Abstract
BACKGROUND:
The aim of these clinical standards is to aid the diagnosis and management of asthma in low-resource settings in low- and middle-income countries (LMICs).
METHODS:
A panel of 52 experts in the field of asthma in LMICs participated in a two-stage Delphi process to establish and reach a consensus on the clinical standards.
RESULTS:
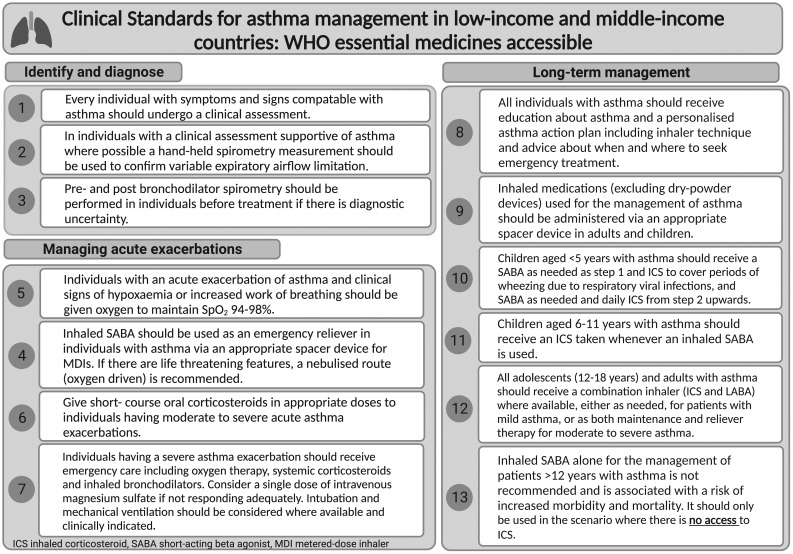
Eighteen clinical standards were defined: Standard 1, Every individual with symptoms and signs compatible with asthma should undergo a clinical assessment; Standard 2, In individuals (>6 years) with a clinical assessment supportive of a diagnosis of asthma, a hand-held spirometry measurement should be used to confirm variable expiratory airflow limitation by demonstrating an acute response to a bronchodilator; Standard 3, Pre- and post-bronchodilator spirometry should be performed in individuals (>6 years) to support diagnosis before treatment is commenced if there is diagnostic uncertainty; Standard 4, Individuals with an acute exacerbation of asthma and clinical signs of hypoxaemia or increased work of breathing should be given supplementary oxygen to maintain saturation at 94–98%; Standard 5, Inhaled short-acting beta-2 agonists (SABAs) should be used as an emergency reliever in individuals with asthma via an appropriate spacer device for metered-dose inhalers; Standard 6, Short-course oral corticosteroids should be administered in appropriate doses to individuals having moderate to severe acute asthma exacerbations (minimum 3–5 days); Standard 7, Individuals having a severe asthma exacerbation should receive emergency care, including oxygen therapy, systemic corticosteroids, inhaled bronchodilators (e.g., salbutamol with or without ipratropium bromide) and a single dose of intravenous magnesium sulphate should be considered; Standard 8, All individuals with asthma should receive education about asthma and a personalised action plan; Standard 9, Inhaled medications (excluding dry-powder devices) should be administered via an appropriate spacer device in both adults and children. Children aged 0–3 years will require the spacer to be coupled to a face mask; Standard 10, Children aged <5 years with asthma should receive a SABA as-needed at step 1 and an inhaled corticosteroid (ICS) to cover periods of wheezing due to respiratory viral infections, and SABA as-needed and daily ICS from step 2 upwards; Standard 11, Children aged 6–11 years with asthma should receive an ICS taken whenever an inhaled SABA is used; Standard 12, All adolescents aged 12–18 years and adults with asthma should receive a combination inhaler (ICS and rapid onset of action long-acting beta-agonist [LABA] such as budesonide-formoterol), where available, to be used either as-needed (for mild asthma) or as both maintenance and reliever therapy, for moderate to severe asthma; Standard 13, Inhaled SABA alone for the management of patients aged >12 years is not recommended as it is associated with increased risk of morbidity and mortality. It should only be used where there is no access to ICS.
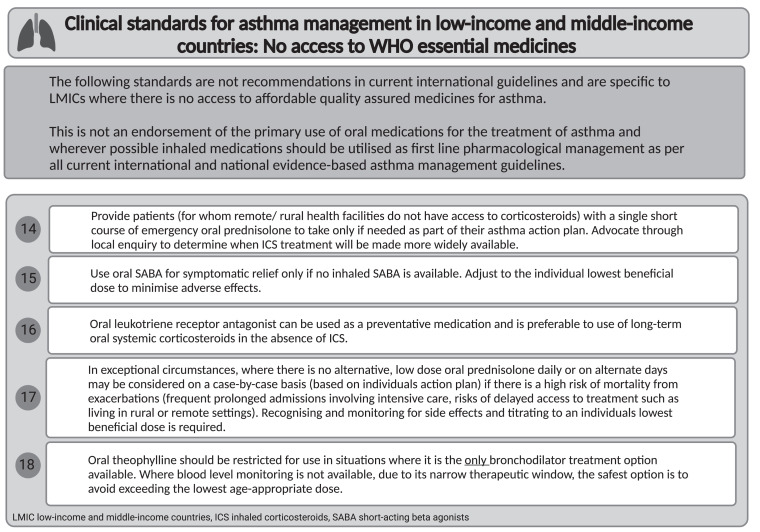
The following standards (14–18) are for settings where there is no access to inhaled medicines. Standard 14, Patients without access to corticosteroids should be provided with a single short course of emergency oral prednisolone; Standard 15, Oral SABA for symptomatic relief should be used only if no inhaled SABA is available. Adjust to the individual’s lowest beneficial dose to minimise adverse effects; Standard 16, Oral leukotriene receptor antagonists (LTRA) can be used as a preventive medication and is preferable to the use of long-term oral systemic corticosteroids; Standard 17, In exceptional circumstances, when there is a high risk of mortality from exacerbations, low-dose oral prednisolone daily or on alternate days may be considered on a case-by-case basis; Standard 18. Oral theophylline should be restricted for use in situations where it is the only bronchodilator treatment option available.
CONCLUSION:
These first consensus-based clinical standards for asthma management in LMICs are intended to help clinicians provide the most effective care for people in resource-limited settings.
Keywords: asthma, chronic respiratory disease, non-communicable disease, clinical standards, low-income and middle-income countries
Abstract
CONTEXTE :
L’objectif de ces normes cliniques est de faciliter le diagnostic et la prise en charge de l’asthme dans les environnements à faibles ressources des pays à revenu faible et intermédiaire (PRFI).
MÉTHODES :
Un groupe de 52 experts dans le domaine de l’asthme dans les PRFI a participé à un processus de Delphi en deux étapes afin d’établir et d’aboutir à un consensus sur les normes cliniques.
RÉSULTATS :
Dix-huit normes cliniques ont été définies : Norme 1, Toute personne présentant des symptômes et des signes compatibles avec l’asthme doit faire l’objet d’une évaluation clinique ; Norme 2, Chez les individus (>6 ans) avec une évaluation clinique soutenant un diagnostic d’asthme, une mesure de la spirométrie à main doit être utilisée pour confirmer une limitation variable du flux expiratoire en démontrant une réponse aiguë à un bronchodilatateur ; Norme 3, Une spirométrie pré- et post-bronchodilatateur doit être réalisée chez les individus (>6 ans) pour soutenir le diagnostic avant le début du traitement en cas d’incertitude diagnostique ; Norme 4, Les individus présentant une exacerbation aiguë de l’asthme et des signes cliniques d’hypoxémie ou de détresse respiratoire doivent recevoir de l’oxygène supplémentaire pour maintenir la saturation entre 94% et 98% ; Norme 5, Les bêta-2-agonistes à courte durée d’action (SABA) inhalés doivent être utilisés en tant que traitement d’urgence chez les individus asthmatiques via un dispositif d’inhalation adapté aux inhalateurs doseurs ; Norme 6, Les corticostéroïdes oraux à courte durée doivent être administrés à des doses appropriées aux individus souffrant d’une exacerbation aiguë modérée à sévère de l’asthme (minimum 3–5 jours) ; Norme 7, Les individus souffrant d’une exacerbation sévère de l’asthme doivent recevoir des soins d’urgence, y compris une oxygénothérapie, des corticostéroïdes systémiques, des bronchodilatateurs inhalés (par exemple, salbutamol avec ou sans bromure d’ipratropium) et une dose unique de sulfate de magnésium intraveineux doit être envisagée ; Norme 8, Tous les individus asthmatiques doivent recevoir une éducation sur l’asthme et un plan d’action personnalisé ; Norme 9, Les médicaments inhalés (à l’exception des dispositifs à poudre sèche) doivent être administrés via un dispositif d’inhalation adapté chez les adultes et les enfants. Les enfants de 0–3 ans auront besoin que le dispositif d’inhalation soit couplé à un masque facial ; Norme 10, Les enfants de moins de 5 ans asthmatiques devraient recevoir un bronchodilatateur à action rapide selon les besoins à l’étape 1, ainsi qu’un corticostéroïde inhalé (CSI) pour couvrir les périodes de respiration sifflante causée par des infections virales respiratoires. À partir de l’étape 2, ils devraient recevoir un bronchodilatateur à action rapide selon les besoins et un CSI quotidien ; Norme 11, Les enfants de 6 à 11 ans asthmatiques devraient recevoir un CSI chaque fois qu’ils utilisent un bronchodilatateur à action rapide inhalé ; Norme 12, Tous les adolescents de 12 à 18 ans et les adultes asthmatiques devraient recevoir un inhalateur combiné (CSI et bêta-agoniste à longue durée d’action [LABA] tel que le budésonide-formotérol ou le CSI/bronchodilatateur à action rapide), si disponible, à utiliser soit selon les besoins (pour l’asthme léger) ou en tant que traitement d’entretien et de secours pour l’asthme modéré à sévère ; Norme 13, L’utilisation d’un bronchodilatateur à action rapide inhalé seul pour la prise en charge des patients de plus de 12 ans n’est pas recommandée car elle est associée à un risque accru de morbidité et de mortalité. Elle devrait être utilisée uniquement en cas d’indisponibilité de CSI. Les normes suivantes (14–18) s’appliquent aux contextes où il n’y a pas d’accès aux médicaments inhalés ; Norme 14, Les patients qui n’ont pas accès aux corticostéroïdes devraient recevoir une seule cure courte de prednisolone oral en cas d’urgence ; Norme 15, Les bronchodilatateurs à action rapide par voie orale pour le soulagement des symptômes ne doivent être utilisés que s’il n’y a pas de bronchodilatateur à action rapide inhalé disponible. Ajuster à la dose minimale bénéfique pour minimiser les effets indésirables ; Norme 16, Les antagonistes des récepteurs des leucotriènes (LTRA) peuvent être utilisés comme médicaments préventifs et sont préférables à l’utilisation de corticostéroïdes systémiques oraux à long terme ; Norme 17, Dans des circonstances exceptionnelles, lorsque le risque de mortalité dû à des exacerbations est élevé, une dose faible de prednisolone oral quotidienne ou en jours alternés peut être envisagée au cas par cas ; Norme 18, La théophylline par voie orale devrait être réservée aux situations où c’est la seule option de traitement bronchodilatateur disponible.
CONCLUSION :
Ces premières normes cliniques basées sur un consensus pour la prise en charge de l’asthme dans les pays à revenu faible ou intermédiaire (LMIC) visent à aider les cliniciens à fournir les soins les plus efficaces aux personnes dans des environnements aux ressources limitées.
Asthma is a common respiratory disease affecting people of all ages and backgrounds, with considerable health and economic consequences.1–5 The majority of people with asthma can be managed effectively provided international asthma management recommendations (e.g., the Global Initiative for Asthma [GINA] strategy) can be implemented.6 Unfortunately, this is often not the case in low- and middle-income countries (LMICs), where limited access to affordable, quality diagnostic tests and treatments restrict the provision of effective asthma care.1,2,7–10 The WHO Package of Essential Non-communicable (PEN) disease interventions for primary healthcare includes step-wise guidance for asthma management based on inhaled short-acting beta-2 agonist (SABA) and inhaled corticosteroids (ICS),11 which are on the WHO Model List of Essential Medicines.12 However, these essential medicines are not always available, or affordable, or used in resource-limited settings.3,5,8,13 There are gaps between what is described in the currently available asthma management recommendations and the reality of what is typically available and affordable in LMICs. We have therefore defined clinical standards tailored to the specific challenges of managing asthma in these settings.
AIM OF THE CLINICAL STANDARDS
Although our aim is to offer suggestions for the diagnosis and management of asthma in LMIC settings, we are not endorsing poor standards of care in LMICs. Instead, we are responding to the severe constraints on the resources available by presenting pragmatic approaches to provide effective clinical care. This consensus-based document describes the following activities:
Identifying and diagnosing asthma in children aged <5 years and 6–11 years, adolescents aged 12–18 years and adults in LMICs, considering the availability of equipment and potential context-specific differential diagnoses (Standards 1–3).
Managing acute exacerbations of asthma in children (<5 years, 6–11 years), adolescents (12–18 years) and adults where only WHO listed essential inhaled medications may be available (Standards 4–7).
Long-term management of asthma in children (<5 years, 6–11 years), adolescents (12–18 years) and adults if only WHO listed essential inhaled medications are available (Standards 8–13).
Long-term management of asthma in children (<5 years, 6–11 years), adolescents (12–18 years) and adults if there is no access to WHO listed essential inhaled medicines (Standards 14–18).
METHODS
A panel of experts in the field of asthma in LMICs were identified from the Global Asthma Network (GAN) and International Union Against Tuberculosis and Lung Disease (The Union). Of the 69 experts initially invited, 56 (81.1%) responded to the first round of the Delphi process and 52 (92.9%) of these to the second round, representing 31 countries (see Supplementary Table S1). All respondents (n = 69) were asked to comment via a Delphi process on several draft standards developed by a core team of 14 researchers. At the first Delphi round, agreement was already high, with a median value of 93.7% (interquartile range [IQR] 85.5–96.9) for all standards. Following the second round of the Delphi process, agreement rose to a median of 96.2% (IQR 94.0–98.5) for all standards. Based on this substantial agreement, a draft document was developed following two rounds of the Delphi process by the expert panel. The document underwent multiple rounds of revisions, and the final version was approved by consensus (100% agreement).
This document uses the GINA definition for asthma: ‘Asthma is a heterogeneous disease, usually characterised by chronic airway inflammation. It is defined by the history of respiratory symptoms, such as wheeze, shortness of breath, chest tightness and cough that vary over time and in intensity, together with variable expiratory airflow limitation.’6
STANDARD 1
Every individual with symptoms and signs compatible with asthma should undergo a clinical assessment.
Diagnosing asthma is based on the history of characteristic respiratory symptoms (wheeze, chest tightness, shortness of breath, dry cough), usually, but not exclusively, starting in childhood with a family history of atopy, confirmed by expiratory airflow limitation. Symptoms tend to be worse at night or early morning, varying in intensity over time and triggered by infections, allergens, irritant exposures, exercise, changes in weather and emotion. Physical examination may be normal but if patients are symptomatic, the most frequent finding is expiratory wheeze on lung auscultation.14 In LMICs, access to lung function testing is limited, and when available, is underused.15 The diagnosis of asthma is often made on clinical grounds, despite the knowledge that asthma may be under or over diagnosed and therefore, under- or over treated using this strategy.16,17 If this assessment is not suggestive of asthma, alternative diagnoses appropriate to the individual’s age and the context should be considered. The differential diagnosis may include other respiratory diseases of LMICs, e.g., TB, non-cystic fibrosis bronchiectasis, parasitic and fungal lung diseases, as well as chronic obstructive pulmonary disease (COPD) due to smoking or biomass exposure, cardiac diseases, dysfunctional breathing (vocal cord dysfunction) and foreign body inhalation.
STANDARD 2
In individuals (<6 years) with a clinical assessment supportive of a diagnosis of asthma, a hand-held spirometry measurement should be used, if possible, to confirm variable expiratory airflow limitation by demonstrating an acute response to a bronchodilator.
Asthma is a clinical diagnosis, based on a history of characteristic symptom patterns and evidence of variable expiratory airflow limitation in lung function measurements, ideally based on spirometry revealing an obstructive pattern with a reduced forced expiratory volume in 1 sec (FEV1) and FEV1/FVC (forced vital capacity) compared to predicted population lower limit of normal (fixed cut-off FEV1 <0.75 in adults and <0.80 in children or fixed ratio of FEV1/FVC of <0.70 where lower limits of normal are unavailable).18,19 Normal spirometry does not exclude asthma and may require repeat testing. Given the lack of availability of spirometry in many primary care settings in LMICs, GINA6 and WHO PEN11 propose a peak expiratory flow (PEF) rate measurement demonstrating either an acute response to a bronchodilator or within-day variability over any 2-week period as an acceptable alternative to confirm variable expiratory airflow limitation. If unable to perform testing, a therapeutic trial is warranted if the history is suggestive of asthma. The likelihood of a diagnosis of asthma increases if there is any one of the following outside of respiratory infections:
A ≥20% improvement in PEF 15 min after the administration of 200–400 mcg inhaled salbutamol;
Excessive variability in twice daily PEF over 2 weeks (>10% and 13% in adults and children, respectively);
A significant increase in lung function after 2–4 weeks of anti-inflammatory treatment (>20% in PEF from baseline).
The greater the variations, or the more occasions excess variation is seen, the more confident the diagnosis.20
STANDARD 3
Pre- and post-bronchodilator spirometry should be performed in individuals (>6 years) to support diagnosis before treatment is commenced in case of diagnostic uncertainty.
As there is no single objective test to diagnose asthma, misdiagnosis is common.21 One factor that contributes to misdiagnosis is failing to confirm reversible airflow obstruction,22 which should be documented prior to treatment commencing, as variability decreases post-treatment. Bronchodilator responsiveness is defined as >10% change relative to an individual’s predicted FEV1 and FVC.23 In LMICs, pre- and post-bronchodilator spirometry should be performed to support the diagnosis if other methods mentioned previously fail to demonstrate variable expiratory airflow limitation, or if there is a lack of response to treatment in children and adults.6,21,24
ACUTE MANAGEMENT
STANDARD 4
Individuals with an acute exacerbation of asthma and clinical signs of hypoxaemia or increased work of breathing should be given supplementary oxygen to maintain SpO2 94–98%.
Oxygen is prescribed for patients with hypoxaemia and is a marker of increased risk of a poor outcome. There are risks associated with both hypoxaemia and hyperoxia, which underlies the importance of prescribing oxygen only if required, and to within a target oxygen saturation range.25,26 A titrated oxygen regime is recommended in the treatment of severe asthma, in which oxygen is administered only to patients with hypoxaemia at a dose that relieves hypoxaemia without causing hyperoxaemia.26 In acute asthma, it is recommended to maintain an oxygen saturation of 93–95% in adults and 94–98% in children aged 6–11 years.6,27
STANDARD 5
Inhaled SABA should be used as an emergency reliver in individuals with asthma via an appropriate spacer device for metered-dose inhalers.
Individuals presenting with an acute asthma exacerbation should receive frequent inhaled SABA therapy. The most efficient and cost-effective delivery method is by pressurised metered-dose inhaler (pMDI) and spacer.28 In LMICs, the cost and availability of commercial spacers may hinder the use of pMDIs. A practical alternative is to create a spacer by adapting clean, used 500 ml plastic drink bottles.29 A Cochrane Review demonstrated no inferiority between home-made and commercial spacers.30 In exacerbations with life-threatening features, oxygen-driven nebulisers are recommended to reduce the risk of oxygen desaturation.31
STANDARD 6
Short-course oral corticosteroids should be administered in appropriate doses to individuals having moderate to severe acute asthma exacerbations (minimum 3–5 days).
Administration of corticosteroids reduces mortality, relapses, hospital admissions and the need for SABA therapy. The earlier the administration, the better the outcome.32 The oral route is as effective as the parenteral route and faster, less invasive and cheaper.33,34 The recommended doses in adults are equivalent to 50 mg prednisolone daily or 200 mg hydrocortisone (in divided doses) for 5–7 days; in children prednisolone 1–2 mg/kg up to maximum 40 mg/day for 3–5 days is advised.6
STANDARD 7
Individuals having a severe asthma exacerbation should receive emergency care including oxygen therapy, systemic corticosteroids, inhaled bronchodilators (e.g., salbutamol with or without ipratropium bromide), and a single dose of intravenous magnesium sulphate should be considered.
The addition of an inhaled short-acting anticholinergic, e.g., ipratropium bromide, to standard initial treatment with oxygen, systemic corticosteroids and inhaled SABA has been shown to significantly decrease hospitalisations in adults and children with severe asthma exacerbations.35–37 The addition of a single infusion of intravenous magnesium sulphate also reduces risk of hospitalisation in adults and children with acute severe asthma,37,38 although evidence in children is limited. Intubation and mechanical ventilatory support should be considered where available and clinically indicated. Intravenous aminophylline increases the risk of side effects (nausea and tremor in children, vomiting, arrhythmias and palpitations in adults) without significant clinical benefit.39,40
LONG-TERM MANAGEMENT
STANDARD 8
All individuals with asthma should receive education about asthma and a personalised action plan.
Asthma education is a key component of asthma guidelines. In children, asthma self-management education programmes have demonstrated positive impact on a wide range of outcome measures.41 In adults, programmes including patient education and self-management support improved asthma-specific quality of life, severity scores and lung function tests.42 Differences in outcome reporting and in programme composition limit comparison between trials and ability for meta-analysis. However, training programmes empowering people to adjust their medication using a written action plan seem more effective than other forms of asthma self-management.35 The action plan (written or pictorial) should include inhaler technique assessment and advice about when and where to seek emergency treatment.
STANDARD 9
Inhaled medications (excluding dry-powder devices) should be administered via an appropriate spacer device in both adults and children. Children aged 0–3 years will require the spacer to be coupled with a face mask.
To optimally manage asthma in both the long-term and during acute exacerbations, it is essential for inhaled medicines to be accessible and delivered effectively using appropriate spacer devices correctly, specifically when using pMDIs. The use of an appropriate spacer device is key to providing optimal pharmacotherapy, permitting maximal therapeutic dose delivery of the respirable aerosol fractions of inhaled medicines.43 Where commercial spacers are unavailable/affordable, spacer devices can be fashioned from plastic drink bottles.29
STANDARD 10
Children aged <5 years with asthma should receive a SABA as needed at step 1 and an ICS to cover periods of wheezing due to respiratory viral infections, and a SABA as needed and daily ICS from step 2 upwards.
The overall goals of management in children younger than 5 years are ensuring good control of symptoms, maintaining normal lung growth, retaining healthy activity levels and minimising the risk of exacerbations, while avoiding adverse effects of medication. When symptoms or wheeze is mild, often only occasionally triggered by upper respiratory tract infections, step 1 management with SABA as needed can be considered as providing relief of symptoms. As symptoms become more frequent or definitive for the clinical diagnosis of asthma, step 2 management with addition of regular daily ICS for at least 3 months to achieve good control is essential.44,45
STANDARD 11
Children aged 6–11 years with asthma should receive an ICS, taken whenever an inhaled SABA is used.
In children 6–11 years of age, the recommendation is the use of an ICS from step 1 whenever a SABA is taken. In a multi-centre study in children aged 5–18 years, the use of combined beclomethasone and salbutamol when symptomatic was found to be comparable to daily ICS use, with salbutamol alone as a reliever in children with mild persistent asthma.46 In a recent pragmatic real-world trial in African-American children aged 6–17 years, the use of beclomethasone and salbutamol when symptomatic showed similar levels of asthma control to use of daily beclomethasone.47 SABA overuse has been well described in adolescents and adults, and is associated with an increased risk of asthma exacerbations, urgent healthcare utilisation and mortality.48,49 Where access to healthcare facilities is limited, children should be provided with an ICS and SABA to prevent worsening asthma control and exacerbations.
STANDARD 12
All adolescents aged 12–18 years and adults with asthma should receive a combination inhaler (ICS and rapid onset of action long-acting beta agonist [LABA] such as budesonide-formoterol), where available, to be used either as needed (for mild asthma) or as both maintenance and reliever therapy, for moderate to severe asthma.
There is a large body of clinical trial evidence from pragmatic and randomised control trials on the efficacy of combination inhalers with rapid onset of action LABA (budesonide-formoterol) as needed. In mild asthma, it prevents exacerbations and improves symptom control compared to use of short-acting beta agonists alone.50–52 A recent systematic review and meta-analysis confirmed the efficacy of this approach in mild asthma.53 Budesonide-formoterol is used as a maintenance and reliever therapy (MART) in moderate and severe asthma to reduce exacerbations.51,54–56 In settings where budesonide-formoterol is not available, other rapid onset of action LABA or ICS-SABA combinations should be used for patients to benefit from an anti-inflammatory medication whilst taking a reliever. This approach has been shown to reduce exacerbations.57 If an ICS-LABA/SABA combination inhaler is unavailable, patients should receive a regular inhaled corticosteroid and a separate inhaled SABA. SABA should never be used alone but in combination with ICS, as this approach reduces mortality and exacerbations.48,58,59
STANDARD 13
Inhaled SABA alone for the management of patients aged >12 years is not recommended as it is associated with a risk of increased morbidity and mortality. It should only be used where there is no access to ICS.
Regular use of SABA, even for 1–2 weeks, is associated with reduced bronchodilator effectiveness, increased allergic response and eosinophilia.60,61 This can lead to a vicious cycle encouraging the overuse of SABA associated with increased numbers of exacerbations and mortality.48,62 Globally, 96% of asthma deaths occur in LMICs, and ensuring the regular use of ICS has the potential to reduce this burden.3
Clinical standards 1–13 for asthma management in LMIC settings where WHO essential medicines are accessible are listed in Table 1.
Table 1.
|
SETTINGS THAT LACK INHALED MEDICATIONS
The following clinical standards for asthma are specific to LMICs and are not recommendations in current international guidelines. These are proposed for scenarios where inhaled medications are not available or affordable, especially ICS.8,63 This is not an endorsement of the primary use of oral medications for the treatment of asthma – wherever possible inhaled medication should be used as first-line pharmacological management as per all current international and national evidence-based asthma management guidelines. Inhaled medicines for asthma are considered best practice and should always be advocated at the primary care level. Oral salbutamol was removed from the WHO Model List of Essential Medicines in 2011. Oral SABA has a slow onset of action and higher doses are needed for bronchodilator effects similar to the inhaled form. In addition, higher rates of adverse effects such as tachycardia, hyperactivity, decreased oxygen saturation and tremors are seen. However, the reality remains that inhaled medications are not available in some LMICs.64
STANDARD 14
Patients without access to corticosteroids should be provided with a single short course of emergency oral prednisolone.
Patients in remote/rural areas with limited access to corticosteroids should be provided with a single short course of emergency oral prednisolone to take only if needed as part of their asthma action plan. All patients should be provided with a written/pictorial action plan appropriate for their level of asthma control, so they know how to recognise and respond to worsening asthma.65,66 The dose of oral prednisolone (preferably given in the morning) is as per Standard 6, tapering is not needed if given for less than 2 weeks. In addition, we strongly recommend advocating to ascertain the timeline for the broader implementation of ICS treatment.
STANDARD 15
Oral SABA should be used for symptomatic relief only if no inhaled SABA is available. Adjust to the individual’s lowest beneficial dose to minimise adverse effects.
The aim of asthma treatment is to improve respiratory symptoms, reduce inflammation and control acute exacerbations using a stepwise approach. Unfortunately, oral SABA is still used in about one in six adolescents and adults with poor asthma control in low-resource settings where access to alternative quality-assured essential asthma medicines is limited.7 Although no specific evidence or guidance is available, in the presence of previous or current adverse events it is useful to reduce the dose of oral SABA to the minimum effective dose, whenever alternative approaches are not available.
STANDARD 16
Oral leukotriene receptor antagonists (LTRA) can be used as a preventive medication and is preferable to the use of long-term oral systemic corticosteroids.
Leukotrienes are inflammatory mediators to prevent and treat mild chronic asthma in association with ICS or as an alternative to ICS. These are less effective than ICS alone, and in 2020 the US Food and Drug Administration released a warning about the serious mental health adverse effects associated with montelukast,67 highlighting mood-related changes, including suicidal ideation and actions. Although in many patients these resolved on discontinuing treatment, they persisted in some patients with or without a history of prior mental illness. Where ICS is not available, as is the case in many LMICs, oral corticosteroid treatment may be effective in preventing future asthma exacerbations, but chronic use often leads to multiple adverse events.68 Oral LTRA may be used as an alternative medication and might be preferable to long-term oral systemic corticosteroids or oral salbutamol, but clinicians should consider the benefits and risks of mental health side effects before prescribing montelukast.
STANDARD 17
In exceptional circumstances when there is a high risk of mortality from exacerbations, low-dose oral prednisolone daily or on alternate days may be considered on a case-by-case basis.
Low-dose oral prednisolone taken daily or on alternate days has been included in asthma treatment recommendations for several decades.6,11 This is typically a final step in recommendations after other asthma treatments with more favourable risk/benefit profiles have been considered. Given the historical context, this standard is included for settings where no inhaled therapies for asthma care are available. Low-dose oral prednisolone is an effective treatment for asthma addressing the underlying inflammatory nature of the disease and providing benefits in terms of symptom control and exacerbation risk reduction. However, in comparison to other asthma treatments, particularly ICS, it is associated with the risk of several dose-related adverse effects.68 Recognising and monitoring for side effects and titrating to an individual’s lowest beneficial dose is required. This standard is appropriate for carefully considered cases where on balance, the benefits outweigh the risks, and the lowest beneficial doses are used.
STANDARD 18
Oral theophylline should be restricted for use in situations where it is the only bronchodilator treatment option available.
Oral theophylline has been included as an oral bronchodilator option in previous international asthma treatment recommendations. It has largely been superseded by other more effective treatments with more favourable side effect profiles. The narrow therapeutic window for theophylline makes its safe use difficult even in well-resourced healthcare settings, where drug levels can be measured and the dose titrated accordingly. Drug-level monitoring is often not an option in LMICs, making the safe use of theophylline even more challenging. When used in the absence of drug-level monitoring, we suggest not exceeding the lowest age-appropriate dose, accepting that this may result in sub-therapeutic dosing; however, this is preferable to potentially toxic dosing. We suggest that oral theophylline only be considered where there are no other oral (or inhaled) bronchodilator options. Oral SABA does not have a narrow therapeutic window in quite the same way, hence our preference for oral SABA over oral theophylline, although both have considerable systemic adverse effects compared to inhaled bronchodilators, which should always be used in preference wherever possible.
Clinical standards 14–18 for asthma management in LMIC settings that lack inhaled medications are listed in Table 2.
Table 2.
|
CONCLUSION
The diagnosis of asthma requires a comprehensive clinical assessment, based on the history of characteristic respiratory symptoms and evidence of variable expiratory airflow limitation. Spirometry and PEF measurements are used to confirm variable expiratory airflow limitation, although in LMICs, where spirometry availability is limited, PEF measurements are commonly used. Maintenance treatment for asthma should include ICS; additional therapy can be added depending on the individual’s response to treatment. Effective management of asthma requires regular monitoring of symptoms and lung function, adherence to treatment and patient education to reduce exposure to environmental triggers.
These clinical standards are intended to assist clinicians with the difficult decisions they face in caring for people with asthma in resource-constrained settings. We also hope that they highlight the major global inequalities in access to basic effective and affordable asthma care. The WHO Global Action Plan for the Prevention and Control of Noncommunicable Diseases includes a target of 80% availability of the essential medicines to treat chronic respiratory diseases. Essential inhaled medicines must be part of universal health coverage to provide effective care for children, adolescents and adults with asthma wherever they live in the world.
Supplementary Material
Acknowledgements
This article is sponsored by the Oskar-Helene-Heim Foundation (OHH; Berlin, Germany) and the Günther Labes Foundation (Berlin, Germany) and available as an Open Access article (subject to CC-BY 4.0 licensing).
Footnotes
Conflicts of interest: EMK reports grants from the National Institute for Health Research Global Health Research Unit on Respiratory Health (RESPIRE); personal fees from AstraZeneca (Cambridge, UK); and is the President of the International Primary Care Respiratory Group, Edinburgh, UK, and the Primary Care Respiratory Group, Kuala Lumpur, Malaysia. KM reports advisory board fees from AstraZeneca and GlaxoSmithKline (Brentford, UK). RM report advisory board and personal fees and research grants from AstraZeneca and honoraria from Organon, Jersey City, NJ, USA. MS was funded by a Wellcome Trust Clinical PhD Fellowship (Grant number 203919/Z/16/Z). The funders had no role in design and conduct of the study; collection, analysis, and interpretation of the data; the writing of the article, or the decision to submit it for publication. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the WHO or the funders. The remaining authors have no conflicts of interests.
References
- 1.Asher MI, et al. Worldwide trends in the burden of asthma symptoms in school-aged children: Global Asthma Network Phase I cross-sectional study. Lancet. 2021;398:1569–1580. doi: 10.1016/S0140-6736(21)01450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Marcos L, et al. The burden of asthma, hay fever and eczema in children in 25 countries: GAN Phase I study. Eur Respir J. 2022;60(3):2102866. doi: 10.1183/13993003.02866-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meghji J, et al. Improving lung health in low-income and middle-income countries: from challenges to solutions. Lancet. 2021;397:928–940. doi: 10.1016/S0140-6736(21)00458-X. [DOI] [PubMed] [Google Scholar]
- 4.Mortimer K, et al. The burden of asthma, hay fever and eczema in adults in 17 countries: GAN Phase I study. Eur Respir J. 2022;60(3):2102865. doi: 10.1183/13993003.02865-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Global Asthma Report Int J Tuberc Lung Dis. 2022;26:S1–S102. doi: 10.5588/ijtld.22.1010. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma Global strategy for asthma management and prevention. Fontana, WI, USA: GINA; 2022. [Google Scholar]
- 7.Garcia-Marcos L, et al. Asthma management and control in children, adolescents, and adults in 25 countries: a Global Asthma Network Phase I cross-sectional study. Lancet Glob Health. 2023;11:e218–e228. doi: 10.1016/S2214-109X(22)00506-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stolbrink M, et al. Improving access to affordable quality-assured inhaled medicines in low- and middle-income countries. Int J Tuberc Lung Dis. 2022;26:1023–1032. doi: 10.5588/ijtld.22.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mortimer K, et al. Asthma management in low and middle income countries: case for change. Eur Respir J. 2022;60(3):2103179. doi: 10.1183/13993003.03179-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortimer K, et al. The reality of managing asthma in sub-Saharan Africa: priorities and strategies for improving care. J Pan Afr Thorac Soc. 2022;3:105–120. [Google Scholar]
- 11.World Health Organisation WHO package of essential noncommunicable (PEN) disease interventions for primary health care. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 12.World Health Organisation World Health Organization Model List of Essential Medicines - 22nd List. Geneva, Switzerland: WHO; 2021. [Google Scholar]
- 13.Rylance S, et al. Key messages and partnerships to raise awareness and improve outcomes for people with asthma and COPD in low- and middle-income countries. Int J Tuberc Lung Dis. 2022;26:1106–1108. doi: 10.5588/ijtld.22.0544. [DOI] [PubMed] [Google Scholar]
- 14.Levy ML, et al. International Primary Care Respiratory Group (IPCRG) Guidelines: diagnosis of respiratory diseases in primary care. Prim Care Respir J. 2006;15:20–34. doi: 10.1016/j.pcrj.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masekela R, Zurba L, Gray D. Dealing with Access to Spirometry in Africa: A Commentary on Challenges and Solutions. Int J Environ Res Public Health. 2018;16(1):62. doi: 10.3390/ijerph16010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aaron SD, et al. Underdiagnosis and Overdiagnosis of Asthma. Am J Respir Crit Care Med. 2018;198:1012–1020. doi: 10.1164/rccm.201804-0682CI. [DOI] [PubMed] [Google Scholar]
- 17.Huang WC, et al. A syndromic approach to assess diagnosis and management of patients presenting with respiratory symptoms to healthcare facilities in Vietnam. ERJ Open Res. 2021;7(1):00572-2020. doi: 10.1183/23120541.00572-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louis R, et al. European Respiratory Society Guidelines for the diagnosis of asthma in adults. Eur Respir J. :2101585. doi: 10.1183/13993003.01585-2021. Jan 2022. [DOI] [PubMed] [Google Scholar]
- 19.Gaillard EA, Moeller A. Evidence-based European guidelines for the diagnosis of asthma in children aged 5-16 years. Lancet Respir Med. 2021;9:558–560. doi: 10.1016/S2213-2600(21)00183-1. [DOI] [PubMed] [Google Scholar]
- 20.de Jong CCM, et al. Diagnosis of asthma in children: findings from the Swiss Paediatric Airway Cohort. Eur Respir J. 2020;56(5):2000132. doi: 10.1183/13993003.00132-2020. [DOI] [PubMed] [Google Scholar]
- 21.Thorley J. NICE issues draft guideline for asthma diagnosis. Lancet Respir Med. 2015;3:184. doi: 10.1016/S2213-2600(15)00038-7. [DOI] [PubMed] [Google Scholar]
- 22.MacNeil J, Loves RH, Aaron SD. Addressing the misdiagnosis of asthma in adults: where does it go wrong? Expert Rev Respir Med. 2016;10:1187–1198. doi: 10.1080/17476348.2016.1242415. [DOI] [PubMed] [Google Scholar]
- 23.Stanojevic S, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022;60(1):2101499. doi: 10.1183/13993003.01499-2021. [DOI] [PubMed] [Google Scholar]
- 24.Saglani S, Menzie-Gow AN. Approaches to asthma diagnosis in children and adults. Front Pediatr. 2019;7:148. doi: 10.3389/fped.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel B, et al. Randomized clinical trial of high concentration versus titrated oxygen use in pediatric asthma. Pediatr Pulmonol. 2019;54:970–976. doi: 10.1002/ppul.24329. [DOI] [PubMed] [Google Scholar]
- 26.Perrin K, et al. Randomised controlled trial of high concentration versus titrated oxygen therapy in severe exacerbations of asthma. Thorax. 2011;66:937–941. doi: 10.1136/thx.2010.155259. [DOI] [PubMed] [Google Scholar]
- 27.O’Driscoll BR, et al. British Thoracic Society Guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir Res. 2017;4:e000170. doi: 10.1136/bmjresp-2016-000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;2013:CD000052. doi: 10.1002/14651858.CD000052.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zar HJ, Asmus MJ, Weinberg EG. A 500-ml plastic bottle: an effective spacer for children with asthma. Pediatr Allergy Immunol. 2002;13:217–222. doi: 10.1034/j.1399-3038.2002.01056.x. [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez C, Sossa M, Lozano JM. Commercial versus home-made spacers in delivering bronchodilator therapy for acute therapy in children. Cochrane Database Syst Rev. 2008;2008:CD005536. doi: 10.1002/14651858.CD005536.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN) BTS/SIGN Guideline for the management of asthma. London, UK: BTS; 2019. [Google Scholar]
- 32.Rowe BH, et al. Corticosteroids for preventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev. 2007:CD000195. doi: 10.1002/14651858.CD000195.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Rowe BH, et al. Early emergency department treatment of acute asthma with systemic corticosteroids. Cochrane Database Syst Rev. 2001:CD002178. doi: 10.1002/14651858.CD002178. [DOI] [PubMed] [Google Scholar]
- 34.Ratto D, et al. Are intravenous corticosteroids required in status asthmaticus? JAMA. 1988;260:527–529. [PubMed] [Google Scholar]
- 35.Griffiths B, Ducharme FM. Combined inhaled anticholinergics and short-acting beta2-agonists for initial treatment of acute asthma in children. Paediatr Respir Rev. 2013;14:234–235. doi: 10.1016/j.prrv.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Kirkland SW, et al. Combined inhaled beta-agonist and anticholinergic agents for emergency management in adults with asthma. Cochrane Database Syst Rev. 2017;1:CD001284. doi: 10.1002/14651858.CD001284.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Craig SS, et al. Interventions for escalation of therapy for acute exacerbations of asthma in children: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2020;8:CD012977. doi: 10.1002/14651858.CD012977.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthma in the emergency department. Cochrane Database Syst Rev. 2014:CD010909. doi: 10.1002/14651858.CD010909.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitra A, et al. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev. 2005;2005:CD001276. doi: 10.1002/14651858.CD001276.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nair P, Milan SJ, Rowe BH. Addition of intravenous aminophylline to inhaled beta(2)-agonists in adults with acute asthma. Cochrane Database Syst Rev. 2012;12:CD002742. doi: 10.1002/14651858.CD002742.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf FM, et al. Educational interventions for asthma in children. Cochrane Database Syst Rev. 2003:CD000326. doi: 10.1002/14651858.CD000326. [DOI] [PubMed] [Google Scholar]
- 42.Peytremann-Bridevaux I, et al. Chronic disease management programmes for adults with asthma. Cochrane Database Syst Rev. 2015:CD007988. doi: 10.1002/14651858.CD007988.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincken W, et al. Spacer devices for inhaled therapy: why use them, and how? ERJ Open Res. 2018;4:00065-2018. doi: 10.1183/23120541.00065-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy ML, et al. Key recommendations for primary care from the 2022 Global Initiative for Asthma (GINA) update. NPJ Prim Care Respir Med. 2023;33:7. doi: 10.1038/s41533-023-00330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. Lancet. 2014;383:1593–1604. doi: 10.1016/S0140-6736(14)60615-2. [DOI] [PubMed] [Google Scholar]
- 46.Martinez FD, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:650–657. doi: 10.1016/S0140-6736(10)62145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumino K, et al. A Pragmatic Trial of Symptom-Based Inhaled Corticosteroid Use in African-American Children with Mild Asthma. J Allergy Clin Immunol Pract. 2020;8:176–185 e2. doi: 10.1016/j.jaip.2019.06.030. [DOI] [PubMed] [Google Scholar]
- 48.Nwaru BI, et al. Overuse of short-acting beta(2)-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi: 10.1183/13993003.01872-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddel HK, et al. Risks associated with managing asthma without a preventer: urgent healthcare, poor asthma control and over-the-counter reliever use in a cross-sectional population survey. BMJ Open. 2017;7:e016688. doi: 10.1136/bmjopen-2017-016688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beasley R, et al. Controlled Trial of Budesonide-Formoterol as Needed for Mild Asthma. N Engl J Med. 2019;380:2020–2030. doi: 10.1056/NEJMoa1901963. [DOI] [PubMed] [Google Scholar]
- 51.O’Byrne PM, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171:129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 52.O’Byrne PM, et al. Inhaled combined budesonide-formoterol as needed in mild asthma. N Engl J Med. 2018;378:1865–1876. doi: 10.1056/NEJMoa1715274. [DOI] [PubMed] [Google Scholar]
- 53.Crossingham I, et al. Combination fixed-dose beta agonist and steroid inhaler as required for adults or children with mild asthma: a Cochrane systematic review. BMJ Evid Based Med. 2022;27:178–184. doi: 10.1136/bmjebm-2021-111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bateman ED, et al. Overall asthma control achieved with budesonide/formoterol maintenance and reliever therapy for patients on different treatment steps. Respir Res. 2011;12:38. doi: 10.1186/1465-9921-12-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatter L, et al. ICS-formoterol reliever versus ICS and short-acting beta(2)-agonist reliever in asthma: a systematic review and meta-analysis. ERJ Open Res. 2021;7:00701-2020. doi: 10.1183/23120541.00701-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rabe KF, et al. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368:744–753. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- 57.Papi A, et al. Albuterol-Budesonide Fixed-Dose Combination Rescue Inhaler for Asthma. N Engl J Med. 2022;386:2071–2083. doi: 10.1056/NEJMoa2203163. [DOI] [PubMed] [Google Scholar]
- 58.Suissa S, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med. 1994;149:604–610. doi: 10.1164/ajrccm.149.3.8118625. [DOI] [PubMed] [Google Scholar]
- 59.Royal College of Physicians The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report. London: RCP; 2014. Why asthma still kills. [Google Scholar]
- 60.Aldridge RE, et al. Effects of terbutaline and budesonide on sputum cells and bronchial hyperresponsiveness in asthma. Am J Respir Crit Care Med. 2000;161:1459–1464. doi: 10.1164/ajrccm.161.5.9906052. [DOI] [PubMed] [Google Scholar]
- 61.Hancox RJ, et al. Bronchodilator tolerance and rebound bronchoconstriction during regular inhaled beta-agonist treatment. Respir Med. 2000;94:767–771. doi: 10.1053/rmed.2000.0820. [DOI] [PubMed] [Google Scholar]
- 62.Spitzer WO, et al. The use of beta-agonists and the risk of death and near death from asthma. N Engl J Med. 1992;326:501–506. doi: 10.1056/NEJM199202203260801. [DOI] [PubMed] [Google Scholar]
- 63.Stolbrink M, et al. The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: a systematic review. Lancet Glob Health. 2022;10:e1423–e1442. doi: 10.1016/S2214-109X(22)00330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.World Health Organisation; 2020. Assessing national capacity for the prevention and control of noncommunicable diseases: report of the 2019 global survey. [Google Scholar]
- 65.Gibson PG, Powell H. Written action plans for asthma: an evidence-based review of the key components. Thorax. 2004;59:94–99. doi: 10.1136/thorax.2003.011858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pinnock H, et al. Systematic meta-review of supported self-management for asthma: a healthcare perspective. BMC Med. 2017;15:64. doi: 10.1186/s12916-017-0823-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Food and Drug Administration (FDA) Drug Saftey Communication U.S. Food and Drug Administration; 2020. FDA requires Boxed Warning about serious mental health side effects for asthma and allergy drug montelukast (Singulair); advises restrciting use for allergic rhinitis. [Google Scholar]
- 68.Price DB, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi: 10.2147/JAA.S176026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


