Dear Editor,
We read with interest the article ‘Treatment outcomes and safety of bedaquiline, delamanid, and linezolid in multidrug-resistant TB’.1 Here we report on our experience using bedaquiline (BDQ) to treat Mycobacterium abscessus (MAB). MAB exhibits extensive resistance patterns and treatment requires individualised regimens based on antimicrobial susceptibility testing (AST), coupled with clinical expertise.2 Current treatment options are limited, particularly for macrolide-resistant MAB, due to the expression of an inducible 23S rRNA methyltransferase erm(41) gene.2 The use of injectable agents such as amikacin, tigecycline or imipenem may be problematic due to logistic issues and the high incidence of adverse effects.3 BDQ is a diarylquinoline that acts through the inhibition of mycobacterial F1F0- adenosine triphosphate synthase, with an effective half-life of more than 5 months, is characterised by excellent intracellular mycobactericidal activity both on replicating and non-replicating strains of Mycobacterium tuberculosis. BDQ also displays impressive in vitro activity against a broad range of non-tuberculous mycobacterial (NTM),4 but clinical data on the efficacy in MAB pulmonary disease (MAB-PD) are lacking.
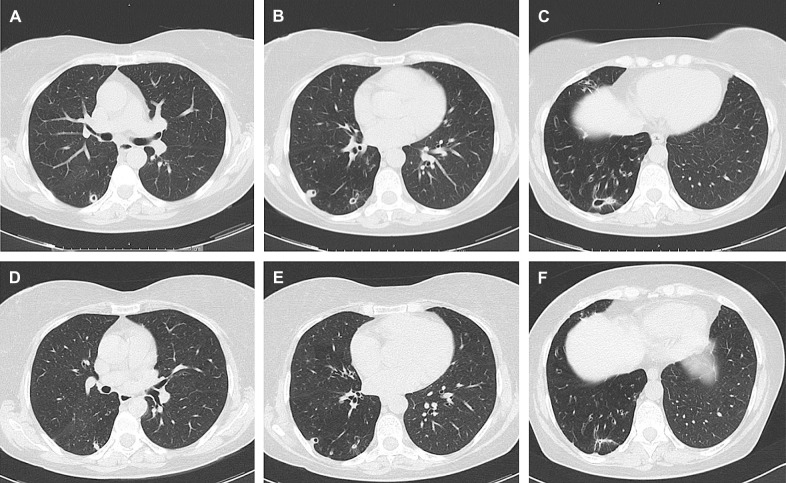
We describe a 58-year-old Italian woman with MAB-PD, whom we successfully treated with an all-oral regime based on BDQ, clofazimine (CFZ) and linezolid (LZD). The patient was a non-smoker with a history of recurrent bronchitis, persistent cough and dyspnoea, diagnosed with non-cystic fibrosis cystic bronchiectasis and a cavitated nodularity in the right lower lobe. Macrolide-susceptible MAB was isolated from bronchoalveolar lavage (BAL) culture, and treatment with daily oral clarithromycin 1,000 mg and doxycycline 200 mg and intravenous amikacin 750 mg from Monday to Saturday and 500 mg on Sunday was started but discontinued after 2 months due to poor tolerance. Four years later, following persistent irritating cough, dyspnoea and weight loss, the patient underwent a thorax computed tomography (CT) scan which showed increased cystic bronchiectasis and enlarged cavitary consolidations in the right middle and lower lobes (Figure panels A, B and C). Sputum culture was negative; however, macrolide-resistant MAB (clarithromycin minimum inhibitory concentration [MIC] 8.0 mg/ml) grew from BAL culture. Therefore, extended AST was performed, showing susceptibility to LZD (MIC 8.0 mg/ml) and BDQ (MIC 0.008 mg/ml). An all-oral regimen with daily LZD 600 mg, CFZ 100 mg and BDQ 400 mg for the first 2 weeks, followed by 200 mg 3 times a week was started, with electrocardiogram (ECG) monitoring of QTc. After 1 month of treatment, the patient reported symptoms resolution, and radiological improvement was observed at a follow-up thorax CT scan. Induced sputum culture was negative after 5 months of treatment. After 6 months, BDQ was suspended while LZD (600 mg three times a week) and CFZ (100 mg per day) were continued for a further 6 months. Although latest guidelines recommend treatment for at least 12 months after culture conversion for NTM-PD,2 treatment was discontinued after a total of 12 months given the optimal clinical response with persistent sputum culture negativity and further radiological improvement (Figure Panels D, E and F). Follow-up BAL was not executed due to the absence of symptoms. No side effects were observed, except for a brownish discoloration of the skin and dental enamel due to CFZ, which resolved with ordinary oral hygiene.
Figure.
Radiological comparison of a patient with Mycobacterium abscessus before and after treatment with bedaquiline-based all-oral regimen. Panels A, B and C show diffuse cystic bronchiectasis, and a cavitated nodularity in the middle lobe and in the right lower lobe at baseline. In panels D, E and F, numerical and dimensional reduction of some of the most appreciable cavitated consolidations to the right is documented after 7 months. The apical segment of the right lower lobe shows no signs of cavitation.
To our knowledge, only one case of an all-oral regimen with BDQ and CFZ has been reported as a salvage therapy for a case of calcaneal MAB osteomyelitis in a haematologic child, in combination with antibiotic-impregnated cement.5 We administered BDQ at the same doses and for the duration recommended for M. tuberculosis.6 Our MAB strain had a lower BDQ MIC (0.008 mg/L) than the reference American Type Culture Collection 19977 strain (0.03 mg/L). CFZ was used at a dose of 100 mg/day. According to previous studies, the combination of BDQ and CFZ exhibits a synergistic effect in in vitro time–kill assay.7 To prevent BDQ-induced resistance,8 LZD was co-administered at daily doses of 600 mg with CFZ. LZD has been reported to be most effective against MAB,2 and although LZD-resistant MAB strains are emerging worldwide, more than 50% of strains remain susceptible in vitro. We observed that switching from a poorly tolerated intravenous regimen to a 12-month, completely oral regimen based on AST led to culture conversion and a favourable clinical and radiological outcome, stable at the 3-month follow-up visit. The regimen was well tolerated, and no major side effects emerged. However, we recommend frequent clinical monitoring, blood-count and ECG to promptly detect drug-related side effects (peripheral neuropathy, anaemia, and QTc prolongation) and adequate follow-up after treatment discontinuation. Although we cannot exclude long-term relapse for our patient, we conclude that an all-oral regimen based on BDQ, CFZ and LZD is a promising therapeutic alternative for severe or relapsing MAB cases.
Footnotes
Conflicts of interest: none declared.
References
- 1.Chung C, et al. Treatment outcomes and safety of bedaquiline, delamanid, and linezolid in multidrug-resistant TB. Int J Tuberc Lung Dis. 2023;27(2):151–153. doi: 10.5588/ijtld.22.0466. [DOI] [PubMed] [Google Scholar]
- 2.Daley CL, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. doi: 10.1183/13993003.00535-2020. Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuter A, et al. The devil we know: is the use of injectable agents for the treatment of MDR-TB justified? Int J Tuberc Lung Dis. 2017;21(11):1114–1126. doi: 10.5588/ijtld.17.0468. [DOI] [PubMed] [Google Scholar]
- 4.Riccardi N, et al. Bedaquiline: a new hope for shorter and better anti-tuberculosis regimens. Recent Pat Antiinfect Drug Discov. 2018;13(1):3–11. doi: 10.2174/1574891X12666170619101904. [DOI] [PubMed] [Google Scholar]
- 5.Chan WY, et al. A child with acute myeloid leukemia complicated by calcaneal osteomyelitis due to Mycobacterium abscessus infection after induction chemotherapy successfully salvaged with bedaquiline and clofazimine. Int J Infect Dis. 2021;103:9–12. doi: 10.1016/j.ijid.2020.10.102. [DOI] [PubMed] [Google Scholar]
- 6.Fox GJ, et al. A review of the evidence for using bedaquiline (TMC207) to treat multi-drug resistant tuberculosis. Infect Dis Ther. 2013;2(2):123–144. doi: 10.1007/s40121-013-0009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruth MM, et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J Antimicrob Chemother. 2019;74(4):935–943. doi: 10.1093/jac/dky526. [DOI] [PubMed] [Google Scholar]
- 8.Asami T, et al. Efficacy estimation of a combination of triple antimicrobial agents against clinical isolates of Mycobacterium abscessus subsp. abscessus in vitro. JAC Antimicrob Resist. 2021;3(1):dlab004. doi: 10.1093/jacamr/dlab004. [DOI] [PMC free article] [PubMed] [Google Scholar]