Abstract
Background and purpose:
The renin-angiotensin system activation, partial ischemia/reperfusion (IR) injury, and hypertension contribute to the development of acute kidney injury. The study aims to look at the vascular responses of angiotensin II (Ang II) during Ang II type 1 receptor (AT1R) blockade (losartan) or co-blockades of AT1R and Mas receptor (A779) in two kidneys one clip (2K1C) hypertensive rats which subjected to partial IR injury with and without ischemia preconditioning (IPC).
Experimental approach:
Thirty-three 2K1C male Wistar rats with systolic blood pressure ≥ 150 mmHg were divided into three groups of sham, IR, and IPC + IR divided into two sub-groups receiving losartan or losartan + A779. The IR group had 45 min partial kidney ischemia, while the IPC + IR group had two 5 min cycles of partial ischemia followed by 10 min of reperfusion and then 45 min of partial kidney ischemia followed by reperfusion. The sham group was subjected to similar surgical procedures except for IR or IPC.
Findings/Results:
Ang II increased mean arterial pressure in all the groups, but there were no significant differences between the sub-groups. A significant difference was observed in the renal blood flow response to Ang II between two sub-groups of sham and IR groups treated with AT1R blockade alone or co-blockades of AT1R + A779.
Conclusion and implications:
These findings demonstrated the significance of AT1R and Mas receptor following partial renal IR in the renal blood flow responses to Ang II in 2K1C hypertensive rats.
Keywords: Angiotensin II, AT1R, MasR, Renal ischemia/reperfusion, Two kidneys-one clip
INTRODUCTION
The kidney's partial ischemia/reperfusion (IR) injury is defined by the limitation of blood supply to the kidney, subsequently regaining reoxygenation and blood flow (1). Renal blood flow (RBF) variations caused by IR injury may be due to microvascular damage and impaired vascular reactivity (2,3). Ischemia preconditioning (IPC) is defined as a transient, brief, and nonlethal ischemia period followed by reperfusion used as an approach to guard the kidney against IR injury (4,5,6,7).
The renin-angiotensin system (RAS) is a paracrine hormonal system that controls blood volume and pressure, electrolyte balance, and systemic vascular resistance (8,9). RAS has two counter-regulatory pressor and depressor arms. The pressor arm comprises angiotensin II (Ang II), angiotensin-converting enzyme (ACE), and Ang II type 1 receptor (AT1R). The depressor arm includes ACE2, Ang 1-7, Ang II type 2 receptor (AT2R), and Mas receptor (MasR) (10). Ang II is the chief active peptide in RAS, which performs its functions by binding to AT1R and AT2R (11). Activation of the RAS and increasing the level of Ang II play an imperative role in IR injury (12).
In IR injury, RAS and Ang II can worsen renal functions by increasing renal vascular resistance (RVR) and reducing RBF. Therefore, inhibiting the production of Ang II and also inhibiting of increasing the RVR and decreasing the RBF caused by Ang II improve the kidney damage caused by IR. Losartan as an AT1R antagonist increases RBF of the ischemic kidney after IR injury (13,14,15,16), possibly by decreasing RVR. Ang 1-7 is another effector peptide of the RAS. Unlike Ang II, which has harmful effects on renal circulation, it can increase RBF and decrease RVR by binding to the MasR, thus protecting the kidney against IR injury (17,18).
Hypertension is common in patients with end-stage renal disease and chronic kidney disease (19). Systemic and local RAS activation through Ang II is essential in initiating and preserving hypertension in two-kidney-one-clip (2K1C) renovascular hypertensive (20,21). 2K1C hypertension and IR injury alter the expression of enzymes (ACE2, ACE, and renin) and receptors of the renal RAS (MasR, AT1R, and AT2R) (13,20,22). The expression of AT1R decreases and AT2R expression increases after IR injury (23,24). In the 2K1C hypertensive rats, the expression of AT1R increases, and AT2R expression decreases in the kidneys' medulla as a result of the increased ratio of AT1R/AT2R.
In addition, 2K1C hypertensive decreases intrarenal MasR expression (25), while IR injury increases MasR expression (26). In Mas-deficient animals, the RBF and RVR decrease and increase, respectively (27). Also, in 2K1C hypertensive rats, MasR blockade increased the response of RBF and RVR to Ang II infusion after partial IR injury. In other words, the block of MasR promotes renal hemodynamic response to Ang II after partial IR injury in 2K1C rats (28). The interaction between the RAS receptors also is documented; MasR can interact with AT1R and AT2R and changes the renal hemodynamic responses to Ang II injection (29,30).
Accordingly, because of the variation of RAS components in IR and 2K1C renovascular hypertensive and the interaction between RAS receptors, we assumed that AT1R and MasR can change renal vascular responses to Ang II infusion in hypertensive rats that suffered from IR injury without and with IPC. To test this theory, 4 weeks after induction of 2K1C hypertension, anesthetized animals were exposed to IR with and without IPC, and vascular responses to Ang II infusion were determined during AT1R blockade or co-blockade of AT1R and MasR.
MATERIALS AND METHODS
Animals
This research used male Wistar rats (n = 33, 246 ± 5 g, 7-8 weeks). The animals were received from an animal lab at the Water and Electrolyte Research Center, Isfahan University of Medical Sciences. The animals were housed in polyacrylic cages and kept under conventional laboratory conditions (23 ± 2 °C temperature, 12/12-h light/dark cycle, and free access to food and water). Isfahan University of Medical Sciences Ethics Committee authorized the experimental procedure for this study (Ethical code: IR.MUI.REC.1397.345).
Surgical procedures
Induction of the 2K1C hypertensive model
After anesthetizing the rats with ketamine (60 mg/kg, intraperitoneally) and xylazine (5 mg/kg, intraperitoneally), a 5-cm longitudinal incision was performed on the right side of the flank to expose the right kidney. Then, the right renal artery was isolated, and 2K1C was induced by placing a silver U-shaped clip (0.2 mm inner diameter) around it (31). The clip was closed so that RBF is not completely cut off and partial RBF is maintained (32,33) (Fig. 1). Finally, the operated animals were housed in cages with standard temperature conditions and free access to water and food for 28 days to recover and increase blood pressure.
Fig. 1.

Induction of the 2K1C hypertensive model. (A) The right renal artery was isolated, and (B) two kidneys one clip was induced by placing a silver U-shaped clip (0.2 mm inner diameter) around it.
Catheterization and measurements
On the day of the experiment; 28 days following clipping and 2K1C renovascular hypertension induction, the rats were anesthetized with urethane (1.7 g/kg, intraperitoneally; Merck, Germany). After tracheal catheterization to facilitate airway and oxygenation during the experiment, the left carotid artery was isolated and catheterized using a polyethylene catheter (Microtube Extrusions Pty Ltd, Australia). Also, the left jugular vein and femoral artery were catheterized (Fig. 2). To collect urine flow during the experiment, a polyethylene catheter was also placed into the bladder (28).
Fig. 2.
Twenty eight days following clipping and two kidneys one clip renovascular hypertension induction, the rats were anesthetized with urethane. (A) After tracheal catheterization, (B) the left carotid artery was isolated and catheterized using a polyethylene catheter. Also, (C) the left jugular vein and (D) femoral artery and bladder were catheterized.
In the next step, the animal was positioned laterally. After making a transverse incision with an electronic surgical cutter, the left kidney was separated from the surrounding tissues and put in the kidney cup. To measure RBF, the renal artery was isolated, and an ultrasonic flow probe (TRANSONIC MAO.7 PSB, Flowprobe, USA) with a diameter of 0.7 mm connected to a flow meter (T402, Transonic Systems Inc., Ithaca, NY 14850 USA) was placed around it. The abdominal aorta also was isolated at the place between the mesenteric and renal arteries, followed by placing an adjustable occluder around it to regulate renal perfusion pressure (RPP) throughout Ang II administration and induction of partial ischemia (28) (Fig. 3).
Fig. 3.

(A-C) The left kidney was isolated and put in the kidney cup. (D) The abdominal aorta was isolated, followed by placing an adjustable occluder around it to regulate renal perfusion pressure throughout angiotensin II administration and induction of partial ischemia. (E) To measure renal blood flow, the renal artery was isolated and (F) an ultrasonic flow probe with a diameter of 0.7 mm connected to a flow meter was placed around it.
Finally, the catheterized femoral and carotid arteries were connected to a Powerlab System (AD Instruments, Australia) to measure RPP, systolic blood pressure, and MAP, respectively. The catheterized jugular vein was linked to the injection pump (New Era Pump System Inc., Farmingdale, NY, USA) for drug administration. Besides, the RBF was measured by the flowmeter and renal vascular resistance was calculated by the RPP/RBF ratio (28).
Experimental protocol
The animals were divided into three main experimental groups: sham, IR, and IPC + IR, and each group was divided into two sub-groups that received losartan alone or a combination of losartan and A779. After 30 min and reaching stable condition (equilibrium phase), the animals with systolic blood pressure ≥ 150 mmHg were considered hypertensive animals. The recorded values for MAP, RPP, RBF, and RVR were regarded as basement data collected during the final 5 min of the equilibrium phase.
The experiment was continued based on the kind of experimental group. The rats in the sham group were subjected to the surgical process without IPC or IR. In the IR group, partial IR was induced by keeping RPP at the range of 25 ± 3 mmHg for 45 min by clamping the abdominal aortic, and after partial IR, reperfusion was permitted by opening the clamp. The IPC group had two 5-min episodes of partial ischemia, two 10-mins episodes of reperfusion, and then 45 min of partial IR (28). The MAP, RPP, RBF, and RVR were recorded 10 min after the beginning of reperfusion (Fig. 4). Animal surgery was performed on a surgical warming plate; thus, the body temperature of the animals was controlled during the experiment.
Fig. 4.
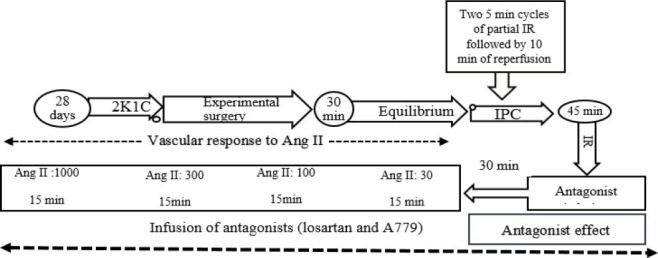
Protocol of study. 2K1C, Two kidney one- clip; IR, ischemia-reperfusion; IPC, ischemia preconditioning.
Antagonist infusion
At 10 min following the start of reperfusion, the AT1R antagonist (losartan: Aburaihan Pharmaceutical Company, Iran) or MasR antagonists (A779) + losartan were given using a microsyringe infusion pump (New Era Pump System Inc., Farmingdale, NY, USA), and 30 min after antagonist infusion, the results for MAP, RPP, RBF, and RVR were measured in the last 3 to 5 min of injection, which were considered an “antagonist effect”. Losartan was infused using a microsyringe infusion pump with a bolus dosage of 5 mg/kg and a continuous infusion rate of 5 mg/kg/h, whereas A779 (Bachem Bioscience Inc., King of Prussia, Pennsylvania, USA) was administered using a bolus dose of 50 μg/kg and a continuous infusion rate of 50 μg/kg/h.
Ang II infusion
In all experimental groups, the vascular responses to graded Ang II (Sigma, St. Louis, MI, USA) administration were determined while the antagonists were still being infused. Each Ang II dosage was administered for 15 min at various levels of 100, 300, and 1000 ng/kg/min. MAP, RPP, and RBF were measured during the final 3-5 min of each Ang II dosage to determine the vascular response to Ang II infusion.
During Ang II injection, RPP was kept in the range before Ang II injection or in the range recorded in the antagonist phase. Finally, an anesthetic overdose of urethane (approximately five times the usual anesthetic dosage; Merck, Germany) was administered to the animals using a left jugular vein catheter.
Statistical analysis
The data are reported as mean ± SEM, and the analysis was carried out using SPSS version 22 software. The MAP, RPP, RBF, and RVR in the basement and antagonist phases of the sham, IR, and IPC + IR groups receiving losartan and losartan + A779 were compared using a one-way ANOVA followed by the LSD post hoc test. Responses to graded Ang II infusion were analyzed by ANOVA for repeated measures followed by the LSD post hoc test. P values ≤ 0.05 for the effect of graded Ang II (Pdose), the comparisons between groups (Pgroup), and the interaction between treatment and groups (Pdose × group) were considered significant.
RESULTS
Measurements for the Basement
No significant differences in MAP (mmHg), RPP (mmHg), RBF (RBF/g LKW, mL/min/g tissue), and RVR (RVR/g LKW, mL/mmHg.min. g tissue) were seen in the basement data (prior to the administration of antagonists) in the sham, IR, and IPC + IR groups in 2K1C renovascular hypertensive rats when AT1R was blocked with losartan alone or in combination with MasR blocked with A779 (losartan + A779) (Table 1).
Table 1.
Basement data. MAP (mmHg), RPP (mmHg), RBF (gLKW, mL/min/g tissue) and RVR (gLKW, mL/mmHg.min.g tissue) in three groups of sham, IR, and IPC + IR in hypertensive rats when AT1R alone and both AT1R and MasR were blocked. No significant difference in the parameters was detected between the groups.
| Groups | AT1R blockade rats | Co-blockade of AT1R and MasR rats | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| n | MAP | RPP | RBF/gLKW | RVR/gLKW | n | MAP | RPP | RBF/gLKW | RVR/gLKW | |
| Sham | 5 | 130.1 ± 6.7 | 123.5 ± 6.5 | 2.37 ± 0.30 | 57.0 ± 9.2 | 7 | 134.3 ± 3.5 | 126.1 ± 3.6 | 2.02 ± 0.30 | 70.9 ± 11.1 |
| IR | 6 | 136.9 ± 7.2 | 129.9 ± 9.2 | 2.53 ± 0.40 | 58.3 ± 9.8 | 4 | 127.9 ± 1.4 | 122.4 ± 4.5 | 2.96 ± 0.60 | 46.9 ± 10.5 |
| IPC + IR | 5 | 132.1 ± 3.1 | 122.4 ± 3.8 | 2.29 ± 0.20 | 54.9 ± 4.7 | 6 | 133.9 ± 7.0 | 124.4 ± 9.9 | 2.83 ± 0.30 | 46.3 ± 5.7 |
| P-values | 0.72 | 0.72 | 0.86 | 0.96 | 0.68 | 0.93 | 0.13 | 0.14 | ||
MAP, Mean arterial pressure; RPP, renal perfusion pressure; RBF, renal blood flow; gLKW, gram left of kidney weight; RVR, renal vascular resistance; IR, ischemia/reperfusion; IPC, ischemia preconditioning; AT1R, angiotensin type 1 receptor; MasR, Mas receptor.
Effect of antagonist
AT1R blockade or co-blockade of AT1R and MasR decreased MAP, RPP, and RVR and increased RBF in all three experimental groups. However, there were no significant differences in the values reported for MAP, RPP, RBF, and RVR between the two subgroups in the sham, IR, and IPC + IR groups (Fig. 5).
Fig. 5.
The effect of antagonist. MAP, RPP, RBF per g kidney weight, and RVR were evaluated 30 min post losartan and losartan + A779 infusion. The P-values were obtained using ANOVA for repeated measures. MAP, Mean arterial pressure; RPP, renal perfusion pressure; RBF, renal blood flow; RVR, renal vascular resistance.
The vascular response to Ang II infusion
The intravenous infusion of graded Ang II elevated MAP in a dose-related manner in all the sub-groups. However, no significant differences were detected between the two sub-groups of each group of sham, IR, or IPC + IR Fig. 6.
Fig. 6.
The effect of graded angiotensin II administration. The percentage change of mean MAP, RPP, RBF, and RVR was evaluated after graded angiotensin II infusion. The P-values were obtained using ANOVA for repeated measures. MAP, Mean arterial pressure; RPP, renal perfusion pressure; RBF, renal blood flow; RVR, renal vascular resistance.
As previously stated, RPP was kept constant at the basal level by an aortic clamp during Ang II administration. As a result, no change in RPP percentage change by Ang II injection was seen between subgroups as expected (Fig. 6).
The percentage change of RBF response to graded Ang II infusion in all the groups significantly increased. However, this response was significantly different between the two sub-groups in the sham and the IR groups, such a difference was not observed in the IPC + IR group. For example, in the sham group, Ang II infusion at a dose of 1000 ng/kg/min increased RBF from the baseline to 16.7 ± 3.17% and 38.76 ± 3.12% in losartan and A779 + losartan sub-groups, respectively (Fig. 6). In addition, in IR group, Ang II infusion at dose 1000 ng/kg/min increased RBF from the baseline to 31.62 ± 3.34%, 19.17 ± 3.83 % in losartan and A779 + losartan sub-groups, respectively (Fig. 6).
The percentage change of RVR response to graded Ang II infusion in all the groups decreased significantly. However, this response was not significantly different between the two sub-groups of all groups (Fig. 6).
DISCUSSION
In the current study, we observed that in the sham group, the RBF response to graded Ang II infusion increased in the sub-groups of losartan and losartan + A779 but, this response was greater in the losartan + A779 sub-group.
There are two types of AT1R including AT1A and AT1B in rodents (34). AT1B is mostly expressed in endocrine organs like the pituitary and adrenal, whereas AT1A is primarily expressed in the cardiovascular system (35). AT1A is important in the development of 2K1C hypertension, as knockout mice do not develop hypertension (36). An acute dose of candesartan, as an AT1R antagonist, increased RBF in the nonclipped kidney of 2K1C (33,37). Due to the high levels of renin and Ang II in 2K1C hypertensive, endogenous Ang II has more accessible sites than exogenous Ang II (33,37,38). Internalization of AT1R and upregulation of nitric oxide (NO) in 2K1C hypertension play a significant role in the reduced RBF response to Ang II. Inhibition of AT1R with losartan in normotensive and 2K1C hypertensive rats increased RBF, which confirms our study (39,40).
In addition, MasR deficiency (Mas-/-) causes changes in renal hemodynamics response, including a decrease in RBF (27,41). In the non-clipped kidney of 2K1C hypertensive rats, blockage of MasR by 7-D-Ala-Ang 1-7 or an ACE2 inhibitor exacerbated the hypertension course and reduced RBF (42). These findings imply the vasodilatory and reno-protective effects of Ang 1-7 in the 2K1C hypertensive by attenuating the vasoconstrictor effects of increased intrarenal Ang II levels.
In the current study during the AT1R + MasR co-blockade (40), the only receptor available is AT2R which Ang II increased RBF by its effect. Losartan raised RBF in the renal wrap hypertension in both the contralateral and wrapped kidneys (43), whereas PD123319 (AT2R antagonist) lowered RBF exclusively in the contralateral kidneys. The decrease in RBF in response to Ang II was amplified by AT2R blockage (44).
The greater response of RBF to Ang II in the sham group treated with losartan + A779 is unknown, but evidences suggest that in hypertensive individuals, MasR and AT2R form a heterodimer with AT1R, reducing AT1R signaling. It is possible that the co-blockade of AT1R and MasR results in the heterodimer formation only between AT1R and AT2R, while the blockade of AT1R causes heterodimer formation between AT2R and MasR with AT1R, as a result, the inhibition of vasoconstrictor AT1R signal is stronger.
The other findings showed the response of increasing RBF and decreasing RVR to Ang II in the renal partial IR group treated with losartan was greater than in the renal partial IR group treated with losartan + A779. There is evidence that RAS components are affected by hypertension and IR injury (20,45). The RAS contributes significantly to the pathophysiology of ischemia-induced kidney damage (46), and the balance of its two axes changes by IR. It has been shown that 60 and 45 min of ischemia and 24 h of reperfusion increase and decrease the levels of Ang II and Ang 1-7 in the ischemic kidney, respectively (47,48). In the renal IR injury, Ang II's vasoconstrictor effects decline which may be because receptors decrease in the vessels and tubules of the ischemic kidney (48). It has been reported that the expression of AT1R decreases in rats with hypertension and ischemic injury (48,49). Furthermore, the expression of vasodilatory receptors (AT2R and MasR) of RAS increases in different parts of the kidney ischemic (26,47). Probably, these variations in the expression of the receptor are the compensatory mechanism to protect kidneys from ischemia (50).
The stimulation of AT2R counteracts the vasoconstrictive functions of AT1R via increasing the formation of NO, kinins (bradykinin, kallikrein), and guanosine cyclic 3‘5’-monophosphate (cGMP) (51). Also, different studies using AT2R antagonists and agonists have shown the role of AT2R in protecting the kidney against IR injury (24,52). As a result, the balance between the AT1R and AT2R is crucial for controlling kidney function (50).
The wide distribution of MasR in the kidney indicates the importance of its role in kidney function (53,54). So, induction of IR in animals Mas-/-causes hemodynamic changes; decreased RBF and increased RVR and activation of the MasR reduced the response caused by AT1R (53). In addition, MasR expression decreases in the clipped kidneys of 2K1C hypertensive rats and A779 limits the AT1R-mediated cellular response (55).
In 2K1C hypertensive rats, the RBF% changes in response to Ang II increased in the presence of losartan. This observation suggests the activation of AT1R by the internal ligand of Ang II leads to vasoconstriction, and as a result, the reduction of RBF. By inhibiting AT1R the vasoconstrictive effect of Ang II was inhibited through this receptor, and as a result, RBF increased.
In our study, in 2K1C hypertensive rats under IR, AT1R blockade increased the RBF response to Ang II when compared with the AT1R + MasR blockade, but in the sham group, the increased RBF response was less in the losartan sub-group compared to losartan + A779 sub-group. This disparity might be attributed to IR and its impact on the renal vascular system.
Inhibition of the vasoconstrictive effect of AT1R by losartan, and reduction of AT1R expression in both 2K1C hypertensive and IR injury make Ang II unable to exert its vasoconstrictive effect through AT1R. In other words, Ang II acts through the vasodilator AT2R which increases both in IR and in 2K1C and causes an increase in RBF. The increase in NO production due to IR is considered a compromise mechanism to maintain RBF and protects the kidney against ischemia damage. Therefore, it is possible that increasing NO is effective in increasing RBF in hypertensive rats. Another finding showed that administration of losartan or losartan + A779 in 2K1C hypertensive rats that underwent IPC had no significant effect on the responses of RBF and RVR to Ang II injection. The vascular response from this study can be affected by three items, including RAS components, hypertension, and IR.
The useful effects of IPC have been reported in the kidneys (56,57,58,59). IPC helps to restore RBF after renal IR injury (60). In contrast, others reported no protective effect of IPC against IR (61). In addition, a single cycle of IPC induction did not have a protective effect against renal IR. It indicates that the resistance of IPC kidneys to IR injury is associated with the change in expression level and localization of Ang II/AT1R. In contrast to losartan, Ang II treatment in IPC mice increased morphological damage, oxidative stress, and inflammatory responses, along with functional impairment (62). The cardioprotective benefits of IPC against I/R injury were improved when losartan was co-administered with IPC (63).
The benefits of raising the expression of endothelial NO synthase (eNOS), inducible NOS (iNOS), and NO levels in IPC kidneys 24 h after reperfusion were eliminated by L-NAME administration (60). IPC contributes to the restoration of RBF most likely via NO generation by eNOS/iNOS (64). In the present research, AT1R blockade or AT1R + MasR blockade in 2K1C hypertensive rats under IPC increased and decreased RBF and RVR responses to Ang II in a non-significant manner. In the current study, for IPC induction, the renal artery was not fully occluded, and it consisted of two cycles of partial ischemia. Therefore, the two cycles of brief partial ischemia in IPC may not be sufficient to protect the kidney against prolonged partial ischemia.
Finally, using the data from this study during kidney hypoperfusion may be useful. For example, after coronary artery bypass surgery, the kidney is exposed to hypoperfusion, while hypertension itself is one of the causes of acute kidney injury after surgery. There are numerous challenges for therapeutic approaches using RAS inhibitors, including angiotensin receptor blockers and ACE inhibitors to decrease kidney problems after coronary artery bypass surgery, and these approaches may enhance the RAS axes' significance. Thus, it appears that therapeutic management of the RAS axes activity can be used to lessen ischemic consequences during hypoperfusion.
CONCLUSION
The percentage change of RBF and RVR responses to graded Ang II infusion increased and decreased, respectively in sub-groups of sham, IR, and IPC + IR groups treated with AT1R or AT1R + A779 blockade. With AT1R blockade, the increased RBF to Ang II in the IR group was greater than in the sham group. This difference in responses may be connected to the kidney's altered vascular function following partial IR. These findings demonstrated the significance of AT1R and MasR following partial renal IR in the RBF and RVR responses to Ang II in 2K1C hypertensive rats.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contributions
M. Nematbakhsh and F. Karimi were involved in the conception and design. Animal experiments and data collection were performed by F. Karimi. M. Nematbakhsh and F. Karimi analyszed and interpreted the data. F. Karimi prepared for the drafting of the paper, M. Nematbakhsh revised the paper critically for intellectual content. The finalized article was approved by all authors.
REFERENCES
- 1.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4(2):20–27. doi: 10.12861/jrip.2015.06. DOI: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Regner KR, Roman RJ. Role of medullary blood flow in the pathogenesis of renal ischemia-reperfusion injury. Curr Opin Nephrol Hypertens. 2012;21(1):33–38. doi: 10.1097/MNH.0b013e32834d085a. DOI: 10.1097/MNH.0b013e32834d085a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karimi F, Kasaei S, Baradaran A, Ashrafi F, Talebi A, Lak Z, et al. Dextrose hydration may promote cisplatin-induced nephrotoxicity in rats: gender-related difference. Indones Biomed J. 2019;11(2):136–144. DOI: 10.18585/inabj.v11i2.502. [Google Scholar]
- 4.Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: from myth to a novel therapeutic option? J Am Soc Nephrol. 2014;25(2):216–224. doi: 10.1681/ASN.2013070708. DOI: 0.1681/ASN.2013070708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veighey K, MacAllister R. Clinical applications of remote ischaemic preconditioning in native and transplant acute kidney injury. Pediatr Nephrol. 2015;30(10):1749–1759. doi: 10.1007/s00467-014-2965-6. DOI: 10.1007/s00467-014-2965-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi HS, Hwang JK, Kim JG, Hwang HS, Lee SJ, Chang YK, et al. The optimal duration of ischemic preconditioning for renal ischemia-reperfusion injury in mice. Ann Surg Treat Res. 2017;93(4):209–216. doi: 10.4174/astr.2017.93.4.209. DOI: 10.4174/astr.2017.93.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granfeldt A, Lefer DJ, Vinten-Johansen J. Protective ischaemia in patients: preconditioning and postconditioning. Cardiovasc Res. 2009;83(2):234–246. doi: 10.1093/cvr/cvp129. DOI: 10.1093/cvr/cvp129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takimoto-Ohnishi E, Murakami K. Renin-angiotensin system research: from molecules to the whole body. J Physiol Sci. 2019;69(4):581–587. doi: 10.1007/s12576-019-00679-4. DOI: 10.1007/s12576-019-00679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saberi S, Dehghani A, Nematbakhsh M. Role of Mas receptor in renal blood flow response to angiotensin-(1-7) in ovariectomized estradiol treated rats. Res Pharm Sci. 2016;11(1):65–72. PMID: 27051434. [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma N, Anders HJ, Gaikwad AB. Fiend and friend in the renin angiotensin system: an insight on acute kidney injury. Biomed Pharmacother. 2019;110:764–774. doi: 10.1016/j.biopha.2018.12.018. DOI: 10.1016/j.biopha.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 11.Ramalingam L, Menikdiwela K, LeMieux M, Dufour JM, Kaur G, Kalupahana N, et al. The renin angiotensin system, oxidative stress and mitochondrial function in obesity and insulin resistance. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1106–1114. doi: 10.1016/j.bbadis.2016.07.019. DOI: 10.1016/j.bbadis.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez-Lara SQ, García-Benavides L, Miranda-Díaz AG. The renin-angiotensin-aldosterone system as a therapeutic target in late injury caused by ischemia-reperfusion. Int J Endocrinol. 2018;2018:3614303,1–19. doi: 10.1155/2018/3614303. DOI: 10.1155/2018/3614303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srisawat U, Kongrat S, Muanprasat C, Chatsudthipong V. Losartan and sodium nitroprusside effectively protect against renal impairments after ischemia and reperfusion in rats. Biol Pharm Bull. 2015;38(5):753–762. doi: 10.1248/bpb.b14-00860. DOI: 10.1248/bpb.b14-00860. [DOI] [PubMed] [Google Scholar]
- 14.Franzén S, Frithiof R. Pre-treatment with the angiotensin receptor 1 blocker losartan protects renal blood flow and oxygen delivery after propofol-induced hypotension in pigs. Sci Rep. 2020;10(1):17924,1–10. doi: 10.1038/s41598-020-74640-6. DOI: 10.1038/s41598-020-74640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori H, Mori H, Masaki T, Nishiyama A. Angiotensin II blockade and renal protection. Curr Pharm Des. 2013;19(17):3033–3042. doi: 10.2174/1381612811319170009. DOI: 10.2174/1381612811319170009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maleki M, Nematbakhsh M. Mas receptor antagonist (A799) alters the renal hemodynamics responses to angiotensin II administration after renal moderate ischemia/reperfusion in rats: gender related differences. Res Pharm Sci. 2019;14(1):12. doi: 10.4103/1735-5362.251848. DOI: 10.4103/1735-5362.251848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nematbakhsh M. Renoprotective impact of angiotensin 1-7: is it certain? J. Nephropathol. 2019;(8)(1):e01,1–6. DOI: 10.15171/jnp.2019.01. [Google Scholar]
- 18.Saberi S, Dehghani A, Nematbakhsh M. Angiotensin 1-7 administration increases renal blood flow in the absence of bradykinin B2 receptor in ovariectomized estradiol treated rats: the role of mas receptor. Acta Medica Iranica. 2019;57(2):103–109. DOI: 10.18502/acta.v57i2.1764. [Google Scholar]
- 19.Bansal N, McCulloch CE, Rahman M, Kusek JW, Anderson AH, Xie D, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: the chronic renal insufficiency cohort study. Hypertension. 2015;65(1):93–100. doi: 10.1161/HYPERTENSIONAHA.114.04334. DOI: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YG, Lee SH, Kim SY, Lee A, Moon JY, Jeong KH, et al. Sequential activation of the intrarenal renin-angiotensin system in the progression of hypertensive nephropathy in Goldblatt rats. Am J Physiol Renal Physiol. 2016;311(1):F195–F206. doi: 10.1152/ajprenal.00001.2015. DOI: 10.1152/ajprenal.00001.2015. [DOI] [PubMed] [Google Scholar]
- 21.Li XC, Zhu D, Zheng X, Zhang J, Zhuo JL. Intratubular and intracellular renin-angiotensin system in the kidney: a unifying perspective in blood pressure control. Clin Sci (Lond) 2018;132(13):1383–401. doi: 10.1042/CS20180121. DOI: 10.1042/CS20180121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang F, Liu GC, Zhou X, Yang S, Reich HN, Williams V, et al. Loss of ACE2 exacerbates murine renal ischemia-reperfusion injury. PloS One. 2013;8(8):e71433,1–17. doi: 10.1371/journal.pone.0071433. DOI: 10.1371/journal.pone.0071433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaschina E, Namsolleck P, Unger T. AT2 receptors in cardiovascular and renal diseases. Pharmacol Res. 2017;125(Pt A):39–47. doi: 10.1016/j.phrs.2017.07.008. DOI: 10.1016/j.phrs.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Ali R, Patel S, Hussain T. Angiotensin type 2 receptor activation limits kidney injury during the early phase and induces Treg cells during the late phase of renal ischemia. Am J Physiol Renal Physiol. 2021;320(5):F814–F825. doi: 10.1152/ajprenal.00507.2020. DOI: 10.1152/ajprenal.00507.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Wang S, Zhao J, Yu C, Hu Y, Tu Y, et al. Naringenin ameliorates renovascular hypertensive renal damage by normalizing the balance of renin-angiotensin system components in rats. Int J Med Sci. 2019;16(5):644–653. doi: 10.7150/ijms.31075. DOI: 10.7150/ijms.31075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silveira KD, Pompermayer Bosco KS, Diniz LR, Carmona AK, Cassali GD, Bruna-Romero O, et al. ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin Sci (Lond) 2010;119(9):385–394. doi: 10.1042/CS20090554. DOI: 10.1042/CS20090554. [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro SV, Ferreira AJ, Kitten GT, Da Silveira KD, Da Silva DA, Santos SH, et al. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kidney Int. 2009;75(11):1184–1193. doi: 10.1038/ki.2009.61. DOI: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 28.Karimi F, Nematbakhsh M. Mas receptor blockade promotes renal vascular response to Ang II after partial kidney ischemia/reperfusion in a two-kidney-one-clip hypertensive rats model. Int J Nephrol. 2021;2021:6618061,1–8. doi: 10.1155/2021/6618061. DOI: 10.1155/2021/6618061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansoori A, Oryan S, Nematbakhsh M. Role of Mas receptor antagonist (A779) in renal hemodynamics in condition of blocked angiotensin II receptors in rats. Physiol Int. 2016;103(1):13–20. doi: 10.1556/036.103.2016.1.2. DOI: 10.1556/036.103.2016.1.2. [DOI] [PubMed] [Google Scholar]
- 30.Safari T, Nematbakhsh M, Hilliard LM, Evans RG, Denton KM. Sex differences in the renal vascular response to angiotensin II involves the M as receptor. Acta Physiol (Oxf) 2012;206(2):150–156. doi: 10.1111/j.1748-1716.2012.02468.x. DOI: 10.1111/j.1748-1716.2012.02468.x. [DOI] [PubMed] [Google Scholar]
- 31.Pereira TdMC, Balarini CdM, Silva IV, Cabral A, Vasquez E, Meyrelles S. Endogenous angiotensin II modulates nNOS expression in renovascular hypertension. Braz J Med Biol Res. 2009;42(7):685–691. doi: 10.1590/s0100-879x2009000700014. DOI: 10.1590/s0100-879x2009000700014. [DOI] [PubMed] [Google Scholar]
- 32.Thongsepee N, Mahabusarakam W, Hiranyachattada S. Diuretic and hypotensive effect of morelloflavone from Garcinia dulcis in two-kidneys-one-clip (2K1C) hypertensive rat. Sains Malaysiana. 2017;46(9):1479–1490. DOI: 10.17576/jsm-2017-4609-17. [Google Scholar]
- 33.Bivol LM, Vågnes OB, Iversen BM. The renal vascular response to ANG II injection is reduced in the nonclipped kidney of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol. 2005;289(2):F393–F400. doi: 10.1152/ajprenal.00319.2004. DOI: 10.1152/ajprenal.00319.2004. [DOI] [PubMed] [Google Scholar]
- 34.Dasgupta C, Zhang L. Angiotensin II receptors and drug discovery in cardiovascular disease. Drug Discov Today. 2011;16(1-2):22–34. doi: 10.1016/j.drudis.2010.11.016. DOI: 10.1016/j.drudis.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Premer C, Lamondin C, Mitzey A, Speth RC, Brownfield MS. Immunohistochemical localization of, and angiotensin II receptor subtypes in the rat adrenal, pituitary, and brain with a perspective commentary. Int J Hypertens. 2013;2013:175428,1–23. doi: 10.1155/2013/175428. DOI: 10.1155/2013/175428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Červenka L, Vaněčková I, Husková Z, Vaňurková Z, Erbanová M, Thumová M, et al. Pivotal role of AT1A receptors in the development of two-kidney, one-clip hypertension: study in AT1A receptor knockout mice. J Hypertens. 2008;26(7):1379–1389. doi: 10.1097/HJH.0b013e3282fe6eaa. DOI: 10.1097/HJH.0b013e3282fe6eaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Červenka L, Horáček V, Vaněčková I, Hubáček JA, Oliverio MI, Coffman TM, et al. Essential role of AT1A receptor in the development of 2K1C hypertension. Hypertension. 2002;40(5):735–741. doi: 10.1161/01.hyp.0000036452.28493.74. DOI: 10.1161/01.hyp.0000036452.28493.74. [DOI] [PubMed] [Google Scholar]
- 38.da Silva GM, da Silva MC, Nascimento DVG, Lima Silva EM, Gouvêa FFF, de França Lopes LG, et al. Nitric oxide as a central molecule in hypertension: focus on the vasorelaxant activity of new nitric oxide donors. Biology (Basel) 2021;10(10):1041. doi: 10.3390/biology10101041. DOI: 10.3390/biology1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navar LG, Ichihara A, Chin SY, Imig JD. Nitric oxide-angiotensin II interactions in angiotensin II-dependent hypertension. Acta physiologica scandinavica. 2000;168(1):139–147. doi: 10.1046/j.1365-201x.2000.00630.x. DOI: 10.1046/j.1365-201x.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 40.Choopani S, Nematbakhsh M. Estradiol supplement or induced hypertension may attenuate the angiotensin II type 1 receptor antagonist-promoted renal blood flow response to graded angiotensin II administration in ovariectomized rats. J Renin Angiotensin Aldosterone Syst. 2022;2022:3223008. doi: 10.1155/2022/3223008. DOI: 10.1155/2022/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1-7) on isolated rabbit afferent arterioles. Hypertension. 2002;39(3):799–802. doi: 10.1161/hy0302.104673. DOI: 10.1161/hy0302.104673. [DOI] [PubMed] [Google Scholar]
- 42.Bürgelová M, Kramer HJ, Teplan V, Thumová M, Červenka L. Effects of angiotensin-(1-7) blockade on renal function in rats with enhanced intrarenal Ang II activity. Kidney Int. 2005;67(4):1453–1461. doi: 10.1111/j.1523-1755.2005.00222.x. DOI: 10.1111/j.1523-1755.2005.00222.x. [DOI] [PubMed] [Google Scholar]
- 43.Siragy HM, Carey RM. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension. 1999;33(5):1237–1242. doi: 10.1161/01.hyp.33.5.1237. DOI: 10.1161/01.hyp.33.5.1237. [DOI] [PubMed] [Google Scholar]
- 44.Brown RD, Hilliard LM, Mirabito KM, Firth LC, Moritz KM, Evans RG, et al. Reduced sensitivity of the renal vasculature to angiotensin II in young rats: the role of the angiotensin type 2 receptor. Pediatr Res. 2014;76(5):448–452. doi: 10.1038/pr.2014.121. DOI: 10.1038/pr.2014.121. [DOI] [PubMed] [Google Scholar]
- 45.Lee SH, Lee YH, Jung SW, Kim DJ, Park SH, Song SJ, et al. Sex-related differences in the intratubular renin-angiotensin system in two-kidney, one-clip hypertensive rats. Am J Physiol Renal Physiol. 2019;317(3):F670–F82. doi: 10.1152/ajprenal.00451.2018. DOI: 10.1152/ajprenal.00451.2018. [DOI] [PubMed] [Google Scholar]
- 46.Chou YH, Chu TS, Lin SL. Role of renin-angiotensin system in acute kidney injury-chronic kidney disease transition. Nephrology (Carlton) 2018;23:121–125. doi: 10.1111/nep.13467. DOI: 10.1111/nep.13467. [DOI] [PubMed] [Google Scholar]
- 47.Kontogiannis J, Burns KD. Role of AT1 angiotensin II receptors in renal ischemic injury. Am J Physiol. 1998;274(1):F79–F90. doi: 10.1152/ajprenal.1998.274.1.F79. DOI: 10.1152/ajprenal.1998.274.1.F79. [DOI] [PubMed] [Google Scholar]
- 48.Allred AJ, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol. 2000;279(4):F636–F45. doi: 10.1152/ajprenal.2000.279.4.F636. DOI: 10.1152/ajprenal.2000.279.4.F636. [DOI] [PubMed] [Google Scholar]
- 49.Amiri F, Garcia R. Renal angiotensin II receptor regulation in two-kidney, one clip hypertensive rats: effect of ACE inhibition. Hypertension. 1997;30(3):337–344. doi: 10.1161/01.hyp.30.3.337. DOI: 10.1161/01.hyp.30.3.337. [DOI] [PubMed] [Google Scholar]
- 50.Matavelli LC, Siragy HM. AT2 receptor activities and pathophysiological implications. J Cardiovasc Pharmacol. 2015;65(3):226–232. doi: 10.1097/FJC.0000000000000208. DOI: 10.1097/FJC.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matavelli LC, Huang J, Siragy HM. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension. 2011;57(2):308–313. doi: 10.1161/HYPERTENSIONAHA.110.164202. DOI: 10.1161/HYPERTENSIONAHA.110.164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samimiat A, Khosravi MS, Hassanshahi J, Nematbakhsh M. The effect of AT2 and Mas receptors antagonists on renal hemodynamic and excretory disorders induced by ischemia/reperfusion in male and female rats. Physiol Pharmacol. 2018;22(2):133–140. [Google Scholar]
- 53.Zimmerman D, Burns KD. Angiotensin-(1-7) in kidney disease: a review of the controversies. Clin Sci (Lond) 2012;123(6):333–346. doi: 10.1042/CS20120111. DOI: 10.1042/CS20120111. [DOI] [PubMed] [Google Scholar]
- 54.Santos RA, Ferreira AJ, Verano-Braga T, Bader M. Angiotensin-converting enzyme 2, angiotensin-(1-7) and Mas: new players of the renin-angiotensin system. J Endocrinol. 2013;216(2):R1–R17. doi: 10.1530/JOE-12-0341. DOI: 10.1530/JOE-12-0341. [DOI] [PubMed] [Google Scholar]
- 55.Alawi LF. Role of Angiotensin II Type 1A Receptors on Renal and Urinary Angiotensin Converting Enzyme 2 (ACE2) and Neprilysin (NEP) in the Two-Kidney One-Clip (2K1C) Model of Renovascular Hypertension. Wright State University. 2015. pp. 1–92. Available from: https://corescholar.libraries.wright. edu/etd_all/2031.
- 56.Jang H-S, Kim J, Kim KY, Kim JI, Cho MH, Park KM. Previous ischemia and reperfusion injury results in resistance of the kidney against subsequent ischemia and reperfusion insult in mice; a role for the Akt signal pathway. Nephrol Dial Transplant. 2012;27(10):3762–3770. doi: 10.1093/ndt/gfs097. DOI: 10.1093/ndt/gfs097. [DOI] [PubMed] [Google Scholar]
- 57.Jang HS, Kim J, Park YK, Park KM. Infiltrated macrophages contribute to recovery after ischemic injury but not to ischemic preconditioning in kidneys. Transplantation. 2008;85(3):447–455. doi: 10.1097/TP.0b013e318160f0d1. DOI: 10.1097/TP.0b013e318160f0d1. [DOI] [PubMed] [Google Scholar]
- 58.Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PloS One. 2012;7(2):e32296,1–10. doi: 10.1371/journal.pone.0032296. DOI: 10.1371/journal.pone.0032296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Livingston MJ, Wang J, Zhou J, Wu G, Ganley IG, Hill JA, et al. Clearance of damaged mitochondria via mitophagy is important to the protective effect of ischemic preconditioning in kidneys. Autophagy. 2019;15(12):2142–2162. doi: 10.1080/15548627.2019.1615822. DOI: 10.1080/15548627.2019.1615822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ge YZ, Wu R, Xin H, Liu H, Lu TZ, Zhao YC, et al. Effects of ischemic preconditioning on the systemic and renal hemodynamic changes in renal ischemia reperfusion injury. Int J Clin Exp Pathol. 2015;8(2):1128–1140. PMID: 25972999. [PMC free article] [PubMed] [Google Scholar]
- 61.Samadi M, Tabibian F, Moradzadeh K, Nassiri SM, Gheisari Y. Evaluating the effect of remote ischemic preconditioning on kidney ischemia-reperfusion injury. J Res Med Sci. 2020;25:6. doi: 10.4103/jrms.JRMS_249_19. DOI: 10.4103/jrms.JRMS_249_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang HS, Kim JI, Kim J, Park JW, Park KM. Angiotensin II removes kidney resistance conferred by ischemic preconditioning. Biomed Res Int. 2014;2014:602149,1–10. doi: 10.1155/2014/602149. DOI: 10.1155/2014/602149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ES ED, Hassan A, Salem S, Fadil S, Taha A. Cardioprotective effect of losartan alone or in combination with remote ischemic preconditioning on the biochemical changes induced by ischemic/reperfusion injury in a mutual prospective study with a clinical and experimental animal arm. Heart Res Open J. 2017;4(3):57–65. doi: 10.1016/j.ijcard.2016.07.178. DOI: 10.17140/HROJ-4-142. [DOI] [PubMed] [Google Scholar]
- 64.Cervenka L, Wang C-T, Mitchell KD, Navar LG. Proximal tubular angiotensin II levels and renal functional responses to AT1 receptor blockade in nonclipped kidneys of Goldblatt hypertensive rats. Hypertension. 1999;33(1):102–107. doi: 10.1161/01.hyp.33.1.102. DOI: 10.1161/01.hyp.33.1.102. [DOI] [PubMed] [Google Scholar]





