Abstract
Background
The long-term risk of coronary heart disease (CHD) and clinical models that predict this risk remain understudied in blood or marrow transplantation (BMT) recipients.
Objectives
This study sought to examine the risk of CHD after BMT and identify the associated risk factors.
Methods
Participants included patients transplanted between 1974 and 2014 at City of Hope, University of Minnesota, or University of Alabama at Birmingham and those who survived ≥2 years after BMT. Multivariable logistic regression models assessed CHD risk in BMT survivors compared with a sibling cohort. A self-reported questionnaire and medical records provided information regarding sociodemographics, comorbidities, and therapeutic exposures, which were used to develop a CHD risk prediction nomogram.
Results
Overall, 6,677 BMT recipients participated; the mean age at BMT was 43.9 ± 17.7 years, 58.3% were male, and 73.3% were non-Hispanic Whites. The median length of follow-up was 6.9 years (range: 2-46.2 years) from BMT. CHD was reported in 249 participants, with a 20-year cumulative incidence of 5.45% ± 0.39%. BMT survivors had a 1.6-fold greater odds of CHD compared with a sibling cohort (95% CI: 1.09-2.40). A nomogram was then developed to predict the risk of CHD at 10 and 20 years after BMT including age at BMT (HR: 1.06/y; 95% CI: 1.04-1.08), male sex (HR: 1.89; 95% CI: 1.15-3.11), a history of smoking (HR: 1.61; 95% CI: 1.01-2.58), diabetes (HR: 2.45; 95% CI: 1.23-4.89), hypertension (HR: 2.02; 95% CI: 1.15-3.54), arrhythmia (HR: 1.90; 95% CI: 0.89-4.06), and pre-BMT chest radiation (yes vs no: HR: 2.83; 95% CI: 1.20-6.67; unknown vs no: HR: 0.88; 95% CI: 0.34-2.28). The C-statistic was 0.77 in the test set (95% CI: 0.70-0.83).
Conclusions
This study identified BMT recipients at high risk for CHD, informing targeted screening for early detection and aggressive control of risk factors.
Key Words: blood or marrow transplantation, cancer survivorship, coronary artery disease, risk prediction
Central Illustration
Coronary heart disease (CHD) is the leading cause of morbidity and mortality in the United States.1 Risk factors for CHD in the general population include hypertension, diabetes, dyslipidemia (referred to as cardiovascular risk factors [CVRFs]), and smoking.1,2 Blood or marrow transplantation (BMT) recipients are vulnerable to new-onset CVRFs because of high-intensity therapeutic exposures including total body irradiation (TBI).3, 4, 5, 6, 7 TBI and chest radiation are associated with accelerated atherosclerosis8,9; endocrinopathies and chronic graft-versus-host disease (cGvHD)-induced inflammation further increase the risk.10, 11, 12 However, the long-term risk of CHD and the associated risk factors in BMT recipients remain understudied. The majority of the previous studies that assessed the risk of CHD in BMT recipients were single-center studies with small sample sizes and a short follow-up or did not examine CVRFs and therapeutic exposures.6,11,13, 14, 15 Using a large cohort study, the BMTSS (Bone Marrow Transplant Survivor Study), we determined the risk of CHD after BMT and sought to identify the association between CHD and sociodemographic characteristics, CVRFs, and other comorbidities as well as pre-BMT and BMT-related therapeutic exposures. We used this information to develop a risk prediction nomogram to identify BMT survivors at risk for clinically overt CHD.
Methods
Study population
BMTSS includes patients transplanted between January 1, 1974, and December 31, 2014, at City of Hope, University of Minnesota, or University of Alabama at Birmingham. BMTSS aims to examine the long-term health outcomes in individuals who survived ≥2 years after undergoing BMT regardless of the current vital status. The University of Alabama at Birmingham Institutional Review Board serves as the single Institutional Review Board of record. The study participants provided informed consent according to the Declaration of Helsinki.
The National Death Index (NDI) and Accurint database were used to determine the date of death for patients who died after surviving ≥2 years after BMT; the NDI Plus was used for determining the cause of death.16,17 Patients who were alive after surviving ≥2 years after BMT were invited to complete the BMTSS survey. Participants were asked to report chronic health conditions diagnosed by their health care provider along with age at diagnosis, medications, history of cGvHD, relapse of primary cancer, and subsequent neoplasms. Participants self-reported key sociodemographic characteristics (sex, race/ethnicity, education, household income, and health insurance), health behaviors (smoking, alcohol use, and physical activity), height, and weight at study participation.18 Information regarding primary cancer diagnosis, therapeutic exposures (pre-BMT and BMT related), donor type (autologous or allogeneic), and stem cell source (bone marrow, cord blood, or peripheral blood stem cells [PBSCs]) was abstracted from medical records. Details regarding these variables are provided in Supplemental Table 1. BMT participants provided information of siblings willing to serve as a non-BMT comparison group. Siblings completed a survey including sociodemographic and health conditions but without BMT-specific questions.
Primary outcome
Alive participants
A diagnosis of clinically overt CHD was based on positive responses to BMTSS survey questions that asked if participants had hardening of the arteries, coronary artery disease, or angina (chest pain caused by a lack of oxygen to the heart requiring nitroglycerin). This was confirmed by self-reported history of percutaneous coronary intervention or coronary artery bypass surgery.
Deceased patients
We considered a deceased patient to have CHD if CHD was included as a cause of death on their death record (NDI or medical records); the age of death served as the age at onset of CHD.
Statistical analysis
We used SAS software v9.4 (SAS Institute Inc). Descriptive statistics were summarized as appropriate and presented as counts with percentages, mean ± SD, and median with range. We used the 2-sample t-test or Wilcoxon rank sum test (for continuous variables) and the chi-square test (for categoric variables) to compare differences between groups. Two-sided tests with P < 0.05 were considered statistically significant.
Risk of CHD in BMT survivors vs siblings
We used logistic regression to examine the risk of CHD in BMT survivors compared with siblings, adjusting for age at survey participation, sex, race/ethnicity, education, annual household income, health behaviors, body mass index (BMI), and comorbidities. The risk was expressed as ORs with 95% CIs.
Risk of CHD in BMT recipients
We calculated the cumulative incidence of CHD in BMT recipients, conditional on surviving ≥2 years after BMT, using Fine and Gray methods, treating death caused by causes other than CHD as competing risk.19 Using proportional subdistribution hazards regression analysis,20 we examined the following factors for association with CHD: age at BMT, sex, race/ethnicity, primary cancer type, relapse risk (high vs standard), BMT type (allogeneic vs autologous), stem cell source, pre-BMT therapeutic exposures, conditioning agents, and cGvHD. We also performed subanalyses restricted to BMT recipients who were alive at the time of the study using multivariable Cox regression to examine the association between CHD and the previously described risk factors, comorbidities, BMI, and health behaviors.
Age at BMT and BMI were treated as continuous variables; the remaining variables were considered categoric. We categorized radiation exposure to the neck, chest, cranium, spine, abdomen, pelvis, and extremities; TBI and total lymphoid irradiation as yes vs no and recorded the total radiation dose for each field. We treated comorbidities, health behaviors, relapse of primary cancer, or the development of subsequent neoplasms and cGvHD (in allogeneic BMT recipients) as time-varying covariates in the association analysis. For patients who did not report their age at the occurrence of the event, we used mean imputation to impute the age of onset with values from patients with the same age, primary diagnosis, and era of BMT.21 Parsimonious models using backward variable selection were created, keeping variables with P < 0.1 from the multivariable analysis in the model.20
Backward variable selection guided by minimizing the Akaike information criterion was used to create a risk prediction nomogram for CHD in alive participants including all variables significantly associated with CHD in the association analyses; comorbidities and health behaviors were considered as risk factors if present at the time of BMT. The data set was split into training and test sets in a 6:4 ratio. The overall calibration of the prediction model was measured by a model-based goodness-of-fit test (Terry’s model) for time-to-event data within the training set.22 The model was developed in the training set, whereas the discrimination of the model was assessed within the test set by the index of concordance, or C-statistic, which was obtained by Harrell’s method and takes censoring into account in time-to-event models. We used the ‘rms’ and ‘DynNom’ package in the RStudio program (RStudio Team) to generate a nomogram for the final predictive model.23
Results
Of the 8,917 eligible BMT recipients, 2,801 (31.4%) died after surviving ≥2 years post-BMT and were included in the study. Among the 6,116 alive at the study initiation, 838 (13.7%) were lost to follow-up. Of the 5,278 BMT survivors approached, 1,340 (25.4%) refused participation, and 3,938 (74.6%) participated by completing the BMTSS survey; 62 (1.6%) had a history of CHD before BMT and were excluded, yielding a sample of 6,677 BMT recipients (alive and deceased) in this analysis (Supplemental Figure 1). The characteristics of BMTSS participants and nonparticipants are shown in Supplemental Table 2. Older, non-Hispanic Whites, autologous BMT recipients, and patients transplanted before 2000 were more likely to participate in the study.
The cohort characteristics overall and by vital status are summarized in Table 1. The mean age at BMT was 43.9 ± 17.7 years; 58.3% were male, and 73.3% were non-Hispanic White. Alive participants were transplanted at an older age compared with those who were deceased, and there were more women and non-Hispanic Whites among the alive participants. The majority of study participants received PBSCs (66.3%) for stem cells, and 4.7% had a history of chest radiation before BMT (56% with Hodgkin lymphoma). More participants in the deceased cohort received PBSCs and myeloablative conditioning compared with the alive participants. The median length of follow-up was 6.9 years (range: 2-46.2 years) from BMT for the 6,677 BMT recipients, whereas it was 9.2 years (range: 2-46 years) for the alive BMT survivors.
Table 1.
Demographic and Clinical Characteristics of BMT Survivors and Siblings
| All Patients (N = 6,677) | Alive Patients (n = 3,876) | Deceased Patients (n = 2,801) | P Value (Alive vs Deceased) | Siblings (n = 1,341) | P Value (Alive vs Siblings) | |
|---|---|---|---|---|---|---|
| Coronary heart disease | 249 (3.7) | 130 (3.4) | 119 (4.3) | <0.001 | 34 (2.5) | 0.14 |
| Age at study participation, y | NA | 52.0 ± 17.27 | NA | NA | 55.4 ± 14.7 | 0.007 |
| Year of BMT | ||||||
| In or before year 2000 | 2,556 (38.3) | 1,431 (36.9) | 1,125 (40.2) | 0.007 | — | — |
| After 2000 | 4,121 (61.7) | 2,445 (63.1) | 1,676 (59.8) | — | ||
| Sex | ||||||
| Male | 3,891 (58.3) | 2,167 (55.9) | 1,724 (61.6) | <0.001 | 530 (39.5) | <0.001 |
| Race/ethnicity | ||||||
| Non-Hispanic Whites | 4,897 (73.3) | 2,909 (75.1) | 1,988 (71.0) | <0.001 | 1,158 (86.4) | <0.001 |
| Hispanic | 847 (12.7) | 460 (11.9) | 387 (13.8) | 91 (6.8) | ||
| Black | 442 (6.6) | 189 (4.9) | 253 (9.0) | 32 (2.4) | ||
| Asian | 339 (5.1) | 210 (5.4) | 129 (4.6) | 35 (2.6) | ||
| Other | 152 (2.3) | 108 (2.8) | 44 (1.6) | 25 (1.9) | ||
| Education | ||||||
| ≤ High school | NA | 856 (22.1) | NA | NA | 161 (12.0) | <0.001 |
| Some college | NA | 1,380 (35.6) | NA | NA | 451 (33.6) | |
| College graduate | NA | 1583 (40.8) | NA | NA | 719 (53.6) | |
| Missing | NA | 856 (22.1) | NA | NA | 10 (0.8) | |
| Annual income | ||||||
| >$20,000 | NA | 2,905 (75.0) | NA | NA | 1,123 (83.7) | <0.001 |
| ≤$20,000 | NA | 434 (11.2) | NA | NA | 82 (6.11) | |
| Missing | NA | 537 (13.9) | NA | NA | 136 (10.1) | |
| Health behaviors | ||||||
| Smoking, yes | NA | 1,301 (33.6) | NA | NA | 432 (32.2) | 0.37 |
| Alcohol, yes | NA | 2,012 (51.9) | NA | NA | 771 (57.5) | <0.001 |
| Age at BMT, y | 43.9 ± 17.7 | 49.7 ± 15.0 | 37.7 ± 18.1 | <0.001 | — | — |
| Type of BMT | ||||||
| Allogeneic | 3,232 (48.4) | 2,067 (53.3) | 1,165 (41.6) | <0.001 | — | — |
| Autologous | 3,445 (51.6) | 1,809 (46.7) | 1,636 (58.4) | — | ||
| Indication for BMT | ||||||
| Non-Hodgkin lymphoma | 1,544 (23.1) | 954 (24.6) | 590 (21.1) | <0.001 | — | — |
| Acute myeloid leukemia/myelodysplasia | 1,420 (21.3) | 920 (23.7) | 500 (17.9) | — | ||
| Plasma cell dyscrasias | 1,326 (19.9) | 581 (15.0) | 745 (26.6) | — | ||
| Chronic myelogenous leukemia | 586 (8.8) | 367 (9.5) | 219 (7.8) | — | ||
| Hodgkin lymphoma | 492 (7.4) | 260 (6.7) | 232 (8.3) | — | ||
| Acute lymphoblastic leukemia | 604 (9.1) | 348 (9.0) | 256 (9.1) | — | ||
| Severe aplastic anemia | 178 (2.7) | 136 (3.5) | 42 (1.5) | — | ||
| Other | 526 (7.9) | 310 (8.0) | 216 (7.7) | — | ||
| Missing | 1 (0) | 0 (0) | 1 (0) | — | ||
| Stem cell source | ||||||
| Cord blood | 370 (5.5) | 261 (6.7) | 109 (3.9) | <0.001 | — | — |
| Peripheral blood stem cells | 4,425 (66.3) | 2,424 (62.5) | 2,001 (71.4) | — | ||
| Bone marrow | 1,882 (28.2) | 1,191 (30.7) | 691 (24.7) | — | ||
| Chronic graft-versus-host disease | ||||||
| Yes | 1,795 (26.9) | 1,049 (27.1) | 746 (26.6) | <0.001 | — | — |
| Select therapeutic exposures | ||||||
| Total body irradiationa | 3,020 (45.4) | 1,850 (47.8) | 1,170 (42.1) | <0.001 | — | — |
| Pre-BMT chest radiationb | 216 (4.7) | 140 (4.3) | 76 (5.7) | 0.048 | — | — |
| Pre-BMT anthracyclinesb | 3,075 (66.8) | 2212 (67.8) | 863 (64.2) | 0.018 | — | — |
| Conditioning intensity | ||||||
| Myeloablative conditioning | 5,156 (77.8) | 2,943 (75.9) | 2,213 (79.0) | <0.001 | — | — |
| Nonmyeloablative or reduced intensity conditioning | 1,468 (22.2) | 927 (23.9) | 541 (19.3) | — | ||
| Missing/other | 53 (0.8) | 6 (0.2) | 47 (1.7) | — | ||
| Body mass index, kg/m2 | NA | 26.26 ± 5.8 | NA | NA | 27.66 ± 5.9 | 0.007 |
| Comorbiditiesc | ||||||
| Diabetes | NA | 486 (13.0) | NA | NA | 62 (4.7) | <0.001 |
| Hypertension | NA | 1011 (29.3) | NA | NA | 295 (23.2) | <0.001 |
| Dyslipidemia | NA | 860 (25.0) | NA | NA | 275 (21.5) | 0.014 |
| Chronic kidney disease | NA | 157 (4.1) | NA | NA | 60 (4.5) | 0.56 |
| Stroke | NA | 125 (3.2) | NA | NA | 21 (1.6) | 0.002 |
| Arrhythmia | NA | 363 (9.6) | NA | NA | 92 (6.9) | 0.004 |
| Venous thromboembolism | NA | 356 (9.3) | NA | NA | 43 (3.2) | <0.001 |
Values are n (%) or mean ± SD.
— = variables that were not relevant to siblings; BMT = blood or marrow transplantation; NA = not available.
Total body irradiation information was missing for 24 participants (3 alive and 21 deceased participants).
Pre-BMT treatment information was missing in 2,072 participants (in 615 alive and 1,457 deceased participants).
Comorbidities information was missing in <5% participants.
CHD risk in BMT survivors compared with siblings
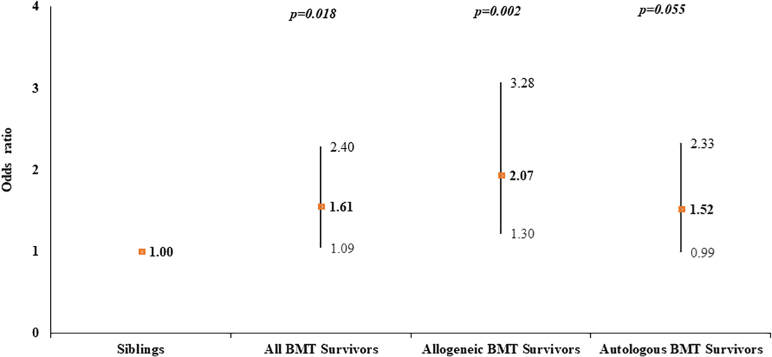
The characteristics of the 3,876 alive BMT survivors and 1,341 siblings are provided in Table 1. Siblings were more likely to be older, female, and non-Hispanic White and have higher education and income. BMT survivors were more likely to have comorbidities including diabetes, hypertension, dyslipidemia, stroke, arrhythmia, and venous thromboembolism. After adjusting for sociodemographics, comorbidities, and health behaviors, BMT survivors were at 1.61-fold greater odds of CHD compared with siblings (95% CI: 1.09-2.40) (Supplemental Table 3). Allogeneic BMT survivors were at 2.07-fold greater odds (95% CI: 1.30-3.28) and autologous BMT recipients at 1.52-fold higher odds (95% CI: 0.99-2.33) of CHD compared with siblings (Figure 1).
Figure 1.
Risk of CHD in BMT Survivors Compared to Siblings
Logistic regression analysis was used to compare the risk of coronary heart disease (CHD) in bone or marrow transplantation (BMT) survivors with the sibling cohort and was adjusted for age, sex, race/ethnicity, education, annual household income, smoking and alcohol history, body mass index, and comorbidities. BMT survivors were at 1.61-fold greater odds of CHD; allogeneic BMT survivors were at 2.07-fold and autologous BMT survivors at 1.52-fold higher odds of CHD compared with siblings.
Risk of CHD in BMT recipients
Overall, 249 BMT recipients developed CHD after a median of 7.1 years (range: 0-32 years) from BMT. The 20-year cumulative incidence of CHD was 5.45% ± 0.39% (Supplemental Figure 2A). Older age at BMT (HR: 1.04/y; 95% CI: 1.03-1.05), male sex (HR: 1.77; 95% CI: 1.35-2.34), diagnosis of chronic myelogenous leukemia (HR: 2.14; 95% CI: 1.01-4.53), and pre-BMT chest radiation (HR: 2.28; 95% CI: 1.37-3.81) were associated with increased CHD risk (Table 2). Among BMT recipients who were alive at study participation, the 20-year cumulative incidence of CHD (5.94% ± 0.64%) was comparable to the entire cohort (Supplemental Figure 3A). Similar to the overall cohort, older age at BMT, male sex, and chest radiation were associated with increased CHD risk (Table 2).
Table 2.
Risk Factors Associated With Coronary Heart Disease in BMT Recipients
| All BMT Recipients (Alive and Deceased) Parsimonious Modela |
All BMT Recipients Parsimonious Modela |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age at BMT | ||||||
| Per year increase in age | 1.04 | 1.03-1.05 | <0.001 | 1.05 | 1.03-1.06 | <0.001 |
| Sex (ref: female) | ||||||
| Male | 1.77 | 1.35-2.34 | <0.001 | 1.80 | 1.22-2.64 | 0.003 |
| Smoking at BMT (ref: no) | ||||||
| Current or past smoking | — | — | — | 1.54 | 1.08-2.20 | 0.018 |
| Comorbidities (ref: absence of specific comorbidity) | ||||||
| Diabetes | — | — | — | 1.76 | 1.15-2.69 | 0.009 |
| Hypertension | — | — | — | 1.52 | 1.04-2.22 | 0.029 |
| Dyslipidemia | — | — | — | 1.83 | 1.23-2.72 | 0.003 |
| Primary cancer diagnosis (ref: acute lymphoblastic leukemia) | ||||||
| Chronic myelogenous leukemia | 2.14 | 1.01-4.53 | 0.046 | NR | NR | NR |
| Non-Hodgkin lymphoma | 1.46 | 0.71-3.00 | 0.30 | NR | NR | NR |
| Acute myeloid leukemia/myelodysplasia | 1.69 | 0.82-3.47 | 0.15 | NR | NR | NR |
| Plasma cell dyscrasias | 1.21 | 0.58-2.53 | 0.61 | NR | NR | NR |
| Hodgkin lymphoma | 2.01 | 0.93-4.36 | 0.078 | NR | NR | NR |
| Severe aplastic anemia | 1.99 | 0.67-5.89 | 0.21 | NR | NR | NR |
| Other | 0.56 | 0.12-2.67 | 0.47 | NR | NR | NR |
| Pre-BMT chest radiation (ref: no chest radiation) | ||||||
| Yes chest radiation | 2.28 | 1.37-3.81 | 0.002 | 3.26 | 1.73-6.14 | <0.001 |
— = variables that were not included in the model including all BMT recipients because there was no information on comorbidities and health behaviors for the deceased patients; BMT = blood or marrow transplant; NR = variable was not retained in the parsimonious model in alive BMT recipients.
Potential covariates examined in univariable analysis included age at BMT, sex, race/ethnicity, cancer type, BMT type (allogeneic vs autologous), stem cell source, disease status, and therapeutic exposures (total body irradiation, conditioning intensity, and pre-BMT chemotherapy and radiation) in all participants. In addition, the following potential covariates were examined among alive participants: education, annual household income, comorbidities (diabetes, hypertension, dyslipidemia, chronic kidney disease, venous thromboembolism, arrhythmia, stroke, and relapse of primary cancer or secondary neoplasm), body mass index at the time of survey, and health behaviors (smoking and alcohol use). Multivariable analysis was obtained using covariates with P < 0.10 in the univariable analysis; we then obtained a parsimonious model using backward variable selection using covariates with P < 0.10 in multivariable analysis. Only risk factors that are statistically significant are shown in this table.
The cohort characteristics for autologous and allogeneic BMT recipients are provided in Supplemental Table 4. Importantly, autologous BMT recipients were older at BMT and more likely to receive pre-BMT chest radiation, myeloablative conditioning, and PBSCs. Over half (55.5%) of the allogeneic BMT recipients carried a history of cGvHD. The median length of follow-up was 8.2 years (range: 2-46.2 years) for allogeneic and 6.3 years (range: 2-35.6 years) for autologous BMT recipients. The 20-year cumulative incidence of CHD was 4.61 ± 0.51% for allogeneic BMT recipients and 6.44 ± 0.63% for autologous BMT recipients (P < 0.001) (Supplemental Figure 2B). Older age at BMT (HRallogeneic: 1.04/y; 95% CI: 1.03-1.05; HRautologous: 1.04/y; 95% CI: 1.02-1.05) and male sex (HRallogeneic: 1.80; 95% CI: 1.18-2.74; HRautologous: 1.64; 95% CI: 1.14-2.35) were associated with increased CHD risk (Supplemental Tables 5 and 6). Chest radiation was associated with increased CHD risk in autologous BMT recipients (HRautologous: 2.92; 95% CI: 1.73-4.92); for every 10-Gy increase in dose of chest radiation, the risk of CHD increased by 21% (HRautologous: 1.21; 95% CI: 1.11-1.30; P < 0.001). Among patients who were alive at study participation, the 20-year cumulative incidence of CHD was similar to the entire cohort (4.13% ± 0.68% for allogeneic and 8.83% ± 1.31% for autologous BMT survivors; P < 0.001) (Supplemental Figure 3B). Risk factors by BMT type in alive patients are shown in Supplemental Tables 7 and 8 and were comparable to those for the entire cohort.
Role of CVRFs in the risk of CHD
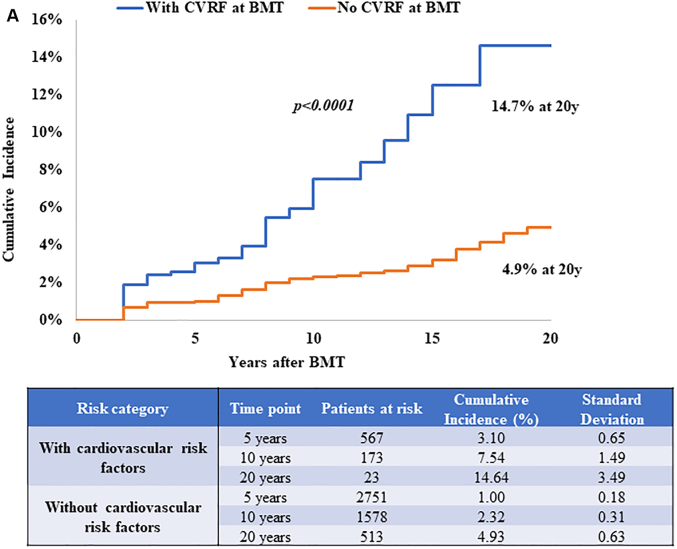
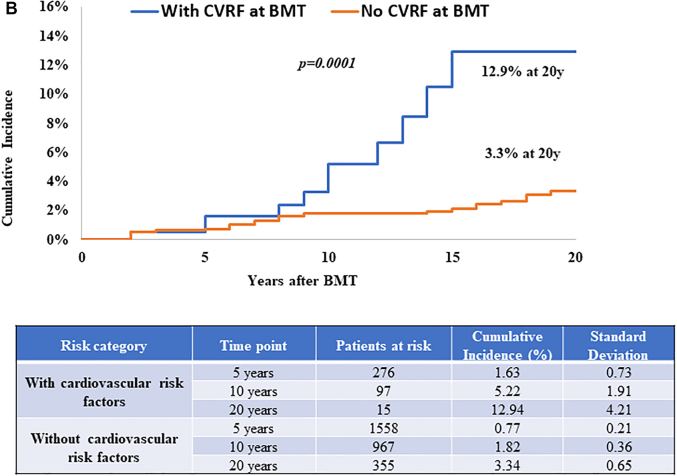
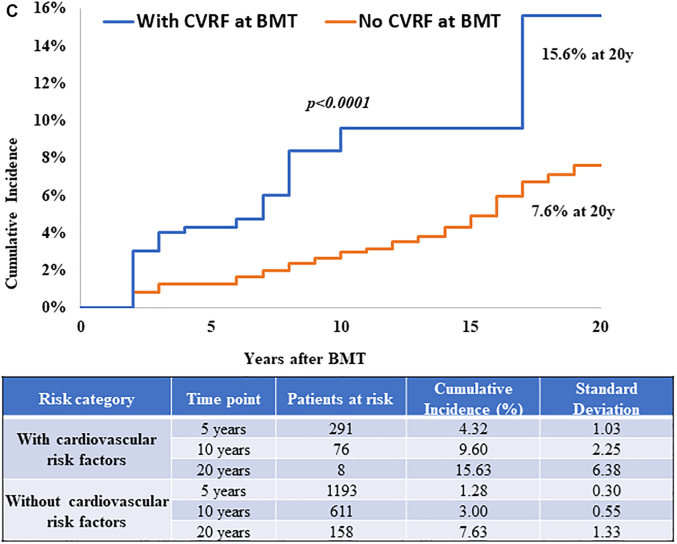
The role of CVRFs was evaluable only among those who were alive at study participation because the source of this information was from self-report. Diabetes (HR: 1.76; 95% CI: 1.15-2.69), hypertension (HR: 1.52; 95% CI: 1.04-2.22), dyslipidemia (HR: 1.83; 95% CI: 1.23-2.72), and smoking (HR: 1.54; 95% CI: 1.08-2.20) were associated with increased CHD risk (Table 2). When examining by BMT type, diabetes (HRallogeneic: 1.89; 95% CI: 1.02-3.48), hypertension (HRallogeneic: 3.25; 95% CI: 1.74-6.06), smoking (HRautologous: 1.78; 95% CI: 1.12-2.84), and dyslipidemia (HRautologous: 1.69; 95% CI: 1.02-2.79) were associated with increased CHD risk (Supplemental Tables 7 and 8). The 20-year cumulative incidence of CHD was 14.7% ± 3.5% for those with at least 1 CVRF at BMT compared with 4.9 ± 0.6% among those without CVRFs (P < 0.001) (Figure 2A). Similar findings were observed when examining this risk by BMT type. The 20-year cumulative incidence of CHD was 12.9% ± 4.2% vs 3.3% ± 0.7% (P < 0.001) among allogeneic BMT survivors (Figure 2B) and 15.6% ± 6.4% vs 7.6% ± 1.3% (P < 0.001) among autologous BMT survivors with and without CVRFs, respectively (Figure 2C). These estimates were similar when CVRFs were assessed at 2 years after BMT (results not shown). Both pre-BMT CVRFs and new-onset CVRFs after BMT were associated with increased CHD risk as shown in Supplemental Table 9.
Figure 2.
CHD Incidence in BMT Survivors With and Without CVRFs
CVRFs included diabetes, hypertension, and dyslipidemia. (A) CHD incidence in alive BMT survivors by CVRFs. Cumulative incidence of CHD is shown in alive BMT survivors with (blue) and without (orange) CVRFs at BMT. (B) CHD incidence by CVRFs in alive allogeneic BMT survivors. The cumulative incidence of CHD is shown in alive allogeneic BMT survivors with (blue) and without (orange) CVRFs at BMT. (C) CHD incidence by CVRFs in alive autologous BMT survivors. The cumulative incidence of CHD is shown in alive autologous BMT survivors with (blue) and without (orange) CVRFs at BMT. Abbreviations as in Figure 1.
Risk prediction nomogram for CHD in BMT survivors
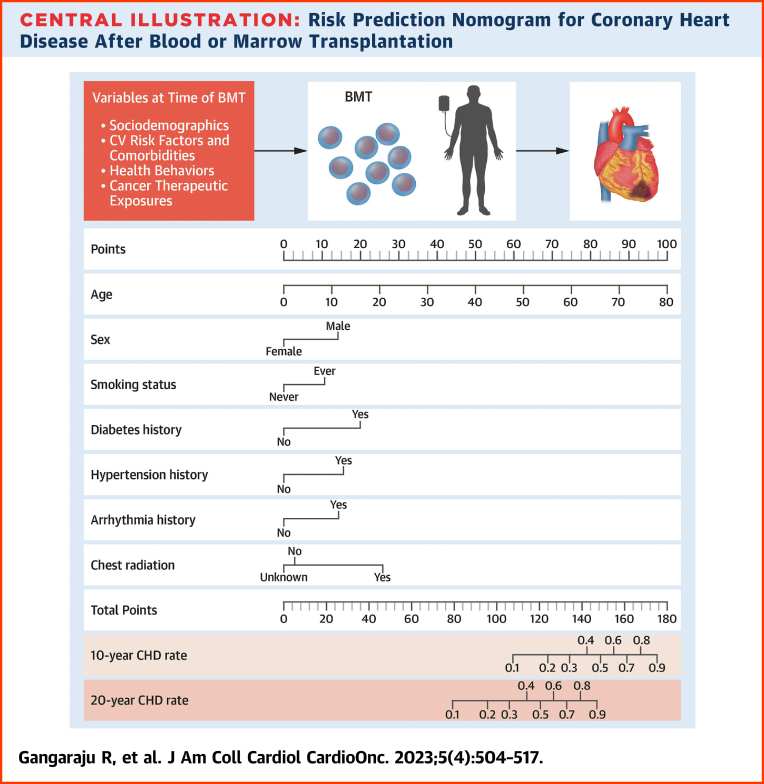
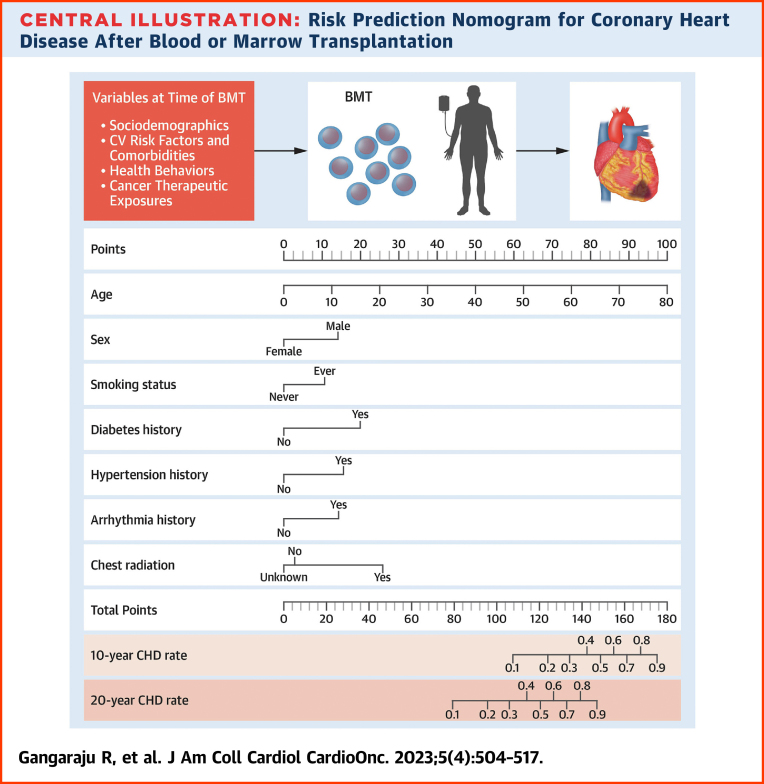
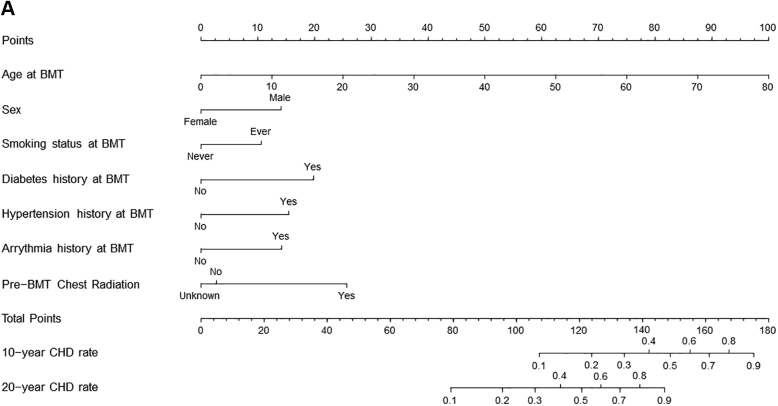
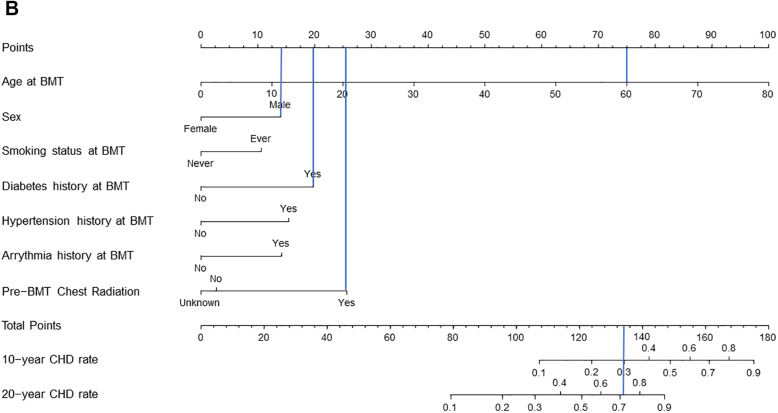
We created a risk prediction nomogram for CHD in alive participants including all variables significantly associated with CHD in the association analyses (Central Illustration); comorbidities and health behaviors were considered as risk factors if present at the time of BMT. The final model for CHD risk applied at BMT included age at BMT (HR: 1.06/y increase in age; 95% CI: 1.04-1.08), male sex (HR: 1.89; 95% CI: 1.15-3.11), history of smoking (HR: 1.61; 95% CI: 1.01-2.58), diabetes (HR: 2.45; 95% CI: 1.23-4.89), hypertension (HR: 2.02; 95% CI: 1.15-3.54), arrhythmia (HR: 1.90; 95% CI: 0.89-4.06), and pre-BMT chest radiation (yes vs no: HR: 2.83; 95% CI: 1.20-6.67; unknown vs no: HR: 0.88; 95% CI: 0.34-2.28) (Supplemental Table 10). The C-statistics were 0.80 (95% CI: 0.74-0.83) for the training set and 0.77 (95% CI: 0.70-0.83) in the test set. The goodness-of-fit statistics had a P value of 0.29, indicating the model was well calibrated when comparing observed and predicted values. Using the variables from the risk prediction model, we developed a nomogram to provide a precise and simple CHD risk analysis tool for each individual. The nomogram is characterized by 1 scale corresponding to each variable, a score scale, a total risk score scale, and a CHD rate scale that shows CHD probability at 10 years and 20 years after BMT (Figure 3A). Using the nomogram, the probability of developing CHD in a hypothetical male patient who underwent BMT at 60 years with a history of diabetes and pre-BMT chest radiation would be 30% at 10 years and 72% at 20 years (Figure 3B).
Central Illustration.
Risk Prediction Nomogram for Coronary Heart Disease After Blood or Marrow Transplantation
Older age at blood or marrow transplantation (BMT), male sex, pre-BMT comorbidities, smoking, and chest radiation increase the risk of coronary heart disease (CHD) and can be used to predict CHD probability at 10 years and 20 years after BMT using the nomogram.
Figure 3.
Risk Prediction Nomogram to Determine the Risk of CHD After BMT
(A) The risk prediction nomogram for post-BMT CHD. The nomogram is characterized by 1 scale corresponding to each variable, a score scale, a total risk score scale, and a CHD rate scale that shows CHD probability at 10 years and 20 years after BMT. (B) The risk prediction nomogram for calculating CHD risk in a hypothetical patient. CHD probability estimation: a line is drawn from the value of each category to the score line. Age at BMT 60 years, score = 75; male sex, score = 14; history of diabetes at BMT, score = 20; and pre-BMT chest radiation, score = 25. The points are then added to determine the total score (134), and a line is drawn downward from the total points scale to find the CHD rate at 10 years (30%) and at 20 years (72%).
Discussion
In this cohort of BMT recipients who had survived ≥2 years, the 20-year cumulative incidence of CHD was 5.5%. Allogeneic BMT survivors were at 2.1-fold and autologous BMT recipients at 1.5-fold higher odds of developing CHD compared with non-BMT controls. Older age at BMT, male sex, history of diabetes, hypertension, smoking, arrhythmia, and pre-BMT chest radiation were associated with increased CHD risk. These variables were used to create a nomogram to predict the individual risk of CHD.
Chest radiation predisposes to atherosclerosis.24, 25, 26 Comorbidities such as hypertension, dyslipidemia, and diabetes further increase the risk of cardiovascular disease (CVD).6,12,13,27 In 548 BMT survivors, Tichelli et al28 observed 20 patients with arterial events (CHD, stroke, or peripheral vascular disease), yielding a 15-year cumulative incidence of 6%; the incidence was higher in older patients and those with CVRFs.28 In a previous BMTSS report in allogeneic BMT recipients, although the prevalence of CVRFs was high, <2% developed arterial events, likely because of a younger patient population and a shorter follow-up.13 With 249 post-BMT CHD events, the current study is the largest to analyze CHD risk in long-term BMT survivors to our knowledge.
In a previous study, the risk of CVD was higher after allogeneic BMT with a 15-year cumulative incidence of 7.5% vs 2.3% after autologous BMT.11 This study compared the risk of CVD in 365 allogeneic BMT survivors with 145 autologous BMT survivors and included cerebrovascular disease, CHD, and peripheral vascular disease in the outcome, with only 10 patients with CHD. The median age at BMT was 27 years for allogeneic and 44.5 years for autologous BMT survivors, which is lower than our study population. In our study, the risk of CHD was higher in autologous BMT survivors and is mediated by older age of autologous BMT survivors compared with allogeneic BMT survivors; older age was associated with CHD risk in both groups. However, allogeneic BMT recipients had a higher magnitude of CHD risk than autologous when compared with siblings in the adjusted analysis. In the general population <50 years of age, the incidence of CHD is higher in men compared with women.29 The median age at BMT was 43.9 years, and the younger study population partly explains the elevated risk of CHD in men in our study. Age is an independent risk factor for CHD30 and is incorporated in the Framingham risk score and the pooled cohort equations for CVD risk assessment.31,32 Aging is also associated with the acquisition of modifiable CVRFs that contribute to CVD risk. Furthermore, the presence of CVRFs accentuates age-associated CVD risk, and the absence of these risk factors results in a reduction of CVD risk.2 A previous study comparing the risk of CVD in 1-year BMT survivors with the general population showed a high risk of CVRFs, including hypertension, dyslipidemia, diabetes, and smoking (all independent risk factors for CHD).14,15 A CVD risk prediction model developed by Armenian et al33 focused on both heart failure and CHD as a CVD outcome; a smaller number of CHD events (n = 43) and the inclusion of anthracycline-related heart failure in the same model presented an opportunity for a risk prediction model focusing only on CHD in a larger sample size with extensive information on sociodemographics, therapeutic exposures, dose of chest radiation, and comorbidities. Chest radiation is associated with atherosclerosis and CHD risk because of sustained inflammation.9,34 Similar to cancer survivors treated with conventional (non-BMT) treatments,35,36 we found a dose-dependent association between chest radiation and CHD. We did not find an association between TBI or cGvHD and CHD risk.
The Center for International BMT Research and the European Group for BMT Late Effects Working Group guidelines provide consensus recommendations for screening and management of CVRFs and CVD in BMT recipients.37 They acknowledge that the low incidence of CHD in BMT survivors is likely caused by under-reporting because of attrition in long-term survivors. They recommend a similar approach for CHD assessment as in the general population. However, the recommendations for the general population do not account for factors that place BMT survivors at high risk for CHD. Jain et al38 have previously shown that the Framingham risk score is an ineffective screening strategy and underestimates CHD compared with the coronary artery calcium score in allogeneic BMT survivors. Our nomogram to determine individual CHD risk is easy to use and could guide surveillance for the detection of CHD early in the course of BMT and identify patients who can be referred for computed tomography angiography or estimation of the coronary artery calcium score such that targeted interventions can be applied. Healthy lifestyle attenuates the CVD risk associated with BMT,15 underscoring the importance of diet and physical activity interventions. Clinical trials evaluating the role of screening for CVRFs, aggressive management of modifiable CVRFs, screening stress tests among older patients who received chest radiation, and the role of antiplatelet therapies and statins for primary CHD prevention are of utmost importance in this population.
Study strengths and limitations
The current study used a large population of BMT recipients with a long follow-up. Siblings served as a comparison cohort, representing the general population. We were able to assess the impact of pre-BMT and BMT-related therapeutic exposures in addition to sociodemographics, chronic health conditions, and health behaviors as risk factors for CHD. Because our study relied on self-report, we could not capture complete details regarding clinical presentation and laboratory abnormalities at the time of CHD development. Although BMTSS collected information regarding medications, the exact time period for medication use such as antihypertensives was not available and not included in the analytical models. Furthermore, recall bias is a limitation because the survey was administered at a single time point several years after BMT. However, we have shown that BMT survivors are able to report adverse medical conditions with accuracy.39 Furthermore, because the non-BMT comparison group also provided self-reported data, there should not be systematic differences by case or control status. Other known risk factors for CHD such as family history were not assessed. Our intention was to determine the risk of CHD in long-term BMT survivors, and BMT recipients who died within the first 2 years were not included in the analysis, potentially resulting in an underestimation of CHD risk after BMT, if fatal CHD events occurred during the first 2 years. In addition, our study included only CHD events confirmed by percutaneous coronary intervention or coronary artery bypass surgery, and we may have excluded subclinical or milder cases of CHD. The CHD outcome was assigned differently for alive BMT survivors and deceased participants; however, both had a similar frequency of CHD events (3.35% vs 4.25%). Our study included BMT recipients from 1974 to 2014, and we acknowledge that transplant practices have changed over time, which may impact the risk of CHD. This risk model needs to be validated in independent cohorts.
Conclusions
These limitations notwithstanding, we showed that BMT survivors are at 61% higher odds of CHD compared with a control group, suggesting a need for increased awareness. Older age at BMT, male sex, and CVRFs are independent risk factors for CHD. Pre-BMT chest radiation further increases this risk in autologous BMT survivors. It is particularly important to understand CHD risk in long-term BMT survivors because of the long latency for development and proven efficacy of early intervention through lifestyle modifications and control of CVRFs.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: BMT survivors have an increased risk of CHD compared with a sibling comparison group without cancer. Older age at BMT, male sex, cardiovascular risk factors, and prior chest radiation increase the risk of CHD. We developed a nomogram to provide a precise and simple CHD risk analysis tool for each individual, which, with further study, can be applied in BMT survivorship or primary care clinics.
TRANSLATIONAL OUTLOOK: Given the elevated risk of CHD, older BMT survivors with cardiovascular risk factors and those exposed to pre-BMT chest radiation should be considered for enhanced screening and aggressive control of cardiovascular risk factors.
Funding Support and Author Disclosures
This study was supported in part by grants from the National Cancer Institute (grants R01 CA078938 and U01 CA213140) (Dr Bhatia); the Leukemia and Lymphoma Society (grant R6502-16) (Dr Bhatia); National Center for Advancing Translational Sciences of the National Institutes of Health under award number KL2TR003097 (Dr Gangaraju); and the National Heart, Lung, and Blood Institute (grant K08 HL159290) (Dr Gangaraju). Dr Gangaraju has served as a consultant for Sanofi and Alexion. Dr Weisdorf has received research funding from Fate and Incyte. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Tsao C.W., Aday A.W., Almarzooq Z.I., et al. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi: 10.1161/CIR.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D.M., Leip E.P., Larson M.G., et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi: 10.1161/circulationaha.105.548206. [DOI] [PubMed] [Google Scholar]
- 3.Majhail N.S., Flowers M.E., Ness K.K., et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43(1):49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turcotte L.M., Yingst A., Verneris M.R. Metabolic syndrome after hematopoietic cell transplantation: at the intersection of treatment toxicity and immune dysfunction. Biol Blood Marrow Transplant. 2016;22(7):1159–1166. doi: 10.1016/j.bbmt.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker K.S., Chow E., Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47(5):619–625. doi: 10.1038/bmt.2011.118. [DOI] [PubMed] [Google Scholar]
- 6.Tichelli A., Bhatia S., Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142(1):11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 7.Tsakiris D.A., Tichelli A. Thrombotic complications after haematopoietic stem cell transplantation: early and late effects. Best Pract Res Clin Haematol. 2009;22(1):137–145. doi: 10.1016/j.beha.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Sun L., Inaba Y., Sogo Y., et al. Total body irradiation causes a chronic decrease in antioxidant levels. Sci Rep. 2021;11(1):6716. doi: 10.1038/s41598-021-86187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby S., McGale P., Peto R., Granath F., Hall P., Ekbom A. Mortality from cardiovascular disease more than 10 years after radiotherapy for breast cancer: nationwide cohort study of 90 000 Swedish women. BMJ. 2003;326(7383):256–257. doi: 10.1136/bmj.326.7383.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majhail N.S., Challa T.R., Mulrooney D.A., Baker K.S., Burns L.J. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(9):1100–1107. doi: 10.1016/j.bbmt.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Tichelli A., Bucher C., Rovo A., et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110(9):3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 12.Armenian S.H., Sun C.L., Vase T., et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120(23):4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker K.S., Ness K.K., Steinberger J., et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109(4):1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow E.J., Mueller B.A., Baker K.S., et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155(1):21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 15.Chow E.J., Baker K.S., Lee S.J., et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32(3):191–198. doi: 10.1200/jco.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Accurint: LexisNexis risk solutions. http://www.accurint.com
- 17.National Death Index https://www.cdc.gov/nchs/ndi/index.htm
- 18.Sun C.L., Francisco L., Kawashima T., et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129–3139. doi: 10.1182/blood-2009-06-229369. quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144. [DOI] [Google Scholar]
- 20.Cox D.R. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 21.Taylor J.M., Munoz A., Bass S.M., Saah A.J., Chmiel J.S., Kingsley L.A. Estimating the distribution of times from HIV seroconversion to AIDS using multiple imputation. Multicentre AIDS Cohort Study. Stat Med. 1990;9(5):505–514. doi: 10.1002/sim.4780090504. [DOI] [PubMed] [Google Scholar]
- 22.Crowson C.S., Atkinson E.J., Therneau T.M. Assessing calibration of prognostic risk scores. Stat Methods Med Res. 2016;25(4):1692–1706. doi: 10.1177/0962280213497434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iasonos A., Schrag D., Raj G.V., Panageas K.S. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi: 10.1200/jco.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 24.Basavaraju S.R., Easterly C.E. Pathophysiological effects of radiation on atherosclerosis development and progression, and the incidence of cardiovascular complications. Med Phys. 2002;29(10):2391–2403. doi: 10.1118/1.1509442. [DOI] [PubMed] [Google Scholar]
- 25.Gujral D.M., Shah B.N., Chahal N.S., Senior R., Harrington K.J., Nutting C.M. Clinical features of radiation-induced carotid atherosclerosis. Clin Oncol (R Coll Radiol) 2014;26(2):94–102. doi: 10.1016/j.clon.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Fonkalsrud E.W., Sanchez M., Zerubavel R., Mahoney A. Serial changes in arterial structure following radiation therapy. Surg Gynecol Obstet. 1977;145(3):395–400. [PubMed] [Google Scholar]
- 27.Armenian S.H., Sun C.L., Mills G., et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16(8):1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tichelli A., Passweg J., Wójcik D., et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93(8):1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 29.Wells G.L. Cardiovascular risk factors: does sex matter? Curr Vasc Pharmacol. 2016;14(5):452–457. doi: 10.2174/1570161114666160722113116. [DOI] [PubMed] [Google Scholar]
- 30.Sniderman A.D., Furberg C.D. Age as a modifiable risk factor for cardiovascular disease. Lancet. 2008;371(9623):1547–1549. doi: 10.1016/s0140-6736(08)60313-x. [DOI] [PubMed] [Google Scholar]
- 31.D’Agostino R.B., Sr., Vasan R.S., Pencina M.J., et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/circulationaha.107.699579. [DOI] [PubMed] [Google Scholar]
- 32.Goff D.C., Lloyd-Jones D.M., Bennett G., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Armenian S.H., Yang D., Teh J.B., et al. Prediction of cardiovascular disease among hematopoietic cell transplantation survivors. Blood Adv. 2018;2(14):1756–1764. doi: 10.1182/bloodadvances.2018019117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halle M., Gabrielsen A., Paulsson-Berne G., et al. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J Am Coll Cardiol. 2010;55(12):1227–1236. doi: 10.1016/j.jacc.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 35.Cheng Y.J., Nie X.Y., Ji C.C., et al. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc. 2017;6(5) doi: 10.1161/JAHA.117.005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bates J.E., Howell R.M., Liu Q., et al. Therapy-related cardiac risk in childhood cancer survivors: an analysis of the childhood cancer survivor study. J Clin Oncol. 2019;37(13):1090–1101. doi: 10.1200/JCO.18.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeFilipp Z., Duarte R.F., Snowden J.A., et al. Metabolic syndrome and cardiovascular disease following hematopoietic cell transplantation: screening and preventive practice recommendations from CIBMTR and EBMT. Bone Marrow Transplant. 2017;52(2):173–182. doi: 10.1038/bmt.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jain N.A., Chen M.Y., Shanbhag S., et al. Framingham Risk Score is an ineffective screening strategy for coronary heart disease in long-term allogeneic hematopoietic cell transplant survivors. Clin Hematol Int. 2020;2(3):109–116. doi: 10.2991/chi.d.200508.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louie A.D., Robison L.L., Bogue M., Hyde S., Forman S.J., Bhatia S. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25(11):1191–1196. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.