Abstract
Background
It is unknown whether a history of childhood cancer modifies the established disparities in cardiovascular risk factors (CVRFs) observed in the general population.
Objectives
We sought to determine if disparities in CVRFs by race/ethnicity are similar among childhood cancer survivors compared with the general population.
Methods
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort with a longitudinal follow-up of 24,084 5-year survivors diagnosed between 1970 and 1999. Multivariable piecewise exponential regression estimated incidence rate ratios (IRRs) for hypertension, hyperlipidemia, diabetes, obesity, and ≥2 CVRFs by race/ethnicity. The CCSS sibling cohort and the National Health and Nutrition Examination Survey cohort were used to compare the sociodemographic-adjusted IRRs for same-race/same-ethnicity disparities.
Results
Non-Hispanic Black (NHB) (n = 1,092) and Hispanic (n = 1,405) survivors compared with non-Hispanic White (NHW) (n = 13,960) survivors reported a higher cumulative incidence of diabetes (8.4%, 9.7%, and 5.1%, respectively); obesity (47.2%, 48.9%, and 30.2%, respectively); multiple CVRFs (17.7%, 16.6%, and 12.3%, respectively); and, for NHB survivors, hypertension (19.5%, 13.6%, and 14.3%, respectively) by 40 years of age (P < 0.001). Controlling for sociodemographic and treatment factors compared with NHW survivors, IRRs for NHB were increased for hypertension (IRR: 1.4; 95% CI: 1.1-1.8), obesity (IRR: 1.7; 95% CI: 1.4-2.1), and multiple CVRFs (IRR: 1.6; 95% CI: 1.2-2.1). IRRs for Hispanic survivors were increased for diabetes (IRR: 1.8; 95% CI: 1.2-2.6) and obesity (IRR: 1.4; 95% CI: 1.2-1.7). The pattern of IRRs for CVRF differences was similar among CCSS sibling and National Health and Nutrition Examination Survey cohorts.
Conclusions
The higher burden of CVRFs among NHB and Hispanic survivors compared with NHW survivors was similar to the general population. The promotion of cardiovascular health equity is critical in this high-risk population.
Key Words: cardiovascular risk factors, childhood cancer survivors, health equity
Central Illustration
Childhood cancer survivors represent a vulnerable population at risk for long-term health problems related to their primary malignancy and late effects of cancer treatment. Marked improvements in pediatric oncology care over the past half century have come at the expense of significant cardiotoxic exposures with subsequent cardiovascular disease (CVD) later in life.1 Despite improvements in overall cancer survival, significant health inequities by race/ethnicity persist in morbidity and mortality.2, 3, 4, 5
CVD is a major cause of late mortality for survivors, second only to subsequent malignancy.6,7 Chemotherapy, most notably anthracyclines, as well as chest radiation are established to be directly cardiotoxic.8,9 Craniospinal and abdominal radiation are also associated with the development or progression of obesity and diabetes.10,11 Previous studies from the Childhood Cancer Survivor Study (CCSS) have reported an increased incidence of serious cardiac events among survivors compared with siblings, including an 11.7% cumulative incidence of heart failure by age 40 for high-risk survivors.9,12 Cardiovascular risk factors (CVRFs) were synergistic with known cardiotoxic therapy in elevating CVD risk in a near-multiplicative fashion.13 Hypertension potentiated the risk for anthracycline-associated heart failure with an estimated relative excess risk of 44.5 because of their interaction. Similarly, multiple CVRFs significantly increased the risk of coronary artery disease after chest-directed radiation. Therefore, early diagnosis and appropriate management of CVRFs and the prevention of major cardiac events represent key targets for interventions such as preventive care and supporting behavior change for a healthy lifestyle.
In the general population, CVD inequities by races/ethnicities persist.14,15 In part, this is caused by disparities in the rate and/or management of CVRFs, particularly the disproportionate burden of hypertension among non-Hispanic Blacks (NHBs) despite controlling for socioeconomic factors.16,17 Similarly, an increased prevalence of diabetes has been observed among Hispanics and NHBs in the United States.18,19 National efforts to curb current trends are vital to prevent long-term cardiovascular sequelae.20, 21, 22 Early diagnosis and optimal treatment of CVRFs are associated with a decreased risk of downstream cardiovascular events in the general population.23 Nevertheless, it is unknown whether a history of childhood cancer modifies disparities in CVRFs because financial toxicity and healthy lifestyles associated with CVRFs may also be disparate for survivors. Ultimately, further investigation of potential disparities will inform specific strategies to promote health equity for all survivors.
This analysis aimed to build on previously identified disparities by race/ethnicity of CVRFs among survivors in the original CCSS cohort, notably the increased risk of diabetes among Hispanics and NHBs as well as the increased risk of hypertension in NHB survivors compared with non-Hispanic White (NHW) survivors, and to test the hypothesis that disparities in the complete CCSS cohort are similar to those observed in the general population.3 Specifically, comparisons with the CCSS sibling cohort and a referent population from the National Health and Nutrition Examination Survey (NHANES) sought to understand whether the pattern of disparities in CVRFs among survivors by race/ethnicity differed from those identified in the general population (Central Illustration).
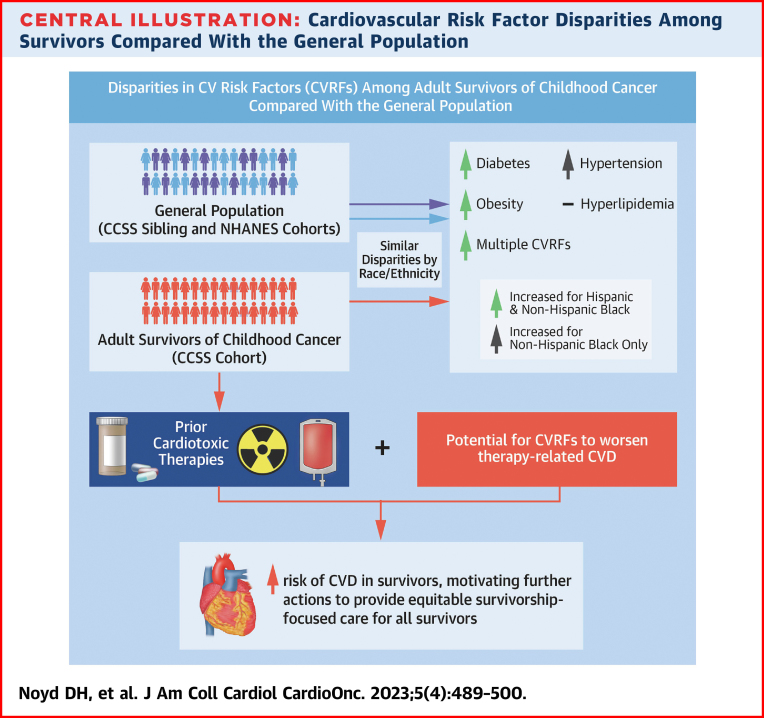
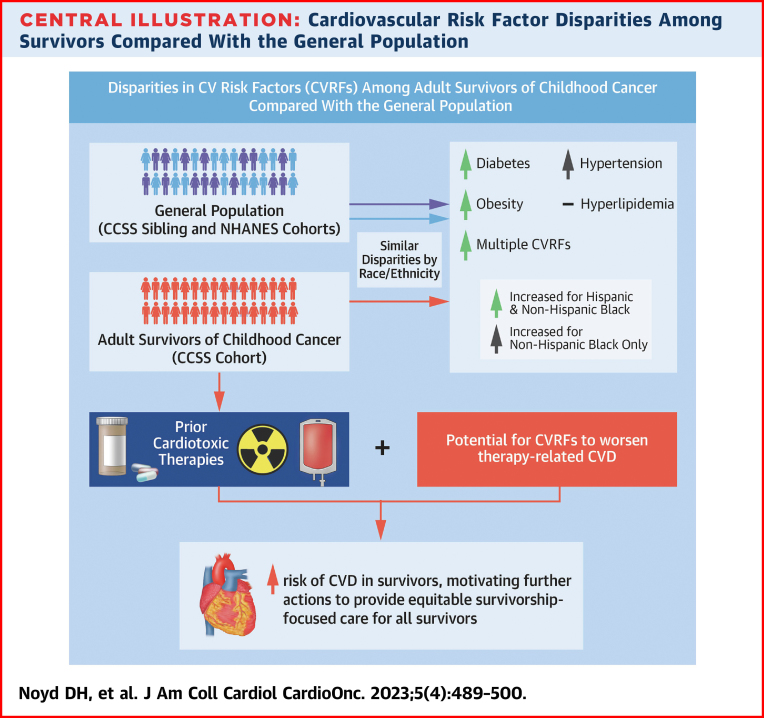
Central Illustration.
Cardiovascular Risk Factor Disparities Among Survivors Compared With the General Population
The burden of diabetes, hypertension, hyperlipidemia, obesity, and multiple cardiovascular risk factors (CVRFs) contribute to the incidence of cardiovascular disease (CVD) across the life span for both survivors and the general population. For survivors, anthracyclines and chest radiation are directly cardiotoxic, whereas CVRFs are known to potentiate risk for CVD beyond the expected additive risk, thus emphasizing the increased importance to mitigate CVRFs among survivors and motivating further actions to provide equitable survivorship-focused care for all survivors. This analysis observed similar disparities by race/ethnicity compared with the general population, with non-Hispanic Black and Hispanic survivors reporting a higher cumulative incidence of diabetes, obesity, and multiple CVRFs. CCSS = Childhood Cancer Survivor Study; CV = cardiovascular; CVD = cardiovascular disease; NHANES = National Health and Nutrition Examination Survey.
Methods
Study populations
The CCSS is a retrospective cohort that includes 25,656 childhood cancer survivors who were diagnosed at 1 of 31 North American centers between 1970 and 1999 who had survived at least 5 years after a diagnosis of leukemia, central nervous system malignancy, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or a bone tumor.24 Survivors with subsequent malignancies or late recurrence occurring before 20 years old, younger than 20 years of age at the baseline questionnaire (to allow marriage status as an adjusted variable for comparison with NHANES data), and with missing race/ethnicity data were excluded, allowing 16,457 survivors for analysis (Figure 1).3 A randomly selected population of siblings (n = 4,738) of survivors in CCSS also completed the same questionnaires and provided a comparison population. All participants provided informed consent, and the protocol was approved by the Institutional Review Board at each study site. NHANES data from 2017-2018 were used to construct a cohort from the general population with the same age range as the CCSS participants. The rationale for the selection of 2 comparison cohorts was to leverage sibling controls and also consider a sample of adults from the general population. The advantage for the CCSS sibling cohort was the same study design and questions to capture CVRFs as survivors. Moreover, given the potential environmental or familial factors associated with CVRFs and having a sibling with a history of childhood cancer, we aimed to control for potential unmeasured confounders through this cohort. The complex study design and quality of NHANES provided a representative sample of the U.S. population to estimate the burden of CVRFs.25 Only NHANES participants 20 to 69 years of age with complete data on all the adjusted variables were included in this analysis (N = 3,047).
Figure 1.
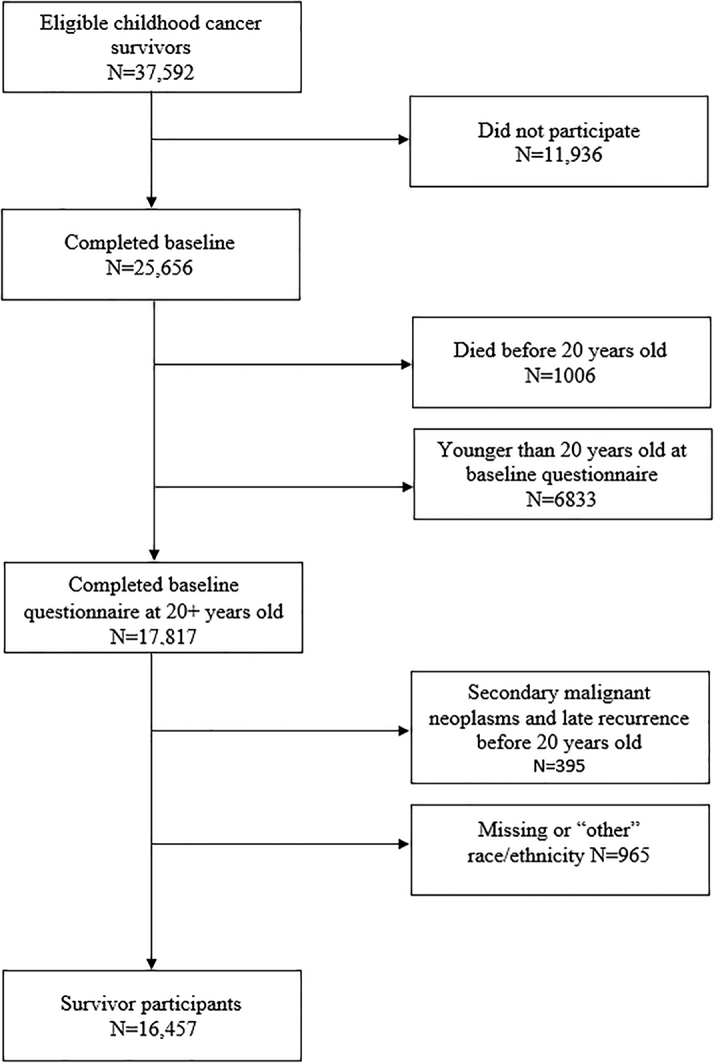
Consort Diagram for the CCSS
The Childhood Cancer Survivor Study is a retrospective cohort that includes 25,656 childhood cancer survivors who were diagnosed at 1 of 31 North American centers between 1970 and 1999 who had survived at least 5 years after a diagnosis of leukemia, central nervous system malignancy, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or a bone tumor. Survivors with subsequent malignancies or late recurrence occurring before 20 years old, younger than 20 years of age at baseline questionnaire, and with missing race/ethnicity data were excluded, allowing 16,457 survivors for analysis.
Demographics and treatment exposures
The CCSS questionnaires included the self-reported race/ethnicity of survivors and siblings as well as categories for sex, educational attainment, marital status, household income, employment, and insurance status. Participants were surveyed longitudinally over time. Chemotherapy agents and cumulative doses during treatment were abstracted from the medical records of consenting participants. Cumulative alkylating agent doses were reported as cyclophosphamide equivalent doses,26 and cumulative anthracycline doses were based on doxorubicin equivalence ratios.27 Chest-directed radiation doses were also abstracted from medical records for each survivor, and a central review was completed for field-specific maximum total doses.24
Outcome measures
We used data from all available CCSS follow-up questionnaires, a total of 6 administered between 2000 and 2017, to measure CVRFs. The primary outcomes for this analysis included hypertension, hyperlipidemia, diabetes, obesity, and multiple (≥2) CVRFs. Each CVRF was determined based on a self-report of both being diagnosed by a physician and on medications. For the calculation of body mass index to classify obesity, the self-reported height and weight were used. Grade ≥2 hypertension, grade ≥2 hyperlipidemia, and diabetes (on medication or, for diabetes, evidence of end organ damage) were defined per the Common Terminology Criteria for Adverse Events (v4.03).28 For the NHANES cohort, hypertension was based on participants who responded yes to taking a prescription for hypertension, hyperlipidemia was defined by taking a prescription medication for cholesterol, and diabetes was defined as being told by a doctor or on medication (oral agent or insulin).25
Statistical analysis
Data are presented using counts (percentages). Sociodemographic and treatment characteristics were tabulated at the CCSS baseline survey and compared across racial/ethnic groups using the chi-square test. The follow-up of CVRF analysis started at cohort entry (5 years after diagnosis) and ended at the first CVRF event of interest for each individual CVRF analysis or at a competing risk event, which included recurrence, subsequent malignant neoplasm (for siblings, we considered any malignancy), and death, or was censored at the completion of the last questionnaire. Recurrence (survivors only) and subsequent malignant neoplasm as well as death were considered competing risk events because they could have exposed survivors to additional cancer treatments that we could not ascertain. Analyses were weighted to account for the undersampling of survivors of acute lymphoblastic leukemia between 1987 and 1999 based on the CCSS study design. Cumulative incidences of CVRFs by attained age with 95% CIs were calculated for survivors stratified by race/ethnicity, with events before age 26 entered as prevalence using Gray’s competing risk method.29 If the 95% CI did not cross one, then this was considered statistically significant at a level of P < 0.05. Multivariable piecewise exponential regression was used to estimate incidence rate ratios (IRRs) of CVRFs by race/ethnicity in survivors and siblings separately, with NHWs as the referent group adjusting for demographics (age, sex, baseline household income, educational attainment, marital status, employment, and insurance) and key treatment exposures (anthracyclines, alkylating agents, and chest-directed radiation therapy for survivors only).
In order to more closely assess the magnitude of the disparities observed among survivors relative to siblings across racial/ethnic groups, we calculated the ratio of the IRRs for each CVRF. Furthermore, a multivariable piecewise exponential model was used to compare IRRs to assess the magnitude of same-race/same-ethnicity survivor-sibling differences between racial and ethnic groups, with modifications by generalized estimating equations to account for possible within-family correlation between survivors and siblings from the same families. Multivariable logistic regression was used in calculating prevalence ORs in NHANES data. Of note, given that CVRFs and sociodemographic factors were only obtained at the time of the NHANES survey, we calculated the prevalence OR for each CVRF rather than the IRR. Statistical analyses were conducted using SAS version 9.4 (SAS Institute). All statistical inferences were 2-sided, and P values <0.05 were considered statistically significant.
Results
Demographic and exposure characteristics by race/ethnicity
Among 16,457 survivors eligible for analysis, 13,960 were NHW, 1,092 were NHB, and 1,405 were Hispanic, which is reflective of the less diverse U.S. population in the 1970s to 1990s (Table 1).5 Sociodemographic and treatment factors differed significantly between groups for education, marital status, employment, household income, insurance status, exposure to anthracyclines, exposure to alkylators, and chest-directed radiotherapy (P < 0.001). For pertinent treatment exposures associated with late CVD, approximately 61% of NHB and Hispanic survivors had an anthracycline exposure compared with 50% of NHW survivors. NHW survivors were more likely to have received chest-directed radiotherapy (26%) and at higher doses compared with 22% of NHB and 20% of Hispanic survivors.
Table 1.
Demographic and Exposure Characteristics by Race/Ethnicity Among Survivors
| White, NH (n = 13,960) | Black, NH (n = 1,092) | Hispanic (n = 1,405) | P Value | |
|---|---|---|---|---|
| Age at diagnosis, y | <0.001 | |||
| 0-4 | 3,127 (26.2) | 259 (24.8) | 391 (33.7) | |
| 5-9 | 3,156 (24.3) | 296 (30.3) | 364 (27.9) | |
| 10-14 | 4,136 (27.0) | 326 (28.0) | 382 (23.1) | |
| ≥15 | 3,541 (22.4) | 211 (17.0) | 268 (15.3) | |
| Sex | 0.85 | |||
| Male | 7,500 (53.6) | 574 (53.3) | 727 (52.9) | |
| Female | 6,460 (46.4) | 518 (46.7) | 678 (47.1) | |
| Age at study, y | <0.001 | |||
| 20-29 | 3,271 (26.6) | 399 (38.4) | 511 (42.2) | |
| 30-39 | 4,949 (37.1) | 441 (41.0) | 581 (39.9) | |
| 40-49 | 3,774 (24.0) | 192 (15.8) | 242 (13.8) | |
| 50-59 | 1,742 (10.9) | 57 (4.5) | 67 (3.8) | |
| 60-69 | 224 (1.4) | 3 (0.2) | 4 (0.2) | |
| Educational attainment | <0.001 | |||
| Less than high school or GED | 932 (7.0) | 114 (11.5) | 158 (11.5) | |
| High school diploma | 2,467 (18.2) | 294 (27.1) | 338 (24.9) | |
| Some college or vocational | 4,857 (36.6) | 448 (42.1) | 533 (41.4) | |
| College graduate or postgraduate degree | 5,182 (38.3) | 202 (19.3) | 326 (22.3) | |
| Marital status | <0.001 | |||
| Married/living as married | 5,870 (43.3) | 253 (25.1) | 526 (37.6) | |
| Married formerly but not currently | 1,069 (8.0) | 128 (12.8) | 122 (8.7) | |
| Never married | 6,294 (48.7) | 588 (62.2) | 669 (53.7) | |
| Employment in the last year | <0.001 | |||
| Employed | 10,804 (85.6) | 676 (76.2) | 948 (78.3) | |
| Unemployed | 1,851 (14.4) | 224 (23.8) | 267 (21.7) | |
| Household incomea | <0.001 | |||
| <$20,000 | 1,153 (9.9) | 246 (30.7) | 181 (17.1) | |
| $20,000-$39,999 | 1,562 (13.4) | 184 (22.5) | 185 (17.2) | |
| $40,000-$59,999 | 1,634 (13.7) | 131 (15.5) | 203 (18.1) | |
| ≥$60,000 | 7,796 (63.1) | 270 (31.3) | 560 (47.5) | |
| Insurance status | <0.001 | |||
| Insured | 11,173 (84.7) | 732 (73.4) | 960 (71.0) | |
| Uninsured | 1,949 (15.3) | 268 (26.6) | 357 (29.0) | |
| Anthracycline doxorubicin equivalent dose, mg/m2 | <0.001 | |||
| None | 6,710 (50.0) | 375 (38.9) | 524 (39.1) | |
| 1-99 | 990 (13.7) | 101 (18.4) | 154 (18.3) | |
| 100-199 | 1,815 (15.3) | 186 (20.1) | 247 (21.7) | |
| 200-299 | 1,058 (7.8) | 97 (9.7) | 116 (8.2) | |
| ≥300 | 1,757 (13.1) | 127 (12.9) | 191 (12.7) | |
| Alkylating agent cyclophosphamide equivalent dose, mg/m2 | <0.001 | |||
| None | 5,789 (48.5) | 404 (45.9) | 519 (43.1) | |
| 1-3,999 | 1,333 (13.9) | 117 (15.2) | 199 (22.5) | |
| 4,000-7,999 | 1,646 (13.1) | 125 (12.9) | 169 (12.7) | |
| 8,000-11,999 | 1,249 (10.9) | 107 (14.3) | 134 (10.2) | |
| 12,000-15,999 | 723 (5.5) | 50 (4.9) | 57 (4.0) | |
| 16,000-19,999 | 415 (3.1) | 22 (2.2) | 39 (2.7) | |
| ≥20,000 | 682 (5.0) | 48 (4.7) | 72 (4.7) | |
| Chest-directed radiotherapy dose, Gy | <0.001 | |||
| None | 8,867 (74.1) | 668 (78.0) | 946 (79.8) | |
| 1-9.9 | 103 (0.9) | 4 (0.7) | 10 (1.0) | |
| 10-19.9 | 616 (4.9) | 50 (5.4) | 74 (5.7) | |
| 20-29.9 | 989 (6.9) | 73 (7.0) | 82 (5.0) | |
| ≥30 | 1,884 (13.1) | 92 (8.9) | 137 (8.4) | |
Values are n (%).
GED = general equivalency diploma; NH = non-Hispanic.
Household income values adjusted to 2020 dollar values.
CVRFs Among Survivors by Race/Ethnicity
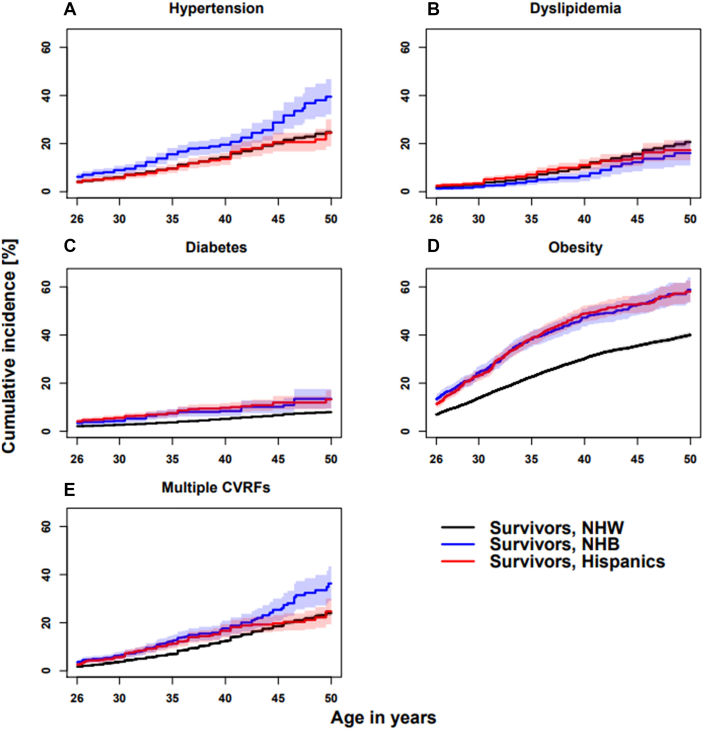
Figure 2 and Supplemental Table 1 show the cumulative incidence of each CVRF in the CCSS survivor cohort. By age 40, 19.5% (95% CI: 16.4%-22.7%) of NHB survivors reported hypertension compared with 14.3% (95% CI: 13.6%-15.0%) of NHW survivors and 13.6% (95% CI: 11.1%-16.1%) of Hispanic survivors (Figure 2A). NHB and Hispanic survivors reported a higher cumulative incidence of diabetes by 40 years of age (8.4% [95% CI: 6.3%-10.5%] and 9.7% [95% CI: 7.8%-11.7%]; P < 0.001, respectively) compared with 5.1% (95% CI: 4.7%-5.6%) of NHW survivors (Figure 2C). Approximately 47.2% of NHB (95% CI: 43.5%-50.9%) and 48.9% (95% CI, 45.6-52.3%) of Hispanic survivors were obese by 40 years of age in contrast to 30.2% (95% CI: 29.3%-31.1%) of NHW survivors (Figure 2D). Finally, 17.7% (95% CI: 14.5%-20.9%) of NHB survivors (P < 0.001) and 16.6% (95% CI: 13.8%-19.4%) of Hispanic survivors (P = 0.002) reported more than 1 CVRF compared with 12.3% (95% CI: 11.7%-13.0%) of NHW survivors by age 40 (Figure 2E).
Figure 2.
Cumulative Incidence (%) of CVRFs by Race/Ethnicity
Age is on the x-axis, and cumulative incidence is on the y-axis (95% CIs shaded). (A) Hypertension, (B) hyperlipidemia, (C) diabetes, (D) obesity, and (E) multiple CVRFs. Non-Hispanic White (NHW), non-Hispanic Black (NHB), and Hispanic cumulative incidence by age are represented in black, blue, and red, respectively. Each cardiovascular risk factor (CVRF) was determined based on a self-report of both being diagnosed by a physician and on medication based on Common Terminology Criteria for Adverse Events v.4.03 criteria. The cumulative incidence for each CVRF started at cohort entry (5 years after diagnosis) and ended at the first CVRF event of interest for each individual CVRF analysis or at a competing risk event, which included recurrence, subsequent malignant neoplasm, and death, or censored at the completion of the last questionnaire.
In multivariable analyses, NHB survivors reported an IRR for hypertension of 1.4 (95% CI: 1.1-1.8; Table 2) compared with NHW survivors. No statistically significant differences in hyperlipidemia were observed by race/ethnicity. For diabetes, NHB survivors and Hispanic survivors reported IRRs of 1.6 (95% CI: 1.0-2.7) and 1.8 (95% CI: 1.2-2.6) compared with NHW survivors. Similarly, NHB and Hispanic survivors reported IRRs of 1.7 (95% CI: 1.4-2.1) and 1.4 (95% CI: 1.2-1.7) for obesity. Finally, NHB survivors also reported an IRR of 1.6 (95% CI: 1.2-2.1) for multiple CVRFs compared with the referent NHW survivor population after controlling for sociodemographic factors.
Table 2.
Adjusteda IRRs of CVRFs Among Survivors by Race/Ethnicity
| Hypertension |
Hyperlipidemia |
Diabetes |
Obesity |
Multiple CVRFs |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of Events (Rateb) | IRR (95% CI) | Number of Events (Rateb) | IRR (95% CI) | Number of Events (Rateb) | IRR (95% CI) | Number of Events (Rateb) | IRR (95% CI) | Number of Events (Rateb) | IRR (95% CI) | |
| White, non-Hispanic, referent | 1,710 (1.85) | 1.00 | 1,385 (2.01) | 1.00 | 491 (0.42) | 1.00 | 3,415 (2.97) | 1.00 | 1,635 (2.35) | 1.00 |
| Black, non-Hispanic | 139 (2.61) | 1.41 (1.10-1.80) | 55 (1.63) | 0.81 (0.54-1.23) | 51 (0.70) | 1.65 (1.04-2.75) | 352 (5.10) | 1.72 (1.43-2.07) | 129 (3.73) | 1.59 (1.20-2.10) |
| Hispanic | 107 (1.64) | 0.89 (0.67-1.17) | 88 (2.12) | 1.06 (0.78-1.44) | 75 (0.76) | 1.77 (1.23-2.56) | 427 (4.29) | 1.44 (1.24-1.68) | 135 (2.70) | 1.15 (0.89-1.49) |
CVRF = cardiovascular risk factor; IRR = incidence rate ratio.
Using multivariable piecewise exponential regression modeling, adjusted for age, sex, age at diagnosis, baseline household income, educational attainment, marital status, employment, insurance, anthracycline, alkylating agents, and chest-directed radiation therapy.
Adjusted incidence rate per 100 person-years at age 40 from the multivariable model by race/ethnicity. The rates shown are for survivors with the following set of covariate values: sex = male, diagnosis age = 5 to 9 years, income ≥$60,000, employed, insured, never married by baseline, some college or vocational, alkylating agent = yes, anthracycline = yes, and chest radiation therapy = no on the basis of the piecewise-exponential model.
Sibling and NHANES cohort comparison for CVRFs by race/ethnicity
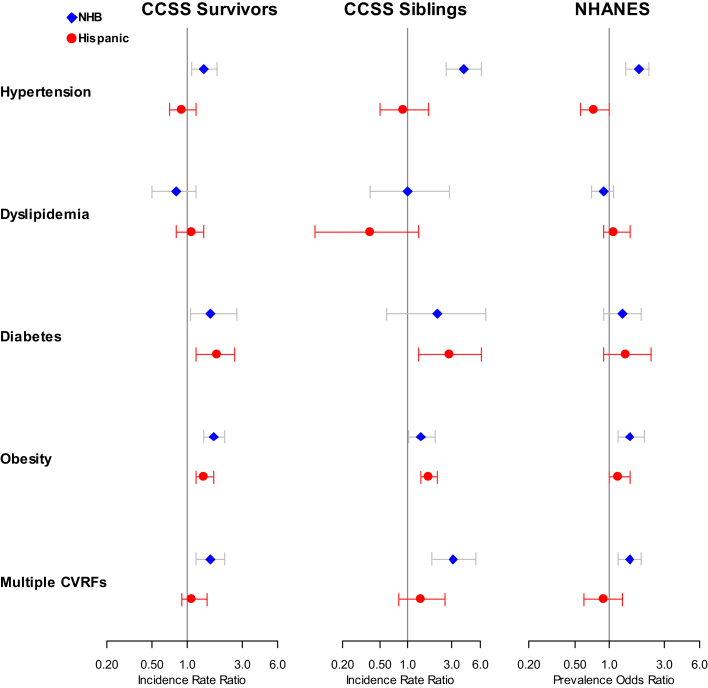
Supplemental Tables 2 and 3 display the demographics for the CCSS sibling and NHANES cohorts, respectively. The multivariable analysis results estimated an IRR of 4.0 (95% CI: 2.6-6.3) for hypertension, 1.4 (95% CI: 1.0-2.0) for obesity, and 3.1 (95% CI: 1.8-5.5) for multiple CVRFs among NHBs compared with the referent NHW sibling population (Supplemental Table 4). For Hispanic siblings, we estimated an IRR of 2.8 (95% CI: 1.3-6.2) for diabetes and 1.7 (95% CI: 1.4-2.1) for obesity compared with NHWs. Supplemental Table 5 displays the estimated adjusted prevalence ORs of 1.8 (95% CI: 1.4-2.4) for hypertension, 1.5 (95% CI: 1.2-2.0) for obesity, and 1.5 (95% CI: 1.2-1.9) for multiple CVRFs among NHB participants compared with NHW participants in the comparison cohort from NHANES. For Hispanic participants, prevalence ORs of 0.7 (95% CI: 0.6-1.0) and 1.4 (95% CI: 0.9-2.3) for hypertension and diabetes were estimated, respectively, compared with NHW participants. Figure 3 provides a visualization of these differences with point estimates and 95% CIs for the CCSS survivor, sibling, and NHANES cohorts. Similar racial/ethnic patterns emerged for hypertension, obesity, and multiple CVRFs.
Figure 3.
CVRFs by Race/Ethnicity for Each Cohort
A multivariable piecewise exponential model was used to compare incidence rate ratios to assess the magnitude of the same-race/same-ethnicity survivor-sibling differences between racial and ethnic groups, with modifications by generalized estimating equations to account for possible within-family correlation between survivors and siblings from the same families. Multivariable logistic regression was used in calculating prevalence odds ratios in National Health and Nutrition Examination Survey (NHANES) data. For all analyses, age, sex, household income, educational attainment, marital status, employment, and insurance were included in the multivariable adjustment. Point estimates and CIs for non-Hispanic Black (NHB) and Hispanic participants are in blue and red, respectively. CCSS = Childhood Cancer Survivor Study; CVRF = cardiovascular risk factor.
Survivor-sibling comparison of cardiovascular risk factors by race/ethnicity
We generally found that the magnitude of the difference between survivors and siblings did not differ by race/ethnicity (expressed as the ratio of the IRRs) (Table 3). The notable exception to this was for hypertension and multiple CVRFs among NHB participants. The ratio of the NHB vs NHW IRR for hypertension among the CCSS survivors compared with the CCSS NHB siblings was 0.4 (95% CI: 0.2-0.6), meaning that the difference among survivors was less than that observed in siblings. This was likely the driver for the lower ratio of the NHB vs NHW IRR for multiple CVRFs observed at 0.5 (95% CI: 0.3-0.9) among CCSS survivors compared with the CCSS NHB siblings.
Table 3.
Adjusteda Incidence Rate Ratios of Cardiovascular Risk Factors by Race/Ethnicity for Survivor-Sibling Comparison
| Hypertension |
Hyperlipidemia |
Diabetes |
Obesity |
Multiple CVRF |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| IRR (Surv vs Sib) | Ratio of IRR (95% CI) | IRR (Surv vs Sib) | Ratio of IRR (95% CI) | IRR (Surv vs Sib) | Ratio of IRR (95% CI) | IRR (Surv vs Sib) | Ratio of IRR (95% CI) | IRR (Surv vs Sib) | Ratio of IRR (95% CI) | |
| White, NH | 1.38 (1.23-1.55) | — | 1.47 (1.28-1.69) | — | 1.61 (1.25 - 2.08) | — | 0.61 (0.57-0.65) | — | 1.26 (1.12 - 1.42) | — |
| Black, NH | 0.53 (0.33-0.84) | 0.38 (0.24-0.61) | 1.24 (0.47-3.28) | 0.84 (0.32-2.25) | 1.71 (0.50-5.91) | 1.06 (0.30-3.74) | 0.69 (0.51-0.93) | 1.13 (0.83-1.53) | 0.69 (0.41-1.15) | 0.54 (0.32-0.92) |
| Hispanic | 1.45 (0.77-2.75) | 1.05 (0.55-2.01) | 4.24 (1.32-13.64) | 2.88 (0.89-9.33) | 1.20 (0.53-2.74) | 0.74 (0.31-1.76) | 0.57 (0.45-0.73) | 0.94 (0.73-1.21) | 1.20 (0.67-2.13) | 0.95 (0.53-1.70) |
Discussion
In this large, sociodemographically diverse population of childhood cancer survivors followed over 3 decades, we observed differences in the incidence of CVRFs based on race/ethnicity that were similar to those observed in the general population. Several important observations in the burden of CVRFs may inform the long-term care of survivors. Childhood cancer survivors who identified as NHB and Hispanic were more likely to have diabetes and obesity compared with NHW survivors. NHB survivors were more likely to have hypertension and multiple CVRFs than NHW survivors. These differences by race/ethnicity persisted despite the adjustment for treatment exposures and socioeconomic factors, thus motivating additional investigation into potential systemic causes of these differences and possibly differences in genetic predisposition to late cardiac outcomes, such as cardiomyopathy.30,31 In the United States, childhood cancer survivors in the CCSS demonstrate a substantial burden of disease for each CVRF with cumulative incidence estimates ranging from 5% (diabetes) to 50% (obesity) by age 40, thus further stressing the opportunity to promote equity in cardiovascular health on a population level.
Disparities in CVRFs by race/ethnicity within the general population are well-documented.14, 15, 16, 17,19 A previous analysis of NHANES data showed only a small proportion of racial and ethnic differences in cardiovascular health were attributable to socioeconomic characteristics.32 Our analyses suggest that the pattern of survivor-sibling differences in CVRFs is similar to that of the general population. This should prompt multilevel interventions to target the prevention and management of CVRFs that span population health, survivorship-focused care, and primary care to adapt evidence-based strategies applied to the general population. Our analysis of the NHANES comparison cohort showed similar differences with increased prevalence ORs of hypertension, obesity, and multiple CVRFs among NHB adults compared with NHW adults. Surprisingly, in contrast to previous reports in the literature, we did not observe a significantly increased prevalence OR for diabetes and obesity among Hispanic adults compared with NHW adults.19 The CCSS sibling cohort allowed direct comparison of incidence rates for CVRFs to quantify disparities within the survivor cohort to the corresponding sibling cohort with consistent data collection methods from the longitudinal questionnaires. Sibling data helped to mitigate unmeasured confounders from genetic, social, or environmental factors shared by families.33,34 In this analysis, the ratio of the IRR for most CVRFs by race/ethnicity suggested that the disparities within the magnitude of the survivor-sibling difference for NHB and Hispanic survivors were no greater than that of NHW survivors and siblings. The notable exception for this was among NHB survivors, who were less likely to report hypertension compared with NHB siblings. This merits additional investigation to validate these findings and explore potential causes for this observation.
Study limitations
The study design with high-quality data from both CCSS and NHANES over the last 2 decades supports their utility to characterize the differences in important CVRFs in each of these populations. Nevertheless, the main limitation of the NHANES data was the use of cross-sectional data that permitted only the estimation of the prevalence of each CVRF rather than the incidence that is possible from the CCSS longitudinal surveys. The different tools to measure CVRFs in CCSS and NHANES also necessitate caution when interpreting our results.25 As with all self-reported outcomes, possible detection bias may lead to underestimation of each CVRF in the CCSS cohort, although this bias could apply to both CCSS survivors and siblings.35 Potential for misclassification based on self-report for CVRFs may occur; however, this is expected to be nondifferential between survivors and siblings and among racial/ethnic groups.36,37 Beyond self-report, potential differences in the diagnosis and the treatment of CVRFs by race/ethnicity represent an additional limitation to this analysis. Moreover, CCSS included a significantly smaller proportion of NHB and Hispanic siblings, which may have limited the power to detect significant differences in CVRFs.38 Area-level measures for social determinants of health were also not considered, which represents another area for additional study.
In the general population, an estimated 70% of cardiac events are attributable to suboptimal cardiovascular health.23,39 Therefore, prompt identification and optimal management of CVRFs are critical in order to reduce the risk of subsequent CVD. Alongside the increased prevalence of diabetes within the general population, NHB and Hispanic adults were significantly less likely to attain adequate control of their diabetes or optimal targets for blood pressure compared with NHW adults.40 The American Heart Association identified structural racism as a major contributor to observed disparities in cardiovascular health.41 Because these analyses considered individual-level sociodemographic factors, area-level data may illuminate possible drivers. Survivors of childhood cancer represent an especially vulnerable population for these racial and ethnic inequities to exacerbate serious cardiac sequelae later in life. In the CCSS cohort, approximately 60% of NHB and Hispanic survivors received anthracyclines, a risk factor that is potentiated by hypertension and multiple CVRFs for the development of heart failure later in life.13 In a previous analysis by the CCSS, CVD disparities among NHB survivors were attenuated after controlling for CVRFs.3 Furthermore, CVD risk models were greatly enhanced by the inclusion of CVRFs to predict CVD.42 The clinical translation of these findings suggests that if inequities in CVRFs are addressed, downstream disparities in CVD could potentially also be mitigated.
Prevention, early detection, and management of modifiable CVRFs are essential to decrease CVD burden. Secondary prevention strategies in the general population provide a framework43, 44, 45, 46; yet, specific strategies for survivors are needed to increase the participation of under-represented minority patients in clinical trials for CVRF interventions.47 The Communicating Health Information and Improving Coordination with Primary Care is an ongoing randomized cardiovascular health promotion trial that targets survivors in the CCSS at high risk for CVD.48 The unraveling of barriers to care, evidence-based interventions for CVRF control, and concerted efforts to dismantle structural racism are vital to reduce disparities in CVRFs observed in the CCSS cohort with the overarching goal to achieve health equity among all survivors of childhood cancer.
Conclusions
We observed an increased burden of CVRFs among NHB and Hispanic survivors in the CCSS compared with NHW survivors. Although similar to disparities in the general population, the potential for these CVRFs to worsen therapy-related CVD motivates further actions to provide equitable survivorship-focused care for all survivors.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Adult survivors of childhood cancer demonstrate an increased burden of late cardiovascular morbidity and mortality potentiated by CVRFs and cardiotoxic therapy. The CCSS cohort showed similar disparities in CVRFs by race/ethnicity as those observed in the general population.
TRANSLATIONAL OUTLOOK: Although disparities in CVRFs by race/ethnicity among survivors were similar compared with the general population, the synergistic effects of cardiotoxic therapy emphasize the importance of primary prevention and the management of CVRFs in this high-risk population. Cardiovascular health equity, from CVRFs to CVD morbidity and mortality, for all survivors is critical. Future interventions are needed to mitigate disparities in CVRFs among NHB and Hispanic survivors.
Funding Support and Author Disclosures
This work was supported by grants from the National Cancer Institute (U24CA55727, PI: Dr Armstrong. Dr Noyd was supported by 5T32 HL007057-44, PI: G.M. Arepally). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support grant (CA21765, PI: C. Roberts) and the American Lebanese-Syrian Associated Charities. All authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Anne Blaes, MD, MS, served as the Guest Associate Editor for this paper. Paaladinesh Thavendiranathan, MD, MSc, served as the Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Hudson M.M., Neglia J.P., Woods W.G., et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatric Blood Cancer. 2012;58(3):334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta S., Wilejto M., Pole J.D., Guttmann A., Sung L. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q., Leisenring W.M., Ness K.K., et al. Racial/ethnic differences in adverse outcomes among childhood cancer survivors: the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(14):1634–1643. doi: 10.1200/jco.2015.66.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai E.W., Ward K.C., Bonaventure A., Siegel D.A., Coleman M.P. Survival among children diagnosed with acute lymphoblastic leukemia in the United States, by race and age, 2001 to 2009: findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5178–5189. doi: 10.1002/cncr.30899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S., Gibson T.M., Ness K.K., et al. Childhood cancer survivorship research in minority populations: a position paper from the Childhood Cancer Survivor Study. Cancer. 2016;122(15):2426–2439. doi: 10.1002/cncr.30072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mertens A.C., Liu Q., Neglia J.P., et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armstrong G.T., Chen Y., Yasui Y., et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;374(9):833–842. doi: 10.1056/NEJMoa1510795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates J.E., Howell R.M., Liu Q., et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37(13):1090–1101. doi: 10.1200/jco.18.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mulrooney D.A., Hyun G., Ness K.K., et al. Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. BMJ. 2020;368:l6794. doi: 10.1136/bmj.l6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meacham L.R., Chow E.J., Ness K.K., et al. Cardiovascular risk factors in adult survivors of pediatric cancer--a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19(1):170–181. doi: 10.1158/1055-9965.Epi-09-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao C., Bhatia S., Xu L., et al. Chronic comorbidities among survivors of adolescent and young adult cancer. J Clin Oncol. 2020;38(27):3161–3174. doi: 10.1200/jco.20.00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow E.J., Chen Y., Kremer L.C., et al. Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol. 2015;33(5):394–402. doi: 10.1200/jco.2014.56.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong G.T., Oeffinger K.C., Chen Y., et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. doi: 10.1200/jco.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–245. doi: 10.2174/1573403x11666141122220003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pool L.R., Ning H., Lloyd-Jones D.M., Allen N.B. Trends in racial/ethnic disparities in cardiovascular health among US adults from 1999-2012. J Am Heart Assoc. 2017;6(9) doi: 10.1161/jaha.117.006027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillespie C.D., Hurvitz K.A. Prevalence of hypertension and controlled hypertension - United States, 2007-2010. MMWR Suppl. 2013;62(3):144–148. [PubMed] [Google Scholar]
- 17.Min Y.I., Anugu P., Butler K.R., et al. Cardiovascular disease burden and socioeconomic correlates: findings from the Jackson Heart Study. J Am Heart Assoc. 2017;6(8) doi: 10.1161/jaha.116.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics–2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 19.Beckles G.L., Chou C.F. Diabetes - United States, 2006 and 2010. MMWR Suppl. 2013;62(3):99–104. [PubMed] [Google Scholar]
- 20.Akhabue E., Perak A.M., Chan C., Greenland P., Allen N.B. Racial differences in rates of change of childhood body mass index and blood pressure percentiles. J Pediatr. 2018;202:98–105.e6. doi: 10.1016/j.jpeds.2018.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietz W.H. The response of the US Centers for Disease Control and Prevention to the obesity epidemic. Annu Rev Public Health. 2015;36(1):575–596. doi: 10.1146/annurev-publhealth-031914-122415. [DOI] [PubMed] [Google Scholar]
- 22.Ogden C.L., Fryar C.D., Hales C.M., Carroll M.D., Aoki Y., Freedman D.S. Differences in obesity prevalence by demographics and urbanization in US children and adolescents, 2013-2016. JAMA. 2018;319(23):2410–2418. doi: 10.1001/jama.2018.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry J.D., Dyer A., Cai X., et al. Lifetime risks of cardiovascular disease. N Engl J Med. 2012;366(4):321–329. doi: 10.1056/NEJMoa1012848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison L.L., Armstrong G.T., Boice J.D., et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/jco.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T.C., Clark J., Riddles M.K., Mohadjer L.K., Fakhouri T.H.I. National Health and Nutrition Examination Survey, 2015-2018: sample design and estimation procedures. Vital Health Stat 2. 2020;184:1–35. [PubMed] [Google Scholar]
- 26.Green D.M., Nolan V.G., Goodman P.J., et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61(1):53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feijen E.A.M., Leisenring W.M., Stratton K.L., et al. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5(6):864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Institute NC. Common Terminology Criteria for Adverse Events. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- 29.Gray R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 30.Sapkota Y., Qin N., Ehrhardt M.J., et al. Genetic variants associated with therapy-related cardiomyopathy among childhood cancer survivors of African ancestry. Cancer Res. 2021;81(9):2556–2565. doi: 10.1158/0008-5472.Can-20-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Pavia P., Kim Y., Restrepo-Cordoba M.A., et al. Genetic variants associated with cancer therapy–induced cardiomyopathy. Circulation. 2019;140(1):31–41. doi: 10.1161/circulationaha.118.037934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teitler J., Wood B.M., Zeng W., Martinson M.L., Plaza R., Reichman N.E. Racial-ethnic inequality in cardiovascular health in the U.S.: does it mirror socioeconomic inequality? Ann Epidemiol. 2021;62:84–91. doi: 10.1016/j.annepidem.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frisell T., Öberg S., Kuja-Halkola R., Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology. 2012;23(5):713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- 34.Li Z., McKeague I.W., Lumey L.H. Optimal design strategies for sibling studies with binary exposures. Int J Biostat. 2014;10(2):185–196. doi: 10.1515/ijb-2014-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado-Rodríguez M., Llorca J. Bias. J Epidemiol Community Health. 2004;58(8):635–641. doi: 10.1136/jech.2003.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dey A.K., Alyass A., Muir R.T., et al. Validity of self-report of cardiovascular risk factors in a population at high risk for stroke. J Stroke Cerebrovasc Dis. 2015;24(12) doi: 10.1016/j.jstrokecerebrovasdis.2015.08.022. 2860-265. [DOI] [PubMed] [Google Scholar]
- 37.Khan A.R., Kim J.H., Ejaz K., et al. Abstract 17197: validity of self reported cardiovascular disease risk factors in African American adults. Circulation. 2019;140(suppl 1) doi: 10.1161/circ.140.suppl_1.17197. A17197-A17197. [DOI] [Google Scholar]
- 38.Leisenring W.M., Mertens A.C., Armstrong G.T., et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. doi: 10.1200/jco.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bundy J.D., Zhu Z., Ning H., et al. Estimated impact of achieving optimal cardiovascular health among US adults on cardiovascular disease events. J Am Heart Assoc. 2021;10(7) doi: 10.1161/jaha.120.019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang L., Li X., Wang Z., et al. Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999-2018. JAMA. 2021;326(8):1–13. doi: 10.1001/jama.2021.9883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Churchwell K., Elkind M.S.V., Benjamin R.M., et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. 2020;142(24):e454–e468. doi: 10.1161/cir.0000000000000936. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y., Chow E.J., Oeffinger K.C., et al. Traditional cardiovascular risk factors and individual prediction of cardiovascular events in childhood cancer survivors. J Natl Cancer Inst. 2020;112(3):256–265. doi: 10.1093/jnci/djz108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebrahim S., Taylor F., Ward K., Beswick A., Burke M., Davey Smith G. Multiple risk factor interventions for primary prevention of coronary heart disease. Cochrane Database Syst Rev. 2011;1:CD001561. doi: 10.1002/14651858.CD001561.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Driel M.L., Morledge M.D., Ulep R., Shaffer J.P., Davies P., Deichmann R. Interventions to improve adherence to lipid-lowering medication. Cochrane Database Syst Rev. 2016;12:CD004371. doi: 10.1002/14651858.CD004371.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akinosun A.S., Polson R., Diaz-Skeete Y., et al. Digital technology interventions for risk factor modification in patients with cardiovascular disease: systematic review and meta-analysis. JMIR Mhealth Uhealth. 2021;9(3) doi: 10.2196/21061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kemp B.J., Thompson D.R., Watson C.J., McGuigan K., Woodside J.V., Ski C.F. Effectiveness of family-based eHealth interventions in cardiovascular disease risk reduction: a systematic review. Prev Med. 2021;149 doi: 10.1016/j.ypmed.2021.106608. [DOI] [PubMed] [Google Scholar]
- 47.Russo C., Stout L., House T., Santana V.M. Barriers and facilitators of clinical trial enrollment in a network of community-based pediatric oncology clinics. Pediatr Blood Cancer. 2020;67(4) doi: 10.1002/pbc.28023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chow E.J., Baldwin L.M., Hagen A.M., et al. Communicating health information and improving coordination with primary care (CHIIP): rationale and design of a randomized cardiovascular health promotion trial for adult survivors of childhood cancer. Contemp Clin Trials. 2020;89 doi: 10.1016/j.cct.2019.105915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.