Abstract
Over the past decade, mRNA modifications have emerged as important regulators of gene expression control in cells. Fueled in large part by the development of tools for detecting RNA modifications transcriptome-wide, researchers have uncovered a diverse epitranscriptome which serves as an additional layer of gene regulation beyond simple RNA sequence. Here, we review the proteins that write, read, and erase these marks, with a particular focus on the most abundant internal modification, m6A. We describe the discovery of the key enzymes that deposit and remove m6A and other modifications and discuss how our understanding of these proteins has shaped our views of modification dynamics. We also review current models for the function of m6A reader proteins and how our knowledge of these proteins has evolved. Finally, we highlight important future directions for the field and discuss key questions that remain unanswered.
Keywords: Epitranscriptome, RNA modifications, m6A, reader protein, methyltransferase, demethylase
1. INTRODUCTION
Cellular RNAs contain over 150 different chemical modifications, most of which reside in non-coding RNAs such as rRNA and tRNA. While the presence of modifications in these abundant RNA species has been known for decades, much of our understanding of post-transcriptional modifications in mRNAs has emerged within just the past several years. These studies have been fueled by new technologies that have enabled the detection and quantification of mRNA modifications and the precise mapping of modification sites transcriptome-wide. These efforts have revealed a growing “epitranscriptome” comprising diverse modifications of varying abundance.
Chemical modifications to mRNAs were initially discovered in the 1970s, when researchers identified m6A and m5C from bulk hydrolyzed nucleosides isolated from oligo(dT)-purified cellular RNA (1, 2). This work revealed m6A to be the most abundant internal modification, and subsequent studies determined that it was present in a GAC or AAC consensus sequence (3, 4). However, due to a lack of methods for profiling m6A transcriptome-wide, it was several decades before individual methylated mRNAs throughout the transcriptome would be discovered. Then, in 2012, the development of antibody-based approaches (MeRIP-seq/m6A-seq) for global m6A detection revealed thousands of methylated mRNAs and identified a distinct distribution of m6A within long internal exons and near the 5’ end of terminal exons (5, 6). These studies, in addition to the discovery that m6A could be reversibly modified through the action of demethylating enzymes (7), sparked a renewed interest in mRNA modifications.
In this review, we provide an overview of the writers, readers, and erasers of mRNA modifications, with a primary focus on m6A. We review the recent advances in our understanding of how these proteins are regulated and their impact on gene expression and cellular function, and we highlight links to human disease. Finally, we discuss key findings as well as controversies and describe emerging areas of importance.
2. WRITERS OF MRNA MODIFICATIONS
2.1. The m6A methyltransferase complex: Discovery of the core METTL3/METTL14 dimer
m6A is deposited co-transcriptionally by a large methyltransferase complex (MTC) (Figure 1a). Efforts to identify the factors responsible for m6A formation began in the early 1990s, when researchers demonstrated that nuclear extracts from HeLa cells were capable of methylating substrate RNAs in vitro (8). A large complex was subsequently purified from HeLa cell nuclear extracts which had multiple components that were required for methyltransferase activity (9, 10). Within this complex, a 70 kilodalton protein with S-adenosyl-methionine (SAM) binding activity was identified and subsequently cloned from HeLa cell cDNA (9). This protein, MT-A70 (10), was found to be catalytically active in vitro, expressed in many tissues, and predominantly localized to the nucleus (9).
Figure 1. Regulation and function of m6A writer proteins.
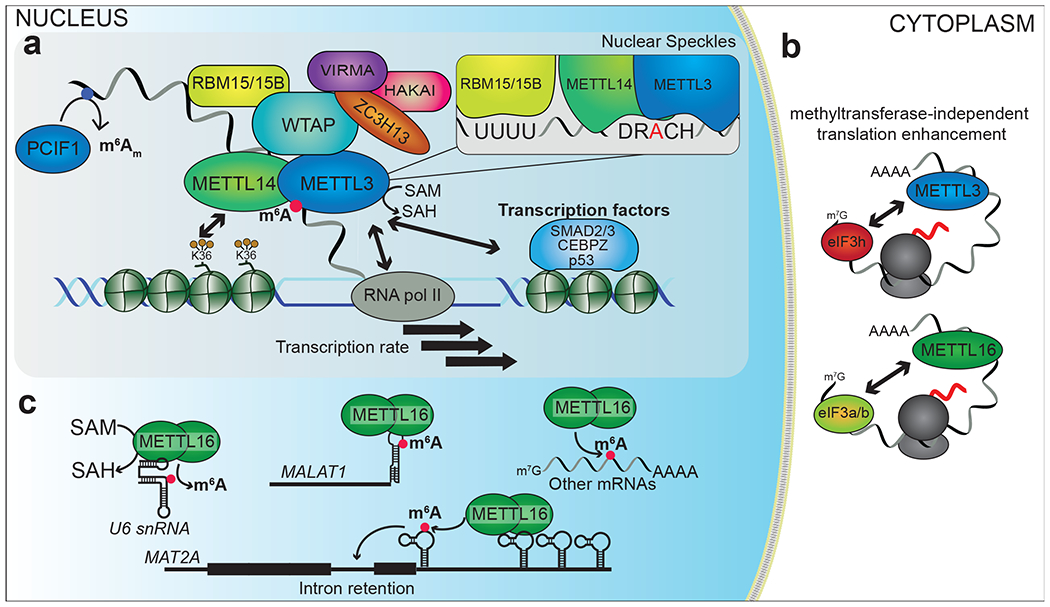
a) m6A is deposited co-transcriptionally by a methyltransferase complex which includes METTL3 as the catalytic subunit and additional proteins METTL14, WTAP, RBM15/15B, VIRMA, HAKAI, and ZC3H13. This complex is recruited to RNAs through interactions with H3K36me3 histone marks, transcription factors, and RNA polymerase II (RNA pol II), and its activity can be influenced by transcription rate. METTL3 deposits m6A within the DRACH consensus sequence, which is where the majority of m6A sites within mRNAs reside. b) In the cytoplasm, METTL3 and METTL16 interact with the translation initiation machinery to promote mRNA translation independently of their methyltransferase activity. c) A subset of mRNAs can be methylated by METTL16, which requires distinct sequence and structural elements. METTL16 also deposits m6A in snRNAs and lncRNAs. At the mRNA 5’ end, PCIF1 deposits m6Am adjacent to the cap.
MT-A70 belongs to the methyltransferase-like (METTL) superfamily of proteins with SAM-binding domains and is now known as METTL3. METTL14, another member of this family with homology to METTL3, has since been demonstrated to be a critical component of the MTC. Initial studies indicated that METTL14 had methyltransferase activity in vitro (11). However, later work showed that this activity was due to co-purification of METTL3, which forms a strong dimer with METTL14 (12). Structural studies further confirmed that only METTL3 binds to SAM, while METTL14 contributes to substrate recognition and stabilizes the interaction between METTL3 and RNA (12–14) (Figure 1a). Thus, although METTL14 does not have methyltransferase activity, it is required for target RNA methylation. Indeed, multiple studies have shown that depletion of either METTL3 or METTL14 alone results in a strong decrease in the abundance of m6A methylation in cellular mRNAs (11, 15).
In addition to its m6A methyltransferase activity, METTL3 has also been shown to have methyltransferase-independent functions. In cancer cells, METTL3 promotes the translation of a subset of mRNAs by binding to the 3’UTR and mediating interactions with eIF3h through mRNA looping (16, 17) (Figure 1b). Through this mechanism, METTL3 enhances the translation of oncogenic transcripts including EGFR, TAZ, and BRD4 and promotes cellular transformation and tumor growth (16, 17).
2.2. Additional MTC components
In 2008, FIP37 was identified in a yeast two-hybrid screen for interactors of MTA, the m6A methyltransferase in Arabidopsis thaliana (18). Subsequent studies showed that the yeast homolog of FIP37, MUM2, also interacts with the yeast m6A methyltransferase IME4 and contributes to m6A deposition (19). FIP37/MUM2 are homologs of mammalian Wilms’ tumor 1-associated protein (WTAP), which in 2014 was shown to bind in complex with METTL3 and METTL14 and to be indispensable for MTC-mediated m6A methylation in mRNAs (11, 15, 20). WTAP regulates the localization of the MTC by recruiting it to nuclear speckles, sites of high transcriptional activity (11, 15, 20) (Figure 1a).
The discovery of other MTC components was facilitated by proteomics studies that sought to identify interacting partners of METTL3, METTL14, and WTAP (15, 21, 22). These studies identified KIAA1429, also known as VIRMA, as an interacting partner of WTAP. This was consistent with previous work showing a similar interaction in Drosophila (23). Biochemical studies further demonstrated that depletion of VIRMA leads to a significant decrease in m6A in poly(A) RNA, indicating its importance for a functional MTC (21). In addition to VIRMA, Horiuchi and colleagues identified RBM15 as a WTAP interactor (22). Later, it was confirmed that RBM15 and its paralog RBM15B interact with the MTC in a WTAP-dependent manner (24). Depletion of RBM15/15B results in a substantial loss of m6A in poly(A) RNA and in the long noncoding RNA XIST, confirming a functional role for RBM15/15B in the methylation of cellular RNAs. Identification of RBM15/15B binding sites using iCLIP revealed that these proteins bind U-rich regions near methylated, but not unmethylated, DRACH motifs (D=A,G,U; R=A,G; H=A,C,U), which are preferred consensus motifs where m6A methylation occurs (24). This suggests that RBM15/15B may help target the MTC to a subset of sites near U-rich sequences (Figure 1a).
HAKAI, an E3 ubiquitin ligase, was also identified within the MTC in proteomics studies (22). Not only has this association been confirmed for the HAKAI homologs in Arabidopsis and Drosophila, but depletion of the HAKAI homolog in each species leads to a reduction of m6A in mRNA (25–27), demonstrating an evolutionarily conserved role in methylation. Interestingly, although HAKAI is an E3 ubiquitin ligase, its catalytic activity is not required for its role in regulating adenosine methylation in flies (26), suggesting that it most likely does not regulate MTC activity by ubiquitination. Indeed, in contrast to its described role as an E3 ligase, Hakai has been shown to stabilize the homologs of core MTC components METTL3, METTL14, and WTAP in flies (26).
The zinc finger protein ZC3H13 is an additional MTC component that was identified using immunoprecipitation and mass spectrometry (21). Knockdown of ZC3H13 leads to reduced m6A deposition and, like other MTC components, it is critical for mESC self-renewal (21, 28). Mechanistically, ZC3H13 regulates the MTC by promoting the nuclear localization of WTAP, VIRMA, and HAKAI (28).
2.3. m6A methylation specificity
Early biochemical studies using enzymatic digestion of radiolabeled cellular RNA indicated that m6A occurs within a GAC or AAC consensus sequence (3, 4). More recent transcriptome-wide m6A mapping studies have further identified the preferred extended DRACH motif (5, 6). However, only a small proportion of DRACH motifs contain m6A, suggesting that other factors contribute to methylation specificity. Methylation of specific residues may be primarily guided by cis-elements within the RNA, giving rise to a hard-wired “m6A code” that is dictated primarily by sequence and, to a lesser extent, RNA structure (29). Additionally, gene architecture appears to contribute to m6A distribution, as m6A is depleted near exon junctions but enriched within long internal exons and in the beginning of terminal exons, the latter giving rise to the appearance of stop codon-proximal enrichment which is a characteristic feature of m6A across diverse cell types and species (5, 6, 30, 31).
A major question has been how m6A is preferentially deposited at specific DRACH motifs. Yue et al. demonstrated that VIRMA depletion leads to selective loss of m6A sites from the vicinity of the stop codon in HeLa cells (21). This effect was not seen with depletion of METTL3, METTL14, or WTAP, suggesting that VIRMA may help confer methylation specificity near the 5’ end of the 3’UTR. However, it remains unclear how VIRMA mediates this specificity, and more studies are needed to determine the mechanism and how generalizable this is across cell types. It is possible that VIRMA bridges interactions between the MTC and transcription-associated proteins, or that it somehow recognizes specific gene architecture elements.
Crosstalk with chromatin marks may be another mechanism for m6A specificity. Studies in mESCs have shown that the MTC is recruited through METTL14 to the H3K36 trimethylation mark (H3K36me3), which is enriched at genomic regions near m6A (32) (Figure 1a). Depletion of the H3K36 methyltransferase SETD2 leads to reduced m6A at approximately 46% of co-regulated H3K36me3/m6A sites, indicating that this may be a mechanism for guiding the MTC to a substantial proportion of RNAs destined for methylation, but also suggesting that other factors exist (32). Indeed, several transcription factors, including SMAD2/3, CEBPZ, and p53, have been shown to bind and recruit the MTC to methylate specific target RNAs (33–35) (Figure 1a). In addition, RNA polymerase II (RNA pol II) transcription rate affects methylation frequency, with slower transcription resulting in more methylation and decreased mRNA stability (36) (Figure 1a). This is reminiscent of other co-transcriptional processes like splicing and 3’ end formation that are also altered by transcription rate (37).
In general, m6A patterns are relatively static across tissues and even species (38), but there are transcripts that exhibit distinct tissue and cell type-specific methylation profiles, even when accounting for differences in gene expression (38, 39). Variability in the expression of specific transcription factors or abundance of H3K36me3 may explain some of this variability. Studies using single-cell m6A profiling have shown that many more consensus motifs are methylated in mRNAs than previously thought based on m6A mapping in bulk cells (40). Most of these sites occur rarely throughout a population of cells, but some RNAs contain high-frequency sites that are methylated in nearly all cells. Thus, chromatin marks and transcription-associated proteins may help recruit the MTC to sites of nascent transcription to facilitate methylation at any suitable motif in the region, but in some cases, there may be other unknown factors that contribute to consistent methylation at the same adenosines in all cells.
Post-translational modifications to MTC components are additional factors that may control methylation. For instance, several lysine residues within METTL3 are susceptible to SUMOylation, which represses its methyltransferase activity (41). Additionally, methylation of arginine residues within the C terminus of METTL14 promotes its interactions with RNA and RNA pol II, leading to enhanced m6A methylation (42). Post-translational modification of the MTC machinery remains a relatively underexplored area, so future studies are needed to better understand how modification of MTC components controls m6A deposition.
2.4. METTL16: An m6A methyltransferase with key roles in cellular metabolism
Although METTL3 is responsible for methylating the majority of cellular mRNAs, a small number of sites are targeted for methylation by METTL16. In addition to depositing m6A in the U6 snRNA (43, 44) and the long noncoding RNAs MALAT1 and XIST (44, 45), METTL16 also methylates the MAT2A mRNA, which encodes the SAM synthetase, as part of a feedback mechanism that helps maintain cellular SAM levels (Figure 1c). When SAM levels are high, METTL16 methylates a conserved hairpin in the MAT2A 3’UTR, leading to intron retention which blocks the production of a functional protein. Depletion of SAM leads to reduced enzymatic turnover of METTL16 and increased occupancy on the MAT2A transcript, which in turn promotes MAT2A splicing and increased MAT2A protein production (43). In C. elegans, the METTL16 homolog METT-10 also regulates SAM levels by methylating the SAM synthase mRNA to regulate its splicing (46, 47). However, the mechanism differs from mammalian cells and involves direct inhibition of the U2AF35 splicing factor by 3’ splice site m6A (46, 47). Nevertheless, this suggests an evolutionarily conserved role for METTL16 as a regulator of SAM homeostasis.
Although both METTL3 and METTL16 use SAM as a methyl donor (Figures 1a,c), they recognize target RNAs through different mechanisms. While METTL3 targets DRACH motifs, METTL16 methylates adenosines within a distinct sequence motif (UACAGAGAA) that resides within a hairpin structural element (44–46). Depletion of METTL16 causes a significant reduction of total m6A levels in poly(A) RNA and loss of m6A in thousands of mRNAs (43, 48). However, due to its role in regulating SAM levels, it is difficult to uncouple METTL16-mediated methylation from changes due to reduced METTL3 activity, and METTL16 is likely to methylate only a few hundred mRNAs or less (48). This is consistent with the fact that the vast majority of m6A sites in mRNAs exist within the DRACH motif (3–6). Interestingly, like METTL3, METTL16 has also been shown to have methyltransferase-independent functions by promoting translation initiation (48) (Figure 1b). METTL16 is predominantly localized to the cytoplasm; therefore, while it clearly directs the nuclear methylation of a subset of cellular RNAs, a major function of METTL16 may be to control gene expression through translation regulation.
2.5. Writers of other mRNA modifications
Pseudouridine (ψ) is a frequent internal mRNA modification, comprising 0.3-0.4% of uridines in mammalian cells (49). ψ profiling studies have identified several hundred to several thousand ψ sites in human and yeast mRNAs (49–52). Several pseudouridine synthase enzymes have been shown to catalyze ψ formation in mRNAs, but PUS1 and PUS7 are the predominant pseudouridine synthases in both mammals and yeast (50–52) (Figure 2). Loss of these enzymes leads to alterations in splicing and 3’ end processing, suggesting a role in regulating co-transcriptional mRNA processing (52). Additionally, pseudouridylation of stop codon uridines can promote translational readthrough, providing a potential mechanism for premature stop codons to bypass nonsense-mediated decay (53).
Figure 2. Writers, readers, and erasers of other mRNA modifications.
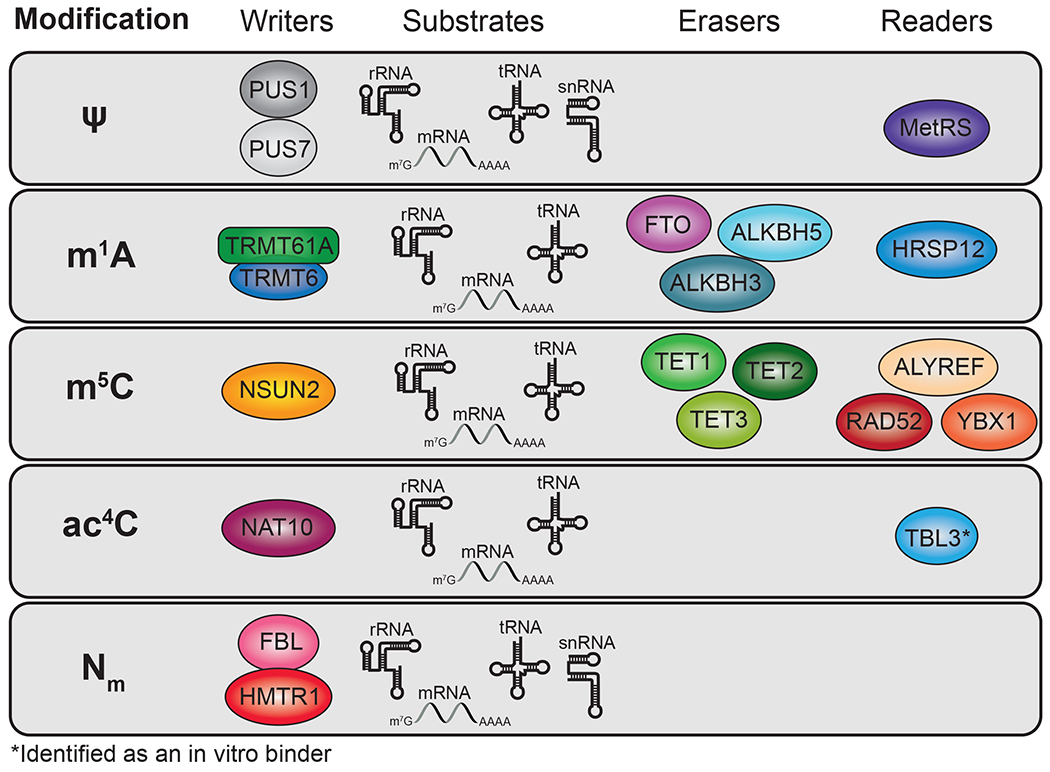
Shown are the major regulatory proteins of pseudouridine, m1A, m5C, ac4C, and Nm as well as the types of RNAs in which these modifications have been identified. Further characterization of many of these regulatory proteins will be important for understanding how these modifications are controlled in cells and their contribution to gene expression. In some cases, eraser and reader proteins have not yet been identified.
In contrast to ψ and m6A, some of the additional internal mRNA modifications that have been identified are less abundant. N1-methyladenosine (m1A) is deposited in mammalian tRNAs by a dimer of TRMT61A and TRMT6 (54) (Figure 2). While there is evidence that this complex can add m1A to mammalian mRNAs (55), the extent to which mRNAs contain m1A remains an active area of research. Some studies have reported hundreds or thousands of sites, while others suggest that m1A is present at only a small number of residues, including in mitochondrial mRNAs, and mostly at low stoichiometry (55–58). Similarly, N4-acetylcytidine (ac4C) is present in human rRNA and tRNAs, but reports of its abundance in mRNAs have been variable, with mass spectrometry estimates varying by an order of magnitude. NAT10 has been identified as the ac4C acetyltransferase (Figure 2), and a recent study using a chemical reduction approach to map ac4C transcriptome-wide identified over 7,000 sites, most of which were eliminated in NAT10 knockout cells (59–61). Interestingly, most of these sites are found in the 5’ UTR and are associated with increased translation initiation (59). Further studies examining the distribution of ac4C and m1A and whether they differ across cell types or cellular conditions will be important. Additionally, although challenging, measures of stoichiometry for these and for all modifications will be critical for a better understanding of their impact on cellular RNAs.
Like m6A, the 5-methylcytidine (m5C) modification was first observed in the 1970s in biochemical studies of polyadenylated RNA (62). This was reported in RNA from cultured hamster cells, but not in other mammalian cell lines (1, 3). In 2012, a bisulfite sequencing approach reported widespread m5C in mammalian mRNAs (63). However, subsequent studies have found more variable numbers of sites, and mass spectrometry data show that m5C is much less abundant than prevalent modifications like m6A (64–67). NSUN2 has been identified as the primary m5C methyltransferase in mammalian mRNAs (63, 68) (Figure 2). Importantly, NSUN2 is upregulated in many cancers, and mutations in NSUN2 can result in intellectual disability, although some of the underlying mechanisms likely relate to its tRNA methyltransferase activity (69, 70).
In addition to base modifications, methylation of the 2’-hydroxyl of the ribose backbone (Nm) is prevalent in tRNAs, rRNAs, and small nucleolar RNAs (snRNAs) (71). Nm is a low-abundance modification in mRNAs, and knowledge of specific transcripts that contain Nm has been limited by difficulties in detecting this mark transcriptome-wide. Dai and colleagues reported nearly 700 Nm sites in HEK293 cells and over 2,100 sites in HeLa cells (72); however, roles for Nm in mRNA regulation and its abundance in other cell types remain poorly understood. Nm in rRNAs is deposited by the methyltransferase fibrillarin (FBL) (73) (Figure 2), and a recent study demonstrated that FBL can also install Nm in the peroxidasin (PXDN) mRNA (74). This results in increased mRNA abundance, but decreased translation efficiency, demonstrating a functional consequence of internal Nm within this transcript.
In addition to internal Nm, the first transcribed nucleotide adjacent to the 5’ cap is also 2′-O-methylated by HMRT1 in mammalian cells (75, 76). 20-30% of these are adenosine residues that are additionally methylated at the N6 position by PCIF1 to form N6,2’-O-dimethyladenosine (m6Am) (77–79) (Figure 1a). Recent mapping studies in mammalian cells have found between 800-3,000 m6Am-containing mRNAs (78–80), with possible differences between cell types (79). Some studies have indicated increased stability of m6Am-containing mRNAs (78, 80, 81), possibly by inhibiting 5’ decapping (81). m6Am may also play roles in translation, with some studies reporting a modest increase in translation of m6Am containing mRNAs (80, 81). However, other studies have found no change in translation efficiency upon PCIF1 depletion (78). Expression of a GFP mRNA with Am or m6Am at the 5’ end in PCIF knockout cells showed decreased translation of the m6Am-containing transcript compared to Am mRNA (79). These discrepancies may indicate biological context-dependent effects of m6Am, since different cell lines were used across studies. This is supported by evidence that m6Am:Am ratios can vary widely across tissues in mice (15:1 in brain, 2:1 in liver) (82). However, this will need to be further explored to better understand the functions of m6Am.
3. m6A READER PROTEINS
m6A exerts many of its biological functions through the regulation of RNA:protein interactions, including the recruitment of m6A “reader” proteins. Reader proteins can either directly recognize m6A or bind indirectly to RNAs through m6A-mediated structural changes. In the past decade, several studies have employed biochemical assays to identify and characterize m6A reader proteins. Although these proteins can recognize methylated RNAs through a variety of RNA binding domains (RBDs), the most robust and specific interaction with m6A is mediated through the YT521-B homology (YTH) domain.
3.1. YTH domain-containing proteins
The first m6A reader proteins were identified with RNA pulldown assays using methylated and unmethylated RNA baits followed by mass spectrometry (6). This revealed two members of the YTH (YT521-B homology) domain-containing family of proteins: YTHDF2 and YTHDF3, as well as the RNA binding protein ELAVL1 (also known as HUR) as proteins with enhanced binding to m6A.
The YTH domain was first identified in the splicing factor YT521-B (now known as YTHDC1) as a novel RNA-binding domain (83, 84). Homologous YTH domain-containing proteins have been identified in diverse organisms, from yeast and plants to mammals, suggesting important functions throughout evolution. Interestingly, while the Saccharomyces cerevisiae YTHDF homolog MRB1 binds to m6A, the Schizosaccharomyces pombe YTH domain protein Mmi1 does not, and the presence of m6A actually reduces its affinity for RNA (85, 86). In mammals, there are five YTH domain-containing proteins: YTHDF1, 2, and 3 (also called DF1, DF2, and DF3), YTHDC1 (DC1), and YTHDC2 (DC2). All share the highly conserved YTH domain and are capable of binding m6A, although studies suggest that YTHDC2 exhibits lower specificity for m6A and may bind non-methylated regions (discussed below). The YTH proteins also differ in size, domain organization, and subcellular localization: while the DF proteins are predominantly cytoplasmic with shared domain architectures, DC1 is nuclear, and DC2 exhibits a nucleocytoplasmic localization with several distinct domains not found in the other YTH proteins (Figure 3a).
Figure 3. m6A reader proteins have diverse functions and mechanisms of m6A recognition.
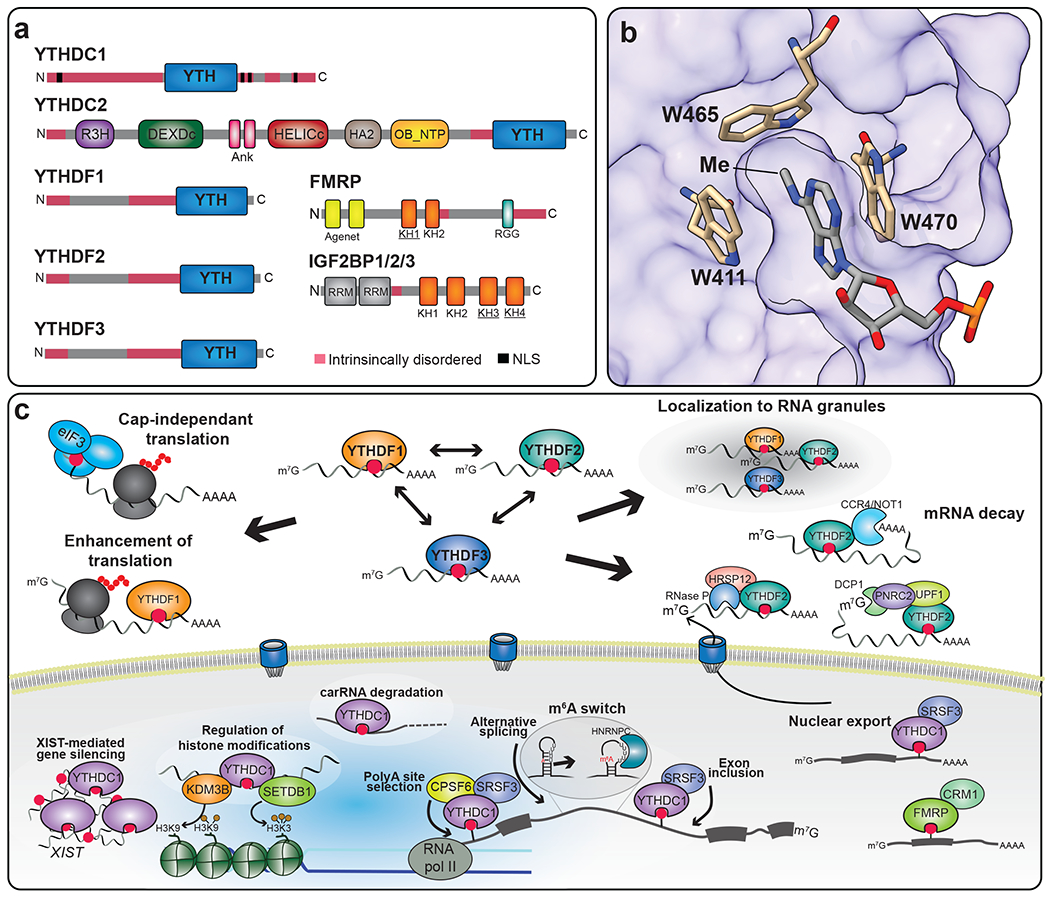
a) Schematic showing the domain structures of direct m6A binding proteins. YTHDF1/2/3, YTHDC1, and YTHDC2 recognize m6A through the highly conserved YTH domain. K-homology (KH) domains mediate m6A binding in FMRP and IGF2BP proteins. The KH domains required for m6A recognition are underlined. (b) Surface representation of the YTH domain of human YTHDF1 in complex with m6A (PDB: 4RCJ). m6A is nested in a hydrophobic pocket of the YTH domain. The three tryptophan residues forming the aromatic cage surrounding m6A are highlighted, as well as the position of the methyl moiety in m6A. c) m6A readers impact several aspects of RNA processing and function. In the nucleus, YTHDC1 regulates chromatin accessibility, promotes degradation of carRNAs, and binds to m6A sites within the lncRNA XIST to promote gene silencing. YTHDC1 also interacts with splicing factors and controls alternative splicing, polyadenylation, and nuclear export. FMRP recognition of methylated transcripts also promotes their export. In the cytoplasm, m6A is recognized by eIF3 to promote cap-independent translation of mRNAs with 5’-UTR m6A sites. YTHDF proteins can localize to stress granules and P-bodies, and all three proteins can promote mRNA decay. The role of YTHDF2 in mRNA destabilization is the most extensively studied and can include recruitment of various effector proteins to promote decapping, deadenylation, or endonucleolytic decay. Binding of YTHDF1 and YTHDF3 can also lead to increased translation of methylated mRNAs in some cell types.
3.2. m6A recognition by the YTH domain
The YTH domain is a highly conserved domain of approximately 130 residues that adopts an open α/β fold containing six β-sheets surrounded by α-helices (87–91). The methylated adenosine is lodged in an aromatic cage consisting of three tryptophans (DF1, DF2, DF3), or two tryptophans and a leucine residue (DC1, DC2). The N6-methyl moiety is nested within a hydrophobic pocket and faces the tryptophan residue forming the base of the cage, and interactions with the adenine base are stabilized by hydrogen bonds between the nucleobase and the sidechains and backbones of neighboring residues (87, 89–91) (Figure 3b).
The reported binding affinities of the YTH domain for methylated RNA are relatively low, with in vitro measurements generally ranging from 100nm to 3µM for the DFs and DC1, and a ~5 to 25 fold lower affinity reported for DC2 (89–93). However, the YTH domain has a 10 to 100-fold higher affinity for m6A versus unmodified A (88, 91, 94). This suggests that YTH proteins may only transiently interact with their targets, or that cooperative interactions with other RBPs are required to stabilize the interaction.
The YTH domain can recognize m6A relatively indiscriminately, as mutation of m6A-adjacent nucleotides at positions −2, −1 and +1 does not significantly alter binding of the YTH domain in vitro (89, 92). An exception is the YTH domain of DC1, which shows a slight preference for G at the −1 position (89, 91, 92). One limitation to in vitro binding assays is that they often use RNAs of fixed length and sequence, and the binding conditions may not mimic the environment of the cell. Methylated RNA may need to be in a favorable conformation to enable m6A recognition by the YTH domain. For example, DF2 preferentially binds to m6A in single-stranded regions, or when flanked by a 5’ bulge when in a duplex (94). In contrast, when m6A is in a Watson-Crick conformation, it remains inaccessible for recognition by the YTH domain. Significantly, 11-30% of all m6A sites are predicted to be in regions inaccessible for binding (94). Similarly, the accessibility of sites can also be impaired by binding of other RBPs. For example, the high stoichiometry m6A sites found in the 18S and 28S rRNAs are not bound by YTH domain proteins in CLIP-seq studies, likely because they are buried within the ribosome (24).
3.3. Functions of YTH domain-containing proteins.
YTHDC1.
DC1 was initially characterized as a spliceosome-interacting protein localized to nuclear speckles (95, 96), and it is unique among the YTH proteins in that it is exclusively nuclear. This localization is driven by a nuclear localization sequence as well as low complexity regions which are required for DC1 condensation in nuclear speckles (Figure 3a) (96–98).
Consistent with its nuclear localization, DC1 regulates several steps of pre-mRNA processing (Figure 3c). Studies in HeLa cells have shown that DC1 is bound competitively by the splicing proteins SRSF3 and SRSF10 to regulate exon inclusion (97) and nuclear export (99). In mouse oocytes, DC1 can interact with CPSF6, SRSF3, and SRSF7 to regulate splicing and alternative polyadenylation of m6A-modified mRNAs (100). DC1 was also found to promote the stability of RNA at sites of double-strand DNA breaks following recruitment and activation of METTL3 (101). Additionally, DC1 binds to the highly methylated long noncoding RNA (lncRNA) XIST and is essential to XIST-mediated gene silencing (24).
The functions of DC1 suggest that it binds to chromatin-associated RNAs rapidly after m6A deposition. For example, in mESCs, DC1 is required for maintenance of stemness and silencing of transposons through the recruitment of SETDB1 and H3K3me3 deposition (102), and in HEK293T cells, m6A and DC1 contribute to the removal of the repressive H3K9Me2 marks through the recruitment of KDM3B (103). DC1 also contributes to transcription regulation by facilitating the degradation of m6A-containing chromatin-associated regulatory RNAs (carRNAs) and limiting their ability to promote open chromatin (104).
DC1 plays important roles in ESCs and is essential for embryonic development (100), so it will be important going forward to understand how DC1 activity is regulated. One possibility is that DC1 function is influenced by the activation of specific signaling pathways. For instance, DC1 contains multiple phosphorylation sites, and its localization to nuclear speckles can be influenced by activation of the Src family kinase FYN (96).
YTHDC2.
DC2 exhibits a unique domain composition compared to the other YTH proteins. DC2 is the largest of the YTH proteins and contains several conserved protein domains in addition to its YTH domain, including ankyrin repeats, an oligonucleotide-binding fold, and DEAD-like helicase domains (Figure 3a). The cellular functions of DC2 are still being elucidated, but it has been shown to be important for spermatogenesis and oogenesis through its regulation of mRNA stability and translation (105–107). CLIP-based studies have revealed that DC2 has a unique binding pattern characterized by enrichment in introns and intergenic regions and within the coding sequence of mRNAs (24), which is distinct from the distribution of m6A and the binding sites of other YTH proteins. Additionally, DC2 binding sites show poor overlap with mapped m6A sites (24, 108), suggesting that DC2 may not function exclusively as an m6A reader. Indeed, DC2’s YTH domain is dispensable for its biological function in spermatogenesis, and DC2 can bind target RNAs in poly(U) sequences in the absence of its YTH domain (108, 109). Consistent with this, the binding affinity and specificity of DC2 toward m6A is much lower than for other YTH proteins (92). Further studies will be necessary to determine the extent of m6A-dependent and m6A-independent RNA regulation by DC2.
YTHDF proteins.
The DF proteins include three cytoplasmic proteins sharing 65-70% sequence identity and a similar global domain organization. The YTH domain is found near the C-terminus and is preceded by a low complexity region of ~350-400 residues which are P/Q/N-rich (Figure 3a). Low complexity regions are known to contribute to protein condensation and liquid-liquid phase separation (LLPS), and consistent with this, the low complexity domain is required for LLPS of DF proteins (110) and mediates their association with stress granules (111) and P-bodies (112). Initially, it was proposed that DF proteins have distinct and partially overlapping functions: DF1 promotes translation of its target mRNAs (113), DF2 promotes their degradation (112) and DF3 promotes both functions (114). However, this model has recently been challenged by a unified model for DF protein function, which proposes that all three DF proteins act redundantly to promote mRNA decay with no effect on translation (93, 115, 116). Interestingly, single-molecule analysis of the RNA targets of DF proteins has revealed that individual mRNAs can be bound more than once by different DF proteins throughout their lifetime (117). This suggests that DF proteins may not immediately promote decay every time they bind to their target RNAs. The precise function of DF proteins has been examined in only a handful of cell types and experimental systems, and future experiments will be necessary to understand the full extent of DF-mediated regulation.
mRNA decay.
DF2 was initially implicated in m6A-mediated mRNA instability following the observation that the abundance of its target mRNAs was increased following DF2 depletion (112). DF2 was also found to traffic to P-bodies and contribute to mRNA deadenylation and decay (112) (Figure 3c). This effect is consistent with metabolic labeling studies performed in the 1970s, which suggested selective instability of m6A-containing mRNAs (118), as well as with the finding that Mettl3 depletion leads to increased mRNA abundance (119, 120). Subsequent work identified a direct interaction between DF2 and the CNOT1 scaffolding subunit of the CCR4-NOT deadenylase complex (121). Interestingly, this study found no direct interaction between CNOT and either DF1 or DF3 and showed that tethering of DF1 or DF3 to a reporter mRNA caused deadenylation, but the magnitude was substantially reduced compared to tethering of DF2 (121). These results suggest that all three DF proteins can mediate mRNA degradation, but that the effect is strongest for DF2. This is consistent with recent studies in mESCs and HeLa cells which demonstrate that the loss of all three DF proteins causes a greater increase in methylated mRNA abundance compared to loss of any single DF protein alone, and that depletion of DF2 has a more pronounced effect on mRNA stability compared to depletion of DF1 or DF3 (93, 115). This is also consistent with the finding that DF2-bound RNAs are less likely to be subsequently bound by other DF proteins compared to DF1- or DF3-bound RNAs, potentially because DF2 targets are more efficiently processed for decay (122).
In addition to recruitment of the CCR4-NOT deadenylase complex, DF2 can interact directly with HRSP12 to recruit RNase P/MRP and elicit endonucleolytic cleavage and degradation of target mRNAs (123) (Figure 3c). DF2 can also promote mRNA decapping through interactions with UPF1 and recruitment of PNRC2 and DCP1A (124). These interactions are not detected with DF1 or DF3, again suggesting that DF2 has a more prominent role in mRNA decay.
Translation control.
A role for DF1 in promoting translation was initially demonstrated in ribosome profiling studies in HeLa cells, which showed that DF1 depletion leads to reduced translation efficiency of its target mRNAs (113). DF1 was also shown to interact with eIF3a/b and eEF2 to promote translation initiation and elongation of methylated mRNAs (113, 125). However, other studies have failed to observe global effects on translation following loss of DF1 and suggest a more limited role for m6A and DF proteins in translational control (93, 110, 115, 126). It is likely that the translation-promoting function of DF1 is influenced by cell type or cellular conditions. For instance, several studies support a role for DF1 in promoting translation in neurons (127–129), and the positive effect of DF1 tethering on translation is most evident during stress recovery (113). In addition to DF1, DF3 has been reported to promote translation of its target mRNAs, but it appears to do so in cooperation with DF1 (114, 130). Future studies examining cell type- and context-dependent effects of DF1/DF3-mediated translation regulation and the mechanisms of their cooperative function will be important for a more complete understanding of how DF proteins impact protein production.
Biological context.
Many studies of DF proteins have been performed in immortalized cells at steady state. It is therefore important to consider how factors like expression level, local subcellular concentration, and cell type-specific interactions may differentially influence DF proteins when proposing general models for their function. For instance, studies in the nervous system have shown that perturbation of distinct DF proteins results in drastically different phenotypes (131). In the developing brain, DF2 is essential for neuronal progenitor cell differentiation and methylated mRNA clearance (132). In contrast, DF1 is important for axonal guidance (133), and in mature neurons it is required for learning and memory through translational control of its target RNAs (127). Moreover, DF1 and DF3, but not DF2, are required for proper synapse morphology (128). Additionally, DF2 and DF3, but not DF1, contribute to the localization of the methylated mRNAs Camk2a and Map2 to neurites in hippocampal neurons (122). Furthermore, depletion of Mettl3 or Mettl14 in mature neurons does not lead to a global increase in m6A-modified mRNAs, suggesting that mRNA decay is not the primary function of m6A in neurons (122, 134, 135). This is an important distinction from the effects of m6A observed in studies in mESCs and other dividing cells and suggests that DF protein function depends on context-dependent factors.
How could DF proteins mediate these differential effects? Analysis of proximity labeling datasets in HeLa cells suggests that DF1, DF2, and DF3 interact with a similar set of proteins (93). In contrast, other studies have reported unique protein interacting partners with the different DF proteins (123, 124, 136). The low complexity region is where most differences occur among the DFs and is important for these unique interactions (123, 124, 136). It is therefore possible that while many DF binding partners are conserved among the three proteins, subtle differences in DF protein sequence can enable unique interactions which then promote distinct functions in certain contexts.
It is also possible that post-translational modifications (PTMs) of DF proteins enable selective regulation of these otherwise highly similar proteins. All three DF proteins contain several phosphorylation sites within their low complexity regions (137). Phosphorylation of these regions could prevent protein condensation in RNA granules and could be an important mechanism to control their activity in response to stress or cellular stimuli. Other modifications have also been described in DF proteins. For example, DF2 is ubiquitinated in a cell-cycle dependent manner, which influences its stability (138). Additionally, stress-dependent SUMOylation of the DF2 C-terminus was found to increase its affinity toward m6A-modified mRNAs (139). Interestingly, the DF2 SUMOylation sites that have been reported are not found in DF1 and DF3. Going forward, it will be important to understand which PTMs are found in the various DF proteins and whether they are differentially regulated in a cell-type or context-specific manner.
3.4. Other direct m6A-binding proteins
In addition to the YTH domain-containing proteins, several other RNA-binding proteins have been identified as m6A readers (6, 140–143). For example, subunits of the translation initiation factor complex eIF3 can bind to m6A sites within 5’UTRs to promote cap-independent translation of cellular mRNAs in response to stress (143). Additionally, eIF3 is required for the translation of m6A-containing circRNAs, further indicating a role for m6A in cap-independent translation, although this mechanism also involves YTHDF3 and eIF4G2 (144).
IGF2BP proteins (IGF2BP1, EGF2BP2 and IGF2BP3) have also been identified as m6A readers. These proteins recognize m6A through their K homology (KH) domains and act to promote mRNA stability and translation (142) (Figure 3a). IGF2BP binds RNA at sequences similar to the m6A consensus sequence, but interestingly these sites are enriched throughout 3’UTRs as opposed to near the stop codon as is typically observed for m6A (142). Moreover, the selectivity of IGF2BP proteins for m6A is much lower than that of YTH proteins (approximately 3-fold increase in binding affinity for m6A compared to A) (142). Another possibility is that IGF2BP proteins are indirect m6A readers that bind RNA through m6A-dependent structural changes (see below), as has been demonstrated for IGF2BP3 (145).
The fragile X mental retardation protein (FMRP) is another protein that displays preferential m6A binding (140). Similar to the IGF2BP proteins, FMRP exhibits a modest preference for binding m6A compared to A (~2.5-fold increase in binding affinity) (146, 147), and evidence from CLIP-based analyses suggests that only a subset of FMRP targets are methylated (146, 148). Nevertheless, FMRP has been shown to regulate some mRNAs through m6A. Many of these roles have been demonstrated in neurons, where FMRP contributes to mRNA export (147, 148), neuronal differentiation (147), and mRNA decay (146). However, FMRP can also directly interact with DF2, and at least part of its function in the control of mRNA stability could be through this interaction (146). Future studies are needed to better understand the m6A-dependent and m6A-independent functions of FMRP.
While the m6A-binding activity of many m6A readers is conserved across cell types (140), it is likely that cell type- and tissue-specific readers exist. For example, PRCC2A has been identified as a reader in oligodendrocytes, where it binds RNAs at DRACH motifs and displays a binding pattern in mRNAs that matches the distribution of m6A (149). PRRC2 plays an important role in oligodendrocyte differentiation and myelination by stabilizing the Olig2 mRNA in an m6A-dependent manner (149). Similarly, RBM45 is a brain-enriched m6A-binding protein that influences splicing and neuronal differentiation (150). It will be interesting to see if PRRC2, RBM45, and other novel readers contribute to m6A-mediated regulation in other cell types, or if there is cell type-specific specialization in reader protein function. Additionally, m6A likely plays important roles in RNA regulation by repelling RBPs. For example, the stress granule protein G3BP1 exhibits preferential binding to non-methylated RNA in some sequence contexts, which in turn impacts RNA stability (140).
3.5. Indirect readers
In addition to direct recognition of m6A by RBPs, m6A can influence RNA:protein interactions by altering RNA structure. m6A can destabilize RNA duplexes by 0.5-1.7 kcal/mol through both a non-favorable trans conformation when paired with U and through improved stacking in its unpaired conformation (151). Thus, m6A has the potential to act as a so-called “m6A-switch”, where it alters local RNA structure to enable, enhance, or prevent the binding of some RBPs (Figure 3c). The first example of this mechanism was the binding of heterogeneous nuclear ribonucleoprotein C (HNRNPC) to a stem-loop within the lncRNA MALAT1 (152). The presence of m6A within this region destabilizes the stem-loop and exposes an HNRNPC binding site. Global analysis revealed nearly 2,800 binding sites of HNRNPC that are altered when m6A is depleted, resulting in changes in alternative splicing of target RNAs (152). Similar observations have since been made for HRNRNPG (153) and HNRNPA2B1 (154), suggesting that the m6A-switch mechanism may play an important role in splicing regulation in the nucleus. Additionally, global profiling of m6A-dependent structural changes has shown that many changes are conserved from the nucleus to the cytoplasm, suggesting that m6A-dependent structural changes are likely to impact mature mRNAs as well (145). Indeed, m6A can indirectly impact RNA recognition by HuR, which in turn mediates miRNA-mediated mRNA targeting to control mESC self-renewal (155).
3.6. Readers of other modifications
Readers of modifications other than m6A have also been identified, although in general these remain less well understood (Figure 2). For example, a recent study identified the metabolic enzyme HRSP12 as an m1A reader that can cooperate with YTHDF2 and m6A to direct RNA degradation (156). However, it remains unclear how HRSP12 recognizes m1A, as it lacks homology for known RNA binding domains. Other studies have identified several m5C readers, including ALYREF, which plays a role in mRNA export (68), YBX1, which stabilizes target RNAs (157), and RAD52, which displays increased binding for m5C at sites of DNA repair (158). The mechanism by which these proteins recognize m5C differs among the proteins, including the involvement of a critical lysine residue in ALYREF and a cold-shock domain in YBX1 (68, 157). Thus, unlike the YTH domain proteins that bind m6A, currently identified m5C readers appear to utilize distinct domains to recognize m5C.
Readers of Ψ are also poorly understood. In S. cerevisiae, methionine aminoacyl tRNAMet synthetase (MetRS) not only binds to Ψ in tRNAs, but also in mRNAs, where it controls the translation of specific transcripts (159). Additionally, Martinez et al. recently found that Ψ sites in pre-mRNAs are enriched for splicing factor binding sites (52). However, direct binding by these proteins was not characterized, and it is possible that they bind indirectly to Ψ-modified RNAs.
Much of the impact of mRNA modifications on gene expression is due to their influence on RNA:protein interactions. Thus, it will be important going forward to identify reader proteins for individual modifications and to determine how specific modified residues alter RNA recognition either directly or indirectly. For many modifications, such studies are still in their infancy. For instance, in vitro RNA pulldown studies identified TBL3 as a potential reader protein for ac4C (160), but this interaction and potential functional implications have yet to be validated in cells.
4. ERASER PROTEINS
4.1. FTO
Shortly before the first transcriptome-wide m6A mapping studies were published, FTO was discovered as an m6A demethylase which could remove m6A from RNA (7). This was an important finding for the still nascent epitranscriptomics field, since it suggested that chemical modifications could potentially be susceptible to dynamic regulation. FTO is a member of the Fe(II) and 2-oxoglutarate-dependent dioxygenase family of proteins, which encode homologs of the bacterial ALKB enzyme that function in DNA and RNA repair (161). In addition to removing m6A from RNA in vitro, FTO was shown to influence m6A levels in mRNAs in cells and in vivo (7, 162). Subsequent studies shed light on the mechanism through which FTO demethylates m6A and found that it does so through the production of N6-hydroxymethyladenosine and N6-formyladenosine intermediates (163). FTO lacks general sequence specificity for m6A, but its demethylation activity can be influenced by m6A-dependent conformational changes, such that much higher activity is seen when m6A is in a single stranded or hairpin structure comparted to a duplex (164, 165). This structural preference may explain why many m6A sites do not seem to be impacted by FTO. Indeed, FTO knockout does not lead to dramatic hypermethylation of all m6A sites (162, 166) and its overexpression in cells often has only small effects on lowering global m6A levels (167).
An important shift in our understanding of FTO came in 2017, when Mauer et. al. reported that FTO can demethylate m6Am (81) (Figure 4). This study further showed that FTO removes m6Am much more efficiently than m6A in vitro and preferentially targets m6Am over m6A in cells. These findings reshaped the view of FTO as exclusively an m6A demethylase and prompted additional studies into the true targets of FTO in cells.
Figure 4. Targets of m6A eraser proteins.
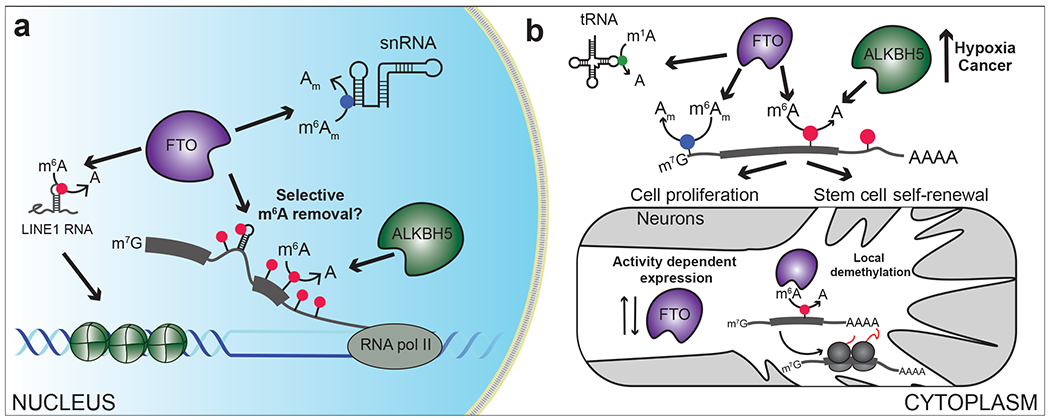
a) In the nucleus, FTO and ALKBH5 demethylate m6A sites in mRNAs. FTO can also remove m6A and m6Am in lncRNAs and snRNAs. b) In the cytoplasm, FTO can remove m6A and m6Am from mRNAs and m1A from tRNA. In the nervous system, FTO expression is regulated by activity and can promote m6A demethylation of specific transcripts in axons. ALKBH5 expression is increased in hypoxic conditions and in cancer cells, resulting in decreased m6A levels of specific mRNAs and enhanced cell proliferation and stem cell self-renewal.
The substrate preference of FTO appears to be affected in part by differences in expression levels and subcellular localization of FTO across different cell types. Initial reports characterized FTO as a nuclear protein (7), but subsequent studies have shown that it can be localized to the cytoplasm in some cell types (168, 169). FTO has also been reported in neuronal synaptic compartments and in axons of cultured neurons, where it may mediate local demethylation of select mRNAs (128, 170) (Figure 4b). Analysis of FTO demethylation in various immortalized cell lines and subcellular regions revealed that FTO has a preference for demethylating m6A in the nucleus and m6A and m6Am in the cytoplasm in polyadenylated RNA, as well as m1A in tRNA (168) (Figure 4a,b). Thus, in some cell lines such as HeLa and HEK293T, where FTO is largely nuclear, the effects of FTO depletion on m6Am are less pronounced, perhaps because access to m6Am is blocked by the nuclear cap binding complex. Indeed, forced expression of FTO in the cytoplasm leads to higher levels of m6Am demethylation compared to m6A (81, 168).
Dynamic regulation of FTO localization may be a mechanism for cells to selectively regulate m6A and m6Am levels. Alteration of FTO subcellular localization has been observed in the brain in response to starvation and fear conditioning (171, 172). FTO post-translational modifications may also impact its subcellular localization in response to stress (173). More research is needed to determine whether other stimuli influence nuclear and cytoplasmic FTO levels and if this corresponds to selective demethylation of m6A or m6Am.
Recent studies also suggest that mRNAs may not be the primary targets of FTO: in line with the predominantly nuclear localization of FTO in many cell types, small nuclear RNAs (snRNAs) have been identified as FTO targets, with FTO depletion causing increased m6Am in snRNAs (166, 174) (Figure 4a). snRNAs are an important class of splicing regulatory transcripts, and reduction in FTO has been shown to result in altered splicing, potentially mediated by its impact on snRNA expression (166, 174, 175). Additionally, m6A in long-interspersed element-1 (LINE1) RNAs has been shown to be targeted by FTO in mESCs (176) (Figure 4a). Loss of FTO causes elevated LINE1 methylation, leading to repression of LINE1 RNA and altered chromatin state and gene expression, which impacts oocyte maturation and embryonic development (176). Thus, removal of m6A and m6Am in nuclear non-coding RNAs appears to be an important aspect of FTO’s demethylase activity.
Numerous studies have linked FTO to human disease. In 2007, genome-wide association studies (GWAS) found that mutations clustering within intron 1 of FTO were associated with increased body mass index and risk for obesity (177). Since then, multiple studies have found associations between genetic variants in FTO and obesity and related phenotypes (178). Importantly, some obesity-associated mutations within FTO have also been found to impact long-range genomic interactions with other genes such as IRX3, suggesting that the disease links of these mutations may not be mediated through the FTO protein itself (179). However, mice with either missense Fto mutations or gain/loss of FTO expression exhibit metabolic phenotypes and developmental defects, so it is likely that misregulation of the FTO protein is a contributing factor to obesity-related diseases (178).
In addition to its links to obesity and metabolism, FTO has been associated with several other diseases, including Alzheimer’s disease (180, 181), neuropsychiatric disorders (182), and various cancers (178, 183). FTO expression is upregulated in some AML subtypes, where it promotes leukemogenesis and inhibits leukemia cell differentiation (184, 185). FTO can also be inhibited by the oncometabolite 2-hydroxyglutarate (2HG) and has been shown to mediate anti-tumor effects in 2HG-sensitive cells through the MYC/CEBPA signaling pathway (185). FTO has also been implicated in glioblastoma, as its inhibition impairs glioblastoma stem cell (GSC) growth and self-renewal and leads to decreased tumor growth in mice. This is accompanied by decreased expression of oncogenes such as ADAM19 (186). Collectively, these studies point to FTO as a potential therapeutic target and suggest cell type-specific pathways that may be regulated by FTO to contribute to disease. However, FTO has been shown to play both positive and negative roles in cancer progression (183), so continued investigation of the cellular target RNAs of FTO will be important for determining its value as a therapeutic target in specific cell types. It will also be important to better understand whether FTO’s effects on a particular RNA’s expression are mediated through m6A or m6Am demethylation.
Finally, recent work has uncovered potential roles for FTO as a mechanism for increasing biomass and yield in plants. Early studies in Arabidopsis first demonstrated that m6A is required for normal plant development (18, 187), indicating that proper m6A regulation is required for plant growth. Although plants lack an FTO homolog, overexpression of human FTO causes m6A demethylation and promotes chromatin accessibility and transcriptional activation, ultimately leading to substantial increases in plant growth (188). This is an exciting area of research with important implications for increasing crop yield.
4.2. ALKBH5
ALKBH5 was the second m6A demethylase enzyme to be discovered and is also a member of the iron and 2-OG dependent family of dioxygenase enzymes (189). ALKBH5 localizes to nuclear speckles and carries out demethylation of nuclear RNA substrates, and ALKBH5 depletion has been shown to impact nuclear mRNA export (189). Structural studies have provided insights into the mechanism of m6A binding and have shown that, like FTO, ALKBH5 prefers single stranded substrates (164, 190–192). However, unlike FTO, ALKBH5 does not have reported activity on m6Am, thus making it likely that m6A is its only substrate (81) (Figure 4a).
The expression pattern of ALKBH5 across tissues is distinct from that of FTO. ALKBH5 is highly abundant in testis, and Alkbh5 knockout mice have impaired spermatogenesis and decreased male fertility (189). Alkbh5 knockout mice otherwise appear normal, suggesting that a primary function for Alkbh5 is to mediate m6A levels during gametogenesis. However, roles for ALKBH5 in other tissues may still be possible, especially through targeting of specific transcripts. For instance, conditional knockout of Alkbh5 in bone marrow mesenchymal stem cells (MSCs) has been shown to regulate MSC differentiation and bone mass through regulation of Prmt6 (193).
Several studies have linked altered levels of ALKBH5 with human disease. For instance, ALKBH5 is upregulated in breast cancer cells in response to a hypoxic environment, which alters m6A levels and abundance of the pluripotency factor NANOG to promote breast cancer stem cell growth (194). ALKBH5 is also elevated in glioblastoma, where it impacts GSC proliferation through modulation of FOXM1 levels (195). Consistent with roles for m6A in AML progression, ALKBH5 levels are also increased in AML and associated with poor prognosis (183) (Figure 4b).
The discovery of m6A demethylase enzymes suggests the possibility that demethylation of mRNAs in the cytoplasm can be a mechanism for dynamic m6A regulation. As discussed above, there are several studies which suggest that this may be true for specific transcripts. However, an alternative model is that demethylases do not dynamically regulate cytoplasmic m6A, but instead act to remove m6A sites in the nucleus shortly after their formation in order to maintain these sites in a persistently unmethylated state. This model is supported by evidence from a nucleotide-resolution m6A mapping study which found that knockout of either ALKBH5 or FTO causes new sites to emerge, instead of leading to hypermethylation of existing sites. This suggests that demethylase-regulated sites are normally present only transiently and thus fail to be detected in wild type cells. However, this idea is countered by several studies which have found that ALKBH5 or FTO depletion is accompanied by only minor increases in m6A (81, 162, 189). Going forward, it will be important to understand whether m6A demethylation serves to “correct” initial transcript methylation or if it acts as a mechanism for dynamic regulation of RNAs. In either case, current evidence suggests that the impact of FTO and ALKBH5 on global m6A is for the most part modest, but that demethylation of specific transcripts may have important outcomes for cellular function in distinct cellular states or cell types.
4.3. Erasers of other modifications
In addition to FTO and ALKBH5, other members of the ALKB family of dioxygenase enzymes have been found to demethylate modified RNAs. For instance, ALKBH1 can remove m1A from tRNAs (196), and ALKBH3 can demethylate m1A residues in mRNA and tRNA (57, 197) (Figure 2). Both of these enzymes have demethylase activity on other modifications as well (198). As discussed above, there have been conflicting reports regarding the prevalence of m1A in cellular mRNAs. Although it seems clear that mitochondrial mRNAs can be m1A-modified, it is unknown whether ALKBH3 acts on these transcripts.
The Ten-eleven translocation (TET) family of dioxygenase proteins (TET1, TET2, and TET3) have been well characterized for their ability to convert 5-methylcytosine in DNA to 5-hydroxymethylcytosine (5hmC) (199). Additionally, TETs are capable of converting m5C to 5hmC in RNA (200, 201) (Figure 2). 5hmC is detectable in cellular mRNAs (201–203), suggesting that it is not a transient product but instead can have its own functions. Indeed, studies in flies indicate roles for 5hmC in promoting translation and regulating brain development (201), and cytosine hydroxymethylation in ESCs leads to reduced stability of pluripotency-related transcripts which may be important during differentiation (203).
5. CONCLUDING REMARKS
Although mRNA modifications were first detected nearly 50 years ago, recent technical advances in identifying modified nucleotides have fueled rapid progress in our understanding of their regulation and biological impact, particularly for m6A. However, important questions remain. For modifications other than m6A, m6Am, and pseudouridine, some discrepancies remain regarding their abundance in cellular mRNAs. The development of more reliable profiling methods will be important for determining the location and stoichiometry of these modifications and enabling consistency across studies. Researchers have made substantial progress in the development of novel modification detection methods over the past few years, including the use of direct RNA sequencing, which holds great promise for the detection of diverse modifications within individual RNA molecules. Still, there are substantial challenges that remain, in particular for mapping modifications with low abundance.
Although many core components of the m6A MTC have been identified, our understanding of the precise mechanism that directs methylation of specific sites remains incomplete. Some evidence suggests that sequence is the major determinant of methylation, with a slight contribution of RNA structure (29). Additionally, it is clear that gene architecture plays a role, as m6A is depleted near exon boundaries and enriched in long internal exons and near the beginning of terminal exons (6, 30, 31). However, transcription factors and histone modifications also help recruit the MTC to specific transcripts (32–35), which suggests that in addition to RNA sequence elements, methylation of transcripts can be guided by cell- and context-specific factors. Additionally, it is important to note that much of our current knowledge of the factors driving mRNA methylation comes from studies in bulk cells. Our group recently developed a method for profiling m6A in single cells, which revealed that mRNAs contain many m6A sites, most of which occur relatively rarely throughout the population of cells (40). This suggests either high variability in methylation site selection between cells, or that the methylation of specific residues may not be as critical as methylation of transcript regions. Continued studies of m6A in single cells, especially in tissues or in response to changing cellular states, will be critical for improving our understanding of m6A distribution and regulation.
Another area of high importance is the study of m6A reader proteins, including the YTHDF proteins. Work in mESCs as well as in HeLa and HEK293 cell lines suggests that all three DF family proteins interact with similar proteins and are functionally redundant in contributing to the degradation of transcripts (93, 115). However, other studies have shown that different DF proteins can have distinct interacting partners, and there is also a body of evidence for DF-specific effects in the nervous system. It will be important to consider the extent to which reader proteins are functionally redundant within different biological contexts. Furthermore, methods for dissecting single-molecule DF:protein interactions will be important for illuminating how the three DF proteins compete or cooperate in cells to bind to m6A sites.
Questions also remain about the functions of m6A eraser proteins, in particular FTO. While m6A was predicted to be a dynamic modification after the discovery of the FTO demethylase, the generality of this assumption has since been challenged. FTO is now known to play important roles in the demethylation of m6Am (81), and its effects on m6A are likely to be transcript and perhaps cell type-specific. ALKBH5 is largely nuclear and plays its most important biological roles in male germ cell development (189). This suggests that m6A may not be as generally dynamic—at least in the sense of widespread active removal—as previously thought. As with so many other aspects of m6A, defining the cell types, cellular states, and specific transcripts that are targeted for demethylation will be important for a more complete understanding of the physiological importance of m6A demethylation and for our views of m6A dynamics.
References
- 1.Desrosiers R, Friderici K, Rottman F. 1974. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci U S A 71: 3971–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry RP, Kelley DE. 1974. Existence of methylated messenger RNA in mouse L cells. Cell 1: 37–42 [Google Scholar]
- 3.Wei CM, Gershowitz A, Moss B. 1976. 5’-Terminal and internal methylated nucleotide sequences in HeLa cell mRNA. Biochemistry 15: 397–401 [DOI] [PubMed] [Google Scholar]
- 4.Wei CM, Moss B. 1977. Nucleotide sequences at the N6-methyladenosine sites of HeLa cell messenger ribonucleic acid. Biochemistry 16: 1672–6 [DOI] [PubMed] [Google Scholar]
- 5.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. 2012. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149: 1635–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, et al. 2012. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485: 201–6 [DOI] [PubMed] [Google Scholar]
- 7.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, et al. 2011. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7: 885–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper JE, Miceli SM, Roberts RJ, Manley JL. 1990. Sequence specificity of the human mRNA N6-adenosine methylase in vitro. Nucleic Acids Res 18: 5735–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bokar JA, Rath-Shambaugh ME, Ludwiczak R, Narayan P, Rottman F. 1994. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. Internal mRNA methylation requires a multisubunit complex. J Biol Chem 269: 17697–704 [PubMed] [Google Scholar]
- 10.Bokar JA, Shambaugh ME, Polayes D, Matera AG, Rottman FM. 1997. Purification and cDNA cloning of the AdoMet-binding subunit of the human mRNA (N6-adenosine)-methyltransferase. RNA 3: 1233–47 [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Yue Y, Han D, Wang X, Fu Y, et al. 2014. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol 10: 93–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sledz P, Jinek M. 2016. Structural insights into the molecular mechanism of the m(6)A writer complex. Elife 5: e18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, Doxtader KA, Nam Y. 2016. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol Cell 63: 306–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Feng J, Xue Y, Guan Z, Zhang D, et al. 2016. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature 534: 575–8 [DOI] [PubMed] [Google Scholar]
- 15.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, et al. 2014. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5’ sites. Cell Rep 8: 284–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin S, Choe J, Du P, Triboulet R, Gregory RI. 2016. The m(6)A Methyltransferase METTL3 Promotes Translation in Human Cancer Cells. Mol Cell 62: 335–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choe J, Lin S, Zhang W, Liu Q, Wang L, et al. 2018. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 561: 556–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong S, Li H, Bodi Z, Button J, Vespa L, et al. 2008. MTA is an Arabidopsis messenger RNA adenosine methylase and interacts with a homolog of a sex-specific splicing factor. Plant Cell 20: 1278–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwala SD, Blitzblau HG, Hochwagen A, Fink GR. 2012. RNA methylation by the MIS complex regulates a cell fate decision in yeast. PLoS Genet 8: e1002732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ping XL, Sun BF, Wang L, Xiao W, Yang X, et al. 2014. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24: 177–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yue Y, Liu J, Cui X, Cao J, Luo G, et al. 2018. VIRMA mediates preferential m(6)A mRNA methylation in 3’UTR and near stop codon and associates with alternative polyadenylation. Cell Discov 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, et al. 2013. Identification of Wilms’ tumor 1-associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem 288: 33292–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega A, Niksic M, Bachi A, Wilm M, Sanchez L, et al. 2003. Biochemical function of female-lethal (2)D/Wilms’ tumor suppressor-1-associated proteins in alternative pre-mRNA splicing. J Biol Chem 278: 3040–7 [DOI] [PubMed] [Google Scholar]
- 24.Patil DP, Chen CK, Pickering BF, Chow A, Jackson C, et al. 2016. m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537: 369–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang L, Ren H, Ma L, Guo J, et al. 2021. Role of Hakai in m(6)A modification pathway in Drosophila. Nat Commun 12: 2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bawankar P, Lence T, Paolantoni C, Haussmann IU, Kazlauskiene M, et al. 2021. Hakai is required for stabilization of core components of the m(6)A mRNA methylation machinery. Nat Commun 12: 3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruzicka K, Zhang M, Campilho A, Bodi Z, Kashif M, et al. 2017. Identification of factors required for m(6) A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol 215: 157–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen J, Lv R, Ma H, Shen H, He C, et al. 2018. Zc3h13 Regulates Nuclear RNA m(6)A Methylation and Mouse Embryonic Stem Cell Self-Renewal. Mol Cell 69: 1028–38 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Campos MA, Edelheit S, Toth U, Safra M, Shachar R, et al. 2019. Deciphering the “m(6)A Code” via Antibody-Independent Quantitative Profiling. Cell 178: 731–47 e16 [DOI] [PubMed] [Google Scholar]
- 30.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, et al. 2015. A majority of m6A residues are in the last exons, allowing the potential for 3’ UTR regulation. Genes Dev 29: 2037–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uzonyi A, Slobodin B, Schwartz S. 2022. Exon-intron architecture determines mRNA stability by dictating m6A deposition. bioRxiv: 2022.06.29.498130 [Google Scholar]
- 32.Huang H, Weng H, Zhou K, Wu T, Zhao BS, et al. 2019. Histone H3 trimethylation at lysine 36 guides m(6)A RNA modification co-transcriptionally. Nature 567: 414–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millan-Zambrano G, et al. 2017. Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature 552: 126–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, et al. 2018. The SMAD2/3 interactome reveals that TGFbeta controls m(6)A mRNA methylation in pluripotency. Nature 555: 256–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raj N, Wang M, Seoane JA, Zhao RL, Kaiser AM, et al. 2022. The Mettl3 epitranscriptomic writer amplifies p53 stress responses. Mol Cell 82: 2370–84 e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slobodin B, Han R, Calderone V, Vrielink J, Loayza-Puch F, et al. 2017. Transcription Impacts the Efficiency of mRNA Translation via Co-transcriptional N6-adenosine Methylation. Cell 169: 326–37 e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonkers I, Lis JT. 2015. Getting up to speed with transcription elongation by RNA polymerase II. Nat Rev Mol Cell Biol 16: 167–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Li K, Cai J, Zhang M, Zhang X, et al. 2020. Landscape and Regulation of m(6)A and m(6)Am Methylome across Human and Mouse Tissues. Mol Cell 77: 426–40 e6 [DOI] [PubMed] [Google Scholar]
- 39.Yin R, Chang J, Li Y, Gao Z, Qiu Q, et al. 2022. Differential m(6)A RNA landscapes across hematopoiesis reveal a role for IGF2BP2 in preserving hematopoietic stem cell function. Cell Stem Cell 29: 149–59 e7 [DOI] [PubMed] [Google Scholar]
- 40.Tegowski M, Flamand MN, Meyer KD. 2022. scDART-seq reveals distinct m(6)A signatures and mRNA methylation heterogeneity in single cells. Mol Cell 82: 868–78 e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du Y, Hou G, Zhang H, Dou J, He J, et al. 2018. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res 46: 5195–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Pan Z, Adhikari S, Harada BT, Shen L, et al. 2021. m(6) A deposition is regulated by PRMT1-mediated arginine methylation of METTL14 in its disordered C-terminal region. EMBO J 40: e106309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, et al. 2017. The U6 snRNA m(6)A Methyltransferase METTL16 Regulates SAM Synthetase Intron Retention. Cell 169: 824–35 e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, et al. 2017. Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep 18: 2004–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown JA, Kinzig CG, DeGregorio SJ, Steitz JA. 2016. Methyltransferase-like protein 16 binds the 3’-terminal triple helix of MALAT1 long noncoding RNA. Proc Natl Acad Sci U S A 113: 14013–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mendel M, Delaney K, Pandey RR, Chen KM, Wenda JM, et al. 2021. Splice site m(6)A methylation prevents binding of U2AF35 to inhibit RNA splicing. Cell 184: 3125–42 e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watabe E, Togo-Ohno M, Ishigami Y, Wani S, Hirota K, et al. 2021. m(6) A-mediated alternative splicing coupled with nonsense-mediated mRNA decay regulates SAM synthetase homeostasis. EMBO J 40: e106434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su R, Dong L, Li Y, Gao M, He PC, et al. 2022. METTL16 exerts an m(6)A-independent function to facilitate translation and tumorigenesis. Nat Cell Biol 24: 205–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Zhu P, Ma S, Song J, Bai J, et al. 2015. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. Nat Chem Biol 11: 592–7 [DOI] [PubMed] [Google Scholar]
- 50.Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. 2014. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature 515: 143–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, et al. 2014. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell 159: 148–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez NM, Su A, Burns MC, Nussbacher JK, Schaening C, et al. 2022. Pseudouridine synthases modify human pre-mRNA co-transcriptionally and affect pre-mRNA processing. Mol Cell 82: 645–59 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karijolich J, Yu YT. 2011. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 474: 395–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozanick S, Krecic A, Andersland J, Anderson JT. 2005. The bipartite structure of the tRNA m1A58 methyltransferase from S. cerevisiae is conserved in humans. RNA 11: 1281–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safra M, Sas-Chen A, Nir R, Winkler R, Nachshon A, et al. 2017. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature 551: 251–55 [DOI] [PubMed] [Google Scholar]
- 56.Grozhik AV, Olarerin-George AO, Sindelar M, Li X, Gross SS, Jaffrey SR. 2019. Antibody cross-reactivity accounts for widespread appearance of m(1)A in 5’UTRs. Nat Commun 10: 5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X, Xiong X, Wang K, Wang L, Shu X, et al. 2016. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat Chem Biol 12: 311–6 [DOI] [PubMed] [Google Scholar]
- 58.Dominissini D, Nachtergaele S, Moshitch-Moshkovitz S, Peer E, Kol N, et al. 2016. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature 530: 441–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arango D, Sturgill D, Yang R, Kanai T, Bauer P, et al. 2022. Direct epitranscriptomic regulation of mammalian translation initiation through N4-acetylcytidine. Mol Cell 82: 2797–814 e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arango D, Sturgill D, Alhusaini N, Dillman AA, Sweet TJ, et al. 2018. Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell 175: 1872–86 e24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo G, Shi X, Wang H, Ye L, Tong X, et al. 2020. Epitranscriptomic N4-Acetylcytidine Profiling in CD4(+) T Cells of Systemic Lupus Erythematosus. Front Cell Dev Biol 8: 842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dubin DT, Taylor RH. 1975. The methylation state of poly A-containing messenger RNA from cultured hamster cells. Nucleic Acids Res 2: 1653–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, et al. 2012. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res 40: 5023–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Legrand C, Tuorto F, Hartmann M, Liebers R, Jacob D, et al. 2017. Statistically robust methylation calling for whole-transcriptome bisulfite sequencing reveals distinct methylation patterns for mouse RNAs. Genome Res 27: 1589–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang T, Chen W, Liu J, Gu N, Zhang R. 2019. Genome-wide identification of mRNA 5-methylcytosine in mammals. Nat Struct Mol Biol 26: 380–88 [DOI] [PubMed] [Google Scholar]
- 66.Khoddami V, Yerra A, Mosbruger TL, Fleming AM, Burrows CJ, Cairns BR. 2019. Transcriptome-wide profiling of multiple RNA modifications simultaneously at single-base resolution. Proc Natl Acad Sci U S A 116: 6784–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang L, Wang W, Li G, Zhang L, Li J, et al. 2020. CIGAR-seq, a CRISPR/Cas-based method for unbiased screening of novel mRNA modification regulators. Mol Syst Biol 16: e10025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang X, Yang Y, Sun BF, Chen YS, Xu JW, et al. 2017. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res 27: 606–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blanco S, Frye M. 2014. Role of RNA methyltransferases in tissue renewal and pathology. Curr Opin Cell Biol 31: 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bohnsack KE, Hobartner C, Bohnsack MT. 2019. Eukaryotic 5-methylcytosine (m(5)C) RNA Methyltransferases: Mechanisms, Cellular Functions, and Links to Disease. Genes (Basel) 10: E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Decatur WA, Fournier MJ. 2003. RNA-guided nucleotide modification of ribosomal and other RNAs. J Biol Chem 278: 695–8 [DOI] [PubMed] [Google Scholar]
- 72.Dai Q, Moshitch-Moshkovitz S, Han D, Kol N, Amariglio N, et al. 2017. Nm-seq maps 2’-O-methylation sites in human mRNA with base precision. Nat Methods 14: 695–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Omer AD, Ziesche S, Ebhardt H, Dennis PP. 2002. In vitro reconstitution and activity of a C/D box methylation guide ribonucleoprotein complex. Proc Natl Acad Sci U S A 99: 5289–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Elliott BA, Ho HT, Ranganathan SV, Vangaveti S, Ilkayeva O, et al. 2019. Modification of messenger RNA by 2’-O-methylation regulates gene expression in vivo. Nat Commun 10: 3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belanger F, Stepinski J, Darzynkiewicz E, Pelletier J. 2010. Characterization of hMTr1, a human Cap1 2’-O-ribose methyltransferase. J Biol Chem 285: 33037–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Furuichi Y, Morgan M, Shatkin AJ, Jelinek W, Salditt-Georgieff M, Darnell JE. 1975. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A 72: 1904–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei C, Gershowitz A, Moss B. 1975. N6, O2’-dimethyladenosine a novel methylated ribonucleoside next to the 5’ terminal of animal cell and virus mRNAs. Nature 257: 251–3 [DOI] [PubMed] [Google Scholar]
- 78.Boulias K, Toczydlowska-Socha D, Hawley BR, Liberman N, Takashima K, et al. 2019. Identification of the m(6)Am Methyltransferase PCIF1 Reveals the Location and Functions of m(6)Am in the Transcriptome. Mol Cell 75: 631–43 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sendinc E, Valle-Garcia D, Dhall A, Chen H, Henriques T, et al. 2019. PCIF1 Catalyzes m6Am mRNA Methylation to Regulate Gene Expression. Mol Cell 75: 620–30 e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ben-Haim MS, Pinto Y, Moshitch-Moshkovitz S, Hershkovitz V, Kol N, et al. 2021. Dynamic regulation of N(6),2’-O-dimethyladenosine (m(6)Am) in obesity. Nat Commun 12: 7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, et al. 2017. Reversible methylation of m(6)A(m) in the 5’ cap controls mRNA stability. Nature 541: 371–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kruse S, Zhong S, Bodi Z, Button J, Alcocer MJ, et al. 2011. A novel synthesis and detection method for cap-associated adenosine modifications in mouse mRNA. Sci Rep 1: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoilov P, Rafalska I, Stamm S. 2002. YTH: a new domain in nuclear proteins. Trends Biochem Sci 27: 495–7 [DOI] [PubMed] [Google Scholar]
- 84.Zhang Z, Theler D, Kaminska KH, Hiller M, de la Grange P, et al. 2010. The YTH domain is a novel RNA binding domain. J Biol Chem 285: 14701–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C, Zhu Y, Bao H, Jiang Y, Xu C, et al. 2016. A novel RNA-binding mode of the YTH domain reveals the mechanism for recognition of determinant of selective removal by Mmi1. Nucleic Acids Res 44: 969–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz S, Agarwala SD, Mumbach MR, Jovanovic M, Mertins P, et al. 2013. High-resolution mapping reveals a conserved, widespread, dynamic mRNA methylation program in yeast meiosis. Cell 155: 1409–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li F, Zhao D, Wu J, Shi Y. 2014. Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res 24: 1490–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu T, Roundtree IA, Wang P, Wang X, Wang L, et al. 2014. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res 24: 1493–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu C, Wang X, Liu K, Roundtree IA, Tempel W, et al. 2014. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 10: 927–9 [DOI] [PubMed] [Google Scholar]
- 90.Luo S, Tong L. 2014. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci U S A 111: 13834–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Theler D, Dominguez C, Blatter M, Boudet J, Allain FH. 2014. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res 42: 13911–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu C, Liu K, Ahmed H, Loppnau P, Schapira M, Min J. 2015. Structural Basis for the Discriminative Recognition of N6-Methyladenosine RNA by the Human YT521-B Homology Domain Family of Proteins. J Biol Chem 290: 24902–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zaccara S, Jaffrey SR. 2020. A Unified Model for the Function of YTHDF Proteins in Regulating m(6)A-Modified mRNA. Cell 181: 1582–95 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu B, Merriman DK, Choi SH, Schumacher MA, Plangger R, et al. 2018. A potentially abundant junctional RNA motif stabilized by m(6)A and Mg(2). Nat Commun 9: 2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nayler O, Hartmann AM, Stamm S. 2000. The ER repeat protein YT521-B localizes to a novel subnuclear compartment. J Cell Biol 150: 949–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hartmann AM, Nayler O, Schwaiger FW, Obermeier A, Stamm S. 1999. The interaction and colocalization of Sam68 with the splicing-associated factor YT521-B in nuclear dots is regulated by the Src family kinase p59(fyn). Mol Biol Cell 10: 3909–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, et al. 2016. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell 61: 507–19 [DOI] [PubMed] [Google Scholar]
- 98.Cheng Y, Xie W, Pickering BF, Chu KL, Savino AM, et al. 2021. N(6)-Methyladenosine on mRNA facilitates a phase-separated nuclear body that suppresses myeloid leukemic differentiation. Cancer Cell 39: 958–72 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roundtree IA, Luo GZ, Zhang Z, Wang X, Zhou T, et al. 2017. YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife 6: e31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kasowitz SD, Ma J, Anderson SJ, Leu NA, Xu Y, et al. 2018. Nuclear m6A reader YTHDC1 regulates alternative polyadenylation and splicing during mouse oocyte development. PLoS Genet 14: e1007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang C, Chen L, Peng D, Jiang A, He Y, et al. 2020. METTL3 and N6-Methyladenosine Promote Homologous Recombination-Mediated Repair of DSBs by Modulating DNA-RNA Hybrid Accumulation. Mol Cell 79: 425–42 e7 [DOI] [PubMed] [Google Scholar]
- 102.Liu J, Gao M, He J, Wu K, Lin S, et al. 2021. The RNA m(6)A reader YTHDC1 silences retrotransposons and guards ES cell identity. Nature 591: 322–26 [DOI] [PubMed] [Google Scholar]
- 103.Li Y, Xia L, Tan K, Ye X, Zuo Z, et al. 2020. N(6)-Methyladenosine co-transcriptionally directs the demethylation of histone H3K9me2. Nat Genet 52: 870–77 [DOI] [PubMed] [Google Scholar]
- 104.Liu J, Dou X, Chen C, Chen C, Liu C, et al. 2020. N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367: 580–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, et al. 2017. Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27: 1115–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R, Pillai RS. 2017. Regulation of m(6)A Transcripts by the 3’-->5’ RNA Helicase YTHDC2 Is Essential for a Successful Meiotic Program in the Mammalian Germline. Mol Cell 68: 374–87 e12 [DOI] [PubMed] [Google Scholar]
- 107.Tanabe A, Tanikawa K, Tsunetomi M, Takai K, Ikeda H, et al. 2016. RNA helicase YTHDC2 promotes cancer metastasis via the enhancement of the efficiency by which HIF-1alpha mRNA is translated. Cancer Lett 376: 34–42 [DOI] [PubMed] [Google Scholar]
- 108.Saito Y, Hawley BR, Puno MR, Sarathy SN, Lima CD, et al. 2022. YTHDC2 control of gametogenesis requires helicase activity but not m(6)A binding. Genes Dev 36: 180–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li L, Krasnykov K, Homolka D, Gos P, Mendel M, et al. 2022. The XRN1-regulated RNA helicase activity of YTHDC2 ensures mouse fertility independently of m(6)A recognition. Mol Cell 82: 1678–90 e12 [DOI] [PubMed] [Google Scholar]
- 110.Ries RJ, Zaccara S, Klein P, Olarerin-George A, Namkoong S, et al. 2019. m(6)A enhances the phase separation potential of mRNA. Nature 571: 424–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fu Y, Zhuang X. 2020. m(6)A-binding YTHDF proteins promote stress granule formation. Nat Chem Biol 16: 955–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang X, Lu Z, Gomez A, Hon GC, Yue Y, et al. 2014. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 505: 117–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, et al. 2015. N(6)-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell 161: 1388–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shi H, Wang X, Lu Z, Zhao BS, Ma H, et al. 2017. YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res 27: 315–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lasman L, Krupalnik V, Viukov S, Mor N, Aguilera-Castrejon A, et al. 2020. Context-dependent functional compensation between Ythdf m(6)A reader proteins. Genes Dev 34: 1373–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kontur C, Jeong M, Cifuentes D, Giraldez AJ. 2020. Ythdf m(6)A Readers Function Redundantly during Zebrafish Development. Cell Rep 33: 108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Flamand MN, Ke K, Tamming R, Meyer KD. 2022. Single-molecule identification of the target RNAs of different RNA binding proteins simultaneously in cells. Genes Dev 36: 1002–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sommer S, Lavi U, Darnell JE Jr. 1978. The absolute frequency of labeled N-6-methyladenosine in HeLa cell messenger RNA decreases with label time. J Mol Biol 124: 487–99 [DOI] [PubMed] [Google Scholar]
- 119.Batista PJ, Molinie B, Wang J, Qu K, Zhang J, et al. 2014. m(6)A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15: 707–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, et al. 2015. Stem cells. m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347: 1002–6 [DOI] [PubMed] [Google Scholar]
- 121.Du H, Zhao Y, He J, Zhang Y, Xi H, et al. 2016. YTHDF2 destabilizes m(6)A-containing RNA through direct recruitment of the CCR4-NOT deadenylase complex. Nat Commun 7: 12626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Flamand MN, Meyer KD. 2022. m6A and YTHDF proteins contribute to the localization of select neuronal mRNAs. Nucleic Acids Res 50: 4464–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park OH, Ha H, Lee Y, Boo SH, Kwon DH, et al. 2019. Endoribonucleolytic Cleavage of m(6)A-Containing RNAs by RNase P/MRP Complex. Mol Cell 74: 494–507 e8 [DOI] [PubMed] [Google Scholar]
- 124.Boo SH, Ha H, Lee Y, Shin MK, Lee S, Kim YK. 2022. UPF1 promotes rapid degradation of m(6)A-containing RNAs. Cell Rep 39: 110861. [DOI] [PubMed] [Google Scholar]
- 125.Lin X, Chai G, Wu Y, Li J, Chen F, et al. 2019. RNA m(6)A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun 10: 2065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Meyer KD. 2019. m(6)A-mediated translation regulation. Biochim Biophys Acta Gene Regul Mech 1862: 301–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shi H, Zhang X, Weng YL, Lu Z, Liu Y, et al. 2018. m(6)A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563: 249–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Merkurjev D, Hong WT, Iida K, Oomoto I, Goldie BJ, et al. 2018. Synaptic N(6)-methyladenosine (m(6)A) epitranscriptome reveals functional partitioning of localized transcripts. Nat Neurosci 21: 1004–14 [DOI] [PubMed] [Google Scholar]
- 129.Weng YL, Wang X, An R, Cassin J, Vissers C, et al. 2018. Epitranscriptomic m(6)A Regulation of Axon Regeneration in the Adult Mammalian Nervous System. Neuron 97: 313–25 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li A, Chen YS, Ping XL, Yang X, Xiao W, et al. 2017. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res 27: 444–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Flamand MN, Meyer KD. 2019. The epitranscriptome and synaptic plasticity. Curr Opin Neurobiol 59: 41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yoon KJ, Ringeling FR, Vissers C, Jacob F, Pokrass M, et al. 2017. Temporal Control of Mammalian Cortical Neurogenesis by m(6)A Methylation. Cell 171: 877–89 e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhuang M, Li X, Zhu J, Zhang J, Niu F, et al. 2019. The m6A reader YTHDF1 regulates axon guidance through translational control of Robo3.1 expression. Nucleic Acids Res 47: 4765–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang Z, Wang M, Xie D, Huang Z, Zhang L, et al. 2018. METTL3-mediated N(6)-methyladenosine mRNA modification enhances long-term memory consolidation. Cell Res 28: 1050–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koranda JL, Dore L, Shi H, Patel MJ, Vaasjo LO, et al. 2018. Mettl14 Is Essential for Epitranscriptomic Regulation of Striatal Function and Learning. Neuron 99: 283–92 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zong X, Xiao X, Shen B, Jiang Q, Wang H, et al. 2021. The N6-methyladenosine RNA-binding protein YTHDF1 modulates the translation of TRAF6 to mediate the intestinal immune response. Nucleic Acids Res 49: 5537–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. 2015. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 43: D512–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fei Q, Zou Z, Roundtree IA, Sun HL, He C. 2020. YTHDF2 promotes mitotic entry and is regulated by cell cycle mediators. PLoS Biol 18: e3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hou G, Zhao X, Li L, Yang Q, Liu X, et al. 2021. SUMOylation of YTHDF2 promotes mRNA degradation and cancer progression by increasing its binding affinity with m6A-modified mRNAs. Nucleic Acids Res 49: 2859–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, et al. 2017. N(6)-methyladenosine (m(6)A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 24: 870–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arguello AE, DeLiberto AN, Kleiner RE. 2017. RNA Chemical Proteomics Reveals the N(6)-Methyladenosine (m(6)A)-Regulated Protein-RNA Interactome. J Am Chem Soc 139: 17249–52 [DOI] [PubMed] [Google Scholar]
- 142.Huang H, Weng H, Sun W, Qin X, Shi H, et al. 2018. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20: 285–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, et al. 2015. 5’ UTR m(6)A Promotes Cap-Independent Translation. Cell 163: 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang Y, Fan X, Mao M, Song X, Wu P, et al. 2017. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res 27: 626–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun L, Fazal FM, Li P, Broughton JP, Lee B, et al. 2019. RNA structure maps across mammalian cellular compartments. Nat Struct Mol Biol 26: 322–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang F, Kang Y, Wang M, Li Y, Xu T, et al. 2018. Fragile X mental retardation protein modulates the stability of its m6A-marked messenger RNA targets. Hum Mol Genet 27: 3936–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Edens BM, Vissers C, Su J, Arumugam S, Xu Z, et al. 2019. FMRP Modulates Neural Differentiation through m(6)A-Dependent mRNA Nuclear Export. Cell Rep 28: 845–54 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hsu PJ, Shi H, Zhu AC, Lu Z, Miller N, et al. 2019. The RNA-binding protein FMRP facilitates the nuclear export of N(6)-methyladenosine-containing mRNAs. J Biol Chem 294: 19889–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu R, Li A, Sun B, Sun JG, Zhang J, et al. 2019. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res 29: 23–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Choi SH, Flamand MN, Liu B, Zhu H, Hu M, et al. 2022. RBM45 is an m(6)A-binding protein that affects neuronal differentiation and the splicing of a subset of mRNAs. Cell Rep 40: 111293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roost C, Lynch SR, Batista PJ, Qu K, Chang HY, Kool ET. 2015. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J Am Chem Soc 137: 2107–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. 2015. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518: 560–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu N, Zhou KI, Parisien M, Dai Q, Diatchenko L, Pan T. 2017. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res 45: 6051–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu B, Su S, Patil DP, Liu H, Gan J, et al. 2018. Molecular basis for the specific and multivariant recognitions of RNA substrates by human hnRNP A2/B1. Nat Commun 9: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. 2014. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol 16: 191–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Boo SH, Ha H, Kim YK. 2022. m(1)A and m(6)A modifications function cooperatively to facilitate rapid mRNA degradation. Cell Rep 40: 111317. [DOI] [PubMed] [Google Scholar]
- 157.Yang Y, Wang L, Han X, Yang WL, Zhang M, et al. 2019. RNA 5-Methylcytosine Facilitates the Maternal-to-Zygotic Transition by Preventing Maternal mRNA Decay. Mol Cell 75: 1188–202 e11 [DOI] [PubMed] [Google Scholar]
- 158.Chen H, Yang H, Zhu X, Yadav T, Ouyang J, et al. 2020. m(5)C modification of mRNA serves a DNA damage code to promote homologous recombination. Nat Commun 11: 2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Levi O, Arava YS. 2021. Pseudouridine-mediated translation control of mRNA by methionine aminoacyl tRNA synthetase. Nucleic Acids Res 49: 432–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xiang Y, Zhou C, Zeng Y, Guo Q, Huang J, et al. 2021. NAT10-Mediated N4-Acetylcytidine of RNA Contributes to Post-transcriptional Regulation of Mouse Oocyte Maturation in vitro. Front Cell Dev Biol 9: 704341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fedeles BI, Singh V, Delaney JC, Li D, Essigmann JM. 2015. The AlkB Family of Fe(II)/alpha-Ketoglutarate-dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond. J Biol Chem 290: 20734–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hess ME, Hess S, Meyer KD, Verhagen LA, Koch L, et al. 2013. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci 16: 1042–8 [DOI] [PubMed] [Google Scholar]
- 163.Fu Y, Jia G, Pang X, Wang RN, Wang X, et al. 2013. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun 4: 1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zou S, Toh JD, Wong KH, Gao YG, Hong W, Woon EC. 2016. N(6)-Methyladenosine: a conformational marker that regulates the substrate specificity of human demethylases FTO and ALKBH5. Sci Rep 6: 25677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang X, Wei LH, Wang Y, Xiao Y, Liu J, et al. 2019. Structural insights into FTO’s catalytic mechanism for the demethylation of multiple RNA substrates. Proc Natl Acad Sci U S A 116: 2919–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koh CWQ, Goh YT, Goh WSS. 2019. Atlas of quantitative single-base-resolution N(6)-methyl-adenine methylomes. Nat Commun 10: 5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jia G, Yang CG, Yang S, Jian X, Yi C, et al. 2008. Oxidative demethylation of 3-methylthymine and 3-methyluracil in single-stranded DNA and RNA by mouse and human FTO. FEBS Lett 582: 3313–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wei J, Liu F, Lu Z, Fei Q, Ai Y, et al. 2018. Differential m(6)A, m(6)A(m), and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol Cell 71: 973–85 e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gulati P, Cheung MK, Antrobus R, Church CD, Harding HP, et al. 2013. Role for the obesity-related FTO gene in the cellular sensing of amino acids. Proc Natl Acad Sci U S A 110: 2557–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yu J, Chen M, Huang H, Zhu J, Song H, et al. 2018. Dynamic m6A modification regulates local translation of mRNA in axons. Nucleic Acids Res 46: 1412–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Vujovic P, Stamenkovic S, Jasnic N, Lakic I, Djurasevic SF, et al. 2013. Fasting induced cytoplasmic Fto expression in some neurons of rat hypothalamus. PLoS One 8: e63694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Walters BJ, Mercaldo V, Gillon CJ, Yip M, Neve RL, et al. 2017. The Role of The RNA Demethylase FTO (Fat Mass and Obesity-Associated) and mRNA Methylation in Hippocampal Memory Formation. Neuropsychopharmacology 42: 1502–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhu T, Yong XLH, Xia D, Widagdo J, Anggono V. 2018. Ubiquitination Regulates the Proteasomal Degradation and Nuclear Translocation of the Fat Mass and Obesity-Associated (FTO) Protein. J Mol Biol 430: 363–71 [DOI] [PubMed] [Google Scholar]
- 174.Mauer J, Sindelar M, Despic V, Guez T, Hawley BR, et al. 2019. FTO controls reversible m(6)Am RNA methylation during snRNA biogenesis. Nat Chem Biol 15: 340–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, et al. 2014. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res 24: 1403–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wei J, Yu X, Yang L, Liu X, Gao B, et al. 2022. FTO mediates LINE1 m(6)A demethylation and chromatin regulation in mESCs and mouse development. Science 376: 968–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, et al. 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lan N, Lu Y, Zhang Y, Pu S, Xi H, et al. 2020. FTO - A Common Genetic Basis for Obesity and Cancer. Front Genet 11: 559138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, et al. 2014. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507: 371–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Reitz C, Tosto G, Mayeux R, Luchsinger JA, Group N-LNFS, Alzheimer’s Disease Neuroimaging I. 2012. Genetic variants in the Fat and Obesity Associated (FTO) gene and risk of Alzheimer’s disease. PLoS One 7: e50354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Keller L, Xu W, Wang HX, Winblad B, Fratiglioni L, Graff C. 2011. The obesity related gene, FTO, interacts with APOE, and is associated with Alzheimer’s disease risk: a prospective cohort study. J Alzheimers Dis 23: 461–9 [DOI] [PubMed] [Google Scholar]
- 182.Chang R, Huang Z, Zhao S, Zou J, Li Y, Tan S. 2022. Emerging Roles of FTO in Neuropsychiatric Disorders. Biomed Res Int 2022: 2677312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li Y, Su R, Deng X, Chen Y, Chen J. 2022. FTO in cancer: functions, molecular mechanisms, and therapeutic implications. Trends Cancer 8: 598–614 [DOI] [PubMed] [Google Scholar]
- 184.Li Z, Weng H, Su R, Weng X, Zuo Z, et al. 2017. FTO Plays an Oncogenic Role in Acute Myeloid Leukemia as a N(6)-Methyladenosine RNA Demethylase. Cancer Cell 31: 127–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Su R, Dong L, Li C, Nachtergaele S, Wunderlich M, et al. 2018. R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m(6)A/MYC/CEBPA Signaling. Cell 172: 90–105 e23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cui Q, Shi H, Ye P, Li L, Qu Q, et al. 2017. m(6)A RNA Methylation Regulates the Self-Renewal and Tumorigenesis of Glioblastoma Stem Cells. Cell Rep 18: 2622–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bodi Z, Zhong S, Mehra S, Song J, Graham N, et al. 2012. Adenosine Methylation in Arabidopsis mRNA is Associated with the 3’ End and Reduced Levels Cause Developmental Defects. Front Plant Sci 3: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yu Q, Liu S, Yu L, Xiao Y, Zhang S, et al. 2021. RNA demethylation increases the yield and biomass of rice and potato plants in field trials. Nat Biotechnol 39: 1581–88 [DOI] [PubMed] [Google Scholar]
- 189.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, et al. 2013. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49: 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aik W, Scotti JS, Choi H, Gong L, Demetriades M, et al. 2014. Structure of human RNA N(6)-methyladenine demethylase ALKBH5 provides insights into its mechanisms of nucleic acid recognition and demethylation. Nucleic Acids Res 42: 4741–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Feng C, Liu Y, Wang G, Deng Z, Zhang Q, et al. 2014. Crystal structures of the human RNA demethylase Alkbh5 reveal basis for substrate recognition. J Biol Chem 289: 11571–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu C, Liu K, Tempel W, Demetriades M, Aik W, et al. 2014. Structures of human ALKBH5 demethylase reveal a unique binding mode for specific single-stranded N6-methyladenosine RNA demethylation. J Biol Chem 289: 17299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li Z, Wang P, Li J, Xie Z, Cen S, et al. 2021. The N(6)-methyladenosine demethylase ALKBH5 negatively regulates the osteogenic differentiation of mesenchymal stem cells through PRMT6. Cell Death Dis 12: 578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang C, Samanta D, Lu H, Bullen JW, Zhang H, et al. 2016. Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A 113: E2047–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, et al. 2017. m(6)A Demethylase ALKBH5 Maintains Tumorigenicity of Glioblastoma Stem-like Cells by Sustaining FOXM1 Expression and Cell Proliferation Program. Cancer Cell 31: 591–606 e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu F, Clark W, Luo G, Wang X, Fu Y, et al. 2016. ALKBH1-Mediated tRNA Demethylation Regulates Translation. Cell 167: 1897. [DOI] [PubMed] [Google Scholar]
- 197.Ueda Y, Ooshio I, Fusamae Y, Kitae K, Kawaguchi M, et al. 2017. AlkB homolog 3-mediated tRNA demethylation promotes protein synthesis in cancer cells. Sci Rep 7: 42271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhang C, Jia G. 2018. Reversible RNA Modification N(1)-methyladenosine (m(1)A) in mRNA and tRNA. Genomics Proteomics Bioinformatics 16: 155–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kohli RM, Zhang Y. 2013. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 502: 472–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Fu L, Guerrero CR, Zhong N, Amato NJ, Liu Y, et al. 2014. Tet-mediated formation of 5-hydroxymethylcytosine in RNA. J Am Chem Soc 136: 11582–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Delatte B, Wang F, Ngoc LV, Collignon E, Bonvin E, et al. 2016. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 351: 282–5 [DOI] [PubMed] [Google Scholar]
- 202.Huber SM, van Delft P, Mendil L, Bachman M, Smollett K, et al. 2015. Formation and abundance of 5-hydroxymethylcytosine in RNA. Chembiochem 16: 752–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lan J, Rajan N, Bizet M, Penning A, Singh NK, et al. 2020. Functional role of Tet-mediated RNA hydroxymethylcytosine in mouse ES cells and during differentiation. Nat Commun 11: 4956. [DOI] [PMC free article] [PubMed] [Google Scholar]


