Abstract
In recent years, a growing number of pre-clinical studies have made use of the social abilities of mice, asking how gene variants (e.g., null, transgenic or mutant alleles) give rise to abnormalities in neurodevelopment. Two distinct courses of research provide the foundation for these studies. One course has mostly focused on how we can assess “sociability” using metrics, often automated, to quantitate mouse approach and withdrawal responses to a variety of social stimuli. The other course has focused on psychobiological constructs that underlie the socio-emotional capacities of mice, including motivation, reward and empathy. Critically, we know little about how measures of mouse sociability align with their underlying socio-emotional capacities. In the present work, we compared the expression of sociability in adolescent mice from several strains versus a precisely defined behavioral model of empathy that makes use of a vicarious fear learning paradigm. Despite substantial strain-dependent variation within each behavioral domain, we found little evidence of a relationship between these social phenotypes (i.e., the rank order of strain differences was unique for each test). By contrast, emission of ultrasonic vocalizations was highly associated with sociability, suggesting that these two measures reflect the same underlying construct. Taken together, our results indicate that sociability and vicarious fear learning are not manifestations of a single, overarching social trait. These findings thus underscore the necessity for a robust and diverse set of measures when using laboratory mice to model the social dimensions of neuropsychiatric disorders.
Keywords: vicarious fear learning, autism; social neuroscience; BTBR; fear conditioning
General Introduction
Laboratory mice are now routinely utilized to model the core features of pervasive developmental disorders (Crawley, 2012; Kazdoba, Leach, & Crawley, 2016) in order to ascertain whether certain alleles associated with these phenomena can alter social phenotypes (Moretti, Bouwknecht, Teague, Paylor, & Zoghbi, 2005; Schoch, Kreibich, Ferri, White, Bohorquez, et al., 2017; Stoppel, et al., 2017; Heun-Johnson & Levitt, 2018). The most commonly-used procedures are assays of sociability, behavioral tests that attempt to quantify approach and withdrawal responses to a variety of social stimuli, the most prominent of which is a an automated 3-chambered test of social proximity/novelty (Moy, Nadler, Perez, Barbaro, Johns, et al., 2004; Sankoorikal, Kaercher, Boon, Lee, & Brodkin, 2006; Moy, Nadler, Young, Perez, & Holloway, 2007). Comparisons reveal differences in the social responsiveness of inbred strains of laboratory mice, and these mice are often described as gregarious, typical or highly social versus less social, impaired, asocial/atypical, indifferent or abnormal.
In the 3-chambered test, there are 3 possible outcomes: [i] socially positive (i.e., more time spent in the chamber paired with a social stimulus), [ii] socially negative (i.e., more time in a contrasting chamber void of social stimuli) or [iii] indifferent (i.e., similar times spent in the experimental chambers). Despite its widespread use, variation in sociability is difficult to assess within this framework because the 3-chambered apparatus does not offer a fine-grained or nuanced picture of social approach/withdrawal behaviors (e.g., mice are separated by metal bars and complex social interactions are often reduced to photobeam breaks). By contrast, less contrived assessments of sociability, which include monitoring freely interacting mice, are sensitive to continuous variation in phenotypic responsiveness, as well as to the preceding circadian conditions and how long an individual has been alone prior to testing (Panksepp, Wong, Kennedy, & Lahvis, 2008). Such testing can therefore be utilized to interrogate polygenic, social traits. Although there are pros and cons to each experimental approach, the extent to which these social phenotypes reflect a similar underlying biological substrate is unknown.
A second course of research has focused on the psychobiological constructs that underlie the social interactions of rodents, including motivation (Panksepp, Jochman, Kim, Koy, Wilson, et al., 2007; Panksepp, Wong, Kennedy, & Lahvis, 2008), vocal communication (Lahvis, Alleva, & Scattoni, 2011; Scattoni, Ricceri, & Crawley, 2011; Wöhr, 2015), reward (Panksepp & Lahvis, 2007; Pearson, Bettis, Meyza, Yamamoto, Blanchard, et al., 2011), and empathy (Chen, Panksepp, & Lahvis, 2009; Jeon, Kim, Chetana, Jo, Ruley, et al., 2011; Panksepp & Lahvis, 2016). Over the last decade, testing procedures have become increasingly sophisticated and the implementation of fear conditioning paradigms has been crucial for assessing whether mice can gather information about the emotional state of conspecifics. For instance, a typical approach will utilize an “observer” who witnesses a “target” receiving an unconditioned stimulus (US)-conditioned stimulus (CS) pairing (Chen, et al., 2009; Jeon, et al., 2011; Panksepp & Lahvis, 2016). Depending on the frequency, duration and intensity of the US parameters, observers may express a “freezing” response in concert with a distressed conspecific (i.e., emotional contagion) or subsequently in response to the CS-only when the target mouse is absent (i.e., vicarious fear learning) (Chen, Panksepp, & Lahvis, 2009; Jeon, Kim, Chetana, Jo, Ruley, et al., 2011; Sanders, Mayford, & Jeste, 2013; Gonzalez-Liencres, Juckel, Tas, Friebe, & Brune, 2014; Panksepp & Lahvis, 2016; Keum, Park, Kim, Park, Kim, et al., 2016; Panksepp & Lahvis, 2018). Additionally, observers can gather affective information from a conspecific either while witnessing the expression of a CS-induced fear response (Bruchey, Jones, & Monfils, 2010; Jones, Riha, Gore, & Monfils, 2014) or following the session during a bout of social interaction (Knapska, Mikosz, Werka, & Maren, 2010; Meyza, Nikolaev, Kondrakiewicz, Blanchard, Blanchard, et al., 2015).
Several investigators have suggested these laboratory assessments tap into the core features of empathy (for reviews, see Panksepp & Lahvis, 2011; Panksepp & Panksepp, 2013; Mogil, 2015; Keum & Shin, 2016; Sivaselvachandran, Acland, Abdallah, & Martin, 2016; Wantanabe, 2016; Keysers & Gazzola, 2017; Lahvis, 2017; Meyza, Ben Ami-Bartal, Monfils, Panksepp, & Knapska, 2017; Panksepp & Lahvis, 2018). Within this context, we use an operational, yet conservative, definition of empathy: “an affective response more appropriate to another’s situation compared to one’s own” (Hoffman, 1975). In this regard, the notion of “empathic motivation” used by others (e.g., Bartal, Decety, & Mason, 2011)—that empathy alone can motivate an altruistic behavior is a contentious proposition that has been repeatedly challenged by some groups (see Silva, Silva, Lima, Meurer, Ceppi, et al., 2021; Blystad, 2021). We have also challenged that assertion, arguing that the expression of helping behavior might have evolved as a manifestation of the “camaraderie effect” (see Lahvis, 2016): a capacity for empathy and a motivation for social reward (see Discussion). From our more conservative view, robust behavioral, physiologic and neural evidence (Chen, et al., 2009, Jeon, et al., 2011; Allsop, Wichmann, Mills, Burgos-Robles, Chang, et al., 2018; Carrillo, Han, Migliorati, Liu, Gazzola, et al., 2019; Keum & Shin, 2019; Smith, Asada, & Malenka, 2021; Terranova, Yokose, Osanai, Marks, Yamamoto, et al., 2022; Zhang, Geng, Chen, Wang, Wang, et al., 2022) supports the contention that rodent vicarious fear learning phenotypes have deep, evolutionary roots. Critically, in humans, vicarious fear learning and empathy are strongly associated (Olsson, McMahon, Papenberg, Zaki, Bolger, et al., 2016), which further supports the hypothesis of a fundamental cross-species relation.
In humans, research on empathy has focused on associations with personality traits, such as extroversion/introversion, and how they may be related to prosocial behavior (Singer & Lamm, 2009). Many of these studies have demonstrated that measurements of extraversion (particularly inter-personal measures) are positively predictive of empathic tendencies (Dymond, 1950; Mehrabian, Young, & Sato, 1988; Barrio, Aluja, & Garcia, 2004; Haas, Brook, Remillard, Ishak, Anderson, et al., 2015), but there are also exceptions (Xin-ping & Tian, 2015; Melchers, Li, Haas, Reuter, Bischoff, et al., 2016; Neumann, Chan, Wang, & Boyle, 2016). Mouse social approach phenotypes bear a resemblance to extraversion; however, remaining unknown are [1] whether varying levels of sociability predict the ability to respond to a conspecific’s affective state in this species and [2] whether vicarious fear learning (viz., responding to another’s distress) relates to direct fear learning (viz., responding to one’s own distress) in this species.
In the present studies, we focused on a large, genetically heterogenous group of adolescent mice to evaluate the extent to which a measure of sociability (Panksepp, et al., 2007; 2008) and vicarious fear learning (Chen, et al., 2009; Panksepp & Lahvis, 2016) are inter-related. We investigated subjects from 5 classical inbred strains (BALB/cJ [“BALB”], BTBR T+Itpr3tfI/J [“BTBR”], C57BL/6J [“B6”], DBA/2J [“DBA”] and FVB/NJ [“FVB”]), an outbred strain (Swiss Webster ND4 [“ND4”]) and a wild-derived inbred strain (MSM/MsJ [“MSM”]). Considerations for selecting these mouse strains included knowledge of their social phenotypes from previous work and our intention to have a high degree of genetic diversity within the overall study population. For example, previous studies of BALB and B6 have documented differences in affiliative behavior and vicarious fear learning (Chen, et al., 2009; Keum, et al., 2016), whereas others have depicted BTBR as a model of idiopathic autism (McFarlane, Kusek, Yang, Phoenix, Bolivar, et al., 2008; Meyza & Blanchard, 2017). The DBA line has a long history in mouse genetics, including a comprehensively annotated genome and copious behavioral comparisons with B6. We also chose to study two strains to expand genetic heterogeneity of our study populations: ND4 (maintained as outbred) and MSM (not been subject to human selection). Our overall goal was to determine the breadth of strain-dependent responsiveness to evaluate potential relationships between sociability and vicarious fear learning in adolescent mice. Because these social phenotypes were poorly correlated at best among strains, our studies suggest that any specific behavioral test will fail to adequately serve as a proxy for the full repertoire of social abilities in mice.
Study 1: Sociability in adolescent mice
Introduction
In the laboratory, when a mouse is released into a cage it immediately begins to explore the new environment. If the cage happens to be the home cage of another mouse, the “resident” can just as quickly begin to investigate the new mouse and attempt to engage in social interaction. The duration and nature of the interaction may depend on the sexual identity, age and previous history of the mice, as well as their genetic makeup. However, during early adolescent development, most of these interactions are amicable in nature, with little influence of sex (Panksepp et al., 2007). Such interactions are driven by a state of reward (Panksepp & Lahvis, 2007) and reflect a mouse’s social motivation, or willingness, to engage its social partner (Panksepp et al., 2008). By contrast, as mice near sexual maturity, social interactions transition into forms more typical of adulthood (e.g., sexual and agonistic tendencies). In the present study, individuals from 7 different genetic lines were tested at 2 time points during adolescence to determine the extent to which genetic variability affects mouse sociability.
Methods
Subjects and husbandry
To establish the colony, individuals from the classical inbred strains of Mus musculus domesticus BALB, BTBR, B6, DBA2 and FVB were procured from the Jackson Laboratory (Bar Harbor, ME, USA). MSM, a wild-derived inbred strain of the Mus musculus molossinus subspecies, was also acquired from Jackson Labs. Outbred Swiss-Webster mice of the ND4 variety were obtained from Harlan Laboratories (Indianapolis, Indiana, USA).
Mice were housed in standard polycarbonate “shoebox” cages (Allentown Inc., Allentown, NJ, USA) lined with pelleted paper bedding (ECOfresh, Absorption Corp., Ferndale, WA, USA) and a nestlet, with ad libitum access to chow (Lab Rodent Diet 5001, Purina Mills LLC, Gray Summit, MO, USA) and water. The colony was maintained under a ‘reversed’ 12:12 h light/dark cycle (‘lights off’ 0900–2100 h) in a room maintained at 21±1°C and 40–60% humidity. Cage changes for the colony were conducted by a senior technician from 0700–0830 h. Litters were weaned on postnatal day (PD) 20–22. At weaning, individuals from 2–4 litters per strain were pooled and randomly assigned into mixed-sex social groups of 2 males and 2 females. Cage changes were conducted by the first author after the completion of each experiment. All experiments were conducted in strict accordance with the guidelines set forth by the institutional care and use committee at OHSU and the National Institutes of Health Guide for the Care and Use of Laboratory Animals (ISBN 978–0-309–15400-0).
Sociability testing
Mice were tested on PD 23–26 (“early adolescence”) and again on PD 44–47 (“late adolescence”). All 4 mice from a social group were individually isolated into a clean cage 24 h prior to testing (see Panksepp, et al., 2008, for rationale). One male and female from each social group were randomly designated as stimulus mice, while the remaining male and female served as test mice. Specific N’s for test mice at the beginning of the experiment were 40 [B6], 32 [BALB], 28 [BTBR], 28 [DBA], 26 [FVB], 12 [MSM] and 38 [ND4]. Cages were transported from the colony to an adjacent testing room (located ~3 m away door-to-door) >30 min prior to testing. All tests were conducted under dim red light (≈15 lux) during the middle of the dark phase (1200–1800 h). Five min prior to the sociability assessment, the top of a test mouse cage was replaced with a transparent piece of Plexiglas that contained a small, central hole where a recording microphone (see below) could be positioned flush with the Plexiglas. Testing commenced when a same-sex, former cage mate (i.e., the stimulus mouse) was added to the cage. Mice were video recorded (3CCD digital camcorder, Sony, Minato-ku, Tokyo, Japan) for 5 min, files were saved as .wmv files on a desktop computer (Precision T3400, Dell, Round Rock, TX, USA) and analyzed offline in a blind manner by the 1st author with behavioral coding software (ButtonBox v.5.0, Behavioral Research Solutions, Madison, WI, USA). Due to experimenter or computer error, 9 mice were not included in measurements for the PD 44–47 time point (2 BTBR, 4 DBA, 1 FVB and 2 ND4) and the 12 MSM mice were not tested at PD 44–47 (see Results).
The total duration of social investigation (SI) that each test mouse directed towards the stimulus mouse was a quantified by the first author during each 5-min testing period. The SI phenotype entails all aspects of social approach and interaction, including pursuit of the stimulus mouse within one body-length, sniffing at any part of its body or allo-grooming. SI was calculated by summing the duration that the test mouse engages in each of these behavioral elements (see Panksepp, et al., 2007; 2008 for additional detail regarding the SI phenotype). A subset of the sociability tests was also rated by a laboratory technician and inter-rater reliability was high (Pearson’s correlation, r=0.96, df=69).
Two additional studies of sociability were conducted with another set of BALB and FVB mice. In a mixed-strain housing study, which was employed to control for the sensory attributes of the stimulus mouse, a male and female mouse of the BALB or FVB strain were weaned into a social group with a B6 male and female. These B6 mice then served as stimulus mice for BALB and FVB test mice during the sociability testing procedures. In the second study, a cross-fostering approach was employed. B6 neonates were replaced with BALB or FVB neonates within 18 h of birth, and raised by a B6 dam. There were 16 test mice per strain in each study. Mice in both studies were weaned, housed and tested for sociability on PD 23–26, as described above.
Ultrasonic vocalization (USV) recording
In the primary study of sociability, USV emission was monitored with an UltraSoundGate CM16 microphone (2–250kHz flat frequency range) that was connected to an UltraSoundGate 416H A/D converter (Avisoft Bioacoustics, Glienicke, Germany). Signals were recorded at a 300kHz (16-bit) sampling rate and saved as .wav files with Avisoft-RECORDER software (v.4.2.31). Spectrograms were generated in SASLab Pro (v.4.52) using a fast Fourier transform (Hamming window, length=256, frame size=100%). Specific settings for USV segmentation using the “whistle tracking” algorithm in SASLab Pro were max change = 6 pixels, hold time = 8ms, minimum duration = 3ms and high-band pass filter = 40kHz. One BALB file was lost for the PD 23–26 time point. At PD 44–47, 19 files were not included in the analysis due to computer failure or file corruption (1 BALB, 5 BTBR, 7 DBA2, 4 FVB and 2 ND4).
Statistics
Three-way ANOVA was used to evaluate SI and USV emission, with genotype and sex as between-group factors and age as a repeated measure. Following each ANOVA, orthogonal contrasts were used to make specific comparisons between the genotypes, sex and age. Relationships between the SI phenotype and USV emission were evaluated with linear regression and Pearson’s product-moment correlation.
Results
Social investigation (SI)
During early adolescence (PD 23–26), expression of the SI phenotype varied considerably across strains (effect of genotype, F[5,171] = 65.8, P<0.0001). As shown in Figure 2A, BALB and FVB mice exhibited an ≈2.5-fold difference in SI, with no overlap between the respective distributions. Flanked by these extremes, the SI phenotype was graded across the other strains, with additional between-strain differences.
Figure 2.
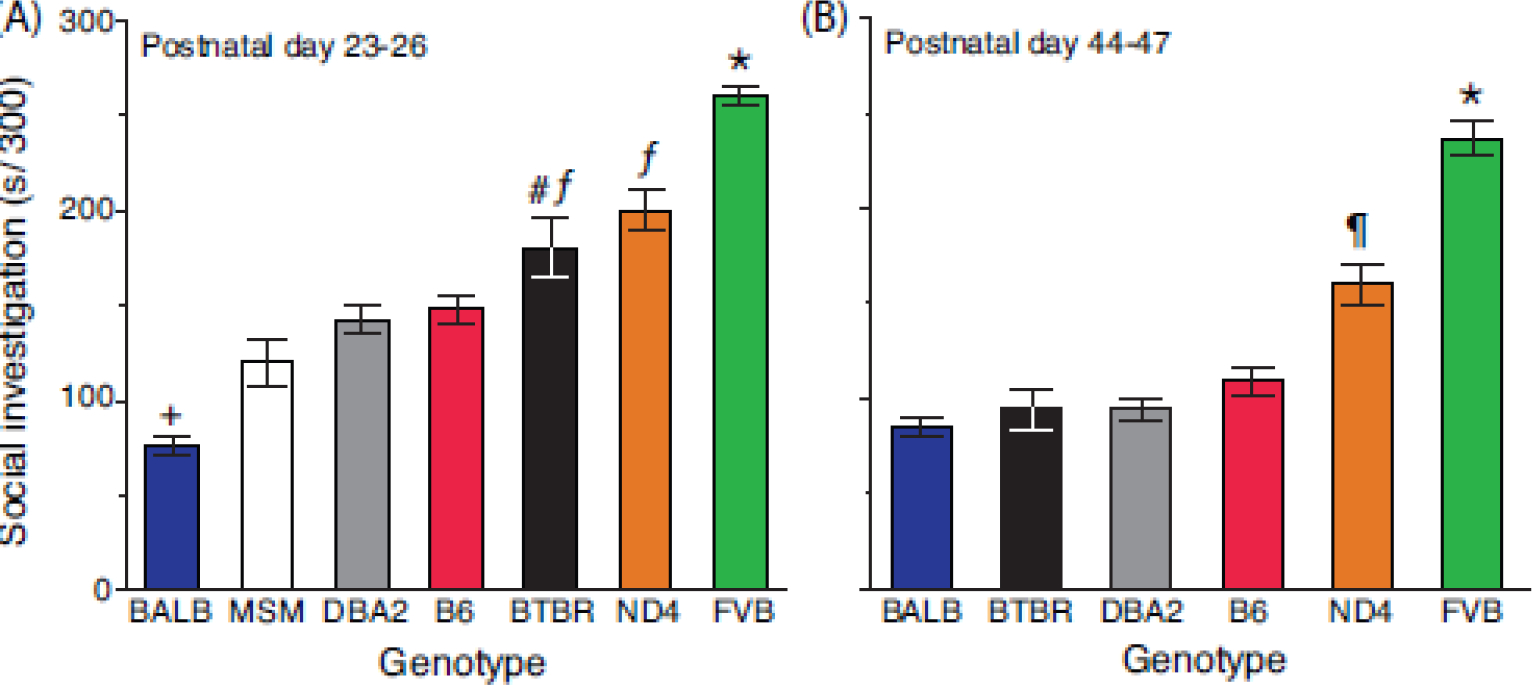
Strain differences in social investigation. Sociability testing was conducted during (A) early and (B) late adolescence. Mice were monitored for 300 s. Data are presented as the mean ± std. error. Genotypes are arranged on the abscissa using the rank-order of means for SI. N=25–40 mice per genotype (balanced across sex) for each time point. N=12 mice for MSM. * = FVB > all other strains, P<0.0001; ƒ = ND4/BTBR > B6/DBA2/MSM/BALB, P<0.0001; # = BTBR > B6, P=0.01; ¶ = ND4 > B6/DBA2/MSM/BTBR/BALB, P<0.0001; + = BALB < all other strains, P<0.0001.
As mice matured, the SI phenotype declined (mean ± std. error, early adolescence [163±5.2 s] vs. late adolescence [129±5.1]; effect of age, F[1,171] = 82.5, P<0.0001) and this change depended on the genetic background of mice (genotype x age interaction, F[1,171] = 8.2, P<0.0001). The data presented in Figure 2B show that the BALB-FVB difference persisted into late adolescence (PD 44–47), but the order of the other between-strain differences changed. For example, BTBR mice expressed more SI than B6 and BALB during early adolescence (orthogonal contrast, P<0.0001), but B6, BALB and BTBR were indistinguishable during late adolescence (orthogonal contrast, P=0.78). Notably, MSM mice became extremely “skittish” following the fear conditioning procedures used in Study 2 and only 12 subjects were tested for SI prior to removing this strain from the study due to their impulsiveness.
A male-female difference in SI was also detected (female [16±5.6] vs. male [138±4.9]; effect of sex, F[1,171] = 11.5, P=0.0009) and this difference was influenced by mouse genotype and age (genotype x sex x age interaction, F[5,171] = 2.4, P=0.04). During early adolescence, the only strain that exhibited a sex difference was BTBR (female [203±13.7] vs. male [158±13.6]; orthogonal contrast, P=0.02). However, during late adolescence B6, BTBR, ND4 and FVB females expressed more SI than males (P<0.05 for each orthogonal contrast).
Stimulus mouse genotype and maternal genotype influences on SI
At weaning, BALB and FVB mice were housed with age-matched B6 mice, respectively and the B6 mice then served as stimulus mice during sociability testing at PD 23–26. Figure 3A shows that the SI phenotype of FVB mice was higher than BALB (effect of genotype, F[1,30] = 182.5, P<0.0001), with no associated sex difference (F[1,30] = 1.3, P=0.26) or an interaction (F[1,30] = 0.4, P=0.54).
Figure 3.
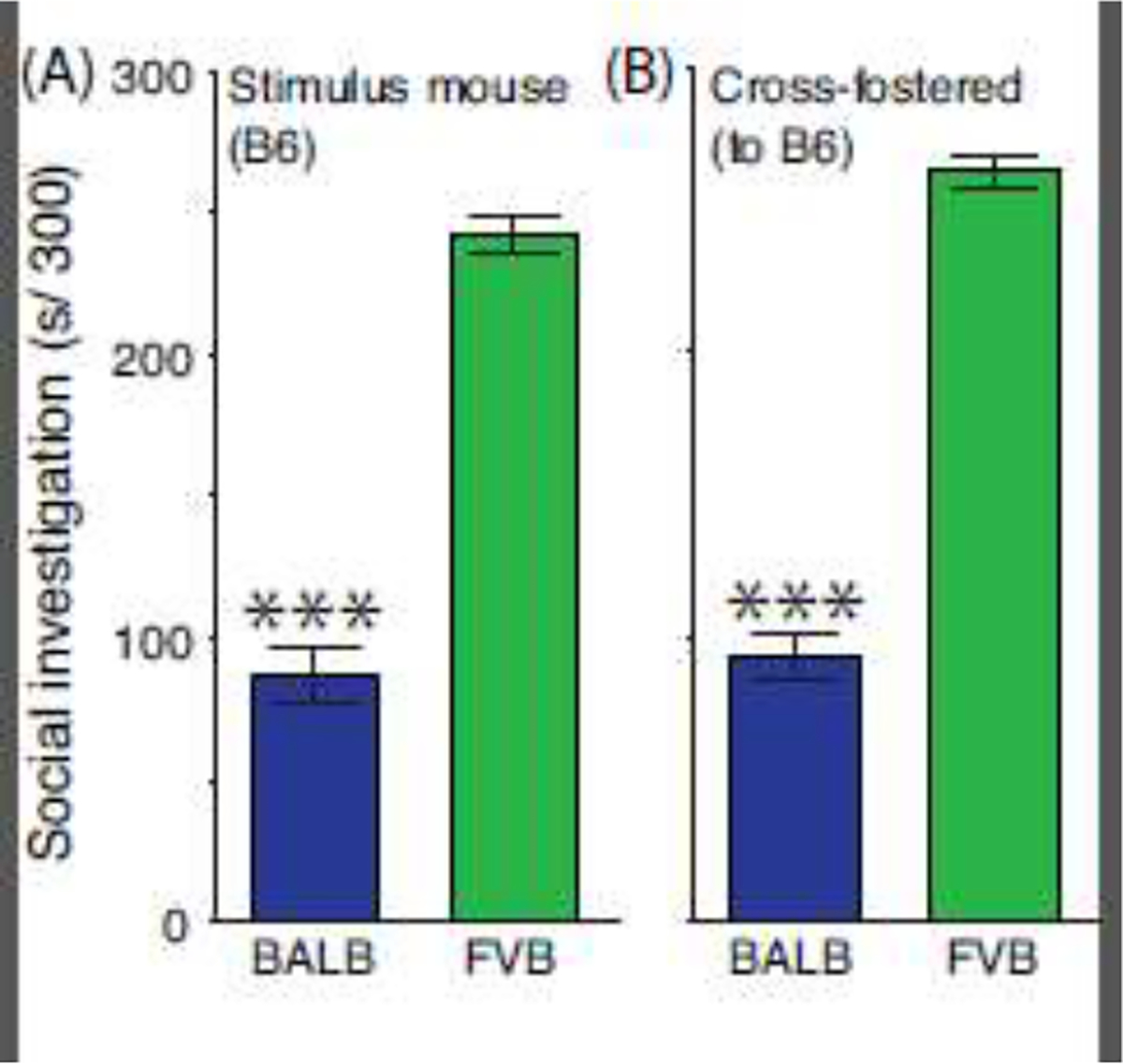
Lack of stimulus mouse genotype and maternal genotype influence on social investigation. Sociability testing was conducted during early adolescence with BALB and FVB test mice. Test mice were reunited (A) with a same-sex B6 cage mate or (B) with a same-sex, same-strain cage mate after being raised by a B6 mother. Mice were monitored for 300 s. Data are presented as the mean ± std. error. N=16 mice per genotype (balanced across sex) for each experiment. *** = main effect of genotype, P<0.0001.
Neonate BALB and FVB mice were raised by surrogate B6 mothers and then evaluated for SI during early adolescence (PD 23–26). As shown in Figure 3B, despite a common maternal influence, FVB mice expressed higher SI than BALB (effect of genotype, F[1,30] = 267.3, P<0.0001), but there was no sex difference (F[1,30] = 0.1, P=0.75) or an interaction (F[1,30] = 0.1, P=0.71).
Ultrasonic vocalizations (USVs)
As a complementary to SI, USV emission was monitored during the sociability tests. Many of the statistical differences for USV emission were similar to those found for the SI phenotype. For example, Figure 4 shows that USV production was affected by genetic background (effect of genotype, F[5,159] = 48.4, P<0.0001) and this influence changed with adolescent maturation (genotype x age interaction, F[5,159] = 7.7, P<0.0001). Similar to the SI phenotype, USV production generally decreased during adolescent development (early adolescence [970±42.2] vs. late adolescence [783±61.1]; effect of age, F[1,159] = 30.3, P<0.0001). This age-dependent alteration was due to a differential response in females versus males (genotype x sex x age interaction, F[5, 159] = 9.5, P<0.0001). For example, males from all strains except BALB exhibited a reduction in USV emission at PD 44–47 relative to PD 23–26 (P<0.05 for each orthogonal contrast). By comparison, USVs for females from three of the strains increased from early to late adolescence (B6 – 953±100.8 vs. 1399±120.9, BALB - 173±28.2 vs. 513±103.6, FVB - 1703±97.2 vs. 2379±70.1; P<0.05 for each orthogonal contrast), while USV emission by females from the other 3 strains did not change.
Figure 4.
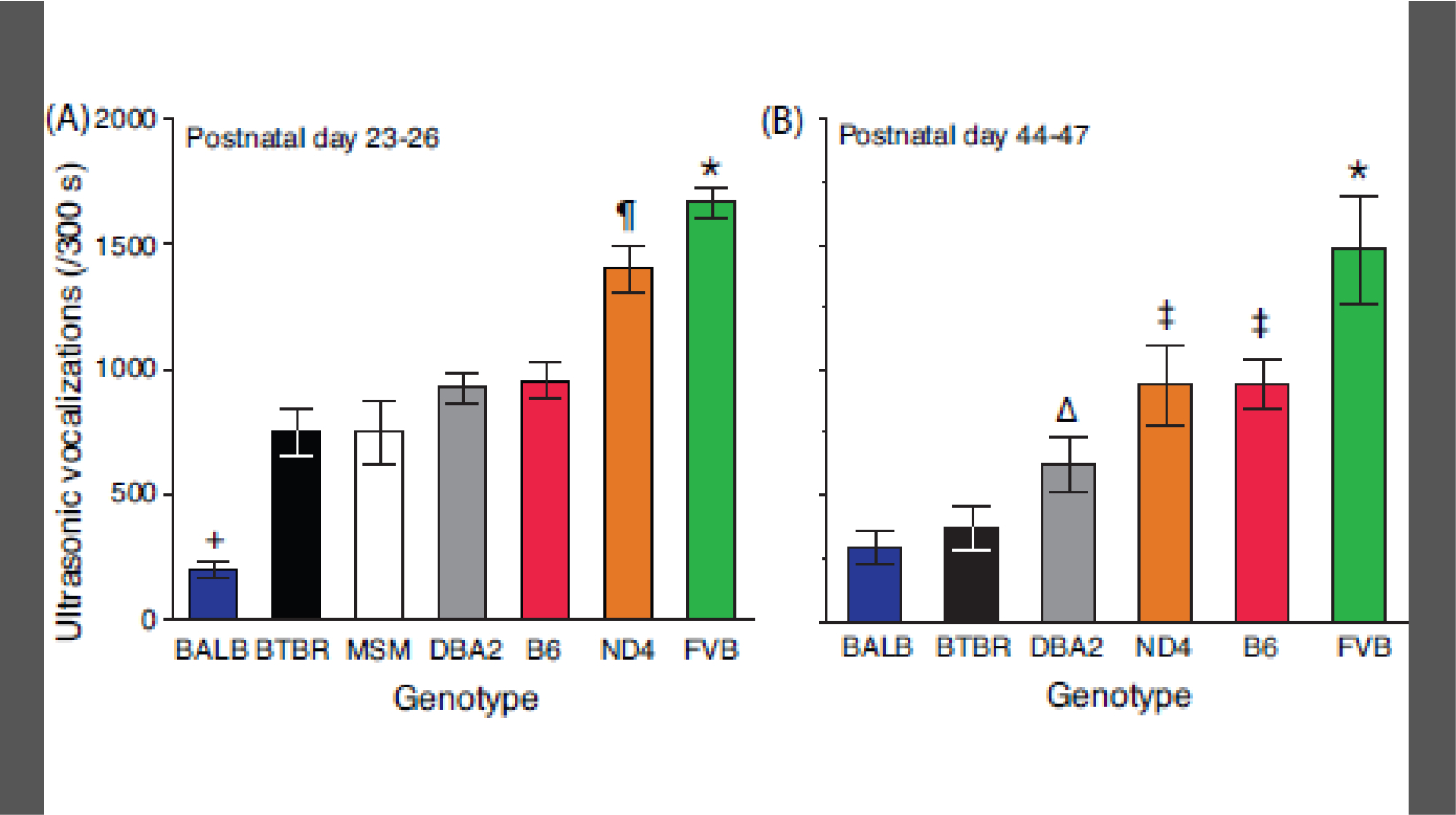
Strain differences in ultrasonic vocalization emission. USV production was monitored during sociability testing on (A) PD 23–26 and (B) PD 44–47. Mice were monitored for 300 s. Data are presented as the mean ± std. error. Genotypes are arranged on the abscissa using the rank-order of means for USV production. N=19–40 mice per genotype (balanced across sex) for each time point. N=12 for MSM. * = FVB > all other strains; = ND4 > B6/DBA2/MSM/BTBR/BALB, P<0.0001; ‡ = B6/ND4 > DBA2, P<0.0001; Δ = DBA2 > BTBR/BALB, P=0.0007; + = BALB < all other strains, P<0.0001.
Association between SI and USV emission
Figure 5A shows the SI-USV relation: the number of USVs emitted during sociability testing was a strong predictor of SI response duration at PD 23–26 (F[1,201] = 372.4, P<0.0001) and PD 44–47 (Figure 5B; F[1,168] = 145.2, P<0.0001), although the effect size was ≈29% higher during early adolescence (R2=0.65 vs. 0.46). At PD 23–26, the relationship between SI and USV emission was significant for all strains (P’s <0.05) except BALB. At PD 44–47, F-values for regression analyses were also significant for all strains (P’s <0.05) except BALB and DBA2. Genetic association between the SI phenotype and USV emission at both ages was high (Pearson’s correlations; early adolescence, r = 0.92, df = 6, P = 0.003; late adolescence, r = 0.91, df = 5, P = 0.01).
Figure 5.
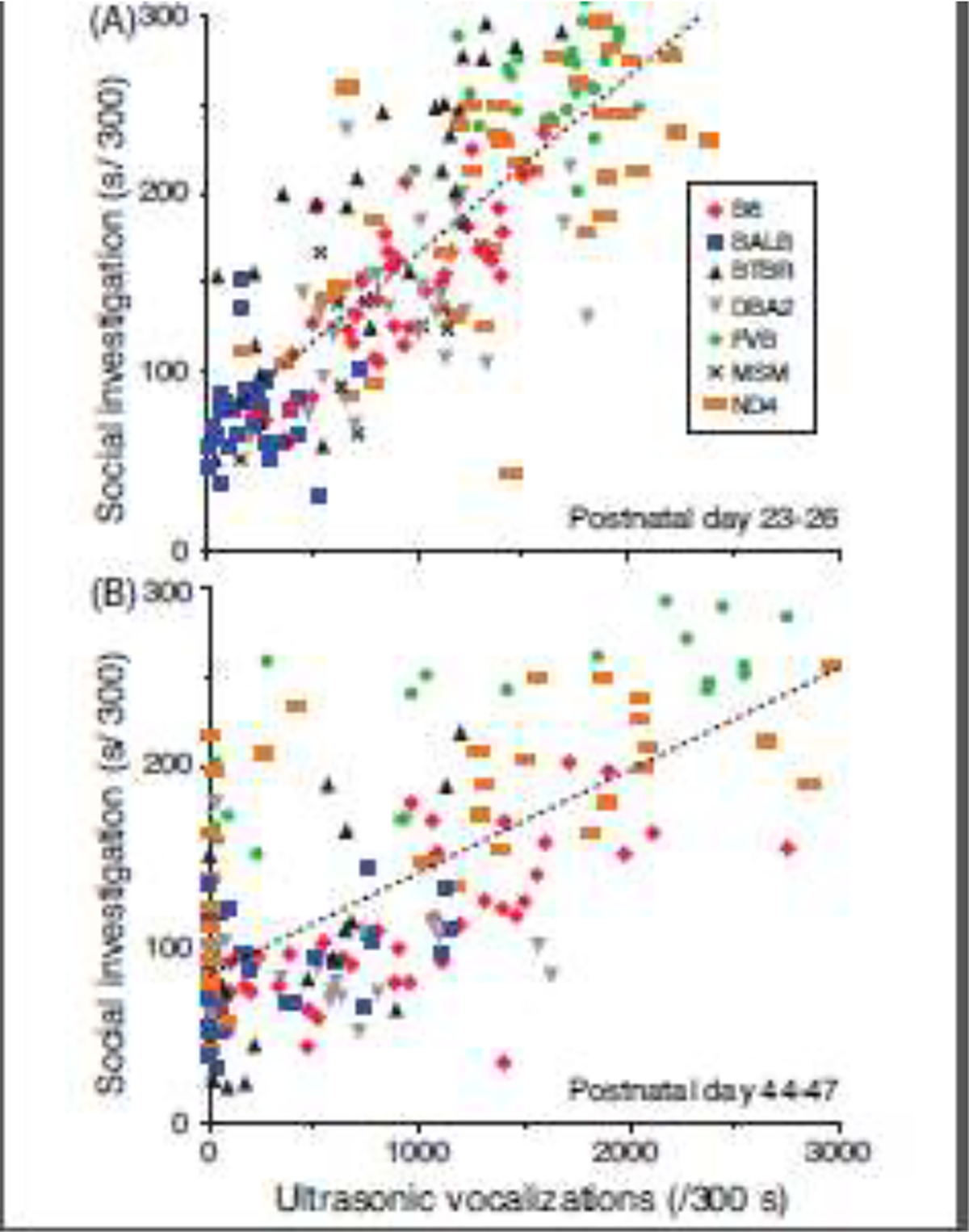
Relationship between social investigation and ultrasonic vocalization emission. Linear regression analysis for (A) PD 23–26 and (B) PD 44–47, with the SI phenotype of each mouse on the abscissa and USV production of each mouse on the ordinate.
Study 2: Vicarious fear learning in adolescent mice
Introduction
Rodents can learn by observing conspecifics (Wolff & Sherman, 2007). In our previous work, we modified a Pavlovian fear conditioning protocol to develop a vicarious fear learning protocol. During this procedure, an observer mouse witnesses a conspecific who experienced a series of aversive foot shocks paired with the presentation of a tone (i.e., a US-CS pairing). Fear is a primary emotion and freezing behavior is one of its objective expressions in animals (Panksepp, 2004; Darwin, 2009). After conditioning, observer mice will freeze in response to the CS presentation (as if they had experienced the shock themselves), which illustrates their ability to adopt the emotional state of the target mouse. Importantly, the expression of mouse “vicarious freezing” by itself does not imply an altruistic intention to help a conspecific nor does it suggest that the observer possesses the ability to cognitively represent the state of the other mouse (i.e., perspective taking). In the present model, observers adopt the affective state of conspecifics by attending to the distress vocalizations that target mice emit during shock presentation (Chen et al., 2009; Panksepp & Lahvis, 2018). The present study was designed to [1] expand upon a previously described difference between BALB and B6 mice (Chen et al., 2009) by including more genetic lines for comparison, and [2] evaluate whether the vicarious learning phenotype is related to the sociability phenotype (Study 1) across these lines.
Methods
Subjects and husbandry
The mice used for the study of sociability where also assessed for fear conditioning In between the sociability tests (PD 31–41), mice were randomly assigned to one of 3 different fear conditioning groups. Subjects were maintained as described above for the sociability study.
Fear conditioning
For this study, all mice from a cage were either individually subjected to a direct, cue-conditioned fear procedure or to a vicarious/joint cue-conditioned fear procedure. For direct conditioning, a single mouse was exposed to a standard fear conditioning procedure (see below). For joint fear conditioning, 2 mice from the same cage were conditioned together. For vicarious fear conditioning, the remaining 2 mice from the cage observed the jointly conditioned mice. Mice in the vicarious conditioning group always observed the same pair of jointly conditioned mice across sessions. Test and stimulus mice from the first sociability testing time point were distributed equally across the direct, vicarious and joint conditioning groups.
Empathy chambers (CleverSys Inc., Reston, Virginia, USA) were used for all of the conditioning procedures. The main chamber (260 × 260 × 210 mm), where direct and joint fear conditioning took place, included a floor and wall of 2 mm stainless steel rods (spaced by 4 mm on-center) where mice could receive a shock. Two peripheral compartments (130 × 130 ×210 mm) had a floor and wall (facing the main chamber) that were lined with inactive, stainless steel rods. Thus, both mice in the main chamber and peripheral compartments could climb the rod-lined walls abutting one another, but mice in the peripheral compartments did not receive a shock (see below). Each chamber was housed within a sound-dampening box constructed from ABS plastic lined with foam rubber. Infrared arrays composed of 96 LEDs were situated above each chamber. Videos were recorded with miniature infrared cameras supplied by CleverSys Inc. and stored as .mpg files on a desktop computer. FreezeScan software was used to automate administration of the US and conditioned stimulus (CS).
Direct conditioning: On Day 1, individual mice were placed into the main chamber. After 120 s, each mouse was subjected to ten 30-s tone (CS) presentations (1kHz, 85dB) that each co-terminated with 1-mA scrambled shock (US) lasting 3 sec. These US-CS pairings were separated by 90-s intervals of silence. On Day 2, mice were exposed to a 2nd iteration of the same conditioning protocol. Mice were then tested 15 min post-conditioning with 5 presentations of the CS-only. On Day 3, mice were tested again (24 h post-conditioning) with 5 presentations of the CS-only.
Joint conditioning: A male-female pair was placed in the main chamber and the procedures for direct conditioning described above were followed exactly. Jointly conditioned mice were tested individually for in the main chamber (both 15 min and 24 h post-conditioning). Sounds were recorded and extracted from spectrograms of jointly conditioned mice. Ultrasonic vocalizations are rarely emitted by mice during fear conditioning, but audible distress vocalizations (DVs)—the “squeaks” that are emitted when a rodent is distressed—are abundant and convey fear to vicariously conditioned mice (Chen, et al., 2009; Panksepp & Lahvis, 2018) DVs were manually identified on spectrograms recorded during the 2nd conditioning sessions (Day 2), using the resonant energy above background noise that characterizes this call type (see Chen, et al., 2009; Panksepp & Lahvis, 2018).
Vicarious conditioning: On Day 1, an individual male or female mouse was first placed in the main chamber for 10 min and halfway through received a single, unconditional scrambled shock (1-mA) lasting 3 sec. This phase of the experiment was employed to familiarize the mice with the testing environment and the US (see Chen, et al., 2009; Sanders, et al., 2013). Fifteen min later, each mouse was placed into one of the peripheral compartments and were allowed to observe the jointly conditioned mice (i.e., target mice) undergo fear conditioning. On Day 2, vicariously conditioned mice again observed the same pair of jointly conditioned mice. They were then tested 15 min and 24 post-conditioning (Day 3), as described above.
Freezing behavior (defined as the complete absence of movement) was scored twice by the first author in a blind manner for all CS presentations and a baseline period (60 s pre-CS) during both test sessions. Intra-rater reliability during CS presentations was r = 0.99 (d.f. = 1,774) and r = 0.99 (d.f. = 1.778) for 15 min post-conditioning and 24 h post-conditioning, respectively. Intra-rater reliability during the baseline period was r = 0.99 (d.f. = 355) and r = 0.99 (d.f. = 351) for 15 min post-conditioning and 24 h post-conditioning, respectively. Interrater reliability was not calculated for the fear conditioning study, but historically it has been high in our laboratory (Chen et al., 2007; Panksepp & Lahvis, 2016). Moreover, videos were recorded in a black-and-white format, so distinguishing among the 3 albino strains (BALB, FVB, ND4) or among the strains with a black coat color was impossible. All data presented in the figures and statistical outcomes are based on an average of the duplicate measurements. Statistics
Four-way ANOVA were used to evaluate CS-induced freezing behavior for each conditioning group, with genotype and sex as between-group factors and test session and trial as a repeated measure. Three-way ANOVA were employed to evaluate baseline freezing within each conditioning group, with genotype, sex and test session as between-group factors. Following each ANOVA, orthogonal contrasts were used to make specific comparisons between genotypes, sexes, test sessions and groups (e.g., direct vs. joint conditioning, baseline/contextual freezing vs. cued freezing). One-way ANOVA was used to analyze DVs for genotype differences followed by pairwise t-tests. Associations between the behavioral variables were evaluated with linear regression and Pearson’s product-moment correlation.
Results
Vicarious fear conditioning
Observer mice that witnessed a pair jointly conditioned targets undergo a cue-conditioned fear procedure subsequently expressed cue-induced freezing and this response was strain dependent (Figure 6; effect of genotype, F[5,608] = 67.8, P<0.0001). As shown in Figure 6, CS-induced freezing was highest in B6 observers both 15 min and 24 h post-conditioning, whereas CS-induced freezing was undetectable in DBA2 and FVB observers at both time points.
Figure 6.
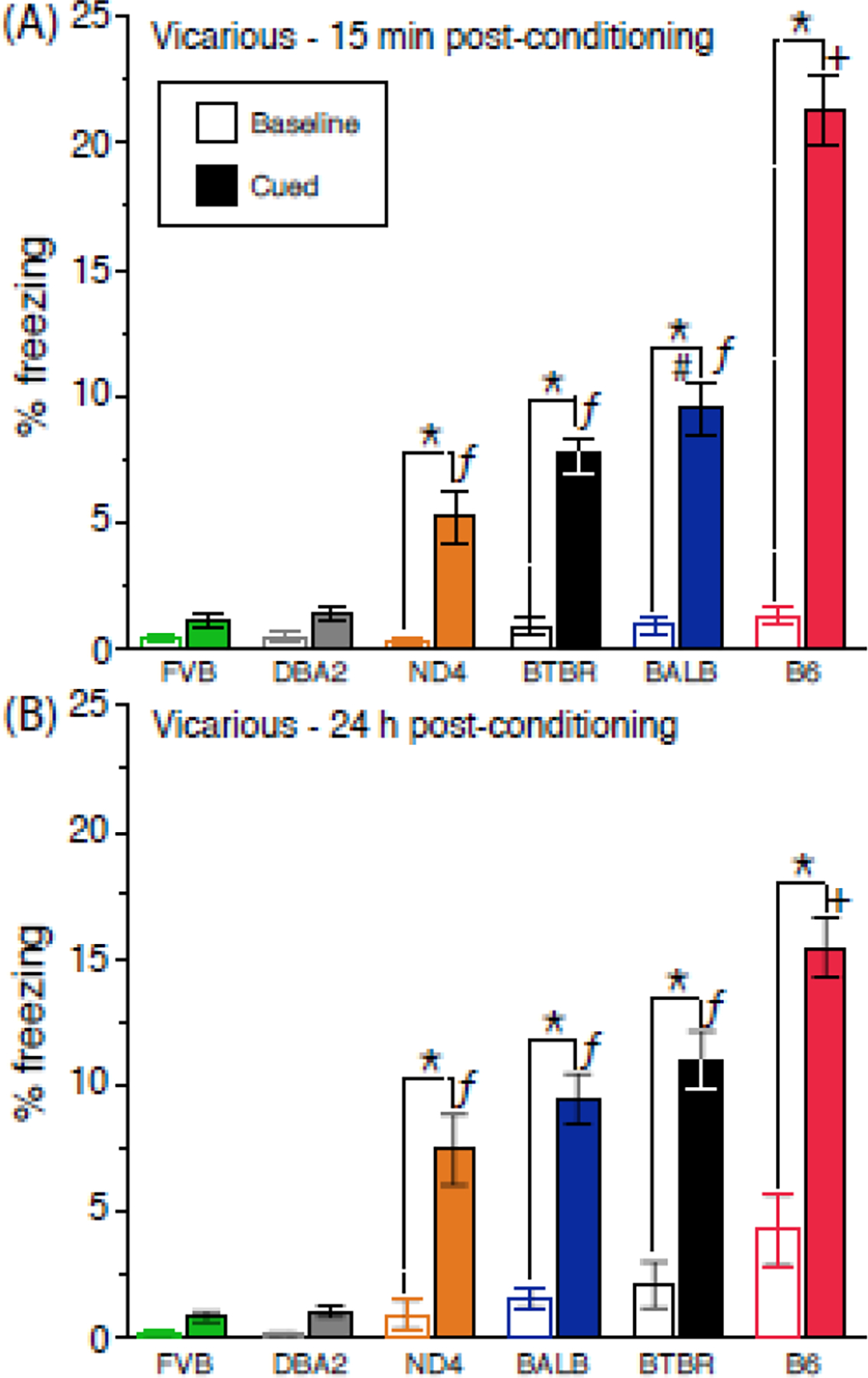
Baseline and cue-induced freezing following vicarious conditioning. Vicarious freezing (A) 15 min and (B) 24 h post-conditioning. Data are presented as the mean ± std. error. Genotypes are arranged on the abscissa using the rank-order of means for cued-induced freezing. N=16–24 mice per genotype (balanced across sex). + = B6 > all other strains, P<0.0001; ƒ = BALB/BTBR/ND4 > DBA2/FVB, P<0.0001; # = BALB>ND4, P=0.002; * = Cued > Baseline, P<0.05.
There was a genotype-by-test session interaction on CS-induced vicarious freezing (F[5,608] = 7.4, P<0.0001). Although B6 freezing was higher than all other strains at both time points, it nevertheless decreased from 15 min to 24 h post-conditioning (21±1.6% vs. 15±1.2%, orthogonal contrast, P<0.0001). By contrast, BTBR freezing exhibited a modest increase across the same time frame (8±0.7% vs. 11±1.2%, orthogonal contrast, P<0.0001).
There also was a genotype-by-sex-by-test session interaction (F[5,608] = 2.6, P=0.02). While the B6 decrease (described above) was uniform for the sexes across testing, only females from the BTBR (6±0.9% vs. 13±2.1%) and ND4 (8±1.9% vs. 12±2.6%) strains increased vicarious freezing at the 24 h time point (P=0.0007 and P=0.02 for each orthogonal contrast, respectively)
Figure 6 also shows that baseline freezing prior to CS presentation was low for all strains, indicating that US pre-exposure (see Methods) did not engender a contextual fear response. There was a small increase in baseline freezing from the 15 min to the 24 h time point (effect of session, F[1,111] = 5.0, P=0.03), with a near-significant genotype-by-session interaction (F[5,111] = 2.3, P<0.06). This change was due to an increase in BALB, BTBR and B6 (orthogonal contrasts; B6>BTBR/BALB, P=0.0009, BTBR/BALB>ND4/DBA2/FVB, P=0.02). Notably, using the baseline measure for standardization (Tipps, Raybuck, Buck, & Lattal, 2014) did not alter the rank order of strain differences for CS-induced freezing (data not shown). Distress vocalizations (DVs) of target mice during vicarious conditioning Audible DVs, which are the primary means through which vicarious fear is acquired by observer mice (Chen, et al., 2009; Panksepp & Lahvis, 2018), were monitored from the jointly conditioned mice used during vicarious conditioning. There was an effect of genotype on DV production (F[5,55] = 3.3, P=0.01). As shown in Table 1, BALB, FVB and ND4 jointly conditioned targets emitted more DVs than BTBR and FVB.
Table 1.
Distress vocalizations of jointly conditioned mice that served as targets for vicariously conditioned observer mice. Recordings were made during the second conditioning session of the experimental procedure (see Methods). Data are rank ordered by genotype and reported as the mean ± std. error.
| Genotype | Distress Vocalizations |
|---|---|
| FVB | 11±1.3 |
| BTBR | 12±1.7 |
| B6 | 13±1.0 |
| BALB | 15±1.7* |
| DBA2 | 15±1.5* |
| ND4 | 15±1.3* |
= BALB, DBA2 and ND4 > BTBR and FVB, respectively, P<0.05.
Relationship between SI (Study 1) and vicarious fear (Study 2)
Population-wide regression analysis did not reveal a relationship between SI during early adolescence and vicarious fear expression 15 min (Figure 7A; F[1,76] = 3.4, P=0.07, R2=0.04) or 24 h post-conditioning (Figure 7C; F[1,76] = 2.6, P=0.11, R2=0.03). By contrast, late adolescent SI was modestly and negatively associated with vicarious freezing 15 min post-conditioning (Figure 7B; F[1,72] = 10.8, P=0.002, R2=0.13) and 24 h post-conditioning (Figure 7D; F[1,72] = 11.8, P=0.001, R2=0.14). All F-values for within-strain regression analyses were not significant at each time point and test session (all P’s > 0.05). Genetic association between the SI phenotype and vicarious freezing did not reach significance 15 min (early adolescence, r = −0.52, df = 5, P = 0.29; late adolescence, r = −0.44, df = 5, P = 0.38) or 24 h post-conditioning (early adolescence, r = −0.38, df = 5, P = 0.44; late adolescence, r = −0.43, df = 5, P = 0.39).
Figure 7.
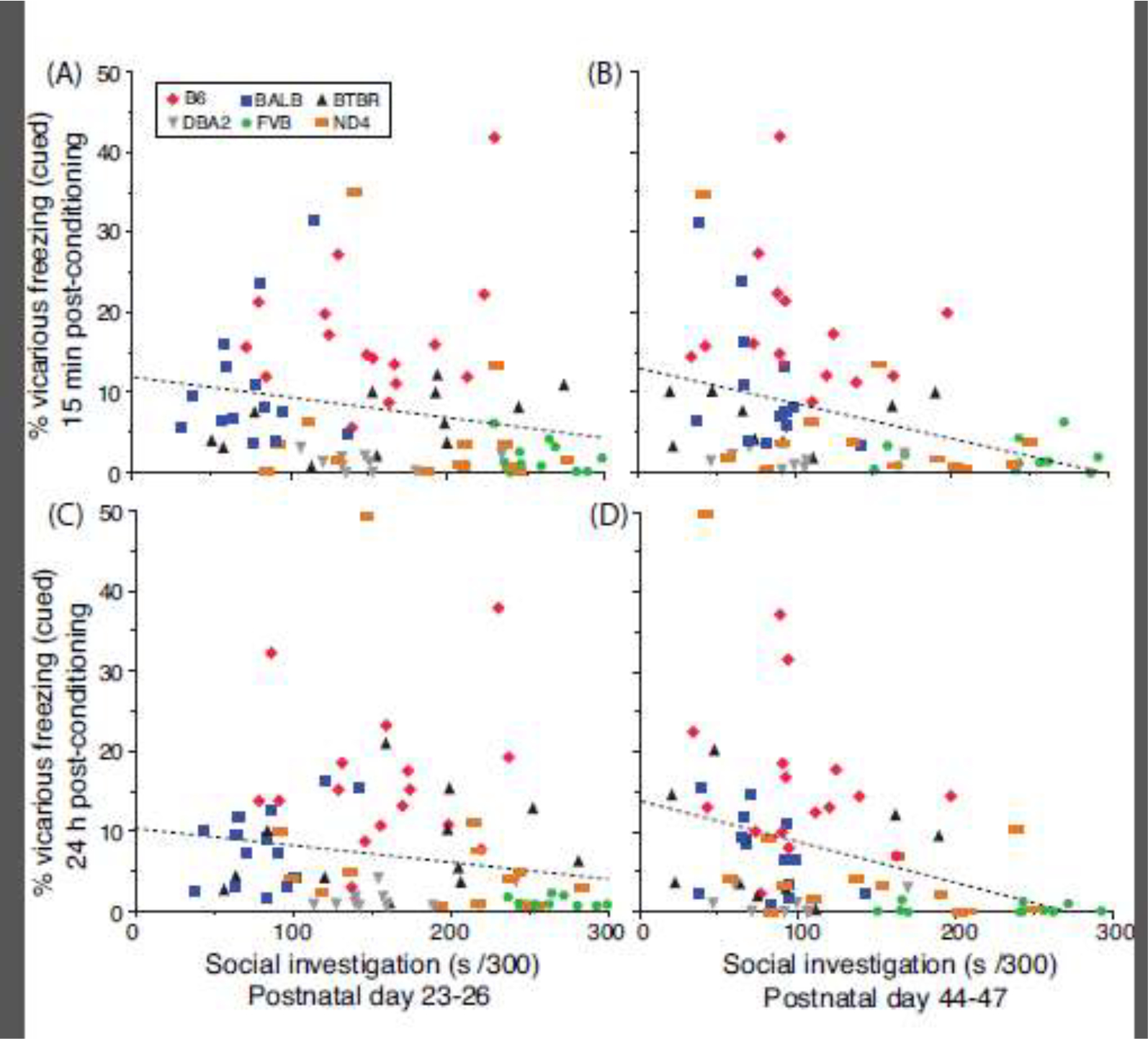
Relationship between social investigation and vicarious freezing. Linear regression analysis for (A, C) PD 23–26 and (B, D) PD 44–47, with the SI phenotype of each mouse on the abscissa and the vicarious freezing response of each mouse on the ordinate.
Direct fear conditioning
As shown in Figure 8, individual mice that were directly conditioned expressed longer freezing responses than vicariously conditioned mice and there was a strain-dependent influence (effect of genotype, F[5,492] = 7.2, P<0.0001), an effect of sex (F[1,492] = 10.6, P<0.01 (female [42±1.1%] vs. male [47±1.1%]) and a near significant genotype-by-sex interaction (F[5,492] = 2.3, P<0.07). There also was an effect of test session (F[1,492] = 27.0, P<0.0001; 15 min [41±1.2%] vs. 24 h post-conditioning [47±1.1%]) and a genotype-by-session interaction (F[5,492] = 11.6, P<0.0001). BALB and ND4 cue-induced freezing increased from 15 min to 24 h post-conditioning whereas DBA2 mice exhibited a decrease (P<0.01 for each orthogonal contrast). A genotype-by-sex-by-session interaction (F[5,492] = 2.8, P=0.02) indicated that ND4 males increased freezing across test sessions (orthogonal contrast, P=0.0004), but females did not.
Figure 8.
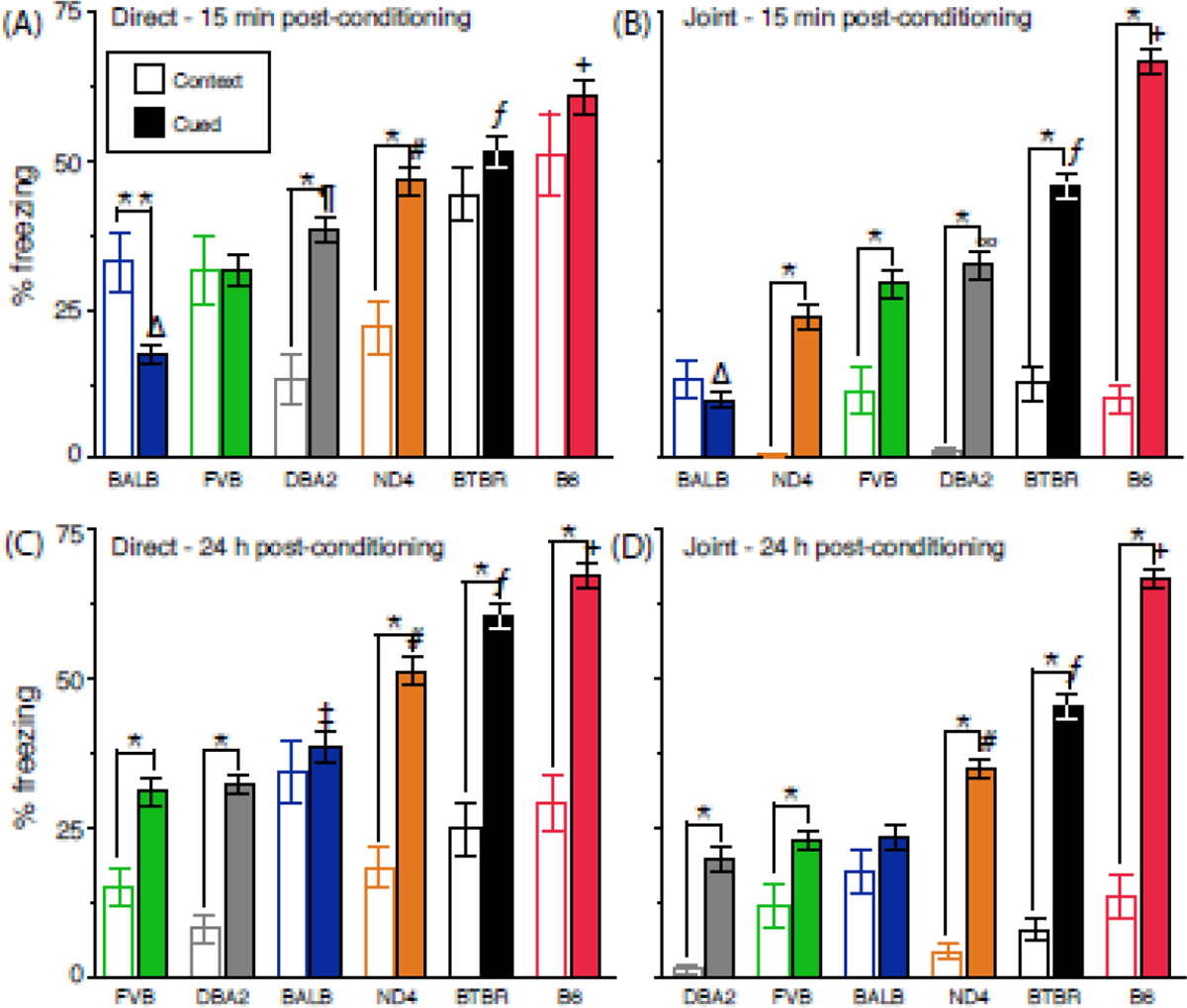
Contextual and cue-induced freezing following direct and joint conditioning. Freezing (A,B) 15 min and (C,D) 24 h post-conditioning for direct and jointly conditioned mice, respectively. Data are presented as the mean ± std. error. Genotypes are arranged on the abscissa using the rank-order of means for the cued component of freezing. N=13–20 and 15–24 mice per genotype (balanced across sex) for the direct and joint groups, respectively. + = B6 > all other strains, P<0.0001; ƒ = BTBR > ND4/DBA2/FVB/BALB, P<0.0001; # = ND4>DBA2/FVB/BALB, P<0.001; ¶ DBA2>FVB/BALB, P=0.0002; Δ= BALB < all other strains, P<0.0001 ‡ = BALB > DBA2/FVB, P<0.0001; ∞ = DBA2>ND4, P=0.0004 * = Cued > Context, P<0.05; ** = Context > Cued, P<0.05
Baseline freezing of directly conditioned mice was higher than the vicariously conditioned groups because this aspect of freezing for this experiment represented a contextual component of fear (i.e., individuals were tested in the same chamber where they received cued conditioning). There was an effect of genotype on this baseline/contextual freezing (F[5,89] = 7.2, P<0.0001). The rank-order of strain differences was B6=BTBR>BALB=FVB>ND4=DBA2 at 15 min post-conditioning (see Figure 8A, each orthogonal contrast, P<0.05). Overall, an effect of session (F[1,89] = 20.2, P<0.0001) indicated that baseline/contextual freezing was reduced across sessions (15 min [33±2.5%] vs. 24 h post-conditioning [22±1.8%]) and there was a near-significant genotype-by-session interaction (F[1,89] = 2.3, P<0.06), with a rank-order of strain differences at 24 h post-conditioning of BALB=B6=BTBR>ND4=FVB>DBA2 (see Figure 8C, each orthogonal contrast, P<0.05). Directly conditioned B6, BTBR and FVB mice reduced baseline/contextual freezing from 15 min to 24 h post-conditioning (each orthogonal contrast, P<0.05), while BALB, ND4 and DBA2 baseline/contextual freezing did not change.
Joint fear conditioning
This group included mouse pairs that were cue-conditioned in the presence of observers and subsequently tested individually in the conditioning chamber. Figure 7 shows an effect of genotype F[5,608] = 148.9, P<0.0001). There also was an effect of sex (F[1,608] = 16.2, P<0.0001; female [39±1.1%] vs. male [34±1.0%]), a genotype-by-sex interaction (F[5,608] = 2.8, P=0.02) and a genotype-by-session interaction (F[5,608] = 14.9, P<0.0001), where jointly conditioned BALB and ND4 mice increased freezing from 15 min to 24 h post-conditioning (each orthogonal contrast, P<0.0001). By contrast, BTBR and FVB decreased freezing over the same time frame (each orthogonal contrast, P<0.0001). A genotype-by-sex-by-session interaction (F[1,608] = 2.5, P=0.03) indicated that the ND4 increase was specific to males (orthogonal contrast, P<0.0001).
Baseline/contextual freezing in jointly conditioned mice also varied across genetic background (effect of genotype, F[5,112]=93.9, P<0.0001), with a rank-order (based on orthogonal contrast) of BALB=B6=FVB=BTBR>ND4=DBA2. An effect of sex (F[1,112] = 4.4, P=0.04) and a genotype-by-sex interaction (F[1,112] = 2.9, P=0.02) indicated that BALB and FVB females expressed higher freezing than males (orthogonal contrasts, P<0.05 and P<0.0001, respectively). Unlike direct conditioning, there were not statistical interactions involving test session.
Comparisons of direct and jointly acquired fear
General inspection of Figure 8A vs. 8B and Figure 8C vs. 8D indicates that baseline/contextual fear and cue-conditioned freezing were respectively lower in jointly conditioned individuals compared to directly conditioned mice. For cued freezing at 15 min post-conditioning, BALB and ND4 freezing was reduced in jointly conditioned mice (each orthogonal contrast, P<0.05) and similar reductions in BTBR and DBA2 mice neared significance (each orthogonal contrast, P=0.09). At 24 h post conditioning, all jointly conditioned strains exhibited reductions in cue-induced freezing compared to directly conditioned mice (each orthogonal contrast, P<0.01) except B6.
During the baseline/contextual assessment of freezing, all jointly conditioned mice exhibited reductions compared to directly conditioned mice (each orthogonal contrast, P<0.001), except DBA2, which neared significance (P=0.07). At 24 h post-conditioning, jointly conditioned BALB, BTBR, B6 and ND4 exhibited reductions relative to direct conditioning (each orthogonal contrast, P<0.01) whereas DBA2 and FVB baseline/contextual freezing remained similar across the time points.
Discussion
The present studies demonstrate a substantial influence of strain-dependent variation on adolescent mouse sociability and empathy, as operationally defined through vicarious fear learning. Although these behavioral phenomena have been explored before, they have never been examined in the same individuals across strains. Our findings collectively indicate that no relationship exists between sociability and vicarious fear learning at an individual level. These findings suggest that the psychological constructs being modeled – social motivation and empathy – have distinct underlying biological substrates. This overall conclusion has critically important implications for how we study and interpret the social endophenotypes of laboratory mice.
Before advancing further into the Discussion, we reiterate our commitment to viewing empathy through a narrowly defined window. We do not see helping behavior or biological altruism as behavioral expressions of empathy alone. In concordance with Blystad (2021), we also disagree with the idea of empathy as a motivation, let alone a single driver of helping behavior. Instead, we continue to argue that empathy is a specific phenomenon – to feel into the emotions of another. This capacity maybe useful for survival, including sensing dangers through the distress of others, or even the weakened resolve of others during competitive interactions (such as mating opportunities). By narrowly focusing empathy in this fashion, we can identify its biological substrates (e.g., see Jeon et al., 2011; Allsop et al., 2018; Smith et al., 2021; Terranova et al., 2022; Zhang et al., 2022).
While the social emotional underpinnings of helping behavior might include capacities for empathy, the motivation to help could be driven by social reward. We have argued that the camaraderie effect (Lahvis, 2017), which incorporates a capacity for empathy and social reward, offers a more tractable and testable model for understanding the psychobiological substrates of helping behavior. In this regard, we cannot overemphasize that a critical problem with the empathy literature is that many authors conflate empathy with altruistic motivation, as well as other distinctive concepts like perspective-taking and sympathy. To avoid the endless controversies that surround big umbrella definitions, we urge for more circumscribed and testable definitions about particular psychological phenomena that can be more widely useful to scientific progress. Indeed, we have similar concerns for the word “sociability” and show here that it does not associate with capacities for empathy.
With respect to sociability, we found a large range of phenotypic variability among 7 strains, along with confirmation of a previously described difference between BALB and B6 (Sankoorikal, et al., 2006; Moy, et al., 2007; Panksepp, et al., 2007). Inclusion of multiple strains revealed that several of them (i.e., BTBR, FVB and ND4) exhibited much higher levels of SI compared to B6 during early adolescence. The social responses of BTBR mice have been described as abnormal and consistent with the symptomology of autism (see Meyza & Blanchard, 2017 for a review). As far as we know, the majority of studies that have evaluated the BTBR strain have done so during late adolescence or adulthood (but see, Scattoni, Gandhy, Ricceri, & Crawley, 2008;. Babineau, Yang, Berman, & Crawley, 2013; Scattoni, Martire, Cartocci, Ferrante, & Ricceri, 2013; Wöhr, 2015). Scattoni et al. (2013) found that BTBR males expressed lower levels of sociability relative to B6 at PD 32. We found that BTBR individuals were considerably less social during late adolescence (PD 44–47) relative to early adolescence (PD 23–26). During early adolescence, SI expression of BTBR was higher than that of B6. Although many of the strains became less social across adolescent development, the decrease in BTBR mice was the sole influence on changing the rank-order of strain-dependent differences. Thus, if the social behavior of BTBR mice expresses some features of autism, the early-to-late adolescent transition appears to be particularly important for determining how a highly social BTBR mouse becomes a substantially less social individual within a relatively narrow time window of development. Inclusion of the finding by Scattoni et al. (2013) would reduce this time frame (≈20-day difference) substantially, at least for males (Bell, 2018).
Despite the emergence of age- and sex-related changes in SI, the difference between BALB and FVB was very robust at both time points during adolescence. High levels of sociability have been previously described for late adolescent and early adult FVB mice (Bolivar, Walters, & Phoenix, 2007; Moy, et al., 2007). Relative to our work describing a difference between BALB and B6 (Panksepp, et al., 2007; 2008; Chen, et al., 2009; and this study), the BALB-FVB difference was of a much larger magnitude, reaching ≈2.5 fold during early adolescence. Given that this strain difference was not affected by the genetic background of the mother or stimulus mouse, and there was not an overlap between the respective distributions of SI, early adolescent BALB and FVB appear to be ideal strains for genetic/genomic or neural assessments of pre-pubertal sociability.
For genotype, age and sex, the pattern of USV production generally followed that of SI. Interestingly, BTBR mice exhibited already low levels of USV emission during early adolescence, similar to BALB, which suggests that decreased vocal production in BTBR mice precedes the decline in the SI phenotype. Consistent with a previous suggestion (Portfors, 2007), there was a very strong association between USV emission and SI expression during early adolescence, across genotypes as well as the entire population of mice irrespective of genetic background. SI expression and USV emission thus appear to represent the same underlying phenomenon (i.e., social motivation) and share similar underlying genetic substrates. Spectrographic analyses have shown that certain characteristics or types of USVs are differentially associated with the genetic background or psychological state of rodents (Panksepp, et al., 2007; Scattoni, et al., 2008; Liu, Lopatina, Higashida, Fujimoto, Akther, et al., 2013; Yang, Loureiro, Kalikhman, & Crawley, 2013). In addition to the number of vocalizations emitted, it will be interesting to assess the spectral characteristics of USVs from these strains, in order to determine whether specific types of calls associate with the between-strain pattern of SI.
The vicarious fear learning phenotype also exhibited a large amount of genotype-dependent variation, which was essentially the same across the strains at both 15 min and 24 h post-conditioning. A high level of vicarious freezing was found for B6 mice and intermediate levels for BALB, BTBR and ND4, while responses of DBA2 and FVB were not detectable above baseline. We therefore replicated the BALB-B6 difference in vicarious freezing (Chen, et al., 2009), but we also found strains that were less responsive than BALB (i.e., DBA2 and FVB). Although BALB vicarious freezing was lower than B6, it was detectable above baseline and appeared to be somewhat higher than in our previous study (Chen, et al., 2009). This could be due to several between-study differences; however, consideration of one particular set of variables is intriguing. In the Chen et al. (2009) study, target mice for BALB and B6 observers came from a separate cage, were novel, and derived from a BALB x B6 cross. In the present study, observers and target mice were cage mates and they shared the same genotype. Thus, targets and observers were familiar and genetically identical, interacting with each other when placed back into their home cage after conditioning. Social familiarity, genetic similarity and post-conditioning social interaction might all have contributed to the apparent increase in BALB vicarious freezing.
Distress vocalizations are the primary signal (i.e., the US) through which distress is communicated from target to observer in this model of vicarious fear (Chen, et al., 2009; Panksepp & Lahvis, 2018), but it remains unknown if there are specific DV features (e.g., vocalization amplitude, number, etc.) that are critical for this communication. In this study, there was strain-dependent variation in the number of DVs emitted by targets (BALB=DBA2=ND4>BTBR=B6=FVB), but it was not clearly predictive of vicarious fear expression by observers (B6>BALB=BTBR=ND4>DBA2=FVB). Thus, either the number of emitted DVs is not important for communicating social distress or between-strain differences in vicarious fear are controlled by additional factors, such as differences in learning or the ability to detect/process DVs. Although there has not been a side-by-side analysis of hearing for these strains during adolescence, previously described differences in the initial stages of auditory processing (Willott, Turner, Carlson, Ding, Seegers Bross, et al., 1998; Willott, Tanner, O’Steen, Johnson, Bogue, et al., 2003; Jones, Jones, Johnson, Yu, Erway et al., 2006; Zhou, Jen, Seburn, Frankel, et al., Zheng, 2006) do not reflect the pattern of strain-dependent variation in vicarious fear learning that we report here.
Mouse cue-induced freezing responses following direct conditioning did not strictly associate with their freezing responses following vicarious conditioning. For example, directly acquired fear expression of BTBR and B6 was similar, but B6 vicarious fear expression was substantially higher than BTBR. Moreover, DBA2 and FVB vicarious freezing was undetectable whereas both strains had measurable directly acquired fear responses. BALB mice had intermediate levels of vicarious freezing relative to the other strains, but their response following direct conditioning was lower. These findings indicate that a combination of variables, including emission of DVs and learning ability, contributes to the expression of vicarious fear for any given strain. In a recent experiment with B6, we have developed an approach that eliminates the learning component and standardized the emission of target DVs (Panksepp & Lahvis, 2018), which should help simplify studies attempting to identify differences in the processing of DVs by observer mice.
During the vicarious conditioning procedure, observers received a single experience with the US in the main chamber prior to being conditioned in adjacent compartments to the targets’ response to the US-CS paring. For testing, observers were placed back into the conditioning chamber and their pre-cue freezing was low (1–4%), indicating very little—if any—effect of the context on freezing. This measure therefore represents a baseline. However, during the other procedures, mice were conditioned and tested in the same chamber; thus, pre-cue freezing for these individuals was equivalent to a contextual component of fear, and in this respect the unusual phenotype of BALB mice is deserving of some additional consideration. Unlike the other strains, directly conditioned BALB mice did not exhibit a cued freezing response larger than their contextual freezing response. At 15 min post-conditioning, the cued response of directly conditioned BALB mice was actually lower than the preceding contextual response. Upon presentation of the tone, BALB mice often exhibited a vigorous flight response prior to freezing, which likely accounted for the discrepancy between cued and contextual freezing for this strain. Additional measures of fear, including active responses such as flight, may be useful for further discriminating the fear responses of these strains (Götz & Janik, 2011).
Another unexpected and intriguing finding were the comparisons of directly conditioned mice in the direct versus joint conditions. These mice were treated in in the same way (including individual testing) except that jointly conditioned mice acquired fear as a dyad whereas directly conditioned mice acquired fear as individuals. Excluding B6, cued freezing responses were universally reduced in jointly conditioned mice relative directly conditioned groups. Moreover, contextual freezing was reduced in nearly all of the jointly conditioned groups. These results resemble a phenomenon known as “social buffering”, where the presence of another conspecific can reduce aversive responses, such as fear or stress, in another individual (Kikusui, Winslow, & Mon, 2006). However, there are several other possible interpretations of this finding: [i] In the joint condition, an individual may have become distracted by the unconditioned response (UR) of their companion, reducing attention to the environment and making conditioning less effective, which would then result in a lower CR during testing. [ii] A second possibility is that a mouse perceives its companion as a part of the environmental context and individual testing eliminates that aspect of the environment, resulting in an altered context and thus reduced context-induced freezing. This explanation, however, would not necessarily explain the reduction found for many strains during the cued phase of testing, although it still could be argued that the presence of a jointly conditioned companion interacts with presentation of the tone during conditioning to affect cue-induced freezing during testing. [iii] Another possibility is that this reduced freezing does indeed represent a social buffering effect. Regardless of a companion’s affective state, perhaps their mere presence during joint conditioning is sufficient to reduce fear acquisition in their partner. It should also be noted that jointly conditioned mice may have perceived the conspecifics that were being vicariously conditioned in the adjacent compartments, and this could have additionally contributed to social buffering and/or an inadvertent change of the context during testing. In addition to vicarious fear learning, which is substantially influenced by genetic variation, these findings demonstrate a distinct form of social modulation on conditioning that appears less sensitive to genetic factors, and set the stage for identifying the variables that contribute to the reduced fear behavior of jointly conditioned mice.
A primary goal of the present study was to examine whether there is an association between vicarious fear learning and sociability at a genetic level in mice. Our data suggests there is no such relation. We found that these phenotypes do not correlate across strains. However, we note that when regression analysis was conducted on the entire of population of mice, negative relation (albeit very weak) were evident between vicarious fear and SI during late adolescence. Another important factor to consider is the degree of variance in the vicarious fear response, which was sizeable for B6, but substantially smaller for strains such as DBA2 and FVB. Strains exhibiting little variation may have contaminated the population-wide analysis and it would be prudent in the future to conduct a similar study with a much larger cohort of mice from a single strain, such as B6. It should also be noted that the fear conditioning procedures could have impacted the sociability phenotype during late adolescence, which complicates comparisons between the different time points. However, many of the developmental changes described for SI here are nevertheless consistent with previous work (e.g., Panksepp & Lahvis, 2007), indicating that a potential influence of fear conditioning on the late adolescent SI phenotype was modest at best.
We also must recognize that the artificial environments used for housing and testing in the present studies imposed unknown influences on the expression of strain-dependent phenotypes. The chosen dimensions of a standard shoebox cage for laboratory mice were arbitrary (Guide for laboratory animal facilities and care, 1963). The environments inside such cages are highly impoverished—even from a social standpoint—as they forbid opportunities for a mouse to find social refuge, a restriction that likely exerts substantial effects on all aspects of mouse sociability (Lahvis, 2016). Likewise, since variations in the environment can skew the maturation of both fear (Lehmann & Herkenham, 2011) and reward circuitry (Bardo, Valone, Robinet, Shaw &, Dwoskin, 1999), one cannot expect that mice who, by the nature of their confinement, experience artificially low daily variations of exposures to risks or access to rewards, would express normal development. In this regard, we recognize, like others (Burrows & Hannan, 2013), the need to develop novel husbandry conditions that include unpredictable challenges, such as changes in food availability, weather, season, lighting and so on (Lahvis, 2017). While these considerations should engender a robust skepticism of the environmental construct validity of the present study, we can be confident that because the measured aspects of social functioning were largely dissociated, they do indeed reflect the existence of distinct psychobiological mechanisms.
The provisional conclusion of no relation between vicarious fear learning and sociability is thus intriguing, suggesting that the social abilities of mice can’t be summarized by a convenient catch-all phrase. This conclusion is bolstered by the work of others (Keum, et al., 2016). They also found no evidence of a relation between “observational fear learning” and social preference across mouse strains. Their study included different procedures and methods, the most prominent being their use of contextual conditioning versus our cue-based approach to assess vicarious fear. Nevertheless, in both studies there was evidence that B6 and BTBR expressed vicarious responses post-conditioning while DBA2 and FVB did not. Thus, our findings together with those of Keum et al. (2016) indicate that genetic differences in empathic responding are stable across strains irrespective of the fear conditioning procedure employed. Moreover, in their model, social distress appears to be mediated through visual communication Jeon, et al, 2011), while in our paradigm visual cues are not required and auditory signals are the primary form of social communication (Chen, et al., 2009; Panksepp & Lahvis, 2016). Taken together, evidence suggests that mouse strain differences in vicarious fear learning are consistent regardless of the of the sensory modality involved to sense distress (visual versus auditory) or the associated learning contingency (context versus cue).
From a broader view, we see a general tendency to oversimplify the multifaceted nature of mouse social emotions. But just as sociability is not a catch-all for social motivation and empathy, we anticipate that even our conservative definition of empathy might also be more multidimensional. For instance, perhaps capacities for vicarious fear learning do not align across mouse strains with more naturalistic and nuanced measures of shared emotional experience, such as the potential use of empathy to learn what foods to eat or what risk-free paths to take (based upon the behaviors of others). If novel models of empathy can be developed, we see strain-dependent responses as a means for determining the extent to which such empathic responses share similar mechanisms with vicarious fear learning.
Figure 1.
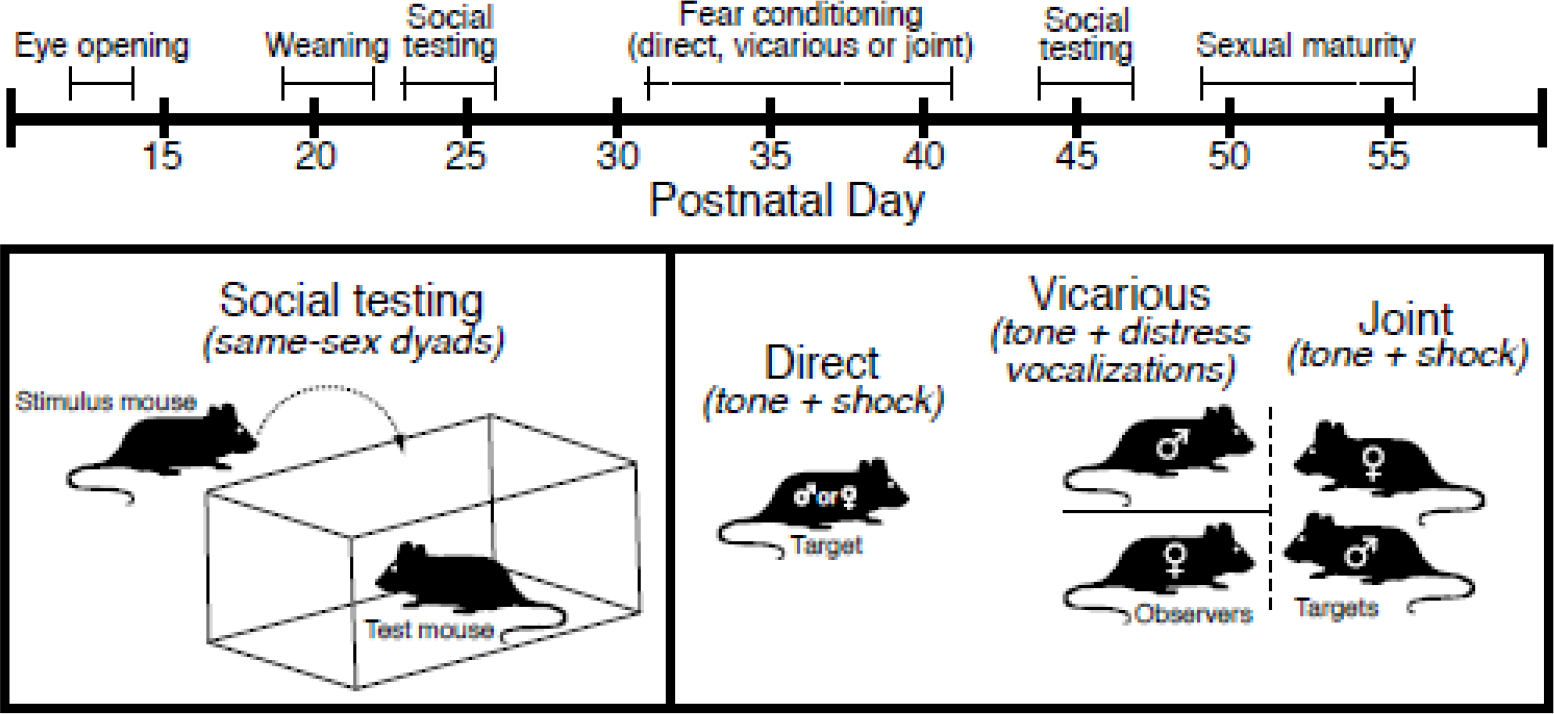
Cartoon depiction of behavioral procedures. The social investigation phenotype of test mice was measured following reunion with a same-sex cage mate after 24 h of isolation on PD 23–26 and PD 44–47. Between these time points on PD 31–41 mice were conditioned and tested for direct, vicarious or jointly acquired fear as described in the Methods section.
Funding:
This work was supported by NIH grants R01DA022543, R21MH106284 and F32MH096475 and in part by a NIH core grant (P50HD105353) awarded to the Waisman Center Intellectual and Developmental Disabilities Research Center
Footnotes
CRediT authorship contribution statement
Garet Lahvis: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Roles/Writing - original draft; Writing - review & editing. Jules Panksepp: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Roles/Writing - original draft; Writing - review & editing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allsop SA, Wichmann R, Mills F, Burgos-Robles A, Chang CJ, Felix-Ortiz AC, Vienne A, Beyeler A, Izadmehr EM, Glober G, Cum MI, Stergiadou J, Anandalingam KK, Farris K, Namburi P, Leppla CA, Weddington JC, Nieh EH, Smith AC, . . . Tye, K. M. (2018). Corticoamygdala Transfer of Socially Derived Information Gates Observational Learning. Cell, 173(6), 1329–1342.e18. 10.1016/j.cell.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babineau BA, Yang M, Berman RF, & Crawley JN (2013). Low home cage social behaviors in BTBR T+tf/J mice during juvenile development. Physiology & Behavior, 114–115, 49–54. 10.1016/j.physbeh.2013.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardo MT, Valone JM, Robinet PM, Shaw WB, & Dwoskin LP (1999). Environmental enrichment enhances the stimulant effect of intravenous amphetamine: Search for a cellular mechanism in the nucleus accumbens. Psychobiology, 27(2), 292–299. 10.3758/bf03332123 [DOI] [Google Scholar]
- Barrio VD, Aluja A, & García LF (2004). Relationship between empathy and the big five personality traits in a sample of spanish adolescents. Social Behavior and Personality: An International Journal, 32(7), 677–681. 10.2224/sbp.2004.32.7.677 [DOI] [Google Scholar]
- Bartal IBA, Decety J, & Mason P (2011). Empathy and Pro-Social Behavior in Rats. Science, 334(6061), 1427–1430. 10.1126/science.1210789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR (2018). Comparing Postnatal Development of Gonadal Hormones and Associated Social Behaviors in Rats, Mice, and Humans. Endocrinology, 159(7), 2596–2613. 10.1210/en.2018-00220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blystad MH (2021). An opinion on the interpretation of social release in rats. Biology Letters, 17(11). 10.1098/rsbl.2021.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar V, Walters S, & Phoenix J (2007). Assessing autism-like behavior in mice: Variations in social interactions among inbred strains. Behavioural Brain Research, 176(1), 21–26. 10.1016/j.bbr.2006.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchey AK, Jones CE, & Monfils MH (2010). Fear conditioning by-proxy: Social transmission of fear during memory retrieval. Behavioural Brain Research, 214(1), 80–84. 10.1016/j.bbr.2010.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows E, & J Hannan A (2013). Towards environmental construct validity in animal models of CNS disorders: optimizing translation of preclinical studies. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 12(5), 587–592. doi: 10.2174/1871527311312050007 [DOI] [PubMed] [Google Scholar]
- Carrillo M, Han Y, Migliorati F, Liu M, Gazzola V, & Keysers C (2019). Emotional Mirror neurons in the rat’s anterior cingulate cortex. Current Biology, 29(8), 1301–1312.e6. 10.1016/j.cub.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, & Lahvis GP (2009). Empathy is moderated by genetic background in mice. PLoS ONE, 4(2), e4387. 10.1371/journal.pone.0004387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN (2012). Translational animal models of autism and neurodevelopmental disorders. Autism and Related Developmental Disorders, 14(3), 293–305. 10.31887/dcns.2012.14.3/jcrawley [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C (2009). The Expression of the Emotions in Man and Animals, Anniversary Edition (4th ed.). Oxford University Press. [Google Scholar]
- Dymond RF (1950). Personality and empathy. Journal of Consulting Psychology, 14(5), 343–350. 10.1037/h0061674 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Liencres C, Juckel G, Tas C, Friebe A, & Brüne M (2014). Emotional contagion in mice: The role of familiarity. Behavioural Brain Research, 263, 16–21. 10.1016/j.bbr.2014.01.020 [DOI] [PubMed] [Google Scholar]
- Götz T, & Janik VM (2011). Repeated elicitation of the acoustic startle reflex leads to sensitisation in subsequent avoidance behaviour and induces fear conditioning. BMC Neuroscience, 12(1). 10.1186/1471-2202-12-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Brook M, Remillard L, Ishak A, Anderson IW, & Filkowski MM (2015). I Know How You Feel: The Warm-Altruistic Personality Profile and the Empathic Brain. PLOS ONE, 10(3), e0120639. 10.1371/journal.pone.0120639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun-Johnson H, & Levitt P (2018). Differential impact of Met receptor gene interaction with early-life stress on neuronal morphology and behavior in mice. Neurobiology of Stress, 8, 10–20. 10.1016/j.ynstr.2017.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman ML (1975). Developmental synthesis of affect and cognition and its implications for altruistic motivation. Developmental Psychology, 11(5), 607–622. 10.1037/0012-1649.11.5.607 [DOI] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, Rabah D, Kinet JP, & Shin HS (2010). Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nature Neuroscience, 13(4), 482–488. 10.1038/nn.2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Jones TA, Johnson KR, Yu H, Erway LC, & Zheng QY (2006). A comparison of vestibular and auditory phenotypes in inbred mouse strains. Brain Research, 1091(1), 40–46. 10.1016/j.brainres.2006.01.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Riha PD, Gore AC, & Monfils MH (2013). Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Animal Cognition, 17(3), 827–834. 10.1007/s10071-013-0711-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdoba TM, Leach PT, & Crawley JN (2015). Behavioral phenotypes of genetic mouse models of autism. Genes, Brain and Behavior, 15(1), 7–26. 10.1111/gbb.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keum S, Park J, Kim A, Park J, Kim KK, Jeong J, & Shin H (2016). Variability in empathic fear response among 11 inbred strains of mice. Genes, Brain and Behavior, 15(2), 231–242. 10.1111/gbb.12278 [DOI] [PubMed] [Google Scholar]
- Keum S, & Shin HS (2016). Rodent models for studying empathy. Neurobiology of Learning and Memory, 135, 22–26. 10.1016/j.nlm.2016.07.022 [DOI] [PubMed] [Google Scholar]
- Keum S, & Shin HS (2019). Neural Basis of Observational Fear Learning: A Potential Model of Affective Empathy. Neuron, 104(1), 78–86. 10.1016/j.neuron.2019.09.013 [DOI] [PubMed] [Google Scholar]
- Keysers C, & Gazzola V (2016). A Plea for Cross-species Social Neuroscience. Social Behavior from Rodents to Humans, 179–191. 10.1007/7854_2016_439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, & Mori Y (2006). Social buffering: relief from stress and anxiety. Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1476), 2215–2228. 10.1098/rstb.2006.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Mikosz M, Werka T, & Maren S (2009). Social modulation of learning in rats. Learning & Memory, 17(1), 35–42. 10.1101/lm.1670910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP, Alleva E, & Scattoni ML (2010). Translating mouse vocalizations: prosody and frequency modulation1. Genes, Brain and Behavior, 10(1), 4–16. 10.1111/j.1601-183x.2010.00603.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP (2016). Social Reward and Empathy as Proximal Contributions to Altruism: The Camaraderie Effect. Social Behavior from Rodents to Humans, 127–157. 10.1007/7854_2016_449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahvis GP (2016). Rodent Models of Autism, Epigenetics, and the Inescapable Problem of Animal Constraint. Animal Models of Behavior Genetics, 265–301. 10.1007/978-1-4939-3777-6_9 [DOI] [Google Scholar]
- Lahvis GP (2017). Unbridle biomedical research from the laboratory cage. ELife, 6. 10.7554/elife.27438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, & Herkenham M (2011). Environmental enrichment confers stress resiliency to social defeat through an infralimbic cortex-dependent neuroanatomical pathway. Journal of Neuroscience, 31(16), 6159–6173. 10.1523/jneurosci.0577-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HX, Lopatina O, Higashida C, Fujimoto H, Akther S, Inzhutova A, Liang M, Zhong J, Tsuji T, Yoshihara T, Sumi K, Ishiyama M, Ma WJ, Ozaki M, Yagitani S, Yokoyama S, Mukaida N, Sakurai T, Hori O, . . . Higashida H. (2013). Displays of paternal mouse pup retrieval following communicative interaction with maternal mates. Nature Communications, 4(1). 10.1038/ncomms2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, & Crawley JN (2008). Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain and Behavior, 7(2), 152–163. 10.1111/j.1601-183x.2007.00330.x [DOI] [PubMed] [Google Scholar]
- Mehrabian A, Young AL, & Sato S (1988). Emotional empathy and associated individual differences. Current Psychology, 7(3), 221–240. 10.1007/bf02686670 [DOI] [Google Scholar]
- Melchers MC, Li M, Haas BW, Reuter M, Bischoff L, & Montag C (2016). Similar personality patterns are associated with empathy in four different countries. Frontiers in Psychology, 7. 10.3389/fpsyg.2016.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyza K, & Blanchard D (2017). The BTBR mouse model of idiopathic autism – Current view on mechanisms. Neuroscience & Biobehavioral Reviews, 76, 99–110. 10.1016/j.neubiorev.2016.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyza K, Nikolaev T, Kondrakiewicz K, Blanchard DC, Blanchard RJ, & Knapska E (2015). Neuronal correlates of asocial behavior in a BTBR T+Itpr3tf/J mouse model of autism. Frontiers in Behavioral Neuroscience, 9. 10.3389/fnbeh.2015.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyza K, Bartal IBA, Monfils M, Panksepp J, & Knapska E (2017). The roots of empathy: Through the lens of rodent models. Neuroscience & Biobehavioral Reviews, 76, 216–234. 10.1016/j.neubiorev.2016.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2015). Social modulation of and by pain in humans and rodents. Pain, 156(Supplement 1), S35–S41. 10.1097/01.j.pain.0000460341.62094.77 [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, & Zoghbi HY (2004). Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Human Molecular Genetics, 14(2), 205–220. 10.1093/hmg/ddi016 [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, & Crawley JN (2004). Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes, Brain and Behavior, 3(5), 287–302. 10.1111/j.1601-1848.2004.00076.x [DOI] [PubMed] [Google Scholar]
- Moy S, Nadler J, Young N, Perez A, Holloway L, Barbaro R, Barbaro J, Wilson L, Threadgill D, & Lauder J (2007). Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behavioural Brain Research, 176(1), 4–20. 10.1016/j.bbr.2006.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann DL, Chan RCK, Wang Y, & Boyle GJ (2016). Cognitive and affective components of empathy and their relationship with personality dimensions in a Chinese sample. Asian Journal of Social Psychology, 19(3), 244–253. 10.1111/ajsp.12138 [DOI] [Google Scholar]
- Olsson A, McMahon K, Papenberg G, Zaki J, Bolger N, & Ochsner KN (2015). Vicarious fear learning depends on empathic appraisals and trait empathy. Psychological Science, 27(1), 25–33. 10.1177/0956797615604124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J (1998). Affective Neuroscience: The Foundations of Human and Animal Emotions (Series in Affective Science) (1st ed.). Oxford University Press. [Google Scholar]
- Panksepp JB, Jochman KA, Kim JU, Koy JJ, Wilson ED, Chen Q, Wilson CR, & Lahvis GP (2007). Affiliative behavior, ultrasonic communication and social reward Are influenced by genetic variation in adolescent mice. PLoS ONE, 2(4), e351. 10.1371/journal.pone.0000351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, & Lahvis GP (2007). Social reward among juvenile mice. Genes, Brain and Behavior, 6(7), 661–671. 10.1111/j.1601-183x.2006.00295.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, Wong JC, Kennedy BC, & Lahvis GP (2008). Differential entrainment of a social rhythm in adolescent mice. Behavioural Brain Research, 195(2), 239–245. 10.1016/j.bbr.2008.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, & Lahvis GP (2011). Rodent empathy and affective neuroscience. Neuroscience & Biobehavioral Reviews, 35(9), 1864–1875. 10.1016/j.neubiorev.2011.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J, & Panksepp JB (2013). Toward a cross-species understanding of empathy. Trends in Neurosciences, 36(8), 489–496. 10.1016/j.tins.2013.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, & Lahvis GP (2016). Differential influence of social versus isolate housing on vicarious fear learning in adolescent mice. Behavioral Neuroscience, 130(2), 206–211. 10.1037/bne0000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp JB, & Lahvis GP (2018). Challenging Convention in empathy research: developing a mouse model and initial neural analyses. Neuronal Correlates of Empathy, 161–176. 10.1016/b978-0-12-805397-3.00013-9 [DOI] [Google Scholar]
- Pearson BL, Bettis JK, Meyza KZ, Yamamoto LY, Blanchard DC, & Blanchard RJ (2012). Absence of social conditioned place preference in BTBR T+tf/J mice: Relevance for social motivation testing in rodent models of autism. Behavioural Brain Research, 233(1), 99–104. 10.1016/j.bbr.2012.04.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV (2007) Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science, 46(1), 28–34. [PubMed] [Google Scholar]
- Sanders J, Mayford M, & Jeste D (2013). Empathic fear responses in mice are triggered by recognition of a shared experience. PLoS ONE, 8(9), e74609. 10.1371/journal.pone.0074609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankoorikal GMV, Kaercher KA, Boon CJ, Lee JK, & Brodkin ES (2006). A mouse model system for genetic analysis of sociability: C57BL/6J versus BALB/cJ inbred mouse strains. Biological Psychiatry, 59(5), 415–423. 10.1016/j.biopsych.2005.07.026 [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Ricceri L, & Crawley JN (2010). Unusual repertoire of vocalizations in adult BTBR T+tf/J mice during three types of social encounters. Genes, Brain and Behavior, 10(1), 44–56. 10.1111/j.1601-183x.2010.00623.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scattoni M, Martire A, Cartocci G, Ferrante A, & Ricceri L (2013). Reduced social interaction, behavioural flexibility and BDNF signalling in the BTBR T+tf/J strain, a mouse model of autism. Behavioural Brain Research, 251, 35–40. 10.1016/j.bbr.2012.12.028 [DOI] [PubMed] [Google Scholar]
- Schoch H, Kreibich AS, Ferri SL, White RS, Bohorquez D, Banerjee A, Port RG, Dow HC, Cordero L, Pallathra AA, Kim H, Li H, Bilker WB, Hirano S, Schultz RT, Borgmann-Winter K, Hahn CG, Feldmeyer D, Carlson GC, . . . Brodkin ES. (2017). Sociability deficits and altered amygdala circuits in mice lacking Pcdh10, an autism associated gene. Biological Psychiatry, 81(3), 193–202. 10.1016/j.biopsych.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva PRR, Silva RH, Lima RH, Meurer YS, Ceppi B, & Yamamoto ME (2020). Are There Multiple Motivators for Helping Behavior in Rats? Frontiers in Psychology, 11. 10.3389/fpsyg.2020.01795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, & Lamm C (2009). The Social Neuroscience of Empathy. Annals of the New York Academy of Sciences, 1156(1), 81–96. 10.1111/j.1749-6632.2009.04418.x [DOI] [PubMed] [Google Scholar]
- Sivaselvachandran S, Acland EL, Abdallah S, & Martin LJ (2018). Behavioral and mechanistic insight into rodent empathy. Neuroscience & Biobehavioral Reviews, 91, 130–137. 10.1016/j.neubiorev.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Smith ML, Asada N, & Malenka RC (2021). Anterior cingulate inputs to nucleus accumbens control the social transfer of pain and analgesia. Science, 371(6525), 153–159. 10.1126/science.abe3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppel LJ, Kazdoba TM, Schaffler MD, Preza AR, Heynen A, Crawley JN, & Bear MF (2017). R-Baclofen reverses cognitive deficits and improves social interactions in two lines of 16p11.2 deletion mice. Neuropsychopharmacology, 43(3), 513–524. 10.1038/npp.2017.236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terranova JI, Yokose J, Osanai H, Marks WD, Yamamoto J, Ogawa SK, & Kitamura T (2022). Hippocampal-amygdala memory circuits govern experience-dependent observational fear. Neuron, 110(8), 1416–1431.e13. 10.1016/j.neuron.2022.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipps ME, Raybuck JD, Buck KJ, & Lattal KM (2014). Delay and trace fear conditioning in C57BL/6 and DBA/2 mice: issues of measurement and performance. Learning & Memory, 21(8), 380–393. 10.1101/lm.035261.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Public Health Service. (1963). Guide for laboratory animal facilities and care. Washington, DC: US Public Health Service [Google Scholar]
- Watanabe S (2016). Evolutionary origins of empathy. In Watt DF& Panksepp J (Eds.), Psychology and neurobiology of empathy (pp. 37–61). Nova Biomedical Books. [Google Scholar]
- Willott JF, Turner JG, Carlson S, Ding D, Seegers Bross L, & Falls WA (1998). The BALB/c mouse as an animal model for progressive sensorineural hearing loss. Hearing Research, 115(1–2), 162–174. 10.1016/s0378-5955(97)00189-5 [DOI] [PubMed] [Google Scholar]
- Willott JF, Tanner L, O’Steen J, Johnson KR, Bogue MA, & Gagnon L (2003). Acoustic startle and prepulse inhibition in 40 inbred strains of mice. Behavioral Neuroscience, 117(4), 716–727. 10.1037/0735-7044.117.4.716 [DOI] [PubMed] [Google Scholar]
- Wöhr M (2015). Effect of social odor context on the emission of isolation-induced ultrasonic vocalizations in the BTBR T+tf/J mouse model for autism. Frontiers in Neuroscience, 9. 10.3389/fnins.2015.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff JO, & Sherman PW (2007). Rodent Societies: An Ecological and Evolutionary Perspective. University of Chicago Press. [Google Scholar]
- Xin-ping L & Tian L (2015) The relationship between empathy and personality characteristics among undergraduate nursing students. Chinese Journal of Chinese Education, 7. [Google Scholar]
- Yang M, Loureiro D, Kalikhman D, & Crawley JN (2013). Male mice emit distinct ultrasonic vocalizations when the female leaves the social interaction arena. Frontiers in Behavioral Neuroscience, 7. 10.3389/fnbeh.2013.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MM, Geng AQ, Chen K, Wang J, Wang P, Qiu XT, Gu JX, Fan HW, Zhu DY, Yang SM, Chen QY, Zhou ZX, Fan BY, Bai Y, Xing KK, Feng JM, Wang JD, Chen Y, Lu YC, . . . Chen T. (2022). Glutamatergic synapses from the insular cortex to the basolateral amygdala encode observational pain. Neuron, 110(12), 1993–2008.e6. 10.1016/j.neuron.2022.03.030 [DOI] [PubMed] [Google Scholar]
- Zhou X, Jen PHS, Seburn KL, Frankel WN, & Zheng QY (2006). Auditory brainstem responses in 10 inbred strains of mice. Brain Research, 1091(1), 16–26. 10.1016/j.brainres.2006.01.107 [DOI] [PMC free article] [PubMed] [Google Scholar]


