Abstract
Background
Late-relapsing hepatitis after yellow fever (LHep-YF) during the convalescent phase of the disease has been described during recent yellow fever (YF) outbreaks in Brazil. LHep-YF is marked by a rebound in liver enzymes and nonspecific clinical manifestations around 46–60 days after YF symptom onset.
Methods
Here we have characterized the clinical course and risk factors for LHep-YF using data from a representative cohort of patients who survived YF in Brazil, 2017–2018. A total of 221 YF-positive patients were discharged from the infectious disease reference hospital in Minas Gerais and were followed up at 30, 45, and 60 days post–symptom onset.
Results
From 46 to 60 days post–symptom onset, 16% of YF patients (n = 36/221) exhibited a rebound of aminotransferases (aspartate aminotransferase or alanine aminotransferase >500 IU/L), alkaline phosphatase, and total bilirubin levels. Other etiologies of liver inflammation such as infectious hepatitis, autoimmune hepatitis, and metabolic liver disease were ruled out. Jaundice, fatigue, headache, and low platelet levels were associated with LHep-YF. Demographic factors, clinical manifestations, laboratory tests, ultrasound findings, and viral load during the acute phase of YF were not associated with the occurrence of LHep-YF.
Conclusions
These findings provide new data on the clinical course of Late-relapsing hepatitis during the convalescent phase of YF and highlight the need for extended patient follow-up after acute YF.
Keywords: yellow fever, hepatitis, convalescent phase, relapsing hepatitis, viral persistence
Late-relapsing hepatitis after yellow fever is a clinical picture described around 60 days after the acute yellow fever phase, presenting with nonspecific symptoms, such as fatigue and rebound in aminotransferases, alkaline phosphatase, and total bilirubin values, with a benign final outcome.
Yellow fever (YF) is a viscerotropic disease caused by the yellow fever virus (YFV) (family: Flaviviridae; genus: Flavivirus). The disease is classically divided into 3 periods: (1) infection, marked by flu-like symptoms and viremia; (2) remission, characterized by clinical improvement and seroconversion; and (3) intoxication, which affects 15%–25% of symptomatic patients, marked by clinical deterioration and onset of hemorrhage, multiorgan dysfunction, jaundice, oliguria, anuria, renal and liver failure, and cardiovascular instability [1]. Although YF has been described for centuries in the Americas, previous outbreaks happened in remote areas with a low number of cases, making it difficult to study the disease. The magnitude of the outbreak in Brazil in 2016–2018 was unprecedented, allowing sufficient case numbers in referral hospitals to be studied in detail [1, 2].
Following the infection or intoxication phases, a subsequent convalescent period is observed when persistent fatigue and moderately elevated aminotransferase levels may be observed [1, 2]. The convalescent phase of YF may last several weeks with slightly abnormal liver function that persists for 60 days or more, together with jaundice or fatigue [1, 3, 4]. During the YF outbreaks in 2017–2018 in Brazil, studies have demonstrated that some patients with YF developed Late-relapsing hepatitis after yellow fever (LHep-YF) [5–7]. Patients with LHep-YF had a rebound in liver enzymes (aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) within 6 months after a previous improvement or normalization of liver function. Given its clinical significance as a potential driver of hospital readmission following acute YF, we aim to characterize the clinical course and risk factors for LHep-YF. Additional risk factors, such as background flavivirus infection (ie, Zika and dengue immunoglobulin M [IgM] and immunoglobulin G [IgG] titers), use of antiviral drugs (sofosbuvir), YFV viral load and viremia, and anti-YFV antibodies neutralization levels, will be presented in subsequent reports.
Brazil experienced a significant YF outbreak from 2016 to 2018, with 2166 confirmed cases and 751 deaths; 45% of cases and deaths were reported in Minas Gerais (MG) state, southeast Brazil [8, 9]. In MG, the Hospital Eduardo de Menezes (HEM) was the primary referral hospital for YF patients in Belo Horizonte, MG, treating approximately 30% of the total cases in the state.
Using data from patients hospitalized at HEM in 2017 and 2018, we identified a cohort of patients meeting the LHep-YF criteria. We then conducted a retrospective study of clinical and laboratory test characteristics of this cohort, using patients with nonfatal YF who did not develop LHep-YF as a control group, to investigate risk factors for the development of LHep-YF and to better characterize the syndrome.
METHODS
Study Design and Data Source
This study was conducted in a retrospective cohort, based on the review of medical records from hospitalized and ambulatory outpatient visits in a referral center for infectious diseases (HEM) in Belo Horizonte, MG, during the 2017–2018 YF outbreaks in Brazil. According to the Secretary of Health of MG guidelines, the criteria to be referred and hospitalized were to present a moderate or severe YF clinical picture, presenting laboratory values of AST >2000 IU/L, international normalized ratio (INR) >1.5, creatinine >2 mg/dL, or platelet count <50 000/µL. Signs and symptoms were also analyzed, such as vomiting, diarrhea, and abdominal pain for moderate cases; and jaundice, oliguria, altered mental status, bleeding, hypotension, difficulty in breathing, and convulsion for severe cases. Patients were referred to HEM and hospitalized there after this initial screening, and a YFV polymerase chain reaction (PCR) test was ordered for each patient. In cases when the patient presented at the hospital with >6 days of symptoms, an enzyme-linked immunosorbent assay test was also ordered, together with a PCR test [10].
Study Population
Two hundred twenty-one patients with laboratory-confirmed YF who were admitted to HEM between January 2017 and June 2018 and discharged. YF diagnosis was performed by detecting IgM anti-YFV (in addition to negative results for IgM against dengue and Zika viruses), YFV RNA by reverse-transcription quantitative polymerase chain reaction (RT-qPCR), or YFV isolation using serum samples. Any patient that presented at least 1 positive result in any of the laboratory tests cited above was included in the study.
LHep-YF Case Definition
LHep-YF was defined as an increase in either aminotransferase level (AST or ALT) to values >500 IU/L and an increase above the normal range for alkaline phosphatase (ALP) (>120 IU/L) and total bilirubin (TBil) (>1.0 mg/dL), 46–60 days after YF symptom onset, following a normalization or 5-fold reduction in either aminotransferase, ALP, or TBil level from the peak observed during acute YF.
YF Patient Follow-up
Following hospital discharge, YF patients were initially advised to return at 30, 45, and 60 days post–symptom onset (dps) for ambulatory follow-up, but in some cases the follow-up period was extended. During follow-up, clinical examination, complete blood cell count, and liver and renal function tests were performed. Serum samples were collected during acute disease hospitalization and ambulatory follow-up and kept at −70°C. The samples were further used for qualitative and quantitative RT-qPCR, for the investigation of YFV RNA, as described below.
YFV RNA Detection and Quantitation
Serum samples collected during the hospitalization period and the follow-up were used for a qualitative RT-qPCR using YFV-specific primers and probes [11]. In brief, total RNA was extracted from 140 µL of serum samples using the QIAmp Viral RNA Mini Kit (Qiagen). Total RNA (5 µL) was used in RT-qPCR targeting the 5′UTR region of the YFV genome [11]. Positive samples were then used for quantitative RT-qPCR, using the Bio Gene Research Yellow Fever PCR kit (Bioclin, Brazil), to determine the YFV RNA genomic viral load. The genomic viral load was expressed as log-transformed genomic copies (GC)/mL. The RNA quantification kit detects at least 20 GC/mL of viral RNA. For RNA quantification, the highest point for the standard curve (provided by the kit) was 2 × 105 GC/mL and diluted up to 2 × 101 GC/mL.
Statistical Analysis
Data were maintained in the Stanford University Research Electronic Data Capture (REDCap) platform. We compared patients with LHep-YF (n = 36) and without LHep-YF (n = up to 185: patients with nonfatal YF attended at HEM, depending on the availability of data for each patient) based on demographics, clinical manifestations, laboratory tests, and ultrasound findings (the full description of variables analyzed is shown in Supplementary Material 1). Analyses were run on R software, version 1.3. For each variable, the relative risk was estimated using the Wald test (with the R package “epitools”). For categorical variables (demographic factors, symptoms and signs, and ultrasound findings), the proportion of LHep-YF and non-LHep-YF patients with a given risk factor were compared using Fisher exact test with Bonferroni correction for multiple comparisons. For continuous variables (initial [admission day, corresponding to 2–5 dps]; maximum and minimum values of inpatient examinations; and postdischarge values at 30, 45, 60, 90, 120, and >150 dps), receiver operating characteristic curves were estimated, and optimal cutoff values by maximizing the sum of sensitivity and specificity were selected (using the R package “cutpointr”). The relative risk associated with dichotomized variables was estimated using the Wald test. LHep-YF and non-LHep-YF patients were compared using the Wilcoxon signed-rank test with Bonferroni correction for multiple comparisons. Viral loads were log-transformed and compared using Student t test. P values < .05 were considered significant.
Ethical Considerations
This study was approved by the Ethics Committee at Instituto René Rachou (FIOCRUZ-MG) and Fundação de Hospitais do Estado de Minas Gerais (protocols Certificado de Apresentação de Apreciação Ética (CAAE) 72569317.2.0000.5091 and CAAE 65910317.0000.5071) and by the institutional review board at Stanford University School of Medicine (eProtocol 53676). No informed consent of study participants was pursued due to the nature of the de-identified data after institutional review board authorization. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
RESULTS
Of the 289 total YF-positive patients attended at HEM during the 2017–2018 YF outbreak, 221 patients survived and were discharged from the hospital. For the first 2 follow-up visits (30 and 45 dps), patients presented with normal or slightly elevated liver function markers (AST and ALT, TBil, and ALP) and did not report any signs or symptoms since hospital discharge.
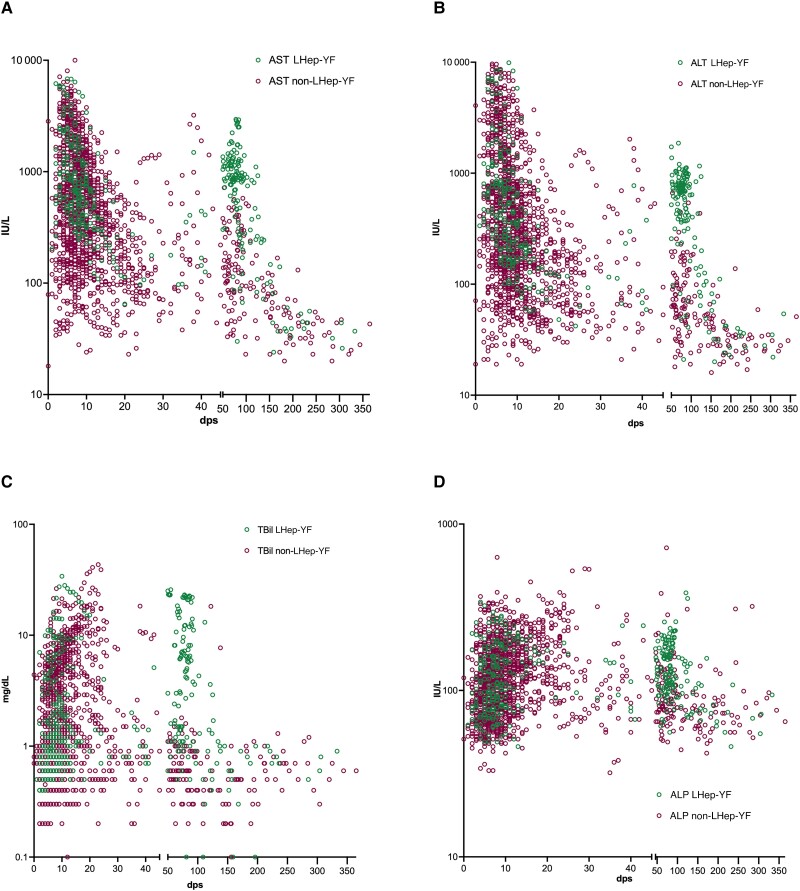
However, from 46 to 60 dps follow-up, some patients returned to HEM exhibiting a rebound of aminotransferases (>500 IU/L), ALP, and TBil levels whereas other patients presented normal or decreasing levels of those markers. According to the definition of LHep-YF, 36 of 221 (16%) patients who survived YF (12% of total YF cases at HEM [n = 36/289]) developed LHep-YF (Table 1, Figure 1). Our cohort consisted of 36 LHep-YF patients, including a previous case report described by our group [5].
Table 1.
Characteristics of 36 Patients With Late-Relapsing Hepatitis After Yellow Fever
| Characteristic | Non-LHep-YF (n = 185) | LHep-YF (n = 36) | Non-LHep-YF dpsa |
LHep-YF dpsa |
|
|---|---|---|---|---|---|
| Male sex, No. | 161 | 26 | NA | NA | |
| Female sex, No. | 24 | 10 | NA | NA | |
| Age, y | 46 (36–56) | 41.5 (36.0–52.0) | NA | NA | |
| dps that patients presented at HEM, YF acute phase | 4 (2.75–5.25) | 4 (3.0–6.0) | NA | NA | |
| Length of hospitalization (d), YF acute phase | 7 (5–10) | 6 (5–8) | NA | NA | |
| AST peakb | 74.5 (50.2–135) | 633 (389–825) | 38.5 (28–51.2) | 68 (62, 76) | |
| ALT peakb | 145 (81.3–270) | 1128 (684–1481) | 39.5 (31–47.5) | 70 (62, 78.5) | |
| TBil peakb | 0.9 (0.6–1.1) | 1.7 (1.1–4.6) | 42 (26.5–74) | 68.5 (53.8, 79) | |
| ALP peakb | 93 (72.8–132) | 158 (131–221.5) | 37 (23–59) | 68.5 (44.8, 83) | |
| Compassionate use of antiviral sofosbuvir, No. (%) | 21 (9.5) | 6 (16.6) | NA | NA | |
| YF 17DD vaccination <15 d before symptom onset, No. (%) | 54 (24.5) | 13 (36.1) | NA | NA | |
| YF 17DD vaccination at least 1 y before symptom onset, No. (%) | 50 (25) | 3 (8.3) | NA | NA |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; dps, days post–onset of symptoms; HEM, Hospital Eduardo de Menezes; LHep-FY, Late-relapsing hepatitis after yellow fever; NA, not applicable; TBil, total bilirubin; YF, yellow fever.
Median days of symptoms after YF onset, at which the highest value was measured.
Normal values: AST and ALT, 10–40 IU/L; TBil, 0.3–1.0 mg/dL; ALP, 30–120 IU/L [12].
Figure 1.
Liver function marker values (A. Aspartate transaminase; B. Alanine transaminase; C. TBil; D. ALP) during hospitalization and follow-up of patients with yellow fever who were admitted to Hospital Eduardo de Menezes and survived (Late-relapsing hepatitis after yellow fever [LHep-YF], n = 36; non-LHep-YF, n = 185). Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; dps, days post–symptom onset; LHep-YF, Late-relapsing hepatitis after yellow fever; TBil, total bilirubin.
The LHep-YF group showed general clinical worsening, including reports of fatigue, headache, myalgia, and arthralgia during the syndrome. Less common symptoms included abdominal pain, postprandial fullness, dyspnea, and rash (Table 2). Nine LHep-YF patients presented with jaundice during the follow-up, including 3 patients who had developed jaundice during the YF acute phase and a recurrence of jaundice during LHep-YF. Six patients with LHep-YF presented with jaundice during the YF acute phase only. Eleven patients were readmitted during the LHep-YF phase (Supplementary Table 1); they received supportive treatment and were discharged after general clinical improvement.
Table 2.
Status of Patients With Late-Relapsing Hepatitis After Yellow Fever, Reported at Any Timepoint of Yellow Fever Follow-up
| Patient Status | LHep-YF (n = 36) | Non-LHep-YF (n = 185) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Myalgia | 33 | 91.7 | 32 | 17.4 |
| Arthralgia | 33 | 91.7 | 12 | 6.5 |
| Weakness | 22 | 61.1 | 25 | 13.6 |
| Jaundice | 9 | 25 | 15 | 8.2 |
| Headache | 9 | 25 | 32 | 17.4 |
| Abdominal pain | 6 | 16.7 | 14 | 7.6 |
| Vomiting | 4 | 11.1 | 1 | 0.5 |
| Dyspnea | 3 | 8.3 | 17 | 9.2 |
| Fever | 2 | 5.6 | 4 | 2.2 |
| Postprandial fullness | 2 | 5.6 | 1 | 0.5 |
| Cough | 2 | 5.6 | 4 | 2.2 |
| Rash | 2 | 5.6 | 1 | 0.5 |
| Confusion | 1 | 2.8 | 1 | 0.5 |
| Hematuria | 1 | 2.8 | 0 | 0.0 |
| Diarrhea | 1 | 2.8 | 2 | 1.1 |
Abbreviation: LHep-YF, Late-relapsing hepatitis after yellow fever.
Serum samples collected during follow-up were tested by RT-qPCR and all were negative for the presence of YFV RNA, indicating no YFV viremia/RNAemia during the LHep-YF (Supplementary Table 2). Other causes of hepatitis were ruled out by the following testing: IgM and IgG anti–hepatitis C virus, anti–hepatitis A virus, anti–hepatitis E virus, anti–Epstein-Barr virus, anti–herpes simplex virus type 1, anti-cytomegalovirus, and anti-toxoplasma; anti–hepatitis B core (HBc); total anti-HBc IgM; hepatitis B surface antigen; anti–human immunodeficiency virus types 1 and 2 IgM; PCR against hepatitis B virus and hepatitis C virus; and RT-qPCR against dengue, Zika, and chikungunya viruses. Laboratory tests for autoimmune hepatitis, including anti–smooth muscle, anti-mitochondrial, anti–nuclear factor HEP2, and anti–neutrophil cytoplasm antibodies (P-ANCA and C-ANCA), were also negative.
Medical records were searched to identify the use of hepatotoxic herbals, drugs, and dietary supplements that could interfere with hepatic function [13]. Among the few patients who reported the use of any medication, the majority used antihypertensives such as angiotensin-converting enzyme inhibitors or angiotensin receptor blockers that are not related to an increase in aminotransferases [13]. The patients denied the continuous use of contraceptives, anticonvulsants, and other hepatotoxic drugs (eg, acetaminophen [paracetamol]).
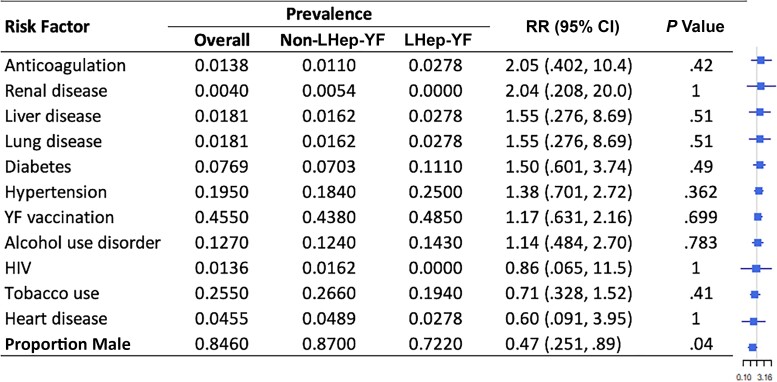
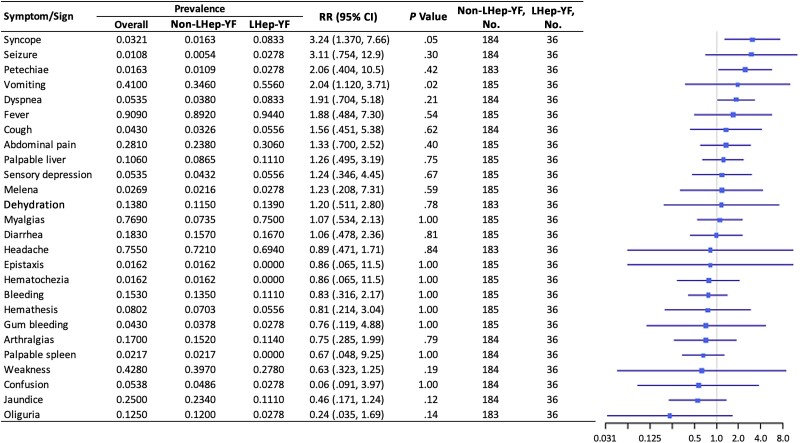
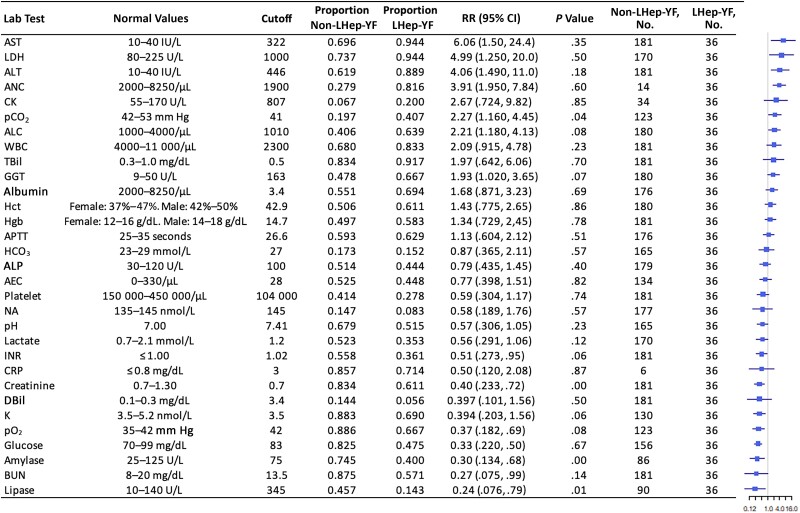
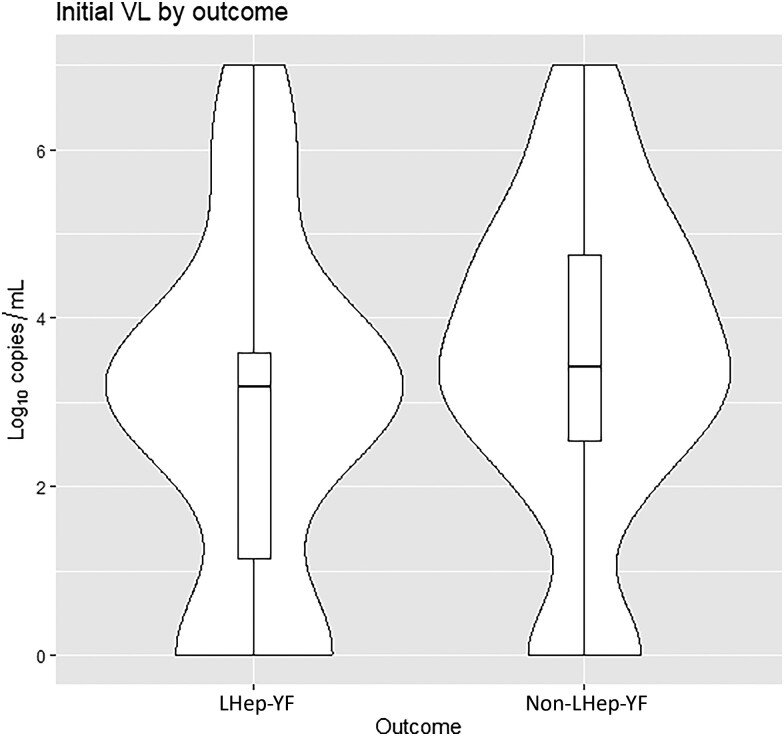
Patients in both cohorts (LHep-YF and non-LHep) presented at the hospital at a median of 4 days after symptom onset (interquartile range [IQR], 2.75–5.25 and 3.0–6.0 respectively) (Table 1). There was no significant difference in median age between LHep-YF (median 41.5 years [IQR 36.0, 52.0], age range: 15–79 years old) and non-LHep-YF patients (46 years, [IQR 36.0, 56.0]; P = .379, age range: 14–65 years old). No difference was observed among underlying medical conditions present before YF diagnosis and development of LHep-YF (Figure 2). There was no significant relationship to symptoms and signs present at the time of initial admission to HEM that could predict subsequent LHep-YF (Figure 3). There is no significant difference between initial Model for End-Stage Liver Disease 3.0 scores [14] for LHep-YF (median 8.5, [IQR 7.25–10.5]) and non-LHep-YF (8.5, [IQR 7.0–14.0]) patients. No initial, minimum, or maximum inpatient laboratory test values during hospitalization at YF acute phase were significantly related to the development of LHep-YF (Figure 4). There was no significant relationship between reported ultrasonographic findings (Supplementary Material 1) and a diagnosis of LHep-YF. There was no significant difference in initial or maximum inpatient genomic viral loads between LHep-YF and non-LHep-YF cases during the YF acute phase (P = .278) (Figure 5).
Figure 2.
Risk factors for Late-relapsing hepatitis after yellow fever (LHep-YF) and non-LHep-YF cases. From left to right, columns list the risk factor of interest (ranked by the magnitude of the relative risk), its overall prevalence, prevalence among non-LHep-YF cases, prevalence among LHep-YF cases, relative risk (RR) with 95% confidence intervals, and a forest plot illustrating the RR (node) and intervals (whiskers) relative to a risk of 1 (vertical line), where nodes to the right of the line indicate increased risk of LHep-YF outcome. (Patients analyzed in the non-LHep-YF cohort: n = 185.) The proportion of males refers to the proportion of LHep-YF or non-LHep-YF patients who were male, not the proportion of male patients with LHep-YF. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; LHep-YF, Late-relapsing hepatitis after yellow fever; RR, relative risk; YF, yellow fever.
Figure 3.
Initial symptoms and signs of Late-relapsing hepatitis after yellow fever (LHep-YF) and non-LHep-YF cases at admission. From left to right, columns list the risk factor of interest (ranked by the magnitude of the relative risk [RR]), its overall prevalence, prevalence among non-LHep-YF cases, prevalence among LHep-YF cases, RR with 95% confidence interval (CI), and a forest plot illustrating the RR (node) and intervals (whiskers) relative to a risk of 1 (vertical line), where nodes to the right of the line indicate increased risk of LHep-YF outcome.
Figure 4.
Initial laboratory test values of Late-relapsing hepatitis after yellow fever (LHep-YF) and non-LHep-YF patients. From left to right, columns list the risk factor of interest (ranked by the magnitude of the relative risk [RR]), normal range values, the cutoff value selected for dichotomization, the proportion of non-LHep-YF cases above the cutoff, the proportion of LHep-YF cases above the cutoff, RR with 95% confidence interval, the P value comparing the 2 populations by Wilcoxon ranked-sign test, the number of patients analyzed in the non-LHep-YF group, and a forest plot illustrating the RR (node) and intervals (whiskers) relative to a risk of 1 (vertical line), where nodes to the right of the line indicate that a value above the cutoff is associated with an LHep-YF outcome. Normal values are presented according to the American Board of Internal Medicine laboratory test reference ranges (January 2022) [12]. Abbreviations: AEC, absolute eosinophil count; ALC, absolute lymphocyte count; ALT, alanine aminotransferase; ALP, alkaline phosphatase; ANC, absolute neutrophil count; APTT, activated partial thromboplastin clotting time; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CI, confidence interval; CK, creatine kinase; CRP, C-reactive protein; DBil, direct bilirubin; GGT, γ-glutamyl transferase; HCO3, Bicarbonate; Hct, hematocrit; Hgb, hemoglobin; INR, international normalized ratio; K, potassium; LDH, lactate dehydrogenase; LHep-YF, Late-relapsing hepatitis after yellow fever; NA, sodium; pCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; RR, relative risk; TBil, total bilirubin; WBC, white blood cell count.
Figure 5.
Initial log-transformed viral load (VL) by outcome shown as violin plot and superimposed boxplot, with a marker indicating median value, hinges indicating quartiles, and whiskers indicating ranges. Mean values for Late-relapsing hepatitis after yellow fever (LHep-YF) and non-LHep-YF cases were 2.86 and 3.42 log10 copies, respectively (P = .278).
Comparing clinical manifestations between patients with LHep-YF (n = 36) and without (n = 185), a significant association with LHep-YF was seen with emesis at >150 dps (P = .0393) jaundice at 30 or 60 dps (P = .0101 and P = .0293, respectively), fatigue at 60 or 150 dps (P = .0139 and P = .0162, respectively), and myalgias at 60 dps (P = .0278), though no results were significant after Bonferroni correction. For laboratory tests, other than liver function tests (AST, ALT, ALP, TBil), significant associations with LHep-YF by Fisher exact test were seen with platelets <245 000/µL or blood urea nitrogen >27 mg/dL at 30 dps (P = .00167 and P = .00228, respectively) and INR >1.08 at 60 dps, though again no results were significant after Bonferroni correction (Figure 4).
DISCUSSION
Late-relapsing hepatitis after YF is a recently described complication of YF defined by recurrent transaminitis following a period of clinical and laboratory test value remission of acute hepatitis secondary to YF [5–7]. This study describes the largest clinical cohort of patients with LHep-YF and demonstrates that this relapsing hepatitis was accompanied by nonspecific clinical manifestations similar to those presenting during the infection and intoxication phases of YF. Symptoms and signs included myalgia, arthralgia, fatigue, and in some cases, jaundice with notable decreases in platelet count, occasionally requiring readmission. All LHep-YF patients survived and recovered clinically with normalization of liver laboratory values (ALT, AST, ALP, and TBil), suggesting a benign prognosis.
Here we describe the occurrence of LHep-YF from 46 to 60 dps, with ALT and AST peaking at around 60 dps. Prior case series from the same YF outbreak have described similar clinical features in patients meeting aminotransferase criteria for LHep-YF, including accompanying fatigue and, in some cases, decreases in platelet counts [6, 7]. LHep-YF onset has previously been observed to occur between 25 and 128 days after onset of YF symptoms [6, 7], with a median interval between onset of acute YF and relapse ALT peak of 75 days in a prior case series of 26 Brazilian patients attended in a hospital in São Paulo state [6].
Due to the 2017–2018 YF outbreak, an intense YF vaccination campaign happened in MG during this time, with >7.1 million doses applied [15]. It is important to bring attention to the fact that around 45% of patients who were attended at HEM and included in this study received 1 dose of the YF vaccine during this mass vaccination campaign (Figure 2). Even though none of these cases were related to adverse events after YF vaccination [16], they still got infected, probably because the time between the YF vaccine and the YFV wild-type infection was too short for the development of an immune response due to the vaccination against YF [17].
A case report described by our group showed that in 1 LHep-YF patient, the levels of most chemokines, proinflammatory cytokines, regulatory cytokines, and growth factors remained elevated at 78 dps, suggesting that altered profiles of circulating inflammatory regulatory mediators could be associated with the LHep-YF outcome [5]. In the case series described by Casadio et al, liver biopsies in 9 patients stained positive for YF antigen and 1 patient had persistence of YFV RNA in serum during this syndrome [6]. While the pathogenesis of relapsing hepatitis is unclear, the presence of YFV RNA in in serum [6], urine, and liver biopsy [5, 18], and the presence of YF antigen in 10 liver biopsies [5, 6] collected during this syndrome, along with the absence of metabolic disorders and other markers of common hepatitis etiologies including autoimmune hepatitis, suggests that LHep-YF could be driven by either direct viral injury [5–7] or a persistent proinflammatory immune response. These data reinforce the potential role of RNA persistence in causing specific late complications, as well as in preventing complete recovery from acute viral infections (reviewed in [19]).
Along with the possible immune response link to LHep-YF occurrence, the 2017–2018 YF outbreak was caused by a new YFV lineage, containing 8 unique mutations in the genome: 1 in the capsid protein and 7 in nonstructural proteins (NS3 and NS5) [20]. These mutations culminated in 8 semi-conservative amino acid changes [20], which could be associated with an advantage for viral fitness and differences in YF pathogenesis. More studies are necessary to show if this YFV lineage difference would help to explain the LHep-YF syndrome.
Notably, hepatitis A has also been associated with a similar relapsing hepatitis syndrome in up to 10% of cases, typically after a remission period of 3 weeks or less [21]. The pathogenesis of this syndrome is also incompletely understood, although in a 14-person case series, hepatitis A virus RNA was detected in the serum of 3 of the patients [22] and has also been isolated from the stool of patients during relapse [23].
Our study has limitations. Due to missing data, it was not possible to analyze the same homogeneous control group for each statistical comparison. Another limitation was that data collection derived from a single hospital; however, it is noteworthy that HEM received about 30% of the total YF cases of MG, suggesting that it is likely a representative cohort. Nevertheless, some LHep-YF cases could not have met the first criteria for admission at HEM, and we are missing this data in our analysis. One more limitation is that we could only analyze patients who return for an ambulatory visit after discharge at Eduardo de Menezes Hospital. Considering that less symptomatic LHep-YF cases may not have come back or come back less frequently for the follow-up visit, we could have overestimated the severity of LHep-YF. Furthermore, other studies conducted with patients from São Paulo and Rio de Janeiro states have also described the same syndrome with similar findings, showing that this clinical syndrome occurred in different regions of Brazil during the same YF outbreak. Another limitation of our study is that we could not precise an exact day as a starting point for the development of LHep-YF. We also did not analyze the interference of sofosbuvir in our cohort. However, other studies have shown that the use of this antiviral did not appear to be associated with further alterations in levels of liver enzymes.
CONCLUSIONS
Here we describe the Late-relapsing hepatitis after YF clinical syndrome, associated with a rebound of AST, ALT, ALP, or TBil levels, 46–60 days after YF symptom onset. LHep-YF was not associated with any epidemiological, clinical, or laboratory factors analyzed in our study. Although LHep-YF seems to be a benign condition, our results show the importance of follow-up of patients with YF after hospital discharge.
Reliable prediction of the syndrome will require a deeper understanding of the disease's pathogenesis and related host immune response. Further studies to investigate the role of possible viral persistence, immune responses, and viral biological characteristics causing LHep-YF are needed to understand the pathogenesis of this syndrome. These results provide new data on the clinical course of YF and highlight the need for extended patient follow-up.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Izabela Mauricio de Rezende, Laboratory of Viruses, Microbiology Department, Federal University of Minas Gerais, Belo Horizonte, Brazil; Division of Infectious Diseases, Department of Pediatrics, Stanford University School of Medicine, California.
Max A McClure, Division of Infectious Diseases, Department of Pediatrics, Stanford University School of Medicine, California.
Leonardo S Pereira, Eduardo de Menezes Hospital, Belo Horizonte, Minas Gerais, Brazil.
Jordana R B Fradico, Integrated Group of Biomarkers Research, René Rachou Institute, Oswaldo Cruz Foundation/FIOCRUZ, Belo Horizonte, Minas Gerais, Brazil.
Adriana R C Cenachi, Eduardo de Menezes Hospital, Belo Horizonte, Minas Gerais, Brazil.
Alexandre S Moura, Eduardo de Menezes Hospital, Belo Horizonte, Minas Gerais, Brazil.
Luísa L de A Paladino, Eduardo de Menezes Hospital, Belo Horizonte, Minas Gerais, Brazil.
Maria Rita T Dutra, Eduardo de Menezes Hospital, Belo Horizonte, Minas Gerais, Brazil.
Pedro A Alves, Immunology of Viral Diseases, René Rachou Institute, Oswaldo Cruz Foundation/FIOCRUZ.
Marcelo A P Xavier, Immunology of Viral Diseases, René Rachou Institute, Oswaldo Cruz Foundation/FIOCRUZ.
Rodrigo F do C Said, Secretaria de Estado de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil.
Dario B Ramalho, Eduardo de Menezes Hospital, Belo Horizonte, Minas Gerais, Brazil.
Thaysa D P Gama, Eduardo de Menezes Hospital, Belo Horizonte, Minas Gerais, Brazil.
Olindo A Martins-Filho, Integrated Group of Biomarkers Research, René Rachou Institute, Oswaldo Cruz Foundation/FIOCRUZ, Belo Horizonte, Minas Gerais, Brazil.
Thomas P Monath, Crozet BioPharma LLC, Lexington, Massachusetts, USA.
Andréa Teixeira-Carvalho, Integrated Group of Biomarkers Research, René Rachou Institute, Oswaldo Cruz Foundation/FIOCRUZ, Belo Horizonte, Minas Gerais, Brazil.
Betânia P Drumond, Laboratory of Viruses, Microbiology Department, Federal University of Minas Gerais, Belo Horizonte, Brazil.
Angelle D LaBeaud, Division of Infectious Diseases, Department of Pediatrics, Stanford University School of Medicine, California.
for the Yellow Fever Collaborative Group:
Alexandre Maurício Castro Bragato, Argus Leão Araújo, Flávio Augusto de Almeida Faria, Indiara Penido, Letícia Menezes, Livia Frota Rabelo, Livia Pamplona, Lívia Fulgêncio da Cunha Melo, Lívia Soares Coelho Fonte Boa, Lívia Zignago Moreira dos Santos, Ludmila de Paula, Marcelle Cardoso Marçal, Natalia Soares Albuquerque, Rodrigo Macedo, and Tayrine Araújo
Notes
Yellow Fever Collaborative Group members. Alexandre Maurício Castro Bragato, Argus Leão Araújo, Flávio Augusto de Almeida Faria, Indiara Penido, Letícia Menezes, Livia Frota Rabelo, Livia Pamplona, Lívia Fulgêncio da Cunha Melo, Lívia Soares Coelho Fonte Boa, Lívia Zignago Moreira dos Santos, Ludmila de Paula, Marcelle Cardoso Marçal, Natalia Soares Albuquerque, Rodrigo Macedo, and Tayrine Araújo.
Acknowledgments . The authors thank their colleagues from Eduardo de Menezes Hospital, Laboratory of Viruses/Federal University of Minas Gerais, and the teams of Ezequiel Dias Foundation and Secretary of Health of MG, Brazil. This work was developed in the context of the research group Grupo de Estudos de Pesquisa e Resposta em Febre Amarela do Estado de Minas Gerais, and the authors thank all members of the group.
Disclaimer. The funders had no role in study design, data collection and analysis, preparation of the manuscript, or the decision to submit the manuscript for publication.
Financial support . This project was funded by the National Institutes of Health (NIH) (grant number R01 AI149614; principal investigators A. D. L. and O. A. M.-F.). P. A. A., B. P. D., A. T.-C., and O. A. M.-F. were supported by Secretaria de Estado de Saúde de Minas Gerais/Secretaria de Estado de Planejamento de Minas Gerais/Instituto René Rachou/Fundação Oswaldo Cruz. I. M. R., P. A. A., B. P. D., A. T.-C., O. A. M.-F., and A. D. L. were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)/NIH (grant number 404192-2019-0/R01 AI149614). P. A. A. was supported by Fundação de Amparo a Pesquisa do Estado de Minas Gerais (grant number APQ-01989-18). This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (grant numbers 88882.348380/2010-1 and 0001/2016). B. P. D., A. T.-C., and O. A. M.-F. are CNPq Research Fellows. A. R. C. C. reports support for this work from Hospital Eduardo de Menezes/Fundação de Hospitais do Estado de Minas Gerais. A. T.-C. also reports support for this work paid to institution from the US Collaborative Biomedical Research Program/CNPq/Ministério da Saúde/NIH. B. P. D. and I. M. R. report support for this work from Hospital Eduardo de Menezes (provision of study materials).
References
- 1. Monath TP. Yellow fever: an update. Lancet Infect Dis 2001; 1:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Quaresma JAS, Pagliari C, Medeiros DBA, Duarte MIS, Vasconcelos PFC. Immunity and immune response, pathology and pathologic changes: progress and challenges in the immunopathology of yellow fever. Rev Med Virol 2013; 23:305–18. [DOI] [PubMed] [Google Scholar]
- 3. Oudart JL, Rey M. Proteinuria, proteinaemia, and serum transaminase activity in 23 confirmed cases of yellow fever. Bull World Health Organ 1970; 42:95–102. [PMC free article] [PubMed] [Google Scholar]
- 4. Francis TI, Moore DL, Edington GM, Smith JA. A clinicopathological study of human yellow fever. Bull World Health Organ 1972; 46:659–67. [PMC free article] [PubMed] [Google Scholar]
- 5. Rezende IM, Pereira LS, Barbosa Fradico JR, et al. Late-relapsing hepatitis after yellow fever. Viruses 2020; 12:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casadio L, Nastri AC, Malta FM, et al. Late-onset relapsing hepatitis associated with yellow fever. New Engl J Med 2020; 382:2059–61. [DOI] [PubMed] [Google Scholar]
- 7. Denis B, Chirio D, Ponscarme D, et al. Hepatitis rebound after infection with yellow fever virus. Emerg Infect Dis 2019; 25:1248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sacchetto L, Drumond BP, Han BA, Nogueira ML, Vasilakis N. Re-emergence of yellow fever in the neotropics—quo vadis? Emerg Top Life Sci 2020; 4:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva NIO, Sacchetto L, de Rezende IM, et al. Recent sylvatic yellow fever virus transmission in Brazil: the news from an old disease. Virol J 2020; 17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brazilian Ministry of Health . Manual de manejo clínico da febre amarela. 1st ed. Brasilia: Brazilian Ministry of Health, 2020. [Google Scholar]
- 11. Domingo C, Patel P, Yillah J, et al. Advanced yellow fever virus genome detection in point-of-care facilities and reference laboratories. J Clin Microbiol 2012; 50:4054–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Board of Internal Medicine . Laboratory test reference ranges.2022:1–22. Available at:https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf. Accessed 2 October 2022.
- 13. Larson AM, Chopra S, Robson KM. Hepatotoxicity due to herbal medications and dietary supplements. Post TW, ed. Waltham, MA: UpToDate, 2021.
- 14. Kim WR, Mannalithara A, Heimbach JK, et al. MELD 3.0: the Model for End-Stage Liver Disease updated for the modern era. Gastroenterology 2021; 161:1887–95.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Secretaria de Estado de Saúde de Minas Gerais . Eventos Adversos Pós-Vacinação Associados à Vacina Febre Amarela (EAPV-VFA) Minas Gerais, 2016 a 2018.2018. Available at:http://www.saude.mg.gov.br/images/noticias_e_eventos/000_2018/BoletinsEpidemiologicos/Boletim_epidemiológico_EAPV_finalv3.pdf. Accessed 9 October 2019.
- 16. Rezende IM, Alves PA, Arruda MS, et al. Yellow fever virus genotyping tool and investigation of suspected adverse events following yellow fever vaccination. Vaccines (Basel) 2019; 7:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Costa-Pereira C, Campi-Azevedo AC, Coelho-Dos-Reis JG, et al. Multi-parameter approach to evaluate the timing of memory status after 17DD-YF primary vaccination. PLoS Negl Trop Dis 2018; 12:e0006462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Rezende IM, Oliveira GFG, Costa TA, et al. Yellow fever molecular diagnosis using urine specimens during acute and convalescent phases of the disease. J Clin Microbiol 2022; 60:e0025422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffin DE. Why does viral RNA sometimes persist after recovery from acute infections? PLoS Biol 2022; 20:e3001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bonaldo MC, Gómez MM, Ac A, et al. Genome analysis of yellow fever virus of the ongoing outbreak in Brazil reveals polymorphisms. Mem Inst Oswaldo Cruz 2017; 112:447–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schiff ER. Atypical clinical manifestations of hepatitis A. Vaccine 1992; 10:S18–20. [DOI] [PubMed] [Google Scholar]
- 22. Glikson M, Galun E, Oren R, Tur-Kaspa R, Shouval D. Relapsing hepatitis A review of 14 cases and literature survey. Medicine 1992; 71:14–23. [DOI] [PubMed] [Google Scholar]
- 23. Sjogren MH, Tanno H, Fay O, et al. Hepatitis A virus in stool during clinical relapse. Ann Intern Med 1987; 106:221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.