Abstract
Background
Human African trypanosomiasis is a parasitic disease caused by trypanosomes among which Trypanosoma brucei gambiense is responsible for a chronic form (gHAT) in West and Central Africa. Its elimination as a public health problem (EPHP) was targeted for 2020. Côte d’Ivoire was one of the first countries to be validated by WHO in 2020 and this was particularly challenging as the country still reported around a hundred cases a year in the early 2000s. This article describes the strategies implemented including a mathematical model to evaluate the reporting results and infer progress towards sustainable elimination.
Methods
The control methods used combined both exhaustive and targeted medical screening strategies including the follow-up of seropositive subjects– considered as potential asymptomatic carriers to diagnose and treat cases– as well as vector control to reduce the risk of transmission in the most at-risk areas. A mechanistic model was used to estimate the number of underlying infections and the probability of elimination of transmission (EoT) was met between 2000–2021 in two endemic and two hypo-endemic health districts.
Results
Between 2015 and 2019, nine gHAT cases were detected in the two endemic health districts of Bouaflé and Sinfra in which the number of cases/10,000 inhabitants was far below 1, a necessary condition for validating EPHP. Modelling estimated a slow but steady decline in transmission across the health districts, bolstered in the two endemic health districts by the introduction of vector control. The decrease in underlying transmission in all health districts corresponds to a high probability that EoT has already occurred in Côte d’Ivoire.
Conclusion
This success was achieved through a multi-stakeholder and multidisciplinary one health approach where research has played a major role in adapting tools and strategies to this large epidemiological transition to a very low prevalence. This integrated approach will need to continue to reach the verification of EoT in Côte d’Ivoire targeted by 2025.
Author summary
Significant efforts to control gambiense human African trypanosomiasis (gHAT) have drastically reduced the prevalence of the disease and elimination of transmission (EoT) is targeted for 2030 by the World Health Organization (WHO). This reduction was particularly challenging in Côte d’Ivoire as it still faced epidemic episodes in the early 2000s. This large epidemiological transition to very low prevalence necessitated the adaptation and evolution of both medical and vector control strategies described in this article. A mathematical model was also used to retrospectively analyse case reporting results, indicating with high probability that local EoT has already been achieved in the four health districts analysed.
With nine gHAT cases detected in two health districts between 2015 and 2019 and less than one case per 10,000 people per year in all health districts at a national level over this five-year period, Côte d’Ivoire received validation by WHO for the achievement of the elimination of the disease as a public health problem in 2020. These results, combined with the modelling, offer encouragement towards the verification of EoT by 2025 in Côte d’Ivoire on condition of maintaining a multidisciplinary, one-health approach including research activities to continuously adapt strategy in the epidemiological transition to zero incidence.
Introduction
Human African trypanosomiasis (HAT) is a parasitic disease caused by trypanosomes that are transmitted to humans by tsetse [1]. Trypanosoma brucei gambiense (T. b. gambiense) is responsible for a chronic form of HAT (gambiense HAT, gHAT) in West and Central Africa accounting for 97% of all reported HAT cases during 2001–2020 [2]. In comparison, T. b. rhodesiense is zoonotic and responsible for an acute form of HAT (rhodesiense HAT, rHAT) in East and South Africa. Both forms can be deadly if left untreated [3]. From the 1970s to the 1990s, HAT experienced a phase of emergence/re-emergence that resulted in a significant increase in the number of cases, peaking at 37,385 reported cases recorded in 1998 [4]. The response to increasing cases, which was organised around national programmes of endemic countries dedicated to HAT control supported by the World Health Organization (WHO) and partners, was effective, and the number of cases reported annually fell below 10,000 in 2009 [5]. gHAT was then included in the WHO roadmap for neglected tropical diseases (NTDs) in 2012, with the goal of elimination as a public health problem (EPHP) by 2020 [6] and subsequently elimination of transmission (EoT) to humans by 2030 [2].
Two main global indicators were subsequently defined to monitor the EPHP process: 1) reducing the annual number of cases to fewer than 2,000 per year by 2020; and 2) a 90% reduction in the area at moderate, high or very high risk (the latter defined as an area that reports in excess of one case/10,000 people/year, averaged over a five-year period). The 90% reduction refers to the period 2016–2020 compared to the 2000–2004 reference period. With fewer than 1,000 cases reported each year since 2018 (565 gHAT and 98 rHAT cases were reported in 2020) the first global indicator was met, however a reduction of 120,000 km2 (83%) in the moderate and high-risk area meant the global 2020 EPHP target was slightly missed but was thought to be achievable by 2022 [2]. Country-specific validation of EPHP by the WHO is conducted through the compilation of a dossier of data to demonstrate that the indicator for national EPHP has been achieved. This indicator is defined as an average of less than one case per 10,000 people per year over a five-year period in each health district. Togo and Côte d’Ivoire were the first countries to be validated by WHO as having achieved EPHP of gHAT [2].
Achieving this goal was challenging in Côte d’Ivoire as the country reported around one hundred gHAT cases a year in the early 2000s [7], the majority of which were in endemic foci in Western-Central forest areas [8]. Since 2009 fewer than 10 gHAT cases were reported annually with the reduction in transmission and detected case numbers driven by active case detection by mobile teams that screen at-risk populations [7,9]. This large epidemiological transition to very low incidence necessitates the adaptation and evolution of control strategies since there are diminishing returns for the same level of effort. This experience is shared by other elimination or eradication programmes such as polio [10] or Guinea worm [11] where previous rapid reductions in case reporting have now been replaced by extremely low but persistent case detections and new intervention approaches have been required. To overcome this end-game challenge, gHAT strategies in Côte d’Ivoire have now shifted to utilise innovative tools both within the classical “screen, diagnose and treat” algorithm and by use of complementary vector control [12]. This article describes the approach led by the Programme National d’Elimination de la Trypanosomiase Humaine Africaine (PNETHA) by focusing on the case reporting results obtained during the period 2015–2019 that enabled the validation of EPHP in Côte d’Ivoire. We also used a mathematical modelling approach to quantitatively evaluate these reporting results and infer progress towards the achievement of EoT of gHAT, demonstrating the importance of an integrated, data-driven approach for sustainable elimination.
Materials and methods
In this section we describe details of the epidemiological context of gHAT in Côte d’Ivoire, the recent activities in intervention and screening by PNETHA, and outline how we have utilised mathematical modelling to retrospectively analyse case reporting results. The paper focuses on the 2015–2019 period on which the EPHP was based, but also takes into account the number of cases reported by PNETHA historically (2000–2014) and after the EPHP dossier was submitted (2020–2021) to further demonstrate the impact of the programme through modelling.
Ethics statement
The gHAT elimination project in Côte d’Ivoire (ElimTrypCI 2016–2019) received ethical clearance from the Comité National d’Ethique de la Recherche, Ministry of Public Health and Hygiene in Côte d’Ivoire (reference 030-18/MSHP/CNER-kp). Prior to inclusion, each potential study participant was informed about the objectives, conduct, benefits and risks of the study in the language of their choice in order to obtain verbal informed consent.
Study area
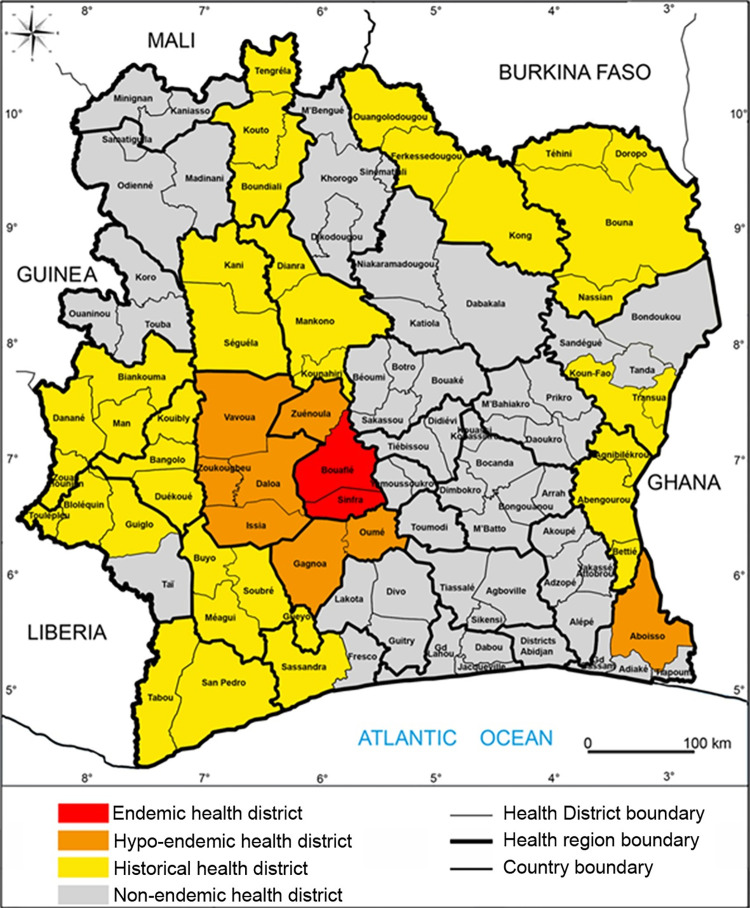
gHAT is characterised in Côte d’Ivoire, as it is throughout Africa, as a focal disease with hotspots of infection [13,14]. Although the focus boundaries are difficult to precisely define, the foci were considered to be epidemiological units until 2014 (S1 Fig). Subsequently, health districts (HD) have been used as the epidemiological units of analysis as a requirement for reporting to WHO for the EPHP. The number of reported cases per HD in Côte d’Ivoire between 2000 and 2014 is given in S1 Table. In 2015 HDs in Côte d’Ivoire can be categorised into four distinct groups (Fig 1):
Fig 1. Study areas and gHAT epidemiological status since 2015.
This figure was created by the mapping service of our team based at Institut Pierre Richet (Bouaké, Côte d’Ivoire). All the base layers are available here: https://data.humdata.org/dataset/cote-d-ivoire-settlements and https://data.humdata.org/dataset/cod-ab-civ.
The endemic HDs of Bouaflé (including the subprefecture of Bonon) and Sinfra which reported cases each year during the period 2000–2010.
The hypo-endemic HDs of Aboisso, Daloa, Issia, Gagnoa, Oumé, Vavoua, Zuénoula and Zoukougbeu which have also reported cases between 2000–2010 but not every year.
The historical HDs in which cases have been reported prior to but not since 2000.
The non-endemic HDs where there has been very little (often unproven) or no past reporting of gHAT before 2000 but where the presence of tsetse is likely (allowing for potential transmission).
Like most other neglected tropical diseases (NTDs), gHAT is not vaccine preventable, so control and elimination activities have been focused on two key parts of the transmission cycle:
Treatment of infected individuals which acts both to prevent disease mortality but also to reduce the time people spend infected and infectious to tsetse vectors.
Targeting the tsetse vector with the goal of reducing the number of transmission events.
Unlike several other NTDs, the treatment pathway for gHAT currently requires confirmation of infection prior to treatment, so mass drug administration or “screen-and-treat” strategies cannot currently be used. The range of medical and vector interventions routinely recommended and deployed to tackle gHAT are presented elsewhere [3]. However, the gHAT control strategy is variable from region to region and over time and so here we outline the different screening, diagnostic and vector control approaches that have formed part of the gHAT elimination strategy in Côte d’Ivoire during the 2015–2019 period.
Active screening
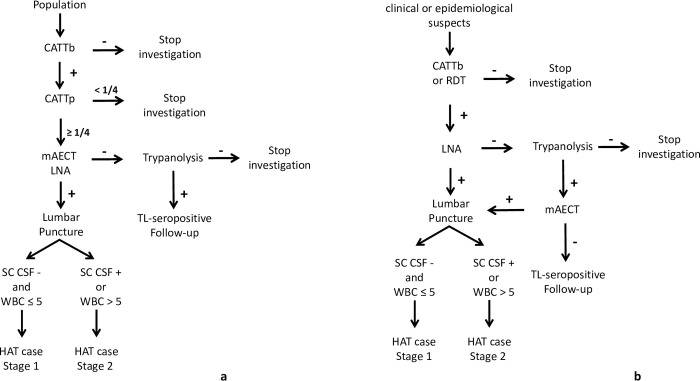
Active screening can be exhaustive for the entire population of a given area, or targeted at a population particularly at risk. In all cases, the first step for active screening carried out by the mobile teams involved informing the administrative and customary authorities and raising awareness among the populations targeted for screening–typically exhaustive active screening has aimed to recruit as many people as possible in villages identified for screening. Diagnosis of exhaustive active screening was made based on the decision algorithm shown in Fig 2A. The card agglutination test for trypanosomiasis (CATT) [15] on whole blood collected by finger puncture (CATTb) was carried out in all the people who presented themselves to the mobile team during the screening activity. In the case of a positive result with CATTb, a sample was taken from the bend of the elbow (5ml of heparinized blood) to perform the CATT on plasma dilution (CATTp). “Seropositives” were defined to be individuals with a positive CATTp at a dilution of at least ¼ (CATTp≥1/4) underwent parasitological examination: mini anion exchange centrifugation test with buffy coat (mAECT BC, [16]) and microscopic examination between slide and coverslip (x400) of lymph node aspirate (LNA) in cases where cervical lymphadenopathy was present. A seropositive who was positive on at least one parasitological examination was confirmed as a gHAT case, denoted by T.
Fig 2. gHAT diagnosis algorithm for active screening and targeted active screening 2015–2019.
a. gHAT diagnosis algorithm for active screening. b. gHAT diagnosis algorithm for targeted active screening conducted during the “identification of villages at risk” strategy. CATTb = card agglutination test for trypanosomiasis performed on whole blood; CATTp = card agglutination test for trypanosomiasis performed on plasma dilution; RDT = rapid diagnostic test; mAECT BC = miniature anion-exchange centrifugation technique performed using buffy coat; LNA = lymph node aspirate; SC-CSF = simple centrifugation of cerebrospinal fluid; WBC = CSF white blood cell/μl;— = negative; + = positive. The “identification of villages at risk” (IVR) strategy was previously described [19].
Seropositives negative in parasitology were tested with the immune trypanolysis test (TL) [17], performed at Centre International de Recherche-Développement sur l’Elevage en zone Subhumide (CIRDES, Bobo-Dioulasso, Burkina-Faso). TL-positive subjects were considered to be “TL-seropositive” (denoted by S) and potential carriers of trypanosomes. A targeted active screening strategy consisted of a follow-up of these TL-seropositive subjects once a year according to the algorithm in Fig 2A until the serological tests were negative or until parasitological confirmation and treatment.
To be more effective in a context of low prevalence, other targeted active screening strategies were put in place. These included door-to-door [8] and spatial follow-up [18] by which it was possible to screen the family in a more friendly fashion as well as the most-at-risk populations that share the same daily spaces as the gHAT cases and TL-seropositive. The diagnostic algorithm of this targeted active screening was the same as the exhaustive active screening (Fig 2A).
Targeted active screening was also performed at villages previously identified as being at greatest risk in a given area based on historical, epidemiological and geographical data as defined by the so-called “identification of villages at risk” (IVR) strategy [19]. This strategy was mainly applied in the historical HDs. During IVR activities, clinical and epidemiological suspects were tested with a simplified algorithm (Fig 2B). Serology was based on CATTb or a rapid diagnostic test (RDT SD1, Abbott Diagnostics, South Korea) [20] and parasitology performed on CATTb or RDT positive subjects, was based only on LNA if enlarged lymph nodes were present. For CATTb or RDT positive subjects, a sample of blood dried on filter paper was collected to perform TL. In case of LNA-positive subjects (confirmed gHAT cases) or when the TL was positive on at least one LNA-negative subjects, the village concerned was automatically selected for exhaustive active screening. The LNA-negative and TL-positive subjects were tested using mAECT BC. The mAECT positive subjects were confirmed gHAT cases and the negative ones were considered as TL-seropositive subjects targeted for follow-up as described above.
Stage diagnosis was performed for all confirmed gHAT cases and was based on the technique of simple centrifugation of cerebrospinal fluid (SC-CSF) to allow visualisation of trypanosomes [21] and in the white blood cell (WBC) count in CSF (as WBC/μl). Cases who were negative for trypanosomes in CSF and with ≤ 5 WBC/μl were classified as having stage 1 disease, typically with mild or no symptoms. Cases who had trypanosomes visualised in CSF or with > 5 WBC/μl were considered to be in stage 2, typically with neurological disorders. People in stage 1 disease were treated with pentamidine and those in stage 2 with nifurtimox-eflornithine combination therapy (NECT) [22]. Post-treatment follow-up including lumbar puncture was performed only in the case of clinical suspicion of relapse as recommended by WHO [3].
Passive screening
The national health system in Côte d’Ivoire is based on a three-level pyramid, with health centres that serve as an entry point at the first and peripheral level, general and regional hospitals at the secondary level and university hospitals and specialized institutes at the tertiary level. These public health structures are complemented by private clinics and hospitals as well as a network of traditional medicine. According to the Ministry of Health in 2018, indicators of human resource availability were nationally at 1.4 physicians per 10,000 population and 2.3 nurses per 5,000 population. The national average rate of service use was 47.5% with wide disparities across health regions.
Passive screening refers to diagnosis made in fixed health facilities and was based on clinical suspicion, with the following symptoms considered suggestive of gHAT disease: 1) long-term fever and no effect of antimalarial treatment, 2) headache for a long period (> 14 days), 3) presence of enlarged lymph nodes in the neck, 4) severe weight loss, 5) weakness, 6) severe pruritus, 7) amenorrhea, abortion(s), or sterility, 8) psychiatric problems (aggressiveness, apathy, mental confusion, unusual increasing hilarity), 9) sleep disturbances (nocturnal insomnia and excessive daytime sleep), 10) motor disorders (abnormal movements, tremor, difficulty walking), 11) speech disorders, 12) convulsion, 13) coma [23]. All subjects in whom at least one of these symptoms was observed were considered clinical suspects. The algorithm used for active screening and described in Fig 2A was applied at the Projet de Recherches Cliniques sur la Trypanosomiase (PRCT, Daloa) reference center for the diagnosis and treatment of gHAT and the only sentinel site for passive screening until 2017.
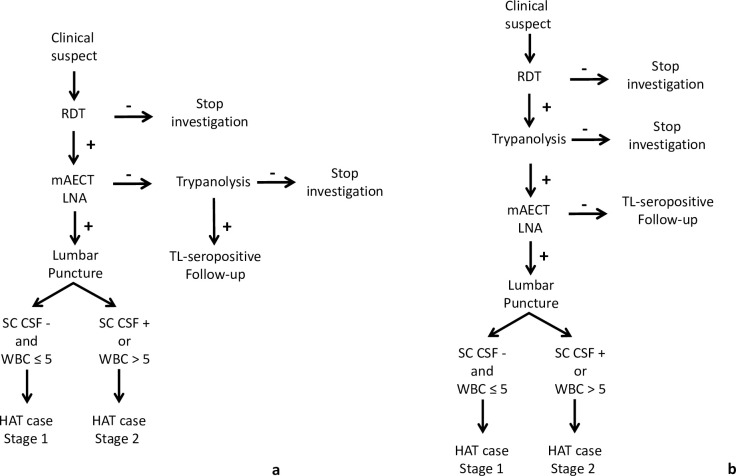
In August 2017, passive screening was set up in 10 health centres of the endemic HDs of Bouaflé (Bonon focus) and Sinfra as part of the DiTECT-HAT research [23]. The diagnostic algorithm that was used is presented in Fig 3A. Clinical suspects were tested with three RDTs (SD1, HAT Sero-K-Set (Coris BioConcept, Belgium), and rHAT Sero-Strip (Coris BioConcept, Belgium)). Subjects who tested positive with at least one RDT (seropositive) were tested by parasitology (mAECT BC and LNA). Staging was performed on confirmed parasitological cases. TL on blood dried on filter paper [24] was then carried out on RDT-positive but parasitology-negative subjects. TL-positive subjects were considered TL-seropositive targeted for targeted active screening as described above.
Fig 3. gHAT diagnosis algorithm for passive screening.
a) gHAT diagnostic algorithm for passive screening in endemic health districts since August 2017. b. gHAT diagnostic algorithm for passive screening in hypo-endemic health districts and national coverage facilities since May 2018. RDT = Rapid Diagnostic Test; mAECT BC = miniature anion-exchange centrifugation technique performed with buffy coat; LNA = lymph node aspirate; SC-CSF = simple centrifugation of cerebrospinal fluid; WBC = CSF white blood cell/μl;— = negative; + = positive.
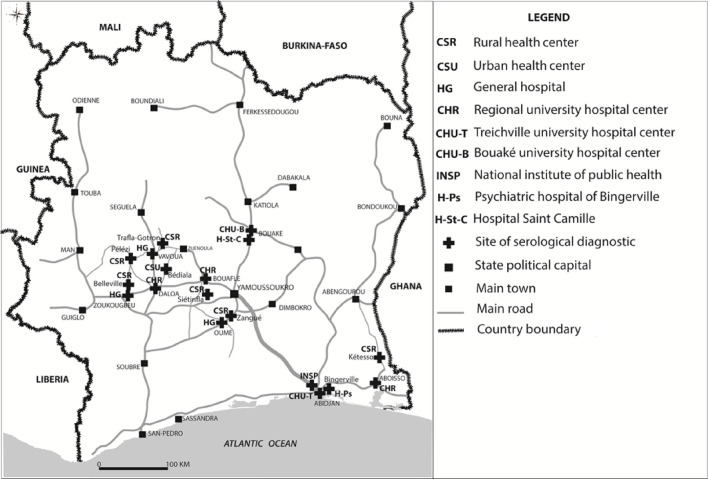
In May 2018, passive screening was also set up in 13 health centres in the hypo-endemic HDs. The 13 health centres were selected based on epidemiological data (geographical distribution of the last cases detected) and their areas of influence, and in five neurology or psychiatry services in Bingerville (1), Bouaké (2) and Abidjan (2) (Fig 4) for national coverage. The diagnostic algorithm used was based on the RDT SD1 (Fig 3B). The 18 sites were supervised every three months and capacity building for doctors, nurses and laboratory technicians was carried out every year to optimise the effectiveness of the gHAT monitoring programme.
Fig 4. Sentinel sites for passive surveillance in hypo-endemic health districts and national coverage facilities 2015–2019.
This figure was created by the mapping service of our team based at Institut Pierre Richet (Bouaké, Côte d’Ivoire). All the base layers regarding administrative data are available in: https://data.humdata.org/dataset/cote-d-ivoire-roads, https://data.humdata.org/dataset/cote-d-ivoire-settlements and https://data.humdata.org/dataset/cod-ab-civ.
Strengthening of the capacities of the health workers, including the gHAT clinical suspicion and diagnosis, preceded the implementation of passive screening in these 28 gHAT sentinel sites.
Vector control
Vector control (VC) provides a complementary method to screening and treatment and has been used to reduce tsetse populations and interrupt gHAT transmission in a variety of geographies (e.g. Chad, Democratic Republic of Congo, Guinea, and Uganda) [25,26,27]. In Côte d’Ivoire, VC mostly using Tiny Targets [28,29] but also Vavoua traps [30] both impregnated with deltamethrin, began in January 2016 in the HD of Bouaflé (Bonon focus). The first three years (until December 2018) of the Bonon intervention has been described elsewhere [31,32] but we summarise this and provide an update on the results obtained until December 2019. The first deployment in Bonon took place in February 2016 with 1,890 Tiny Targets. During annual redeployments in February 2017, February 2018 and February 2019, additional Tiny Targets were added to reach a total of 2,016 deployed in February 2019 (Fig 5A). It is during this redeployment that 57 Vavoua traps were set to reinforce VC in areas where tsetse were still being caught during periodic entomological assessments.
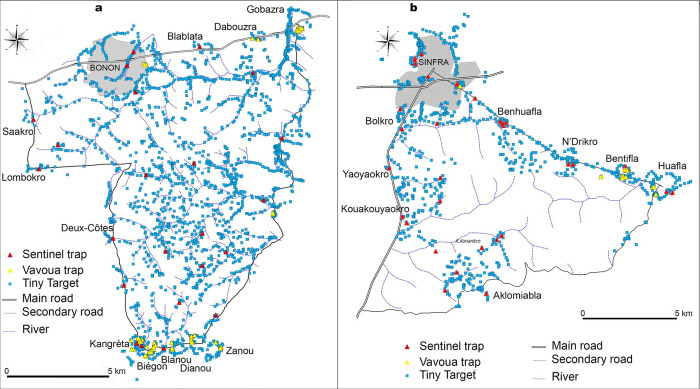
Fig 5. Distribution of control devices in endemic health districts.
a. Distribution of Tiny Targets and Vavoua traps in December 2019 and sentinel traps in the Bouaflé health district (Bonon focus). b. Distribution of Tiny Targets and Vavoua traps in December 2019 and sentinel traps in the Sinfra health district. Vavoua traps and Tiny Targets are impregnated control devices. Sentinel traps are unimpregnated Vavoua traps for capture. This figure was created by the mapping service of our team based at Institut Pierre Richet (Bouaké, Côte d’Ivoire). All the base layers regarding administrative data are available in: https://data.humdata.org/dataset/cote-d-ivoire-roads, https://data.humdata.org/dataset/cote-d-ivoire-settlements and https://data.humdata.org/dataset/cod-ab-civ.
A more targeted VC began in May 2017 in the HD of Sinfra; the intervention aimed to control tsetse densities by deploying Tiny Targets supplemented by Vavoua traps at human/tsetse contact points in areas where the risk of transmission was believed to be highest. A total of 736 Tiny Targets were deployed in May 2017 and redeployed in July 2018 and July 2019. In July 2018, 115 additional Tiny Targets and 44 Vavoua traps were also deployed to reinforce VC in areas where tsetse were still being caught during periodic entomological assessments. In July 2019, the 44 Vavoua traps were replaced by Tiny Targets but 12 other additional Vavoua traps were set. At the end of 2019, 895 Tiny Targets and 12 Vavoua traps were deployed in the Sinfra HD (Fig 5B).
A T0 entomological survey using unimpregnated Vavoua traps for capture was conducted in the two foci before the first deployment of Tiny Targets. This T0 survey made it possible, in addition to sensitising local communities, (i) to delineate the intervention areas, (ii) to characterise the tsetse populations (species, densities, distribution) and (iii) to determine the distribution of control devices to be deployed. All traps were set for 48 or 96 hours and georeferenced using a GPS. Fly collection was made for two or four consecutive days and captured tsetse were identified by species with a magnifying glass and an identification key [33]. Apparent densities of flies per trap per day (ADTs) were determined.
To monitor the results of the VC campaign, the locations of sentinel traps (unimpregnated Vavoua traps for capture) were selected from those set during the T0 survey, on the basis of the ADT and to ensure homogeneous spatial coverage of the study area. These sentinel traps represented 10% of the total traps in T0. Quarterly entomological assessments used 30 sentinel traps in Bonon and 35 sentinel traps in Sinfra. All traps were set for 48 hours. Fly collection was made for two consecutive days and captured tsetse were identified by species [33]. ADTs were compared with those of T0.
Modelling
For the modelling approach, we report on data for the period 2000–2021. S1 Data provides detailed active and passive case data partitioned by stage for the locations where there are sufficient data to conduct model fitting (Bonon, Bouaflé subprefecture, Daloa, Oumé and Sinfra); only HDs with 10 or more data points (one data point is counted as a year with at least one passive case detected, or one active screening) were included as fewer data points were not deemed sufficient to provide a robust model fit. Other HDs are not included in that file and are omitted from model fitting, but aggregate case data (totals per year per location) are included in S1 Table. Following implementation of the different interventions and data collection we used mathematical modelling to assess these data and provide quantitative analysis of progress in Côte d’Ivoire. The modelling was used to estimate the underlying number of new infections each year between 2000 and 2021 and therefore assess the reduction in transmission over this time period. To achieve this, a previously developed mechanistic model (the “Warwick gHAT model”), originally published in [34], and more recently updated for the DRC [35] and Chad [36], was adapted for the context in Côte d’Ivoire and fitted to the annual time series data collected by PNETHA. The model has recently been described in detail elsewhere [35], and model equations and parameters are given in the S1 Text. Briefly, this model captures the natural history of infection in humans from exposure to the parasites and the relatively long progression through stage 1 and, if not treated, stage 2 infection. We simulate detection and treatment of cases via both active and passive screening, with the number of active cases identified within a year linked to the number of people tested by mobile teams. Tsetse are explicitly included in the model to capture human-tsetse-human transmission cycles and, furthermore, it is assumed that there is differential risk in exposure to tsetse bites between different people. As described in the vector control section, from 2016 and 2017, vector control was implemented in Bonon (in Bouaflé HD) and Sinfra, respectively, and the corresponding reduction in tsetse populations is included in the transmission model for these foci. Tsetse trap data were used to inform the parameters associated with observed tsetse population reduction by fitting the tsetse population dynamic sub-model to these data via maximum likelihood estimation (see S1 Text).
To fit the full deterministic epidemiological model to data we use a Markov chain Monte Carlo (MCMC) methodology (see S1 Text) which compares annual active and passive case reporting simulated in the model to those observed in the data for each year for each HD. We carried out fitting for the two endemic HDs–Bouaflé and Sinfra–and two hypo-endemic HDs–Daloa and Oumé. Other listed hypo-endemic HDs and the historical and non-endemic HDs were not included in model fitting due to insufficient data points. As Bouaflé HD is comprised of two epidemiological foci–Bouaflé subprefecture and Bonon–and only one of these had the vector control intervention deployed there, for the Bouaflé HD fitting we considered these small geographical units independently before aggregating to HD level.
The fitted model parameters are found in Table D in S1 Text and include the basic reproduction number (R0), the relative risk of high-risk people being bitten by tsetse compared to low-risk people (r), the proportion of the population who are low risk (k1), the proportion of stage 2 cases that go on to be reported (as opposed to those that die undetected) (u), and passive detection rates (ηH, γH). In some years the number of people tested in active screening was unknown, consequently this value was inferred during fitting. All these parameters were fitted independently in each region as they are assumed to be geographically variable. Prior parameter distributions for these fitted parameters and fixed parameter values can be found in the Tables D and C in S1 Text, respectively.
In addition to inferring model parameters, the fitting process enabled us to estimate the expected number of annual new infections in each HD during the data period (2000–2021) and therefore to quantify the reduction in transmission over time. It also allowed us to calculate the probability that each location had achieved local EoT by a given year.
Results
gHAT situation during the 2000–2014 period
With the results of this article focusing on the 2015–2019 period, it is apt to describe the epidemiological situation observed before this period, based on the number of cases reported between 2000 and 2014 by the PNETHA (S1 Table). A total of 650 gHAT cases were reported, most of them from the Bonon subprefecture (323) and Sinfra HD (176) where the last two epidemics were recorded: early 2000s for Bonon [37,38] and mid-1990s for Sinfra [39,40]. During this period, 151 cases were recorded in the hypo-endemic HD (from one case in Gagnoa, Issia and Zuénoula HD to 50 cases in the Daloa HD). In all HDs, the number of cases gradually decreased over time. The last cases were recorded before 2006 in Aboisso, Gagnoa, Issia and Zuénoula HD, in 2008 in Zoukougbeu, in 2012 in Vavoua and in 2013 in Bouaflé subprefecture, while some cases were still reported in 2014 in the Bonon subprefecture, Sinfra, Daloa and Oumé.
Active screening during 2015–2019
The results of exhaustive active screening activities carried out between 2015 and 2019 in the endemic HDs of Bouaflé and Sinfra and the hypo-endemic HD of Aboisso are presented in Table 1. A total of 13,074 people were tested, a single confirmed case of gHAT was detected in the HD of Bouaflé in 2015 and four TL-seropositives were identified (three in Bouaflé and one in Sinfra). The results of the exhaustive active screening activities carried out between 2017 and 2019 in the historical HDs are presented in S2 Table. A total of 28,796 people were tested and no gHAT cases or TL-seropositives were identified.
Table 1. Results of active screening activities in endemic and hypo-endemic health districts.
| Health district (year) | PEx | S | T |
|---|---|---|---|
| Bouaflé (2015) | 5744 | 3 | 1 |
| Sinfra (2016) | 3415 | 1 | 0 |
| Aboisso (2018) | 3915 | 0 | 0 |
| Total | 13074 | 4 | 1 |
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases
Table 2 presents the results of targeted active screening conducted on populations sharing the same spaces as the last gHAT cases and TL-seropositives identified mainly in the HDs of Sinfra and Bouaflé, considering the results of spatial follow-ups in particular. A total of 3,105 people were tested between 2017 and 2019 but no cases or TL-seropositive individuals were identified. The results of targeted active screening activities using the IVR method by HD between 2017 and 2019 are presented in S3 Table. A total of 5,093 clinical and epidemiological suspects were tested but no cases or TL-seropositives were identified.
Table 2. Results of targeted active screening on populations sharing the same spaces as the last gHAT cases and TL-seropositives.
| Year | 2017 | 2018 | 2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Health District | PEx | S | T | PEx | S | T | PEx | S | T |
| Bouaflé | 121 | 0 | 0 | 204 | 0 | 0 | 1327 | 0 | 0 |
| Sinfra | 558 | 0 | 0 | 112 | 0 | 0 | 678 | 0 | 0 |
| Vavoua | 25 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| Daloa | 64 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 0 |
| Total | 768 | 0 | 0 | 316 | 0 | 0 | 2021 | 0 | 0 |
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases
Follow-up results of TL-seropositive subjects are shown in Table 3. A total of 97 subjects were followed and tested. One case was detected in 2017 in the HD of Bouaflé and one in 2019 in Sinfra. In 2019, 18 subjects were still serologically positive and four of them were positive for both CATTp and TL. The case detected in Sinfra had been monitored for more than 20 years while living in Abidjan for 15 years. The case of Bouaflé was first identified as a TL-seropositive in 2014 and he stayed in his village.
Table 3. Results of follow-up of TL-seropositive subjects.
| Year | 2015 | 2017 | 2018 | 2019 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health district | PEx | S | T | PEx | S | T | PEx | S | T | PEx | S | T |
| Bouaflé | 13 | 10 | 0 | 18 | 13 | 1 | 16 | 9 | 0 | 17 | 9 | 0 |
| Sinfra | 0 | 0 | 0 | 9 | 7 | 0 | 2 | 1 | 0 | 13 | 8 | 1 |
| Vavoua | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Daloa | 2 | 2 | 0 | 2 | 2 | 0 | 2 | 1 | 0 | 2 | 1 | 0 |
| Total | 15 | 12 | 0 | 29 | 22 | 1 | 20 | 11 | 0 | 33 | 18 | 1 |
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases
Passive screening during 2015–2019
Between 2015 and 2019 a total of 169 people were tested and two cases of gHAT detected at the PRCT of Daloa, the reference centre for the diagnosis and treatment of gHAT recognized as such for decades by the populations of the gHAT foci of the West Central Côte d’Ivoire [9]. Both of the cases were detected in 2015 from the 33 people tested that year. One of the two cases was from the HD of Sinfra and the other from the HD of Bouaflé. No further cases were detected from the 136 people tested at PRCT Daloa between 2016 and 2019.
Table 4 presents the results of the passive screening implemented between 2017 and 2019 in the endemic HDs of Sinfra and Bouaflé. A total of 3,433 clinical suspects were tested and two cases were reported in 2017, one in Sinfra and one in Bouaflé. They were diagnosed as stage 2 infections (the case in Sinfra was very advanced) as already described [23]. A third person, positive with the three RDTs and TL but negative in parasitology identified in Bouaflé HD in 2018, died following a sudden neurological deterioration without it being possible to confirm the gHAT diagnosis using further parasitological investigations. Given the strong clinical and serological suspicion then confirmed by other serological and molecular tests, this case was considered a serological gHAT case, i.e. a confirmed case, in the PNETHA registers. No cases or TL-seropositives were identified in 2019.
Table 4. Results of passive screening in the endemic health districts.
| Year | 2017 | 2018 | 2019 | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health District | PEx | S | T | PEx | S | T | PEx | S | T | PEx | S | T |
| Bouaflé | 175 | 2 | 1 | 695 | 2 | 1* | 736 | 0 | 0 | 1606 | 4 | 2 |
| Sinfra | 180 | 2 | 1 | 675 | 0 | 0 | 972 | 0 | 0 | 1827 | 2 | 1 |
| Total | 355 | 4 | 2 | 1370 | 2 | 1 | 1708 | 0 | 0 | 3433 | 6 | 3 |
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases
*The serological case identified in Bouaflé HD in 2018
The results of the passive screening implemented in 2018 and 2019 as part of sentinel site surveillance are shown in S4 Table. A total of 605 clinical suspects were tested, including 84 in national coverage facilities and 521 in hypo-endemic HDs. While five individuals were RDT-positive in hypo-endemic HDs, no cases or TL-seropositives were identified.
A case of gHAT was confirmed and treated in 2018 in Koudougou in Burkina Faso as part of the passive surveillance set up there. The epidemiological investigation showed that this case was most likely infected near Bonon where he lived from 2001 (his birth) to 2018 before moving to Koudougou for health reasons. The clinical questionnaire revealed significant neurological damage linked to an infection dating back several years. The case was included in the PNETHA registers as a confirmed case in 2018 from the HD of Bouaflé.
Therefore, in total, nine cases of gHAT were detected between 2015 and 2019 that were likely to have been infected in Côte d’Ivoire, six in the HD of Bouaflé and three in that of Sinfra (Table 5). Of these nine cases, three were found in active screening (Table 6), including one in exhaustive active screening, and two during the follow-up of TL-seropositives. The six other cases were detected passively, including two at the PRCT in Daloa, three at the HDs of Sinfra and Bouaflé and one at Koudougou in Burkina Faso. The nine cases were diagnosed as stage 2 infections. Those detected in Côte d’Ivoire were treated with NECT. The case detected in Koudougou was treated with α-difluoromethylornithine (DFMO) according to the national procedure in Burkina Faso.
Table 5. Number of gHAT cases detected per year between 2015 and 2019.
| Number of gHAT cases / year | ||||||
|---|---|---|---|---|---|---|
| Health District | 2015 | 2016 | 2017 | 2018 | 2019 | Total |
| Bouaflé | 2 | 0 | 2 | 2* | 0 | 6 |
| Sinfra | 1 | 0 | 1 | 0 | 1 | 3 |
| Total | 3 | 0 | 3 | 2 | 1 | 9 |
* including the gHAT case diagnosed in Burkina Faso
Table 6. Method of screening of the gHAT cases detected between 2015 and 2019.
| Number of gHAT cases | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Year | 2015 | 2016 | 2017 | 2018 | 2019 | |||||
| Health District | A | P | A | P | A | P | A | P | A | P |
| Bouaflé | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 2* | 0 | 0 |
| Sinfra | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
* including the gHAT case diagnosed in Burkina Faso
A = Active detection; P = Passive detection
Data according to the national indicator
Table 7 gives data for the national indicator for EPHP as defined by the WHO (average number of gHAT cases per year over 5 consecutive years/10,000 inhabitants, by HD [2]). Only the HDs of Bouaflé and Sinfra are shown as these are the only HDs in which cases were reported between 2015 and 2019. Relative to the total population of the two HDs, the indicator was far below 1/10,000, a necessary condition for validating the EPHP.
Table 7. Number of gHAT cases according to the national indicator as defined by the WHO.
| Health District | gHAT cases 2015 | gHAT cases 2016 | gHAT cases 2017 | gHAT cases 2018 | gHAT cases 2019 | Average cases/year | Average population* | n/10,000 inhabitants |
|---|---|---|---|---|---|---|---|---|
| Bouaflé | 2 | 0 | 2 | 2 | 0 | 1.2 | 725,778 | 0.017 |
| Sinfra | 1 | 0 | 1 | 0 | 1 | 0.6 | 354,954 | 0.017 |
* Source: Institut National de Statistique, Recensement Général de la Population et de l’Habitat (RGPH) 1998 and 2014
Vector control
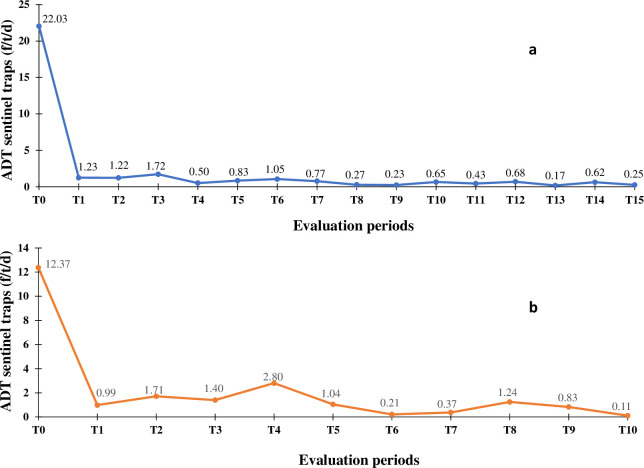
In Bonon, 267 traps were set during the T0 survey carried out in June 2015. In total, 1,894 flies of the species Glossina palpalis palpalis were captured, i.e. an average ADT of 3.54 flies/trap/day. No other tsetse species was caught. The average ADT of the 30 sentinel traps (1,322 tsetse captured) was 22.03 flies/trap/day. It was 0.25 flies/trap/day (15 tsetse captured) during the 15th entomological evaluation (T15, December 2019), i.e. a reduction of 98.9% in tsetse density (Fig 6A). In Sinfra, the T0 survey was carried out in November 2016 with 339 traps set and 988 flies of the species G. p. palpalis captured (ADT 1.45 flies/trap/day). No other tsetse species were caught. The average ADT of the 35 sentinel traps was 8.99 flies/trap/day with 866 tsetse captured. It was 0.11 flies/trap/day (8 tsetse captured) during the 10th entomological evaluation (T10, December 2019), i.e. a reduction of 98.8% in tsetse density (Fig 6B).
Fig 6. Evolution of the tsetse apparent density per trap during the entomological evaluations.
a. Evolution of the tsetse apparent density per trap during the entomological evaluations from T0 (June 2015) to T15 (December 2019) in the Bouaflé health district (Bonon focus). b. Evolution of the tsetse apparent density per trap during the entomological evaluations from T0 (November 2016) to T10 (December 2019) in the Sinfra health district. ADT = Apparent Density per Trap; f/t/d = tsetse flies/trap/day.
Modelling
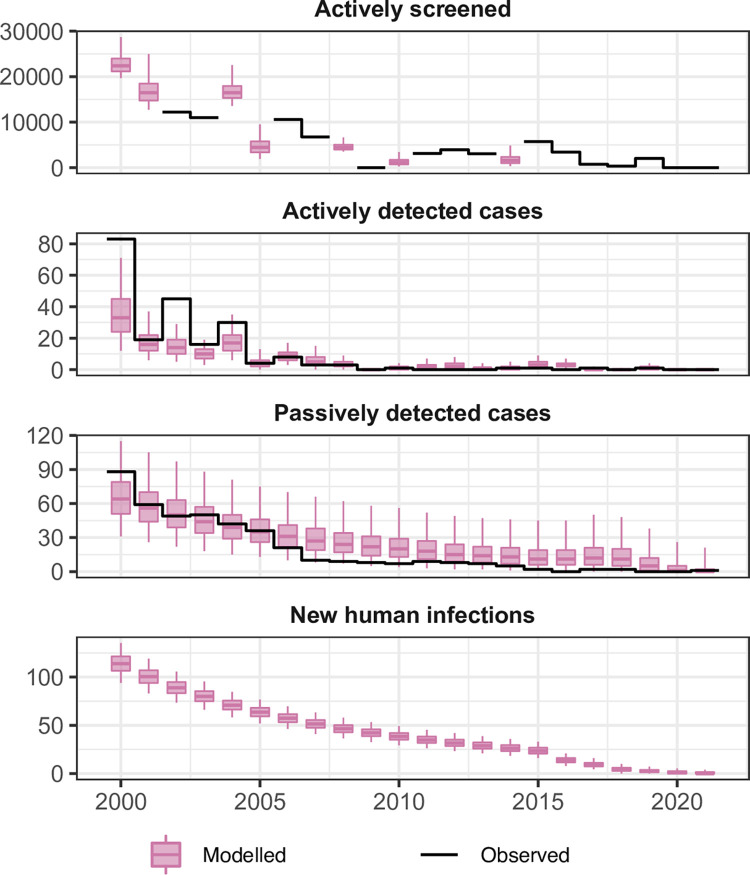
Fig 7 shows the aggregated results for fitting the deterministic model to the longitudinal gHAT case data for Bouaflé, Sinfra, Oumé and Daloa HDs for the 2000–2021 period. Individual HD fits (or subprefecture fits in the case of Bouaflé HD) can be found in the S1 Text. Much of the case reporting dynamics appear to be driven by Bonon subprefecture of Bouaflé HD which accounted for between 19 and 100% of all actively reported cases and 11–62% of passively reported cases during 2000–2008 for the fitted foci. Similarly, Bonon subprefecture was estimated to contribute 25–56% of annual new infections. Passive case reporting in Sinfra occurred at a similar level to Bonon (24–67% of cases between 2000–2008) and saw a slow but steady decline. Active case reporting in Sinfra mirrored the pattern in active screening coverage during the 2000s. Daloa and Oumé HDs and Bouaflé subprefecture all had very low case reporting, never reaching more than 14 cases per year per foci across 2000–2021; correspondingly the model fitting produced very low estimates of annual new infections.
Fig 7. Aggregate fits to the data from Bouaflé, Sinfra, Oumé and Daloa health districts between 2000–2021.
The black lines show the observed data (either number of people screened or cases) and the pink box and whisker plots show the deterministic model with stochastic sampling (centre line is the median, boxes contain 50% credible intervals (CIs) and whiskers show 95% CIs). Some years of data are missing the total number of people actively screened so this was estimated during fitting with results shown as box and whiskers. New infections are not directly observable and are estimated through the model based on case reporting.
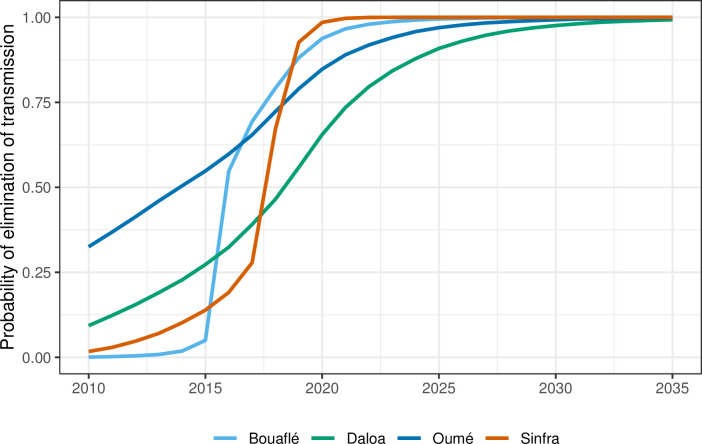
By 2011 there was extremely little case reporting across all foci. Fig 8 shows the year in which transmission was estimated to be interrupted for each HD–for this calculation we utilised the analogous stochastic version of our model and the posterior parameterisation to better factor in chance events around EoT and remove the need to use a proxy threshold to compute EoT using deterministic outputs (see S1 Text for more details). In the Bouaflé subprefecture, Daloa, and Oumé, we computed that there was a moderate probability of EoT occurring in 2015 or earlier, however there is some considerable uncertainty in the year of EoT in these locations. In Bonon and Sinfra the use of highly impactful VC (from 2016 and 2017, respectively) coupled with the low or zero case reporting in recent years, results in model estimates of 2016 and 2018 for the average year of EoT (see Fig 8 and Fig M in S1 Text) and these estimates have less uncertainty than the other HDs. Our modelling estimated that 52–71% of the transmission reduction across HDs likely occurred between 2000–2010, however it was after 2010 that transmission was likely interrupted (See Table 8 and Table H in S1 Text).
Fig 8. Estimated probability of elimination of transmission (EoT) by year for Bouaflé, Sinfra, Oumé and Daloa health districts.
The probability is computed by assessing the proportion of stochastic model simulations where there are zero new transmission events for that year and the subsequent years for each year for each location. For disaggregation of Bouaflé HD into Bonon and Bouaflé subprefectures see S1 Text.
Table 8. Estimated transmission reduction for Bouaflé, Sinfra, Oumé and Daloa health districts.
| Health district | Transmission reduction | ||
|---|---|---|---|
| 2000–2010 | 2011–2021 | 2000–2021 | |
| Bouaflé | 70.9% [60.0–79.6%] | 100% [100–100%] | 100% [100–100%] |
| Daloa | 54.9% [24.4–73.9%] | 100% [61.1–100%] | 100% [74.9–100%] |
| Oumé | 52.0% [20.6–73.5%] | 100% [62.8–100%] | 100% [75.4–100%] |
| Sinfra | 67.0% [47.8–80.0%] | 100% [100–100%] | 100% [100–100%] |
The reductions are computed through model fitting to historical data with the deterministic model. Medians and 95% credible intervals are given.
Discussion
gHAT control activities in Côte d’Ivoire have been based on an integrated approach, consisting of a combination of medical interventions (active and passive screening followed by treatment) and vector control. The results of active screening and identification of villages at risk have shown that there is most likely very little or no transmission of T. b. gambiense in historical HDs. Indeed, no gHAT cases or TL-seropositives were identified out of nearly 34,000 people tested between 2017 and 2019. Exhaustive and targeted active screening and passive screening activities also support the hypothesis of low or no transmission in hypo-endemic HDs with no cases detected even in the PRCT of Daloa.
The results of active screening have shown a clear reduction in the reported prevalence of the disease in the HDs of Bouaflé and Sinfra. They have also justified the gradual abandonment of exhaustive active screening in favour of targeted active screening and passive screening already described in several gHAT foci [41]. These strategies, however, confirmed that two HDs still had an extremely low number of cases, all in second stage, during 2015–2019. The results shown in the present study confirm the continued trend of a decrease in case reporting already observed since the beginning of the 2000s and the discovery of the last active focus of gHAT in Côte d’Ivoire [42,43], and this is despite the socio-political crisis that Côte d’Ivoire went through between 2002 and 2012 [9,38]. Monthly supervision and annual retraining of the health workers involved in this project have contributed greatly to the effectiveness of the implemented strategy and to the reliability of data.
Modelling suggests that there is a corresponding decrease in underlying transmission, and all HDs have a very high probability that EoT has already occurred in Côte d’Ivoire. Collected data confirm the importance of having adapted screening strategies by targeting areas and populations at risk and which made it possible to detect the majority of the remaining gHAT cases [8,23,44,45]. The fact that all the notified cases were in stage 2 of the disease indicates that these are likely to be relatively old infections and there is probably an absence of recent transmission.
The vector control carried out in the HDs of Bouaflé (Bonon focus) and Sinfra led to a sharp drop in tsetse densities from the first deployment of Tiny Targets and/or traps. A tsetse density reduction of more than 90% was rapidly achieved in each focus and maintained until the end of 2019. The presence of residual populations of tsetse was maintained in conserved forests consisting essentially of sacred forests (often on the outskirts of villages) in which the laying of screens and traps was often forbidden. These forests constitute favourable biotopes for tsetse, due to the presence of free ranging domestic pigs which frequent them regularly and constitute an ideal source of food [31,46,47], in addition to other possible hosts such as reptiles. Pigs have already been described as a preferential feeding host for G. p. palpalis [48,49], the only tsetse species present in the two vector control areas. Nevertheless, vector control is believed to have had a substantial impact on the risk of transmission, as has already been described for the Bonon focus [31] and is supported for both Bonon and Sinfra by the modelling analysis conducted as part of the present study.
The gHAT epidemiology in Côte d’Ivoire also depends on the gHAT situation in neighbouring countries. Côte d’Ivoire has a border with five endemic gHAT countries: Liberia, Guinea, Mali, Burkina Faso and Ghana (Fig 1) with large cross-border mobilities that pose a risk of spreading gHAT from border countries to Côte d’Ivoire but also from Côte d’Ivoire to neighbouring countries. In the past, most of Côte d’Ivoire’s historic foci were in direct contact with foci in neighbouring countries [50]. But since 2000, no gHAT cases have been detected in a cross-border foci and no cases in Côte d’Ivoire appear to have been infected in a neighbouring country, although we cannot rule that out. Since 2015, very few cases have been reported from neighbouring countries in which there no longer seem to be active foci except on the Guinean coast [2], which is very far from Côte d’Ivoire. The risk of gHAT spreading in Côte d’Ivoire from a neighbouring country is therefore very low. Cases imported from Côte d’Ivoire have been regularly reported in Burkina Faso due to the large historical and recent population movements between these two countries [9,51,52]. However, the decrease in prevalence in Côte d’Ivoire has reduced the risk of spread to Burkina Faso and the case detected in Koudougou in 2018 (infected in the Bouaflé HD) is the latest reported.
It is important in this article to mention other phenomena that have not prevented the achievement of the EPHP of gHAT in Côte d’Ivoire but which should be considered as key in regard to the EoT. This is particularly the case of the role of a domestic or wild animal reservoir in the T. b. gambiense epidemiology that is still under debate [53]. In Côte d’Ivoire, free-ranging pigs have been identified in the Sinfra, Bonon and Vavoua foci as a multi-reservoir of T. brucei and/or T. congolense with mixed infections of different strains [46,47]. This trypanosome diversity hinders the easy and direct detection of T. b. gambiense. It is important to stress both the lack of tools to prove or exclude with certainty the presence of T. b. gambiense, and the need of technical improvements to explore the role of pigs and animals in general, in the epidemiology of gHAT.
A residual human reservoir of T. b. gambiense could also compromise the EoT in areas where tsetse are still present. TL-seropositive individuals (positive with either CATT or RDTs and with the highly specific TL test, but negative with parasitological tests) have been identified in both endemic HD (Bouaflé and Sinfra), and in some hypo-endemic HD. If we have already shown that some of them experienced a spontaneous cure (and no longer pose a risk of transmission), we also observed that others are potential latent infections [22,54] and this is well illustrated by the two cases detected in 2017 and 2019 in Bouaflé and Sinfra HD, respectively. The case detected in Sinfra had been monitored for more than 20 years. Fortunately, he had been living in Abidjan for 15 years and probably did not pose any risk of transmission. This is not so for the Bouaflé case who stayed for three years in his village before being parasitologically confirmed. Fortunately, no other cases could be detected during the targeted active screening conducted between 2017 and 2019 on populations sharing the same spaces. The living area of this case was included in the VC campaign implemented in January 2016 in the Bouaflé HD, that may have limited the risk of transmission. In addition to these cases where infection is tolerated and diagnosis is difficult, there is also the difficulty of detecting gHAT cases in a context where the prevalence has become very low to the point that the disease is no longer considered a threat by the communities or by health workers. This is well illustrated by the complex health seeking pathway of the case passively diagnosed in 2017 in the Sinfra HD in which the first disease symptoms appeared three years earlier and the patient had visited several health care centers and hospitals in different cities [45].
The modelling analysis presented here used a previously developed mechanistic model which explicitly incorporated human-tsetse contact and parasite transmission as well as heterogeneities in exposure of people to tsetse blood feeding. Longitudinal case data was used to parameterise the model for each geographical location and the resultant model fits align well with reported active and passive cases. Nevertheless, it is acknowledged that this model variant does not incorporate the possibility for non-human animal-tsetse transmission cycles, nor potentially long-term asymptomatic human carriers. Either of these two possibilities could lead to more transmission events per detected case, and therefore to more pessimistic model outcomes [55,56]. Despite this, the extremely low case reporting across several years in Côte d’Ivoire may indicate that these transmission cycles (if they exist) are not sustaining transmission to humans; modelling analyses in the low-prevalence regions of the former Equateur province of the Democratic Republic of Congo [55] and the Mandoul focus of Chad [36] have found this kind of persistent low or zero reporting is suggestive of very limited or no infection contribution from non-human animals. Furthermore, in the foci with vector control, the large reduction in tsetse population density will have reduced transmission between tsetse and any potential infection source (animal or human).
The dynamic tsetse population sub-model used here includes the pupal stage of development as well as adult flies; this enabled us to model some resurgence of fly populations between Tiny Target deployments. This type of bounceback was included in the model to capture a plausible biological mechanism for tsetse population growth between vector control deployment and this model matched fly catches well. We acknowledge that it is possible for bounceback to also occur through reinvasion of flies from neighbouring regions with no control and that other sources of tsetse-related data including habitat or climate data might be useful in trying to elucidate drivers of bounceback in different locations, especially after target deployments are stopped, or to predict potential pockets of high tsetse density, however these data require the use of alternative geostatistical modelling [57] which is beyond the scope of the present study.
While we use a stochastic simulation to model the human population, we have used a deterministic ODE-based approach to model tsetse dynamics. In general, a stochastic model would be preferred, especially at very low prevalence, however due to lack of data on the total tsetse population and inability to uncouple the size of tsetse population from the probability of infection per bite, we must instead fit a relative vector density [58]. This means that we are no longer modelling a discrete population of vectors, but a continuous density so a stochastic model is infeasible. Due to the slow dynamics of gHAT and short life-span of tsetse, however, we expect this to have minimal impact on our estimates of elimination. In this study the focus was on past transmission, however we do provide illustrative projections for the probability of EoT in Fig 8 and Fig M in S1 Text. These projections assume the continuation of the current strategy in all health districts, however further work should be done to explore a range of plausible future strategies including scaling back. We recommend that these type of model projections are also coupled with health economic evaluations which could be used to assess what, how much and where investment is needed for the gHAT programme in Côte d’Ivoire to quantify pathway to country-wide EoT, verification of EoT, and also to consider what constitutes an efficient package of interventions to reach this target. As a preliminary study, a recent paper examined the costs of vector control using Tiny Targets in the Bonon focus from 2016 to 2017 [32].
This article summarises the information provided in the dossier that led to the WHO’s validation of EPHP in December 2020 [59]. This success was achieved through an integrated approach combining medical screening and vector control interventions [12] and an integrated multi-stakeholder and multidisciplinary approach often needed in the fight against other infectious diseases including NTDs [60]. Research has played a major role in adapting tools and strategies to new epidemiological realities that present novel challenges. Moving towards the future, the strategies that will be put in place will have to be increasingly effective by targeting the areas and populations most at risk, to diagnose the last cases and minimise the risk of transmission via restriction of the human-tsetse and tsetse- T. b. gambiense contact.
The objective in Côte d’Ivoire is now to reach EoT by 2025. This requires continuing to adapt the control strategies. For the 2023–2025 step, focus will be on passive screening at the national scale and on reactive and targeted active screening including the follow-up of TL-seropositive subjects and people who share their places of life. Medical and entomological capacities for reaction will be maintained, should any case be identified in the country. It is also crucial to consider some new challenges, including (i) the potential pig reservoir of T. b. gambiense and its consequences on gHAT transmission, and (ii) community engagement to continue implementing suitable control strategies in a context where rare cases, if any, will be diagnosed. All the activities will be carried out in order to be able to compile the necessary information for the request for verification of EoT that may be submitted by the Ministry of Health to WHO in 2025.
Supporting information
The Bouaflé HD includes the Bonon gHAT endemic foci. To be more precise, we present separately the data of the Bonon subprefecture (Bouaflé-Bonon subprefecture that includes the Bonon focus) and the rest of the Bouaflé HD (Bouaflé subprefecture).
(XLSX)
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases.
(XLSX)
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases.
(XLSX)
PEx = population examined; RDT pos = number of positive rapid diagnostic tests; HD = Health District; CHU = Centre Hospitalier Universitaire; INSP = Institut National de Santé Publique; CHR = Centre Hospitalier Régional; HG = Hôpital Général; CSU = Centre de Santé Urbain; CSR = Centre de Santé Rural. Locations for each are indicated in Fig 4.
(XLSX)
This figure was created by the mapping service of our team based at Institut Pierre Richet (Bouaké, Côte d’Ivoire). All the base layers regarding administrative data are available in: https://data.humdata.org/dataset/cote-d-ivoire-roads, https://data.humdata.org/dataset/cote-d-ivoire-settlements and https://data.humdata.org/dataset/cod-ab-civ
(TIF)
Details on the model definition, parameter inputs, fitting methodology, posterior parameter distributions following fitting, fits against the data.
(PDF)
Data as used for the model fitting. This file does not include HDs with insufficient data for model fitting.
(XLSX)
Data used for fitting the tsetse population model in regions which implemented vector control.
(XLSX)
Acknowledgments
The authors wish to thank their institutions, and all contributors to the control of gHAT in Côte d’Ivoire, whatever their status. The PNETHA and partners thank WHO for their support to collect and consolidate data on reported gHAT cases in the frame of the WHO Atlas. The authors wish to pay tribute to Pierre Cattand who significantly contributed to the HAT control in Côte d’Ivoire as head of the research laboratory as well as training within the WHO Clinical Research Project on Trypanosomiasis based in Daloa.
Data Availability
All data are fully available without restriction. All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Bill and Melinda Gates Foundation (www.gatesfoundation.org) through the Trypa-NO! Project (grant number INV-001785, DK, PS, VJ) and through the Human African Trypanosomiasis Modelling and Economic Predictions for Policy (HAT MEPP) project (grant numbers OPP1177824 and INV-005121, KSR). It was also supported by the DiTECT-HAT project, part of the EDCTP2 programme supported by the European Union (grant number DRIA-2014-306-DiTECT-HAT, DK, VJ). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Büscher P, Cecchi G, Jamonneau V, Priotto G. Human African trypanosomiasis. The Lancet. 2017;390: 2397–2409. [DOI] [PubMed] [Google Scholar]
- 2.Franco JR, Cecchi G, Paone M, Diarra A, Grout L, Ebeja AK, et al. The elimination of human African trypanosomiasis: Achievements in relation to WHO road map targets for 2020. PLoS Negl Trop Dis. 2022;16: e0010047. doi: 10.1371/journal.pntd.0010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Control and surveillance of human African trypanosomiasis: Report of a WHO Expert Committee. World Health Organization. 2013. Available: https://apps.who.int/iris/handle/10665/95732 [Google Scholar]
- 4.Simarro PP, Jannin J, Cattand P. Eliminating human African trypanosomiasis: Where do we stand and what comes next? PLoS Med. 2008;5: e55. doi: 10.1371/journal.pmed.0050055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simarro PP, Diarra A, Ruiz Postigo JA, Franco JR, Jannin JG. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: The way forward. PLoS Negl Trop Dis. 2011;5: e1007. doi: 10.1371/journal.pntd.0001007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Accelerating work to overcome the global impact of Neglected Tropical Diseases. A roadmap for implementation. Executive Summary. 2012: 1–22. [Google Scholar]
- 7.Franco JR, Cecchi G, Priotto G, Paone M, Diarra A, Grout L, et al. Monitoring the elimination of human African trypanosomiasis: Update to 2014. PLoS Negl Trop Dis. 2017;11: e0005585. doi: 10.1371/journal.pntd.0005585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koffi M, N’Djetchi M, Ilboudo H, Kaba D, Coulibaly B, N’Gouan E, et al. A targeted door-to-door strategy for sleeping sickness detection in low-prevalence settings in Côte d’Ivoire. Parasite. 2016;23: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kambiré R, Lingué K, Courtin F, Sidibé I, Kiendrébéogo D, N’gouan KE, et al. La trypanosomose humaine africaine dans l’espace ivoiro-burkinabé: optimisation des stratégies de surveillance épidémiologique. Parasite. 2012;19: 389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson KM, Kalkowska DA. An updated economic analysis of the global Polio eradication initiative. Risk Anal. 2021;41: 393–406. doi: 10.1111/risa.13665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molyneux D, Sankara DP. Guinea worm eradication: Progress and challenges—should we beware of the dog? PLoS Negl Trop Dis. 2017;11: e0005495. doi: 10.1371/journal.pntd.0005495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ndung’u JM, Boulangé A, Picado A, Mugenyi A, Mortensen A, Hope A, et al. Trypa-NO! contributes to the elimination of gambiense human African trypanosomiasis by combining tsetse control with “screen, diagnose and treat” using innovative tools and strategies. PLoS Negl Trop Dis. 2020;14: e0008738. doi: 10.1371/journal.pntd.0008738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laveissiere C, Challier A. La répartition des glossines en Côte d’Ivoire: cartes à 1/2 000 000 et note explicative. Orstom, Paris, France. 1981. 33p. Available: https://docplayer.fr/20436256-La-repartition-des-glossines-en-cote-d-ivoire.html [Google Scholar]
- 14.Laveissiere C, Hervouët JP. La trypanosomiase humaine en Afrique de l’Ouest: épidémiologie et contrôle. IRD Editions; 1991. 164p. [Google Scholar]
- 15.Magnus E, Vervoort T, Van Meirvenne N. A card-agglutination test with stained trypanosomes (CATT) for the serological diagnosis of T. b. gambiense trypanosomiasis. Ann Soc Belg Med Trop. 1978;58: 169–176. [PubMed] [Google Scholar]
- 16.Camara M, Camara O, Ilboudo H, Sakande H, Kaboré J, N’Dri L, et al. Sleeping sickness diagnosis: use of buffy coats improves the sensitivity of the mini anion exchange centrifugation test. Trop Med Int Health. 2010;15: 796–799. doi: 10.1111/j.1365-3156.2010.02546.x [DOI] [PubMed] [Google Scholar]
- 17.Jamonneau V, Bucheton B, Kaboré J, Ilboudo H, Camara O, Courtin F, et al. Revisiting the immune trypanolysis test to optimise epidemiological surveillance and control of sleeping sickness in West Africa. PLoS Negl Trop Dis. 2010;4: e917. doi: 10.1371/journal.pntd.0000917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Courtin F, Jamonneau V, Camara M, Camara O, Coulibaly B, Diarra A, et al. A geographical approach to identify sleeping sickness risk factors in a mangrove ecosystem. Trop Med Int Health. 2010;15: 881–889. doi: 10.1111/j.1365-3156.2010.02559.x [DOI] [PubMed] [Google Scholar]
- 19.Courtin F, Camara O, Camara M, Kagbadouno M, Bucheton B, Solano P, et al. Sleeping sickness in the historical focus of forested Guinea: update using a geographically based method. Parasite. 2019;26: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisser S, Lumbala C, Nguertoum E, Kande V, Flevaud L, Vatunga G, et al. Sensitivity and specificity of a prototype rapid diagnostic test for the detection of Trypanosoma brucei gambiense infection: A Multi-centric Prospective Study. PLoS Negl Trop Dis. 2016;10: e0004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miezan TW, Meda HA, Doua F, Dje NN, Lejon V, Büscher P. Single centrifugation of cerebrospinal fluid in a sealed pasteur pipette for simple, rapid and sensitive detection of trypanosomes. Trans R Soc Trop Med Hyg. 2000;94:293. doi: 10.1016/s0035-9203(00)90327-4 [DOI] [PubMed] [Google Scholar]
- 22.Kaba D, N’Gouan KE, Djohan V, Berte D, Ble SL, Kouakou L, et al. Diagnostic clinique et traitement de la trypanosomiase humaine africaine dans le contexte d’élimination. Bull Assoc Anc Elèves Inst Pasteur. 2020;62:240. [Google Scholar]
- 23.Koné M, Kaba D, Kaboré J, Thomas LF, Falzon LC, Koffi M, et al. Passive surveillance of human African trypanosomiasis in Côte d’Ivoire: Understanding prevalence, clinical symptoms and signs, and diagnostic test characteristics. PLoS Negl Trop Dis. 2021;15: e0009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camara O, Camara M, Lejon V, Ilboudo H, Sakande H, Léno M, et al. Immune trypanolysis test with blood spotted on filter paper for epidemiological surveillance of sleeping sickness. Trop Med Int Health. 2014;19: 828–831. doi: 10.1111/tmi.12316 [DOI] [PubMed] [Google Scholar]
- 25.Courtin F, Camara M, Rayaisse J-B, Kagbadouno M, Dama E, Camara O, et al. Reducing human-tsetse contact significantly enhances the efficacy of sleeping sickness active screening campaigns: A promising result in the context of elimination. PLoS Negl Trop Dis. 2015;9: e0003727. doi: 10.1371/journal.pntd.0003727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahamat MH, Peka M, Rayaisse JB, Rock KS, Toko MA, Darnas J, et al. Adding tsetse control to medical activities contributes to decreasing transmission of sleeping sickness in the Mandoul focus (Chad). PLoS Negl Trop Dis. 2017;11: e0005792. doi: 10.1371/journal.pntd.0005792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tirados I, Hope A, Selby R, Mpembele F, Miaka EM, Boelaert M, et al. Impact of tiny targets on Glossina fuscipes quanzensis, the primary vector of human African trypanosomiasis in the Democratic Republic of the Congo. PLoS Negl Trop Dis. 2020;14: e0008270. doi: 10.1371/journal.pntd.0008270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rayaisse JB, Esterhuizen J, Tirados I, Kaba D, Salou E, Diarrassouba A, et al. Towards an optimal design of target for tsetse control: comparisons of novel targets for the control of Palpalis group tsetse in West Africa. PLoS Negl Trop Dis. 2011;5: e1332. doi: 10.1371/journal.pntd.0001332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw APM, Tirados I, Mangwiro CTN, Esterhuizen J, Lehane MJ, Torr SJ, et al. Costs of using “Tiny Targets” to control Glossina fuscipes fuscipes, a vector of gambiense sleeping sickness in Arua district of Uganda. PLoS Negl Trop Dis. 2015;9: e0003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laveissiere C, Grebaut P. Recherches sur les pièges à glossines (Diptera: Glossinidae). Mise au point d’un modèle économique: Le piège «Vavoua». Trop med parasitol. 1990;41: 185–192. [PubMed] [Google Scholar]
- 31.Kaba D, Djohan V, Berté D, Ta BTD, Selby R, Kouadio KADM, et al. Use of vector control to protect people from sleeping sickness in the focus of Bonon (Côte d’Ivoire). PLoS Negl Trop Dis. 2021;15: e0009404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Courtin F, Kaba D, Rayaisse JB, Solano P, Torr SJ, Shaw APM. The cost of tsetse control using ‘Tiny Targets’ in the sleeping sickness endemic forest area of Bonon in Côte d’Ivoire: Implications for comparing costs across different settings. PLoS Negl Trop Dis. 2022;16: e0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pollock JN. Manuel de lutte contre la mouche tsé-tsé: Biologie, systématique et répartition des tsé-tsé. FAO. 1982;1: 274. Available: https://policycommons.net/artifacts/3363701/manuel-de-lute-contre-la-mouche-tse-tse-volume-1/4162347/fragments/?page=1 [Google Scholar]
- 34.Rock KS, Stone CM, Hastings IM, Keeling MJ, Torr SJ, Chitnis N. Chapter three—mathematical models of human African trypanosomiasis epidemiology. Adv Parasitol. Academic press. 2015. pp. 53–133. [DOI] [PubMed] [Google Scholar]
- 35.Crump RE, Huang CI, Knock ES, Spencer SEF, Brown PE, Miaka EM, et al. Quantifying epidemiological drivers of gambiense human African trypanosomiasis across the Democratic Republic of Congo. PLoS Comput Biol. 2021;17: e1008532. doi: 10.1371/journal.pcbi.1008532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rock KS, Huang CI, Crump RE, Bessell PR, Brown PE, Tirados I, et al. Update of transmission modelling and projections of gambiense human African trypanosomiasis in the Mandoul focus, Chad. Infect Dis Poverty. 2022;11: 11. doi: 10.1186/s40249-022-00934-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solano P, Kone A, Garcia A, Sane B, Michel V, Michel JF, et al. Role of patient travel in transmission of human African trypanosomiasis in a highly endemic area of the Ivory Coast. Med Trop Rev. 2003;63: 577–582. [PubMed] [Google Scholar]
- 38.Kaba D, Dje NN, Courtin F, Oke E, Koffi M, Garcia A, et al. L’impact de la guerre sur l’évolution de la THA dans le Centre-Ouest de la Cote d’Ivoire. Trop Med Int Health. 2006;11: 136–143. [DOI] [PubMed] [Google Scholar]
- 39.Laveissière C, Sane B, Diallo PB, Truc P, Meda AH. Le risque épidémiologique dans un foyer de la maladie du sommeil en Côte d’Ivoire. Trop Med Int Health.1997;2:729–732. [DOI] [PubMed] [Google Scholar]
- 40.Dje NN, Miezan TW, N’guessan P, Brika P, Doua F, Boa F. Distribution géographique des trypanosomés pris en charge en Côte d’Ivoire de 1993 à 2000. Bull Soc Pathol Exot. 2002;95: 359–361. [PubMed] [Google Scholar]
- 41.Mulenga P, Lutumba P, Coppieters Y, Mpanya A, Mwamba-Miaka E, Luboya O, et al. Passive screening and diagnosis of sleeping sickness with new tools in primary health services: an operational research. Infect Dis Ther. 2019;8: 353–367. doi: 10.1007/s40121-019-0253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Solano P, Jamonneau V, N’Guessan P, N’Dri L, Dje NN, Miezan TW, et al. Comparison of different DNA preparation protocols for PCR diagnosis of human African trypanosomosis in Côte d’Ivoire. Acta Trop. 2002;82: 349–356. [DOI] [PubMed] [Google Scholar]
- 43.Courtin F, Dupont S, Zeze DG, Jamonneau V, Sané B, Coulibaly B, et al. Human African trypanosomiasis: urban transmission in the focus of Bonon (Côte d’Ivoire). Trop Med Int Health. 2005;10: 340–346. [DOI] [PubMed] [Google Scholar]
- 44.Jamonneau V, Ilboudo H, Kaboré J, Kaba D, Koffi M, Solano P, et al. Untreated human infections by Trypanosoma brucei gambiense are not 100% fatal. PLoS Negl Trop Dis. 2012;6: e1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koné M, N’Gouan EK, Kaba D, Koffi M Kouakou L, N’Dri L, et al. The complex health seeking pathway of a human African trypanosomiasis patient in Côte d’Ivoire underlines the need of setting up passive surveillance systems. PLoS Negl Trop Dis. 2020;14: e0008588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.N’Djetchi MK, Ilboudo H, Koffi M, Kaboré J, Kaboré JW, Kaba D, et al. The study of trypanosome species circulating in domestic animals in two human African trypanosomiasis foci of Cote d’Ivoire identifies pigs and cattle as potential reservoirs of Trypanosoma brucei gambiense. PLoS Negl Trop Dis. 2017;11: e0005993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Traoré BM, Koffi M, N’Djetchi MK, Kaba D, Kaboré J, Ilboudo H, et al. Free-ranging pigs identified as a multi-reservoir of Trypanosoma brucei and Trypanosoma congolense in the Vavoua area, a historical sleeping sickness focus of Côte d’Ivoire. PLoS Negl Trop Dis. 2021;15: e0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sané B, Laveissière C, Meda HA. Diversity of feeding behavior of Glossina palpalis palpalis in the forest belt of the Ivory Coast: relation to the prevalence of human African trypanosomiasis. Trop Med Int Health. 2000;5: 73–78. [DOI] [PubMed] [Google Scholar]
- 49.Simo G, Njiokou F, Mbida Mbida JA, Njitchouang GR, Herder S, Asonganyi T, et al. Tsetse fly host preference from sleeping sickness foci in Cameroon: Epidemiological implications. Infect Genet Evol. 2008;8: 34–39. doi: 10.1016/j.meegid.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 50.Courtin F, Jamonneau V, Duvallet G, Garcia A, Coulibaly B, Doumenge JP, et al. Sleeping sickness in West Africa (1906–2006): changes in spatial repartition and lessons from the past. Trop Med Int Health. 2008;13: 334–344. doi: 10.1111/j.1365-3156.2008.02007.x [DOI] [PubMed] [Google Scholar]
- 51.Kiendrébéogo D, Kambiré R, Jamonneau V, Lingué K, Solano P, Courtin F. Histoire d’un itinéraire épidémiologique entre le Burkina Faso et la Côte d’Ivoire: le cas des foyers de maladie du sommeil de Koudougou. Parasite. 2012;19: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dama E, Drabo A, Kaboré J, Ouédraogo E, Coulibaly B, Ilboudo H, et al. Description of the first native sleeping sickness case diagnosed in Burkina Faso in the over two decades. 2018;12: e0006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Büscher P, Bart J-M, Boelaert M, Bucheton B, Cecchi G, Chitnis N, et al. Do cryptic reservoirs threaten gambiense-sleeping sickness elimination? Trends Parasitol. 2018;34: 197–207. doi: 10.1016/j.pt.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia A, Jamonneau V, Magnus E, Laveissière C, Lejon V, N’Guessan P, et al. Follow-up of Card Agglutination Trypanosomiasis Test (CATT) positive but apparently aparasitaemic individuals in Côte d’Ivoire: evidence for a complex and heterogeneous population. Trop Med Int Health. 2000;5: 786–793. [DOI] [PubMed] [Google Scholar]
- 55.Crump RE, Huang C-I, Spencer SEF, Brown PE, Shampa C, Miaka EM, et al. Modelling to infer the role of animals in gambiense human African trypanosomiasis transmission and elimination in the DRC. PLoS Negl Trop Dis. 2022;16: e0010599. doi: 10.1371/journal.pntd.0010599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aliee M, Keeling MJ, Rock KS. Modelling to explore the potential impact of asymptomatic human infections on transmission and dynamics of African sleeping sickness. PLoS Comput Biol. 2021;17: e1009367. doi: 10.1371/journal.pcbi.1009367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stanton MC, Esterhuizen J, Tirados I, Betts H, Torr SJ. The development of high resolution maps of tsetse abundance to guide interventions against human African trypanosomiasis in northern Uganda. Parasit Vectors. 2018;11: 340. doi: 10.1186/s13071-018-2922-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis CN, Rock KS, Miaka EM, Keeling MJ. Village-scale persistence and elimination of gambiense human African trypanosomiasis. PLoS Negl Trop Dis. 2019;13: e0007838. doi: 10.1371/journal.pntd.0007838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.WHO. WHO validates Cote d’Ivoire for eliminating sleeping sickness as a public health problem. 2021. Available: https://www.who.int/news/item/25-03-2021-who-validates-cote-d-ivoire-for-eliminating-sleeping-sickness-as-a-public-health-problem [Google Scholar]
- 60.Rotureau B, Waleckx E, Jamonneau V, Solano P, Molia S, Debré P, et al. Enhancing research integration to improve One Health actions: learning lessons from neglected tropical diseases experiences. BMJ Glob Health. 2022;7: e008881. doi: 10.1136/bmjgh-2022-008881 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Bouaflé HD includes the Bonon gHAT endemic foci. To be more precise, we present separately the data of the Bonon subprefecture (Bouaflé-Bonon subprefecture that includes the Bonon focus) and the rest of the Bouaflé HD (Bouaflé subprefecture).
(XLSX)
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases.
(XLSX)
PEx = population examined; S = number of TL-seropositive subjects; T = number of confirmed gHAT cases.
(XLSX)
PEx = population examined; RDT pos = number of positive rapid diagnostic tests; HD = Health District; CHU = Centre Hospitalier Universitaire; INSP = Institut National de Santé Publique; CHR = Centre Hospitalier Régional; HG = Hôpital Général; CSU = Centre de Santé Urbain; CSR = Centre de Santé Rural. Locations for each are indicated in Fig 4.
(XLSX)
This figure was created by the mapping service of our team based at Institut Pierre Richet (Bouaké, Côte d’Ivoire). All the base layers regarding administrative data are available in: https://data.humdata.org/dataset/cote-d-ivoire-roads, https://data.humdata.org/dataset/cote-d-ivoire-settlements and https://data.humdata.org/dataset/cod-ab-civ
(TIF)
Details on the model definition, parameter inputs, fitting methodology, posterior parameter distributions following fitting, fits against the data.
(PDF)
Data as used for the model fitting. This file does not include HDs with insufficient data for model fitting.
(XLSX)
Data used for fitting the tsetse population model in regions which implemented vector control.
(XLSX)
Data Availability Statement
All data are fully available without restriction. All relevant data are within the manuscript and its Supporting Information files.