Abstract
Apoptosis contributes to the loss of CD4 cells during human immunodeficiency virus type 1 (HIV-1) infection. Although the product of the env gene, gp160/gp120, is known to play a role in cell death mediated by HIV-1, the role of other HIV-1 genes in the process is unclear. We found that HIV-1 lacking the env gene (HIVΔenv) still induced apoptosis in T-cell lines and primary CD4 T cells. The ability to induce apoptosis was attributable to Tat, a viral regulatory protein. Tat induction of apoptosis was separate from the transactivation function of Tat, required expression of the second exon of Tat, and was associated with the increased expression and activity of caspase-8 (casp-8), a signaling molecule in apoptotic pathways. Moreover, induction of apoptosis could be prevented by treating cells with an inhibitor of casp-8. In addition, we show that HIV-1Δenv infection and Tat expression increased the sensitivity of cells to Fas-mediated apoptosis, an apoptotic pathway that signals via casp-8. The up-regulation of casp-8 by HIV-1 Tat expression may contribute to the increased apoptosis and sensitivity to apoptotic signals observed in the cells of HIV-1-infected persons.
Human immunodeficiency virus type 1 (HIV-1) disease is characterized by the progressive loss of CD4+ T cells, and apoptosis has been proposed to be the primary mechanism (1, 2). Results from in vivo and in vitro studies indicate that the loss of CD4 cells occurs by direct killing of the infected cell by HIV-1 as well as by indirect killing of uninfected bystander cells (13, 14). In addition, the peripheral blood mononuclear cells (PBMC) of infected persons are significantly more sensitive to apoptotic signals than the cells of uninfected individuals (reviewed in reference 2). Although the mechanisms responsible for the increased sensitivity to apoptotic stimuli, the induction of apoptosis in infected cells, and the indirect induction of apoptosis in uninfected cells are likely to be multifactorial, HIV-1 gene products themselves may contribute in part to the increased apoptosis associated with HIV-1 disease.
Among the HIV-1 proteins that have been implicated in regulating apoptosis are gp120 (reviewed by Siliciano [36]), Vpr (3, 8, 38, 44), Nef (30), and Tat (11, 17, 22, 25, 31, 33, 35, 41, 45–47). Tat is essential for viral replication because of its function in transactivating the viral long terminal repeat (LTR) (reviewed in reference 10). Mechanisms by which Tat has been reported to induce apoptosis include up-regulation of the apoptosis effector molecule Fas ligand (FasL) (41), inhibition of expression of manganese-dependent superoxide dismutase (42), and activation of cyclin-dependent kinases (22). However, the ability of Tat to regulate apoptosis in the context of the infected cell has not been investigated.
The proteins responsible for transmitting the apoptotic signal within the cell have recently been identified. External apoptotic stimuli, as well as signals generated from within the cell, result in the activation of signal transduction pathways that converge on a protease (termed caspase) cascade (reviewed in reference 34) resulting in the execution of apoptotic cell death. Signaling through the death receptor, Fas, results in recruitment of an adaptor protein, FADD (7). The binding of FADD to Fas recruits the protease FLICE/caspase-8 (casp-8), which results in autoproteolytic activation of casp-8 (6, 20, 27, 28, 43). Casp-8 cleaves downstream caspases, such as casp-3, initiating the caspase cascade (9, 37, 39). Viruses have evolved a number of strategies to both inhibit and activate apoptosis at various steps in apoptotic pathways (reviewed in reference 40).
In this study, we used a genetic approach to identify HIV-1 gene products that regulate apoptosis during a single round of infection. The HIV-1 Tat protein was found to induce apoptosis both when expressed from the HIV-1 provirus and when expressed alone. Moreover, HIV-1Δenv-infected and Tat-expressing cells were more sensitive to an apoptotic stimulus. The ability of Tat to induce apoptosis and increase sensitivity to apoptotic signals was observed in both primary CD4 T cells and T-cell lines. In addition, using Tat mutants, we were able to genetically separate induction of apoptosis from other Tat functions, i.e., transactivation and FasL induction. Tat expression was found to up-regulate casp-8, a protease involved in apoptotic pathways. The up-regulation of casp-8 by Tat may result in the induction of apoptosis and the increased sensitivity of cells to apoptotic stimuli.
MATERIALS AND METHODS
Cells.
Jurkat and MT4 cells were maintained in RPMI with 10% fetal calf serum. 293T cells were grown in Dulbecco modified Eagle medium containing 10% fetal calf serum. All cells lines were propagated at 37°C–5% CO2 in a humidified chamber. All cells lines were obtained from the American Type Culture Collection or the NIH AIDS Repository Reagent Program.
To establish primary CD4 cell cultures, PBMC (107), isolated by banding on Ficoll, were stained with a monoclonal antibody (MAb) to CD4 (Leu3A) and sorted by flow cytometry; 105 CD4 cells were used to establish primary CD4 cell lines. CD4 cells were cultured in T-25 flasks with 25 × 106 irradiated (3,000 rads) PBMC as feeders and stimulated with anti-CD3 (OKT3) at 30 ng/ml in RPMI supplemented with streptomycin (50 μg/ml), penicillin (50 U/ml), 5 μM β-mercaptoethanol, and 10% human serum. Interleukin-2 (IL-2) was added the next day to 20 U/ml and then added every 2 to 3 days. The cells were reexpanded every 2 to 3 weeks by restimulation with anti-CD3, followed by IL-2 every 2 to 3 days as described for the initial expansion. The CD4 cells were used for infection at days 10 to 14 after stimulation with anti-CD3.
Plasmids.
Proviral plasmids derived from pLai (32) and mutations of the regulatory and accessory genes (21) have been previously described.
Proviruses with a stop codon after the first exon of Tat (Tatex1) were constructed by using overlapping oligonucleotides and PCR. The 5′ primer was 5′CTCTATCAAAGTAGTAAGTAGTAC3′, and the 3′ primer was 5′GTACTACTTACTGCTTTGATAGAG3′. Insertion of the stop codon was confirmed by sequencing.
The HIV-1 tat gene (HXB2 strain) and the Tat C22G and Tat K41A constructs were directly cloned into the LXSN retroviral vector (26). Tat C22G and Tat K41A were obtained from Andrew Rice (Baylor College of Medicine, Houston, Tex.) (16). The first exon of Tat (Tat72) was amplified by PCR using primers to Tat that inserted a stop codon after the codon for amino acid 72. The 5′ primer was 5′CCGGAATTCCGGATGGAACCAGTA3′, and the 3′ primer was 5′CGCGGATCCGCGCTATTGCTTTGATAGAGA3′. The 5′ primer contains an EcoRI site, and the 3′ primer contains a BamHI site, which allowed direct cloning into LXSN.
Virus stocks and infections.
Preparation of the HIV (by transient cotransfection of proviral plasmid and vesicular stomatitis virus G glycoprotein [VSV G] expression plasmid) and murine leukemia virus (MLV) (by transient cotransfection of the vector, MLV packaging plasmid, and VSV G expression plasmid) stocks has previously been described (4, 5). HIV-1ΔenvΔrev stocks were made by cotransfection of the proviral plasmid, the VSV G expression plasmid, and a rev expression plasmid. The concentrated virus stocks were aliquoted and stored at −70°C; titers were determined by the MAGI assay (18).
Jurkat and MT4 cells were infected at a multiplicity of infection of 10 as described previously (4, 5).
Jurkat cells infected with Tat-expressing retroviral vectors (LXSN backbone) were selected in G418 at 0.8 mg/ml. Tat-expressing cells were analyzed when all cells in mock-infected cultures treated with G418 were no longer viable.
Flow cytometry and detection of apoptosis.
Induction of apoptosis through the Fas receptor was initiated by using MAb CH-11 (PanVera Corp., Madison, Wis.) at 50 ng/ml for 2 h.
Apoptosis was detected using a Boehringer Mannheim (Indianapolis, Ind.) in situ cell death detection kit, which is based on the TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) method. The cells were prepared for labeling by fixing with 1% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C for 1 h or overnight. The cells were permeabilized with 0.5% Tween 20 in PBS and then labeled with fluorescein isothiocyanate (FITC)-conjugated dUTP according to the manufacturer’s instructions. After labeling, the cells were analyzed by flow cytometry.
The staining for intracellular p24 antigen has been previously described (5).
PCR.
RNA was isolated from cells by using Tri-reagent (Molecular Research Center, Inc., Cincinnati, Ohio). The reverse transcription (RT) reaction for the synthesis of cDNA was performed as described previously (12). Sequences of the primers used for detection of actin and FasL have been published elsewhere (29). The primers span introns to ensure mRNA-specific amplification.
Western blot analysis.
Total cellular lysates were prepared from uninfected and infected cells at 2 days postinfection. The lysates were resolved on a 10% polyacrylamide gel and then transferred to an Immobilon P membrane (Millipore, Bedford, Mass.), using a semidry transfer apparatus. The membranes were blocked with 5% dried milk in PBS and then probed with a MAb to casp-8 (Pharmingen, San Diego, Calif.) at 1:1,000 and a MAb to tubulin (Sigma, St. Louis, Mo.) at 1:1,000 in 5% milk in PBS. The membranes were washed three times with PBS before the addition of the anti-mouse horseradish peroxidase-conjugated secondary antibody (Sigma) at 1:10,000 in 5% milk in PBS. Bound antibody was detected by enhanced chemiluminescence (Amersham, Arlington Heights, Ill.) and exposure to X-ray film. Bands densities were quantified with NIH Image software.
RPA.
Two days postinfection, total RNA was isolated from uninfected and HIV-infected cells by using Tri-reagent (Molecular Research Center). Two micrograms of total RNA was hybridized to the in vitro-transcribed hAPO-3 probe set as instructed by the manufacturer (Pharmingen). The RNA-probe hybrids were digested by using an RNase protection assay (RPA) kit according to manufacturer’s instructions for the RiboQuant assay system (Pharmingen). After samples were resolved on a 40-cm 5% polyacrylamide gel, the gel was dried and analyzed with a PhosphorImager (Molecular Dynamics).
Casp-8 assay and inhibition of casp-8 activity.
Casp-8 protease activity was detected by using the tetrapeptide substrate IETD-AFC (7-amino-4-trifluormethyl coumarin) (ApoAlert FLICE/Casp-8 fluorescent assay; Clontech, Palo Alto, Calif.). Upon cleavage of the substrate by casp-8, free AFC was detected by using a fluorometer with a 400-nm excitation filter and a 505-nm emission filter. At 2 days postinfection, 1 × 106 to 2 × 106 cells were lysed in 50 μl of the supplied lysis buffer and then analyzed according to the manufacturer’s instructions. To verify that the detected activity was due to casp-8, duplicate lysates were incubated with the casp-8 inhibitor IETD-fmk (Clontech) prior to incubation with the AFC substrate.
Casp-8 activity was inhibited by treating cells with the specific casp-8 inhibitor IETD-fmk (Clontech) at 50 μM for 16 h. After the incubation, the cells were fixed in 1% paraformaldehyde and analyzed for apoptosis as described above.
RESULTS
HIV-1Δenv induces apoptosis.
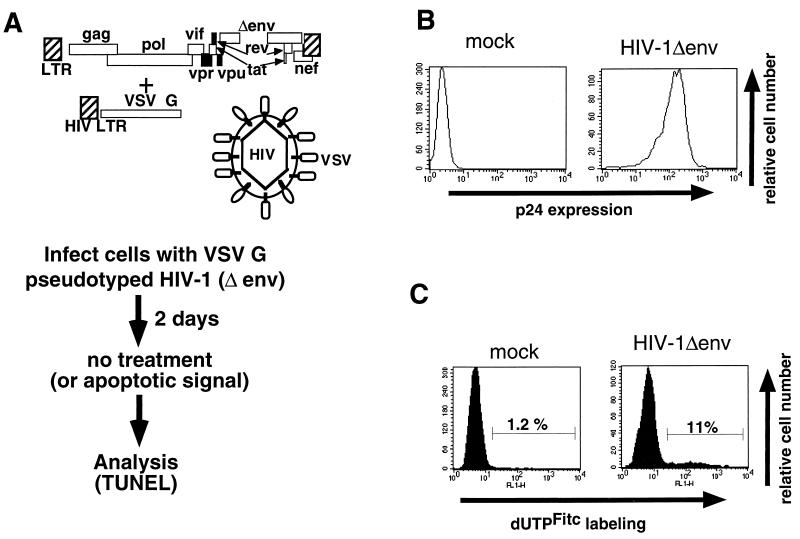
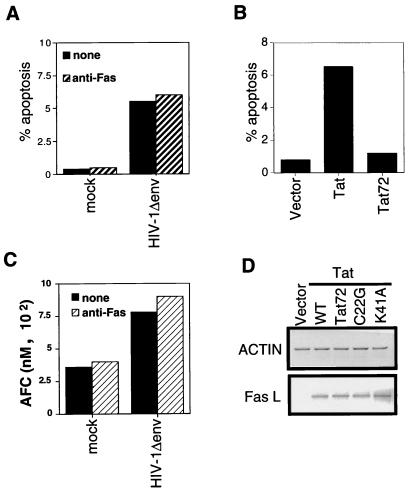
We wished to evaluate the contribution of HIV-1 gene products other than gp160/120 in apoptosis after HIV-1 infection. To do this, cells were infected with high-titer VSV G pseudotypes of HIV-1 with a deletion of the env gene (HIV-1Δenv) (Fig. 1A). We have previously demonstrated (4) that this protocol establishes infection in essentially 100% of the culture during a single round of infection (Fig. 1B). Jurkat cells infected with pseudotyped HIV-1Δenv or mock infected were then analyzed by the TUNEL assay for the percentage of cells undergoing apoptosis. Substantially higher numbers of HIV-1Δenv-infected cells (11%) than mock-infected cells (1%) were apoptotic (Fig. 1C). The observed apoptosis was not due to VSV G because not all VSV G-pseudotyped mutant virions induce apoptosis (Fig. 2C, 1 exon Δenv). In addition, high-titered MLV vector stocks pseudotyped with VSV G did not induce apoptosis (data not shown). Thus, HIV-1 gene products other than env are able to induce apoptosis of the infected cell.
FIG. 1.
Method used to analyze HIV-1-induced apoptosis. HIV-1Δenv was pseudotyped with VSV G (A) and used to infect cells at multiplicity of infection that establishes 100% infection (B). (C) Two days postinfection, the cells were analyzed for apoptosis by the TUNEL method using FITC-UTP labeling.
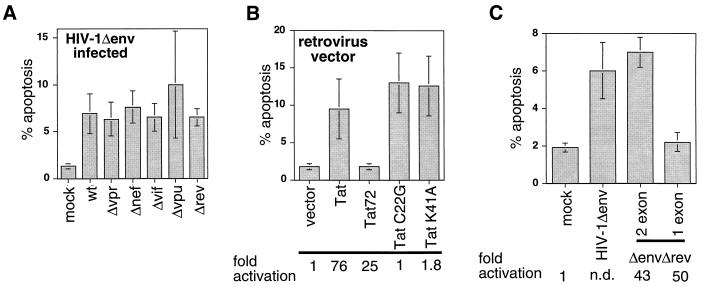
FIG. 2.
Genetic analysis of HIV-1- and HIV-1 Tat-induced apoptosis. (A) Mutation of vif, vpr, vpu, rev, and nef does not affect HIV-1-induced apoptosis. Cells were infected with HIV-1Δenv/VSV G pseudotypes, which were neither wild type (wt) or mutated in one of the regulatory or accessary genes, and analyzed for apoptosis by TUNEL 2 days postinfection. (B) Full-length HIV-1 Tat but not Tatex1 expressed in the absence of other HIV-1 proteins is able to induce apoptosis. Cells were infected with retroviral vectors that express wild-type or mutant tat genes. Cells expressing Tat were subsequently analyzed for apoptosis by the TUNEL method and for the ability to transactivate the viral LTR. The ability to transactivate the LTR was measured by transfection of an LTR-luciferase construct and is expressed at the bottom as fold activation relative to the value for the empty vector. (C) Full-length Tat but not Tatex1 is able to induce apoptosis when expressed from the HIV-1 provirus. Jurkat cells were infected with an HIV-1Δenv/VSV G pseudotype that was either wild type or Δrev and expressed one- or two-exon Tat. Two days postinfection, the cells were analyzed for apoptosis. The ability of the Δrev one- and two-exon Tat viruses to transactivate the LTR was assessed by infecting Jurkat cells containing an LTR-luciferase construct. Transactivation ability is expressed at the bottom as fold activation relative to the value for uninfected cells. N.d., not determined. Data in all panels are means of at least three independent experiments.
A genetic approach was used to determine which HIV-1 gene(s) was responsible for inducing apoptosis in the absence of env. The regulatory and accessory genes of HIV-1 were mutated in the provirus containing the deletion of env, and the viruses were pseudotyped with VSV G. Infected cells were analyzed at 2 days postinfection for the number of apoptotic cells. Mutation of vpr, nef, vif, vpu, or rev alone did not impair the ability of the infecting virus to induce apoptosis (Fig. 2A). The rev mutant is deficient in transport of unspliced and singly spliced viral mRNA from the nucleus and therefore expresses only tat and nef (24). The results indicate that either multiple viral proteins are capable of inducing apoptosis or that tat alone could be responsible for the induction of apoptosis.
Therefore, we investigated directly the ability of Tat to induce apoptosis, using a murine retroviral vector to express Tat alone in the absence of other HIV-1 proteins. Jurkat cells infected with retroviral vectors containing tat constructs were selected in G418 and analyzed for apoptosis between days 7 and 10 postinfection. All mock-infected cultures selected in G418 were no longer viable at these time points, as determined by trypan blue exclusion (data not shown). Indeed, Tat expression alone was found to induced significant numbers of apoptotic cells (Fig. 2B). In addition, Tat’s ability to induce apoptosis was associated with slower growth of Tat-expressing cells than of vector-infected cells (data not shown).
Next, we used Tat mutants to determine if the induction of apoptosis could be segregated from the essential function of Tat in transactivation of the viral LTR. Two Tat transactivation mutants (Tat C22G and Tat K41A) and a Tat construct that was truncated at the end of the first exon (Tat72) were assayed for the ability to induce apoptosis. The C22G and K41A mutations disrupt binding with a kinase complex essential for Tat transactivation activity (15, 16). Deletion of the second exon of Tat has been reported to have little effect on transactivation (19).
The ability of the Tat mutants to transactivate the viral LTR was confirmed by transfecting the Tat-expressing cells with an LTR-luciferase reporter plasmid (Fig. 2B). As anticipated, the C22G and K41A mutations did not transactivate the viral LTR, whereas Tat72 performed nearly as well as wild-type Tat. When the cells were analyzed for apoptosis, both Tat C22G and Tat K41A induced apoptosis but Tat72 did not. Therefore, the induction of apoptosis is separate from the function of Tat in transactivation and requires expression of the second exon.
To confirm that the second exon of tat was required to induce apoptosis in the context of a viral infection, we constructed a provirus that expressed only the first exon of tat in the ΔenvΔrev backbone. Jurkat cells were infected with equal numbers of infectious units of HIV-1Δenv, HIV-1ΔenvΔrev, or HIV-1ΔenvΔrev tatex1 and then analyzed for apoptosis as described above. Only viruses that expressed two-exon Tat proteins were able to induce apoptosis (Fig. 2C). The amount of apoptosis induced by the Tatex1 provirus was comparable to that for mock-infected cells. To ascertain that the difference in the amount of apoptosis between Δrev viruses with one-exon versus two-exon tat genes was not due to different levels of infection, Jurkat cells containing an LTR-luciferase construct were infected with each virus. Nearly equivalent levels of activation of luciferase activity were observed from equal numbers of cells for each virus (Fig. 2C, bottom), which indicates equal levels of infection because luciferase activity is dependent on transactivation of the LTR by Tat following infection. Therefore, these results suggest that the second exon of Tat is required for Tat to induce apoptosis.
Tat up-regulates FLICE/casp-8.
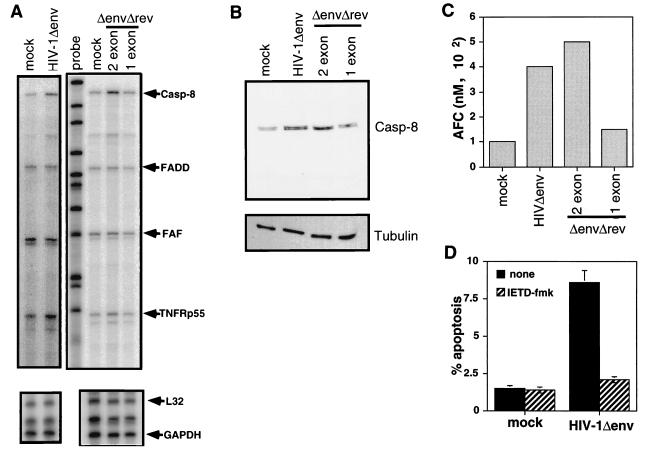
To determine how Tat induced apoptosis, we examined the expression of a number of proteins involved in apoptotic pathways. To determine the relative amounts of RNA of proteins associated with apoptotic pathways, we used a multiprobe RPA that allows the simultaneous analysis of several RNA species. The RPA was performed on RNA isolated from mock-infected cells and cells infected with viruses that express one- or two-exon Tat proteins. Tat was found to up-regulate the level of casp-8 RNA 2.5- to 4-fold relative to L32 and GAPDH control RNAs. The increase in casp-8 was observed in cells infected with HIV-1Δenv that expressed two-exon Tat but not one-exon Tat (Fig. 3A). The increase in casp-8 levels was apparently specific to casp-8 because the amounts of other RNAs such as FADD and Fas-associated factor (FAF) were equivalent (1- –1.5-fold) in uninfected and HIV-1Δenv-infected cells. In some experiments a slight (1.5- to 2-fold) increase in the 55-kDa tumor necrosis factor receptor was observed. However, it is unlikely that this increase in TNFRp55 contributed to the apoptosis observed in our system, as we observed no induction of apoptosis in Jurkat cells treated with high concentrations of TNF for up to 48 h.
FIG. 3.
HIV-1 Tat up-regulates casp-8 mRNA and protein activity. (A) The amount of casp-8 RNA is increased in HIV-1-infected cells which express full-length Tat proteins. Jurkat cells were infected with an HIV-1Δenv/VSV G pseudotype that was were wild type, Δrev, or Δrev/Tatex1. Two days postinfection, the RNA was isolated and subjected to RPA analysis. Two micrograms of total RNA was analyzed for each sample. Positions of protected RNA fragments for casp-8, FADD, FAF, TNFRp55, L32, and GAPDH are indicated. The gel was analyzed with a PhosphorImager, and the fold increase in specific RNA relative to value for L32 and GAPDH was determined. The L32 and GAPDH blots are shorter exposures of the same gel. The probe lane is undigested probe used for hybridization. Results representative of the analysis of three individual infections are shown. (B) HIV-1-infected cells expressing full-length Tat but not Tatex1 have elevated casp-8 protein levels. Lysates of uninfected and infected Jurkat cells were prepared 2 days postinfection and analyzed for casp-8 protein levels by Western blotting. Each lane was loaded with 1.5 × 105 cells. The fold increase in casp-8 levels was set relative to the tubulin level. The lysates are from the same cells analyzed by RPA in panel A. (C) Cells infected with HIV-1 which express two-exon Tat proteins have elevated levels of casp-8 activity. At 2 days postinfection, 106 infected and uninfected Jurkat cells were lysed and assayed for casp-8 activity by using a substrate that releases a fluorophore upon cleavage. The activity is expressed as the amount of AFC released from the AFC-substrate conjugate. The experiment was repeated on at least three separate infections. The results shown are from cells analyzed by RPA and Western blotting. (D) Treatment of HIV-1-infected cells with a casp-8 inhibitor inhibits induction of apoptosis. At 1 day postinfection, mock- or HIV-1Δenv-infected cells were treated with IETD-fmk for 16 h and then analyzed for apoptosis.
We next assessed casp-8 protein levels in infected cells to determine if the increase in casp-8 RNA resulted in increased casp-8 protein. Cell lysates from mock-infected cells and cells infected with viruses that express one- or two-exon Tat proteins were analyzed by Western blotting using a MAb directed to casp-8. Levels of casp-8 protein were approximately threefold higher in cells infected with viruses which express two-exon Tat proteins than in mock-infected cells or cells infected with a virus expressing Tatex1 (Fig. 3B). Thus, there is also an increase in casp-8 protein.
To determine if an increase in the cellular content of casp-8 resulted in higher casp-8 enzymatic activity, we used using the casp-8 substrate IETD-AFC to assay mock- or HIV-1Δenv-infected Jurkat cells for the amount of casp-8 protease activity. Cleavage of IETD-AFC by casp-8 releases AFC, which is detected by fluorometry. Casp-8 activity was found to be elevated in HIV-1Δenv-infected cells (Fig. 3C). To confirm that this increased activity was attributable to Tat and specifically to the second exon of Tat, Jurkat cells were infected with the Δrev, and one- and two-exon tat viruses described above. Cells infected with viruses expressing full-length two-exon Tat had increased casp-8 activity (Fig. 3C).
To demonstrate that casp-8 activity was required for HIV-1Δenv to induce apoptosis, we treated HIV-1Δenv-infected cells with a specific inhibitor of casp-8 (IETD-fmk). Treatment of cells with the casp-8 inhibitor almost completely reduced apoptosis in HIV-1-infected cells to the levels observed in mock-infected cells (Fig. 3D). Thus, Tat induced apoptosis is likely due to the increase in casp-8 activity.
HIV-1Δenv-infected and Tat-expressing cells are more susceptible to an apoptotic stimulus.
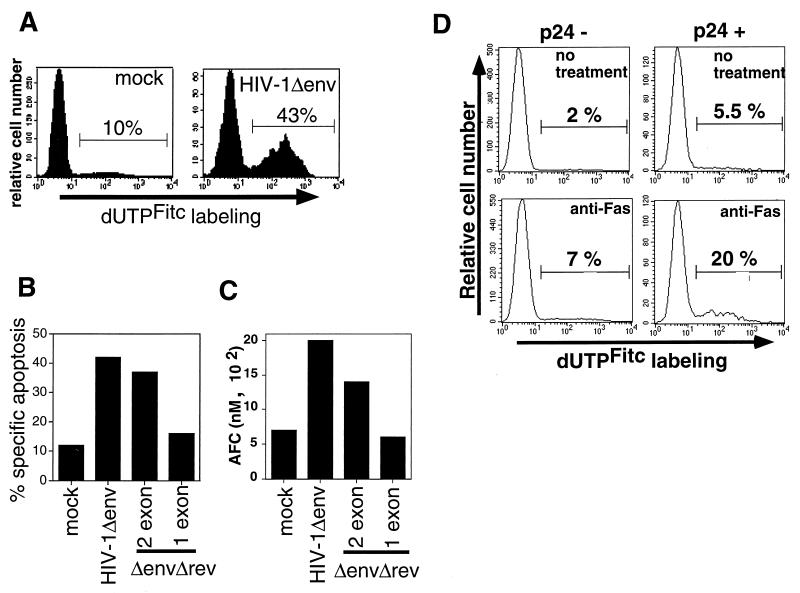
Casp-8 is coupled to the Fas apoptotic pathway via the adaptor protein FADD. Signaling through Fas recruits FADD to the receptor, and then FADD recruits casp-8. Activation of casp-8 results in cleavage of downstream caspases and apoptosis (6, 7, 27). We next tested the sensitivity of HIV-1Δenv-infected cells to Fas-mediated apoptosis to examine whether increased casp-8 levels would increase sensitivity to apoptotic signals that progress through casp-8. At 2 days postinfection, mock-infected and HIV-infected cells were treated with a MAb to Fas that is able to induce apoptosis. HIV-infected cells were more susceptible than uninfected cells to Fas-mediated apoptosis (Fig. 4A). In addition, the increased sensitivity to anti-Fas was associated with Tat proteins that induce casp-8 expression. HIV-1Δenv that expressed two-exon Tat proteins sensitized cells to Fas-mediated apoptosis, whereas HIV-1Δenv that expressed only the one-exon Tat did not (Fig. 4B). Analysis of casp-8 activity in lysates of anti-Fas-treated cells mirrored the results obtained for cells analyzed for apoptosis by TUNEL. Infected and uninfected Jurkat cells were treated with anti-Fas (50 ng/ml) and then lysed and assayed for casp-8 activity by using a casp-8 substrate that releases a fluorophore upon cleavage. Following treatment with equal amounts of anti-Fas, more casp-8 activity was detected in cells infected with viruses expressing full-length Tat than in cells expressing one-exon Tat or in mock-infected cells (Fig. 4C).
FIG. 4.
HIV-1 Tat increases sensitivity to Fas-mediated apoptosis. (A) Jurkat cells were infected with HIV-1Δenv/VSV G pseudotypes and treated with anti-Fas antibody 2 days postinfection. The amount of apoptosis was detected by TUNEL with FITC-dUTP labeling. (B) HIV-1 expressing full-length but not Tatex1 induces increased sensitivity to Fas-mediated apoptosis. Jurkat cells infected with an HIV-1Δenv/VSV G pseudotype that was either wild type, Δrev, or Δrev/Tatex1 were exposed to anti-Fas and analyzed as described above. The data are expressed as percent specific apoptosis, calculated as (amount of apoptosis due to anti-Fas − amount of apoptosis due to no treatment/100 − amount of apoptosis due to no treatment) × 100. (C) Cells infected with HIV-1 which express two-exon Tat proteins have increased casp-8 activity following anti-Fas stimulation. At 2 days postinfection, 106 infected and uninfected Jurkat cells treated with anti-Fas were lysed and assayed for casp-8 activity by using a substrate that releases a fluorophore upon cleavage. The activity is expressed as the amount of AFC released from the AFC-substrate conjugate. The analysis of casp-8 activity was performed on lysates of cells analyzed in panel B. Results of a representative experiment of three independent trials are shown. (D) HIV-1 induces apoptosis and increased sensitivity to Fas-mediated apoptosis in primary CD4 T cells. Two days after infection with HIV-1Δenv/VSV G, primary CD4 cells were analyzed for expression of HIV-1 p24 antigen and apoptosis. Twenty percent of the cells were infected. Events were acquired until the acquisition of 5,000 p24-positive events. The histograms are from p24-positive and p24-negative cells in the same culture.
We also analyzed cells that expressed Tat in the absence of other viral proteins and confirmed the results obtained for HIV-1Δenv infection. The expression of Tat, Tat C22G, or K41A alone increased sensitivity to Fas-mediated cell death, while the expression of Tat72 had no effect on Fas sensitivity (data not shown).
Increased apoptosis in HIV-1Δenv-infected primary CD4 cells in the absence of env.
We also examined whether HIV Δenv caused apoptosis and increased sensitivity to anti-Fas in primary T cells. Primary CD4 T cells, which were expanded by treatment with anti-CD3 and maintained with IL-2, were infected with the VSV G-pseudotyped HIVΔenv and analyzed for apoptosis at 2 days postinfection. Because infection of primary cells with the pseudotyped HIV-1 does not result in 100% infection, the cells were analyzed both for p24 antigen expression with an anti-p24 MAb and for apoptosis by TUNEL. The data shown for the uninfected cells are from cells in the infected culture that were negative for p24 antigen expression. The amount of apoptosis observed in the p24-negative cells in the infected culture was equivalent to the amount of apoptosis observed in cells from a culture not exposed to virus (data not shown). Similar to the results obtained with a T-cell line, HIVΔenv induced apoptosis and increased sensitivity to Fas-mediated apoptosis in primary CD4 T cells (Fig. 4D). Moreover, increased apoptosis was observed only in p24-positive cells and not uninfected cells in the same culture, suggesting that soluble products were not initiating apoptosis.
Induction of apoptosis and induction of FasL expression are genetically separable functions of Tat.
Tat has previously been reported to induce the expression of FasL (41). Therefore, it remained possible that the increased casp-8 activity was due to FasL interactions with Fas in HIV-1Δenv-infected cell cultures. To determine whether Tat required FasL-Fas interactions to induce apoptosis, we assessed the ability of Tat and HIV-1Δenv to induce apoptosis in a Fas-resistant cell line. MT4 cells are not sensitive to Fas-mediated apoptosis (Fig. 5A), and treatment of MT4 cells with anti-Fas does not induce casp-8 activity (Fig. 5C). Nonetheless, HIV-1Δenv infection (Fig. 5A) and Tat expression (Fig. 5B) were able to induce apoptosis in MT4 cells. HIV-1Δenv-infected MT4 cells were also analyzed for increased casp-8 activity, and as observed for Jurkat cells, HIV-1Δenv infection up-regulated casp-8 activity (Fig. 5C) in the absence of an external stimulus. Because MT4 cells are resistant to Fas-mediated signaling, there was no increase in casp-8 activity following anti-Fas treatment. Thus, Tat does not require FasL-Fas interaction to induce apoptosis, and the increase in casp-8 activity in HIV-1Δenv-infected cells does not require FasL-Fas interaction.
FIG. 5.
Tat-induced apoptosis is independent of Tat-mediated FasL induction. HIV-1Δenv infection (A) or Tat expression (B) induces apoptosis in Fas-resistant MT4 cells. MT4 cells that were infected with HIV-1Δenv/VSV G were analyzed for apoptosis by TUNEL 2 days postinfection. Treatment with anti-Fas had no effect on the level of apoptosis in uninfected or infected cells. Tat- or Tat72-expressing cells were analyzed for apoptosis by TUNEL. Results of a representative experiment of three individual repetitions are shown. (C) HIV-1 induces increased casp-8 activity in Fas-resistant MT4 cells. Two days postinfection, 106 infected or uninfected MT4 cells were assayed for casp-8 activity by using a substrate that releases a fluorophore upon cleavage. The results shown are from the analysis of lysates for cells analyzed for apoptosis in panel A. Activity is expressed as the amount of AFC released. (D) Induction of FasL by Tat is separate from the functions of transactivation and induction of apoptosis. RNA from Jurkat cells expressing Tat, Tat72, Tat C22G, or Tat K41A was analyzed for the expression of FasL and actin mRNA by RT-PCR. One microgram of total RNA was used in the RT reaction. WT, wild type.
That Tat did not require FasL-Fas interactions to induce apoptosis suggested that the induction of apoptosis and the induction of FasL expression were genetically separable. Therefore, the Tat mutants described above were examined for the ability to induce expression of FasL. Cells expressing Tat and each of the mutants C22G, K41A, and Tat72 were analyzed for FasL expression by RT-PCR. All tat alleles examined were able to induce FasL (Fig. 5D). Tat72 can induce FasL but is deficient in inducing apoptosis. Thus, FasL induction and induction of apoptosis map to different regions of the Tat protein. The data obtained with MT4 cells and Tat mutants indicate that Tat does not require FasL-Fas interactions to induce apoptosis. Rather, the ability of Tat to induce apoptosis correlates with the up-regulation of casp-8 expression.
DISCUSSION
Here we demonstrate that HIV Tat sensitizes infected cells to apoptotic signals. It is likely that the increased levels of casp-8 protein in HIV-1Δenv-infected and Tat-expressing cells directly contributes to apoptosis and increased sensitivity to apoptotic signals in both T-cell lines and primary CD4 cells. The up-regulation of casp-8 requires the second exon of Tat, and this function is genetically separable from both transactivation of the viral LTR and induction of FasL expression. Increases in casp-8 mediated by Tat may contribute to the increased apoptosis associated with HIV-1 disease.
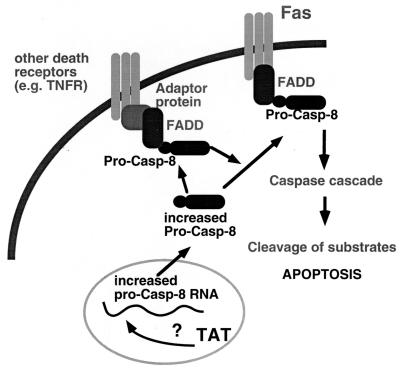
Casp-8, the most proximal caspase to the death receptors, is believed to be responsible for initiating the stepwise activation of multiple caspases, resulting in the signaling cascade that leads to the apoptotic death of the cell (9, 37, 39). We propose the following model for Tat-mediated apoptosis and increased sensitivity to apoptotic signals (Fig. 6). Tat increases the levels of casp-8 RNA, with a resulting increase in casp-8 protein. The increased cellular content of casp-8 leads to an increase in casp-8 activity, resulting in the induction of apoptosis. In addition, the increased casp-8 levels may allow for an increase in the recruitment of casp-8 to the occupied death receptor with a concomitant increased sensitivity to the apoptotic stimulus. This would explain the elevated sensitivity to anti-Fas, as Fas is a death receptor that signals through casp-8. This model is consistent with the observation that increased expression of casp-8 can result in apoptosis (27). Furthermore, increased expression of some caspases has been demonstrated to increase sensitivity to apoptotic signals (23). Because a number of apoptotic stimuli proceed through casp-8, Tat may increase sensitivity to a variety of apoptotic signals. This may in part explain the observation that the PBMC of HIV-infected persons are more susceptible to apoptosis by a number of stimuli.
FIG. 6.
Model of Tat-mediated induction of apoptosis and increased sensitivity to Fas-mediated apoptosis. Tat increases the expression of pro-casp-8 RNA by an undefined mechanism, resulting in increased pro-casp-8 protein, which can result in apoptosis. Sensitivity to Fas-mediated apoptosis is elevated because increased amounts of pro-casp-8 allows for increased recruitment to the occupied receptor.
Tat has been reported to both inhibit (11, 25, 45, 47) and induce (17, 22, 25, 31, 33, 35, 41) apoptosis. The observed ability of Tat to inhibit apoptosis may result from selecting stable cell lines expressing Tat, which would coselect cells with increased resistance to the apoptosis mediated by Tat. An earlier study attributed Tat-induced apoptosis to the up-regulation of the apoptosis effector molecule FasL, which resulted in FasL-Fas interactions in the culture and apoptosis (41). Although we confirmed that Tat induces FasL mRNA expression (Fig. 5D), we found that Tat does not require FasL-Fas interactions to induce apoptosis, and Tat induction of apoptosis is genetically separable from the induction of FasL. Nonetheless, the induction of FasL by Tat could contribute to the apoptosis attributable to Tat in cell culture. Indeed, Tat would heighten the sensitivity of cells to FasL because of increased casp-8 expression.
How Tat up-regulates casp-8 expression remains to be defined. Moreover, it has not been determined if the induction of casp-8 and the resulting apoptosis is a bona fide function of Tat or a consequence of an unidentified function. However, we were able to genetically separate the induction of apoptosis from the function of Tat in inducing FasL expression and the essential function of Tat in transactivating the viral promoter. We have also found that the HIV-2 Tat protein induces apoptosis (data not shown). Therefore, because the function of inducing apoptosis is conserved in the evolutionarily distinct but related HIV-2, this finding suggests that it may fulfill an important role in viral replication.
HIV infection is characterized by the loss of CD4 T cells, and apoptosis may be the major mechanism of CD4 cell elimination. The mechanisms that contribute to the apoptotic death of CD4 cells are likely multifactorial. HIV-1 Tat is able to induce apoptosis and increase sensitivity to an apoptotic stimulus in a time frame equivalent to the half-life of infected cells in vivo. Other HIV-1 proteins (gp120, Vpr, and Nef) have also been reported to regulate apoptosis. Understanding the relative contribution of each viral component to the apoptosis associated with HIV-1 infection will be important in determining the role of HIV-induced cell death in AIDS pathogenesis.
ACKNOWLEDGMENTS
We thank W. C. Goh, M. Linial, P. Nieman, and M. A. Vodicka for comments on the manuscript and the FHCRC Flow Cytometry, Image Analysis, and Biotechnology Laboratories.
This work was supported by Elizabeth Glaser Pediatric AIDS Foundation grant PF-77330 to S.R.B. and NIH grant AI 30927 to M.E.
REFERENCES
- 1.Ameisen J C. Programmed cell death and AIDS: from hypothesis to experiment. Immunol Today. 1992;13:388–391. doi: 10.1016/0167-5699(92)90086-M. [DOI] [PubMed] [Google Scholar]
- 2.Ameisen J C, Estaquier J, Idziorek T, De Bels F. Programmed cell death and AIDS pathogenesis: significance and potential mechanisms. Curr Top Microbiol Immunol. 1995;200:195–211. doi: 10.1007/978-3-642-79437-7_14. [DOI] [PubMed] [Google Scholar]
- 3.Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams W V, Green D R, Weiner D B. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- 4.Bartz S R, Rogel M E, Emerman M. Human immunodeficiency virus type 1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartz S R, Vodicka M A. Production of high-titer human immunodeficiency virus type 1 pseudotyped with vesicular stomatitis virus glycoprotein. Methods. 1997;12:337–342. doi: 10.1006/meth.1997.0487. [DOI] [PubMed] [Google Scholar]
- 6.Boldin M P, Goncharov T M, Goltsev Y V, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 7.Chinnaiyan A M, O’Rourke K, Tewari M, Dixit V M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 8.Conti L, Rainaldi G, Matarrese P, Varano B, Rivabene R, Columba S, Sato A, Belardelli F, Malorni W, Gessani S. The HIV-1 vpr protein acts as a negative regulator of apoptosis in a human lymphoblastoid T cell line: possible implications for the pathogenesis of AIDS. J Exp Med. 1998;187:403–413. doi: 10.1084/jem.187.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes-Alnemri T, Armstrong R C, Krebs J, Srinivasula S M, Wang L, Bullrich F, Fritz L C, Trapani J A, Tomaselli K J, Litwack G, Alnemri E S. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc Natl Acad Sci USA. 1996;93:7464–7469. doi: 10.1073/pnas.93.15.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaynor R B. Regulation of HIV-1 gene expression by the transactivator protein tat. Curr Top Microbiol Immunol. 1995;193:51–77. doi: 10.1007/978-3-642-78929-8_3. [DOI] [PubMed] [Google Scholar]
- 11.Gibellini D, Caputo A, Celeghini C, Bassini A, La Placa M, Capitani S, Zauli G. Tat-expressing Jurkat cells show an increased resistance to different apoptotic stimuli, including acute human immunodeficiency virus-type 1 (HIV-1) infection. Br J Haematol. 1995;89:24–33. doi: 10.1111/j.1365-2141.1995.tb08915.x. [DOI] [PubMed] [Google Scholar]
- 12.Goh W C, Rogel M E, Kinsey C M, Michael S F, Fultz P N, Nowak M A, Hahn B H, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- 13.Gougeon M-L, Laurent-Crawford A G, Hovanessian A G, Montagnier L. Direct and indirect mechanisms mediating apoptosis during HIV infection: contribution to in vivo CD4 T cell depletion. Semin Immunol. 1993;5:187–194. doi: 10.1006/smim.1993.1022. [DOI] [PubMed] [Google Scholar]
- 14.Herbein G, Van Lint C, Lovett J L, Verdin E. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J Virol. 1998;72:660–670. doi: 10.1128/jvi.72.1.660-670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann C H, Rice A P. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J Virol. 1995;69:1612–1620. doi: 10.1128/jvi.69.3.1612-1620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann C H, Rice A P. Specific interaction of the human immunodeficiency virus Tat proteins with a cellular protein kinase. Virology. 1993;197:601–608. doi: 10.1006/viro.1993.1634. [DOI] [PubMed] [Google Scholar]
- 17.Katsikis P D, Garcia-Ojeda M E, Torres-Roca J F, Greenwald D R, Herzenberg L A. HIV type 1 Tat protein enhances activation but not Fas (CD95)-induced peripheral blood T cell apoptosis in healthy individuals. Int Immunol. 1997;9:835–841. doi: 10.1093/intimm/9.6.835. [DOI] [PubMed] [Google Scholar]
- 18.Kimpton J, Emerman M. Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated β-galactosidase gene. J Virol. 1992;66:2232–2239. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuppuswamy M, Subramanian T, Srinivasan A, Chinnadurai G. Multiple functional domains of Tat, the trans-activator of HIV-1, defined by mutational analysis. Nucleic Acids Res. 1989;17:3551–3561. doi: 10.1093/nar/17.9.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuwana T, Smith J J, Muzio M, Dixit V, Newmeyer D D, Kornbluth S. Apoptosis induction by caspase-8 is amplified through the mitochondrial release of cytochrome c. J Biol Chem. 1998;273:16589–16594. doi: 10.1074/jbc.273.26.16589. [DOI] [PubMed] [Google Scholar]
- 21.Lewis P, Hensel M, Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992;11:3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. . (Erratum, 11:4249.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C J, Friedman D J, Wang C, Metelev V, Pardee A B. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 23.Los M, Van de Craen M, Penning L C, Schenk H, Westendorp M, Baeuerle P A, Droge W, Krammer P H, Fiers W, Schulze-Osthoff K. Requirement of an ICE/CED-3 protease for Fas/APO-1-mediated apoptosis. Nature. 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- 24.Malim M H, Cullen B R. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol. 1993;13:6180–6189. doi: 10.1128/mcb.13.10.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCloskey T W, Ott M, Tribble E, Khan S A, Teichberg S, Paul M O, Pahwa S, Verdin E, Chirmule N. Dual role of HIV Tat in regulation of apoptosis in T cells. J Immunol. 1997;158:1014–1019. [PubMed] [Google Scholar]
- 26.Miller A D, Rosman G J. Improved retroviral vectors for gene transfer and expression. BioTechniques. 1989;7:980–982. [PMC free article] [PubMed] [Google Scholar]
- 27.Muzio M, Chinnaiyan A M, Kischkel F C, O’Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz J D, Zhang M, Gentz R, Mann M, Krammer P H, Peter M E, Dixit V M. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 28.Muzio M, Stockwell B R, Stennicke H R, Salvesen G S, Dixit V M. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- 29.O’Connell J, O’Sullivan G C, Collins J K, Shanahan F. The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med. 1996;184:1075–1082. doi: 10.1084/jem.184.3.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okada H, Takei R, Tashiro M. HIV-1 Nef protein-induced apoptotic cytolysis of a broad spectrum of uninfected human blood cells independently of CD95(Fas) FEBS Lett. 1997;414:603–606. doi: 10.1016/s0014-5793(97)01080-6. [DOI] [PubMed] [Google Scholar]
- 31.Patki A H, Lederman M M. HIV-1 Tat protein and its inhibitor Ro 24-7429 inhibit lymphocyte proliferation and induce apoptosis in peripheral blood mononuclear cells from healthy donors. Cell Immunol. 1996;169:40–46. doi: 10.1006/cimm.1996.0088. [DOI] [PubMed] [Google Scholar]
- 32.Peden K, Emerman M, Montagnier L. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology. 1991;185:661–672. doi: 10.1016/0042-6822(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 33.Purvis S F, Jacobberger J W, Sramkoski R M, Patki A H, Lederman M M. HIV type 1 Tat protein induces apoptosis and death in Jurkat cells. AIDS Res Hum Retroviruses. 1995;11:443–450. doi: 10.1089/aid.1995.11.443. [DOI] [PubMed] [Google Scholar]
- 34.Salvesen G S, Dixit V M. Caspases: intracellular signaling by proteolysis. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 35.Sastry K J, Marin M C, Nehete P N, McConnell K, el-Naggar A K, McDonnell T J. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene. 1996;13:487–493. [PubMed] [Google Scholar]
- 36.Siliciano R F. The role of CD4 in HIV envelope-mediated pathogenesis. Curr Top Microbiol Immunol. 1996;205:159–179. doi: 10.1007/978-3-642-79798-9_8. [DOI] [PubMed] [Google Scholar]
- 37.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart S A, Poon B, Jowett J B, Chen I S. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi A, Hirata H, Yonehara S, Imai Y, Lee K K, Moyer R W, Turner P C, Mesner P W, Okazaki T, Sawai H, Kishi S, Yamamoto K, Okuma M, Sasada M. Affinity labeling displays the stepwise activation of ICE-related proteases by Fas, staurosporine, and CrmA-sensitive caspase-8. Oncogene. 1997;14:2741–2752. doi: 10.1038/sj.onc.1201131. [DOI] [PubMed] [Google Scholar]
- 40.Teodoro J G, Branton P E. Regulation of apoptosis by viral gene products. J Virol. 1997;71:1739–1746. doi: 10.1128/jvi.71.3.1739-1746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 42.Westendorp M O, Shatrov V A, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer P H, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-κB activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Chang H Y, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell. 1998;1:319–325. doi: 10.1016/s1097-2765(00)80032-5. [DOI] [PubMed] [Google Scholar]
- 44.Yao X J, Mouland A J, Subbramanian R A, Forget J, Rougeau N, Bergeron D, Cohen E A. Vpr stimulates viral expression and induces cell killing in human immunodeficiency virus type 1-infected dividing Jurkat T cells. J Virol. 1998;72:4686–4693. doi: 10.1128/jvi.72.6.4686-4693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zauli G, Gibellini D, Caputo A, Bassini A, Negrini M, Monne M, Mazzoni M, Capitani S. The human immunodeficiency virus type-1 Tat protein upregulates Bcl-2 gene expression in Jurkat T-cell lines and primary peripheral blood mononuclear cells. Blood. 1995;86:3823–3834. [PubMed] [Google Scholar]
- 46.Zauli G, Gibellini D, Celeghini C, Mischiati C, Bassini A, La Placa M, Capitani S. Pleiotropic effects of immobilized versus soluble recombinant HIV-1 Tat protein on CD3-mediated activation, induction of apoptosis, and HIV-1 long terminal repeat transactivation in purified CD4+ T lymphocytes. J Immunol. 1996;157:2216–2224. [PubMed] [Google Scholar]
- 47.Zauli G, Gibellini D, Milani D, Mazzoni M, Borgatti P, La Placa M, Capitani S. Human immunodeficiency virus type 1 Tat protein protects lymphoid, epithelial, and neuronal cell lines from death by apoptosis. Cancer Res. 1993;53:4481–4485. [PubMed] [Google Scholar]