Abstract
Background
Nontuberculous mycobacteria (NTM) cause pulmonary (PNTM) and extrapulmonary (ENTM) disease. Infections are difficult to diagnose and treat, and exposures occur in healthcare and community settings. In the United States, NTM epidemiology has been described largely through analyses of microbiology data from health departments, electronic health records, and administrative data. We describe findings from a multisite pilot of active, laboratory- and population-based NTM surveillance.
Methods
The Centers for Disease Control and Prevention’s Emerging Infections Program conducted NTM surveillance at 4 sites (Colorado, 5 counties; Minnesota, 2 counties; New York, 2 counties; and Oregon, 3 counties [PNTM] and statewide [ENTM]) from 1 October 2019 through 31 March 2020. PNTM cases were defined using published microbiologic criteria. ENTM cases required NTM isolation from a nonpulmonary specimen, excluding stool and rectal swabs. Patient data were collected via medical record review.
Results
Overall, 299 NTM cases were reported (PNTM: 231, 77%); Mycobacterium avium complex was the most common species group. Annualized prevalence was 7.5/100 000 population (PNTM: 6.1/100 000; ENTM: 1.4/100 000). Most patients had signs or symptoms in the 14 days before positive specimen collection (ENTM: 62, 91.2%; PNTM: 201, 87.0%). Of PNTM cases, 145 (62.8%) were female and 168 (72.7%) had underlying chronic lung disease. Among ENTM cases, 29 (42.6%) were female, 21 (30.9%) did not have documented underlying conditions, and 26 (38.2%) had infection at the site of a medical device or procedure.
Conclusions
Active, population-based NTM surveillance will provide data for monitoring the burden of disease and characterize affected populations to inform interventions.
Keywords: nontuberculous mycobacteria, surveillance, epidemiology
Surveillance for nontuberculous mycobacteria (NTM) infections is necessary to better define the disease burden, patient characteristics, and factors associated with infections. We piloted active, laboratory- and population-based surveillance for pulmonary and extrapulmonary NTM infections in 4 geographic areas.
Nontuberculous mycobacteria (NTM) are common environmental organisms that can cause pulmonary (PNTM) and extrapulmonary (ENTM) disease. People can be exposed to NTM via soil and water, including in building plumbing systems and community and healthcare environments [1]. Population-level risk has been associated with climatic factors and trace elements in source water [2, 3], and community-associated NTM infections have been linked to exposures such as tattoos and hot tubs [4–6]. Healthcare outbreaks have been associated with exposure to NTM-contaminated water, including via dental procedures, invasive medical devices, and heater–cooler devices used during cardiac surgery [7–12]. NTM infections acquired through water exposure can lead to a high burden of hospitalizations and high healthcare costs [13]. NTM disease can be difficult to diagnose and treat due to factors such as slow growth in culture, presence of underlying conditions, lengthy clinical course, treatment complexity, and antibiotic resistance.
Most descriptions of US NTM epidemiology come from analyses of microbiology data reported to health departments, electronic health records, and administrative data [4, 14–23]. Some studies have shown increases in PNTM disease burden over the past 2 decades [16, 19, 20]. However, administrative data have variable accuracy for identifying NTM disease [24], and available data may have limited information on individual patient characteristics. We sought to address these challenges by piloting active, laboratory- and population-based NTM surveillance in 4 areas of the United States to describe the epidemiology and assess the feasibility of and improve methods for ongoing surveillance.
METHODS
Surveillance System
Surveillance was conducted from 1 October 2019 through 31 March 2020 by the Centers for Disease Control and Prevention's (CDC's) Emerging Infections Program (EIP) [25] in Colorado (5 counties), Minnesota (2 counties), New York (2 counties), and Oregon (3 counties, PNTM; statewide, ENTM). The total population under surveillance was 7 531 331 for PNTM and 9 916 434 for ENTM (Supplementary Table).
The human subjects advisor in the CDC's National Center for Emerging and Zoonotic Infectious Diseases reviewed the project and determined that it constituted a nonresearch public health surveillance activity. CDC institutional review board (IRB) review was not required. The project was reviewed at participating EIP sites and was deemed a nonresearch public health activity or received IRB approval.
Case Definitions
Case-patients were surveillance area residents who met the case definitions described below and had ≥1 NTM-positive clinical specimen collected during the surveillance period. The first NTM-positive clinical specimen during the surveillance period was considered the index specimen. Species excluded from surveillance are listed in the Supplementary Material.
PNTM cases were defined based on the microbiologic component of US and European clinical practice guideline criteria for PNTM disease diagnosis (Supplementary Methods) [26]. Whether case-patients had medical record documentation of clinician-diagnosed NTM disease and met the clinical and radiologic components of the PNTM diagnostic criteria were also assessed (Supplementary Methods).
ENTM cases were defined by NTM isolated from any nonpulmonary body site, excluding stool and rectal swabs. PNTM and ENTM cases identified in the same patient were reported separately.
Cases were considered prevalent if NTM were detected in specimens collected before the surveillance period and ≤6 months before the date of index specimen collection (DISC) or if medical records indicated NTM disease was present before the surveillance period. Otherwise, cases were considered incident.
Data Collection
EIP site staff worked with the clinical and public health laboratories serving surveillance area residents to obtain reports of NTM-positive clinical specimens along with relevant patient information, with the goal of identifying all cases in residents of the surveillance area during the pilot period. Demographic and clinical data, information on selected community and healthcare exposures, and laboratory data were abstracted from case-patient medical records using standardized case report forms (Supplementary Methods).
Data were collected and managed using Research Electronic Data Capture (REDCap) tools hosted at the CDC [27, 28]. REDCap is a secure, web-based software platform designed to support data capture for research studies.
Data Analyses
To calculate crude annual prevalence per 100 000 population, total numbers of PNTM and ENTM cases from the 6-month pilot were multiplied by 2 and divided by US Census Bureau bridged-race vintage post-census population estimates for 2019 [29]. Prevalence was calculated by EIP site, sex, race and ethnicity, and age group for PNTM and ENTM. Crude annual incidence per 100 000 population was determined using the same approach but limiting numerators to incident cases. We summarized case-patient data and determined whether clinical guideline diagnostic criteria were met and antibiotics administered or prescribed within 30 days after the index specimen culture result date were aligned with current guidelines [26]. Data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Prevalence and Incidence
Among 486 patients with NTM-positive specimens, 420 had pulmonary and 68 had extrapulmonary specimens. Two patients had both pulmonary and extrapulmonary NTM-positive specimens. A total of 189 patients were excluded because they had only 1 NTM-positive sputum, tracheal, or endotracheal specimen reported during the surveillance period and did not meet the criteria for prevalent infection.
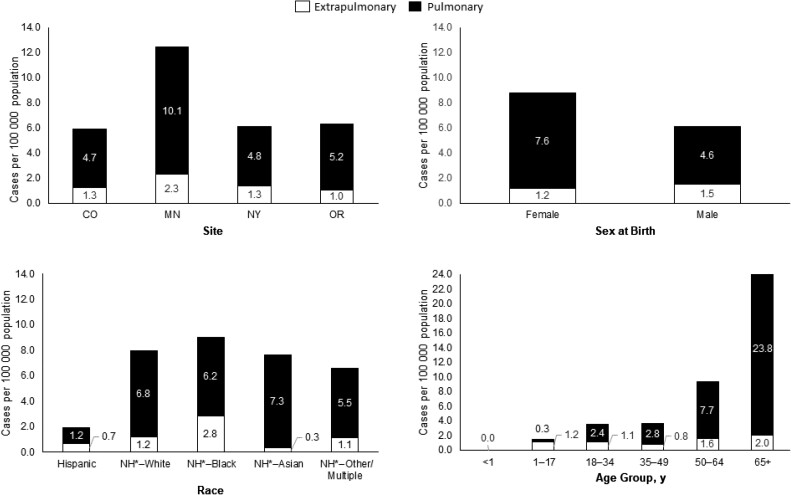
A total of 299 NTM cases in 297 patients were reported. Among 231 PNTM cases, 141 (61.0%) were incident and 90 (39.0%) were prevalent. Among 68 ENTM cases, 55 (80.9%) were incident and 13 (19.1%) were prevalent. The annualized prevalence was 6.1 PNTM and 1.4 ENTM cases per 100 000 population, with an overall prevalence of 7.5 cases per 100 000 population. PNTM prevalence was highest in Minnesota and among females, persons who were Asian, persons who were not known to be Hispanic, and persons aged ≥65 years. ENTM prevalence was also highest in Minnesota and in persons aged ≥65 years but higher in males than in females and in persons who were Black or not known to be Hispanic compared with persons of other races and ethnicities (Figure 1). The annualized incidence was 3.7 PNTM and 1.1 ENTM cases per 100 000 population, with an overall incidence of 4.8 cases per 100 000 population.
Figure 1.
Pulmonary and extrapulmonary nontuberculous mycobacteria annualized prevalence per 100 000 population by site, sex at birth, race, and age group. NH*: Not known to be Hispanic; records indicated either ethnicity was non-Hispanic or ethnicity was not known. Abbreviations: CO, Colorado; MN, Minnesota; NY, New York; OR, Oregon.
Pulmonary NTM Infections
Most of the 231 case-patients were female (145, 62.8%), White and not known to be Hispanic (170, 73.6%), and had chronic underlying lung disease (168, 72.7%; Table 1). Most patients’ index specimens were sputum (156, 67.5%) or from bronchoalveolar lavage (67, 29.0%); 7 specimens were lung tissue and 2 were bronchial washes (1 case had 2 index specimens). Pulmonary or systemic symptoms in the 14 days before the DISC were documented for 201 patients (87.0%). Chest imaging with nodules or cavities in the 90 days before or after the DISC was documented for 146 patients (63.2%; Table 2). Overall, 76 cases (32.9%) met the clinical practice guideline PNTM disease diagnostic criteria [26], and 127 cases (55.0%) met modified criteria allowing for nodules or cavities on a chest computed tomography (CT) scan or chest radiograph. Almost 80% of case-patients (181) had documented clinician-diagnosed PNTM disease in their medical records (Table 2, Supplementary Figure).
Table 1.
Demographic Characteristics and Underlying Conditions of Persons With Extrapulmonary or Pulmonary Nontuberculous Mycobacteria Infection
| Characteristic | Extrapulmonary N = 68 |
Pulmonary N = 231 |
|---|---|---|
| Age, median (range), y | 41 (1–90) | 67 (16–93) |
| Female, n (%) | 29 (42.6) | 145 (62.8) |
| Race and ethnicity, n (%) | ||
| Hispanic, any race | 5 (7.4) | 7 (3.0) |
| Not known to be Hispanica–Whiteb | 41 (60.3) | 170 (73.6) |
| Not known to be Hispanica–Black or African Americanc | 9 (13.2) | 19 (8.2) |
| Not known to be Hispanica–Asian | 1 (1.5) | 19 (8.2) |
| Not known to be Hispanica–other or multiple races | 2 (2.9) | 7 (3.0) |
| Not known to be Hispanica,d–unknown race | 10 (14.7) | 9 (3.9) |
| Underlying conditions, n (%) | ||
| Cardiovascular disease | 8 (11.8) | 31 (13.4) |
| Chronic kidney disease | 5 (7.4) | 11 (4.8) |
| Chronic lung disease | 7 (10.3) | 168 (72.7) |
| Bronchiectasis | – | 117 (50.6) |
| Chronic obstructive pulmonary disease | – | 49 (21.2) |
| Cystic fibrosis | 0 | 21 (9.1) |
| Emphysema | – | 9 (3.9) |
| Connective tissue disease | 6 (8.8) | 11 (4.8) |
| Diabetes mellitus | 9 (13.2) | 35 (15.2) |
| Gastrointestinal disease | 5 (7.4) | 83 (35.9) |
| Gastroesophageal reflux disease | – | 79 (34.2) |
| Immunocompromising condition | 13 (19.1) | 10 (4.3) |
| Human immunodeficiency virus infection | 9 (13.2) | 8 (3.5) |
| AIDS/CD4 count <200 cells/mm3 | 6 (8.8) | 2 (0.9) |
| Primary immunodeficiency | 2 (2.9) | 0 |
| Transplant, hematopoietic stem cell | 0 | 0 |
| Transplant, solid organ | 2 (2.9) | 2 (0.9) |
| Liver disease | 2 (2.9) | 20 (8.7) |
| Malignancy | 6 (8.8) | 44 (19.0) |
| Hematologic | 0 | 2 (0.9) |
| Solid organ (nonmetastatic) | 5 (7.4) | 33 (14.3) |
| Solid organ (metastatic) | 1 (1.5) | 10 (4.3) |
| Neurologic condition | 4 (5.9) | 23 (10.0) |
| Plegias or paralysis | 1 (1.5) | 2 (0.9) |
| Skin condition | 5 (7.4) | 8 (3.5) |
| Other | 7 (10.3) | 32 (13.9) |
| Chest wall deformity (eg, pectus excavatum) | – | 2 (0.9) |
| Cough suppression disorder | – | 2 (0.9) |
| History of tuberculosis | – | 20 (8.7) |
| Scoliosis | – | 4 (1.7) |
| None | 21 (30.9) | 11 (4.8) |
| Unknown | 2 (2.9) | 2 (0.9) |
– indicates variable not collected.
Records indicated either ethnicity was non-Hispanic or ethnicity was not known.
Includes 1 extrapulmonary nontuberculous mycobacteria (NTM) case-patient and 15 pulmonary NTM case-patients with unknown ethnicity.
Includes 1 extrapulmonary NTM case-patient with unknown ethnicity.
Of case-patients with unknown race, 6 extrapulmonary and 4 pulmonary NTM case-patients had unknown ethnicity.
Table 2.
Clinical Characteristics and Community and Healthcare Exposures of Persons With Extrapulmonary or Pulmonary Nontuberculous Mycobacteria Infection
| Characteristic | Extrapulmonary N = 68 |
Pulmonary N = 231 |
|---|---|---|
| Days from index specimen collection to culture result date, median (interquartile range) | 30 (20.5–41) | 47 (31–68) |
| Location of index specimen collection, n (%) | ||
| Inpatient | 35 (51.5) | 74 (32.0) |
| Outpatient | 33 (48.5) | 154 (66.7) |
| Unknown | 0 | 3 (1.3) |
| Signs and symptoms in the 14 d before the DISC,a n (%) | ||
| Any | 62 (91.2) | 201 (87.0)b |
| Chest pain | – | 31 (13.4) |
| Cough | – | 159 (68.8) |
| Dyspnea | – | 63 (27.3) |
| Fatigue or malaise | 14 (20.6) | 50 (21.6) |
| Fever | 16 (23.5) | 29 (12.6) |
| Hemoptysis | – | 32 (13.9) |
| Night sweats | 3 (4.4) | 17 (7.4) |
| Pain at infection site | 26 (38.2) | – |
| Positive imaging | 4 (5.9) | – |
| Pus, drainage, abscess | 22 (32.4) | – |
| Redness or swelling | 23 (33.8) | – |
| Weight loss | 10 (14.7) | 37 (16.0) |
| Other | 14 (20.6) | 35 (15.2) |
| None | 2 (2.9) | 18 (7.8) |
| Unknown | 3 (4.4) | 10 (4.3) |
| Chest imaging in the 90 d before or after the DISC, n (%) | ||
| Chest CT only | – | 115 (49.8) |
| Bronchiectasis only | 16/115 (13.9) | |
| Nodules only | … | 41/115 (35.7) |
| Cavity or cavitation only | … | 7/115 (6.1) |
| Bronchiectasis plus nodules | … | 31/115 (27.0) |
| Nodules or cavities | … | 87/115 (75.7) |
| None of the above | … | 12/115 (10.4) |
| Unknown | … | 0 |
| Chest radiograph only | – | 40 (17.3) |
| Bronchiectasis only | … | 6/40 (15.0) |
| Nodules only | … | 7/40 (17.5) |
| Cavity or cavitation only | … | 5/40 (12.5) |
| Bronchiectasis plus nodules | … | 7/40 (17.5) |
| Nodules or cavities | … | 20/40 (50.0) |
| None of the above | … | 14/40 (35.0) |
| Unknown | … | 0 |
| Chest CT and chest radiograph | – | 47 (20.3) |
| Bronchiectasis only | … | 3/47 (6.4) |
| Nodules only | … | 14/47 (29.8) |
| Cavity or cavitation only | … | 2/47 (4.3) |
| Bronchiectasis plus nodules | … | 15/47 (31.9) |
| Nodules or cavities | … | 39/47 (83.0) |
| None of the above | … | 5/47 (10.6) |
| Unknown | … | 0 |
| None | – | 27 (11.7) |
| Unknown | – | 2 (0.9) |
| Clinician-diagnosed NTM disease,c n (%) | 52 (76.5) | 181 (78.4) |
| Diagnostic criteria for pulmonary NTM disease [26], n (%) | ||
| Both clinical and radiologic criteria were met | – | 76 (32.9) |
| Clinical criterion only was met | – | 101 (43.7) |
| Radiologic criterion only was met | – | 8 (3.5) |
| Neither clinical nor radiologic criteria were metd | – | 12 (5.2) |
| Unknown or data not available | – | 34 (14.7) |
| Referral to specialist, n (%)e | 59 (86.8) | 219 (94.8) |
| Infectious diseases specialist | 47/59 (79.7) | 144/219 (65.8) |
| Pulmonary specialist | 2/59 (3.4) | 151/219 (68.9) |
| Infectious diseases and pulmonary specialists | 0/59 | 79/219 (36.1) |
| Surgeon | 27/59 (45.8) | 5/219 (2.3) |
| Otherf | 6/59 (10.2) | 1/219 (0.5) |
| NTM-related hospitalization in the year before or on the DISC,g n (%) | 33 (48.5) | 41 (17.7) |
| Hospitalized on the DISC,h n (%) | 31 (45.6) | 72 (31.2) |
| Documented exposures in the year before or on the DISC,i n (%) | … | … |
| Community | 21 (31.8) | 60 (26.0) |
| Acupuncture | 1 (1.5) | – |
| Bird contact | 1 (1.5) | 2 (0.9) |
| Construction | 0 | 4 (1.7) |
| Fish tank | 1 (1.5) | 1 (0.4) |
| Gardening or landscaping | 1 (1.5) | 9 (3.9) |
| Homelessness | 3 (4.5) | 10 (4.3) |
| Hot tub | 1 (1.5) | 6 (2.6) |
| Humidifier use | 0 | 2 (0.9) |
| Incarceration | 2 (3.0) | 6 (2.6) |
| Livestock | 0 | 2 (0.9) |
| Nail salon | 1 (1.5) | 0 |
| Neti pot | 3 (4.5) | 0 |
| Nebulizer | – | 6 (2.6) |
| Swimming pool | 0 | 4 (1.7) |
| Tattoo | 1 (1.5) | – |
| Trauma | 7 (10.6) | – |
| Other | 8 (12.1) | 25 (10.8) |
| Healthcare | 28 (42.4) | 74 (32.0) |
| Bronchoscopy | – | 46 (19.9) |
| Cystic fibrosis clinic | – | 14 (6.1) |
| Dental procedure | 2 (3.0) | 1 (0.4) |
| Injection or infusion | 10 (15.2) | 10 (4.3) |
| Medical device | 1 (1.5) | 2 (0.9) |
| Nursing home residence | 2 (3.0) | 7 (3.0) |
| Surgical procedure | 19 (28.8) | 5 (2.2) |
| Other | 0 | 3 (1.3) |
| None | 21 (31.8) | 90 (39.0) |
| Unknown | 3 (4.5) | 20 (8.7) |
– indicates variable not collected.
Date of index specimen collection.
Includes patients with ≥1 specified pulmonary or systemic symptom: chest pain, cough, dyspnea, fatigue or malaise, fever, hemoptysis, or night sweats.
Defined as a diagnosis of NTM disease recorded in the medical record; unknown for 4 extrapulmonary and 3 pulmonary NTM cases.
Two pulmonary NTM cases did not meet the imaging criterion, and clinical criterion data were unknown; 1 pulmonary NTM case did not meet the clinical criterion and imaging was not performed.
Referral to specialist was unknown for 1 extrapulmonary and 3 pulmonary NTM cases.
Examples of other specialists include dermatologist, neurologist, hematologist/oncologist, otolaryngologist, hepatologist, chiropractor.
Five extrapulmonary and 6 pulmonary NTM cases had unknown overall hospitalization status in the year before the DISC, and 1 extrapulmonary and 1 pulmonary NTM case had unknown NTM-related hospitalization status.
Five extrapulmonary and 6 pulmonary NTM cases had unknown overall hospitalization status in the year before the DISC, and 1 extrapulmonary and 1 pulmonary NTM case had unknown admission and/or discharge dates.
Among 66 extrapulmonary NTM cases not known to be associated with an outbreak (2 outbreak-associated extrapulmonary NTM cases were excluded). Multiple exposures could be reported for a case.
Abbreviations: CT, computed tomography; DISC, date of index specimen collection; NTM, nontuberculous mycobacteria.
Most patients with available data (216 of 228, 94.7%) were referred to an infectious diseases specialist or pulmonologist within 30 days of the DISC, and 41 of 224 patients with available data (18.3%) had an NTM-related hospitalization in the year before or on the DISC (Table 2). Information on community and healthcare exposures in the year before or on the DISC was available in the medical record for 211 case-patients (91.3%). Community exposures were documented for 60 of 211 (28.4%), including homelessness (10 case-patients, 4.7%), followed by gardening or landscaping (9 case-patients, 4.3%). Healthcare exposures were documented for 74 case-patients (35.1%). Ninety of 211 case-patients (42.7%) had none of the collected community or healthcare exposures documented (Table 2).
Mycobacterium avium complex (MAC) was isolated from 166 (71.9%) cases (Table 3). Of these, 79 case-patients (47.6%) were prescribed or administered ≥1 antimicrobial within 30 days of the index specimen culture result date. Twenty-nine case-patients with MAC (17.5%) were prescribed or received guideline-recommended [26] antibiotics: rifampin or rifabutin, ethambutol, and azithromycin or clarithromycin (25 cases); and rifampin or rifabutin, ethambutol, azithromycin or clarithromycin, and amikacin or streptomycin (4 cases). Among the remaining MAC case-patients, 78 (47.0%) did not receive antimicrobials, and treatment data were unknown or unavailable for 9 (5.4%) case-patients. Treatment data for PNTM cases due to other species are provided in the Supplementary Material.
Table 3.
Nontuberculous Mycobacteria Species Identified From Pulmonary and Extrapulmonary Index Specimens
| Organism | Extrapulmonary N = 68 |
Pulmonary N = 231 |
|---|---|---|
| Mycobacterium avium complex, n (%) | 28 (41.2) | 166 (71.9) |
| M. avium | 16 (23.5) | 96 (41.6) |
| Mycobacterium intracellulare subsp. chimaera | 2 (2.9) | 2 (0.9) |
| M. intracellulare subsp. intracellulare | 5 (7.4) | 43 (18.6) |
| Other | 0 | 1 (0.4) |
| Not otherwise specified | 5 (7.4) | 24 (10.4) |
| Non-M. avium complex, n (%) | 39 (57.4) | 67 (29.0) |
| Mycobacterium abscessus complex | 9 (13.2) | 20 (8.7) |
| Mycobacterium chelonae complex | 13 (19.1) | 4 (1.7) |
| Mycobacterium fortuitum group | 8 (11.8) | 19 (8.2) |
| Mycobacterium kansasii | 4 (5.9) | 6 (2.6) |
| Other | 5 (7.4) | 17 (7.4) |
| Not otherwise specified | 0 | 1 (0.4) |
| Not Mycobacterium tuberculosis, not characterized further, n (%) | 1 (1.5) | 0 |
Two pulmonary index specimens had >1 species reported: M. abscessus complex and M. chelonae complex (1) and M. avium and M. abscessus complex (1).
Extrapulmonary NTM Infections
Twenty-nine of 68 ENTM case-patients were female (42.6%), and 41 (60.3%) were White, not known to be Hispanic (Table 2). The most common underlying condition was human immunodeficiency virus infection (9, 13.2%). Twenty-one case-patients (30.9%) were not documented to have any of the collected underlying conditions (Table 1). The most common specimens from which NTM were isolated were lymph nodes (13, 19.1%), blood (12, 17.6%), and skin (11, 16.2%; Table 4). Among 65 case-patients with available data, common signs or symptoms were pain at the infection site (26, 40.0%); redness or swelling (23, 35.4%); and pus, drainage, or abscess (22, 33.8%; Table 2).
Table 4.
Index Specimen Types Positive for Nontuberculous Mycobacteria for Extrapulmonary Cases
| Specimen Type | Total (N = 68) n (%) |
|---|---|
| Lymph node | 13 (19.1) |
| Blood | 12 (17.6) |
| Skin | 11 (16.2) |
| Wound | 9 (13.2) |
| Soft tissue | 4 (5.9) |
| Joint/synovial fluid | 3 (4.4) |
| Muscle | 3 (4.4) |
| Peritoneal fluid | 2 (2.9) |
| Pleural fluid | 1 (1.5) |
| Othera | 11 (16.2) |
Includes 8 sinus, 1 gastric lavage, 1 heart tissue, 1 vascular prosthesis specimen. One case had 2 index specimen types.
Information on community and healthcare exposures was available for 65 case-patients (95.6%); data for 2 case-patients who were part of a known outbreak were excluded. Of the remaining 63 case-patients, 21 (33.3%) had community exposures, most commonly trauma (7, 11.1%), and 28 (44.4%) had healthcare exposures, such as from medical devices, healthcare procedures, or residence in a nursing home (Table 2). Twenty-six of 68 (38.2%) cases involved infection at the site of a medical device or healthcare procedure, including infections at surgical sites (17), injection or infusion sites (8), dental procedure sites (1), and a medical device site (1). One patient had an ENTM infection at both a surgical and injection/infusion site.
MAC was the most common species group (28 cases, 41.2%), followed by Mycobacterium chelonae complex (13, 19.1%; Table 3). Fifty-two of 64 case-patients with available data (81.3%) had documented clinician-diagnosed NTM disease; 47 of 67 patients with available data (70.1%) were referred to an infectious diseases specialist within 30 days of the DISC; and 33 of 62 case-patients with available data (53.2%) had an NTM-related hospitalization in the year before or on the DISC. Most case-patients with available data were prescribed ≥1 antimicrobial within 30 days of the index specimen culture result date (43 of 63; 68.3%). Among 28 MAC case-patients, 6 (21.4%) received a macrolide and ethambutol and 3 (10.7%) received a macrolide, ethambutol, and rifampin.
DISCUSSION
In this multisite, active, laboratory- and population-based NTM disease surveillance pilot conducted for 6 months in 2019–2020, we observed annualized prevalence and incidence rates of 7.5 cases and 4.8 cases per 100 000 population, respectively. PNTM disease rates were approximately 4 times higher than ENTM rates, and prevalence of both PNTM and ENTM disease varied by location and demographic group.
Reports of US NTM epidemiology have used surveys of public health laboratories, analyses of microbiology data in electronic health record datasets, and assessments of data gathered under various states’ past or current reportable condition requirements [4, 14–23, 30]. Many of these studies are older, used passive surveillance data, or lack detailed clinical information [4, 14, 21, 23, 30]. Other studies have relied on administrative data [19, 20], which have been shown to have limited sensitivity for identifying NTM disease [19, 24, 31].
Despite these limitations, we observed that NTM prevalence and incidence rates in our pilot, using a reference standard surveillance approach, were similar to those reported in older studies that used similar case definitions based on the microbiologic component of clinical practice guideline diagnostic criteria published in 2007 [32]. For example, Henkle and colleagues analyzed public health surveillance data from Oregon and reported an annualized PNTM prevalence of 5.9 cases per 100 000 population in 2011–2012, PNTM incidence of 5.6 cases per 100 000 population in 2012 [33], and ENTM incidence of 1.3 cases per 100 000 population in 2012 [14]. Another study using electronic health record data from integrated healthcare delivery systems in 4 states in 2004–2006 reported an average annual, age-adjusted PNTM disease prevalence of 5.5 cases per 100 000 population [22]. In contrast, rates observed in our surveillance were much lower than those found in an analysis of Kaiser Permanente Hawaii electronic health record data, which yielded an overall PNTM infection incidence rate of 36 cases per 100 000 person-years from 2005 to 2019 [34], likely reflecting geographic variability in the distribution and prevalence of NTM species in the environment as well as the prevalence of host risk factors for disease [20, 34]. NTM prevalence has been reported to be higher in warmer climates, such as the southeastern United States [20], which was not represented in our pilot effort.
Unlike many prior studies, we were able to incorporate clinical and radiologic data to determine the percentage of PNTM cases who met current clinical practice guideline diagnostic criteria published in 2020 [26]. These criteria are similar to those published in 2007 [32], except that the clinical component can be met with pulmonary or systemic symptoms. We found that while 87% of case-patients had pulmonary or systemic symptoms and 78% of case-patients had a diagnosis of PNTM disease documented in their medical records, only 33% met the full diagnostic criteria. This is lower than in a previous report in which 86% of patients who met the microbiologic component of the diagnostic criteria also met the 2007 version of the full diagnostic criteria [35]. That only 33% met full diagnostic criteria in our surveillance is largely due to the radiologic component of the criteria that requires findings of both bronchiectasis and nodules for case-patients whose only chest imaging was a CT scan. Since we only collected chest imaging findings from the period defined by the 90 days before and after the DISC, we may have underestimated the percentage of case-patients who met the full diagnostic criteria. When we relaxed the radiologic component to allow for a finding of nodules or cavities on either a chest radiograph or CT scan, 55% of case-patients met the full diagnostic criteria.
Some investigators have expressed concerns about potential limited sensitivity of the PNTM diagnostic criteria in the clinical practice guidelines [36, 37]. Plotinsky and colleagues found that 43% of patients diagnosed and treated for PNTM disease by specialists did not meet the diagnostic criteria, largely because they had only a single positive culture for NTM that did not fulfill the microbiologic component (55% of those not meeting full criteria) or because they did not meet the radiologic component (45% of those not meeting full criteria) [36]. The authors proposed modified definitions that incorporated relaxed microbiologic and radiologic components and classified cases as definite or probable [36]. More recently, other investigators reported similar findings, with 34% of patients with ≥1 pulmonary NTM isolate meeting the full diagnostic criteria. The investigators proposed broadening the radiologic component to include findings such as infiltrates, opacities, and consolidation [37].
In addition to diagnostic criteria, the clinical practice guidelines provide treatment recommendations for PNTM disease caused by the most common species [26]. Treating patients with PNTM disease is generally recommended; however, relatively low percentages of PNTM case-patients identified in our surveillance pilot received recommended antibiotic treatment in the 30 days after the result date of the index specimen culture. For example, although most PNTM case-patients identified in this surveillance pilot had documented clinician-diagnosed NTM disease, fewer than 1 in 5 patients with MAC infections were documented to have been prescribed or received recommended treatment within 30 days after the index specimen culture result date. It is important to note that clinical practice guidelines acknowledge that the isolation of NTM from respiratory specimens may not always represent progressive clinical disease and sometimes is the result of environmental contamination [26]. Clinical judgment is paramount in deciding whether and when to initiate treatment. The low proportion of case-patients treated in this surveillance pilot may reflect overall low illness severity. Alternatively, it may reflect the limited time period during which treatment data were collected.
More than 40% of ENTM case-patients had healthcare exposures documented during the year before or on the DISC. Of these, 93% had NTM infection at the body site involved in the healthcare exposure, such as the site of a medical device or procedure. These data highlight the importance of preventing healthcare-associated NTM infections. A review of CDC consultations involving transmission of water-related organisms in healthcare from 2014 to 2017 showed NTM were involved in the greatest number of investigations: 40, representing 549 patients [9]. Additionally, NTM were associated with the largest number of hospitalizations and deaths among waterborne infectious diseases in the United States in 2014, estimated at 51 400 and 3800, respectively [13]. While NTM are widespread in the environment, exposures in healthcare settings can be mitigated by focusing on facility water system design and management [10, 38, 39]. Continued surveillance will allow a better understanding of potential healthcare-associated NTM infections, enhance detection of exposures of concern, and inform focused prevention strategies.
This pilot had limitations. First, surveillance was conducted in a limited geographic area and therefore is not nationally representative. Second, the duration of the pilot was 6 months. Since NTM infection rates may not be constant throughout the year, our crude annualized rates may be over- or underestimates. Third, we limited the identification of prevalent cases to those with NTM-positive specimens collected no more than 6 months before the DISC. This may have underestimated prevalent infections and overestimated incident infections. The case definition has been updated for ongoing surveillance, initiated in 2021, with prevalent cases defined by isolation of NTM in the 12 months before the DISC. Fourth, it was challenging to obtain complete medical records for case-patients who were receiving care from multiple providers. The quality and completeness of medical record documentation varied greatly, particularly among outpatient providers. This may have affected our ability to determine whether PNTM cases met clinical practice guideline diagnostic criteria and to describe disease characteristics and treatment. With ongoing surveillance, relationships between state health department or academic partner personnel and outpatient providers will continue to be strengthened, which may improve medical record access.
In conclusion, data from this active, laboratory- and population-based surveillance pilot provide a detailed description of NTM disease epidemiology in 4 geographically diverse areas of the United States. The pilot experience was used to modify methods for ongoing population-based surveillance, which began in 2021. Data gathered through ongoing active surveillance will be used to monitor incidence and prevalence over time and to generate a detailed description of the demographic and clinical characteristics of persons affected by NTM disease that can be used to inform prevention strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Cheri Grigg, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Kelly A Jackson, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Devra Barter, Division of Disease Control and Public Health Response, Colorado Department of Public Health and Environment, Denver, Colorado, USA.
Christopher A Czaja, Division of Disease Control and Public Health Response, Colorado Department of Public Health and Environment, Denver, Colorado, USA.
Helen Johnston, Division of Disease Control and Public Health Response, Colorado Department of Public Health and Environment, Denver, Colorado, USA.
Ruth Lynfield, Minnesota Department of Health, St. Paul, Minnesota, USA.
Paula Snippes Vagnone, Minnesota Department of Health, St. Paul, Minnesota, USA.
Laura Tourdot, Minnesota Department of Health, St. Paul, Minnesota, USA.
Nancy Spina, New York State Department of Health, Albany, New York, USA.
Ghinwa Dumyati, University of Rochester Medical Center, Rochester, New York, USA.
P Maureen Cassidy, Public Health Division, Oregon Health Authority, Portland, Oregon, USA.
Rebecca Pierce, Public Health Division, Oregon Health Authority, Portland, Oregon, USA.
Emily Henkle, Oregon Health and Science University, Portland, Oregon, USA.
D Rebecca Prevots, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, Maryland, USA.
Max Salfinger, University of South Florida College of Public Health & Morsani College of Medicine, Tampa, Florida, USA.
Kevin L Winthrop, Oregon Health and Science University, Portland, Oregon, USA.
Nadege Charles Toney, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Shelley S Magill, Division of Healthcare Quality Promotion, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Notes
Acknowledgments. We thank the healthcare facilities and laboratory staff and the patients in the surveillance areas as well as all staff at participating Emerging Infections Program sites who contributed to this work.
Disclaimer. The findings and conclusions presented here are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH).
Financial support. This work was supported by the CDC through the Emerging Infections Program cooperative agreement [CK17-1701] and by the Division of Intramural Research, National Institute for Allergy and Infectious Diseases (NIAID) at the NIH, via subject matter expertise provided by Dr Prevots. R. P. reports support from CDC EIP cooperative agreement funding (funding to Oregon Health Authority). C. A. C., P. M. C., R. L., and P. S. V. report support from the CDC (the Colorado Department of Public Health and Environment receives funding from the CDC to conduct EIP surveillance projects).
References
- 1. Centers for Disease Control and Prevention . Nontuberculous mycobacteria (NTM) infections. 2019. Available at:https://www.cdc.gov/hai/organisms/nontuberculous-mycobacteria.html. Accessed 8 March 2023.
- 2. Lipner EM, French JP, Falkinham JO, et al. Nontuberculous mycobacteria infection risk and trace metals in surface water: a population-based ecologic epidemiologic study in Oregon. Ann Am Thorac Soc 2022; 19:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adjemian J, Olivier KN, Seitz AE, Falkinham JO, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med 2012; 186:553–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shih DC, Cassidy PM, Perkins KM, Crist MB, Cieslak PR, Leman RL. Extrapulmonary nontuberculous mycobacterial disease surveillance–Oregon, 2014–2016. MMWR Morb Mortal Wkly Rep 2018; 67:854–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fjällbrant H, Akerstrom M, Svensson E, Andersson E. Hot tub lung: an occupational hazard. Eur Respir Rev 2013; 22:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffin I, Schmitz A, Oliver C, et al. Outbreak of tattoo-associated nontuberculous mycobacterial skin infections. Clin Infect Dis 2019; 69:949–55. [DOI] [PubMed] [Google Scholar]
- 7. Baker AW, Lewis SL, Alexander BD, et al. Two-phase hospital-associated outbreak of Mycobacterium abscessus: investigation and mitigation. Clin Infect Dis 2017; 64:902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyman MM, Grigg C, Kinsey CB, et al. Invasive nontuberculous mycobacterial infections among cardiothoracic surgical patients exposed to heater-cooler devices. Emerg Infect Dis 2017; 23:796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Perkins KM, Reddy SC, Fagan R, Arduino MJ, Perz JF. Investigation of healthcare infection risks from water-related organisms: summary of CDC consultations, 2014-2017. Infect Control Hosp Epidemiol 2019; 40:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crist MN, Perz JF. Modern healthcare versus nontuberculous mycobacteria: who will have the upper hand? Clin Infect Dis 2017; 64:912–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sax H, Bloemberg G, Hasse B, et al. Prolonged outbreak of Mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis 2015; 61:67–75. [DOI] [PubMed] [Google Scholar]
- 12. Peralta G, Tobin-D’Angelo M, Parham A, et al. Notes from the field: Mycobacterium abscessus infections among patients of a pediatric dentistry practice—Georgia, 2015. MMWR Morb Mortal Wkly Rep 2016; 65:355.–. [DOI] [PubMed] [Google Scholar]
- 13. Collier SA, Deng L, Adam EA, et al. Estimate of burden and direct healthcare cost of infectious waterborne disease in the United States. Emerg Infect Dis 2021; 27:140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henkle E, Hedberg K, Schafer S, Winthrop KL. Surveillance of extrapulmonary nontuberculous mycobacteria infections, Oregon, USA, 2007–2012. Emerg Infect Dis 2017; 23:1627–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis 2009; 49:e124–129. [DOI] [PubMed] [Google Scholar]
- 16. Adjemian J, Daniel-Wayman S, Ricotta E, Prevots DR. Epidemiology of nontuberculous mycobacteriosis. Semin Respir Crit Care Med 2018; 39:325–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ricotta EE, Adjemian J, Blakney RA, et al. Extrapulmonary nontuberculous mycobacteria infections in hospitalized patients, United States, 2009–2014. Emerg Infect Dis 2021; 27:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spaulding AB, Lai YL, Zelazny AM, et al. Geographic distribution of nontuberculous mycobacterial species identified among clinical isolates in the United States, 2009–2013. Ann Am Thorac Soc 2017; 14:1655–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of nontuberculous mycobacterial lung disease in a large U.S. managed care health plan, 2008–2015. Ann Am Thorac Soc 2020; 17:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med 2012; 185:881–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donohue MJ. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect Dis 2018; 18:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Resp Crit Care Med 2010; 182:970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Good RC, Snyder DE. Isolation of nontuberculous mycobacteria in the United States, 1980. J Infect Dis 1982; 146:829–33. [DOI] [PubMed] [Google Scholar]
- 24. Mejia-Chew C, Yaeger L, Montes K, Bailey TC, Olsen MA. Diagnostic accuracy of health care administrative diagnosis codes to identify nontuberculous mycobacteria disease: a systematic review. Open Forum Infect Dis 2021; 8:ofab035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention . Emerging Infections Program. Available at:https://www.cdc.gov/ncezid/dpei/eip/index.html. Accessed 8 March 2023.
- 26. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis 2020; 71:905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention . Bridged-race population estimates—data files and documentation. 2019. Available at:https://www.cdc.gov/nchs/nvss/bridged_race/data_documentation.htm#Vintage2019. Accessed 8 March 2023.
- 30. Smith GS, Ghio AJ, Stout J, et al. Epidemiology of nontuberculous mycobacteria isolations among central North Carolina residents 2006–2010. J Infect 2016; 72:678–86. [DOI] [PubMed] [Google Scholar]
- 31. Hill AR. The quest for systematic epidemiology of nontuberculous mycobacterial lung disease in the United States: closing in on an elusive goal. Ann Am Thorac Soc 2020; 17:169–77. [DOI] [PubMed] [Google Scholar]
- 32. Griffith DE, Aksamit T, Brown-Elliott B, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007; 175:367–416. [DOI] [PubMed] [Google Scholar]
- 33. Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based incidence of pulmonary nontuberculous mycobacterial disease in Oregon 2007 to 2012. Ann Am Thorac Soc 2015; 12:642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Adjemian J, Frankland TB, Daida YG, et al. Epidemiology of nontuberculous mycobacterial lung disease and tuberculosis, Hawaii, USA. Emerg Infect Dis 2017; 23:439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med 2010; 182:977–82. [DOI] [PubMed] [Google Scholar]
- 36. Plotinsky RN, Talbot EA, von Reyn CF. Proposed definitions for epidemiologic and clinical studies of Mycobacterium avium complex pulmonary disease. PLoS One 2013; 8:e77385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghio AJ, Smith GS, DeFlorio-Baker S, et al. Application of diagnostic criteria for non-tuberculous mycobacterial disease to a case series of mycobacterial-positive isolates. J Clin Tuberc Other Mycobact Dis 2019; 17:100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention . Reduce risk from water. 2019. Available at:https://www.cdc.gov/hai/prevent/environment/water.html. Accessed 8 March 2023.
- 39. Centers for Disease Control and Prevention . Nontuberculous mycobacteria: healthcare facilities. 2019. Available at:https://www.cdc.gov/hai/organisms/ntm/healthcare-facilities.html. Accessed 8 March 2023.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.