Abstract
Solute binding proteins (SBPs) are of central physiological relevance for prokaryotes. These proteins present substrates to transporters, but they also stimulate different signal transduction receptors. SBPs form a superfamily of at least 33 protein Pfam families. To assess possible links between SBP sequence and the ligand recognized, we have inspected manually all SBP three‐dimensional structures deposited in the protein data bank and retrieved 748 prokaryotic structures that have been solved in complex with bound ligand. These structures were classified into 26 SBP Pfam families. The analysis of the ligands recognized revealed that most families possess a preference for a compound class. There were three families each that bind preferentially saccharides and amino acids. In addition, we identified families that bind preferentially purines, quaternary amines, iron and iron‐chelating compounds, oxoanions, bivalent metal ions or phosphates. Phylogenetic analyses suggest convergent evolutionary events that lead to families that bind the same ligand. The functional link between chemotaxis and compound uptake is reflected in similarities in the ligands recognized by SBPs and chemoreceptors. Associating Pfam families with ligand profiles will be of help to design experimental strategies aimed at the identification of ligands for uncharacterized SBPs.
Solute binding proteins (SBPs) are of central physiological relevance for bacteria and form a super‐family composed of at least 33 Pfam families. Based on the analysis of the totality of currently available 3D holo‐SBP structures, we show that most families preferentially bind members of a ligand family. This information will be helpful to identify the ligands recognized by uncharacterised SBPs.

INTRODUCTION
Solute binding proteins (SBPs) form a protein superfamily that is composed of at least 33 individual protein families in the Pfam database (Mistry et al., 2021). SBPs are found in all kingdoms of life (Scheepers et al., 2016). In Gram‐positive bacteria and archaea, they are tethered to the external face of the membrane, whereas in Gram‐negative bacteria they are present as diffusible proteins in the periplasm (Chu & Vogel, 2011). SBPs differ largely in size (20–65 kDa), but share the same topology consisting of two lobes connected by a hinge (Fukamizo et al., 2019). SBPs bind different ligands at a conserved binding site that is formed by both lobes of the protein.
The type of ligand recognized by SBPs is almost unlimited, including amino‐ and organic acids, peptides, sugars, polyamines, quaternary amines, purines, metal ions, oxoanions, vitamins, quorum sensing signals and different siderophores (Matilla et al., 2021). Bacteria typically contain many SBP genes. We have recently published the SBP repertoire of 49 bacterial model strains that possess on average 60 ± 49 SBP genes, which frequently represent several percent of their genomes (Ortega et al., 2022). In addition, a proteomics study has shown that the cellular level of many SBPs is very high (Matilla et al., 2023).
This abundance of SBPs is a reflection of the multiple functions of this protein superfamily. First, the main function of SBPs is to present different solutes to transmembrane transporters to initiate compound uptake (Gautom et al., 2021; Luo et al., 2021; Sakanaka et al., 2019). Second, solute‐loaded SBPs also bind to different transmembrane receptors such as chemoreceptors, sensor histidine kinases, protein kinases or diguanylate cyclases/phosphodiesterases to stimulate signal transduction cascades (Bhattacharyya et al., 2018; Carey et al., 2018; Lopes & Sourjik, 2018), permitting the coordination of transport with signal transduction. SBP‐mediated receptor stimulation appears to be a universal and widespread mechanism, and the elucidation of the role of SBPs in signal transduction is currently a major research effort (Matilla et al., 2021). Third, there is strong evidence that SBPs carry out additional functions that are currently poorly understood. One such example is the phosphate‐specific PstS SBP of Pseudomonas aeruginosa, which apart from presenting inorganic phosphate to the PstABC transporter (Peng et al., 2017) and stimulating the phosphate‐specific chemoreceptor CtpL (Rico‐Jimenez et al., 2016), also form an appendage‐like structure on the cell surface that contributes to multi‐drug resistance (Zaborina et al., 2008) and biofilm formation (Neznansky et al., 2014). SBPs are also of significant biotechnological relevance. They can serve as precursors for enzyme evolution (Clifton et al., 2018; Noda‐Garcia & Tawfik, 2020) and are used for biosensor construction (Smith et al., 2022; Tavares & van der Meer, 2021; Vongsouthi et al., 2021).
In a previous study, we have reported the ligands that are recognized by sensor domains of chemoreceptors, sensor kinases and transcriptional regulators belonging to 127 Pfam families, information that has been included into the Pfam database (Matilla, Velando, Martín‐Mora, et al., 2022). This study has been very well received by the scientific community and the primary objective of the present study is to define the ligand profiles of the individual SBP Pfam families.
Previous studies have shown that structural alignments of all available 3D SBP structures resulted in the identification of clusters that showed a preference for a ligand or family of ligands (Berntsson et al., 2010; Scheepers et al., 2016). Here, we have assessed whether the ligand specificity is reflected in sequence similarity, as defined by the classification of SBPs into different Pfam families (Mistry et al., 2021). In fact, the inspection of the information currently associated with the 33 SBP families in Pfam, provides either no or little information on the ligands recognized by family members. In the present study, we have manually inspected all of SBP three‐dimensional (3D) structures deposited in the protein data bank (pdb) (Burley et al., 2019), retrieved the ligands bound to all prokaryotic proteins and classified proteins according to their Pfam family. We show here that many SBP families possess a clear preference for a ligand family. This work will help to annotate the function of other members of this important protein superfamily.
EXPERIMENTAL PROCEDURES
Identification and classification of ligands bound to SBP 3D structures
The pdb ID codes of 3D structures for each of the SBP families deposited in the pdb (Burley et al., 2019) were retrieved from the Pfam database (Mistry et al., 2021). Each of the structures was inspected, and the ligand bound to the ligand‐binding site between the two lobes of the structure was identified. The publications (if available) associated with each structure in pdb were consulted to confirm that a given ligand is of functional relevance.
Ligands have then been classified into groups and subgroups (Table S1). To calculate the relative abundance of ligands for a given protein family, the totality of ligands observed in a given family was set as 100%. If a given ligand was bound to multiple structures, each of the structures was included for the total ligand count. The relative abundance of ligands as shown in Figures 1, 2, 3, 4 is the ratio of the number of individual ligands or members of ligand families relative to the total number of ligands per family.
FIGURE 1.

SBP families that preferentially recognize saccharides. The ligands that bind to the 3D structures of family members deposited in the protein data bank are shown. Data for the SBP_bac_1, Peripla_BP_4 and SBP_bac_8 families are based on 37, 44 and 30 different proteins respectively (Table S1).
FIGURE 2.
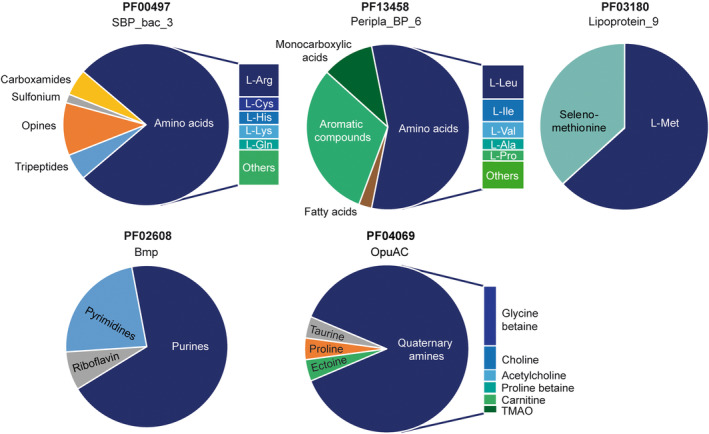
SBP families that preferentially bind different amines. The ligands that bind to the 3D structures of family members deposited in the protein data bank are shown. Data on the SBP_bac_3, Peripla_BP_6, Lipoprotein_9, Bmp and OpuAC families are based on 40, 21, 19, 7 and 14 different proteins respectively (Table S1).
FIGURE 3.

SBP families that preferentially recognize iron and iron‐containing compounds, oxoanions, bivalent metal anions and phosphates. The ligands that bind to the 3D structures of family members deposited in the protein data bank are shown. Data on the Peripla_BP_2, SBP_bac_6, SBP_bac_11, ZnuA, PBP_like_2 and Phosphonate_bd are based on 25, 6, 12, 20, 13 and 7 different proteins respectively (Table S1).
FIGURE 4.

The SBP families without clear ligand preferences. The ligands that bind to the 3D structures of family members deposited in the protein data bank are shown. Data on the DctP and SBP_bac_5 families are based on 58 and 28 proteins respectively (Table S1).
Construction of the phylogenetic tree
SBP sequences were extracted from UniprotKB (UniProt Consortium, 2023) with a custom Python script implementing the Bioservices (Cokelaer et al., 2013) and Biopython (Cock et al., 2009) packages. For each protein, a query from the main pdb code was made to Uniprot. FASTA sequence files, the Uniprot entry ID, gene locus and PFAM family were extracted for each protein. Sequences were then aligned using the MAFFT NS‐I algorithm (Katoh et al., 2019) and subsequently used for the construction of the phylogenetic tree using Fast Tree (JTT + CAT) with a Shimodaira‐Hasegawa test for phylogeny (Price et al., 2010). Both tools were obtained from the TREND pipeline (Gumerov & Zhulin, 2020). The phylogenetic tree was represented by iTOL (Letunic & Bork, 2021).
RESULTS AND DISCUSSION
The superfamily of SBPs is comprised of at least 33 individual Pfam protein families (Table S2). The individual pdb entries were inspected manually to extract structures of prokaryotic stand‐alone SBPs that have been solved in complex with bound ligand (holo‐structures). Protein families from eukaryotes (e.g. ANF_receptor, ABC_transp_aux) and families in which SBPs form sensor domains of receptors (e.g. Peripla_BP_3) were excluded (Table S2). For another four families, no holo‐structures were available (Table S2). In total, 748 prokaryotic holo‐SBP structures belonging to 26 SBP families were retrieved (Tables S1 and S2). A number of structures were of the same protein crystallized with different ligands (Table S1). In total, ligand‐bound 3D structures were obtained for 403 different proteins that belonged to 175 different organisms. Protein ligands were extracted manually (Table S1) and SBPs classified according to their protein family. The ligand range of populated SBP families is depicted in Figures 1, 2, 3, 4, whereas ligands that bind to less‐populated families are listed in Table 1.
TABLE 1.
List of less‐populated SBP families.
| Pfam protein family | No. of proteins | Ligands bound | |
|---|---|---|---|
| Code | Family name | ||
| PF00532 | Peripla_BP_1 | 4 | Monosaccharides, quaternary amines |
| PF03401 | TctC | 5 | Malate, citrate, L‐Asp, L‐Glu, 2‐oxoadipic acid, hexanedionic acid |
| PF04392 | ABC_sub_bind | 3 | L‐Trp, L‐Tyr, L‐Phe |
| PF05048 | NosD | 2 | Difructose, L‐kestose |
| PF06646 | CypI | 2 | Vitamin B1 |
| PF09084 | NMT1 | 6 | Thiamine, aminopyrimidine |
| PF12727 | PBP_like | 2 | Phosphate |
| PF13433 | Peripla_BP_5 | 1 | Amide |
| PF16868 | NMT1_3 | 1 | Glutamic acid |
| PF18610 | Peripla_BP_7 | 1 | D‐Glucose |
Note: The ligands bound to the corresponding 3D structures are listed.
A significant number of populated SBP families showed a clear preference for a compound family. These families bind preferentially saccharides (Figure 1), amino acids, purines, quaternary amines (Figure 2), iron and iron‐chelating compounds, oxoanions, metal ions or phosphate (Figure 3). In contrast, the well‐populated families, DctP and SBP_bac_5, did not show a clear ligand preference (Figure 4).
Three SBP families have a ligand preference for mono‐, di‐ and oligosaccharides
Saccharides and amino acids appear to be the primary ligand families for SBPs (Table 2). Members of 10 different SBP families were found to bind saccharides (Table 2). There were three SBP families, SBP_bac_1, SBP_bac_8 and Peripla_BP_4, that showed a clear ligand preference for saccharides (Figure 1, Table S1). With 37, 30 and 44 proteins, respectively, these three families were well‐populated. The families SBP_bac_1 and SBP_bac_8 showed a wide ligand spectrum as they bind mono‐, di‐ and oligosaccharides, whereas family Perpla_BP_4 binds exclusively monosaccharides and the quorum sensing signal autoinducer‐2.
TABLE 2.
Link between SBP‐mediated compound uptake and chemotaxis.
| SBP | Chemoreceptor (selection) | ||||
|---|---|---|---|---|---|
| Ligand family | Protein families | Name | Species | Ligand recognized | Reference |
| Mono‐, di‐ and oligosaccharides | SBP_bac_1, SBP_bac_5, SBP_bac_6, SBP_bac_8, SBP_bac_11, Peripla_BP_1, Peripla_BP_4, Peripla_BP_7, DctP, NosD | Tlp2‐4 | Campylobacter jejuni | Mannose, fucose, galactose | Taha et al. (2022) |
| Tlp11 | C. jejuni | Galactose | Feng et al. (2022) | ||
| McpA | Bacillus velezensis | Ribose, ribitol, mannose, fucose | Day et al. (2016) | ||
| Tar | Escherichia. coli | Maltose (via MBP SBP) | Zhang et al. (1999) | ||
| Monocarboxylic acids | DctP, Peripla_BP_6 | McpV | Sinorhizobium meliloti | Propionate, acetate, glycolate | Compton et al. (2018) |
| Dicarboxylic acids | DctP, TctC | McpS | Pseudomonas putida | Malate, fumarate, oxaloacetate a | Lacal et al. (2010) |
| Amino acids (including non‐proteinogenic amino acids) | SBP_bac_3, SBP_bac_8, Peripla_BP_6, Lipoprotein_9, TctC, ABC_sub_bind, NMT1_3, DctP, Peripla_BP_2, SBP_bac_11, OpuAC | PctA/PctB | Pseudomonas aeruginosa | Multiple amino acids | Gavira et al. (2020) |
| McpC | Bacillus subtilis | Multiple amino acids | Glekas et al. (2012) | ||
| McpU | S. meliloti | Multiple amino acids | Webb, Compton, Del Campo, et al. (2017) | ||
| Phosphate and derivatives | PBP_like_2, Phosphonate_bd, PBP_like | CtpH | P. aeruginosa | Phosphate | Rico‐Jimenez et al. (2016) |
| CtpL | P. aeruginosa | Phosphate (via PstS SBP) | Rico‐Jimenez et al. (2016) | ||
| Purines/pyrimidines | Bmp, NMT1 | McpH | P. putida | Uric acid, purine, adenine a | Fernández et al. (2016) |
| Tap | E. coli | Thymine, uracil (probably via SBP) | Liu and Parales (2008) | ||
| Oxoanions | SBP_bac_1, SBP_bac_11, Peripla_BP_2, PBP_like_2, Phosphonate_bd | AFM38850 | Agrobacterium tumefaciens | Arsenite | Shi et al. (2017) |
| SO_1056 | Shewanella oneidensis | Chromate | Boyeldieu et al. (2021) | ||
| Quaternary amines | OpuAC, Peripla_BP_1, SBP_bac_1 | McpX | S. meliloti | Choline, glycine betaine a | Webb, Compton, Castañeda Saldaña, et al. (2017) |
| PctD | P. aeruginosa | Choline, acetylcholine, betaine | Matilla, Velando, Tajuelo, et al. (2022) | ||
| Polyamines | SBP_bac_8 | TlpQ | P. aeruginosa | Spermidine, putrescine a | Corral‐Lugo et al. (2018) |
| Metal ions and metal chelating compounds | Peripla_BP_2, SBP_bac_1, SBP_bac_5, SBP_bac_6, SBP_bac_8, SBP_bac_11, ZnuA, DctP | SO_1056 | S. oneidensis | Ni2+ (repellent) | Boyeldieu et al. (2022) |
| Peptides | SBP_bac_5, SBP_bac_3, DctP | Tap | E. coli | Various dipeptides (via DppA SBP) | Manson et al. (1986) |
| Autoinducer‐2 | Peripla_BP_4 | PctA/TlpQ | P. aeruginosa | Autoinducer‐2 | Zhang et al. (2020) |
| Tsr | E. coli | Autoinducer‐2 (via LsrB SBP) | Hegde et al. (2011) | ||
Note: The major ligand families of SBPs and the corresponding SBP protein families are shown on the left. Representative examples for chemoreceptors that respond to SBP ligand families are listed on the right.
A selection is given, compounds with highest affinity.
With 44 different proteins, Peripla_BP_4 is one of the most populated families. Although it is specific for monosaccharides, family members bind 27 different sugars. Thus, a diversity of monosaccharides are recognized. The most abundant monosaccharide recognized by this family was glucose, with seven SBPs (Table S1). Around 15% of the Peripla_BP_4 structures had autoinducer‐2 bound (Figure 1 and Table S1). Autoinducer‐2 shows structural similarities with furanose, which may explain its recognition by this SBP family. Autoinducer‐2 is a central quorum sensing signal (Pereira et al., 2013), and SBPs that recognize this compound may interact with signal transduction receptors.
Three SBP families bind preferentially amino acids
Proteins belonging to 11 SBP families were found to bind amino acids (Table 2 and Table S1). Among them, families SBP_bac_3 and Peripla_BP_6 bind a range of different amino acids, whereas Lipoprotein_9 binds exclusively L‐Met and its close derivative selenomethionine (Figure 2 and Table S1). Clear amino acid preferences emerged for the SBP_bac_3 and Peripla_BP_6 families. Whereas SBP_bac_3 proteins bind preferentially amino acids with positive change (L‐Arg, L‐Lys and L‐His), Peripla_BP_6 binds preferentially the three amino acids with a branched side chain (L‐Leu, L‐Val and L‐Ile). Lipoprotein_9 was one of the families with the narrowest ligand spectrum (Figure 2 and Table S1). All 19 proteins of this family recognize exclusively L‐Met or selenomethionine.
The bacterial hosts for these proteins show a wide phylogenetic distribution, including different Gram‐positive and Gram‐negative strains (Table S1). Selenomethionine is a naturally occurring amino acid, and several bacteria metabolize this compound (Stolz et al., 2006). Selenomethionine mimics closely the properties of L‐Met, and selenomethionine‐containing proteins are frequently used to resolve the crystallographic phase problem using multi‐wavelength anomalous diffraction techniques (Wenzel et al., 2019).
Almost all bacterial species possess biosynthetic pathways for methionine (Ferla & Patrick, 2014). L‐Met is the only amino acid that is specifically recognized by a SBP protein family, suggesting a particular physiological relevance that may be related to the fact that this compound is also required for the synthesis of S‐adenosylmethionine, the main cellular carrier of methyl groups (Ferla & Patrick, 2014). L‐Met appears to be sensed almost exclusively by this family since there was only one other family, Peripla_BP_6, that recognizes L‐Met (Table S1). Based on the evidence that this is a methionine‐specific family, we propose to rename the Lipoprotein_9 family SBP_Met.
Many bacteria possess amino acid racemases that interconvert the L‐ and D‐ forms of amino acids (Miyamoto & Homma, 2021). Of the 61 amino acid‐responsive SBPs, there were only three proteins that bind a D‐isomer [pdb ID 4F3S & 4JB7, unpublished; pdb ID 4NAP, (Vetting et al., 2015)]. This preference for L‐amino acids agrees with data on amino acid‐responsive chemoreceptors that also recognize primarily L‐isomers (Matilla, Velando, Martín‐Mora, et al., 2022).
Families bmp and OpuAC bind preferentially purine/pyrimidine containing compounds or quaternary amines
Although the Bmp family comprises only seven members, there was a clear ligand preference for purines (adenine, guanine) and pyrimidines (uridine, thymidine) (Figure 2 and Table S1). In addition, there were several purine nucleosides (adenosine, guanosine and inosine) among the ligands (Table S1). Riboflavin (vitamin B2) was the only ligand that did not match this pattern. However, part of the riboflavin structure shares strong similarities with the purine skeleton. Two additional families, Peripla_BP_2 and NMT1, were also found to bind riboflavin and thymidine, respectively (Table S1).
Quaternary amines contain a positively charged nitrogen atom linked to four carbon atoms. Many physiologically relevant quaternary amines possess a trimethylated amino group. Family OpuAC showed a clear preference for quaternary amines (Figure 2). Most abundant are family members that recognize glycine betaine, followed by choline, acetylcholine, proline and carnitine (Table S1). These compounds are important osmo‐ and thermo‐protectants (Caldas et al., 1999; Chen & Beattie, 2008; Meadows & Wargo, 2015) or intermediates in their synthesis (Yang et al., 2022). Glycine betaine can reach a cellular concentration of up to 1 M in cells subjected to osmotic stress, and it has been shown to stabilize proteins (Singh et al., 2009). A single protein of the SBP_bac_1 family [pdb ID 6R1B, (Fenn et al., 2019)] binds quaternary amines (Table S1).
Two SBP families for iron acquisition
Iron is a critical nutrient for the growth and survival of most bacterial species (Frawley & Fang, 2014). There are two well‐populated SBP families for the acquisition of iron, Peripla_BP_2 and SBP_bac_6. Whereas most Peripla_BP_2 members bind a range of different iron‐chelating compounds, SBP_bac_6 members bind iron ions directly (Figure 3 and Table S1). The most abundant ligand (seven proteins) of the Peripla_BP_2 family is protoporphyrin IX, which is ubiquitously present in small amounts in all living cells as a precursor for haeme synthesis (Sachar et al., 2016). The other large group of ligands for this family are siderophores, which are iron‐scavenging molecules produced by many bacteria (Kramer et al., 2020). Examples of such compounds recognized by Peripla_BP_2 members are ferrichrome, gallichrome, staphyloferrin B, ferrioxamine B and E, staphyloferrin A and ferri‐Bacillibactin (Table S1).
Families SBP_bac_11 and ZnuA bind preferentially metal oxoanions or metal ions
The primary ligands for family SBP_bac_11 are different metal oxoanions like molybdate, tungstate, chromate and perrhenate (Figure 3). About half of the family members recognize molybdate and/or tungstate. Despite the close chemical and physical similarities of both of these oxoanions, there are SBPs that were able to recognize specifically one or the other, whereas other SBPs recognize both of them (Aguilar‐Barajas et al., 2011; Andreesen & Makdessi, 2008). Several family members bind the structurally related sulphate and phosphate ions. Furthermore, a number of phosphorylated compounds like 3‐phosphoglycerate, phosphoenolpyruvate and glucose‐6‐phosphate were identified as ligands (Table S1), suggesting that the recognition of the phosphate moiety is essential for binding. SBP_bac_1, DctP, PBP_like_2 and Phosphonate_bd are additional families that recognize oxoanions like tungstate, molybdate and arsenate (Figure 3 and Table S1).
Twenty different proteins of the ZnuA family were retrieved (Table S1). The ligand profile of this family is very well defined since all proteins recognize metal ions. Almost half of the family members bind Zn2+ ions. Whereas Zn2+, Mn2+ and Fe2+ had a similar molecular weight, Cd2+ ions are significantly heavier. Many ZnuA family members bind specifically a single metal ion. Some proteins of the families SBP_bac_5, Peripla_BP‐2, SBP‐bac_1, DctP, SBP_bac_6, SBP_bac_8 and SBP_bac_11 also bind different metal ions, such as Ni2+, Co2+, Zn2+, Fe3+ or Zr4+ (Table S1).
Two SBP families recognize phosphorous containing compounds
Phosphorous is an essential macroelement and is required for many different biological processes. Almost all PBP_like_2 family members recognize inorganic phosphate, phosphate, whereas two of them also bind arsenate and tungstate (Figure 3). As indicated above, both of these oxoanions are structurally similar to phosphate and the toxicity of arsenate is due to its binding to phosphate‐binding proteins (Dani, 2011). According to the Pfam database, the Phosphonate_bd family binds alkylphosphonates. Our analysis shows that the ligand range of this family is much larger and includes different phosphates, phosphites and pyrophosphates (Table S1, Figure 3). There were only two proteins, PhnD1 and PhnD, that recognize the alkylphosphonates ethylphosphonate and 2‐aminoethylphosphonate, respectively. One protein in the low‐populated family PBP_like also binds phosphate (Table S1).
DctP and SBP_bac_5 lack a clear ligand preference
Not all SBP families possess a clear ligand preference. This is exemplified by the DctP family, which with 58 members, is the most populated protein family. As shown in Figure 4, there was no clear ligand preference. In addition to different sugars and carboxylic acids, the DcpT family binds ligands as diverse as zinc and calcium ions, dipeptides, ectoine, the auxin indole‐3‐acetic acid, glycerol‐3‐phosphate and N‐acetyl‐neuraminic acid (Table S1). In total, there were three families that bind peptides (Table 2). However, the primary family for peptide recognition was SBP_bac_5, for which peptides represent almost half of the ligands recognized (Figure 4). SBP_bac_5 family members are rather permissive for peptide length, with ligands ranging from dipeptides to nonapeptides (Table S1). In addition, members of this family bind a number of structurally very diverse ligands including Ni2+, glutathione, different sugars, haemin or EDTA (Figure 4 and Table S1).
Evidence for convergent SBP evolution
We constructed a phylogenetic tree of the proteins analysed in this study (Figure 5). Among the three families that recognize preferentially saccharides, members of the SBP_bac_1 and SBP_bac_8 families were interwoven (Figure 5). In contrast, the third saccharide binding family, Peripla_BP_4, was phylogenetically distant from the former two families, suggesting convergent evolution for saccharide binding. Similar observations were made for the amino acid sensing SBPs, in which Peripla_BP_6 was distant to the two vicinal families, Lipoprotein_9 and SBP_bac_3. In addition, the phosphate‐binding families PBP_like_2 and Phosphonate_bd, were also phylogenetically distant. The tree shown in Figure 5 is based on sequence similarity. The inspection of a tree constructed on structural similarity (Scheepers et al., 2016) is also consistent with the notion of convergent evolutionary processes.
FIGURE 5.

Phylogenetic tree of SBPs analysed in this study. Branches have been coloured according to the corresponding PFAM family. Families that show a preference for ligands in the same compound family are boxed: red: saccharides, orange: amino acids, green: phosphate and related compounds. The phylogenetic tree was taken from iTOL.
Parallelism between SBP and chemoreceptor ligand profiles
The primary function of SBPs is to present solutes to transport proteins to initiate their uptake. Chemotaxis permits bacteria to access compounds of interest to promote their uptake. Data support that accessing nutrients and, more generally, locating niches that are optimal for growth are the major functions of bacterial chemotaxis (Colin et al., 2021). Typically, chemotaxis is initiated by the direct recognition of chemoeffectors by chemoreceptor sensor domains or the binding of signal‐loaded SBPs (Matilla et al., 2021). Because gaining access via chemotaxis and uptake of compounds are functionally linked, we compared SBP and chemoreceptor ligand profiles. In Table 2, we show that for every major SBP ligand family, there are chemoreceptors that respond to the same ligands. In most cases, chemoreceptors recognize chemoeffectors directly, indicating that there are sensor domains with similar ligand specificities as SBPs. Relatively few chemoreceptors rely on the recognition of ligand‐loaded SBPs (Table 2).
CONCLUSIONS
Previous studies have established a link between SBP structure and ligand range (Berntsson et al., 2010; Scheepers et al., 2016). In structural alignments of all available SBP 3D structures, clusters were defined that are specific for members of a ligand family. In the present study, we have investigated whether there is a link between SBP sequence and the type of ligand recognized. For the majority of SBP Pfam families, we report here the ligand profile. Currently, there is no or very scare information on the ligands recognized in the SBP family entries at the Pfam database (Mistry et al., 2021). In analogy to our previous study on ligand recognition by chemoreceptors, sensor kinases and transcriptional regulators (Matilla, Velando, Martín‐Mora, et al., 2022), the information on ligands recognized by SBPs will be introduced into the Pfam database, which will permit the scientific community to gain rapid first information on the ligands that may be recognized by an uncharacterized SBP. Such information will permit to rationally orient efforts to experimentally identify the ligand of an uncharacterized SBP. Chemotaxis permits bacteria to assess compounds of interest that are subsequently taken up by transporters. Chemotaxis and transport are thus functionally linked. Although the structural basis for ligand recognition at SBPs and chemoreceptors is different, we show here that for every major class of SBP ligands, there are characterized chemoreceptors that bind to the same ligands, revealing major environmental cues that have driven the co‐evolution of transport systems and chemoreceptors.
AUTHOR CONTRIBUTIONS
Jean Paul Cerna‐Vargas: Data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing – review and editing (equal). Beatriz Sánchez‐Romera: Data curation (equal); formal analysis (equal); writing – review and editing (equal). Miguel A. Matilla: Conceptualization; funding acquisition; project administration; writing – review and editing. Álvaro Ortega: Data curation (equal); formal analysis (equal); funding acquisition (equal); investigation (equal); project administration (equal); writing – review and editing (equal). Tino Krell: Conceptualization (equal); funding acquisition (equal); project administration (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
This work was supported by the Spanish Ministry for Science and Innovation/Agencia Estatal de Investigación 10.13039/501100011033 (grants PID2020‐112612GB‐I00 to TK, PID2019‐103972GA‐I00 to MAM and PID2021‐122202OB‐I00 to AO) and the Junta de Andalucía (grant P18‐FR‐1621 to TK). JPCV was supported by the grant Unión Europea‐NextGeneration EU RD 289/2021 UPM‐Recualifica Margarita Salas.
CONFLICT OF INTEREST STATEMENT
The authors do not declare a conflict of interest.
Supporting information
Table S1
Table S2
Cerna‐Vargas, J.P. , Sánchez‐Romera, B. , Matilla, M.A. , Ortega, Á. & Krell, T. (2023) Sensing preferences for prokaryotic solute binding protein families. Microbial Biotechnology, 16, 1823–1833. Available from: 10.1111/1751-7915.14292
Contributor Information
Jean Paul Cerna‐Vargas, Email: jeanpaul.cerna@eez.csic.es.
Tino Krell, Email: tino.krell@eez.csic.es.
REFERENCES
- Aguilar‐Barajas, E. , Díaz‐Pérez, C. , Ramírez‐Díaz, M.I. , Riveros‐Rosas, H. & Cervantes, C. (2011) Bacterial transport of sulfate, molybdate, and related oxyanions. Biometals, 24, 687–707. [DOI] [PubMed] [Google Scholar]
- Andreesen, J.R. & Makdessi, K. (2008) Tungsten, the surprisingly positively acting heavy metal element for prokaryotes. Annals of the new York Academy of Sciences, 1125, 215–229. [DOI] [PubMed] [Google Scholar]
- Berntsson, R.P. , Smits, S.H. , Schmitt, L. , Slotboom, D.J. & Poolman, B. (2010) A structural classification of substrate‐binding proteins. FEBS Letters, 584, 2606–2617. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya, N. , Nkumama, I.N. , Newland‐Smith, Z. , Lin, L.‐Y. , Yin, W. , Cullen, R.E. et al. (2018) An aspartate‐specific solute‐binding protein regulates protein kinase G activity to control glutamate metabolism in mycobacteria. mBio, 9, e00931‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyeldieu, A. , Ali Chaouche, A. , Mejean, V. & Jourlin‐Castelli, C. (2021) Combining two optimized and affordable methods to assign chemoreceptors to a specific signal. Analytical Biochemistry, 620, 114139. [DOI] [PubMed] [Google Scholar]
- Boyeldieu, A. , Poli, J. , Ali Chaouche, A. , Fierobe, H. , Giudici‐Orticoni, M. , Méjean, V. et al. (2022) Multiple detection of both attractants and repellents by the dCache‐chemoreceptor SO_1056 of Shewanella oneidensis. The FEBS Journal, 289, 6752–6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley, S.K. , Berman, H.M. , Bhikadiya, C. , Bi, C. , Chen, L. , Di Costanzo, L. et al. (2019) RCSB Protein Data Bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Research, 47, D464–D474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldas, T. , Demont‐Caulet, N. , Ghazi, A. & Richarme, G. (1999) Thermoprotection by glycine betaine and choline. Microbiology (Reading), 145(Pt 9), 2543–2548. [DOI] [PubMed] [Google Scholar]
- Carey, J.N. , Mettert, E.L. , Roggiani, M. , Myers, K.S. , Kiley, P.J. & Goulian, M. (2018) Regulated stochasticity in a bacterial signaling network permits tolerance to a rapid environmental change. Cell, 173, 196–207.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. & Beattie, G.A. (2008) Pseudomonas syringae BetT is a low‐affinity choline transporter that is responsible for superior osmoprotection by choline over glycine betaine. Journal of Bacteriology, 190, 2717–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, B.C. & Vogel, H.J. (2011) A structural and functional analysis of type III periplasmic and substrate binding proteins: their role in bacterial siderophore and heme transport. Biological Chemistry, 392, 39–52. [DOI] [PubMed] [Google Scholar]
- Clifton, B.E. , Kaczmarski, J.A. , Carr, P.D. , Gerth, M.L. , Tokuriki, N. & Jackson, C.J. (2018) Evolution of cyclohexadienyl dehydratase from an ancestral solute‐binding protein. Nature Chemical Biology, 14, 542–547. [DOI] [PubMed] [Google Scholar]
- Cock, P.J.A. , Antao, T. , Chang, J.T. , Chapman, B.A. , Cox, C.J. , Dalke, A. et al. (2009) Biopython: freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics, 25, 1422–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cokelaer, T. , Pultz, D. , Harder, L.M. , Serra‐Musach, J. & Saez‐Rodriguez, J. (2013) BioServices: a common Python package to access biological Web Services programmatically. Bioinformatics, 29, 3241–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin, R. , Ni, B. , Laganenka, L. & Sourjik, V. (2021) Multiple functions of flagellar motility and chemotaxis in bacterial physiology. FEMS Microbiology Reviews, 45, fuab038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton, K.K. , Hildreth, S.B. , Helm, R.F. & Scharf, B.E. (2018) Sinorhizobium meliloti chemoreceptor McpV senses short‐chain carboxylates via direct binding. Journal of Bacteriology, 200, e00519‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral‐Lugo, A. , Matilla, M.A. , Martín‐Mora, D. , Silva Jiménez, H. , Mesa Torres, N. , Kato, J. et al. (2018) High‐affinity chemotaxis to histamine mediated by the TlpQ chemoreceptor of the human pathogen Pseudomonas aeruginosa. MBio, 9, e01894‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani, S.U. (2011) The arsenic for phosphorus swap is accidental, rather than a facultative one, and the question whether arsenic is nonessential or toxic is quantitative, not a qualitative one. Science of the Total Environment, 409, 4889–4890. [DOI] [PubMed] [Google Scholar]
- Day, C.J. , King, R.M. , Shewell, L.K. , Tram, G. , Najnin, T. , Hartley‐Tassell, L.E. et al. (2016) A direct‐sensing galactose chemoreceptor recently evolved in invasive strains of Campylobacter jejuni. Nature Communications, 7, 13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, H. , Lv, Y. , Krell, T. , Fu, R. , Liu, Y. , Xu, Z. et al. (2022) Signal binding at both modules of its dCache domain enables the McpA chemoreceptor of Bacillus velezensis to sense different ligands. Proceedings of the National Academy of Sciences of the United States of America, 119, e2201747119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn, J.S. , Nepravishta, R. , Guy, C.S. , Harrison, J. , Angulo, J. , Cameron, A.D. et al. (2019) Structural basis of glycerophosphodiester recognition by the mycobacterium tuberculosis substrate‐binding protein UgpB. ACS Chemical Biology, 14, 1879–1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferla, M.P. & Patrick, W.M. (2014) Bacterial methionine biosynthesis. Microbiology, 160, 1571–1584. [DOI] [PubMed] [Google Scholar]
- Fernández, M. , Morel, B. , Corral‐Lugo, A. & Krell, T. (2016) Identification of a chemoreceptor that specifically mediates chemotaxis toward metabolizable purine derivatives. Molecular Microbiology, 99, 34–42. [DOI] [PubMed] [Google Scholar]
- Frawley, E.R. & Fang, F.C. (2014) The ins and outs of bacterial iron metabolism. Molecular Microbiology, 93, 609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukamizo, T. , Kitaoku, Y. & Suginta, W. (2019) Periplasmic solute‐binding proteins: structure classification and chitooligosaccharide recognition. International Journal of Biological Macromolecules, 128, 985–993. [DOI] [PubMed] [Google Scholar]
- Gautom, T. , Dheeman, D. , Levy, C. , Butterfield, T. , Alvarez Gonzalez, G. , Le Roy, P. et al. (2021) Structural basis of terephthalate recognition by solute binding protein TphC. Nature Communications, 12, 6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavira, J.A. , Gumerov, V.M. , Rico‐Jiménez, M. , Petukh, M. , Upadhyay, A.A. , Ortega, A. et al. (2020) How bacterial chemoreceptors evolve novel ligand specificities. MBio, 11, e03066‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glekas, G.D. , Mulhern, B.J. , Kroc, A. , Duelfer, K.A. , Lei, V. , Rao, C.V. et al. (2012) The Bacillus subtilis chemoreceptor McpC senses multiple ligands using two discrete mechanisms. The Journal of Biological Chemistry, 287, 39412–39418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumerov, V.M. & Zhulin, I.B. (2020) TREND: a platform for exploring protein function in prokaryotes based on phylogenetic, domain architecture and gene neighborhood analyses. Nucleic Acids Research, 48, W72–W76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde, M. , Englert, D.L. , Schrock, S. , Cohn, W.B. , Vogt, C. , Wood, T.K. et al. (2011) Chemotaxis to the quorum‐sensing signal AI‐2 requires the Tsr chemoreceptor and the periplasmic LsrB AI‐2‐binding protein. Journal of Bacteriology, 193, 768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , Rozewicki, J. & Yamada, K.D. (2019) MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, J. , Özkaya, Ö. & Kümmerli, R. (2020) Bacterial siderophores in community and host interactions. Nature Reviews. Microbiology, 18, 152–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal, J. , Alfonso, C. , Liu, X. , Parales, R.E. , Morel, B. , Conejero‐Lara, F. et al. (2010) Identification of a chemoreceptor for tricarboxylic acid cycle intermediates: differential chemotactic response towards receptor ligands. The Journal of Biological Chemistry, 285, 23126–23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic, I. & Bork, P. (2021) Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Research, 49, W293–W296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. & Parales, R.E. (2008) Chemotaxis of Escherichia coli to pyrimidines: a new role for the signal transducer tap. Journal of Bacteriology, 190, 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, J.G. & Sourjik, V. (2018) Chemotaxis of Escherichia coli to major hormones and polyamines present in human gut. The ISME Journal, 12, 2736–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, Z. , Morey, J.R. , Deplazes, E. , Motygullina, A. , Tan, A. , Ganio, K. et al. (2021) A Trap‐Door Mechanism for Zinc Acquisition by Streptococcus pneumoniae AdcA. MBio, 12, e01958‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson, M.D. , Blank, V. , Brade, G. & Higgins, C.F. (1986) Peptide chemotaxis in E. coli involves the Tap signal transducer and the dipeptide permease. Nature, 321, 253–256. [DOI] [PubMed] [Google Scholar]
- Matilla, M.A. , Genova, R. , Martín‐Mora, D. , Maaβ, S. , Becher, D. & Krell, T. (2023) The cellular abundance of chemoreceptors, chemosensory signaling proteins, sensor histidine kinases, and solute binding proteins of Pseudomonas aeruginosa provides insight into sensory preferences and signaling mechanisms. International Journal of Molecular Sciences, 24, 1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla, M.A. , Ortega, Á. & Krell, T. (2021) The role of solute binding proteins in signal transduction. Computational and Structural Biotechnology Journal, 19, 1786–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla, M.A. , Velando, F. , Martín‐Mora, D. , Monteagudo‐Cascales, E. & Krell, T. (2022) A catalogue of signal molecules that interact with sensor kinases, chemoreceptors and transcriptional regulators. FEMS Microbiology Reviews, 46, fuab043. [DOI] [PubMed] [Google Scholar]
- Matilla, M.A. , Velando, F. , Tajuelo, A. , Martín‐Mora, D. , Xu, W. , Sourjik, V. et al. (2022) Chemotaxis of the human pathogen Pseudomonas aeruginosa to the neurotransmitter acetylcholine. MBio, 13, e0345821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows, J.A. & Wargo, M.J. (2015) Carnitine in bacterial physiology and metabolism. Microbiology, 161, 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry, J. , Chuguransky, S. , Williams, L. , Qureshi, M. , Salazar, G.A. , Sonnhammer, E.L.L. et al. (2021) Pfam: the protein families database in 2021. Nucleic Acids Research, 49, D412–D419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, T. & Homma, H. (2021) D‐Amino acid metabolism in bacteria. Journal of Biochemistry, 170, 5–13. [DOI] [PubMed] [Google Scholar]
- Neznansky, A. , Blus‐Kadosh, I. , Yerushalmi, G. , Banin, E. & Opatowsky, Y. (2014) The Pseudomonas aeruginosa phosphate transport protein PstS plays a phosphate‐independent role in biofilm formation. FASEB Journal, 28, 5223–5233. [DOI] [PubMed] [Google Scholar]
- Noda‐Garcia, L. & Tawfik, D.S. (2020) Enzyme evolution in natural products biosynthesis: target‐ or diversity‐oriented? Current Opinion in Chemical Biology, 59, 147–154. [DOI] [PubMed] [Google Scholar]
- Ortega, Á. , Matilla, M.A. & Krell, T. (2022) The repertoire of solute‐binding proteins of model bacteria reveals large differences in number, type, and ligand range. Microbiology Spectrum, 10, e02054‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, Y.C. , Lu, C. , Li, G. , Eichenbaum, Z. & Lu, C.D. (2017) Induction of the pho regulon and polyphosphate synthesis against spermine stress in Pseudomonas aeruginosa. Molecular Microbiology, 104, 1037–1051. [DOI] [PubMed] [Google Scholar]
- Pereira, C.S. , Thompson, J.A. & Xavier, K.B. (2013) AI‐2‐mediated signalling in bacteria. FEMS Microbiology Reviews, 37, 156–181. [DOI] [PubMed] [Google Scholar]
- Price, M.N. , Dehal, P.S. & Arkin, A.P. (2010) FastTree 2—approximately maximum‐likelihood trees for large alignments. PLoS One, 5, e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico‐Jimenez, M. , Reyes‐Darias, J.A. , Ortega, A. , Diez Pena, A.I. , Morel, B. & Krell, T. (2016) Two different mechanisms mediate chemotaxis to inorganic phosphate in Pseudomonas aeruginosa. Scientific Reports, 6, 28967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachar, M. , Anderson, K.E. & Ma, X. (2016) Protoporphyrin IX: the good, the bad, and the ugly. The Journal of Pharmacology and Experimental Therapeutics, 356, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka, M. , Hansen, M.E. , Gotoh, A. , Katoh, T. , Yoshida, K. , Odamaki, T. et al. (2019) Evolutionary adaptation in fucosyllactose uptake systems supports bifidobacteria‐infant symbiosis. Science Advances, 5, eaaw7696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers, G.H. , Lycklama, A.N.J.A. & Poolman, B. (2016) An updated structural classification of substrate‐binding proteins. FEBS Letters, 590, 4393–4401. [DOI] [PubMed] [Google Scholar]
- Shi, K. , Fan, X. , Qiao, Z. , Han, Y. , McDermott, T.R. , Wang, Q. et al. (2017) Arsenite oxidation regulator AioR regulates bacterial chemotaxis towards arsenite in agrobacterium tumefaciens GW4. Scientific Reports, 7, 43252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, L.R. , Dar, T.A. , Rahman, S. , Jamal, S. & Ahmad, F. (2009) Glycine betaine may have opposite effects on protein stability at high and low pH values. Biochimica et Biophysica Acta, 1794, 929–935. [DOI] [PubMed] [Google Scholar]
- Smith, D.D. , Girodat, D. , Abbott, D.W. & Wieden, H.‐J. (2022) Construction of a highly selective and sensitive carbohydrate‐detecting biosensor utilizing Computational Identification of Non‐disruptive Conjugation sites (CINC) for flexible and streamlined biosensor design. Biosensors and Bioelectronics, 200, 113899. [DOI] [PubMed] [Google Scholar]
- Stolz, J.F. , Basu, P. , Santini, J.M. & Oremland, R.S. (2006) Arsenic and selenium in microbial metabolism. Annual Review of Microbiology, 60, 107–130. [DOI] [PubMed] [Google Scholar]
- Taha, N. , Elgamoudi, B.A. , Andrianova, E.P. , Haselhorst, T. , Day, C.J. , Hartley‐Tassell, L.E. et al. (2022) Diverse sensory repertoire of paralogous chemoreceptors Tlp2, Tlp3, and Tlp4 in Campylobacter jejuni. Microbiol Spectr, 10, e0364622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares, D. & van der Meer, J.R. (2021) Ribose‐binding protein mutants with improved interaction towards the non‐natural ligand 1,3‐cyclohexanediol. Frontiers in Bioengineering and Biotechnology, 9, 705534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UniProt Consortium . (2023) UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Research, 51, D523–D531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetting, M.W. , Al‐Obaidi, N. , Zhao, S. , San Francisco, B. , Kim, J. , Wichelecki, D.J. et al. (2015) Experimental strategies for functional annotation and metabolism discovery: targeted screening of solute binding proteins and unbiased panning of metabolomes. Biochemistry, 54, 909–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vongsouthi, V. , Whitfield, J.H. , Unichenko, P. , Mitchell, J.A. , Breithausen, B. , Khersonsky, O. et al. (2021) A rationally and computationally designed fluorescent biosensor for d‐serine. ACS Sensors, 6, 4193–4205. [DOI] [PubMed] [Google Scholar]
- Webb, B.A. , Compton, K.K. , Del Campo, J.S.M. , Taylor, D. , Sobrado, P. & Scharf, B.E. (2017) Sinorhizobium meliloti chemotaxis to multiple amino acids is mediated by the chemoreceptor McpU. Molecular Plant‐Microbe Interactions, 30, 770–777. [DOI] [PubMed] [Google Scholar]
- Webb, B.A. , Karl Compton, K. , Castaneda Saldana, R. , Arapov, T.D. , Keith Ray, W. , Helm, R.F. et al. (2017) Sinorhizobium meliloti chemotaxis to quaternary ammonium compounds is mediated by the chemoreceptor McpX. Molecular Microbiology, 103, 333–346. [DOI] [PubMed] [Google Scholar]
- Wenzel, S. , Imasaki, T. & Takagi, Y. (2019) A practical method for efficient and optimal production of Seleno‐methionine‐labeled recombinant protein complexes in the insect cells. Protein Science, 28, 808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, N. , Ding, R. & Liu, J. (2022) Synthesizing glycine betaine via choline oxidation pathway as an osmoprotectant strategy in Haloferacales. Gene, 847, 146886. [DOI] [PubMed] [Google Scholar]
- Zaborina, O. , Holbrook, C. , Chen, Y. , Long, J. , Zaborin, A. , Morozova, I. et al. (2008) Structure‐function aspects of PstS in multi‐drug‐resistant Pseudomonas aeruginosa. PLoS Pathogens, 4, e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Li, S. , Liu, X. , Wang, Z. , Jiang, M. , Wang, R. et al. (2020) Sensing of autoinducer‐2 by functionally distinct receptors in prokaryotes. Nature Communications, 11, 5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Gardina, P.J. , Kuebler, A.S. , Kang, H.S. , Christopher, J.A. & Manson, M.D. (1999) Model of maltose‐binding protein/chemoreceptor complex supports intrasubunit signaling mechanism. Proceedings of the National Academy of Sciences of the United States of America, 96, 939–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Table S2


