intrinsic affinity of p17MA for membranes was shown to be three- to fivefold lower than that of Pr55gag, despite the fact that both proteins contain the same combination of N-terminal myristate and basic residues. Sequential removal of alpha-helical regions of p17MA restored plasma membrane binding (39). It was proposed that the myristate moiety was exposed within the context of Pr55gag but became sequestered within p17MA. This proposal is consistent with a myristoyl switch mechanism in which a conformational change regulates exposure of the myristate moiety and the membrane binding affinity of p17MA during the viral life cycle. A similar conclusion was reached by other investigators (30).
The myristoyl group within p17MA can be artificially reexposed by internal or C-terminal deletions within p17MA (30, 39). However, we propose that the physiological “trigger” for the myristoyl switch is cleavage of Pr55gag by HIV-1 protease. Here, we test this hypothesis by studying the membrane binding properties of newly generated p17MA derived directly from Pr55gag by proteolysis with HIV-1 protease. We show that p17MA generated in situ from membrane-bound Pr55gag initially is membrane bound but is slowly released from the membrane into the soluble fraction. Additional experiments with Gag incorporated into sealed right-side-out membrane vesicles provide insight into the nature of the membrane association of p17MA inside the viral particle. These studies effectively reproduce critical steps of viral maturation in the test tube and serve as a model for understanding the regulation of differential membrane binding of HIV-1 Gag.
MATERIALS AND METHODS
Plasmids and reagents.
Plasmid pGEM7Z-Pr55, which contains the HXB2 HIV-1 gag open reading frame, was a kind gift from L. Parent and J. W. Wills (Pennsylvania State University Medical School, Hershey) and was used for reticulocyte lysate synthesis. Plasmid pHXB2gtpΔBal-D25S, with an internal deletion in the HIV-1 Pol open reading frame and an inactivating point mutation in the protease domain, has been described elsewhere (38). Purified HIV-1 HXB2 protease and anti-p24CA polyclonal antibody were obtained from the NIH AIDS Research and Reference Reagent Program. Anti-p17MA monoclonal antibody was purchased from Applied Biotechnologies, Inc.
Reticulocyte lysates.
Pr55gag was synthesized in vitro by use of a TNT-SP6-coupled rabbit reticulocyte lysate system (Promega) programmed with pGEM7Z-Pr55 in the presence of 35S-cysteine (50 μCi; ICN). Reaction mixtures of 50 μl were adjusted to 0.05% Triton X-100 for 20 min at room temperature as described previously (38), and insoluble material was removed by ultracentrifugation at 100,000 × g. The supernatant was diluted fourfold with NTE buffer (100 mM NaCl, 10 mM Tris [pH 7.4], 1 mM EDTA, 1 mM dithiothreitol [DTT] and incubated with COS-1 cell plasma membranes (25 μg/ml) for 2 h at 4°C. Membrane-bound Pr55gag was isolated by ultracentrifugation at 100,000 × g and used for in vitro protease assays. For experiments involving myristoylation, reticulocyte lysates were programmed with pGEM7Z-Pr55 or pGEM3Z-p17 in the presence of 3H-myristic acid (80 μCi; New England Nuclear Corp.), and the protein was treated as described above. The products were analyzed by sodium dodecyl sulfate (SDS) gel electrophoresis, and the gels were soaked in 1 M sodium salicylate prior to fluorography.
Membrane preparations.
COS-1 cells were maintained in Dulbecco’s modified minimal essential medium (Gibco) supplemented with 10% fetal bovine serum (Gemini). Cells were seeded in 10-cm dishes, and confluent cells were harvested as described previously (27) to obtain a crude plasma membrane fraction. Briefly, the cell medium was aspirated, and the cells were washed twice with cold saline. The cells were scraped off the dishes and swollen in 0.8 ml of hypotonic buffer, containing 10 mM HEPES (pH 7.5), 5 mM KCl, 1.5 mM MgCl2, and 1 mM DTT. The cells were homogenized, and the nuclear and mitochondrial fractions were removed by centrifugation at 10,000 × g for 10 min at 4°C. The S-10 supernatant was fractionated by ultracentrifugation at 100,000 × g, and the P-100 pellet was recovered, resuspended in NTE buffer to 0.2 mg of protein/ml, and stored at −80°C until further use.
Transfections and metabolic labeling.
Transfections with plasmid pHXB2 were performed by the DEAE-dextran method (2). At 48 h after transfection, cells in 10-cm dishes were starved for 1 h in Dulbecco’s modified minimal essential medium without methionine or cysteine but supplemented with 2% dialyzed fetal bovine serum (Gibco) and then were labeled for 2 to 4 h in the same medium containing 50 μCi of 35S-methionine and 35S-cysteine (Tran35S-Label; Amersham) per ml. The medium was aspirated, and the cells were washed three times with cold saline and homogenized in hypotonic buffer as described above. Membrane-bound Pr55gag was recovered by ultracentrifugation as described above.
In vitro HIV-1 protease assays.
Membrane preparations containing Pr55gag were centrifuged at 100,000 × g for 45 min at 4°C, and the membranes were resuspended in protease assay buffer, containing 50 mM sodium acetate (pH 5.5 at 4°C), 0.1 mM EDTA, 10% glycerol, 5% ethylene glycol, and 1 mM DTT (20). Typically, 50 μl of reticulocyte lysate (original volume) was resuspended in 25 μl of protease assay buffer. Pr55gag was then incubated with 4 μM HIV-1 protease for various times. The reaction was stopped with protease inhibitor cocktail, containing 1.5 μg each of leupeptin, pepstatin A, and aprotinin (Boehringer) per ml, 20 μg each of AEBSF, Nα-β-tosyl-l-lysine chloromethyl ketone, tolylsulfonyl phenylalanyl chloromethyl ketone, and benzamidine (Calbiochem) per ml, and 10 mM DTT. The proteolytic products were fractionated by ultracentrifugation at 100,000 × g for 45 min into soluble and membrane-bound fractions. For rabbit reticulocyte lysate-synthesized Pr55gag, the proteolytic products were directly resolved by SDS gel electrophoresis and autoradiography. For Pr55gag expressed in transfected COS-1 cells, the individual fractions were resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and Pr55gag, p24CA, and p17MA were detected by Western blotting with anti-p24CA and anti-p17MA antibodies. Quantitation of the autoradiographs was performed with a Storm PhosphorImager analyzer (Molecular Dynamics).
RESULTS
Proteolysis of membrane-bound Pr55gag in situ.
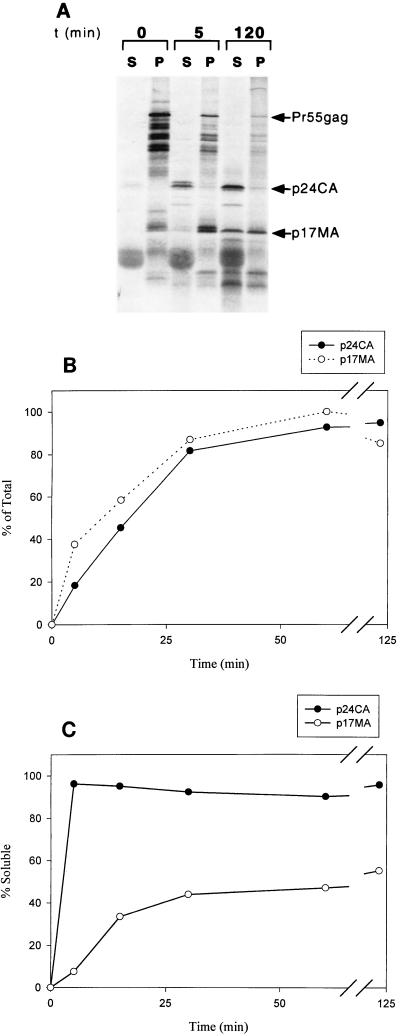
We first analyzed the membrane binding behavior of p17MA generated in situ from the Pr55gag precursor. Pr55gag protein was synthesized in rabbit reticulocyte lysates and bound to COS-1 plasma membranes as described in Materials and Methods. Membrane-bound Pr55gag was resuspended in protease assay buffer and incubated with HIV-1 protease for various times. The proteolytic products were separated into soluble and membrane-bound fractions and analyzed by SDS gel electrophoresis and autoradiography. The results are illustrated in Fig. 1. Proteolysis occurred rapidly and was visualized by the loss of Pr55gag and the appearance of the p17MA and p24CA cleavage products (Fig. 1A and B). The formation of the processed Gag products was apparent within 2 min after the addition of the protease and appeared to be complete by 1 h (Fig. 1B). Both p17MA and p24CA showed the same time-dependent kinetics of accumulation, consistent with the fact that they arose by proteolytic cleavage of a common precursor, p41 (Fig. 1B).
FIG. 1.
Proteolysis of reticulocyte lysate-synthesized Pr55gag with HIV-1 protease. (A) Pr55gag was synthesized in reticulocyte lysates as described in Materials and Methods, bound to COS-1 plasma membranes, and incubated with purified HIV-1 protease for 0, 5, or 120 min. The proteolytic products were fractionated by ultracentrifugation into soluble (S) and membrane bound (P), analyzed by SDS gel electrophoresis, and visualized with a PhosphorImager. Note the presence of p17MA in the membrane fraction and that of the p24-p2 intermediate in the soluble fraction at 5 min. After 2 h, 65% of p17MA has shifted to the soluble fraction. t, time. (B) Generation of p17MA and p24CA after treatment of Pr55gag with HIV-1 protease as a function of time. The time dependence of p24CA and p17MA generation follows the same kinetics, since they arise from a common first cleavage intermediate of Pr55gag, p39-p41. (C) Time course of p17MA and p24CA release from the membrane. The membrane dissociation of p17MA is slow in comparison to the release of p24CA.
During the time course of the assay, Pr55gag remained entirely membrane bound, while p24CA and a transient p24-p2 intermediate appeared rapidly and exclusively in the soluble fraction (Fig. 1A and C). In contrast, the membrane binding properties of p17MA varied with time. At early times, the protein was predominantly membrane bound, like Pr55gag (Fig. 1A and C). After an initial lag time, the distribution of p17MA shifted toward the soluble fraction. The time course of the release of p17MA from the membrane was sigmoidal, with approximately 65% of the protein being released from the membrane by 2 h. This steady-state distribution of p17MA was similar to that found for p17MA expressed exogenously in vitro and in vivo (30, 39).
Myristoylation of Pr55gag and p17MA.
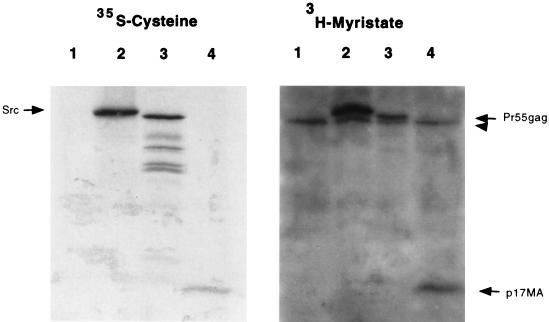
Control experiments were performed to ensure that an alteration of myristoylation levels was not responsible for the release of p17MA from the membrane. One possibility was that a subpopulation of Pr55gag was not myristoylated and that soluble p17MA was being generated from a nonmyristoylated Pr55gag precursor. To ensure that this was not the case, we compared the level of myristoylation of Pr55gag and p17MA to that of pp60v-src in reticulocyte lysates. Proteins were synthesized in the presence of 35S-cysteine to monitor total protein levels or 3H-myristate to monitor myristoylation. When adjusted for the number of cysteines in each protein (10 in v-Src, 10 in Pr55gag, and 2 in p17MA), the levels of incorporation of the 3H label on a mole-per-mole basis were similar for all proteins. Based on previous estimates of a stoichiometry of 1 mol of myristate incorporated per mol of Src protein (6) and the similarity between Src and Gag myristoylation levels depicted in Fig. 2, it is likely that Pr55gag is fully myristoylated.
FIG. 2.
Myristoylation of Pr55gag and p17MA. Synthesis of pp60v-src, Pr55gag, and p17MA in reticulocyte lysates was carried out in the presence of 35S-cysteine to normalize for protein levels (left panel) and with 3H-myristic acid to determine relative myristoylation levels (right panel). Lane 1, unprogrammed lysate; lane 2, v-Src; lane 3, Pr55gag; lane 4, p17MA. A nonspecific myristoylated band present in the reticulocyte lysates was evident in all lanes (arrowhead) and migrated just below Src and Pr55gag.
An alternative explanation for the release of p17MA from the membrane could be a loss of the myristate moiety from the N terminus of Gag during proteolysis by HIV-1 protease. To rule out this possibility, Pr55gag was synthesized in reticulocyte lysates in the presence of 3H-myristic acid and cleaved with protease, and the products were examined by SDS gel electrophoresis and fluorography. Although the bands on the fluorograph were weak, both Pr55gag and p17MA generated from Pr55gag by protease treatment retained the 3H-myristate label (data not shown).
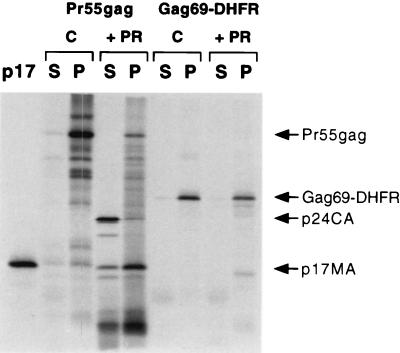
Taken together, the results shown in Fig. 1 and 2, coupled with previous work by Zhou and Resh (39), are consistent with the hypothesis that the cleavage of Pr55gag by HIV protease triggers a myristoyl switch in which the myristoyl moiety is sequestered and is no longer accessible for membrane binding. As a result of this switch, or conformational change, the newly generated p17MA protein dissociates from the lipid bilayer and is released into the soluble fraction. One would therefore expect that p17MA mutants that do not undergo a myristoyl switch would be resistant to the action of protease and should not be released from the membrane. To verify this hypothesis, p17MA mutants lacking the C-terminal alpha helix (Gag69-DHFR and Gag97-DHFR) (39) were synthesized in reticulocyte lysates and bound to membranes. Membrane-bound material was then treated with HIV-1 protease and reisolated by ultracentrifugation. As predicted, both Gag69-DHFR (Fig. 3) and Gag97-DHFR (data not shown) were resistant to the action of protease and were not released from the membrane. This experiment also ruled out any potential indirect effects of HIV-1 protease on membrane structure, which might indirectly have affected the distribution of p17MA.
FIG. 3.
Gag69-DHFR is not released from the membrane by HIV protease. Pr55gag and Gag69-DHFR, a p17MA mutant containing the first 69 amino acids of Gag fused to dihydrofolate reductase (DHFR), were synthesized in reticulocyte lysates, bound to membranes, and treated (+ PR) or not treated (C) with HIV-1 protease for 2 h. Membranes were reisolated, and membrane-bound (P) and soluble (S) fractions were analyzed by SDS-PAGE and autoradiography. Reticulocyte lysate-synthesized 35S-p17MA was used as a molecular weight marker (p17, left lane).
Electrostatic forces contribute to the residual membrane binding of p17MA.
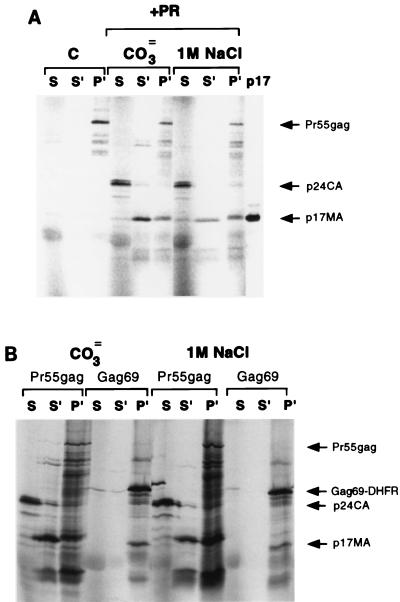
Two forces have been shown to mediate the membrane binding of Pr55gag: hydrophobic insertion of myristate into the lipid bilayer and electrostatic interactions of the basic domain with negatively charged head groups of acidic phospholipids (38). If a myristoyl switch sequesters the myristate moiety, then only electrostatic forces should contribute to the residual membrane binding of p17MA. We therefore subjected p17MA to extraction with either high pH or high salt, conditions that have been used before to extract nonintegral membrane proteins from membranes (3, 31). Membrane-bound p17MA was generated after 10 min of digestion of Pr55gag with HIV-1 protease as described above, and the membrane fraction was subjected to extraction as follows. For high salt, the p17MA-containing pellet was resuspended in protease assay buffer containing 1 M NaCl for 30 min on ice. For high pH, the pellet was resuspended in 0.1 M Na2CO3 (pH 9.0) for 30 min on ice. The extraction mixtures were fractionated by ultracentrifugation into soluble and membrane-bound fractions, and the results were analyzed by SDS gel electrophoresis and autoradiography. As depicted in Fig. 4A, 50 to 70% of the p17MA that was membrane bound after 10 min of protease treatment could be released from the membrane by extraction with either carbonate or high salt. In contrast, Gag69-DHFR, the p17MA mutant in which the myristate moiety is constitutively exposed, was resistant to extraction from the membrane by carbonate or salt (Fig. 4B). Pr55gag remained membrane bound under all conditions, while all of p24CA was released into the initial soluble fraction. These results imply that newly generated p17MA behaves as a peripheral membrane protein which is loosely attached to the membrane bilayer.
FIG. 4.
Extraction of p17MA from membranes. (A) Pr55gag was synthesized in reticulocyte lysates, incubated with COS-1 cell membranes, and treated or not treated (C lanes) with HIV-1 protease for 10 min to generate membrane-bound p17MA. The products were fractionated by ultracentrifugation into soluble (S) and membrane bound. The membrane fraction was subsequently extracted with 0.1 M Na2CO3 (pH 9) (CO3= lanes) or with buffer containing 1 M NaCl (1 M NaCl lanes). The extraction mixtures were then refractionated into soluble (S′) and membrane bound (P′), and the products were resolved by SDS-PAGE and visualized by PhosphorImager analysis. Note that under these conditions, Pr55gag was not completely digested and could be detected in the membrane fraction. A marker for 35S-labeled p17MA is included in the last lane. (B) Membranes containing Pr55gag or Gag69-DHFR were treated with HIV-1 protease and either carbonate or salt as described in panel A. Note that Gag69-DHFR did not undergo proteolysis and was not released from the membrane fraction (P′).
COS-1 cell transfections.
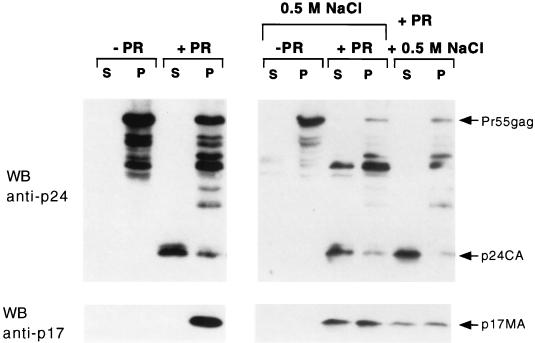
We also studied the properties of p17MA generated from Pr55gag in transfected cells. COS-1 cells were transfected with plasmid pHXB2, and plasma membrane fractions were isolated 48 h later. The membrane fractions were incubated as described above with HIV-1 protease, and the products were isolated by centrifugation. Surprisingly, no cleavage of Pr55gag was observed with either HIV-1 protease or trypsin (data not shown). Since Pr55gag is localized to the cytosolic side of the cell plasma membrane, we reasoned that the isolated membrane preparation contained sealed membranes, with Pr55gag bound to the inner surface of the membrane vesicles. The orientation of Pr55gag inside these sealed vesicles is thus identical to that of Pr55gag in virus particles. Access of externally added protease to Pr55gag should be achieved by gentle permeabilization of the vesicles. As depicted in Fig. 5, the addition of 0.1% Triton X-100 to the membranes resulted in the proteolytic cleavage of Pr55gag and the generation of p24CA and p17MA. All of the residual Pr55gag remained in the membrane fraction, while most of p24CA was released into the soluble fraction. However, little release of p17MA from the membrane was observed under these conditions.
FIG. 5.
Proteolytic cleavage of Pr55gag in transfected COS-1 cell membranes. COS-1 cells were transfected with pHXB2gtpΔBal-D25S, and the plasma membrane-enriched fraction was isolated as described in Materials and Methods. Membranes were incubated (+ PR) or not incubated (− PR) with HIV-1 protease in the presence of 0.1% Triton X-100. The reactions were performed in the absence of salt addition or with 0.5 M NaCl added during or immediately after (last two lanes) proteolysis. The products were fractionated into soluble (S) and membrane bound (P) and resolved by SDS gel electrophoresis. p17MA was visualized by Western blotting (WB) with anti-p17MA monoclonal antibody (bottom panel). The blot was stripped and reprobed with anti-p24CA antibody to detect Pr55gag and p24CA (top panel).
Since p17MA was being generated on the inner surface of a closed membrane vesicle, it was possible that any released protein was rapidly reattaching to the membrane. We therefore included 0.5 M NaCl during the proteolysis and centrifugation steps in order to neutralize electrostatic interactions that could contribute to reattachment of p17MA to the membrane. As depicted in Fig. 5, 50% of the p17MA was now released from the membrane. Pr55gag remained entirely membrane bound, while nearly all of p24CA was soluble. The same results were obtained when 0.5 M salt was added to the reaction mixtures between proteolysis and subsequent fractionation. Thus, the effect of the salt is to enhance the release of already soluble, but trapped, p17MA from the inner surface of sealed plasma membrane vesicles. The release of p17MA from detergent- and salt-treated membrane vesicles is identical to the results obtained in Fig. 1; in that experiment, Pr55gag and HIV protease were added to the external surface of membrane vesicles. We conclude that cleavage of Pr55gag by HIV-1 protease generates a form of p17MA that is loosely attached to the membrane bilayer by electrostatic interactions. In the context of a closed system, namely, a sealed plasma vesicle or a virion particle, p17MA may remain mostly membrane associated because diffusion is limited and the local p17MA concentration is high. Once infection and fusion with a host cell membrane have occurred, p17MA is likely to be released from the membrane into the cytosol.
DISCUSSION
A myristoyl switch regulates the membrane binding of Gag.
Pr55gag plays a crucial role in the late stages of the HIV-1 life cycle by promoting virus assembly and budding at the cell plasma membrane. Plasma membrane binding is conferred by the combination of two signals: myristate and a positively charged basic residue domain in the N-terminal region of Pr55gag. Studies of several N-myristoylated proteins have shown that neither signal alone is sufficient to stably anchor a protein to a lipid bilayer (5, 24). However, when present together in the same polypeptide chain, myristate and the basic domain act in synergy to promote strong membrane binding. Studies from this laboratory have demonstrated that Pr55gag is membrane bound because both the myristate and the basic domain are exposed (38, 39). We propose that a myristoyl switch results in the sequestration of the myristoyl moiety in p17MA, leaving only the basic domain exposed. Nuclear magnetic resonance and X-ray crystallographic studies have confirmed that the basic domain of p17MA is arranged as a β-pleated sheet exposed to the solvent (16, 23). The disposition of the myristate moiety in the three-dimensional structure of p17MA remains unknown, as only nonmyristoylated p17MA has been analyzed. However, cryoelectron microscopy of immature HIV-1 particles has indicated that a conformational change occurs within the matrix region upon Gag cleavage and that the structure of the matrix domain in Pr55gag is different from that of isolated p17MA (10).
Cleavage by protease triggers the myristoyl switch.
In this study, we present evidence to support the hypothesis that the cleavage of Pr55gag by HIV-1 protease is the trigger for the myristoyl switch. In previous studies involving p17MA, exogenously produced protein which has already undergone the myristoyl switch was examined (30, 39). The advantage of generating p17MA from Pr55gag in situ is that it allows a direct comparison of differences in membrane affinity between the precursor and the newly processed product. This comparison provides a unique view of the dynamic interactions of p17MA with the membrane, since it is within this context that the changes in p17MA membrane affinity become apparent as a function of time.
Proteolytic treatment of Pr55gag for various times allowed us to perform kinetic analyses of the membrane binding behaviors of both the precursor and the newly generated cleavage products. During the course of the assays, Pr55gag remained membrane bound at all times, while p24CA was immediately released from the membrane. Thus, p24CA and Pr55gag served as internal controls for soluble and membrane fractions, respectively. An intermediate cleavage product, p39-p41, corresponding to the N-terminal MA-CA-p2 fragment of Pr55gag, was transiently seen in the membrane fraction (data not shown). Initially, p17MA remained with the membrane fraction, consistent with the fact that it is generated from a membrane-bound precursor. In time, however, p17MA underwent a slow dissociation to become 65% soluble. The time course of p17MA membrane dissociation proceeded with an initial lag at early times to give an overall sigmoidal shape to the curve, consistent with a slow event occurring before the membrane dissociation step. It is likely that this slow event corresponds to the conformational changes associated with the myristoyl switch.
Electrostatic forces hold p17MA in the membrane.
After undergoing the myristoyl switch, p17MA could be detached from membranes further by use of conditions that neutralize the electrostatic component of membrane binding. This neutralization was accomplished by increasing the pH or by adding high salt to the buffer. (Fig. 4). The results indicated that the forces which hold p17MA to the bilayer are predominantly electrostatic, in accord with the study of Ehrlich and coworkers (9). In contrast, Pr55gag remained membrane bound at all times during the extractions, indicating that additional factors contribute to its membrane binding. Insertion of myristate into the lipid bilayer aids in maintaining Pr55gag in the membrane and in conferring resistance of Pr55gag to salt extraction (31). In addition, the stronger binding of Pr55gag to membranes likely is mediated by regions of Gag downstream from the membrane binding domain. Other investigators have described regions of Gag outside of the matrix domain which contribute to Gag membrane binding (25, 28). Moreover, the higher-order protein-protein interactions that take place during viral assembly through the capsid and the nucleocapsid domains (15) likely further stabilize the membrane binding of Pr55gag.
p17MA remains membrane bound in a closed vesicular system.
In reticulocyte lysates, Pr55gag is synthesized as a soluble protein and is then bound to exogenously added membranes. In vitro-translated Pr55gag is therefore bound to the external surface of membrane vesicles. However, in cells, newly synthesized Pr55gag is inserted into the inner leaflet of the plasma membrane which, after particle formation, becomes the inner leaflet of the virion. In order to monitor the cleavage of Gag localized to its physiologic site, we examined the membrane binding behavior of Pr55gag derived from cell plasma membranes. Isolation of plasma membrane fractions from transfected COS-1 cells revealed that Pr55gag was resistant to proteolytic cleavage unless low concentrations of detergent were present. This result implies that the membranes were present as sealed vesicles, with all of the Pr55gag located inside the vesicles. A similar result was obtained with Moloney murine leukemia virus Gag (33) and is consistent with the finding that homogenization of the plasma membrane from cultured cells mainly yields “right-side-out” vesicles (7). The orientation of Pr55gag in plasma membrane vesicles thus mimics that which occurs in viral particles.
The persistent binding of p17MA to permeabilized COS-1 cell plasma membranes (Fig. 5A) was in clear contrast to the results obtained with the reticulocyte lysate system (Fig. 1). Longer incubation in the presence of detergent but without salt was insufficient to release p17MA into the soluble fraction (data not shown), indicating that the time of incubation was not limiting for the release of p17MA. Instead, it is likely that protein-protein interactions contribute to maintaining the binding of p17MA to the inner leaflet of vesicles and viral particles. The high level of Pr55gag overexpression in transfected COS-1 cells coupled with the relatively small size of a plasma membrane vesicle (7) mimic the environment of a viral particle. Assuming an average diameter of 140 nm for viruslike particles (13, 34) and the localization of p17MA within a sector underneath the membrane of 70 Å, as seen by cryoelectron microscopy (10), one can calculate the internal accessible volume of a particle to be 10−15 ml. With an estimated 1,200 capsid subunits per particle in electron micrographs (14), the concentration of p17MA in viral particles is approximately 2 mM. A similar calculation, assuming a bilayer width of 30 Å and an average phospholipid surface area of 70 Å2 (35), reveals that the effective phospholipid concentration inside a virion is on the order of 100 mM. The relatively weak electrostatic component contributed by one p17MA monomer could be amplified if p17MA were to oligomerize in the presence of a high phospholipid concentration. Indeed, the crystal structure of p17MA reveals the presence of a p17MA trimer with a large membrane binding surface composed of exposed basic residues (16). Thus, when situated within a small closed system (virion or membrane vesicle), even though p17MA has undergone a myristoyl switch, the protein is unlikely to dissociate from the membrane.
Several studies have now examined the subcellular localization of p17MA expressed in different contexts. When p17MA is expressed alone in transfected cells, approximately 60 to 70% of the protein is cytosolic (30, 39). A similar distribution is observed when the binding of p17MA to artificial phospholipid vesicles is monitored (9, 39). While some studies with HIV-1-infected cells have demonstrated that the majority of p17MA remains membrane associated and that only a small percentage is released into the cytosol (4, 11, 12), others (30) have detected significant levels of p17MA in the cytoplasm of the HIV-1-infected H9/IIIB cell line. The differences in the absolute levels of soluble, cytoplasmic p17MA among these reports may be due to the use of different cell types, different membrane preparation methods, or differences in the levels of Gag protein expression and/or phosphorylation in the various systems. In addition, unique properties of HIV-1-infected cells may contribute to the preferential retention of p17MA in the plasma membrane. However, the common feature of all these studies is the finding that the membrane binding affinity of p17MA is weaker than that of its parent Pr55gag precursor.
A model for reversible membrane binding of HIV-1 Gag.
The results of this study and of others can be incorporated into a model for the membrane interactions of Pr55gag and p17MA. Pr55gag is targeted to and binds to the plasma membrane during the late stage of the HIV-1 life cycle by a combination of a myristate and a basic residue motif. As the local concentration of Pr55gag molecules in the membrane increases, viral assembly is initiated. The dramatic increase in the local concentration of Pr55gag introduces rigidity to the plasma membrane, which bulges outward to form a budding particle. Gag-Gag interactions occurring during viral particle assembly contribute further to stabilizing Pr55gag in the membrane and to facilitating the incorporation of other viral components via interactions with domains of the Pr55gag polypeptide downstream from the matrix domain. When assembly is completed, a nearly spherical virion pinches off the cell membrane. During or after assembly, the viral protease becomes activated and processes Pr55gag to give rise to the structural proteins. At some point during this process, p17MA undergoes a conformational change, thereby internalizing its myristate moiety. p17MA remains attached to the viral membrane envelope by electrostatic interactions, which are reinforced by protein-protein interactions due to high protein concentrations. As the virus infects a new cell and fuses with the cell plasma membrane, the particle opens and p17MA becomes exposed to a relatively large dilution of cytosol, compared to the constraints encountered within the viral particle. The effective p17MA protein and phospholipid concentrations are reduced, and p17MA then dissociates from the membrane into the cytosol.
ACKNOWLEDGMENTS
We thank Raisa Louft-Nisenbaum for expert technical assistance and Lori Klausner for excellent secretarial and graphics assistance.
This work was supported by NIH grant CA 72309. M.D.R. is an established investigator of the American Heart Association.
REFERENCES
- 1.Accola M A, Hoglund S, Gottlinger H G. A putative α-helical structure which overlaps the capsid-p2 boundary in the human immunodeficiency virus type 1 Gag precursor is crucial for viral particle assembly. J Virol. 1998;72:2072–2078. doi: 10.1128/jvi.72.3.2072-2078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. pp. 16.13.1–16.13.7. [Google Scholar]
- 3.Berthiaume L, Resh M D. Biochemical characterization of a palmitoyl acyl transferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinskaya A G, Ghorpade A, Heinzinger N K, Smithgall T E, Lewis R E, Stevenson M. Phosphorylation-dependent human immunodeficiency virus type 1 infection and nuclear targeting of viral DNA. Proc Natl Acad Sci USA. 1996;93:367–371. doi: 10.1073/pnas.93.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buser C, Sigal C T, Resh M D, McLaughlin S. Membrane binding of myristylated peptides corresponding to the N-terminal region of pp60src is mediated by acidic phospholipids. Biochemistry. 1994;33:13093–13101. doi: 10.1021/bi00248a019. [DOI] [PubMed] [Google Scholar]
- 6.Deichaite I, Casson L P, Ling H-P, Resh M D. In vitro synthesis of pp60v-src: myristylation in a cell-free system. Mol Cell Biol. 1988;8:4295–4301. doi: 10.1128/mcb.8.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devaney E, Howell K E. Immuno-isolation of a plasma membrane fraction from the Fao cell. EMBO J. 1985;4:3123–3130. doi: 10.1002/j.1460-2075.1985.tb04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorfman T, Mammano F, Haseltine W A, Gottlinger H G. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J Virol. 1994;68:1689–1696. doi: 10.1128/jvi.68.3.1689-1696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrlich L S, Fong S, Scarlata S, Zybarth G, Carter C. Partitioning of HIV-1 Gag and Gag-related proteins to membranes. Biochemistry. 1996;35:3933–3943. doi: 10.1021/bi952337x. [DOI] [PubMed] [Google Scholar]
- 10.Fuller S D, Wild T, Gowen B E, Krausslich H-G, Vogt V M. Cryo-electron microscopy reveals ordered domains in the immature HIV-1 particle. Curr Biol. 1997;7:729–738. doi: 10.1016/s0960-9822(06)00331-9. [DOI] [PubMed] [Google Scholar]
- 11.Gallay P, Swingler S, Aiken C, Trono D. HIV-1 infection of nondividing cells: C-terminal tyrosine phosphorylation of the viral matrix protein is a key regulator. Cell. 1995;80:379–388. doi: 10.1016/0092-8674(95)90488-3. [DOI] [PubMed] [Google Scholar]
- 12.Gallay P, Swingler S, Song J, Bushman F, Trono D. HIV nuclear import is governed by the phosphotyrosine-mediated binding of matrix to the core domain of integrase. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 13.Gelderblom H R. Assembly and morphology of HIV: potential effect of structure on viral function. AIDS. 1991;5:617–638. [PubMed] [Google Scholar]
- 14.Gelderblom H R. HIV sequence compendium of the Los Alamos National Laboratory. Los Alamos, N.Mex: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1997. Fine structure of HIV and SIV. [Google Scholar]
- 15.Gross I, Hohenberg H, Kuckhagel C, Krausslich H-G. N-terminal extension of human immunodeficiency virus capsid protein converts the in vitro assembly phenotype from tubular to spherical particles. J Virol. 1998;72:4798–4810. doi: 10.1128/jvi.72.6.4798-4810.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill C P, Worthylake D, Bancroft D P, Christensen A M, Sundquist W I. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc Natl Acad Sci USA. 1996;93:3099–3104. doi: 10.1073/pnas.93.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang M, Martin M A. Incorporation of Pr160gag-pol into virus particles requires the presence of both the major homology region and adjacent C-terminal capsid sequences within the Gag-Pol polyprotein. J Virol. 1997;71:4472–4478. doi: 10.1128/jvi.71.6.4472-4478.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang M, Orenstein J M, Martin M A, Freed E O. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J Virol. 1995;69:6810–6818. doi: 10.1128/jvi.69.11.6810-6818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan A J, Manchester M, Swanstrom R. The activity of the protease of human immunodeficiency virus type 1 is initiated at the membrane of infected cells before the release of viral proteins and is required for release to occur with maximum efficiency. J Virol. 1994;68:6782–6786. doi: 10.1128/jvi.68.10.6782-6786.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karlstrom A R, Levine R L. Copper inhibits the protease from human immunodeficiency virus 1 by both cysteine-dependent and cysteine-independent mechanisms. Proc Natl Acad Sci USA. 1991;88:5552–5556. doi: 10.1073/pnas.88.13.5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondo E, Mammano F, Cohen E A, Gottlinger H G. The p6gag domain of human immunodeficiency virus type 1 is sufficient for the incorporation of Vpr into heterologous viral particles. J Virol. 1995;69:2759–2764. doi: 10.1128/jvi.69.5.2759-2764.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee Y-M, Tang X-B, Cimakasky L M, Hildreth J E K, Yu X-F. Mutations in the matrix protein of human immunodeficiency virus type 1 inhibit surface expression and virion incorporation of viral envelope glycoproteins in CD4+ T lymphocytes. J Virol. 1997;71:1443–1452. doi: 10.1128/jvi.71.2.1443-1452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massiah M A, Starich M R, Paschall C, Summers M F, Christensen A M, Sundquist W I. Three dimensional structure of the human immunodeficiency virus type 1 matrix protein. J Mol Biol. 1994;244:198–223. doi: 10.1006/jmbi.1994.1719. [DOI] [PubMed] [Google Scholar]
- 24.Peitzsch R M, McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- 25.Platt E J, Haffar O K. Characterization of human immunodeficiency virus type 1 Pr55gag membrane association in a cell-free system: requirement for a C-terminal domain. Proc Natl Acad Sci USA. 1994;91:4594–4598. doi: 10.1073/pnas.91.10.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poon D T K, Li G, Aldovini A. Nucleocapsid and matrix protein contributions to selective human immunodeficiency virus type 1 genomic RNA packaging. J Virol. 1998;72:1983–1993. doi: 10.1128/jvi.72.3.1983-1993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resh M D, Erikson R L. Highly specific antibody to Rous sarcoma virus src gene product recognizes a novel population of pp60v-src and pp60c-src molecules. J Cell Biol. 1985;100:409–417. doi: 10.1083/jcb.100.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandefur S, Varthakavi V, Spearman P. The I domain is required for efficient plasma membrane binding of human immunodeficiency virus type 1 Pr55gag. J Virol. 1998;72:2723–2732. doi: 10.1128/jvi.72.4.2723-2732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigal C T, Zhou W, Buser C, McLaughlin S, Resh M D. The N-terminal basic residues of Src mediate membrane binding through electrostatic interaction with acidic phospholipids. Proc Natl Acad Sci USA. 1994;91:12253–12257. doi: 10.1073/pnas.91.25.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spearman P, Horton R, Ratner L, Kuli-Zade I. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J Virol. 1997;71:6582–6592. doi: 10.1128/jvi.71.9.6582-6592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spearman P, Wang J-J, Vander Heyden N, Ratner L. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J Virol. 1994;68:3232–3242. doi: 10.1128/jvi.68.5.3232-3242.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevenson M. Portals of entry: uncovering HIV nuclear transport pathways. Trends Cell Biol. 1996;6:9–15. doi: 10.1016/0962-8924(96)81032-4. [DOI] [PubMed] [Google Scholar]
- 33.Suomalainen M, Hultenby K, Garoff H. Targeting of Moloney murine leukemia virus Gag precursor to the site of virus budding. J Cell Biol. 1996;135:1841–1852. doi: 10.1083/jcb.135.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wills J W, Craven R C. Form, function, and use of retroviral Gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Yeagle P. Membrane models and model membranes. The membranes of cells. Orlando, Fla: Academic Press, Inc.; 1987. [Google Scholar]
- 36.Yu X, Yu Q-C, Lee T-H, Essex M. The C terminus of human immunodeficiency virus type 1 matrix protein is involved in early steps of the virus life cycle. J Virol. 1992;66:5667–5670. doi: 10.1128/jvi.66.9.5667-5670.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuan X, Yu X, Lee T-H, Essex M. Mutations in the N-terminal region of human immunodeficiency virus type 1 matrix protein block intracellular transport of the Gag precursor. J Virol. 1993;67:6387–6394. doi: 10.1128/jvi.67.11.6387-6394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou W, Parent L J, Wills J W, Resh M D. Identification of a membrane binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W, Resh M D. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J Virol. 1996;70:8540–8548. doi: 10.1128/jvi.70.12.8540-8548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]