Abstract
Foragers use several senses to locate food, and many animals rely on vision and smell. It is beneficial not to rely on a single sense, which might fail under certain conditions. We examined the contribution of vision and smell to foraging and maze exploration under laboratory conditions using Cataglyphis desert ants as a model. Foraging intensity, measured as the number of workers entering the maze and arriving at the target as well as target arrival time, were greater when food, blue light, or both were offered or presented in contrast to a control. Workers trained to forage for a combined food and light cue elevated their foraging intensity with experience. However, foraging intensity was not higher when using both cues simultaneously than in either one of the two alone. Following training, we split between the two cues and moved either the food or the blue light to the opposite maze corner. This manipulation impaired foraging success by either leading to fewer workers arriving at the target cell (when the light stayed and the food was moved) or to more workers arriving at the opposite target cell, empty of food (when the food stayed and the light was moved). This result indicates that ant workers use both senses when foraging for food and readily associate light with food.
Keywords: binary-tree maze, desert ants, diet choice, foraging, sensual modality, spatial learning
Foraging is a fundamental activity because animals depend on food for their survival and reproduction. Foraging is also a dangerous, and time- and energy-consuming activity (Fewell 1988; Brown and Kotler 2004), forcing efficiency. Foraging comprises many components that could be improved with experiences, such as food handling, tool use, optimal diet choice, and navigation to and from food patches (Hughes 1979; Dyer 1996; Punzo 2005; Truskanov and Lotem 2017). Animals use many sensory modalities to locate food and/or avoid predation, such as visual, auditory, olfactory, and tactile ones (Müller and Wehner 2007; Roth et al. 2008; Heimbauer et al. 2012; Schneider et al. 2014). Across the Animalia kingdom, olfactory cues are especially useful when visual ones are not available or yet unlearned. For instance, naïve Trigona bees did not show a preference for color-coded feeders but showed a clear preference for odor-marked ones (Villa and Weiss 1990).
Animals can choose between several sensory-based navigation strategies or use them simultaneously to reduce navigation and foraging errors (Collett and Graham 2004; Cunningham et al. 2009; Buehlmann et al. 2020). Sensory modalities also play a role in learning. Information is usually learned better when several sensory modalities are involved in the learning process (van Swinderen and Greenspan 2003; Martuzzi et al. 2007; Alpert et al. 2008; Shams and Seitz 2008). This is especially beneficial to central place foragers, such as ants and bees, which need not only to learn to navigate to food patches that are often only temporarily available (Krakauer and Rodriguez-Girones 1995) but also to maximize efficiency in bringing food back to their nest (Giraldeau and Kramer 1982; Traniello 1989; Weier and Feener 1995). For example, visual cues in honeybees can be learned but olfactory cues are learned even faster and are more resilient to interference (Lindauer 1971; Robacker and Ambrose 1979). In ants, olfactory cues are acquired easily and are resilient to extinction (Piqueret et al. 2019). Lasius niger ants, for instance, can learn olfactory cues after one trial, while more trials are necessary when using spatial cues (Oberhauser et al. 2019).
Cataglyphis species are solitary foragers, not relying on pheromone trails, and not recruiting other workers while foraging. Being an individual forager requires locating food sources independently over long distances in a changing environment and bringing them along to the nest. Desert ants are capable of foraging in convoluted routes for scattered food and return to the nest via the shortest route (Wolf and Wehner 2000; Sommer et al. 2008; Wystrach et al. 2011; Mangan and Webb 2012). This process is called path integration, home-vectoring, or dead-reckoning (Wolf and Wehner 2000; Wehner and Srinivasan 2003; Webb 2019). The ants use an array of mechanisms to achieve this. Visual cues, mostly the sun position, wind direction, the earth’s geomagnetic field, and light polarization are used for navigation over long distances while olfactory cues help to find food over shorter ones (Müller and Wehner 2007; Bregy et al. 2008; Chu et al. 2008; Steck et al. 2009a, 2011; Fleischmann et al. 2018). After a food patch has been located, “counting steps,” exploitation of self-induced optic flow, and adding vectors, are used for path integration (Ronacher and Wehner 1995; Collett and Collett 2000; Cheng and Wehner 2002; Wittlinger et al. 2006). Finally, when reaching the vicinity of the nest, olfactory cues are again utilized to locate it (Wolf and Wehner 2000; Steck et al. 2010, 2011), or other means, such as “systematic search”—a search pattern based on loops intended to find the nest next to its approximate position (Merkle et al. 2006; Schultheiss and Cheng 2011). In short, although desert ants often rely on vision while foraging and navigating (e.g., Zollikofer et al. 1995; Schwarz et al. 2011), they also use olfactory cues when foraging and locating the nest on their way back (Wolf and Wehner 2000; Cardé and Willis 2008; Wolf 2011).
Little research, however, has been done to discover which of the two senses, the visual or olfactory sense, is dominant while foraging in C. niger (but see Steck et al. 2011 for a study on the combination of visual and olfactory cues in a congeneric species). This is important to understand the foraging efficiency of this and similar species. We have examined: (a) if a simultaneous exposure to two different sensory cues (visual and olfactory) facilitates faster spatial learning, and (b) the effects of separating these two cues after learning in order to tease out the relative influence of each. To do so, we performed three experiments using two maze types. Generally, mazes have been proved useful in studies of ant behavior and especially foraging, learning, and navigation (e.g., Jaffé et al. 1990; Chameron et al. 1998; Czaczkes et al. 2013; Saar et al. 2017).
In the first experiment, a baseline was established by testing the ant workers’ preference in two basic situations in a two-choice maze: Smell emitting food (fish liver) in the target cell vs. an empty dish with no food, and visible light (blue led) in the target cell vs. the regular light in the laboratory (white neon light, external to the maze). In the second experiment, a complex maze was used to record further aspects of foraging behavior. Workers were trained to solve the maze in four scenarios: Food only, light only, combined cues (food and blue light), and empty maze (no food, laboratory white light). In the third experiment, all workers were trained to solve a complex maze with combined cues (food and blue light) at its end over three runs. During the fourth run, the workers’ performance was tested when the light remained in the original position and the food was moved to the opposite corner, and when the food remained in the original position and the light moved to the opposite corner. This was done to tease out the dominant sensory modality after acquiring a combined cue.
We expected: (1) workers to prefer food over an empty dish but no blue light over neon white light, if blue light is not associated with food presence (experiment 1); (2) the combined food and blue light treatment to lead to higher foraging intensity than any of the other treatments (experiment 2); and (3) the workers to reach faster and in higher numbers the treatment in which both food and blue light remain in the original maze cell than the other treatments, in which either the food or the blue light were moved to the opposite maze corner (experiment 3).
Materials and Methods
Ant collection and maintenance
We collected in total 61 Cataglyphis niger colonies from 2/2021 to 2/2022 from Tel-Baruch sand dunes (32.132°N, 34.788°E), which is a small natural habitat in north-west Tel Aviv (see Saar et al. 2018 for further information regarding its flora and fauna). Colonies were kept in the laboratory at ~28 °C and 12:12 L:D conditions (Figure 1). Within seven days after collection, colonies were split into sub-colonies for each one of the three experiments. Neither queens nor brood were included in the newly formed sub-colonies. We kept each sub-colony in an artificial nest of 20 × 20 × 5 cm for a 7-day-acclimation period before starting each experiment, during which the ants had free access to water in glass tubes sealed with cotton wool, but not to food.
Figure 1.
Schemes of the nest and experimental arena. (A) The nest (N) and the maze, where the experiment itself took place, were connected by a corridor with holes (circles) enabling the workers to move through. The maze comprises three levels. The cells in the two first levels split into two cells in the next level (a binary tree maze). The target (food, blue light, or both; T) was placed on the extreme right or left. The opposite-target cell (OT) is at the other extreme corner. (B) In experiment 1, we allowed access only to the first two levels of the maze. (C) C. niger workers attending pupae under semi-natural conditions.
Experimental arena
All experiments were conducted in the laboratory in a modular experimental arena of 50 × 20 × 5 cm (l × w × h) made of transparent Plexiglas. The arena was divided into 3 parts (Figure 1A): (a) Nest (20 × 20 cm); (b) corridor (20 × 5 cm), which connects the nest and the maze; and (c) the maze area (25 × 20 cm). The maze comprised 15 cells in 4 levels. At each level, the number of cells is doubled, as in a binary-tree maze (Figure 1A; similar to Saar et al. 2017). The 3 arena parts as well as the maze cells were linked with round holes (1 cm in diameter), which could be open or close.
Experiment 1: basic food and light preferences
The first experiment was limited to the first and second maze levels (Figure 1B), whereas the two last maze levels were not accessible. First, we examined if the ant workers preferred blue led light (Cree 5 mm super focus; hereafter, “blue light”) to the regular laboratory white neon light, external to the maze (hereafter, “white light”). Some ant species are known to prefer some colors or learn specific colors and associate them with food (Riabinina et al. 2011; Carbaugh et al. 2020). Other studies on ants searching in mazes used similar light sources (Jaffé et al. 1990; Bernadou and Heinze 2013). We were interested not in blue light per se but rather aimed at choosing some light color, which is sufficiently different from the laboratory white Neon light. We placed the blue light in one cell of the maze and nothing on the opposite side. We documented the number of ant workers entering the blue-lit cell or the white-lit cell. This procedure was repeated 12 times with different groups of ~20 ant workers (20.5 ± 1.0; mean ± 1 SD). Second, we examined whether the workers preferred smell-emitting food over no food (empty Eppendorf lead) using the same design. As food, we used 0.25 mg (±0.13; 1 SD) canned fish-liver (Royal fish, Grindavik, Iceland) placed in an Eppendorf lead. The procedure was repeated 10 times with groups of 20–30 ant workers (26.2 ± 4.9; mean ± 1 SD). Each test began when the doors connecting the nest to the corridor and maze were opened, and lasted 20 min., during which we counted the number of workers entering each maze cell. The target cell was switched between the right and left between tests.
Statistical analysis
: The number of workers entering each of the two cells in each test was compared using a Wilcoxon Signed-Rank Test.
Experiment 2: assessing the role of sensory modalities in maze solving
We split 13 colonies into 4 groups of ~33 (32.9 ± 2.5; mean ± 1 SD) workers each. Splitting colonies in this species result in worker groups that search readily for food (Gilad et al. 2022) and are common in other systems of social insects (Schmid-Hempel and Schmid-Hempel 1998; Modlmeier et al. 2014). Each group was trained to solve an identical 15-cell maze, with a different cue at its end: (a) fish liver combined with blue light, (b) only fish liver, (c) only blue light, and (d) a control, an empty dish, no food, and white light. The training was comprised of 3 runs with 30 min rest intervals between successive runs (similar to Saar et al. 2017; Bega et al. 2020; Gilad et al. 2022). Each run started by opening the doors connecting the nest part to the corridor and the maze to allow workers to enter. Each run was concluded 10 min after the arrival of the first worker at the target cell (the food, light, or empty dish), or if no worker solved the maze—after 50 min. We did our best to standardize the experiment and to conduct all replicates of the experiments as similarly as possible. As the light source and mazes are identical, and as the food quantity is very similar across replications, the smell and light should reach similar parts of the maze in all replications. We recorded three variables during the first and third run: (a) the number of workers that entered the maze (maze entries); (b) the time from the experiment beginning until the first worker arrived at the target cell (target-arrival time); and (c) and the number of workers reaching the target cell (target arrivals).
Statistical analysis
: Maze entries, target arrivals, and target-arrival times were inter-correlated (|r| > 0.335). Therefore, and to reduce the number of statistical tests, we conducted first a principal component analysis (PCA) on the three variables. The first principal component (PC1) explained 58.3% of the variance and was the only axis with an eigenvalue larger than 1 (λ = 1.748). PC1 represented a trade-off between target-arrival time, loading negatively (−0.6394) and maze entries and target arrivals, loading positively (0.4213 and 0.6431). In other words, higher PC1 values indicate that more workers entered the maze, reached the target, and did so faster. Then, we used a mixed linear model with the colony as the random variable, treatment (a–d) and run (1 and 3) as the fixed variables, and PC1 as the response variable.
Experiment 3: assessing the roles of light and smell after training
We split 15 colonies into groups of ~37 ant workers (37.1 ± 4.5; mean ± 1 SD). Each group was trained for three successive runs to solve an identical maze with combined food and blue light at its end. This design enabled examining the change in foraging behavior with successive runs, as all treatments during the first three runs were identical. At the end of the training, in the last and fourth run, the workers were tested in one of four different scenarios: (a) both food and blue light remained in their original position; (b) food stayed in the same place but the blue light was moved to the opposite corner of the maze; (c) the blue light stayed in the same place but the food was moved to the opposite corner; and (d) the blue light stayed in its original position and the food was removed completely. We recorded maze entries, target-arrival times, and target arrivals in the first, third, and fourth runs. In the fourth run, we also recorded the number of workers arriving at the opposite side of the maze (opposite-target arrivals). In treatments a and d, there was an empty dish on that side, whereas in treatments b and c the blue light and food moved there, respectively.
Statistical analysis
: Maze entries, target arrivals, and target-arrival times were inter-correlated (|r| > 0.528). We conducted therefore a principal component analysis (PCA) on the three variables in the first and third run. The first principal component (PC1) explained 75.2% of the variance and was the only axis with an eigenvalue larger than 1 (λ = 2.257). PC1 represented a tradeoff between target-arrival time, loading negatively (−0.5601) and maze entries and target arrivals, loading positively (0.5574 and 0.6128). Then, we used a mixed linear model with the colony as the random variable, run (1 and 3) as the fixed variable, and PC1 as the response variable. The treatments came into play in the fourth run. Here, we focused on worker arrivals and used two separate linear mixed models, both with colony and treatment as the random and fixed explanatory variables. Target arrivals were used as the response variable in the first test and opposite-target arrivals were used in the second. Both were log10-transformed because they deviated from a normal distribution (skewed to the right).
Results
Experiment 1: basic food and light preferences
There was no difference between the number of workers entering the blue-lit cell and the white-lit cell (Z = −1.065, P = 0.287, N = 12). However, the number of workers entering the food cell was more than four times larger than the entries to the empty cell (Z = −2.393, P = 0.017, N = 10; Figure 2), suggesting that they can smell the food from a distance.
Figure 2.
Experiment 1: The number of workers entering the cell, which contained the food or blue light (Treatment) vs. an empty dish or white light (Control). Whereas more workers entered the cell containing food than the control, there was no attraction to blue light in this experiment. Medians, quartiles, the range, and all data points are presented.
Experiment 2: assessing the role of sensory modalities in maze solving
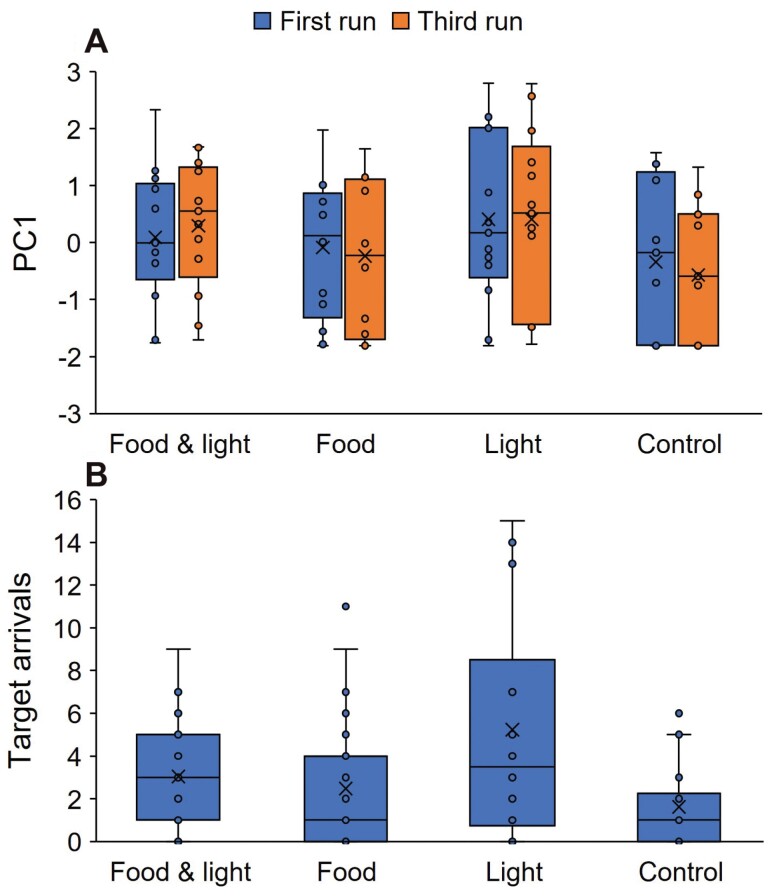
PC1 was higher in the combined food and light treatment than in the control (t = −2.250, P = 0.027; Figure 3) indicating a higher foraging intensity in the former case. However, there was no difference between the combined food and light treatment and either the food only treatment or the blue light only treatment (t = −1.178, P = 0.242 and t = 0.750, P = 0.455, respectively). There was also no difference in PC1 between the first and third runs (t = −0.187, P = 0.852).
Figure 3.
Experiment 2: (A) PC1, which stands for foraging intensity (higher values indicate more workers entering the maze and arriving at the target cell and doing so faster). There was no difference between the first and third runs. Foraging intensity on food and light was greater than the control (empty dish). (B) The number of worker arrivals at the target cell in the four different treatments (both runs are lumped together). Medians, quartiles, the range, and all datapoints are presented.
Experiment 3: assessing the roles of light and smell after training
PC1 was higher in the third run than in the first run (t = 4.045, P < 0.001; Figure 4A,B) indicating that more workers entered the maze in the third run and that workers reached the target faster and in higher numbers. Regarding the fourth run, more workers arrived at the target in the combined food and blue light treatment than when the blue light stayed and food was moved to the opposite corner (t = −2.031, P = 0.049; Figure 4C). In parallel, more workers arrived at the opposite corner when the blue light was moved there and food stayed in the original cell than in the combined food and blue light treatment (t = 3.890, P < 0.001; Figure 4C). As the results in this part of the experiment are based only on worker arrivals, which are relatively low in number, we might have underestimated some of the differences among treatments.
Figure 4.
Experiment 3: (A) PC1 (higher values indicate more workers entering the maze and arriving at the target cell and doing so faster) was higher in the third run than in the first run, indicating an intensifying level of foraging. (B) The same pattern, presented by worker arrivals at the target cell. (C) The effect of moving either the food or the blue light from the target cell to the opposite target cell in the fourth run. When the blue light was moved, more workers arrived at the opposite target cell, whereas when food was moved fewer workers arrived at the target cell. Medians, quartiles, the range, and all data points are presented. In three of the four treatments in (C) the median of the opposite-target arrivals is zero (and hence the orange column is absent).
Discussion
We examined how the two main sensory modalities, vision, and smell, affect foraging in desert ants under laboratory conditions. In the first experiment, we demonstrate that workers are attracted to food compared to an empty dish, indicating they can smell the food from a distance (Figure 2). In parallel, workers are not attracted to blue light per se, when given a choice between blue and white light (Figure 2). In the second experiment, however, maze entries and target arrivals were higher in the combined food and light treatment only compared to the control, from which both food and blue light were absent (Figure 3). In other words, blue light alone led to foraging intensity comparable to both food alone and food and blue light combined, which somewhat contradicts the results of the first experiment as well as our prediction. In the third experiment, we demonstrated first that foraging intensifies between the first and third runs (Figure 4a,b). More importantly, shifting either the food or blue light to the opposite cell led to confusion or impaired foraging performance (Figure 4c). When the food remained and the blue light was moved the number of workers arriving at the opposite cell was as high as the number of workers arriving at the original target. When the blue light remained and the food moved fewer workers arrived at the original target cell than in the control. The three experiments demonstrate the complex interaction of food smell and light as cues indicating the presence of food and workers are probably assisted by both while foraging.
The first experiment, where ant workers were given a choice between food and an empty dish and between blue light and white light was an important preliminary test. It demonstrated that the ant workers can smell the food and approach it in higher numbers. Although Cataglyphis spp. rely on vision while foraging, they also use smell when foraging or searching for their nest (Wolf and Wehner 2000; Steck et al. 2009b; Buehlmann et al. 2014). As desert ants often feed on dead arthropods that are scattered over large areas, their smell probably contributes to their efficient discovery, at least in the last stages of the search (Buehlmann et al. 2015). Furthermore, such ants can associate a specific odor with food and be later attracted to a similar odor (Huber and Knaden 2018). That said, desert ants tested in mazes in the laboratory can detect food and learn the way leading towards it without the smell (Bega et al. 2020). In the first experiment, the ant workers demonstrated no preference for blue light per se, when it was not associated with the food reward. While some animals possess innate preferences for specific colors (Franks et al. 1964; Carbaugh et al. 2020), it seems not to hold true here.
The second experiment does not fully agree with the first experiment, as blue light triggered intense foraging, similar to food presence (or food and blue light combined). Despite the discrepancy between experiments 1 and 2 regarding the attraction to blue light without food, experiment 3 demonstrates that the ants learned to associate food and blue light and reached the cell to which the light was moved in the fourth run (see below). Similarly, Steck et al. (2011) demonstrate that the congeneric C. fortis can use both visual and olfactory cues and use each cue in isolation as efficiently as both combined. The only significant result of the second experiment was the lower foraging intensity in the control treatment when only an empty plate was offered. That said, the second experiment differed from the first one in at least three important aspects: (1) a whole maze was used and not only two cells; (2) the experiment comprised three successive runs rather than only one; and (3) we measured foraging intensity (i.e., maze entries, target arrival times, and target arrivals) rather than choice.
Animals often explore their nearby habitat without obtaining an immediate reward (Hughes 1997; Gómez-Laplaza and Gerlai 2010). Such exploration may assist animals to orient better in their habitat, and recognize specific dangers, or they can be driven by mere “curiosity” (Renner 1988; Wang and Hayden 2021). Furthermore, exploration may be more necessary in a complex habitat than in a simple one or more relevant when encountering novel objects than familiar ones (Mettke-Hofmann et al. 2002, 2006). This perhaps explains what took place here: Blue light within a maze might be perceived as more complex than blue light close to the nest. Finally, as the maze in the second experiment was larger, it could be that vision gains more important in searching for longer distances than smell (Hattingh and Samways 1995; Tang et al. 2013).
The third experiment pointed to an increase in foraging intensity from the first to the third run: More workers entered the maze and reached the target (food and light) and did so faster. This indicated that either spatial learning took place here, an increase in search drive after exposure to food, or a mixture of both. This result is similar to previous studies with the same species (Razin et al. 2013; Bega et al. 2020; Gilad et al. 2022). In the fourth run, the food and blue light were uncoupled, as one remained in the original location whereas another was moved to the opposite corner. In both cases the worker’s ability to reach the target cell diminished. When the blue light remained and food was moved, fewer workers arrived at the target cell. When the food remained and the blue light moved, more workers reached the cell opposite the target, to which the light was moved. We therefore suggest that the workers use both senses when searching for food. We suggest that the workers relied both on the current cues (blue light and food smell) and their spatial memory of food location. Past studies demonstrated that other insects could associate specific food types with odors or colors (Banschbach 1994; Giurfa et al. 1995; Raubenheimer and Tucker 1997; Gadd and Raubenheimer 2000). We suggest that a similar association between food and blue light took place here.
A good question is why the two treatments of the food stay and light moves vs. light stays and food moves differed from one another, as moving light attracted more workers to the opposite target cell than moving food. It could be that light is perceived from a larger distance than the smell and attracts workers there or that it directs the workers to a more specific location than smell (as in other systems: Hattingh and Samways 1995; Steck et al. 2009b; Tang et al. 2013). The smell in the “wrong” location, as placed in the fourth run here, probably resulted in confusion: Fewer workers arrived at both the target and opposite target cells. Future studies should perhaps focus on this difference and search for more exact explanations. Cues in nature can vary on a temporal and spatial basis, and it is therefore important to understand how such changes affect foraging behavior. Finally, in contrast to other systems, where providing both visual and olfactory cues improved foraging intensity over providing only a single cue (Steck et al. 2011; Johannesen et al. 2012; Johnston and Martini 2020), this did not hold true here. Furthermore, we could not point to the superiority of either one of the two cues in the current design, in contrast to other studies demonstrating one cue is more influential than another (Legge et al. 2014; Wystrach et al. 2015; Barragán-Fonseca et al. 2020; Ren et al. 2020). In the congeneric C. fortis, Steck et al. (2011) demonstrated that learning the nest location using only visual or olfactory cues is slower than when relying on both. With experience, however, workers returning to the nest find it equally well when relying on only a single cue.
In summary, we demonstrated here the combined effect of visual and olfactory cues on the foraging intensity of desert ants in mazes in the laboratory and suggest that both are similarly important. Future studies should separate not only the visual and olfactory cues but also both cues and spatial memory. For example, a shift of either one of the two cues to the opposite corner should be compared not only to a control, in which both cues remain in the same place but rather to a control, in which both move together to the opposite cell. Thus, one could expect tension between spatial memory of the former food location and the combined cues pointing to a new location.
Contributor Information
Tomer Gilad, School of Zoology, George S Wise Faculty of Life Sciences, Tel Aviv University, 69978 Tel Aviv, Israel.
Ori Bahar, School of Zoology, George S Wise Faculty of Life Sciences, Tel Aviv University, 69978 Tel Aviv, Israel.
Malak Hasan, School of Zoology, George S Wise Faculty of Life Sciences, Tel Aviv University, 69978 Tel Aviv, Israel.
Adi Bar, School of Zoology, George S Wise Faculty of Life Sciences, Tel Aviv University, 69978 Tel Aviv, Israel.
Aziz Subach, School of Zoology, George S Wise Faculty of Life Sciences, Tel Aviv University, 69978 Tel Aviv, Israel.
Inon Scharf, School of Zoology, George S Wise Faculty of Life Sciences, Tel Aviv University, 69978 Tel Aviv, Israel.
Funding
We thank the German Research Foundation for funding this research project (DFG; grant no. FO 298/31-1).
Conflict of Interest
We declare no conflict of interest. No permissions were required to conduct this study.
References
- Alpert GF, Hein G, Tsai N, Naumer MJ, Knight RT, 2008. Temporal characteristics of audiovisual information processing. J Neurosci 28:5344–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banschbach VS, 1994. Colour association influences honey bee choice between sucrose concentrations. J Comp Physiol A 175:107–114. [Google Scholar]
- Barragán-Fonseca KY, Van Loon JJ, Dicke M, Lucas-Barbosa D, 2020. Use of visual and olfactory cues of flowers of two brassicaceous species by insect pollinators. Ecol Entomol 45:45–55. [Google Scholar]
- Bega D, Samocha Y, Yitzhak N, Saar M, Subach Aet al. , 2020. Non-spatial information on the presence of food elevates search intensity in ant workers, leading to faster maze solving in a process parallel to spatial learning. PLoS One 15:e0229709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernadou A, Heinze J, 2013. Mating-associated changes in the behaviour of Leptothorax gredleri ant queens. Ethology 119:634–643. [Google Scholar]
- Bregy P, Sommer S, Wehner R, 2008. Nest-mark orientation versus vector navigation in desert ants. J Exp Biol 211:1868–1873. [DOI] [PubMed] [Google Scholar]
- Brown JS, Kotler BP, 2004. Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014. [Google Scholar]
- Buehlmann C, Graham P, Hansson BS, Knaden M, 2014. Desert ants locate food by combining high sensitivity to food odors with extensive crosswind runs. Curr Biol 24:960–964. [DOI] [PubMed] [Google Scholar]
- Buehlmann C, Graham P, Hansson BS, Knaden M, 2015. Desert ants use olfactory scenes for navigation. Anim Behav 106:99–105. [Google Scholar]
- Buehlmann C, Mangan M, Graham P, 2020. Multimodal interactions in insect navigation. Anim Cogn 23:1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbaugh JR, Renthal RD, Vinson SB, Medina RF, 2020. Color discrimination and preference in the fire ant Solenopsis invicta Buren. Insect Soc 67:167–178. [Google Scholar]
- Cardé RT, Willis MA, 2008. Navigational strategies used by insects to find distant, wind-borne sources of odor. J Chem Ecol 34:854–866. [DOI] [PubMed] [Google Scholar]
- Chameron S, Schatz B, Pastergue-Ruiz I, Beugnon G, Collett TS, 1998. The learning of a sequence of visual patterns by the ant Cataglyphis cursor. Proc R Soc B 265:2309–2313. [Google Scholar]
- Cheng K, Wehner R, 2002. Navigating desert ants Cataglyphis fortis learn to alter their search patterns on their homebound journey. Physiol Entomol 27:285–290. [Google Scholar]
- Chu J, Zhao K, Zhang Q, Wang T, 2008. Construction and performance test of a novel polarization sensor for navigation. Sens Actuators A 148:75–82. [Google Scholar]
- Collett M, Collett TS, 2000. How do insects use path integration for their navigation? Biol Cybern 83:245–259. [DOI] [PubMed] [Google Scholar]
- Collett TS, Graham P, 2004. Animal navigation: Path integration, visual landmarks and cognitive maps. Curr Biol 14:R475–R477. [DOI] [PubMed] [Google Scholar]
- Cunningham SJ, Castro I, Potter MA, 2009. The relative importance of olfaction and remote touch in prey detection by North Island brown kiwis. Anim Behav 78:899–905. [Google Scholar]
- Czaczkes TJ, Grüter C, Ellis L, Wood E, Ratnieks FL, 2013. Ant foraging on complex trails: Route learning and the role of trail pheromones in Lasius niger. J Exp Biol 216:188–197. [DOI] [PubMed] [Google Scholar]
- Dyer F, 1996. Spatial memory and navigation by honeybees on the scale of the foraging range. J Exp Biol 199:147–154. [DOI] [PubMed] [Google Scholar]
- Fewell JH, 1988. Energetic and time costs of foraging in harvester ants Pogonomyrmex occidentalis. Behav Ecol Sociobiol 22:401–408. [Google Scholar]
- Fleischmann PN, Grob R, Müller VL, Wehner R, Rössler W, 2018. The geomagnetic field is a compass cue in Cataglyphis ant navigation. Curr Biol 28:1440–1444.e2. [DOI] [PubMed] [Google Scholar]
- Franks RE, Burns EC, England NC, 1964. Color preference of the horn fly Haematobia irritans on beef cattle. J Econ Entomol 57:371–372. [Google Scholar]
- Gadd CA, Raubenheimer D, 2000. Nutrient-specific learning in an omnivorous insect: The American cockroach Periplaneta americana L. learns to associate dietary protein with the odors citral and carvone. J Insect Behav 13:851–864. [Google Scholar]
- Gilad T, Dorfman A, Subach A, Libbrecht R, Foitzik Set al., 2022. Evidence for the effect of brief exposure to food, but not learning interference, on maze solving in desert ants. Integr Zool (in press). doi: 10.1111/1749-4877.12622 [DOI] [PubMed] [Google Scholar]
- Giraldeau LA, Kramer DL, 1982. The marginal value theorem: A quantitative test using load size variation in a central place forager, the eastern chipmunk Tamias striatus. Anim Behav 30:1036–1042. [Google Scholar]
- Giurfa M, Nunez J, Chittka L, Menzel R, 1995. Colour preferences of flower-naive honeybees. J Comp Physiol A 177:247–259. [Google Scholar]
- Gómez-Laplaza LM, Gerlai R, 2010. Latent learning in zebrafish Danio rerio. Behav Brain Res 208:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattingh V, Samways MJ, 1995. Visual and olfactory location of biotopes, prey patches, and individual prey by the ladybeetle Chilocorus nigritus. Entomol Exp Appl 75:87–98. [Google Scholar]
- Heimbauer LA, Antworth RL, Owren MJ, 2012. Capuchin monkeys Cebus apella use positive, but not negative, auditory cues to infer food location. Anim Cogn 15:45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R, Knaden M, 2018. Desert ants possess distinct memories for food and nest odors. Proc Nat Acad Sci 115:10470–10474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RN, 1979. Optimal diets under the energy maximization premise: The effects of recognition time and learning. Am Nat 113:209–221. [Google Scholar]
- Hughes RN, 1997. Intrinsic exploration in animals: Motives and measurement. Behav Proc 41:213–226. [DOI] [PubMed] [Google Scholar]
- Jaffé K, Ramos C, Lagalla C, Parra L, 1990. Orientation cues used by ants. Insect Soc 37:101–115. [Google Scholar]
- Johannesen A, Dunn AM, Morrell LJ, 2012. Olfactory cue use by three-spined sticklebacks foraging in turbid water: Prey detection or prey location? Anim Behav 84:151–158. [Google Scholar]
- Johnston N, Martini X, 2020. The influence of visual and olfactory cues in host selection for Bemisia tabaci Biotype B in the presence or absence of Tomato yellow leaf curl virus. Insects 11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer DC, Rodríguez-Gironés MA, 1995. Searching and learning in a random environment. J Theor Biol 177:417–429. [Google Scholar]
- Legge EL, Wystrach A, Spetch ML, Cheng K, 2014. Combining sky and earth: Desert ants Melophorus bagoti show weighted integration of celestial and terrestrial cues. J Exp Biol 217:4159–4166. [DOI] [PubMed] [Google Scholar]
- Lindauer M, 1971. Communication Among Social Bees. Cambridge, MA, USA: Harvard University Press. [Google Scholar]
- Mangan M, Webb B, 2012. Spontaneous formation of multiple routes in individual desert ants Cataglyphis velox. Behav Ecol 23:944–954. [Google Scholar]
- Martuzzi R, Murray MM, Michel CM, Thiran JP, Maeder PPet al. , 2007. Multisensory interactions within human primary cortices revealed by BOLD dynamics. Cereb Cortex 17:1672–1679. [DOI] [PubMed] [Google Scholar]
- Merkle T, Knaden M, Wehner R, 2006. Uncertainty about nest position influences systematic search strategies in desert ants. J Exp Biol 209:3545–3549. [DOI] [PubMed] [Google Scholar]
- Mettke-Hofmann C, Rowe KC, Hayden TJ, Canoine V, 2006. Effects of experience and object complexity on exploration in garden warblers Sylvia borin. J Zool 268:405–413. [Google Scholar]
- Mettke-Hofmann C, Winkler H, Leisler B, 2002. The significance of ecological factors for exploration and neophobia in parrots. Ethology 108:249–272. [Google Scholar]
- Modlmeier AP, Keiser CN, Shearer TA, Pruitt JN, 2014. Species-specific influence of group composition on collective behaviors in ants. Behav Ecol Sociobiol 68:1929–1937. [Google Scholar]
- Müller M, Wehner R, 2007. Wind and sky as compass cues in desert ant navigation. Naturwissenschaften 94:589–594. [DOI] [PubMed] [Google Scholar]
- Oberhauser FB, Schlemm A, Wendt S, Czaczkes TJ, 2019. Private information conflict: Lasius niger ants prefer olfactory cues to route memory. Anim Cogn 22:355–364. [DOI] [PubMed] [Google Scholar]
- Piqueret B, Sandoz JC, D’Ettorre P, 2019. Ants learn fast and do not forget: Associative olfactory learning, memory and extinction in Formica fusca. R Soc Open Sci 6:190778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punzo F, 2005. Experience affects hunting behavior of the wasp Pepsis mildei Stål (Hymenoptera: Pompilidae). J N Y Entomol Soc 113:222–229. [Google Scholar]
- Raubenheimer D, Tucker D, 1997. Associative learning by locusts: Pairing of visual cues with consumption of protein and carbohydrate. Anim Behav 54:1449–1459. [DOI] [PubMed] [Google Scholar]
- Razin N, Eckmann JP, Feinerman O, 2013. Desert ants achieve reliable recruitment across noisy interactions. J R Soc Interface 10:20130079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Wu S, Xing Z, Xu R, Cai Wet al. , 2020. Behavioral responses of western flower thrips Frankliniella occidentalis to visual and olfactory cues at short distances. Insects 11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner MJ, 1988. Learning during exploration: The role of behavioral topography during exploration in determining subsequent adaptive behavior in the Sprague-Dawley rat Rattus norvegicus. Int J Comp Psychol 2:43–56. [Google Scholar]
- Riabinina O, de Ibarra NH, Howard L, Collett TS, 2011. Do wood ants learn sequences of visual stimuli? J Exp Biol 214:2739–2748. [DOI] [PubMed] [Google Scholar]
- Robacker DC, Ambrose JT, 1979. Effects of number of reinforcements and interference on visual and olfactory learning modalities of the honey bee (Hymenoptera: Apidae). Ann Entomol Soc 72:775–780. [Google Scholar]
- Ronacher B, Wehner R, 1995. Desert ants Cataglyphis fortis use self-induced optic flow to measure distances travelled. J Comp Physiol A 177:21–27. [Google Scholar]
- Roth TC, Cox JG, Lima SL, 2008. Can foraging birds assess predation risk by scent? Anim Behav 76:2021–2027. [Google Scholar]
- Saar M, Gilad T, Kilon-Kallner T, Rosenfeld A, Subach Aet al. , 2017. The interplay between maze complexity, colony size, learning and memory in ants while solving a maze: A test at the colony level. PLoS One 12:e0183753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saar M, Subach A, Reato I, Liber T, Pruitt JNet al. , 2018. Consistent differences in foraging behavior in 2 sympatric harvester ant species may facilitate coexistence. Curr Zool 64:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid-Hempel R, Schmid-Hempel P, 1998. Colony performance and immunocompetence of a social insect Bombus terrestris in poor and variable environments. Funct Ecol 12:22–30. [Google Scholar]
- Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JBet al. , 2014. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc Nat Acad Sci 111:14941–14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss P, Cheng K, 2011. Finding the nest: Inbound searching behaviour in the Australian desert ant Melophorus bagoti. Anim Behav 81:1031–1038. [Google Scholar]
- Schwarz S, Narendra A, Zeil J, 2011. The properties of the visual system in the Australian desert ant Melophorus bagoti. Arthropod Struct Dev 40:128–134. [DOI] [PubMed] [Google Scholar]
- Shams L, Seitz AR, 2008. Benefits of multisensory learning. Trends Cogn Sci 12:411–417. [DOI] [PubMed] [Google Scholar]
- Sommer S, von Beeren C, Wehner R, 2008. Multiroute memories in desert ants. Proc Nat Acad Sci 105:317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck K, Hansson BS, Knaden M, 2009b. Smells like home: Desert ants Cataglyphis fortis use olfactory landmarks to pinpoint the nest. Front Zool 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck K, Hansson BS, Knaden M, 2011. Desert ants benefit from combining visual and olfactory landmarks. J Exp Biol 214:1307–1312. [DOI] [PubMed] [Google Scholar]
- Steck K, Knaden M, Hansson BS, 2010. Do desert ants smell the scenery in stereo? Anim Behav 79:939–945. [Google Scholar]
- Steck K, Wittlinger M, Wolf H, 2009a. Estimation of homing distance in desert ants Cataglyphis fortis remains unaffected by disturbance of walking behaviour. J Exp Biol 212:2893–2901. [DOI] [PubMed] [Google Scholar]
- van Swinderen B, Greenspan RJ, 2003. Salience modulates 20–30 Hz brain activity in Drosophila. Nat Neurosci 6:579–586. [DOI] [PubMed] [Google Scholar]
- Tang YC, Zhou CL, Chen XM, Zheng H, 2013. Visual and olfactory responses of seven butterfly species during foraging. J Insect Behav 26:387–401. [Google Scholar]
- Traniello JFA, 1989. Foraging strategies of ants. Annu Rev Entomol 34:191–210. [Google Scholar]
- Truskanov N, Lotem A, 2017. Trial-and-error copying of demonstrated actions reveals how fledglings learn to ‘imitate’ their mothers. Proc R Soc B 284:20162744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa JD, Weiss MR, 1990. Observations on the use of visual and olfactory cues by Trigona spp. foragers. Apidologie 21:541–545. [Google Scholar]
- Wang MZ, Hayden BY, 2021. Latent learning, cognitive maps, and curiosity. Curr Opin Behav Sci 38:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb B, 2019. The internal maps of insects. J Exp Biol 222:jeb188094. [DOI] [PubMed] [Google Scholar]
- Wehner R, Srinivasan MV, 2003. Path integration in insects. In: Jeffrey KJ, editor. The Neurobiology of Spatial Behaviour. New York, NY, USA: Oxford; University Press, 9–30. [Google Scholar]
- Weier JA, Feener DH, 1995. Foraging in the seed-harvester ant genus Pogonomyrmex: Are energy costs important? Behav Ecol Sociobiol 36:291–300. [Google Scholar]
- Wittlinger M, Wehner R, Wolf H, 2006. The ant odometer: Stepping on stilts and stumps. Science 312:1965–1967. [DOI] [PubMed] [Google Scholar]
- Wolf H, 2011. Odometry and insect navigation. J Exp Biol 214:1629–1641. [DOI] [PubMed] [Google Scholar]
- Wolf H, Wehner R, 2000. Pinpointing food sources: Olfactory and anemotactic orientation in desert ants Cataglyphis fortis. J Exp Biol 203:857–868. [DOI] [PubMed] [Google Scholar]
- Wystrach A, Mangan M, Webb B, 2015. Optimal cue integration in ants. Proc R Soc B 282:20151484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wystrach A, Schwarz S, Schultheiss P, Beugnon G, Cheng K, 2011. Views, landmarks, and routes: How do desert ants negotiate an obstacle course? J Comp Physiol A 197:167–179. [DOI] [PubMed] [Google Scholar]
- Zollikofer CPE, Wehner R, Fukushi T, 1995. Optical scaling in conspecific Cataglyphis ants. J Exp Biol 198:1638–1646. [DOI] [PubMed] [Google Scholar]