Abstract
Recently, it was shown that actin molecules are present in human immunodeficiency virus type 1 (HIV-1) particles. We have examined the basis for incorporation and the location of actin molecules within HIV-1 and murine retrovirus particles. Our results show that the retroviral Gag polyprotein is sufficient for actin uptake. Immunolabeling studies demonstrate that actin molecules localize to a specific radial position within the immature particle, clearly displaced from the matrix domain underneath the viral membrane but in proximity to the nucleocapsid (NC) domain of the Gag polyprotein. When virus or subviral Gag particles were disrupted with nonionic detergent, actin molecules remained associated with the disrupted particles. Actin molecules remained in a stable complex with the NC cleavage product (or an NC-RNA complex) after treatment of the disrupted HIV-1 particles with recombinant HIV-1 protease. In contrast, matrix and capsid molecules were released. The same result was obtained when mature HIV-1 particles were disrupted with detergent. Taken together, these results indicate that actin molecules are associated with the NC domain of the viral polyprotein.
Retrovirus morphogenesis requires transport of virion components to the site of assembly and subsequent release by budding at the plasma membrane. The major internal structural proteins of all retroviruses are initially translated as the Gag precursor polyprotein (Pr65gag in Moloney murine leukemia virus [MoMuLV] and Pr55gag in human immunodeficiency virus type 1 [HIV-1]), containing the domains matrix (MA), capsid (CA), and nucleocapsid (NC). Each Gag precursor also contains another small domain which is not conserved between the viruses. Domain p12 (MoMuLV) lies between MA and CA, while domain p6 (HIV) lies carboxy terminal to NC. Particle formation does not absolutely require the presence of these small domains or the incorporation of other viral components, such as those of the envelope, although they may play some role in assembly and budding (11).
The Gag polyproteins of type C retroviruses, including lentiviruses, are transported to the inner face of the plasma membrane, where assembly occurs concomitantly with budding. Budding virions have an immature morphology, characterized by an electron-opaque ring surrounding an electron-lucent center. During or shortly after release, the domains of the Gag polyprotein are separated by the proteolytic activity of the viral protease, allowing a morphological rearrangement and the generation of mature virions, characterized by an electron-opaque center with an electron-lucent periphery.
The cell membrane is the location of type C particle formation. It is supported from the inside by a specialized region of the cytoskeleton (18) defining a microenvironment in which the assembling Gag polyproteins are inserted. It seems likely that a number of interactions of viral proteins with the surrounding cytoskeletal elements which may help to stabilize the assembling virus particle have evolved. Several studies have suggested a functional role of the actin cytoskeleton in virus assembly and budding (reviewed in reference 3). Rausch murine leukemia virus Gag was found to be associated with the cytoskeleton after detergent extraction of infected cells (5). Further, colocalization of MoMuLV structural proteins with actin was observed after microfilament disruption with cytochalasin D (12). Treatment with cytochalasin D also resulted in a marked decrease in MoMuLV (12), mouse mammary tumor virus (13), and HIV-1 (22) particle release. Type B and C retroviruses have been observed at the tips of long actin-containing projections in occasional images (4, 13, 14, 22). Recent data from cosedimentation assays showed a direct interaction between in vitro-translated HIV-1 Gag polyprotein and F-actin filaments (19).
While several lines of evidence support a functional role of the cytoskeleton at the stage of assembly and budding, less is known about its fate at late stages of budding and in released virus particles. Detection of the cleavage products of vimentin, desmin, and actin in the lysate of HIV-1-infected cells suggests that the barrier of the submembrane network is overcome by partial cleavage of these structures (10, 11, 23, 24). However, incorporation of considerable amounts of uncleaved cytoskeletal proteins, particularly actin, into retroviral particles opens the possibility of a continuing functional and structural role for cytoskeletal proteins within the virion (1, 4, 17, 26). A continuing functional role would require specific interaction with the viral structural proteins and hence a specific location for actin within the virion.
Localization has been proposed on the basis of morphological evidence (15). It has been suggested that an actin layer is intermediate between the viral envelope and the MA protein and accounts for the poorly staining region just inside the MoMuLV and HIV-1 membranes (16).
Here, we present experiments that define the location of actin within the retroviral particle and identify the viral component responsible for its incorporation.
MATERIALS AND METHODS
Preparation of Gag particles.
Gag particles were produced by expression of HIV-1 Gag with the recombinant baculoviruses AcNPVgag12myr (for full-length HIV-1 Gag expression [21]), AcNPVgag13myr (for HIV-1Δp6 Gag expression), and AcNPVgagd140-143myr (for HIV-1 ΔMA Gag expression), which were generously provided by P. Boulanger (INSERM, Montpellier, France), or MoMuLV Gag (ecotropic MoMuLV Gag), which was kindly provided by S. Morikawa (National Institute of Health, Tokyo, Japan) in BTI-TN-5B1-4 cells (“High 5”). Virus-containing cell culture supernatants were harvested at 22 to 24 h postinfection and treated as described previously (7, 28).
Preparation of mature and immature HIV-1 from MT-4 cells.
HIV particles were produced by infection of MT-4 cells with the infectious clone pNL4-3 for 20 to 24 h in the presence or absence of 5 nM protease inhibitor Ro31-8959 (20, 28). The medium was cleared by two debris spins (10 min at 2,000 rpm followed by 30 min at 6,000 rpm in a Heraeus Christ centrifuge) at 4°C. The particles were then isolated by layering the supernatant above 2 ml of 30% (wt/wt) sucrose in phosphate-buffered saline (PBS) in an SW40 tube and spinning at 40,000 rpm for 3 h. Pellets were resuspended either in ice-cold PBS containing 1% paraformaldehyde (wild type mature) or in 0.5% Triton X-100 (wild type immature) and allowed to stand for 30 min on ice prior to further biochemical analysis.
Rate zonal centrifugation.
One hundred microliters of concentrated particles was carefully loaded above a 1.2-ml sucrose gradient (20 to 55% or 30 to 60% [wt/wt] in PBS) and spun in a Beckman Tabletop TL 100 ultracentrifuge (TLS55 rotor) for 30 min at 4°C at 260,000 × g. Fractions were collected from the top to the bottom of the gradient. The refractive index of each fraction was determined. Sodium dodecyl sulfate sample buffer (4×) was added to each fraction before electrophoresis (27).
Detergent treatment and in vitro proteolytic processing.
For particle disruption, the virus suspension was incubated for 30 min at room temperature (RT) in the presence of 0.5% (wt/vol) Triton X-100 (in a 100-μl final volume) prior to centrifugation. For proteolytic processing with recombinant HIV-1 protease (kindly provided by Hans-Georg Kräusslich, Hamburg, Germany), samples were incubated at 37°C in the presence of 0.05 μg of recombinant protease per ml in 0.5% Triton X-100 in PBS.
Western blotting.
Particle lysates were separated on Laemmli gels and blotted onto nitrocellulose, essentially as described previously (27). Viral and cellular proteins were detected by using a specific sheep antiserum against the HIV-1 MA protein (AIDS Research and Reference Reagent Program, National Institutes of Health, Bethesda, Md.), specific rabbit antisera to the HIV-1 CA and NC proteins (kindly provided by Hans-Georg Kräusslich), mouse monoclonal anti-CA (“Kal-1”; ScheBoTech, Wettenburg, Germany), or actin (A-2066; Sigma-Aldrich Chemie GmbH, Deisenhofen, Germany) at a 1:500 dilution for 3 h at RT. The bound antibodies were visualized by using the appropriate secondary antibody labeled with alkaline phosphatase (Sigma-Aldrich Chemie GmbH), according to the manufacturer’s instructions.
Immunocytochemistry and microscopy.
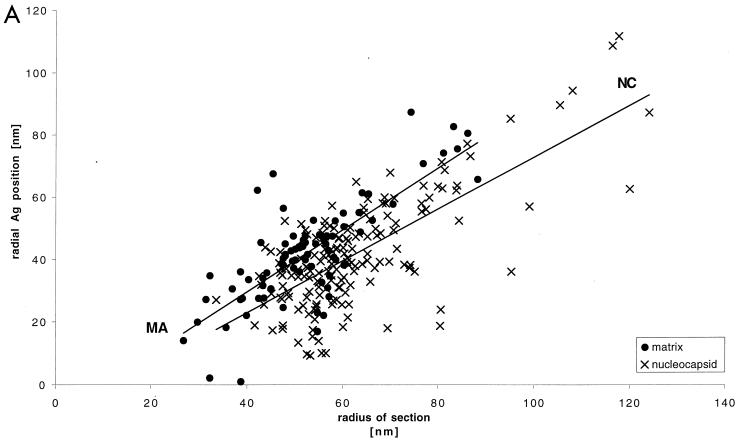
The location of viral or cellular antigens was analyzed by using the polyclonal antisera described above. The protocol was based on our postsectioning fixation technique for freeze-substituted samples (6, 8). Primary antibodies (described above) were visualized by labeling with protein A-colloidal gold conjugates (5 and 10 nm in diameter). Electron microscopy was performed as described previously (6, 8) by using either a Philips EM400 microscope operated at 80 kV or a Philips CM20 microscope operated at 80 kV at a magnification of either ×27,000 or ×33,000. The measurements shown in Fig. 3 were taken from images digitized on a Zeiss SCAI scanner (Oberkochen) with a step size of 14 μm. The positions of the gold-labeled antibodies were measured with respect to the particle center and the particle membrane. This was done by drawing a line through the particle center and the gold and recording its length (Rg) and the distance from the center to the membrane (Rm). Plots of Rm versus Rg could be fit with lines that had slopes equal to 1 and intercepts which varied with the label. This is the behavior expected from sections of structures of variable diameters in which the gold and the membrane have a constant separation. The variability due to variations in particle diameter was compensated for by presenting the data in reduced coordinates (Rm − Rg) as described in the legend to Fig. 3.
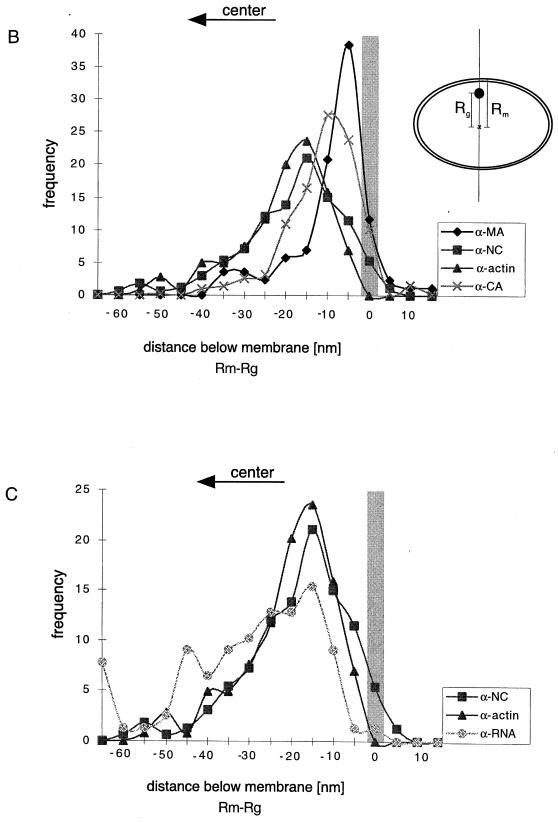
FIG. 3.
Quantitation of immunogold-labeled viral and cellular antigens. The variable diameter of the retrovirus particle (6) motivates the use of reduced coordinates for comparison of the positions of label in different particles. Our previous work indicated that the positions of Gag protein domains relative to the membrane are invariant between particles of different diameters (6). Hence, we measured the distances from the center of the virus particle to the center of the gold particle corresponding to the specific label (Rg) and to the position of the membrane (Rm) along a line passing through the gold particle and the center of the virus particle, as shown in the inset in panel B. (A) A plot of Rg versus Rm for HIV-1 MA and NC revealed a straight line with a slope close to 1 and an intercept which varied with the antigen labeled. Similar data were obtained for the other antigens of HIV-1 and MoMuLV (not shown in this representation but vide infra). The labeling data plotted in reduced coordinates (Rm − Rg) reveal the variations in intercept for the different labels and hence their positions relative to the average position of the membrane. This is shown for HIV-1 MA, CA, NC, and actin (B) and for NC, actin, and RNA (C). The application of Student’s t test to the reduced coordinates confirms the visual impression of the significance of the variation in the average positions of the label. The mean position of the MA label is different from that of NC and actin (equal means with P of 2.29 × 10−10 and 1.26 × 10−15, respectively), as is CA (equal means with P of 10−10 and 10−23, respectively). The NC, RNA, and actin means are not distinguishable. The shaded bars show the position of the membrane.
Negative staining of detergent-treated particles was performed with carbon-coated Formvar grids and a 1.0% (wt/vol) solution of uranyl acetate (Sigma-Aldrich Chemie GmbH).
RESULTS
Actin is incorporated into retroviral particles.
Western blotting of mature HIV-1 particles revealed the presence of actin (not shown), confirming recent observations (17). Immature HIV-1 particles (i.e., produced in the presence of protease inhibitor) also contained actin, indicating that the activity of the protease is not required for actin incorporation. We incubated HIV-1 particles in the nonionic detergent Triton X-100 to investigate this association and the location of actin within the virus particle. Under these conditions, immature retroviral particles lose the enveloping lipid bilayer yet retain their characteristic size and shape (see below), a behavior which has also been reported also for avian retroviruses (25). The detergent-treated immature HIV-1 particles were separated on sucrose gradients, and the protein content of the individual fractions was analyzed by Western blotting (Fig. 1). The majority of detectable Gag antigen migrated in sodium dodecyl sulfate-polyacrylamide gels at a position corresponding to the uncleaved precursor polyprotein Pr55gag (Fig. 1A) due to the presence of protease inhibitor. The minor bands of lower molecular weight seen in Fig. 1A reflect residual protease activity. Approximately 50% of the actin was released from the particles and found as soluble protein in the top fractions of the gradient. The remaining actin was resistant to detergent treatment and comigrated with the structural proteins of the virus, consistent with a tight association.
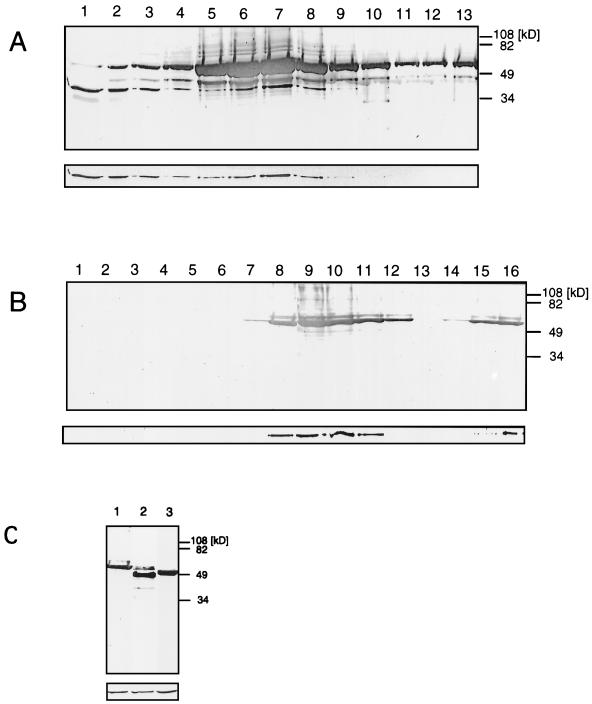
FIG. 1.
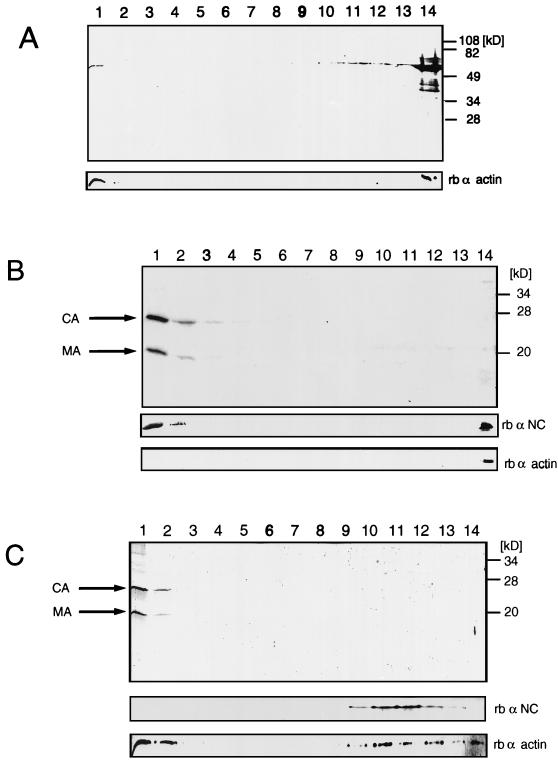
Immature HIV-1 and subviral HIV-1 Gag particles incorporate actin molecules. (A) Immature HIV-1 particles were harvested after treatment of infected T cells with protease inhibitor and then incubated with 0.5% Triton X-100 prior to centrifugation on a 30 to 60% (wt/wt) sucrose gradient for 30 min. The lower panel shows a Western blot probed with rabbit antiactin, and the upper (larger) panel shows the same blot subsequently probed with mouse anti-CA. 1 and 13 indicate the top and bottom fractions, respectively. The peak fraction containing p55 and actin corresponded to 42.5% sucrose on this rate zonal gradient. (B) HIV-1 Gag particles were harvested after expression of the Gag open reading frame in insect cells and separated on a 30 to 60% (wt/wt) sucrose gradient for 30 min. The lower panel shows a Western blot probed with rabbit antiactin, and the upper (larger) panel shows a parallel blot probed with mouse anti-CA. 1 and 16 indicate the top and bottom fractions, respectively. The peak fraction containing p55 and actin corresponded to 37.5% sucrose on this rate zonal gradient. (C) Gradient-purified Gag particles from an experiment similar to that shown in panel B using the wild type (lane 1), ΔMA (lane 2), and Δp6 (lane 3). The lower panel shows a Western blot probed with rabbit antiactin, and the upper (larger) panel shows a parallel blot probed with mouse anti-CA.
We used the production of particles in an insect cell system to determine the minimal requirements for incorporation. Particles produced by expression of the gag gene in insect cells with recombinant baculovirus were analyzed in a similar experiment. Expression of the Gag polyprotein resulted in efficient release of Gag particles. The particles remain immature since no protease is expressed, and hence no mature viral structural proteins were detected. Actin molecules cofractionated with the Gag polyprotein of HIV-1 (Fig. 1B) and MoMuLV (not shown) in a sucrose gradient. We took advantage of the availability of systems which express mutant Gag particles to examine the requirements for actin incorporation more closely. The fact that MoMuLV has no domain corresponding to p6 of HIV suggests that this domain plays no role in actin incorporation. Further, the lack of a requirement for the MA and p6 domains was demonstrated by the presence of actin in particles produced by expression of Δp6 and ΔMA Gag (Fig. 1C). Similar analysis of mock-infected supernatants by gradient centrifugation and Western blotting revealed no actin (data not shown).
The presence of actin in both the viral and subviral Gag particle samples reveals that the retroviral Gag polyprotein is sufficient for actin incorporation; the presence and activity of the protease have no major effect on this phenomenon. Its presence in Δp6 and ΔMA Gag indicates that these domains play no role in incorporation.
Immunolabeling of immature MoMuLV and HIV-1 particles.
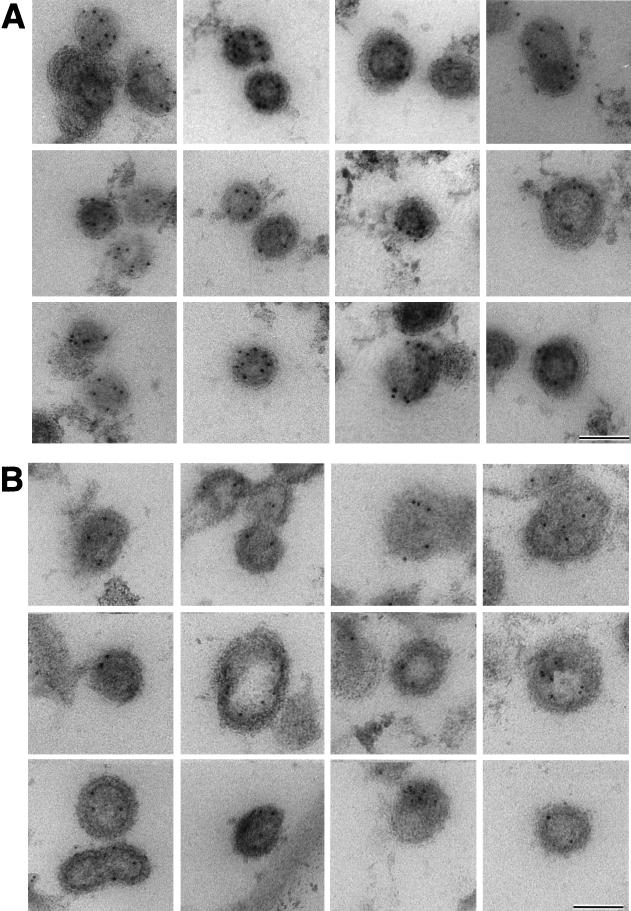
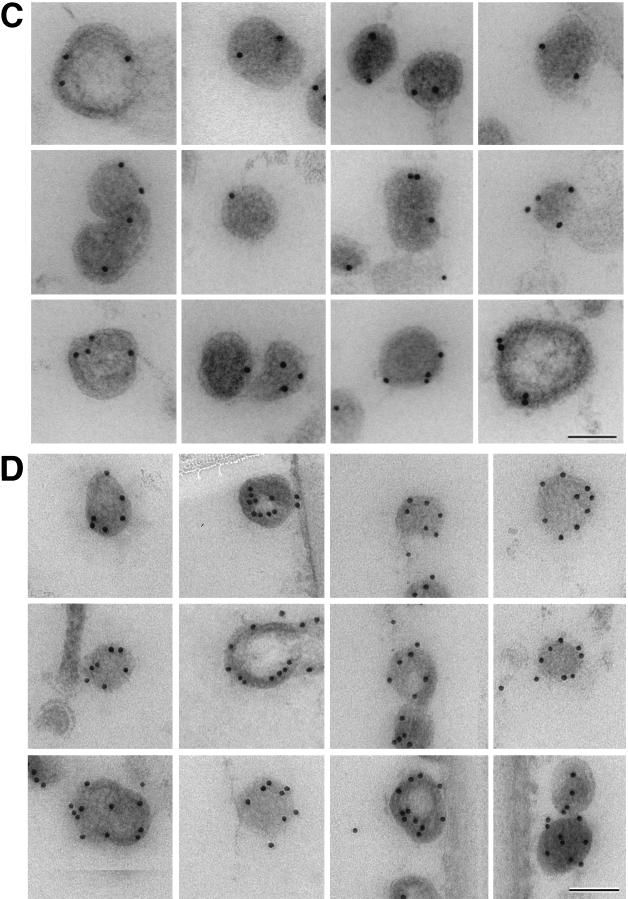
Biochemical results indicated that actin molecules are associated with the Gag polyprotein of retroviral particles; however, it was still unclear whether actin is distributed throughout the particle or localized to a specific site. We used immunocytochemistry to determine the distribution of actin within the immature virus particle. Previous work (17) yielded an estimate of ∼200 actin molecules for each virus particle; therefore, only a few (<10) molecules can be expected to be present in an individual thin section of a fixed particle (9). We employed an antigen-preserving, postembedding immunolabeling procedure (6, 8) to enhance labeling efficiency. Figure 2A shows ultrathin sections of actin-immunolabelled immature MoMuLV Gag particles. An average of three to eight gold particles per virion were detected in a section, reflecting efficient immunological detection by actin-specific antibodies. Most of the actin was found to be clearly displaced from the membrane and close to an electron-dense structure, presumably the NC-RNA complex. When several gold labels were present in a section, their position at a specific radius was more apparent (Fig. 2A). When the same antibody was used to detect actin molecules in ultrathin sections of immature HIV-1 particles, an average of two to five gold particles per virion were detected. As expected, the radial position of the antigens varied with the diameter of the particle in the section (which in turn reflects the height of the section within the particle), yet the position of the label was relatively constant with respect to the viral membrane. This perception is confirmed by plotting the gold position (distance of gold from the center [Rg]) as a function of particle size (distance of the membrane from the center [Rm]) (Fig. 3). Despite this reduced labeling efficiency, most particles contained gold label which was well inside the virus membrane (Fig. 2B). We investigated the radial positions of the domains of the Gag polyprotein by immunolabeling of HIV Gag particles with antibodies directed against the MA, CA, and NC domains and against RNA. Again, when several gold particles were present, their azimuthal arrangement was apparent. While MA antibodies resulted in gold labeling near the virus membrane, the average label corresponding to the other antigens lay at lower radii, closer to the center of the particle. The graphs in Fig. 3B and C show the distribution of antigen labeling as a function of the distance below the membrane. The length of the detecting antibody or protein A-gold complexes and presentation of the antigen in different orientations spread the label around the true position of the antigen so that the means of the distributions must be compared. This is reflected in the widths of the distributions seen in Fig. 3. It confirms the visual impression of the antigen location and shows that MA antigens are predominantly detected in proximity to the virus membrane, while NC antigens are found further inside the virus particle. The separation of NC, CA, and MA (Fig. 3) is confirmed by a Student’s t test (MA/NC, t = 6.6; MA/CA, t = 2.4; NC/CA, t = 6.59; P = 3 × 10−11, 1 × 10−2, and 3 × 10−12, respectively, for equal means). The t test indicates that only the colocalization of actin with domain NC is significant (MA/actin, t = 8.67; CA/actin, t = 10.43; however, t was 1.544 for NC/actin, with corresponding P values of 3.0 × 10−15, 1.8 × 10−23, and 0.12, respectively). Figure 3C shows the overlap of the distribution of actin and NC with the much broader distribution of RNA labeling. Although the overlap is apparent, the platykurtic RNA distribution does not lend itself to a simple Student’s t test of association.
FIG. 2.
Immunogold detection of viral and cellular antigens in Gag particles. The images reveal individual Gag particles labeled with specific antibodies and visualized with colloidal gold-protein A (5 nm in panels A and B and 10 nm in panels C and D). (A) MoMuLV Gag particles labeled with antiactin. (B to D) HIV-1 Gag particles labeled with antiactin (B), anti-MA (C), and anti-NC (D). Bars, 100 nm.
Actin cofractionates with the NC cleavage product.
The immunolabeling experiments showed that actin molecules localize to a specific position within the immature retroviral particle but did not clarify the basis for this spatial arrangement. It could reflect either an interaction between actin and the Gag polyprotein or nonspecific trapping between two protein layers. Therefore, Gag particles were treated with 0.5% nonionic detergent Triton X-100, and the release of actin molecules from the disrupted particles was analyzed by separating the viral proteins in a sucrose gradient. While detergent treatment of immature (protease inhibitor-treated) wild-type viral particles had little effect on the organization of the internal immature core, the subviral Gag particles were clearly disrupted by the treatment (7).
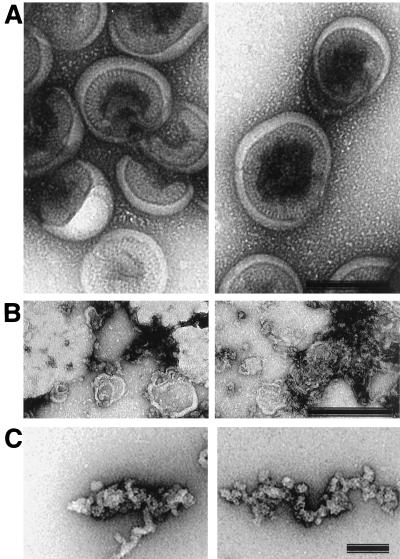
This difference can be seen in electron micrographs of negatively stained detergent-treated particles (Fig. 4). Detergent treatment of protease inhibitor-treated virions leaves a large fraction in a compact form (Fig. 4A) similar in size and shape to the immature membranous particle visualized by cryoelectron microscopy (7). Faults between the hemispherical domains are visible, but the particle remains relatively intact. In contrast, removal of the membrane from the subviral Gag particles causes their dissociation (Fig. 4B), and elongated strings of RNP can be visualized (Fig. 4C). Detergent treatment compromises the particle’s integrity; however, it does not disrupt the interaction of Gag molecules with the RNA of the particle itself. The Gag polyprotein easily penetrated the high-sucrose-concentration regions of the gradient and was found almost exclusively in the bottom fractions (Fig. 5A). Trace amounts of Pr55gag protein were detected in the top fractions. Detergent treatment of Gag particles still allowed a considerable portion of the actin molecules to cofractionate with the unprocessed Gag precursor protein, suggesting that these molecules were still bound to the disrupted immature particles. Significant but variable amounts of actin molecules were also released from the treated particles and were found in the top fractions of the gradient. Typically, 50% of the total actin was lost from the disrupted HIV-1 Gag particles. The components of the detergent-lysed immature viral particles were detected in lighter fractions of the gradient than in the parallel experiment with Gag particles. This difference between immature viral particles (produced in the presence of protease inhibitor) and the Gag particles may be related to their differing stabilities upon detergent treatment. The reason for this differential effect on immature particles may be that proteins from the pol open reading frame confer added stability on immature viral particles.
FIG. 4.
Effects of detergent treatment on immature HIV-1 particles and Gag particles. Negatively stained preparations of detergent-treated immature (protease inhibitor-treated) HIV-1 particles (A) and of HIV-1 Gag particles treated with detergent (0.5% [wt/vol] Triton X-100) under the same conditions (B and C). Note the relatively intact appearance of the particles in panel A and the fact that the Gag proteins appear to remain linked, presumably due to the presence of RNA, in the more dissociated Gag particle preparations in panels B and C. Bars, 100 nm in A and B; 10 nm in C.
FIG. 5.
Actin cofractionates with the NC cleavage product. Sucrose density (30 to 60%, wt/wt) gradients of HIV-1 Gag particles disrupted with 0.5% (wt/vol) Triton X-100 (A), HIV-1 Gag particles incubated with recombinant HIV-1 protease in the presence of 0.5% (wt/vol) Triton X-100 (B), and mature HIV-1 particles disrupted with 0.5% (wt/vol) Triton X-100 (C). 1 and 14 indicate the top and bottom fractions, respectively. Large panels, Coomassie-stained 12% polyacrylamide gels; small panels, Western blots with polyclonal rabbit antisera directed against actin or the HIV-1 NC protein. The NC protein stains poorly with Coomassie blue but is easily visualized with antibody in the Western blots.
Detergent treatment of Gag particles showed that a considerable fraction of the actin was still tightly associated with the disrupted particles (Fig. 5A). To extend this observation, Gag particles were disrupted with 0.5% Triton X-100 and incubated with recombinant HIV-1 protease. The detergent completely removes the virus membrane so that the Pr55gag precursor protein is processed efficiently by the viral protease. The cleavage products were separated on a sucrose gradient, and gradient fractions were analyzed in Coomassie-stained gels and Western blots with polyclonal antisera specific for actin and the NC protein. Addition of exogenous HIV-1 protease resulted in proper proteolytic processing to produce mature MA, CA, and NC (Fig. 5B). No proteolytic processing was observed in the absence of either HIV-1 protease or detergent (data not shown). After centrifugation of detergent- and HIV-1 protease-treated particles, MA and CA remained in the top fractions of the gradient, indicating that both cleavage products had been released from the disrupted particles. In contrast, roughly three-fourths of the NC protein accumulated at the bottom of the gradient, presumably because it remained bound to the RNA of the subviral particle. Western blotting with polyclonal antiserum specific for actin (Fig. 5B, lower panel) demonstrated that actin cofractionated with the NC protein with only minor amounts at the top of the gradient.
The gradient result demonstrates that actin is associated with the NC protein or an NC-RNA complex. It suggests that actin incorporation is mediated through an interaction with the NC domain (or an NC-RNA complex) within the viral polyprotein, which is retained after cleavage. Further experimentation is required to define the nature of the association. Exogenous addition of viral protease only mimics the process of proteolytic modification, which takes place naturally within the well-ordered environment of the enveloped immature virus particle. Similar experiments were performed with particles released after an infection of CD4-positive MT-4 cells to determine whether actin is also associated with the NC cleavage product in the mature HIV-1 virion. Mature virions were collected through a cushion of sucrose, inactivated for 30 min in 1% paraformaldehyde, and banded on a sucrose gradient. Prolonged treatment with high concentrations of paraformaldehyde could result in cross-linking of the internal proteins of the virus. No evidence for significant cross-linking was found under our conditions of treatment. Cross-linking sufficient to cause adventitious colocalization of actin and Gag would result in a dramatic reduction in the levels of both proteins on the gels as well as a visible cross-linked product. Banded particles were isolated and incubated in 0.5% Triton X-100 for 30 min at RT prior to rate zonal centrifugation. Gradient fractions were analyzed by gel electrophoresis followed by Coomassie staining and Western blotting (Fig. 5C). As in the detergent- and protease-treated Gag particles, most of MA and CA remained at the top of the gradient. The NC protein and roughly half of the actin molecules comigrated to high-density fractions. The components of the mature particles were detected in lighter fractions of the gradient than in the parallel experiment with protease-treated Gag particles. Whatever the origin of the shift, the fact that the actin and NC distributions shift together indicates their association and rules out the possibility that they comigrate adventitiously under our gradient conditions.
DISCUSSION
Several experiments suggest that the actin cytoskeleton may be important for retroviral particle morphogenesis (3, 11, 19, 22). While this work provides some insight into the role of the actin cytoskeleton in the transport and assembly of Gag molecules, it does not characterize its role at late stages of budding and in the virus particle after release. Actin and actin-associated proteins are present within HIV-1, mouse mammary tumor virus (3, 17), and several other enveloped viruses (14, 26), opening the possibility of a structural role in the virion. An interaction between in vitro-translated HIV-1 Gag polyprotein and actin has been reported previously (19).
Our experiments demonstrate actin incorporation into MoMuLV and HIV-1 Gag particles and so rule out a crucial role for the products of the env and pol open reading frames in this process. Our experiments with mutant Gag particles deleted in MA or p6 indicated that neither of these domains was involved in incorporation.
Immunolocalization within the particle was based on our previous conclusion that the domains of Gag in the immature particle are arrayed radially (7). This was originally based on an interpretation of the radial density distribution of particles in cryoelectron micrographs and is now confirmed by our immunolocalization of the domains within the particle. Our immunolabeling and quantitative analysis of the results (6, 8, 9) demonstrate that actin colocalizes with the NC domain of the Gag polyprotein. It is clearly separated from the virus membrane and the underlying MA protein layer (Fig. 3).
An internal actin protein layer has been proposed for immature and mature wild-type MoMuLV virions by Nermut and Hockley (16). They hypothesize that actin contributes to a layer between the virus membrane and an internal Gag protein shell which is responsible for the poorly staining “intermediate layer,” a characteristic of images of sectioned MoMuLV (14a). Their proposal is not supported by our observations with immature HIV-1 Gag particles. Our images and distance measurements on immature MoMuLV particles (Fig. 2A) clearly indicate a similar arrangement of actin molecules in these two retroviral systems.
The proximity of actin and the NC domain as revealed by immunolabeling studies suggests a direct or indirect association of these proteins in immature retroviral particles. Indeed, when disrupted immature HIV-1 Gag particles were treated with recombinant HIV-1 protease, actin molecules remained in a stable complex with the NC cleavage product, whereas MA and CA molecules were released (Fig. 5B). Interestingly, the separation of detergent-treated mature HIV-1 virions led to similar results, suggesting that the actin-NC interaction is preserved in mature virions (Fig. 5C). Whether it has a functional role there which is distinct from its role in immature particles remains open.
None of the evidence presented here distinguishes between the interaction of actin with NC and with an NC-RNA complex. RNase treatment of detergent-solubilized Gag particles does not cause release of actin (26a); however, the interaction of RNA with NC appears to protect RNA from efficient digestion and hence is not definitive.
The role of actin-binding proteins in this interaction cannot be ruled out, since ezrin and moesin, which are actin membrane associated, and cofilin, a small actin-severing protein, are present in pure preparations of HIV-1 (17). However, these proteins are present in small amounts relative to actin (17), and hence their role in efficient incorporation would demand a multimeric arrangement of actin molecules. We have performed electron microscopy of detergent-disrupted immature and mature HIV-1 virions under a variety of conditions and found no evidence for a filamentous arrangement of actin molecules (26a). The role of cytoskeletal elements in virus particles is unclear. They may even be a residue of an interaction during budding, as the presence of the actin membrane components ezrin and moesin suggest. The submembrane network is generally regarded as a barrier that has to be overcome before virus particles can be released from the infected cells. In a wild-type infection, the cortical cytoskeleton may be weakened by the proteolytic activity of the viral protease. The identification of several cytoskeletal proteins (e.g., actin, moesin, and vimentin) as targets for the HIV-1 protease (10, 23, 24) and the detection of small amounts of related cleavage products in HIV-1 particles (17) support this model. As a consequence, it could be argued that detection of cytoskeletal proteins reflects only the adventitious uptake of a fragmented cytoskeleton present at the location of virus assembly. However, treatment of HIV-1-infected cells with an inhibitor of the viral protease had no negative effect on actin incorporation, demonstrating that the presence of actin in virions does not rely on the disrupting activity of the viral protease. Furthermore, actin incorporation was also observed when the Gag polyprotein alone was assembled into immature retroviral particles (Fig. 1B). MoMuLV and HIV-1 assembly and budding proceed at the inner face of the plasma membrane. It is conceivable that the Gag polyprotein is integrated into a well-structured submembrane network, displacing existing interactions between cytoskeletal proteins by competing for binding sites while allowing a remaining network to support the viral organization. The formation of type B and D particles deep within the cytoplasm probably rules out such an interaction with the submembrane network as a necessary part of assembly for these retroviruses.
The specific localization of actin molecules within the virion suggests a structural role of cytoskeletal proteins in immature retroviral particles. Nevertheless, it is well established that particles resembling the internal portion of immature virions can be assembled from Gag proteins produced in bacteria (2), arguing against an absolute requirement for actin in particle assembly. Work is in progress to better characterize this interaction and its role in virion formation and function.
ACKNOWLEDGMENTS
We thank Hans-Georg Kräusslich for many useful discussions and for use of the P3 facility at the Heinrich-Pette Institut (HPI) in Hamburg. We thank Reinhold Welker for help with the preparation of mature and immature wild-type HIV-1 particles. We also thank Pierre Boulanger (INSERM, Montpellier, France) and Shigeru Morikawa (National Institute of Health, Tokyo, Japan) for providing recombinant baculoviruses. Further, we are pleased to acknowledge our colleagues at the European Molecular Biology Laboratory (EMBL) and the HPI for helpful discussions. We also appreciate the willingness of Milan Nermut (NIMS, London, United Kingdom) to discuss his model for actin localization in retrovirus particles. The continuous encouragement and interest by Michael Way (EMBL) was an important driving force for the project. We thank Cesare Rossi (EMBL) for critical reading of the manuscript.
REFERENCES
- 1.Arthur L O, Bess J W, Jr, Sowder R C, Benveniste R E, Mann D L, Chermann J-C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 2.Campbell S J, Vogt V M. Self-assembly in vitro of purified CA-NC proteins from Rous sarcoma virus and human immunodeficiency virus type 1. J Virol. 1995;69:6487–6497. doi: 10.1128/jvi.69.10.6487-6497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cudmore S, Reckmann I, Way M. Viral manipulations of the actin cytoskeleton. Trends Microbiol. 1997;5:142–148. doi: 10.1016/S0966-842X(97)01011-1. [DOI] [PubMed] [Google Scholar]
- 4.Damsky C H, Sheffield J B, Tuszynski G P, Warren L. Is there a role for actin in virus budding? J Cell Biol. 1977;75:593–605. doi: 10.1083/jcb.75.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edbauer C, Naso R. Cytoskeleton-associated Pr65gag and retrovirus assembly. Virology. 1983;130:415–426. doi: 10.1016/0042-6822(83)90096-x. [DOI] [PubMed] [Google Scholar]
- 6.Fuller S D, Gowen B E, Buendia B, Karsenti E. The core of the mamalian centriole contains γ-tubulin. Curr Biol. 1995;5:1384–1393. doi: 10.1016/s0960-9822(95)00276-4. [DOI] [PubMed] [Google Scholar]
- 7.Fuller S D, Wilk T, Gowen B E, Kräusslich H-G, Vogt V E. Cryo-electron microscopy reveals ordered domains within the immature HIV-1 particle. Curr Biol. 1997;7:729–738. doi: 10.1016/s0960-9822(06)00331-9. [DOI] [PubMed] [Google Scholar]
- 8.Gowen B E, Buendia B, Karsenti E, Fuller S D. Post-embedding α-tubulin immunolabelling of isolated centrosomes. Histochem J. 1995;27:240–246. [PubMed] [Google Scholar]
- 9.Howell K E, Reuter-Carlson U, Devaney E, Luzio J P, Fuller S D. One antigen, one gold? A quantitative analysis of immuno-gold labelling of plasma membrane 5′ nucleotidase in frozen-thin sections. Eur J Cell Biol. 1987;44:318–327. [PubMed] [Google Scholar]
- 10.Konvalinka J, Heuser A M, Hruskova-Heidingfeldova O, Vogt V M, Sedlacek J, Strop P, Kräusslich H-G. Proteolytic processing of particle-associated retroviral polyproteins by homologous and heterologous viral proteinases. Eur J Biochem. 1995;228:191–198. doi: 10.1111/j.1432-1033.1995.tb20249.x. [DOI] [PubMed] [Google Scholar]
- 11.Kräusslich H-G, Welker R. Intracellular transport of retroviral capsid components. In: Kräusslich H-G, editor. Morphogenesis and maturation of retroviruses. Berlin, Germany: Springer-Verlag; 1996. pp. 25–63. [DOI] [PubMed] [Google Scholar]
- 12.Luftig R, Lupo L. Viral interactions with the host-cell cytoskeleton: the role of retroviral proteases. Trends Microbiol. 1994;2:178–182. doi: 10.1016/0966-842x(94)90669-6. [DOI] [PubMed] [Google Scholar]
- 13.Maldarelli F, King N W, Jr, Yagi M J. Effects of cytoskeletal disrupting agents on mouse mammary tumor virus replication. Virus Res. 1987;7:281–295. doi: 10.1016/0168-1702(87)90043-8. [DOI] [PubMed] [Google Scholar]
- 14.Mortara R A, Koch G L E. Analysis of pseudopodial structure and assembly with viral projections. J Cell Sci Suppl. 1986;5:129–144. doi: 10.1242/jcs.1986.supplement_5.8. [DOI] [PubMed] [Google Scholar]
- 14a.Nermut, M. V. Personal communication.
- 15.Nermut M V, Frank H, Schäfer W. Properties of mouse leukemia viruses. III. Electron microscopic appearance as revealed after conventional preparation techniques as well as freeze drying and freeze etching. Virology. 1972;49:345–358. doi: 10.1016/0042-6822(72)90487-4. [DOI] [PubMed] [Google Scholar]
- 16.Nermut M V, Hockley D J. Comparative morphology and structural classification of retroviruses. In: Kräusslich H-G, editor. Morphogenesis and maturation of retroviruses. Berlin, Germany: Springer-Verlag; 1996. pp. 1–24. [DOI] [PubMed] [Google Scholar]
- 17.Ott D E, Coren L V, Kane B P, Busch L K, Johnson D G, Sowder R C, Chertova E N, Arthur L O, Henderson L E. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J Virol. 1996;70:7734–7743. doi: 10.1128/jvi.70.11.7734-7743.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pumplin D W, Bloch R J. The membrane skeleton. Trends Cell Biol. 1993;3:113–117. doi: 10.1016/0962-8924(93)90173-x. [DOI] [PubMed] [Google Scholar]
- 19.Rey O, Canon J, Krogstad P. HIV-1 GAG protein associates with F-actin present in microfilaments. Virology. 1996;220:530–534. doi: 10.1006/viro.1996.0343. [DOI] [PubMed] [Google Scholar]
- 20.Roberts N A, Martin J A, Kinchington D, Broadhurst A V, Craig J C, Duncan I S, Galpin S A, Handa B K, Kay J, Kröhn A, Lambert R W, Merrett J H, Mills J S, Parkes K E B, Redshaw S, Ritchie A J, Taylor D L, Thomas G J, Machin P J. Rational design of peptide-based HIV protease inhibitors. Science. 1990;248:358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- 21.Royer M, Cerutti M, Gray B, Hong S-S, Devauchelle G, Boulanger P. Functional domain of HIV-1 gag polyprotein expressed in baculovirus infected cells. Virology. 1991;184:417–422. doi: 10.1016/0042-6822(91)90861-5. [DOI] [PubMed] [Google Scholar]
- 22.Sasaki H, Nakamura M, Ohno T, Matsuda Y, Yuda Y, Nonomura Y. Myosin-actin interaction plays an important role in human immunodeficiency virus type 1 release from host cells. Proc Natl Acad Sci USA. 1995;92:2026–2030. doi: 10.1073/pnas.92.6.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoeman R, Honer B, Stoller T, Kesselmeier C, Miedel M, Traub P, Graves M. Human immunodeficiency virus type 1 protease cleaves the intermediate filament proteins vimentin, desmin, and glial fibrillary acidic protein. Proc Natl Acad Sci USA. 1990;87:6336–6340. doi: 10.1073/pnas.87.16.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoeman R, Kesselmeier C, Mothes E, Honer B, Traub P. Non-viral cellular substrates for human immunodeficiency virus type 1 protease. FEBS Lett. 1991;278:199–203. doi: 10.1016/0014-5793(91)80116-k. [DOI] [PubMed] [Google Scholar]
- 25.Stewart L, Schatz G, Vogt V M. Properties of avian retrovirus particles defective in viral protease. J Virol. 1990;64:5076–5092. doi: 10.1128/jvi.64.10.5076-5092.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang E, Wolf T, Lamb R A, Choppin P W, Goldberg A R. The presence of actin in enveloped viruses. In: Goldman R D, editor. Cell motility. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1976. pp. 589–599. [Google Scholar]
- 26a.Wilk, T. Unpublished data.
- 27.Wilk T, Pfeiffer T, Bosch V. Retained in vitro infectivity and cytopathogenicity of HIV-1 despite truncation of the C-terminal tail of the env gene product. Virology. 1992;189:167–177. doi: 10.1016/0042-6822(92)90692-i. [DOI] [PubMed] [Google Scholar]
- 28.Wilk T, Pfeiffer T, Bukovsky A, Moldenhauer G, Bosch V. Glycoprotein incorporation and HIV-1 infectivity despite exchange of the gp160 membrane-spanning domain. Virology. 1996;218:269–274. doi: 10.1006/viro.1996.0190. [DOI] [PubMed] [Google Scholar]