Abstract
Background and Objectives
Autoimmune-associated epilepsy (AAE) with antiglutamic acid decarboxylase 65 (GAD65) antibodies is considered a T-cell–mediated encephalitis that evolves to drug-resistant epilepsy. We do not have an effective therapeutic strategy for these patients. Because the GAD enzyme is primarily responsible for the conversion of glutamate to GABA, the mechanism of epileptogenesis in this condition predicts decreased levels of GABA content in synaptic vesicles. Cenobamate (CNB) acts as a positive allosteric modulator at synaptic and extra synaptic GABAA receptors, producing increased inhibitory neurotransmission in the brain. This mechanism could be especially beneficial in AAE with anti-GAD65 antibodies because it would be able to correct the imbalance due to the GABAergic stimulation deficit in postsynaptic neurons.
Methods
We recruit a retrospective multicentric consecutive case series of AAE with anti-GAD65 antibodies from 5 epilepsy units in Spain who have received treatment with CNB.
Results
A total of 8 patients were recruited. This cohort of highly refractory patients have failed a mean of 9.50 (SD = 3.20) ASM without control of seizures for sustained periods of time. The average number of seizures per month during the previous 3 months before CNB treatment was 19.63 (SD = 17.03). After the introduction of CNB improvement was achieved in all our patients, with a median reduction in the number of seizures of 92.22% (interquartile range [IQR]: 57.25–98.75). The mean follow-up was 156.75 days (SD = 68.23). In patients with concomitant treatment with clobazam (CLB), the median percentage of seizure reduction was higher than those not taking CLB: 94.72% (IQR: 87.25–100) vs 41.50% (p = 0.044) and also higher than the control group of patients with refractory epilepsy not related to anti-GAD65 treated with the same combination: 94.72% (IQR: 87.25–100) vs 45.00% (IQR: 25.00–87.00) (p = 0.019).
Discussion
Treatment with the combination CNB + CLB could be a type of personalized medicine in patients with AAE with anti-GAD65. Our preliminary data will need to be endorsed with new prospective and controlled studies.
Introduction
Epilepsy is a long-lasting predisposition to generate seizures, due to hyperexcitability and hypersynchrony of neuron networks, with a neurobiological, cognitive, psychological, and social impact.1 Significant work has been performed in recent years to increase the accuracy of epilepsy diagnosis, including immunologic, genetic, and structural causes, followed by the development of personalized treatments in what has been called "precision medicine."
This is especially important for some epilepsies, such as autoimmune epilepsy (AE), where existing antiseizure medications (ASMs) have not been able to significantly improve clinical outcomes. For this reason, one of the hottest areas of epileptology research now is AE. The conceptual characteristics of these entities have been the subject of an interesting discussion in recent years.2 So, a proposal by the ILAE Autoimmunity and Inflammation Working Group suggests replacing the term AE with “acute symptomatic seizures secondary to autoimmune encephalitis” and “autoimmune-associated epilepsy” (AAE).3 This distinction better reflects whether there is a lasting predisposition to seizures, as would be expected in a chronic epilepsy.
One of these situations of AAE occurs in “chronic autoimmune encephalitis” where T-cell–mediated autoimmunity predominates4 limbic encephalitis associated with intracellular antineuronal antibodies, onconeuronal antibodies,5 or antiglutamic acid decarboxylase 65 (GAD65).6-8
From a therapeutic perspective, most of these individuals develop drug-resistant epilepsy (DRE) and demand the use of immunomodulatory medications, which are occasionally unsafe.6,7 So, at the present time, we do not have a highly effective therapeutic strategy for these patients and a new therapeutic alternative is highly needed.8,9
AAE associated with anti-GAD65 antibodies can be considered the paradigm of this type of disease, and its clinical expression includes memory impairment, DRE with temporal lobe seizures, and alteration of behavior.
The primary inhibitory neurotransmitter in the brain is gamma-aminobutyric acid (GABA). The enzyme GAD limits the rate at which glutamate is converted to GABA.10 The brain contains 2 forms of GAD: GAD65 and GAD67.11 Antibodies against the b78 epitope have been shown to prevent GAD65 from binding to the cytosolic face of synaptic vesicles that contain GABA, inhibiting GABA production in nerve terminals, and blocking the exocytosis of GABA in synaptic vesicles.12,13 As a result, there would be less GABA available in the synaptic cleft and more presynaptic glutamate.14
Cenobamate (CNB) is an ASM approved by the US FDA and EMA for the treatment of focal onset seizures with or without secondary generalization in adult patients with epilepsy who have not been adequately controlled despite a history of treatment with at least 2 ASM.15 It has been demonstrated that CNB act as a positive allosteric modulator at synaptic and extra synaptic GABA-A receptors, altering both phasic (Iphasic) and tonic (Itonic) currents, thus increasing inhibitory neurotransmission in the brain. This particular GABA-modulating effect distinguishes CNB from other GABAergic medications.16
From a theoretical point of view, this mechanism of action could be especially beneficial in AAE with Anti-GAD65 antibodies because CNB could potentiate the imbalanced GABA inhibition currents at postsynaptic levels.
We present 8 cases of AAE with anti-GAD65 antibodies with a very good response to CNB treatment.
Methods
We recruit a consecutive case series collected retrospectively from the databases of patients with DRE at the hospitals included in 2 research networks in Spain, the Clinical and Translational Research Network of Andalusia (Neuro-RECA), and the autoimmune pathology working group of the Spanish Society of Epilepsy (SEEP).
Inclusion Criteria
(1) To meet the diagnostic criteria for AAE with anti-GAD65 antibodies and (2) to have undergone CNB treatment for at least 90 days from the start of the treatment up until March 1, 2023, the date chosen for the database's closure.
Patients with other possible etiologies for their epilepsy were excluded from the study.
Determination of Anti-GAD65 Antibodies
Serum and CSF anti-GAD65 autoantibodies were estimated by ELISA or immunoblot.
An anti-GAD65 ELISA kit (RSR Ltd., Cardiff, UK) was used for the detection of autoantibodies with an optimum cut-off value of 1250 U/mL. The samples, calibrators, and controls were incubated for at least half an hour in GAD65-coated ELISA plate wells followed by washing to remove the remaining unfixed antibodies. GAD65 biotin was added to each well, and the bound complex was then quantitated by the addition of streptavidin peroxidase and tetramethyl benzidine as colorogenic peroxidase substrates. The absorbance was measured using an ELISA plate reader at 405 nm and then 450 nm after the addition of stop solution (0.25M H2SO4) to obtain the calibration curve. The international standard WHO Reference Preparation NIBSC 97/550 was used as a calibrator. The assay had a measurement range of 5–2,000 U/mL, a sensitivity of 92%, and a specificity of 99%.
Immunoblots were performed using a commercial kit with blot strips (EUROIMMUN, Lübeck, Germany). The patient samples were diluted 1:100 with sample buffer. The test strips were incubated with the samples for 30 minutes after being coated with parallel lines of highly purified antigens and antigen fragments. After washing, the detection of bound antibodies requires a second step of incubation with a solution of alkaline phosphatase–labeled anti-human IgG. Nitroblue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) was used as the substrate to produce the color reaction. The test strips were air-dried, and the bands were scanned and evaluated using EUROLineScan (EUROIMMUN). Samples were considered positive when presenting signal intensities >10. The positive samples were randomized and anonymized and sent to EUROIMMUN for blind testing with 2 confirmation tests using indirect immunofluorescence on frozen rat brain sagittal sections, either using a cell-based assay or a standard immunofluorescence assay with cells transfected with the plasmid of interest. Serum strong positivity (titer >1:3,000) was considered valid.
Titration of CNB
Titration of CNB was performed similarly according to the summary product characteristics (SmPC) in all patients starting at 12,5 mg per day and increasing every 2 weeks to the initial target dose (25, 50, 100, 150, 200, 250, or 300 mg).
Variables
Efficacy variable: Average reduction in seizures measured by quotient between last month and 3 months before treatment with CNB.
-
Other variables:
Demographic and clinical variables: sex, age, duration of epilepsy, epileptogenic focus, history of encephalitis, previous or concomitant immunotherapy, previous surgical procedures, previous ASM, concomitant ASM, mean seizures/month before CNB, follow-up, mean of seizures/month (after CNB), adverse effects, and dose of CNB last follow-up and % of seizure reduction were collected for all patients included.
Ancillary test results: 3T brain MRI, brain FDG PET-TC, EEG or video EEG, serum autoimmunity study, and CSF autoimmunity study were collected for all patients included.
Neuropsychological profile: Cases 1 and 2 were enrolled in an unpublished prospective trial with the aim of evaluating the effect of CNB on cognitive functions. We gathered information on these patients' executive functions (Trail-Making Test B17 and Rey-Osterrieth complex figure test18), attention (Trail-Making Test A17), and episodic memory (measured by the Free and Cued Selective Reminding Test18 and Rey-Osterrieth complex figure test18) before the start of treatment and 6 months later.
Control Group
To make our study more statistically significant, we chose to compare our cases with a control group of patients who were given the CNB + CLB combination taken from the cenobamate early access program. All patients in the database of refractory epilepsy from only one of the epilepsy units that participated in the study (Unit of the Regional University Hospital of Málaga) who received concurrent treatment with the CNB + CLB combination and whose etiology of refractory epilepsy was unrelated to the presence of anti-GAD antibodies 65 were without exception included in the control group.
Statistical Analysis
The Kolmogorov-Smirnov and Shapiro-Wilk tests were used to determine the normality of the distribution of the data for numerical data. The mean, SD, and/or median of continuous variables are used to summarize them. The percentage of patients is used to represent categorical variables. Depending on the sample's normality, either the Student t test or the Mann-Whitney/Kruskal-Wallis test was applied. The Levene test was used to analyze the equivalence of variances. Owing to the descriptive nature of the study, which was a retrospective case series, sample size and study power were not calculated. Data were analyzed using version 25.0 of IBM SPSS (Statistical Package for Social Sciences) (IBM Corp., Armonk, NY).
Standard Protocol Approvals, Registrations, and Patient Consents
The Declaration of Helsinki was followed when conducting the study. Because this study is retrospective in nature, ethical review and approval were waived. Each patient signed the Neurology Service's general informed permission form allowing us to use their data for research.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Results
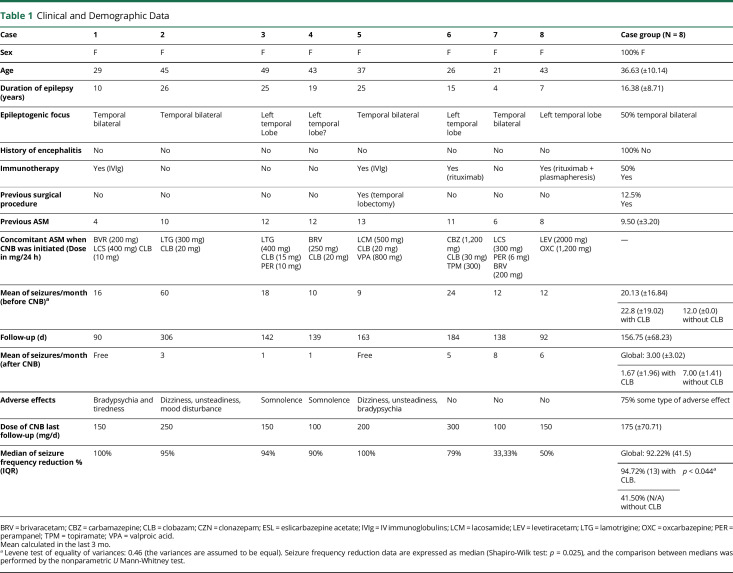
A total of 8 patients who met the inclusion criteria from 5 Neurology Services (Regional University Hospital of Málaga, Hospital Clínic of Barcelona, Center for Advanced Neurology of Seville, Virgen de la Victoria University Hospital of Málaga, and Vithas Málaga Hospitals) were recruited. We include a brief clinical description of each of them. Table 1 shows us the patient's baseline features.
Table 1.
Clinical and Demographic Data
Case 1
A 29-year-old woman with autoimmune hypothyroidism as a comorbidity and no other relevant antecedents. In 2012, during a period of increased stress, she had a generalized tonic-clonic seizure. From that moment on, she began with repetitive episodes of feeling familiarity (déjà vu) with subsequent loss of awareness for one or 2 minutes.
The patient received 4 ASM with no improvements in seizure frequency before the start of CNB. The seizure frequency ranged from 12 to 20 seizures per month. Treatment with oral prednisone and IVIG was performed, which slightly improved the frequency of seizures. The patient initiated treatment at a dose of 12,5 mg and increased it progressively every 2 weeks following the SmPc recommendations of up to 150 mg per day. After 3 months since the treatment onset, the patient has been seizure-free for the last 2 months.
Case 2
A 45-year-old woman with no relevant antecedents. At age 19, she began having seizures triggered by music, consisting of hearing human voices, and a subsequent loss of awareness and automatic behaviors. Sometimes she had generalized tonic-clonic seizures. In recent years, she began to have other daytime episodes in which she was blocked from staring at what was in front of her without knowing what she must do at that moment, followed by contractions in the right hemiface, eye revulsion, and a fall. The frequency of the seizures was variable, ranging from several per day to several days without seizures.
Treatment with CNB was started with the usual dose escalation schedule, up to 250 mg per day. An approximate 95% reduction in seizure frequency was achieved after 10 months of follow-up.
Case 3
A 49-year-old woman with no history of interest. Comorbidity: hypothyroidism and vitiligo. At age 24, she began having generalized tonic-clonic seizures during sleep. After those, other stereotyped seizures that began with epigastric knots, speech arrest, and loss of awareness. Sometimes the autonomic symptoms are more profuse, with anguish, fear, and other reflex seizures to musical stimulation that implies an emotion with the same semiology.
Antiseizure treatment was started with different strategies, controlling generalized tonic-clonic seizures, but not focal seizures. Approximate cadence of 18 seizures per month that worsen with menstruation.
Treatment with CNB was started with the usual dose escalation schedule, up to 150 mg per day. An approximate reduction of 94% in the number of seizures was achieved after 4.5 months of follow-up.
Case 4
A 43-year-old woman, with no antecedents of interest, who had a first generalized tonic-clonic seizure during sleep at the age of 24. A few months later, she began to present with psychic and dyscognitive seizures with loss of awareness, sometimes in a clustered way, which have been repeated with a frequency of 2–3 a week despite the different treatments. She has been developing a mild-moderate cognitive impairment, mainly in episodic and declarative memory. In October 2022, CNB treatment was added in the usual scheme of ascending regimens up to 100 mg, with a 90% reduction in the number of seizures in the last 3 months.
Case 5
A 37-year-old woman with no relevant antecedents at the age of 12 years with focal seizures with loss of awareness and a semiological profile of medial temporal lobe epilepsy. Psychiatric comorbidity includes psychotic episodes and somatic comorbidity with type I diabetes mellitus and seropositive myasthenia gravis. At age 20 years, she underwent a right temporary lobectomy without benefiting from seizure control.
Treatment with CNB was started with the usual dose escalation schedule, up to 200 mg per day. At 5 months of onset, the patient has been seizure-free for the last 3 months.
Case 6
A 26-year-old woman with a personal history of type I diabetes mellitus. At the age of 11 years, she began to present seizures that started with stereotyped nonspecific cephalic discomfort that can be followed or not by disconnection with oral and manual automatisms and left arm dystonia. She speaks during seizures, although she is not legible. The average frequency of these seizures was 24 per month. She received multiple ASMs and immunomodulatory treatments, without positive results. Treatment with CNB was started with the usual dose escalation schedule, up to 300 mg per day. An approximate reduction of 79% in the number of seizures was achieved after 5 months of follow-up.
Case 7
A 21-year-old normal woman with onset at the age of 17 with seizures with psychic aura (déjà vu) or no aura, loss of awareness, manual automatisms, and postictal dysphasia. The frequency of seizures was variable, but the frequency when CNB started was around 3 per week. Comorbidities: type I diabetes. Of interest, this patient also has positive anti-Ma2 antibodies in serum and CSF. No tumor has been found. She received previous immunotherapy with high-dose corticotherapy (worsened diabetes), IVIg (no effect), rituximab (no effect), and plasma exchange (no effect).
Treatment with CNB was started with the usual dose escalation schedule, up to 100 mg per day. An approximate reduction of 33,3% in the number of seizures was achieved after more than 4 months of follow-up.
Case 8
A 43-year-old university woman with episodes of epigastric and psychic (déjà vu/fear) or autonomic symptoms, followed by loss of awareness with oral and manual automatisms, and postictal dysphasia that appeared for the first time in 2004. Sometimes she retains awareness, but she is unable to speak or understand spoken or written language. Very rarely, it evolves to focal to bilateral tonic-clonic seizures. Type I diabetes was diagnosed in 2011 (after seizure onset). Depressive syndrome treated with antidepressive drug in the past. She has received immunotherapy without benefit: rituximab, plasma exchange, and IVIg.
Treatment with CNB was started with the usual dose escalation schedule, up to 100 mg per day. An approximate reduction of 50% in the number of seizures was achieved after 5 months of follow-up.
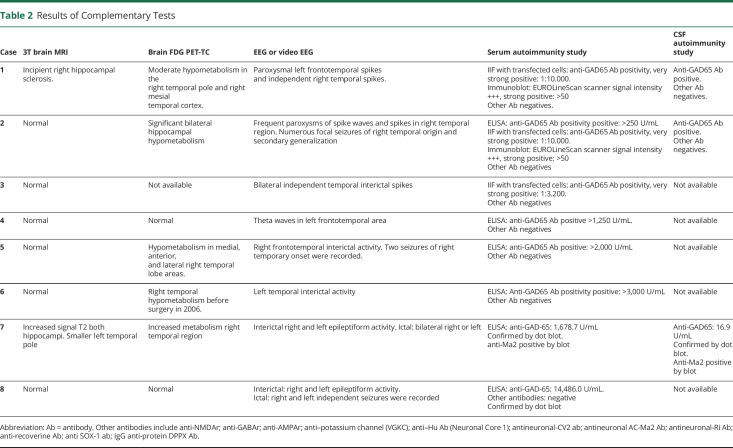
The main clinical and demographic variables of all patients and the most significant results in ancillary tests are shown in Tables 1 and 2, respectively.
Table 2.
Results of Complementary Tests
As can be seen in Table 1, our patients were highly refractory. Specifically, the average evolution of their epilepsy was 16.38 years (SD = 8.71), and they had failed a mean of 9.50 (SD = 3.20) previous ASMs before the attempt at treatment with CNB. 7/8 of our patients met absolute DRE criteria, defined as the failure of at least 6 ASM.19 The average number of seizures per month during the previous 3 months was 19.63 (SD = 17.03). Four of them (50%) had previously received immunomodulatory treatment (IVIg, rituximab, plasmapheresis, or a combination of treatments), and in case 5, a previous temporal lobectomy had been performed, but with unsuccessful results. All these data are indicative of the extreme degree of refractoriness of these patients, as expected in patients diagnosed with AAE with anti-GAD65 antibodies. Not surprisingly, as published in the scientific literature, it is known that only 8% of patients with GAD65 epilepsy responded to ASM.20
However, after the introduction of CNB at a medium dose of 175 mg per day (SD = 70.71) with a mean follow-up of 156.75 (SD = 68.23) days, improvement was achieved in all our patients, with an average seizure frequency reduction of 80.18% (SD = 25.19) and a median of 92.22%. If we consider as responders, patients who reduce the number of seizures to 50%, the percentage of responders in our series was 87.5%.
The fact that 75% of our patients were also receiving therapy for concurrent CLB is an important consideration. The median percentage of seizure reduction in these patients (cases 1 to 6) was larger than in those who were not taking CLB (cases 7 and 8): 94.72% (interquartile range [IQR]: 87.25–100.00) vs 41.50% (p = 0.044). In particular, patients receiving CLB simultaneously experienced a reduction in seizures from an average of 22.8 per month (SD = 19.02) to 1.67 per month (SD = 1.96), with 2 of these patients experiencing seizure-free (see Table 1).
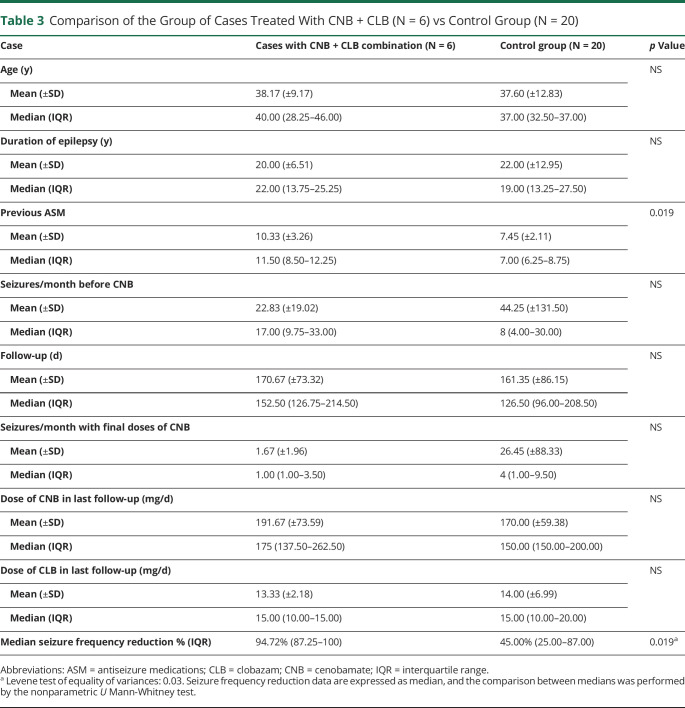
Table 3 shows the comparison of the main clinical and efficacy variables of the subgroup of cases treated with CNB + CLB vs the control group. Median seizure frequency decrease for the case subgroup was 94.72 percent (IQR: 87.25–100) vs 45.00 percent (IQR: 25.00–87.00) (p = 0.019), demonstrating the case group's continued greater efficacy in comparison with this control group. In comparison with other types of refractory epilepsy, these data indicate that this combination has a particularly high efficacy in AAE with anti-GAD65 antibodies.
Table 3.
Comparison of the Group of Cases Treated With CNB + CLB (N = 6) vs Control Group (N = 20)
In the section of Supplementary Material, the full clinical data of the control group (eTable 1, links.lww.com/NXI/A894) and the data from the neuropsychological study of cases 1 and 2 are presented (eFigure 1, links.lww.com/NXI/A892 and eFigure 2, links.lww.com/NXI/A893).
Discussion
In this study, we present 8 patients with AAE with anti-GAD65 antibodies who were retrospectively collected in 5 epilepsy units of the Neuro-RECA and the autoimmune working group of the SEEP networks. These patients demonstrated that adjunctive therapy with CNB at doses between 100-300 mg/d during routine clinical practice resulted in significant decreases in seizure frequency. In addition, the subgroup of 6 patients who received the CNB + CLB combination demonstrated an especially positive response, achieving a median percentage of seizure reduction of 94.72 percent, noticeably higher than patients in the case group treated only with CNB and that of patients treated with CNB + CLB but whose refractory epilepsy was unrelated to anti-GAD65 antibodies.
According to the available epidemiologic data, AAE with anti-GAD65 may represent 8.7% of cases of nonlesional temporal lobe epilepsy21 and has always caused serious therapeutic problems given its proven refractoriness to ASMs and immunomodulatory treatments.6,22 In addition, a recent review of developing therapeutic resources for autoimmune-based epileptic syndromes did not identify any specifically targeting AAE with anti-GAD65 antibodies.23 For this reason, any new therapeutic resource for these patients is of special relevance.
The mechanisms of epileptogenesis in AAE with anti-GAD65 antibodies have been the subject of intense research in the last years. There is evidence that the GAD65 enzyme is crucial for the direct synthesis of GABA from astrocytic glutamine using glutamate.24 In addition, anti-GAD65 antibodies are shown to cause a decrease in cortical GABA levels in human magnetic resonance spectroscopy studies.25 Finally, it has long been understood that decreased GABA levels are linked to higher seizure rates and epilepsy.26 Overall, these experimental data enables us to draw the inference that, while we cannot definitively state that anti-GAD65 antibodies are pathogenic in the full AAE syndrome, they are probably connected to the epileptogenesis of the syndrome. The main current hypothesis suggests that inhibition of this GAD65 by antibodies may not only decrease the GABAergic tone in the synaptic cleft but also increase the amount of excitatory glutamate, which is no longer metabolized to GABA.25 As a result, its immunologic blockage by antibodies would eventually lead to a situation where GABA-glutamic acid was permanently out of balance at the synaptic level.
In comparison with the ASMs that have been made so far, CNB is a new ASM that offers several unique qualities. Efficacy findings from clinical trials on patients with DRE have been particularly encouraging, showing a response rate of 50% in the number of seizures of 64% and a seizure-free patient rate of 21% when we use dosages of 400 mg per day.27 Its mechanism of action is dual. On the one hand, it is a powerful blocker of the permanent current of the sodium channels (INaP), which makes it different from the rest of the ASM that act on voltage-gated sodium channels (VGSC).15 In addition, it acts as a positive allosteric modulator at GABAA receptors both inside and outside of synapses,16 which we consider of special relevance to our work.16,28 This potent GABAergic action could theoretically be able to compensate for the GABAergic neurotransmission deficit in AAE with anti-GAD65 antibodies.
Recent studies suggest that concurrent administration of CNB and CLB may significantly raise the serum levels of N-desmethylclobazam (N-CLB) through a pharmacokinetic interaction at CYP2C19 level.29 In addition, this combination leads to simultaneous inhibition of GABA-A receptor at 2 different sites leading to complementary mechanisms of action.16
We can assume that this synergistic impact between the 2 medications could support a higher efficacy independent of the underlying cause. If that were the case, it would be impossible to support the idea that anti-GAD65 antibodies are a form of tailored treatment for AAE. To testing this theory, we made a comparison with a control group that shared clinical and demographic traits with the 6 individuals treated with CNB + CLB but whose etiology was unrelated to anti-GAD65 antibodies showing that the response was also greater than in this other group of patients.
In a more speculative way, it could be cited as a possible adjuvant mechanism, the immunomodulatory and anti-inflammatory properties that have been attributed in general to VGSC blockers.30
The arguments presented allow us to consider treatment with the combination CNB + CLB, a type of personalized medicine, in patients with AAE with anti-GAD65 antibodies because the mechanism of action of the drug seems fully aimed at compensating for the GABAergic deficit that is supposed to be crucial in the process of epileptogenesis of these patients.
In any event, it is important to remember that AAE with anti-GAD65 antibodies is a syndrome that encompasses both epileptic seizures and other neurologic symptoms (cognitive impairment, psychiatric manifestations, rigid person syndrome, etc). We are unaware of how CNB might affect these other syndrome symptoms. We know that this ASM had an impact on mice's performance in the Morris water maze, novel object recognition, and object location memory, according to a recently published study on the behavioral, synaptic, and cellular actions of CNB.31
Two of our patients (Cases 1 and 2) were a part of an unpublished prospective study to assess the effects of CNB therapy on her cortical functions. The individual data of these patient showed that after 6 months of treatment with CNB, the scores of executive functions improved in both cases and episodic memory improved in case 2 and remained unchanged in case 1. These data could suggest some effect on other aspects of the disease of treatment with the CNB + CLB combination. Detailed neuropsychological assessment is provided as supplementary material (eFigure 1, links.lww.com/NXI/A892 and eFigure 2, links.lww.com/NXI/A893).
The fact that our study was retrospective, the small number of patients, and the brief follow-up period are its primary limitations. However, this is the first time data demonstrating CNB's usefulness for patients with AEE have been published. New prospective and controlled investigations will be required to support this retrospective study of clinical data.
Glossary
- AAE
autoimmune-associated epilepsy
- AE
autoimmune epilepsy
- ASM
antiseizure medications
- CLB
clobazam
- CNB
cenobamate
- DRE
drug-resistant epilepsy
- GABA
gamma-aminobutyric acid
- GAD65
glutamic acid decarboxylase 65
- IQR
interquartile range
- SEEP
Spanish Society of Epilepsy
- SmPC
summary product characteristics
- VGSC
voltage-gated sodium channels
Appendix. Authors
Study Funding
This study has been funded by Andalusian Network of Clinical and Translational Research in Neurology (Neuro-RECA) of the Ministry of Health and Consumer Affairs of Andalusia (Code: RIC-0111-2019), Nicolas Monardes Program of the Andalusian Health Public System, SAS (Code: RC-0002-2021 Action C), Clinical-Electroencephalographic and Immunological Characterization of Epilepsy Associated with Autoimmunity (Exp.: NEURORECA- 0003-2022), Neuro-RECA grants for Scientific Research in Neurology, Ministry of Health and Consumer Affairs, and Junta de Andalucía 2022.
Disclosure
P.J. Serrano-Castro has received consultant and/or speaker honoraria from Angelini-Pharma, Bial, Eisai, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), Roche Pharmaceuticals, UCB Pharma, Zambon, and Zogenix España; J.J. Rodríguez-Uranga has received consultant and/or speaker honoraria from Angelini-Pharma, Bial, Eisai, Biopas, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), and UCB Pharma; P. Cabezudo-García has received consultant and/or speaker honoraria from Angelini-Pharma, Bial, Eisai, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), UCB Pharma, Exeltis, and Neuraxpharm; G. Garcia-Martin has received consultant and/or speaker honoraria from Bial, Eisai, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), and UCB Pharma; M. Carreño has received consultant and/or speaker honoraria from Angelini-Pharma, Bial, Eisai, GW Pharmaceuticals (now a part of Jazz Pharmaceuticals), and UCB Pharma; J. Romero-Godoy, N.L. Ciano-Petersen, G. Estivill-Torrús, B. Oliver, J. Ortega-Pinazo, Y. López-Moreno, M.J. Aguilar-Castillo, A.L. Gutierrez-Cardo, T. Ramirez-Garcia, and L. Sanchez-Godoy do not declare conflicts of interest. Go to Neurology.org/NN for full disclosures.
References
- 1.Fisher RS, Van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the international Bureau for epilepsy (IBE). Epilepsia. 2005;46(4):470-472. doi: 10.1111/j.0013-9580.2005.66104.x [DOI] [PubMed] [Google Scholar]
- 2.Budhram A, Burneo JG. Acute symptomatic seizures, epilepsy, and autoimmune encephalitis: clarifying terminology in neural antibody-associated disease. Epilepsia. 2023;64(2):306-310. doi: 10.1111/epi.17478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steriade C, Britton J, Dale RC, et al. Acute symptomatic seizures secondary to autoimmune encephalitis and autoimmune-associated epilepsy: conceptual definitions. Epilepsia. 2020;61(7):1341-1351. doi: 10.1111/epi.16571 [DOI] [PubMed] [Google Scholar]
- 4.Gillinder L, Britton J. Autoimmune-associated seizures. Continuum (Minneap Minn). 2022;28(2):363-398. [DOI] [PubMed] [Google Scholar]
- 5.Serafini A, Lukas RV, VanHaerents S, et al. Paraneoplastic epilepsy. Epilepsy Behav. 2016;61:51-58. doi: 10.1016/j.yebeh.2016.04.046 [DOI] [PubMed] [Google Scholar]
- 6.Li X, Guo Q, Zheng Z, Wang X, Liu S. Immune-mediated epilepsy with GAD65 antibodies. J Neuroimmunol. 2020;341:577189. doi: 10.1016/j.jneuroim.2020.577189 [DOI] [PubMed] [Google Scholar]
- 7.Joubert B, Belbezier A, Haesebaert J, et al. Long-term outcomes in temporal lobe epilepsy with glutamate decarboxylase antibodies. J Neurol. 2020;267(7):2083-2089. doi: 10.1007/s00415-020-09807-2 [DOI] [PubMed] [Google Scholar]
- 8.Vogrig A, Gigli GL, Nilo A, Pauletto G, Valente M. Seizures, epilepsy, and NORSE secondary to autoimmune encephalitis: a practical guide for clinicians. Biomedicines. 2023;11(1):44. doi: 10.3390/biomedicines11010044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seifert-Held T, Eberhard K, Lechner C, et al. Functional recovery in autoimmune encephalitis: a prospective observational study. Front Immunol 2021;12:641106. doi: 10.3389/fimmu.2021.641106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei J, Wu JY. Post-translational regulation of L-glutamic acid decarboxylase in the brain. Neurochem Res. 2008;33(8):1459-1465. doi: 10.1007/s11064-008-9600-5 [DOI] [PubMed] [Google Scholar]
- 11.Martin DL, Liu H, Martin SB, Wu SJ. Structural features and regulatory properties of the brain glutamate decarboxylases. Neurochem Int. 2000;37(2-3):111-119. doi: 10.1016/s0197-0186(00)00014-0 [DOI] [PubMed] [Google Scholar]
- 12.Manto M, Honnorat J, Hampe CS, et al. Disease-specific monoclonal antibodies targeting glutamate decarboxylase impair GABAergic neurotransmission and affect motor learning and behavioral functions. Front Behav Neurosci. 2015;9:78. doi: 10.3389/fnbeh.2015.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishida K, Mitoma H, Song SY, et al. Selective suppression of cerebellar GABAergic transmission by an autoantibody to glutamic acid decarboxylase. Ann Neurol. 1999;46(2):263-267. doi: [DOI] [PubMed] [Google Scholar]
- 14.Flammer J, Neziraj T, Rüegg S, Pröbstel AK. Immune mechanisms in epileptogenesis: update on diagnosis and treatment of autoimmune epilepsy syndromes. Drugs. 2023;83(2):135-158. doi: 10.1007/s40265-022-01826-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberti R, de Caro C, Iannone LF, Zaccara G, Lattanzi S, Russo E. Pharmacology of cenobamate: mechanism of action, pharmacokinetics, drug–drug interactions and tolerability. CNS Drugs. 2021;35(6):609-618. doi: 10.1007/s40263-021-00819-8 [DOI] [PubMed] [Google Scholar]
- 16.Sharma R, Nakamura M, Neupane C, et al. Positive allosteric modulation of GABAA receptors by a novel antiepileptic drug cenobamate. Eur J Pharmacol. 2020;879:173117. doi: 10.1016/j.ejphar.2020.173117 [DOI] [PubMed] [Google Scholar]
- 17.Peña-Casanova J, Quiñones-Úbeda S, Quintana-Aparicio M, et al. Spanish multicenter normative studies (NEURONORMA project): norms for verbal span, visuospatial span, letter and number sequencing, trail making test, and symbol digit modalities test. Arch Clin Neuropsychol. 2009;24(4):321-341. doi: 10.1093/arclin/acp038 [DOI] [PubMed] [Google Scholar]
- 18.Peña-Casanova J, Gramunt-Fombuena N, Quiñones-Úbeda S, et al. Spanish multicenter normative studies (NEURONORMA project): norms for the rey-osterrieth complex figure (copy and memory), and free and cued selective reminding test. Arch Clin Neuropsychol. 2009;24(4):371-393. doi: 10.1093/arclin/acp041 [DOI] [PubMed] [Google Scholar]
- 19.Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs effect of past treatment history. Neurology. 2008;70(1):54-65. doi: 10.1212/01.wnl.0000286959.22040.6e [DOI] [PubMed] [Google Scholar]
- 20.Cabezudo-García P, Mena-Vázquez N, Villagrán-García M, Serrano-Castro PJ. Efficacy of antiepileptic drugs in autoimmune epilepsy: a systematic review. Seizure. 2018;59:72-76. doi: 10.1016/j.seizure.2018.05.004 [DOI] [PubMed] [Google Scholar]
- 21.Falip M, Carreño M, Miró J, et al. Prevalence and immunological spectrum of temporal lobe epilepsy with glutamic acid decarboxylase antibodies. Eur J Neurol. 2012;19:827-833. doi: 10.1111/j.1468-1331.2011.03609.x [DOI] [PubMed] [Google Scholar]
- 22.Daif A, Lukas RV, Issa NP, et al. Antiglutamic acid decarboxylase 65 (GAD65) antibody-associated epilepsy. Epilepsy Behav. 2018;80:331-336. doi: 10.1016/j.yebeh.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 23.Aguilar-Castillo MJ, Cabezudo-García P, Ciano-Petersen NL, et al. Immune Mechanism of Epileptogenesis and Related Therapeutic Strategies. Biomedicines. 2022;10(3):716. doi: 10.3390/biomedicines10030716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls AB, Eyjolfsson EM, Smeland OB, et al. Knockout of GAD65 has major impact on synaptic GABA synthesized from astrocyte-derived glutamine. J Cereb Blood Flow Metab. 2011;31(2):494-503. doi: 10.1038/jcbfm.2010.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stagg CJ, Lang B, Best JG, et al. Autoantibodies to glutamic acid decarboxylase in patients with epilepsy are associated with low cortical GABA levels. Epilepsia. 2010;51(9):1898-1901. doi: 10.1111/j.1528-1167.2010.02644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathews GC. The dual roles of GABA in seizures and epilepsy generate more excitement. Epilepsy Curr. 2007;7(1):28-30. doi: 10.1111/j.1535-7511.2007.00159.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38-48. doi: 10.1016/S1474-4422(19)30399-0 [DOI] [PubMed] [Google Scholar]
- 28.Guignet M, Campbell A, White HS. Cenobamate (XCOPRI): can preclinical and clinical evidence provide insight into its mechanism of action?. Epilepsia. 2020;61(11):2329-2339. doi: 10.1111/EPI.16718 [DOI] [PubMed] [Google Scholar]
- 29.Elakkary S, Hagemann A, Klimpel D, Bien CG, Brandt C. A retrospective non‐interventional study evaluating the pharmacokinetic interactions between cenobamate and clobazam. Epilepsia. 2023;64(4):e36-e42. doi: 10.1111/epi.17515 [DOI] [PubMed] [Google Scholar]
- 30.Roselli F, Livrea P, Jirillo E. Voltage-Gated Sodium Channel Blockers as Immunomodulators. Recent Pat CNS Drug Discov. 2006;1(1):83-91. doi: 10.2174/157488906775245255 [DOI] [PubMed] [Google Scholar]
- 31.Song WS, Cho YS, Oh SP, Yoon SH, Kim YS, Kim MH. Cognitive and behavioral effects of the anti-epileptic drug cenobamate (YKP3089) and underlying synaptic and cellular mechanisms. Neuropharmacology. 2022;221:109292. doi: 10.1016/j.neuropharm.2022.109292 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.