Summary
Neuronal loss and axonal demyelination underlie long-term functional impairments in patients affected by brain disorders such as ischemic stroke. Stem cell-based approaches reconstructing and remyelinating brain neural circuitry, leading to recovery, are highly warranted. Here, we demonstrate the in vitro and in vivo production of myelinating oligodendrocytes from a human induced pluripotent stem cell (iPSC)-derived long-term neuroepithelial stem (lt-NES) cell line, which also gives rise to neurons with the capacity to integrate into stroke-injured, adult rat cortical networks. Most importantly, the generated oligodendrocytes survive and form myelin-ensheathing human axons in the host tissue after grafting onto adult human cortical organotypic cultures. This lt-NES cell line is the first human stem cell source that, after intracerebral delivery, can repair both injured neural circuitries and demyelinated axons. Our findings provide supportive evidence for the potential future use of human iPSC-derived cell lines to promote effective clinical recovery following brain injuries.
Keywords: Oligodendrocyte, Oligodendrogenesis, Remyelination, Demyelination, iPS cells, Cell therapy, Stroke, Myelin, Human brain
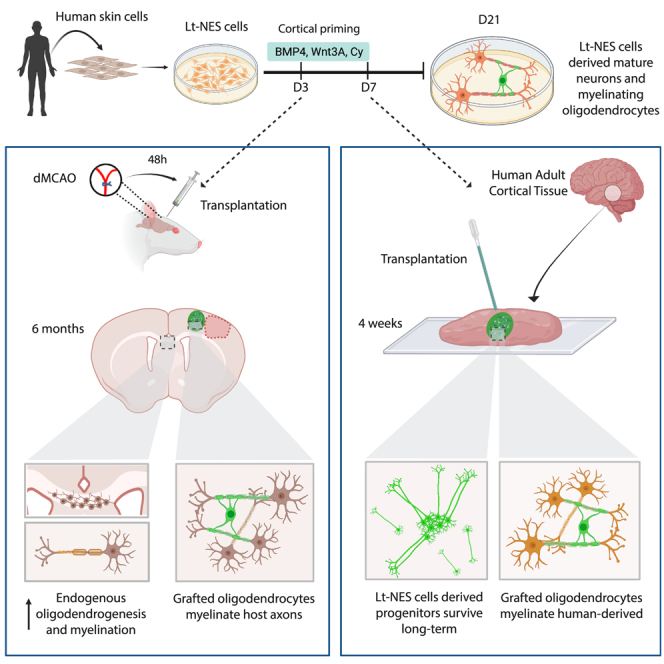
Graphical abstract
Highlights
-
•
Human iPSC line-generating neurons also produces myelinating oligodendrocytes
-
•
Human iPSC line-derived cells myelinate axons in stroke-injured rat brain
-
•
Human iPSC line-derived cells myelinate axons in adult human cortical slices
Kokaia and colleagues describe the first human iPSC-derived cell line with the capacity for concomitant generation of both mature functional neurons and myelinating oligodendrocytes after intracerebral transplantation. This cell source could provide the basis for a novel stem cell-based approach to reconstruct and remyelinate injured neural circuitry and to promote functional recovery also in human brain disorders.
Introduction
Several disorders affecting the human brain, such as ischemic stroke, head trauma, and multiple sclerosis, lead to both neuronal loss and axonal demyelination, which underlie the functional deficits (Benedict et al., 2020; Kuhn et al., 2019; Nasrabady et al., 2018). A major cause of demyelination is the death of oligodendrocytes, i.e., the cell population producing myelin. The brain reacts to demyelination by two compensatory mechanisms: first, through the production of new myelin from oligodendrocytes in the area surrounding the lesion (Duncan et al., 2018), resulting in fewer and, in some cases, mistargeted myelin sheets (Neely et al., 2022); second, by increased endogenous oligodendrogenesis (Franklin et al., 2021). However, this response is limited and most newly generated oligodendrocytes fail to differentiate and remyelinate (Hughes et al., 2018; Marin and Carmichael, 2019).
Transplantation of remyelinating cells derived from stem cells has the potential to become a novel approach for treating myelin loss in the human brain. Oligodendrocytes can be generated from human induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs) using different reprogramming and differentiation protocols (Ehrlich et al., 2017; Garcia-Leon et al., 2018; Shaker et al., 2021; Wang et al., 2013). Moreover, grafted oligodendrocytes produced from human pluripotent stem cells are capable of remyelinating host axons in the rodent’s central nervous system. Human ESC-derived oligodendrocyte progenitor cells (OPCs), transplanted into the demyelinated mouse spinal cord and irradiated rat brain, remyelinated host-derived demyelinated axons (Nistor et al., 2005; Piao et al., 2015). Also, human iPSC-derived OPCs and isolated O4+ population (containing immature and mature oligodendrocytes), grafted into mouse injured spinal cord and demyelinated corpus callosum, remyelinated axons (Ehrlich et al., 2017; Kawabata et al., 2016). Besides replacing myelinating cells, human stem cell-derived transplants can promote remyelination in animal models by other mechanisms. For example, intracerebral transplantation of human astrocytic-fated iPSC-derived progenitors increased endogenous oligodendrogenesis and remyelination via the release of growth factors in mice with white matter stroke (Llorente et al., 2021). Whether human oligodendroglial progenitors, derived from iPSCs or ESCs, can remyelinate demyelinated tissue after transplantation in the adult human brain is unknown.
Effective repair in brain disorders using cell transplantation will require the replacement of both neurons and oligodendrocytes, capable of remyelinating host and grafted neurons. Currently, little is known whether the same human stem cell source can give rise to both functional neurons and myelinating oligodendrocytes after transplantation into the adult brain (Baker et al., 2017; Nori et al., 2011). We recently showed that human iPSC-derived long-term neuroepithelial stem (lt-NES) cells, fated toward a cortical neuronal phenotype and transplanted into the rat cortex adjacent to an ischemic lesion, gave rise to functional cortical neurons. These neurons sent projections to both hemispheres, became integrated into host neural circuitry, and reversed sensorimotor deficits (Palma-Tortosa et al., 2020; Tornero et al., 2017). We also found that 40% of cells in the transplant, a substantial number of graft-derived cells in the corpus callosum, and a few of them in the thalamus and striatum, expressed the oligodendrocyte marker SOX10. Moreover, human-derived myelin basic protein (MBP) was observed close to the transplant, providing suggestive evidence that the graft-derived SOX10+ cells could be oligodendrocytes contributing to remyelination.
Here, we demonstrate that the human iPSC-derived lt-NES cells, primed toward a cortical neuronal phenotype, produce bona fide oligodendrocytes both in vitro and in vivo. The generated cells display the structural, molecular, and functional characteristics of human mature oligodendrocytes and myelinate lt-NES cell-derived axons in culture as well as host-derived axons after xenotransplantation into rat stroke-injured cortex and allotransplantation into human adult cortical organotypic cultures.
Results
Cortically fated human lt-NES cells form myelinating oligodendrocytes in cell culture
In our previous study (Palma-Tortosa et al., 2020), we obtained some evidence for the presence not only of neurons but also of myelin-forming oligodendrocytes in the grafts after intracerebral transplantation of cortically primed human lt-NES cells in a rat stroke model. Here, we wanted to determine, first in vitro, whether the lt-NES cells can form genuine functional oligodendrocytes in addition to neurons. We, therefore, differentiated lt-NES cells according to our cortical priming protocol for up to 21 days in the cell culture (Tornero et al., 2013), and analyzed protein and gene expression of different oligodendrocytes and neuronal markers.
Using immunocytochemistry, we found that undifferentiated lt-NES cells (at day 0) were positive for SOX10, a marker known to be expressed in both neuroectodermal cells and oligodendrocytes, but did not express the neuroblast marker doublecortin (DCX) or the pan-oligodendrocyte marker OLIG2 (Figure S1A). While SOX10 expression decreased with time, the expression of DCX and OLIG2 started following 4 days of differentiation with a tendency to increase (Figure S1A). Expression of MBP started on day 8 in cells displaying the bipolar morphology typical of OPCs. At later time points, we observed an increase in morphological complexity (branching) of MBP-expressing cells, characteristic of pre-myelinating and myelinating oligodendrocytes (Figure S1A). Flow cytometry showed increased O4 (a marker for pre-myelinating and myelinating oligodendrocytes) expression over time (Figure S1D). Similarly, using quantitative reverse-transcription polymerase chain reaction (qRT-PCR), we observed that our cortical priming protocol gave rise to increased DCX, OLIG2, and MBP gene expression starting at day 8 (Figure S1B).
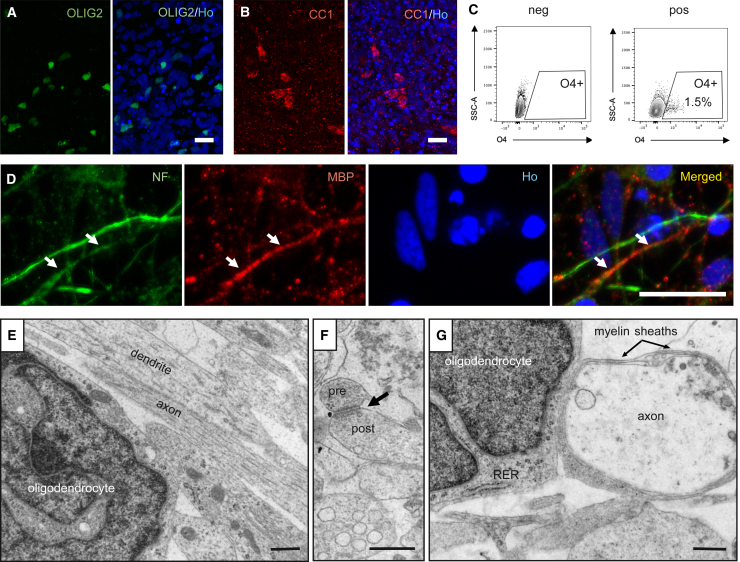
We then asked if the cortically primed human lt-NES cell-derived oligodendrocytes, differentiated for 21 days, can myelinate human lt-NES cell-derived axons. At this time point, immunocytochemistry demonstrated the presence of 5% OLIG2- and 3% CC1 (a marker of mature oligodendrocytes)-expressing cells in the cultures (Figures 1A and 1B). To verify that the observed CC1 expression was not due to the presence of astrocytes in the culture, we performed double staining of CC1 with the astrocytic markers GFAP and S100β. No colocalization was found (data not shown). Moreover, flow cytometry showed that 1.5% of the cells expressed O4 (Figure 1C, 1.5% ± 0.28%, n = 4). Importantly, we found MBP expression adjacent to the axonal marker Neurofilament, suggesting that the oligodendrocytes in the culture could be involved in axonal myelination (Figure 1D).
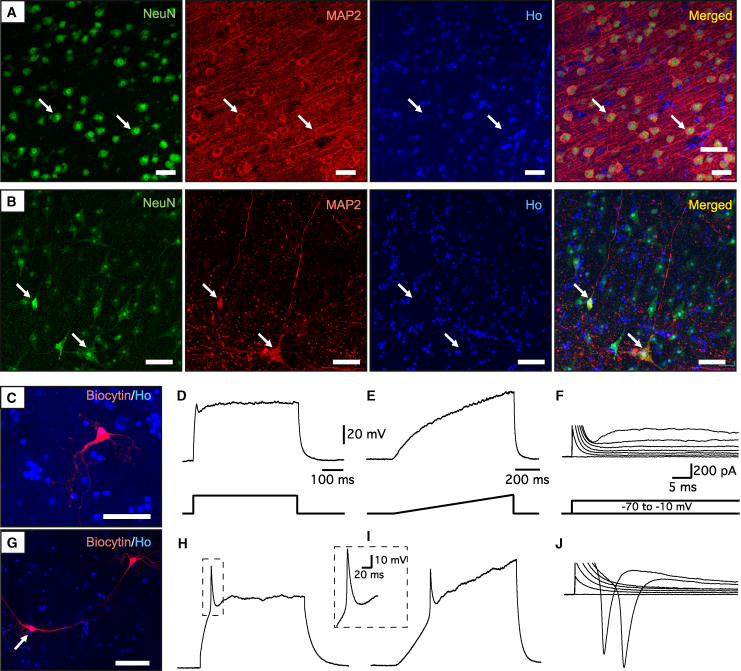
Figure 1.
Cortically primed human lt-NES cells generate mature neurons and myelinating oligodendrocytes after 21 days of in vitro differentiation
(A and B) Confocal images of lt-NES cells differentiated for 21 days showing the expression of (A) the pan-oligodendrocyte marker OLIG2 and (B) the mature oligodendrocyte marker CC1. Representative images of n = 4 independent experiments (ind. exp.). Scale bars, 20 μm.
(C) Flow cytometry analysis of O4+ cells, including pre-myelinating and myelinating oligodendrocytes, after 21 days of differentiation (n = 4 ind. exp.).
(D) Confocal immunohistochemical images showing axons immunoreactive for Neurofilament and myelin basic protein (MBP) expressed in closed proximity in culture (arrows depict proximity between both markers). Ho, Hoechst. Scale bars, 20 μm.
(E–G) EM images showing (E) a mature lt-NES cell-derived oligodendrocyte and neural processes, (F) an axodendritic contact with structural characteristics of the asymmetric synapse (arrow indicates a synapsis containing pre- and post-synaptic components), and (G) lt-NES cell-derived myelin loosely wrapped around an lt-NES cell-derived axon. Representative images of n = 8 ind. exp. RER, rough endoplasmic reticulum. Scale bars, 500 nm (E–G).
We used electron microscopy (EM) to provide further evidence for the presence of lt-NES cell-derived oligodendrocytes and their ability to myelinate human axons in the cultures. In addition to neurons (Gronning Hansen et al., 2020), we detected a cell population exhibiting the ultrastructural features of mature oligodendrocytes, i.e., irregularly shaped dark nucleus with clumped chromatin near the inner nuclear membrane (Figure 1E). The cytoplasm was electron dense and contained short cisternae of rough endoplasmic reticulum with short mitochondria. A large number of dendrites and axons was observed in the neuropil (Figure 1E). The neural processes formed axodendritic contacts with structural characteristics of asymmetric synapses (Figure 1F), suggesting that the lt-NES cell-derived neurons had established a network already at 21 days in culture. Importantly, the lt-NES cell-derived oligodendrocytes formed loose myelin sheaths around axons, which could be the initial stage of myelination (Figure 1G).
Cortically fated human lt-NES cells form oligodendrocytes and myelinate host axons after transplantation in stroke-injured rat cortex
We then explored whether intracerebrally transplanted, cortically fated lt-NES cell-derived progenitors could remyelinate axons after stroke, i.e., an injury-causing axonal demyelination (Zuo et al., 2019). Rats were subjected to cortical stroke, implanted with human lt-NES cells close to the injury after 48 h, and sacrificed 6 months later. In all the animals, the stroke-induced neuronal loss, as determined by lack of NeuN (marker of mature neurons) immunoreactivity, was restricted to the cortex (mostly somatosensory cortex [S1FF and S1BL] and motor area [M1]), sparing subcortical structures (Palma-Tortosa et al., 2020).
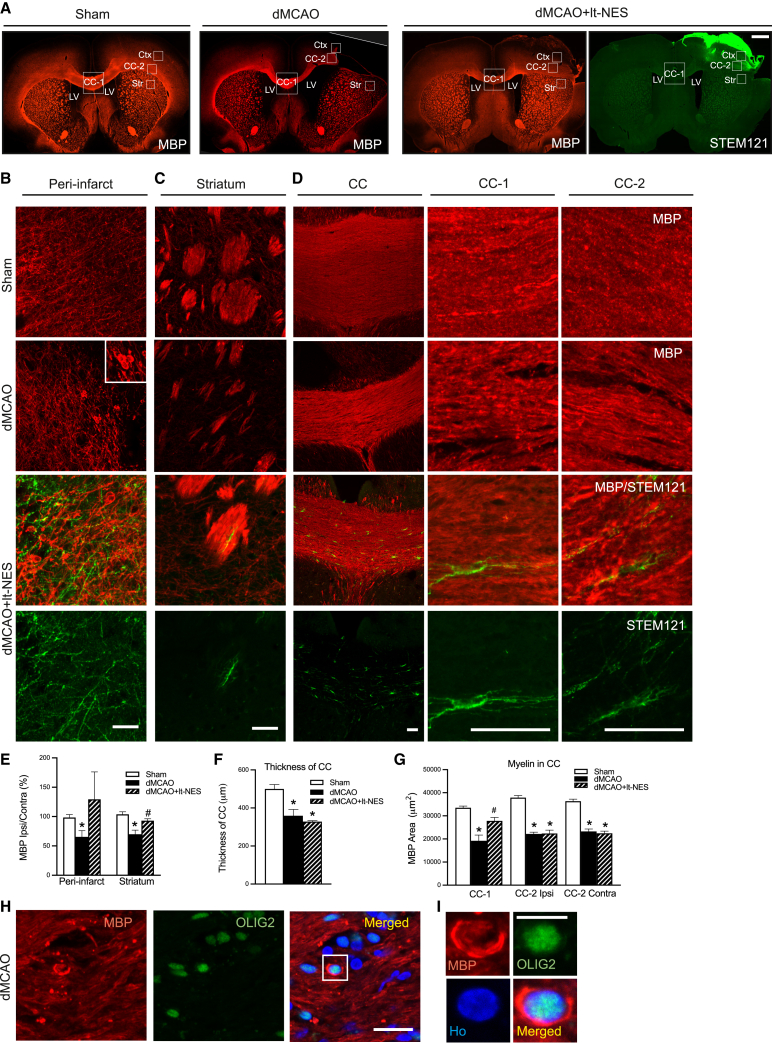
To study the overall distribution of axonal demyelination following stroke, we quantified the expression of MBP as a measure of myelin density (Figure 2). The ischemic insult caused decreased MBP expression in the dorsal striatum, the middle and lateral parts of the corpus callosum and its thickness and peri-infarct areas (Figures 2A–2G). Ectopic MBP expression was detected around OLIG2+ cell bodies close to the injury in stroke animals (Figures 2H and 2I). Transplantation of lt-NES cells resulted in increased MBP expression in the middle part of the corpus callosum and dorsal striatum and a similar tendency in the peri-infarct area compared with non-grafted rats, whereas the thickness of corpus callosum did not differ between the groups of stroke-affected animals (Figures 2A–2G). The presence of lt-NES cells in areas of demyelination was mainly restricted to the peri-infarct zone and corpus callosum, illustrating that the contribution of grafted cells to remyelination was limited and indicating that other myelination mechanisms were most likely triggered by the transplantation (Figures 2A–2D).
Figure 2.
Intracortical transplantation of human lt-NES cell-derived progenitors improves myelination in stroke-injured rat brains
(A) Coronal overview of MBP+ area in the rat brain of sham-treated animals (Sham) and in animals subjected only to stroke (dMCAO) or stroke followed by transplantation (dMCAO+lt-NES). An overview of the area positive for the human cell cytoplasmic marker STEM121+ is also included in the transplanted group. White squares show the areas where images were taken and quantification has been performed. Scale bar, 1 mm. CC-1, middle part of the corpus callosum; CC-2, ipsilateral part of the corpus callosum; Ctx, cortex; LV, lateral ventricle; Str: striatum.
(B–G) Confocal immunohistochemical images and quantification of MBP+ area in (B and E) peri-infarct area, (C and E) dorsal striatum, and (D, F, and G) middle and lateral (ipsilateral and contralateral) parts of the corpus callosum of Sham, dMCAO, or dMCAO+lt-NES. STEM121 staining shows the contribution of graft-derived cells in different areas. Scale bars, 100 μm (B–D). n = 4–5 animals per group.
(H and I) Image showing myelin surrounding OLIG2+ cell (higher magnification in H). Nuclear staining (Hoechst, blue) is included in the merged panel. Scale bars, 20 μm. ∗p < 0.05 vs. Sham. #p < 0.05 vs. dMCAO. Data are shown as mean ± SEM.
Our previous study indicated that the grafted lt-NES cell-derived neurons regulate motor behavior in stroke-injured rats through transcallosal connections to the contralateral hemisphere (Palma-Tortosa et al., 2020). We therefore analyzed in more detail the effect of stroke and lt-NES cell transplantation on the number of oligodendrocytes in the corpus callosum. Immunohistochemical analysis showed more OLIG2+ cells in the middle part of the corpus callosum in animals subjected to stroke compared with sham-treated animals. In transplanted rats, the increase of OLIG2+ cells was even more pronounced. The majority of OLIG2+ cells did not co-express the human nuclear marker STEM101 and were most likely rat-derived cells. Some human cells were also found, even if they contributed only to a fraction of the total number of OLIG2+ cells (Figures S2A–S2C).
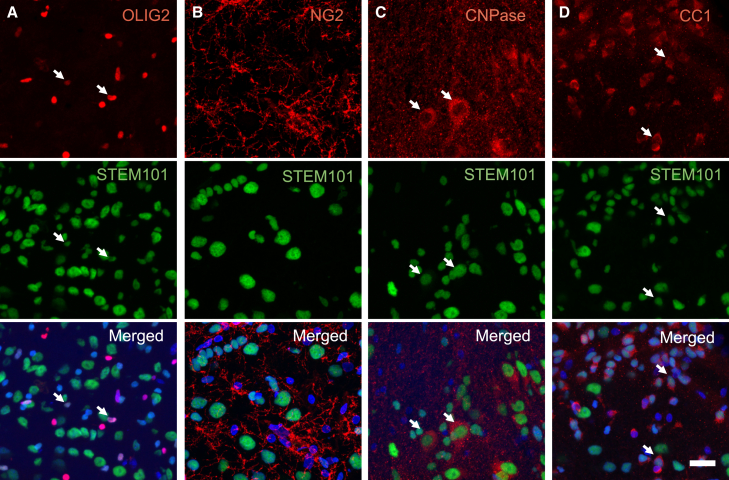
Since we detected OLIG2+ cells of human origin in the corpus callosum, we hypothesized that part of the transplanted lt-NES cell-derived progenitors had become oligodendrocytes and contributed to the remyelination. Arguing for the formation of mature oligodendrocytes in vivo, we found that, at 6 months after transplantation, 15%–20% of the STEM101+ cells in the core of the graft expressed OLIG2 (Figure 3A) and about 20% expressed CNPase (marker for pre-myelinating and myelinating oligodendrocytes) (Figure 3C). We also observed human-derived cells expressing the mature oligodendrocyte marker, CC1, with varying density through the graft (Figure 3D). Hardly any grafted cells expressed neuron-glial antigen 2 (Figure 3B), a marker for OPCs.
Figure 3.
Cortically primed human lt-NES cell-derived progenitors give rise to mature oligodendrocytes 6 months after intracortical transplantation into stroke-injured rat somatosensory cortex
Representative confocal images of the transplantation area showing the expression of (A) the pan-oligodendrocyte marker OLIG2, (B) OPC marker neuron-glial antigen 2 (NG2), (C) marker for immature and mature oligodendrocytes CNPase, and (D) mature oligodendrocyte marker CC1. Arrows indicate the colocalization of respective markers with STEM101, a human nuclear marker. Nuclear staining (Hoechst, blue) is included in the merged panel. Scale bar, 20 μm.
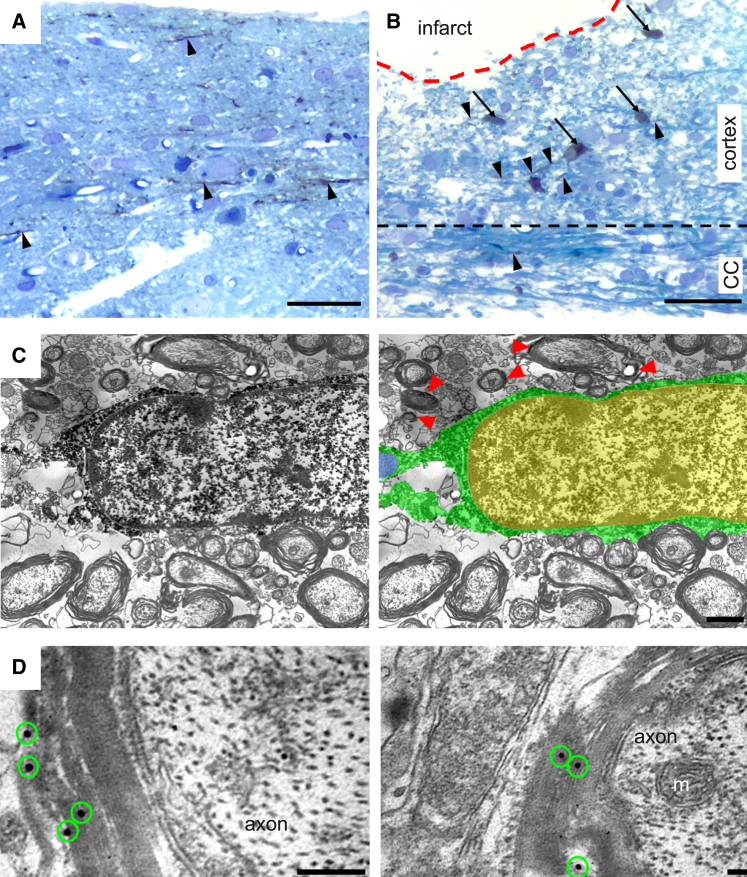
To provide further evidence for the formation of mature myelinating oligodendrocytes, we performed the ultrastructural analysis 6 months after transplantation in stroke-affected animals with grafts of GFP-labeled, cortically fated human lt-NES cells. Following the validation of GFP expression in myelin sheets in our cell line (data not shown), immunoperoxidase reaction or immunogold staining using anti-GFP antibodies was carried out to localize the grafted GFP+ lt-NES cells and their processes, which were easily identified due to the brown DAB reaction product in cytoplasm and processes (Figures 4A and 4B). Most GFP+ cells were located in the peri-infarct area, while some were found in the corpus callosum and contralateral cortex. Some GFP+ cells exhibited the morphology of mature myelinating oligodendrocytes (Figure 4C): dark electron-dense rectangular-shape cytoplasm, heterogeneous nuclear chromatin pattern, and short and wide endoplasmic reticulum cisternae organized in the vicinity of the nucleus.
Figure 4.
Grafted human lt-NES cell-derived oligodendrocytes myelinate axons in the rat injured cortex
(A and B) Light micrographs of a toluidine blue-stained plastic-embedded section from the cortex of stroke-subjected rats contralateral (A) and ipsilateral (B) to the lesion. GFP/DAB-positive lt-NES cell-derived cells were visualized in the peri-infarct area (ipsilateral cortex), corpus callosum, and contralateral cortex. Arrows highlight GFP/DAB-positive cells (brown color), and arrowheads indicate GFP/DAB-positive processes (brown color).
(C) Representative iEM image showing that, at 6 months after transplantation, GFP/DAB-positive cells in the corpus callosum exhibit the morphology of mature myelinating oligodendrocytes (for better visualization in the EM images, GFP/DAB-positive oligodendrocyte cytoplasm and processes are colored green and the nucleus is yellow). The host axon myelinated by the GFP/DAB-positive oligodendrocyte is colored blue. Red arrowheads indicate areas of GFP/DAB-positive myelin.
(D) Electron micrographs of GFP+ immunogold particles (green circles) associated with compact myelin sheaths. CC, corpus callosum; m, mitochondrion. Scale bars, 50 μm (A and B), 2 μm (C), and 0.1 μm (D).
To determine if the human lt-NES cell-derived oligodendrocytes could form myelin sheaths around host axons, we performed post-embedding immunogold labeling of GFP. In support of host axonal myelination by the graft-derived human oligodendrocytes, immuno-EM (iEM) demonstrated individual or clusters of gold particles within the membranous sheets of myelin (i.e., graft-derived myelin) surrounding host unlabeled axons (Figure 4D).
Grafted human lt-NES cell-derived oligodendrocytes myelinate host axons in adult human cortical tissue
We have previously shown that cortically fated lt-NES cell-derived progenitors differentiate to cortical neurons and establish functional connections with host neurons after transplantation onto organotypic cultures of adult human cortex obtained from epileptic patients undergoing surgery (Gronning Hansen et al., 2020). Here, we used this model to determine whether grafted lt-NES cells also give rise to mature oligodendrocytes that can myelinate axons in the adult human cortical environment.
We first assessed the preservation of the cortical tissue during the organotypic culture. In accordance with our previous findings (Gronning Hansen et al., 2020; Palma-Tortosa et al., 2020), we found that the expression of the neuronal marker NeuN was visually unaffected after 4 weeks in culture compared with acute tissue, whereas MAP2 expression had decreased by more than 90% (Figures 5A and 5B). OLIG2, CNPase, and CC1 were expressed in acute tissue (Figures S3A and S3B), and MBP expression resembled the typical aligned axonal distribution in the adult human cortex (Figure S3A). After 4 weeks in culture, the expression of OLIG2, CNPase, and CC1 was unaffected (Figures S4A–S4C). Overall MBP expression was diminished compared with acute tissue (Figure S3A).
Figure 5.
The functionality of neuronal circuitry is compromised in human adult cortical organotypic tissue after 4 weeks in culture
(A and B) Representative confocal images of (A) acute and (B) 4-week-cultured human cortical tissue showing the expression of the neuronal markers NeuN and MAP2. Arrows indicate colocalization. Nuclear staining (Hoechst, blue) is included in the merged panel.
(C–J) Examples of cortical neurons recorded in 4-week-cultured human cortical tissue. (C and G) Biocytin labeling of the recorded neurons. (D–F) Whole-cell patch-clamp recording traces from the cell in (C). The cell is not able to fire AP either at step (250 pA) (D) or at the ramp (0–300 pA) (E) current injection in current clamp mode. (F) The same cell has a very small inward sodium current upon 10 mV depolarization step in voltage-clamp mode at −70 mV holding potential. (H–J) Patch-clamp recording of another cell, shown in (G), that was able to generate a single AP in the similar protocol as above (H and I), with a bigger sodium current in voltage-clamp mode (J). Scale bars are the same for the upper and lower panels of the traces unless otherwise indicated. Ho, Hoechst. Scale bars, 50 μm.
Electrophysiological analysis showed that two out of seven recorded neurons were able to fire a single action potential (AP) (Figures 5G–5I), while the majority were unable to generate any (five out of seven) (Figures 5C–5E). Inability to fire AP corresponded with small inward sodium currents, indicating that sodium conductance, which is essential for normal neuronal function and an initial phase of AP, was compromised (Figures 5F and 5J). However, passive membrane properties, such as membrane resistance (301 ± 67 MΩ) and resting membrane potential (−74.7 ± 2.5 mV), were still comparable with the values reported in fresh adult human cortical tissue (Avaliani et al., 2014), indicating that the neurons are still alive and relatively viable, although not very active.
Taken together, our data indicate that the architecture of the adult human cortical tissue is largely preserved but the functionality of the neuronal network is decreased after 4 weeks in culture.
To assess their capacity to differentiate into myelinating oligodendrocytes in the adult human cortical environment, cortically fated GFP+ lt-NES cells were then transplanted onto the organotypic slice cultures (Figure S5). We found graft-derived (GFP+ cells) with extensive arborizations throughout the organotypic culture after 4 weeks (Figure 6A). About 40%–50% of the GFP+ cells colocalized with the pan-oligodendrocyte marker OLIG2 (Figures 6B and 6C). Importantly, colocalization of GFP+ cells with either CNPase or CC1 were also found, arguing for the presence of both immature and mature graft-derived oligodendrocytes (Figures 6D–6G). Due to the cytoplasmic nature of CNPase and CC1 staining and, since a human cell marker cannot be used to identify human cells transplanted into human tissue, the percentage of grafted cells positive for these markers could not be evaluated.
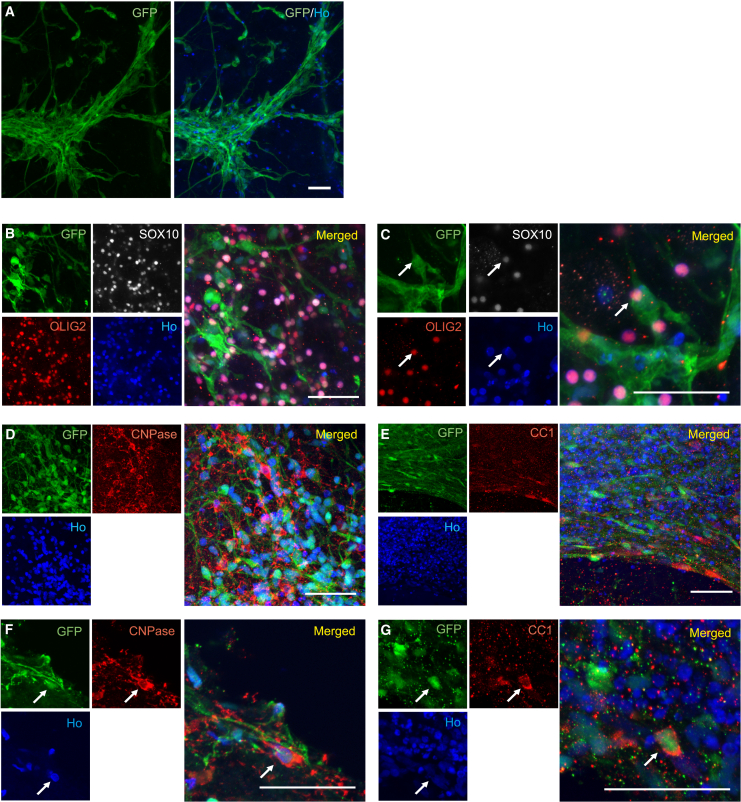
Figure 6.
Human lt-NES cell-derived progenitors survive long-term and become mature oligodendrocytes 4 weeks after ex vivo transplantation into organotypic cultures of the human adult cortex
(A) Overview of grafted GFP+ lt-NES cells 4 weeks after transplantation into the human cortical culture.
(B–G) Confocal images showing GFP+ lt-NES cells after ex vivo transplantation expressing (B and C) SOX10 and OLIG2, (D and F) CNPase, and (E and G) CC1. (B, D, and E) Low-magnification images. (C, F, and G) High-magnification images. Ho, Hoechst. Arrows indicate colocalization. Scale bars, 50 μm.
We finally determined if the grafted human GFP+ lt-NES cell-derived oligodendrocytes had formed myelin sheaths. Anti-GFP antibodies and immunogold labeling combined with iEM demonstrated the presence of individual or clusters of gold particles within the membranous sheets of myelin (signifying graft-derived myelin) surrounding non-GFP-labeled host axons (Figures 7A and 7B). These results provide strong evidence that lt-NES cell-derived oligodendrocytes can myelinate human-derived axons after transplantation into adult human cortical tissue.
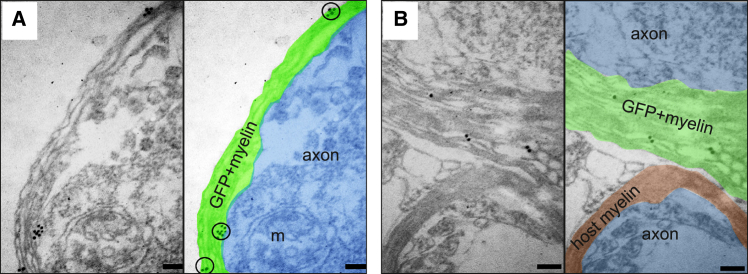
Figure 7.
Human lt-NES cell-derived oligodendrocytes myelinate human-derived axons 4 weeks after ex vivo transplantation into organotypic cultures of the human adult cortex
(A and B) iEM images showing human grafted GFP+ lt-NES cell-derived myelin wrapping human axons in organotypic cultures. For better visualization in the EM images, GFP/gold-positive myelin is colored green, GFP-negative myelin is colored red, and axons are colored blue. Black circles depict immunogold particles associated with compact myelin sheaths. m, mitochondrion. Scale bars, 0.1 μm.
Discussion
Here, we describe the in vitro and in vivo production of myelinating oligodendrocytes from a human iPSC-derived cell line, which also gives rise to neurons with the capacity to integrate into the adult human neural circuitry (Palma-Tortosa et al., 2020). Most importantly, the generated oligodendrocytes survive and form myelin-ensheathing human axons in the host tissue after grafting onto adult human cortical organotypic cultures. We have previously shown that the human iPSC-derived cortical neurons establish connections with the human host neurons in these slice cultures (Gronning Hansen et al., 2020). Taken together, we report for the first time a human iPSC-derived cell line, primed toward a cortical neuronal phenotype, with the capacity to generate both neurons and oligodendrocytes reconstructing neural circuitry and myelinating axons in the adult human cerebral cortex.
Our conclusion that the generated cells are bona fide functional oligodendrocytes is inferred from the following findings: first, the lt-NES cell-derived progenitors expressed oligodendrocyte lineage markers, such as OLIG2, O4, CNPase, and CC1, as evidenced by immunocytochemistry and flow cytometry; second, EM showed the presence of mature oligodendrocytes and myelin around lt-NES cell-derived axons after 21 days of in vitro differentiation; third, 6 months after intracortical transplantation into stroke-injured rat cortex, 20% of grafted cells had become oligodendrocytes, expressing OLIG2, CNPase, and CC1, and graft-derived myelin was present in areas of demyelination, as observed with MBP immunohistochemistry and EM; fourth, 1 month after transplantation onto adult human cortical cultures, grafted cells expressed markers for mature oligodendrocytes (CNPase and CC1), and graft-derived myelin was found around host human axons using immunohistochemistry and EM.
Generally, human neural progenitor cell- or stem cell-derived OPCs are used to generate myelinating oligodendrocytes (Chanoumidou et al., 2020) and neural differentiation protocols to produce functional neurons (Tornero et al., 2013; Zhao et al., 2020). Rapid concomitant generation of both mature neurons and myelinating oligodendrocytes from human stem cells in culture is a challenge. For example, differentiation of human iPSC-derived lt-NES cells into both neuronal and glial lineages gave rise to mature neurons but, even if oligodendrocytic markers were expressed, no oligodendrocyte-like morphology or axonal myelination was identified after 4 weeks (Isoda et al., 2016). Oligodendrocyte generation in a neuronally committed culture can be improved by the addition of factors that promote glial proliferation and differentiation. Thus, Ehrlich et al. (2017) reported that overexpression of SOX10, OLIG2, and NKX6.2 in human iPSC-derived neural progenitors, combined with platelet-derived growth factor (PDGF-AA), smoothened agonist, and thyroid hormone (T3), accelerates oligodendroglial differentiation, reaching 70% oligodendrocytes and 20% neurons after 28 days in vitro. Importantly, MBP expression was observed at day 35 of the differentiation (Ehrlich et al., 2017). Shaker et al. (2021) have shown that the generation of organoids from human neuroectodermal cells in the presence of T3, neurotrophin 3, hepatocyte growth factor, insulin growth factor, PDGF-AA, and cAMP gives rise to 29% neurons, 20% immature oligodendrocytes, and 40% myelinating oligodendrocytes at day 42 of differentiation. We found here a relatively low percentage of oligodendrocytes (5%) using our cortical differentiation protocol in vitro. However, functional myelination was observed already after 21 days of differentiation, i.e., earlier as compared with previous protocols (Ehrlich et al., 2017; Shaker et al., 2021). It seems possible that, also in our cultures, increased numbers of oligodendrocytes could be obtained using some of the factors mentioned above.
Previous studies with human pluripotent stem cells have reported the production of either neurons or oligodendrocytes after transplantation. In grafts of human iPSC-derived O4+ cells in the spinal cord of demyelinating mice, around 80% of the cells were oligodendrocytes and only 2% expressed neuronal markers (Ehrlich et al., 2017). Similarly, human ESC-derived OPCs, transplanted in spinal cord injured rats gave rise to 94% oligodendrocytes (Sharp et al., 2010). Conversely, other intracerebral ESC- or iPSC-derived grafts have mainly contained neurons. At 60 weeks after transplantation of human ESC-derived lt-NES cells in mouse dentate gyrus and motor cortex, about 80% of cells in the grafts were neurons and no oligodendrocytes were found (Steinbeck et al., 2012). Likewise, transplantation of iPSC-derived lt-NES cells in spinal cord injured mice gave rise to 75% mature neurons and less than 1% oligodendrocytes after 7 weeks (Fujimoto et al., 2012).
Whether substantial numbers of both functional neurons and myelinating oligodendrocytes can be produced from the same intracerebral human stem cell grafts is not well studied. Nori et al. (2011) found a majority of neurons and 9% oligodendrocytes in grafts of human iPSC-derived neurospheres at 112 days after transplantation in spinal cord injured mice. Intracortical transplantation of human iPSC-derived neural stem cells in a pig model of cortical stroke gave rise to 75% neurons and 25% oligodendrocytes (Baker et al., 2017). These percentages are consistent with our observations of 40% mature neurons and 20% pre-myelinating and myelinating oligodendrocytes after intracortical transplantation of cortically primed lt-NES cells into stroke-injured rat cortex. Compared with the studies of Nori et al. (2011) and Baker et al. (2017), we show here for the first time that grafted human pluripotent stem cell-derived oligodendrocytes can be functional and myelinate axons. Most importantly, we demonstrate the survival of a large number of these oligodendrocytes and their capacity for myelination also after transplantation in an ex vivo model of adult human cerebral cortex. It should be pointed out, though, that our study was only designed to show proof-of-principle that grafted human iPSC-derived oligodendrocytes can myelinate human axons. Further analysis needs to be done to determine the extent of this myelination in the human system.
We found increased endogenous oligodendrogenesis in response to stroke, as reported previously (Hernandez et al., 2021). Transplantation of human iPSC-derived progenitors further enhanced endogenous oligodendrogenesis and increased axonal remyelination in several demyelinated brain areas. Such potentiation of oligodendrogenesis, attributed to a so-called bystander effect, has been observed also following administration of other human stem cell types, e.g., after intracerebral transplantation of human iPSC-derived immature astroglia in a mouse model of neonatal white matter injury (Jiang et al., 2016) or intravenous delivery of human adipose-derived stem cells in a rat demyelinated model (Bakhtiari et al., 2021). Only a minor portion of the new oligodendrocytes originated from the grafted, cortically primed human lt-NES cells. This finding indicates that the migration of graft-derived progenitors from the transplant core to demyelinated areas and the capacity to become myelinating oligodendrocytes need to be increased to optimize functional recovery. It is important to point out, though, that the low contribution to remyelination by the grafted human-derived oligodendrocytes and the dominant role of endogenous oligodendrogenesis observed here in the rat stroke model may not reflect the outcome in a hypothetical clinical setting. Since endogenous oligodendrogenesis and remyelination efficiency is decreased by aging (Segel et al., 2019; Shen et al., 2008), replacement by grafted oligodendrocytes would probably be more critical for remyelination in older patients, who are most often affected by stroke.
Current stem cell-based approaches, which have reached patient application in stroke, aim at stimulating plasticity and dampening inflammation (de Celis-Ruiz et al., 2021; Gong et al., 2021; Hess et al., 2017), but only minor or no improvements have been so far been observed. From a clinical perspective, a human cell source that, after intracerebral delivery, has the capacity both for the functional repair of injured neural circuitries and for remyelination could be a step forward toward effective stem cell therapy for patients not only with stroke but also other brain injuries. The present findings provide supportive evidence that the further development of such a cell source is a realistic possibility.
Experimental procedures
Resource availability
Corresponding author
Further information and requests for resources and reagents should be directed to and will be fulfilled by the corresponding author, Zaal Kokaia (zaal.kokaia@med.lu.se).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The data that support the findings of this study are available on request from the corresponding author.
Generation of iPSC-derived oligodendrocytes and cortical neurons
Human iPSC-derived lt-NES cells were produced as described previously (Falk et al., 2012; Gronning Hansen et al., 2020) (see supplemental experimental procedures). lt-NES cells used for transplantation onto ex vivo human tissue were transduced with a lentiviral vector carrying GFP under constitutive promoter (GFP+ lt-NES cells). Differentiation of lt-NES cells to oligodendrocytes and neurons with a cortical phenotype was performed as described previously (Gronning Hansen et al., 2020; Tornero et al., 2013). In brief, growth factors (EGF, bFGF) and B27 were omitted and lt-NES cells were cultured at low density in a differentiation-defined medium in the presence of bone morphogenetic protein 4 (10 ng/mL, R&D Systems), wingless-type MMTV integration site family, member 3A (Wnt3A) (10 ng/mL, R&D Systems), and cyclopamine (1 μM, Calbiochem) for 7 days. On day 7, the medium was changed to Brain Phys (STEMCELL Technologies) supplemented with B27 without retinoid acid (1:50, Invitrogen) until day 21 of differentiation.
Animals and surgical procedures
All procedures were conducted following the European Union Directive (2010/63/EU) and were approved by the ethical committee for the use of laboratory animals at Lund University and the Swedish Board of Agriculture (Dnr. 5.8.18-07222/2021).
Adult athymic, nude male rats (220 g, n = 18; Charles River) were used (five sham-treated animals, five stroke-subjected animals, and eight for stroke and cell transplantation). Among transplanted animals, five were chosen for immunological characterization of the grafts and three for iEM. Details for cortical ischemic injury and cell transplantation in the somatosensory cortex are described in supplemental experimental procedures.
Organotypic cultures of the human adult cortex
Healthy human neocortical tissue was obtained with informed consent by resection of a small piece of the middle temporal gyrus from patients undergoing elective surgery for temporal lobe epilepsy (n = 3) according to guidelines approved by the Regional Ethical Committee, Lund (Dnr. 2021-07006-01). The tissue was delivered and handled as described previously (Gronning Hansen et al., 2020; Miskinyte et al., 2017). Details in the processing of the human slices are described in supplemental experimental procedures.
Transplantation of lt-NES cells onto human organotypic cortical slices
The lt-NES cell transplantation was performed as described previously (Gronning Hansen et al., 2020). In brief, GFP+ lt-NES cells were detached at day 7 of differentiation and resuspended at a concentration of 100,000 cells/μL in pure cold Matrigel Matrix (Corning). After part of the medium was removed from the top of the insert, the suspension mix was collected into a cold glass capillary and injected as small drops stabbing the semi-dry slice at various sites. After the Matrigel was solidified, additional medium was carefully added to completely immerse the tissue. The medium were changed once a week and co-culture was maintained for 4–6 weeks before fixation.
Immunostainings and quantifications
Details of the immunocytochemistry in cultured cells and immunohistochemistry in rat slices and human organotypic slices are described in supplemental experimental procedures. Antibodies used are included in Table S1.
Overview images of rat brain slices stained with OLIG2 and MBP were taken using a Virtual Slide Scanning System (VS-120-S6-W, Olympus, Germany).
CNPase and OLIG2 quantification in the core of the transplant in rat slices was performed in 10 μm thick 40× confocal images (LSM 780, Zeiss, Germany) and positive cells were counted by sampling different areas ((CNPase+-STEM101+)/total STEM101+ and (OLIG2+-STEM101+)/total STEM101+).
Quantification of OLIG2+ cells in the corpus callosum in rat slices was performed in 20× confocal images (10 μm thick z stack). For analysis of myelination, confocal images (5 μm thick z stack) were taken in the corpus callosum (40×), peri-infarct area (20×), and striatum (20×). For analysis of the dorsal lateral striatum, starting 700 μm lateral to the dorsal part of the lateral ventricle, two 20× images (x = 700 and 1,400 μm) were taken. To measure the thickness of the corpus callosum, confocal images were taken with a 20× Zoom 0.6. All quantifications were performed on maximum-intensity projection images using ImageJ software by blinded researchers.
Quantification of GFP+ OLIG2+ cells in the human organotypic slices was carried out using 20× confocal images in areas where transplantation was observed, and the number of double-positive cells counted through a z stack by sampling different single planes.
Flow cytometry
The lt-NES cell cultures were harvested and washed before antibody incubation. Anti-O4 APC-conjugated antibody (Miltenyi, no. 130-119-155) was diluted 1:200 in FACS buffer (PBS + 2% fetal bovine serum + 2 nM ethylenediaminetetraacetic acid). Cells were incubated for 30 min at +4°C in darkness, followed by wash and incubation with propidium iodide (Life Technologies), a viability marker, diluted 1:1,000 in FACS buffer at least 5 min before acquisition. Cells were analyzed in an LSR II flow cytometer (Becton Dickinson) and results were analyzed with BD FACSDiva 9.0 software (BD Biosciences) and FlowJo v.10.8 Software (BD Life Sciences). The gating strategy is shown in Figure S1C.
qRT-PCR
qRT-PCR was performed on RNA extracted from cells at different time points of differentiation (D0, D4, D8, D12, and D15). RNA extraction was performed with the RNeasy Mini Kit (QIAGEN) following the protocol described by the manufacturer. RNA purity and concentration of samples were determined using a NanoDrop spectrophotometer (ND-1000). RNA (1 μg) was used for cDNA synthesis with qScript cDNA SuperMix (QuantaBio). TaqMan probes (Thermo Fisher Scientific; OLIG2, HS00300164_s1; MBP, HS00921945_m1; DCX, HS00167057_m1; GAPDH, HS02786624_g1) were used and qRT-PCR was run in triplicate samples on an iQ5 real-time cycler (Bio-Rad) with GAPDH as the housekeeping gene.
EM
For in vitro EM, lt-NES cell cultures (n = 8 wells) were fixed with 2% formaldehyde and 0.2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). Samples were frozen and cut into ultrathin sections with a diamond knife. Ultrathin sections were examined and photographed using a transmission electron microscope FEI Tecnai Biotwin 120 kv.
For in vivo iEM, 6 months after transplantation, three rats were deeply anesthetized with pentobarbital and transcardially perfused with 0.1 M PBS followed by ice-cold 2% formaldehyde, containing 0.2% glutaraldehyde, in 0.1 M PBS (pH 7.4). Brains were removed, post-fixed for 1 h and washed in 0.1 M PBS. For ex vivo iEM the same fixation was performed for 30 min. Both rat and human tissue were processed as described in supplemental experimental procedures.
Electrophysiological recordings
Electrophysiological recordings were performed on 4-week-old human adult cortical organotypic slices as described previously (Andersson et al., 2016). Details are described in supplemental experimental procedures.
Statistical analysis
Statistical analysis was performed using Prism 9 software (GraphPad). An unpaired t test was used when data were normally distributed, whereas a Mann-Whitney U test was used when data did not pass the normality test. When different independent groups were compared, a one-way ANOVA plus Tukey’s multiple comparison tests were performed. Significance was set at p < 0.05. Data are mean ± SEM.
Author contributions
S.P.-T., O.L., and Z.K. conceived the project. R.M.-C., L.J., C.A.-M., N.A., O.T., I.H., E.M., and S.P.-T. conducted the experiments and analyzed the data. S.P.-T., O.L., and Z.K. wrote the manuscript. J.B. provided human material. R.M.-C., N.A., E.M., G.S., and O.T. were involved in the collection and/or assembly of data, data analysis, and interpretation. All authors reviewed and edited the manuscript.
Acknowledgments
This work is supported by grants from the Swedish Research Council, Swedish Brain Foundation, Swedish Stroke Foundation, Region Skåne, Neurofonden, the Thorsten and Elsa Segerfalk Foundation, Rut och Erik Hardebo Foundation, and the Swedish Government Initiative for Strategic Research Areas (StemTherapy).
Conflict of interests
The authors declare no competing interests.
Published: May 25, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2023.04.010.
Supplemental information
References
- Andersson M., Avaliani N., Svensson A., Wickham J., Pinborg L.H., Jespersen B., Christiansen S.H., Bengzon J., Woldbye D.P.D., Kokaia M. Optogenetic control of human neurons in organotypic brain cultures. Sci. Rep. 2016;6 doi: 10.1038/srep24818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avaliani N., Sørensen A.T., Ledri M., Bengzon J., Koch P., Brüstle O., Deisseroth K., Andersson M., Kokaia M. Optogenetics reveal delayed afferent synaptogenesis on grafted human-induced pluripotent stem cell-derived neural progenitors. Stem Cell. 2014;32:3088–3098. doi: 10.1002/stem.1823. [DOI] [PubMed] [Google Scholar]
- Baker E.W., Platt S.R., Lau V.W., Grace H.E., Holmes S.P., Wang L., Duberstein K.J., Howerth E.W., Kinder H.A., Stice S.L., et al. Induced pluripotent stem cell-derived neural stem cell therapy enhances recovery in an ischemic stroke pig model. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-10406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhtiari M., Ghasemi N., Salehi H., Amirpour N., Kazemi M., Mardani M. Evaluation of Edaravone effects on the differentiation of human adipose derived stem cells into oligodendrocyte cells in multiple sclerosis disease in rats. Life Sci. 2021;282 doi: 10.1016/j.lfs.2021.119812. [DOI] [PubMed] [Google Scholar]
- Benedict R.H.B., Amato M.P., DeLuca J., Geurts J.J.G. Cognitive impairment in multiple sclerosis: clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020;19:860–871. doi: 10.1016/S1474-4422(20)30277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanoumidou K., Mozafari S., Baron-Van Evercooren A., Kuhlmann T. Stem cell derived oligodendrocytes to study myelin diseases. Glia. 2020;68:705–720. doi: 10.1002/glia.23733. [DOI] [PubMed] [Google Scholar]
- de Celis-Ruiz E., Fuentes B., Moniche F., Montaner J., Borobia A.M., Gutiérrez-Fernández M., Díez-Tejedor E. Allogeneic adipose tissue-derived mesenchymal stem cells in ischaemic stroke (AMASCIS-02): a phase IIb, multicentre, double-blind, placebo-controlled clinical trial protocol. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-051790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I.D., Radcliff A.B., Heidari M., Kidd G., August B.K., Wierenga L.A. The adult oligodendrocyte can participate in remyelination. Proc. Natl. Acad. Sci. USA. 2018;115:E11807–E11816. doi: 10.1073/pnas.1808064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich M., Mozafari S., Glatza M., Starost L., Velychko S., Hallmann A.L., Cui Q.L., Schambach A., Kim K.P., Bachelin C., et al. Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc. Natl. Acad. Sci. USA. 2017;114:E2243–E2252. doi: 10.1073/pnas.1614412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk A., Koch P., Kesavan J., Takashima Y., Ladewig J., Alexander M., Wiskow O., Tailor J., Trotter M., Pollard S., et al. Capture of neuroepithelial-like stem cells from pluripotent stem cells provides a versatile system for in vitro production of human neurons. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R.J.M., Frisén J., Lyons D.A. Revisiting remyelination: towards a consensus on the regeneration of CNS myelin. Semin. Cell Dev. Biol. 2021;116:3–9. doi: 10.1016/j.semcdb.2020.09.009. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y., Abematsu M., Falk A., Tsujimura K., Sanosaka T., Juliandi B., Semi K., Namihira M., Komiya S., Smith A., Nakashima K. Treatment of a mouse model of spinal cord injury by transplantation of human induced pluripotent stem cell-derived long-term self-renewing neuroepithelial-like stem cells. Stem Cell. 2012;30:1163–1173. doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- García-León J.A., Kumar M., Boon R., Chau D., One J., Wolfs E., Eggermont K., Berckmans P., Gunhanlar N., de Vrij F., et al. SOX10 single transcription factor-based fast and efficient generation of oligodendrocytes from human pluripotent stem cells. Stem Cell Rep. 2018;10:655–672. doi: 10.1016/j.stemcr.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P., Zhang W., He Y., Wang J., Li S., Chen S., Ye Q., Li M. Classification and characteristics of mesenchymal stem cells and its potential therapeutic mechanisms and applications against ischemic stroke. Stem Cell. Int. 2021;2021 doi: 10.1155/2021/2602871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grønning Hansen M., Laterza C., Palma-Tortosa S., Kvist G., Monni E., Tsupykov O., Tornero D., Uoshima N., Soriano J., Bengzon J., et al. Grafted human pluripotent stem cell-derived cortical neurons integrate into adult human cortical neural circuitry. Stem Cells Transl. Med. 2020;9:1365–1377. doi: 10.1002/sctm.20-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez I.H., Villa-Gonzalez M., Martin G., Soto M., Perez-Alvarez M.J. Glial cells as therapeutic approaches in brain ischemia-reperfusion injury. Cells. 2021;10 doi: 10.3390/cells10071639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D.C., Wechsler L.R., Clark W.M., Savitz S.I., Ford G.A., Chiu D., Yavagal D.R., Uchino K., Liebeskind D.S., Auchus A.P., et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. doi: 10.1016/S1474-4422(17)30046-7. [DOI] [PubMed] [Google Scholar]
- Hughes E.G., Orthmann-Murphy J.L., Langseth A.J., Bergles D.E. Myelin remodeling through experience-dependent oligodendrogenesis in the adult somatosensory cortex. Nat. Neurosci. 2018;21:696–706. doi: 10.1038/s41593-018-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoda M., Kohyama J., Iwanami A., Sanosaka T., Sugai K., Yamaguchi R., Matsumoto T., Nakamura M., Okano H. Robust production of human neural cells by establishing neuroepithelial-like stem cells from peripheral blood mononuclear cell-derived feeder-free iPSCs under xeno-free conditions. Neurosci. Res. 2016;110:18–28. doi: 10.1016/j.neures.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Jiang P., Chen C., Liu X.B., Pleasure D.E., Liu Y., Deng W. Human iPSC-derived immature astroglia promote oligodendrogenesis by increasing TIMP-1 secretion. Cell Rep. 2016;15:1303–1315. doi: 10.1016/j.celrep.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata S., Takano M., Numasawa-Kuroiwa Y., Itakura G., Kobayashi Y., Nishiyama Y., Sugai K., Nishimura S., Iwai H., Isoda M., et al. Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016;6:1–8. doi: 10.1016/j.stemcr.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Gritti L., Crooks D., Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8 doi: 10.3390/cells8111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorente I.L., Xie Y., Mazzitelli J.A., Hatanaka E.A., Cinkornpumin J., Miller D.R., Lin Y., Lowry W.E., Carmichael S.T. Patient-derived glial enriched progenitors repair functional deficits due to white matter stroke and vascular dementia in rodents. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.aaz6747. [DOI] [PubMed] [Google Scholar]
- Marin M.A., Carmichael S.T. Mechanisms of demyelination and remyelination in the young and aged brain following white matter stroke. Neurobiol. Dis. 2019;126:5–12. doi: 10.1016/j.nbd.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Miskinyte G., Devaraju K., Grønning Hansen M., Monni E., Tornero D., Woods N.B., Bengzon J., Ahlenius H., Lindvall O., Kokaia Z. Direct conversion of human fibroblasts to functional excitatory cortical neurons integrating into human neural networks. Stem Cell Res. Ther. 2017;8:207. doi: 10.1186/s13287-017-0658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrabady S.E., Rizvi B., Goldman J.E., Brickman A.M. White matter changes in Alzheimer's disease: a focus on myelin and oligodendrocytes. Acta Neuropathol. Commun. 2018;6:22. doi: 10.1186/s40478-018-0515-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely S.A., Williamson J.M., Klingseisen A., Zoupi L., Early J.J., Williams A., Lyons D.A. New oligodendrocytes exhibit more abundant and accurate myelin regeneration than those that survive demyelination. Nat. Neurosci. 2022;25:415–420. doi: 10.1038/s41593-021-01009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistor G.I., Totoiu M.O., Haque N., Carpenter M.K., Keirstead H.S. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- Nori S., Okada Y., Yasuda A., Tsuji O., Takahashi Y., Kobayashi Y., Fujiyoshi K., Koike M., Uchiyama Y., Ikeda E., et al. Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc. Natl. Acad. Sci. USA. 2011;108:16825–16830. doi: 10.1073/pnas.1108077108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma-Tortosa S., Tornero D., Grønning Hansen M., Monni E., Hajy M., Kartsivadze S., Aktay S., Tsupykov O., Parmar M., Deisseroth K., et al. Activity in grafted human iPS cell-derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc. Natl. Acad. Sci. USA. 2020;117:9094–9100. doi: 10.1073/pnas.2000690117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao J., Major T., Auyeung G., Policarpio E., Menon J., Droms L., Gutin P., Uryu K., Tchieu J., Soulet D., Tabar V. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16:198–210. doi: 10.1016/j.stem.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segel M., Neumann B., Hill M.F.E., Weber I.P., Viscomi C., Zhao C., Young A., Agley C.C., Thompson A.J., Gonzalez G.A., et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. 2019;573:130–134. doi: 10.1038/s41586-019-1484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker M.R., Pietrogrande G., Martin S., Lee J.H., Sun W., Wolvetang E.J. Rapid and efficient generation of myelinating human oligodendrocytes in organoids. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.631548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J., Frame J., Siegenthaler M., Nistor G., Keirstead H.S. Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants improve recovery after cervical spinal cord injury. Stem Cell. 2010;28:152–163. doi: 10.1002/stem.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Sandoval J., Swiss V.A., Li J., Dupree J., Franklin R.J.M., Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat. Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeck J.A., Koch P., Derouiche A., Brüstle O. Human embryonic stem cell-derived neurons establish region-specific, long-range projections in the adult brain. Cell. Mol. Life Sci. 2012;69:461–470. doi: 10.1007/s00018-011-0759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornero D., Tsupykov O., Granmo M., Rodriguez C., Grønning-Hansen M., Thelin J., Smozhanik E., Laterza C., Wattananit S., Ge R., et al. Synaptic inputs from stroke-injured brain to grafted human stem cell-derived neurons activated by sensory stimuli. Brain. 2017;140:692–706. doi: 10.1093/brain/aww347. [DOI] [PubMed] [Google Scholar]
- Tornero D., Wattananit S., Grønning Madsen M., Koch P., Wood J., Tatarishvili J., Mine Y., Ge R., Monni E., Devaraju K., et al. Human induced pluripotent stem cell-derived cortical neurons integrate in stroke-injured cortex and improve functional recovery. Brain. 2013;136:3561–3577. doi: 10.1093/brain/awt278. [DOI] [PubMed] [Google Scholar]
- Wang S., Bates J., Li X., Schanz S., Chandler-Militello D., Levine C., Maherali N., Studer L., Hochedlinger K., Windrem M., Goldman S.A. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–264. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Duan K., Ai Z., Niu B., Chen Y., Kong R., Li T. Generation of cortical neurons through large-scale expanding neuroepithelial stem cell from human pluripotent stem cells. Stem Cell Res. Ther. 2020;11:431. doi: 10.1186/s13287-020-01939-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo M., Guo H., Wan T., Zhao N., Cai H., Zha M., Xiong Y., Xie Y., Ye R., Liu X. Wallerian degeneration in experimental focal cortical ischemia. Brain Res. Bull. 2019;149:194–202. doi: 10.1016/j.brainresbull.2019.04.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.