Abstract
Background
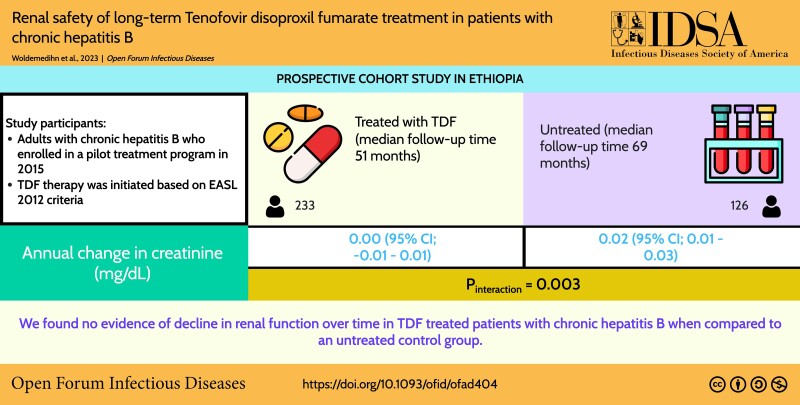
Data on renal safety of tenofovir disoproxil fumarate (TDF) treatment among individuals with chronic hepatitis B (CHB) are inconsistent. The current study aimed to assess the effect of long-term TDF treatment on renal outcomes in adult patients with CHB.
Methods
From a CHB cohort in Ethiopia, we included 233 patients treated with TDF and 126 untreated controls. Levels of creatinine and creatinine clearance over time were described in patients with and without TDF treatment. Linear mixed effects models with a treatment × time interaction were used to investigate the effect of TDF on creatinine and creatinine clearance. In treated patients only, change in creatinine and creatinine clearance was estimated separately in the first year as compared with subsequent years via linear mixed effects models.
Results
Median follow-up in the treated group was 51 months (IQR, 27–72), and 75% of patients were male (median age, 33 years; IQR, 26–40). Median follow-up in the untreated group was 69 months (IQR, 66–72), and 48% of participants were male (median age, 33 years; IQR, 27–41). We found no change in creatinine over time in TDF-treated patients as compared with a slight increase in untreated patients (P interaction = .003). There was a decrease in creatinine clearance over time in both groups, which was stronger in patients without TDF treatment (P interaction = .007). In TDF-treated patients, changes in creatinine and creatinine clearance occurred mainly within the first 12 months after treatment initiation.
Conclusions
This study showed no evidence of long-term renal toxicity of TDF treatment in patients with CHB.
Keywords: antiviral treatment, cohort study, nephrotoxicity, sub-Saharan Africa, viral hepatitis
Graphical Abstract
Graphical Abstract.
Hepatitis B virus (HBV) infection is a global health challenge. It is estimated that 296 million people are living with chronic hepatitis B (CHB) worldwide and approximately 820 000 die annually of HBV-related causes [1]. Between 1990 and 2013, there was a 33% rise in the number of HBV-related deaths as a result of liver cirrhosis and/or hepatocellular carcinoma globally [2]. Sustained suppression of viral replication with antiviral therapy prevents progression of CHB toward cirrhosis and hepatocellular carcinoma [3, 4]. The advances in antiviral therapy have significantly improved the prognosis of HBV infection, and regression of fibrosis and cirrhosis with long-term antiviral treatment has been demonstrated [4].
Tenofovir disoproxil fumarate (TDF) is an orally bioavailable prodrug of tenofovir, a nucleotide analogue reverse transcriptase inhibitor with potent efficacy against hepadna and retroviruses [5, 6]. TDF has a high barrier to resistance and is a first-line drug for CHB in international liver society guidelines [1, 7–9]. Despite its excellent antiviral activity against HBV, TDF is unable to clear the viral reservoir; therefore, lifelong maintenance therapy is required [1, 7]. With an increasing number of people with CHB being treated for years and even decades, there has been a growing concern about potential long-term adverse effects of TDF. Indeed, previous studies have illustrated renal safety concerns with long-term use of TDF, mainly through proximal tubular injury [10–14].
To date, most of the evidence of renal tubular dysfunction in individuals taking TDF has been reported from studies on HIV. However, people with HIV always use TDF in combination with other antiretroviral drugs, and it is therefore hard to isolate the effect of TDF itself in this group [13, 15, 16]. Yet, patients with CHB receive TDF in monotherapy, and data on renal safety in this group are inconsistent. Furthermore, very little is known about long-term renal safety in Africans with CHB, which is an important knowledge gap as African countries aim for hepatitis elimination by 2030 [17].
The aim of this study was to assess the effect of long-term TDF treatment on renal outcomes in a large CHB cohort in Ethiopia. Specifically, we aimed to (1) describe creatinine and creatinine clearance over time in treated and untreated CHB; (2) investigate the effect of TDF treatment on creatinine and creatinine clearance over time; and (3) examine the effect of sex, decompensated liver cirrhosis, and time since treatment start on changes in creatinine and creatinine clearance.
METHODS
Study Setting
Between 9 February and 14 December 2015, a cohort of 1303 people with CHB was established at St Paul's Hospital Millennium Medical College in Addis Ababa, Ethiopia. St Paul's Hospital is a tertiary hospital delivering medical services to patients referred from all over the country. Consenting adults (≥18 years of age) with CHB were eligible for enrollment in the cohort, in the absence of hepatocellular carcinoma and other terminal illnesses. People with HIV coinfection were transferred to the nearest HIV care and treatment center. The clinical and operational aspects of the cohort have been published previously [18, 19].
Patient Assessment and Treatment
The baseline assessment included clinical examination, sociodemographic information, medical history, and previous use of antiviral agents and other medications. Transient elastography (FibroScan 402; Echosens) was done in all nonpregnant patients after minimum 2 hours fasting, as recommended by the manufacturer. The following laboratory tests were performed at enrollment: complete blood count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, hepatitis B surface antigen (HBsAg), hepatitis B e-antigen (HBeAg), HBV DNA, HIV test, hepatitis C virus (HCV) antibody, and hepatitis D virus (HDV) antibody.
Within this cohort, TDF therapy was initiated per the 2012 guidelines of the European Association for the Study of the Liver, with some modifications as previously described [18, 19]. Patients who fulfilled any of the following criteria were considered eligible for treatment:
Decompensated cirrhosis: defined as past or present evidence of ascites, esophageal varices, encephalopathy, or jaundice
Compensated cirrhosis: confirmed with ultrasound and/or transient elastography >9.9 kPa
Significant fibrosis (transient elastography >7.9 kPa) and viral load >2000 IU/mL
Moderate/severe liver inflammation (ALT >80 U/L) and viral load >2000 IU/mL
Hepatocellular carcinoma among first-degree relatives and viral load >2000 IU/mL
Among individuals who initiated TDF therapy, HBsAg, HIV, complete blood count, ALT, AST, creatinine, and HBV DNA were measured at 3- to 6-month intervals. Individuals who did not meet the treatment eligibility criteria were followed up at 6- to 12-month intervals with measurement of HBsAg, complete blood count, ALT, AST, and HBV DNA—but not creatinine.
Selection of Study Participants
From this cohort, 290 patients had started TDF therapy at the time of the study. For the current analysis, we sampled 233 patients who were treated with TDF for at least 12 months, who had a creatinine measurement ≤12 months before starting TDF therapy, and who had at least 1 follow-up measurement of creatinine after treatment initiation. For comparison, we included 126 consecutive untreated patients who came for a scheduled follow-up visit between 15 September and 15 December 2020 (ie, patients who did not meet the treatment criteria); creatinine was measured at baseline in all these patients and rechecked at their last follow-up visit. The untreated controls were temporally contiguous with the TDF-treated patients. According to the predefined follow-up protocol, TDF-treated patients—but not untreated patients—had multiple creatinine measurements between baseline and the last study visit.
Laboratory Tests
Standard biochemistry, including creatinine, was measured with the Humalyzer 3000 assay (Human) and standard hematology with the Huma-Count 30 (Human). HIV screening was performed with a World Health Organization–approved rapid diagnostic test (HIV 1 + 2 Antibody Colloidal Gold; KHB Shanghai Kehua Bio-engineering Co), and a positive result was confirmed with the HIV 1/2 STAT-PAK rapid diagnostic test (Chembio Diagnostics). HBsAg status was assessed with a World Health Organization–approved rapid diagnostic test (Determine; Alere Inc). HBeAg was analyzed with an enzyme-linked fluorescent immunoassay (VIDAS HBe/anti-HBe; BioMérieux). Enzyme-linked immunosorbent assays were used to detect HCV antibodies (Elisys Uno; Human) and HDV antibodies (ETI-AB-DELTAK-2; DiaSorin). A second anti-HDV enzyme-linked immunosorbent assay (Dia.Pro Diagnostic Bioprobes Srl) was used to confirm indeterminate or weak positive results obtained with the DiaSorin assay. HBV DNA viral load testing was done with the Abbott RealTime HBV assay (Abbott Molecular).
Outcome Variables
Outcomes in the current study included creatinine (mg/dL) and creatinine clearance (mL/min). Creatinine clearance was calculated with the Cockcroft-Gault equation: (140 – age) ×(weight, kg) × (0.85 if female)/(72 × creatinine) [20].
Explanatory Variable and Covariates
For each participant, the start date of TDF treatment was registered (and, if applicable, the end date). For patients receiving TDF, the start of treatment was set as the baseline. For patients without treatment, cohort enrollment was set as the baseline. Patients who stopped treatment (n = 34) where retained in the longitudinal analyses until the date of stopping.
Baseline covariates included sex, age, decompensated liver cirrhosis (vs compensated/no cirrhosis), HBeAg positivity, HCV positivity, HDV positivity, body mass index (kg/m2), HBV DNA viral load (IU/mL), bilirubin (μmol/L), platelet count, transient elastography (kPa), ALT (U/L), and AST (U/L). Decompensated liver cirrhosis was defined as described earlier. Body mass index was calculated as body weight in kilograms divided by height in meters squared. HBV DNA viral load was logarithmically transformed with base 10.
Statistical Analysis
Mean with SD and range or median with IQR and range was used for continuous variables and number with proportion for categorical variables. Descriptive statistics (mean with SD and individual trajectories) were used to present creatinine and creatinine clearance levels over time for treated and untreated participants and for participants undergoing TDF treatment, as stratified by sex and baseline decompensated liver cirrhosis. To investigate the effect of TDF treatment on creatinine and creatinine clearance, linear mixed effects models were run with the patient as the random intercept and with a treatment × time interaction. We further performed linear mixed effects models with the random intercept and time as fixed effects in treated patients only, overall and stratified for months 1 to 12 and 13 to 75. All models were first run unadjusted and then adjusted for age, sex and decompensated cirrhosis. In the adjusted overall model in treated patients only, we also tested for the interaction between time and sex or decompensated cirrhosis. Analyses were performed in R (version 4.2.1) and Stata SE (version 17.0; StataCorp). P < .05 was considered statistically significant.
Patient Consent Statement
Written informed consent was obtained from all study participants. The design of the work was approved by the National Research Ethics Review Committee in Ethiopia and by the Regional Committees for Medical and Health Research Ethics in Norway.
RESULTS
Characteristics of Study Participants
We included 359 participants: 233 receiving TDF treatment (75% male; median age, 33 years [IQR, 26–40; range, 18–65]) and 126 without TDF treatment (48% male; median age, 33 years [IQR, 27–41; range, 18–61]; Table 1). Approximately one-third (35%, n = 82) had decompensated cirrhosis among those receiving TDF treatment as compared with none in the untreated group. In patients undergoing TDF treatment, 33%, 2%, and 3% were positive for HBeAg, anti-HCV, and anti-HDV, respectively, vs 5%, 4%, and 0% in the untreated group. The median follow-up in months was 51 (range, 3–75) for the treated group and 69 (range, 57–75) for the untreated group. The mean creatinine level at baseline was 0.8 mg/dL (SD, 0.2; range, 0.1–1.4) in the treated group and 0.8 mg/dL (SD, 0.2; range, 0.4–2.0) in the untreated group.
Table 1.
Baseline Characteristics of Study Subjects by TDF Treatment Status
| TDF Treatment | ||||
|---|---|---|---|---|
| Treated (n = 233) | Untreated (n = 126) | |||
| No. | % | No. | % | |
| Sex | ||||
| Male | 174 | 75 | 60 | 48 |
| Female | 59 | 25 | 66 | 52 |
| Liver cirrhosis | ||||
| Decompensated cirrhosis | 82 | 35 | 0 | 0 |
| No/compensated cirrhosis | 151 | 65 | 126 | 100 |
| Hepatitis B e-antigen | ||||
| Negative | 153 | 66 | 110 | 87 |
| Positive | 77 | 33 | 5 | 4 |
| Missing | 3 | 1 | 11 | 9 |
| Hepatitis C antibody | ||||
| Negative | 212 | 91 | 109 | 86 |
| Positive | 5 | 2 | 5 | 4 |
| Missing | 16 | 7 | 12 | 10 |
| Hepatitis D antibody | ||||
| Negative | 221 | 95 | 126 | 100 |
| Positive | 7 | 3 | 0 | 0 |
| Missing | 5 | 2 | 0 | 0 |
| Median | IQR (Range) | Median | IQR (Range) | |
|---|---|---|---|---|
| Follow-up time (mo) | 51 | 27–72 (3–75) | 69 | 66–72 (57–75) |
| Age (y) | 33 | 26–40 (18–65) | 33 | 27–41 (18–61) |
| Mean | SD (Range) | Mean | SD (Range) | |
|---|---|---|---|---|
| BMI, kg/m2 | 21.4 | 3.8 (14–35) | 24.1 | 4.4 (16–40) |
| Missing | 5 | 2 | ||
| HBV DNA viral load, log10 IU/mL | 4.9 | 2.2 (1–9) | 3.2 | 1.2 (1–8) |
| Missing | 19 | 2 | ||
| Platelet count, × 109/L | 239 | 120 (61–637) | 291 | 71 (56–507) |
| Missing | 16 | 9 | ||
| Liver stiffness, kPa | 20.8 | 19 (3.4–75) | 5.4 | 1.4 (3–12) |
| Missing | 18 | 12 | ||
| Alanine aminotransferase, U/L | 57 | 72 (11–493) | 26 | 15 (9–105) |
| Missing | 0 | 0 | ||
| Aspartate aminotransferase, U/L | 53 | 57 (10–576) | 26 | 12 (10–95) |
| Missing | 0 | 0 | ||
| Creatinine, μmol/L | 0.8 | 0.2 (0.1–1.4) | 0.8 | 0.2 (0.4–2.0) |
| Missing | 0 | 0 | ||
| Creatinine clearance, mL/min | 115 | 57 (52–783) | 117 | 42 (35–411) |
| Missinga | 4 | 0 |
Abbreviations: BMI, body mass index; TDF, tenofovir disoproxil fumarate.
Baseline body weight, needed to calculate creatinine clearance, was missing in 4 patients.
Description of Creatinine and Creatinine Clearance Over Time in Treated and Untreated Participants
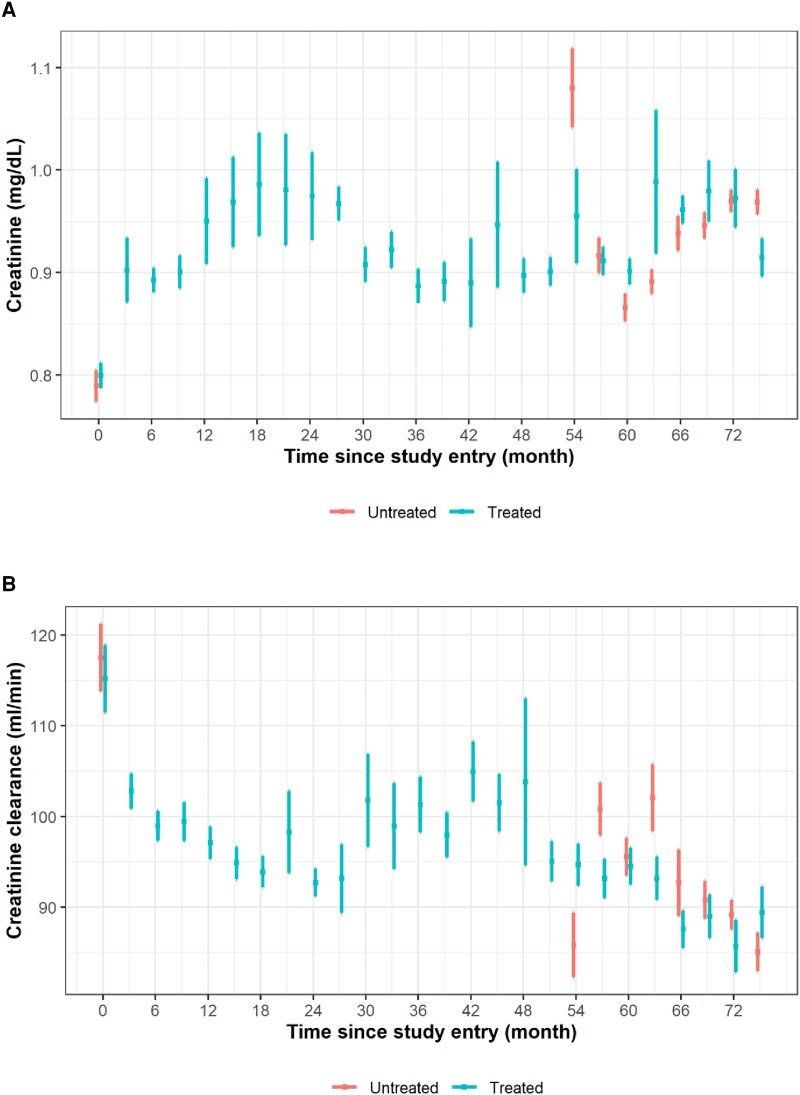
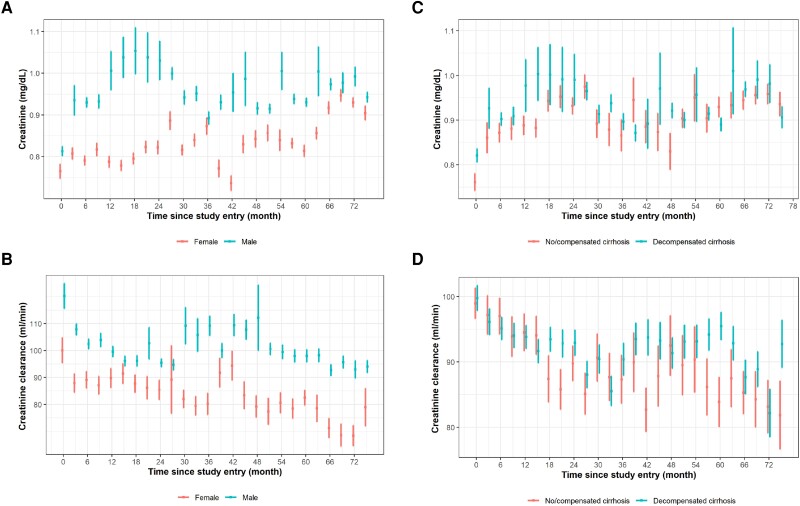
There was an increase in mean creatinine levels and a decrease in creatinine clearance in the treated and untreated participants over time (Figure 1, Supplementary Figure 1). Among patients undergoing TDF treatment, the changes in creatinine and creatinine clearance over time looked similar for men and women but with generally lower levels of creatinine and creatinine clearance in women (Figure 2A and 2B, Supplementary Figure 2A and 2B). Levels of creatinine and creatinine clearance were similar over time for patients with and without decompensated cirrhosis (Figures 2C and 2D, Supplementary Figure 2C and 2D). Supplementary Table 1 shows, for each time point, the number of participants in the study, number of creatinine assessments, and mean creatinine level.
Figure 1.
Means and SD are presented for (A) creatinine and (B) creatinine clearance in relation to time since study entry for participants receiving and not receiving TDF treatment. For participants treated with TDF, treatment started at time point 0 (baseline). We included all time points with at least 5 measurements of creatinine/creatinine clearance. Creatinine clearance values >200 were set to missing. TDF, tenofovir disoproxil fumarate.
Figure 2.
Means and SDs are presented for creatinine and creatinine clearance for participants receiving TDF treatment in relation to time since study entry, stratified by (A, B) sex and (C, D) decompensated cirrhosis at baseline. Treatment started at time point 0 (baseline). We included all time points with at least 5 measurements of creatinine/creatinine clearance. Creatinine clearance values >200 were set to missing. TDF, tenofovir disoproxil fumarate.
Effect of TDF Treatment on Creatinine and Creatinine Clearance
Overall we found no change in creatinine (average change per year, 0.00 μmol/L; 95% CI, −.01 to .01; P = .674) in patients with TDF and a slight increase (0.02 μmol/L; 95% CI, .01–.03; P < .001) in patients without TDF (P interaction = .003; Table 2). Creatinine clearance decreased in both groups (average change per year; patients with TDF, −1.8 mL/min; 95% CI, −2.6 to −1.1; P < .001; patients without TDF, −4.0; 95% CI, −5.5 to −2.6; P < .001) but with a significantly larger decrease in patients without TDF (P interaction = .007).
Table 2.
Linear Mixed Effects Model: Effect of TDF Treatment on Creatinine and Creatinine Clearance
| Unadjusted Estimates | Adjusted Estimatesa | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient b | 95% CI | P Value | Interactionc | Coefficient b | 95% CI | P Value | Interactionc | |
| Creatinine, mg/dL (n = 359) | ||||||||
| Average change per year | ||||||||
| With TDF treatment | 0.00 | .00 to .01 | .166 | .003 | 0.00 | −.01 to .01 | .674 | .003 |
| Without TDF treatment | 0.03 | .01 to.04 | <.001 | … | 0.02 | .01 to .03 | <.001 | … |
| Creatinine clearance, mL/min (n = 355) d | ||||||||
| Average change per year | ||||||||
| With TDF treatment | −2.2 | −3.0 to −1.5 | <.001 | .007 | −1.8 | −2.6 to −1.1 | <.001 | .007 |
| Without TDF treatment | −4.4 | −5.8 to −3.0 | <.001 | … | −4.0 | −5.5 to −2.6 | <.001 | … |
Abbreviation: TDF, tenofovir disoproxil fumarate.
Estimates adjusted for decompensated liver cirrhosis, age, and sex.
Marginal mean estimates extracted from the linear mixed effects model with random intercept.
P value from interaction between treatment and time, showing whether the coefficients differ between patients with and without TDF treatment.
Four missing values because of missing baseline body weight.
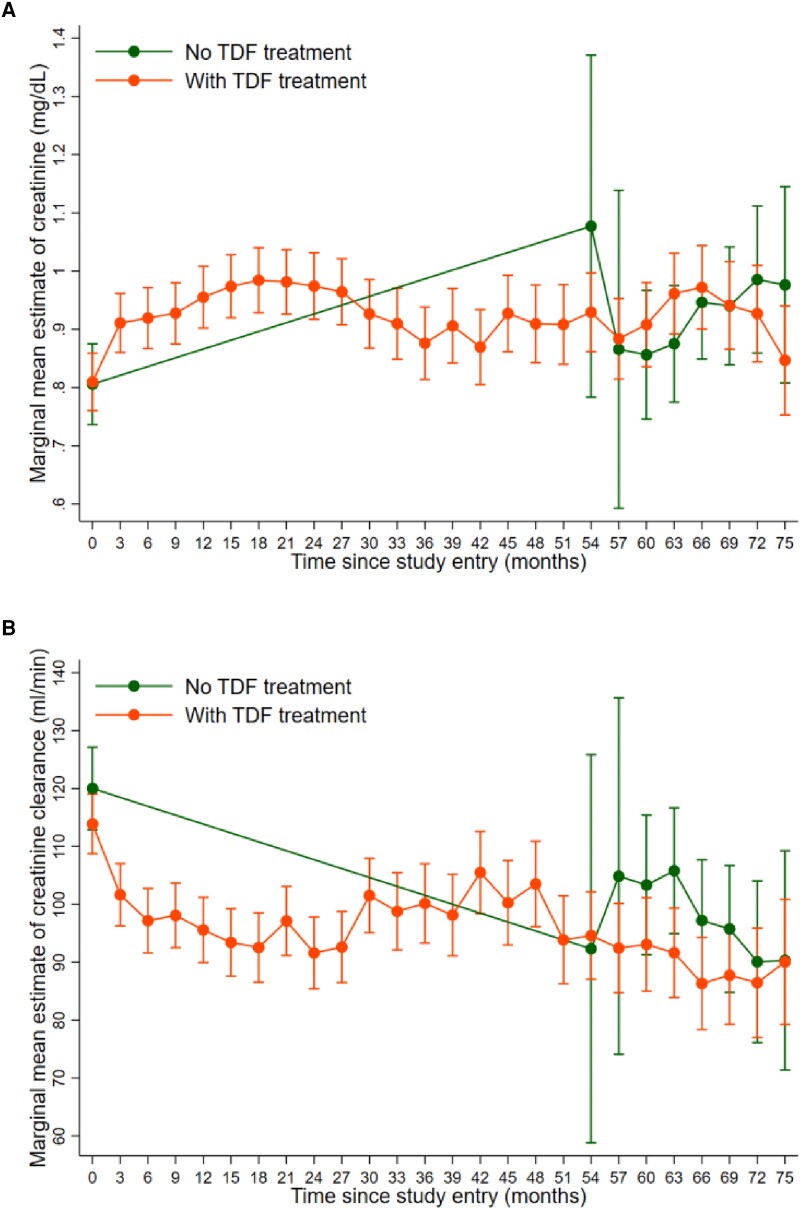
When looking at the change in patients with TDF treatment only, we found that the increase in creatinine (average change per month, 0.010; 95% CI, .006–.014; P < .001) and decrease in creatinine clearance (average change per month, −1.38; 95% CI, −1.84 to −.93; P < .001) happened within the first 12 months after treatment initiation, followed by a slight decrease in creatinine (−0.001; 95% CI, −.002 to .000; P = .006) and no change in creatinine clearance (−0.07; 95% CI, −.17 to .02; P = .143) for the remaining follow-up time (Table 3, Figure 3).
Table 3.
Linear Mixed Effects Model: Estimated Change in Creatinine and Creatinine Clearance Since Start of TDF Treatment, Years 1 vs 2–6
| Unadjusted Estimates | Adjusted Estimatesa | P Value Interactionc | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficientb | 95% CI | P Value | Coefficientb | 95% CI | P Value | Time × Sex | Time × Cirrhosis | |
| Creatinine, mg/dL (n = 233) | ||||||||
| Average change per month | ||||||||
| Overall (1–75 mo) | 0.000 | .000 to .001 | .144 | 0.000 | −.001 to .001 | .964 | .004 | .092 |
| Men | … | … | … | −0.001 | −.001 to .000 | .127 | … | … |
| Women | … | … | … | 0.001 | .001 to .002 | <.001 | … | … |
| Year 1 (1–12 mo) | 0.011 | .007 to .015 | <.001 | 0.010 | .006 to .014 | <.001 | … | … |
| Years 2–6 (13–75 mo) | −0.001 | −.002 to .000 | .045 | −0.001 | −.002 to −.000 | .006 | … | … |
| Creatinine clearance, mL/min (n = 229)d | ||||||||
| Average change per year | ||||||||
| Overall (1–75 mo) | −0.19 | −.25 to −.12 | <.001 | −0.15 | −.22 to −.09 | <.001 | .215 | .310 |
| Year 1 (1–12 mo) | −1.42 | −1.88 to −.97 | <.001 | −1.38 | −1.84 to −.93 | <.001 | … | … |
| Years 2–6 (13–75 mo) | −0.10 | −1.20 to −.01 | .032 | −0.07 | −.17 to .02 | .143 | … | … |
Interaction with sex and liver cirrhosis is described.
Abbreviations: TDF, tenofovir disoproxil fumarate.
Estimates adjusted for decompensated liver cirrhosis, age, and sex.
Marginal mean estimates extracted from the linear mixed effects model with random intercept.
P value from interaction between time and sex or cirrhosis, included in the overall and adjusted models. Stratified results are presented in case of significant interaction.
Four missing values because of missing body weight.
Figure 3.
Marginal mean values and 95% CIs of (A) creatinine and (B) creatinine clearance from baseline to month 75 in patients with and without TDF treatment. Estimates are extracted from linear mixed effects models adjusted for age, sex, and decompensated cirrhosis at baseline. TDF, tenofovir disoproxil fumarate.
There was a significant interaction between sex and change in creatinine from 1 to 75 months: while there was no change in creatinine for males during this period (P = .127), there was a significant increase for females (P < .001, P interaction = .004; Table 3, Supplementary Figure 3A). There was no significant interaction between sex and change in creatinine clearance as well as between liver cirrhosis and change in creatinine and creatinine clearance (Supplementary Figure 3B–3D).
DISCUSSION
In this study, we assessed renal function over time among TDF-treated and untreated CHB in a well-characterized cohort in Ethiopia. We found no significant change in creatinine over time in TDF-treated patients, as compared with a slight increase in untreated patients. We further found a slight decrease in creatinine clearance in both groups over time, which was less pronounced in patients undergoing TDF treatment. Notably, biochemical changes in TDF-treated patients happened in the first 12 months after treatment initiation with stable levels thereafter.
The comparison with untreated patients in our study was an important strength, as previous observational studies often reported only changes in renal markers over time in TDF-treated patients. For example, a large cohort analysis conducted in Korea among 640 people with CHB treated with TDF revealed a significant increase in creatinine and decrease in estimated glomerular filtration rate (eGFR) after 5 years [12]. In an observational study of 437 people with CHB, Buti et al found that only 1.7% had elevation of serum creatinine >0.5 mg/dL above baseline after 7 years of TDF therapy [21]. In observational studies such as these—without a control group—it is impossible to distinguish drug-induced renal toxicity from physiologic decline in renal function with aging. In our study, with a slight decline in creatinine clearance in TDF-treated and untreated patients and with a significantly steeper decline among untreated patients, we can argue that this was not related to the treatment itself. This is supported by a recent longitudinal study from the United Kingdom of 60 TDF-treated and 146 untreated people with CHB, where there was no significant difference in renal impairment after an average follow-up of 3.3 years [22].
In patients receiving TDF treatment, changes in creatinine and creatinine clearance were mainly observed during the initial 12 months after treatment initiation. Indeed, we found a significant increase in creatinine (0.12 mg/dL) and a corresponding decrease in creatinine clearance (16.6 mL/min) over the first year after treatment initiation. Over the following 5 years, however, no further deterioration was observed. This is in keeping with a recent systematic review and meta-analysis that compared the renal safety of tenofovir and entecavir (ETV) in patients with CHB [23]. The authors found that after 12 and 18 to 24 months, a greater reduction in eGFR was observed in the TDF group as compared with the ETV group, but no significant difference was seen in patients treated for >24 months. Of 16 studies included in this meta-analysis, it is worth noting that only 2 had an observation period >60 months, 4 were prospective, and none described African cohorts; thus, our study adds important data to the total body of evidence.
Studies reporting renal impairment of TDF therapy in CHB have usually used patients treated with ETV as the comparator. A consortium of 25 international centers conducted a retrospective analysis of 6189 treatment-naïve adults with CHB who initiated therapy with TDF (n = 2482) or ETV (n = 3707) to compare longitudinal changes in eGFR. Over the 10-year follow-up, TDF was associated with a higher risk of worsening renal function (adjusted hazard ratio, 1.26) as compared with ETV [24]. Of note, this was a study of Asian patients who were, on average, substantially older (50 years) than patients in our study (33 years), which makes a direct comparison difficult. Interestingly, a recent hospital-based study among people with CHB who received either TDF (n = 257) or ETV (n = 514) therapy revealed a comparable decline in eGFR in the 2 groups; however, in multivariable analysis, TDF was associated with an increased risk of renal dysfunction in individuals aged ≥60 years [25].
Our study with previous data suggests that long-term TDF therapy is safe in younger individuals, including those with cirrhosis, but other alternatives might be preferred for the elderly and for people with other risk factors for renal impairment. In our study, only 1 patient—an overweight middle-aged man with diabetes, hypertension, cardiomyopathy, alcohol misuse, and gout—discontinued TDF because of a progressive creatinine increase. This patient was under treatment with several other potentially nephrotoxic drugs, and the role of TDF in the development of renal failure was unclear.
Our study had some strengths. First, it had longer follow-up than most previous studies and a relatively large sample size. Second, the analysis was done in a prospective cohort, thus avoiding inherent biases in retrospective analyses. Third, our study was, to the best of our knowledge, the first to be carried out in sub-Saharan Africa, which is the current epicenter of the HBV epidemic. Data from sub-Saharan Africa will be key to inform elimination efforts on the continent, since African CHB cohorts are generally younger and infected with other HBV genotypes and thus might not be comparable to Asian cohorts.
Our study also had its limitations. First, we assessed glomerular function markers, such as serum creatinine level and creatinine clearance, which might underestimate TDF-associated kidney injury, as this occurs mainly through proximal tubular dysfunction. More sensitive markers of early renal dysfunction, such as serum phosphorus and urine protein, were not recorded in our study. Second, untreated patients in our study had fewer creatinine measurements than TDF-treated patients, which makes a direct comparison less robust. Third, there was some difference in clinical characteristics at baseline between the treated and untreated groups, which is inherent to observational studies of this type (ie, confounding by indication). In our study, the treated group had more advanced liver disease and would, if anything, be expected to have increased risk of renal injury, which was not observed despite exposure to TDF.
In conclusion, our study showed no significant decline in renal function among TDF-treated vs untreated people with CHB during a follow-up period of up to 75 months. Changes in creatinine and creatinine clearance among TDF-treated patients were mainly observed during the initial 12 months after treatment initiation. Our study indicates that TDF is safe in patients with HBV monoinfection for at least 6 years.
Supplementary Material
Contributor Information
Gezahegn M Woldemedihn, Department of Statistics, Hawassa University, Hawassa, Ethiopia; Oslo Centre for Biostatistics and Epidemiology, Oslo University Hospital, Oslo, Norway.
Hanna Aberra, Medical Department, St Paul's Hospital Millennium Medical College, Addis Ababa, Ethiopia.
Hailemichael Desalegn, Medical Department, St Paul's Hospital Millennium Medical College, Addis Ababa, Ethiopia; Department of Infectious Diseases, Vestfold Hospital Trust, Tønsberg, Norway.
Nega Berhe, Department of Infectious Diseases, Vestfold Hospital Trust, Tønsberg, Norway; Regional Centre for Imported and Tropical Diseases, Oslo University Hospital–Ullevål, Oslo, Norway; Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia.
Denekew Bitew Belay, Department of Statistics, Bahir Dar University, Bahir Dar, Ethiopia.
Corina S Rueegg, Oslo Centre for Biostatistics and Epidemiology, Oslo University Hospital, Oslo, Norway.
Asgeir Johannessen, Department of Infectious Diseases, Vestfold Hospital Trust, Tønsberg, Norway; Regional Centre for Imported and Tropical Diseases, Oslo University Hospital–Ullevål, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Conception or design of the work: A. J., N. B., and C. S. R. Data collection: H. D. and H. A. Data analysis: G. M. W. and C. S. R. Interpretation of results: G. M. W., C. S. R., A. J., H. A., D. B. B. Drafting the manuscript: G. M. W., H. A., A. J., and C. S. R. Critical revision of the manuscript: all authors. Final approval of the manuscript: all authors.
Acknowledgments. We are indebted to the patients who participated in the study. We acknowledge the staff at the liver clinic at St Paul's Hospital Millennium Medical College and the laboratory staff at Aklilu Lemma Institute of Pathobiology for their dedication and hard work. We thank the Norwegian Research Council, the South-Eastern Norway Regional Health Authority, and the NORHED project at Hawassa University for financial support.
Financial support . This work was supported by the Norwegian Research Council (grant 220622/H10) and the South-Eastern Norway Regional Health Authority (grant 2011068). Antiviral drugs were donated by Gilead Sciences, Inc. The sponsors had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- 1. World Health Organization . Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization, 2015. [PubMed] [Google Scholar]
- 2. Stanaway JD, Flaxman AD, Naghavi M, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet 2016; 388:1081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim WR, Loomba R, Berg T, et al. Impact of long-term tenofovir disoproxil fumarate on incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Cancer 2015; 121:3631–8. [DOI] [PubMed] [Google Scholar]
- 4. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet 2013; 381:468–75. [DOI] [PubMed] [Google Scholar]
- 5. Gallant JE, Deresinski S. Tenofovir disoproxil fumarate. Clin Infect Dis 2003; 37:944–50. [DOI] [PubMed] [Google Scholar]
- 6. Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin Pharmacokinet 2004; 43:595–612. [DOI] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver . Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67:372–91. [DOI] [PubMed] [Google Scholar]
- 8. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016; 10:1–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Terrault NA, Bzowej NH, Chang KM, et al. AASLD Guidelines for treatment of chronic hepatitis B. Hepatology 2016; 63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jung WJ, Jang JY, Park WY, et al. Effect of tenofovir on renal function in patients with chronic hepatitis B. Medicine (Baltimore) 2018; 97:e9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chon YE, Park SY, Kim SU, et al. Long-term renal safety between patients with chronic hepatitis B receiving tenofovir vs entecavir therapy: a multicenter study. J Viral Hepat 2022; 29:289–96. [DOI] [PubMed] [Google Scholar]
- 12. Lim TS, Lee JS, Kim BK, et al. An observational study on long-term renal outcome in patients with chronic hepatitis B treated with tenofovir disoproxil fumarate. J Viral Hepat 2020; 27:316–22. [DOI] [PubMed] [Google Scholar]
- 13. Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol 2013; 24:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee HY, Oh H, Park CH, Yeo YH, Nguyen MH, Jun DW. Comparison of renal safety of tenofovir and entecavir in patients with chronic hepatitis B: systematic review with meta-analysis. World J Gastroenterol 2019; 25:2961–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goicoechea M, Liu S, Best B, et al. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J Infect Dis 2008; 197:102–8. [DOI] [PubMed] [Google Scholar]
- 16. Sise ME, Hirsch JS, Canetta PA, Herlitz L, Mohan S. Nonalbumin proteinuria predominates in biopsy-proven tenofovir nephrotoxicity. AIDS 2015; 29:941–6. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Global progress report on HIV, viral hepatitis and sexually transmitted infections. Geneva: World Health Organization, 2021. [Google Scholar]
- 18. Desalegn H, Aberra H, Berhe N, et al. Treatment of chronic hepatitis B in sub-Saharan Africa: 1-year results of a pilot program in Ethiopia. BMC Med 2018; 16:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aberra H, Desalegn H, Berhe N, et al. Early experiences from one of the first treatment programs for chronic hepatitis B in sub-Saharan Africa. BMC Infect Dis 2017; 17:438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16:31–41. [DOI] [PubMed] [Google Scholar]
- 21. Buti M, Tsai N, Petersen J, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci 2015; 60:1457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang T, Smith DA, Campbell C, et al. Hepatitis B virus (HBV) viral load, liver and renal function in adults treated with tenofovir disoproxil fumarate (TDF) vs untreated: a retrospective longitudinal UK cohort study. BMC Infect Dis 2021; 21:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yang X, Yan H, Zhang X, Qin X, Guo P. Comparison of renal safety and bone mineral density of tenofovir and entecavir in patients with chronic hepatitis B: a systematic review and meta-analysis. Int J Infect Dis 2022; 124:133–42. [DOI] [PubMed] [Google Scholar]
- 24. Mak LY, Hoang J, Jun DW, et al. Longitudinal renal changes in chronic hepatitis B patients treated with entecavir versus TDF: a REAL-B study. Hepatol Int 2022; 16:48–58. [DOI] [PubMed] [Google Scholar]
- 25. Tsai HJ, Chuang YW, Yang SS, Chang YZ, Chang HR, Lee TY. Evaluating the renal safety of tenofovir disoproxil fumarate in hepatitis B patients without chronic kidney disease. J Viral Hepat 2021; 28:1579–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.