Symptoms of osteoarthritis (OA) evolve over years with variable rate and course. While studies have focused on understanding long-term trajectories of OA [e.g.,1], how these relate to shorter-term fluctuations in symptoms is not well-understood. Yet for many people, the lived experience of OA is recurrent fluctuations in episodic pain of varying frequency, intensity and duration; it is these episodes of increased pain and associated disability that often drive primary healthcare consultation. Historically, the term ‘exacerbation’ and ‘flare-up’ have been used interchangeably to describe sudden-onset episodes of increased pain; the term ‘flare-up’ has been adopted herein2. Patients describe flare-ups as being unpredictable and distressing, particularly in advanced stages3. Because of the recognized importance of flare-ups to the patient experience, there have been efforts to develop diagnostic criteria and consensus definition for OA flare-ups4,5, though a specific definition has not yet been widely accepted or validated. It is also important to differentiate between flare-ups and ‘complications’ that may result in symptoms (e.g., subchondral insufficiency micro-fracture), as treatment strategies will differ.
While flare-ups are of clear importance to patients and occur throughout the life-course of OA, research efforts to date have not provided insights about how flare-ups may relate to the longer-term course of OA. Rather than focusing solely upon one aspect (e.g., long-term trajectory) or the other (e.g., flare-ups), we propose reconceptualising OA as an ‘acute-on-chronic’ symptomatic condition6, akin to other variable pathologies such as chronic obstructive pulmonary disease (COPD) and gout. Both COPD and gout have an underlying chronic course punctuated by symptom exacerbations or flares that vary from person-to-person and throughout the disease course. Both diseases also have similar management strategies involving three elements; i) longer term therapy aimed at modifying the underlying disease process, ii) specific flare management, and iii) trigger avoidance. For a heterogeneous condition like OA, core recommendations of exercise and weight loss aim primarily to manage the longer-term course of the disease. How these approaches should be maintained during acute flare-ups is unclear. Unlike for COPD or gout in which patients can continue to take the underlying treatment for these diseases while managing their flares, OA flare-ups can disrupt adherence to the long-term management strategy of physical activity. Although evidence is emerging to support reduced acute pain from an exercise session with adherence to neuromuscular exercise programs7, it is unknown whether such programs should be continued while in the midst of a flare-up. Depending on the underlying cause of the flare-up, resting the joint in the short-term (e.g., using knee braces or modifying painful activities) may limit joint-loading to help with tissue healing and/or continued physical activity engagement. Whether complete rest or reduced volume/distribution of load is needed remains uncertain, and how to determine the appropriate course of action when a flare-up occurs without understanding the etiology is difficult. Further, advice regarding trigger avoidance for OA flare-ups is more challenging because the impact of exposures are not well-understood, and are likely to evolve more insidiously (low-level and non-traumatic) over time. More studies are needed to fully understand if any longer-term management strategies can directly mitigate OA flare-ups, and what should be done to manage them.
Cumulative low-level micro-trauma, in response to repetitive or aberrant mechanical loading is thought to be central to OA etiopathogenesis8. Although strength and control through exercise is beneficial for joints and may potentially reduce acute pain due to exercise [e.g.,7], perturbations in local joint stress could hasten flare-ups. If some flare-ups are the joint’s response to mechanical load, do subsequent inflammatory responses lead to further joint damage and need to be stopped or shortened, or are flare-ups the joint’s attempt to restore homeostasis and therefore necessary? Understanding this process has implications for whether it is safe to pharmacologically reduce the duration of an OA flare-up and/or short-term inflammation that may be present, or safer to help patients adopt ways to minimise or prevent their occurrence instead. There are some data to suggest, for example, that the inflammatory process with resolution phase may actually be necessary for healing post-joint injury9. More broadly, a better understanding of mechanisms underlying OA flare-ups are needed to develop appropriate management approaches. For example, we need to understand how flare-ups relate to excessive load in healthy joints, or to ‘normal’ load in vulnerable joints.
In addition to management implications, understanding how flare-ups may relate to long-term trajectories of symptomatic OA would also provide greater insights into the inter-individual variability in the OA symptom experience. Currently, neither radiographic severity or symptom severity provide adequate understanding of an individual’s disease stage or likely course. This is complicated further by OA progression following a ‘pattern of inertia’, whereby stable structural disease predominates, with triggers for additional rapid progression often unclear10. Perhaps, as noted in other diseases such as gout, examination of the pattern of flare-ups over time may provide greater understanding of OA disease stage (Fig. 1). Conceptually, occurrence of flare-ups, their frequency, predictability and time to episode resolution, may diminish ‘organ reserve’ e the capacity of an organ to return to homeostasis following a perturbation/challenge11 (Fig. 1). Understanding aspects of flare-ups that may contribute to reducing organ reserve would be important to mitigate long-term adverse effects. A model of diminishing organ reserve would complement the characterisation of OA as a disease of organ failure of the joint, with constant pain as a later stage manifestation of the complex multifactorial, multi-structural OA etiopathogenesis8. For other pathologies like chronic kidney disease, biomarkers such as glomerular filtration rate represent renal function reserve. Developing similar proxies of organ reserve in OA may prove beneficial. Measuring time to acute flare-up resolution, time between flareups, and persistence of symptoms, relative to potential triggers, may reflect where in the continuum of organ reserve the joint lies (Fig. 1). A better understanding of each individual’s OA experience may be gained by considering the multidimensional nature of OA, with synovial joint pathologic age (e.g., structural severity) that is distinct from chronological age, and separate dimensions reflecting symptom stage and organ reserve (Fig. 2). The intersection of these different elements may prove to be a valid proxy of ‘disease stage’. To test this model, one would need to examine the relation of flare-up characteristics, such as duration of flare-up episodes and time between flare-ups, to disease course/severity.
Fig.1.
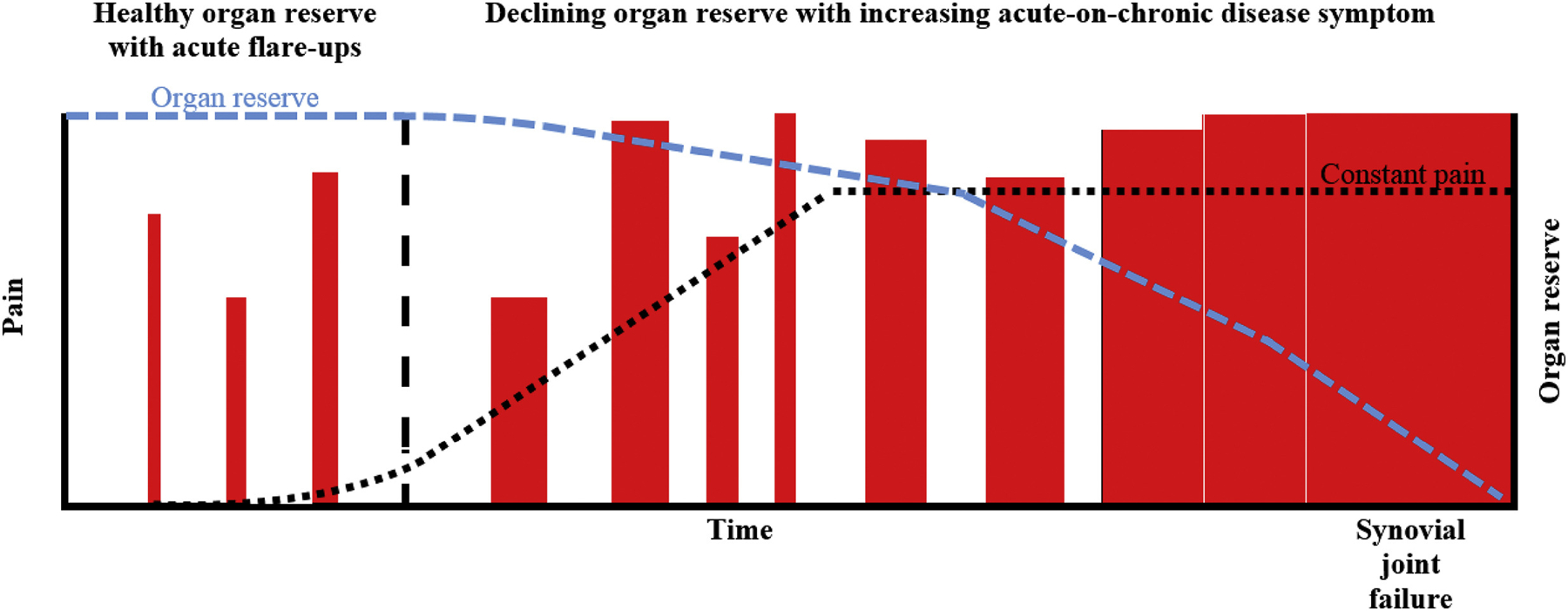
Theoretical natural history of symptomatic OA progression. The impact of intermittent discrete (potentially benign) flare-up episodes (red bars) progress in frequency, intensity (height of bars) and duration (width of bars), with reducing periods of remission and capacity for complete symptom resolution. These acute symptom events drive the underlying disease process, eventually resulting in constant pain, complete loss of organ reserve (capacity to restore homeostasis) and synovial joint failure. Each red bar representing a flare-up is preceded by potential exposure flare-up triggers. The black vertical dashed line represents a period of time after which flare-ups may no longer be potentially benign. The black dotted line represents the course of pain over time. The blue dotted line represents the organ reserve over time, which theoretically may diminish with repeated and/or frequent flare-ups.
Fig.2.
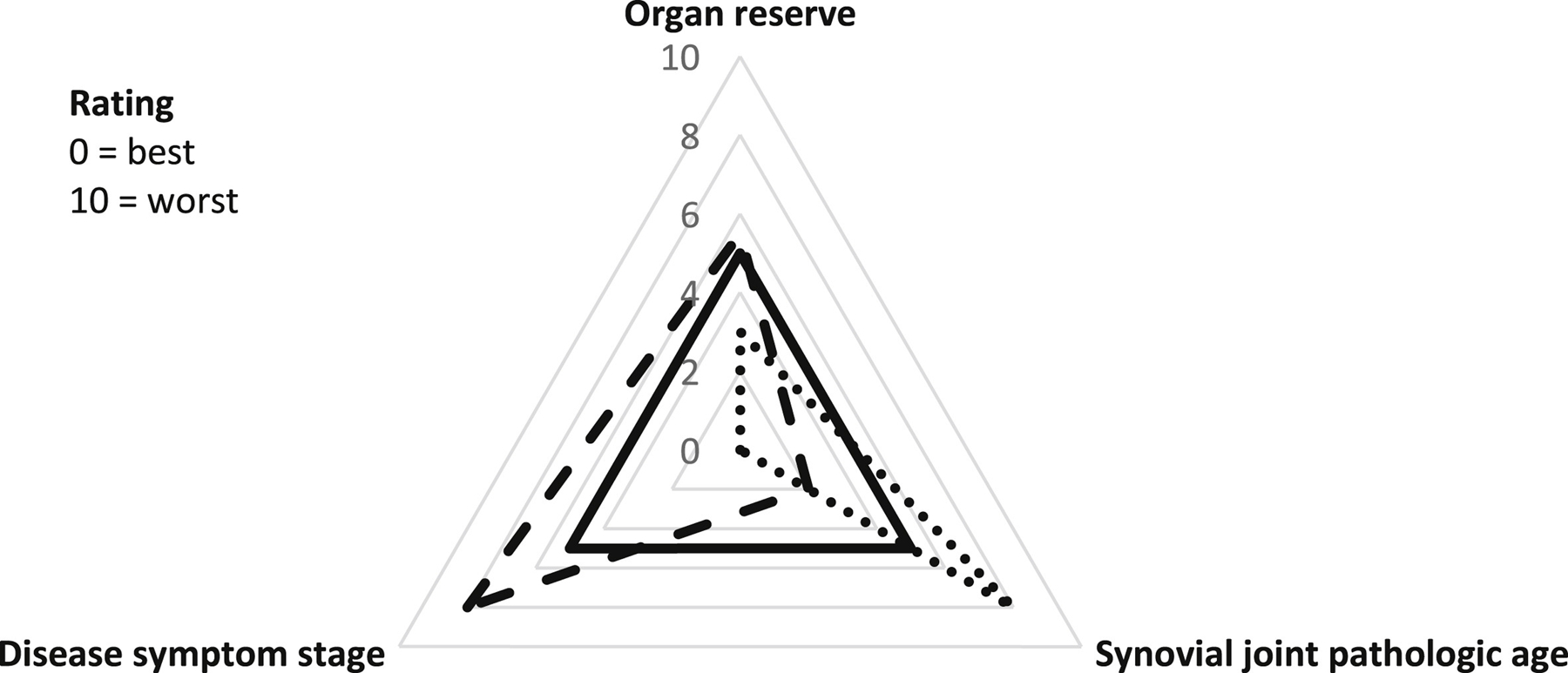
Organ reserve vs synovial joint pathologic age vs disease symptom stage in OA, including illustrative examples; Solid line: observation where organ reserve, synovial joint pathologic age and symptom disease stage are congruent. Dotted line: observation of relatively preserved organ reserve despite advanced synovial joint pathologic age with absence of disease symptoms. Dashed line: observation of severe disease symptoms with moderate diminished organ reserve and relatively preserved synovial joint pathologic age.
Under this paradigm, OA management may best be targeted to each individual’s disease stage rather than a ‘one-size-fits-all’ approach regardless of where one is along the continuum. It may even be relevant to make distinctions between discrete (potentially benign) acute flare-up management early in the disease symptom course from more persistent ‘acute-on-chronic’ flare-ups that might have longer lasting consequences and become more unpredictable as organ reserve diminishes.
Irrespective of whether flare-ups are discrete changes in state, consciously perceived by patients and detectable by clinicians, a more empirically-based understanding of how OA symptom variability relates to etiopathogenesis may also be important for long-term management. For example, when monitoring hypertension as a cardiovascular risk factor, blood pressure variability over time is a strong predictor of stroke12. In longitudinal studies of OA, where pain and function are often measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)13, patient trajectories are largely stable over time [e.g.,1]. This long-term stability is inconsistent with patients’ day-to-day experiences, which for many has considerable variability14 and deteriorates over time. This discordance may be due to imprecise or infrequent repeated pain measurement, or inadequacy of standard self-reported pain instruments to truly capture longer term changes. A particular issue with standard pain instruments is that patients often accommodate to their pain and adjust their frame of reference over time, resulting in ‘response shift’15. For example, a patient may report pain as being 4/10 at baseline, but a few years later they again report their pain as currently being 4/10 despite feeling worse than they were a few years prior because they now consider their initial pain level was 2/10 given their current experience. More intensive daily measurements over repeated periods that balance participant burden with richer insights into long-term pain patterns are warranted.
Existing tools and traditional study designs may be insufficient to investigate flare-ups. Studies should consider the use of self-controlled methodologies (such as web-based case-crossover studies16) to remove between-person variability and facilitate real-time data capture. Novel studies combining patient-reported data, biomarkers and biomechanical measures, that can be ascertained quickly, are required to optimally investigate ‘acute-on-chronic’ OA flare-ups.
Improved means of studying short-term pain variability and longer-term symptom course in OA is needed to better inform disease prognosis and appropriate management strategies. Understanding what optimal flare-up management should look like in the short-term, in addition to current chronic disease management strategies, can help minimise the disability caused by OA in daily living and help people continue to work and engage in social participation. Whether flare-ups and symptom variability are merely random events or cumulative phenomena that also matter in the long term is an important research agenda for the OA community.
Role of the funding
MJT is currently funded by an Integrated Clinical Academic Programme Clinical Lectureship from the National Institute for Health Research (NIHR) and Health Education England (HEE) (ICA-CL-2016-02-014). TN is funded by NIH K24 AR070892, R01 AG066010 and P30 AR072571.
The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, HEE or the Department of Health and Social Care.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
Contributor Information
M.J. Thomas, Primary Care Centre Versus Arthritis, School of Primary, Community and Social Care, Keele University, Keele, Staffordshire, ST5 5BG, United Kingdom, Haywood Academic Rheumatology Centre, Midlands Partnership NHS Foundation Trust, Haywood Hospital, Burslem, Staffordshire, ST5 5BG, United Kingdom.
T. Neogi, Section of Rheumatology, Department of Medicine, Boston University School of Medicine, Boston, MA, 02118, United States
References
- 1.Nicholls E, Thomas E, van der Windt DA, Croft PR, Peat G. Pain trajectory groups in persons with, or at high risk of, knee osteoarthritis: findings from the Knee Clinical Assessment Study and the Osteoarthritis Initiative. Osteoarthritis Cartilage 2014;22:2041–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parry EL, Thomas MJ, Peat G. Defining acute flares in knee osteoarthritis: a systematic review. BMJ Open 2018;8:e019804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawker GA, Stewart L, French MR, Cibere J, Jordan JM, March L, et al. Understanding the pain experience in hip and knee osteoarthritis – an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008;16:415–22. [DOI] [PubMed] [Google Scholar]
- 4.Marty M, Hilliquin P, Rozenberg S, Valat JP, Vignon E, Coste P, et al. Validation of the KOFUS (Knee Osteoarthritis Flare-Ups Score). Joint Bone Spine 2009;76:268–72. [DOI] [PubMed] [Google Scholar]
- 5.Guillemin F, Ricatte C, Barcenilla-Wong A, Schoumacker A, Cross M, Alleyrat C, et al. Developing a preliminary definition and domains of flare in knee and hip osteoarthritis (OA): consensus building of the flare-in-OA OMERACT Group. J Rheumatol 2019;46:1188–91. [DOI] [PubMed] [Google Scholar]
- 6.Tsai CL, Camargo CA Jr. Methodological considerations, such as directed acyclic graphs, for studying “acute on chronic” disease epidemiology: chronic obstructive pulmonary disease example. J Clin Epidemiol 2009;62:982–90. [DOI] [PubMed] [Google Scholar]
- 7.Sandal LF, Roos EM, Bøgesvang SJ, Thorlund JB. Pain trajectory and exercise-induced pain flares during 8 weeks of neuromuscular exercise in individuals with knee and hip pain. Osteoarthritis Cartilage 2016;24:589–92. [DOI] [PubMed] [Google Scholar]
- 8.Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am 2008;34:531–59. [DOI] [PubMed] [Google Scholar]
- 9.Lieberthal J, Sambamurthy N, Scanzello CR. Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage 2015;23:1825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felson D, Niu J, Sack B, Aliabadi P, McCullough C, Nevitt MC. Progression of osteoarthritis as a state of inertia. Ann Rheum Dis 2013;72:924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neustadt J, Pieczenik S. Organ reserve and healthy aging. Integr Med 2008;7:50–2. [Google Scholar]
- 12.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlo€f’ B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet 2010;357:895–905. [DOI] [PubMed] [Google Scholar]
- 13.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- 14.Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis Cartilage 2009;17:1275–82. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz CE, Sprangers MA. Methodological approaches for assessing response shift in longitudinal health-related quality-of-life research. Soc Sci Med 1999;48:1531–48. [DOI] [PubMed] [Google Scholar]
- 16.Makovey J, Metcalf B, Zhang Y, Chen JS, Bennell K, March L, et al. Web-based study of risk factors for pain exacerbation in osteoarthritis of the knee (SPARK-Web): design and rationale. JMIR Res Protoc 2015;4:e80. [DOI] [PMC free article] [PubMed] [Google Scholar]


