Abstract
Epstein-Barr virus (EBV), the only known human lymphocryptovirus (LCV), displays a remarkable degree of genetic and biologic identity to LCVs that infect Old World primates. Within their natural hosts, infection by these viruses recapitulates many key aspects of EBV infection, including the establishment of long-term latency within B lymphocytes, and is therefore a potentially valuable animal model of EBV infection. However, it is unclear whether these LCVs have adopted or maintained the same mechanisms used by EBV to express essential viral proteins, such as EBNA-1, in the face of cell-mediated repression of EBV gene expression that occurs upon establishment of the asymptomatic carrier state. To address this issue, we determined whether the endogenous LCVs of baboon (Cercopithecine herpesvirus 12) and rhesus macaque (Cercopithecine herpesvirus 15) have the functional equivalent of the EBV promoter Qp, which mediates exclusive expression of EBNA-1 during the restricted programs of EBV latency associated with the carrier state. Our results indicate that (i) both the baboon and rhesus macaque LCVs have a genomic locus that is highly homologous to the EBV Qp region, (ii) key cis-regulatory elements of Qp are conserved in these LCV genomes and compose promoters that are functionally indistinguishable from EBV Qp, and (iii) EBNA-1 transcripts identical in structure to EBV Qp-specific EBNA-1 mRNAs are present in nonhuman LCV-infected cells, demonstrating that these Qp homologs are indeed utilized as alternative EBNA-1 promoters. These observations indicate that the molecular mechanisms which regulate EBV gene expression during restricted latency have been conserved among the LCVs. The contribution of these mechanisms to viral persistence in vivo can now be experimentally tested in nonhuman primate models of LCV infection.
Epstein-Barr virus (EBV), the only known human herpesvirus of the Lymphocrytovirus genus, establishes a latent infection within B lymphocytes that is maintained for the life of its host. Upon primary infection of a B lymphocyte, EBV induces cellular proliferation through the concerted actions of several of the viral latency-associated genes. These genes encode six nuclear proteins (EBNA-1, -2, -3A, -3B, -3C, and -LP), three plasma membrane proteins (LMP-1, -2A, and -2B), the RK-BARF0 protein, and two highly expressed noncoding small nuclear RNAs (EBER-1 and EBER-2) (16, 26). In vitro, such latently infected B cells are immortal and can be propagated indefinitely as lymphoblastoid cell lines (LCLs) that continue to express the full complement of EBV latency-associated genes, a program of EBV gene expression referred to as type III latency. Following acute infection in vivo, however, there is a host-mediated repression of a subset of the EBV genes expressed during the initial growth or type III latency program, namely those for EBNA-2, -3A, -3B, -3C, and -LP and the viral oncoprotein LMP-1 (19, 37, 46, 72). Several of these, most notably the EBNA-3 proteins, are also the predominant targets for the developing cellular immunity to EBV-infected B cells (25, 41, 50). Because B lymphocytes are potentially long-lived and dynamic cells that are readily accessible to the host antiviral immune surveillance, alternative programs of EBV latency are viewed as critical adaptations of EBV to the B-cell environment that ensure long-term survival of the latently infected cell and thus the pathogenic potential of EBV.
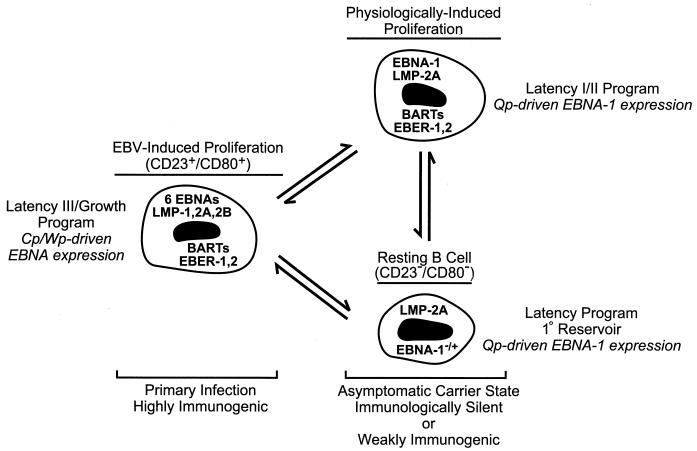
That EBV infection might persist in the face of a strong anti-EBV immune surveillance through maintenance of a less active state of latency was first suggested from studies of the EBV-associated B-cell tumor Burkitt lymphoma (BL). BL cells, which are resistant to killing by EBV-specific cytotoxic T lymphocytes (52), maintain a restricted program of latent gene expression (type I latency) that includes EBNA-1 (essential for EBV genome maintenance), the BamHI-A rightward transcripts (BARTs) that encode RK-BARF0 and potentially other proteins, and the EBERs (2, 7, 18, 53). PCR-based analyses of EBV infection in peripheral blood lymphocytes have confirmed that a program of EBV gene expression that is similar to type I latency, but which also includes LMP-2A, occurs during the asymptomatic carrier phase of EBV infection (9, 10, 37, 46, 72). Moreover, such analyses have indicated that the principal site of EBV latency in the peripheral blood is a resting B cell (37, 38). Thus, although EBV is capable of promoting sustained B-cell proliferation in vitro and has appreciable oncogenic potential, latent infection within an immunocompetent host is largely asymptomatic due to the restricted expression of EBV proteins that directly promote lymphoproliferation (Fig. 1).
FIG. 1.
Model for the maintenance of EBV latency in B lymphocytes. In primary infection, a rapid EBV-induced expansion of infected B cells that express the full complement of known latency-associated genes (see text) serves to establish a pool of infected cells. These are equivalent to EBV-immortalized LCLs which characteristically express each of the six EBNA proteins through the Cp or Wp promoter, and they are susceptible to the developing cellular immunity directed toward EBV proteins expressed exclusively during type III latency (50). In the asymptomatic carrier state, resolution of primary infection is concomitant with the establishment of resting B cells as the primary reservoir of EBV in the peripheral blood (37, 38). These cells display a restricted pattern of EBV gene expression more characteristic of EBV-associated tumors such as BL (type I latency) or nasopharyngeal carcinoma (type II latency), in which Cp and Wp are silent and EBNA gene transcription is limited to EBNA-1 driven by the promoter Qp (6, 7, 9–11, 18, 22, 37, 45, 46, 53, 63, 67, 72, 75). Hypothetically, latently infected B cells are periodically subjected to EBV- or physiologically induced proliferation, e.g., in response to mitogenic signals such as CD40 ligand on activated T cells, that may serve to sustain a critical pool of infected B cells. Note that the precise pattern of EBV gene expression in the resting B cell is currently undefined, as expression of only LMP-2A and EBNA-1 has been evaluated for this population (37); however, studies of EBV gene expression in unfractionated B-lymphocyte populations (9, 10, 46, 72) have indicated that a broader pattern of expression including the BARTs and EBERs occurs in healthy carriers, and thus EBV gene expression in the resting B cell may be indistinguishable from that in the hypothetical infected B cell shown here that is proliferating in response to physiological signals.
Although tumor cell lines such as those derived from BL have been a useful in vitro model of restricted latency, ultimately aspects of this important component of the EBV life cycle will need to be addressed in the context of nonmalignant B cells within a latently infected host, preferably an animal model that either duplicates or closely simulates the natural course of EBV infection. Unfortunately, EBV has an extremely limited host range, and although nonhuman primates such as cottontop marmosets are able to be infected with EBV, these experimental infections do not recapitulate key aspects of EBV infection of its natural host, particularly the establishment of a persistent latent infection in B lymphocytes (65). Attempts to infect Old World primates with EBV have likewise been unsuccessful for several possible reasons, including cross-serologic reactivity between EBV and the endogenous lymphocryptoviruses (LCVs) of these species (13, 17, 23), the use of a nonimmortalizing strain of EBV (30), and an apparent species-specific restriction for efficient B-cell immortalization (39). An alternative model, however, is the infection of Old World primates with the LCVs that naturally infect these species. Comparative analyses of EBV and the endogenous LCVs of chimpanzee, baboon, and rhesus macaque have indicated that these viruses have highly homologous colinear genomes with very similar if not identical coding potentials, suggesting that they are biologically equivalent pathogens within their respective host species (17, 19–21, 29, 32, 33, 54, 74). This prediction is supported by findings that each of the EBV-related LCV proteins examined thus far is functionally equivalent to its EBV homolog, namely, EBNA-1, EBNA-2, LMP-1, and LMP-2A (14, 15, 31, 32, 74), and that these LCVs share with EBV the ability to immortalize B lymphocytes of their natural host (12, 17, 39, 40, 47, 48). However, the most compelling support for this potential model of EBV infection is the recent demonstration that infection of rhesus macaques with the LCV of rhesus macaques (RhLCV) duplicates key aspects of a natural EBV infection, notably (i) an oral route of transmission; (ii) the atypical lymphocytosis, lymphadenopathy, and elevated proportion of CD23+ B cells in the peripheral blood that are observed in acute EBV infection associated with infectious mononucleosis; (iii) sustained serologic responses to lytic- and latent-infection antigens; (iv) establishment of a persistent latent infection in peripheral blood B cells; and (v) intermittent shedding of virus in oropharyngeal secretions following resolution of the acute infection (40).
Because of the high degree of genetic identity that exists between the LCVs and because of their parallel courses of infection, maintenance of long-term latency in their respective LCV-immune hosts is almost certainly dependent on an ability of all LCVs to exist in a restricted program(s) of latency. Of particular importance with regard to the utility of the proposed LCV model of EBV infection, however, is whether the same regulatory mechanisms that dictate EBV gene expression during restricted latency are also operational in the animal model. A hallmark of restricted EBV latency is the utilization of the viral promoter Qp to drive transcription of the EBNA-1 gene (45, 63, 72). This is in contrast to the type III latency program, during which Qp is silent and expression of all six EBNAs is driven by one of two promoters (Wp or Cp) located approximately 50 kb upstream of Qp (Fig. 1) (5, 58, 59, 73). Here we report that the LCV of baboon (BaLCV) and RhLCV possess promoters that are functionally equivalent to EBV Qp. Specifically, these LCV promoters, like their EBV homolog, are dependent on interferon regulatory factors (IRFs) for activation and are negatively autoregulated by their respective EBNA-1 proteins. Furthermore, we demonstrate that when active these promoters, which lie approximately 46 kb upstream of the EBNA-1-coding region, give rise to EBNA-1 transcripts identical in exon structure to EBV Qp-specific EBNA-1 transcripts. These data, therefore, provide strong evidence for the existence of restricted latency programs in the nonhuman primate LCV infections that are identical to those believed to be essential to maintenance of a persistent EBV infection, and they thus further validate these animal models of EBV infection.
MATERIALS AND METHODS
Cell culture.
Lymphoid cells were maintained in RPMI 1640 medium supplemented with 2 mM l-glutamine and 10% defined fetal bovine serum (HyClone). S594 is a BaLCV-infected B-cell line derived by spontaneous outgrowth from baboon peripheral blood lymphocytes (47). Mm278LCL is an RhLCV-infected cell line derived by infection of rhesus macaque B lymphocytes in vitro with virus from the LCL8664 cell line (48). IB4 is an EBV-immortalized human LCL, and Louckes, P3HR-1 (clone 16), and Akata are human BL cell lines. Induction of virus replication in P3HR-1 cells was accomplished by treatment with 20 ng of 12-O-tetradecanoylphorbol-13-acetate (Sigma) per ml and 4 mM sodium butyrate for 48 h prior to cell harvest, at which time approximately 90% of the cells are routinely positive for expression of EBV capsid antigen as determined by immunofluorescence staining. Murine embryonic fibroblasts (MEFs) nullizygous for IRF-1 and IRF-2 (IRF-1,2−/−) (70) were maintained in Dulbecco’s modified Eagle medium supplemented with glucose (4.5 g/liter), l-glutamine (2 mM), and defined fetal bovine serum (10%).
Isolation, cloning, and nucleotide sequence analysis of viral DNA.
DNA extracted from S594 cells by a modified Hirt procedure (49) was used as a source of BaLCV genomic DNA. Briefly, 107 cells were lysed in 2 ml of lysis buffer (0.6% sodium dodecyl sulfate, 10 mM EDTA, 10 mM Tris-HCl [pH 7.5]) and incubated for 2 h at 37°C. The bulk of cellular DNA was removed by the addition of NaCl to a final concentration of 1 M and incubation overnight at 4°C, followed by centrifugation at 12,000 × g for 20 min at 4°C. The supernatant was extracted once with phenol-chloroform and once with chloroform, followed by precipitation of the DNA with 2 volumes of ethanol. This DNA was digested with BamHI and used to generate a genomic library in the lambda phage vector ZAP Express (Stratagene). The library was screened with a 32P-labeled EBV SalI-I restriction fragment, which spans the Qp promoter region and hybridized to an ∼6-kbp DNA fragment on Southern blots of BamHI-digested S594 DNA. Following plaque purification, the pBK-CMV phagemid within the ZAP Express vector and containing the 6-kbp BaLCV BamHI fragment was excised in vivo according to the protocol of the manufacturer (Stratagene). To subclone DNA fragments spanning the putative BaLCV Qp, the 6-kbp BamHI fragment was ligated to itself and sheared by sonication until the average size of DNA fragments was 800 to 1,000 bp. The randomized DNA fragments were treated with T4 DNA polymerase in the presence of deoxynucleoside triphosphates to repair the ends and then ligated into the SmaI restriction site of pBluescript KS(+) II (Stratagene). Clones containing BaLCV DNA homologous to Qp were identified by colony hybridization with an EBV SalI-I probe and then subjected to DNA sequence analysis with T7 and T3 sequencing primers. The nucleotide sequence data obtained from 12 clones were assembled to yield the DNA sequence spanning positions −537 to +76 relative to the transcription initiation site (+1) of the EBV Qp.
RhLCV DNA was isolated from the B-cell line LCL8664 (48), partially digested with Sau3AI, and then ligated into the BamHI cloning site of the cosmid SuperCos1 (Stratagene). An EBV BamHI-Q probe was used to isolate clone QA15, which contained an insert corresponding to EBV nucleotides 58,036 to 108,534 (the genomic coordinate of Qp is ca. 62,400). The nucleotide sequence of the putative RhLCV Qp was determined directly from this cloned viral DNA, initially by employing oligonucleotide primers that were designed based on the regions surrounding EBV Qp that demonstrated the highest degree of sequence conservation between EBV and BaLCV.
Plasmids.
Reporter gene plasmids were generated by ligating viral DNA fragments into the multiple cloning site of pOGH, which contains a promoterless human growth hormone (hGH) gene (64). The EBV Qp-hGH reporter plasmid used contains viral DNA from positions −143 to +75 (SalI to PvuII site) relative to the Qp transcription start site; in mtQp-hGH (also positions −143 to +75) the IRF binding element of Qp (alternatively referred to as QRE-2) has been inactivated by replacement with a BamHI linker (42). The BaLCV and RhLCV Qp fragments, equivalent to EBV DNA spanning positions −143 to +75, were generated by PCR with the respective cloned viral DNA as the template. Reporter plasmids lacking a functional IRF binding site (mtQp) were generated by replacing the hexanucleotide sequence AACGAA (see Fig. 2) with a BamHI recognition site (GGATCC) by site-directed mutagenesis with the QuikChange system (Stratagene). To inactivate the putative EBNA-1 binding domain of the BaLCV and RhLCV promoters, a 34-bp deletion (nucleotides +10 to +43; see Fig. 2) was introduced by recombinant PCR as previously described for the EBV promoter (57). BaLCV and RhLCV EBNA-1 expression vectors were generated by ligating the respective EBNA-1 coding sequence (to be described elsewhere) into pSG5 (Stratagene). Rhesus macaque EBNA-1 expression was confirmed by transfection into Cos cells, followed by immunoblotting with rhesus macaque immune sera. The IRF-2 expression plasmid pSG.IRF-2 has been described previously (43).
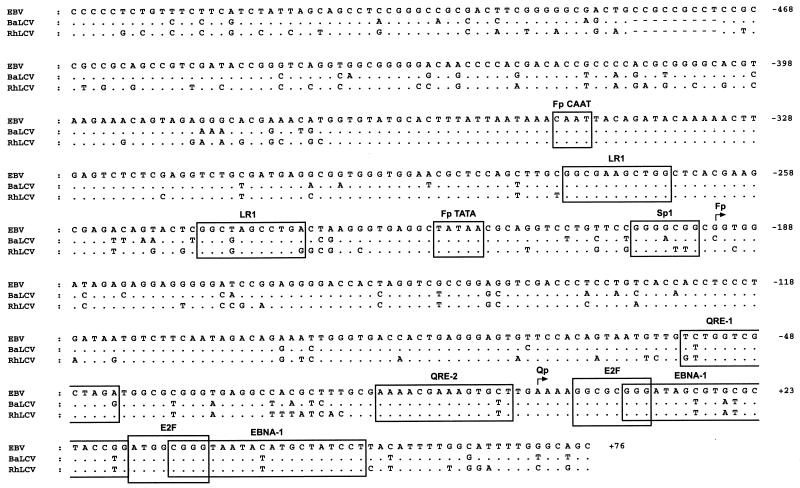
FIG. 2.
DNA sequence conservation in the Qp promoter region. The Qp promoter region of the B95-8 EBV genome sequence (coordinates 61,886 to 62,498) (4) is aligned with the DNA sequences of the homologous regions of the BaLCV and RhLCV genomes and represents nucleotides −537 to +76 relative to the major transcription start site (+1) within the EBV Qp (45, 63). Known and predicted promoter-regulatory elements (see text) are boxed and include those for the lytic cycle-specific EBNA-1 promoter Fp (CAAT, LR-1, TATA, and Sp1) (8, 56) as well as for Qp (QRE-1, QRE-2, E2F, and EBNA-1) (42–44, 57, 60, 61, 68). Sites of transcription initiation are denoted by bent arrows. Differences in the BaLCV and RhLCV sequences relative to that of EBV are indicated; dots and dashes represent identical and deleted nucleotides, respectively.
Transfections and reporter gene assays.
Prior to transfection by electroporation as described previously (57), Louckes, S594, and IB4 cells were maintained in roller bottle cultures for at least two feedings. Transfections were done in triplicate (8 × 106 cells per transfection) with 10 μg of Qp-hGH reporter plasmid and, where indicated, also with 5 μg of either the empty expression vector pSG5 or the appropriate pSG5-based EBNA-1 expression vector, pSG.E1. The level of hGH in the culture medium was determined in duplicate approximately 40 h posttransfection by using a radioimmunoassay kit (Nichols Institute). IRF-1,2−/− MEFs were transfected by using the modified calcium phosphate procedure with N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES) (Calbiochem) as the buffer (3). One day prior to transfection, 8 × 105 cells were plated per 100-mm-diameter tissue culture dish. Cells were cotransfected in triplicate with 10 μg of reporter plasmid and 5 μg of either pSG5 or pSG.IRF-2. The calcium phosphate-DNA precipitate was allowed to remain on the cells for 18 h at 35°C in a 3% CO2 atmosphere, after which the cells were rinsed twice with 10 ml of phosphate-buffered saline and fed with 10 ml of fresh growth medium. Cells were then maintained at 37°C in 5% CO2 for an additional 48 h prior to assay of hGH expression. All transfections included 1 μg of a β-galactosidase expression vector (pCMV-βgal), and all hGH values were normalized to β-galactosidase activity (adjusted for total protein assayed) in transfected-cell extracts to correct for differences in transfection efficiency.
Analysis of RNA.
For Northern (RNA) blot analysis, 10 μg of poly(A)+ RNA, isolated as described previously (43), was fractionated by electrophoresis in a 1.2% agarose–2.2 M formaldehyde gel and transferred to a GeneScreen Plus membrane (DuPont). RNA Millennium markers (Ambion) were used as size standards. RNA blots were subjected to hybridization to a 32P-labeled (by nick translation) DNA probe containing the BaLCV or RhLCV EBNA-1 open reading frame (ORF), washed, and processed by autoradiography as previously reported (59).
Generation of EBNA-1 cDNAs by reverse transcription-PCR (RT-PCR) was accomplished as follows. Five hundred nanograms of poly(A)+ RNA was reverse transcribed at 42°C with Superscript-II reverse transcriptase (GibcoBRL) and 10 pmol of the relevant RT primer in a 20-μl reaction mixture as recommended by the manufacturer. Primers were chosen based on selection by the program Primer Designer (Scientific and Educational Software) and so that they would anneal to the respective EBNA-1 mRNA approximately 250 nucleotides downstream of the EBNA-1 translation initiation codon. RT primer sequences were as follows: EBV, 5′-GTGGGTCCCTTTGCAGCCAA-3′; BaLCV, 5′-GCTTCCTCCTGATGTACCAC-3′; and RhLCV, 5′-TGCTTCCTCCAGTGCCACCT-3′. Control reactions without reverse transcriptase were run in parallel. Following cDNA synthesis, samples were incubated at 70°C for 15 min and then diluted with TE buffer (10 mM Tris-HCl [pH 8.0], 0.1 mM EDTA) to 500 μl, from which 10 μl was used as a template for amplification by PCR. The PCR primers used for each sample were a nested 3′ primer specific for the exon encoding EBNA-1 and one of three 5′ primers (SP1, SP2, or SP3) to distinguish the origin of EBNA-1-specific transcription (see Results). These 5′ primers were as follows: for EBV, 5′-TATAACGCAGGTCCTGTTCC-3′ (SP1), 5′-CCTGTCACCACCTCCCTGAT-3′ (SP2), and 5′-AAGGCGCGGGATAGCGTGCG-3′ (SP3); for BaLCV, 5′-GTGAGGCTATAACGCAGGTC-3′ (SP1), 5′-ACCAGCCACAACCTCCCTGA-3′ (SP2), and 5′-AAGGCGCGGGATAGTGTATG-3′ (SP3); and for RhLCV, 5′-GTGAGGCTATAACGCATGTC-3′ (SP1), 5′-CCACCTCCCTAATAGTGTCT-3′ (SP2), and 5′-AAGGCGCGGGATAGTGTATG-3′ (SP3). The nested 3′ PCR primers were as follows: EBV, 5′-GTCTTGGCCCTGATCCTGAG-3′; BaLCV, 5′-TTGCGCCACTGCCTCCTTTG-3′; and RhLCV, 5′-CCATTGCCATGTCTTGTCTC-3′. PCR was done with 50-μl reaction mixtures containing 25 pmol of each primer; 1 mM each dATP, dCTP, dGTP, and dTTP; 10% dimethyl sulfoxide; 10 mM Tris-HCl (pH 9.0); 2.5 mM MgCl2; 50 mM KCl; and 2.5 U of Taq DNA polymerase. DNA was amplified for 35 cycles (95°C for 40 s, 55°C for 2 min, and 72°C for 3 min), followed by a final extension at 72°C for 15 min. One-tenth of each reaction mixture was then electrophoresed in a 1.5% agarose gel, transferred to a GeneScreen Plus membrane (DuPont), and processed by standard Southern blot hybridization techniques (55). The probes utilized were EBV, BaLCV, and RhLCV Qp-specific EBNA-1 cDNAs that had been generated by RT-PCR, cloned, and subjected to DNA sequence analysis.
RESULTS
DNA sequence conservation in the EBNA-1 promoter region.
To determine whether the baboon and rhesus macaque LCV genomes were likely to contain a homolog of the EBV EBNA-1 promoter Qp, DNA was isolated from the BaLCV-infected S594 and RhLCV-infected Mm278LCL B-cell lines and subjected to Southern blot analysis with a 0.9-kbp EBV DNA probe (SalI-I) that contains Qp. Using relatively nonstringent conditions for the hybridization and washing of Southern blots, we obtained results indicating that both viruses contain DNA homologous to the EBV Qp promoter region (data not shown). We therefore cloned and determined the nucleotide sequences of the BaLCV and RhLCV DNAs homologous to the EBV Qp region (see Materials and Methods). An alignment of the BaLCV and RhLCV nucleotide sequence data with the sequence of the homologous EBV DNA (4) is presented in Fig. 2 and represents the DNA spanning positions −537 to +76 (EBV genomic coordinates 61,886 to 62,498) relative to the major transcription start site (+1) of Qp.
The degree of identity observed between the two primate LCVs and EBV within this locus was 86% for BaLCV and 80% for RhLCV. BaLCV and RhLCV were 86% identical. Although BaLCV demonstrated a 6% greater identity to EBV than did RhLCV, BaLCV and RhLCV appeared to be more closely related to each other than to EBV, since the primate LCVs shared a number of differences from EBV that were clearly nonrandom. For example, both primate LCVs had an identical 9-bp deletion relative to EBV at positions −456 to −464. Furthermore, of 132 nucleotide substitutions noted in the BaLCV and RhLCV DNAs relative to EBV, 60 (46%) occurred at the same position in BaLCV and RhLCV, and of these, 45 (75%) represented an identical substitution.
IRF-2 activates the BaLCV and RhLCV Qp promoters.
In general, the known and putative regulatory elements of Qp were well conserved, as were those of Fp, a lytic-cycle EBNA-1 promoter (28, 45, 62) located immediately upstream of Qp (Fig. 2). This suggested that BaLCV and RhLCV each contain a promoter that is functionally equivalent to Qp. However, in both BaLCV and RhLCV nucleotide substitutions were detected within or adjacent to three previously defined regulatory elements of Qp: QRE-1, a positive regulatory element and potential binding site for the cellular transcription factor LBP-1 (42); QRE-2, a positive as well as potentially negative regulatory element targeted by members of the IRF family of transcription factors (43, 60, 76); and the region III EBNA-1 binding domain, which contains two binding sites for EBNA-1 that act in trans with EBNA-1 to negatively autoregulate Qp (57, 61, 68). Two putative E2F binding sites located immediately downstream of the transcription start site (68) and shown previously to be required for full promoter activity (44) were completely conserved.
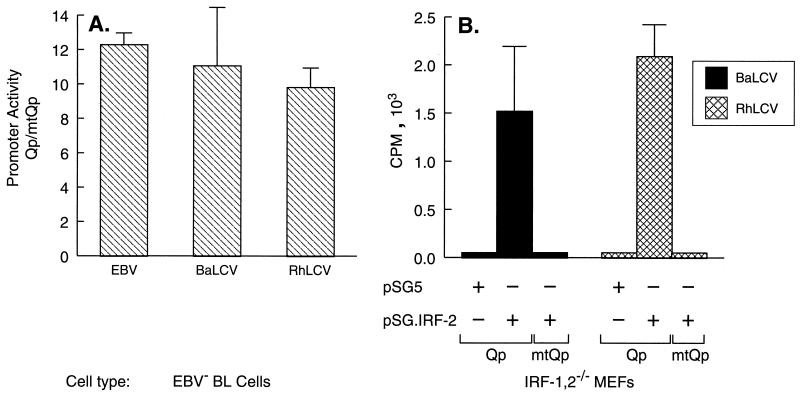
Previous studies have established that Qp is constitutively activated by either IRF-1 or IRF-2 through binding to QRE-2 (43, 60). Therefore, to determine whether this region of the BaLCV and RhLCV genomes did indeed contain a promoter functionally equivalent to Qp, we assessed the ability of BaLCV and RhLCV DNAs to direct QRE-2-dependent transcription in a reporter gene assay. BaLCV and RhLCV DNA fragments equivalent to positions −143 to +75 of EBV Qp were generated by PCR and cloned into a promoterless hGH reporter plasmid. Reporter constructs in which the putative QRE-2 element of each was mutated (mtQp) by substitution with a BamHI recognition sequence as previously described for EBV Qp (43) were also generated, and the promoter activities of the constructs were then compared to those of the analogous EBV-derived plasmids in transient-transfection assays with EBV-negative Louckes BL cells. As demonstrated in Fig. 3A, both the BaLCV and RhLCV reporter plasmids were transcriptionally active, and mutation of the putative QRE-2 element in each resulted in a 90% reduction in promoter activity, virtually identical to the results obtained with the analogous EBV reporter plasmids. To confirm that transcriptional activation of the BaLCV and RhLCV promoters could be driven by either IRF-1 or IRF-2, cotransfection experiments were performed with MEFs nullizygous for IRF-1 and IRF-2 (IRF-1,2−/−). As shown in Fig. 3B, in the absence of cotransfection with an IRF-2 expression plasmid, neither the BaLCV nor the RhLCV promoter was active in IRF-1,2−/− cells. However, both promoters could be activated in response to cotransfection with an IRF-2 expression plasmid, whereas the promoters within reporter constructs containing a mutated QRE-2 element (mtQp) were unresponsive to IRF-2 (Fig. 3B). Similar results were obtained with coexpression of IRF-1 (data not shown). Thus, both primate LCVs contain a promoter that is functionally indistinguishable from the EBV Qp with respect to dependence on IRF-1 or IRF-2 for activation of transcription.
FIG. 3.
Activation of the BaLCV and RhLCV Qp promoters is IRF dependent. (A) The dependence of the BaLCV and RhLCV Qp promoters on the IRF binding site QRE-2, which is essential for EBV Qp function, was evaluated by an hGH reporter gene assay with EBV-negative Louckes BL cells. Promoter activity is presented as the ratio of hGH expression achieved from a wild-type promoter sequence (Qp) to that achieved from the respective LCV Qp in which the IRF binding site had been mutated (mtQp). (B) The BaLCV and RhLCV Qp promoters require IRF expression for activation. MEFs nullizygous for IRF-1 and IRF-2 were cotransfected with a Qp or mtQp hGH reporter plasmid and either an empty expression vector (pSG5) or the IRF-2 expression vector pSG.IRF-2. Promoter activity was measured by radioimmunoassay of hGH expression. The data shown are from a representative experiment in which all transfections were done in triplicate and hGH values were corrected for transfection efficiency. The 5′ and 3′ coordinates of the promoter DNA in each reporter plasmid were −143 to +75 relative to the EBV Qp transcription start site. Error bars indicate standard deviations.
Negative autoregulation of the BaLCV and RhLCV Qp promoters.
An additional feature of the Qp EBNA-1 promoter is the presence of two binding sites for EBNA-1 immediately downstream of the transcription start site through which EBNA-1 is capable of repressing transcription from Qp (57, 61, 68). It is believed that this function of EBNA-1 is largely responsible for silencing Qp during type III latency, as well as for regulating EBNA-1 levels through Qp during the restricted EBV latency programs in normal B lymphocytes in vivo and in EBV-infected tumor cells. Within the baboon and rhesus macaque LCVs, four nucleotide changes were detected relative to EBV in the upstream-most EBNA-1 binding site, and one change was detected within the downstream binding site (Fig. 2). This degree of conservation is similar to that observed between the EBNA-1 binding sites within the respective EBV and BaLCV origins of DNA replication (33). The same substitutions (relative to EBV Qp) occurred in both BaLCV and RhLCV, and four of these five substitutions were nucleotide transitions as opposed to transversions. To determine whether the BaLCV and RhLCV promoters were responsive to their respective EBNA-1 proteins, the BaLCV and RhLCV EBNA-1 ORFs were cloned into mammalian expression vectors and used in cotransfection assays to assess the effect of EBNA-1 on promoter function. As demonstrated in Fig. 4 (left panel), both the BaLCV and RhLCV promoters were repressed in the presence of their respective EBNA-1 protein. Furthermore, repression was indeed mediated through the EBNA-1 binding sites, since introduction of a 34-bp deletion that removed the entire proximal binding site and several nucleotides of the downstream EBNA-1 binding site resulted in unresponsiveness to EBNA-1 (Fig. 4, right panel). Thus, the BaLCV and RhLCV promoters, like the EBV Qp, are subject to autoregulation. As a result of the 34-bp deletion, relative promoter activity increased three- to fivefold in these cells in the absence of EBNA-1 (Fig. 4). An identical but less dramatic effect of this deletion on EBV Qp activity has also been observed (57), suggesting that cellular factors also negatively regulate Qp. Such repression may be mediated by pRB that has been targeted to Qp through E2F transcription factors bound to low-affinity E2F binding sites (68) within the EBNA-1 binding domain (see Fig. 2).
FIG. 4.
The BaLCV and RhLCV Qp promoters are negatively autoregulated. (Left panel) EBV-negative Louckes BL cells were cotransfected with a BaLCV or RhLCV Qp reporter plasmid and either an empty expression vector (pSG5) or an EBNA-1 expression vector (pSG.E1) encoding BaLCV or RhLCV EBNA-1, respectively. (Right panel) BaLCV and RhLCV Qp reporter plasmids lacking a functional EBNA-1 binding domain (QpΔ34) are unresponsive to the respective LCV EBNA-1. Data are from a representative experiment in which all transfections were done in triplicate and hGH values were corrected for transfection efficiency. The reporter plasmids used in these experiments had the same 5′ and 3′ coordinates as indicated for Fig. 3. Error bars indicate standard deviations.
Qp-specific EBNA-1 transcription in latently infected B lymphocytes.
The data presented above indicated that the baboon and rhesus macaque LCVs possess a promoter that is functionally indistinguishable from EBV Qp. However, because Qp is approximately 46 kbp upstream of the exon containing the EBNA-1 ORF, the appropriate splicing events must occur to generate an EBNA-1 mRNA. Therefore, before it could be concluded that the baboon and rhesus LCV homologs of Qp are functional EBNA-1 promoters, it was necessary to demonstrate that transcripts that initiate from these promoters are spliced to generate an mRNA from which EBNA-1 could be expressed. Within cells latently infected by EBV, Qp is active only within tumor cells and normal B cells in vivo that maintain a restricted latency program (45, 62, 72). Thus, we did not expect to observe Qp-driven expression of EBNA-1 in LCLs latently infected with either BaLCV or RhLCV, in which the Cp EBNA promoter is active (16a). Northern blot analysis of EBNA-1 expression in BaLCV-infected S594 cells and RhLCV-infected Mm278LCL cells revealed the presence of transcripts consistent with transcription initiating from Cp (or Wp), as expected, and also smaller transcripts indicative of either Fp- or Qp-specific transcription (Fig. 5).
FIG. 5.
Northern blot analysis of EBNA-1 expression in BaLCV- and RhLCV-infected B lymphocytes. Each blot contained 10 μg of poly(A)+ RNA isolated from the BaLCV- and RhLCV-infected B-cell lines S594 and Mm278LCL, respectively. The positions to which the RNA size markers migrated in the gel (kilobases) are indicated to the left of the blots. Based on the sizes of the transcripts detected, those consistent with transcription initiating from either Fp or Qp (Fp/Qp) or from either Cp or Wp (Cp/Wp) are bracketed. Blots were reprobed for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA to monitor RNA loading. The specific activities of the DNA probes were as follows: BaLCV, 2.9 × 108 cpm/μg; RhLCV, 2.6 × 108 cpm/μg; and GAPDH, 2.5 × 108 cpm/μg. Autoradiographic exposure times were 7 days (EBNA-1) and 5 h (GAPDH). Data are representative of those from three experiments.
To determine whether Qp was active in these cells or whether these smaller transcripts were merely the result of activation of Fp in a small proportion of cells supporting lytic infection, we performed an RT-PCR-based assay capable of distinguishing Qp- from Fp-specific EBNA-1 transcripts (45). As illustrated in Fig. 6 (bottom), cDNA synthesis was primed with a primer that annealed within the EBNA-1 ORF, followed by amplification of cDNAs by PCR with a nested EBNA-1-specific 3′ primer (E1) and one of three 5′ primers (SP1, SP2, or SP3) that would anneal within the 5′ exon of a cDNA derived from an EBNA-1 mRNA that initiated from either Fp or Qp (SP3) or exclusively from Fp (SP2); the primer SP1 was included to detect any transcripts that might initiate upstream of Fp (56).
FIG. 6.
The BaLCV and RhLCV Qp promoters are functional within latently infected B lymphocytes. RT-PCR analysis was employed to detect, and distinguish between, Qp- and Fp-specific EBNA-1 gene transcription in S594 (BaLCV-infected) and Mm278LCL (RhLCV-infected) cells. The exon structure of an EBNA-1 mRNA (not to scale) derived from either Fp (active during lytic infection) or Qp is illustrated at the bottom; Fp and Qp transcription start sites (bent arrows) and the relative annealing sites of the oligonucleotide primers used for RT and subsequent amplification of cDNAs by PCR are depicted with respect to the mRNA structure. The upper two panels demonstrate detection of Qp-specific EBNA-1 transcription in latently infected BL cells (Akata), in which Fp is silent, and detection of Fp activity in lytically infected BL cells (P3HR-1 BL cells treated with 12-O-tetradecanoylphorbol-13-acetate and sodium butyrate), respectively. By comparison, the predominate origin of EBNA-1 transcription detected in the BaLCV- and RhLCV-infected cells was attributable to Qp. PCR products were detected by Southern blot hybridization with an EBV, BaLCV, or RhLCV cDNA probe that had been generated by the respective SP3-E1 primer pair and sequenced to confirm that they were EBNA-1 cDNAs. +/−, presence and absence, respectively, of reverse transcriptase in the cDNA synthesis reaction.
Surprisingly, although the BaLCV-infected S594 cells exhibited some Fp/lytic cycle activity, the majority of transcripts detected within these cells, and the only transcripts detected in the RhLCV-infected cells, initiated from Qp, not Fp. The BaLCV and RhLCV Fp-specific primers (SP2) were fully functional when tested for the ability to amplify genomic DNA (data not shown), and thus the smaller amount of Fp-specific transcripts detected could not be attributed to inefficiency of the SP2 primers. Therefore, none of the RhLCV and only a minority of the BaLCV cDNAs amplified with the SP3 primer could be attributed to Fp- or lytic cycle-specific transcription. Moreover, even though our RT-PCR assay was not necessarily quantitative, the relative levels of Qp-specific transcription predicted from this assay were in good agreement with the data obtained by Northern blot analysis of EBNA-1 mRNA expression in these two cell lines (Fig. 5). The sizes of the amplified BaLCV and RhLCV cDNAs, furthermore, suggested that the equivalent of the 172-nucleotide noncoding exon from the BamHI-U fragment present in EBV EBNA-1 mRNAs was also contained in the primate LCV EBNA-1 mRNAs. Sequence analysis of the BaLCV and RhLCV cDNAs confirmed that the U exon, as well as the 5′ splice site of the EBNA-1 coding exon, is conserved in both viruses (data not shown).
Finally, to address whether the endogenous Qp activity detected in the baboon and rhesus macaque LCLs was due to the specific host cell environment or to inherent differences in the regulation of these promoters relative to EBV Qp, we compared the activities of the BaLCV and EBV Qp promoters in baboon (S594) and human (IB4) LCLs by a reporter gene assay. The RhLCV-infected LCLs were excluded because of the very low transfection efficiency achieved with these cells. As illustrated in Fig. 7, both the BaLCV and EBV Qp promoters containing their respective autoregulatory domains were active in S594 cells, consistent with the detection of endogenous Qp activity in these BaLCV-infected cells (Fig. 6). By contrast, both promoters were virtually inactive in IB4 cells, in agreement with previous observations of a lack of EBV Qp-driven reporter activity in human B cells that maintain type III latency (57, 61). Interestingly, EBV Qp activity in the S594 cells was fivefold greater than that of BaLCV Qp. This is consistent with our recent observation that BaLCV EBNA-1 may not be as efficient as EBV EBNA-1 in the repression of EBV Qp (data not shown). Upon deletion of the EBNA-1 binding domain in each construct, however, we observed only minor differences in Qp activity, regardless of the cell line used. These data suggest, therefore, that the endogenous Qp activity detected in the nonhuman primate LCLs predominantly reflects the cellular environment (most likely a low level of EBNA-1 expression) and not an inherent difference in the regulation of these promoters relative to EBV Qp. Thus, the Qp homologs present within the baboon and rhesus macaque LCV genomes are indeed alternative promoters of EBNA-1 gene transcription functionally equivalent to EBV Qp.
FIG. 7.
The EBV and BaLCV Qp promoters are similarly regulated in baboon and human LCLs. S595 and IB4 cells were transfected in triplicate with EBV and BaLCV Qp-hGH reporter plasmids (Fig. 4) that did (Qp) or did not (QpΔ34) contain a functional EBNA-1 autoregulatory domain. Data have been corrected for differences in transfection efficiency. Error bars indicate standard deviations.
DISCUSSION
EBV, as well as other herpesviruses, is characterized by the ability to persist for the life of its host as a latent infection in which expression of viral genes is highly restricted. A defining property of the restricted programs of EBV latency is the exclusive expression of EBNA-1 via the promoter Qp. Here we have shown that the endogenous LCVs of baboons and rhesus macaques contain a promoter that is functionally indistinguishable from EBV Qp. Consequently, the BaLCV and RhLCV promoters, as previously demonstrated for EBV Qp (43, 57, 60, 61, 68), are dependent on constitutively expressed IRF-1 or IRF-2 for activation and can be autoregulated. Furthermore, the presence of transcripts that initiate from these Qp homologs within LCV-infected B cells and which have the same exon structure as the analogous EBV mRNA confirms that these are indeed functional promoters that regulate transcription of the BaLCV and RhLCV EBNA-1 genes. This work, therefore, provides the first direct evidence that Old World primate LCVs utilize restricted latency programs to maintain a persistent infection within their LCV-immune host and that the same mechanisms that regulate EBV gene expression during restricted latency are also likely to be operational in the nonhuman primate models of EBV infection.
One difference between these EBNA-1 promoters that we did note was that whereas EBV Qp is normally silent within an LCL (type III latency), active BaLCV and RhLCV Qp promoters were detected in the LCLs examined here (Fig. 5 and 6), even though these cells maintain a type III latency program as indicated by the presence of an active Cp (30a) and expression of EBNA-2 (40). This may indicate that EBNA transcription driven by Cp or Wp in the BaLCV- and RhLCV-infected LCLs is insufficient to express the level of EBNA-1 needed to completely silence Qp during type III latency. Indeed, we repeatedly found that the levels of EBNA-1 mRNA were very low in these LCLs, and in particular in the RhLCV-infected LCL (Fig. 5), requiring autoradiographic exposure times of several days. By contrast, detection of Cp- or Wp-specific EBV EBNA-1 transcripts within an LCL such as IB4, which does not exhibit Qp activity, requires only several hours of exposure (59). Furthermore, both BaLCV and EBV Qp reporter plasmids were more active in S594 cells than in IB4 cells, as one would expect if EBNA-1 levels were critical in determining the degree of Qp activity during a type III latency.
An additional observation of note is the presence of a block of nucleotide substitutions within BaLCV and RhLCV that result in an AT-rich region relative to the EBV Qp between positions −21 and −27 (Fig. 2), the position at which one would expect a TATA box in a classical RNA polymerase II promoter. Although it is unlikely that these are functional TATA boxes, it is interesting to speculate that Qp may have evolved from a promoter that originally contained a TATA box and that the BaLCV and RhLCV promoters still contain remnants of this element. Because Qp is a relatively simple promoter, not unlike those of many housekeeping genes, loss of a TATA box may have contributed positively to the efficiency of Qp-mediated EBNA-1 expression by releasing this promoter from a higher order of regulation that may be generally inherent in TATA-containing promoters (66).
Previous studies have established that EBV and the Old World primate LCVs are virtually identical with respect to their B-cell tropism and effects on the growth properties of latently infected cells (LCLs) in vitro (12, 13, 17, 39, 40, 47, 48). Demonstrations that the EBNA-1, EBNA-2, LMP-1, and LMP-2 genes of EBV not only are genetically conserved among the LCVs but also are functional homologs (14, 15, 31, 32, 74), furthermore, are strong evidence that identical molecular mechanisms and biochemical pathways are employed by these viruses to promote cell growth associated with type III latency. Infection of primates such as rhesus macaques with RhLCV, therefore, provides an excellent model of this aspect of EBV infection, as we have recently shown (40). An equally important component of the EBV life cycle, however, is the restricted latency program(s) that presumably enables the virus to persist within the B-cell population of a host that is in all classical respects immune to EBV infection. The demonstration here that the mechanism employed by EBV to express EBNA-1 during restricted latency is conserved in BaLCV and RhLCV infections suggests that this animal model of EBV infection will be equally representative of the events that directly promote long-term infection.
Although it is generally accepted that restricted latency is an important mechanism for immune evasion and maintenance of persistent infection (36, 71), this remains to be formally tested. Conserved mechanisms for restricted latency in nonhuman LCV infections suggest that this is an essential component for virus survival, and such infections now provide an experimental system for testing in vivo. Based on an analysis of the minimal genetic information necessary for B-cell immortalization by EBV (24), one would predict that deletion of Qp in RhLCV would not affect B-cell immortalization in vitro associated with the type III latency program but would prevent establishment of the restricted latency program in vivo due to the inability to express EBNA-1 upon repression of Cp and Wp. Naive rhesus macaques infected with RhLCV lacking Qp may well develop an acute mononucleosis-like syndrome upon primary infection, and immune responses to both viral lytic- and latent-infection proteins may develop normally, but an inability to sustain a restricted latency program may preclude immune evasion and prevent establishment of a persistent latent infection. These experiments would formally test the importance of restricted latency programs in vivo and potentially provide attenuated viruses for vaccination against EBV or heterologous proteins.
An authentic animal model of EBV infection would also be of great utility in addressing the fundamental aspects of restricted latency itself. None of the EBV gene products associated with restricted latency in vivo (EBNA-1, LMP-2, the BARTs, and EBERs) (Fig. 1) are known to directly influence cell growth, and with the exception of EBNA-1, which is required for maintenance of the viral genome, none are essential for cell proliferation and sustained growth in vitro (27, 34, 35, 51, 69). The contribution of these EBV gene products to persistence in vivo, therefore, is unclear. Infection with recombinant viruses carrying mutations in the LMP-2, BART, or EBER genes or with these genes deleted may provide a means to define the roles of these genes in vivo and elucidate additional strategies for disrupting persistent herpesvirus infections.
ACKNOWLEDGMENTS
We thank Patricia Vaughan, Gary Stein, and Tadatsugu Taniguchi for the IRF-1,2−/− MEFs, George Miller for clone 16 P3HR-1 cells, and Daniel Henson for excellent technical assistance.
This work was supported by Public Health Service grants CA56639 and CA73544 (to J.S.) and CA65319 and CA68051 (to F.W.), Cancer Center Support (CORE) grant CA21765 from the National Cancer Institute, and the American Lebanese Syrian Associated Charities (ALSAC). F.W. is the recipient of an Established Investigator Award from the American Heart Association. I.K.R. was supported by Public Health Service grant T32-AI07372.
REFERENCES
- 1.Ablashi D V, Gerber P, Easton J. Oncogenic herpesviruses of nonhuman primates. Comp Immunol Microbiol Infect Dis. 1979;2:229–241. doi: 10.1016/0147-9571(79)90011-0. [DOI] [PubMed] [Google Scholar]
- 2.Arrand J R, Rymo L. Characterization of the major Epstein-Barr virus-specific RNA in Burkitt lymphoma-derived cells. J Virol. 1982;41:376–389. doi: 10.1128/jvi.41.2.376-389.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons, Inc.; 1989. [Google Scholar]
- 4.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T G, Hatfull G, Hudson G S, Satchwell S C, Sequin C, Tufnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 5.Bodescot M, Perricaudet M, Farrell P. A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J Virol. 1987;61:3424–3430. doi: 10.1128/jvi.61.11.3424-3430.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks L, Yao Q Y, Rickinson A B, Young L S. Epstein-Barr virus latent gene transcription in nasopharyngeal carcinoma cells: coexpression of EBNA1, LMP1, and LMP2 transcripts. J Virol. 1992;66:2689–2697. doi: 10.1128/jvi.66.5.2689-2697.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks L A, Lear A L, Young L S, Rickinson A B. Transcripts from the Epstein-Barr virus BamHI A fragment are detectable in all three forms of virus latency. J Virol. 1993;67:3182–3190. doi: 10.1128/jvi.67.6.3182-3190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulfone-Paus S, Dempsey L A, Maizels N. Host factors LR1 and Sp1 regulate the Fp promoter of Epstein-Barr virus. Proc Natl Acad Sci USA. 1995;92:8293–8297. doi: 10.1073/pnas.92.18.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen F, Zou J Z, di Renzo L, Winberg G, Hu L F, Klein E, Klein G, Ernberg I. A subpopulation of normal B cells latently infected with Epstein-Barr virus resembles Burkitt lymphoma cells in expressing EBNA-1 but not EBNA-2 or LMP-1. J Virol. 1995;69:3752–3758. doi: 10.1128/jvi.69.6.3752-3758.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, H., P. Smith, R. Ambinder, and S. D. Hayward. Expression of Epstein-Barr virus BamHI-A rightward transcripts (BARTs) in latently infected B cells from peripheral blood. Blood, in press. [PubMed]
- 11.Fahraeus R, Li-Fu H, Ernberg I, Finke J, Rowe M, Klein G, Falk K, Nilsson E, Yadaf M, Busson P, Tursz T, Kallin B. Expression of the Epstein-Barr virus genome in nasopharyngeal carcinoma. Int J Cancer. 1988;42:329–338. doi: 10.1002/ijc.2910420305. [DOI] [PubMed] [Google Scholar]
- 12.Falk L A, Henle G, Henle W, Deinhardt F, Schudel A. Transformation of lymphocytes by Herpesvirus papio. Int J Cancer. 1977;20:219–266. doi: 10.1002/ijc.2910200209. [DOI] [PubMed] [Google Scholar]
- 13.Frank A, Andiman W A, Miller G. Epstein-Barr virus and nonhuman primates: natural and experimental infection. Adv Cancer Res. 1976;23:171–201. doi: 10.1016/s0065-230x(08)60546-1. [DOI] [PubMed] [Google Scholar]
- 14.Franken M, Annis B, Ali A N, Wang F. 5′ coding and regulatory region sequence divergence with conserved function of the Epstein-Barr virus LMP2A homolog in herpesvirus papio. J Virol. 1995;69:8011–8019. doi: 10.1128/jvi.69.12.8011-8019.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franken M, Devergne O, Rosenzweig M, Annis B, Kieff E, Wang F. Comparative analysis identifies conserved tumor necrosis factor receptor- associated factor 3 binding sites in the human and simian Epstein-Barr virus oncogene LMP1. J Virol. 1996;70:7819–7826. doi: 10.1128/jvi.70.11.7819-7826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fries K L, Sculley T B, Webster-Cyriaque J, Rajadurai P, Sadler R H, Raab-Traub N. Identification of a novel protein encoded by the BamHI A region of the Epstein-Barr virus. J Virol. 1997;71:2765–2771. doi: 10.1128/jvi.71.4.2765-2771.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.Fuentes-Panana E M, Swaminathan S, Ling P D. Transcriptional activation signals found in the Epstein-Barr virus (EBV) latency C promoter are conserved in the latency C promoter sequences from baboon and rhesus monkey EBV-like lymphocryptoviruses (cercopithecine herpes virus 12 and 15) J Virol. 1999;73:826–833. doi: 10.1128/jvi.73.1.826-833.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerber P, Kalter S S, Schidlovsky G, Peterson W J, Daniel M D. Biologic and antigenic characteristics of Epstein-Barr virus-related herpesviruses of chimpanzees and baboons. Int J Cancer. 1977;20:448–459. doi: 10.1002/ijc.2910200318. [DOI] [PubMed] [Google Scholar]
- 18.Gregory C D, Rowe M, Rickinson A B. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt’s lymphoma cell line. J Gen Virol. 1990;71:1481–1495. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- 19.Heller M, Gerber P, Kieff E. DNA of herpesvirus papio, a third member of the Epstein-Barr virus herpesvirus papio group. J Virol. 1982;41:931–939. doi: 10.1128/jvi.41.3.931-939.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heller M, Gerber P, Kieff E. Herpesvirus papio DNA is similar in organization to Epstein-Barr virus DNA. J Virol. 1981;37:698–709. doi: 10.1128/jvi.37.2.698-709.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heller M, Kieff E. Colinearity between the DNAs of Epstein-Barr virus and herpesvirus papio. J Virol. 1981;37:821–826. doi: 10.1128/jvi.37.2.821-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitt M M, Allday M J, Hara T, Karran L, Jones M D, Busson P, Tursz T, Ernberg I, Griffin B E. EBV gene expression in an NPC-related tumor. EMBO J. 1989;8:2639–2651. doi: 10.1002/j.1460-2075.1989.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalter S S, Herberling R L, Ratner J J. EBV antibody in sera of non-human primates. Nature. 1972;238:353–354. doi: 10.1038/238353a0. [DOI] [PubMed] [Google Scholar]
- 24.Kempkes B, Pich D, Zeidler R, Sugden B, Hammerschmidt W. Immortalization of human B lymphocytes by a plasmid containing 71 kilobase pairs of Epstein-Barr virus DNA. J Virol. 1995;69:231–238. doi: 10.1128/jvi.69.1.231-238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna R, Burrows S R, Kurilla M G, Jacob C A, Misko I S, Sculley T B, Kieff E, Moss D J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992;176:160–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, et al., editors. Fields virology. 3rd ed. Vol. 2. New York, N.Y: Raven Press; 1996. pp. 2343–2396. [Google Scholar]
- 27.Kim O J, Yates J L. Mutants of Epstein-Barr virus with a selective marker disrupting the TP gene transform B cells and replicate normally in culture. J Virol. 1993;67:7634–7640. doi: 10.1128/jvi.67.12.7634-7640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lear A L, Rowe M, Kurilla M G, Lee S, Henderson S, Kieff E, Rickinson A B. The Epstein-Barr virus (EBV) nuclear antigen 1 BamHI F promoter is activated on entry of EBV-transformed B cells into the lytic cycle. J Virol. 1992;66:7461–7468. doi: 10.1128/jvi.66.12.7461-7468.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y S, Tanaka A, Lau R Y, Nonoyama M, Rabin H. Comparative studies of herpesvirus papio (baboon herpesvirus) DNA and Epstein-Barr virus DNA. J Gen Virol. 1980;51:245–253. doi: 10.1099/0022-1317-51-2-245. [DOI] [PubMed] [Google Scholar]
- 30.Levine P H, Leiseca S A, Hewetson J F, Traul K A, Andrese A P, Granlund D J, Fabrizio P, Stevens D A. Infection of rhesus monkeys and chimpanzees with Epstein-Barr virus. Arch Virol. 1980;66:341–351. doi: 10.1007/BF01320630. [DOI] [PubMed] [Google Scholar]
- 31.Ling P D, Hayward S D. Contribution of conserved amino acids in mediating the interaction between EBNA2 and CBF1/RBPJk. J Virol. 1995;69:1944–1950. doi: 10.1128/jvi.69.3.1944-1950.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling P D, Ryon J J, Hayward S D. EBNA-2 of herpesvirus papio diverges significantly from the type A and type B EBNA-2 proteins of Epstein-Barr virus but retains an efficient transactivation domain with a conserved hydrophobic motif. J Virol. 1993;67:2990–3033. doi: 10.1128/jvi.67.6.2990-3003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loeb D D, Sung N S, Pesano R L, Sexton C J, Hutchison III C, Pagano J S. Plasmid origin of replication of herpesvirus papio: DNA sequence and enhancer function. J Virol. 1990;64:2876–2883. doi: 10.1128/jvi.64.6.2876-2883.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longnecker R, Miller C, Miao X-Q, Marchini A, Kieff E. The only domain which distinguishes Epstein-Barr virus latent membrane protein 2A (LMP2A) from LMP2B is dispensable for lymphocyte infection and growth transformation in vitro. J Virol. 1992;66:6461–6469. doi: 10.1128/jvi.66.11.6461-6469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longnecker R, Miller C L, Miao X-Q, Tomkinson B, Kieff E. The last seven transmembrane and carboxy-terminal cytoplasmic domains of Epstein-Barr virus latent membrane protein 2 are dispensable for lymphocyte infection and growth transformation in vitro. J Virol. 1993;67:2006–2013. doi: 10.1128/jvi.67.4.2006-2013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masucci M G, Ernberg I. Epstein-Barr virus: adaptation to a life within the immune system. Trends Microbiol. 1994;2:125–130. doi: 10.1016/0966-842x(94)90599-1. [DOI] [PubMed] [Google Scholar]
- 37.Miyashita E M, Yang B, Babcock G J, Thorley-Lawson D A. Identification of the site of Epstein-Barr virus persistence in vivo as a resting B cell. J Virol. 1997;71:4882–4891. doi: 10.1128/jvi.71.7.4882-4891.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyashita E M, Yang B, Lam K M C, Crawford D H, Thorley-Lawson D A. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell. 1995;80:593–601. doi: 10.1016/0092-8674(95)90513-8. [DOI] [PubMed] [Google Scholar]
- 39.Moghaddam A, Koch J, Annis B, Wang F. Infection of human B lymphocytes with lymphocryptoviruses related to Epstein-Barr virus. J Virol. 1998;72:3205–3212. doi: 10.1128/jvi.72.4.3205-3212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moghaddam A, Rosenzweig M, Lee-Parritz D, Annis B, Johnson R P, Wang F. An animal model for acute and persistent Epstein-Barr virus infection. Science. 1997;276:2030–2033. doi: 10.1126/science.276.5321.2030. [DOI] [PubMed] [Google Scholar]
- 41.Murray R, Kurilla M, Brooks J, Thomas W, Rowe M, Kieff E, Rickinson A. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV positive malignancies. J Exp Med. 1992;176:157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nonkwelo C, Henson E B D, Sample J. Characterization of the Epstein-Barr virus Fp promoter. Virology. 1995;206:183–195. doi: 10.1016/s0042-6822(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 43.Nonkwelo C, Ruf I K, Sample J. Interferon-independent and -induced regulation of Epstein-Barr virus EBNA-1 gene transcription in Burkitt lymphoma. J Virol. 1997;71:6887–6897. doi: 10.1128/jvi.71.9.6887-6897.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nonkwelo C, Ruf I K, Sample J. The Epstein-Barr virus EBNA-1 promoter Qp requires an initiator-like element. J Virol. 1997;71:354–361. doi: 10.1128/jvi.71.1.354-361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nonkwelo C, Skinner J, Bell A, Rickinson A, Sample J. Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol. 1996;70:623–627. doi: 10.1128/jvi.70.1.623-627.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu L, Rowe D T. Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol. 1992;66:3715–3724. doi: 10.1128/jvi.66.6.3715-3724.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rabin H, Neubauer R H, Hopkins R D, Dzhikidze E K, Shevtsova Z V, Lapin B A. Transforming activity and antigenicity of an Epstein-Barr-like virus from lymphoblastoid cell lines of baboons with lymphoid disease. Intervirology. 1977;8:240–249. doi: 10.1159/000148899. [DOI] [PubMed] [Google Scholar]
- 48.Rangan S R, Martin L N, Bozelka B E, Wang N, Gormus B J. Epstein-Barr virus-related herpesvirus from a rhesus monkey (Macaca mulatta) with malignant lymphoma. Int J Cancer. 1986;38:425–432. doi: 10.1002/ijc.2910380319. [DOI] [PubMed] [Google Scholar]
- 49.Redemann B E, Mendelson E, Carter B J. Adeno-associated virus Rep protein synthesis during productive infection. J Virol. 1989;63:873–882. doi: 10.1128/jvi.63.2.873-882.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rickinson A B, Kieff E. Epstein-Barr virus. In: Fields B N, et al., editors. Fields virology. 3rd ed. Vol. 2. New York, N.Y: Raven Press; 1996. pp. 2397–2446. [Google Scholar]
- 51.Robertson E S, Tomkinson B, Kieff E. An Epstein-Barr virus with a 58-kilobase-pair deletion that includes BARF0 transforms B lymphocytes in vitro. J Virol. 1994;68:1449–1458. doi: 10.1128/jvi.68.3.1449-1458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rooney C, Rowe M, Wallace L, Rickinson A. Epstein-Barr virus-positive Burkitt’s lymphoma cells not recognized by virus-specific T-cell surveillance. Nature. 1985;317:629–631. doi: 10.1038/317629a0. [DOI] [PubMed] [Google Scholar]
- 53.Rowe D T, Rowe M, Evan G I, Wallace L E, Farrell P J, Rickinson A B. Restricted expression of EBV latent genes and T-lymphocyte-detected membrane antigen in Burkitt’s lymphoma cells. EMBO J. 1986;5:2599–2607. doi: 10.1002/j.1460-2075.1986.tb04540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryon J J, Fixman E D, Houchens C, Zong J, Lieberman P M, Chang Y-N, Hayward G S, Hayward S D. The lytic origin of herpesvirus papio is highly homologous to Epstein-Barr virus ori-Lyt: evolutionary conservation of transcriptional activation and replication signals. J Virol. 1993;67:4006–4016. doi: 10.1128/jvi.67.7.4006-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 56.Sample J, Brooks L, Sample C, Young L, Rowe M, Gregory C, Rickinson A, Kieff E. Restricted Epstein-Barr virus protein expression in Burkitt lymphoma is due to a different Epstein-Barr nuclear antigen 1 transcriptional initiation site. Proc Natl Acad Sci USA. 1991;88:6343–6347. doi: 10.1073/pnas.88.14.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sample J, Henson E B D, Sample C. The Epstein-Barr virus nuclear protein 1 promoter active in type I latency is autoregulated. J Virol. 1992;66:4654–4661. doi: 10.1128/jvi.66.8.4654-4661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sample J, Hummel M, Braun D, Birkenbach M, Kieff E. Nucleotide sequences of messenger RNAs encoding Epstein-Barr virus nuclear proteins: a probable transcriptional initiation site. Proc Natl Acad Sci USA. 1986;83:5096–5100. doi: 10.1073/pnas.83.14.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sample J, Kieff E. Transcription of the Epstein-Barr virus genome during latency in growth-transformed lymphocytes. J Virol. 1990;64:1667–1674. doi: 10.1128/jvi.64.4.1667-1674.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaefer B C, Paulson E, Strominger J L, Speck S H. Constitutive activation of Epstein-Barr virus (EBV) nuclear antigen 1 gene transcription by IRF1 and IRF2 during restricted EBV latency. Mol Cell Biol. 1997;17:873–886. doi: 10.1128/mcb.17.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schaefer B C, Strominger J L, Speck S H. Host-cell-determined methylation of specific Epstein-Barr virus promoters regulates the choice between distinct viral latency programs. Mol Cell Biol. 1997;17:364–377. doi: 10.1128/mcb.17.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaefer B C, Strominger J L, Speck S H. The Epstein-Barr virus BamHI F promoter is an early lytic promoter: lack of correlation with EBNA 1 gene transcription in group 1 Burkitt’s lymphoma cell lines. J Virol. 1995;69:5039–5047. doi: 10.1128/jvi.69.8.5039-5047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaefer B C, Strominger J L, Speck S H. Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci USA. 1995;92:10565–10569. doi: 10.1073/pnas.92.23.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Selden R F, Howie K B, Rowe M E, Goodman H M, Moore D D. Human growth hormone as a reporter gene in regulation studies employing transient gene expression. Mol Cell Biol. 1986;6:3173–3179. doi: 10.1128/mcb.6.9.3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shope T, Dechairo D, Miller G. Malignant lymphoma in cottontop marmosets after inoculation with Epstein-Barr virus. Proc Natl Acad Sci USA. 1973;70:2487–2491. doi: 10.1073/pnas.70.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smale S T. Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes. Biochim Biophys Acta. 1997;1351:73–88. doi: 10.1016/s0167-4781(96)00206-0. [DOI] [PubMed] [Google Scholar]
- 67.Smith P, Griffin B. Differential expression of Epstein-Barr viral transcripts for two proteins (TP1 and LMP) in lymphocyte and epithelial cells. Nucleic Acids Res. 1991;19:2435–2440. doi: 10.1093/nar/19.9.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sung N S, Wilson J, Davenport M, Sista N D, Pagano J S. Reciprocal regulation of the Epstein-Barr virus BamHI-F promoter by EBNA-1 and an E2F transcription factor. Mol Cell Biol. 1994;14:7144–7152. doi: 10.1128/mcb.14.11.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBERS) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka N, Ishihara M, Kitagawa M, Harada H, Kimura T, Matsuyama T, Lamphier M S, Aizawa S, Mak T W, Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994;77:829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 71.Thorley-Lawson D A, Miyashita E M, Khan G. Epstein-Barr virus and the B cell: that’s all it takes. Trends Microbiol. 1996;4:204–208. doi: 10.1016/s0966-842x(96)90020-7. [DOI] [PubMed] [Google Scholar]
- 72.Tierney R J, Steven N, Young L S, Rickinson A B. Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol. 1994;68:7374–7385. doi: 10.1128/jvi.68.11.7374-7385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Woisetschlaeger M, Yandava C N, Furmanski L A, Strominger J L, Speck S H. Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci USA. 1990;87:1725–1729. doi: 10.1073/pnas.87.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yates J L, Camiolo S M, Ali S, Ying A. Comparison of the EBNA-1 proteins of Epstein-Barr virus and herpesvirus papio in sequence and function. Virology. 1996;222:1–13. doi: 10.1006/viro.1996.0392. [DOI] [PubMed] [Google Scholar]
- 75.Young L S, Dawson C W, Clark D, Rupani H, Busson P, Tursz T, Johnson A, Rickinson A B. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988;69:1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L, Pagano J S. IRF-7, a new interferon regulatory factor associated with Epstein-Barr virus latency. Mol Cell Biol. 1997;17:5748–5757. doi: 10.1128/mcb.17.10.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]