Key Teaching Points.
-
•
Although pacemaker implantation is a well-established therapy for swallow syncope, it is generally deemed inappropriate for young patients (aged <40 years). Cardioneuroablation targeting ganglionated plexuses (GPs) located on the epicardial side of the right and left atria is an attractive therapeutic option for swallow syncope, especially in young patients.
-
•
The posteromedial left GP is considered the final direct pathway of the parasympathetic nerve input to the atrioventricular (AV) node. Therefore, ablation of the posteromedial left GP is critically important to denervate the AV node in the treatment of functional AV block.
-
•
When the sinus rate is maintained (>60 beats/min) at the time of functional AV block, minimal cardioneuroablation selectively targeting the posteromedial left GP may be sufficient to eliminate syncopal episodes.
Introduction
Swallow syncope is a rare form of neurally mediated syncope (NMS), specifically of the cardioinhibitory type, that is associated with sinus arrest and/or functional atrioventricular (AV) block. It is caused by overactivation of the vagus nerve during swallowing of meals.1,2 Because of the limited efficacy and possible adverse effects of medications and pacemakers,3,4 cardioneuroablation has been proposed as an alternative therapeutic strategy for cardioinhibitory NMS, with promising preliminary results.5, 6, 7 To date, however, there have been only a limited number of case reports on cardioneuroablation for swallow syncope.8,9 Here, we present a case of drug-resistant swallow syncope with transient advanced AV block, successfully treated by selective ablation of the posteromedial left ganglionated plexus (GP).
Case report
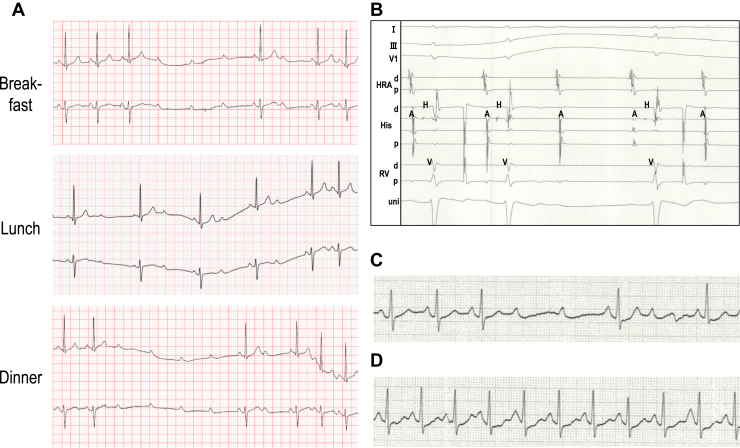
The patient was a 23-year-old female subject with drug-resistant daily swallow syncope. She reported faintness and chest discomfort while swallowing meals from the age of 15, with the frequency of symptoms increasing from a few times per week initially to several times a day and complete loss of consciousness occurring at age 22. There were no apparent triggers for symptom worsening. Although diet modification, such as avoiding potatoes and carbonated drinks, was somewhat effective in reducing symptom duration, it did not reduce the frequency of symptoms. The patient visited a local doctor and was referred to a regional hospital center, where a 12-lead electrocardiography (ECG) and echocardiography revealed no abnormalities. However, Holter monitoring revealed advanced AV blocks at the time of symptoms during meals (Figure 1A). The sinus rates during the AV blocks were maintained at >60 beats/min. Further evaluation with an electrophysiological study under awake conditions showed an AH interval of 129 ms, an HV interval of 52 ms, and normal sinus node recovery time (941 ms). A transient AV block was observed during the ingestion of a rice ball, but not after intravenous administration of atropine (0.5 mg). An intracardiac ECG revealed that the AV block was a suprahisian block (Figure 1B). Upper gastrointestinal fibroscopy revealed no abnormal organ findings in the esophagus, stomach, or duodenum. Balloon dilatation in the middle of the esophagus under endoscopic guidance induced a transient advanced AV block (Figure 1C), but not after intravenous injection of atropine (0.5 mg) (Figure 1D). These results strongly suggest that the transient AV blocks during meals were suprahisian blocks associated with vagus nerve stimulation by esophageal dilatation during swallowing. Thus, the patient was diagnosed with swallow syncope and referred to our hospital for disease treatment.
Figure 1.
A: Holter monitoring revealed transient advanced atrioventricular (AV) block during swallowing of meals. Sinus rate was maintained (>60 beats/min) at the time of the AV block. B: Intracardiac electrocardiography showed AH block during ingestion of a rice ball. C: Dilation of esophageal balloon under endoscopic guidance induced transient advanced AV block. D: After intravenous administration of atropine (0.5 mg), the AV block was no longer induced by esophageal balloon dilatation.
First, we administered oral atropine sulfate before meals, which was effective in relieving the patient’s symptoms but was not well tolerated, as evidenced by a low-grade fever. Likewise, cilostazol was effective but not tolerated owing to headaches and nausea. We also tested disopyramide, tulobuterol, pimobendan, and docarpamine, but none of them was effective. Given the drug-resistant nature of the disease and the frequency of symptoms severely impairing the patient’s quality of life, invasive treatment was deemed necessary. Considering her young age and the lifelong risk of device complications, implantation of a conventional transvenous pacemaker seemed inappropriate. Therefore, we recommended cardioneuroablation rather than leadless pacemaker implantation. The patient preferred cardioneuroablation and was admitted to our hospital.
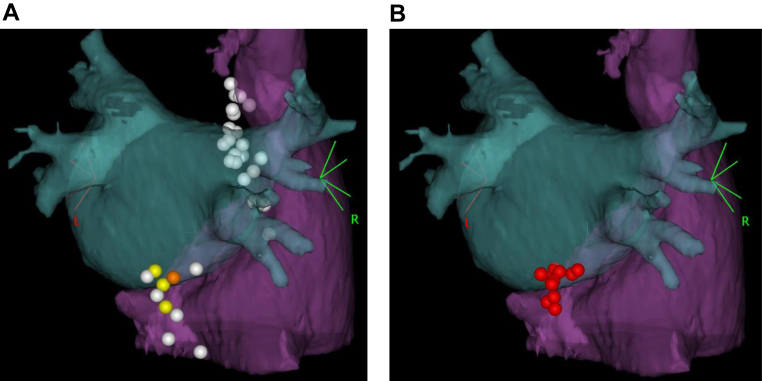
Cardioneuroablation was performed under deep conscious sedation with intravenous administration of midazolam and dexmedetomidine, and ventilation support using bilevel positive airway pressure. A bolus of 5000 units of unfractionated heparin was administered after the insertion of 3 sheaths via the right femoral vein. Two decapolar catheters were placed in the coronary sinus (CS) and His bundle regions. An esophageal balloon was inserted through the right nasal cavity and dilated in the middle of the esophagus under fluoroscopic guidance. The dilatation of the esophageal balloon did not induce an AV block, presumably owing to deep conscious sedation, but reproducibly prolonged the AH interval under CS pacing with a cycle length of 700 ms (Figure 2A). Thus, the procedural endpoint was set as the disappearance of AH prolongation induced by esophageal balloon dilatation. Transseptal puncture was performed under intracardiac ultrasound guidance, and the activated clotting time was maintained above 300 seconds by continuous intravenous infusion of unfractionated heparin. Previously acquired enhanced computed tomography images were integrated into a 3D mapping system (CARTO3; Biosense Webster Inc, Irvine, CA) (Figure 3). High-frequency stimulation (HFS) with a frequency of 20 Hz and an amplitude of 30 mA was delivered from the distal tip of an ablation catheter (ThermoCool SmartTouch™ Surround Flow; Biosense Webster) for 5 seconds to both the right and left sides of the atrial septum, including the CS ostium and inferior vena cava, to identify the locations of the GPs. A positive parasympathetic response to the HFS was defined as an R-R prolongation of ≥50%. Three points at the CS ostium and 1 point at the posteroseptal mitral annulus showed a positive parasympathetic response to the HFS, all of which were located in the posteromedial left GP area. No parasympathetic responses were observed in the superior right GP area (Figure 3A and Supplemental Figure 1). Radiofrequency (RF) energy was delivered to the sites with a positive parasympathetic response (Figure 3B), with a target ablation index at 500–600 for the CS ostium and 550–650 for the posteroseptal mitral annulus. After 10 RF energy applications, the prolongation of the AH interval by esophageal balloon dilation disappeared (Figure 2B), reaching the endpoint of the ablation procedure.
Figure 2.
Intracardiac electrocardiography at the time of esophageal balloon dilatation under coronary sinus (CS) pacing (cycle length = 700 ms). The AH interval was reproducibly prolonged at baseline (A) but not after ablation (B).
Figure 3.
Three-dimensional anatomical images of cardioneuroablation targeting the posteromedial left ganglionated plexus from the reverse left anterior oblique view. Previously acquired enhanced computed tomography images were integrated into the 3-D mapping system. A: Yellow tags indicate points with positive parasympathetic response to high-frequency stimulation located at the coronary sinus ostium and orange tags at the posteroseptal mitral annulus. White tags represent the points without positive parasympathetic response. B: Red tags represent the points of radiofrequency energy applications.
Following the cardioneuroablation, the patient’s symptoms completely resolved. Holter monitoring on day 3 showed accelerated junctional beats surpassing the sinus rhythm and no bradycardia during meals (Supplemental Figures 2 and 3). The patient was discharged on day 4 after the procedure and has been free of any symptoms for 11 months. Holter monitoring performed 3 months after ablation showed no bradyarrhythmia.
Discussion
Swallow syncope is a rare form of cardioinhibitory NMS that occurs when esophageal dilatation during swallowing triggers vagus nerve overstimulation, which leads to sinus arrest and/or transient AV block, resulting in syncope.1,2 Although some investigators have emphasized the importance of lifestyle changes, including avoidance of triggers and diet modification,2 there seemed to be no apparent triggers for worsening symptoms in the present case. In addition, the patient had already undergone diet modification, avoiding potatoes and carbonated drinks. Further food restrictions, such as avoidance of solid foods, would have likely impaired her quality of life in another way. Pharmacological therapy with antibradycardia drugs, such as atropine sulfate, has been used for treating swallow syncope.1 However, its long-term efficacy is unclear, and side effects are a matter of concern. Indeed, in this case, both atropine and cilostazol were effective but could not be continued owing to intolerable side effects.
Although pacemaker implantation is often considered as a treatment for cardioinhibitory NMS, its efficacy remains controversial. Even in recent randomized controlled trials that enrolled highly selected cardioinhibitory NMS patients aged ≥40 years, syncope recurred in 8.7%–21% of patients receiving active DDD pacing.3 This was likely owing to a considerable number of patients diagnosed with cardioinhibitory NMS also having vasodepressor syncope, a so-called mixed-type NMS. In contrast, swallow syncope is considered a pure cardioinhibitory NMS because it occurs instantly during swallowing associated with sinus arrest and/or transient AV block. Therefore, pacemaker implantation for the treatment of swallow syncope is theoretically promising. Indeed, among case reports of swallow syncope, pacemaker implantation successfully relieved symptoms in 52 of 53 cases (98.1%).1 However, implantation of a conventional transvenous pacemaker in young patients poses a lifelong risk of device complications, as well as cosmetic issues. Therefore, in the present case, we recommended leadless pacemaker implantation to the patient as a second-line therapy when cardioneuroablation would be unsuccessful.
Cardioneuroablation was first reported by Pachon and colleagues5 in 2005. The study population comprised 21 patients with NMS, intermittent high-degree AV block, and/or sinus node dysfunction. They targeted 3 GPs from the right atrium: the aortic-superior vena cava GP located between the superior vena cava and aortic root, the superior right GP located between the right superior pulmonary vein and right atrium, and the posteromedial left GP located between the inferior vena cava and right and left atria. The RF energy was delivered to the GP areas under the guidance of spectral and anatomical mapping. During a mean follow-up period of 9.2 months, all patients experienced symptom relief.5 Thereafter, cardioneuroablation has been performed not only for NMS and bradyarrhythmias5, 6, 7, 8, 9 but also for atrial fibrillation.10 In addition to the 3 GP sites in the right atrium, 5 GP sites in the left atrium have been targeted.10 Also, HFS and/or simple anatomical approach has been proposed to identify the locations of GPs instead of spectral mapping.6, 7, 8, 9, 10 Recently, a first randomized controlled trial evaluating the efficacy of cardioneuroablation for cardioinhibitory NMS was reported.7 Cardioneuroablation was superior to conventional nonpharmacologic therapy (education and lifestyle modification) in terms of lower rate of recurrent syncope during 25 months of follow-up (8% vs 54%, P = .0004).
Although the results of previous studies on cardioneuroablation for NMS are encouraging, targeting of all GP sites in the right and left atria seems time-consuming. It has been reported that the superior right GP innervates the sinus node, whereas the posteromedial left GP innervates the AV node.11,12 Also given its anatomical proximity to the AV node, the posteromedial left GP is likely to be the final direct pathway of the parasympathetic nerve input to the AV node. Indeed, in a recent report by Aksu and colleagues13 treating 31 patients with functional AV block, ablation of the posteromedial left GP was critically important for successful vagal denervation of the AV node. In the case presented here, the sinus rate was maintained (>60 beats/min) at the time of the AV block during swallowing (Figure 1A). Therefore, to selectively denervate the AV node without affecting the sinus node, we targeted the posteromedial left GP only. Interestingly, in a recent case report by Ascione and colleagues,14 cardioneuroablation targeting the superior right GP in an NMS patient with sinus arrest resulted in recurrent syncope with advanced AV block, which necessitated a second ablation procedure targeting the posteromedial left GP. In patients with cardioinhibitory NMS with sinus arrest, suppressed AV node conduction at the time of sinus arrest may be masked, and so cardioneuroablation should target both the superior right GP and posteromedial left GP for denervation of the sinus and AV nodes. However, selective ablation of the posteromedial left GP seems sufficient for a functional AV block with a maintained sinus rate. This minimal ablation strategy can avoid the risk of inappropriate sinus tachycardia6,9 and may reduce the potential risk of procedural complications. At the same time, it may be associated with accelerated junctional beats surpassing the sinus rhythm, which can be symptomatic in sensitive patients. Actually, in the present case, junctional beats competing with the sinus rhythm during meals were documented on Holter monitoring after the ablation procedure (Supplemental Figure 2), although the patient was completely asymptomatic throughout the follow-up period.
This study has several limitations. First, the procedural endpoint used in this case—that is, the disappearance of AH prolongation induced by esophageal balloon dilatation—may not have been a strong enough indicator of AV nodal denervation. Second, we should have checked the procedural endpoints immediately after applying RF energy to the 4 HFS-positive sites before proceeding with the anatomical safety applications.
Conclusion
Here, we present a case of successful cardioneuroablation for swallow syncope with a functional AV block. The posteromedial left GP was selectively ablated by applying RF energy from the CS ostium and posteroseptal mitral annulus, which eliminated the functional AV block during swallowing and completely relieved the associated symptoms for 11 months. When the sinus rate is maintained at the time of the functional AV block, ablation of the posteromedial left GP, the final pathway of the parasympathetic nerve input to the AV node, is crucial and may be sufficient. Future large-scale studies should evaluate the safety and efficacy of this minimal ablation strategy selectively targeting the posteromedial left GP in the treatment of functional AV block with maintained sinus rate.
Footnotes
Funding Sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures: The authors have no conflicts to disclose.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.hrcr.2023.04.022.
Appendix. Supplementary Data
References
- 1.Siew K.S.W., Tan M.P., Hilmi I.N., Loch A. Swallow syncope: a case report and review of literature. BMC Cardiovasc Disord. 2019;19:191. doi: 10.1186/s12872-019-1174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dani M., Dirksen A., Taraborrelli P., et al. “Be careful what you swallow”: a case series of swallow syncope. JACC Case Rep. 2021;3:469–473. doi: 10.1016/j.jaccas.2020.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behnoush A.H., Yazdani K., Khalaji A., et al. Pharmacologic prevention of recurrent vasovagal syncope: a systematic review and network meta-analysis of randomized controlled trials. Heart Rhythm. 2023;20:448–460. doi: 10.1016/j.hrthm.2022.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Sutton R., de Jong JSY, Stewart J.M., Fedorowski A., de Lange F.J. Pacing in vasovagal syncope: physiology, pacemaker sensors, and recent clinical trials-precise patient selection and measurable benefit. Heart Rhythm. 2020;17:821–828. doi: 10.1016/j.hrthm.2020.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pachon J.C., Pachon E.I., Pachon J.C., et al. “Cardioneuroablation”--new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. 2005;7:1–13. doi: 10.1016/j.eupc.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Aksu T., Golcuk E., Yalin K., Guler T.E., Erden I. Simplified cardioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol. 2016;39:42–53. doi: 10.1111/pace.12756. [DOI] [PubMed] [Google Scholar]
- 7.Piotrowski R., Baran J., Sikorska A., Krynski T., Kulakowski P. Cardioneuroablation for reflex syncope: efficacy and effects on autonomic cardiac regulation-a prospective randomized trial. JACC Clin Electrophysiol. 2023;9:85–95. doi: 10.1016/j.jacep.2022.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Štiavnický P., Wichterle D., Hrošová M., Kautzner J. Cardioneuroablation for the treatment of recurrent swallow syncope. Europace. 2020;22:1741. doi: 10.1093/europace/euaa060. [DOI] [PubMed] [Google Scholar]
- 9.Miranda-Arboleda A.F., Burak C., Abdollah H., Baranchuk A., Aksu T., Enriquez A. Cardioneuroablation for swallowing-induced syncope: to pace or to ablate, that is the question. Heart Rhythm Case Rep. 2023;9:P283–P286. doi: 10.1016/j.hrcr.2023.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katritsis D.G., Pokushalov E., Romanov A., et al. Autonomic denervation added to pulmonary vein isolation for paroxysmal atrial fibrillation: a randomized clinical trial. J Am Coll Cardiol. 2013;62:2318–2325. doi: 10.1016/j.jacc.2013.06.053. [DOI] [PubMed] [Google Scholar]
- 11.Ardell J.L., Randall W.C. Selective vagal innervation of sinoatrial and atrioventricular nodes in canine heart. Am J Physiol. 1986;251:H764–H773. doi: 10.1152/ajpheart.1986.251.4.H764. [DOI] [PubMed] [Google Scholar]
- 12.Chiou C.W., Eble J.N., Zipes D.P. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation. 1997;95:2573–2584. doi: 10.1161/01.cir.95.11.2573. [DOI] [PubMed] [Google Scholar]
- 13.Aksu T., Gopinathannair R., Bozyel S., Yalin K., Gupta D. Cardioneuroablation for treatment of atrioventricular block. Circ Arrhythm Electrophysiol. 2021;14 doi: 10.1161/CIRCEP.121.010018. [DOI] [PubMed] [Google Scholar]
- 14.Ascione C., Benabou L., Hocini M., Jaïs P., Haïssaguerre M., Duchateau J. Cardioneuroablation: don’t underestimate the posteromedial left atrial ganglionated plexus. HeartRhythm Case Rep. 2022;9:67–69. doi: 10.1016/j.hrcr.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.