Abstract
Background
Myeloproliferative neoplasms (MPNs) are hematopoietic stem cell neoplasms with a high risk of thrombosis, including acute myocardial infarction (AMI). However, outcomes after AMI have not been thoroughly characterized.
Objectives
The purpose of this study was to characterize outcomes after AMI in patients with MPNs compared with patients without MPNs.
Methods
Patients with a primary admission of AMI from January 2006 to December 2018 were identified using the National Inpatient Sample. Outcomes of interest included in-hospital death or cardiac arrest (CA) and major bleeding. Propensity score weighting was used to compare outcomes between MPN and non-MPN groups.
Results
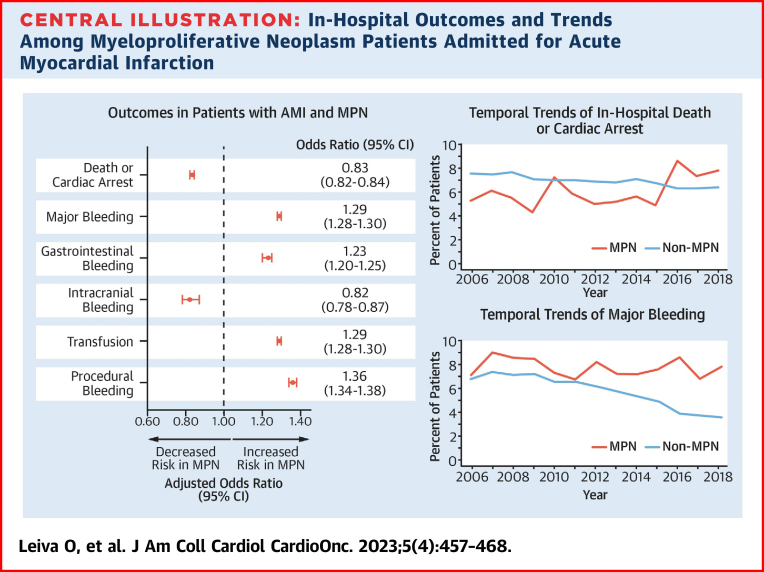
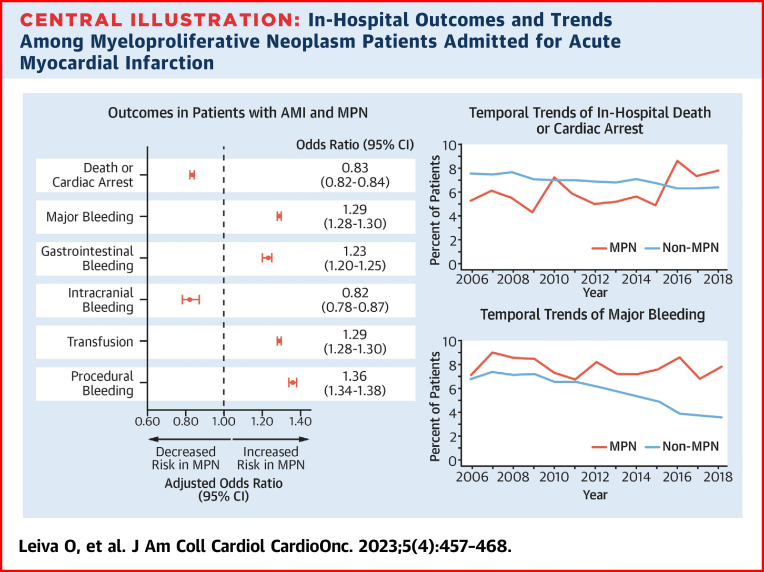
A total of 1,644,304 unweighted admissions for AMI were included; of these admissions, 5,374 (0.3%) were patients with MPNs. After propensity score weighting, patients with MPNs had a lower risk of in-hospital death or CA (OR: 0.83; 95% CI: 0.82-0.84) but a higher risk of major bleeding (OR: 1.29; 95% CI: 1.28-1.30) compared with non-MPN patients. There was a decreasing temporal rate of in-hospital death or CA and bleeding in patients without MPNs (Ptrend < 0.001 for both). However, there was an increasing temporal rate of in-hospital death or CA (Ptrend < 0.001) and a stable rate of major bleeding (Ptrend = 0.48) in patients with MPNs.
Conclusions
Among patients hospitalized with AMI, patients with MPNs have a lower risk of in-hospital death or CA compared with patients without MPNs, although they have a higher risk of bleeding. More investigation is needed in order to improve post-AMI bleeding outcomes in patients with MPN.
Key Words: acute myocardial infarction, bleeding complications, myeloproliferative neoplasms, outcomes, percutaneous coronary intervention
Central Illustration
Myeloproliferative neoplasms (MPNs) are hematopoietic stem cell neoplasms that include polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF) and are associated with an increased risk of cardiovascular disease especially thrombosis.1 Arterial thrombosis, including acute myocardial infarction (AMI), is common, with a pooled prevalence of 16.2% in patients with MPNs.2 Additionally, experimental mouse models have suggested that sequence variations in the JAK2 gene, the most common driver mutation in MPNs, is associated with accelerated atherosclerosis and increased plaque sizes.3
AMI, including ST-segment elevation myocardial infarction (STEMI) and non–ST-segment elevation acute coronary syndrome, is responsible for over 1 million hospitalizations in the United States and has an in-hospital mortality of approximately 5%.4, 5, 6 Patients with neoplastic and malignant disorders, including MPNs, remain at risk of AMI.7 Prior studies have suggested that patients with malignancy have increased all-cause mortality and major bleeding compared with patients without malignancy.8,9 Additionally, patients with malignancy admitted for AMI are less likely to undergo percutaneous coronary intervention (PCI) compared with patients without malignancy despite benefits with revascularization.10 However, unlike most patients with other malignancies, patients with MPNs tend to have an indolent course with a median life expectancy ranging from months to 20 years depending on risk factors and phenotype.11 Despite their rarity, an estimated 20,000 patients are diagnosed with MPNs each year, and there are more than 200,000 patients with MPNs in the United States.12 Additionally, in-hospital characteristics and outcomes of patients with MPNs and AMI hospitalization have not been thoroughly investigated; thus, an unmet need remains. Therefore, we investigated the impact of MPNs on in-hospital outcomes of patients admitted for AMI.
Methods
Study design and population
Hospitalizations for AMI were identified using the National Inpatient Sample (NIS). The NIS is part of the Healthcare Cost and Utilization Project and is the largest inpatient database in the United States, capturing approximately 20% of hospitalizations nationwide. Data in the NIS are derived from billing data submitted by hospitals to statewide data organizations and contain demographic and clinical characteristics. The NIS reports data using the International Classifications of Diseases-9th Revision (ICD-9) until September 2015 and International Classification of Diseases-10th Revision (ICD-10) afterward. This study was deemed exempt by the New York University Grossman School of Medicine Institutional Review Board given that the data are publicly available and deidentified.
All hospitalizations with a primary diagnosis of AMI between January 1, 2006, and December 31, 2018, were included. Patients with ET (ICD-9 238.71, ICD-10 D47.3), PV (ICD-9 238.4 and 207.10-12, ICD-10 D45), and PMF (ICD-9 238.76 and 289.83, ICD-10 D47.1, D75.81, and D47.4) were identified using ICD-9 and ICD-10 codes.13,14 Procedures, including left heart catheterization, PCI, mechanical circulatory support (MCS), and coronary artery bypass grafting (CABG), were captured using ICD-9 and ICD-10 procedure codes. Comorbidities were captured via ICD-9 and ICD-10 codes and Elixhauser comorbidities.15 The ICD-9 and ICD-10 codes used for this study are listed in Supplemental Table 1.
Outcomes
In-hospital outcomes were evaluated for patients with MPNs compared with patients without MPNs. Our primary outcome of interest was in-hospital death or cardiac arrest (CA). Our secondary outcome of interest was major bleeding defined as a need for transfusion of blood products, gastrointestinal bleeding, intracranial bleeding, and procedure-related bleeding. Additional secondary outcomes were individual components of major bleeding. Outcomes were abstracted using ICD-9 and ICD-10 codes (Supplemental Table 1).
Statistical analysis
Patients with and without MPNs were compared, and the standardized mean difference (SMD) was calculated for variables before and after propensity score weighting. Continuous variables were presented using the mean and SD, and categoric variables were presented as counts and percentages. Imbalances between groups were considered to be insignificant if the SMD for a given covariable was <0.10. A propensity score (the predicted probability of MPN status) was calculated using a nonparsimonious multivariable logistic regression. We included age, sex, race, smoking history, coronary artery disease (CAD), prior myocardial infarction, prior PCI, prior CABG, heart failure, anemia, chronic lung disease, atrial fibrillation, hypertension, liver disease, peripheral vascular disease, chronic kidney disease, Charlson comorbidity index, STEMI, chronic total occlusion, left heart catheterization, PCI, CABG, MCS use, and cardiogenic shock as covariables. We used the propensity score to perform propensity score weighted analysis.16 Weights were calculated using the propensity score with 1/propensity score being assigned to patients with MPNs and 1/(1 − propensity score) for patients without MPN. The MPN and non-MPN patients were compared using univariable or multivariable logistic regression analysis with results presented as ORs or adjusted ORs (aORs) with 95% CIs. Temporal trends in PCI use, in-hospital death or CA, and major bleeding were examined using the Mann-Kendall trend test.
To identify risk factors for the composite of in-hospital death, CA, or major bleeding in patients with MPNs who were hospitalized with AMI, we compared the characteristics of patients with MPNs who had experienced in-hospital death, CA, or major bleeding with those who did not. We excluded patients with multiple MPN types given the limitations of being able to verify the MPN phenotype per World Health Organization criteria. Characteristics that were significantly different between groups (P < 0.10) were included in a multivariable logistic regression.
We also conducted an analysis of in-hospital outcomes by race (White, Black, Hispanic, and Asian race) among patients with MPNs in order to identify any racial differences in outcomes. We focused on in-hospital death, CA, or major bleeding as the primary outcome in this analysis and in-hospital death and major bleeding as secondary outcomes. We used a multivariable logistic regression to estimate the risk of outcomes in different races compared with White race using age, sex, smoking history, CAD, prior myocardial infarction, prior PCI, prior CABG, anemia, peripheral vascular disease, liver disease, diabetes, chronic lung disease, chronic kidney disease, Charlson comorbidity index, MPN type, STEMI, cardiogenic shock, invasive management, expected primary payer type, and MCS use as covariates.
Analyses were conducted using SPSS version 27.0 (IBM) and Stata version 15 (STATA corporation). A 2-tailed P value < 0.05 was considered significant.
Results
Patient characteristics
A total of 1,644,304 unweighted admissions for AMI were included with a mean age of 67.2 ± 14.1 years, and 639,716 (38.9%) were female. Of the patients included, 5,374 (0.3%) patients had MPNs. Among the patients with MPNs, 2,622 (48.8%), 2,569 (47.8%), and 312 (5.8%) had PV, ET, and PMF, respectively. There were 84 patients with multiple MPN diagnoses (2 or more); therefore, PV, ET, and PMF counts are not mutually exclusive. There was no difference in age (mean 67.6 vs 67.2 years, SMD = 0.025), female sex (39.9% vs 38.9%, SMD = 0.02), prior CAD (76.3% vs 79.2%, SMD = 0.069), prior PCI (10.4% vs 12.3%, SMD = 0.059), or Charlson comorbidity index (mean 3.9 vs 3.9, SMD < 0.001) between MPN and non-MPN patients. Variables were adequately balanced between MPN and non-MPN patients after propensity score weighting. Patient characteristics before and after propensity score weighting are summarized in Table 1.
Table 1.
Unweighted and Propensity Score Weighted Baseline Characteristics
| Unweighted |
Propensity Score Weighted |
||||||
|---|---|---|---|---|---|---|---|
| All (N = 1,644,304) | MPN (n = 5,374) | Non-MPN (n = 1,638,930) | SMD | MPN | Non-MPN | SMD | |
| Age | 67.2 ± 14.1 | 67.6 ± 14.7 | 67.2 ± 14.1 | 0.03 | 67.4 ± 14.6 | 67.2 ± 14.1 | 0.013 |
| Female | 639,716 (38.9) | 637,573 (38.9) | 2,143 (39.9) | 0.02 | 38.2 | 38.9 | 0.014 |
| Race | |||||||
| White | 1,098,021 (66.8) | 3818 (71.0) | 1,094,203 (66.8) | 0.11 | 67.3 | 66.8 | 0.003 |
| Black | 152,408 (9.3) | 472 (8.8) | 151,936 (9.3) | 8.5 | 9.3 | ||
| Hispanic | 113,890 (6.9) | 317 (5.9) | 113,573 (6.9) | 6.6 | 6.9 | ||
| Asian | 35,485 (2.2) | 125 (2.3) | 35,360 (2.2) | 2.8 | 2.2 | ||
| Other/unknown | 244,730 (14.9) | 642 (11.9) | 244,088 (14.9) | 14.7 | 14.9 | ||
| Smoking history | 419,665 (25.5) | 1435 (26.7) | 418,230 (25.5) | 0.03 | 25.6 | 25.5 | 0.002 |
| Comorbidities | |||||||
| CAD | 1,302,345 (79.2) | 4103 (76.3) | 1,298,242 (79.2) | 0.07 | 79.3 | 79.2 | 0.002 |
| Prior MI | 186,205 (11.3) | 610 (11.4) | 185,595 (11.3) | < 0.001 | 11.7 | 11.3 | 0.013 |
| Prior PCI | 201,917 (12.3) | 560 (10.4) | 201,357 (12.3) | 0.06 | 12.5 | 12.3 | 0.006 |
| Prior CABG | 188,946 (11.5) | 438 (8.2) | 188,508 (11.5) | 0.11 | 11.8 | 11.5 | 0.009 |
| Heart failure | 17,810 (1.1) | 60 (1.1) | 17,750 (1.1) | 0.003 | 1.1 | 1.1 | 0 |
| Anemia | 256,948 (15.6) | 1,178 (21.9) | 255,770 (15.6) | 0.16 | 15.6 | 15.6 | 0 |
| Chronic lung disease | 342,741 (20.8) | 1428 (26.6) | 341,313 (20.8) | 0.14 | 21.0 | 20.8 | 0.005 |
| Diabetes | 588,550 (35.8) | 1,517 (28.2) | 587,033 (35.8) | 0.16 | 36.4 | 35.8 | 0.012 |
| Atrial fibrillation | 244,056 (14.8) | 842 (15.7) | 243,214 (14.8) | 0.02 | 15.4 | 14.8 | 0.017 |
| Hypertension | 1,071,478 (65.2) | 3,439 (64.0) | 1,068,039 (65.2) | 0.02 | 65.2 | 65.2 | 0 |
| Liver disease | 25,975 (1.6) | 113 (2.1) | 25,862 (1.6) | 0.04 | 1.7 | 1.6 | 0.008 |
| Peripheral vascular disease | 181,515 (11.0) | 693 (12.9) | 180,822 (11.0) | 0.06 | 10.9 | 11.0 | 0.003 |
| Chronic kidney disease | 312,853 (19.0) | 968 (18.0) | 311,885 (19.0) | 0.03 | 19.8 | 19.0 | 0.02 |
| CCI | 3.9 ± 2.4 | 3.9 ± 2.3 | 3.9 ± 2.4 | < 0.001 | 4.0 ± 2.3 | 3.9 ± 2.4 | 0.022 |
| MPN typea | |||||||
| PV | 2,622 (0.2) | 2,622 (48.8) | 0 | — | 51.5 | 0 | — |
| ET | 2,569 (0.2) | 2,569 (47.8) | 0 | — | 45.5 | 0 | — |
| PMF | 312 (0.02) | 312 (5.8) | 0 | — | 5.5 | 0 | — |
| AMI characteristics and treatment | |||||||
| STEMI | 519,727 (31.6) | 1,652 (30.7) | 518,075 (31.6) | 0.02 | 31.3 | 31.6 | 0.006 |
| Chronic total occlusion | 107,336 (6.5) | 306 (5.7) | 107,030 (6.5) | 0.03 | 6.4 | 6.5 | 0.004 |
| Left heart catheterization | 1,108,306 (67.4) | 3,494 (65.0) | 1,104,812 (67.4) | 0.05 | 67.3 | 67.4 | 0.002 |
| PCI | 710,984 (43.2) | 2,059 (38.3) | 708,925 (43.2) | 0.10 | 42.9 | 43.2 | 0.006 |
| CABG | 144,623 (8.8) | 476 (8.9) | 144,147 (8.8) | 0.002 | 9.3 | 8.8 | 0.017 |
| MCS use | 83,016 (5.1) | 293 (5.5) | 82,723 (5.0) | 0.018 | 5.2 | 5.0 | 0.009 |
| Cardiogenic shock | 64,659 (3.9) | 191 (3.6) | 64,468 (3.9) | 0.02 | 4.2 | 3.9 | 0.015 |
| Length of stay, mean days | 4.7 ± 5.5 | 5.3 ± 7.1 | 4.7 ± 5.5 | 0.10 | 4.8 ± 5.3 | 4.7 ± 5.6 | 0.055 |
Values are mean ± SD, n (%), or % unless otherwise indicated.
AMI = acute myocardial infarction; CABG = coronary artery bypass grafting; CAD = coronary artery disease; CCI = Charlson comorbidity index; ET = essential thrombocythemia; MCS = mechanical circulatory support; MI = myocardial infarction; MPN = myeloproliferative neoplasm; PCI = percutaneous coronary intervention; PMF = primary myelofibrosis; PV = polycythemia vera; SMD = standardized mean difference; STEMI = ST-segment elevation myocardial infarction.
Not mutually exclusive given 84 patients had multiple (2 or more) MPN types recorded.
Invasive management in patients with MPNs and without MPNs
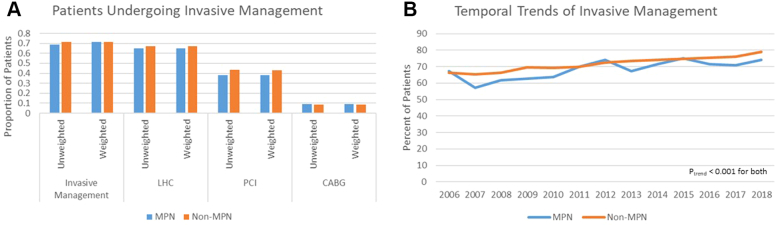
Invasive management (left heart catheterization, PCI, or CABG) was lower, although not significantly so, in patients with MPNs than in those without (68.8% vs 71.6%, SMD = 0.06). After propensity score weighting, the difference between patients with and without MPNs was smaller (71.3% vs 71.6%, SMD = 0.007). Additionally, patients with MPNs were less likely to undergo PCI (38.3% vs 43.2%, SMD = 0.10) but not CABG (8.9% vs 8.8%, SMD = 0.002). The use of MCS (5.5% vs 5.0%, SMD = 0.018) and the prevalence of cardiogenic shock (3.6% vs 3.9%, SMD = 0.02) were similar between patients with and without MPNs.
The proportion of patients who underwent invasive management increased significantly for both patients with MPNs (from 67.3% in 2006 to 74.1% in 2018; Ptrend < 0.001) and without MPNs (from 66.2% in 2006 to 79.0% in 2018; Ptrend < 0.001) (Figure 1).
Figure 1.
Trends in Invasive Management of AMI by MPN Status
(A) Rates of patients undergoing invasive management, left heart catheterization (LHC), percutaneous coronary intervention (PCI), and coronary artery bypass grafting (CABG) by myeloproliferative neoplasm (MPN) status in unweighted and weighted analyses. (B) Temporal trends of invasive management in patients with and without MPN.
Outcomes of patients with MPNs compared with patients without MPNs
In the unweighted cohort, patients with MPNs had a decreased frequency of in-hospital death or CA (6.0% vs 6.9% P = 0.009) compared with patients without MPNs. However, patients with MPNs had increased major bleeding (12.5% vs 9.3%; P < 0.001), gastrointestinal bleeding (1.9% vs 1.5%; P = 0.025), and procedural bleeding (2.1% vs 1.7%; P = 0.010). There was no difference in intracranial bleeding (0.2% vs 0.2%; P = 0.87) (Table 2).
Table 2.
Outcomes of Unweighted and Propensity Score Weighted Patients With AMI by MPN Status
| All (N = 1,644,534) | Unweighted MPN (n = 5,374) |
Non-MPN (n = 1,638,930) | P Value | MPN | Propensity Score Weighted Non-MPN |
P Value | |
|---|---|---|---|---|---|---|---|
| All patients | |||||||
| Death or cardiac arrest | 113,930 (6.9) | 324 (6.0) | 113,606 (6.9) | 0.009 | 5.8 | 6.4 | <0.001 |
| Major bleeding | 152,725 (9.3) | 670 (12.5) | 152,055 (9.3) | <0.001 | 11.4 | 9.3 | <0.001 |
| Secondary outcomes | |||||||
| GI bleeding | 24,978 (1.5) | 102 (1.9) | 24,876 (1.5) | 0.025 | 1.8 | 1.5 | <0.001 |
| Intracranial bleeding | 3,053 (0.2) | 10 (0.2) | 3,043 (0.2) | 0.87 | 0.2 | 0.2 | <0.001 |
| Transfusion | 113,454 (6.9) | 514 (9.6) | 112,940 (6.9) | <0.001 | 8.5 | 6.9 | <0.001 |
| Procedural hemorrhage | 27,536 (1.7) | 115 (2.1) | 27,421 (1.7) | 0.010 | 2.2 | 1.7 | <0.001 |
| Invasive management | N = 1,177,644 | n = 3,698 | n = 1,173,946 | ||||
| Death or cardiac arrest | 61,633 (5.2) | 171 (4.6) | 61,462 (5.2) | 0.096 | 4.5 | 5.2 | <0.001 |
| Major bleeding | 105,063 (8.9) | 443 (12.0) | 104,620 (8.9) | <0.001 | 11.1 | 8.9 | <0.001 |
| GI bleeding | 12,696 (1.1) | 61 (1.6) | 12,635 (1.1) | 0.001 | 1.6 | 1.1 | <0.001 |
| Intracranial bleeding | 1,592 (0.1) | 9 (0.2) | 1,583 (0.1) | 0.11 | 0.2 | 0.1 | <0.001 |
| Transfusion | 74,645 (6.3) | 315 (8.5) | 74,330 (6.3) | <0.001 | 8.5 | 6.9 | <0.001 |
| Procedural bleeding | 26,278 (2.2) | 107 (2.9) | 26,171 (2.2) | 0.009 | 2.9 | 2.2 | <0.001 |
| Noninvasive management | N = 466,890 | n = 1,676 | n = 465,214 | ||||
| Death or cardiac arrest | 52,297 (11.2) | 153 (9.1) | 52,144 (11.2) | 0.007 | 9.1 | 11.2 | <0.001 |
| Major bleeding | 47,662 (10.2) | 227 (13.5) | 47,435 (10.2) | <0.001 | 12.0 | 10.2 | <0.001 |
| GI bleeding | 12,282 (2.6) | 41 (2.4) | 12,241 (2.6) | 0.70 | 2.4 | 2.6 | <0.001 |
| Intracranial Bleeding | 1,461 (0.3) | 1 (0.1) | 1,460 (0.3) | 0.074 | 0.1 | 0.3 | <0.001 |
| Transfusion | 38,809 (8.3) | 199 (11.9) | 38,610 (8.3) | <0.001 | 10.4 | 8.3 | <0.001 |
| Procedural bleeding | 1,258 (0.3) | 8 (0.5) | 1,250 (0.3) | 0.098 | 0.5 | 0.3 | <0.001 |
Values are n (%) or % unless otherwise indicated.
GI = gastrointestinal; MPN = myeloproliferative neoplasm.
After propensity score weighting, patents with MPNs had decreased odds of in-hospital death or CA (OR: 0.83; 95% CI: 0.82-0.84) but increased odds of major bleeding (OR: 1.29; 95% CI: 1.28-1.30), including transfusion (OR: 1.29; 95% CI: 1.28-1.30), procedural bleeding (OR: 1.36; 95% CI: 1.34-1.38), and gastrointestinal bleeding (OR: 1.23; 95% CI: 1.20-1.25), but lower odds of intracranial bleeding (OR: 0.82; 95% CI: 0.78-0.87) (Table 3). Unweighted ORs are shown in Supplemental Table 2.
Table 3.
Propensity Score Weighted OR of Outcomes of MPN Compared With Non-MPN Patients
| All patients | |
| In-hospital death or cardiac arrest | 0.83 (0.82-0.84) |
| Bleeding | 1.29 (1.28-1.30) |
| Gastrointestinal bleeding | 1.23 (1.20-1.25) |
| Intracranial bleeding | 0.82 (0.78-0.87) |
| Transfusion | 1.29 (1.28-1.30) |
| Procedural hemorrhage | 1.36 (1.34-1.38) |
| Invasive management | |
| In-hospital death or cardiac arrest | 0.86 (0.85-0.87) |
| Bleeding | 1.32 (1.31-1.33) |
| Gastrointestinal bleeding | 1.51 (1.48-1.55) |
| Intracranial bleeding | 1.43 (1.34-1.52) |
| Transfusion | 1.29 (1.27-1.30) |
| Procedural hemorrhage | 1.33 (1.30-1.35) |
| Noninvasive management | |
| In-hospital death or cardiac arrest | 0.79 (0.78-0.80) |
| Bleeding | 1.23 (1.22-1.25) |
| Gastrointestinal bleeding | 0.92 (0.90-0.95) |
| Intracranial bleeding | 0.17 (0.15-0.20) |
| Transfusion | 1.31 (1.29-1.33) |
| Procedural hemorrhage | 2.10 (1.96-2.24) |
MPN = myeloproliferative neoplasm.
Values are propensity score weighted OR (95% CI).
Among patients who underwent invasive management, there was no difference in the risk of in-hospital death or CA in patients with MPNs compared with patients without MPNs in unweighted analysis (OR: 0.88; 95% CI: 0.75- 1.02), but there was a decreased risk in propensity score weighted analysis (OR: 0.86; 95% CI: 0.85-0.87). In propensity score weighted analysis, patients with MPNs who underwent invasive management had an increased risk of major bleeding (OR: 1.32; 95% CI: 1.31-1.33), including gastrointestinal bleeding (OR: 1.51; 95% CI: 1.48-1.55), intracranial bleeding (OR: 1.43; 95% CI: 1.34-1.52), transfusion (OR: 1.29; 95% CI: 1.27-1.30), and procedural bleeding (OR: 1.33; 95% CI: 1.30-1.35), compared with patients without MPNs.
Among patients who did not undergo invasive management, patients with MPNs had a lower risk of in-hospital death in propensity score weighted analysis (OR: 0.79; 95% CI: 0.78-0.80). However, patients with MPNs remained at higher risk of major bleeding (OR: 1.23; 95% CI: 1.22-1.25) including transfusion (OR: 1.31; 95% CI: 1.29-1.33) but not gastrointestinal (OR: 0.92; 95% CI: 0.90-0.95), intracranial (OR: 0.17; 95% CI: 0.15-0.20), or procedural bleeding (OR: 2.10; 95% CI: 1.96-2.24) in the propensity score weighted analysis (Table 3).
Trends in in-hospital death or CA and major bleeding in patients with and without MPNs
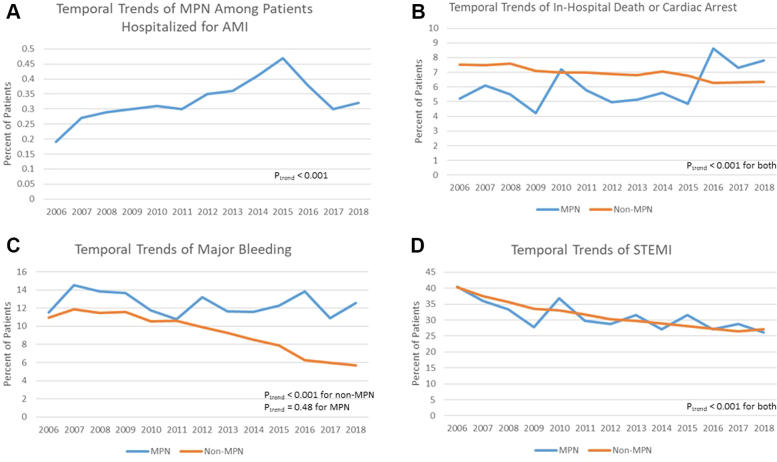
Among patients hospitalized with AMI, the proportion of patients with MPNs increased from 0.19% in 2006 to 0.32% in 2018 (Ptrend < 0.001). Among patients with MPNs, in-hospital death or CA increased significantly from 5.2% in 2006 to 7.8% in 2018 (Ptrend < 0.001). On the contrary, among patients without MPNs, in-hospital death or CA decreased from 7.5% in 2006 to 6.3% in 2018 (Ptrend < 0.001). Major bleeding remained high in patients with MPNs from 11.5% in 2006 to 12.6% in 2018 (Ptrend = 0.48). However, in patients without MPNs, major bleeding significantly decreased from 10.9% in 2006 to 5.7% in 2018 (Ptrend < 0.001). There was a temporal decrease in the rates of STEMI in both MPN (40.5% in 2006 to 26.1% in 2018) and non-MPN patients (40.3% in 2006 to 27.1% in 2018; Ptrend for both < 0.001). Temporal trends of MPN patients admitted for AMI and in-hospital death or CA, major bleeding, and STEMI for MPN and non-MPN patients are shown in Figure 2.
Figure 2.
Temporal Trends in In-Hospital Death, Major Bleeding, and STEMI
Temporal trends of rates of (A) MPN patients admitted for AMI, (B) in-hospital death or cardiac arrest, (C) major bleeding, and (D) ST-segment elevation myocardial infarction (STEMI) presentation in patients with and without MPN. Other abbreviations as in Figure 1.
Risk factors for in-hospital death or CA and bleeding in patients with MPNs
After excluding 84 patients with multiple MPN diagnosis codes, a total of 5,290 patients had 1 diagnosis of MPN, 936 (17.7%) of whom had in-hospital death, CA, or bleeding. Patients with death, CA, or bleeding were more likely to be older (mean age 71.0 ± 13.7 years vs 66.8 ± 14.8 years; P < 0.001); to be female (43.4% vs 39.1%; P = 0.015); and to have lower rates of coronary artery disease including prior myocardial infarction, prior PCI, and prior CABG. They were more likely to have ET (56.5% vs 44.2%) or PMF (13.4% vs 4.3%) and less likely to have PV (30.1% vs 51.5%) compared with patients who did not have death, CA, or bleeding. Patients who presented with STEMI or cardiogenic shock, required MCS, or underwent CABG were more likely to suffer CA, bleeding, or death. They were also less likely to have undergone invasive management (62.2% vs 70.2%; P < 0.001). Patient characteristics between patients with and without in-hospital death, CA, or bleeding are summarized in Table 4.
Table 4.
Risk Factors for Death, Cardiac Arrest, or Bleeding in Patients With MPNs
| Death, Cardiac Arrest, Bleeding (n = 936) | No Death, Cardiac Arrest, Bleeding (n = 4,354) | P Value | |
|---|---|---|---|
| Age, y | 71.0 ± 13.7 | 66.8 ± 14.8 | <0.001 |
| Female | 406 (43.4) | 1,701 (39.1) | 0.015 |
| Race | 0.47 | ||
| White | 645 (68.9) | 3,110 (71.4) | |
| Black | 86 (9.2) | 383 (8.8) | |
| Hispanic | 64 (6.8) | 246 (5.6) | |
| Asian | 21 (2.2) | 103 (2.4) | |
| Other/unknown | 120 (12.8) | 512 (11.8) | |
| Smoking history | 152 (16.2) | 1,267 (29.1) | <0.001 |
| Comorbidities | |||
| CAD | 673 (71.9) | 3,365 (77.3) | 0.001 |
| Prior MI | 78 (8.3) | 521 (12.0) | 0.001 |
| Prior PCI | 68 (7.3) | 486 (11.2) | <0.001 |
| Prior CABG | 62 (6.6) | 366 (8.4) | 0.074 |
| Heart failure | 24 (2.6) | 35 (0.8) | <0.001 |
| Anemia | 343 (36.7) | 820 (18.8) | <0.001 |
| Chronic lung disease | 269 (28.7) | 1,147 (26.3) | 0.14 |
| Diabetes | 292 (31.2) | 1,210 (27.8) | 0.038 |
| Hypertension | 549 (58.6) | 2,833 (65.1) | <0.001 |
| Liver disease | 18 (1.9) | 94 (2.2) | 0.71 |
| Peripheral vascular disease | 160 (17.1) | 524 (12.0) | <0.001 |
| Chronic kidney disease | 223 (23.8) | 726 (16.7) | <0.001 |
| CCI | 4.5 (2.3) | 3.8 (2.3) | <0.001 |
| MPN type | <0.001 | ||
| PV | 282 (30.1) | 2,242 (51.5) | |
| ET | 529 (56.5) | 1,925 (44.2) | |
| PMF | 125 (13.4) | 187 (4.3) | |
| Thrombocytopenia | 61 (6.5) | 148 (3.4) | <0.001 |
| Splenomegaly | 9 (1.0) | 21 (0.5) | 0.091 |
| AMI characteristics and treatment | |||
| STEMI | 322 (34.4) | 1,300 (29.9) | 0.007 |
| Chronic total occlusion | 54 (5.8) | 243 (5.6) | 0.81 |
| Invasive management | 582 (62.2) | 3,057 (70.2) | <0.001 |
| PCI | 275 (29.4) | 1,753 (40.3) | <0.001 |
| CABG | 159 (17.0) | 314 (7.2) | <0.001 |
| MCS use | 127 (13.6) | 163 (3.7) | <0.001 |
| Cardiogenic shock | 102 (10.9) | 86 (2.0) | <0.001 |
Values are mean ± SD or n (%).
Abbreviations as in Table 1.
After multivariable logistic regression, anemia (aOR: 1.72; 95% CI: 1.44-2.05), peripheral vascular disease (aOR: 1.29; 95% CI: 1.04-1.61), and Charlson comorbidity index (aOR: 1.09; 95% CI: 1.02-1.16) were associated with an increased risk of in-hospital death, CA, or bleeding. Additionally, ET (aOR: 1.62; 95% CI: 1.35-1.94) and PMF (aOR: 3.98; 95% CI: 2.98-5.32) phenotypes were associated with a higher risk of death, CA, or bleeding compared with patients with PV. Patients undergoing invasive management had a decreased risk of death, CA, or bleeding (aOR: 0.75; 95% CI: 0.60-0.94). STEMI presentation (aOR: 1.44; 95% CI: 1.20-1.72), MCS use (aOR: 2.16; 95% CI: 1.60-2.92), cardiogenic shock (aOR: 4.26; 95% CI: 3.02-6.01), and CABG (aOR: 2.90; 95% CI: 2.20-3.82) were associated with an increased risk of death, CA, or bleeding. aORs of risk factors for in-hospital death, CA, or bleeding are shown in Table 5, and unadjusted ORs are shown in Supplemental Table 3.
Table 5.
Adjusted Odds of Death, Cardiac Arrest, or Bleeding in Patients With MPN
| Age, y | 1.00 (0.99-1.01) |
| Female | 1.08 (0.92-1.27) |
| Smoking history | 0.75 (0.61-0.92) |
| CAD | 0.84 (0.69-1.03) |
| Prior MI | 0.87 (0.66-1.15) |
| Prior PCI | 0.70 (0.52-0.94) |
| Prior CABG | 0.80 (0.59-1.09) |
| Heart failure | 1.70 (0.96-3.03) |
| Anemia | 1.72 (1.44-2.05) |
| Diabetes | 0.96 (0.79-1.15) |
| Hypertension | 0.84 (0.72-0.99) |
| Peripheral vascular disease | 1.29 (1.04-1.61) |
| Chronic kidney disease | 0.99 (0.77-1.23) |
| CCI | 1.09 (1.02-1.16) |
| MPN type | |
| PV | Ref |
| ET | 1.62 (1.35-1.94) |
| PMF | 3.98 (2.98-5.32) |
| Thrombocytopenia | 1.32 (0.92-1.88) |
| Splenomegaly | 1.19 (0.50-2.84) |
| STEMI | 1.44 (1.20-1.72) |
| Invasive management | 0.75 (0.60-0.94) |
| PCI | 0.94 (0.75-1.17) |
| CABG | 2.90 (2.20-3.82) |
| MCS use | 2.16 (1.60-2.92) |
| Cardiogenic shock | 4.26 (3.02-6.01) |
Values are adjusted OR (95% CI).
Abbreviations as in Table 1.
Adjusted for age, sex, smoking history, coronary artery disease, prior myocardial infarction, prior PCI, prior CABG, heart failure, anemia, diabetes, hypertension, peripheral vascular disease, Charlson comorbidity index, MPN type, thrombocytopenia, splenomegaly, STEMI presentation, invasive management, PCI, CABG, MCS use, and cardiogenic shock.
Race differences in outcomes of patients with MPNs admitted for AMI
We also investigated race differences on outcomes in patients with MPNs and AMI. Among patients with MPNs and AMI, a total of 3,755 (71.0%) were White, 469 (8.9%) were Black, 310 (5.9%) were Hispanic, 124 (2.3%) were Asian, and 632 (11.9%) were of other or unknown race. The rates of left heart catheterization and PCI were similar across race groups. Black (9.4%), Hispanic (10.6%), and Asian (8.1%) patients had higher rates of self-pay or no charge as the expected primary payer compared with White patients (5.0%; Supplemental Table 4). In-hospital death, CA, or bleeding occurred in 17.2% of White patients, 18.3% of Black patients, 20.6% of Hispanic patients, and 16.9% of Asian patients. In-hospital death occurred in 4.7% of White, 4.5% of Black, 6.4% of Hispanic, and 4.8% of Asian patients. Bleeding occurred in 12.1% of White, 14.5% of Black, 13.5% of Hispanic, and 11.3% of Asian patients.
After adjusting for age, sex, smoking history, CAD, prior myocardial infarction, prior PCI, prior CABG, anemia, peripheral vascular disease, liver disease, diabetes, chronic lung disease, chronic kidney disease, Charlson comorbidity index, MPN type, STEMI, cardiogenic shock, invasive management, expected primary payer type, and MCS use, there was no difference in the composite outcome of in-hospital death, CA, or bleeding in Black (aOR: 1.09; 95% CI: 0.84-1.43), Hispanic (aOR: 1.22; 95% CI: 0.90-1.67), or Asian patients (aOR: 0.99; 95% CI: 0.60-1.65) compared with Whites. After adjusting for the same covariates, Hispanic patients had an increased risk of in-hospital death compared with White patients (aOR: 1.68; 95% CI: 1.00-2.82), although Black (aOR: 1.21; 95% CI: 0.74-1.99) and Asian (aOR: 1.39; 95% CI: 0.58-3.34) patients did not. There was also no difference in the risk of bleeding in Black (aOR: 1.15; 95% CI: 0.85-1.54), Hispanic (aOR: 1.01; 95% CI: 0.70-1.44), or Asian (aOR: 0.87; 95% CI: 0.48-1.56) patients compared with White patients after adjustment (Supplemental Table 5).
Discussion
Cardiovascular disease and arterial thrombosis are responsible for substantial morbidity and mortality in patients with MPNs. Although reports of acute coronary syndrome and myocardial infarction in patients with MPNs have been described in the literature, little is known about the short-term outcomes and risk factors in this patient population. Our study results suggest that among patients admitted for AMI, MPN is associated with an increased risk of bleeding but a decreased risk of in-hospital mortality or CA compared with patients without MPNs (Central Illustration). However, although the rates of in-hospital death, CA, or bleeding are decreasing over time in patients admitted with AMI without MPNs, our results suggest that these rates are increasing in patients with MPNs. Additionally, our study suggests that patients with ET and PMF have worse in-hospital outcomes compared with patients with PV and that invasive management with either left heart catheterization, PCI, or CABG in this patient population is associated with decreased in-hospital death, CA, or bleeding.
Central Illustration.
In-Hospital Outcomes and Trends Among Myeloproliferative Neoplasm Patients Admitted for Acute Myocardial Infarction
Among patients admitted for acute myocardial infarction (AMI), patients with myeloproliferative neoplasm (MPN) had lower rates of in-hospital death or cardiac arrest but higher rates of major bleeding, gastrointestinal bleeding, transfusion, and procedural bleeding compared to non-MPN patients. Patients with MPN had increased temporal rates of in-hospital death or cardiac arrest and major bleeding, whereas patients without had decreased rates over time.
Patients with MPNs are at increased risk of cardiovascular events including thrombosis and AMI. Unlike patients with solid malignancies, our study suggests that patients with MPNs who present with myocardial infarction may be at a similar or lower risk of in-hospital mortality and CA compared with patients without MPNs.17 However, patients with MPNs admitted for thrombosis of any kind (including AMI) have increased in-hospital mortality compared with patients admitted for other reasons.14 One interesting finding is the temporal trend of in-hospital death or CA and bleeding in patients with and without MPNs. Patients without MPNs have had decreasing in-hospital mortality or CA, whereas patients with MPNs have had increasing death or CA despite a decreased temporal trend of STEMI in both groups. This decrease in in-hospital mortality among the general population has been described both in the United States and in other countries (ie, Germany).18 The increasing rates of in-hospital mortality or CA in patients with MPNs is unclear. There was no difference in the age trend across years in patients with MPNs (data not shown) that would explain this. Current guidelines on the management of MPNs recommend normalization of blood counts and aspirin in patients with PV or ET; however, guidance on the management of patients with MPNs in AMI is sparse.19 Additionally, data in trends of cardiovascular disease outcomes and burden in patients with MPN are lacking. This remains fertile ground for further investigation and would shed light on improving cardiovascular outcomes in MPNs.
Our study identified potential risk factors associated with an increased risk of in-hospital death, CA, or bleeding in patients with MPNs admitted for AMI. Among the risk factors associated with an increased risk of death, CA, or bleeding were peripheral vascular disease, anemia, STEMI presentation, and an ET or PMF MPN phenotype. Similar to previous literature in the general AMI population, a history of smoking and invasive management were associated with a decreased risk of adverse in-hospital events.20,21 Additionally, peripheral vascular disease and anemia have also been shown to be associated with an increased risk of adverse events after AMI in the general population.22,23 Additionally, our study did not reveal a significant difference in outcomes between different races with the exception of an increased risk of in-hospital death in Hispanic patients compared with White patients. In other studies that investigated racial differences in outcomes among the general AMI population, similar rates of adverse outcomes (including in-hospital death) have been described in White and non-White patients.24,25 However, our study only investigated in-hospital outcomes but not long-term ones. Indeed, Black and Hispanic patients have been shown to have worse long-term outcomes after AMI compared with White patients.26, 27, 28 Among patients with MPNs, an analysis of the Surveillance, Epidemiology, and End Results database showed an association with an increased risk of 1-year cardiovascular and all-cause mortality death in non-Hispanic Black patients compared with non-Hispanic White patients.29 Further investigation is warranted in characterizing health disparities in this high-risk population. Bleeding is a common complication in both MPNs and in patients with AMI. Our study suggests that patients hospitalized for AMI with ET or PMF have an increased risk of in-hospital bleeding compared with patients with PV. This is in line with prior studies showing an increased risk of bleeding in ET and PMF patients. One meta-analysis of 29 studies involving 13,436 patients with MPNs suggested that the long-term prevalence of bleeding is higher in patients with PMF (8.9%; 95% CI: 6.5%-12.2%) than ET (7.3%; 95% CI: 5.3%-10.0%) or PV (6.9%; 95% CI: 5.5%-8.7%).2 Additionally, extreme thrombocytosis (platelets >1,000 × 109/L) is associated with an increased risk of bleeding in patients with MPNs likely because of acquired von Willenbrand disease.30 In addition, patients with PMF may have thrombocytopenia and altered platelet function, leading to an increased risk of bleeding. This may, in part, explain the increased risk of bleeding in patients with MPNs compared with patients without MPNs in this cohort.1,31 Although invasive management was associated with a reduced risk of death or bleeding in patients with MPNs, patients with MPNs were at an increased risk of bleeding, including gastrointestinal and procedural bleeding, compared with patients without MPNs. These results stress the importance of bleeding risk when treating patients with MPNs and AMI, especially with invasive management. In 1 study of patients with PV, patients treated with aspirin in addition to anticoagulation (indication not captured in the study) had a 5-fold increased risk of bleeding compared with patients treated with aspirin alone.32 Additionally, patients with MPNs and thrombocytosis may have high platelet turnover and therefore reduce the efficacy of aspirin and other antiplatelet agents.33,34 Another study found an increased risk of bleeding in patients with MPNs treated with P2Y12 inhibitor, although it did not reach statistical significance (OR: 2.829; 95% CI: 0.998-8.021).35 The efficacy of dual antiplatelet therapy for post-AMI therapy and the risk of bleeding events has not been characterized and therefore remains an unanswered question. Additionally, unlike patients without MPNs, the rates of major bleeding have increased among patients with MPNs, highlighting the need for further investigation in order to identify therapeutic strategies to minimize the risk of bleeding in patients with AMI and MPNs.
Study limitations
This study has limitations to consider. One limitation is the retrospective nature of our study, which makes it prone to unmeasured confounding. Additionally, the data in the NIS are abstracted from ICD-9 and ICD-10 billing codes, which are prone to errors because they rely on coding. Data on MPN treatment, blood counts, duration of disease, and genetic testing are not reported and may affect cardiovascular outcomes in this patient population.1 For example, JAK2 gene sequence variant and acute myocardial infarction occurring within 12 months of MPN diagnosis were associated with an increased risk of major adverse cardiovascular events in patients with MPNs after AMI hospitalization.36 Therefore, further investigation with more granular details of MPN treatment and genotyping is needed. Possible confounders that are not captured by the NIS and may lead to residual bias include prior cardiovascular therapies, disease severity, and adherence to medications. Additionally, patients with MPNs were less likely to undergo PCI compared with patients without MPNs. An increased risk of bleeding may have contributed to lower use of PCI in patients with MPNs, and the unequal use of PCI is another potential source of confounding in our analysis. The appropriateness of PCI and invasive management of AMI could not be evaluated using the NIS database and thus remains an important gap in knowledge in this patient population. The NIS does not distinguish if diagnoses occurred before or during hospitalization; therefore, CA outcomes in our cohort may have occurred before hospitalization. Granular data on the details of revascularization, including disease severity and vessels revascularized, are not reported in the database. Additionally, the NIS captures hospitalizations and not unique patients; therefore, whether patients with MPNs are readmitted more frequently for AMI or other cardiovascular etiologies is unclear and merits further investigation. Therefore, given these limitations, our study is hypothesis generating; thus, further studies are needed to further characterize outcomes in patients with MPNs and AMI.
Conclusions
Patients with MPN are at an increased risk of thrombotic complications including AMI. Our study suggests that among patients admitted with AMI, in-hospital mortality or CA of patients with MPNs is lower compared with patients without MPNs. However, temporal trends show an increase in in-hospital mortality or CA in patients with MPNs admitted for AMI despite a similar reduction in STEMI presentations over time. Additionally, patients with MPNs are associated with an increased risk of in-hospital bleeding, which represents a clinical conundrum that will require further investigation to resolve.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with MPN are at high risk of thrombotic complications. Among patients admitted with acute myocardial infarction, patients with MPN are associated with decreased in-hospital death or cardiac arrest but higher rates of bleeding compared with the non-MPN population.
TRANSLATIONAL OUTLOOK: Further studies on the mechanisms behind increased thrombotic risk in patients with MPN are needed. Additionally, novel approaches for balancing thrombosis and bleeding risk in patients with MPN are deserving of further study.
Funding Support and Author Disclosures
Dr Hobbs is on the advisory boards of Incyte, Novartis, AbbVie, Constellation, and Blupring; has received research support from Incyte and Constellation; and has received grants from ASH-AMFDP and K12 Paul Calabresi award. Dr Bangalore has done ad hoc consulting and speaking for Abbott Vascular, Biotronik, Boston Scientific, Amgen, Pfizer, Merck, and Inari.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Leiva O., Hobbs G., Ravid K., Libby P. Cardiovascular disease in myeloproliferative neoplasms. J Am Coll Cardiol CardioOnc. 2022;4(2):166–182. doi: 10.1016/j.jaccao.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rungjirajittranon T., Owattanapanich W., Ungprasert P., Siritanaratkul N., Ruchutrakool T. A systematic review and meta-analysis of the prevalence of thrombosis and bleeding at diagnosis of Philadelphia-negative myeloproliferative neoplasms. BMC Cancer. 2019;19(1):184. doi: 10.1186/s12885-019-5387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang W., Liu W., Fidler T., et al. Macrophage inflammation, erythrophagocytosis, and accelerated atherosclerosis in Jak2 (V617F) mice. Circ Res. 2018;123(11):e35–e47. doi: 10.1161/CIRCRESAHA.118.313283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt D.L., Lopes R.D., Harrington R.A. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662–675. doi: 10.1001/jama.2022.0358. [DOI] [PubMed] [Google Scholar]
- 5.Reed G.W., Rossi J.E., Cannon C.P. Acute myocardial infarction. Lancet. 2017;389(10065):197–210. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 6.Virani S.S., Alonso A., Benjamin E.J., et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 7.Leiva O., AbdelHameid D., Connors J.M., Cannon C.P., Bhatt D.L. Common pathophysiology in cancer, atrial fibrillation, atherosclerosis, and thrombosis. J Am Coll Cardiol CardioOnc. 2021;3(5):619–634. doi: 10.1016/j.jaccao.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharadwaj A., Potts J., Mohamed M.O., et al. Acute myocardial infarction treatments and outcomes in 6.5 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. 2020;41(23):2183–2193. doi: 10.1093/eurheartj/ehz851. [DOI] [PubMed] [Google Scholar]
- 9.Iannaccone M., D’Ascenzo F., Vadala P., et al. Prevalence and outcome of patients with cancer and acute coronary syndrome undergoing percutaneous coronary intervention: a BleeMACS substudy. Eur Heart J Acute Cardiovasc Care. 2018;7(7):631–638. doi: 10.1177/2048872617706501. [DOI] [PubMed] [Google Scholar]
- 10.Kwok C.S., Wong C.W., Kontopantelis E., et al. Percutaneous coronary intervention in patients with cancer and readmissions within 90 days for acute myocardial infarction and bleeding in the USA. Eur Heart J. 2021;42(10):1019–1034. doi: 10.1093/eurheartj/ehaa1032. [DOI] [PubMed] [Google Scholar]
- 11.Tefferi A., Guglielmelli P., Larson D.R., et al. Long-term survival and blast transformation in molecularly annotated essential thrombocythemia, polycythemia vera, and myelofibrosis. Blood. 2014;124(16):2507–2513. doi: 10.1182/blood-2014-05-579136. quiz 2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deadmond M.A., Smith-Gagen J.A. Changing incidence of myeloproliferative neoplasms: trends and subgroup risk profiles in the USA, 1973-2011. J Cancer Res Clin Oncol. 2015;141(12):2131–2138. doi: 10.1007/s00432-015-1983-5. [DOI] [PubMed] [Google Scholar]
- 13.Polednak A.P. Recent decline in the U.S. death rate from myeloproliferative neoplasms, 1999-2006. Cancer Epidemiol. 2012;36(2):133–136. doi: 10.1016/j.canep.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 14.Ulanja M.B., Beutler B.D., Antwi-Amoabeng D., et al. Patient outcomes in myeloproliferative neoplasm-related thrombosis: insights from the National Inpatient Sample. Thromb Res. 2020;194:72–81. doi: 10.1016/j.thromres.2020.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Elixhauser A., Steiner C., Harris D.R., Coffey R.M. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Desai R.J., Franklin J.M. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ. 2019;367:l5657. doi: 10.1136/bmj.l5657. [DOI] [PubMed] [Google Scholar]
- 17.Pothineni N.V., Shah N.N., Rochlani Y., et al. Temporal trends and outcomes of acute myocardial infarction in patients with cancer. Ann Transl Med. 2017;5(24):482. doi: 10.21037/atm.2017.11.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann J.T., Gossling A., Sorensen N.A., Blankenberg S., Magnussen C., Westermann D. Temporal trends in incidence and outcome of acute coronary syndrome. Clin Res Cardiol. 2020;109(9):1186–1192. doi: 10.1007/s00392-020-01612-1. [DOI] [PubMed] [Google Scholar]
- 19.Barbui T., Tefferi A., Vannucchi A.M., et al. Philadelphia chromosome-negative classical myeloproliferative neoplasms: revised management recommendations from European LeukemiaNet. Leukemia. 2018;32(5):1057–1069. doi: 10.1038/s41375-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandes G.C., Kovacs R., Abbott J.D., et al. Determinants of early and late in-hospital mortality after acute myocardial infarction: a subanalysis of the OBTAIN registry. Can J Cardiol. 2023;39(4):531–537. doi: 10.1016/j.cjca.2022.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Gong W., Yan Y., Wang X., et al. Risk factors for in-hospital cardiac arrest in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2022;80(19):1788–1798. doi: 10.1016/j.jacc.2022.08.797. [DOI] [PubMed] [Google Scholar]
- 22.Kobo O., Contractor T., Mohamed M.O., et al. Impact of pre-existent vascular and poly-vascular disease on acute myocardial infarction management and outcomes: an analysis of 2 million patients from the National Inpatient Sample. Int J Cardiol. 2021;327:1–8. doi: 10.1016/j.ijcard.2020.11.051. [DOI] [PubMed] [Google Scholar]
- 23.Anker S.D., Voors A., Okonko D., et al. Prevalence, incidence, and prognostic value of anaemia in patients after an acute myocardial infarction: data from the OPTIMAAL trial. Eur Heart J. 2009;30(11):1331–1339. doi: 10.1093/eurheartj/ehp116. [DOI] [PubMed] [Google Scholar]
- 24.Edmund Anstey D., Li S., Thomas L., Wang T.Y., Wiviott S.D. Race and sex differences in management and outcomes of patients after ST-elevation and non-ST-elevation myocardial infarct: results from the NCDR. Clin Cardiol. 2016;39(10):585–595. doi: 10.1002/clc.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yong C.M., Ungar L., Abnousi F., Asch S.M., Heidenreich P.A. Racial differences in quality of care and outcomes after acute coronary syndrome. Am J Cardiol. 2018;121(12):1489–1495. doi: 10.1016/j.amjcard.2018.02.036. [DOI] [PubMed] [Google Scholar]
- 26.Graham G.N., Jones P.G., Chan P.S., Arnold S.V., Krumholz H.M., Spertus J.A. Racial disparities in patient characteristics and survival after acute myocardial infarction. JAMA Netw Open. 2018;1(7) doi: 10.1001/jamanetworkopen.2018.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hess C.N., Kaltenbach L.A., Doll J.A., Cohen D.J., Peterson E.D., Wang T.Y. Race and sex differences in post-myocardial infarction angina frequency and risk of 1-year unplanned rehospitalization. Circulation. 2017;135(6):532–543. doi: 10.1161/CIRCULATIONAHA.116.024406. [DOI] [PubMed] [Google Scholar]
- 28.Garcia M., Almuwaqqat Z., Moazzami K., et al. Racial disparities in adverse cardiovascular outcomes after a myocardial infarction in young or middle-aged patients. J Am Heart Assoc. 2021;10(17) doi: 10.1161/JAHA.121.020828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leiva O., How J.C., Brunner A.M., Hobbs G.S. Trends in all-cause and cardiovascular mortality among patients with myeloproliferative neoplasms: a surveillance, epidemiology, and end-results database analysis. Blood. 2022;140(suppl 1):6808–6809. [Google Scholar]
- 30.Tefferi A., Barbui T. Polycythemia vera and essential thrombocythemia: 2021 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(12):1599–1613. doi: 10.1002/ajh.26008. [DOI] [PubMed] [Google Scholar]
- 31.Matsuura S., Thompson C.R., Belghasem M.E., et al. Platelet dysfunction and thrombosis in JAK2(V617F)-mutated primary myelofibrotic mice. Arterioscler Thromb Vasc Biol. 2020;40(10):e262–e272. doi: 10.1161/ATVBAHA.120.314760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zwicker J.I., Paranagama D., Lessen D.S., Colucci P.M., Grunwald M.R. Hemorrhage in patients with polycythemia vera receiving aspirin with an anticoagulant: a prospective, observational study. Haematologica. 2021;107(5):1106–1110. doi: 10.3324/haematol.2021.279032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gremmel T., Gisslinger B., Gisslinger H., Panzer S. Response to aspirin therapy in patients with myeloproliferative neoplasms depends on the platelet count. Transl Res. 2018;200:35–42. doi: 10.1016/j.trsl.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Pedersen O.H., Larsen M.L., Kristensen S.D., Hvas A.M., Grove E.L. Recurrent cardiovascular events despite antiplatelet therapy in a patient with polycythemia vera and accelerated platelet turnover. Am J Case Rep. 2017;18:945–948. doi: 10.12659/AJCR.904148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaifie A., Kirschner M., Wolf D., et al. Bleeding, thrombosis, and anticoagulation in myeloproliferative neoplasms (MPN): analysis from the German SAL-MPN-registry. J Hematol Oncol. 2016;9:18. doi: 10.1186/s13045-016-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leiva O., Jenkins A., Rosovosky R., Leaf R.K., Goodarzi K., Hobbs G. Risk factors for major adverse cardiovascular events post-acute coronary syndrome hospitalization in patients with myeloproliferative neoplasms. Circulation. 2022;146(suppl 1) A12866-A12866. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.