Abstract
BACKGROUND
Intestinal parasite Giardia can affect children’s physical development mainly stunting even in asymptomatic cases. The protozoa G. lamblia is divided into assemblages A-H. However, it is still unclear whether clinical manifestations and pathogenesis may vary according to the infecting assemblage.
OBJECTIVES
To investigate whether G. lamblia assemblages influence differently the physical development of preschoolers from a community of Rio de Janeiro, Brazil.
METHODS
Anthropometric parameters were analysed from children attending a daycare centre and stool samples were obtained for the G. lamblia diagnosis. G. lamblia isolates from positive samples were genotyped. Data were analysed in order to verify whether there is a relationship between G. lamblia infection and the physical development of children according to the assemblage.
FINDINGS
Herein we demonstrated that although eutrophic, G. lamblia-infected daycare preschoolers from a low-income community presented growth delay compared to non-infected ones. This effect was observed for the three assemblages (A, B or E) found infecting humans.
MAIN CONCLUSION
G. lamblia causes growth delays on children independent of infecting assemblage (A, B or E).
Key words: Giardia lamblia, height for age, weight for age, pre-schoolers, genotyping
Giardia lamblia is the most common flagellate intestinal protozoan in human infections. The species is phylogenetically divided into assemblages from A to H. 1 The assemblages A and B present a high zoonotic potential and are classically reported in humans. 1 The other assemblages (C-H) are considered host-specific, however this affinity for the host is discussed because the circulation of these assemblages in atypical hosts has grown. 2 , 3 The assemblage E is associated with infection in farm animals, however several reports of human infection were reported. 2 , 4 , 5 ) Although the genetic divergences of these assemblages and their affinity for groups of hosts are known, there is still a lack of knowledge if the pathogenesis, clinical manifestations or drug resistance predisposition can vary according to the infecting assemblage.
Infection by assemblages A and B can be asymptomatic or show a wide spectrum of clinical features and may vary depending on the characteristics of the population evaluated. 6 - 13 The clinical presentation directly related to assemblage E in human infection has not yet been reported. There are growing evidences that giardiasis impact on children development. G. lamblia-infected children present growth delays and weight reduction, even without apparent clinical symptoms as diarrheal disease. 14 - 20
Malnutrition is commonly observed in G. lamblia-infected children, 21 who present reduced weight, serum iron and zinc levels. 17 Malabsorption is reported in at least 50% of symptomatic giardiasis patients, 14 - 16 which can cause growth delays of up to 4 months after an episode of diarrhoea. 14 , 15 However, most studies looking at the physical impacts associated with G. lamblia infection are carried out with individuals from areas of social vulnerability where other factors such as malnutrition associated with economic conditions may represent confounding factors. 22 , 23 It is unknown whether G. lamblia infection can also harm apparently healthy and asymptomatic children in subclinical infections and whether the impact of infection is related to the infecting assemblage. In this context, this study aims to investigate whether G. lamblia assemblages differently affect the physical development of asymptomatic preschoolers attended at a municipal daycare centre located in a low-income community of Rio de Janeiro, Brazil.
MATERIALS AND METHODS
Study design, collection of faecal samples and parasitological examinations - A cross-sectional survey was carried out in the years 2014 and 2015 in a public municipal daycare in a low-income community of Rio de Janeiro. The daycare hosts children aged 10 months to 4 years, for a period of 9 hours (7:30 am to 16:30 pm), and provides five meals balanced by nutritionist a day. The demographic information regarding sex and age (months) were also collected.
A single stool sample was collected from 194 out 220 subjects. For the diagnosis of geohelminths and protozoa, the samples were submitted to parasitological examination by the Ritchie method. In parallel, stool aliquots were conditioned at -20oC until molecular diagnosis and genotyping.
All children infected with intestinal parasites were monitored by physicians from the Brazilian program Saúde da Família (PSF) at Centro de Saúde Heitor Beltrão, Secretaria Municipal de Saúde, Prefeitura da Cidade do Rio de Janeiro and were treated with medications recommended by the Brazilian Ministry of Health.
Ethics - The subjects were included in the research after responsible agreement and of the free and informed consent form signature, according to the ethical requirements. This study was approved by the ethics review board in human research from Instituto Oswaldo Cruz, Fundação Oswaldo Cruz-Fiocruz (CAAE 24712319.6.0000.5248).
Molecular diagnosis and genotyping of G. lamblia - DNA extraction from faeces was performed using the QIAamp DNA Stool Mini Kit (Qiagen GmbH, Hilden, Germany), according to the manufacturer’s instructions, with the exception of the lysis temperature, which was increased to 95°C, and the volume of AE buffer used for DNA elution, which was decreased to 100 μL. The isolated DNA was stored at -20°C until the time of use.
For the diagnosis and genotyping of G. lamblia, the conserved genes coding for the protein glutamate dehydrogenase (gdh) and beta-giardin (βgia) were used, as described by Fantinatti et al. 24
Anthropometric measurements - Anthropometric data (weight and height) of all children included were obtained after the completion of stool sample collection and before treatment. The measurements were taken at the daycare centre with the support of a nursery team of the local municipal health department (PSF), in a single moment per year. The weight was measured in the range of 0.01 kg using a digital scale and height in the nearest 0.1 cm using with a millimetrered tape. Standard deviation scores (Z-scores) of weight-for-height (WHZ), height-for-age (HAZ) and weight-for-age (WAZ) were calculated using the NutStat Module on EpiInfo 2000 (according to Centers for Disease Control and Prevention, Atlanta, USA) and the World Health Organization’s 1978 growth chart. The Z-score corresponds to a dispersion measure with standard deviation (SD) in a reference population. Stunting, wasting and underweight were defined by -2 SD from mean HAZ, WHZ and WAZ, respectively. 25
Statistical analyses - The statistical analyses were performed using GraphPad Prism software (version 6.0, San Diego, USA). The Z-scores of positive-infected individuals with distinct G. lamblia assemblages and negative-infected individuals were compared using the Mann-Whitney U non-parametric test. Statistical significance was established at p < 0.05.
RESULTS
Frequency of G. lamblia infection and their assemblages among pre-schoolers - A total of 194 faecal samples were collected from children, of which 86 samples (44.3%) were positive for G. lamblia. Regarding gender, there was no statistical difference among Giardia infected preschoolers, infection rate reaching 51/109 (46.8%) in females and 35/85 (41.2%) in males (p = 0.7).
In addition to G. lamblia, the stool examination revealed the following intestinal parasites and their respective frequencies: Entamoeba histolytica/dispar/moshkovskii/bangladeshi (4/194 - 2.06%), Entamoeba coli (5/194 - 2.58%), Endolimax nana (13/194 - 6.70%) and Ascaris lumbricoides (15/194 - 7.73%).
G. lamblia-positive stool DNA samples were extracted and the parasite was genotyped using gdh and βgia markers with the following distribution: 42 (48.8%) assemblage A, 21 (24.4%) assemblage B, 19 (22.1%) assemblage E and four (4.7%) assemblage A/E (Supplementary Table). 24 There were no differences in assemblages frequencies through genotyping with distinct markers.
G. lamblia infection and children’s nutritional status - The anthropometric parameters were evaluated in 167 children. The vast majority of children was classified as eutrophic for HAZ (n = 130), WAZ (n = 152) and WHZ (n = 154).
Although eutrophic for the HAZ and WAZ parameters, children infected by G. lamblia had significantly lower Z-score medians (interquartile range) (-1.36 [-1.79 to -0.48], n = 60 and -0.35 [-1.36 to +0.34], n = 71) (A in Figure) values than non-infected ones (-0.47 [-1.10 to -0.11], n = 81 and 0.02 [-0.58 to -0.53], n = 96), p = 0.0001 and p = 0.017, respectively (C in Figure). For the WHZ parameter, there was no difference in Z-score between groups (Giardia-positive: 0.47 [+0,42 to +0,93] n = 71, Giardia-negative: 0,55 [-0,20 to +0,94] n = 95), p = 0.65 (E in Figure) (Supplementary Table (341.8KB, pdf) ).
Influence of Giardia lamblia infection and its assemblages on anthropometric parameters measured on preschool children. A and B: height-for-age (HAZ); C and D: weight-for-age (WAZ); E and F: weight-for-height (WHZ). The insert in A, C and E is a dot plot representation of individual’s Z-score values. The column bar represents the median values with interquartile range. Asterisks denote statistically significant differences between groups compared by Mann-Whitney U non-parametric test. * p < 0.05; ** p < 0.005. Giardia-pos: Giardia-positive; Giardia-neg: Giardia-negative.
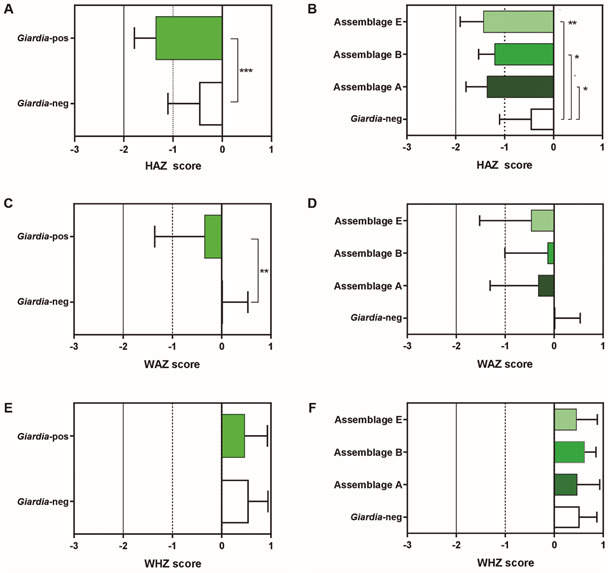
G. lamblia infected children were subdivided according to the infecting assemblage (A, B or E). Individuals infected with any of the three G. lamblia assemblage A, B or E showed significantly lower HAZ score values compared to Giardia-negative individuals (p = 0.03, p = 0.03, p = 0.003, respectively) (B in Figure). No difference was seen for HAZ score values among the subgroups of children infected by the different assemblages. In addition, although G. lamblia positive children did present lower weight, no significant difference was observed for WAZ among individuals infected by assemblages A, B and E compared to the group of Giardia-negative (D in Figure). Regarding the WHZ parameter, no differences were observed between the group Giardia-negative children compared with the subgroups of children infected by the different assemblages (F in Figure) (Supplementary Table (341.8KB, pdf) ).
DISCUSSION
In this study, a high infection rate of G. lamblia was demonstrated, with almost half of the children in the daycare centre evaluated as positive for this infection, as well as a high genotypic diversity of the parasite, in which A, B and E assemblages were detected. The main finding was the influence of G. lamblia infection on the nutritional parameters of children, specifically HAZ and WAZ. The malabsorptive syndrome induced by G. lamblia can cause nutritional deficits and growth faltering in childhood as a result of chronic damage, even in asymptomatic individuals. 18 - 20 , 26 , 27
The biological properties, besides distinct antigenic composition of different G. lamblia assemblages, can affect the parasite-host relationship, possibly accounting to the clinical diversity of the infection. 3 In the present study, we evaluated putative differences in G. lamblia assemblage’s pathogenicity showing that, despite eutrophic, children infected by G. lamblia presented growth retardation regardless the assemblage (A, B or E) they were infected.
In Brazil, a significant reduction in malnutrition besides no increase in overweight cases have been observed in children under 5 years of age. 28 The preschoolers studied herein stay in the daycare 9-hours by day, where they have a balanced nutritional support (five meals). Therefore, as expected, most children were eutrophic. It points that the physical impairment observed herein is probably a direct consequence of the parasite infection, as already proven in a murine experimental model. 29 In this study, the nutritional indicators influenced by G. lamblia infection were HAZ and WAZ, confirming previous results evidencing an insidious effect of subclinical infections, not associated with acute or persistent diarrheal disease. 20
Experimental model studies have shown that Giardia infection can lead to changes in small intestine physiology, causing disruption of tight junctions, enterocyte apoptosis, microvilli shortening, altered trypsin activity of enterocyte and interfering with Na/Cl and in the metabolism of glucose and minerals. 30 Interference in biliary activity impairs lipid metabolism leading, consequently, to the reduction of fat-soluble vitamins (A, D, E and K). These changes may be associated with deficits in deficits in children’s development, especially with the reduction in HAZ indices.
G. lamblia-associated growth delays have also been observed in others studies. 19 , 31 - 35 This is in line with the hypothesis that G. lamblia is one of the major contributors to decrement on length in young children. 23 , 32 Whether any assemblage of G. lamblia is faster to induce intestinal damage, impair nutritional intake and consequently physical development is unknown.
All the three G. lamblia assemblages found infecting humans (A, B and E) 2 , 4 were detected in our previous study area. 24 ) Importantly, there was no specific assemblage associated with decrease in length.
Many studies have been carried out in an attempt to associate assemblages A and B with a symptom or a set of symptoms. However, the studies show divergence between the findings, suggesting that this association is multifactorial, and should be considered characteristics of the parasite, host and environment (endemic strains and newly introduced strains). 3 There are reports in the literature of the assemblage E infecting humans but, as these findings are recent, the association of this genotype with the pathogenesis of human giardiasis is still unknown. 4 , 5 , 24 , 36 - 40
We also identified the presence of other pathogenic intestinal parasites (A. lumbricoides and E. histolytica) that could also impair the physical development of these children. 41 - 45 As previous results have already shown an association between Giardia infection and growth impair, 23 besides the high frequency of this parasite in our casuistic, we decided to investigate it.
In a limiting way, this was a cross-sectional study and, therefore, it is not possible to exclude the possibility of reverse causality. In other words, the low incoming socioeconomic conditions observed in rural areas and slums could favour inadequate nutrition for these children, which could increase the risk of infection. The preschoolers studied herein present a high frequency (44.3%) of G. lamblia infection and are exposed to frequent reinfections. 24 Chronic parasitism leading to inflammatory disorders of the proximal small intestine, condition known as environmental enteric dysfunction, 46 can be related to G. lamblia infection and underlies growth faltering in our study population.
In conclusion, G. lamblia is associated with deficits in physical development in pre-school children living the study area, independently of the infecting assemblage. Giardiasis should be targeted by specific control measures, with improved diagnosis at the community level and access to treatment.
ACKNOWLEDGEMENTS
To Elizabeth Salgado and the nursery employees, for supporting the access to the preschoolers, to health care team of Programa Saúde da Família, Centro de Saúde Heitor Beltrão, Secretaria Municipal de Saúde of Rio de Janeiro, to Dr A. R. Bello and MSci E. Verissimo, for parasitological studies, to Dr K. Rabelo, for reviewing this manuscript, and to Plataforma de Sequenciamento de DNA por Eletroforese Capilar (RPT01A)/Fiocruz.
REFERENCES
- 1.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev. 2011 doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan U, Zahedi A. Molecular epidemiology of giardiasis from a veterinary perspective. Adv Parasitol. 2019 doi: 10.1016/bs.apar.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Fantinatti M, Gonçalves-Pinto M, Da-Cruz AM. Can Giardia lamblia assemblages drive the clinical outcome of giardiasis? Curr Trop Med Rep. 2022 [Google Scholar]
- 4.Fantinatti M, Bello AR, Fernandes O, Da-Cruz AM. Identification of Giardia lamblia assemblage E in humans points to a new anthropozoonotic cycle. J Infect Dis. 2016 doi: 10.1093/infdis/jiw361. [DOI] [PubMed] [Google Scholar]
- 5.Zahedi A, Field D, Ryan U. Molecular typing of Giardia duodenalis in humans in Queensland - First report of assemblage E. Parasitology. 2017 doi: 10.1017/S0031182017000439. [DOI] [PubMed] [Google Scholar]
- 6.Crotti D, D'Annibale ML, Fonzo G, Lalle M, Cacciò SM, Pozio E. Dientamoeba fragilis is more prevalent than Giardia duodenalis in children and adults attending a day care centre in Central Italy. Parasite. 2005 doi: 10.1051/parasite/2005122165. [DOI] [PubMed] [Google Scholar]
- 7.Minvielle MC, Molina NB, Polverino D, Basualdo JA. First genotyping of Giardia lamblia from human and animal feces in Argentina, South America. Mem Inst Oswaldo Cruz. 2008 doi: 10.1590/s0074-02762008000100015. [DOI] [PubMed] [Google Scholar]
- 8.Kohli A, Bushen OY, Pinkerton RC, Houpt E, Newman RD, Sears CL, et al. Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Trans R Soc Trop Med Hyg. 2008 doi: 10.1016/j.trstmh.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelayo L, Nuñez FA, Rojas L, Furuseth Hansen E, Gjerde B, Wilke H, et al. Giardia infections in Cuban children: the genotypes circulating in a rural population. Ann Trop Med Parasitol. 2008 doi: 10.1179/136485908X355247. [DOI] [PubMed] [Google Scholar]
- 10.Tungtrongchitr A, Sookrung N, Indrawattana N, Kwangsi S, Ongrotchanakun J, Chaicumpa W. Giardia intestinalis in Thailand: identification of genotypes. J Health Popul Nutr. 2010 doi: 10.3329/jhpn.v28i1.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira FS, Machado Sá da Bandeira RA, Constantino CA, da Fonseca AM, Gomes Jda G, Rodrigues RM, et al. Molecular and clinical characterization of Giardia duodenalis infection in preschool children from Lisbon, Portugal. J Parasitol Res. 2013 doi: 10.1155/2013/252971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rafiei A, Roointan ES, Samarbafzadeh AR, Shayesteh AA, Shamsizadeh A, Pourmahdi Borujeni M. Investigation of possible correlation between Giardia duodenalis genotypes and clinical symptoms in Southwest of Iran. Iran J Parasitol. 2013;8(3):389–395. [PMC free article] [PubMed] [Google Scholar]
- 13.Fahmy HM, El-Serougi AO, El Deeb HK, Hussein HM, Abou-Seri HM, Klotz C, et al. Giardia duodenalis assemblages in Egyptian children with diarrhea. Eur J Clin Microbiol Infect Dis. 2015 doi: 10.1007/s10096-015-2389-7. [DOI] [PubMed] [Google Scholar]
- 14.Simsek Z, Zeyrek FY, Kurcer MA. Effect of Giardia infection on growth and psychomotor development of children aged 0-5 years. J Trop Pediatr. 2004 doi: 10.1093/tropej/50.2.90. http://doi.org/ 10.1093/tropej/50.2.90 [DOI] [PubMed] [Google Scholar]
- 15.Celiksöz A, Aciöz M, Degerli S, Cinar Z, Elaldi N, Erandaç M. Effects of giardiasis on school success, weight and height indices of primary school children in Turkey. Pediatr Int. 2005 doi: 10.1111/j.1442-200x.2005.02110.x. [DOI] [PubMed] [Google Scholar]
- 16.Behera B, Mirdha BR, Makharia GK, Bhatnagar S, Dattagupta S, Samantaray JC. Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Dig Dis Sci. 2008 doi: 10.1007/s10620-007-9927-9. [DOI] [PubMed] [Google Scholar]
- 17.Abou-Shady O, El Raziky MS, Zaki MM, Mohamed RK. Impact of Giardia lamblia on growth, serum levels of zinc, copper, and iron in Egyptian children. Biol Trace Elem Res. 2011 doi: 10.1007/s12011-010-8673-6. [DOI] [PubMed] [Google Scholar]
- 18.Halliez MC, Buret AG. Extra-intestinal and long term consequences of Giardia duodenalis infections. World J Gastroenterol. 2013 doi: 10.3748/wjg.v19.i47.8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coronato-Nunes B, Calegar DA, Monteiro KJL, Hubert-Jaeger L, Reis ERC, Xavier SCDC, et al. Giardia intestinalis infection associated with malnutrition in children living in northeastern Brazil. J Infect Dev Ctries. 2017 doi: 10.3855/jidc.8410. [DOI] [PubMed] [Google Scholar]
- 20.MAL-ED Network Investigators Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: The MAL-ED Longitudinal Birth Cohort Study. PLoS Med. 2017 doi: 10.1371/journal.pmed.1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva RR, da Silva CA. de Jesus Pereira CA.de Carvalho Nicolato RL.Negrão-Corrêa D.Lamounier JA Association between nutritional status, environmental and socio-economic factors and Giardia lamblia infections among children aged 6-71 months in Brazil. Trans R Soc Trop Med Hyg. 2009;103:512–519. doi: 10.1016/j.trstmh.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 22.de Onís M, Monteiro C, Akré J, Glugston G. The worldwide magnitude of protein-energy malnutrition an overview from the WHO Global Database on Child Growth. Bull World Health Organ. 1993;71(6):703–712. [PMC free article] [PubMed] [Google Scholar]
- 23.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED Cohort Study. Lancet Glob Health. 2018 doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fantinatti M, Lopes-Oliveira LAP, Cascais-Figueredo T, Austriaco-Teixeira P, Verissimo E, Bello AR, et al. Recirculation of Giardia lamblia assemblage A after metronidazole treatment in an area with assemblages A, B, and E sympatric circulation. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waterlow JC, Buzina R, Keller W, Lane JM, Nichaman MZ, Tanner JM. The presentation and use of height and weight data for comparing the nutritional status of groups of children under the age of 10 years. Bull World Hlth Org. 1977;55:489–498. [PMC free article] [PubMed] [Google Scholar]
- 26.Farthing MJ. Giardiasis. Gastroenterol Clin North Am. 1996 doi: 10.1016/s0889-8553(05)70260-0. [DOI] [PubMed] [Google Scholar]
- 27.Carvalho-Costa FA, Gonçalves AQ, Lassance SL, Silva LM, Neto, Salmazo CA, Bóia MN. Giardia lamblia and other intestinal parasitic infections and their relationships with nutritional status in children in Brazilian Amazon. Rev Inst Med Trop Sao Paulo. 2007 doi: 10.1590/s0036-46652007000300003. [DOI] [PubMed] [Google Scholar]
- 28.Conde WL, Monteiro CA. Nutrition transition and double burden of under nutrition and excess of weight in Brazil. Am J Clin Nutr. 2014 doi: 10.3945/ajcn.114.084764. [DOI] [PubMed] [Google Scholar]
- 29.Riba A, Hassani K, Walker A, van Best N, von Zezschwitz D, Anslinger T, et al. Disturbed gut microbiota and bile homeostasis in Giardia-infected mice contributes to metabolic dysregulation and growth impairment. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aay7019. [DOI] [PubMed] [Google Scholar]
- 30.Fink MY, Singer SM. The intersection of immune responses, microbiota, and pathogenesis in giardiasis. Trends Parasitol. 2017 doi: 10.1016/j.pt.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heimer J, Staudacher O, Steiner F, Kayonga Y, Havugimana JM, Musemakweri A, et al. Age-dependent decline and association with stunting of Giardia duodenalis infection among schoolchildren in rural Huye district, Rwanda. Acta Trop. 2015 doi: 10.1016/j.actatropica.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez-Jiménez J, Luna-Cázares LM, Cruz LM, De Aquino-López JA, Sandoval-Gómez D, León-Ortiz AT, et al. Children from a rural region in The Chiapas Highlands, Mexico, show an increased risk of stunting and intestinal parasitoses when compared with urban children. Bol Med Hosp Infant Mex. 2019 doi: 10.24875/BMHIM.18000069. [DOI] [PubMed] [Google Scholar]
- 33.Shrestha A, Schindler C, Odermatt P, Gerold J, Erismann S, Sharma S, et al. Intestinal parasite infections and associated risk factors among schoolchildren in Dolakha and Ramechhap districts, Nepal: a cross-sectional study. Parasit Vectors. 2018 doi: 10.1186/s13071-018-3105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehto KM, Fan YM, Oikarinen S, Nurminen N, Hallamaa L, Juuti R, et al. Presence of Giardia lamblia in stools of six- to 18-month old asymptomatic Malawians is associated with children's growth failure. Acta Paediatr. 2019 doi: 10.1111/apa.14832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berendes D, Capone D, Knee J, Holcomb D, Sultana S, Pickering AJ, et al. Associations between enteric pathogen carriage and height-for-age, weight-for-age and weight-for-height in children under 5 years old in urban Dhaka, Bangladesh. Epidemiol Infect. 2020 doi: 10.1017/S0950268820000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel-Moein KA, Saeed H. The zoonotic potential of Giardia intestinalis assemblage E in rural settings. Parasitol Res. 2016 doi: 10.1007/s00436-016-5081-7. [DOI] [PubMed] [Google Scholar]
- 37.Iwashita H, Sugamoto T, Takemura T, Tokizawa A, Vu TD, Nguyen TH, et al. Molecular epidemiology of Giardia spp. in northern Vietnam: potential transmission between animals and humans. Parasite Epidemiol Control. 2020 doi: 10.1016/j.parepi.2020.e00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-R JC, Ogbuigwe P, Pita AB, Velathanthiri N, Knox MA, Biggs PJ, et al. First report of novel assemblages and mixed infections of Giardia duodenalis in human isolates from New Zealand. Acta Trop. 2021 doi: 10.1016/j.actatropica.2021.105969. [DOI] [PubMed] [Google Scholar]
- 39.Ogbuigwe P, Biggs PJ, Garcia-Ramirez JC, Knox MA, Pita A, Velathanthiri N, et al. Uncovering the genetic diversity of Giardia intestinalis in isolates from outbreaks in New Zealand. Infect Dis Poverty. 2022 doi: 10.1186/s40249-022-00969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Yao L, Chen H, Zhang W, Jiang Y, Yang F, et al. Giardia duodenalis in patients with diarrhea and various animals in northeastern China: prevalence and multilocus genetic characterization. Parasit Vectors. 2022 doi: 10.1186/s13071-022-05269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kightlinger LK, Seed JR, Kightlinger MB. Ascaris lumbricoides aggregation in relation to child growth status, delayed cutaneous hypersensitivity, and plant anthelmintic use in Madagascar. J Parasitol. 1996;82(1):25–33. [PubMed] [Google Scholar]
- 42.Saldiva SR, Silveira AS, Philippi ST, Torres DM, Mangini AC, Dias RM, et al. Ascaris-Trichuris association and malnutrition in Brazilian children. Paediatr Perinat Epidemiol. 1999 doi: 10.1046/j.1365-3016.1999.00145.x. [DOI] [PubMed] [Google Scholar]
- 43.Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- 44.Mondal D, Petri WA, Jr, Sack RB, Kirkpatrick BD, Haque R. Entamoeba histolytica-associated diarrheal illness is negatively associated with the growth of preschool children: evidence from a prospective study. Trans R Soc Trop Med Hyg. 2006 doi: 10.1016/j.trstmh.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Oliveira D, Ferreira FS, Atouguia J, Fortes F, Guerra A, Centeno-Lima S. Infection by intestinal parasites, stunting and anemia in school-aged children from Southern Angola. PLoS One. 2015 doi: 10.1371/journal.pone.0137327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marie C, Ali A, Chandwe K, Petri WA, Jr, Kelly P. Pathophysiology of environmental enteric dysfunction and its impact on oral vaccine efficacy. Mucosal Immunol. 2018 doi: 10.1038/s41385-018-0036-1. [DOI] [PubMed] [Google Scholar]


