Abstract
Background
WNT signaling is a cellular pathway that has been implicated in the development and progression of prostate cancer. Oligometastatic castration-sensitive prostate cancer (omCSPC) represents a unique state of disease in which metastasis-directed therapy (MDT) has demonstrated improvement in progression-free survival. Herein, we investigate the clinical implications of genomic alterations in the WNT signaling cascade in men with omCSPC.
Methods
We performed an international multi-institutional retrospective study of 277 men with metachronous omCSPC who underwent targeted DNA sequencing of their primary/metastatic tumor. Patients were classified by presence or absence of pathogenic WNT pathway mutations (in the genes APC, RNF43, and CTNNB1). Pearson’s χ2 and Mann-Whitney U were used to determine differences in clinical factors between genomic strata. Kaplan-Meier survival curves were generated for radiographic progression-free survival (rPFS) and overall survival (OS), stratified according to WNT pathway mutation status.
Results
A pathogenic WNT pathway mutation was detected in 11.2% of patients. Patients with WNT pathway mutations were more likely to have visceral metastases (22.6% vs 2.8%; p<0.01) and less likely to have regional lymph node metastases (29.0% vs 50.4%; p=0.02). At time of oligometastasis, these patients were treated with MDT alone (33.9%), MDT + limited course of systemic therapy (20.6%), systemic therapy alone (22.4%), or observation (defined as no treatment for ≥6 months following metastatic diagnosis). Multivariable cox regression demonstrated WNT pathway mutations associated with significantly worse OS (HR=3.87, 95%CI 1.25–12.00).
Conclusion
Somatic WNT pathway alterations are present in approximately 11% of patients with omCSPC and are associated with an increased likelihood of visceral metastases. While these patients have a worse natural history, they may benefit from MDT.
Introduction
Within the United States, prostate cancer represents the most common malignancy in men with nearly 250,000 new cases accounting for 34,000 deaths in 20211. Although the majority of patients with prostate cancer have an indolent clinical course, there is a subset with more aggressive disease with progression to metastatic disease and ultimately lethal castration-resistance. Within castrate-resistant prostate-cancer (CRPC), genomic alterations have been extensively examined2–5.
Mutations within the WNT signaling pathway have been identified in up to 20% of patients with CRPC6,7. The canonical WNT pathway is a evolutionarily conserved embryonic pathway that regulates key aspects of cell fate, cell-cycle progression, proliferation, and migration8,9 and has been implicated in the pathogenesis of therapeutic resistance in several malignancies 10–12 . Within prostate cancer, WNT pathway activating genomic alterations are associated with inferior outcomes with androgen deprivation13 possibly due to an androgen receptor independent proliferation through increased canonical WNT/β-catenin signaling14. Despite emerging evidence of the role of WNT pathway activating mutations within CRPC, significantly less is understood within castration-sensitive prostate cancer (CSPC)15.
Within metastatic CSPC, there is a state of limited metastatic disease, termed oligometastasis (omCSPC) in which metastasis-directed therapy (MDT) may play an integral role in therapy. Oligometastatic disease, typically defined as no more than 3–5 metastases, has demonstrated improved outcomes with metastasis directed therapy over standard of care in a variety of malignancies16,17. Within metachronous (development of metastasis >6 months after initial diagnosis) omCSPC, randomized clinical data has also demonstrated that MDT improves both progression-free and ADT-free survival compared to observation18–20 . Despite this improvement, there remains heterogeneity of outcomes within this population and tumor genomics may provide crucial information to better understand the disease course. Given the important role of WNT pathway mutations within mCRPC, it is a candidate pathway to evaluate within omCSPC. Herein we aim to describe the incidence and type of WNT pathway mutations observed in patients with omCSPC and evaluate for associations with clinical outcomes.
Methods
Following IRB approval, we performed an international multi-institutional retrospective review of men with metachronous omCSPC who underwent next generation sequencing (NGS) of either their primary tumor or metastatic site. NGS was performed either through Foundation One CDx (324-gene panel), Personal Genome Diagnostics CancerSELECT 125 (125-gene panel), or Tempus xT tissue assay (648-gene panel) platforms. Patients included those treated at Johns Hopkins Hospital and Ghent Hospital receiving standard of care therapy (SOC) as well as those enrolled on the STOMP18 and ORIOLE19 clinical trials. Both clinical trials had IRB approval and all participants provided informed consent. All patients on STOMP and ORIOLE trials were evaluated for NGS however 14 patients had unavailable tissue (supplemental Figure 1). Patients treated as SOC underwent NGS at the discretion of their treating physician. Patients with samples that underwent NGS but failed were excluded from analysis. For this study, omCSPC was defined as ≤ 5 lesions on conventional imaging (CT/radionuclide bone scan) or enhanced (PET) imaging.
Patients were stratified according to presence of WNT pathway mutation status. Initial treatments included MDT alone (SABR or metastasectomy), MDT + limited course of systemic therapy, systemic therapy alone (ADT, androgen receptor signaling inhibitors or docetaxel), or observation defined as no therapy ≥ 6 months after diagnoses of omCSPC. Patients treated with MDT were required to have receive local therapy to all sites of conventional imaging detected metastases. WNT pathway mutations included pathogenic mutations of APC, RNF43, or CTNNB1. Available follow-up data from serial physical examinations, imaging, and prostate-specific antigen (PSA) measurements were obtained.
The primary endpoint of interest was to describe the frequency and type as well as identify clinical features associated with WNT pathway mutations. Clinical features were compared with a t-test or Mann-Whitney U test and Pearson’s χ2 test or Fisher’s exact test for continuous and categorical variables, respectively. Our secondary endpoints included overall survival (OS) and radiographic progression-free survival (rPFS). OS was defined as time from oligometastasis to death of any cause. rPFS was defined as time from oligometastasis to development of new or enlarging metastasis on conventional (per RECIST criteria21) or enhanced imaging (per radiologist discretion). Patients underwent imaging at time of PSA or symptomatic progression. Patients were censored at last follow-up. Survival analyses were performed with the Kaplan-Meier Method. A backward conditional multivariable cox regression model was built using statistically significant variables on univariable regression. For all analyses, a p-value ≤ 0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics, version 25.
Results
A total of 277 patients with metachronous omCSPC who underwent NGS were included with a median follow-up of 37.2 (IQR 20.2–57.1) months. Baseline tumor characteristics can be found in Table 1. At time of oligometastatic recurrence, median PSA was 3.8 ng/mL (IQR 1.2–11.1), the majority of patients had either one (46.6%) or two (29.2%) metastases, most commonly in either pelvic lymph nodes (47.7%) or bone (42.2%) with a minority presenting with visceral metastases (5.1%).
Table 1.
Demographic, tumor, and treatment characteristics of the entire cohort
| Characteristic | N=277 |
|---|---|
| Initial Characteristics | |
| iPSA (IQR) | 7.5 (4.6–13.4) |
| Primary Therapy | 236 (85.2%) |
| Surgery | 36 (13.0%) |
| RT | 5 (1.8%) |
| Unavailable | |
| T Stage | 15 (5.4%) |
| T1 | 87 (31.4%) |
| T2 | 165 (59.6%) |
| T3 | 5 (1.8%) |
| T4 | 5 (1.8%) |
| Unavailable | |
| N Stage | 213 (76.9%) |
| N0 | 32 (11.6%) |
| N1 | 32 (11.6%) |
| Nx | |
| Gleason | 14 (5.1%) |
| 6 | 118 (42.6%) |
| 7 | 43 (15.5%) |
| 8 | 92 (33.2%) |
| 9 | 8 (2.9%) |
| 10 | 2 (0.7%) |
| Unavailable | |
| Characteristics at oligometastasis | |
| PSA at Oligomet (IQR) | 3.8 (1.2–11.1) |
| Number of Mets | |
| 1 | 129 (46.6%) |
| 2 | 81 (29.2%) |
| 3 | 43 (15.5%) |
| 4 | 18 (6.5%) |
| 5 | 6 (2.2%) |
| Location of Mets | |
| Pelvic LN | 132 (47.7%) |
| Distant LN | 65 (23.5%) |
| Bone | 117 (42.2%) |
| Visceral | 14 (5.1%) |
| Initial Treatment (First treatment within 6 months oligomet) | |
| MDT | 94 (33.9%) |
| MDT+ defined course systemic therapy | 57(20.6%) |
| Systemic Therapy | 62 (22.4%) |
| Observation | 60 (21.7%) |
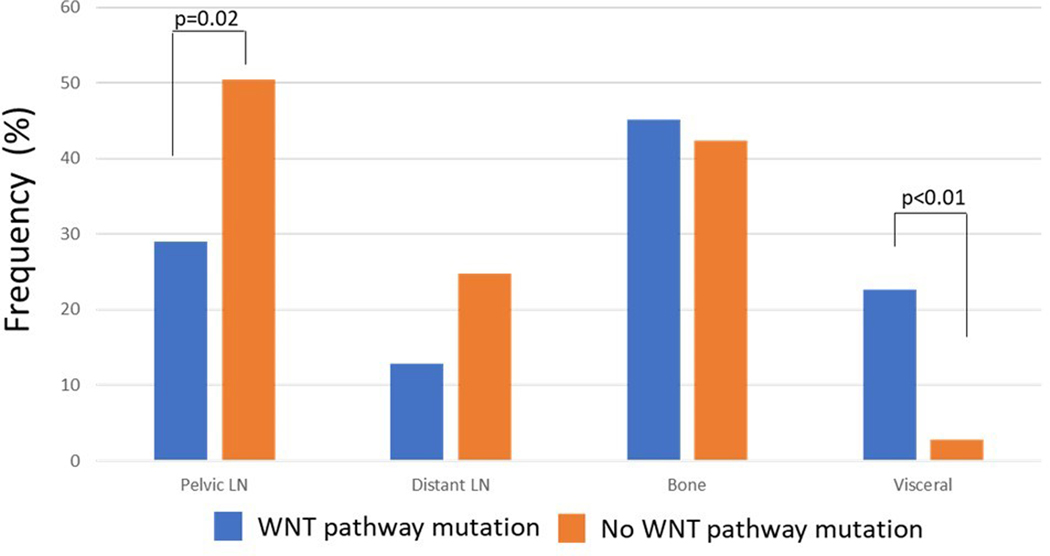
Within the entire cohort 11.2% (n=31) of tumors harbored pathogenic WNT pathway mutations which included mutations in APC (5.4%, n=15), RNF43 (2.9%, n=8), and CTNNB1 (4.7%, n=13) (Table 2). 1.4% of patients had more than one WNT pathway alteration in the same tumor. A complete list of specific mutations can be found in supplemental Table 1. Patients with WNT pathway mutations were found to have a faster time to oligometastasis (42.1 vs 54.0 months, p=0.05), less likely to present with pelvic lymph nodes at time of oligometastatic recurrence (29.0% vs 50.4%; p=0.02) and more likely to present with both visceral (22.6% vs 2.8%; p<0.01) and more specifically lung (16.1% vs 1.6%; p<0.01) metastases (Figure 1). No differences in Gleason score, PSA, number of metastases, or frequency of distant lymph node or bone metastses were identified (Supplemental Table 2). Patient samples with WNT pathway mutations were found to be enriched for SPOP mutations (22.6% vs 9.3%, p=0.03) but not significantly associated with mutations in TP53, Rb1, homologous recombination deficiency genes, or PI3K pathway genes (supplemental Table 2, supplemental Figure 2).
Table 2.
Frequency of WNT pathway mutations and specific WNT pathway genes
| N=277 | |
|---|---|
| WNT Pathway Mutation | |
| Yes | 31 (11.2%) |
| No | 246 (88.8%) |
| APC | |
| Yes | 15 (5.4%) |
| No | 262 (94.6%) |
| RNF43 | |
| Yes | 8 (2.9%) |
| No | 269 (97.1%) |
| CTNNB1 | |
| Yes | 13 (4.7%) |
| No | 264 (95.3%) |
Figure 1.
Frequency of metastatic lesion location at time of oligometastsis stratified by presence or absence of WNT pathway mutation.
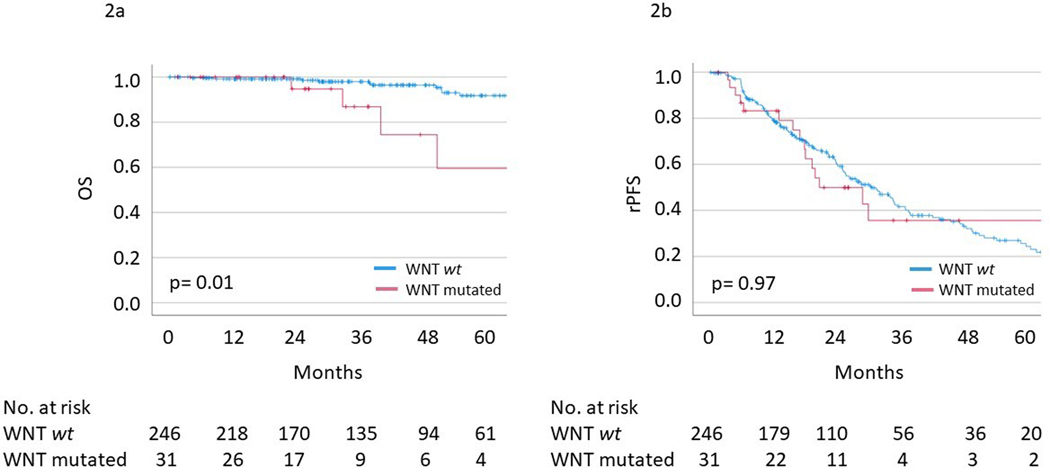
Within the entire cohort, median and 5-yr OS were not met and 89%, respectively. Multivariable analysis demonstrated number of metastases (HR=1.83, 95%CI 1.26–2.67; p<0.01) and WNT pathway mutation (HR=3.87, 95%CI 1.25–12.00; p=0.02) (Table 3, Figure 2a) associated with inferior OS. Patients with WNT pathway mutations also experienced worse prostate-cancer specific survival (5-yr PCSM 59% vs 95%, p<0.01) from oligometastsis and median OS from time of initial prostate cancer diagnosis (172.9 months vs not reached, p=0.01; supplemental Figure 3).
Table 3.
Univariable and Multivariable Cox Regression of characteristics associated with OS and rPFS
| Univariate | Multivariate | |||
|---|---|---|---|---|
|
| ||||
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| OS | ||||
| Gleason Grade Group ≥4 | 1.60(0.62–4.12) | 0.33 | ||
| iPSA | 1.00 (0.99–1.02) | 0.54 | ||
| PSA at Oligometastasis | 1.00 (0.97–1.02) | 0.99 | ||
| Number of Metastases | 1.84 (1.26–2.69) | <0.01 | 1.83 (1.26–2.67) | <0.01 |
| Visceral metastases | 4.35 (1.25–15.13) | 0.02 | Term removed by model | |
| WNT Mutation | 3.861.26–11.83) | 0.02 | 3.87 (1.25–12.00) | 0.02 |
| rPFS | ||||
| Gleason Grade Group ≥4 | 0.98 (0.72–1.35) | 0.91 | ||
| iPSA | 1.00 (1.00–1.01) | 0.22 | ||
| PSA at Oligometastasis | 1.0 (1.00–1.01) | 0.60 | ||
| Number of Metastases | 1.26 (1.08–1.48) | <0.01 | ||
| Visceral metastases | 0.79 (0.37–1.68) | 0.53 | ||
| WNT Mutation | 1.01 (0.59–1.73) | 0.97 | ||
OS: overall survival, rPFS: radiographic progression-free survival
Figure 2.
Kaplan Meier survival curves of (a) overall survival and (b) radiographic progression-free survival stratified by WNT pathway mutational status.
Within the entire cohort, median and 5-year rPFS were 29.9 (95%CI 24.5–35.2) months and 25% respectively. Multivariable cox regression was not performed as number of metastases was the only variable significantly associated with rPFS on univariable analysis (HR = 1.26, 95%CI 1.08–1.48; p<0.01). On univariable analysis, WNT pathway mutation status was not associated with rPFS (HR=1.01, 95%CI 0.59–1.73; p=0.53) (Table 3, Figure 2b).
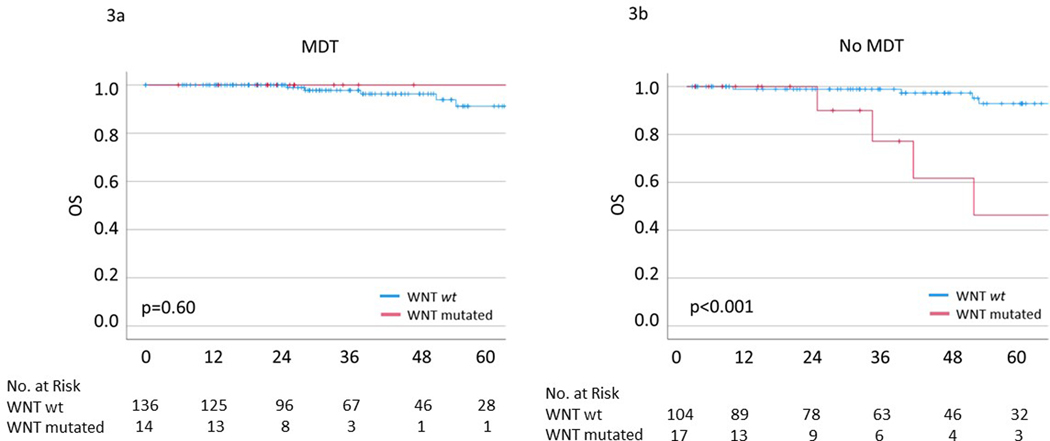
We then compared outcomes stratified by whether patients received MDT. Patients who received MDT were found to have significantly lower Gleason scores (p=0.05) and PSA at oligometastasis (2.5 vs 7.4, p<0.01) however there were no differences in any other clinical or genomic features (supplemental Table 3). Among patients who received MDT, pathogenic WNT pathway mutations were not associated with worse 5-year OS (100% vs 91%, p=0.60) (Figure 3a). Conversely, among patients who did not receive MDT, patients with WNT pathway mutations exhibited significantly worse OS (46% vs 93%, p<0.001) (Figure 3b). Similar results were observed for rPFS however did not reach statistical significance (supplemental Figure 4). Further, MDT demonstrated an improvement in 5-yr OS (100% vs 48%, p=0.03) in WNT pathway mutated patients however had no significant effect on 5-yr OS among patients without a WNT pathway mutation (91% vs 93%, p=0.90) (supplemental Figure 5). A detailed list of clinical and genomic characteristics comparing patients treated with and without MDT among patients with WNT pathway mutations can be found in supplemental Figure 4.
Figure 3.
Kaplan Meier survival curves of overall survival stratified by WNT pathway mutational status in (a) patients treated with metastasis-directed therapy and (b) patients not treated with metastasis directed therapy.
Discussion
We report the incidence and type of WNT pathway mutations within metachronous omCSPC and their association with clinical outcomes. Specifically, we have demonstrated WNT activating mutations are present in approximately 11% of patients with omCSPC which is concordant with prior work22,23, demonstrating increasing gene mutational burden in the WNT pathway with more advanced disease24. Additionally, WNT pathway mutations are associated increased likelihood of visceral metastases and decreased likelihood of regional lymph nodes metastases. Finally, we have identified WNT pathway mutations to be associated with worse overall survival in omCSPC and that outcomes may be improved if men receive MDT.
The WNT pathway has been previously implicated as a driver of visceral metastases in several primary malignancies through a variety of cellular mechanisms22. Most notably, both canonical and non-canonical WNT signaling has been implicated in the epithelial-mesenchymal transition (EMT) 25,26. The sEMT allows for enhanced migratory capacity and invasiveness of epithelial cells and is widely realized as a cellular mechanism for the development of metastasis27,28. We and others have shown that similar epithelial plasticity programs are sufficient and seem to be important clinically for prostate cancer metastasis29–32 and may serve as a mechanism by which omCSPC harboring WNT pathway activating mutations have a significantly higher incidence of visceral metastases.
The findings presented above are partially concordant with those observed in CRPC. Velho et al. demonstrated activating WNT pathway mutations were associated with inferior PFS (6.5 vs 9.6 months) and OS (23.6 vs 27.7 months) in patients with CRPC treated with first line abiraterone/enzaluatimde13. The authors hypothesize the differential outcomes may be related to an underlying WNT pathway induced androgen-independent growth and resistance to androgen receptor antagonism14,33. However, other studies in CRPC have not demonstrated significant differences in disease progression according to WNT pathway status (by cell-free DNA)34.
Within CSPC treated with ADT, Geng et al. demonstrated single nucleotide polymorphisms (SNPs) associated with decreased APC expression was associated with more advanced stage cancer. Further, patients with SNPs associated with increased APC mRNA expression seemed protective with improved PFS35. Interestingly, although the results presented here demonstrate inferior OS, counterintuitively, no difference in rPFS was observed. This may be a result of WNT pathway mutations exerting its effect on OS through propensity to spread to viscera rather than rate of progression or anti-androgen resistance. Importantly, the patient population included here is distinctly different from that in prior reports, as over 50% of the population presented here was treated with consolidative metastasis-directed therapy (MDT) which may limit interpretation of androgen-resistance within these patients.
Another particularly interesting finding of this study is the frequency of concurrent WNT pathway and SPOP mutations. As we have demonstrated, patients with WNT pathway mutations appear to experience worse outcomes with increased visceral metastasis. Conversely, mutations in SPOP have demonstrated improved time to castration resistance and overall survival in synchronous mCSPC when treated with androgen receptor axis targeted therapies36. Interestingly, this work by Swami et al36. as well as work by Nakazawa et al.37 have similarly observed an association between WNT and SPOP mutations. It is possible these mutations represent a spectrum of clinical outcomes with concurrent mutations experiencing an intermediate prognosis between the unfavorable WNT pathway mutations alone and favorable SPOP mutations alone. Unfortunately, our study and others do not have sufficient numbers to fully characterize this spectrum of concurrent mutations however future work should aim to clarify this finding.
This present study further adds to prior work evaluating for potential prognostic and predictive biomarkers in omCSPC treated with MDT. Deek et al. recently reported on a high-risk mutational signature consisting of TP53, ATM, Rb1, BRCA1/2, which demonstrated significantly worse PFS and rPFS in a combined analysis of the STOMP and ORIOLE clinical trials20. Further, this work identified a potential greater PFS benefit with MDT over observation in patients with a high-risk mutation (p-interaction of 0.12). Given small numbers of WNT pathway aberrations in this cohort we were unable to evaluate the effect of concurrent high-risk mutations. Importantly, however there was no significant association of WNT pathway mutations with mutations in TP53, RB1, or homologous recombination deficiency (HRD - ATM, BRCA1/2, CHEK2) indicating the results observed here are unlikely confounded by an enrichment of other high-risk mutations within the WNT mutated subgroup.
Our study has several limitations. First, while the majority of patients included were from 2 randomized clinical trials, this was a retrospective non-pre-specified analysis and also included patients managed off these trials. Additionally, although all patients had metachronous omCSPC there was significant heterogeneity in how these patients were managed ranging from observation, anti-androgen monotherapy, MDT monotherapy, or combination therapy. Second, the genomic profiling was performed through multiple vendors which may have affected how pathogenic mutations were determined. Genomic profiling was also primarily from the primary tumor rather than the metastatic sites which may underestimate mutations acquired during disease progression. Further limitations related to primary prostate cancer multifocality and sampling of tissue via biopsies may have led to missing of relevant clonal alterations. Third, our definition of a WNT pathway mutation was not comprehensive; rather it focused on 3 specific genes (APC, RNF and CTNNB1) that have been implicated in an activated WNT pathway phenotype. Finally, our results of WNT pathway mutations as a prognostic variable of OS but not rPFS should be interpreted cautiously. Although, this may have been a result of the association of WNT pathway mutations with visceral metastases, the lack of difference in rPFS does raise the possibility this could represent a Type I statistical error.
Conclusion
Within metachronous omCSPC, WNT pathway activating mutations are present in approximately 11% of patients and are significantly associated with the presence of visceral metastases. Our data suggest that mutations within this pathway may be negatively prognostic for overall survival similar to observations in CRPC, however MDT in the omCSPC state may improve these outcomes. This work underscores the influence of genomics on the disease course of patients with omCSPC receiving MDT.
Supplementary Material
Funding:
PTT was funded by an anonymous donor, Movember Foundation-Distinguished Gentlemen’s Ride-Prostate Cancer Foundation, Babara’s Fund, National Capitol Cancer Research Fund and the NIH/NCI (U01CA212007, U01CA231776 and U54CA273956) and DoD (W81XWH-21-1-0296) and the STOMP trial was supported by Kom op tegen Kanker, a Belgian non-profit organization.
Conflicts of Interest
Emmanuel Antonarakis : Patent holder/licenser for Qiagen; Consultant for Amgen, Astellas, AstraZeneca, Bayer, Clovis, Dendreon, Eli Lilly and Co. ESSA, GlaxoSmithKline, Jannsen, Medivation, Merck, and Sanofi; Research grant recipient from AstraZeneca, Bristol Myers-Squibb, Celgene, Clovis, Dendreon, Genentech, Janssen, Johnson & Johnson, Merck, Novartis, Sanofi, Tokai
Piet Ost: Consultant for Bayer, Janssen, Curium; Research grant recipient from Varian, Bayer
Phuoc Tran: Consultant for Natsar Pharm, Janssen-Taris Biomedical and RefleXion Medical, Personal fees from Noxopharm, Janssen-Taris Biomedical, Myovant and AstraZeneca; Holds a patent 9114158- Compounds and Methods of Use in Ablative Radiotherapy licensed to Natsar Pharm.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Hamid AA, Gray KP, Shaw G, et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur Urol. 2019;76(1):89–97. [DOI] [PubMed] [Google Scholar]
- 3.Abida W, Cyrta J, Heller G, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proceedings of the National Academy of Sciences. 2019;116(23):11428–11436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abida W, Armenia J, Gopalan A, et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091–2102. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama NN, Shao S, Hoang BH, Mercola D, Zi X. Wnt signaling in castration-resistant prostate cancer: implications for therapy. Am J Clin Exp Urol. 2014;2(1):27–44. [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croce JC, McClay DR. Evolution of the Wnt pathways. Methods Mol Biol. 2008;469:3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murillo-Garzón V, Kypta R. WNT signalling in prostate cancer. Nat Rev Urol. 2017;14(11):683–696. [DOI] [PubMed] [Google Scholar]
- 10.Teng Y, Wang X, Wang Y, Ma D. Wnt/beta-catenin signaling regulates cancer stem cells in lung cancer A549 cells. Biochem Biophys Res Commun. 2010;392(3):373–379. [DOI] [PubMed] [Google Scholar]
- 11.Pohl SG, Brook N, Agostino M, Arfuso F, Kumar AP, Dharmarajan A. Wnt signaling in triple-negative breast cancer. Oncogenesis. 2017;6(4):e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishisho I, Nakamura Y, Miyoshi Y, et al. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253(5020):665–669. [DOI] [PubMed] [Google Scholar]
- 13.Isaacsson Velho P, Fu W, Wang H, et al. Wnt-pathway Activating Mutations Are Associated with Resistance to First-line Abiraterone and Enzalutamide in Castration-resistant Prostate Cancer. Eur Urol. 2020;77(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee E, Ha S, Logan SK. Divergent Androgen Receptor and Beta-Catenin Signaling in Prostate Cancer Cells. PLoS One. 2015;10(10):e0141589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van der Eecken K, Vanwelkenhuyzen J, Deek MP, et al. Tissue- and Blood-derived Genomic Biomarkers for Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review. Eur Urol Oncol. 2021;4(6):914–923. [DOI] [PubMed] [Google Scholar]
- 16.Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol. 2019;37(18):1558–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058. [DOI] [PubMed] [Google Scholar]
- 18.Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol. 2018;36(5):446–453. [DOI] [PubMed] [Google Scholar]
- 19.Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deek MP, Van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022:Jco2200644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 22.Deek MP, Van der Eecken K, Phillips R, et al. The Mutational Landscape of Metastatic Castration-sensitive Prostate Cancer: The Spectrum Theory Revisited. Eur Urol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutera P, Van Der Eecken K, Kishan AU, et al. Definitions of disease burden across the spectrum of metastatic castration-sensitive prostate cancer: comparison by disease outcomes and genomics. Prostate Cancer Prostatic Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koushyar S, Meniel VS, Phesse TJ, Pearson HB. Exploring the Wnt Pathway as a Therapeutic Target for Prostate Cancer. Biomolecules. 2022;12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zucchini-Pascal N, Peyre L, Rahmani R. Crosstalk between beta-catenin and snail in the induction of epithelial to mesenchymal transition in hepatocarcinoma: role of the ERK1/2 pathway. Int J Mol Sci. 2013;14(10):20768–20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B, Tang Z, Gong H, Zhu L, Liu X. Wnt5a promotes epithelial-to-mesenchymal transition and metastasis in non-small-cell lung cancer. Biosci Rep. 2017;37(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malek R, Wang H, Taparra K, Tran PT. Therapeutic Targeting of Epithelial Plasticity Programs: Focus on the Epithelial-Mesenchymal Transition. Cells Tissues Organs. 2017;203(2):114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gajula RP, Chettiar ST, Williams RD, et al. The twist box domain is required for Twist1-induced prostate cancer metastasis. Mol Cancer Res. 2013;11(11):1387–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gajula RP, Chettiar ST, Williams RD, et al. Structure-function studies of the bHLH phosphorylation domain of TWIST1 in prostate cancer cells. Neoplasia. 2015;17(1):16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malek R, Gajula RP, Williams RD, et al. TWIST1-WDR5-Hottip Regulates Hoxa9 Chromatin to Facilitate Prostate Cancer Metastasis. Cancer Res. 2017;77(12):3181–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo UG, Lee CF, Lee MS, Hsieh JT. The Role and Mechanism of Epithelial-to-Mesenchymal Transition in Prostate Cancer Progression. Int J Mol Sci. 2017;18(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyamoto DT, Zheng Y, Wittner BS, et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349(6254):1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Annala M, Vandekerkhove G, Khalaf D, et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov. 2018;8(4):444–457. [DOI] [PubMed] [Google Scholar]
- 35.Geng JH, Lin VC, Yu CC, et al. Inherited Variants in Wnt Pathway Genes Influence Outcomes of Prostate Cancer Patients Receiving Androgen Deprivation Therapy. Int J Mol Sci. 2016;17(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swami U, Graf RP, Nussenzveig RH, et al. SPOP mutations as a Predictive Biomarker for Androgen Receptor-Axis-Targeted Therapy in De Novo Metastatic Castration-Sensitive Prostate Cancer. Clin Cancer Res. 2022. [DOI] [PubMed] [Google Scholar]
- 37.Nakazawa M, Fang M, C HM, Lotan TL, Isaacsson Velho P, Antonarakis ES. Clinical and genomic features of SPOP-mutant prostate cancer. Prostate. 2022;82(2):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.