Abstract
Recombinant human growth hormone (rhGH) and GH receptor antagonists (GHAs) are used clinically to treat a range of disorders associated with GH deficiency or hypersecretion, respectively. However, these biotherapeutics can be difficult and expensive to manufacture with multiple challenges from recombinant protein generation through to the development of long‐acting formulations required to improve the circulating half‐life of the drug. In this review, we summarize methodologies and approaches used for making and purifying recombinant GH and GHA proteins, and strategies to improve pharmacokinetic and pharmacodynamic properties, including PEGylation and fusion proteins. Therapeutics that are in clinical use or are currently under development are also discussed.
Keywords: growth hormone, antagonist, recombinant protein production, long‐acting, PEGylation, fusion protein, biotherapeutic
1. BACKGROUND
Human growth hormone (hGH) is a nonglycosylated 22 kDa single‐chain peptide hormone secreted from the anterior pituitary that functions as a key promoter of postnatal longitudinal growth by inducing bone growth and affecting protein, lipid, and carbohydrate metabolism. Recombinant hGH (rhGH) therapy has been shown to be beneficial for the treatment of GH deficiency (GHD) in both adults and children and has been in clinical use since the 1980s for a range of disorders. rhGH is administered to adults with GHD, adults with catabolic illnesses, older adults with decreased GH secretion, and children with GHD, idiopathic short stature, chronic renal insufficiency, Prader–Willi syndrome, small for gestational age, short stature due to homeobox gene deficiency, and short bowel syndrome (Danowitz & Grimberg, 2022; Franklin & Geffner, 2011; Kirk, 2012; Vance & Mauras, 1999). rhGH has a very short half‐life in the circulation due to renal clearance, and thus daily subcutaneous injections are required (Webster et al., 2008). The current standard daily regimen for rhGH presents challenges in terms of adherence, especially for long‐term use and patient adherence to daily rhGH therapy may decline over time. In recent years, several long‐acting rhGH formulations have been released to the market, which have the potential to decrease the burden of daily injections and enhance patients' compliance with the therapy (Steiner et al., 2023). The global market for hGH was valued at 3.8 billion USD in 2020 and is expected to increase to 9.2 billion USD by 2030.
Conversely, GH hypersecretion results in gigantism in childhood and a condition called acromegaly in adults (Colao et al., 2019) that can be treated with the GH receptor (GHR) antagonist, pegvisomant (described below). Increased GH signaling has also been implicated in tumor growth and progression and is elevated in certain cancers. As a result, there has been increasing interest in developing GH antagonists (GHAs) as potential cancer therapies (Brooks & Waters, 2010; Chesnokova & Melmed, 2019; Kopchick et al., 2022; Lu et al., 2019).
The aim of this review is to summarize the approaches that have been used to generate long‐acting GHR agonists and antagonists in the lab and clinical setting. Strategies to increase recombinant protein expression and purification, and to improve pharmacokinetic/pharmacodynamic properties are summarized including protein PEGylation (i.e. covalently attaching polyethylene glycol [PEG]) and fusion. We also discuss clinical applications and therapeutics that are in clinical use or are currently under development.
1.1. GH structure
22 kDa hGH consists of 191 amino acids with two intramolecular disulfide bonds at Cys53–Cys165 and Cys182–Cys189 (Kopchick, 2003). A single hGH ligand binds to two receptor molecules (Figure 1a). The hGH molecule contains two distinct binding sites with different affinities for the GHR; Site 1 is a high‐affinity binding site whereas Site 2 has lower affinity for the receptor. Upon GH‐GHR binding, a rotational change in the transmembrane domain of the receptor occurs, resulting in transphosphorylation and activation of the downstream signaling pathways, such as the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (Brooks et al., 2014). From the crystal structure, the tertiary structure of this hormone is an antiparallel four‐helix bundle molecule organized in an up–up–down–down manner (de Vos et al., 1992; Figure 1b). A disulfide bridge between Cys53 and Cys165 links the connection between Helices I and II and Helix IV. The second disulfide bridge between Cys182 and Cys189 forms a small loop in the C‐terminus (Junnila & Kopchick, 2013). Both disulfide bridges are well conserved across species and important for protein folding and stability (Connors et al., 1973). Some studies have suggested that the disulfide bond between Cys53 and Cys165 is important for the biological potency of hGH and is required for activation of the JAK/STAT pathway, whereas the disulfide bond between Cys182 and Cys189 may only modestly impact the biological activity of hGH. But, removal of the Cys182‐Cys189 disulfide bridge does decrease protein stability and binding affinity for the GHR (Junnila et al., 2013; Junnila & Kopchick, 2013).
FIGURE 1.
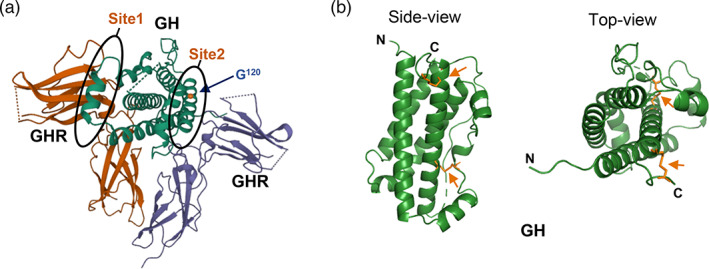
Crystal structure human growth hormone (hGH). (a) 1:2 complex of hGH (green) associated with a growth hormone receptor (GHR) homodimer (orange and purple), with binding sites and residue G120 indicated (PDB ID 1HWG). (b) Side and top view of hGH (PDB ID 1HGU). Disulfide bonds are indicated by an arrow.
1.2. Pegvisomant, a GHR antagonist
The GHR antagonist, pegvisomant (Somavert; Pfizer), was initially discovered during attempts to improve the growth‐promoting activity of bovine Gh. The crystal structure of GH highlighted the amphiphilic properties of the third α‐helix (109–126 in bovine Gh and 110–127 in hGH; Kopchick et al., 2014). A peptide containing the third α‐helix was found to promote growth (Hara et al., 1978). However, three amino acids at Positions 117, 119, and 122 of bovine Gh were not ideal for amphiphilic helix formation. The initial hypothesis was that substituting these three residues would improve the amphiphilic property of the third helix and would increase the bioactivity of GH. Pulsatile secretion of hGH from the pituitary normally stimulates the production and secretion of hepatic insulin‐like growth factor 1 (IGF‐1), but surprisingly, transgenic mice expressing a mutant bovine Gh gene with the substitution of E117L, G119R, and A122D had reduced circulating IGF‐1 concentrations and exhibited a dwarf phenotype (Yang et al., 1993). Further studies confirmed that G119 of bovine Gh was the critical amino acid involved in growth promotion (Campbell et al., 1993). The glycine residue at Position 120 of hGH corresponds to G119 of bovine Gh, and substitution of glycine 120 to lysine/arginine in hGH resulted in similar inhibitory activity (Chen et al., 1990; Thirone et al., 2002). This amino acid substitution is the basis of pegvisomant, which is a PEGylated protein antagonist (Kopchick et al., 2002; van der Lely & Kopchick, 2006). The protein component of pegvisomant is called B2036. It incorporates the G120 K substitution and 8 additional mutations at binding site 1 (H18D, H21N, R167N, K168A, D171S, K172R, E174S, and I179T). These substitutions serve two functions; they increase the binding affinity at site 1 and remove two potential PEGylation sites in the binding site (K168A and K172R), as PEGylation at these sites would be expected to interfere with ligand binding. B2036 can competitively bind to the GHR, but does not activate it, thereby inhibiting the GHR signal transduction.
1.3. Current and potential therapeutic indications for GH and GHA
rhGH was originally used for replacement therapy in children and adults deficient for hGH. GHD in children is a rare disorder that can arise from genetic causes (congenital) or acquired through damage or injury, but often also arises spontaneously with no known cause (idiopathic GHD; Ayuk, 2006).
GHD in adults and children is treated using GH replacement. Currently, this involves daily injection of rhGH (Yuen et al., 2021). In children with GHD, rhGH treatment induces linear growth and the aim of treatment is for the patients to be within the normal height range by adulthood (Pfäffle, 2015; Yuen et al., 2021). GH replacement has many beneficial effects on body composition and bone turnover in adults (Carroll et al., 2000). It has also been shown to help improve quality of life, reduce cardiovascular risk factors, increase left ventricular mass, and improve cardiac performance (Carroll et al., 2000).
More recently, the number of clinical applications for hGH has expanded considerably. rhGH has also been used to treat adults with catabolic illnesses, older adults with decreased GH secretion, chronic renal insufficiency, idiopathic short stature, short stature due to homeobox gene deficiency, Prader–Willi syndrome, Noonan Syndrome, small for gestational age, short bowel syndrome, and women undergoing in vitro fertilization (Danowitz & Grimberg, 2022; Franklin & Geffner, 2011; Kirk, 2012; Shang et al., 2022; Vance & Mauras, 1999).
Hypersecretion of GH in adults, after epiphyseal closure causes acromegaly (Colao et al., 2019; Petrossians et al., 2017). Acromegaly is characterized by disproportionate skeletal, tissue, and organ growth and patients with acromegaly present with changes to their limbs and facial structure, increased perspiration, headaches, paresthesia (pins and needles), sexual dysfunction, hypertension, and goiter (Colao et al., 2019; Petrossians et al., 2017). In around 98% of cases acromegaly is the result of GH hypersecretion by benign pituitary adenomas (Hannah‐Shmouni et al., 2016). Pituitary adenomas arise from clonal expansion of a mutated anterior pituitary cell and most acromegaly‐causing pituitary adenomas consist of mature somatotrophs that only produce GH (Chanson & Salenave, 2008). Treatment for acromegaly includes somatostatin receptor ligands, dopamine agonists, and the GHR antagonist, pegvisomant.
There has also been increasing interest in the application of GHAs as cancer therapeutics (Wang et al., 2023). B2036 and pegvisomant have been shown to have anticancer activity in cancer cells and in vivo in cancer xenograft models (Bougen et al., 2012; Dagnaes‐Hansen et al., 2004; Divisova et al., 2006; Evans et al., 2016; Friend et al., 1999; Kaulsay et al., 2001; Lempereur et al., 2003; Unterberger et al., 2022). Pegvisomant has not been assessed in clinical trials for oncology applications. However, a Phase I study evaluated and compared the efficacy of pegvisomant and octreotide by assessing pharmacodynamic biomarkers associated with GH activity (Yin et al., 2007). Pegvisomant dosed at high s.c. doses daily for 14 days was found to be well tolerated and was more efficacious than octreotide in suppressing the GH axis (Yin et al., 2007). Given the wealth of preclinical data supporting GH‐signaling as an anticancer target, further clinical trials in this area will be of interest. The clinical and preclinical indications for rhGH and GHA are summarized in Figure 2.
FIGURE 2.

Clinical and experimental indications for recombinant human growth hormone (GH; Danowitz & Grimberg, 2022; Isgaard et al., 2015; Shang et al., 2022) and GH receptor antagonist (Colao et al., 2019; Lu et al., 2019).
2. RECOMBINANT GH/GHA PRODUCTION
Recombinant proteins have been widely used in biological and biomedical sciences. However, expression and purification can be challenging because every protein is different, and expression strategies and purification protocols need to be tailored for individual proteins and their intended use. Expression and purification of GH and GHAs from both prokaryotic and eukaryotic host systems have been described (summarized in Figure 3).
FIGURE 3.

Outline of the different approaches used for expression and purification of growth hormone (GH) and GH receptor antagonist. Image created using BioRender. PEG, polyethylene glycol. IMAC, immobilized‐metal affinity chromatography.
2.1. GH/GHA expression and purification from Escherichia coli
Endogenous GH is a nonglycosylated protein and is therefore very suitable for production in prokaryotic expression systems. However, it tends to accumulate as insoluble protein aggregates in inclusion bodies when expressed in the cytoplasm of E. coli. Different approaches to overcome this and improve expression have been described, including periplasmic expression, cytoplasmic expression of fusion proteins, and refolding the insoluble protein from inclusion bodies.
2.1.1. Periplasmic expression
Periplasmic expression is widely used to facilitate purification and enable disulfide bond formation for high‐value therapeutic proteins. It is an optimal approach for many recombinant proteins that has significant advantages in downstream processing, such as reduced release of cytoplasmic proteins, membrane components, and DNA, reduced micronization of cellular debris, and low endotoxin contamination, making downstream purification less complicated (Balasundaram et al., 2009). This approach is ideal for proteins containing disulfide bonds as the periplasmic space is an oxidizing environment, which facilitates formation, but it tends to result in much lower expression levels compared with cytoplasmic expression. Recombinant proteins are usually engineered with an N‐terminal signal peptide, which allows the protein to be exported to the periplasm via the general secretion (Sec) protein export pathway (Georgiou & Segatori, 2005). The signal peptide is cleaved by a signal peptidase during or shortly after substrate translocation, and the mature protein is released on the trans‐side of the membrane (Freudl, 2018).
Periplasmic secretion has been widely used for the production of rhGH (Alanen et al., 2015; Amaranto et al., 2021; Bagherinejad et al., 2016, 2018; Becker & Hsiung, 1986; Browning et al., 2017; Chang et al., 1987; Chang et al., 1989; Ghorpade & Garg, 1993; Gray et al., 1985; Guerrero Montero et al., 2019; Jeiranikhameneh et al., 2017; Li et al., 2004; Matos et al., 2014; Menezes et al., 2017; Perez‐Perez et al., 2020; Rigi et al., 2021; Soares et al., 2003; Soares et al., 2008; Sockolosky & Szoka, 2013; Teresa et al., 2000; Uchida et al., 1997; Zamani et al., 2016; Zhou et al., 2021). A common approach is to fuse the signal sequence to the N‐terminus of rhGH for transport, and a His‐tag to the C‐terminus for subsequent purification. Different signal peptides have been evaluated with varying success (Table 1). In a recent report, a modified Staphylococcus aureus protein A signal peptide fused to the mature hGH coding region was utilized that allowed rhGH to be secreted through the Sec pathway. This increased expression 3‐fold compared with cytoplasmic expression (Rigi et al., 2021). In addition, Perez‐Perez et al. (2020) developed a novel expression method using a signal peptide from PelB fused to small metal‐binding protein (PelB‐SmbP) that combines the benefits of periplasmic expression with purification via immobilized Ni affinity chromatography. hGH expression was improved 10‐fold compared with His‐tagged hGH protein, with a yield of 15.5 mg hGH from 1 L culture, the highest periplasmic production reported.
TABLE 1.
Periplasmic expression of GH and GHA.
| Protein | Inoculation scale | Sec or Tat pathway | Signal peptide | His tag | His tag removal | Chromatographic purification | Yield or expression level | Ref. |
|---|---|---|---|---|---|---|---|---|
| hGH | Shake flask | Sec | PhoA | No | N/A | No | 280 ng of hGH/mL/L A550 unit of cells | (Gray et al., 1985) |
| hGH | Shake flask | Sec | OmpA | No | N/A | Anion‐exchange size‐exclusion | 20 mg from 1 L culture (A600 = 1.6) | (Becker & Hsiung, 1986) |
| hGH | Shake flask | Sec | STII | No | N/A | IMAC (containing anti‐GH coupled to Affigel 10) | 5–25 μg of hGH/mL/L A550 unit of cells | (Chang et al., 1987) |
| hGH | Shake flask | Sec | LTB | No | N/A | No | N/A | (Ghorpade & Garg, 1993) |
| 20K hGH | Shake flask | Sec | npr | No | N/A | Anion‐exchange size‐exclusion reversed phase‐HPLC | N/A | (Uchida et al., 1997) |
| hGH | Shake flask | Sec | DsbA; Npr; STII | No | N/A | No |
DsbA‐hGH 2.80 μg/mL/A600 Npr‐hGH 2.2 μg/mL/A600 STII‐hGH 0.47 μg/mL/A600 |
(Soares et al., 2003) |
| hGH | Shake flask | Sec | PelB or OmpA | Yes (C‐terminal) | No | IMAC |
0.64‐2.57 mg from 1 L culture for pelB‐hGH; 0.32–2.29 mg from 1 L culture for ompA‐hGH |
(Sockolosky & Szoka, 2013) |
| hGH | Shake flask | Sec | L‐asparaginase II | No | N/A | N/A | hGH protein is expressed as inclusion bodies | (Zamani et al., 2016) |
| hGH | Shake flask | Sec | pelB‐SmbP (SmbP tag was removed subsequently) | Yes (C‐terminal) | No | IMAC (two‐steps) | 15.5 mg from 1 L culture | (Perez‐Perez et al., 2020) |
| hGH | Shake flask | Sec | SpA | Yes (C‐terminal) | No | IMAC | The amount of active hGH in the periplasm was ~28% of the total proteins | (Rigi et al., 2021) |
| hGH | Shake flask | Sec | OmpA | Yes (between OmpA/hGH) | Yes | IMAC | 800 μg/mL | (Zhou et al., 2021) |
| hGH | Shake flask | Tat | TorA | No | N/A | N/A | N/A | (Matos et al., 2014) |
| hGH | Shake flask | Tat | TorA | Yes (C‐terminal) | No | IMAC | N/A | (Alanen et al., 2015) |
| hGH | Shake flask | Tat | TorA | Yes (C‐terminal) | No | No | TatExpress BL21 cells export 31 mg/L−1 hGH to the periplasm, (OD600 = 2.5 AU) | (Browning et al., 2017) |
| hGH | Fed‐batch fermentation | Tat | TorA | Yes (C‐terminal) | No | IMAC | 5.4 g hGH from 1 L fed‐batch culture | (Guerrero Montero et al., 2019) |
| hGH | Shake flask | Tat |
TorA SufI |
Yes (C‐terminal) | No | No | N/A (coexpression of periplasmic chaperone, DsbA or DnaK/J‐GrpE) | (Bagherinejad et al., 2016, 2018) |
| G120R‐hGH | Shake flask | Sec | DsbA | No | N/A | Anion‐exchange size‐exclusion | 0.79 mg G120R‐hGH/g of wet weight cells | (Menezes et al., 2017) |
| GHA1 GHA2 | Fed‐batch fermentation | Sec | OmpA3 | No | N/A | Anion‐exchange | 800 mg hGHA1 and 1.2 g hGHA2 from 200 L culture | (Li et al., 2004) |
Abbreviations: GH, growth hormone; GHA, GH receptor antagonist; GHA1, Cys‐hGH‐del1‐4, G120R, K168A, E174A, C182S, del186‐191; GHA2, hGH‐H21A, G120R, E174A; hGH, human growth hormone; IMAC, immobilized‐metal affinity chromatography; N/A, not available.
The Sec pathway exports proteins across the cytoplasmic membrane in an unfolded state, and the oxidization of disulfide bonds occurs in the periplasm in wild‐type bacteria. An alternative transport pathway called the twin‐arginine translocation (Tat) pathway, transports correctly folded proteins across the membrane (Natale et al., 2008). This pathway is not able to export proteins containing disulfide bonds since these are normally only formed in the periplasm. Theoretically, the reduced cytoplasmic proteins are recognized as incorrectly folded and tend to be excluded by the Tat pathway. Despite this, studies have also employed the Tat pathway to export hGH to the periplasm (Alanen et al., 2015; Bagherinejad et al., 2016; Browning et al., 2017; Guerrero Montero et al., 2019; Matos et al., 2014). To overcome this, Robinson laboratory developed a series of bacterial strains called CyDisCo which can oxidize disulfide bonds in the cytoplasm and allow hGH to be transported by the Tat pathway (Alanen et al., 2015; Matos et al., 2014). However, interestingly, the Tat system has been shown to export hGH even when it lacks disulfide bonds, with bonds forming after translocation into the periplasm (Alanen et al., 2015). Gram‐negative bacteria possess a TatABC‐type Tat translocase, which comprises three proteins, TatA, TatB, and TatC (Sargent et al., 1998). However, these Tat components are expressed at relatively low levels in wild‐type E. coli which can lead to saturation of the system by high substrate expression levels (Barrett et al., 2003). Co‐expression of TatABC proteins from a second plasmid can overcome this and increases export of proteins to the periplasm via the Tat pathway (Matos et al., 2012). Accordingly, over‐expression of the TatABC genes from the E. coli chromosome resulted in a series of super‐secreting strains that drive protein expression via Tat pathway, and these “TatExpress” strains significantly improved Tat‐dependent secretion of hGH when compared with a wild‐type strain (Browning et al., 2017). A follow‐up study from the Robinson lab reported that several g/L hGH could be exported into the periplasm of a W3110 TatExpress strain if a Tat signal peptide was used. The yield of purified His‐tagged hGH was 5.4 g from 1 L extended fed‐batch fermentation culture (Guerrero Montero et al., 2019).
2.1.2. Cytoplasmic expression
Attempts to express rhGH in the cytoplasm of E. coli usually result in its aggregation as inclusion bodies. Many strategies have been developed to improve soluble cytoplasmic expression of proteins in E. coli. These include inducing protein expression at lower temperatures (15°C–25°C), low IPTG concentration, coexpression of molecular chaperones, modification of the protein with fusion tags, and modification of the host strain.
Lowering the rate of protein synthesis by controlling the transcriptional and translational rates, increases the amount of soluble hGH expression (Koo & Park, 2007). The proportion of soluble protein was increased when the expression rate decreased, and by using a T7 transcription terminator‐deleted expression system, more than 90% of hGH was expressed in a soluble form (Koo & Park, 2007). Induction at 16°C–20°C also significantly improved the solubility of rhGH, whereas reducing the IPTG concentration was not as effective (Kim, Park, et al., 2013). Ruddock et al. developed a system named CyDisCo (cytoplasmic disulfide bond formation in E. coli), which involves coexpression of a target protein with a sulfhydryl oxidase and a disulfide bond isomerase. Using the CyDisCo system and fed‐batch fermentation culture, they were able to express soluble hGH in the cytoplasm with a yield of 0.97 ± 0.12 g/L (Gaciarz et al., 2017).
Creating fusion proteins is another option to increase protein solubility in the cytoplasm. Fusion proteins play an important role in improving recombinant protein production in E. coli as they may improve protein solubility, correct protein folding, and can be used to facilitate protein purification. For example, fusing hGH to a thioredoxin tag (Trx–hGH) significantly improves soluble cytoplasmic expression with up to 1 g/L of Trx–hGH soluble fusion protein expressed in E. coli using flask cultivations or fed‐batch fermentation (Levarski et al., 2014). Many alternative fusion partners have also been described. Nguyen et al. (2014) fused hGH with seven different fusion partners (Trx, His6, GST, maltose binding protein [MBP], protein disulfide bond isomerase [PDI], N‐utilization substance protein A [NusA], and the b′a′ domain of PDI [PDIb′a′]) and assessed solubility of the fusion proteins in E. coli. With the exception of the His6 tag fusion, all the fusion proteins exhibited ≥90% solubility when expressed at 18°C, with the Trx tag resulting in the highest yield (~37 mg purified hGH obtained from 0.5 L culture). However, when expressed at 37°C, only the MBP and PDI fusion proteins were soluble (up to 70%). Our lab also used a fusion tag approach to increase the solubility of a series of GHAs and found that an N‐terminal Trx–His fusion partner increased the solubility when the proteins were expressed at 18°C or 30°C (Tamshen et al., 2020; Wang et al., 2020; Wang et al., 2021). Another recent study fused the Fc domain of human Immunoglobulin G (IgG) 1 to the C‐terminal of an hGH super‐agonist to enhance soluble cytoplasmic expression of the chimeric protein (Mirbaha et al., 2022). Several studies have reported the insolubility of bovine Gh in E. coli expression systems (George et al., 1985; Langley et al., 1987; Wingfield et al., 1987). We used a Trx tag fused to the bovine GHA, G119R, in an attempt to improve soluble expression, but found that the majority of the fusion protein aggregated as inclusion bodies (unpublished data). However, using alternative fusion tags may be more appropriate for bovine Gh. For example, when bovine GH was fused with an NusA tag, 89% of the fusion protein was expressed in the soluble fraction (Davis et al., 1999). Moreover, a recent study reported that coexpression with a molecular chaperone increased the soluble expression of Trx‐tagged ovine GH with a final yield of 22 mg soluble protein/L (Liu et al., 2022). A summary of the approaches for soluble expression of GH or GHA is listed in Table 2.
TABLE 2.
Summary of the approaches for soluble expression of GH or GHA
| Protein | Inoculation scale | Induction conditions | Fusion partner or purification tag | Tag removal | Chromatographic purification | Yield | Ref. |
|---|---|---|---|---|---|---|---|
| hGH | Shake flask | 16°C for 16 h |
Untagged His‐tagged |
No | His‐hGH: IMAC, anion‐exchange (MonoQ), size‐exclusion, untagged hGH: two steps of anion‐exchange (DEAE and MonoQ), size‐exclusion |
40 mg His‐hGH from 1 L culture 34 mg untagged hGH from 1 L culture |
(Kim, Park, et al., 2013) |
| hGH | Shake flask | 0.02 or 1 mM IPTG | His‐tagged | No | No | 44 mg soluble hGH/OD.L | (Koo & Park, 2007) |
| hGH | Fed‐batch fermentation | 30°C | His‐tagged | No | IMAC | 0.97 ± 0.12 g/L | (Gaciarz et al., 2017) |
| TMP‐hGH | Shake flask | 0.5 mM IPTG, 25°C for 16 h | MBP‐TMP | Yes | Hydrophobic interaction chromatography, size‐exclusion | Not described | (Wang et al., 2013) |
| hGH | Shake flask | 0.5 mM IPTG, 18°C for 18 h | Trx; His6; GST; MBP; NusA; PDI; PDIb'a' | Yes | IMAC (two steps), size‐exclusion |
37 mg hGH from 0.5 L culture expressing Trx‐hGH; 12 mg from 0.5 L culture expressing MBP‐hGH; 7 mg from 0.5 L culture expressing PDIb′a′‐hGH |
(Nguyen et al., 2014) |
| hGH |
Shake flask Fed‐batch fermentation |
1 mM IPTG, 29°C | Trx | Yes | IMAC (two steps), anion‐exchange | 511 mg from 1 L culture (fed‐batch fermentation) | (Levarski et al., 2014) |
| hGH | Shake flask | 18°C, 0.2 mM IPTG for 18 h | Cleavable self‐aggregating tags (L6KD/α3‐peptide/EFK8/ELK16) | Yes | Anion‐exchange | 8–57 mg from 1 L culture | (Lin, Liu, et al., 2021) |
| Fc‐GH super‐agonist | Shake flask | 1 mM IPTG, 15°C for 20 h | His tag | No | IMAC | Not described | (Mirbaha et al., 2022) |
| B2036 | Shake flask | 0.1 mM IPTG; 18°C for 18 h | Trx‐His tag | Yes | IMAC, anion‐exchange | 24 mg from 1 L culture | (Wang et al., 2020) |
| B2036‐S144C | Shake flask | 0.1 mM IPTG, 30°C for 4 h | Trx‐His tag | Yes | IMAC, anion‐exchange | Not described | (Wang et al., 2021) |
| Bovine GH | Shake flask | 1 mM IPTG, 37°C for 3 h | NusA | No | No | 89% NusA‐bovine GH expressed in the soluble fraction | (Davis et al., 1999) |
| Ovine GH | Shake flask | 0.025 mM IPTG, 20°C for 20 h | Trx‐His tag coexpression with molecular chaperone GroEL‐GroES | Yes | IMAC | 22 mg soluble ovine GH from 1 L culture | (Liu et al., 2022) |
Abbreviations: GH, growth hormone; GHA, GH receptor antagonist; GST, glutathione S‐transferase; hGH, human growth hormone; His, histidine; IMAC, immobilized‐metal affinity chromatography; MBP, maltose binding protein; NusA, N‐utilization substance A; PDI, predelivery inspection; Trx, thioredoxin.
2.1.3. Refolding the protein from inclusion bodies
The accumulation of insoluble hGH protein aggregates as inclusion bodies is a commonly described issue following overexpression in E. coli and reduces the efficiency of hGH and GHA production. Although the formation of inclusion bodies has certain advantages, such as protecting the protein from proteolysis and ease of isolation, precipitation as inclusion bodies poses a major hurdle in the recovery of bioactive proteins (Kim, Park, et al., 2013; Nguyen et al., 2014). Numerous studies have attempted to refold GH orthologues originating from different species from inclusion bodies (e.g., human, bovine, ovine, porcine, and fish; Aramvash et al., 2018; Choi & Geletu, 2018; Chung et al., 2015; Crivelli et al., 1991; Fradkin et al., 2010; Funkenstein et al., 2005; George et al., 1985; Jeh et al., 1998; Keshavarz et al., 2021; Khan et al., 1998; Mahmoud et al., 1998; Mukhija et al., 1995; Mukhopadhyay & Sahni, 2002; Ocłoń et al., 2018; Paduel et al., 1999; Panda et al., 1999; Patra et al., 2000; Poen & Pornbanlualap, 2013; Promdonkoy et al., 2004; Rao et al., 1997; Sereikaite et al., 2007; Shin et al., 1998; Singh et al., 2009, 2012; Sonoda & Sugimura, 2008; Upadhyay et al., 2012; Wallis & Wallis, 1990; Wingfield et al., 1987; Zomorrodipour et al., 2004). A summary of the approaches for refolding GH or GHA from inclusion bodies is listed in Table 3.
TABLE 3.
Summary of the approaches for refolding GH or GHA from inclusion bodies.
| Protein | Inoculation scale | Denaturant buffers (pH) | Fusion tag | Tag removal | Chromatographic purification | Separate monomer from dimer | Final yield | Ref. |
|---|---|---|---|---|---|---|---|---|
| hGH | Shake flask | 6 M guanidine hydrochloride | N‐terminal His‐tag | No | IMAC | No | 30 mg from 1 L culture | (Mukhija et al., 1995) |
| hGH | Fed‐batch fermentation | 20 mM Tris–HCl, pH 11.5 | TNF‐α and His10 tag | Yes | IMAC, anion‐exchange | Not described | 2 g from 1 L high cell density culture (overall yield 25%) | (Shin et al., 1998) |
| hGH | Fed‐batch fermentation | 100 mM Tris buffer, 2 M urea, pH 12.5 | No | N/A | Anion‐exchange, size‐exclusion | Yes | 52 mg from 104 mg inclusion bodies | (Patra et al., 2000) |
| hGH‐V 20 and 22K; hGH‐N 20K | Shake flask | 40 mM Tris base with 4.5 M urea and 50 mM cysteine, pH 11.0 | No | N/A | Anion‐exchange, size‐exclusion | Yes | 400–700 mg from 5 L culture | (Solomon et al., 2006) |
| hGH | Fed‐batch fermentation | 100 mM Tris buffer, 2 M GnHCl, pH 12.5 | No | N/A | Anion‐exchange | Not described | 50% of the initial inclusion bodies | (Sonoda & Sugimura, 2008) |
| hGH | Fed‐batch fermentation | 50 mM Tris, 2 M urea, 0.5 mM EDTA, pH 12.0 | No | N/A | Anion‐exchange, size‐exclusion | Not described | 105 mg from 500 mg inclusion bodies | (Singh et al., 2009) |
| hGH | Shake flask | 6 M n‐propanol and 2 M urea, pH 8.5 | No | N/A | Anion‐exchange, size‐exclusion | Not described | 24 mg from 60 mg inclusion bodies | (Singh et al., 2012) |
| hGH | Shake flask | 6–8 M urea, 50 mM phosphate buffer, and 5 mM β‐mercaptoethanol, pH 12.0 | ubiquitin (Ub) tag | Yes | Anion‐exchange, HIC | Not described | 20 mg from 1 L culture | (Wojtowicz‐Krawiec et al., 2014) |
| hGH | Shake flask | 30% trifluoroethanol, 3 M urea, 1 mM DTT | No | N/A | Anion‐exchange size‐exclusion | Not described | 36 mg from 79 mg inclusion bodies | (Upadhyay et al., 2016) |
| hGH | Shake flask | 2% deoxycholate, pH 12.5 | FLAG | Yes | Cation‐exchange, anion‐exchange, size‐exclusion | Yes | Not described | (Aramvash et al., 2018) |
| hGH | Shake flask | 2 M urea, 1 mM DTT, pH 8.5 (Note: freeze‐thaw method) | No | N/A | Anion‐exchange, size‐exclusion | Yes | 10 mg from 72 mg IBs | (Singhvi et al., 2021) |
| hGH |
Shake flask Fed‐batch fermentation |
100 mM Tris‐HCl, 2 M urea, 10% glycerol, 2% sucrose, 1% Triton X100 and 1 mM EDTA | No | N/A | Anion‐exchange, size‐exclusion | Yes | Maximum biomass productivity was 4.17 g/L | (Keshavarz et al., 2021) |
| hGH | Shake flask | N/A | Cleavable self‐aggregating tags | Yes | No | No | 8–57 mg from 1 L culture | (Lin, Amesso Ndengue, et al., 2021) |
| hGH | Shake flask | Nondenaturing conditions, 0.25 M arginine, pH 10, 10 mM DTT, and 2.4 kbar | No | N/A | No | No | Refolding yield: 81% with ~73% monomer | (Chura‐Chambi et al., 2022) |
| B2036 | Shake flask | 8 M urea (pH 9.5) | No | N/A | Anion‐exchange | Yes | 3.7 mg from 1 L of cell culture | (Wang et al., 2020) |
| bGH | Unclear | 8 M guanidinium chloride | No | N/A | Anion‐exchange, size‐exclusion | No | 95 mg from 375 mg IBs | (Wingfield et al., 1987) |
|
Buffalo GH Goat GH |
Shake flask | 8 M guanidinium hydrochloride, pH 8.5 | No | N/A | Anion‐exchange | Yes | Goat GH 5.8 mg from 11 mg IBs | (Mukhopadhyay & Sahni, 2002) |
| oGH | Shake flask | 6 M guanidine hydrochloride | N‐terminal His‐tag | No | IMAC | No | 32 mg from 1 L culture | (Rao et al., 1997) |
| oGH | Fed‐batch fermentation | 2 M Tris‐HCl buffer, 2 M urea, pH 12.0 | N‐terminal His‐tag | No | Size‐exclusion | Yes | More than 60% of the initial IBs | (Khan et al., 1998) |
| fGH | Fed‐batch fermentation | 0.5% SDS, 0.001% 2‐mercaptoethanol, pH 10.5 | No | N/A | No, butanol treatment | No | 4.8 g from 5 L culture | (Jeh et al., 1998) |
| fGH | Shake flask | 0.1% N‐lauroylsarcosine, 4 mM DTT, pH 10.0 | N‐terminal His‐tag | No | No | No | 450 mg from 1 L culture | (Choi & Geletu, 2018) |
|
gfGH‐II cGH |
Shake flask | 0.1 M NaOH | HisL‐gfGH‐II GST‐cGH | Yes | IMAC | Not described | Not described | (Mahmoud et al., 1998) |
| mkGH | Shake flask | 8 M urea, 20 mM reduced glutathione, pH 9.0 | Not described | N/A | Anion‐exchange, HIC | Not described | 25–30 mg from 4 g biomass | (Sereikaite et al., 2007) |
| rfGH | Shake flask | 4.5 M urea, 0.2 mM cysteine, pH 11.3 | No | N/A | Anion‐exchange | Yes | 2.5 mg from 1 L culture | (Funkenstein et al., 2005) |
| scGH | Shake flask | 2M urea, 1% Triton X‐100, pH 11.0 | N‐terminal His‐tag | No | IMAC | No | 31.3 mg from 1 L culture | (Poen & Pornbanlualap, 2013) |
| ggGH | Fed‐batch fermentation | PBS, 3 M urea, 0.1 mM DTT, pH 12.0 | N‐terminal His‐tag | No | IMAC | No | 5.7 mg from 10 mL fed‐batch culture (~2 g wet cell weight) | (Chung et al., 2015) |
| dfH | Shake flask | 4.5 M urea, pH 11.3 | No | N/A | Anion‐exchange | Yes | 35 mg form 2.5 L culture | (Paduel et al., 1999) |
|
zfGH rtGH |
Shake flask | 4.5 M urea, 0.1 mM cysteine, pH 11.0 | No | N/A | Anion‐exchange, size‐exclusion | Yes |
18 mg zfGH from 5 L culture 12 mg rtGH from 5 L culture |
(Ocłoń et al., 2018) |
| mGH | Fed‐batch fermentation | 100 mM Tris, 10 mM reduced glutathione, 1 mM oxidized glutathione, 2 M urea, pH 9.0 | No | N/A | Anion‐exchange, HIC | Yes |
Expression level 3.8 g/L Yield after chromatograph 22% |
(Fradkin et al., 2010) |
Abbreviations: bGH, bovine GH; cGH, carp GH; dfGH, dolphin fish GH; fGH, flounder GH; gfGH‐II, goldfish type II GH; ggGH, giant grouper; GH, growth hormone; GHA, GH receptor antagonist; HIC, hydrophobic interaction chromatography; IBs, inclusion bodies; mGH, murine GH; mkGH, Mink GH; oGH, ovine GH; rfGH, rabbit fish GH; rtGH, rainbow trout GH; scGH, striped catfish GH; zfGH, zebrafish GH; Tumour necrosis factor alpha (TNF‐α).
To obtain soluble protein from inclusion bodies, the inclusion bodies are first solubilized in denaturant and then subjected to a refolding process. Therefore, having an efficient solubilization and refolding method is a critical step. The protocol to solubilize inclusion bodies usually uses a strong denaturing buffer containing 8 M urea or 6 M guanidine HCl. Two variants of bovine Gh have been successfully refolded from inclusion bodies using a strong denaturing buffer with 8 M guanidinium chloride. Following dialysis, anion‐exchange and size‐exclusion chromatography, the overall recovery was ~25% of total bovine Gh present prior to refolding (Wingfield et al., 1987). His‐tagged hGH has been extracted and solubilized from inclusion bodies using 6 M guanidine hydrochloride, with a yield of 30 mg His‐tagged hGH from 1 L culture media following immobilized‐metal affinity chromatography (IMAC; Mukhija et al., 1995). In addition, ovine GH was successfully purified from inclusion bodies using 6 M guanidine hydrochloride to solubilize the inclusion bodies with 4.5 M urea pH 11.5 used to refold the denatured protein following IMAC. The yield of His‐tagged ovine GH was 32 μg/mL at shake‐flask level and a dimeric form was observed (Rao et al., 1997). GH contains two internal disulfide bonds, and misfolding of these disulfide bonds during the inclusion body refolding process can result in the formation of dimers or multimers. Another study used 6–8 M urea, pH 12 to solubilize hGH from inclusion bodies and extracted 20 mg of GH from 1 L culture following chromatography purification (Wojtowicz‐Krawiec et al., 2014).
Milder solubilization strategies using lower urea concentrations have been shown to help retain a more native‐like secondary structure and improve the recovery of bioactive protein when compared with using higher concentrations of urea (Singh et al., 2015). Patra et al. refolded hGH from inclusion bodies produced in E. coli after 10 h of fed‐batch fermentation using different urea and pH conditions. With 100 mM Tris buffer at pH 12.5 containing 2 M urea, the solubilized amount of hGH from inclusion bodies was comparable to Tris buffer with 8 M urea at pH 8. Following ion‐exchange and size‐exclusion purification, the overall yield of hGH purified from inclusion bodies was ~50%. In this study, they also observed that extraction of hGH from inclusion bodies at alkaline pH increased the presence of a dimeric form (Patra et al., 2000). However, another study found that a solubilization buffer containing 2 M urea at alkaline pH was ineffective in their production system, whereas 100 mM Tris buffer with 2 M GnHCl at pH 12.5 resulted in 95% solubility of hGH from inclusion bodies (Sonoda & Sugimura, 2008). Refolding protein from inclusion body has also been used to isolate different hGH isoforms. For example, the 20 and 22 kDa isoforms of human pituitary GH and placental GH were expressed as inclusion bodies and resolved in 4.5 M urea, pH 11. After refolding and anion‐exchange chromatography, the yields were between 400 and 700 mg from a 5 L culture (Solomon et al., 2006). In another study, a mutated hGH variant, hGH des(1–6,14), which exhibited antagonistic activity, was expressed in E. coli as inclusion body, and solubilized using 4.5 M urea buffer, pH 11.3. Following anion‐exchange and size‐exclusion chromatography, circular‐dichroism spectroscopy analysis of the purified mutant demonstrated that the α‐helix content of the analogue was similar to the wild‐type hGH, suggesting the three‐dimensional structure was preserved (Tchelet et al., 1997). A recent study demonstrated that hGH inclusion bodies contained a native‐like secondary and tertiary structure, and mild and nondenaturing conditions (a combination of alkaline pH and high pressure) preserved the structure. After decompression, using a redox pair formed by 2 mM Glutathione (GSH) and 1 mM oxidised gluathione (GSSG) in the presence of Dithiothreitol (DTT) was important for the refolding process of hGH and resulted in a good yield of 81% with ~73% of monomer (Chura‐Chambi et al., 2022).
Several studies have reported inclusion bodies protocols for isolating GH from different species of fish. Rabbitfish GH was purified from inclusion bodies and refolded in buffer containing 4.5 M urea in the presence of cysteine at pH 11.3. Subsequent purification by Q‐Sepharose chromatography resulted in a yield of ~2.5 mg monomeric GH from 1 L bacterial culture (Funkenstein et al., 2005). Striped catfish GH was solubilized from inclusion bodies with 2 M urea solution in the present of 1% Trixton X‐100, pH 11, and yielded 31 mg from 1 L of cell culture following IMAC purification (Poen & Pornbanlualap, 2013). Flounder GH with an N‐terminal His‐tag was not soluble, even when induced at 18°C. But it was possible to solubilize it from inclusion bodies by including 0.1% N‐lauroylsarcosine in the denaturant buffer. This resulted in a yield of 450 mg flounder GH from 1 L culture medium after removing the denaturant regents, but without chromatographic purification (Choi & Geletu, 2018). Similarly, giant grouper GH was found to be expressed as inclusion bodies even when protein expression was induced at 16°C (Chung et al., 2015). Initially, 4 g/L protein was produced via mid‐log phase induction in a large‐scale fed‐batch culture and inclusion bodies were then solubilized in Phosphate‐buffered saline (PBS) buffer containing 3 M urea and 0.1 mM DTT, pH 12. Following refolding and IMAC chromatography, 5.7 mg of giant grouper GH was recovered from 10 mL of fed‐batch culture (45% recovery; Chung et al., 2015).
Apart from denaturant and pH parameters, other elements also play a role in the production of GH from inclusion body. For example, organic solvents such as trifluoroethanol are mild solubilization agents that stabilize the secondary structure of the protein in the inclusion body aggregates while destabilizing tertiary structure. Combining 30% trifluoroethanol with 3 M urea was shown to be an efficient method to solubilize hGH with ~36 mg hGH recovered from 79 mg inclusion bodies following chromatographic purification (Upadhyay et al., 2016). Using a single freeze–thaw cycle of the inclusion body is a simple and low‐cost approach to improve refolding efficiency. An additional benefit of this approach is that lower urea concentrations can be used (Qi & Chilkoti, 2015). We used this approach to refold bovine GHA (bG119R) from inclusion bodies and found that a single freeze–thaw cycle combined with 2 M urea buffer, pH 8.0, was as efficient as using 8 M urea with the traditional denaturation method (unpublished). hGH has also been solubilized from inclusion bodies in 2 M urea with 1 mM DTT using a freeze–thaw method, with ~10 mg hGH obtained from 72 mg inclusion bodies following anion‐exchange and size‐exclusion chromatography (Singhvi et al., 2021). Furthermore, fusing GH with self‐aggregating peptide tags has also been reported to improve the recovery from inclusion bodies. Four aggregating tags were used to drive an hGH fusion protein into active protein aggregates. Following cleavage of the tags, hGH was released into the supernatant. This approach avoids requirement of the traditional refolding steps and resulted in purification of 57 mg/L hGH from inclusion bodies, with 92% of the bioactivity of commercial hGH (Lin, Amesso Ndengue, et al., 2021).
2.2. GH/GHA production in other species, such as yeast
In addition to E. coli, a wide range of eukaryotic host systems have been applied to produce recombinant GH (Table 4), including yeast Saccharomyces cerevisiae (Jin et al., 1999; Jung et al., 2005) and Pichia pastoris (Apte‐Deshpande et al., 2009; Ascacio‐Martínez & Barrera‐Saldaña, 2004; Azadi et al., 2017; Azadi et al., 2018; Calik et al., 2008; Deng et al., 2020; Li et al., 2009; Orman et al., 2009; Rothan et al., 2014; Wang et al., 2003; Wu, Liu, et al., 2014; Xu et al., 2008). Pichia pastoris is a commonly used eukaryotic host for the manufacturing of recombinant proteins. Compared with other eukaryotic hosts, P. pastoris has several advantages, such as its capability to grow at high cell densities, its effective secretion system, and its proficiency in executing posttranslational modifications. However, yeast cells may not be able to perform all posttranslational modifications necessary for proper protein folding and activity (Ahmad et al., 2014). Other eukaryotic hosts have also been used to produce recombinant GH, such as mammalian cell lines CHO (Aghili & Zarkesh‐Esfahani, 2018; Rezaei et al., 2013) and VERO (Lupker et al., 1983; Ohno et al., 1991), and insect cell systems (Jing et al., 2002). Mammalian expression systems are often preferred for producing recombinant proteins because they are capable of performing complex posttranslational modifications that are necessary for the proper folding, stability, and activity of many proteins. Mammalian cells can also secrete the protein into the culture medium, making downstream processing and purification easier. In addition, recombinant proteins produced in mammalian cells are often less immunogenic, making them more suitable for therapeutic applications in humans. However, the production of GH in mammalian cells is generally low and the high cost associated with large‐scale production is another limitation (Deng et al., 2020). Insect cell systems have also been used to produce recombinant proteins. Depending on the protein of interest, insect expression systems can have a higher protein expression capacity than mammalian cells; however, this is not always the case. Insect cells can perform some posttranslational modifications, but they may produce proteins that are more immunogenic than those produced in mammalian cells (Liu et al., 2013). All in all, each expression system has its advantages and disadvantages, and the choice of system depends on several factors, such as the desired yield, cost of production, protein activity, immunogenicity, and downstream processing requirements. Mammalian cells and insect cells have not been widely used to produce recombinant GH due to lower yields. This section focuses on the production of GH from the P. pastoris system.
TABLE 4.
Expression of GH from P. pastoris yeast
| Protein | Inoculation scale | Protein secretion location | Codon optimization | Fusion tag | Tag removal | Chromatographic purification | Productivity | Ref. |
|---|---|---|---|---|---|---|---|---|
| hGH | Shake flask | Culture broth | His‐tag | Yes, using a factor Xa protease | IMAC | Expression level: 115 mg/L | (Calik et al., 2008) | |
| hGH | Shake flask | Culture broth | No | N/A | Anion‐exchange | Expression level: 240 mg/L with addition of Tween 20 | (Apte‐Deshpande et al., 2009) | |
| hGH | Fed‐batch fermentation | Culture broth | No | No | N/A | No | Total protein 0.807 g/L; 108 g/L (DCW) | (Azadi et al., 2017) |
| hGH | Fed‐batch fermentation | Culture broth | No | No | N/A | Two steps of anion‐exchange (weak and strong), HIC | Total protein 1.14 g/L; 162.5 g/L (DCW) | (Azadi et al., 2018) |
| pGH | Shake flask | Intracellular production | Yes | His‐tag | No | IMAC | Total protein in cells 340 mg/L; intracellular soluble protein 70 mg/L | (Deng et al., 2020) |
| Fish GH | Shake flask | Culture broth | Yes | No | N/A | No | Code optimized protein expression 2.8 mg/L | (Rothan et al., 2014) |
| Ailuropoda.melanoleuca GH | Shake flask | Culture broth | Yes | No | N/A | No | Expression level ~100 mg/L | (Xu et al., 2008) |
| Canine GH | Shake flask | Culture broth | Yes | No | N/A | No | 40 μg/mL | (Ascacio‐Martínez & Barrera‐Saldaña, 2004) |
| HSA/GH fusion Protein | Fed‐batch fermentation | Culture broth | No | N/A | No | Secretion level 3‐4 g/L | (Wu, Ji, et al., 2014) |
Abbreviations: DCW, dry cell weight; GH, growth hormone; hGH, human growth hormone; HIC, hydrophobic interaction chromatography; HSA, human serum albumin.
Pichia pastoris is an established industrial platform for producing recombinant proteins. The major advantages of P. pastoris over bacterial expression systems such as E. coli are their ability to secrete recombinant protein into the culture medium and the absence of endotoxin contamination. Different strategies to improve the expression of heterogeneous protein in P. pastoris expression systems have been developed, such as optimization of fermentation conditions, gene code optimization, coexpression of chaperones, and selection for high gene copy host (Gao & Shi, 2013; Juturu & Wu, 2018; Murasugi, 2010). Fed‐batch fermentation strategies have been evaluated for producing rhGH in P. pastoris (Azadi et al., 2017; Azadi et al., 2018). A study reported that when using a sorbitol/methanol mixed feed strategy, the cell biomass of rhGH achieved was 108 g/L (dry cell weight [DCW]) and total protein 0.807 g/L (Azadi et al., 2017). Addition of 10 mmol ascorbic acid to sorbitol/methanol co‐feeding significantly increased the biomass of rhGH to 162.5 g/L (DCW) and total protein 1.14 g/L (Azadi et al., 2018). To express other GH from other species in the P. pastoris system (e.g., porcine, canine, Ailuropoda melanoleuca, and fish), codon optimized gene sequences are often used (Cho et al., 1987; Ascacio‐Martínez & Barrera‐Saldaña, 2004; Deng et al., 2020; Rothan et al., 2014). For example, porcine GH was produced more effectively in P. pastoris when using an optimized gene sequence, with expression levels reaching 10% of the total intracellular protein (Cho et al., 1987). Similarly, codon optimization of the giant grouper GH gene also improved expression in P. pastoris, compared with native gene (2.80 ± 0.27 vs. 1.75 ± 0.25 mg from 1 L culture; Rothan et al., 2014).
Coexpression of molecular chaperone proteins has been demonstrated to improve the intracellular soluble expression of GH in the P. pastoris system. Molecular chaperones are a class of molecules that interact with unfolded or partially folded protein, that play an important role in facilitating correct folding of proteins (Camberg et al., 2013). Ssa1 and Sis1 are molecular chaperones which belong to the heat shock protein (Hsp)70 and Hsp40 family of molecular chaperones, respectively, that assist with the formation of correct native conformation of peptides. Coexpression with Ssa1 and Sis1 proteins in P. pastoris enhanced the intracellular soluble expression of porcine GH resulting in a yield of 340 mg/L, of which 70 mg/L was soluble and 270 mg/L was insoluble protein (Deng et al., 2020). Increased protein expression can also be obtained by engineering P. pastoris strains that contain multiple copies of a gene of interest. For example, a P. pastoris strain carrying two to three copies of a human serum albumin (HSA)–GH fusion gene had significant increased protein secretion than a strain with only one copy. The secretion level can reach to 3–4 g/L in the strain carrying three copies of HSA–GH fusion gene and two copies of chaperone protein disulfide isomerase, whereas the strain carry one copy of HSA–GH fusion gene only express recombinant protein at 400–500 mg/L in the same fed‐batch fermentation condition (Wu, et al., 2014).
3. GENERATION OF LONG‐ACTING GH OR GHA THERAPEUTICS
GH and GHAs are proteins of a relatively small size (22 kDa) with a short circulating half‐life of ~20 min due to renal clearance. A number of strategies have been used to overcome this obstacle, including glycosylation, protein fusion, and albumin conjugation (AlQahtani et al., 2019). One strategy for increasing the serum half‐life of proteins is to generate poly(ethylene glycol) (PEG)‐protein conjugates which increase the molecular weight and hydrodynamic volume (Dozier & Distefano, 2015; Turecek et al., 2016), thus preventing the biomolecules from being excreted through kidney filtration (Abuchowski et al., 1977). There are many FDA‐approved PEG conjugates as a result (Alconcel et al., 2011; Sanchez Armengol et al., 2022). PEGylation can be accomplished through chemical and enzymatic techniques to attach PEG molecules to proteins on the thiol‐group of cysteine, carboxyamide group of glutamine, ε‐amino group of lysine, or alcohol group of serine, and threonine. However, PEGylation may lead to a reduction in binding affinity, and consequently activity, due to steric interference with the drug‐receptor binding interaction. This loss in drug potency is compensated for by a longer circulating half‐life. The resulting change in pharmacokinetic—pharmacodynamic profile has enabled development of drugs that otherwise would not have been feasible and has led to improvements in other existing drugs. Thus, while most drug development approaches seek to specifically increase the activity of the drug, the focus of PEGylated drugs is to balance pharmacokinetic and pharmacodynamic properties to produce a therapy that has both increased efficacy and greater compliance in the clinical setting (Fishburn, 2008). In this section, we will discuss strategies for the construction of long‐acting GH or GHA, such as PEGylation and development of fusion proteins, and will discuss recent advances in the field.
3.1. Conjugation of GH/GHA with PEG
3.1.1. Chemistries used for GH/GHA PEGylation
Various chemistries have been employed to PEGylate GH/GHA and these have advantages and disadvantages. For example, N‐hydroxysuccinimide (NHS) ester PEGs react with the protein amines. The polymers are readily commercially available; however, the disadvantage is the lack of site specificity and the quick hydrolysis of the NHS group. The latter typically requires a large excess of PEG that must be removed from the product. But the resulting bond is a stable amide. Aldehyde PEG has been employed to react with amines on both GH and GHA. Aldehydes are less prone to hydrolysis than NHS groups. However, the resulting imine is unstable and therefore the bond must be reduced to the stable amine. This reaction can be slow, depending on the aldehyde used and is typically conducted at mildly acidic pH. Maleimide PEGs have also been employed to react with thiol groups. These groups are more stable than NHS groups in solution and have the advantage of specific reaction with the free cysteines. The disadvantage is that the resulting thiol ether group has been known to reverse in physiological conditions, which can change pharmacokinetic profiles over time (Lyon et al., 2014; Shen et al., 2012). Polymers with either azide or alkyne groups have been applied for click chemistry reactions. These chemistries are site selective and the PEGs and products are stable in solution. However, the partner reactive group is not found on the native protein. In addition, unless strained alkynes are employed, the reaction requires copper, which can be damaging to some proteins. Other conjugation chemistries that have not yet been utilized on GH or GHA have been reviewed (Ko & Maynard, 2018).
3.1.2. Nonspecific PEGylation
A common approach for protein PEGylation is through nonspecific conjugation to amine groups with reagents containing activated esters, such as NHS. As described above, while this method results in higher conjugation yields when commercially available reagents are used, its nonspecific nature often disrupts protein–ligand binding due to steric interference. Furthermore, conjugates produced using this method are heterogenous, creating challenges in characterization and reproducibility.
Non‐specific PEGylation was the approach used to produce the long‐acting GHR antagonist, pegvisomant, which is approved by the Food and Drug Administration (FDA) for the treatment of acromegaly. In the case of pegvisomant, 4–6 5 kDa PEG moieties are attached via nine amine groups present in the protein core of B2036 (eight lysines and the N‐terminal amine group; Clark et al., 1996). PEGylation dramatically decreases the affinity of B2036 for the GHR, resulting in reduced bioactivity in vitro (Muller et al., 2004) However, it also significantly improves the half‐life of B2036 in the circulation to ~72 h and reduces antigenicity, thus improving the bioactivity of the drug in vivo (Pradhananga et al., 2002). PEGylated variants of B2036 have been described, including, B20, which contains a G120R substitution instead of G120K. This removes a potential PEGylation site in binding site 2 of the protein and improves in vitro bioactivity following PEGylation. PEGylation with amine‐reactive NHS‐PEG results in a heterogeneous mixture of conjugates containing four to seven PEG moieties (Wang et al., 2020).
This approach has also been used to generate long‐acting GH therapeutics. To extend the circulating half‐life of GH, Clark et al. (1996) produced hGH derivatives containing up to seven 5 kDa PEG moieties to primary amines by reaction with amine‐reactive NHS‐PEG. Separation of PEGylated species that differ by one 5 kDa PEG chain is challenging and not achievable by size exclusion, as the relative size difference between variants with PEGylation of N and (N + 1) moieties reduces as N increases (Fee & Van Alstine, 2011). To overcome this, a series of chromatographic methods, including SP‐Sepharose high performance chromatography and High Performance Liquid Chromatography (HPLC), was used to separate amine PEGylated GH derivatives. This resulted in a series of purified GH conjugates, GH‐(PEG)2, GH‐(PEG)5, and GH‐(PEG)7, with purity of ~85% (Clark et al., 1996). However, the purified GH‐PEG derivatives were modified at different amino acid sites due to the random nature of amine PEGylation.
3.1.3. Site‐specific PEGylation
As mentioned above, one of the issues with nonspecific PEGylation is that it yields heterogenous products. One way to avoid this is to use a site‐specific conjugation approach. Controlled attachment of PEG to specific residues distal to an active or binding site of a protein can reduce steric hindrance of these sites and improve bioactivity. Site‐specific protein conjugation strategies are widely used and have been reviewed elsewhere (Dozier & Distefano, 2015; Ko & Maynard, 2018; Veronese & Mero, 2008; Zhang et al., 2012). Here, key strategies used to achieve site‐specific PEGylation of GH and GHA are discussed (Figure 4 and Table 5).
FIGURE 4.

Summary of PEGylation strategies for growth hormone (GH) modification. Positions of the alpha helices are indicated by a blue line. (a) human GH (hGH) amino acid sequence showing sites of polyethylene glycol (PEG) conjugation. Image created using BioRender. (b) Topology representation of hGH structure (PBD ID 1HGU, Pymol). Positions of the four main alpha helices are indicated by colored lines and/or numbers in (a,b).
TABLE 5.
Site specific PEGylation of GH or GHA
| Protein core | PEGylation sites | PEGylation type | PEG (kDa) | pharmacokinetic character | Bioactivity | Ref. |
|---|---|---|---|---|---|---|
| hGH variant | T3 | Maleimide‐thiol | 20 | Terminal half‐life was 9 h in male SD rats |
In vitro: hGH‐T3C possesses similar bioactivity to recombinant GH or pituitary GH, while EC50 of PEG‐T3C was significantly higher than GH control proteins or unmodified T3C in mouse FDC‐P1 cells stably expressing the rabbit GHR In vivo: every second day or every third day administration of PEG‐T3C stimulates increases in body weight and tibial epiphysis growth comparable with that produced by daily administration of GH in rats |
(Cox et al., 2007) |
| hGH variant | T3, S144, T135, T148 and stp192 |
Maleimide‐thiol Thiol‐vinyl sulfone |
5 | Not described | The EC50s for the T3C, S144C, and T148C variants were measured by cell proliferation assay and were found to be similar to that of pituitary hGH and rhGH. The EC50 for PEG‐T3C and PEG‐S144C was 0.07 and 2 nM, respectively | (Cox et al., 2004) |
| hGH variants | Y35, F92, G131, R134, Y143, and K145 | Click chemistry (pAcF‐substituted) | 30 | Terminal half‐life of 30 K conjugates ranged from 4.41 to 6.02 h in SD rats, which varies depending on the conjugation site |
In vitro: near equivalent EC50 in BA/F3 cells expressing rat GHR among the six variants In vivo: PEGylated Y35pAcFhGH had favourable pharmacokinetic and pharmacodynamic properties in rats. ( |
(Cho et al., 2011) |
| hGH variants | Y35, G131, and K145 | Click chemistry (azido‐bearing unnatural amino acid, NEAK‐ substituted) | 5, 10, 20, 40 |
The clearance half‐life was increased in the range of 7‐ to 40‐fold in male SD rats t β1/2‐Y35‐20K = 11.3 ± 1.2 h; t β1/2‐Y35‐40K = 29.0 ± 4.4 h |
In vitro: IM‐9 cell‐based STAT5 phosphorylation assays demonstrated that EC50 of hGH modified with mono‐linear PEG increased slightly with 5–20 kDa PEG and increased significantly in protein modified with 40 kDa branched PEG In vivo: di‐PEGylated hGH was superior to mono‐PEGylated hGH for long‐term use in vivo |
(Wu et al., 2017) |
| hGH variants | N‐terminal, Gln141 | N‐terminal amine; enzymatic site‐specific PEGylation | 20 | The half‐lives (h) of hGH, PEG‐Nter‐hGH and PEG‐Gln141‐hGH was 1.6 ± 0.2, 7.6 ± 2.7, 7.2 ± 2.6, respectively, in female SD rats |
In vitro: not described In vivo: cumulative body weight gain |
(da Silva et al., 2013; Grigoletto et al., 2016) |
| hGH variants | N‐terminal | Aldehyde reductive amination two linkers: phenyl amide and ethyl moieties | 20 | The half‐lives (h) of hGH‐phenyl‐PEG and hGH‐prop‐PEG was 9.4 and 7.3, respectively |
In vitro: not described In vivo: circulating IGF‐1 in the hGH‐ phenyl‐PEG group was significantly increased and reached a peak at 24 h |
(Wu, Ji, et al., 2014) |
| hGH | Gln40 | Enzymatic site‐specific PEGylation | 20 | Not described | In vitro: In Nb2‐11 cells, PEG‐hGH had a residual proliferative activity of 4.6% (EC50 = 5 ± 0.12 ng/mL) taken as 100% of hGH activity (EC50 = 0.2 ± 0.01 ng/mL) | (Khameneh et al., 2015) |
| hGH | Gln141 | Enzymatic site‐specific PEGylation | 0.556, 10 | Not described | Not described | (Mero et al., 2011) |
| GHA | N‐terminal | N‐terminal amine | 20, 40 | Not described |
In vitro: conjugation of 20 kDa PEG may decrease the binding ability of GHA with its receptor, conjugation of 40 kDa PEG almost abolished the binding of GHA with its receptor In vivo: The IGF‐1 level in the 20 kDa PEG conjugated at 2 mg/kg treated group was decreased by 30%–43% compared with the saline group in male SD rats |
(Wu et al., 2013) |
| B2036 | S144 | Maleimide‐thiol | 20, 30, 40 | Half‐life of 40K PEG‐B2036 was 58.3 h in CD‐1 female mice |
In vitro: Following PEGylation, EC50 was reduced by 6.5, 10.5, and 12.6‐fold, respectively, for 20, 30, or 40 kDa PEG‐S144C conjugates compared to unconjugated S144C in Ba/F3 cells stably expressing human GHR In vivo: administration of 40 kDa PEG‐S144C conjugate at 10 mg/kg/day reduced serum IGF‐1 concentrations by 50.6% in mice |
(Wang et al., 2020) |
| B2036 | Y35 | Click chemistry (pglY‐substituted) | 5, 10, 20 | Not described |
In vitro: IC50 values were 17.7, 54.0, 68.8, 103.3, and 1289 nM for B2036‐Alkyne, B2036‐5K PEG, B2036‐10K PEG, B2036‐20K PEG, and Pegvisomant, respectively, in Ba/F3 cells stably expressing human GHR In vivo: not described |
(Tamshen et al., 2020) |
Abbreviations: GH, growth hormone; GHA, GH receptor antagonist; GHR, growth hormone receptor; hGH, human growth hormone; NEAK, Nε‐2‐azideoethyloxycarbonyl‐l‐lysine; PEG, polyethylene glycol; SD, Sprague–Dawley.
Chemical modification on naturally occurring amino acids
Alkylation of the N‐terminal amino group of a protein by reductive amination can be achieved, even if lysine residues are present in the protein. This strategy works due to the slight difference in the pKa values between these two types of amino groups. The pKa for the N‐terminal α‐amine group is ~7.8, whereas the pKa for an ε‐amine group on lysine is 10.1. When the chemical reaction is performed at a lower pH, for example, at pH 5, the lysine amine group is mostly protonated and are therefore unable to react with the aldehyde group, thus the free amine on the N‐terminus is the only site able to be modified. However, these approaches are rarely completely site‐specific as a small amount of modification of lysines usually still occurs.
N‐terminal mono‐PEGylation of GH and GHA has been achieved using this approach (da Silva et al., 2013; Grigoletto et al., 2016; Wu et al., 2013; Wu, Ji, et al., 2014). N‐terminal PEGylation of GHA with 20 or 40 kDa PEG propionaldehyde was used to generate a long‐acting GHR antagonist. Administration of the 20 kDa conjugate (2 mg/kg) to rats reduced serum IGF‐1 by 30%–43%, whereas the larger 40 kDa conjugate had no activity in vivo (as described above, pulsatile secretion of hGH from the pituitary stimulates the production and secretion of hepatic IGF‐1, and normalization of circulating IGF‐1 concentrations is the key biochemical criterion by which treatment efficacy is assessed in patients with acromegaly; Renehan & Brennan, 2008). The authors speculated that this may result from interference by the larger 40 kDa PEG with GHR binding sites (Wu et al., 2013). Another study prepared N‐terminal PEGylated hGH using two different linkers (phenyl amide and ethyl moieties). PEGylated hGH using the phenyl amide linker performed better than the conjugate generated using the propyl linker, in terms of proteolytic sensitivity, immunogenicity, pharmacokinetic parameters, and pharmacodynamic behavior. hGH‐phenyl‐PEG administration in Sprague–Dawley rats significantly increased IGF‐1 concentrations compared with hGH, with circulating levels peaking at 24 h (Wu, Ji, et al., 2014).
Chemical modification on engineered cysteines
A common method for site‐specific PEGylation of proteins is to conjugate the PEG chain through the thiol group on a cysteine residue. In this instance, an unpaired cysteine residue is often genetically encoded into the target protein. Many thiol‐specific reagents, such as the maleimide groups, are commercially available. The modification is achieved by reacting a free cysteine with a maleimide group attached to a PEG moiety. If protein engineering tools are available, amino acid substitution to install a cysteine at a defined site works well as a method for site‐specific PEGylation (Ko & Maynard, 2018; Paluck et al., 2016). Notably, there is no guarantee that the modified protein will fold properly and not form an undesired disulfide dimer. Alternatively, native disulfide bonds can be reduced to provide cysteines available for conjugation (Dozier & Distefano, 2015; Ko & Maynard, 2018).
The key to designing site‐specific biotherapeutics with sustained activity is to control the site where the polymer is conjugated, and usually involves conjugation to a “nonessential” residue. This approach minimizes any effects of the polymer on bioactivity and results in a homogeneous PEGylated protein (Ko & Maynard, 2018). Many studies have investigated the impact of the conjugation site on GH or GHA retention and bioactivity. For example, Cox et al. (2004) evaluated three site‐specific PEGylated GH variants (hGH‐T3C‐PEG, hGH‐S144C‐PEG, and hGH‐T148C‐PEG) and demonstrated that the PEGylated variants had substantially improved bioactivity over nonspecific amine‐PEGylated hGH. Another study from the group demonstrated that attachment of a 20 kDa PEG at amino acid site T3C increased the in vitro bioactivity ~100‐fold compared with amine‐PEGylated GH with five to six 5 kDa PEGs (Clark et al., 1996; Cox et al., 2007). The half‐life of the 20 kDa PEG‐T3C‐GH conjugate was increased to 9 h. PEG‐T3C‐GH was shown to stimulate dose‐dependent increases in body weight and tibial epiphyses width in HYPOX rats (Cox et al., 2007).
We used site‐specific conjugation to PEGylate B2036 via an introduced cysteine at amino acid Position 144 which is away from either of the binding sites (Wang et al., 2021). To avoid the formation of dimers at the engineered cysteine residue (Cys144), the maleimide‐thiol reaction was performed whilst the B2036‐thioredoxin fusion protein was immobilized on a solid support (nickel resin). This site‐specific PEGylation method combined protein purification, PEGylation, and removal of unreactive mPEG into one step, which significantly improved the yield of the conjugate and saved time. Site‐specific PEGylation of B2036 also minimized the impact on the potency of the conjugates. Attachment of 20, 30, or 40 kDa PEG at amino acid Cys144 reduced the in vitro bioactivity of B2036 by 6.5‐, 10.5‐, and 12.6‐fold, respectively. However, this was a marked improvement compared with amine PEGylated B2036 (with four to six 5 kDa PEGs) which resulted a 143‐fold reduction in activity. Subcutaneous administration of 40 kDa mPEG conjugate (10 mg/kg/day) reduced serum IGF‐1 concentrations by 50.6% (Wang et al., 2021). Although conjugation with a larger mPEG chain is usually accompanied by loss of bioactivity, the longer serum half‐life compensates for this in vivo.
Chemical modification on noncanonical amino acids
In recent years, advances in the genetic code expansion field have allowed for polymer attachment at alternative sites. Particularly, noncanonical amino acids which contain a polymer initiator can be substituted into any position in the amino acid sequence, and these provide functional handles that can be modified as required (Tamshen et al., 2020). Incorporation of unnatural amino acids containing azide or alkyne functional groups that are compatible with copper‐catalyzed “click” cycloaddition has been commonly used (Lee et al., 2016). Incorporation of the unnatural amino acid, p‐acetylphenylalanine (pAcF), into hGH allowed site‐specific conjugation with PEG‐Oxyamine, resulting in 80%–97% conjugation efficiency depending on the site of attachment. However, the expression level of pAcF‐modified hGH in E. coli ranged from 20% to 70% of wild‐type GH, depending on the incorporation site. Six pAcF‐hGH variants (Y35, F92, Q131, R134, Y143, and K145) out of 20, had similar in vitro bioactivity, and their conjugates exhibited longer half‐life than native hGH. PEGylated Y35pAcF‐hGH displayed greater pharmacodynamic behavior compared with other variants in terms of the ability to induce weight gain in hypophysectomized rats, which underlined the importance of the location of the PEGylation sites (Cho et al., 2011).
hGH variants have also been generated that contain the non‐natural amino acid Nε‐2‐azideoethyloxycarbonyl‐l‐lysine at selected positions (Y35, G131, and K145), to investigate the impact of controlled PEG attachment at defined sites. The half‐lives of 20 kDa PEG hGH conjugates (Y35, G131, and K145) were 11.3, 6.4, and 8.0 h, respectively, and the half‐lives of the 40 kDa PEG conjugates were 29.0, 7.6, and 8.6 h, respectively, demonstrating that the site of conjugation impacts on the circulating half‐life. Furthermore, site‐specific PEGylation on more than one of the sites reduced immunogenicity and improved the pharmacokinetic profile while retaining bioactivity, when compared with PEGylation at a single site (Wu et al., 2017).
We developed a platform for preparation of a site‐specific B2036‐PEG conjugate with improved in vitro activity compared with nonspecific anime‐PEGylated B2036 (pegvisomant). In this study, an unnatural amino acid propargyl tyrosine (pglY) was incorporated into B2036 at amino acid Y35 for site‐specific PEGylation using copper‐catalyzed click chemistry. The alkyne on pglY can react selectively with azides under mild, copper‐catalyzed conditions. A 20 kDa B2036‐PEG conjugate had 5.8‐fold reduction in bioactivity when compared with pegvisomant, for which a 72.8‐fold reduction was observed. Using this approach also led to a high conjugation efficiency of 90% (Tamshen et al., 2020). Notably, although studies using click in chemistry for site‐specific PEGylation of GH or GHA reported high protein–polymer conjugation efficiency (>80%), incorporation of an unnatural amino acid inevitably resulted in reduced protein expression yield and in some cases was associated with a by‐product (truncated protein).
Enzymatic modification on naturally occurring amino acids
Enzymatic labelling is another method for conjugating polymers to proteins. This method utilizes an enzyme which generally recognizes a specific amino acid sequence. For example, enzymatic site‐specific PEGylation mediated by microbial transglutaminase (mTGase) has been used to produce a long‐acting hGH conjugate. TGases (protein‐glutamine γ‐glutamyltransferase [E.C.2.3.2.13]), catalyze acyl‐transfer reactions between the γ‐carboxyamide group of the glutamine residues of a protein and a primary amine, normally the ε‐amino group from lysine. TGases have been used to create protein conjugates derived from hGH. hGH contains 13 glutamine residues (Gln, Q), but only Gln141 and Gln40 can be transglutaminated by mTGase (Doerwald et al., 2006; Hu et al., 2017).
One problem for TGase‐mediated PEGylation is to control which glutamine residue will be modified and obtain a high yield of a homogeneous mono‐PEGylated protein. Several studies were attempted to improve this by screening highly selective enzymes or increasing the selectivity of TGase during reaction. Zhao et al. (2010) generated a mTGase mutant library and screened an mTGase with a superior specific activity on Gln141 of hGH. Mero et al. (2009) developed a method to directly identify the Gln residue conjugated with a monodisperse Boc‐PEG‐NH2. They demonstrated that the Gln residues conjugated with a monodisperse polymer were identified easily by electrospray ionization mass spectrometry (MS) and tandem MS analyses, and this method has been tested on hGH, horse heart apomyoglobin, and human granulocyte colony‐stimulating factor. Subsequently, they investigated an approach to increase the specificity of mTGase, resulting in yielding monoconjugated isomer for some proteins presenting more than one Gln as mTGase substrates, such as hGH and salmon calcitonin. This study demonstrated that the enzymatic PEGylation yielded single mono‐PEGylated conjugates in the present of cosolvents in the reaction mixture, which the authors speculated to be a result of the influence on the secondary structure of the protein and the activity of mTGase. In the presence of 50% (v/v) ethanol, for example, conjugation with either low or high molecular weight PEG‐NH2 yielded mono‐PEGylated hGH on Gln141 (Mero et al., 2011). Interestingly, a study from Khameneh et al. (2015) showed that hGH was site‐specifically PEGylated on Gln40 using mTGase under optimized PEG: protein ratio, pH value, and reaction time, which contrasted with the conjugated site Gln141 reported by Mero et al. The authors found that cosolvent solution improved the selectivity of mTGase but discussed that the secondary structure of hGH was not changed in the presence of ethanol or methanol, but its tertiary structure was perturbed. A study from Henrik et al. introduced Q141N mutation to hGH to allow site‐selective modification at position Gln40 (Ramírez‐Andersen et al., 2018).
The way the PEG chain is arranged around the protein as well as the site where conjugation takes place are fundamental because the PEG chain can sterically interrupt the protein/receptor recognition process. N‐terminal chemical PEGylation (PEG‐Nter‐GH) and enzymatic labelling of hGH on Gln141 using TGase (PEG‐Gln141‐GH) improve the pharmacokinetics of these two mono‐PEGylated proteins compared with unconjugated GH, and there was no significant difference in the pharmacokinetic parameters between these two conjugates (da Silva et al., 2013). Administration of a single dose in rats of either PEG‐Gln141‐GH or PEG‐Nter‐GH had better or comparable potency compared with daily doses of hGH over 7 days, in terms of weight gain, femoral length, and tibial diaphysis width. No significant differences were observed between the two conjugation sites when attached with 20 kDa PEG (Grigoletto et al., 2016).
This method has been used to produce hGH‐PEGylated at amino acid site Gln141 for pharmaceutical applications. For example, NNC126‐0083 is a long‐acting hGH molecule, in which a 43 kDa PEG residue was attached to Gln141 on hGH. This PEGylation results in prolonged in vivo circulating half‐life by increasing the absorption time and slowing the elimination phase (De Schepper et al., 2011; Rasmussen et al., 2010; Rasmussen et al., 2010; Søndergaard et al., 2011). GH formulations using PEGylation technology have been reviewed recently (Steiner et al., 2023) and some of these will be discussed in Section 4.
In summary, PEGylation is a common approach used to improve pharmacokinetic profiles and reduce immunogenicity of therapeutic proteins; however, optimizing the PEGylation strategy to minimize any negative effects on the biological activity of the protein can be a time‐consuming trial and error process. With the development of new polymer chemistries and conjugation methods, more options have become available. For example, the use of degradable polymers may avoid common issues associated with PEG such as production of anti‐PEG antibodies, and potential accumulation in tissues with long‐term treatment (Hoang Thi et al., 2020; Ibrahim et al., 2022; Zhang et al., 2016). There is also a growing interest in exploring the potential for using stimuli‐responsive/self‐immolative smart polymers to modify the pharmacological activity of therapeutic proteins (Fogueri & Singh, 2009). For example, a polymer might be engineered to achieve preprogramed pulsatile release of GH over a period of time, mimicking the natural pattern of pituitary GH secretion. However, we note that the production of new polymer materials for human use can be complex and expensive, and there may be still safety concerns related to the long‐term use of these materials.
3.2. Fusion proteins
Protein fusion is another approach to increase the half‐life of biotherapeutics and advances in fusion technology have led to the generation of new classes long‐acting hGH biotherapeutics. For example, fusing HSA to the N‐terminus of hGH led to the development of albutropin which has improved pharmacokinetic properties (Osborn et al., 2002). Compared with hGH, albutropin exhibits 4‐ and 6‐fold increased serum half‐life in rats and monkeys, respectively. In addition, a single administration of albutropin had comparable bioactivity to seven consecutive daily injections of hGH. The pharmacokinetic and pharmacodynamic profile in rats and monkeys suggest that albutropin can be administrated less frequently than hGH to achieve similar therapeutic effects in patients (Osborn et al., 2002).
Albumin has also been linked to hGH by N‐terminal modification with pseudo‐bifunctional PEG‐hexadecane (3.5 or 10 kDa PEG) as the linker (Wu et al., 2015). hGH‐PEG3.5 fused to albumin exhibited longer half‐life (19.2 ± 1.0 h) than hGH (1.9 ± 0.1 h) and hGH‐PEG3.5‐hexadecane (13.7 ± 0.3 h; Wu et al., 2013). Another study described a series of hGH–albumin fusion conjugates generated using a range of different linkers and conjugation sites and found that conjugation was most easily achieved through reductive alkylation or by alkylation to introduced cysteine residues using functionalized albumin‐binding side chains. Position L101C on hGH proved to be the optimal position for conjugation, with improved pharmacodynamic properties observed with a once‐weekly dosing regimen (Ramírez‐Andersen et al., 2018).
Fusion with antibodies is another approach to improve pharmacokinetic properties of hGH. For example, hGH was fused to a hybrid Fc fragment containing partial Fc domains of human Immunoglobulin D (IgD) and IgG4 without any site‐directed mutagenesis. This hGH–Fc fusion protein was called GX–H9 and was codeveloped by Genexine and Handok. Fusion to the Fc domain increased the hydrodynamic diameter from 4.8 ± 0.9 to 10.5 ± 2.1 nm and increased the molecular weight from 20 to 130 kDa (Kim et al., 2013; Kim et al., 2013). A Phase II study reported that GX–H9 has the potential for twice‐monthly administration (Ku et al., 2018). Long‐acting GH formulations using protein fusion technology have been reviewed (Cawley et al., 2013; Høybye et al., 2015; Miller et al., 2020; Steiner et al., 2023; Yuen et al., 2018) and some of these formations will be discussed in the next section.
4. LONG‐ACTING GH PHARMACEUTICAL FORMULATIONS IN CLINICAL DEVELOPMENT
Long‐acting GH has the potential to improve patient compliance rates due to less frequent dosing. The first long‐acting GH formulation employed a microsphere technique which creates a depot for rhGH upon injection (Johnson et al., 1997; Kemp et al., 2004). Under normal physiological conditions, anterior pituitary released GH aggregates for compact storage in secretory granules and this process is assisted by abundant zinc ions (Miletta et al., 2014; Thorlacius‐Ussing, 1987). To mimic the native microenvironment, rhGH is complexed with zinc prior to being encapsulated in biocompatible and biodegradable polylactide co‐glycolic acid (PLGA) microspheres. Upon subcutaneous injection, GH release occurs in two steps: the initial phase of diffusion, followed by the prolonged‐release phase of diffusion and polymer degradation. Emptied PLGA microspheres degrade by hydrolysis and the remnant biocompatible lactic and glycolic acids are promptly cleared by the kidneys (Park, 1995). The overall process of microsphere degradation is slow, allowing prolonged GH release, which is sustained from 1 week up to 1 month. Hence, the need for daily injections is eliminated. In 1999, Nutropin Depot was the first long‐acting GH to be approved for use in GHD patients and was found to improve serum IGF‐1 levels to a normal range for up to 17 days (Cook et al., 2002; Kemp et al., 2004). However, in 2004, Nutropin Depot was withdrawn from the market due to costs involved in manufacturing the product and formulation issues including viscosity which made it difficult to administer with large administration volumes required (Lal & Hoffman, 2018). A newer version of depot formulation is LB03002 (Eutropin Plus) which offers a sustained release of rhGH using sodium hyaluronate microspheres. Upon injection, tissue hyaluronidase at the site of injection breaks the microspheres to release hGH (Peter et al., 2009). Previously, the use of fine needles was difficult due to the larger size of the PLGA microparticles but the small particle size and low viscous medium chain triglycerides of sodium hyaluronate microspheres mitigated this issue (Kim et al., 2005). A randomized, controlled study confirmed 0.7 mg/kg/week administration was noninferior to daily rhGH with a total 0.37 mg/kg/week dose (Hwang et al., 2018). LB03002 is currently marketed for clinical use in South Korea for GHD children and is also approved in Europe.
Several PEGylated formulation of GH have been made and tested by pharmaceutical companies (Høybye et al., 2015). PHA‐794428 (Pfizer) is an earlier version of PEGylated GH with a branched 40 kDa PEG attached to the N terminus. This increases the molecular weight of the PHA‐794428 conjugate to ~62 kDa. Human pharmacokinetic data comparing equivalent doses of somatropin (rhGH) and PHA‐794428 (60–100 μg/kg) indicated that PHA‐794428 has an ~6‐ to 7‐fold increase in half‐life compared with somatropin (Webster et al., 2008). However, a significant number of adult GHD patients who received PHA‐794428, displayed lipoatrophy, which provoked discontinuation of pegylated GH formulations for some time (Touraine et al., 2009). Another pegylated‐GH formulation NNC126‐0083 (Novo Nordisk) was prepared by conjugation of 43 kDa PEG to glutamine 141 of the GH. This formulation was well tolerated and no lipoatrophy was observed (Søndergaard et al., 2011), but it was discontinued as dosing did not achieve a satisfactory weekly IGF‐1 profile (De Schepper et al., 2011). A more recent example of a long‐acting GH, Jintrolong (GeneScience Pharmaceuticals), consists of a branched 40 kDa PEG molecule attached to amino groups of rhGH. This formulation is well tolerated and no injection‐site lipoatrophy was observed (Luo et al., 2017). Clinical studies in patients with GHD demonstrated that once‐weekly Jintrolong treatment is effective and safe (Du et al., 2022; Hou et al., 2023; Wu et al., 2022).
Reversible attachment of a long‐acting carrier to rhGHs forms an inactive prodrug which can be designed to release GH across an appropriate time frame. TransCon hGH (SKYTROFA, lonapegsomatropin‐tcgd; Ascendis Pharma) is an rhGH transiently bound to PEG using a hydrolysable linker to achieve an extended serum half‐life. The linker is designed with specific characteristics to allow hydrolysis in a controlled manner under physiologic pH and temperature so the fully release of unmodified rhGH occurs over a 1‐week period. Tolerance at injection sites was satisfactory and dosing at 0.14–0.3 mg/kg/week led to a dose‐related IGF‐1 elevation to normal physiological range (Chatelain et al., 2017). A Phase III trial demonstrated greater height velocity in GHD children who received weekly TransCon hGH treatment for a year, compared with daily rhGH injections (Thornton et al., 2019). TransCon hGH is approved by the US FDA and European Commission as a once‐weekly subcutaneous injection for children with growth failure.
Alternative approaches include fusion protein therapeutics. A novel formulation of long‐acting GH targets its affinity for endogenous albumin, the most abundant serum protein in the blood. Somapacitan (Sogroya; Novo Nordisk) is a rhGH covalently attached to a 1.2 kDa fatty acid which facilitates noncovalent and reversible binding of GH to albumin. The extended half‐life allows for once‐weekly dosing (Johannsson et al., 2018). In both GHD adults and children, IGF‐1 levels were raised to normal physiological levels throughout the week upon single dosage (Juul et al., 2019). Somapacitan has recently been approved for GHD in adults and children. Another clinically approved fusion protein is Somatrogon (Ngenla, MOD‐4023; OpKo health and Pfizer). Somatrogon is a long‐acting GH fused to three copies of the carboxyl‐terminal peptides derived from the human chorionic gonadotropin, currently used for treating pediatric GHD as a once‐weekly subcutaneous injection (Deal et al., 2022; Horikawa et al., 2022). Other versions of fusion proteins are currently under development at various stages of clinical trials: GX–H9 (Genexine and Handok), a long‐acting GH fused to a hybrid Fc which consists of noncytolytic IgD and IgG4 (Ku et al., 2018; Malievskiy et al., 2020); JR‐142 (JCR Pharmaceuticals), a long‐acting GH fused to a modified HSA (Japan Registry of Clinical Trials, 2021); LAPS rhGH (HM10560A; Hanmi Pharmaceutical Co), a long‐acting GH conjugated to recombinant immunoglobulin G4 Fc fragment (clinicaltrials.gov, HM10560A).
One of the primary advantages of long‐acting GH formulations lies in their ability to reduce the burden of frequent injections, as conventional rhGH necessitates daily administration. The ultimate goal for these formulations is to further decrease the dosing frequency to a weekly or even monthly basis. However, GHA administration requires more frequent dosing in order to be effective. For example, pegvisomant which is currently the only clinically available long‐acting GHA formulation requires daily dose even though it has a 72 h serum half‐life in humans. This highlights the challenges for developing long‐acting GHAs. Unlike long‐acting GH agonists, an antagonist would need to compete with circulating GH which is released in pulses from the pituitary and may therefore require a longer circulating half‐life and higher dosing to effectively compete. Ongoing research and advances in drug delivery systems and formulation technologies such as sustained‐release formulations, depot injections, or implantable devices may lead to the development of new approaches to improve the pharmacokinetic properties of GHA formulations, thus reducing the need for frequent dosing. It is important to note that the development of long‐acting GH or GHAs is a complex and challenging task, and it may require a multidisciplinary approach involving expertise in drug design, protein/peptide and polymer technologies, pharmacokinetics, and pharmacodynamics, and formulation science. Ongoing research efforts, collaborations between academia and industry, and advancements in biotechnology will likely play a crucial role in overcoming these challenges and advancing the field.
5. CONCLUSION
The GH axis is an important target for medical therapy that is indicated for GHD, acromegaly and may also be applicable in other diseases. Expression of recombinant GH or GHA has been demonstrated in various host systems, such as prokaryotic (E. coli) and eukaryotic (i.e., yeast and mammalian cells) expression systems. However, large‐scale production of recombinant GH and GHA is still challenging. Furthermore, the short serum half‐life for unmodified GH and GHA is an obstacle for in vivo applications. As discussed here, a variety of strategies have been used to modify the protein core to extend the serum half‐life, with varying success. Several rhGH formulations have been released into the market, but only one GHA (pegvisomant) is available for clinical use, and there is still a need for the development of novel long‐acting GHAs.
AUTHOR CONTRIBUTIONS
Yue Wang: Writing—original draft; conceptualization; writing—review and editing; methodology. Minah Kim: Writing—original draft; writing—review and editing. Chantal Buckley: Writing—original draft; writing—review and editing. Heather D. Maynard: Writing—review and editing. Ries J. Langley: Writing—review and editing. Jo K. Perry: Conceptualization; writing—review and editing; methodology; supervision.
ACKNOWLEDGMENTS
We thank Theo Portlock for assistance with figures. Open access publishing facilitated by The University of Auckland, as part of the Wiley ‐ The University of Auckland agreement via the Council of Australian University Librarians.
Wang Y, Kim M, Buckley C, Maynard HD, Langley RJ, Perry JK. Growth hormone receptor agonists and antagonists: From protein expression and purification to long‐acting formulations. Protein Science. 2023;32(9):e4727. 10.1002/pro.4727
Review Editor: Aitziber L. Cortajarena
Contributor Information
Yue Wang, Email: wang.yue@auckland.ac.nz.
Jo K. Perry, Email: j.perry@auckland.ac.nz.
REFERENCES
- Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252(11):3582–3586. [PubMed] [Google Scholar]
- Aghili Z, Zarkesh‐Esfahani S. Taguchi experimental design for optimization of recombinant human growth hormone production in CHO cell lines and comparing its biological activity with prokaryotic growth hormone. Drug Res (Stuttg). 2018;68(2):80–88. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Hirz M, Pichler H, Schwab H. Protein expression in Pichia pastoris: recent achievements and perspectives for heterologous protein production. Appl Microbiol Biotechnol. 2014;98(12):5301–5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alanen HI, Walker KL, Lourdes Velez Suberbie M, Matos CFRO, Bönisch S, Freedman RB, et al. Efficient export of human growth hormone, interferon α2b and antibody fragments to the periplasm by the Escherichia coli Tat pathway in the absence of prior disulfide bond formation. Biochim Biophys Acta. 2015;1853(3):756–763. [DOI] [PubMed] [Google Scholar]
- Alconcel SNS, Baas AS, Maynard HD. FDA‐approved poly(ethylene glycol)–protein conjugate drugs. Polym Chem. 2011;2(7):1442. [Google Scholar]
- AlQahtani AD, O'Connor D, Domling A, Goda SK. Strategies for the production of long‐acting therapeutics and efficient drug delivery for cancer treatment. Biomed Pharmacother. 2019;113:108750. [DOI] [PubMed] [Google Scholar]
- Amaranto M, Vaccarello P, Correa EME, Barra JL, Godino A. Novel intein‐based self‐cleaving affinity tag for recombinant protein production in Escherichia coli . J Biotechnol. 2021;332:126–134. [DOI] [PubMed] [Google Scholar]
- Apte‐Deshpande A, Rewanwar S, Kotwal P, Raiker VA, Padmanabhan S. Efficient expression and secretion of recombinant human growth hormone in the methylotrophic yeast Pichia pastoris: potential applications for other proteins. Biotechnol Appl Biochem. 2009;54(4):197–205. [DOI] [PubMed] [Google Scholar]
- Aramvash A, Sabet A, Mansurpur M, Azizi A, Bahrami A, Kamali N. Comparison of purification processes for recombinant human growth hormone produced in E. coli . Iran J Sci Technol Trans A Sci. 2018;42(4):1697–1705. [Google Scholar]
- Ascacio‐Martínez JA, Barrera‐Saldaña HA. Production and secretion of biologically active recombinant canine growth hormone by Pichia pastoris . Gene. 2004;340(2):261–266. [DOI] [PubMed] [Google Scholar]
- Ayuk J. Growth hormone and its disorders. Postgrad Med J. 2006;82(963):24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azadi S, Mahboubi A, Naghdi N, Solaimanian R, Mortazavi SA. Evaluation of sorbitol‐methanol co‐feeding strategy on production of recombinant human growth hormone in Pichia Pastoris . Iran J Pharm Res. 2017;16(4):1555–1564. [PMC free article] [PubMed] [Google Scholar]
- Azadi S, Sadjady S, Mortazavi S, Naghdi N, Mahboubi A, Solaimanian R. Bioprocess and downstream optimization of recombinant human growth hormone in Pichia pastoris . Res Pharm Sci. 2018;13(3):222–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherinejad M, Sadeghi HM, Abedi D, Moazen F, Rabbani M. Effect of twine‐arginine translocation‐signaling fusion system and chaperones co‐expression on secretory expression of somatropin. Adv Biomed Res. 2018;7(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagherinejad MR, Sadeghi HMM, Abedi D, Chou CP, Moazen F, Rabbani M. Twin arginine translocation system in secretory expression of recombinant human growth hormone. Res Pharm Sci. 2016;11(6):461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasundaram B, Harrison S, Bracewell DG. Advances in product release strategies and impact on bioprocess design. Trends Biotechnol. 2009;27(8):477–485. [DOI] [PubMed] [Google Scholar]
- Barrett CML, Ray N, Thomas JD, Robinson C, Bolhuis A. Quantitative export of a reporter protein, GFP, by the twin‐arginine translocation pathway in Escherichia coli . Biochem Biophys Res Commun. 2003;304(2):279–284. [DOI] [PubMed] [Google Scholar]
- Becker GW, Hsiung HM. Expression, secretion and folding of human growth hormone in Escherichia coli . FEBS Lett. 1986;204(1):145–150. [DOI] [PubMed] [Google Scholar]
- Bougen NM, Steiner M, Pertziger M, Banerjee A, Brunet‐Dunand SE, Zhu T, et al. Autocrine human GH promotes radioresistance in mammary and endometrial carcinoma cells. Endocr Relat Cancer. 2012;19(5):625–644. [DOI] [PubMed] [Google Scholar]
- Brooks AJ, Dai W, O'Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, et al. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344(6185):1249783. [DOI] [PubMed] [Google Scholar]
- Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and clinical implications. Nat Rev Endocrinol. 2010;6(9):515–525. [DOI] [PubMed] [Google Scholar]
- Browning DF, Richards KL, Peswani AR, Roobol J, Busby SJW, Robinson C. Escherichia coli “TatExpress” strains super‐secrete human growth hormone into the bacterial periplasm by the tat pathway. Biotechnol Bioeng. 2017;114(12):2828–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calik P, Orman MA, Celik E, Halloran SM, Calik G, Ozdamar TH. Expression system for synthesis and purification of recombinant human growth hormone in Pichia pastoris and structural analysis by MALDI‐ToF mass spectrometry. Biotechnol Prog. 2008;24(1):221–226. [DOI] [PubMed] [Google Scholar]
- Camberg JL, Doyle SM, Johnston DM, Wickner S. Molecular chaperones. Brenner's Encyclopedia of Genetics. Elsevier; 2013. p. 456–460. [Google Scholar]
- Campbell RM, Chen WY, Wiehl P, Kelder B, Kopchick JJ, Scanes CG. A growth hormone (GH) analog that antagonizes the Lipolytic effect but retains full insulin‐like (Antilipolytic) activity of GH. Exp Biol Med. 1993;203(3):311–316. [DOI] [PubMed] [Google Scholar]
- Carroll PV, Christ ER, Sönksen PH. Growth hormone replacement in adults with growth hormone deficiency: assessment of current knowledge. Trends Endocrinol Metab. 2000;11(6):231–238. [DOI] [PubMed] [Google Scholar]
- Cawley P, Wilkinson I, Ross RJ. Developing long‐acting growth hormone formulations. Clin Endocrinol (Oxf). 2013;79(3):305–309. [DOI] [PubMed] [Google Scholar]
- Chang CN, Key M, Bochner B, Heyneker H, Gray G. High‐level secretion of human growth hormone by Escherichia coli . Gene. 1987;55(2–3):189–196. [DOI] [PubMed] [Google Scholar]
- Chang JYH, Pai RC, Bennett WF, Bochner BR. Periplasmic secretion of human growth hormone by Escherichia coli . Biochem Soc Trans. 1989;17(2):335–337. [DOI] [PubMed] [Google Scholar]
- Chanson P, Salenave S. Acromegaly. Orphanet J Rare Dis. 2008;3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatelain P, Malievskiy O, Radziuk K, Senatorova G, Abdou MO, Vlachopapadopoulou E, et al. A randomized phase 2 study of long‐acting TransCon GH vs daily GH in childhood GH deficiency. J Clin Endocrinol Metab. 2017;102(5):1673–1682. [DOI] [PubMed] [Google Scholar]
- Chen WY, Wight DC, Wagner TE, Kopchick JJ. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc Natl Acad Sci U S A. 1990;87(13):5061–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokova V, Melmed S. Growth hormone in the tumor microenvironment. Arch Endocrinol Metab. 2019;63(6):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Daniel T, Buechler YJ, Litzinger DC, Maio Z, Putnam AMH, et al. Optimized clinical performance of growth hormone with an expanded genetic code. Proc Natl Acad Sci U S A. 2011;108(22):9060–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JM, Lee TH, Chung HH, Lee YB, Lee TG, Park YW, et al. Method for the production of porcine growth hormone using a synthetic gene in yeast cells. Eur Pat. 1987:EP0295287B1. Published online December 28. [Google Scholar]
- Choi TJ, Geletu TT. High level expression and purification of recombinant flounder growth hormone in E. coli . J Genet Eng Biotechnol. 2018;16(2):347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WJ, Huang CL, Gong HY, Ou TY, Hsu JL, Hu SY. Recombinant production of biologically active giant grouper (Epinephelus lanceolatus) growth hormone from inclusion bodies of Escherichia coli by fed‐batch culture. Protein Expr Purif. 2015;110:79–88. [DOI] [PubMed] [Google Scholar]
- Chura‐Chambi RM, Farah CS, Morganti L. Human growth hormone inclusion bodies present native‐like secondary and tertiary structures which can be preserved by mild solubilization for refolding. Microb Cell Fact. 2022;21(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Olson K, Fuh G, Marian M, Mortensen D, Teshima G, et al. Long‐acting growth hormones produced by conjugation with polyethylene glycol. J Biol Chem. 1996;271(36):21969–21977. [DOI] [PubMed] [Google Scholar]
- clinicaltrials.gov. Safety and efficacy study of recombinant human growth hormone in adult growth hormone deficiency patients (HM10560A).
- Colao A, Grasso LFS, Giustina A, Melmed S, Chanson P, Pereira AM, et al. Acromegaly. Nat Rev Dis Prim. 2019;5(1):20. [DOI] [PubMed] [Google Scholar]
- Connors MH, Kaplan SL, Li CH, Grumbach MM. Retention of biologic activity of human growth hormone in man after reduction and alkylation. J Clin Endocrinol Metab. 1973;37(4):499–504. [DOI] [PubMed] [Google Scholar]
- Cook DM, Biller BMK, Vance ML, Hoffman AR, Phillips LS, Ford KM, et al. The pharmacokinetic and pharmacodynamic characteristics of a long‐acting growth hormone (GH) preparation (nutropin depot) in GH‐deficient adults. J Clin Endocrinol Metab. 2002;87(10):4508–4514. [DOI] [PubMed] [Google Scholar]
- Cox GN, Rosendahl MS, Chlipala EA, Smith DJ, Carlson SJ, Doherty DH. A long‐acting, mono‐PEGylated human growth hormone analog is a potent stimulator of weight gain and bone growth in Hypophysectomized rats. Endocrinology. 2007;148(4):1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox NG, Doherty DH, Rosendahl MS. US6753165B1 – methods for making proteins containing free cysteine residues – Google patents. US pat no 6753165. Published online 2004. https://patents.google.com/patent/US6753165B1/en.
- Crivelli E, Cardamone M, Puri NK. A single step method for the solubilization and refolding of recombinant protein from E. coli inclusion bodies. Aust J Biotechnol. 1991;5(2):78–80, 86. [PubMed] [Google Scholar]
- da Silva FD, Mero A, Pasut G. Chemical and enzymatic site specific PEGylation of hGH. Bioconjug Chem. 2013;24(3):456–463. [DOI] [PubMed] [Google Scholar]
- Dagnaes‐Hansen F, Duan H, Rasmussen LM, Friend KE, Flyvbjerg A. Growth hormone receptor antagonist administration inhibits growth of human colorectal carcinoma in nude mice. Anticancer Res. 2004;24(6):3735–3742. [PubMed] [Google Scholar]
- Danowitz M, Grimberg A. Clinical indications for growth hormone therapy. Adv Pediatr. 2022;69(1):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GD, Elisee C, Newham DM, Harrison RG. New fusion protein systems designed to give soluble expression in Escherichia coli . Biotechnol Bioeng. 1999;65(4):382–388. [PubMed] [Google Scholar]
- De Schepper J, Rasmussen MH, Gucev Z, Eliakim A, Battelino T. Long‐acting pegylated human GH in children with GH deficiency: a single‐dose, dose‐escalation trial investigating safety, tolerability, pharmacokinetics and pharmacodynamics. Eur J Endocrinol. 2011;165(3):401–409. [DOI] [PubMed] [Google Scholar]
- de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255(5042):306–312. [DOI] [PubMed] [Google Scholar]
- Deal CL, Steelman J, Vlachopapadopoulou E, Stawerska R, Silverman LA, Phillip M, et al. Efficacy and safety of weekly Somatrogon vs daily Somatropin in children with growth hormone deficiency: a phase 3 study. J Clin Endocrinol Metab. 2022;107(7):e2717–e2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Li J, Ma M, Zhao P, Ming F, Lu Z, et al. Co‐expressing GroEL–GroES, Ssa1–Sis1 and Bip–PDI chaperones for enhanced intracellular production and partial‐wall breaking improved stability of porcine growth hormone. Microb Cell Fact. 2020;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divisova J, Kuiatse I, Lazard Z, Weiss H, Vreeland F, Hadsell DL, et al. The growth hormone receptor antagonist pegvisomant blocks both mammary gland development and MCF‐7 breast cancer xenograft growth. Breast Cancer Res Treat. 2006;98(3):315–327. [DOI] [PubMed] [Google Scholar]
- Doerwald FZ, Iversen LF, Johansen NL. Transglutaminase mediated conjugation of growth hormone. 2006. Pat WO2006134148. Published online June 15, 2006.
- Dozier J, Distefano M. Site‐specific PEGylation of therapeutic proteins. Int J Mol Sci. 2015;16(10):25831–25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Wu D, Yi P, Bai X, Luo Y, Yang H, et al. Evaluation of efficacy and safety of long‐acting PEGylated recombinant human growth hormone (Jintrolong) for patients with growth hormone deficiency. J Pediatr Endocrinol Metab. 2022;35(4):511–517. [DOI] [PubMed] [Google Scholar]
- Evans A, Jamieson SM, Liu DX, Wilson WR, Perry JK. Growth hormone receptor antagonism suppresses tumour regrowth after radiotherapy in an endometrial cancer xenograft model. Cancer Lett. 2016;379(1):117–123. [DOI] [PubMed] [Google Scholar]
- Fee CJ, Van Alstine JM. Purification of PEGylated proteins. Protein purification: principles, high resolution methods, and applications. 3rd ed. Wiley Blackwell; 2011. p. 339–362. [DOI] [PubMed] [Google Scholar]
- Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97(10):4167–4183. [DOI] [PubMed] [Google Scholar]
- Fogueri L, Singh S. Smart polymers for controlled delivery of proteins and peptides: a review of patents. Recent Pat Drug Deliv Formul. 2009;3(1):40–48. [DOI] [PubMed] [Google Scholar]
- Fradkin AH, Boand CS, Eisenberg SP, Rosendahl MS, Randolph TW. Recombinant murine growth hormone from E. coli inclusion bodies: expression, high‐pressure solubilization and refolding, and characterization of activity and structure. Biotechnol Prog. 2010;26(3):743–749. [DOI] [PubMed] [Google Scholar]
- Franklin SL, Geffner ME. Growth hormone: the expansion of available products and indications. Pediatr Clin North Am. 2011;58(5):1141–1165. [DOI] [PubMed] [Google Scholar]
- Freudl R. Signal peptides for recombinant protein secretion in bacterial expression systems. Microb Cell Fact. 2018;17(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend KE, Radinsky R, McCutcheon IE. Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg. 1999;91(1):93–99. [DOI] [PubMed] [Google Scholar]
- Funkenstein B, Dyman A, Lapidot Z, de Jesus‐Ayson EG, Gertler A, Ayson FG. Expression and purification of a biologically active recombinant rabbitfish (Siganus guttatus) growth hormone. Aquaculture. 2005;250(1–2):504–515. [Google Scholar]
- Gaciarz A, Khatri NK, Velez‐Suberbie ML, Saaranen MJ, Uchida Y, Keshavarz‐Moore E, et al. Efficient soluble expression of disulfide bonded proteins in the cytoplasm of Escherichia coli in fed‐batch fermentations on chemically defined minimal media. Microb Cell Fact. 2017;16(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Shi Z. Process control and optimization for heterologous protein production by methylotrophic Pichia pastoris . Chinese J Chem Eng. 2013;21(2):216–226. [Google Scholar]
- George HJ, L'italien JJ, Pilacinski WP, Glassman DL, Krzyzek RA. High‐level expression in Escherichia coli of biologically active bovine growth hormone. DNA. 1985;4(4):273–281. [DOI] [PubMed] [Google Scholar]
- Georgiou G, Segatori L. Preparative expression of secreted proteins in bacteria: status report and future prospects. Curr Opin Biotechnol. 2005;16(5):538–545. [DOI] [PubMed] [Google Scholar]
- Ghorpade A, Garg LC. Efficient processing and export of human growth hormone by heat labile enterotoxin chain B signal sequence. FEBS Lett. 1993;330(1):61–65. [DOI] [PubMed] [Google Scholar]
- Gray GL, Baldridge JS, McKeown KS, Heyneker HL, Chang CN. Periplasmic production of correctly processed human growth hormone in Escherichia coli: natural and bacterial signal sequences are interchangeable. Gene. 1985;39(2–3):247–254. [DOI] [PubMed] [Google Scholar]
- Grigoletto A, Mero A, Zanusso I, Schiavon O, Pasut G. Chemical and enzymatic site specific PEGylation of hGH: the stability and in vivo activity of PEG‐N‐terminal‐hGH and PEG‐Gln141‐hGH conjugates. Macromol Biosci. 2016;16(1):50–56. [DOI] [PubMed] [Google Scholar]
- Guerrero Montero I, Richards KL, Jawara C, Browning DF, Peswani AR, Labrit M, et al. Escherichia coli “TatExpress” strains export several g/L human growth hormone to the periplasm by the tat pathway. Biotechnol Bioeng. 2019;116(12):3282–3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah‐Shmouni F, Trivellin G, Stratakis CA. Genetics of gigantism and acromegaly. Growth Horm IGF Res. 2016;30‐31:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Chen CJH, Sonenberg M. Recombination of the biologically active peptides from a tryptic digest of bovine growth hormone. Biochemistry. 1978;17(3):550–556. [DOI] [PubMed] [Google Scholar]
- Hoang Thi TT, Pilkington EH, Nguyen DH, Lee JS, Park KD, Truong NP. The importance of poly(ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers (Basel). 2020;12(2):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikawa R, Tanaka T, Hasegawa Y, Yorifuji T, Ng D, Rosenfeld RG, et al. Efficacy and safety of once‐weekly Somatrogon compared with once‐daily Somatropin (Genotropin®) in Japanese children with pediatric growth hormone deficiency: results from a randomized phase 3 study. Horm Res Paediatr. 2022;95(3):275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Huang K, Gong C, Luo F, Wei H, Liang L, et al. Long‐term pegylated GH for children with GH deficiency: a large, prospective. Real‐World Study. J Clin Endocrinol Metab. 2023;00:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høybye C, Cohen P, Hoffman AR, Ross R, Biller BM, Christiansen JS, et al. Status of long‐acting‐growth hormone preparations – 2015. Growth Horm IGF Res. 2015;25(5):201–206. [DOI] [PubMed] [Google Scholar]
- Hu S, Zhao X, Wang J, Chang C, Sen L, Su J. Transglutaminase variants with improved specificity. 2017;63(October):132. Available from: https://patentimages.storage.googleapis.com/34/7f/ca/13cf303baedee7/WO2017023866A1.pdf. Accessed 7 Sept 2021. [Google Scholar]
- Hwang JS, Lee HS, Lee KH, Yoo HW, Lee DY, Suh BK, et al. Once‐weekly administration of sustained‐release growth hormone in Korean prepubertal children with idiopathic short stature: a randomized, controlled phase II study. Horm Res Paediatr. 2018;90(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M, Ramadan E, Elsadek NE, Emam SE, Shimizu T, Ando H, et al. Polyethylene glycol (PEG): the nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J Control Release. 2022;351:215–230. [DOI] [PubMed] [Google Scholar]
- Isgaard J, Arcopinto M, Karason K, Cittadini A. GH and the cardiovascular system: an update on a topic at heart. Endocrine. 2015;48(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeh HS, Kim CH, Lee HK, Han K. Recombinant flounder growth hormone from Escherichia coli: overexpression, efficient recovery, and growth‐promoting effect on juvenile flounder by oral administration. J Biotechnol. 1998;60(3):183–193. [DOI] [PubMed] [Google Scholar]
- Jeiranikhameneh M, Moshiri F, Falasafi SK, Zomorodipour A. Designing signal peptides for efficient periplasmic expression of human growth hormone in Escherichia coli . J Microbiol Biotechnol. 2017;27(11):1999–2009. [DOI] [PubMed] [Google Scholar]
- Jin M, Junjie B, Xinhui L, Jianren L, Qing J, Hongjun Z. Expression of rainbow trout growth hormone cDNA in Saccharomyces cerevisiae . Chin J Biotechnol. 1999;15(4):219–224. [PubMed] [Google Scholar]
- Jing O, Qingxin L, Lin Y, Xunzhang W. Secretion expression of porcine growth hormone gene in insect cells. Xu Mu Shou Yi Xue Bao Acta Vet Zootech Sin. 2002;33(5):482–485. [Google Scholar]
- Johannsson G, Feldt‐Rasmussen U, Håkonsson IH, Biering H, Rodien P, Tahara S, et al. Safety and convenience of once‐weekly somapacitan in adult GH deficiency: a 26‐week randomized, controlled trial. Eur J Endocrinol. 2018;178(5):491–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson OL, Jaworowicz W, Cleland JL, Bailey L, Charnis M, Duenas E, et al. The stabilization and encapsulation of human growth hormone into biodegradable microspheres. Pharm Res. 1997;14(6):730–735. [DOI] [PubMed] [Google Scholar]
- Jung C, Lee YP, Jeong YR, Kim JY, Kim YH, Kim HS. Characterization of Nα‐acetyl methionyl human growth hormone formed during expression in Saccharomyces cerevisiae with liquid chromatography and mass spectrometry. J Chromatogr B. 2005;814(1):53–59. [DOI] [PubMed] [Google Scholar]
- Junnila RK, Kopchick JJ. Significance of the disulphide bonds of human growth hormone. Endokrynol pol. 2013;64(4):300–304. [DOI] [PubMed] [Google Scholar]
- Junnila RK, Wu Z, Strasburger CJ. The role of human growth hormone's C‐terminal disulfide bridge. Growth Horm IGF Res. 2013;23(3):62–67. [DOI] [PubMed] [Google Scholar]
- Juturu V, Wu JC. Heterologous protein expression in Pichia pastoris: latest research Progress and applications. Chembiochem. 2018;19(1):7–21. [DOI] [PubMed] [Google Scholar]
- Juul RV, Rasmussen MH, Agersø H, Overgaard RV. Pharmacokinetics and pharmacodynamics of once‐weekly Somapacitan in children and adults: supporting dosing rationales with a model‐based analysis of three phase I trials. Clin Pharmacokinet. 2019;58(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulsay KK, Zhu T, Bennett W, Lee KO, Lobie PE. The effects of autocrine human growth hormone (hGH) on human mammary carcinoma cell behavior are mediated via the hGH receptor. Endocrinology. 2001;142(2):767–777. [DOI] [PubMed] [Google Scholar]
- Kemp SF, Fielder PJ, Attie KM, Blethen SL, Reiter EO, Ford KM, et al. Pharmacokinetic and pharmacodynamic characteristics of a long‐acting growth hormone (GH) preparation (nutropin depot) in GH‐deficient children. J Clin Endocrinol Metab. 2004;89(7):3234–3240. [DOI] [PubMed] [Google Scholar]
- Keshavarz R, Babaeipour V, Mohammadpour‐Aghdam M, Deldar AA. Overexpression, overproduction, purification, and characterization of rhGH in Escherichia coli . Biotechnol Appl Biochem. 2021;68(1):122–135. [DOI] [PubMed] [Google Scholar]
- Khameneh B, Jaafari MR, Hassanzadeh‐Khayyat M, Varasteh AR, Chamani JK, Iranshahi M, et al. Preparation, characterization and molecular modeling of PEGylated human growth hormone with agonist activity. Int J Biol Macromol. 2015;80:400–409. [DOI] [PubMed] [Google Scholar]
- Khan RH, Appa Rao KBC, Eshwari ANS, Totey SM, Panda AK. Solubilization of recombinant ovine growth hormone with retention of native‐like secondary structure and its refolding from the inclusion bodies of Escherichia coli . Biotechnol Prog. 1998;14(5):722–728. [DOI] [PubMed] [Google Scholar]
- Kim ES, Jang DS, Yang SY, Lee MN, Jin KS, Cha HJ, et al. Controlled release of human growth hormone fused with a human hybrid fc fragment through a nanoporous polymer membrane. Nanoscale. 2013;5(10):4262–4269. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Park HS, Seo KH, Yang HJ, Kim SK, Choi JH. Complete solubilization and purification of recombinant human growth hormone produced in Escherichia coli. Mulvenna J, ed. PLoS One. 2013;8(2):e56168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Hahn SK, Kim MJ, Kim DH, Lee YP. Development of a novel sustained release formulation of recombinant human growth hormone using sodium hyaluronate microparticles. J Control Release. 2005;104(2):323–335. [DOI] [PubMed] [Google Scholar]
- Kirk J. Indications for growth hormone therapy in children. Arch Dis Child. 2012;97(1):63–68. [DOI] [PubMed] [Google Scholar]
- Ko JH, Maynard HD. A guide to maximizing the therapeutic potential of protein–polymer conjugates by rational design. Chem Soc Rev. 2018;47(24):8998–9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo TY, Park TH. Expression of recombinant human growth hormone in a soluble form in Escherichia coli by slowing down the protein synthesis rate. J Microbiol Biotechnol. 2007;17(4):579–585. [PubMed] [Google Scholar]
- Kopchick JJ. History and future of growth hormone research. Horm Res Paediatr. 2003;60(3):103–112. [DOI] [PubMed] [Google Scholar]
- Kopchick JJ, Basu R, Berryman DE, Jorgensen JOL, Johannsson G, Puri V. Covert actions of growth hormone: fibrosis, cardiovascular diseases and cancer. Nat Rev Endocrinol. 2022;18(9):558–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick JJ, List EO, Kelder B, Gosney ES, Berryman DE. Evaluation of growth hormone (GH) action in mice: discovery of GH receptor antagonists and clinical indications. Mol Cell Endocrinol. 2014;386(1–2):34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopchick JJ, Parkinson C, Stevens EC, Trainer PJ. Growth hormone receptor antagonists: discovery, development, and use in patients with acromegaly. Endocr Rev. 2002;23(5):623–646. [DOI] [PubMed] [Google Scholar]
- Ku CR, Brue T, Schilbach K, Ignatenko S, Magony S, Chung YS, et al. Long‐acting FC‐fusion rhGH (GX‐H9) shows potential for up to twice‐monthly administration in GH‐deficient adults. Eur J Endocrinol. 2018;179(3):169–179. [DOI] [PubMed] [Google Scholar]
- Lal RA, Hoffman AR. Long‐acting growth hormone preparations in the treatment of children. Pediatr Endocrinol Rev. 2018;16(Suppl 1):162–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley KE, Berg TF, Strickland TW, Fenton DM, Boone TC, Wypych J. Recombinant‐DNA‐derived bovine growth hormone from Escherichia coli. 1. Demonstration that the hormone is expressed in reduced form, and isolation of the hormone in oxidized, native form. Eur J Biochem. 1987;163(2):313–321. [DOI] [PubMed] [Google Scholar]
- Lee TC, Kang M, Kim CH, Schultz PG, Chapman E, Deniz AA. Dual unnatural amino acid incorporation and click‐chemistry labeling to enable single‐molecule FRET studies of p97 folding. Chembiochem. 2016;17(11):981–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempereur L, Brambilla D, Maria Scoto G, D'Alcamo M, Goffin V, Crosta L, et al. Growth hormone protects human lymphocytes from irradiation‐induced cell death. Br J Pharmacol. 2003;138(8):1411–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levarski Z, Šoltýsová A, Krahulec J, Stuchlík S, Turňa J. High‐level expression and purification of recombinant human growth hormone produced in soluble form in Escherichia coli . Protein Expr Purif. 2014;100:40–47. [DOI] [PubMed] [Google Scholar]
- Li W, Li X, Zhong F, Jin H, Xie M, Liu Y, et al. Cloning and expression of fox growth hormone gene in Pichia pastoris . Sheng Wu Gong Cheng Xue Bao. 2009;25(10):1470–1476. [PubMed] [Google Scholar]
- Li W, Zhu LH, Wang EB, Ye ZC, Lin J, Guo LH, et al. Effect of two human growth hormone receptor antagonists on glomerulosclerosis in streptozotocin‐induced diabetic rats. Acta Pharmacol Sin. 2004;25(4):490–495. [PubMed] [Google Scholar]
- Lin CC, Liu TW, Yeh ML, Tsai YS, Tsai PC, Huang CF, et al. Significant down‐regulation of growth hormone receptor expression revealed as a new unfavorable prognostic factor in hepatitis C virus‐related hepatocellular carcinoma. Clin Mol Hepatol. 2021;27(2):313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Amesso Ndengue PP, Jing Y, Zhao L, Yang X. Facile expression and purification of active human growth hormone in E. coli by a cleavable self‐aggregating tag scheme. Protein Expr Purif. 2021;188:105974. [DOI] [PubMed] [Google Scholar]
- Liu F, Wu X, Li L, Liu Z, Wang Z. Use of baculovirus expression system for generation of virus‐like particles: successes and challenges. Protein Expr Purif. 2013;90(2):104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Li J, Liu M, Hou J. Molecular chaperone GroEL‐GroES enhances the soluble expression of biologically active ovine growth hormone in the prokaryotic system. Protein Expr Purif. 2022;195–196:106097. [DOI] [PubMed] [Google Scholar]
- Lu M, Flanagan JU, Langley RJ, Hay MP, Perry JK. Targeting growth hormone function: strategies and therapeutic applications. Signal Transduct Target Ther. 2019;4(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Hou L, Liang L, Dong G, Shen S, Zhao Z, et al. Long‐acting PEGylated recombinant human growth hormone (Jintrolong) for children with growth hormone deficiency: phase II and phase III multicenter, randomized studies. Eur J Endocrinol. 2017;177(2):195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupker JH, Roskam WG, Miloux B, Liauzun P, Yaniv M, Jouannau J. Abundant excretion of human growth hormone by recombinant‐plasmid‐transformed monkey kidney cells. Gene. 1983;24(2–3):281–287. [DOI] [PubMed] [Google Scholar]
- Lyon RP, Setter JR, Bovee TD, Doronina SO, Hunter JH, Anderson ME, et al. Self‐hydrolyzing maleimides improve the stability and pharmacological properties of antibody‐drug conjugates. Nat Biotechnol. 2014;32(10):1059–1062. [DOI] [PubMed] [Google Scholar]
- Mahmoud SS, Wang S, Moloney MM, Habibi HR. Production of a biologically active novel goldfish growth hormone in Escherichia coli . Comp Biochem Physiol Part B Biochem Mol Biol. 1998;120(4):657–663. [DOI] [PubMed] [Google Scholar]
- Malievskiy O, Mykola A, Zelinska N, Bolshova E, Senatorova G, Oroszlán G, et al. SAT‐LB15 24‐month efficacy and safety of once weekly and every other week administration of GX‐H9, hybrid FC‐fused long‐acting human growth hormone: a phase 2 study in children with growth hormone deficiency. J Endocr Soc. 2020;4(Suppl 1). [Google Scholar]
- Matos CFRO, Branston SD, Albiniak A, Dhanoya A, Freedman RB, Keshavarz‐Moore E, et al. High‐yield export of a native heterologous protein to the periplasm by the Tat translocation pathway in Escherichia coli . Biotechnol Bioeng. 2012;109(10):2533–2542. [DOI] [PubMed] [Google Scholar]
- Matos CFRO, Robinson C, Alanen HI, Prus P, Uchida Y, Ruddock LW, et al. Efficient export of prefolded, disulfide‐bonded recombinant proteins to the periplasm by the Tat pathway in Escherichia coli CyDisCo strains. Biotechnol Prog. 2014;30(2):281–290. [DOI] [PubMed] [Google Scholar]
- Menezes ACSC, Suzuki MF, Oliveira JE, Ribela MTCP, Furigo IC, Donato J Jr, et al. Expression, purification and characterization of the authentic form of human growth hormone receptor antagonist G120R‐hGH obtained in Escherichia coli periplasmic space. Protein Expr Purif. 2017;131:91–100. [DOI] [PubMed] [Google Scholar]
- Mero A, Schiavon M, Veronese FM, Pasut G. A new method to increase selectivity of transglutaminase mediated PEGylation of salmon calcitonin and human growth hormone. J Control Release. 2011;154(1):27–34. [DOI] [PubMed] [Google Scholar]
- Mero A, Spolaore B, Veronese FM, Fontana A. Transglutaminase‐mediated PEGylation of proteins: direct identification of the sites of protein modification by mass spectrometry using a novel monodisperse PEG. Bioconjug Chem. 2009;20(2):384–389. [DOI] [PubMed] [Google Scholar]
- Miletta MC, Schöni MH, Kernland K, Mullis PE, Petkovic V. The role of zinc dynamics in growth hormone secretion. Horm Res Paediatr. 2014;80(6):381–389. [DOI] [PubMed] [Google Scholar]
- Miller BS, Velazquez E, Yuen KCJ. Long‐acting growth hormone preparations – current status and future considerations. J Clin Endocrinol Metab. 2020;105(6):e2121–e2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbaha S, Rezaei M, Emamzadeh R, Zarkesh ES. Design and production of a novel chimeric human growth hormone superagonist fused to human Fc domain. Res Pharm Sci. 2022;17(3):284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhija R, Rupa P, Pillai D, Garg LC. High‐level production and one‐step purification of biologically active human growth hormone in Escherichia coli . Gene. 1995;165(2):303–306. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay UK, Sahni G. Production of recombinant buffalo (Bubalus bubalis) and goat (Capra hircus) growth hormones from genetically modified E. coli strains. J Biotechnol. 2002;97(3):199–212. [DOI] [PubMed] [Google Scholar]
- Muller AF, Kopchick JJ, Flyvbjerg A, van der Lely AJ. Growth hormone receptor antagonists. J Clin Endocrinol Metab. 2004;89(4):1503–1511. [DOI] [PubMed] [Google Scholar]
- Murasugi A. Secretory expression of human protein in the yeast Pichia pastoris by controlled fermentor culture. Recent Pat Biotechnol. 2010;4(2):153–166. [DOI] [PubMed] [Google Scholar]
- Natale P, Brüser T, Driessen AJM. Sec‐ and Tat‐mediated protein secretion across the bacterial cytoplasmic membrane‐distinct translocases and mechanisms. Biochim Biophys Acta. 2008;1778(9):1735–1756. [DOI] [PubMed] [Google Scholar]
- Nguyen MT, Koo BK, Thi Vu TT, Song JA, Chong SH, Jeong B, et al. Prokaryotic soluble overexpression and purification of bioactive human growth hormone by fusion to thioredoxin, maltose binding protein, and protein disulfide isomerase. Cotterill S, ed. PLoS One. 2014;9(3):e89038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocłoń E, Solomon G, Hayouka Z, Gertler A. Preparation of biologically active monomeric recombinant zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss) recombinant growth hormones. Fish Physiol Biochem. 2018;44(4):1215–1222. [DOI] [PubMed] [Google Scholar]
- Ohno T, Wang X, Kurashima J, Saijo‐Kurita K, Hirono M. A novel Vero cell line for use as a mammalian host‐vector system in serum‐free medium. Cytotechnology. 1991;7(3):165–172. [DOI] [PubMed] [Google Scholar]
- Orman MA, Çalık P, Özdamar TH. The influence of carbon sources on recombinant‐human‐growth‐hormone production by Pichia pastoris is dependent on phenotype: a comparison of Muts and Mut+ strains. Biotechnol Appl Biochem. 2009;52(3):245–255. [DOI] [PubMed] [Google Scholar]
- Osborn BL, Sekut L, Corcoran M, Poortman C, Sturm B, Chen G, et al. Albutropin: a growth hormone–albumin fusion with improved pharmacokinetics and pharmacodynamics in rats and monkeys. Eur J Pharmacol. 2002;456(1–3):149–158. [DOI] [PubMed] [Google Scholar]
- Paduel A, Chapnik‐Cohen N, Gertler A, Elizur A. Preparation and characterization of recombinant dolphin fish (Coryphaena hippurus) growth hormone. Protein Expr Purif. 1999;16(3):417–423. [DOI] [PubMed] [Google Scholar]
- Paluck SJ, Nguyen TH, Maynard HD. Heparin‐mimicking polymers: synthesis and biological applications. Biomacromolecules. 2016;17(11):3417–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda AK, Khan RH, Appa Rao KBC, Totey SM. Kinetics of inclusion body production in batch and high cell density fed‐batch culture of Escherichia coli expressing ovine growth hormone. J Biotechnol. 1999;75(2–3):161–172. [DOI] [PubMed] [Google Scholar]
- Park TG. Degradation of poly(lactic‐co‐glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16(15):1123–1130. [DOI] [PubMed] [Google Scholar]
- Patra AK, Mukhopadhyay R, Mukhija R, Krishnan A, Garg LC, Panda AK. Optimization of inclusion body solubilization and renaturation of recombinant human growth hormone from Escherichia coli . Protein Expr Purif. 2000;18(2):182–192. [DOI] [PubMed] [Google Scholar]
- Perez‐Perez DA, Pioquinto‐Avila E, Arredondo‐Espinoza E, Morones‐Ramirez JR, Balderas‐Renteria I, Zarate X. Engineered small metal‐binding protein tag improves the production of recombinant human growth hormone in the periplasm of Escherichia coli . FEBS Open Bio. 2020;10(4):546–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter F, Savoy C, Ji HJ, Juhasz M, Bidlingmaier M, Saenger P. Pharmacokinetic and pharmacodynamic profile of a new sustained‐release GH formulation, LB03002, in children with GH deficiency. Eur J Endocrinol. 2009;160(3):349–355. [DOI] [PubMed] [Google Scholar]
- Petrossians P, Daly AF, Natchev E, Maione L, Blijdorp K, Sahnoun‐Fathallah M, et al. Acromegaly at diagnosis in 3173 patients from the Liège acromegaly survey (LAS) database. Endocr Relat Cancer. 2017;24(10):505–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfäffle R. Hormone replacement therapy in children: the use of growth hormone and IGF‐I. Best Pract Res Clin Endocrinol Metab. 2015;29(3):339–352. [DOI] [PubMed] [Google Scholar]
- Poen S, Pornbanlualap S. Growth hormone from striped catfish (Pangasianodon hypophthalmus): genomic organization, recombinant expression and biological activity. Gene. 2013;518(2):316–324. [DOI] [PubMed] [Google Scholar]
- Pradhananga S, Wilkinson I, Ross R. Pegvisomant: structure and function. J Mol Endocrinol. 2002;29(1):11–14. [DOI] [PubMed] [Google Scholar]
- Promdonkoy B, Warit S, Panyim S. Production of a biologically active growth hormone from giant catfish (Pangasianodon gigas) in Escherichia coli . Biotechnol Lett. 2004;26(8):649–653. [DOI] [PubMed] [Google Scholar]
- Qi Y, Chilkoti A. Protein‐polymer conjugation‐moving beyond PEGylation. Curr Opin Chem Biol. 2015;28:181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez‐Andersen HS, Behrens C, Buchardt J, Fels JJ, Folkesson CG, Jianhe C, et al. Long‐acting human growth hormone analogue by noncovalent albumin binding. Bioconjug Chem. 2018;29:3129–3143. [DOI] [PubMed] [Google Scholar]
- Rao KBCA, Garg LC, Panda AK, Totey SM. High‐level expression of ovine growth hormone in Escherichia coli: single‐step purification and characterization. Protein Expr Purif. 1997;11(2):201–208. [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Bysted BV, Anderson TW, Klitgaard T, Madsen J. Pegylated long‐acting human growth hormone is well‐tolerated in healthy subjects and possesses a potential once‐weekly pharmacokinetic and Pharmacodynamic treatment profile. J Clin Endocrinol Metab. 2010;95(7):3411–3417. [DOI] [PubMed] [Google Scholar]
- Rasmussen MH, Jensen L, Anderson TW, Klitgaard T, Madsen J. Multiple doses of pegylated long‐acting growth hormone are well tolerated in healthy male volunteers and possess a potential once‐weekly treatment profile. Clin Endocrinol (Oxf). 2010;73(6):769–776. [DOI] [PubMed] [Google Scholar]
- Renehan AG, Brennan BM. Acromegaly, growth hormone and cancer risk. Best Pr Res Clin Endocrinol Metab. 2008;22(4):639–657. [DOI] [PubMed] [Google Scholar]
- Rezaei M, Zarkesh‐Esfahani SH, Gharagozloo M. The effect of different media composition and temperatures on the production of recombinant human growth hormone by CHO cells. Res Pharm Sci. 2013;8(3):211–217. [PMC free article] [PubMed] [Google Scholar]
- Rigi G, Rostami A, Ghomi H, Ahmadian G, Mirbagheri VS, Jeiranikhameneh M, et al. Optimization of expression, purification and secretion of functional recombinant human growth hormone in Escherichia coli using modified staphylococcal protein a signal peptide. BMC Biotechnol. 2021;21(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan HA, Ser Huy T, Mohamed Z. Effect of codon optimisation on the production of recombinant fish growth hormone in Pichia pastoris. Sci World J. 2014;2014:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Armengol E, Unterweger A, Laffleur F. PEGylated drug delivery systems in the pharmaceutical field: past, present and future perspective. Drug Dev Ind Pharm. 2022;48(4):129–139. [DOI] [PubMed] [Google Scholar]
- Sargent F, Bogsch EG, Stanley NR, Wexler M, Robinson C, Berks BC, et al. Overlapping functions of components of a bacterial sec‐independent protein export pathway. EMBO J. 1998;17(13):3640–3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereikaite J, Statkute A, Morkunas M, Radzevicius K, Borromeo V, Secchi C, et al. Production of recombinant mink growth hormone in E. coli . Appl Microbiol Biotechnol. 2007;74(2):316–323. [DOI] [PubMed] [Google Scholar]
- Shang Y, Wu M, He R, Ye Y, Sun X. Administration of growth hormone improves endometrial function in women undergoing in vitro fertilization: a systematic review and meta‐analysis. Hum Reprod Update. 2022;28(6):838–857. [DOI] [PubMed] [Google Scholar]
- Shen BQ, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody‐drug conjugates. Nat Biotechnol. 2012;30(2):184–189. [DOI] [PubMed] [Google Scholar]
- Shin NK, Kim DY, Shin CS, Hong MS, Lee J, Shin HC. High‐level production of human growth hormone in Escherichia coli by a simple recombinant process. J Biotechnol. 1998;62(2):143–151. [DOI] [PubMed] [Google Scholar]
- Singh A, Upadhyay V, Upadhyay AK, Singh SM, Panda AK. Protein recovery from inclusion bodies of Escherichia coli using mild solubilization process. Microb Cell Fact. 2015;14(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SM, Sharma A, Panda AK. High throughput purification of recombinant human growth hormone using radial flow chromatography. Protein Expr Purif. 2009;68(1):54–59. [DOI] [PubMed] [Google Scholar]
- Singh SM, Sharma A, Upadhyay AK, Singh A, Garg LC, Panda AK. Solubilization of inclusion body proteins using n‐propanol and its refolding into bioactive form. Protein Expr Purif. 2012;81(1):75–82. [DOI] [PubMed] [Google Scholar]
- Singhvi P, Verma J, Panwar N, Wani TQ, Singh A, Qudratullah M, et al. Molecular attributes associated with refolding of inclusion body proteins using the freeze–thaw method. Front Microbiol. 2021;12:618559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares CRJ, Gomide FIC, Ueda EKM, Bartolini P. Periplasmic expression of human growth hormone via plasmid vectors containing the PL promoter: use of HPLC for product quantification. Protein Eng Des Sel. 2003;16(12):1131–1138. [DOI] [PubMed] [Google Scholar]
- Soares CRJ, Ueda EKM, Oliveira TL, Gomide FIC, Heller SR, Bartolini P. Distinct human prolactin (hPRL) and growth hormone (hGH) behavior under bacteriophage lambda PL promoter control: temperature plays a major role in protein yields. J Biotechnol. 2008;133(1):27–35. [DOI] [PubMed] [Google Scholar]
- Sockolosky JT, Szoka FC. Periplasmic production via the pET expression system of soluble, bioactive human growth hormone. Protein Expr Purif. 2013;87(2):129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon G, Reicher S, Gussakovsky EE, Jomain JB, Gertler A. Large‐scale preparation and in vitro characterization of biologically active human placental (20 and 22K) and pituitary (20K) growth hormones: placental growth hormones have no lactogenic activity in humans. Growth Horm IGF Res. 2006;16(5–6):297–307. [DOI] [PubMed] [Google Scholar]
- Søndergaard E, Klose M, Hansen M, Hansen BS, Andersen M, Feldt‐Rasmussen U, et al. Pegylated long‐acting human growth hormone possesses a promising once‐weekly treatment profile, and multiple dosing is well tolerated in adult patients with growth hormone deficiency. J Clin Endocrinol Metab. 2011;96(3):681–688. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Sugimura A. Improved solubilization of recombinant human growth hormone inclusion body produced in Escherichia coli . Biosci Biotechnol Biochem. 2008;72(10):2675–2680. [DOI] [PubMed] [Google Scholar]
- Steiner M, Frank J, Saenger P. Long‐acting growth hormone in 2022. Pediatr Investig. 2023;7(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamshen K, Wang Y, Jamieson SMF, Perry JK, Maynard HD. Genetic code expansion enables site‐specific PEGylation of a human growth hormone receptor antagonist through click chemistry. Bioconjug Chem. 2020;31(9):2179–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchelet A, Vogel T, Helman D, Guy R, Neospouolus C, Goffin V, et al. Selective modification at the N‐terminal region of human growth hormone that shows antagonistic activity. Mol Cell Endocrinol. 1997;130(1–2):141–152. [DOI] [PubMed] [Google Scholar]
- Teresa M, Ribela CP, Camargo IMC, Oliveira JE, Bartolini P. Single‐step purification of recombinant human growth hormone (hGH) directly from bacterial osmotic shock fluids, for the purpose of 125I‐hGH preparation. Protein Expr Purif. 2000;18(2):115–120. [DOI] [PubMed] [Google Scholar]
- Thirone ACP, Carvalho CRO, Saad MJA. G120K‐PEG, a human GH antagonist, decreases GH signal transduction in the liver of mice. Mol Cell Endocrinol. 2002;192(1–2):65–74. [DOI] [PubMed] [Google Scholar]
- Thorlacius‐Ussing O. Zinc in the anterior pituitary of rat: a histochemical and analytical work. Neuroendocrinology. 1987;45(3):233–242. [DOI] [PubMed] [Google Scholar]
- Thornton P, Hofman P, Maniatis A, Aghajanova E, Chertok E, Korpal‐Szczyrska M, et al. OR17‐4 Transcon growth hormone In the treatment of pediatric growth hormone deficiency: results of the phase 3 height trial. J Endocr Soc. 2019;3(Suppl 1):OR17‐4. [Google Scholar]
- Touraine P, D'Souza GA, Kourides I, Abs R, Barclay P, Xie R, et al. Lipoatrophy in GH deficient patients treated with a long‐acting pegylated GH. Eur J Endocrinol. 2009;161(4):533–540. [DOI] [PubMed] [Google Scholar]
- Turecek PL, Bossard MJ, Schoetens F, Ivens IA. PEGylation of biopharmaceuticals: a review of chemistry and nonclinical safety information of approved drugs. J Pharm Sci. 2016;105(2):460–475. [DOI] [PubMed] [Google Scholar]
- Uchida H, Naito N, Asada N, Wada M, Ikeda M, Kobayashi H, et al. Secretion of authentic 20‐kDa human growth hormone (20K hGH) in Escherichia coli and properties of the purified product. J Biotechnol. 1997;55(2):101–112. [DOI] [PubMed] [Google Scholar]
- Unterberger CJ, Maklakova VI, Lazar M, Arneson PD, Mcilwain SJ, Tsourkas PK, et al. GH action in prostate cancer cells promotes proliferation, limits apoptosis, and regulates cancer‐related gene expression. Endocrinology. 2022;163(5):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay AK, Murmu A, Singh A, Panda AK. Kinetics of inclusion body formation and its correlation with the characteristics of protein aggregates in Escherichia coli. Herman C, ed. PLoS One. 2012;7(3):e33951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay V, Singh A, Jha D, Singh A, Panda AK. Recovery of bioactive protein from bacterial inclusion bodies using trifluoroethanol as solubilization agent. Microb Cell Fact. 2016;15(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lely AJ, Kopchick JJ. Growth hormone receptor antagonists. Neuroendocrinology. 2006;83(3–4):264–268. [DOI] [PubMed] [Google Scholar]
- Vance ML, Mauras N. Growth hormone therapy in adults and children. N Engl J Med. 1999;341(16):1206–1216. [DOI] [PubMed] [Google Scholar]
- Veronese FM, Mero A. The impact of PEGylation on biological therapies. BioDrugs. 2008;22(5):315–329. [DOI] [PubMed] [Google Scholar]
- Wallis OC, Wallis M. Purification and properties of a recombinant DNA‐derived ovine growth hormone analogue (oGH1) expressed in Escherichia coli . J Mol Endocrinol. 1990;4(1):61–69. [DOI] [PubMed] [Google Scholar]
- Wang S, Shen M, Xu Y, Chen F, Chen M, Chen S, et al. Rational and efficient preparation of a chimeric protein containing a tandem dimer of thrombopoietin mimetic peptide fused to human growth hormone in Escherichia coli . Appl Microbiol Biotechnol. 2013;97(7):2885–2894. [DOI] [PubMed] [Google Scholar]
- Wang W, Sun YH, Wang YP, Zhu ZY. Expression of grass carp growth hormone in the yeast Pichia pastoris . Yi Chuan Xue Bao. 2003;30(4):301–306. [PubMed] [Google Scholar]
- Wang Y, Jamieson SMF, Perry JK. Targeting growth hormone in cancer: future perspectives. Endocr Relat Cancer. 2023:30(9). Published online June 2023. [DOI] [PubMed] [Google Scholar]
- Wang Y, Langley RJ, Tamshen K, Harms J, Middleditch MJ, Maynard HD, et al. Enhanced bioactivity of a human GHR antagonist generated by solid‐phase site‐specific PEGylation. Biomacromolecules. 2021;22(2):299–308. [DOI] [PubMed] [Google Scholar]
- Wang Y, Langley RJ, Tamshen K, Jamieson SM, Lu M, Maynard HD, et al. Long‐acting human growth hormone receptor antagonists produced in E. coli and conjugated with polyethylene glycol. Bioconjug Chem. 2020;31(6):1651–1660. [DOI] [PubMed] [Google Scholar]
- Webster R, Xie R, Didier E, Finn R, Finnessy J, Edgington A, et al. PEGylation of somatropin (recombinant human growth hormone): impact on its clearance in humans. Xenobiotica. 2008;38(10):1340–1351. [DOI] [PubMed] [Google Scholar]
- Wingfield PT, Graber P, Buell G, Rose K, Simona MG, Burleigh BD. Preparation and characterization of bovine growth hormones produced in recombinant Escherichia coli . Biochem J. 1987;243(3):829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz‐Krawiec A, Sokolowska I, Smorawinska M, Chojnacka‐Puchta L, Mikiewicz D, Lukasiewicz N, et al. Use of Ubp1 protease analog to produce recombinant human growth hormone in Escherichia coli . Microb Cell Fact. 2014;13(1):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Chen J, Wu Y, Zhang B, Cai X, Zhang Z, et al. Precise and combinatorial PEGylation generates a low‐immunogenic and stable form of human growth hormone. J Control Release. 2017;249:84–93. [DOI] [PubMed] [Google Scholar]
- Wu L, Ho SV, Wang W, Gao J, Zhang G, Su Z, et al. N‐terminal mono‐PEGylation of growth hormone antagonist: correlation of PEG size and pharmacodynamic behavior. Int J Pharm. 2013;453(2):533–540. [DOI] [PubMed] [Google Scholar]
- Wu L, Ji S, Hu T. N‐terminal modification with pseudo‐Bifunctional PEG‐hexadecane markedly improves the pharmacological profile of human growth hormone. Mol Pharm. 2015;12(5):1402–1411. [DOI] [PubMed] [Google Scholar]
- Wu L, Ji S, Shen L, Hu T. Phenyl amide linker improves the pharmacokinetics and pharmacodynamics of N‐terminally mono‐PEGylated human growth hormone. Mol Pharm. 2014;11(9):3080–3089. [DOI] [PubMed] [Google Scholar]
- Wu M, Liu W, Yang G, Yu D, Lin D, Sun H, et al. Engineering of a Pichia pastoris expression system for high‐level secretion of HSA/GH fusion protein. Appl Biochem Biotechnol. 2014;172(5):2400–2411. [DOI] [PubMed] [Google Scholar]
- Wu W, Zhou J, Wu C, Zhou Q, Li X, Zhang Y, et al. PEGylated recombinant human growth hormone Jintrolong® exhibits good long‐term safety in Cynomolgus monkeys and human pediatric growth hormone deficiency patients. Front Endocrinol (Lausanne). 2022;13:821588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wu X, Hou R, Liao M, Il KH, Shou J, et al. High‐level secretory expression, purification and characterization of Ailuropoda melanoleuca growth hormone in Pichia pastoris. Protein Expr Purif. 2008;60(2):182–187. [DOI] [PubMed] [Google Scholar]
- Yang CW, Striker LJ, Pesce C, Chen WY, Peten EP, Elliot S, et al. Glomerulosclerosis and body growth are mediated by different portions of bovine growth hormone. Studies in transgenic mice. Lab Invest. 1993;68(1):62–70. [PubMed] [Google Scholar]
- Yin D, Vreeland F, Schaaf LJ, Millham R, Duncan BA, Sharma A. Clinical pharmacodynamic effects of the growth hormone receptor antagonist pegvisomant: implications for cancer therapy. Clin Cancer Res. 2007;13(3):1000–1009. [DOI] [PubMed] [Google Scholar]
- Yuen KCJ, Miller BS, Biller BMK. The current state of long‐acting growth hormone preparations for growth hormone therapy. Curr Opin Endocrinol Diabetes Obes. 2018;25(4):267–273. [DOI] [PubMed] [Google Scholar]
- Yuen KCJ, Miller BS, Boguszewski CL, Hoffman AR. Usefulness and potential pitfalls of long‐acting growth hormone analogs. Front Endocrinol (Lausanne). 2021;12:637209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani M, Nezafat N, Ghasemi Y. Evaluation of recombinant human growth hormone secretion in E. coli using the L‐asparaginase II signal peptide. Avicenna J Med Biotechnol. 2016;8(4):182–187. [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Yang XL, Yuan YH, Pu J, Liao F. Site‐specific PEGylation of therapeutic proteins via optimization of both accessible reactive amino acid residues and PEG derivatives. BioDrugs. 2012;26(4):209–215. [DOI] [PubMed] [Google Scholar]
- Zhang P, Sun F, Liu S, Jiang S. Anti‐PEG antibodies in the clinic: current issues and beyond PEGylation. J Control Release. 2016;244(Pt B):184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Shaw AC, Wang J, Chang CC, Deng J, Su J. A novel high‐throughput screening method for microbial transglutaminases with high specificity toward Gln141 of human growth hormone. J Biomol Screen. 2010;15(2):206–212. [DOI] [PubMed] [Google Scholar]
- Zhou ZR, Huang W, Liu KJ, Lin FL, Wang XL, Wang F, et al. Soluble expression, one‐step purification and characterization of recombinant human growth hormone fused with ompA3 in Escherichia coli . Protein Pept Lett. 2021;28(5):533–542. [DOI] [PubMed] [Google Scholar]
- Zomorrodipour A, Yakhchali B, Khodabandeh M, Dizaji AK, Hosseini MS, Ahmad ZR, et al. The over‐expression of biologically active human growth hormone in a T5‐based system in Escherichia coli, studying temperature effect. Biotechnology. 2004;15(1):27–32. [Google Scholar]


