Abstract
Background
We observed an increase in the frequency of false-positive (FP) human immunodeficiency virus (HIV) test results that correlated with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) prevalence. We measured FP rates of laboratory-based fourth-generation HIV antigen/antibody test among those with polymerase chain reaction (PCR)-confirmed infection with SARS-CoV-2 compared with FP rate of those who tested SARS-CoV-2 PCR-negative.
Methods
All patients PCR tested for SARS-CoV-2 within 2 weeks of an HIV fourth-generation assay were selected. Positive HIV fourth-generation assays were reviewed and divided into groups of FP, true positive (TP), and presumptive negative (PN). Variables included age, race, ethnicity, gender, pregnancy, and Coronavirus Disease 2019 (COVID-19) immunization status. Associations with positive SARS-CoV-2 tests were assessed using linear logistic regression. Multivariate logistic regression was used to assess sets of variables.
Results
There were 31 910 medical records that met criteria. The frequency of SARS-CoV-2 positive tests was calculated in groups of HIV TP, FP, and PN. In total, 31 575 patients had PN HIV test result, 248 patients had TP, and 87 patients had FP. Those with HIV FP tests had the highest percentage of COVID-19–positive test results at 19.5%, which was significantly higher than HIV PN (11.3%; P = .016) and HIV TP (7.7%; P = .002). After adjustment for all covariates, only FP HIV was significantly associated with COVID-19 (odds ratio, 4.22; P = .001).
Conclusions
This study reveals that patients with positive SARS-CoV-2 PCR tests are significantly more likely to have an FP fourth-generation HIV test than those with negative SARS-CoV-2 PCR tests.
Keywords: SARS-CoV-2, HIV-2, HIV fourth-generation test
This study reveals that patients with positive severe acute respiratory syndrome coronavirus 2 polymerase chain reaction tests are significantly more likely to have a false-positive fourth-generation immunodeficiency virus test.
Immunoassays that rely on antibody (Ab) or antigen (Ag) detection are subject to a variety of interferences that can lead to false-negative or false-positive (FP) results [1, 2]. There are exogenous causes such as sample integrity, errors in assay performance, interpretation and reporting, and methodologic errors. Endogenous causes within the sample being tested include rheumatoid factors, autoantibodies, hyperglobulinemia, heterophile Abs, human anti-animal Abs, lysozyme, cross-reacting Ags, paraproteins, and biotin [1]. FP tests for human immunodeficiency virus (HIV) have been reported in a variety of inflammatory conditions and possibly secondary to cross-reactivity to other infectious agents [3, 4]. The occurrence of an FP HIV test is not without consequences for patients, providers, and laboratories [2, 5].
The expansion of an emergency department (ED)-based HIV counseling, testing, and referral (CTR) program occurred several months before the 2020 surge in ED visits associated with Coronavirus Disease 2019 (COVID-19). Observations from this expansion led us to examine the performance of a fourth-generation HIV 1/2 Ag/Ab assay among persons presenting with and without polymerase chain reaction (PCR)-confirmed COVID-19 infection.
METHODS
Study Design
This was a retrospective, cross-sectional study from March 2020 to January 2022 at Henry Ford Hospital in Detroit, Michigan. Through electronic medical record extraction, all patients who were PCR tested for Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and had a result within 2 weeks of an HIV fourth-generation assay (Elecsys HIV Duo, Roche Diagnostics, Indianapolis, IN) were selected. All positive HIV fourth-generation assays were independently reviewed and divided into groups: FP (fourth-generation assay positive, HIV-1/HIV-2 antibody differentiation immunoassay enzyme immunoassay negative/indeterminant, and HIV-1 nucleic acid amplification test (NAT) negative); true positive (TP; fourth-generation assay positive, enzyme immunoassay positive); and presumptive negative (PN; fourth-generation assay negative). We developed these groups because, in the absence of suspected acute HIV infection or recent HIV exposure, negative fourth-generation tests were not systematically followed up to determine true-negative vs false-negative status. FP, TP, and PN were then subdivided by SARS CoV-2 PCR status, positive or negative. In the case of duplicate patients, only the first encounter was counted; however, if an individual initially tested SARS-CoV-2 PCR-negative but was then positive on a repeat test within the 2-week window, that individual was counted as having COVID-19 in the dataset. To gain insight into possible mechanisms for the observed FP results, the numeric signal-to-cutoff ratio (cutoff indices [COIs]) readings for both Ag and Ab that were available were then selected for analyses from this subset of individuals based on availability.
HIV Screening
Routine HIV testing was performed following the Centers for Disease Control and Prevention (CDC) testing guidelines using a fourth-generation HIV Ag/Ab test following CDC/Association of Public Health Laboratories laboratory algorithmic recommendations [6, 7]. In addition, individuals suspected of acute HIV infection and those newly diagnosed underwent NAT testing. Screening was also done in the ED as part of an HIV CTR program. ED-based screening uses an algorithmic approach, generating a best practice alert to order a laboratory-based fourth-generation HIV test upon ordering a complete blood count for individuals aged 18–65 years. CTR staff are responsible for follow-up of patients with a positive fourth-generation test and those who may test negative with suspected acute HIV.
Assays
The Roche Elecsys HIV Duo assay is a fourth-generation automated combination immunoassay that uses separate, simultaneous reactions to detect HIV Ag (p24) and HIV-1/2 Abs. The COIs are converted to qualitative results and reported as nonreactive (COI < 1.0) or reactive (COI ≥ 1.0) by the system.
Bio-Rad Geenius HIV 1/2 Supplemental Assay was used for the detection and differentiation of individual antibodies to HIV-1 and HIV-2. The Aptima HIV-1 Quant Dx assay (Hologic Panther system), through transcription-mediated amplification, uses multiple, long primers that target several regions of the HIV-1 genome (pol and long terminal repeat [LTR]) independently to provide a quantitative result.
Presence or absence of SARS-CoV-2 infection was determined by real-time PCR testing for SARS-CoV-2 viral RNA from nasopharyngeal swabs tested at the Henry Ford Hospital microbiology laboratory with commercial PCR systems validated for clinical use under emergency use authorization. To provide redundancy during reagent supply interruptions, we routinely use multiple commercial real-time PCR systems (NeuMoDx 288, NeuMoDx Medical, Ann Arbor, MI; Cepheid GeneXpert and Infinity, Sunnyvale, CA; Hologic Panther, Marlborough, MA; and Diasorin Liaison MDX, Diasorin Molecular LLC, Cypress, CA).
Statistical Analyses
Each patient with multiple visits had their initial visit selected to ensure the assumption of independence. χ2 tests for discrete data and 2-sample Wilcoxon tests for ordinal and continuous data were used. A Fligner–Policello technique was used to adjust for unequal variances. Associations with positive COVID-19 tests were assessed using linear logistic regression. Multivariate logistic regression was used to assess sets of variables. When 2 characteristics were evaluated on the same set of individuals with overlap, McNamar's test was used for comparison. A scatterplot with medians included was used to illustrate distributions of the data. All percentages are presented with the exact 95% confidence interval (CI).
All procedures followed were in accordance with the ethical standards of the institutional committee responsible for human experimentation and with the Helsinki Declaration.
RESULTS
Frequency of FP HIV Test Results
A total of 31 910 medical records that met the eligibility criteria were identified. In total, 31 575 patients had a PN HIV fourth-generation assay test result, 248 patients had a TP (79%; 95% CI, .69–.90), and 87 patients had an FP (28%; 95% CI, .22–.35; Table 1). Among FP patients with active COVID-19 infection, the mean age was 45.6 years (95% CI, 38.5–52.4; standard deviation, 15.0), and 52.9% (95% CI, 27.8–77.01) were female, 70.6% (95% CI, 44.0–84.7) were Black, 17.7% (95% CI, 3.8–43.4) were White, and 94.1% (95% CI,71.3–99.8) were not immunized against SARS-CoV-2 (Table 2). After adjustment for all covariates that included age, sex, race, ethnicity, pregnancy, and SARS-CoV-2 immunization status (Table 3), only FP HIV test results were significantly associated with COVID-19 (odds ratio, 4.22; 95% CI, 1.84–9.67; P = .001). Although pregnancy, per se, is not considered a cause of FP fourth-generation HIV tests, analyses repeated with pregnancy removed were without significant changes [8]. The frequency of both TP and PN results did not differ significantly by COVID-19 status. Using logistic regression analysis to predict an FP HIV test, we found that an FP HIV result was 2.93 more likely to occur in those with COVID-19 compared with those without COVID-19 (odds ratio, 2.93; 95% CI, 1.44–5.94; P = .003). The median interval between the 17 positive SARS-CoV-2 PCR and FP HIV tests was 0.98 days (range, 0.09–13.84; interquartile range [IQR], 0.1–9.15).
Table 1.
χ 2 Test of Coronavirus Disease 2019–Positive Tests in Groups of Human Immunodeficiency Virus Fourth-Generation True-Positives, False-Positives, and True-Negatives
| Severe Acute Respiratory Syndrome Coronavirus 2 Polymerase Chain Reaction Test | Human Immunodeficiency Virus Fourth-Generation Test (n = 31 910) N (%) |
||
|---|---|---|---|
| True Positive | False Positive | Presumptive Negative | |
| Positive | 19 (7.7) | 17 (19.5) | 3577 (11.3) |
| Negative | 229 (92.3) | 70 (80.5) | 27 998 (88.7) |
| Total | 248 | 87 | 31 575 |
Chi-squared Statistic = 9.16, P value = .01.
Table 2.
Demographic Characteristics of the Study Population
| Characteristic | COVID-19 Positive | COVID-19 Negative | ||||
|---|---|---|---|---|---|---|
| TP HIV (n = 19) |
FP HIV (n = 17) |
PN HIV (n = 3340) |
TP HIV (n = 224) |
FP HIV (n = 61) |
PN HIV (n = 26 694) |
|
| Age, mean ± standard deviation, y | 46.7 ± 15.0 | 45.6 ± 15.0 | 47.7 ± 17.8 | 42.7 ± 13.5 | 39.0 ± 18.1 | 40.2 ± 16.0 |
| Sex, n (%) | ||||||
| Female | 4 (21.1) | 9 (52.9) | 2084 (58.3) | 58 (25.3) | 50 (71.4) | 19 405 (69.3) |
| Male | 15 (79) | 8 (47.1) | 41.7 (1492) | 171 (74.7) | 28.6 (20) | 8589 (30.7) |
| Race, n (%) | ||||||
| Black | 14 (73.7) | 12 (70.6) | 1928 (53.9) | 187 (81.7) | 33 (47.5) | 12 162 (43.4) |
| White | 1 (5.3) | 3 (17.7) | 912 (25.5) | 27 (11.8) | 26 (37.1) | 11 434 (40.8) |
| Other | 4 (21.1) | 2 (11.8) | 737 (20.6) | 15 (6.6) | 11 (15.7) | 4402 (15.7) |
| Ethnicity, n (%) | ||||||
| Hispanic | 1 (5.3) | 1 (5.9) | 308 (8.6) | 4 (1.8) | 3 (4.3) | 1954 (7.0) |
| Non-Hispanic | 16 (84.2) | 15 (88.2) | 3080 (84.7) | 218 (95.2) | 62 (88.6) | 24 736 (88.4) |
| Other | 2 (10.5) | 1 (5.9) | 241 (6.7) | 7 (3.1) | 5 (7.1) | 1308 (4.7) |
| Immunization for COVID,a n (%) | ||||||
| No | 16 (84.2) | 16 (94.1) | 3276 (91.6) | 203 (88.7) | 65 (92.9) | 25 031 (89.4) |
| Yes | 3 (15.8) | 1 (5.9) | 301 (8.4) | 26 (11.4) | 5 (7.1) | 2967 (10.5) |
Abbreviations: COVID-19, Coronavirus Disease 2019; FP, false positive; HIV, human immunodeficiency virus; PN, presumptive negative; TP, true positive.
Yes was defined as having received 2 or more doses of Pfizer BioNTech or Moderna messenger RNA type vaccine or 1 or more doses of Janssen vaccine.
Table 3.
Multivariate Analysis of Coronavirus Disease 2019 Patients Including and Excluding Pregnancy
| Variable | All Patients | Excluding Pregnant Patients | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| False-positive human immunodeficiency virus test result | 4.22 (1.84–9.67) | .001 | 4.07 (1.76–9.42) | .001 |
| Age | 1.02 (1.00–1.05) | .087 | 1.02 (1.00–1.05) | .086 |
| Black | 1.53 (.62–3.79) | .353 | 1.47 (0.59–3.65) | .408 |
| Hispanic | 3.06 (.51–18.23) | .220 | 2.96 (.50–17.65) | .233 |
| Female | 0.60 (.26–1.39) | .234 | 0.61 (.26–1.41) | .246 |
| Coronavirus Disease 2019 vaccination | 1.18 (.37–3.73) | .778 | 1.22 (.39–3.90) | .732 |
| Pregnancy | 0.92 (.10–9.52) | .938 | – | – |
Abbreviations: CI, confidence interval; OR, odds ratio.
Determinants of Reactive HIV Tests
The COIs of the HIV fourth-generation assays that were available were analyzed for 256 patients (Table 4). FP HIV results were associated with the Ag portion of the HIV assay in 87.1% (95% CI, 76.1–99.3) vs 33.9% (95% CI, 22.3–47.01) for the Ab portion, P = .001. Overall, 66.1% (95% CI, 77.7–153.0) of FP results were solely based on a reactive Ag and a nonreactive Ab, whereas only 2.8% (95% CI, 77.3–94.0) of TP results were Ag reactive and Ab nonreactive (P = .001). As previously reported, 87.3% of TP results were associated with Ab reactivity only. Thus, most FP results were triggered by Ag only, while most TP results were due to Ab only (P = .001). The presence or absence of COVID-19 did not significantly impact these proportions.
Table 4.
Description of Antigen and Antibody Signal Comparing True-Positive and False-Positive Human Immunodeficiency Virus With and Without Coronavirus Disease 2019
| COVID-19 | Ag ≥ 1 and Ab < 1 | Ag < 1 and Ab ≥ 1 | Ag ≥ 1 and Ab ≥ 1 | Total |
|---|---|---|---|---|
| True positives | ||||
| Positive | 0 | 9 | 2 | 11 |
| Negative | 2 | 53 | 5 | 60 |
| Total | 2 | 62 | 7 | 71 |
| False positives | ||||
| Positive | 10 | 0 | 6 | 16 |
| Negative | 31 | 8 | 7 | 46 |
| Total | 41 | 8 | 13 | 62 |
Abbreviations: Ab, antibodies; AG, antigen; COVID-19, Coronavirus Disease 2019.
The Ab portion of the HIV fourth-generation test was reactive in 21 of 62 (33.9%; 95% CI, 22.3–47.01) FP tests and in 6 of 16 (37.5%; 95% CI, 15.2–69.6) and 15 of 46 (32.6%; 95% CI, 19.5–48.0) FP tests in those with and without COVID-19 infection, respectively. A reactive Ab was the sole determinant of HIV test positivity in none of the COVID-19–associated FP results and 8 of 46 (17.4%; 95% CI, 7.8–31.3) of those that were SARS-CoV-2 PCR-negative. Among TP HIV tests, Ab was reactive in 69 of 71 (97.2%; 95% CI, 90.2–99.7) samples. Two SARS-CoV-2 PCR-negative individuals with early HIV infection were Ab nonreactive and Ag reactive, consistent with early HIV infection. A significant effect of COVID-19 was not observed in the distribution of Abs reactivity within each subset of FP, TP, and PN. Overall, a reactive Ab was significantly less prevalent among FP results (21 of 62; 33.9%; 95% CI, 22.3–47.0) compared with TP results (69 of 71; 97.2%; 95% CI, 90.2–99.71; P = .001).
HIV Ag and Ab Reactivity and COVID-19
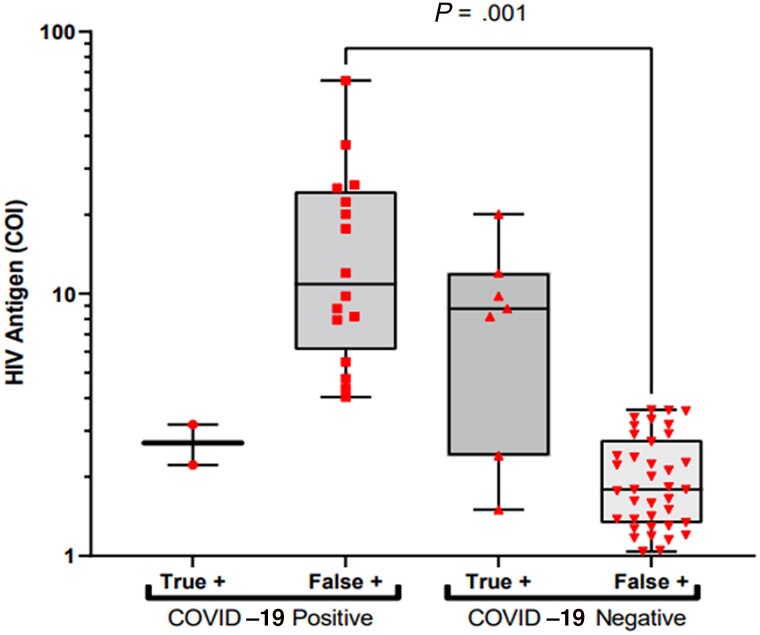
Among FP results, a significantly higher median COI for reactive Ags was associated with the presence of COVID-19 (Figure 1), with a median of 10.90 (IQR, 6.72–23.85) vs 1.79 (IQR, 1.34–2.73; P = .001). In contrast, the TP median COI of reactive Ags was lower in those with SARS-CoV-2 compared with those who were SARS-CoV-2 PCR-negative, 2.70 (IQR, 2.22–3.17) vs 8.77 (IQR, 2.41–12.00; P = .119). No effect of COVID-19 was found on Ag magnitude when both reactive and nonreactive PN Ag COIs were considered; median 0.16 (IQR, 0.15–0.16) for COVID-19 positives compared with 0.17 (IQR, 0.17–0.18; P = .001).
Figure 1.
HIV antigen COIs of true-positive and false-positive results in patients with and without COVID-19 infection. Gray box represents interquartile limits; horizontal line indicates the median. Abbreviations: COI, cutoff indices; COVID-19, Coronavirus Disease 2019; HIV, human immunodeficiency virus.
The median COIs of reactive Abs differed, but not significantly, between FP HIV in COVID-19 infection as opposed to no COVID-19 infection (618 and 1050, respectively; P = .312). The IQRs for the 2 groups had a great deal of overlap (17–1304 and 397–2621, respectively). Overall, these findings indicate FP results due to the Ab portion of the HIV fourth-generation test are less commonly seen in COVID-19 infections, but the magnitudes of COIs in these FP results are not different.
DISCUSSION
Based on our findings, patients with active COVID-19 appear significantly more likely to have an FP fourth-generation HIV test. The mechanism for this is unknown but may reflect a cross-reaction with the fourth-generation's Ag component in acute COVID-19 infection and, to a lesser degree, Ab cross-reactivity. During the first severe acute respiratory syndrome pandemic in 2003, Kliger and Levanon showed via sequence analysis that HIV and SARS-CoV-1 viral proteins shared sequence motifs that construct their active conformation [9]. Zhang et al confirmed that 4 insertions unique to the 2019 novel coronavirus spike protein that are part of the receptor binding site of the 2019 novel coronavirus share similarities with HIV-1 proteins as well, suggesting potential cross-reactivity between Ags of the 2 viruses [10]. Zhang et al showed that these insertions were short, 6–8 amino acid segments and considered the relationship to HIV-1 as “coincidental” and not specific, shared by other viruses. However, due to the nature of the test, an exact amino acid sequence homology to HIV is not required to yield an FP test result, which requires only enough antigenic similarity for a detectable amount of false signal. In fact, the absence of strict homology and the short length may help to explain the idiopathic occurrence of FP HIV results in some individuals in our cohort.
FP COIs for the Ag portion of the assay were significantly greater in magnitude in those with COVID-19 infection compared with those without COVID-19 infection. There was no corresponding increase in the magnitude of Ag COIs for TP results in COVID-19 infection nor in the Abs portion of the assay. This finding reveals a possible reason for the greater number of reactive COIs with the Ag portion of the assay in FP HIV COVID-19 positives. The response to COVID-19 infection likely produces a SARS-CoV-2 peptide or protein that mimics the p24 protein of HIV, causing an FP HIV test result. Conversely, an FP SARS-CoV-2 rapid nucleocapsid Ag test was reported from an individual with acute HIV-1 infection [11]. In this context, at the time of hospitalization for COVID-19, SARS-CoV-2 nucleocapsid antigenemia was detected in 95% of patients and was ≥100 ng/mL in 41% [12]. Although antigenic homology may be a driving factor, the relationship to SARS-CoV-2 Ags is undetermined. Yang et al recently published results of an HIV screening program using the same Roche assay and analyzer that we used. They reported that of the 578 participants who screened with a positive HIV result, 13.3% were both Ag and Ab positive, 77.7% were Ab only positive, and 9.0% were Ag only positive [13]. The authors are not aware of similar behavior in other serology assays in concurrent viral infections. Additional research would be needed to construct models that provide evidence to further support these hypotheses with empirical data in future work.
The current literature is limited on this topic, but there have been multiple case reports published with similar findings. Salih et al documented a 32-year-old female with mild COVID-19 with multiple FP fourth-generation tests undergoing a thyroidectomy [14]. Tan et al reported 2 cases of patients with COVID-19 with multiple FP HIV screening tests and negative immunoblot tests [15]. Papamanoli and Psevdos reported an FP HIV screening test in a patient with pulmonary embolism due to SARS-CoV-2 as well [16].
Our study has some limitations. First, it is inherently limited by its retrospective nature, making it difficult to determine true correlation. Furthermore, our designation of PN HIV results is limited by the study design in that those who tested negative on the fourth-generation assay did not undergo viral load testing unless acute HIV infection was suspected; hence, a true negative rate could not be ascertained. It is well known that some individuals with COVID-19 will test negative by real-time PCR. If retested within the healthcare system, repeated tests would be captured and appropriately counted as positive or negative. Narrowly, our results are confined to those with PCR-positive COVID-19 [12, 17]. In addition, only 1 platform for HIV and COVID-19 testing was used, thus limiting the generalizability of our results, although there have been isolated reports of FP HIV results with other platforms [14–16]. The predominant locally circulating SARS-CoV-2 variants during the period of study were Alpha, Delta, and Omicron; however, we have no specific data on the infecting variants encountered in this study. Therefore, we cannot rule out that the occurrence and frequency of FP results may be variant-dependent.
While all potential confounding variables could not be eliminated, neither COVID-19 vaccination nor pregnancy changed the association of FP HIV results with concurrent COVID-19 infection, although we did not control for time from vaccination or period of gestation. We manually chart-reviewed all FP tests to determine if there was another etiology for their result. No other known alternative explanations were found.
The exact biochemistry of falsely reactive tests is often unknown and attributed to a “general” inflammatory process, to which COVID-19 is no stranger. While we were unable to link the degree of inflammation to false HIV positivity, we were able to show that HIV Ag reactivity was a primary trigger for an FP result on the test used in this study. A major strength of our study is that it included many patients, and all positive fourth-generation assay results, that is, TP, FP, and selected PN (in suspected acute HIV), were tracked by CTR program staff to confirm or reject an HIV diagnosis.
In conclusion, acute COVID-19 should be considered as a potential etiology for an FP fourth-generation HIV test.
Contributor Information
Smitha Gudipati, Department of Internal Medicine, Wayne State University, Detroit, Michigan, USA; Department of Internal Medicine, Michigan State University, East Lansing, Michigan, USA; Division of Infectious Diseases, Henry Ford Hospital, Detroit, Michigan, USA.
Anita Shallal, Department of Internal Medicine, Wayne State University, Detroit, Michigan, USA; Department of Internal Medicine, Michigan State University, East Lansing, Michigan, USA; Division of Infectious Diseases, Henry Ford Hospital, Detroit, Michigan, USA.
Edward Peterson, Division of Infectious Diseases, Henry Ford Hospital, Detroit, Michigan, USA.
Bernard Cook, Division of Pathology, Henry Ford Hospital, Detroit, Michigan, USA.
Norman Markowitz, Department of Internal Medicine, Wayne State University, Detroit, Michigan, USA; Division of Infectious Diseases, Henry Ford Hospital, Detroit, Michigan, USA.
Notes
Author contributions. All authors contributed to the design, writing, and analysis of the study.
Acknowledgments. The authors acknowledge the nurse, Kim Mumby, who helped identify the increase in false-positive HIV fourth-generation tests in their system and brought the concern to the authors' attention.
References
- 1. Hardie DR, Korsman SN, Hsiao NY, Morobadi MD, Vawda S, Goedhals D. Contamination with HIV antibody may be responsible for false positive results in specimens tested on automated platforms running HIV 4th generation assays in a region of high HIV prevalence. PLoS One 2017; 12:e0182167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reid J, Van Zyl G, Linstrom M, Korsman S, Marais G, Preiser W. High positive HIV serology results can still be false positive. IDCases 2020; 21:e00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dodig S. Interferences in quantitative immunochemical methods. Biochemia Medica 2009; 19:50–62. [Google Scholar]
- 4. Tate J, Ward G. Interferences in immunoassay. Clin Biochem Rev 2004; 25:105–20. [PMC free article] [PubMed] [Google Scholar]
- 5. Bhattacharya R, Barton S, Catalan J. When good news is bad news: psychological impact of false positive diagnosis of HIV. AIDS Care 2008; 20:560–4. [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention, Association of Public Health Laboratories . Quick reference guide—laboratory testing for the diagnosis of HIV infection: updated recommendations. Atlanta, GA: CDC, 2014. [Google Scholar]
- 7. Centers for Disease Control and Prevention . HIV testing. Available at:https://www.cdc.gov/hiv/testing/index.html. Accessed 14 February 2023.
- 8. Adhikari EH, Macias D, Gaffney D, et al. Diagnostic accuracy of fourth-generation ARCHITECT HIV Ag/Ab Combo assay and utility of signal-to-cutoff ratio to predict false-positive HIV tests in pregnancy. Am J Obstet Gynecol 2018; 219:408.e1–e9. [DOI] [PubMed] [Google Scholar]
- 9. Kliger Y, Levanon EY. Cloaked similarity between HIV-1 and SARS-CoV suggests an anti-SARS strategy. BMC Microbiol 2003; 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang C, Zheng W, Huang X, Bell EW, Zhou X, Zhang Y. Protein structure and sequence reanalysis of 2019-nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV-1. J Proteome Res 2020; 19:1351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamaniha K, Kinjo T, Akamine M, Setoguchi M, Tateyama M, Fujita J. False-positive for SARS-CoV-2 antigen test in a man with acute HIV infection. J Infect Chemother 2021; 27:1112–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ACTIV3-TICO Bamlanivimab Study Group; Lundgren JD, Grund B, et al. Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: a randomized controlled trial. Ann Intern Med 2022; 175:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang M, Yang W, Shi W, Tao C. Clinical application evaluation of Elecsys® HIV duo assay in southwest China. Front Cell Infect Microbiol 2022; 12:877643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salih RQ, Salih GA, Abdulla BA, et al. False-positive HIV in a patient with SARS-CoV-2 infection; a case report. Ann Med Surg (Lond) 2021; 71:103027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tan SS, Chew KL, Saw S, Jureen R, Sethi S. Cross-reactivity of SARS-CoV-2 with HIV chemiluminescent assay leading to false-positive results. J Clin Pathol 2021; 74:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Papamanoli A, Psevdos G. False-positive HIV screening test in a patient with pulmonary embolism because of severe acute respiratory syndrome coronavirus 2 infection. AIDS 2021; 35:1521–2. [DOI] [PubMed] [Google Scholar]
- 17. Sule WF, Oluwayelu DO. Real-time RT-PCR for COVID-19 diagnosis: challenges and prospects. Pan Afr Med J 2020; 35(Suppl 2):121. [DOI] [PMC free article] [PubMed] [Google Scholar]