ABSTRACT
BACKGROUND:
It was aimed to determine whether Alpinia officinarum (AO) (galangal), which has been regarded to be effective on wound healing, is healing on experimental contact type burns and compare its effects with silver sulfadiazine (SSD).
METHODS:
Thirty-five rats were divided into five groups of seven rats each group. Superficial second degree burns were formed by contacting a 1×1 cm copper tip which was kept at 100°C constant temperature to the three shaved areas on the back of rats without applying any pressure for 10 s. All groups were irrigated with a 100 cc saline solution for 2 min. Any procedure or treatment was not applied to Group I (Control). Group II (Burn Control) was only irrigated, Group III (SSD) was applied topical SSD 4 times, with 6-h intervals (at h 0, 6, 12 and 18), Group IV (Galangal) was applied topical AO 4 times, and Group V (Gel) was applied placebo topical material, used for the preparation of topical AO, 4 times. Wound healing findings were evaluated histopathologically.
RESULTS:
In the galangal group, it was found that collagen discoloration didn’t penetrate into deep dermis compared to other groups; epidermis, hair follicles, and sebaceous glands remained protected compared to the burn control group, and there was a thicker layer of epidermis. It was found that the galangal group was the closest group to the control group histologically. In the galangal group, it was determined that the number of vessels and total hair follicles were significantly higher in the 8th h and 4th h respectively (p<0.05), while epidermal thickness and number of degenerated hair follicles were significantly higher in all hours compared to other three groups (p<0.05). It was determined that galangal group had the lowest scores in the evaluation of edema, polymorphonuclear leukocytes infiltration, collagen discoloration, injury of vessels, hair follicles and sebaceous glands in comparisons between groups and within groups’ own processes.
CONCLUSION:
Administrating AO containing gel 4 times a day within the first 24 h is effective in the experimental contact type second degree burn model. It is significantly superior to SSD treatment, especially in the first 8 h of administration.
Keywords: Alpinia officinarum, burn, galangal, silver sulfadiazine, wound healing
INTRODUCTION
The plant Alpinia officinarum (AO) (Galangal, Lesser galangal, smaller galangal) is a native of China but now cultivated in India in the plains of West Bengal, Assam and Eastern Himalayas. Galangal has been used in both Ayurvedic and Chinese medicine since very early times (A.D. 500) and in Europe since the middle ages.[1] The rhizome of galangal is reported to be a very effective herb that acts mainly on the digestive system. It also relieves pain, reduces fever and controls bacterial and fungal infections.[2] Plants have the dietary flavonoid “galangin’’ naturally, and it is present in high concentrations in the rhizomes of AO, which has been used as a spice and as an herbal medicine in traditional Chinese medicine for centuries.[3] Galangin is one of the two major bioactive constituents of the AO.[4,5] Galangin possesses several biological activities and has an effect on many cell systems. Antioxidant, antimutagenic, antitumor, antimicrobial, anti-inflammatory, and antiviral activities of this flavonoid have been shown in both in vitro and in vivo systems.[6–8] It also has vasodilator and anti-ischemic effects that reduce the risk and improves endothelial cell functions in coronary artery diseases.[9]
According to the American Burn Association data, 486,000 people receive treatment for burns in the United States each year. A total of 40,000 requires hospitalization, of which 30,000 are to burn units, and the majority of patients are treated in emergency departments.[10] In the epidemiological study conducted by Haberal et al.,[11] 84.9% of the patients were treated as outpatients. In the study conducted by Türegün et al.,[12] 84.6% of the patients had minor burns in Turkey. The aim of burn treatment is to obtain the best structural and aesthetic results as soon as possible and at the lowest cost by avoiding infections, especially in outpatients. A topical agent to be used for this purpose should reduce the mortality and morbidity rates by preventing bacterial contamination and also should be easily accessible. An ideal agent with these properties has not been on the market yet.[13]
In the literature, it is conspicuous that no clinical or experimental studies have been present about the effects of galangal in patients with burns and experimental burn models. In this study, we aimed to investigate whether galangal, which has been regarded to be effective on wound healing for a long while, heals the experimental contact type burns and to compare it with silver sulfadiazine (SSD), the most commonly used topical agent worldwide.[14]
MATERIALS AND METHODS
Experimental Procedure
This study was started with the ethical approval of protocol numbered 62/2012 of the Dokuz Eylul University Faculty of Medicine (DEUFM) Animal Experiments Local Ethics Committee based on the criteria (Principles of Laboratory Animal Care) determined by the National Institute of Health for animal rights during the experiment. The experimental part of this study was carried out in the Experimental Research and Animal Laboratory of the DEUFM, and histological studies were performed in the Department of Histology and Embryology of the Ege University Faculty of Medicine. During the experiment, rats were fed with the standard laboratory diet, and their water uptake was released. They kept in 24±1°C at room temperature, in appropriate lighting conditions, in 12 h light- dark cycle and in cages cleaned once every day. Bait and water were given as ad libitum. 12 h before this study; they were given only water. Controls and upkeep were done non-stop and regularly.
Experimental Protocol
Adult female Wistar-albino species rats, weighing 195 to 245 g in average that were administered 200 mg/kg paracetamol (Perfalgan®, Bristol-Myers Squibb) through intraperitoneal 28 G insulin injection 25 min before the process for their analgesia were then anesthetized by administering a combination of 10 mg/kg xylazine and 50 mg/kg ketamine intraperitoneally and then the experimental stage was started. In the thoracodorsal regions of the rats, 1×1 cm of three separate areas were shaved using a razor blade. A soldering iron kit, operating with electrical energy, which can be kept at a constant temperature of 100°C, was designed. This kit has a copper extension, which is 18 cm long, with a sensitive thermometer on its handle and a copper tip square-shaped 1×1 cm in size. The produced heat on the copper tip was measured and displayed on the digital display of the thermometer. This temperature was lower due to loss with heat convection. While the temperature of the soldering iron kit was 276 to 314°C, the temperature of the copper tip was 100±5°C on the sensitive thermometer with repeated measurements. The burning model was standardized, with increasing the temperature of the copper tip back to 100°C after applying each burn. Experimental contact type burns were formed by contacting the copper tip for 10 s without applying extra pressure to the shaved areas. One minute later, burns were provided with irrigation for two minutes with a 100 cc saline solution.
Grouping of the Subjects
Thirty-five rats were divided into five groups. Any procedure or treatment was not applied to Group I (Control Group). Group II (Burn Control Group) was only irrigated with saline after the burn. Topical SSD treatment was applied 4 times, with 6-h intervals (at h 0, 6, 12 and 18) to Group III (SSD Group) after the irrigation with saline. The galangal extract containing gel was applied 4 times, with 6-h intervals to Group IV (Galangal Group). The plain (placebo) gel was applied 4 times, with 6-h intervals to Group V (Gel Group).
The wound site was closed with sterile and dry gauze dressing, which did not contain any topical agent and secured with specially prepared burn clothing for rats not to put pressure on the wound after topical treatment and irrigation with saline. In addition, 500 mg paracetamol was added to every 500 ml of drinking water of the rats to provide analgesia.
Microscopic Material-Method and Histopathological Evaluation
Incisional biopsies of the square-shaped 1×1 cm in size were taken from the dorsal skin of the rats at h 4, 8 and 24. For light microscopic sections, sections of 5 μm in Leica RM 2145 model microtome were taken into a 45°C water bath. A microwave oven (Arcelik MD554) was used for adhering to the sections to the blades thoroughly and removing the paraffin. After fixation with 4% paraformaldehyde, skin tissue was stained with hematoxylin-eosin and Mallory-azan following standard procedures and examined by histologists under a light microscope (Olympus BX51). Biopsies were evaluated using the modified verhofstad histopathological scoring[15] system (Table 1) by two blinded histologists who were unaware of the groups from which the biopsies were taken. Results obtained from this scoring were compared by taking the arithmetic average of the scores given. In addition, the number of vessels degenerated, and total hair follicles and epidermal thickness were also included in the histopathological evaluation.
Table 1.
Modified verhofstad histopathological scoring[15]
| Score | Edema | PMNL infiltration | Collagen discoloration | Vascular injury | Hair follicle damage | Gl. sebasea damage |
|---|---|---|---|---|---|---|
| 0 | None | Normal | None | None | None | None |
| 1 | Mild | Mild | Mild | Mild | Mild | Mild |
| 2 | Significant | Significant | Significant | Significant | Significant | Significant |
| 3 | Intense | Massive | Intense | Intense | Intense | Intense |
PMNL: Polymorphonuclear leukocytes.
Preparation of the AO Extract
The extraction method was determined as a result of the literature review.[4] High-efficiency values, failure to work at temperatures that would cause deterioration of active substances, short processing time, and availability of tools were considered when choosing the method. The rhizome parts of the plant were dried in a closed and shade place. The dry herbal material was homogeneously grounded with the mechanical grinder. 5 g of powdered herbal material from the ground parts of the plant were weighed into the glass balloon and the extraction was completed with hexane at 45°C with siphoning of the Soxhlet apparatus 6 times for every 15 min. Chloroform extraction was performed on the same material separated by hexane extract under the same conditions. The extraction process was completed by performing methanol extraction on the same material after chloroform extraction. Unwanted molecules and oils in the rhizome of the plant were removed by hexane and chloroform extraction, so methanol extraction was determined as the main part to be used. All the glass materials used were covered with aluminum foil to protect the plant contents from the light effect. Methanol was removed by the evaporator. The extract was freeze dried at –80°C overnight and lyophilized (Christ). Eventually, the obtained dry extract was placed in stability testing environments in bottles with lid.
High-Performance Liquid Chromatography (HPLC)
During the analysis, the extraction scanning process was completed by calibrating the standard molecules as six points at 0.4, 0.5, 1.0, 1.3, 1.5, and 2.0 mg/ml concentrations. Analyses were performed with the HPLC system consisting of Shimadzu SCL-10VP, manual sampler, column oven, and DAD detector elements. The analyses with 0.8 ml/min-1 flow rate, UV 252 nm, 40°C temperature, and 10 μl injection were completed using a zorbax, eclipse SB-C18 and 5 μm 4.6×250 mm column with an isocratic mobile phase and methanol-water-phosphoric acid (60: 38: 2 v/v/v).
The active components of AO are galangin and 3-O-methyl galangin, which are known as its most active molecules and found intensely in the rhizome of the plant[4] (Fig. 1).
Figure 1.

Spectrum of active substances in galangal extract used in the study by high-performance liquid chromatography method (A) Galangin, (B) 3-O-methyl galangin.
Preparation of the Placebo Gel
Placebo gel formulation was prepared by dispersing 7.5% (w/w) sodium carboxymethyl cellulose (Na-CMC, average Mw ~ 90.000, Sigma, USA) and 2% ethanol (TEKEL, Turkey) in distilled water and stirring continuously until forming a homogeneous gel.
Preparation of the Gel Containing AO Extract
Firstly, 3% AO extract was dissolved in 2% ethanol (TEKEL, Turkey). The gel formulation was prepared by dispersing 7.5% (w/w) sodium carboxymethyl cellulose (Na-CMC, average Mw ~ 90.000, Sigma, USA) in half of the water and 3% AO-ethanol solution was added to the other half of distilled water. Then, the parts were mixed while stirring continuously until forming a homogeneous gel.
Statistical Analysis
Data obtained from this study were entered into the database of Statistical Package for Social Sciences 15.0 software also through which the statistical analyses were performed. Median, minimum and maximum values of continuous variables were presented, and the compliance of these variables with the normal distribution was investigated. It was concluded that requirements of compliance with the normal distribution were not met for all the variables. Thus, non-parametric methods must be preferred. Comparisons of the independent groups were performed using the Kruskal-Wallis and Mann-Whitney U test methods, while comparisons of dependent groups were performed using the Friedman test method. The difference between the groups was considered statistically significant in the case of the p-value being <0.05.
RESULTS
Results of the Weight Analysis
All subjects in the groups were weighed before the process. Data were collected by weighing the rats after each biopsy taken for post-burn evaluation, adding these values to their dry weights, and also weighing just before being sacrificed. No statistically significant difference in weight was found among the groups.
Results of the Histopathological Analysis
Histopathological examination of Burn Control Group (2b, 3b, 4b, 5b, 6b, 7b), SSD Group (2c, 3c, 4c, 5c, 6c, 7c), and Gel Group (2e, 3e, 4e, 5e, 6e, 7e) biopsies taken at the 4th, 8th and 24th h revealed that they were affected by contact type burns (Figs. 2-7). The epidermis was thinned. Degeneration findings were detected in the hair follicles, in the lower sebaceous gland structure and in the cells forming this structure. Discoloration of collagen fibers and deformation findings in the dermis were noted. Collagen discoloration was very noticeable, especially in biopsies stained with Mallory-Azan (Figs. 3a-e, 5a-e, 7a-e), and it was less common in the deep dermis in the galangal group biopsies (Figs. 2d-7d) when it was compared to the burn control, gel and SSD groups. The epidermis, hair follicles, and sebaceous gland structures were more preserved in the galangal treatment group, and also a noticeably thicker layer of the epidermis was observed compared to the burn control group. It was the closest group to the control group histologically.
Figure 2.

(a-e) 4 h, all groups biopsies (H and E staining). Blue arrow indicates epidermal layer, black arrow indicates dermal collagens,while red arrow indicates gl. sebacea and hair follicle (Magnification scale ×10=500 μm).
Figure 7.

(a-e) 24 h, all groups biopsies (Mallory-Azan staining). Blue arrow indicates epidermal layer, black arrow indicates dermal collagens, while red arrow indicates gl. sebacea (Magnification scale ×10=500 μm).
Figure 3.

(a-e) 4 h, all groups biopsies (Mallory-Azan staining). Blue arrow indicates epidermal layer, black arrow indicates dermal collagens, while red arrow indicates gl. sebacea (Magnification scale ×10=500 μm, (a): ×20=250 μm).
Figure 5.

(a-e) 8 h, all groups biopsies (Mallory-Azan staining). Blue arrow indicates epidermal layer, black arrow indicates dermal collagens (Magnification scale ×10=500 μm).
Figure 4.
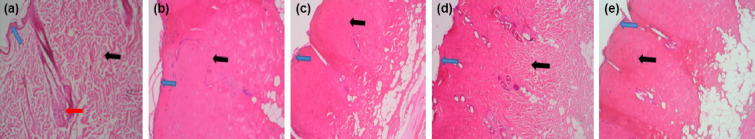
(a-e) 8 h, all groups biopsies (H and E staining). Blue arrow indicates epidermal layer, black arrow indicates dermal collagens, while red arrow indicates gl. sebacea and hair follicle (Magnification scale ×10=500 μm).
Figure 6.

(a-e) 24 h, all groups biopsies (H and E staining). Blue arrow indicates epidermal layer, black arrow indicates dermal collagens, while red arrow indicates gl. sebacea and hair follicle (Magnification scale ×10=500 μm).
Results of the Statistical Analysis
Evaluation of the Epidermal Thickness and Number of Vessels
After the binary comparisons of the groups (Table 2), the epidermal thickness value was significantly higher in the galangal group compared to the other groups (p<0.05). The number of vessels in all three treatment groups was significantly higher at all hours compared to burn control group (p<0.05), while it was significantly higher only at the 4th h in the galangal group compared to SSD and gel groups (p<0.05) (Table 3). When the number of vessels was analyzed within the groups’ own processes, it only showed a significant difference in the galangal group (p<0.05) (Figs. 8 and 9).
Table 2.
Intergroup comparison of epidermal thicknesses of 4th, 8th and 24th h biopsies
| Epidermal thickness (μm) | Mean | Standard deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| 4th h | |||||
| Control | 30.6739 | 0.7509 | 30.6749 | 29.6826 | 31.6453 |
| Burn control | 10.7701 | 0.7339 | 10.9214 | 9.7846 | 11.9099 |
| Silver sulfadiazine | 11.1212 | 0.8922 | 11.1223 | 9.9133 | 12.7255 |
| Galangal | 19.5424 | 0.9462 | 19.5439 | 17.9857 | 20.9013 |
| Gel | 12.8723 | 1.0177 | 12.8722 | 10.9517 | 13.7827 |
| 8th h | |||||
| Control | 30.6895 | 0.7609 | 30.6926 | 29.7021 | 31.7103 |
| Burn control | 11.1996 | 0.8373 | 11.3146 | 9.8547 | 12.4999 |
| Silver sulfadiazine | 11.6532 | 0.9026 | 11.6500 | 10.4326 | 13.2405 |
| Galangal | 20.8943 | 1.0477 | 20.8933 | 19.2893 | 22.2815 |
| Gel | 13.0107 | 0.9987 | 13.0638 | 11.1211 | 13.8824 |
| 24th h | |||||
| Control | 30.6798 | 0.7555 | 30.6781 | 29.7110 | 31.6913 |
| Burn control | 11.4686 | 0.9240 | 11.5659 | 9.9851 | 12.9556 |
| Silver sulfadiazine | 11.9848 | 0.8975 | 11.9857 | 10.7957 | 13.5713 |
| Galangal | 21.9930 | 0.9954 | 21.9939 | 20.2805 | 23.4371 |
| Gel | 13.1604 | 1.0029 | 13.2612 | 11.2713 | 14.0102 |
Table 3.
Intergroup comparison of the numbers of vessels of 4th, 8th and 24th h biopsies
| Number of vessels | Mean | Standard deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| 4th h | |||||
| Control | 40 | 2 | 39 | 36 | 43 |
| Burn control | 8 | 1 | 8 | 6 | 10 |
| Silver sulfadiazine | 14 | 4 | 13 | 10 | 21 |
| Galangal | 28 | 4 | 28 | 22 | 33 |
| Gel | 15 | 3 | 16 | 11 | 18 |
| 8th h | |||||
| Control | 38 | 2 | 38 | 36 | 41 |
| Burn control | 8 | 2 | 7 | 5 | 11 |
| Silver sulfadiazine | 18 | 3 | 18 | 12 | 22 |
| Galangal | 18 | 4 | 17 | 14 | 24 |
| Gel | 15 | 3 | 14 | 10 | 20 |
| 24th h | |||||
| Control | 40 | 2 | 39 | 38 | 43 |
| Burn control | 9 | 2 | 9 | 5 | 12 |
| Silver sulfadiazine | 15 | 3 | 15 | 11 | 19 |
| Galangal | 18 | 4 | 17 | 14 | 24 |
| Gel | 15 | 3 | 15 | 11 | 19 |
Figure 8.

Epidermal thickness measurements of all groups by biopsy hours.
Figure 9.

Number of vessels of all groups by biopsy hours.
Evaluation of the Number of Degenerated Hair Follicles and Total Number of Hair Follicles
After the binary comparisons of the groups (Table 4), the number of degenerated hair follicles showed a significant difference in the galangal group compared to the other groups (p<0.05), while it showed a significant difference in SSD group only at the 8th h compared to burn control group (p<0.05). The total number of hair follicles was significantly higher in the galangal group only at the 8th h compared to SSD and gel groups (p<0.05) (Table 5). When the total number of hair follicles was analyzed within the groups’ own processes, it was significantly higher only in the galangal group (p<0.05) (Figs. 10 and 11).
Table 4.
Intergroup comparison of the numbers of degenerated hair follicles of 4th, 8th and 24th h biopsies
| Number of degenerated hair follicles | Mean | Standard deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| 4th h | |||||
| Burn control | 24 | 4 | 23 | 18 | 29 |
| Silver sulfadiazine | 23 | 5 | 22 | 16 | 30 |
| Galangal | 11 | 2 | 11 | 9 | 14 |
| Gel | 19 | 2 | 19 | 16 | 23 |
| 8th h | |||||
| Burn control | 26 | 3 | 26 | 23 | 31 |
| Silver sulfadiazine | 20 | 5 | 18 | 15 | 28 |
| Galangal | 11 | 3 | 12 | 7 | 14 |
| Gel | 22 | 4 | 21 | 18 | 27 |
| 24th h | |||||
| Burn control | 24 | 4 | 25 | 18 | 29 |
| Silver sulfadiazine | 24 | 5 | 22 | 18 | 30 |
| Galangal | 11 | 2 | 12 | 8 | 13 |
| Gel | 24 | 5 | 23 | 18 | 29 |
Table 5.
Intergroup comparison of total number of hair follicles of 4th, 8th and 24th h biopsies
| Total number of hair follicles | Mean | Standard deviation | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| 4th h | |||||
| Control | 41 | 4 | 41 | 32 | 46 |
| Burn control | 28 | 6 | 29 | 19 | 35 |
| Silver sulfadiazine | 31 | 6 | 32 | 21 | 39 |
| Galangal | 30 | 3 | 30 | 25 | 33 |
| Gel | 33 | 5 | 34 | 26 | 38 |
| 8th h | |||||
| Control | 42 | 3 | 41 | 38 | 46 |
| Burn control | 32 | 4 | 30 | 26 | 38 |
| Silver sulfadiazine | 29 | 4 | 31 | 22 | 33 |
| Galangal | 35 | 3 | 34 | 33 | 41 |
| Gel | 31 | 3 | 30 | 26 | 35 |
| 24th h | |||||
| Control | 40 | 3 | 40 | 35 | 43 |
| Burn control | 32 | 6 | 34 | 22 | 39 |
| Silver sulfadiazine | 30 | 4 | 28 | 23 | 36 |
| Galangal | 34 | 4 | 34 | 29 | 41 |
| Gel | 33 | 6 | 34 | 25 | 40 |
Figure 10.

Number of degeneratedhair follicles of all groups by biopsy hours.
Figure 11.

Total number of hair follicles of all groups by biopsy hours.
Evaluation by the Modified Verhofstad Histopathological Scoring[15] System
Comparisons among the groups were not statistically significant because the parameters (edema, vascular damage, polymorphonuclear leukocytes (PMNL) infiltration, collagen discoloration, hair follicle damage, and glandula sebacea damage) were evaluated using this system showed few variabilities (0, 1, 2, 3). Therefore, comments were made according to the calculated mean scores of the groups. In the comparisons both among the groups and within the groups’ own processes, it was observed that the galangal group had the lowest scores in all parameters, especially in glandula sebaceous damage (Table 6 and Fig. 12).
Table 6.
Group averages by the Modified Verhofstad Histopathological Scoring[15] System
| Histopathological classification | Burn Control Mean | SSD Mean | Galangal Mean | Gel Mean |
|---|---|---|---|---|
| Edema | ||||
| 4th h | 2.85 | 2.71 | 1.42 | 2.71 |
| 8th h | 2.71 | 2.42 | 1.28 | 2.71 |
| 24th h | 2.42 | 2 | 1.28 | 2.71 |
| PMNL infiltration | ||||
| 4th h | 1.85 | 1.57 | 1.28 | 1.71 |
| 8th h | 2.57 | 2.57 | 1.28 | 2.57 |
| 24th h | 2.28 | 2 | 1.28 | 2.57 |
| Collagen discoloration | ||||
| 4th h | 2.71 | 2.71 | 1.42 | 2.28 |
| 8th h | 2.57 | 2.28 | 1.42 | 2.28 |
| 24th h | 2.28 | 1.71 | 1.57 | 2.42 |
| Vascular injury | ||||
| 4th h | 2.71 | 2.42 | 1.42 | 2.28 |
| 8th h | 2.28 | 2.14 | 1.42 | 2.14 |
| 24th h | 2.14 | 1.85 | 1.42 | 2.14 |
| Hair follicle damage | ||||
| 4th h | 2.71 | 2.57 | 1.28 | 2.57 |
| 8th h | 2.57 | 2.28 | 1.28 | 2.42 |
| 24th h | 2.57 | 1.85 | 1.57 | 2.42 |
| Glandula sebacea damage | ||||
| 4th h | 2.71 | 2.57 | 1 | 2.57 |
| 8th h | 2.71 | 2.71 | 0.57 | 2.71 |
| 24th h | 2.85 | 2.57 | 0.71 | 2.71 |
PMNL: Polymorphonuclear leukocytes; SSD: Silver sulfadiazine.
Figure 12.

Histopathological scoring of all groups by biopsy hours.
DISCUSSION
Among the topically used drugs to burn wound healing, 1% SSD cream is the most commonly used agent worldwide.[14] SSD has been shown to reduce bacterial contamination, hasten epithelialization, and delay wound contraction.[16–21] SSD is also known to delay wound healing, cause atrophic and hypertrophic scars, cause renal toxicity and leukopenia when it is applied for more than 3 weeks and be at risk of developing resistance.[13]
In a study conducted by Subrahmanyam, fifty patients with partial burns divided into two groups. While pure and untreated honey was applied topically to one group, SSD cream was applied topically to the other group. Biopsies taken from the wound site on the 7th and 21st days of the treatment showed that clinically granulation and epithelialization rate was 84% and 100%, respectively, in the honey treated group. On the 21st day, wound healing was completed in all patients in the honey-treated group.[22] The results of another study by Subrahmanyam showed the antibacterial activity of honey. It was superior to SSD in wound healing and preventing bacterial growth in the burn wound.[23]
The flavonoid galangin, the most active molecule found in the rhizome of the galangal (AO) (Fig. 1), is also be found in the nature in the contents of honey and propolis.[24] These results suggest that it may be more appropriate to use another simple, inexpensive, alternative, natural and possibly superior topical agent, such as honey, when treating minor burn wounds. To our knowledge, there is not any research into the effects of the AO in experimental contact type burns. Thus, we could not compare all of the data results obtained in the previous studies.
From the past to the present day, many experimental models have been developed using animals of different sizes, shapes, species, and weights to gain knowledge about the burn pathophysiology, burn tissue and the mechanism of action of therapeutic agents.[25] It is extremely important to establish an appropriate burn model to monitor the physiological process after the burn and to evaluate the effects of different topical or systemic treatments properly. In vivo burn model must be simple, safe, consistent and repeatable for the benefit of the researchers. The instruments used for the standardization of the burn model, method, localization of the burn, size of the burn area, the temperature used, and the duration of exposure to the heat should be defined. The burned area should be as small as possible to avoid a major systemic response and also be large enough for multiple biopsies. It is important to choose a sufficiently smooth surface (e.g., paravertebral area) on the animal skin to create a uniform contact type burn, so that both the pressure and contact areas would be equal for all the burned tissues.[26] The post-burn epidermal layer stimulates regeneration and spontaneous healing of the burned epithelium. The structures of the skin remaining in the dermoepidermal junction also play a key role in post-burn healing.[27] Most of the experimental animal models reported for scald or contact type burns are risky to the researcher and may not be consistent concerning adjusting the depth and width of the burn. Gurfinkel et al.[28] developed a novel device used in pigs and rats to create an in vivo burn model that overcomes these shortcomings. This model was not preferred in this study due to the difficulty in providing that device and the quick death of the rats. Li et al.[29] created deep second-degree burn wounds in Wistar rats and evaluated the healing efficacy of crocodile oil. This method was also not preferred because it was considered to be risky for the safety of the researchers. Begieneman et al.[30] burned the shaved areas of 12-week-old female Wistar rats with a copper stamp heated at 100°C, as preferred in this study. It can be concluded that the burn type in this study was standardized to second degree burn with superficial partial thickness. Because the histopathological examination revealed that the epidermal integrity was maintained in all groups, the dermis was not affected to a great extent. The subcutaneous structure was not affected and adnexal structures (hair root, sweat gland, sebaceous gland, and vascular structures) were relatively protected.
Epidermal thickness, the number of vessels and number of hair follicles decreased in the burn control group compared to the control group in this study. Furthermore, degeneration findings, such as edema, vascular damage, PMNL infiltration, collagen discoloration, hair follicle damage, and glandula sebacea damage, were observed histopathologically. It is reported in the literature that the most important physiopathological change in the second degree thermal injuries is the loss of vascular viability.[31–33] In this study, the total number of preserved vessels in the galangal group was significantly higher than the burn control group. SSD and placebo gel treatments also showed a protective effect on the number of vessels. However, the effect of galangal at the 4th h was statistically significant compared to the other two treatment groups. Galangal was also more effective at the 4th h in the analysis of decreasing of vessel damage. The duration of this study and dose of bioactive material may not be sufficient to observe the long term anti-ischemic, anti-oxidant, anti-inflammatory, and vasoprotective effects of galangal.[2,6,34] This may shed light on the clinicians investigating the effects of galangal on protecting the vascular structure in the acute and chronic phases of the burn wound.
Collagen, which is the most important protein of skin tissue, makes up approximately 75% of skin weight. Collagen tissue damage is most common in burns. After the injury, Type IV-V collagen becomes dominant in the 1st h while type III and type I become dominant at 24th and 60th h, respectively.[35] Collagenase is a specific proteinase with a limited number of substrates.[36] However, elastase is the only enzyme capable of degrading all proteins of the connective tissue, including elastin, an elastic fibrous protein.[37] Elastin is the main component of elastic fibers of connective tissue and elastic fibers together with collagen fibers form a network under the epidermis.[38] It is claimed that elastase released from PMNLs damages elastin and collagen fibers as a result of rapid inflammation of the dermis layer after UV exposure to the skin.[39] In a study conducted by Lee et al.,[40] one hundred and fifty plants used in medicine production were examined, and eighty of them, including AO rhizome, were shown to inhibit elastase enzyme at different levels. In another study carried out by Kanashiro et al.,[41] spectrophotometric measurements showed that four different flavonoid compounds, including galangin, inhibited the catalytic activity of elastase released from neutrophils. In this study, discoloration of collagen fibers and deformation findings in the dermis was very noticeable, especially in biopsies stained with Mallory–Azan (Figs. 3, 5, 7), and it was less common in the deep dermis in the galangal group biopsies (Figs. 2d-7d) when it is compared to the burn control, gel and SSD groups. Furthermore, the protection of sebasea glands was the most significant morphological parameter. It can be said that this effect of galangal may be secondary to inhibition of the elastase enzyme causing the prevention of contact of the inflammation with sebasea glands and collagen fibers in the deep dermis. Further research is required for this subject.
Polymeric hydrogels are wound dressings used for burns and wounds. The high water content of hydrogels makes them ideal pharmaceutical forms to treat burn wounds.[42,43] The wound-healing effect of AO extract containing Na-CMC gel was investigated in this study. The most of the healing effects of the plain gel, which is in the form of a hydrogel on the burn wound, were limited and statistically insignificant. Although the positive effects on the epidermal thickness and the total number of vessels were found to be statistically significant compared to the burn control group, it showed a lesser effect than galangal and SSD groups. In a study conducted by Daniels and Knie,[44] the hydrogel was compared with other carrier agents prepared with water and alcohol and it stood up by its features, such as moistening, anti-inflammatory effect, faster drying and cooling of the skin. Based on these data, histopathological findings in the gel group may be thought to be related to these effects.
In conclusion, administrating AO containing gel 4 times a day within the first 24 h is effective in the experimental contact type second degree burn model. It is significantly superior to SSD treatment, especially in the first 8 h of administration. However, when the conditions, methods and findings of this study were evaluated, some limitations were found. This study was conducted on an experimental burn model, not on humans. Biochemical markers were not used to evaluate wound healing. No cytotoxicity studies were performed. Pain, pain response and antibacterial efficacy of the treatments were not evaluated. Only the acute phase effects were examined. Long-term local and systemic complications of wound healing were not evaluated. The findings obtained from this study will contribute to the literature on the use of galangal in practice in addition to its widespread use among the public.
Acknowledgment
This study was supported by the Ege University Scientific Research Projects Fund as project 2013TIP-027.
Footnotes
Ethics Committee Approval: This study was approved by the Dokuz Eylul University Faculty of Medicine Animal Experimental Ethics Committee (Date: 02.11.2012, Decision No: 62/2012).
Peer-review: Internally peer-reviewed.
Authorship Contributions: Concept: S.K.; Design: S.K.; Supervision: S.K.; Data: K.K., Y.U., E.Ö.Ç., F.K.; Analysis: K.K., Y.U., E.Ö.Ç.; Literature search: K.K., Y.U., E.Ö.Ç.; Writing: K.K.; Critical revision: S.K.
Conflict of Interest: None declared.
REFERENCES
- 1.Indrayan A, Agrawal P, Rathi AK, Shatru A, Agrawal NK, Tyagi DK. Nutritive value of some indigenous plant rhizomes resembling Ginger. Nat Prod Rad. 2009;8:507–13. [Google Scholar]
- 2.Ghosh S, Rangan L. Alpinia:The gold mine of future therapeutics. 3 Biotech. 2013;3:173–85. doi: 10.1007/s13205-012-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciolino H, Yeh G. The flavonoid galangin is an inhibitor of CYP1A1 activity and an agonist/antagonist of the aryl hydrocarbon receptor. Br J Cancer. 1999;79:1340. doi: 10.1038/sj.bjc.6690216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tao L, Wang ZT, Zhu EY, Lu YH, Wei DZ. HPLC analysis of bioactive flavonoids from the rhizome of Alpinia officinarum. South Afr J Bot. 2006;72:163–6. [Google Scholar]
- 5.Matsuda H, Ando S, Kato T, Morikawa T, Yoshikawa M. Inhibitors from the rhizomes of Alpinia officinarum on production of nitric oxide in lipopolysaccharide-activated macrophages and the structural requirements of diarylheptanoids for the activity. Bioorg Med Chem. 2006;14:138–42. doi: 10.1016/j.bmc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Sivakumar AS, Anuradha CV. Effect of galangin supplementation on oxidative damage and inflammatory changes in fructose-fed rat liver. Chem Biol Interact. 2011;193:141–8. doi: 10.1016/j.cbi.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Chen JC, Ho FM, Chao PD, Chen CP, Jeng KC, Hsu HB, et al. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Blonska M, Bronikowska J, Pietsz G, Czuba Z, Scheller S, Krol W. Effects of ethanol extract of propolis (EEP) and its flavones on inducible gene expression in J774A. 1 macrophages. J Ethnopharmacol. 2004;91:25–30. doi: 10.1016/j.jep.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Khalil M, Sulaiman S. The potential role of honey and its polyphenols in preventing heart disease:A review. Afr J Tradit Complement Alternat Med. 2010;7:315–21. doi: 10.4314/ajtcam.v7i4.56693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Association AB. Burn Incidence Fact Sheet. 2016. Available from: http://www.ameriburnorg/who-we-are/media/burn-incidence-fact-sheet .
- 11.Haberal M, Oner Z, Bayraktar U, Bilgin N. Epidemiology of adults and childrens burns in a Turkish burn center. Burns. 1987;13:136–40. doi: 10.1016/0305-4179(87)90103-3. [DOI] [PubMed] [Google Scholar]
- 12.Türegün M, Sengezer M, Selmanpakoglu N, Çeliköz B, Nişanci M. The last 10 years in a burn centre in Ankara, Turkey:An analysis of 5264 cases. Burns. 1997;23:584–90. doi: 10.1016/s0305-4179(97)00081-8. [DOI] [PubMed] [Google Scholar]
- 13.Arslan K, Karahan O, Okuş A, Unlü Y, Eryılmaz M, Ay S, et al. Comparison of topical zinc oxide and silver sulfadiazine in burn wounds:An experimental study. Turk J Trauma Emerg Surg. 2012;18:376–83. doi: 10.5505/tjtes.2012.45381. [DOI] [PubMed] [Google Scholar]
- 14.Papini R, Wilson A, Steer J, McGrouther D, Parkhouse N. Wound management in burn centres in the United Kingdom. Br J Surg. 1995;82:505–9. doi: 10.1002/bjs.1800820423. [DOI] [PubMed] [Google Scholar]
- 15.Akdemir O, Hede Y, Zhang F, Lineaweaver WC, Arslan Z, Songur E. Effects of taurine on reperfusion injury. J Plast Reconst Aesthetic Surg. 2011;64:921–8. doi: 10.1016/j.bjps.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Vloemans A, Soesman A, Suijker M, Kreis R, Middelkoop E. A randomised clinical trial comparing a hydrocolloid-derived dressing and glycerol preserved allograft skin in the management of partial thickness burns. Burns. 2003;29:702–10. doi: 10.1016/s0305-4179(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 17.Warriner R, Burrell R. Infection and the chronic wound:A focus on silver. Adv Skin Wound Care. 2005;18:2–12. doi: 10.1097/00129334-200510001-00001. [DOI] [PubMed] [Google Scholar]
- 18.Atiyeh BS, Costagliola M, Hayek SN, Dibo SA. Effect of silver on burn wound infection control and healing:Review of the literature. Burns. 2007;33:139–48. doi: 10.1016/j.burns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Wright JB, Lam K, Buret AG, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds:Effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis, and healing. Wound Repair Regen. 2002;10:141–51. doi: 10.1046/j.1524-475x.2002.10308.x. [DOI] [PubMed] [Google Scholar]
- 20.Demling RH, DeSanti L. The rate of re-epithelialization across meshed skin grafts is increased with exposure to silver. Burns. 2002;28:264–6. doi: 10.1016/s0305-4179(01)00119-x. [DOI] [PubMed] [Google Scholar]
- 21.Lansdown A. Silver. 2:Toxicity in mammals and how its products aid wound repair. J Wound Care. 2002;11:173–7. doi: 10.12968/jowc.2002.11.5.26398. [DOI] [PubMed] [Google Scholar]
- 22.Subrahmanyam M. A prospective randomised clinical and histological study of superficial burn wound healing with honey and silver sulfadiazine. Burns. 1998;24:157–61. doi: 10.1016/s0305-4179(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 23.Subrahmanyam M. Topical application of honey in treatment of burns. Br J Surg. 1991;78:497–8. doi: 10.1002/bjs.1800780435. [DOI] [PubMed] [Google Scholar]
- 24.Patel D, Patel K, Gadewar M, Tahilyani V. Pharmacological and bioanalytical aspects of galangin-a concise report. Asian Pac J Trop Biomed. 2012;2:S44–55. doi: 10.1016/S2221-1691(12)60239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitsunaga JK, Jr, Gragnani A, Ramos ML, Ferreira LM. Rat an experimental model for burns:A systematic review. Acta Cir Bras. 2012;27:417–23. doi: 10.1590/s0102-86502012000600010. [DOI] [PubMed] [Google Scholar]
- 26.Singer AJ, Berruti L, Thode HC, Jr, McClain SA. Standardized burn model using a multiparametric histologic analysis of burn depth. Acad Emerg Med. 2000;7:1–6. doi: 10.1111/j.1553-2712.2000.tb01881.x. [DOI] [PubMed] [Google Scholar]
- 27.Wolf S, Herndon D. Burn Care: Landes Bioscience. United States: CRC Press; 1999. pp. 21–6. [Google Scholar]
- 28.Gurfinkel R, Singer AJ, Cagnano E, Rosenberg L. Development of a novel animal burn model using radiant heat in rats and swine. Acad Emerg Med. 2010;17:514–20. doi: 10.1111/j.1553-2712.2010.00736.x. [DOI] [PubMed] [Google Scholar]
- 29.Li HL, Chen LP, Hu YH, Qin Y, Liang G, Xiong YX, et al. Crocodile oil enhances cutaneous burn wound healing and reduces scar formation in rats. Acad Emerg Med. 2012;19:265–73. doi: 10.1111/j.1553-2712.2012.01300.x. [DOI] [PubMed] [Google Scholar]
- 30.Begieneman MP, Kubat B, Ulrich MM, Hahn NE, Stumpf-Stolker Y, Tempelaars M, et al. Prolonged C1 inhibitor administration improves local healing of burn wounds and reduces myocardial inflammation in a rat burn wound model. J Burn Care Res. 2012;33:544–51. doi: 10.1097/BCR.0b013e31823bc2fc. [DOI] [PubMed] [Google Scholar]
- 31.Madri J. Inflammation and healing. Andersons Pathol. 1990;1:67–110. [Google Scholar]
- 32.Yenerman M. Genel Patoloji. 1st ed. İstanbul: İstanbul Üniversitesi; 1986. pp. 271–94. [Google Scholar]
- 33.Atiyeh BS, Hayek SN, Gunn SW. New technologies for burn wound closure and healing-review of the literature. Burns. 2005;31:944–56. doi: 10.1016/j.burns.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Morello S, Vellecco V, Alfieri A, Mascolo N, Cicala C. Vasorelaxant effect of the flavonoid galangin on isolated rat thoracic aorta. Life Sci. 2006;78:825–30. doi: 10.1016/j.lfs.2005.05.072. [DOI] [PubMed] [Google Scholar]
- 35.Madden JW, Anem AJ. Wound healing. In: Townsend CM Jr, editor. Sabiston Textbook of Surgery. Philadelphia, PA: WB Saunders Co; 1991. p. 164. [Google Scholar]
- 36.Gadek JE, Fells GA, Wright DG, Crystal RG. Human neutrophil elastase functions as a Type III collagen “collagenase”. Biochem Biophys Res Commun. 1980;95:1815–22. doi: 10.1016/s0006-291x(80)80110-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhi-Ying Y, Guo-Xiong G, Wei-Qin Z, Zhe-Fu L. Elastolytic activity from'Flavobacterium odoratum'microbial screening and cultivation, enzyme production and purification. Proc Biochem. 1994;29:427–36. [Google Scholar]
- 38.Meyer W, Neurand K, Radke B. Elastic fibre arrangement in the skin of the pig. Arch Dermatol Res. 1981;270:391–401. doi: 10.1007/BF00403783. [DOI] [PubMed] [Google Scholar]
- 39.Motoyoshi K, Takenouch MR. 053. Proceedings of the 19th IFSCC Congress. Sydney, Australia. IFSCC. 1996 [Google Scholar]
- 40.Lee KK, Kim JH, Cho JJ, Choi JD. Inhibitory effects of 150 plant extracts on elastase activity, and their anti-inflammatory effects. Int J Cosmetic Sci. 1999;21:71–82. doi: 10.1046/j.1467-2494.1999.181638.x. [DOI] [PubMed] [Google Scholar]
- 41.Kanashiro A, Souza JG, Kabeya LM, Azzolini A, Lucisano-Valim YM. Elastase release by stimulated neutrophils inhibited by flavonoids:Importance of the catechol group. Z Naturforsch C J Biosci. 2007;62:357–61. doi: 10.1515/znc-2007-5-607. [DOI] [PubMed] [Google Scholar]
- 42.Madaghiele M, Demitri C, Sannino A, Ambrosio L. Polymeric hydrogels for burn wound care:Advanced skin wound dressings and regenerative templates. Burns Trauma. 2014;2:153–61. doi: 10.4103/2321-3868.143616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nascimento EG, Sampaio TB, Medeiros AC, Azevedo EP. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cir Bras. 2009;24:460–5. doi: 10.1590/s0102-86502009000600007. [DOI] [PubMed] [Google Scholar]
- 44.Daniels R, Knie U. Galenics of dermal products-vehicles, properties and drug release. J Dtsch Dermatol Ges. 2007;5:367–83. doi: 10.1111/j.1610-0387.2007.06321.x. [DOI] [PubMed] [Google Scholar]


