Abstract
Significance:
Organisms adapt to changing environments by engaging cellular stress response pathways that serve to restore proteostasis and enhance survival. A primary adaptive mechanism is the integrated stress response (ISR), which features phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2). Four eIF2α kinases respond to different stresses, enabling cells to rapidly control translation to optimize management of resources and reprogram gene expression for stress adaptation. Phosphorylation of eIF2 blocks its guanine nucleotide exchange factor, eIF2B, thus lowering the levels of eIF2 bound to GTP that is required to deliver initiator transfer RNA (tRNA) to ribosomes. While bulk messenger RNA (mRNA) translation can be sharply lowered by heightened phosphorylation of eIF2α, there are other gene transcripts whose translation is unchanged or preferentially translated. Among the preferentially translated genes is ATF4, which directs transcription of adaptive genes in the ISR.
Recent Advances and Critical Issues:
This review focuses on how eIF2α kinases function as first responders of stress, the mechanisms by which eIF2α phosphorylation and other stress signals regulate the exchange activity of eIF2B, and the processes by which the ISR triggers differential mRNA translation. To illustrate the synergy between stress pathways, we describe the mechanisms and functional significance of communication between the ISR and another key regulator of translation, mammalian/mechanistic target of rapamycin complex 1 (mTORC1), during acute and chronic amino acid insufficiency. Finally, we discuss the pathological conditions that stem from aberrant regulation of the ISR, as well as therapeutic strategies targeting the ISR to alleviate disease.
Future Directions:
Important topics for future ISR research are strategies for modulating this stress pathway in disease conditions and drug development, molecular processes for differential translation and the coordinate regulation of GCN2 and other stress pathways during physiological and pathological conditions. Antioxid. Redox Signal. 39, 351–373.
Keywords: integrated stress response, translational control, translation initiation, eIF2, eIF2 kinases
Introduction
The ability to survive and adapt to a changing environment contributes to the length and quality of life. Foundational to stress adaptation are cellular processes that regulate homeostatic control of the proteome, known as proteostasis. Loss of proteostasis shortens health span, that is, the length of time spent free of chronic diseases or age-related disabilities (Taylor and Dillin, 2011). The proteostasis network includes the processes of protein synthesis, folding, trafficking, and degradation. Among these processes, protein synthesis and its regulation are central for sensing and managing the type, magnitude, and duration of a cellular threat. This review focuses on an early warning system coined the Integrated stress response (ISR), which triggers a mechanism of translational control that responds to diverse environmental stresses, lowering consumption of energy and nutrients while reprogramming gene expression to boost cellular defenses and protection (Harding et al., 2003).
The ISR features phosphorylation of the α subunit of eukaryotic translation initiation factor 2 (eIF2) by a family of protein kinases that are each activated by distinct cellular threats or environmental stressors (Fig. 1). To appreciate the importance of this signaling event, a basic understanding of translational control at the initiation step is necessary. In the unphosphorylated state, eIF2 rapidly binds GTP and delivers initiator transfer RNA (tRNA) to ribosomes. With each round of translation initiation, the GTP associated with eIF2 is hydrolyzed to guanosine diphosphate (GDP), necessitating the activity of a guanine nucleotide exchange factor (GEF), eIF2B, to replenish eIF2 to its active GTP-bound state. Phosphorylation of eIF2α (p-eIF2α) blocks the eIF2B-directed exchange, consequently reducing the levels of eIF2•GTP that are required for delivery of methionyl initiator tRNA (Met-tRNAiMet) to the small ribosomal subunit and formation of the 43S preinitiation complex (PIC) during the initiation phase of protein synthesis. Thus, with a single signaling event, global synthesis is rapidly and efficiently slowed.
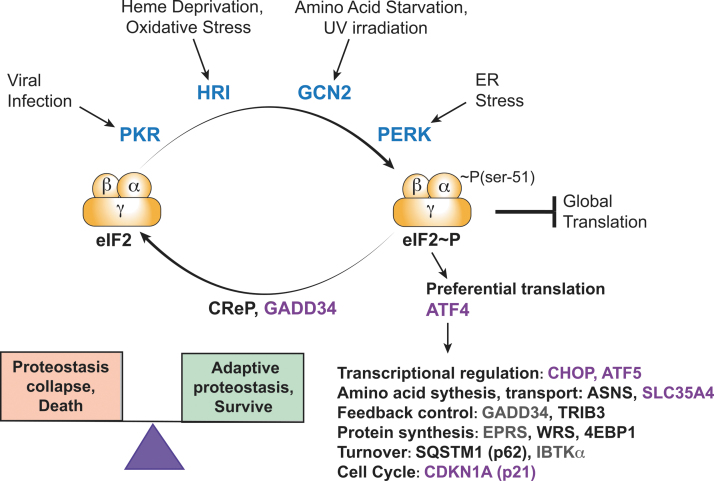
FIG. 1.
p-eIF2α triggers global and gene specific translation during the ISR. Four protein kinases phosphorylate the α subunit of eIF2 at serine-51 in response to different stress conditions. Enhanced levels of p-eIF2α inhibit the GEF eIF2B, lowering eIF2•GTP levels and global translation. The constitutive expressed CReP and ISR-induced GADD34 combine with PP1 to dephosphorylate p-eIF2α, ensuring that the levels of p-eIF2α are appropriate for the stress condition. Enhanced levels of p-eIF2α also elicits preferential translation of select mRNAs, including that encoding transcriptional regulator ATF4. Increased levels of ATF4 induce the transcriptional expression of a collection of ISR genes, including those encoding additional transcription factors (CHOP and ATF5), amino acid synthesis and transport [ASNS (Chen et al., 2004) and SLC35A4 (Andreev et al., 2015)], feedback control [GADD34 and TRIB3 (Jousse et al., 2007; Nikonorova et al., 2018; Ohoka et al., 2005)], protein synthesis [EPRS and WRS (Baird et al., 2014; Harding et al., 2003; Young et al., 2016a) and 4EBP1], protein turnover [IBTKα (Baird et al., 2014; Willy et al., 2017) and SQSTM1 (p62) (B'chir et al., 2013)], and cell cycle [CDK1A (p21) (Collier et al., 2018; Lehman et al., 2015)]. Many of the ISR genes that are transcriptionally induced by ATF4 are also preferentially translated during enhanced p-eIF2α (indicated in purple), ensuring their enhanced expression during the ISR. The ISR is critical for maintenance of proteostasis during stress, ensuring survival. However, during chronic induction of the ISR can instead be maladaptive, which can lead to proteostatic collapse and cell death. Hence, the magnitude and duration of the ISR can determine the balance between cell survival and death. 4EBP1, 4E binding protein 1; ASNS, asparagine synthetase; ATF, activating transcription factor; CReP, constitutive repressor of eIF2α phosphorylation; eIF, eukaryotic initiation factor; GADD34, growth arrest and DNA damage-inducible gene 34; GEF, guanine nucleotide exchange factor; ISR, integrated stress response; mRNA, messenger RNA; p-eIF2α, phosphorylation of eIF2α; PP1, protein phosphatase 1; WRS, Wolcott-Rallison syndrome.
While bulk translation can be sharply lowered by stress-induced p-eIF2α, there are other gene transcripts whose translation efficiencies are largely unchanged or even enhanced by lowered eIF2 function. In this way, p-eIF2α directs a gradient of protein synthesis changes, with translation of most messenger RNAs (mRNAs) being dampened, whereas others are resistant or preferentially translated by increased p-eIF2α during stress (Andreev et al., 2015; Baird et al., 2014) (Fig. 1). Among the preferentially translated products are transcription factors, such as activating transcription factor 4 (ATF4) (Harding et al., 2000a; Lu et al., 2004; Vattem and Wek, 2004).
ATF4 is a central to guide transcription of ISR-targeted genes involved in stress mitigation and restoration of proteostasis. Increased levels of ATF4 induced by p-eIF2α facilitate ATF4 coupling with other basic leucine zipper (bZIP) DNA binding proteins, such as isoforms of C/EBP, to directly induce the transcription of genes involved in mRNA synthesis, amino acid synthesis and transport, protein synthesis and turnover, cell cycle progression, and feedback regulation (Fig. 1) (Ebert et al., 2020; Hai and Curran, 1991; Han et al., 2013; Harding et al., 2003; Huggins et al., 2015; Vallejo et al., 1993). Therefore, the induction of p-eIF2α during the ISR elicits both translational and transcriptional mechanisms, along with turnover processes, that collectively contribute to the reprogramming of gene expression toward stress adaptation (Fig. 1).
To maximize stress adaptation, the ISR implements a cascade of steps, each triggered in a precise and timed fashion upon the onset of a stress. It should be emphasized that the adaptive gene expression involves catabolic processes, such as those triggering degradation of proteins and reclamation of amino acids, along with anabolic processes, including synthesis of amino acids and associated metabolic products. These ISR-directed processes are integrated into the biology of the cell type and serve to enhance cell survival or in some cases function in the cell program of differentiation and proliferation.
At the start of the ISR, upstream eIF2α kinases sense stress conditions that confer preferential translation of key effector proteins. These effector proteins can then amplify or suppress transcriptional expression of additional regulators, which alter precise cellular events and biological processes to alleviate stress damage. Among the induced ISR regulators is growth arrest and DNA damage-inducible gene 34 (GADD34; PPP1R15a) that serves to target protein phosphatase 1 (PP1) dephosphorylation of p-eIF2α, allowing for restoration of eIF2B-directed guanine nucleotide exchange and protein synthesis (Brush et al., 2003; Connor et al., 2001; Lee et al., 2009; Ma and Hendershot, 2003; Novoa et al., 2003; Novoa et al., 2001; Young et al., 2015) (Fig. 1). Further, ATF4 induces expression of TRIB3, which serves to dampen ATF4 activity by mechanisms suggested to involve targeted degradation of the transcription factor or interference between ATF4 and the transcriptional apparatus (Jousse et al., 2007; Ohoka et al., 2005). As a consequence, the ISR is self-limiting, establishing an “adaptive zone” where p-eIF2α and associated translational and transcriptional activities function at precise levels and durations of time (Wek and Anthony, 2009). If the levels of p-eIF2α occur outside the adaptive zone, the configuration of ISR effectors can instead trigger maladaptive processes and subsequently cell death. Thus, the timing and amplitude of the ISR can hold the balance between health and disease, with dysregulation of the ISR upon inherited or pharmacological changes, or during periods of chronic stress, triggering damage among multiple tissues (Fig. 1).
Although the described features of the ISR model are well established, many of the mechanistic features have only recently emerged. It should be emphasized that by design there is some plasticity in the execution of the ISR. Depending on the cell type and the nature of the stress, p-eIF2α and translational control can trigger expression of different effector proteins and facilitate different viability outcomes. We are only beginning to appreciate the underlying mechanisms for the versatility in ISR-directed gene expression and outcomes, but an important reason is suggested to be the differences in the transcriptome available for translational control among different cell types and the integration of the ISR with other response pathways that are more narrowly restricted to certain stresses.
The following narrative will focus on five important topics in the ISR with an eye toward recent mechanistic insights. First, we address mechanisms by which eIF2α kinases sense and are activated by different stress conditions, triggering the ISR. Second, we discuss the mechanisms by which p-eIF2α and other stress arrangements inhibit eIF2B exchange activity to confer translational control. Third, we highlight mechanisms by which p-eIF2α during the ISR direct ribosomes to differentially translate mRNAs, including that encoding the central ISR effector ATF4, and how these processes can be adjusted to tailor gene expression to confer optimal adaptation for each stress condition. Fourth, we describe how the ISR can interface with other stress response pathways to coordinate and optimize their implementation. Here, we focus on the integration of the ISR and mammalian/mechanistic target of rapamycin complex 1 (mTORC1), another central regulator of translation, and how their coordinated activities optimize adaptation to nutrient insufficiency. Finally, we describe pathological conditions that stem from aberrant regulation of the ISR and how possible therapeutic interventions involving the ISR may alleviate disease.
The eIF2α Kinases Serve as First Responders in the ISR
The mammalian ISR is hierarchical, with four related eIF2α kinases serving as first responders to detect early signs of cell stress. Toward this goal, each eIF2α kinase senses distinct stress conditions via different regulatory regions juxtaposed to a related kinase catalytic domain (Fig. 1). These regulatory regions then engage with specific ligands or interactive proteins whose levels abruptly change during a given stress, triggering ordered mechanisms of activation that are broadly shared among the eIF2α kinases (Fig. 2). The activation mechanisms include dimerization of the kinase catalytic domains, trans intra-dimer phosphorylation at the kinase activation loop, and eventual eIF2α substrate recognition and phosphorylation. For example, expression of protein kinase RNA-activated (PKR; EIF2AK2) is transcriptionally induced by interferon and this eIF2α kinase participates in cellular protection against viral infection. Increased levels of PKR then monitor accumulation of double-stranded RNAs (dsRNAs), which are produced during replication of different viruses by direct association with dsRNA-binding motifs located in the PKR amino terminus (Dey et al., 2005; Green and Mathews, 1992; Lemaire et al., 2008; Nanduri et al., 2000; Nanduri et al., 1998). Bound dsRNA is believed to form a bridge between PKR polypeptides, triggering oligomerization and an ordered activation mechanism (Dar et al., 2005; Dey et al., 2005; Lemaire et al., 2008; Nanduri et al., 2000; Nanduri et al., 1998; Zappa et al., 2022). Induced p-eIF2α by PKR then triggers translation repression in the infected host cells and a path toward apoptosis, thwarting further replication of the virus and infection into neighboring tissues.
FIG. 2.
The eIF2α kinases monitor stress arrangements via different regulatory regions juxtaposed to their kinase catalytic domains. Each of the eIF2α kinases shares a related protein kinase domain (orange bars) that is juxtaposed to distinct regulatory and targeting regions that function to monitor ligands that are modulated by different stress conditions (green bars). The size of the eIF2α kinase domains varies among the family members due to the different lengths of insert sequences in the N-terminal lobe of the kinase domains. Regulatory regions include two dsRBMs in PKR and heme-binding regions in its N-terminus and kinase insert of HRI. PERK has a SS for entry into the ER, an IRE1-related region, and an ER TM segment. GCN2 regulatory regions include the RWD, partial kinase, HARS, and CTD that facilitates oligomerization and contributes to the eIF2α kinase association with ribosomes. For a review of the structures of eIF2α kinase catalytic and regulatory domains, see Rothenburg et al. (2016). CTD, carboxy terminal domain; dsRBM, double-stranded RNA-binding motif; ER, endoplasmic reticulum; GCN, general control nonderepressible; HARS, histidyl-tRNA synthetase; HRI, heme regulated inhibitor; IRE1, inositol requiring enzyme 1; PERK, PKR-like ER kinase; PKR, protein kinase RNA-activated; RNA, ribonucleic acid; SS, signal sequence; TM, transmembrane; tRNA, transfer RNA.
In the example of heme regulated inhibitor (HRI; EIF2AK1), this eIF2α kinase is basally repressed by heme, which binds two regulatory motifs in HRI (Figs. 1 and 2). HRI is most abundantly expressed in erythroid cells, and iron depletion sharply reduces heme levels, releasing the inhibitor from HRI, culminating in enhanced p-eIF2α (Han et al., 2001; Rafie-Kolpin et al., 2000; Suragani et al., 2012). The ensuing translation repression ensures that globin, the predominant protein product in erythroid cells, is not synthesized during heme deficiency. Unfettered synthesis of globin without heme can lead to precipitation of globin polypeptides, creating proteotoxicity and severe anemia (Han et al., 2005; Han et al., 2001; Zhang et al., 2019).
HRI is also activated by inhibition of oxidative phosphorylation in the mitochondria (Fessler et al., 2020; Guo et al., 2020; Zhang et al., 2019). Mitochondrial dysfunction stimulates OMA1, a mitochondrial-associated protease, leading to cleavage of DAP3 binding cell death enhancer 1 (DELE1) protein that is situated in the inner mitochondrial membrane (Fessler et al., 2022; Fessler et al., 2020; Guo et al., 2020). Cleavage of DELE1 releases its carboxy terminal portion containing tetratricopeptide repeat (TPR) motifs from the mitochondria into the cytosol where the shorter version, DELE1s, directly binds HRI, leading to enhanced p-eIF2α and translational control that serves to alleviate the mitochondrial dysfunction (Fessler et al., 2020; Guo et al., 2020). Of importance, the presence of heme does not interfere with DELE1s activation of HRI (Guo et al., 2020). Therefore, there are multiple strategies for activating HRI, each involving HRI binding to different ligands during distinct stress conditions.
Cellular location of the eIF2α kinases is also instrumental for rapid detection of stress signals. Illustrating this idea, PKR-like ER kinase (PERK; EIF2AK3) is a transmembrane protein situated in the endoplasmic reticulum (ER), with its amino terminal regulatory motifs located in the lumen, adjoined to a cytosolic eIF2α kinase domain (Harding et al., 1999; Shi et al., 1998; Sood et al., 2000) (Fig. 2). In this way, PERK monitors the intra-ER environment.
Two models are proposed for describing how PERK measures ER stress via accumulating unfolded proteins. Both of these models are derived in part from studies of the related ER stress sensor, inositol requiring enzyme 1 (IRE1)α. It should be emphasized that these models are not mutually exclusive and they may work in conjunction depending on the nature and timing of the ER stress condition.
The first is the direct binding model that centers on the discovery that the structure of the ER lumenal portion of PERK has a hydrophobic groove that can directly bind unfolded polypeptides (Credle et al., 2005; Gardner and Walter, 2011; Karagoz et al., 2017; Wang et al., 2018). Binding of unfolded proteins facilitates PERK oligomerization involving another lumenal domain of this eIF2α kinase, which then triggers phosphorylation at the kinase activation loop and increases p-eIF2α. The resulting reduction of translation lowers the influx of nascent polypeptides into the ER, accompanied by the induction of genes encompassing the unfolded protein response (UPR) that serve to expand the processing capacity of the ER (reviewed in Walter and Ron, 2011).
A second model by which PERK senses increasing levels of unfolded protein is a chaperone competition model. This is an indirect model for detection of unfolded proteins and features the molecular chaperone binding immunoglobulin protein (BiP; heat shock protein A5 [HSPA5]/glucose regulated protein 78 [GRP78]) serving as a PERK repressor (Bertolotti et al., 2000; Kimata et al., 2007; Ma et al., 2002a; Oikawa et al., 2009). Typically, BiP functions to refold misfolded protein in stressed cells and to aid in this process, BiP has a substrate binding domain that associates with unfolded polypeptides and a nucleotide binding domain that ensures cycling between open ATP and closed ADP-bound states. BiP is suggested to bind to the lumenal portion of PERK, likely with the assistance of ER-localized J-domain co-chaperones, such as endoplasmic reticulum-localized DnaJ4 (ERdj4) (Amin-Wetzel et al., 2017).
PERK binding with BiP blocks PERK oligomerization, which is required for its activation. Unfolded or misfolded proteins that accumulate during ER stress are believed to compete for BiP and titrate the repressor from PERK, allowing for PERK oligomerization and induced p-eIF2α. In this way, the recycling of PERK binding to and release from BiP serves to monitor changes in the amounts of unfolded protein in the secretory pathway. A variation of the chaperone competition model is proposed where PERK instead binds to the nucleotide binding domain of BiP, and accumulating misfolded protein during ER stress engages with the BiP substrate binding domain, directing release from PERK and activation of the eIF2α kinase (Carrara et al., 2015; Kopp et al., 2019).
Another example of cell targeting being instrumental for stress activation of an eIF2α kinase involves activation of general control nonderepressible 2 (GCN2; EIF2AK4) that occurs during amino acid depletion and on exposure to ultraviolet (UV) irradiation. GCN2 contains a regulatory domain homologous with histidyl-tRNA synthetases (HARS) and can bind uncharged tRNAs that accumulate during starvation for different amino acids (Dong et al., 2000; Wek et al., 1995; Wek et al., 1989; Zaborske et al., 2009) (Fig. 2).
It is proposed that GCN2 association with ribosomes is critical for its recognition and binding of uncharged tRNAs, and this process involves accessory proteins GCN1 and the ribosome P-stalk, uL-10 and P1 (Harding et al., 2019; Inglis et al., 2019; Marton et al., 1997; Pochopien et al., 2021; Ramirez et al., 1991; Sattlegger and Hinnebusch, 2005; Sattlegger and Hinnebusch, 2000; Zhu and Wek, 1998). Further, the stalling of elongating ribosomes, which can create collisions with subsequent translating ribosomes, may be a direct or indirect trigger for activation of GCN2 (Ishimura et al., 2016; Wu et al., 2020; Yan and Zaher, 2021). In the case of UV-C exposure, damaged mRNAs are suggested to create stall points for translating ribosomes that culminate in activation of the protein kinase sterile alpha motif and leucine zipper containing kinase (ZAKα), which then engages with ribosome-associated GCN2/GCN1 complex to induce p-eIF2α (Wu et al., 2020). Induced p-eIF2α by GCN2 by UV irradiation regulates the cell cycle and enhances the synthesis, uptake, and reclamation of amino acids, which collectively helps restore amino acid homeostasis in cells (Collier et al., 2018). Although the HARS-regulatory domain is required to activate GCN2 during different stress conditions, it is not currently clear whether ribosome engagement and ZAKα functions are also obligatory or contribute only during certain stress arrangements (Snieckute et al., 2022).
The complement of four eIF2α kinases provides for a range of stress-regulated ligands to be monitored, thus ensuring rapid induction of p-eIF2α and stress alleviation in mammals. Among eukaryotes, only GCN2 appears to be conserved among animals, plants, fungi and protist, supporting the idea that adaptive responses to nutrient limitations are universal in nature. It is noteworthy that certain eukaryotes have adopted new eIF2α kinases with new regulatory regions, suggesting that these organisms require additional stress sensing strategies to implement and optimize the ISR. For example, protein kinase Z (PKZ) is expressed only in fish and is most closely related to PKR, containing Z-deoxynucleic acid/ribonucleic acid (Z-DNA/RNA) binding domains instead of double-stranded RNA-binding motifs present in PKR (Liu et al., 2011; Rothenburg et al., 2005). Together, PKZ and PKR are believed to expand the spectrum of viruses that are recognized by the ISR, broadening its antiviral effects. In the case of the parasitic protozoan Toxoplasma gondii, T. gondii translation initiation factor 2K-B (TgIF2K-B) displays regulatory regions that are distinct from other studied eIF2α kinases and affords parasite protection from oxidative stress and optimizes pathogenicity (Augusto et al., 2021).
p-eIF2α Inhibits eIF2B Guanine Nucleotide Exchange Function
The eIF2 delivers initiator Met-tRNAiMet to ribosomes and aids in the recognition of start codons during the initiation phase of protein synthesis (Fig. 3). As highlighted earlier, eIF2 is a guanine-nucleotide binding protein and its GDP/GTP-bound status determines its affinity for Met-tRNAiMet. The eIF2 consists of three different subunits, designated α, β, and γ, with eIF2γ being the core that binds GDP/GTP and Met-tRNAiMet. The α subunit also aids with initiator tRNA binding and start codon recognition, and it serves as a critical phosphorylation target in the ISR.
FIG. 3.
Scheme depicting the function and regulation of 2B GEF activity. (A) During the process of translation initiation, GTP bound to eIF2 is hydrolyzed to GDP, and the GEF eIF2B functions to recycle eIF2•GDP to eIF2•GTP to facilitate Met-tRNAiMet binding to ribosomes for further rounds of translation initiation. The GAP and GDI functions of bound eIF5 are also depicted and show that eIF2B must displace eIF5 to facilitate nucleotide exchange and form the new TC. p-eIF2α converts it from a substrate to an inhibitor of eIF2B exchange activity. eIF2B exchange activity is inhibited by both p-eIF2α and by direct phosphorylation by GSK-3 as indicated in red. The metabolite F6P acts as an allosteric activator of eIF2B GEF activity indicated by the green arrow. ISRIB and 2BAct (eIF2B activator) function as inhibitors to block p-eIF2 binding to eIF2B, rendering the GEF insensitive to its effects also indicated in red. F6P, fructose-6-phosphate; GAP, GTPase activator protein; GCP, guanosine diphosphate; GDI, guanine nucleotide dissociation inhibitor; GSK-3, glycogen synthase kinase 3; GTP, guanosine trisphosphate; ISRIB, ISR inhibitor; Met-tRNAiMet, methionyl initiator tRNA; TC, ternary complex.
The GTP bound form of eIF2 has high affinity for Met-tRNAiMet, and the combination of eIF2•GTP•Met-tRNAiMet is often referred to as the ternary complex (TC). The TC, along with additional translation initiation factors, associates with the small ribosomal subunit to form the PIC. The PIC then combines with the 5′-cap of mRNAs and processively scans unidirectionally 5′ to 3′ along the 5′-leader of mRNA transcripts in search of an initiation codon for appropriate base-pairing and initiation (Fig. 3). Scanning is aided by eIF4A and related helicases that serve to resolve RNA secondary structures (Hinnebusch, 2014; Wang et al., 2022). In addition, scanning requires an open PIC conformation stabilized by multiple initiation factors, including the TC and eIF1, which helps to ensure recognition of optimal initiation codons (Hinnebusch, 2014; Llacer et al., 2015; Zhou et al., 2020).
During the scanning process, the GTP associated with eIF2 is hydrolyzed to GDP, aided by the GTPase activator protein (GAP) eIF5. The inorganic phosphate (Pi) is released from the eIF2•GDP on engagement between the start codon and the Met-tRNAiMet is situated in the ribosome P-site. The subsequent dissociation of eIF2•GDP and other associated initiation factors facilitates joining of the large ribosomal subunit to the small subunit, yielding the competent 80S ribosome that is now ready to begin the elongation phase of translation.
Subsequent rounds of translation initiation require recycling of eIF2•GDP to eIF2•GTP, which is initially blocked by eIF5, ensuring an orderly regulation of this exchange event (Fig. 3). The eIF5 can compete with eIF2B for eIF2•GDP binding, and eIF5 is suggested to function as a guanine nucleotide dissociation inhibitor (GDI) that prevents premature GDP release from eIF2 (Jennings and Pavitt, 2010; Singh et al., 2006). Engagement with eIF2B causes both the release of eIF5 and the bound GDP from eIF2, facilitating the binding of eIF2 with GTP, which is essential for the formation of the TC and subsequent rounds of translation initiation (Jennings and Pavitt, 2014; Williams et al., 2001). The p-eIF2α converts the initiation factor from a substrate to an inhibitor of eIF2B GEF function and thwarts exchange of eIF2•GDP to eIF2•GTP. In addition, eIF2B can function to destabilize existing p-eIF2•GTP•Met-tRNAiMet by outcompeting eIF5 binding (Jennings et al., 2017). As a consequence, p-eIF2α lowers the levels of TC that can sharply reduce bulk translation initiation (Fig. 3). Whether or not stress conditions differentially regulate the distinct GAP and GDI functions of eIF5 (Bogorad et al., 2018) and how this would affect the ISR, is not clear. Of interest, phosphorylation of eIF2β by GCN2 is reported to enhance binding of eIF5 and its GDI function, potentially adding an additional layer of regulation to reduce eIF2B-directed nucleotide exchange during stress (Dokladal et al., 2021). However, it is anticipated that the GAP function of eIF5 would influence translation start site selection, whereas eIF5 competition with eIF2B for association with eIF2 and the eIF5 GDI function would deter premature formation of TC.
The p-eIF2α functions to inhibit the guanine nucleotide exchange activity of eIF2B. The eIF2B is a large atypical GEF consisting of five different subunits (named α-ɛ) assembled into a pentamer that arranges into a symmetrical decameric structure (Gordiyenko et al., 2014). The α, β, and δ subunits of eIF2B function as a regulatory subcomplex, with the γ and ɛ subunits forming the catalytic portion of eIF2B (Pavitt et al., 1998). Recent structural studies indicate that eIF2 and p-eIF2 bind to different locations on the eIF2B complex (Adomavicius et al., 2019; Gordiyenko et al., 2019; Kashiwagi et al., 2019; Kashiwagi et al., 2016; Kenner et al., 2019), suggesting that eIF2 binding to eIF2B forms two alternative complexes: a largely inactive (or inhibited) and a productive (or active) complex.
Binding of p-eIF2 is suggested to allosterically change the conformation of eIF2B, which reduces its interaction with eIF2 substrate. The inactive state of eIF2B would consequently have lowered nucleotide exchange activity (for detailed reviews see Pavitt, 2018; Schoof et al., 2021) (Fig. 3). It is important to emphasize that the p-eIF2 has a higher affinity for eIF2B than its unphosphorylated counterpart. Further, although expression of eIF2B can vary among cell types, the amounts of the exchange factor in cells is substantially lower than levels of eIF2 and eIF5 (Singh et al., 2007). Therefore, even low amounts of p-eIF2α can bind to eIF2B and deter its activity and confer translational control. Depending on the levels of p-eIF2α induced during stress, there can be a gradient of lowered translation initiation in the ISR. High levels of p-eIF2α can lead to maximal reductions in bulk protein synthesis, whereas low amounts of p-eIF2α contribute to only modest reductions in global translation along with attendant preferential translation of ISR-targeted gene transcripts (Fig. 3).
In addition to control by p-eIF2α, eIF2B is regulated by direct phosphorylation (Fig. 3). The catalytic eIF2Bɛ subunit is phosphorylated at Ser-540 by glycogen synthase kinase 3 (GSK-3), which inhibits eIF2B exchange activity (Wang et al., 2001). Phosphorylation of an adjacent site by dual specificity tyrosine-phosphorylation-regulated kinase (DYRK) is suggested to prime phosphorylation by GSK-3, indicating that eIF2B regulation can be a node for multiple signaling pathways (Woods et al., 2001). It is also suggested that phosphorylated eIF2B is more susceptible to inhibition by p-eIF2α (Cagnetta et al., 2019). Interestingly, activation of the ISR and the attendant increase in p-eIF2α increases GSK-3 activity (Baltzis et al., 2007; Yuan et al., 2022), which suggests that an amplification process occurs during certain stresses. Conversely, mitogens such as insulin inactivate GSK-3, providing one explanation for the ability of mitogens to stimulate translation through enhanced eIF2B function (Welsh and Proud, 1993).
To identify small molecules that alleviated translational control and the ISR, Walter and colleagues pursued a screen for compounds that blocked p-eIF2α-dependent translation using an ATF4 translational reporter (Sidrauski et al., 2013). The synthetic bisglycolamide ISRIB (ISR inhibitor) was identified and shown to directly bind to eIF2B, rendering the GEF largely independent of p-eIF2α control (Fig. 3). Structural studies indicate that ISRIB bound to eIF2B β and δ regulatory subunits, bridged the interface of two eIF2B (β-ɛ) tetramers, and promoted decamer holoenzyme formation (Sekine et al., 2015; Tsai et al., 2018).
The ISRIB binding to eIF2B was first proposed to facilitate interaction with eIF2 and increase eIF2B exchange activity. In a competing, and now more widely accepted model, it is suggested that ISRIB binding functions by rendering eIF2B insensitive to p-eIF2α inhibition (Schoof et al., 2021; Zyryanova et al., 2021). Binding of ISRIB with eIF2B is mutually exclusive with its binding to p-eIF2α. The basis for this mutual antagonism is that ISRIB counteracts conformational changes in eIF2B induced by binding of p-eIF2α rather than competition for the same binding site (Schoof et al., 2021).
The antagonism between ISRIB and p-eIF2α for association with eIF2B helps to explain the observation that the activity of ISRIB can be lowered when there are high levels of induced p-eIF2α in cells (Halliday et al., 2015; Rabouw et al., 2019). Whether or not natural ligands with mechanism(s) of action similar to that of ISRIB exist in nature is not currently known. This premise is likely given that the binding pocket for ISRIB on eIF2B is conserved between yeast and humans, along with the observation that sugar metabolites stimulate eIF2B exchange activity in in vitro systems, as described further next.
Recent studies suggest additional allosteric regulation of eIF2B activity by both synthetic and natural small-molecule ligands. Each of the regulatory subunits of eIF2B shares sequence homology to ribose 1,5-bisphosphate isomerase (RBPI) and other sugar-phosphate metabolizing enzymes, pointing to a potential for interaction with and regulation by sugar metabolites (Bogorad et al., 2014; Kuhle et al., 2015; Nakamura et al., 2012). A library of over 400 naturally occurring human metabolites was screened for binding to homodimeric eIF2Bα and several sugar phosphates, including fructose-6-phosphate (F6P), were identified with the highest affinities (Hao et al., 2021). Importantly, F6P increased eIF2B exchange activity in a concentration-dependent manner (Fig. 3). It is proposed that sugar-phosphate metabolites bind to eIF2Bα to promote decameric holoenzyme assembly and increase eIF2B activity, thus linking glycolytic flux to increases in protein synthesis (Hao et al., 2021). However, it should be emphasized that these experiments featured purified components and cell extracts.
Future studies are needed to discern whether stimulation of holoenzyme assembly occurs in cells to stimulate eIF2B activity in response to metabolic changes. An alternative explanation for sugar phosphate activation of eIF2B activity is that these metabolites allosterically increase exchange activity similar to that described for eIF2B binding of ISRIB (Zyryanova et al., 2021). Metabolite activation of eIF2B suggests that direct enhancement of eIF2B may occur in response to certain changes in cell metabolism, which would enhance eIF2B activity even during induced p-eIF2α. In this way, metabolite regulation of eIB2B would circumvent the attendant translational control via the ISR.
Upstream Open Reading Frames Function in mRNA Bar Codes to Direct Differential Translation
On induced p-eIF2α, scanning ribosomes delineate mRNAs that are preferentially translated by “bar codes” embedded with the 5′-leaders of the gene transcripts. A central element of the bar code is upstream open reading frames (uORFs), which are typically viewed as short coding sequences (CDS) that encode polypeptides at least two residues in length. It is noteworthy that uORFs are prevalent among eukaryotes and are predicted to be present in upwards to half of human genes (Andreev et al., 2022; Calvo et al., 2009; Zhang et al., 2021).
Informatic analyses and ribosome profiling reveal that some transcripts possess uORFs with non-canonical start codons such as CUG, GUG, and UUG, further expanding the potential of this type of regulatory mechanism (Andreev et al., 2022; Ingolia et al., 2011; Lee et al., 2012). As described earlier, the stringency of initiation codon recognition is enhanced by the levels of certain translation factors, including eIF1 and eIF5 mimic protein (5MP/BZW) (Hinnebusch, 2014; Kozel et al., 2016; Singh et al., 2021; Zhou et al., 2020). Further, uORFs consisting of only an optimal start codon, followed by a stop codon- hence encoding only a single residue product and devoid of peptide bonds, are functional elements in translational control (Schleich et al., 2017; Schleich et al., 2014). Therefore, uORFs are prevalent among mRNAs and the mere presence of an uORF is not sufficient to direct preferential translation, rather the sequence and placement of the uORF is critical to confer translational control.
Typically, translated uORFs function as barriers to translation of the downstream CDS (Fig. 4). The degree of uORF repression depends in part on whether it contains an optimal start codon, which will enhance translation of the uORFs, along with the length of the uORF (Kozak, 2001; Luukkonen et al., 1995). Long uORFs are often repressing, whereas shorter versions can allow for ribosome to reacquire eIF2•GTP•Met-tRNAiMet and initiate at a downstream CDS. It is suggested that certain translation initiation factors, such as eIF3, eIF4G1, and eIF4E, are retained for a limited number of catalyzed peptide bonds after ribosome initiation, and the retention of these initiating factors enhances the efficiency of reinitiation (Bohlen et al., 2020a; Mohammad et al., 2021; Poyry et al., 2007; Poyry et al., 2004; Wagner et al., 2020).
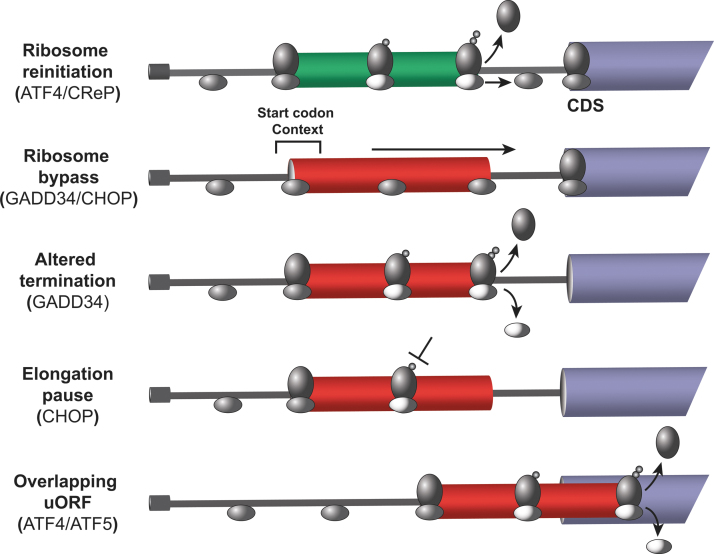
FIG. 4.
The uORFs function in the bar code for translational control in the ISR. The mRNAs are indicated as a solid line with the 5′-cap. The CDS for each transcript are indicated as a blue bar, with uORFs that allow for reinitiation (green) and repressing uORFs (red) indicated by bars. The small and large ribosomal subunits (ovals) are shown to scan, elongate, and terminate and release during the processes of uORF-directed translational control. During translation initiation, the eIF2 is released from ribosomes (light gray oval) and reinitiating ribosomes resume scanning and reacquire TC (dark oval). Gene transcripts that have the indicated uORF configurations as listed and are further described in detail in the text. CDS, downstream coding sequence; uORF, upstream open reading frame.
Another property that contributes to the repressing functions of uORFs is the ability to elicit a pause or stall of elongating or terminating ribosomes (Cao and Geballe, 1996; Kozak, 2001; Lin et al., 2019; Young et al., 2016b; Young et al., 2015). In this way, ribosomes translating the uORF require an extended period to elongate and terminate, which expedites release of initiation factors and reduces the efficiency of reinitiation at downstream CDS (Fig. 4). Ribosome pausing during elongation is also suggested to cause formation of a ribosome queue involving 40S subunits scanning the 5′-leader of an mRNA that encounter stalled ribosomes (Andreev et al., 2018; Ivanov et al., 2018; Kearse et al., 2019). The queued 40S subunit would then spend an extended period on start codons of poor context or non-canonical start codons, which increases the likelihood of initiator tRNA recognition and uORF translation, thus creating a more inhibitory uORF even with a poor initiation context. An uORF can also be inhibitory when it overlaps out-of-frame with a CDS (Lu et al., 2004; Vattem and Wek, 2004). Ribosomes translating the uORF will then terminate downstream of the CDS start codon, blocking the ability of ribosomes to translate the CDS.
Finally, RNA stem-loops situated downstream of uORFs can function to impair ribosome reinitiation, supporting the idea that RNA structures can also be important elements of the bar code by which ribosomes delineate mRNAs for preferential translation (Amin et al., 2022). While many of the repressing uORFs are expressed in a constitutive fashion, as described more fully later, others can be bypassed in a prescribed fashion, such as on induction of p-eIF2α during stress. Overall, uORFs can possess diverse functional properties and can be integrated alone or in combination in the 5′-leader of mRNAs to direct specific mechanisms of translational during the ISR.
Preferential Translation of ATF4 by Delayed Reinitiation Mechanism
Delayed translation reinitiation is an important mechanism by which two or more uORFs direct preferential translation during the ISR. As presented in Figure 5, the 5′-leader of the mammalian ATF4 transcript encodes two uORFs with different functions. The 5′-proximal uORF1 encodes a 3 amino acid residue polypeptide and allows for efficient reinitiation at downstream CDS, whereas uORF2 overlaps out-of-frame with the ATF4 CDS and is inhibitory (Harding et al., 2000a; Lu et al., 2004; Vattem and Wek, 2004).
FIG. 5.
Delayed reinitiation mechanism for preferential translation of ATF4 mRNA in response to p-eIF2α during stress. In absence of stress there are low levels of p-eIF2α and ample eIF2•GTP for delivery of initiator tRNA to ribosomes. Ribosomes (ovals represents large and small subunits) initiate translation at the 5′-proximal uORF1 (green bar), and following termination rapidly reacquire new TC and reinitiate at the inhibitory uORF2 (red bar). The uORF2 extends out-of-frame into the ATF4 CDS (blue bar) and after translation termination of the inhibitory uORF, there are low amounts of ATF4 and its target genes expressed in the absence of stress. An abbreviated uORF consisting of only start-stop codons can be situated upstream of uORF1, and the shortened uORF is suggested to also allow for ribosome reinitiation. During stress, induced p-eIF2α and lowered eIF2 TC is suggested to delay ribosome reinitiation after translation of uORF1, enabling 40S ribosomes (light gray oval) to scan through uORF2 before reacquiring a new eIF2 TC (dark gray oval). The translation competent 40S ribosome can instead initiate at the ATF4 CDS and the resulting increased synthesis of ATF4 protein directly enhances transcriptional expression of ISR-targeted genes.
During non-stressed conditions, ribosomes adjoined with 2•GTP•Met-tRNAiMet associate with the 5′-end of the ATF4 transcript and initiate translation at uORF1. After translation of uORF1, the 40S ribosome resumes scanning and rapidly reacquires the TC to reinitiate translation at the next CDS- uORF2 (Fig. 5) (Vattem and Wek, 2004). After following translation of the inhibitory uORF2, the ribosome dissociates from ATF4 mRNA, thereby reducing the translation of the ATF4 CDS.
During cellular stress and induced p-eIF2α, there is lowered eIF2 TC due to inhibition of eIF2B GEF activity. Therefore, after translating uORF1, the 40S ribosome resumes scanning, but it is believed to take longer to reacquire the limiting eIF2•GTP•Met-tRNAiMet needed to recognize the next initiation codon. Because of the delay in reacquiring the TC, portions of the 40S ribosomes scan through the inhibitory uORF2 and instead reinitiate at the next initiation codon for the ATF4 CDS (Vattem and Wek, 2004) (Fig. 5). This delayed reinitiation results in preferential translation of the ATF4 CDS and increased ATF4 protein that directly induces the transcription of ISR genes involved in amino acid metabolism and transport, alleviation of oxidative damage, and additional transcription factors (Ebert et al., 2020; Hai and Curran, 1991; Han et al., 2013; Harding et al., 2003; Huggins et al., 2015; Vallejo et al., 1993).
One of the ATF4-target genes ATF5, which encodes a related bZIP transcription factor, is also subject to preferential translation via the delayed reinitiation mechanism featuring two uORFs (Watatani et al., 2008; Zhou et al., 2008). Both ATF4 and ATF5 share hallmark features of the related GCN4 translational control that Hinnebusch and colleagues have described for the general amino acid control pathway in budding yeast (Abastado et al., 1991a; Abastado et al., 1991b; Hinnebusch, 2005).
A key feature of the delayed reinitiation model is the ability of a 5′-proximal uORF to allow translation reinitiation. Although the reinitiation process is not fully clear, it is believed to involve retained ribosome association with mRNA, followed by renewed scanning and recruitment of Met-tRNAiMet for recognition of a start codon at a downstream CDS. One mechanism may involve the regulated association of eIF3 with elongating ribosomes. It is suggested that O-linked N-acetylglucosamine modification (O-GlcNAc) of eIF3a reduces association of the multisubunit initiation factor with elongating ribosomes (Shu et al., 2022). In response to amino acid deprivation, there is lowered O-GlcNAcylation, thus allowing for eIF3 to be retained with ribosomes following translation of ATF4 uORF1, which would enable reinitiation at a downstream CDS.
A complex consisting of density regulated re-initiation and release factor protein (DENR) and malignant T-cell amplified sequence 1 (MCTS1) proteins, in conjunction with eIF2D, also serves to recycle post-termination ribosomes (Ahmed et al., 2018). Recent studies suggest that DENR-MCTS1 is important for efficient reinitiation of ribosomes, and deletion of either DENR or MCTS1 reduces translation of the ATF4 CDS (Bohlen et al., 2020b; Vasudevan et al., 2020). It is noteworthy that ATF4 can have an additional uORF upstream of uORF1, which consists only of a start codon followed by a stop codon. In this case, both the abbreviated upstream ORF and uORF1 may allow for ribosome reinitiation (Schleich et al., 2017; Schleich et al., 2014).
While in humans, the DENR-MCTS1 were believed to largely function to enhance reinitiation after translation of an abbreviated uORF (start-stop codons), it was subsequently determined that uORFs encoding polypeptides could effectively lead to reinitiation if the codon preceding the stop codon (penultimate codon) was GCGAla, a feature shared in uORF1 among ATF4 gene orthologs (Bohlen et al., 2020b; Schleich et al., 2017). It is suggested that DENR-MCTS1 expedite ribosome eviction of certain deacylated tRNAs during ribosome elongation at the penultimate codons of uORFs. This process would help elongating ribosomes retain association with selected initiation factors as ribosomes terminate uORF translation and resume scanning along the mRNA for subsequent reinitiation.
As emphasized earlier, the timing of the reacquisition of Met-tRNAiMet, which is delayed by p-eIF2α and lowered eIF2•GTP levels, determines whether the scanning 40S ribosome recognizes the next start codon at inhibitory uORF2 or is delayed and instead reinitiates at the subsequent ATF4 CDS. Notably, eIF2D in conjunction with DENR-MCTS1 is able to recruit initiator tRNA independent of eIF2 (Bohlen et al., 2020b; Vasudevan et al., 2020). Thus, the delay in the reinitiation process invoked during the delayed ribosome scanning triggered by p-eIF2α may include the eIF2D delivery of Met-tRNAiMet to the reinitiating ribosome. Further, Cyclin/Cdk phosphorylation of DENR is critical for stabilization of DENR-MCTS1 during mitosis, which can elevate translation of many genes involved in cell mitosis (Clemm von Hohenberg et al., 2022). It is proposed that ATF4 translation can be enhanced by the phosphorylation and stabilization of DENR independent of stress and the ISR, which may provide another avenue for regulation of ATF4 expression and function (Clemm von Hohenberg et al., 2022).
Preferential Translation Can Also Occur by a Bypass Mechanism Involving a Single uORF
Preferential translation during the ISR is also regulated by a ribosome bypass mechanism involving only a single inhibitory uORF. For example, CHOP encodes a bZIP transcription factor whose expression features both induced gene transcription via ATF4 and preferential translation induced by p-eIF2α (Fawcett et al., 1999; Harding et al., 2000a; Ma et al., 2002b; Palam et al., 2011). Translation of the CHOP uORF results in a ribosome elongation pause that thwarts reinitiation at the downstream CDS (Baird et al., 2014; Young et al., 2016b).
During stress and increased p-eIF2α, a portion of ribosomes scan through the inhibitory uORF and instead translate the CHOP CDS. Although the mechanistic features for the ribosome bypass of the CHOP inhibitory uORF are not clear, it is suggested that the poor initiation codon context of the uORF is a significant contributor (Baird et al., 2014; Young et al., 2016b). An uORF with an optimal initiation codon is less likely to be bypassed and consequently lowers CHOP CDS translation more substantially. As noted earlier, ribosome pausing during elongation can cause a queue of scanning 40S ribosomes that would increase the dwell time and enhance recognition of start codons of poor context. Lowered levels of eIF2•GTP•Met-tRNAiMet during p-eIF2α would then lower the numbers of ribosomes binding to the mRNA and scanning, thus reducing the queue of ribosomes and their dwell time and recognition of the uORF initiation codon (Acevedo et al., 2018; Andreev et al., 2021; Andreev et al., 2018). These changes in scanning ribosome flux would allow for a subset of ribosomes to bypass the uORF and instead increase translation of the optimal CHOP CDS.
Expression of GADD34 is also regulated by preferential translation and a ribosome bypass mechanism (Fig. 6) (Lee et al., 2009; Young and Wek, 2016). As noted earlier, GADD34 functions in feedback dephosphorylation of p-eIF2α. The 5′-leader of GADD34 encodes two uORFs, with a 5′-proximal uORF1 that is not well translated, functioning only as a modest dampening element that is independent of stress conditions. By comparison, the GADD34 uORF2 can be well translated, and in the absence of stress and low amounts of p-eIF2α, the uORF2 serves as a potent inhibitor of GADD34 CDS translation (Fig. 6). In this case, Pro-Pro-Gly-Stop encoded in uORF2 leads to inefficient termination, reducing ribosome reinitiation at downstream GADD34 CDS (Young et al., 2015). On stress and induced p-eIF2α, a portion of scanning ribosomes bypass uORF2 due in part to its poor start codon context and instead initiates translation at the downstream GADD34 CDS.
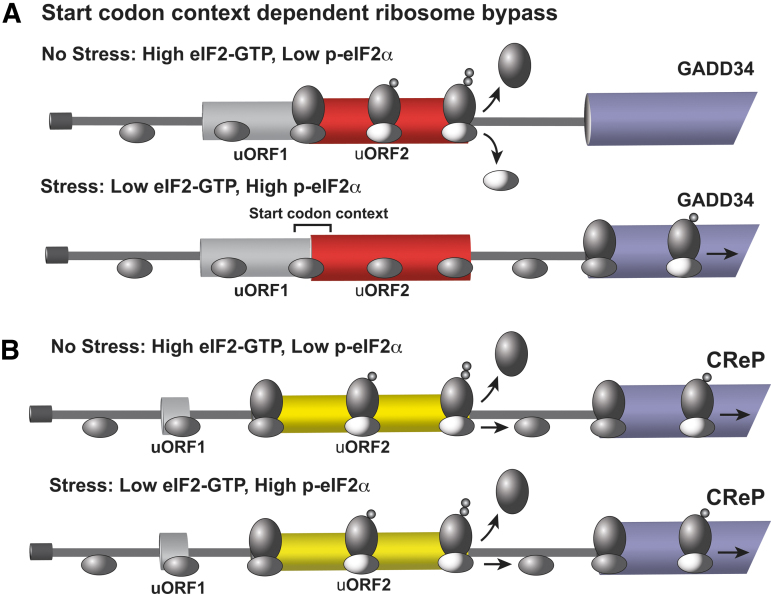
FIG. 6.
Preferential translation of GADD34 occurs by a bypass mechanism while CReP translation is constitutive. The GADD34 and CReP mRNA encode targeting subunits that facilitates protein phosphatase 1 dephosphorylation of p-eIF2α. As illustrated in this figure, both gene transcripts contain two uORFs, with GADD34 being preferentially translated by ribosomes (ovals represents large and small subunits) in response to induced p-eIF2α, whereas translation of CrEP occurs independent of p-eIF2α status. (A) The GADD34 mRNA contains two upstream uORFs that overlap in part and are out-of-frame. The start codon context is shown for the uORF2. The uORF1 (gray bar) is nominally translated, and the inhibitory uORF2 (red bar) can be bypassed in response to induced p-eIF2α during stress. In the absence of stress and low p-eIF2α levels, there is abundant eIF2 TC and scanning ribosomes (dark gray oval) readily initiate translation at uORF2. During the initiation phase of translation, the eIF2 is released from ribosomes (light gray oval). Elongating ribosomes translate the uORF2, and the encoded Pro-Pro-Gly-Stop is suggested to lead to inefficient translation termination, facilitating release of ribosomes from the mRNA and lowering ribosome reinitiation at the downstream GADD34 CDS. During stress, induced p-eIF2α and the resulting lowered TC are suggested to allow for a portion of the scanning ribosomes to proceed through the uORF2 and instead initiate translation at the downstream CDS (blue bar). ISR-directed transcription of the GADD34 gene, along with preferential translation, potently increases GADD34 protein that facilitates feedback dephosphorylation of p-eIF2α, allowing for resumption of bulk translation. (B) The CReP transcript also has two uORFs, with only uORF2 (red bar) being well translated. However, uORF2 allows for ribosomes to resume scanning and reacquire TC (dark oval), enabling reinitiation at the downstream CReP CDS. Therefore, CReP is well translated independent of stress and serves to help maintain appropriate p-eIF2α levels during both nonstressed and stressed conditions.
Illustrating the idea that uORFs can be present in many gene transcripts but not confer preferential translation, CReP has two uORFs analogous to GADD34 and also functions in PP1-targeted dephosphorylation of p-eIF2α (Fig. 6) (Andreev et al., 2015; Jousse et al., 2001; Young et al., 2015). The CReP uORF1 is modestly translated and uORF2 more robustly. However, uORF2 allows for efficient ribosome reinitiation at the downstream CReP CDS. In this way, constitutive repressor of eIF2α phosphorylation (CReP) is well translated independent of stress (Andreev et al., 2015; Young et al., 2015). However, if the GADD34 uORF sequences encoding Pro-Pro-Gly-Stop are substituted into the uORF2 of CREP, the chimeric CReP mRNA is subject to preferential translation in response to p-eIF2α (Young et al., 2015). These results emphasize that there can be modular elements within uORFs that confer different translational control outcomes during the ISR.
The ISR Differentially Regulates Gene Expression Depending on Stress Condition
While p-eIF2α and translational control is induced in response a range of different stresses, the ISR can specifically tailor gene expression for optimal adaptation to each stress condition. One mechanism for the tailored gene expression is that the ISR can function in conjunction with other stress-specific pathways. For example, in response to ER stress, the eIF2α kinase PERK functions with ER-resident sensory protein, IRE1α and ATF6α, to invoke the UPR that serves to alleviate accumulation of unfolded protein and expand the processing capacity of this organelle (introduced above and reviewed in Walter and Ron, 2011).
PERK is fully enmeshed in the UPR, with induced p-eIF2α serving to dampen synthesis of nascent secretory proteins that will further exacerbate the underlying ER stress (Harding et al., 2000b). Further, PERK and induced ATF4 enhance the expression and activation of ATF6α and control the duration of IRE1 activation (Chang et al., 2018; Teske et al., 2011). Together, the UPR controls the synthesis and degradation of mRNAs and proteins expressed from a broad swath of genes to effectively adapt to ER perturbations.
Another mechanism for the ISR tailoring gene expression to the stress condition involves regulation of transcription and pre-mRNA splicing to reconfigure the transcriptome for optimal p-eIF2α-directed translational control. In response to UV irradiation, induced p-eIF2α by GCN2 can halt cell progression at the G1 checkpoint, which allows cells to invoke DNA repair and enhance cell survival (Collier et al., 2018; Deng et al., 2002). Although induced ATF4 is typically central to the ISR, exposure to UV-B or UV-C represses the transcription of the ATF4 gene by a mechanism involving the liver-enriched inhibitor protein isoform of C/EBPβ (Dey et al., 2012; Dey et al., 2010). ATF4 mRNA is short-lived and as a consequence, ATF4 mRNA is rapidly depleted in response to UV treatment and therefore not available for preferential translation despite robust p-eIF2α (Collier et al., 2015; Dey et al., 2010). If an mRNA encoding the 5′-leader of ATF4 adjoined to a reporter gene is exogenously expressed and therefore available in cells, it is preferentially translated after UV treatment, showing that UV-induced p-eIF2α is fully capable of triggering the delayed reinitiation mechanism (Dey et al., 2010). Further, forced expression of ATF4 or its downstream target CHOP through genetic or pharmacological strategies triggers cell death in UV-treated cells (Collier et al., 2015; Dey et al., 2010). These results indicate that optimal ISR adaptation to UV exposure involves critical modifications that omit expression of the central ATF4 and its target genes.
A mechanism by which p-eIF2α triggers cell cycle arrest and UV adaptation involves preferential translation of CDKN1A (cyclin-dependent kinase inhibitor 1A; p21; p21Cip1/Waf1), encoding the potent cyclin-dependent kinase inhibitor p21 (Collier et al., 2018). In human keratinocytes, splice variant 4 of CDKN1A mRNA is highly expressed, and this transcript encodes an inhibitory uORF that is bypassed in response to p-eIF2α, enhancing CDKN1A CDS translation (Collier et al., 2018). Increased p21 levels direct cell cycle arrest required for cell survival after UV stress.
Another mechanism by which the ISR may tailor gene expression for specific stress conditions involves reversible mRNA methylation that can occur at the 5-leader of mRNAs. It was reported that N6-methyladenosine (m6A) of uORF2 at A225 in the ATF4 mRNA can enhance the dwell time of scanning ribosomes at uORF2, thus increasing the start codon recognition and translation of the inhibitory uORF that would, in turn, reduce expression of ATF4 CDS (Zhou et al., 2018). On starvation for all amino acids, there is a threefold reduction in the m6A modification, which is suggested to lower uORF2 translation and increase ATF4 synthesis (Zhou et al., 2018). This dynamic regulation of m6A in ATF4 mRNA may vary among different cell types depending on the availability of methyltransferases and demethylases. We analyzed ATF4 translational control using reporters with mutations (A225 changed to G) that are devoid of m6A modification and did not see any appreciable differences in induced ATF4 translation in MEF cell on pharmacological induction of ER stress (Wek, unpublished study). This result may indicate that the contributions of m6A in the delayed reinitiation model may also differ among stress conditions.
Coregulation Between eIF2α Kinase GCN2 and mTORC1 Signaling
The ISR can function in combination with other stress response pathways for optimal adaptation to each stress condition. In addition to p-eIF2α and the ISR, another major mechanism regulating translation initiation involves the mTORC1, which serves to promote growth when nutrients and energy are ample. During nutrient sufficiency, mTORC1 phosphorylates multiple substrates, many of which promote ribosome biogenesis and translation initiation (reviewed in Liu and Sabatini, 2020). However, on nutrient depletion, there is reciprocal coregulation between the ISR and mTORC1 pathways, with increased p-eIF2α by GCN2 coinciding with lowered phosphorylation of mTORC1 target proteins, 4E binding protein 1 (4EBP1), and ribosomal protein S6 kinase (S6K1) (Fig. 7). Coregulation of these signaling events ensures they are in sync to lower bulk translation and increase protein degradation, which together conserve energy and maximize stress remediation and prevention of cellular injury. In this way, reduced mTORC1 signaling functions cooperatively with ISR activation to determine the equilibrium between anabolic and catabolic cellular processes that support proteostasis. Discordant regulation of the two signaling pathways renders cells unable to appropriately balance and manage metabolic state and need, disrupting proteostasis and triggering cell death (Misra et al., 2021) (Fig. 7).
FIG. 7.
Reciprocal coregulation of GCN2 and mTORC1 during amino acid insufficiency affects translational and transcriptional expression. (Upper green panel) During conditions of amino acid insufficiency, such as leukine deprivation or asparaginase exposure, there is reciprocal regulation of GCN2 and mTORC1 and their corresponding phosphorylation of target proteins. With wild-type GCN2 (GCN2 WT), p-eIF2α is increased alongside reduced mTORC1 phosphorylation of 4EBP1 (p-4EBP1) and S6K1 (p-S6K1), slowing translation initiation at the eIF2B and eIF4F steps. Diminished GEF activity of eIF2B lowers bulk protein synthesis and promotes preferential translation of ATF4 and other ISR target genes. Reduced S6K1 activity suppresses translation of gene transcripts with TOP tracts. Together, these changes slow anabolic capacity, facilitating proteostasis recovery. (Upper red panel) In cells or tissues lacking GCN2 (GCN2 KO), the activities of eIF2B, eIF4F, and S6K1 continue, resulting in a proteostasis mismatch, with sustained anabolic capacity in a time of nutrient scarcity. (Middle green panel) Maintenance of adaptive proteostasis during chronic amino acid insufficiency promotes ATF4 synthesis, which executes targeted changes to the transcriptome to resolve the nutrient stress and limit ISR activation (e.g., ASNS, GADD34). Other ATF4 target genes (e.g., SESTRIN2, REDD1) function to repress mTORC1, increasing protein breakdown and reducing anabolism. (Lower red panel) A disrupted GCN2-ATF4 axis during prolonged amino acid insufficiency alters or amplifies ISR target gene execution, which fails to appropriately trigger mTORC1 repression, leading to discordant ISR and mTORC1 regulation of protein synthesis, promoting ER stress and cell injury and premature death. KO, knockout; mTORC1, mammalian/mechanistic target of rapamycin complex 1; REDD1, regulated in development and DNA damage response 1; S6K1, ribosomal protein S6 kinase; TOP, 5′-terminal oligopyrimidine.
Among the eIF2α kinases, GCN2 has the clearest presence of cross-talk with mTORC1. This relationship is observed from humans to yeast, although the mechanistic details differ. During amino acid starvation in yeast, GCN2 transiently interacts with KOG1 (controller of growth; yeast homolog of mammalian Raptor), the unique regulatory subunit of TORC1, downregulating its phosphorylation of SCH9, the functional ortholog of mammalian S6K1 (Yuan et al., 2017). Loss of GCN2 in yeast renders TORC1 nonresponsive to amino acid starvation. Control in the opposite direction, where GCN2 activation is downstream of TORC1 inhibition, is also present in yeast. Pharmacological inhibition of TORC1 in yeast triggers GCN2 activation via a pathway involving two A phosphatase associated protein-protein phosphatase 2A (TAP42-PP2A) phosphatase-mediated dephosphorylation of GCN2 at Ser577 (Cherkasova and Hinnebusch, 2003). Release of the inhibitory Ser577 phosphorylation is suggested to enhance GCN2 association with uncharged tRNA, leading to activation of GCN2 and induced p-eIF2α. Therefore, in yeast there are signaling networks emanating from GCN2 and from mTORC1 to ensure their reciprocal coregulation.
In mammals, Anthony et al. (2004) reported the seminal observation that GCN2 was required for mTORC1 suppression in livers of mice fed diets devoid of individual amino acids. The phosphorylation states of both 4EBP1 and S6K1 were sustained or elevated in the liver of GCN2 knockout mice despite being fed a leucine-devoid diet (Anthony et al., 2004). Subsequent work confirmed GCN2-dependency for mTORC1 suppression during starvation for several different amino acids in both normal physiology and cancer models (Bunpo et al., 2009; Misra et al., 2021; Nakamura et al., 2018; Nikonorova et al., 2018; She et al., 2013).
The mechanisms by which activated GCN2 contributes to repression of mTORC1 is not fully understood, but there are suggested to be early (≤2 h) and late regulatory events. Early mechanisms by which GCN2 contributes to mTORC1 repression during starvation for certain amino acids are not well understood, but they are known to be a consequence of induced p-eIF2α and independent of ATF4 (Averous et al., 2016; Nikonorova et al., 2018). Importantly, PERK and p-eIF2α do not contribute to mTORC1 suppression during acute ER stress (Nikonorova et al., 2018), indicating that early GCN2 repression of mTORC1 involves nutrient-specific signaling processes. Perhaps GCN2 communication with mTORC1 involves events linked to nutrient limitation, such as ribosome stalling or modulation of aminoacyl tRNA synthetases (Darnell et al., 2018; Han et al., 2012). GCN2 is also suggested to change the expression and locations of transporters (Cordova et al., 2022; Gauthier-Coles et al., 2021), which could alter the flux of certain amino acids at the plasma membrane and lysosomes and as consequence regulate mTORC1.
The early regulatory mechanism ensures that GCN2 and mTORC1 function together to not only lower bulk protein synthesis but also control gene-specific translation. A key example of how GCN2 and mTORC1 pathways cooperate to alter translation of targeted genes is 5′-terminal oligopyrimidine (TOP) motif-containing mRNAs (TOP mRNAs). TOP mRNAs encode for ribosomal proteins and associated translation factors, along with those involved energy production and mitochondrial function (reviewed in Cockman et al., 2020).
During nutrient stress, repressed mTORC1 diminishes eIF4E binding to TOP mRNAs to a greater extent than other mRNAs. Depleted mTORC1 phosphorylation of LARP1 triggers LARP1 association with TOP mRNAs, where it thwarts assembly of eIF4F onto the 5′-capped TOP transcripts, inhibiting their translation (Fuentes et al., 2021; Lahr et al., 2017; Philippe et al., 2018; Tcherkezian et al., 2014). Although most TOP mRNAs are responsive to mTORC1 activity, some TOP mRNAs are instead regulated in an mTORC1-independent manner during amino acid starvation. In this case, these TOP mRNAs bind with stress granule-associated T-cell-restricted intracellular antigen; cytotoxic granule associated RNA binding protein (TIA1) and TIA-1 related protein (TIAR), which arrests initiation of translation (Damgaard and Lykke-Andersen, 2011). Interestingly, the mTORC1-independent mechanism is also controlled by GCN2 (Damgaard and Lykke-Andersen, 2011). These TOP regulatory mechanisms illustrate how appropriate reciprocal coregulation of GCN2 and mTORC1 during amino acid limitations is critical for lowering the cellular anabolic capacity via gene-specific translational control.
Loss of GCN2 permits continued translation of TOP mRNAs in the liver of mice exposed to nutrient stress, resulting in steatosis, liver injury, and premature cell death (Al-Baghdadi et al., 2017; Anthony et al., 2001; Nikonorova et al., 2018). There are also mechanisms reported in which acute activation of mTORC1 can target dephosphorylation of eIF2α that bolsters TC formation (Gandin et al., 2016). Thus, proper coregulation of GCN2 and TORC1 activities, separate from the downstream impact of ATF4-mTORC1 crosstalk, is important to conserve ribosome anabolic capacity that impacts maintenance of proteostasis and subsequent health outcomes.
Longer-term amino acid deprivation sustains mTORC1 suppression by ATF4-directed transcriptional induction of key mTORC1 repressors, such as SESTRIN2 (Xu et al., 2020; Ye et al., 2015) and regulated in development and DNA damage response 1 (REDD1) (Jin et al., 2009; Whitney et al., 2009; Xu et al., 2020) that engage with upstream regulators of mTORC1, and 4EBP1 (Kang et al., 2017; Yamaguchi et al., 2008), which would lower mTORC1-induced translation initiation (Fig. 7). Repression of mTORC1 signaling enhances catabolic processes that are critical to adapt to nutrient insufficiency. It is curious that in fed conditions in certain cell types, ATF4 can instead function as a downstream effector of anabolic mTORC1 signaling, which would then promote strategic amino acid uptake and synthesis (Byles et al., 2021; Torrence et al., 2021). This bidirectional relationship implicates ATF4 as a communication fulcrum supporting increased amino acid supply when inward flux is insufficient to meet cellular demands. In this way, the ATF4-directed uptake of amino acids can arise from either catabolic conditions triggered by starvation for nutrients or enhanced anabolic demands, which can occur during tumor proliferation or feeding-induced anabolism.
Dysregulation of the ISR Contributes to Human Diseases
The induction of p-eIF2α during the ISR, followed by feedback dephosphorylation of the initiation by GADD34, ensures that the magnitude and timing of the pathway are tightly controlled and are optimized to maintain proteostasis. We refer to this window of regulated p-eIF2α and reciprocal repression of bulk translation as the adaptive zone (Fig. 8A). If the levels of p-eIF2α are aberrantly heightened or lowered outside the adaptive zone, the configuration of ISR effectors can instead become maladaptive and trigger cell death processes.
FIG. 8.
A dynamic range of the duration and magnitude of p-eIF2α and translational control ensures successful adaptation to stress. The ISR features stress-activation of eIF2α kinase(s), which induces bulk translation repression and attendant ISR gene expression that helps to ameliorate the stress, followed by feedback dephosphorylation of p-eIF2α by GADD34-directed PP1 and resumption of protein synthesis (A) The schematic illustrates the reciprocal regulation between increasing p-eIF2α (in black) and decreasing levels of mRNA translation (in blue) that are featured in the ISR. The adaptive zone (yellow) represents the dynamic range of the translational control response during successful adaptation to stress. Cells encounter stress, triggering p-eIF2α, which signals an adaptive response that alleviates cell damage and mitigates the stress. As indicated, the successful adaptation to stress by ISR largely functions in the adaptive zone. However, in situations of chronic stress or with genetic changes that dysregulate the ISR, there can be hypo- or hyperphosphorylation of eIF2α (orange to dark red zones), and these extremes in translational control can trigger cell damage and death. (B) In the example of hypoinduction of the ISR, mutations in PERK and GCN2 can sharply reduce basal and stress-induced p-eIF2α and attendant control of mRNA translation, shifting the dynamic range of the ISR into the danger zone, as illustrated by the box shifting to the left in the illustration. The consequences of loss of PERK and GCN2 functions are WRS and PVOD, respectively. (C) Genetic changes can also lead to hyperinduction of the ISR and disease, as described more fully in the text. For example, mutations in CReP reduce PP1 dephosphorylation of p-eIF2α, leading to constitutive induction of the ISR that is further activated on stress-induction of an eIF2α kinase. The hyperinduction of p-eIF2α and the ISR shifts the dynamic range of the ISR into the danger zone, as illustrated by the box shifting to the right in the illustration. Likewise, certain mutations in aminoacyl tRNA synthetase genes and BDKDK-deficiency lead to hyperinduction of p-eIF2α, which shifts the ISR responses to the danger zone. In the case of mutations in EIF2S3 (eIF2γ) or genes encoding the eIF2B subunits, there is impaired eIF2 TC formation independent of and p-eIF2α that can be further exacerbated upon induced p-eIF2α. Features of the adaptive zone diagram are adapted from a prior article (Wek and Anthony, 2009). PVOD, pulmonary veno-occlusive disease.
There are a number of genetic changes reported to disrupt the ISR-directed adaptation to stress. The genetic lesions can be in genes encoding the eIF2α kinases or phosphatases, which would dysregulate p-eIF2α levels, or in eIF2 or eIF2B subunits, which disrupt formation of eIF2 TC independent of p-eIF2α. Further, genetic changes can trigger stress conditions that constitutively induce p-eIF2α and the ISR. As described here, these genetic lesions shift the ISR to the danger zone that contributes to a range of diseases, emphasizing that the ISR is critical for the health of many different organ systems (Fig. 8).
Loss-of-function mutations are reported in PERK, leading to Wolcott-Rallison syndrome (WRS) that presents with neonatal diabetes, osteoporosis, digestive dysfunctions, and hepatic complications, and contributes to early death (Delepine et al., 2000; Senee et al., 2004). The inability of PERK to appropriately induce p-eIF2α and translational control in WRS leads to inappropriately high protein synthesis, which can overwhelm the processing capacity of the ER. Further, preclusion of preferential translation of key ISR genes, and their attendant transcriptional control, thwarts expression of genes that contribute to protein folding, processing, and trafficking. Specialized secretory tissues, such as those in the pancreas, are especially vulnerable to loss of PERK function with distended ER and aberrant accumulation of proinsulin in the islet β cells. In this way, the ISR in cells derived from WRS patients would have low amounts of p-eIF2α and diminished translational control, shifting proteostasis outside the adaptive zone of the ISR (Fig. 8B).
Loss-of-function mutations in GCN2 have been linked to pulmonary disorders, including pulmonary veno-occlusive disease (PVOD), pulmonary arterial hypertension (PAH), and pulmonary capillary haemangiomatosis (PCH) (Best et al., 2014; Emanuelli et al., 2020; Eyries et al., 2014). The rationale for why GCN2 deficiency culminates in pulmonary disorders in humans is not clear, but lungs can be challenged by an assortment of stress agents, including inhaled toxicants and microbes. GCN2 and the ISR may provide for cell resistance to these insults and for remodeling of the pulmonary vasculature. GCN2 and ATF4 induce expression of autophagic genes (B'chir et al., 2013) and these processes contribute to vascular remodeling (Chen et al., 2018). It is also noteworthy that the ISR and ATF4 provide for antioxidation responses, along with angiogenesis through enhanced expression of VEGF (Harding et al., 2003; Longchamp et al., 2018; Roybal et al., 2004). GCN2 also participates in cell differentiation and proliferation, which would be important for the health of pulmonary tissues and immune system. Finally, it is suggested that loss of GCN2 may disrupt signaling through bone morphogenetic protein receptor type II (BMPR2) and mutations in BMPR2 have been reported for a majority of familial PAH (Eichstaedt et al., 2016).
Mutations in PPP1R15B (CReP), which contributes to constitutive dephosphorylation of p-eIF2α, leads to diabetes, growth defects, bone and kidney deformities, and microcephaly (Abdulkarim et al., 2015). In this case, the basal p-eIF2α levels would be expected to be enhanced in these afflicted patients, which, combined with further stressed induced activation of eIF2α kinases, would be predicted to hyperactivate the ISR, shifting the pathway outside the adaptive zone (Fig. 8C).
Genetic disruption of eIF2 or eIF2B also shifts the ISR outside the adaptive zone (Fig. 8). Mutations in the 2S3 gene, encoding the γ-subunit of eIF2, inappropriately activate the ISR, resulting in mental retardation, epileptic seizures, hypogenitalism, microcephaly and obesity (MEHMO) (Young-Baird et al., 2020) (Fig. 8C). Mutations in genes encoding one of the five eIF2B subunits can diminish the exchange of eIF2•GDP to 2•GTP and lower TC. As a consequence, when combined with stress induction of p-eIF2α, the deficient eIF2B enhances the amplitude of the ISR and extends the duration of the response (Fig. 8), culminating in Vanishing white matter (VWM or Childhood ataxia with central nervous system hypomyelination) (Leegwater et al., 2001; Li et al., 2004; Richardson et al., 2004) (Fig. 8C). VWM presents with severe white matter abnormalities, including myelin and cystic degeneration. In considering treatment strategies for the maladies resulting from mutations directly afflicting eIF2 or eIF2B, ISRIB (described above) is reported to enhance TC levels and rescue protein synthesis (Wong et al., 2018; Young-Baird et al., 2020). A molecule similar to ISRIB with improved pharmacological properties (eIF2B activator [2BAct]) also shows promise as a treatment in a mouse model of VWM caused by the R191H mutation in the catalytic ɛ subunit of 2B (Wong et al., 2019).
Genetic changes that create unremitting stress in cells lead to hyperphosphorylation of eIF2α, shifting the ISR outside of the adaptive zone (Fig. 8). Dominant gain-of-function mutations in genes encoding aminoacyl-tRNA synthetases result in Charcot-Marie-Tooth (CMT) disease, a family of genetic disorders where peripheral nerves undergo progressive degeneration (Ishimura et al., 2016; Mendonsa et al., 2021). These mutations result in high levels of uncharged tRNAs that can lead to ribosomal elongation stalling and robust activation of GCN2 and the ISR. However, because of nature of the synthetase gene mutations, induction of the GCN2 cannot resolve the stalled elongation complexes, shifting ISR outside the adaptive zone (Fig. 8). Supporting the idea that activation of GCN2 and a maladaptive ISR contributes to this neurodegenerative CMT disease, genetic loss of GCN2 or use of a small-molecule inhibitor improved disease symptoms in mouse models (Ishimura et al., 2016; Spaulding et al., 2021).
Loss-of-function mutations in the BCKDK gene encoding the branched-chain keto acid dehydrogenase kinase lead to unregulated catabolism of branched-chain amino acids (BCAAs), which constitutively induces p-eIF2α by GCN2 and the ISR (Fig. 8C). Patients with BCKDK deficiency present with autism and epilepsy, which can be remedied in part with dietary supplements of BCAAs (Garcia-Cazorla et al., 2014; Novarino et al., 2012). GCN2-mediated p-eIF2α and ISR activation are documented in the brains of mice lacking BCKDK (Joshi et al., 2006; She et al., 2013).
However, these events are protective to white matter because knocking out GCN2 in these mice precludes p-eIF2α and ISR activation but triggers a lethal leukodystrophy soon after birth due to inflammatory-mediated cell death of developing oligodendrocytes (She et al., 2013). Unresolved stress leading to constitutive activation of the ISR is also reported for cancers. For example, androgen-sensitive and castration-resistant prostate cancers are addicted to GCN2 to maintain amino acid transporter gene expression that is required for essential amino acids to fuel tumor growth and metabolism (Cordova et al., 2022). Genetic loss of GCN2 or pharmacological inhibition of the eIF2α kinase reduce proliferation of prostate cancer cells in vitro and sharply curtail tumor growth in xenograft models.
Future Directions
There is still much to be learned about the mechanisms and functions of the ISR and the processes by p-eIF2α and attendant translation and gene expression are maintained or disrupted among organ systems subjected to different stress conditions. Although we understand key features by which each of the eIF2α kinases sense certain stress conditions, the processes by which a range of different stresses can induce a single eIF2α kinase and their ability to compensate for other eIF2α kinase as secondary sensors of stress is not yet fully clear.
Understanding these regulatory processes will be important for optimizing strategies for modulating the ISR in disease conditions and development of drugs that efficiently target a given 2α kinase or combinations of these ISR sensors. Another important topic for future research involves the biochemical processes by which p-eIF2α allows for bypass of certain uORF elements and the associated participation of RNA structures and RNA-binding proteins in preferential translation. Finally, future studies need to better delineate the early and late mechanisms for reciprocal coregulation of GCN2 and mTORC1 and the physiological and pathological conditions by which this regulation is maintained or disrupted.
Acknowledgments
The authors thank members of the Anthony and Wek laboratories for helpful discussions and Sheree Wek for preparation of the figure artwork. They also thank the anonymous reviewers for their constructive evaluations of the organization and content of this review article.
Abbreviations Used
- 4EBP1
4E binding protein 1
- ASNS
asparagine synthetase
- ATF
activating transcription factor
- BCAA
branched-chain amino acid
- BiP
binding immunoglobulin protein
- BMPR2
bone morphogenetic protein receptor type II
- bZIP
basic leucine zipper
- CDKN1A
cyclin-dependent kinase inhibitor 1A; p21; p21Cip1/Waf1
- CDS
coding sequence
- CMT
Charcot-Marie-Tooth
- CReP
constitutive repressor of eIF2α phosphorylation
- CTD
carboxy terminal domain
- DELE1
DAP3 binding cell death enhancer 1
- DENR
density regulated re-initiation and release factor protein
- dsRBM
double-stranded RNA-binding motif
- dsRNA
double-stranded RNA
- eIF
eukaryotic translation initiation factor
- ER
endoplasmic reticulum
- F6P
fructose-6-phosphate
- GADD34
growth arrest and DNA damage-inducible gene 34
- GAP
GTPase activator protein
- GCN
general control nonderepressible
- GDI
guanine nucleotide dissociation inhibitor
- GDP
guanosine diphosphate
- GEF
guanine nucleotide exchange factor
- GSK-3
glycogen synthase kinase 3
- GTP
guanosine trisphosphate
- HARS
histidyl-tRNA synthetase
- HRI
heme regulated inhibitor
- IRE1
inositol requiring enzyme 1
- ISR
integrated stress response
- ISRIB
ISR inhibitor
- m6A
N6-methyladenosine
- MCTS1
malignant T-cell amplified sequence 1
- Met-tRNAiMet
methionyl initiator tRNA
- mRNA
messenger RNA
- mTORC1
mammalian/mechanistic target of rapamycin complex 1
- PAH
pulmonary arterial hypertension
- p-eIF2α
phosphoryation of eIF2α
- PERK
PKR-like ER kinase
- PIC
preinitiation complex
- PKR
protein kinase RNA-activated
- PKZ
protein kinase Z
- PP1
protein phosphatase 1
- PVOD
pulmonary veno-occlusive disease
- REDD1
regulated in development and DNA damage response 1
- RNA
ribonucleic acid
- S6K1
ribosomal protein S6 kinase
- SS
signal sequence
- TC
ternary complex
- TM
transmembrane
- TOP
5′-terminal oligopyrimidine
- tRNA
transfer RNA
- uORF
upstream open reading frame
- UPR
unfolded protein response
- UV
ultraviolet
- VWM
vanishing white matter
- WRS
Wolcott-Rallison syndrome
- ZAKα
sterile alpha motif and leucine zipper containing kinase
- Z-DNA
Z-deoxynucleic acid
Authors' Contributions
R.C.W., T.G.A., and K.A.S. conceptualized, wrote and edited the article, and prepared the figures.
Author Disclosure Statement
R.C.W. is a member of the advisory board in HiberCell, Inc. K.A.S. and T.G.A. are consultants for HiberCell, Inc., and K.A.S. receives research support from HiberCell, Inc.
Funding Information
The authors acknowledge support from NIH grants GM136331 (R.C.W.) and DK109714 (T.G.A., R.C.W.), the Showalter Trust (R.C.W.), and the Indiana University Melvin and Bren Simon Comprehensive Cancer Center P30CA082709 (K.A.S.).
References
- Abastado JP, Miller PF, Hinnebusch AG. A quantitative model for translational control of the GCN4 gene of Saccharomyces cerevisiae. New Biol 1991a;3(5):511–524. [PubMed] [Google Scholar]
- Abastado JP, Miller PF, Jackson BM, et al. Suppression of ribosomal reinitiation at upstream open reading frames in amino acid-starved cells forms the basis for GCN4 translational control. Mol Cell Biol 1991b;11(1):486–496; doi: 10.1128/mcb.11.1.486-496.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulkarim B, Nicolino M, Igoillo-Esteve M, et al. A missense mutation in PPP1R15B causes a syndrome including diabetes, short stature, and microcephaly. Diabetes 2015;64(11):3951–3962; doi: 10.2337/db15-0477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo JM, Hoermann B, Schlimbach T, et al. Changes in global translation elongation or initiation rates shape the proteome via the Kozak sequence. Sci Rep 2018;8(1):4018; doi: 10.1038/s41598-018-22330-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adomavicius T, Guaita M, Zhou Y, et al. The structural basis of translational control by eIF2 phosphorylation. Nat Commun 2019;10(1):2136; doi: 10.1038/s41467-019-10167-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed YL, Schleich S, Bohlen J, et al. DENR-MCTS1 heterodimerization and tRNA recruitment are required for translation reinitiation. PLoS Biol 2018;16(6):e2005160; doi: 10.1371/journal.pbio.2005160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Baghdadi RJT, Nikonorova IA, Mirek ET, et al. Role of activating transcription factor 4 in the hepatic response to amino acid depletion by asparaginase. Sci Rep 2017;7(1):1272; doi: 10.1038/s41598-017-01041-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin PH, Carlson KR, Wek RC. An RNA stem-loop functions in conjunction with an upstream open reading frame to direct preferential translation in the integrated stress response. J Biol Chem 2022;299(2):102864; doi: 10.1016/j.jbc.2022.102864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin-Wetzel N, Saunders RA, Kamphuis MJ, et al. A J-protein co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 2017;171(7):1625.e13–1637.e13; doi: 10.1016/j.cell.2017.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, Arnold M, Kiniry SJ, et al. TASEP modelling provides a parsimonious explanation for the ability of a single uORF to derepress translation during the integrated stress response. Elife 2018;7:e32563; doi: 10.7554/eLife.32563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, Baranov PV, Milogorodskii A, et al. A deterministic model for non-monotone relationship between translation of upstream and downstream open reading frames. Math Med Biol 2021;38(4):490–515; doi: 10.1093/imammb/dqab015 [DOI] [PubMed] [Google Scholar]
- Andreev DE, Loughran G, Fedorova AD, et al. Non-AUG translation initiation in mammals. Genome Biol 2022;23(1):111; doi: 10.1186/s13059-022-02674-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, O'Connor PB, Fahey C, et al. Translation of 5′ leaders is pervasive in genes resistant to eIF2 repression. Elife 2015;4:e03971; doi: 10.7554/eLife.03971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TG, McDaniel BJ, Byerley RL, et al. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J Biol Chem 2004;279:36553–36561. [DOI] [PubMed] [Google Scholar]
- Anthony TG, Reiter AK, Anthony JC, et al. Deficiency of dietary EAA preferentially inhibits mRNA translation of ribosomal proteins in liver of meal-fed rats. Am J Physiol Endocrinol Metab 2001;281(3):E430–E439; doi: 10.1152/ajpendo.2001.281.3.E430 [DOI] [PubMed] [Google Scholar]
- Augusto L, Martynowicz J, Amin PH, et al. TgIF2K-B Is an eIF2alpha kinase in Toxoplasma gondii that responds to oxidative stress and optimizes pathogenicity. mBio 2021;12(1):e03160-20; doi: 10.1128/mBio.03160-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averous J, Lambert-Langlais S, Mesclon F, et al. GCN2 contributes to mTORC1 inhibition by leucine deprivation through an ATF4 independent mechanism. Sci Rep 2016;6:27698; doi: 10.1038/srep27698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- B'chir W, Maurin AC, Carraro V, et al. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res 2013;41:7683–7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TD, Palam LR, Fusakio ME, et al. Selective mRNA translation during eIF2 phosphorylation induces expression of IBTKalpha. Mol Biol Cell 2014;25(10):1686–1697; doi: 10.1091/mbc.E14-02-0704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltzis D, Pluquet O, Papadakis AI, et al. The eIF2alpha kinases PERK and PKR activate glycogen synthase kinase 3 to promote the proteasomal degradation of p53. J Biol Chem 2007;282(43):31675–31687; doi: 10.1074/jbc.M704491200 [DOI] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, et al. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2000;2(6):326–332; doi: 10.1038/35014014 [DOI] [PubMed] [Google Scholar]
- Best DH, Sumner KL, Austin ED, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest 2014;145(2):231–236; doi: 10.1378/chest.13-2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad AM, Lin KY, Marintchev A. eIF2B mechanisms of action and regulation: A thermodynamic view. Biochemistry 2018;57(9):1426–1435; doi: 10.1021/acs.biochem.7b00957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad AM, Xia B, Sandor DG, et al. Insights into the architecture of the eIF2Balpha/beta/delta regulatory subcomplex. Biochemistry 2014;53(21):3432–3445; doi: 10.1021/bi500346u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen J, Fenzl K, Kramer G, et al. Selective 40S footprinting reveals cap-tethered ribosome scanning in human cells. Mol Cell 2020a;79(4):561–574.e5; doi: 10.1016/j.molcel.2020.06.005 [DOI] [PubMed] [Google Scholar]
- Bohlen J, Harbrecht L, Blanco S, et al. DENR promotes translation reinitiation via ribosome recycling to drive expression of oncogenes including ATF4. Nat Commun 2020b;11(1):4676; doi: 10.1038/s41467-020-18452-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush MH, Weiser DC, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol 2003;23(4):1292–1303; doi: 10.1128/MCB.23.4.1292-1303.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunpo P, Dudley A, Cundiff JK, et al. GCN2 protein kinase is required to activate amino acid deprivation responses in mice treated with the anti-cancer agent L-asparaginase. J Biol Chem 2009;284(47):32742–32749; doi: 10.1074/jbc.M109.047910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byles V, Cormerais Y, Kalafut K, et al. Hepatic mTORC1 signaling activates ATF4 as part of its metabolic response to feeding and insulin. Mol Metab 2021;53:101309; doi: 10.1016/j.molmet.2021.101309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnetta R, Wong HH, Frese CK, et al. Noncanonical modulation of the eIF2 pathway controls an increase in local translation during neural wiring. Mol Cell 2019;73(3):474.e5–489.e5; doi: 10.1016/j.molcel.2018.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci U S A 2009;106(18):7507–7512; doi: 10.1073/pnas.0810916106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Geballe AP. Inhibition of nascent-peptide release at translation termination. Mol Cell Biol 1996;16(12):7109–7114; doi: 10.1128/MCB.16.12.7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrara M, Prischi F, Nowak PR, et al. Noncanonical binding of BiP ATPase domain to Ire1 and Perk is dissociated by unfolded protein CH1 to initiate ER stress signaling. Elife 2015;4:e03522; doi: 10.7554/eLife.03522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TK, Lawrence DA, Lu M, et al. Coordination between two branches of the unfolded protein response determines apoptotic cell fate. Mol Cell 2018;71(4):629.e5–636.e5; doi: 10.1016/j.molcel.2018.06.038 [DOI] [PubMed] [Google Scholar]
- Chen H, Pan YX, Dudenhausen EE, et al. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive basic region/leucine zipper transcription factors as well as localized histone acetylation. J Biol Chem 2004;279(49):50829–50839; doi: 10.1074/jbc.M409173200 [DOI] [PubMed] [Google Scholar]
- Chen R, Jiang M, Li B, et al. The role of autophagy in pulmonary hypertension: A double-edge sword. Apoptosis 2018;23(9–10):459–469; doi: 10.1007/s10495-018-1477-4 [DOI] [PubMed] [Google Scholar]
- Cherkasova VA, Hinnebusch AG. Translational control by TOR and TAP42 through dephosphorylation of eIF2alpha kinase GCN2. Genes Dev 2003;17:859-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemm von Hohenberg K, Muller S, Schleich S, et al. Cyclin B/CDK1 and Cyclin A/CDK2 phosphorylate DENR to promote mitotic protein translation and faithful cell division. Nat Commun 2022;13(1):668; doi: 10.1038/s41467-022-28265-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockman E, Anderson P, Ivanov P. TOP mRNPs: Molecular mechanisms and principles of regulation. Biomolecules 2020;10(7):969; doi: 10.3390/biom10070969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AE, Spandau DF, Wek RC. Translational control of a human CDKN1A mRNA splice variant regulates the fate of UVB-irradiated human keratinocytes. Mol Biol Cell 2018;29(1):29–41; doi: 10.1091/mbc.E17-06-0362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier AE, Wek RC, Spandau DF. Translational repression protects human keratinocytes from UVB-induced apoptosis through a discordant eIF2 kinase stress response. J Invest Dermatol 2015;135(10):2502–2511; doi: 10.1038/jid.2015.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, et al. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol 2001;21(20):6841–6850; doi: 10.1128/MCB.21.20.6841-6850.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova RA, Misra J, Amin PH, et al. GCN2 eIF2 kinase promotes prostate cancer by maintaining amino acid homeostasis. bioRxiv 2022; 10.1101/2022.06.17.496598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Credle JJ, Finer-Moore JS, Papa FR, et al. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci U S A 2005;102(52):18773–18784; doi: 10.1073/pnas.0509487102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard CK, Lykke-Andersen J. Translational coregulation of 5′TOP mRNAs by TIA-1 and TIAR. Genes Dev 2011;25(19):2057–2068; doi: 10.1101/gad.17355911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar AC, Dever TE, Sicheri F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell 2005;122(6):887–900; doi: 10.1016/j.cell.2005.06.044 [DOI] [PubMed] [Google Scholar]
- Darnell AM, Subramaniam AR, O'Shea EK. Translational control through differential ribosome pausing during amino acid limitation in mammalian cells. Mol Cell 2018;71(2):229.e11–243.e11; doi: 10.1016/j.molcel.2018.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delepine M, Nicolino M, Barrett T, et al. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet 2000;25(4):406–409; doi: 10.1038/78085 [DOI] [PubMed] [Google Scholar]
- Deng J, Harding HP, Raught B, et al. Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol 2002;12(15):1279–1286; doi: 10.1016/s0960-9822(02)01037-0 [DOI] [PubMed] [Google Scholar]
- Dey M, Cao C, Dar AC, et al. Mechanistic link between PKR dimerization, autophosphorylation, and eIF2alpha substrate recognition. Cell 2005;122:901–913. [DOI] [PubMed] [Google Scholar]
- Dey S, Baird TD, Zhou D, et al. Both transcriptional regulation and translational control of ATF4 are central to the integrated stress response. J Biol Chem 2010;285(43):33165–33174; doi: 10.1074/jbc.M110.167213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey S, Savant S, Teske BF, et al. Transcriptional repression of ATF4 gene by CCAAT/enhancer-binding protein beta (C/EBPbeta) differentially regulates integrated stress response. J Biol Chem 2012;287(26):21936–21949; doi: 10.1074/jbc.M112.351783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokladal L, Stumpe M, Pillet B, et al. Global phosphoproteomics pinpoints uncharted Gcn2-mediated mechanisms of translational control. Mol Cell 2021;81(9):1879.e6–1889.e6; doi: 10.1016/j.molcel.2021.02.037 [DOI] [PubMed] [Google Scholar]
- Dong J, Qiu H, Garcia-Barrio M, et al. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol Cell 2000;6(2):269–279; doi: 10.1016/s1097-2765(00)00028-9 [DOI] [PubMed] [Google Scholar]
- Ebert SM, Bullard SA, Basisty N, et al. Activating transcription factor 4 (ATF4) promotes skeletal muscle atrophy by forming a heterodimer with the transcriptional regulator C/EBPbeta. J Biol Chem 2020;295(9):2787–2803; doi: 10.1074/jbc.RA119.012095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichstaedt CA, Song J, Benjamin N, et al. EIF2AK4 mutation as “second hit” in hereditary pulmonary arterial hypertension. Respir Res 2016;17(1):141; doi: 10.1186/s12931-016-0457-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli G, Nassehzadeh-Tabriz N, Morrell NW, et al. The integrated stress response in pulmonary disease. Eur Respir Rev 2020;29(157):200184; doi: 10.1183/16000617.0184-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyries M, Montani D, Girerd B, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet 2014;46(1):65–69; doi: 10.1038/ng.2844 [DOI] [PubMed] [Google Scholar]
- Fawcett TW, Martindale JL, Guyton KZ, et al. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 1999;339(Pt 1):135–141. [PMC free article] [PubMed] [Google Scholar]
- Fessler E, Eckl EM, Schmitt S, et al. A pathway coordinated by DELE1 relays mitochondrial stress to the cytosol. Nature 2020;579(7799):433–437; doi: 10.1038/s41586-020-2076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler E, Krumwiede L, Jae LT. DELE1 tracks perturbed protein import and processing in human mitochondria. Nat Commun 2022;13(1):1853; doi: 10.1038/s41467-022-29479-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes P, Pelletier J, Martinez-Herraez C, et al. The 40S-LARP1 complex reprograms the cellular translatome upon mTOR inhibition to preserve the protein synthetic capacity. Sci Adv 2021;7(48):eabg9275; doi: 10.1126/sciadv.abg9275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandin V, Masvidal L, Cargnello M, et al. mTORC1 and CK2 coordinate ternary and eIF4F complex assembly. Nat Commun 2016;7:11127; doi: 10.1038/ncomms11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cazorla A, Oyarzabal A, Fort J, et al. Two novel mutations in the BCKDK (branched-chain keto-acid dehydrogenase kinase) gene are responsible for a neurobehavioral deficit in two pediatric unrelated patients. Hum Mutat 2014;35(4):470–477; doi: 10.1002/humu.22513 [DOI] [PubMed] [Google Scholar]
- Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 2011;333(6051):1891–1894; doi: 10.1126/science.1209126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Coles G, Vennitti J, Zhang Z, et al. Quantitative modelling of amino acid transport and homeostasis in mammalian cells. Nat Commun 2021;12(1):5282; doi: 10.1038/s41467-021-25563-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordiyenko Y, Llacer JL, Ramakrishnan V. Structural basis for the inhibition of translation through eIF2alpha phosphorylation. Nat Commun 2019;10(1):2640; doi: 10.1038/s41467-019-10606-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordiyenko Y, Schmidt C, Jennings MD, et al. eIF2B is a decameric guanine nucleotide exchange factor with a gamma2epsilon2 tetrameric core. Nat Commun 2014;5:3902; doi: 10.1038/ncomms4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SR, Mathews MB. Two RNA-binding motifs in the double-stranded RNA-activated protein kinase, DAI. Genes Dev 1992;6(12B):2478–2490; doi: 10.1101/gad.6.12b.2478 [DOI] [PubMed] [Google Scholar]
- Guo X, Aviles G, Liu Y, et al. Mitochondrial stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway. Nature 2020;579(7799):427–432; doi: 10.1038/s41586-020-2078-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A 1991;88(9):3720–3724; doi: 10.1073/pnas.88.9.3720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday M, Radford H, Sekine Y, et al. Partial restoration of protein synthesis rates by the small molecule ISRIB prevents neurodegeneration without pancreatic toxicity. Cell Death Dis 2015;6:e1672; doi: 10.1038/cddis.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han AP, Fleming MD, Chen JJ. Heme-regulated eIF2alpha kinase modifies the phenotypic severity of murine models of erythropoietic protoporphyria and beta-thalassemia. J Clin Invest 2005;115(6):1562–1570; doi: 10.1172/JCI24141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han AP, Yu C, Lu L, et al. Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J 2001;20:6909–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Back SH, Hur J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol 2013;15(5):481–490; doi: 10.1038/ncb2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JM, Jeong SJ, Park MC, et al. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149(2):410–424; doi: 10.1016/j.cell.2012.02.044 [DOI] [PubMed] [Google Scholar]
- Hao Q, Heo JM, Nocek BP, et al. Sugar phosphate activation of the stress sensor eIF2B. Nat Commun 2021;12(1):3440; doi: 10.1038/s41467-021-23836-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 2000a;6(5):1099–1108; doi: 10.1016/s1097-2765(00)00108-8 [DOI] [PubMed] [Google Scholar]
- Harding HP, Ordonez A, Allen F, et al. The ribosomal P-stalk couples amino acid starvation to GCN2 activation in mammalian cells. Elife 2019;8:e50149; doi: 10.7554/eLife.50149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, et al. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 2000b;5(5):897–904; doi: 10.1016/s1097-2765(00)80330-5 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999;397(6716):271–274; doi: 10.1038/16729 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 2003;11(3):619–633; doi: 10.1016/s1097-2765(03)00105-9 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. Translational regulation of Gcn4 and the general amino acid control of yeast. Annu Rev Microbiol 2005;59:407–450; doi: 10.1146/annurev.micro.59.031805.133833 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG. The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem 2014;83:779–812; doi: 10.1146/annurev-biochem-060713-035802 [DOI] [PubMed] [Google Scholar]
- Huggins CJ, Mayekar MK, Martin N, et al. C/EBPgamma is a critical regulator of cellular stress response networks through heterodimerization with ATF4. Mol Cell Biol 2015;36(5):693–713; doi: 10.1128/MCB.00911-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis AJ, Masson GR, Shao S, et al. Activation of GCN2 by the ribosomal P-stalk. Proc Natl Acad Sci U S A 2019;116(11):4946–4954; doi: 10.1073/pnas.1813352116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 2011;147(4):789–802; doi: 10.1016/j.cell.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimura R, Nagy G, Dotu I, et al. Activation of GCN2 kinase by ribosome stalling links translation elongation with translation initiation. Elife 2016;5:e14295; doi: 10.7554/eLife.14295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov IP, Shin BS, Loughran G, et al. Polyamine control of translation elongation regulates start site selection on antizyme inhibitor mRNA via ribosome queuing. Mol Cell 2018;70(2):254.e6–264.e6; doi: 10.1016/j.molcel.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings MD, Kershaw CJ, Adomavicius T, et al. Fail-safe control of translation initiation by dissociation of eIF2alpha phosphorylated ternary complexes. Elife 2017;6:e24542; doi: 10.7554/eLife.24542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings MD, Pavitt GD. eIF5 has GDI activity necessary for translational control by eIF2 phosphorylation. Nature 2010;465(7296):378–381; doi: 10.1038/nature09003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings MD, Pavitt GD. A new function and complexity for protein translation initiation factor eIF2B. Cell Cycle 2014;13(17):2660–2665; doi: 10.4161/15384101.2014.948797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HO, Seo SK, Woo SH, et al. Activating transcription factor 4 and CCAAT/enhancer-binding protein-beta negatively regulate the mammalian target of rapamycin via Redd1 expression in response to oxidative and endoplasmic reticulum stress. Free Radic Biol Med 2009;46(8):1158–1167; doi: 10.1016/j.freeradbiomed.2009.01.015 [DOI] [PubMed] [Google Scholar]
- Joshi MA, Jeoung NH, Obayashi M, et al. Impaired growth and neurological abnormalities in branched-chain alpha-keto acid dehydrogenase kinase-deficient mice. Biochem J 2006;400(1):153–162; doi: 10.1042/BJ20060869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C, Bruhat A, Carraro V, et al. Inhibition of CHOP translation by a peptide encoded by an open reading frame localized in the chop 5′UTR. Nucleic Acids Res 2001;29(21):4341–4351; doi: 10.1093/nar/29.21.4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C, Deval C, Maurin AC, et al. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem 2007;282(21):15851–15861; doi: 10.1074/jbc.M611723200 [DOI] [PubMed] [Google Scholar]
- Kang MJ, Vasudevan D, Kang K, et al. 4E-BP is a target of the GCN2-ATF4 pathway during Drosophila development and aging. J Cell Biol 2017;216(1):115–129; doi: 10.1083/jcb.201511073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagoz GE, Acosta-Alvear D, Nguyen HT, et al. An unfolded protein-induced conformational switch activates mammalian IRE1. Elife 2017;6:e30700; doi: 10.7554/eLife.30700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi K, Takahashi M, Nishimoto M, et al. Crystal structure of eukaryotic translation initiation factor 2B. Nature 2016;531(7592):122–125; doi: 10.1038/nature16991 [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Yokoyama T, Nishimoto M, et al. Structural basis for eIF2B inhibition in integrated stress response. Science 2019;364(6439):495–499; doi: 10.1126/science.aaw4104 [DOI] [PubMed] [Google Scholar]
- Kearse MG, Goldman DH, Choi J, et al. Ribosome queuing enables non-AUG translation to be resistant to multiple protein synthesis inhibitors. Genes Dev 2019;33(13–14):871–885; doi: 10.1101/gad.324715.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenner LR, Anand AA, Nguyen HC, et al. eIF2B-catalyzed nucleotide exchange and phosphoregulation by the integrated stress response. Science 2019;364(6439):491–495; doi: 10.1126/science.aaw2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y, Ishiwata-Kimata Y, Ito T, et al. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J Cell Biol 2007;179(1):75–86; doi: 10.1083/jcb.200704166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp MC, Larburu N, Durairaj V, et al. UPR proteins IRE1 and PERK switch BiP from chaperone to ER stress sensor. Nat Struct Mol Biol 2019;26(11):1053–1062; doi: 10.1038/s41594-019-0324-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Constraints on reinitiation of translation in mammals. Nucleic Acids Res 2001;29(24):5226–5232; doi: 10.1093/nar/29.24.5226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel C, Thompson B, Hustak S, et al. Overexpression of eIF5 or its protein mimic 5MP perturbs eIF2 function and induces ATF4 translation through delayed re-initiation. Nucleic Acids Res 2016;44(18):8704–8713; doi: 10.1093/nar/gkw559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhle B, Eulig NK, Ficner R. Architecture of the eIF2B regulatory subcomplex and its implications for the regulation of guanine nucleotide exchange on eIF2. Nucleic Acids Res 2015;43(20):9994–10014; doi: 10.1093/nar/gkv930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahr RM, Fonseca BD, Ciotti GE, et al. La-related protein 1 (LARP1) binds the mRNA cap, blocking eIF4F assembly on TOP mRNAs. Elife 2017;6:e24146; doi: 10.7554/eLife.24146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Liu B, Lee S, et al. Global mapping of translation initiation sites in mammalian cells at single-nucleotide resolution. Proc Natl Acad Sci U S A 2012;109(37):E2424–E2432; doi: 10.1073/pnas.1207846109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YY, Cevallos RC, Jan E. An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2α phosphorylation. J Biol Chem 2009;284:6661–6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater PA, Vermeulen G, Konst AA, et al. Subunits of the translation initiation factor eIF2B are mutant in leukoencephalopathy with vanishing white matter. Nat Genet 2001;29(4):383–388; doi: 10.1038/ng764 [DOI] [PubMed] [Google Scholar]
- Lehman SL, Cerniglia GJ, Johannes GJ, et al. Translational upregulation of an individual p21Cip1 transcript variant by GCN2 regulates cell proliferation and survival under nutrient stress. PLoS Genet 2015;11(6):e1005212; doi: 10.1371/journal.pgen.1005212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire PA, Anderson E, Lary J, et al. Mechanism of PKR activation by dsRNA. J Mol Biol 2008;381(2):351–360; doi: 10.1016/j.jmb.2008.05.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wang X, Van Der Knaap MS, et al. Mutations linked to leukoencephalopathy with vanishing white matter impair the function of the eukaryotic initiation factor 2B complex in diverse ways. Mol Cell Biol 2004;24(8):3295–3306; doi: 10.1128/MCB.24.8.3295-3306.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, May GE, Kready H, et al. Impacts of uORF codon identity and position on translation regulation. Nucleic Acids Res 2019;47(17):9358–9367; doi: 10.1093/nar/gkz681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 2020;21(4):183–203; doi: 10.1038/s41580-019-0199-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TK, Zhang YB, Liu Y, et al. Cooperative roles of fish protein kinase containing Z-DNA binding domains and double-stranded RNA-dependent protein kinase in interferon-mediated antiviral response. J Virol 2011;85(23):12769–12780; doi: 10.1128/JVI.05849-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llacer JL, Hussain T, Marler L, et al. Conformational differences between open and closed states of the eukaryotic translation initiation complex. Mol Cell 2015;59(3):399–412; doi: 10.1016/j.molcel.2015.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longchamp A, Mirabella T, Arduini A, et al. Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell 2018;173(1):117.e14–129.e14; doi: 10.1016/j.cell.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol 2004;167(1):27–33; doi: 10.1083/jcb.200408003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkonen BG, Tan W, Schwartz S. Efficiency of reinitiation of translation on human immunodeficiency virus type 1 mRNAs is determined by the length of the upstream open reading frame and by intercistronic distance. J Virol 1995;69(7):4086–4094; doi: 10.1128/JVI.69.7.4086-4094.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Vattem KM, Wek RC. Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem 2002a;277(21):18728–18735; doi: 10.1074/jbc.M200903200 [DOI] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, et al. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 2002b;318(5):1351–1365; doi: 10.1016/s0022-2836(02)00234-6 [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem 2003;278(37):34864–34873; doi: 10.1074/jbc.M301107200 [DOI] [PubMed] [Google Scholar]
- Marton MJ, Vazquez de Aldana CR, Qiu H, et al. Evidence that GCN1 and GCN20, translational regulators of GCN4, function on elongating ribosomes in activation of eIF2alpha kinase GCN2. Mol Cell Biol 1997;17(8):4474–4489; doi: 10.1128/MCB.17.8.4474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonsa S, von Kuegelgen N, Bujanic L, et al. Charcot-Marie-Tooth mutation in glycyl-tRNA synthetase stalls ribosomes in a pre-accommodation state and activates integrated stress response. Nucleic Acids Res 2021;49(17):10007–10017; doi: 10.1093/nar/gkab730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra J, Holmes MJ, Mirek ET, et al. Discordant regulation of eIF2 kinase GCN2 and mTORC1 during nutrient stress. Nucleic Acids Res 2021;49(10):5726–5742; doi: 10.1093/nar/gkab362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad MP, Smirnova A, Gunisova S, et al. eIF4G is retained on ribosomes elongating and terminating on short upstream ORFs to control reinitiation in yeast. Nucleic Acids Res 2021;49(15):8743–8756; doi: 10.1093/nar/gkab652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Fujihashi M, Aono R, et al. Dynamic, ligand-dependent conformational change triggers reaction of ribose-1,5-bisphosphate isomerase from Thermococcus kodakarensis KOD1. J Biol Chem 2012;287(25):20784–20796; doi: 10.1074/jbc.M112.349423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Nambu T, Ebara S, et al. Inhibition of GCN2 sensitizes ASNS-low cancer cells to asparaginase by disrupting the amino acid response. Proc Natl Acad Sci U S A 2018;115(33):E7776–E7785; doi: 10.1073/pnas.1805523115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri S, Carpick BW, Yang Y, et al. Structure of the double-stranded RNA-binding domain of the protein kinase PKR reveals the molecular basis of its dsRNA-mediated activation. EMBO J 1998;17(18):5458–5465; doi: 10.1093/emboj/17.18.5458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri S, Rahman F, Williams BR, et al. A dynamically tuned double-stranded RNA binding mechanism for the activation of antiviral kinase PKR. EMBO J 2000;19(20):5567–5574; doi: 10.1093/emboj/19.20.5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonorova IA, Mirek ET, Signore CC, et al. Time-resolved analysis of amino acid stress identifies eIF2 phosphorylation as necessary to inhibit mTORC1 activity in liver. J Biol Chem 2018;293(14):5005–5015; doi: 10.1074/jbc.RA117.001625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novarino G, El-Fishawy P, Kayserili H, et al. Mutations in BCKD-kinase lead to a potentially treatable form of autism with epilepsy. Science 2012;338(6105):394–397; doi: 10.1126/science.1224631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, et al. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 2001;153(5):1011–1022; doi: 10.1083/jcb.153.5.1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zhang Y, Zeng H, et al. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J 2003;22(5):1180–1187; doi: 10.1093/emboj/cdg112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohoka N, Yoshii S, Hattori T, et al. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J 2005;24(6):1243–1255; doi: 10.1038/sj.emboj.7600596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa D, Kimata Y, Kohno K, et al. Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp Cell Res 2009;315(15):2496–2504; doi: 10.1016/j.yexcr.2009.06.009 [DOI] [PubMed] [Google Scholar]
- Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem 2011;286(13):10939–10949; doi: 10.1074/jbc.M110.216093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavitt GD. Regulation of translation initiation factor eIF2B at the hub of the integrated stress response. Wiley Interdiscip Rev RNA 2018;9(6):e1491; doi: 10.1002/wrna.1491 [DOI] [PubMed] [Google Scholar]
- Pavitt GD, Ramaiah KV, Kimball SR, et al. eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev 1998;12(4):514–526; doi: 10.1101/gad.12.4.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe L, Vasseur JJ, Debart F, et al. La-related protein 1 (LARP1) repression of TOP mRNA translation is mediated through its cap-binding domain and controlled by an adjacent regulatory region. Nucleic Acids Res 2018;46(3):1457–1469; doi: 10.1093/nar/gkx1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochopien AA, Beckert B, Kasvandik S, et al. Structure of Gcn1 bound to stalled and colliding 80S ribosomes. Proc Natl Acad Sci U S A 2021;118(14):e2022756118; doi: 10.1073/pnas.2022756118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyry TA, Kaminski A, Connell EJ, et al. The mechanism of an exceptional case of reinitiation after translation of a long ORF reveals why such events do not generally occur in mammalian mRNA translation. Genes Dev 2007;21(23):3149–3162; doi: 10.1101/gad.439507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyry TA, Kaminski A, Jackson RJ. What determines whether mammalian ribosomes resume scanning after translation of a short upstream open reading frame? Genes Dev 2004;18(1):62–75; doi: 10.1101/gad.276504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouw HH, Langereis MA, Anand AA, et al. Small molecule ISRIB suppresses the integrated stress response within a defined window of activation. Proc Natl Acad Sci U S A 2019;116(6):2097–2102; doi: 10.1073/pnas.1815767116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafie-Kolpin M, Chefalo PJ, Hussain Z, et al. Two heme-binding domains of heme-regulated eukaryotic initiation factor-2alpha kinase. N terminus and kinase insertion. J Biol Chem 2000;275(7):5171–5178; doi: 10.1074/jbc.275.7.5171 [DOI] [PubMed] [Google Scholar]
- Ramirez M, Wek RC, Hinnebusch AG. Ribosome association of GCN2 protein kinase, a translational activator of the GCN4 gene of Saccharomyces cerevisiae. Mol Cell Biol 1991;11(6):3027–3036; doi: 10.1128/mcb.11.6.3027-3036.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JP, Mohammad SS, Pavitt GD. Mutations causing childhood ataxia with central nervous system hypomyelination reduce eukaryotic initiation factor 2B complex formation and activity. Mol Cell Biol 2004;24(6):2352–2363; doi: 10.1128/MCB.24.6.2352-2363.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenburg S, Deigendesch N, Dittmar K, et al. A PKR-like eukaryotic initiation factor 2alpha kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc Natl Acad Sci U S A 2005;102(5):1602–1607; doi: 10.1073/pnas.0408714102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenburg S, Georgiadis MS, Wek RC. Evolution of eIF2α Kinases: Adapting Translational Control to Diverse Stresses. In: Evolution of the Protein Synthesis Machinery and Its Regulation. (Hernández G, Jagus R. eds.) Springer; Cham: 2016; pp. 235–260. [Google Scholar]
- Roybal CN, Yang S, Sun CW, et al. Homocysteine increases the expression of vascular endothelial growth factor by a mechanism involving endoplasmic reticulum stress and transcription factor ATF4. J Biol Chem 2004;279(15):14844–14852; doi: 10.1074/jbc.M312948200 [DOI] [PubMed] [Google Scholar]
- Sattlegger E, Hinnebusch AG. Separate domains in GCN1 for binding protein kinase GCN2 and ribosomes are required for GCN2 activation in amino acid-starved cells. EMBO J 2000;19(23):6622–6633; doi: 10.1093/emboj/19.23.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattlegger E, Hinnebusch AG. Polyribosome binding by GCN1 is required for full activation of eukaryotic translation initiation factor 2{alpha} kinase GCN2 during amino acid starvation. J Biol Chem 2005;280(16):16514–16521; doi: 10.1074/jbc.M414566200 [DOI] [PubMed] [Google Scholar]
- Schleich S, Acevedo JM, Clemm von Hohenberg K, et al. Identification of transcripts with short stuORFs as targets for DENR*MCTS1-dependent translation in human cells. Sci Rep 2017;7(1):3722; doi: 10.1038/s41598-017-03949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleich S, Strassburger K, Janiesch PC, et al. DENR-MCT-1 promotes translation re-initiation downstream of uORFs to control tissue growth. Nature 2014;512(7513):208–212; doi: 10.1038/nature13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof M, Boone M, Wang L, et al. eIF2B conformation and assembly state regulate the integrated stress response. Elife 2021;10:e65703; doi: 10.7554/eLife.65703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Zyryanova A, Crespillo-Casado A, et al. Stress responses. Mutations in a translation initiation factor identify the target of a memory-enhancing compound. Science 2015;348(6238):1027–1030; doi: 10.1126/science.aaa6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senee V, Vattem KM, Delepine M, et al. Wolcott-Rallison syndrome: Clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes 2004;53(7):1876–1883; doi: 10.2337/diabetes.53.7.1876 [DOI] [PubMed] [Google Scholar]
- She P, Bunpo P, Cundiff JK, et al. General control nonderepressible 2 (GCN2) kinase protects oligodendrocytes and white matter during branched-chain amino acid deficiency in mice. J Biol Chem 2013;288(43):31250–31260; doi: 10.1074/jbc.M113.498469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol 1998;18(12):7499–7509; doi: 10.1128/MCB.18.12.7499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu XE, Mao Y, Jia L, et al. Dynamic eIF3a O-GlcNAcylation controls translation reinitiation during nutrient stress. Nat Chem Biol 2022;18(2):134–141; doi: 10.1038/s41589-021-00913-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidrauski C, Acosta-Alvear D, Khoutorsky A, et al. Pharmacological brake-release of mRNA translation enhances cognitive memory. Elife 2013;2:e00498; doi: 10.7554/eLife.00498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CR, Glineburg MR, Moore C, et al. Human oncoprotein 5MP suppresses general and repeat-associated non-AUG translation via eIF3 by a common mechanism. Cell Rep 2021;36(2):109376; doi: 10.1016/j.celrep.2021.109376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CR, Lee B, Udagawa T, et al. An eIF5/eIF2 complex antagonizes guanine nucleotide exchange by eIF2B during translation initiation. EMBO J 2006;25(19):4537–4546; doi: 10.1038/sj.emboj.7601339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CR, Udagawa T, Lee B, et al. Change in nutritional status modulates the abundance of critical pre-initiation intermediate complexes during translation initiation in vivo. J Mol Biol 2007;370(2):315–330; doi: 10.1016/j.jmb.2007.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snieckute G, Genzor AV, Vind AC, et al. Ribosome stalling is a signal for metabolic regulation by the ribotoxic stress response. Cell Metab 2022;34(12):2036.e8–2046.e8; doi: 10.1016/j.cmet.2022.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood R, Porter AC, Ma K, et al. Pancreatic eukaryotic initiation factor-2alpha kinase (PEK) homologues in humans, Drosophila melanogaster and Caenorhabditis elegans that mediate translational control in response to endoplasmic reticulum stress. Biochem J 2000;346(Pt 2):281–293. [PMC free article] [PubMed] [Google Scholar]
- Spaulding EL, Hines TJ, Bais P, et al. The integrated stress response contributes to tRNA synthetase-associated peripheral neuropathy. Science 2021;373(6559):1156–1161; doi: 10.1126/science.abb3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suragani RN, Zachariah RS, Velazquez JG, et al. Heme-regulated eIF2alpha kinase activated Atf4 signaling pathway in oxidative stress and erythropoiesis. Blood 2012;119(22):5276–5284; doi: 10.1182/blood-2011-10-388132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb Perspect Biol 2011;3(5):a004440; doi: 10.1101/cshperspect.a004440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tcherkezian J, Cargnello M, Romeo Y, et al. Proteomic analysis of cap-dependent translation identifies LARP1 as a key regulator of 5′TOP mRNA translation. Genes Dev 2014;28(4):357–371; doi: 10.1101/gad.231407.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske BF, Wek SA, Bunpo P, et al. The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell 2011;22(22):4390–4405; doi: 10.1091/mbc.E11-06-0510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrence ME, MacArthur MR, Hosios AM, et al. The mTORC1-mediated activation of ATF4 promotes protein and glutathione synthesis downstream of growth signals. Elife 2021;10:e63326; doi: 10.7554/eLife.63326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai JC, Miller-Vedam LE, Anand AA, et al. Structure of the nucleotide exchange factor eIF2B reveals mechanism of memory-enhancing molecule. Science 2018;359(6383):eaaq0939; doi: 10.1126/science.aaq0939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejo M, Ron D, Miller CP, et al. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci U S A 1993;90(10):4679–4683; doi: 10.1073/pnas.90.10.4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan D, Neuman SD, Yang A, et al. Translational induction of ATF4 during integrated stress response requires noncanonical initiation factors eIF2D and DENR. Nat Commun 2020;11(1):4677; doi: 10.1038/s41467-020-18453-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 2004;101(31):11269–11274; doi: 10.1073/pnas.0400541101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Herrmannova A, Hronova V, et al. Selective translation complex profiling reveals staged initiation and co-translational assembly of initiation factor complexes. Mol Cell 2020;79(4):546.e7–560.e7; doi: 10.1016/j.molcel.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011;334(6059):1081–1086; doi: 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- Wang J, Shin BS, Alvarado C, et al. Rapid 40S scanning and its regulation by mRNA structure during eukaryotic translation initiation. Cell 2022;185(24):4474.e17–4487.e17; doi: 10.1016/j.cell.2022.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Li J, Tao J, et al. The luminal domain of the ER stress sensor protein PERK binds misfolded proteins and thereby triggers PERK oligomerization. J Biol Chem 2018;293(11):4110–4121; doi: 10.1074/jbc.RA117.001294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Paulin FE, Campbell LE, et al. Eukaryotic initiation factor 2B: Identification of multiple phosphorylation sites in the epsilon-subunit and their functions in vivo. EMBO J 2001;20(16):4349–4359; doi: 10.1093/emboj/20.16.4349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watatani Y, Ichikawa K, Nakanishi N, et al. Stress-induced translation of ATF5 mRNA is regulated by the 5′-untranslated region. J Biol Chem 2008;283(5):2543–2553; doi: 10.1074/jbc.M707781200 [DOI] [PubMed] [Google Scholar]
- Wek RC, Anthony TG. Beta testing the antioxidant function of eIF2alpha phosphorylation in diabetes prevention. Cell Metab 2009;10(1):1–2; doi: 10.1016/j.cmet.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Wek RC, Jackson BM, Hinnebusch AG. Juxtaposition of domains homologous to protein kinases and histidyl-tRNA synthetases in GCN2 protein suggests a mechanism for coupling GCN4 expression to amino acid availability. Proc Natl Acad Sci U S A 1989;86(12):4579–4583; doi: 10.1073/pnas.86.12.4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wek SA, Zhu S, Wek RC. The histidyl-tRNA synthetase-related sequence in the eIF-2 alpha protein kinase GCN2 interacts with tRNA and is required for activation in response to starvation for different amino acids. Mol Cell Biol 1995;15:4497–4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh GI, Proud CG. Glycogen synthase kinase-3 is rapidly inactivated in response to insulin and phosphorylates eukaryotic initiation factor eIF-2B. Biochem J 1993;294(Pt 3):625–629; doi: 10.1042/bj2940625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney ML, Jefferson LS, Kimball SR. ATF4 is necessary and sufficient for ER stress-induced upregulation of REDD1 expression. Biochem Biophys Res Commun 2009;379(2):451–455; doi: 10.1016/j.bbrc.2008.12.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DD, Price NT, Loughlin AJ, et al. Characterization of the mammalian initiation factor eIF2B complex as a GDP dissociation stimulator protein. J Biol Chem 2001;276(27):24697–24703; doi: 10.1074/jbc.M011788200 [DOI] [PubMed] [Google Scholar]
- Willy JA, Young SK, Mosley AL, et al. Function of inhibitor of Bruton's tyrosine kinase isoform alpha (IBTKalpha) in nonalcoholic steatohepatitis links autophagy and the unfolded protein response. J Biol Chem 2017;292(34):14050–14065; doi: 10.1074/jbc.M117.799304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YL, LeBon L, Basso AM, et al. eIF2B activator prevents neurological defects caused by a chronic integrated stress response. Elife 2019;8:e42940; doi: 10.7554/eLife.42940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YL, LeBon L, Edalji R, et al. The small molecule ISRIB rescues the stability and activity of Vanishing White Matter Disease eIF2B mutant complexes. Elife 2018;7:e32733; doi: 10.7554/eLife.32733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods YL, Cohen P, Becker W, et al. The kinase DYRK phosphorylates protein-synthesis initiation factor eIF2Bepsilon at Ser539 and the microtubule-associated protein tau at Thr212: Potential role for DYRK as a glycogen synthase kinase 3-priming kinase. Biochem J 2001;355(Pt 3):609–615; doi: 10.1042/bj3550609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Peterson A, Zinshteyn B, et al. Ribosome collisions trigger general stress responses to regulate cell fate. Cell 2020;182(2):404.e14–416.e14; doi: 10.1016/j.cell.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Dai W, Kutzler L, et al. ATF4-mediated upregulation of REDD1 and Sestrin2 suppresses mTORC1 activity during prolonged leucine deprivation. J Nutr 2020;150(5):1022–1030; doi: 10.1093/jn/nxz309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Ishihara H, Yamada T, et al. ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab 2008;7(3):269–276; doi: 10.1016/j.cmet.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Yan LL, Zaher HS. Ribosome quality control antagonizes the activation of the integrated stress response on colliding ribosomes. Mol Cell 2021;81(3):614.e4–628.e4; doi: 10.1016/j.molcel.2020.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JB, Palm W, Peng M, et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev 2015;29(22):2331–2336; doi: 10.1101/gad.269324.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Baird TD, Wek RC. Translation regulation of the glutamyl-prolyl-tRNA synthetase gene EPRS through bypass of upstream open reading frames with noncanonical initiation codons. J Biol Chem 2016a;291(20):10824–10835; doi: 10.1074/jbc.M116.722256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Palam LR, Wu C, et al. Ribosome elongation stall directs gene-specific translation in the integrated stress response. J Biol Chem 2016b;291(12):6546–6558; doi: 10.1074/jbc.M115.705640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Wek RC. Upstream open reading frames differentially regulate gene-specific translation in the integrated stress response. J Biol Chem 2016;291(33):16927–16935; doi: 10.1074/jbc.R116.733899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SK, Willy JA, Wu C, et al. Ribosome reinitiation directs gene-specific translation and regulates the integrated stress response. J Biol Chem 2015;290(47):28257–28271; doi: 10.1074/jbc.M115.693184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Baird SK, Lourenco MB, Elder MK, et al. Suppression of MEHMO syndrome mutation in eIF2 by small molecule ISRIB. Mol Cell 2020;77(4):875.e7–886.e7; doi: 10.1016/j.molcel.2019.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yu Z, Gao J, et al. Inhibition of GCN2 alleviates hepatic steatosis and oxidative stress in obese mice: Involvement of NRF2 regulation. Redox Biol 2022;49:102224; doi: 10.1016/j.redox.2021.102224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Guo S, Gao J, et al. General control nonderepressible 2 (GCN2) kinase inhibits target of rapamycin complex 1 in response to amino acid starvation in Saccharomyces cerevisiae. J Biol Chem 2017;292(7):2660–2669; doi: 10.1074/jbc.M116.772194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborske JM, Narasimhan J, Jiang L, et al. Genome-wide analysis of tRNA charging and activation of the eIF2 kinase Gcn2p. J Biol Chem 2009;284(37):25254–25267; doi: 10.1074/jbc.M109.000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa F, Muniozguren NL, Wilson MZ, et al. Signaling by the integrated stress response kinase PKR is fine-tuned by dynamic clustering. J Cell Biol 2022;221(7):e202111100; doi: 10.1083/jcb.202111100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Y, Wu X, et al. Determinants of genome-wide distribution and evolution of uORFs in eukaryotes. Nat Commun 2021;12(1):1076; doi: 10.1038/s41467-021-21394-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Macias-Garcia A, Ulirsch JC, et al. HRI coordinates translation necessary for protein homeostasis and mitochondrial function in erythropoiesis. Elife 2019;8:e46976; doi: 10.7554/eLife.46976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Palam LR, Jiang L, et al. Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J Biol Chem 2008;283(11):7064–7073; doi: 10.1074/jbc.M708530200 [DOI] [PubMed] [Google Scholar]
- Zhou F, Zhang H, Kulkarni SD, et al. eIF1 discriminates against suboptimal initiation sites to prevent excessive uORF translation genome-wide. RNA 2020;26(4):419–438; doi: 10.1261/rna.073536.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wan J, Shu XE, et al. N(6)-methyladenosine guides mRNA alternative translation during integrated stress response. Mol Cell 2018;69(4):636.e7–647.e7; doi: 10.1016/j.molcel.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Wek RC. Ribosome-binding domain of eukaryotic initiation factor-2 kinase GCN2 facilitates translation control. J Biol Chem 1998;273(3):1808–1814; doi: 10.1074/jbc.273.3.1808 [DOI] [PubMed] [Google Scholar]
- Zyryanova AF, Kashiwagi K, Rato C, et al. ISRIB blunts the integrated stress response by allosterically antagonising the inhibitory effect of phosphorylated eIF2 on eIF2B. Mol Cell 2021;81(1):88.e6–103.e6; doi: 10.1016/j.molcel.2020.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]