ABSTRACT
BACKGROUND:
Many predictive factors and scoring systems associated with Fournier’s gangrene have been proposed, including comorbidities, vital signs, biochemical and hematological parameters, and demographic characteristics of the patient. The aim of this study was to determine the strengths of the scoring systems that have been formed by revealing these factors from a wider perspective and in a larger patient population.
METHODS:
The patient population included 144 patients, 21 of whom died. Age, biochemical and hematological parameters, Uludag Fournier’s Gangrene Severity Index (UFGSI), Fournier’s Gangrene Severity Index (FGSI), and Age-Adjusted Charlson Comorbidity Index (ACCI) scores were analyzed using the Mann Whitney U-test due to their non-parametric distribution. Categorical data such as comorbidities, gender, need for positive inotropes, diversion ostomy status, and UFGSI grading status was analyzed with the Chi-square test, and independent risk factors were determined from the significant data emerging from univariate and multivariate logistic regression analysis. Their strengths were compared using the logistic regression model (Fournier’s Gangrene Mortality Prediction Model: FGMPM) created through ROC analysis of the FGSI, UFGSI, and ACCI scores.
RESULTS:
The results of the statistical analyses showed that albumin (p<0.001) and need for positive inotropic support (p<0.001) were independent risk factors for mortality and ROC analysis revealed that the created FGMPM regression model (AUC: 0.995) was a stronger model than the FGSI (AUC: 0.874), UFGSI (0.893), and ACCI (0.788) scoring systems.
CONCLUSION:
The results of this study revealed that albumin and the need for positive inotropic support are independent risk factors for mortality. It is thought that the determination of these two parameters can be used to predict mortality more practically than the parameters used in the UFGSI and FGSI.
Keywords: Fournier’s gangrene, Fournier’s gangrene mortality prediction models, mortality
INTRODUCTION
Fournier’s gangrene (FG) was first described by Jean-Alfred Fournier in the penis and scrotum in young men.[1] It pathophysiologically begins with gangrenous skin caused by polymicrobial infections of urogenital, anorectal, and gynecological origin that form microthrombi in the small submucosal vessels of the perineal region and show rapid spread.[2]
Diabetes, chronic alcoholism, immunosuppression, and malignancy have been shown as risk factors for the development of FG.[3] Despite the early diagnosis, aggressive debridement, and broad-spectrum antibiotics, the mortality rate is around 20–30% due to the presence of comorbidities.[4]
FG is a multifactorial disease. Therefore, the Fournier’s Gangrene Severity Index (FGSI),[5] the Uludag Fournier’s Gangrene Severity Index (UFGSI),[6] and the Age-Adjusted Charlson Comorbidity Index (ACCI) are used to estimate mortality. The FGSI was first developed by Laor et al.[5] in 1995 with a modification of the APACHE II scoring system, by adding some criteria, such as age and speed of disease to the UFGSI and ACCI, which are scoring systems with which patients who will develop mortality can be determined with relatively higher accuracy.[6] The aim of this study was to create a new mortality estimation model by revealing the factors affecting mortality in FG and to compare the new model’s strength with existing nomograms.
MATERIALS AND METHODS
Approval for the study was granted by the Local Ethics Committee (Ethics Committee approval number: E-19-2507) and all the procedures were in compliance with the Helsinki Declaration. A retrospective evaluation was made of 144 patients, aged >18 years, who underwent FG surgery in the General Surgery Department of a tertiary level hospital between 01.01.2010 and 01.1.2019. Data were collected from patient records, computer system records, operating notes, clinic follow-up forms and the anamnesis related to surgical indications, intensive care unit forms and the surgical procedures applied. Written informed consent was obtained from all patients pre-operatively on surgical procedures.
All patients with or without extrapelvic extension originating from the perineal region were included in the study and patients with necrotizing fasciitis originating from the extrapelvic region were excluded. Patients with chronic renal failure were excluded from the study, and patients with acute renal failure due to sepsis were included.
Age, gender, and comorbid conditions of the patients included in the study were determined. Laboratory parameters were obtained from the hospital automation system and the clinical course of each patient was examined through the clinical records. The UFGSI grade status was determined and UFGSI, FGSI, and ACCI scores were calculated. The Fournier’s Gangrene Mortality Estimation Model (FGMPM) score determined using some of the parameters in the FGSI, the UFGSI, and the Age-ACCI, and parameters such as the need for intensive care, the requirement for inotropes and neutrophil-lymphocyte ratio. The parameters used in the FGMPM score and reference values are shown in Table 1.
Table 1.
Fournier’s gangrene mortality prediction model
| Variables | +4 | +3 | +2 | +1 | 0 | +1 | +2 | +3 | +4 |
|---|---|---|---|---|---|---|---|---|---|
| Physiological parameters | |||||||||
| Heart rate | >180 | 140–179 | 110–139 | – | 70–109 | – | 55–69 | 40–54 | <39 |
| Respiratory rate | >50 | 35–49 | – | 25–34 | 12–24 | 10–11 | 6–9 | – | <5 |
| Biochemical variables | |||||||||
| Albümin (g/dL) | <1 | 1.1-2 | 2.1-3 | 3.1–3.3 | 3.4–5 | – | – | – | – |
| Serum urea (mg/dL) | |||||||||
| (*2 for acute renal failure) | >150.1 | 100.1–150 | 50.1–100 | 25.1–50 | 8–25 | <7.9 | – | – | – |
| Serum sodium (mmol/L) | >180 | 160–179 | 155–159 | 150–154 | 130–149 | – | 120–129 | 110–119 | <110 |
| Neurophil to lymphocyte ratio | >30.1 | 20.1–30 | 10.1–20 | 5.1–10 | <5 | – | – | – | – |
| Dissemination score | |||||||||
| Urogenital and/or anorectal region, add ‘‘1’’ | |||||||||
| Pelvic region, add ‘‘2’’ | |||||||||
| Beyond the pelvic region, add ‘‘6’’ | |||||||||
| Histological examination of necrosis tissue score, | |||||||||
| Grade 1, necrosis in the skin and subcutaneous tissue, add ‘‘1’’ | |||||||||
| Grade 2, skin, subcutaneous and fascia involvement, add ‘‘2’’ | |||||||||
| Grade 3, affected muscle tissue in addition to fascia involvement, add ‘‘6’’ | |||||||||
| Age ≥60 years, add ‘‘1’’ | |||||||||
| Age <60 years, add ‘‘0’’ | |||||||||
| Need for intensive care | |||||||||
| Positive add ‘‘1’’ | |||||||||
| Negative add ‘‘0’’ | |||||||||
| Need for inotropic support | |||||||||
| Positive add ‘‘1’’ | |||||||||
| Negative add ‘‘0’’ |
In the histological examination of necrosis tissue, patients were evaluated as Grade 1 with necrosis in the skin and subcutaneous tissue but the fascia was not affected, Grade 2 with skin, subcutaneous and fascia involvement, and Grade 3 with affected muscle tissue in addition to fascia involvement.
Statistical Analysis
Power analysis was applied using the power.roc.test function in the pROC package. The minimum sample size was determined to be 140 patients for an area under the curve (AUC) of 0.80 on the receiving operating characteristic (ROC) curve to provide 95% power and alpha at 0.05.
Data obtained in the study were analyzed statistically using SPSS version 23 software (IBM Corp., Armonk, NY, USA). Numerical data were checked for compliance with normal distribution using the Kolmogorov Smirnov test. No variables were found to meet the normal distribution assumption. Continuous numerical variables were analyzed using the Mann Whitney U-test. These variables were stated as mean, standard deviation, median, minimum, and maximum values. Chi-square analysis was applied to categorical variables, which were stated as frequency and percentage values. For similar variables, a simple logistic regression analysis was performed for each variable by taking the variables with a statistically significant P value.
The Hosmer Lemeshow test was used to evaluate the goodness of fit. To evaluate the success of the obtained logistic regression model, ROC curve analysis was performed and performance measures were calculated. In the definition of the predictive model, the AUC values were determined as 0.90–1.00 = excellent, 0.80–0.90 = good, 0.70–0.80 = moderate, 0.60–0.70 = weak, and 0.50–0.60 = unsuccessful. A value of p<0.05 was considered statistically significant.
RESULTS
Evaluation was made of a total of 144 patients, comprising 101 (70.13%) males and 43 (29.87%) females. Mortality developed in 21 (14.5%) patients; 11 males and ten females. There was no statistically significant difference between gender and mortality due to FG (p=0.054). The median age was 53 years (range: 19–78 years) in those with mortality, and 61 years (range: 38–80 years) in those without mortality and a statistically significant relationship was found between age and mortality (p<0.001). When the relationships between mortality and the presence of diabetes mellitus (DM), coronary artery disease (CAD), chronic obstructive pulmonary disease, cerebrovascular disease, and concomitant malignancy were examined, a statistically significant relationship was found only in respect of CAD (p=0.049) and malignancy (p<0.001). A diversion colostomy was performed for 43 (29.86%) patients with an average hospitalization time of 21.57±15.56 days, but no statistically significant difference was observed between mortality and hospitalization time or not performing a diversion colostomy. During the follow-up period, 21 (14.58%) patients required positive inotropic support and a statistically significant difference was determined in mortality in this regard (p<0.001).
When the relationships between biochemical and complete blood count parameters and mortality were examined, statistically significant relationships were found for albumin (p<0.001), urea (p=0.048), adjusted calcium (p=0.009), red cell distribution width (RDW) (p=0.007), and hematocrit (p=0.003). A statistically significant relationship was also found between FG grading as specified by the UFGSI and mortality (p=0.014) (Tables 2 and 3).
Table 2.
The statistical relationships between continuous numerical data and mortality
| Mortality | N | Mean | Standard deviation | Median | Minimum | Maximum | p | |
|---|---|---|---|---|---|---|---|---|
| Age | Absent | 123 | 51.44 | 14.04 | 53.00 | 19.00 | 79.00 | 0.006 |
| Present | 21 | 59.90 | 9.23 | 61.00 | 38.00 | 80.00 | ||
| Total | 144 | 52.68 | 13.75 | 54.00 | 19.00 | 80.00 | ||
| Albumin | Absent | 123 | 2.68 | 0.62 | 2.60 | 1.40 | 4.60 | <0.001 |
| Present | 21 | 1.91 | 0.49 | 1.80 | 1.08 | 2.90 | ||
| Total | 144 | 2.57 | 0.65 | 2.50 | 1.08 | 4.60 | ||
| Urea | Absent | 123 | 47.45 | 35.00 | 35.00 | 8.00 | 203.00 | 0.048 |
| Present | 21 | 68.62 | 46.06 | 66.00 | 16.00 | 187.00 | ||
| Total | 144 | 50.53 | 37.38 | 36.50 | 8.00 | 203.00 | ||
| Creatinine | Absent | 123 | 1.21 | 0.89 | 0.92 | 0.47 | 5.45 | 0.702 |
| Present | 21 | 1.22 | 0.94 | 0.85 | 0.39 | 4.42 | ||
| Total | 144 | 1.21 | 0.89 | 0.92 | 0.39 | 5.45 | ||
| Glucose | Absent | 123 | 194.60 | 138.31 | 128.00 | 64.00 | 836.00 | 0.287 |
| Present | 21 | 225.33 | 150.40 | 212.00 | 72.00 | 680.00 | ||
| Total | 144 | 199.09 | 140.01 | 132.50 | 64.00 | 836.00 | ||
| Adjusted | Absent | 116 | 15.59 | 20.06 | 7.00 | 0.00 | 90.00 | 0.009 |
| Calcium | Present | 94 | 19.98 | 18.94 | 15.00 | 0.00 | 80.00 | |
| Total | 144 | 8.21 | 0.75 | 8.20 | 6.10 | 10.10 | ||
| Sodium | Absent | 123 | 135.00 | 5.00 | 135.00 | 120.00 | 144.00 | 0.064 |
| Present | 21 | 133.00 | 6.00 | 133.00 | 124.00 | 146.00 | ||
| Total | 144 | 134.82 | 4.74 | 134.50 | 120.00 | 146.00 | ||
| Potassium | Absent | 123 | 4.08 | 0.53 | 4.05 | 2.84 | 5.50 | 0.745 |
| Present | 21 | 4.18 | 0.91 | 3.91 | 3.22 | 7.23 | ||
| Total | 144 | 4.09 | 0.59 | 4.03 | 2.84 | 7.23 | ||
| Hemoglobin | Absent | 123 | 11.95 | 2.29 | 12.00 | 6.50 | 17.00 | 0.083 |
| Present | 21 | 10.66 | 2.85 | 11.40 | 5.30 | 15.20 | ||
| Total | 144 | 11.76 | 2.41 | 11.95 | 5.30 | 17.00 | ||
| Hematocrit | Absent | 123 | 35.93 | 6.68 | 35.40 | 19.60 | 49.90 | 0.003 |
| (%) | Present | 21 | 30.13 | 7.84 | 29.50 | 15.20 | 42.40 | |
| Total | 144 | 35.08 | 7.12 | 35.20 | 15.20 | 49.90 | ||
| RDW | Absent | 123 | 14.35 | 2.17 | 13.80 | 11.10 | 21.20 | 0.007 |
| Present | 21 | 16.47 | 3.64 | 15.00 | 11.90 | 24.60 | ||
| Total | 144 | 14.65 | 2.53 | 14.00 | 11.10 | 24.60 | ||
| Leukocyte | Absent | 123 | 17.19 | 6.77 | 16.20 | 1.50 | 36.80 | 0.861 |
| Count | Present | 21 | 17.19 | 9.49 | 17.70 | 1.00 | 32.90 | |
| Total | 144 | 17.17 | 7.19 | 16.50 | 1.00 | 36.80 | ||
| Neutrophil | Absent | 123 | 14.46 | 6.59 | 13.30 | 0.80 | 35.80 | 0.521 |
| Count | Present | 21 | 15.14 | 8.26 | 16.20 | 0.20 | 29.50 | |
| Total | 144 | 14.55 | 6.83 | 13.55 | 0.20 | 35.80 | ||
| Lymphocyte | Absent | 123 | 1.49 | 1.00 | 1.30 | 0.01 | 6.90 | 0.109 |
| Count | Present | 21 | 1.22 | 0.96 | 1.00 | 0.20 | 4.20 | |
| Total | 144 | 1.45 | 0.99 | 1.25 | 0.20 | 6.90 | ||
| Platelets | Absent | 123 | 295.26 | 129.27 | 276.00 | 79.00 | 823.00 | 0.530 |
| Present | 21 | 308.42 | 167.09 | 303.00 | 13.00 | 599.00 | ||
| Total | 144 | 297.18 | 134.84 | 278.00 | 13.00 | 823.00 | ||
| Length of | Absent | 123 | 20.75 | 14.48 | 18 | 3 | 98 | 0.596 |
| stay (days) | Present | 21 | 26.33 | 20.62 | 19 | 2 | 67 | |
| Total | 144 | 21.57 | 15.56 | 18 | 2 | 98 | ||
| ACCI | Absent | 123 | 2.30 | 2.21 | 2.00 | 0.00 | 11.00 | <0.001 |
| Present | 21 | 5.04 | 2.78 | 5.00 | 1.00 | 11.00 | ||
| Total | 144 | 2.70 | 2.49 | 2.00 | 0.00 | 11.00 | ||
| FGSI | Absent | 123 | 2.69 | 2.40 | 2.00 | 0.00 | 11.00 | <0.001 |
| Present | 21 | 8.00 | 3.68 | 8.00 | 1.00 | 15.00 | ||
| Total | 144 | 3.47 | 3.22 | 2.50 | 0.00 | 15.00 | ||
| UFGSI | Absent | 123 | 5.95 | 3.67 | 6.00 | 1.00 | 15.00 | <0.001 |
| Present | 21 | 13.52 | 4.60 | 15.00 | 5.00 | 21.00 | ||
| Total | 144 | 7.06 | 4.65 | 7.00 | 1.00 | 21.00 |
#In the Mann-Whitney U test, p<0.05 was accepted as statistically significant.
Table 3.
The relationships between categorical variables and mortality
| Mortality | p | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| None | Present | Total | ||||||
|
|
|
|
||||||
| N | % | N | % | N | % | |||
| Sex | Male | 90 | 89.10 | 11 | 11.90 | 101 | 70.13 | 0.054 |
| Female | 33 | 76.7 | 10 | 23.3 | 43 | 29.87 | ||
| Diabetes mellitus | Absent | 67 | 87.00 | 10 | 13.00 | 77 | 53.47 | 0.561 |
| Present | 56 | 83.60 | 11 | 16.40 | 67 | 46.53 | ||
| CAD | Absent | 108 | 87.80 | 15 | 12.20 | 123 | 85.42 | 0.049 |
| Present | 15 | 71.40 | 6 | 28.60 | 21 | 14.58 | ||
| HT | Absent | 92 | 85.20 | 16 | 14.80 | 108 | 75.00 | 0.892 |
| Present | 31 | 86.10 | 5 | 13.90 | 36 | 25.00 | ||
| Malignancy | Absent | 112 | 89.60 | 13 | 10.40 | 85 | 59.03 | <0.001 |
| Present | 11 | 57.90 | 8 | 42.10 | 19 | 40.97 | ||
| COPD | Absent | 111 | 84.70 | 20 | 15.30 | 131 | 90.97 | 0.692* |
| Present | 12 | 92.30 | 1 | 7.70 | 13 | 9.03 | ||
| CVE | Absent | 119 | 86.20 | 19 | 13.80 | 138 | 95.83 | 0.211* |
| Present | 4 | 66.70 | 2 | 33.30 | 6 | 4.17 | ||
| Need for inotropes | Absent | 121 | 98.40 | 2 | 1.60 | 123 | 85.42 | <0.001* |
| Present | 2 | 9.50 | 19 | 90.50 | 21 | 14.58 | ||
| Diversion colostomy | Absent | 87 | 86.10 | 14 | 13.90 | 101 | 70.14 | 0.707 |
| Present | 36 | 83.70 | 7 | 16.30 | 43 | 29.86 | ||
| UFGSI grade | I | 56 | 94.90 | 3 | 5.10 | 59 | 40.97 | 0.014 |
| II | 23 | 85.20 | 4 | 14.80 | 27 | 18.75 | ||
| III | 44 | 75.90 | 14 | 24.10 | 58 | 40.28 | ||
Pearson chi-square test.
In the Fisher exact test, p<0.05 was considered significant. CAD: Coronary artery disease; HT: ???????????????; COPD: Chronic obstructive pulmonary disease; CVE: ?????????????????; UFGSI: Uludag Fournier’s Gangrene Severity Index.
The relationships between mortality and the continuous numerical values of age, albumin, RDW, hematocrit, adjusted calcium, and urea values and the categorical variables of CAD, concomitant malignancy, UFGSI grade, and need for positive inotropic support were found to be significant, and these relationships were further evaluated using univariate and multivariate logistic regression analysis. According to the univariate logistic regression analysis, the values found for age (p=0.011), albumin (p=0.001), urea (p=0.022), adjusted calcium (p=0.028), hematocrit (p=0.001), RDW (p=0.001), UFGSI grade (p=0.001), concomitant malignancy (p=0.001), and need for positive inotropic support (p<0.001) showed statistically significant relationships with mortality, while the multivariate logistic regression analysis showed that only albumin (p=0.001) and need for positive inotropic support (p=0.001) were independent risk factors for the development of mortality (Table 4).
Table 4.
Univariate and multivariate logistic regression analysis results
| Simple logistic regression | Multivariate logistic regression | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| OR | 95.00% CI | p | OR | 95.00% CI | p | |
| Age | 1.054 | 1.012–1.098 | 0.011 | |||
| Albumin | 0.051 | 0.014–0.189 | <0.001 | 0.008 | 0.01–0.325 | 0.001 |
| Urea | 1.013 | 1.002–1.024 | 0.022 | |||
| D. Calcium | 0.483 | 0.253–0.924 | 0.028 | |||
| Hematocrit (%) | 0.886 | 0.824–0.953 | 0.001 | |||
| RDW | 1.307 | 1.112–1.537 | 0.001 | |||
| Histological grade | 0.001 | |||||
| I | 1 | |||||
| II | 3.246 | 0.666–17.584 | 0.001 | |||
| III | 5.939 | 1.803–26.935 | 0.001 | |||
| Malignancy | ||||||
| Absent | 1 | |||||
| Present | 6.266 | 2.100–18.536 | 0.001 | |||
| CAD | ||||||
| Absent | 1 | |||||
| Present | 0.347 | 0.117–1.033 | 0.057 | |||
| Need for inotropic support | ||||||
| Absent | 1 | 1 | ||||
| Present | 574.71 | 76.335–4329.00 | <0.001 | 4098 | 25.327–500000 | 0.001 |
OR: Odds Ratio; CI: Confidence Interval; RDW: Red cell distribution width; CAD: Coronary artery disease. *P<0.05 was considered significant.
Comparing the strengths of the previously created FGSI, UFGSI, and ACCI scoring systems with the newly developed FGMPM using ROC analysis, the AUC and confidence interval (%) were found to be 0.788 (0.687–0.890) for the ACCI, 0.893 (0.823–0.963) for the UFGSI, 0.874 (0.781–0.967) for the FGSI, and 0.995 (0.987–1.000) for the newly developed model, the FGMPM (Fig. 1).
Figure 1.
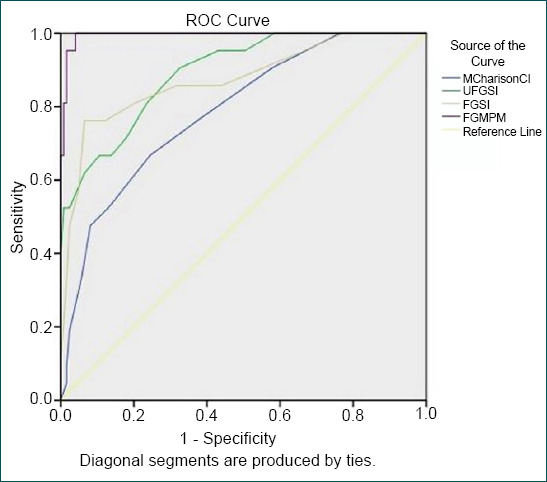
Comparisons of the FGSI, UFGSI and ACCI scoring systems with FGMPM. FGMPM: Fournier’s gangrene mortality prediction model; UFGSI: Uludag Fournier’s gangrene severity index; FGSI: Fournier’s gangrene severity index; ACCI: Age-adjusted charlson comorbidity index.
DISCUSSION
Many studies have investigated the factors causing mortality in FG and many predictive factors have been revealed. Significant relationships were found between mortality and BUN levels of >50 mg/dL by Clayton et al.;[7] FGSI score of >9 by Laor et al.;[5] body surface area by Janane et al.;[8] low hemoglobin level, high BUN level, and low albumin level by Tuncel et al.;[9] and DM, CRF, leukocytosis, shock findings, a high FGSI/ACCI score, and impaired INR levels by Garg et al.[10]
In a multicentric study involving 17 years of experience, a strong correlation was found between mortality and comorbid conditions such as DM, CAD, and kidney diseases and failure, while no statistically significant relationship was found between HT, lung diseases, liver diseases, and concomitant malignancy and mortality.[11] The results of the current study demonstrated a statistically significant relationship between comorbid conditions such as malignancy (p<0.001) and CAD (p=0.049) and mortality. However, Yeniyol et al.[12] emphasized that mortality is more frequently seen in patients with concomitant conditions such as heart failure, hypertension, and kidney failure.
The manifestation of all these predisposing factors led to the need to demonstrate the effect of FG on mortality in various clinics through scoring systems, and the FGSI was first described by Laor et al. in 1995.[5] Considering that homeostasis was affected by the severity of the disease, changes were made to the APACHE II scoring system, to form the FGSI, and it was concluded that the FGSI could predict mortality at the rate of 75% and survival at 78%. In a recent report published in Turkey, Ersay et al.[13] found that the FGSI score was an independent risk factor for mortality in 70 patients with FG. In a study of 80 patients by Yilmazlar et al.[6] in addition to the FGSI parameters, age and the extent of disease spread were specified, and a new scoring system, the UFGSI was developed, which is stronger than the FGSI for the prediction of mortality.
In a study conducted in China in 2015 comparing the FGSI, UFGSI, ACCI, and sAPGAR scoring systems for FG, it was found that the UFGSI and ACCI were useful in predicting prognosis, and the ACCI was more sensitive and specific.[14]
According to the current study univariate logistic regression analysis based on the demographics, laboratory data, physiological parameters, and findings of physical examination, the significant parameters were found to be age (p=0.011), albumin (p=0.001), urea (p=0.022), adjusted calcium (p=0.028), hematocrit (p=0.001), RDW (p=0.001), UFGSI grade (p=0.001), concomitant malignancy (p=0.001), and need for positive inotropic support (p<0.001) and these had statistically significant relationships with mortality. In the multivariate logistic regression analysis, however, only albumin (p=0.001) and need for positive inotropic support (p=0.001) were independent risk factors in determining mortality (Table 3). According to the logistic regression analysis results of the current study, in addition to the factors with an effect on mortality such as albumin, urea, and histological grade, some factors in the FGSI, UFGSI, and ACCI scores were included in the FGMPM scoring system. Although previous studies have shown a relationship between mortality and the extent of gangrene,[6,15] this is the first study to have shown a relationship between histological grade and mortality. This shows that the depth of necrosis is as important as the width in determining the spread and that it contributes to mortality, and is an important point to which attention must be paid during debridement.
In the study conducted by Yilmazlar et al.,[6] it was found that the AUC of the UFGSI was 0.947 (0.873–0.94), while the AUC of the FGSI was 0.843 (0.744–0.914), thereby demonstrating that the UFGSI was a stronger model. In the current study, when the strength of the proposed FGMPM was compared with the previously created FGSI, UFGSI, and ACCI models through ROC analysis, the AUC and confidence interval (%) were found to be 0.788 (0.687–0.890) for the ACCI, 0.893 (0.823–0.963) for the UFGSI, and 0.874 (0.78–0.967) for the FGSI. Thus, the FGMPM model was stronger with a value of 0.985 (0.998–1.000).
Conclusion
With the examination of 144 cases of FG, the current study is among the largest such series in the literature. The proposed model was concluded to be a stronger model than the existing models in determining predictive factors more accurately. It was also considered that the ability to utilize a decrease in albumin value as an independent risk factor and to predict high mortality in patients who need positive inotropic support, both of which are easy variables to detect, makes it more advantageous than other time-consuming models.
Footnotes
Ethics Committee Approval: This study was approved by the Ankara Numune Training and Research Hospital Ethics Committee (Date: 07.02.2019, Decision No: E-19-2507).
Peer-review: Internally peer-reviewed.
Authorship Contributions: Concept: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Design: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Supervision: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Resource: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Materials: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Data: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Analysis: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Literature search: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Writing: B.Ç., C.C., B.A.Ö., İ.A., F.A.; Critical revision: B.Ç., C.C., B.A.Ö., İ.A., F.A.
Conflict of Interest: None declared.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Fournier JA. Gangrene foudroyante de la verge. Semain Med. 1883;3:345–8. doi: 10.1007/BF02554904. [DOI] [PubMed] [Google Scholar]
- 2.Johnin K, Nakatoh M, Kadowaki T, Kushima M, Koizumi S, Okada Y. Fournier's gangrene caused by Candida species as the primary organism. Urology. 2000;56:153. doi: 10.1016/s0090-4295(00)00527-6. [DOI] [PubMed] [Google Scholar]
- 3.Morpurgo E, Galandiuk S. Fournier's gangrene. Surg Clin North Am. 2002;82:1213–24. doi: 10.1016/s0039-6109(02)00058-0. [DOI] [PubMed] [Google Scholar]
- 4.Pawłowski W, Wroński M, Krasnodebski IW. Zgorzel fourniera [Fournier's gangrene. Pol Merkur Lekarski. 2004;17:85–7. [PubMed] [Google Scholar]
- 5.Laor E, Palmer LS, Tolia BM, Reid RE, Winter HI. Outcome prediction in patients with Fournier's gangrene. J Urol. 1995;154:89–92. [PubMed] [Google Scholar]
- 6.Yilmazlar T, Ozturk E, Ozguc H, Ercan I, Vuruskan H, Oktay B. Fournier's gangrene: An analysis of 80 patients and a novel scoring system. Tech Coloproctol. 2010;14:217–23. doi: 10.1007/s10151-010-0592-1. [DOI] [PubMed] [Google Scholar]
- 7.Clayton MD, Fowler JE, Jr, Sharifi R, Pearl RK. Causes, presentation and survival of fifty-seven patients with necrotizing fasciitis of the male genitalia. Surg Gynecol Obstet. 1990;170:49–55. [PubMed] [Google Scholar]
- 8.Janane A, Hajji F, Ismail TO, Chafiqui J, Ghadouane M, Ameur A, et al. Hyperbaric oxygen therapy adjunctive to surgical debridement in management of Fournier's gangrene: Usefulness of a severity index score in predicting disease gravity and patient survival. Actas Urol Esp. 2011;35:332–8. doi: 10.1016/j.acuro.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Tuncel A, Aydin O, Tekdogan U, Nalcacioglu V, Capar Y, Atan A. Fournier's gangrene: Three years of experience with 20 patients and validity of the Fournier's gangrene severity index score. Eur Urol. 2006;50:838–43. doi: 10.1016/j.eururo.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Garg G, Singh V, Sinha RJ, Sharma A, Pandey S, Aggarwal A. Outcomes of patients with Fournier's gangrene: 12-year experience from a tertiary care referral center. Turk J Urol. 2019;45(Suppl 1):S111–6. doi: 10.5152/tud.2019.39586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Qushayri AE, Khalaf KM, Dahy A, Mahmoud AR, Benmelouka AY, Ghozy S, et al. Fournier's gangrene mortality: A 17-year systematic review and meta-analysis. Int J Infect Dis. 2020;92:218–25. doi: 10.1016/j.ijid.2019.12.030. [DOI] [PubMed] [Google Scholar]
- 12.Yeniyol CO, Suelozgen T, Arslan M, Ayder AR. Fournier's gangrene: Experience with 25 patients and use of Fournier's gangrene severity index score. Urology. 2004;64:218–22. doi: 10.1016/j.urology.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 13.Ersay A, Yilmaz G, Akgun Y, Celik Y. Factors affecting mortality of Fournier's gangrene: Review of 70 patients. ANZ J Surg. 2007;77:43–8. doi: 10.1111/j.1445-2197.2006.03975.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhu XD, Ding F, Wang GD, Shao Q. Different scoring systems to evaluate the prognosis of Fournier's gangrene: A comparative study. Zhonghua Nan Ke Xue. 2015;21:720–3. [PubMed] [Google Scholar]
- 15.Yilmazlar T, Ozturk E, Alsoy A, Ozguc H. Necrotizing soft tissue infections: APACHE II score, dissemination, and survival. World J Surg. 2007;31:1858–62. doi: 10.1007/s00268-007-9132-1. [DOI] [PubMed] [Google Scholar]


