Summary
This review aims to evaluate the current preclinical state of liver bioengineering, the clinical context for liver cell therapies, the cell sources, the delivery routes, and the results of clinical trials for end-stage liver disease. Different clinical settings, such as inborn errors of metabolism, acute liver failure, chronic liver disease, liver cirrhosis, and acute-on-chronic liver failure, as well as multiple cellular sources were analyzed; namely, hepatocytes, hepatic progenitor cells, biliary tree stem/progenitor cells, mesenchymal stromal cells, and macrophages. The highly heterogeneous clinical scenario of liver disease and the availability of multiple cellular sources endowed with different biological properties make this a multidisciplinary translational research challenge. Data on each individual liver disease and more accurate endpoints are urgently needed, together with a characterization of the regenerative pathways leading to potential therapeutic benefit. Here, we critically review these topics and identify related research needs and perspectives in preclinical and clinical settings.
Keywords: acute liver failure, cell therapy, cirrhosis, hepatocytes, macrophages, progenitors, scaffolds, stem cells
Cardinale, Ciccocioppo, Uygun and colleagues review the context and the results of liver cell therapies, and identify research needs and perspectives. The highly heterogeneous clinical scenario of liver disease and the availability of multiple cellular sources endowed with different biological properties make this a multidisciplinary translational research challenge.
Introduction
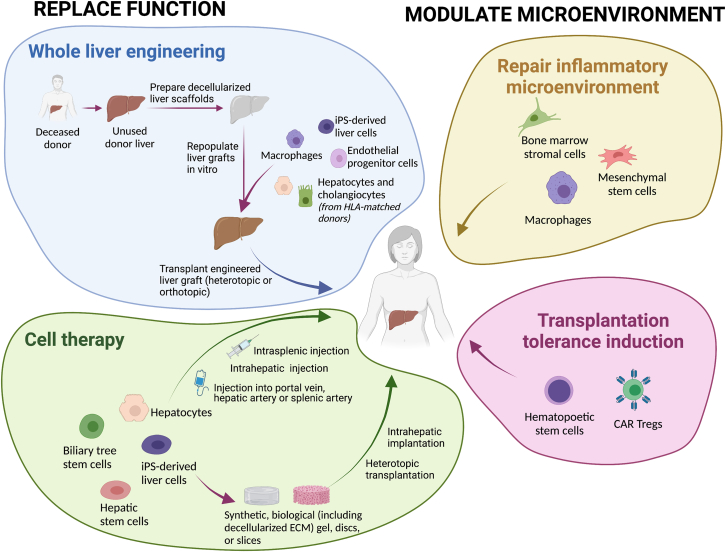
Over the past decades, liver diseases have risen to become leading causes of death and illness worldwide. The only therapeutic option for end-stage liver disease (ESLD) is orthotopic liver transplantation (OLT). However, the complexity of this procedure and the paucity of donor organs limit this option to a minority of patients. Therefore, new therapeutic strategies are required. Regenerative medicine holds the promise of revolutionizing the care of these patients. Regenerative medicine and cell-based therapies for the liver have emerged as key research areas. Liver cell therapies, mainly based on hepatocytes, mesenchymal stromal cells (MSCs) and macrophages, have increasingly been applied in clinical trials. However, evidence of clinical efficacy is still scarce, and long-term effects are insufficiently explored. In addition, there is a need for groundbreaking approaches and techniques in regenerative hepatology, including introduction of innovative cell sources, such as hepatic progenitor cells (HPCs), biliary tree stem/progenitor cells (BTSCs), or induced pluripotent stem cells (iPSCs); technologies to enhance cell engraftment; and advanced liver bioengineering (Figure 1). This review aims to evaluate the current preclinical state of liver bioengineering; the clinical context for liver cell therapies; the involved cell types, including their sources and delivery routes; and first results of clinical trials in ESLD. It ultimately aims to provide updated guidance for basic scientists and clinicians. Moreover, promising novel areas on which research could be prospectively focused are highlighted.
Figure 1.
Therapeutic approaches in liver regenerative medicine
iPSC, induced pluripotent stem cell; Treg cell, regulatory T cell.
Liver cell atlas and pathways of liver regeneration and fibrosis
The human liver represents a paradigm for organ and tissue regeneration. The liver parenchyma contains two main mature epithelial cell types: hepatocytes and cholangiocytes.
Hepatocytes constitute most of the parenchymal mass and are arranged in cellular cords and organized in hepatic lobules. Single-cell transcriptome studies support the concept of hepatocyte zonation, which implies that groups of hepatocytes show peculiar biological and functional properties based on their position within the lobule and relative to the vasculature. Because of their proximity to the hepatic artery, hepatocytes located around the portal tract (periportal or zone 1) receive oxygen-rich hepatic arterial blood and nutrient-rich portal venous blood; they are diploid and show prominent glucose uptake, glycogen synthesis, albumin synthesis, and bile formation. On the other side of the lobule, hepatocytes around central veins (pericentral or zone 3) are exposed to low oxygen and metabolite concentrations; pericentral hepatocytes are polyploid and implicated in glycolysis, alcohol and drug metabolism, and fatty acid β-oxidation. Mid-lobular (zone 2) hepatocytes express high levels cytochrome P450 species relevant for xenobiotic metabolism (Aizarani et al., 2019; Ramachandran et al., 2020; Richter et al., 2021).
Hepatocyte zonation was the basis for the streaming liver theory, which postulates a predominantly proliferative activity of periportal hepatocytes/hepatocyte precursors, leading to cellular patches streaming toward the pericentral vein, being central to the renewal and physiological turnover of liver parenchyma (Font Burgada et al., 2015; Zajicek et al., 1985). This theory was challenged by identification of a pericentral (Axin+) population able to fuel homeostatic renewal of the hepatocyte mass, resulting in the two-stream hypothesis (Bird and Forbes, 2015; Sun et al., 2020; Wang et al., 2015). More recently, mid-lobular hepatocytes have also been implicated in regenerative pathways (He et al., 2021; Wei et al., 2021). In sum, a modest local proliferation of hepatocytes in all zones seems to be responsible for maintaining the homeostatic renewal of the functional hepatocyte mass (Chen et al., 2020; Lin et al., 2018).
Defined zones of the liver lobule are activated by different noxious stimuli or involved diseases. Severe damage to or loss of pericentral or periportal hepatocytes triggers proliferation of adjacent hepatocytes, eventually supported by hepatocytes located away from the injury site (Chen et al., 2020; Lin et al., 2018); along these lines, mid-lobular hepatocytes are well-positioned to contribute to regenerating hepatocytes in zones 1 or 3 (He et al., 2021; Wei et al., 2021). In prolonged chronic liver injury, hepatocyte clones moving into the injury zone become increasingly exhausted and dysfunctional, and an increased rate of apoptotic cell death is observed; this can overwhelm hepatocyte regeneration capacity by inducing cellular senescence (Ramachandran et al., 2020). Interestingly, in this context, cells of biliary origin in the canals of Hering can support liver regeneration by differentiating into hepatocytes (Deng et al., 2018; Manco et al., 2019). Indeed, these specialized epithelial cells have been shown to act as naive/undifferentiated progenitor cells (HPCs) in rodent models (Aizarani et al., 2019; Huch et al., 2015). There is less evidence for this regenerative mechanism in humans because of the inability to perform cell tracking experiments. However, pathological studies show that HPCs can give rise to new hepatocytes and also play a beneficial role in human liver regeneration (Lanthier and Spahr, 2019). Moreover, experiments with human intrahepatic cholangiocyte organoids have provided indirect evidence for ductal-to-hepatocyte differentiation (Roos et al., 2022).
Cholangiocytes are the other epithelial cells of the liver, forming the surface epithelium of the bile ductules and the biliary tree (Banales et al., 2019). The intrahepatic biliary tree is a 3D network of anastomosing conduits of increasing size, starting from the canals of Hering to segmental ducts (Lanzoni et al., 2016; Overi et al., 2018). Schematically, the intrahepatic biliary tree can be divided into the canals of Hering, small and larger bile ducts (Banales et al., 2019); based on the location in the biliary tree in which they reside, biliary epithelial cells exhibit morphological, functional, and replicative heterogeneity. This heterogeneity is the basis for understanding the pathogenesis of chronic biliary diseases; i.e., cholangiopathies (Lanzoni et al., 2016). Four main cholangiocyte subpopulations have been identified: (1) HPCs in the canals of Hering and adjacent bile ductules; (2) small cholangiocytes lining bile ductules and interlobular bile ducts (Banales et al., 2019; Han et al., 2013), (3) large cholangiocytes lining large bile ducts (Banales et al., 2019; Han et al., 2013), and (4) peribiliary gland (PBG) cells associated with large bile ducts (Carpino et al., 2020). Small and large cholangiocytes show proliferative capability, able to support the very slow physiological turnover of the bile duct epithelium (Banales et al., 2019; Han et al., 2013). However, as for hepatocytes, the proliferative capability of cholangiocytes can be exhausted by chronic inflammation (cholangiopathies), and in these clinical conditions, mainly activated cholangiocytes/HPCs support biliary regeneration (Carbone et al., 2018; Carpino et al., 2015, 2018; Gigliozzi et al., 2004; Wang et al., 2013).
Liver bioengineering
Different cell therapies and bio-artificial livers have been used to treat ESLD of different etiologies (Giancotti et al., 2019; Qi et al., 2015; Struecker et al., 2014; Figure 1). One important factor in the success of cell-based therapies in liver diseases and liver failure is the route of administration of cells, which would determine cell engrafting and function upon transplantation. Different protocols have been proposed to maximize the effectiveness of transplanted cells. Intravascular administration of cells into the portal vein, hepatic artery, or splenic artery allows access to the liver with minimal risk but is technically demanding and may lead to cell damage and poor engraftment because of shear stress (Dwyer et al., 2021). Intrahepatic injection is another route for cell delivery to the liver, but it may lead to tissue injury and blockage of the hepatic vascular system (Habeeb et al., 2015). Intrasplenic injection of cells with or without subsequent splenectomy is solely an experimental method not feasible for patients but is equivalent to portal vein injection in humans (Ogasawara et al., 2021). Approaches to protect cells from the host immune system and improve engraftment with any of these delivery routes have also been proposed. These include coating of cells with extracellular matrix molecules, such as heparin or hyaluronan, or encapsulating the cells in synthetic or natural biomaterials (Bhatia et al., 2014). In 2008, a new method to generate whole-heart scaffolds by perfusion decellularization was introduced, which triggered renewed interest in the field of solid organ bioengineering (Ott et al., 2008). By 2010, this technology was successfully adopted for whole livers of rats, mice, ferrets, rabbits, and pigs (Baptista et al., 2011; Mazza et al., 2015; Uygun et al., 2010; Yagi et al., 2013) opening the door to human-size liver bioengineering. However, the enthusiasm was mitigated by reports published in 2015 and 2019, demonstrating that re-endothelialization of whole-liver scaffolds is paramount to allow engraftment of hepatocytes and effective transplantation of the whole bioengineered liver (Ko et al., 2015; Shaheen et al., 2020). Thus, the lack of a functional liver endothelium leads to development of thrombosis after surgical anastomosis, usually occurring after a few hours, with progressive clotting developing in the revascularized scaffolds (Ko et al., 2015; Shaheen et al., 2020). Hence, greater efforts are required, especially regarding optimization of the revascularization process.
Whole-organ engineering is a research area aiming to solve organ shortage, especially for the liver. The native organ may come from an animal source under certain restrictions or from a human organ that was deemed unfit for transplantation because long ischemic time, massive hepatic steatosis, or other restrictions (e.g., previously undetected donor neoplasm) (Maghsoudlou et al., 2016; Mazza et al., 2015). Porcine livers are attractive because they are considerably close in size to human livers (Peloso et al., 2015). However, porcine livers show important microarchitectural differences from human livers, such as fibrotic septa between acinar units and differences in vascular resistance at the origin of sinusoids, which limit the re-cellularization efficacy and vascular compliance post-transplantation (i.e., early development of portal hypertension) (Ko et al., 2015). Whether this truly hinders their final application in human liver bioengineering will have to be determined in the future.
Scaffolds are prepared using a process called decellularization, which aims to eliminate the cellular content of the donor organ without significantly affecting the biochemical and biomechanical features of the extracellular matrix scaffold through different physical, chemical, or enzymatic protocols. Major progress has been made in the past decade to improve decellularization protocols of sections of liver tissue and whole livers (Guyette et al., 2014; Li et al., 2016; Maghsoudlou et al., 2016; Mayorca-Guiliani et al., 2017). However, an in-depth evaluation of the vascular network integrity and overall microarchitecture after decellularization is critical to ensure that the scaffolds can be efficiently and homogeneously recellularized. This should be supplemented by proteomics analysis (scaffold matrisome) and DNA remnant quantification to ensure adequate decellularization in every scaffold.
When the decellularized liver scaffold has been obtained and characterized, the next challenge is the recellularization process. For this, an appropriate number of different cell types is required, including endothelial cells, liver parenchymal cells (hepatocytes), and other non-parenchymal cells, such as Kupffer cells/macrophages and mesenchymal cells, particularly hepatic stellate cells. Importantly, a large number of cells is required, involving massive large-scale ex vivo cell expansion. This is difficult when using primary cells, but protocols now exist (Hu et al., 2018; Schneeberger et al., 2020). Alternatively, iPSCs are promising because protocols are already available for their large-scale differentiation and expansion, including vascular cell phenotypes (Chen et al., 2018; Sampaziotis et al., 2015, 2017; Takeishi et al., 2020). Finally, integration of all of these cell types (vascular, hepatic, and non-parenchymal cells) into a single functional tissue remains an ongoing challenge, being key to successful bioengineering of a transplantable liver.
Besides whole-organ engineering, three dimensional bottom-up approaches in bioengineering have emerged as an attractive technique to build complex tissues such as the liver in vitro in recent years (Xiang et al., 2022). In addition, organoids are complex 3D structures that can potentially be candidates for several applications in regenerative medicine of the liver. In 2013 and 2015, Huch et al. (2013, 2015) described isolation of LGR5+ stem cells from murine and human liver, respectively. The development of a 3D culture system with defined growth factors allowed their in vitro propagation in the form of organoids. When medium conditions are adapted to a differentiation medium, in which wingless/integrated (WNT) and neurogenic locus notch homolog (NOTCH) signaling are inhibited, organoid differentiation toward hepatocyte-like cells can be induced. Since then, multiple other liver organoids have been described, with different features and functional levels; e.g., bile duct-derived organoids (Huch et al., 2015; Tysoe et al., 2019; Rimland et al., 2021), extrahepatic bile duct-derived organoids (Sampaziotis et al., 2017; Rimland et al., 2021), gallbladder-derived organoids (Lugli et al., 2016; Rimland et al., 2021), and hepatocyte-derived organoids (Hu et al., 2018). “Liver bud organoids” have been obtained by co-culturing iPSC-derived endodermal cells, endothelial progenitors, and mesenchymal progenitors (Koike et al., 2019; Takebe et al., 2013). Modulating signaling pathways regulating hepatocyte renewal in vivo, strategies have been developed for establishing primary hepatocyte organoids derived from either adult or fetal liver (Peng et al., 2018, 2021). Compared with fetally derived hepatocytes or adult mouse primary hepatocytes, the proliferative capacity of mature hepatocytes appeared to be limited (2–2.5 months) (Hu et al., 2018). Indeed, use of human adult hepatocyte-derived organoids is controversial because current protocols mainly work with fetal specimens (Hu et al., 2018). Notably, hepatocyte organoids can adopt either a proliferative or a metabolic state, depending on the culture conditions. Furthermore, the metabolic gene expression profile can be modulated based on the principles that govern liver zonation (Peng et al., 2018, 2021). Of note is the work of Vyas et al. (2018), who generated liver organoids with simultaneous and parallel differentiation of fetal hepatoblast progenitor cells in bile ducts and hepatocytes in liver decellularized extracellular matrix (ECM) scaffolds. However, in currently existing liver organoid models, there is no possibility to perform vascular anastomosis. This delays their clinical translation, which makes future work in organoid vascularization a priority.
The ability to build the tissue microarchitecture with high resolution using viable cells and biomaterials using 3D bioprinting allows generation of transplantable tissues from the ground up to replace the functions of a failing liver. Advances in 3D printing technology enabled generation of normal or diseased liver microtissues to be used as drug toxicity testing platforms (Gori et al., 2020; Nguyen et al., 2016). Yang et al. (2021) used 3D printing to generate hepatorganoids using Rumin and Gripon (HepaRG) cells, subsequently implanted into the abdominal cavity of Fah−/−Rag2−/− mice, and showed that the survival of the animals significantly increased after 4 weeks of transplantation. While these results are promising, 3D bioprinted constructs lack vasculature and biliary ductules, and printing of whole organs has yet to be achieved (Ali et al., 2018). Takebe et al. (2013) described for the first time generation of a liver bud with hiPSC-derived hepatic endodermal cells in association with human umbilical vein endothelial cells (hUVECs) and human MSCs. Generation of an endothelial cell network organized with more mature hepatic tissue was a large step forward and allowed swift anastomosis of these liver buds with the vascular system of mice after implantation. However, their size and lack of a truly functional vascular network hinders their transplantation in large quantities or in a clinical setup (Takebe et al., 2013).
Liver cell therapy
OLT is the only curative treatment for severe acute or chronic liver disease (CLD) of various etiologies (Forbes et al., 2015). Notably, the long-term outcome of OLT reaches its maximum potential when etiologies of liver diseases and co-morbidities are targeted simultaneously; e.g., hepatitis C virus (HCV) infection, hepatitis B virus (HBV) infection, non-alcoholic fatty liver disease (NAFLD)/metabolic dysfunction-associated fatty liver disease (MAFLD), alcohol-related liver disease (ALD), or autoimmune hepatitis (AIH). Different cell therapies and bio-artificial livers have been attempted and used not only for cirrhosis but also for acute liver failure (ALF), inborn errors of metabolism, chronic cholestatic and autoimmune diseases, and NAFLD (Giancotti et al., 2019; Qi et al., 2015; Struecker et al., 2014; Figure 2). Cell therapy should be accompanied by the standard of care according to the etiologies of liver diseases and comorbidities. A central aspect of liver regenerative medicine is the clinical setting: ALF vs. CLD/cirrhosis. The distinction between acute versus chronic is sometimes less clear. For example, (acute) alcoholic hepatitis most often develops on a background of pre-existing cirrhosis (defined as acute-on-chronic) and carries a very high risk of morbidity and mortality. Few treatments are effective, and several strategies, including those aimed at liver regeneration, have been studied.
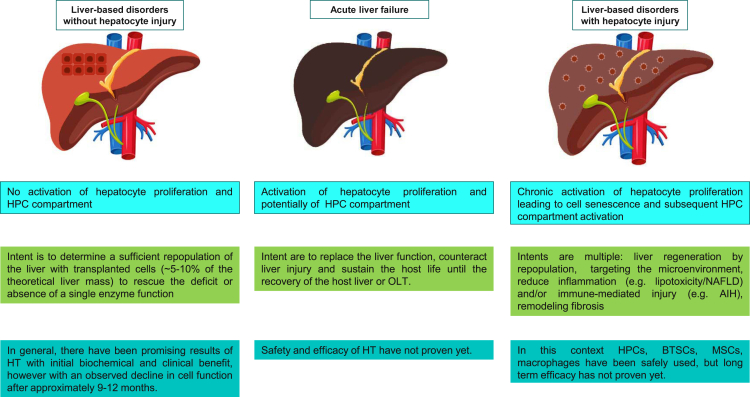
Figure 2.
An overview of the types of liver diseases that can potentially be treated with cell therapy
The efficiency of engraftment and repopulation of donor cells is dictated by the host tissue microenvironment. The number of donor cells needed to provide therapeutic benefits is dependent on the recipient liver pathophysiology. Shown are pathological features (top row), the intent and targets of cell transplantation-based therapy (center row), and the overall results of the different cell sources used (bottom row). AIH, autoimmune hepatitis; BTSC, biliary tree stem cell; HPC, hepatic progenitor cell; HT, hepatocyte transplantation; MSC, mesenchymal stem cell; NAFLD, non-alcoholic fatty liver disease; OLT, orthotopic liver transplantation.
In liver cell therapy, two main approaches and related cell sources have been attempted: replacing the tissue by using epithelial cell sources and modulation of tissue repair by using mesenchymally derived cells capable to modify the microenvironment. As far as the liver tissue and function, replacement human hepatocytes, HPCs, and BTSCs have already been used in clinical studies. Each cell source has specific unique properties. The pros and cons of each source are closely related to the specific disease targeted, and no direct comparison among the sources has been attempted. In the sections below, we focused on matching defined epithelial cell sources and their specific properties with diverse clinical settings. A separate section is reserved for cell sources targeting the liver microenvironment; e.g., macrophages and MSCs.
Cell therapy: Replace function
Human hepatocytes in inborn error of metabolism/liver-based metabolic disorders (LBMDs)
In LBMDs, the aim of cell therapy is to allow sufficient repopulation of the liver with transplanted cells, estimated at ∼5%–10% of the theoretical liver mass, to rescue the deficit or absence of a single enzyme function (Nguyen et al., 2020). Hepatocyte transplantation (HT) in LBMDs has been reported in less than 40 cases so far (Crigler-Najjar syndrome type I, 12 subjects; familial hypercholesterolemia, 5 subjects; factor VII deficiency, 2 subjects; glycogen storage disease type I, 3 subjects; infantile Refsum’s disease, 1 subject; progressive familial intrahepatic cholestasis type 2, 2 subjects; Ornithine transcarbamylase (OTC) deficiency, 8 subjects; argininosuccinate lyase (ASL) and carbamoyl phosphate synthetase (CPS1) deficiency, 1 and 3 subjects, respectively; citrullinemia, 1 subject) (Dwyer et al., 2021; Han et al., 2013). Notably, a few subjects with LBMD have been treated with epithelial cell adhesion module (EpCAM)-sorted fetal HPCs (1 case of Crigler-Najjar syndrome type 1 and one case of biliary atresia) (Khan et al., 2008a, 2008b).
The clinical results of HT in LBMDs have been reviewed recently (Nguyen et al., 2020). In general, the major outcomes for HT in LBMDs showed that there have been promising results with initial biochemical and clinical benefits but scarce sustained responses, with an observed decline in cell function after approximately 9–12 months (Nguyen et al., 2020).
Key general factors affecting the poor outcomes of clinical HT include sub-optimal cell engraftment and difficulties in diagnosing and managing rejection. Strategies to improve primary hepatocyte engraftment into the liver have been developed for LBMDs, such as through a selective and highly proliferative stimulus following partial hepatectomy (Nguyen et al., 2020). Lack of spontaneous recruitment of the residing or transplanted cells into the liver microenvironment in LBMDs necessitates reconditioning of the host liver to stimulate proliferation of the transplanted cells. Although quite successful in animal models, this approach failed to show a similar level of efficacy when applied to humans (Nguyen et al., 2020).
The major disadvantages associated with HT include the limited supply of donor organs to isolate good-quality cells, low cell engraftment, cryopreservation difficulties, and the need for long-term immunosuppression (Giancotti et al., 2019; Nguyen et al., 2020).
In this context, the great availability of fetal livers from spontaneous or legal therapeutic abortions and their biological properties, including scarce or null immunogenicity and tumorigenicity, make fetal liver cells an ideal source of easily isolatable and cultivable cells for liver regenerative medicine (Giancotti et al., 2019). Indeed, compared with adult liver, the cell isolation procedure in fetal and neonatal livers also provides cell suspensions with a higher proportion of EpCAM+ HPCs (Schmelzer et al., 2007). Nevertheless, the higher number of viable functional hepatocytes isolated from adult liver compared with fetal liver still represents a positive characteristic of the adult source (Giancotti et al., 2019; Nguyen et al., 2020). Use of fetal liver from spontaneous or legal therapeutic abortions in regenerative medicine still faces legal, ethical, and religious obstacles. In November 2019, the Trump government published a regulation banning fetal cell use in medical research. In response, scientists reported the high quantity of discoveries obtained with these resources and the importance of continuing research in this area (McCune and Weissman, 2019). In April 2021, the National Institutes of Health removed restrictions on research using fetal tissue, allowing US university researchers and government scientists to use material from elective abortions for biomedical research purposes. Europe is the most regulated area on this issue in the world, and heterogeneity exists among countries, as reported in detail elsewhere (Kawasaki et al., 2020). To date, there is no law regulating research use of human aborted fetuses in Japan (Kawasaki et al., 2020).
Transplantation of in vitro-expanded hepatocytes holds great potential for large-scale clinical application to treat liver diseases, comprising attempts of gene editing through different methods (Hu et al., 2018; Peng et al., 2018, 2021). Recently, several protocols for establishing monolayer cultures of human hepatocytes have been reported (Fu et al., 2019; Kim et al., 2019; Zhang et al., 2018; Xiang et al., 2019). These and previous reports highlight the challenges in long-term expansion of adult human hepatocytes.
Human hepatocytes in ALF
In ALF, the therapeutic window is narrow; thus, urgent organ procurement along with maximal life support are major determinants of survival (Wendon et al., 2017). In this condition, the sudden collapse of the liver parenchyma because of acute massive hepatocyte injury has been treated by HT with the intent to replace and support liver function until the host liver recovers, driven by self-regeneration, or a donor liver is available (Dhawan et al., 2020; Fisher and Strom, 2006; Fisher et al., 2000; Habibullah et al., 1994; Khan et al., 2004; Meyburg et al., 2010; Nguyen et al., 2020; Schneider et al., 2006; Sterling and Fisher, 2001; Strom et al., 1999).
The clinical results of HT in patients with ALF have been reviewed recently (Nguyen et al., 2020), and there has been a large heterogeneity in the administration route, number of transplanted cells, and use of adult versus fetal liver cells. A total of 41 subjects with ages ranging from 1 day to 69 years and multiple underlying etiologies of liver failure have been treated with human hepatocytes and 8 with unsorted fetal hepatocytes (Habibullah et al., 1994; Khan et al., 2004). The latest advance in the field of HT is intraperitoneal administration of alginate microencapsulated human primary hepatocytes for treatment of ALF in children (Dhawan et al., 2020).
Overall, the major outcomes for HT in ALF showed that full recovery of liver function has been occasionally obtained after treatment, but because there are no reliable prognostic models or randomized controlled trials in ALF, definitive conclusions regarding the efficacy of HT in ALF cannot be drawn. Critical features limiting the success of HT, especially in ALF, are the large number of hepatocytes needed (>10% host liver mass) and the quality of the liver graft used for hepatocyte isolation (Nguyen et al., 2020).
Human pluripotent stem cells, such as embryonic stem cells (ESCs) or iPSCs, are very promising sources of functional mature hepatocytes to use in liver regenerative medicine or cell therapy applications. Use of ESCs causes ethical concerns regarding their origin from human embryos, and they bear a clinical risk because their pluripotent nature makes undifferentiated ESC capable to form malignant teratocarcinomas in vivo (Giancotti et al., 2019). Significant advancements have been made in defining protocols for differentiation of human iPSCs into functional mature hepatocytes; e.g., induced multipotent progenitor cell-derived hepatocytes (Zhu et al., 2014), direct reprogramming of fibroblasts or MSCs (Du et al., 2014; Huang et al., 2014; Rezvani et al., 2016), utilization of human gastric epithelial cells differentiated into endodermal progenitors (Wang et al., 2016), or co-culturing with endothelial cells (Pettinato et al., 2019) (Figure 1). Although human iPSCs could, in theory, represent a valid alternative to primary cells in regenerative medicine because of the potentially inexhaustible source of autologous stem cells they can provide, low scalability and partial phenotypic immaturity still represent major limitations to their use in the clinic. Forward programming by direct overexpression of transcriptional factors in human iPSCs could bypass these limitations, leading to direct cellular conversion while preserving the capacity for proliferation. This approach has been successfully used to generate neurons, skeletal myocytes, and oligodendrocytes (Pawlowski et al., 2017). Recently, the same platform has been exploited to produce hepatocytes by forward programming, based on overexpression of three hepatocyte nuclear factors (HNF1A, HNF6, and FOXA3) in combination with different nuclear receptors expressed in the adult liver. Forward programming allows rapid production of hepatocytes (FoP-Heps) with functional characteristics and could offer a versatile alternative to direct differentiation for generating hepatocytes in vitro (Tomaz et al., 2022).
While the approaches described so far are quite promising for the large-scale expansion of primary human hepatocytes, they were developed using not fully good manufacturing practice (GMP)-compliant reagents (e.g., using fetal bovine serum and Matrigel); hence, they will need to be optimized to be successfully translated for clinical use. Per se, in vitro expansion is considered a manipulation. For this reason, human hepatocytes, HPCs, and BTSCs are not considered “medicinal products for advanced therapy,” while, sources that require in vitro expansion manipulation need to comply with the European rules for drug development and production (Iglesias-Lopez C et al., 2021).
HPCs and BTSCs in CLD
In CLD, the extent of liver regeneration decreases progressively and finally becomes insufficient to maintain liver homeostasis (Nguyen et al., 2020; Overi et al., 2018, 2020). The progression from early-stage tissue fibrosis to advanced histological cirrhosis and, clinically, from clinically compensated to decompensated cirrhosis and liver failure may be considered key landmarks of progressively insufficient liver regeneration (Thuluvath et al., 2018). In this pathophysiological context, liver replacement strategies could have a clinical rationale. In a situation of hepatocyte senescence and preexisting stimulation of residing progenitor cells, typical of CLD, contributing to liver regeneration by using liver progenitor cell sources could be a rational approach because of the higher capacity of self-replication vs. adult hepatocytes and natural commitment of liver progenitor cells (Fisher et al., 2000; Khan et al., 2008a, 2008b; Lanzoni et al., 2016; Stéphenne et al., 2006). Human fetal liver was the cell source used in clinical studies of cell therapy in liver cirrhosis. Human fetal liver (from 10 weeks of gestation) contains two progenitor cell niches: the ductal plates/canals of Hering, which contain HPCs (Schmelzer et al., 2007; Semeraro et al., 2012), and the PBG, which contains BTSCs (Cardinale et al., 2011; Giancotti et al., 2019; Figure 2). In a controlled trial of subjects with liver cirrhosis receiving fetal EpCAM+ HPC infusion via the hepatic artery (Khan et al., 2010), there was a significant decrease in patient model for end-stage liver disease (MELD) scores in the treated group (N = 25) at the 6-month follow-up. Pietrosi et al. (2015) and Gridelli et al. (2012) treated nine and one patients, respectively, by intrasplenic infusion of a total fetal liver cell population and demonstrated positive effects on clinical scores and encephalopathy. Preliminary results have been reported for a phase I/II clinical trial consisting of hepatic artery transplantation of fetal BTSCs in patients with advanced cirrhosis (Cardinale et al., 2014).
Overall, there exists a large heterogeneity of cell isolation and selection protocols, which hinders the ability to pool data and perform a meta-analysis. The few clinical reports where patients with CLD have received cells from fetal liver (n = 4) showed an extremely small sample size, although there was a fairly long follow-up (median, 12 months). There were no randomized controlled trials, and therefore no study was stratified as being of good methodological quality. No conclusions can be drawn regarding the major outcomes when using HPCs and BTSCs in the clinical setting of CLDs.
Remarkably, in all trials employing fetal liver-derived progenitor cells, immune suppression was not required even though donors and recipients were not matched for histocompatibility antigens. Attempts to improve cell engraftment have also been made with stem/progenitor cells (Nevi et al., 2019), such as use of a hyaluronic acid-based coating of BTSCs (Nevi et al., 2017a, 2017b).
Logistic limitations related to use of adult or fetal liver have been reduced by advanced cell cryopreservation methods (Giancotti et al., 2019; Nguyen et al., 2020; Nevi et al., 2017a, 2017b). Cryopreservation is a key step for routine use of cell products in clinical cell therapies. A number of different cryopreservation techniques have been proposed, including use of cryopreservation agents, cell coating techniques, preconditioning techniques, and gradual freezing. Unfortunately, with regard to the cell types isolated from solid organs, such as liver cells, a large variability in cell viability and engraftment efficiency after thawing has been reported (Giancotti et al., 2019; Nevi et al., 2017a, 2017b, 2019; Nguyen et al., 2020).
Cell therapy: Tissue repair
As stated above, during homeostasis or moderate hepatic injury, liver regeneration is mediated by hepatocyte mitosis. However, during chronic injury, impaired hepatocyte regeneration occurs because of cellular senescence (Wiemann et al., 2002) or inhibition of mitosis. Regeneration could then, at least partly, be mediated by HPCs, capable of transforming into cholangiocytes but also hepatocytes. Numerous data in the literature clearly indicate the existence of remarkable cellular plasticity in liver epithelial cells during liver injury, with the consequence that, under specific circumstances, HPCs can change their cell fate to promote liver regeneration through a regenerative or metaplasia-like response. Robust evidence of this “rescue” phenomenon exists in animals through cell tracking experiments (Lu et al., 2015; Manco et al., 2019), but only indirect proof is available in human studies (Deng et al., 2018; Lanthier and Spahr, 2019; Lanthier et al., 2015; Lin et al., 2010; Yoon et al., 2011). However, despite this HPC activation, there is no argument for effective regeneration in some severe human diseases, as shown in patients with severe alcoholic steatohepatitis and an unfavorable Lille score. In these patients with a poor prognosis, a massive expansion of HPCs is demonstrated, which does not lead to beneficial liver regeneration (Dubuquoy et al., 2015). This is probably due to the deleterious effect of the inflammatory microenvironment not only on hepatocyte replication but also on HPC replication and survival. An alternative approach is therefore to act on the microenvironment so that it is favorable for proliferation of hepatocytes or proliferation and differentiation toward hepatocytes of HPCs. Indeed, important data indicate that the microenvironment is needed for functional rescue (Boulter et al., 2012; van Hul et al., 2009, 2011). While Notch signaling is important for HPC proliferation, Wnt signaling is essential for HPC differentiation into hepatocytes (Boulter et al., 2012; Minnis-Lyons et al., 2021). In this setting, two cell populations deserve special attention to ameliorate the injury niche: macrophages and mesenchymal stromal cells (MSCs) (Figure 2).
Hepatic macrophages are the most abundant liver immune cells. They consist of resident macrophages in the liver, called Kupffer cells (KCs), and recruited macrophages from circulating monocytes, called monocyte-derived macrophages (MDMs) (Wen et al., 2021). One of the main functions of KCs is defense against pathogens, playing a key role in the gut-liver axis. KCs also play a central role in liver regeneration (Wen et al., 2021). Indeed, stimuli other than bacterial signals could activate KCs, such as signals associated with liver injury and parenchymal loss, called damage-associated molecular patterns (DAMPs). Macrophages have a dual role, seemingly opposite at first sight (Gao and Tsukamoto, 2016). On one hand, KCs contribute to hepatic inflammation by recruiting proinflammatory MDMs and other host immune cells in the early phase of liver injury (Gadd et al., 2014). They also induce fibrosis through activation of hepatic stellate cells. This proinflammatory role is well described in the context of MAFLD (Gadd et al., 2014) and ALD (Stärkel et al., 2019). On the other hand, their importance in repair mechanisms and liver regeneration is also reported (Wen et al., 2021). A switch from proinflammatory MDMs to restorative MDMs constitutes a key event of macrophage-dependent repair mechanisms. Restorative MDMs express MMPs and phagocytosis-related genes, promoting clearance of dead cells and wound healing. MDMs also secrete growth factors, such as hepatocyte growth factor (HGF), that promote hepatocyte proliferation. Several findings support this concept. In mice, KC depletion reduces production of the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) but also hepatocyte proliferation, resulting in delayed regeneration after partial hepatectomy (Meijer et al., 2000). In humans, high macrophage content is associated with higher liver serine peptidase inhibitor Kazal type I (SPINK1) expression (a promotor of hepatocyte cell proliferation) (Chang et al., 2017), hepatocyte proliferation, and better outcome in ASH (Lanthier et al., 2015). The prognostic value of this inflammation status is also evidenced on standard liver histology (Altamirano et al., 2014). Finally, the role of macrophages in liver regeneration also involves HPC-supported liver repair. KC depletion is associated not only with reduced hepatocyte proliferation but also with reduced differentiation of HPCs toward hepatocytes (Boulter et al., 2012; van Hul et al., 2011). Adoptive transfer of monocytes stimulates HPC proliferation by activating TNF-like weak inducer of apoptosis (TWEAK) as well as differentiation (Elsegood et al., 2015; Bird et al., 2013). In humans, liver macrophage activation has also been linked to higher fibroblast growth factor inducible 14 (the receptor for TWEAK) expression and increased HPC proliferation (Lanthier et al., 2015). However, the restorative macrophage phenotype that combines anti-inflammation with antifibrotic/anti-cancer properties remains elusive. In this context, a first-in-human phase 1 trial of autologous macrophage peripheral infusion in cirrhotic patients has been performed and shown to meet its primary outcome of safety and feasibility (Moroni et al., 2019). A phase 2 randomized controlled trial is planned (Brennan et al., 2021).
MSCs are multipotent fibroblast-like cells originating from the bone marrow, umbilical tissue, or adipose tissue, to mention the main tissues. They produce high levels of an array of bioactive molecules, including growth factors, cytokines, and enzymes, when activated. Indeed, in animals, they improve engraftment of hepatocytes in the case of co-transplantation (Joshi et al., 2012). They have immunomodulatory effects on all cells involved in the immune response and could also inhibit hepatic stellate cells and ECM synthesis. The interest in MSCs lies in this possible modulation of immune response and acting on suppressing the inflammatory part of liver diseases rather than contributing directly as a cell source advancing regeneration. Numerous results from animal experiments using cells from bone marrow or adipose tissue, for example, attest to the potential of these cells to secrete exosomes and other effectors that could act on adaptive and innate immunity (Wu and Meng, 2021). Modulation of the inflammatory microenvironment might enhance their clinical application for treatment of different pathological processes. So far, MSCs have been demonstrated to exert immunoregulatory mechanisms in a paracrine fashion through release of soluble factors and by cell-cell contact with immune cells of adaptive and innate immunity. A full comprehension of their immunomodulatory mechanisms is still lacking and represents one of the most unresolved issues for their therapeutic use (Gao and Tsukamoto, 2016). The broad variety of potential soluble mediators involved in paracrine mechanisms of immunomodulation includes transforming growth factor β1 (TGF-β1), prostaglandin E2 (PGE2), HGF, indoleamine-pyrrole 2,3-dioxygenase (IDO), nitric oxide (NO), and IL-10. On the other hand, suppression of immune cell activity driven by direct cell-cell contact is exerted by upregulating molecules that are critical for T cell activation and leukocyte recruitment to the inflammation site. As a matter of fact, in vitro and in vivo studies have demonstrated that MSCs promote immunomodulatory effects through activation of the Fas/FasL, PD-1/PD-L1, and Notch/FOXP3 pathways (Zhao et al., 2012; Di Tinco et al., 2021). These mechanisms are shared features with stem cells owning different embryological origin (Riccio et al., 2014). It is also well known that the immunomodulatory abilities of MSCs are critically affected by the surrounding microenvironment and that the inflammatory status can condition MSCs’ immune response (Wang et al., 2014). However, there is little evidence of this in humans, and many concerns remain to be addressed in clinical use of MSCs for liver diseases, such as types, numbers, and routes of administration (Wu and Meng 2021).
Several clinical trials on MSC infusion provide conflicting or only weak results (Zhu et al., 2021; Lanthier, 2018; Liu et al., 2022). In a randomized controlled trial on patients with cirrhosis, MSCs did not have any beneficial effect on MELD score improvement or patient survival (Mohamadnejad et al., 2013). A transient effect on liver biological parameters was evidenced in another study on patients with post-HCV ESLD when MSCs were administered with granulocyte colony-stimulating factor (G-CSF) (Salama et al., 2014). In patients with hepatitis B, the long-term outcomes were also not markedly improved in one study (Peng et al., 2011), while effects on mortality are described in another trial (Lin et al., 2017). When administered with bone marrow mononuclear cells in patients with cirrhosis and alcoholic hepatitis, MSCs did not provide any clinical effect (Spahr et al., 2013). However, MSCs and bone mononuclear cells could have an effect on the hepatic microenvironment, inducing liver macrophage activation, as evidenced on liver histology obtained with a second liver biopsy performed 4 weeks after infusion. This was associated with significant changes in hepatic transcriptome analysis, such as increased SPINK1 and HGF mRNA expression (Lanthier et al., 2017). In a randomized controlled trial in patients with cirrhosis, administration of CD133+ cells in combination with G-CSF did not provide any benefit compared with the control group (Newsome et al., 2018). These results are in line with those from another randomized controlled trial that also found that G-CSF and bone marrow-derived cells (hematopoietic stem cells [HSCs] and MSCs) had no effect in the context of severe decompensated ALD cirrhosis (Spahr et al., 2013). A recent systematic review and meta-analysis of randomized controlled trials (RCTs) of MSC-based therapy for patients with CLD, accounting for 12 studies and 846 patients, with 411 patients receiving MSCs therapy and 435 patients undergoing traditional supportive therapy, showed that, overall, the major outcomes of this therapy was improvement of liver function, including MELD score, albumin levels, and coagulation function, but no beneficial effects on survival rate were demonstrated. Interestingly, the meta-analysis indicated a similar efficacy of autologous bone marrow-derived MSCs (n = 8 studies) and umbilical cord-derived MSCs (n = 4 studies), and no serious adverse events and side effects were reported (Liu et al., 2022). Of note, only 3 of the 12 RCTs were of high quality.
Finally, a peculiar MSC source consists of adult-derived human liver mesenchymal-like cells obtained from healthy donors and expanded in a current good manufacturing practice (cGMP)-compliant environment (human allogeneic liver-derived progenitor cells [HALPCs]; HepaStem). HALPCs were used in an open-label phase II multicentric European study in patients with acute-on-chronic liver failure (ACLF) or acute decompensation of cirrhosis (AD). The 24 treated patients (mean age, 51 years) were mostly male with alcohol-related cirrhosis. Two of the 3 initial patients treated with high doses had severe adverse bleeding events attributed to treatment. Their efficacy will require a randomized controlled trial because of the favorable disease course on cessation of alcohol (23 of 24 patients had ACLF attributed to ALD in this study) (Nevens et al., 2021). HALPCs were also used in a phase I/II prospective, open-label, multicenter, randomized trial primarily aiming to evaluate safety in pediatric patients with urea cycle disorders (UCDs) or Crigler-Najjar (CN) syndrome 6 months post transplantation (Smets et al., 2019). Fourteen patients with UCDs and 6 with CN syndrome were divided into 3 cohorts by body weight and infused intraportally with 3 doses of HALPCs. This study led to European clinical trial authorization for a phase II study with repeated infusions and intermediate doses.
Cell-based approaches modulating the microenvironment of liver grafts: Human studies
The liver’s innate immunoregulatory microenvironment makes liver transplantation (LT) the optimal setting to explore new strategies for induction of operational tolerance, defined as stable graft function in the absence of immunosuppression for more than 1 year without any features of rejection (Dai et al., 2020; Manzia et al., 2018; Orlando et al., 2009). Cell-based therapies are some of the most promising tools to achieve this goal by infusion of the recipient’s autologous immune cells or allogenic liver donor cells (Ellias et al., 2021; Kholodenko et al., 2018). Notwithstanding, of a variety of cell products were tested in preclinical models, only three have been applied in clinical studies, including induction of chimerism by HSCs, adoptive transfer of regulatory cells, and infusion of MSCs (Crispe et al., 2006). Induction of mixed chimerism by transfer of donor HSCs together with the liver graft, leading to donor-specific tolerance, was the first to be explored and proved to guarantee long-term tolerance (Donckier et al., 2006; Kim et al., 2009; Tryphonopoulos et al., 2005); however, conditioning therapy seems to be indispensable for engraftment of donor HSCs, carrying the risk of graft-versus-host disease (Perruche et al., 2006). Autologous HSC infusion might be an alternative because it may reset the immune system into a tolerant status by generating new auto-tolerant T and B cells, but results from an ongoing clinical trial are anticipated(ClinicalTrials.gov: NCT02549586). Adoptive transfer of cells with immunoregulatory activity (such as regulatory T [Treg] cells and regulatory dendritic cells [DCregs]) are considered promising alternatives with long-term efficacy and low toxicity, not requiring a myeloablative conditioning protocol. So far, Treg cell-induced immune regulation has been the best-studied mechanism of cell-based tolerance; Treg cells migrate to the site of inflammation and exert immunosuppressive effects on CD4+ and CD8+ T cells directly or through production of inhibitory cytokines (Crispe et al., 2006; Dai et al., 2020). Two preliminary studies have demonstrated the safety and efficacy of Treg cell infusion to achieve immunosuppression weaning after LT (Todo et al., 2016; Sánchez-Fueyo et al., 2020), and several clinical trials are currently exploring the long-term effectiveness of Treg cell therapy. Further promising Treg cell-based strategies under evaluation include generation of antigen-specific Treg cells, such as chimeric antigen receptor transduction or CRISPR-Cas9 technology, aiming to enhance Treg cells’ regulatory activity (Raffin et al., 2020). Also, use of DCregs, a cell population involved in the early stage of immune response, seems promising because several preclinical experiences showed their ability to blunt immune response memory and prevent hyper-acute rejection (Zhou et al., 2016). A phase I/II trial is currently testing the effectiveness of donor-derived DCreg infusion before living-donor LT (Thomson et al., 2018); the results are anticipated in 2023. Finally, despite MSCs seeming to be a useful strategy to dampen anti-donor immune response (Podestà et al., 2019), the few experiences reported are still not consistent to establish MSC infusion as a safe and effective tolerogenic cell therapy, and further studies defining the timing and dosing of MSC administration are needed (Detry et al., 2017).
Conclusions: Research needs and perspectives
Bioengineering approaches to replace liver functions has been long underway, but it was not until decellularization and recellularization techniques were suggested that a human-size transplantable liver graft could be realized. Recent advances in the upscaling of this approach to human livers have demonstrated great potential for clinical transplantation. In addition, advancement of 3D bioprinting to create functional liver units has brought much-needed control over the placement of cells in the constructs to recreate the cellular microarchitecture. However, many challenges still remain. Complete vascularization of transplantable liver grafts has yet to be achieved to accomplish undisrupted blood circulation and functioning of the cells. In 3D bioprinting, maintaining the viability of cells in constructs sufficiently large to maintain the functions of a whole organ has been problematic. Future studies should address these challenges and potentially integrate both approaches for successful translation of bioengineered liver grafts to the clinic.
Progression of liver disease is characterized by several pathobiology instances that may be considered innovative targets for regenerative medicine. Tailored preclinical models are expected. From a clinical point of view, in the context of liver cell therapy, RCTs are needed to evaluate the long-term safety and efficacy of the different cell sources (hepatocyte, HPC, BTSC, macrophage, or MSC transplantation) in the variable clinical settings of liver diseases of different etiologies. Data on each individual liver disease and more accurate clinical endpoints are urgently needed. Assessment of the liver tissue before and after treatment will be crucial to provide a gold standard for non-invasive cell tracing and, more importantly, characterize regenerative pathways responsible for the therapeutic benefits. Logistic limitations related to use of adult or fetal liver have been reduced by advanced cell cryopreservation methods. Innovative and technically advanced effective cryopreservation applicable in clinical trials is needed.
The highly heterogeneous and dynamic clinical scenario of liver disease and the existence of multiple sources with different properties determine the need to define the patient setting, the expected prognosis, the therapeutic window, and the expected results and, most importantly, to match the cell type (endowed with a defined property or more properties; e.g., hepatic function or immunomodulation) with the patient’s clinical setting (inborn errors of metabolism, ALF, CLD with parenchymal injury). Such complexity needs to be faced by multidisciplinary teams of experts at research and clinical levels.
Author contributions
V.C., R.C., and B.E.U. conceived, drafted, and edited the manuscript. V.C. homogenized all contributions and drafted Figure 2. B.E.U. drafted Figure 1. N.L., P.M.B., G. Carpino, G. Carnevale, G.O., R.A., and T.M.M. drafted and edited the manuscript. G. Carnevale formatted the references. D.S., M.P., and D.A. revised and edited the manuscript. All Authors approved the final version.
Acknowledgments
A research grant from Minister of Universty and Research under PNRR M4C2I1.3 Heal Italia project PE00000019 CUP Sapienza University of Rome was received by D.A. and V.C. to contribute to this article.
Conflict of interests
The authors declare no competing interests.
Contributor Information
Vincenzo Cardinale, Email: vincenzo.cardinale@uniroma1.it.
Rachele Ciccocioppo, Email: rachele.ciccocioppo@univr.it.
Basak E. Uygun, Email: buygun@mgh.harvard.edu.
References
- Aizarani N., Saviano A., Sagar, Mailly L., Durand S., Herman J.S., Pessaux P., Baumert T.F., Grün D. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature. 2019;572:199–204. doi: 10.1038/S41586-019-1373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M., Pr A.K., Lee S.J., Jackson J.D. Three-dimensional bioprinting for organ bioengineering: promise and pitfalls. Curr. Opin. Organ Transplant. 2018;23:649–656. doi: 10.1097/MOT.0000000000000581. [DOI] [PubMed] [Google Scholar]
- Altamirano J., Miquel R., Katoonizadeh A., Abraldes J.G., Duarte-Rojo A., Louvet A., Augustin S., Mookerjee R.P., Michelena J., Smyrk T.C., et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–1239.e1-6. doi: 10.1053/J.GASTRO.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banales J.M., Huebert R.C., Karlsen T., Strazzabosco M., LaRusso N.F., Gores G.J. Cholangiocyte pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019;16:269–281. doi: 10.1038/S41575-019-0125-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista P.M., Siddiqui M.M., Lozier G., Rodriguez S.R., Atala A., Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology. 2011;53:604–617. doi: 10.1002/hep.24067. [DOI] [PubMed] [Google Scholar]
- Bhatia S.N., Underhill G.H., Zaret K.S., Fox I.J. Cell and tissue engineering for liver disease. Sci. Transl. Med. 2014;6:245sr2. doi: 10.1126/SCITRANSLMED.3005975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T.G, Forbes S.J. Two Fresh Streams to Fill the Liver’s Hepatocyte Pool. Cell Stem Cell. 2015;17 doi: 10.1016/j.stem.2015.09.007. 377–8. [DOI] [PubMed] [Google Scholar]
- Bird T.G., Lu W.Y., Boulter L., Gordon-Keylock S., Ridgway R.A., Williams M.J., Taube J., Thomas J.A., Wojtacha D., Gambardella A., et al. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc. Natl. Acad. Sci. USA. 2013;110:6542–6547. doi: 10.1073/PNAS.1302168110/-/DCSUPPLEMENTAL/PNAS.201302168SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter L., Govaere O., Bird T.G., Radulescu S., Ramachandran P., Pellicoro A., Ridgway R.A., Seo S.S., Spee B., Van Rooijen N., et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat. Med. 2012;18:572–579. doi: 10.1038/NM.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan P.N., Macmillan M., Manship T., Moroni F., Glover A., Graham C., Semple S., Morris D.M., Fraser A.R., Pass C., et al. Study protocol: a multicentre, open-label, parallel-group, phase 2, randomised controlled trial of autologous macrophage therapy for liver cirrhosis (MATCH) BMJ Open. 2021;11 doi: 10.1136/BMJOPEN-2021-053190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M., Nardi A., Flack S., Carpino G., Varvaropoulou N., Gavrila C., Spicer A., Badrock J., Bernuzzi F., Cardinale V., et al. Pretreatment prediction of response to ursodeoxycholic acid in primary biliary cholangitis: development and validation of the UDCA Response Score. Lancet. Gastroenterol. Hepatol. 2018;3:626–634. doi: 10.1016/S2468-1253(18)30163-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V., Carpino G., Gentile R., Napoletano C., Rahimi H., Franchitto A., Semeraro R., Nuti M., Onori P., Berloco P.B., et al. Transplantation of human fetal biliary tree stem/progenitor cells into two patients with advanced liver cirrhosis. BMC Gastroenterol. 2014;14:204. doi: 10.1186/S12876-014-0204-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V., Wang Y., Carpino G., Cui C.B., Gatto M., Rossi M., Berloco P.B., Cantafora A., Wauthier E., Furth M.E., et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/HEP.24590. [DOI] [PubMed] [Google Scholar]
- Carpino G., Cardinale V., Folseraas T., Overi D., Floreani A., Franchitto A., Onori P., Cazzagon N., Berloco P.B., Karlsen T.H., et al. Hepatic Stem/Progenitor Cell Activation Differs between Primary Sclerosing and Primary Biliary Cholangitis. Am. J. Pathol. 2018;188:627–639. doi: 10.1016/J.AJPATH.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Carpino G., Cardinale V., Renzi A., Hov J.R., Berloco P.B., Rossi M., Karlsen T.H., Alvaro D., Gaudio E. Activation of biliary tree stem cells within peribiliary glands in primary sclerosing cholangitis. J. Hepatol. 2015;63:1220–1228. doi: 10.1016/J.JHEP.2015.06.018. [DOI] [PubMed] [Google Scholar]
- Carpino G., Nevi L., Overi D., Cardinale V., Lu W.Y., di Matteo S., Safarikia S., Berloco P.B., Venere R., Onori P., et al. Peribiliary Gland Niche Participates in Biliary Tree Regeneration in Mouse and in Human Primary Sclerosing Cholangitis. Hepatology. 2020;71:972–989. doi: 10.1002/HEP.30871. [DOI] [PubMed] [Google Scholar]
- Chang C., Zhao W., Luo Y., Xi L., Chen S., Zhao C., Wang G., Guo J., Xu C. Serine peptidase inhibitor Kazal type I (SPINK1) promotes BRL-3A cell proliferation via p38, ERK, and JNK pathways. Cell Biochem. Funct. 2017;35:339–348. doi: 10.1002/CBF.3288. [DOI] [PubMed] [Google Scholar]
- Chen C., Soto-Gutierrez A., Baptista P.M., Spee B. Biotechnology Challenges to In Vitro Maturation of Hepatic Stem Cells. Gastroenterology. 2018;154:1258–1272. doi: 10.1053/J.GASTRO.2018.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Jimenez R.J., Sharma K., Luu H.Y., Hsu B.Y., Ravindranathan A., Stohr B.A., Willenbring H. Broad Distribution of Hepatocyte Proliferation in Liver Homeostasis and Regeneration. Cell Stem Cell. 2020;26:27–33.e4. doi: 10.1016/J.STEM.2019.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe I.N., Giannandrea M., Klein I., John B., Sampson B., Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol. Rev. 2006;213:101–118. doi: 10.1111/J.1600-065X.2006.00435.X. [DOI] [PubMed] [Google Scholar]
- Dai H., Zheng Y., Thomson A.W., Rogers N.M. Transplant Tolerance Induction: Insights From the Liver. Front. Immunol. 2020;11:1044–1114. doi: 10.3389/FIMMU.2020.01044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Zhang X., Li W., Feng R.X., Li L., Yi G.R., Zhang X.N., Yin C., Yu H.Y., Zhang J.P., et al. Chronic Liver Injury Induces Conversion of Biliary Epithelial Cells into Hepatocytes. Cell Stem Cell. 2018;23:114–122.e3. doi: 10.1016/J.STEM.2018.05.022. [DOI] [PubMed] [Google Scholar]
- Detry O., Vandermeulen M., Delbouille M.H., Somja J., Bletard N., Briquet A., Lechanteur C., Giet O., Baudoux E., Hannon M., et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: A phase I-II, open-label, clinical study. J. Hepatol. 2017;67:47–55. doi: 10.1016/J.JHEP.2017.03.001. [DOI] [PubMed] [Google Scholar]
- Dhawan A., Chaijitraruch N., Fitzpatrick E., Bansal S., Filippi C., Lehec S.C., Heaton N.D., Kane P., Verma A., Hughes R.D., Mitry R.R. Alginate microencapsulated human hepatocytes for the treatment of acute liver failure in children. J. Hepatol. 2020;72:877–884. doi: 10.1016/J.JHEP.2019.12.002. [DOI] [PubMed] [Google Scholar]
- Di Tinco R., Bertani G., Pisciotta A., Bertoni L., Pignatti E., Maccaferri M., Bertacchini J., Sena P., Vallarola A., Tupler R., et al. Role of PD-L1 in licensing immunoregulatory function of dental pulp mesenchymal stem cells. Stem Cell Res. Ther. 2021;12:598. doi: 10.1186/s13287-021-02664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donckier V., Troisi R., le Moine A., Toungouz M., Ricciardi S., Colle I., Van Vlierberghe H., Craciun L., Libin M., Praet M., et al. Early immunosuppression withdrawal after living donor liver transplantation and donor stem cell infusion. Liver Transpl. 2006;12:1523–1528. doi: 10.1002/LT.20872. [DOI] [PubMed] [Google Scholar]
- Du Y., Wang J., Jia J., Song N., Xiang C., Xu J., Hou Z., Su X., Liu B., Jiang T., et al. Human hepatocytes with drug metabolic function induced from fibroblasts by lineage reprogramming. Cell Stem Cell. 2014;14:394–403. doi: 10.1016/J.STEM.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Dubuquoy L., Louvet A., Lassailly G., Truant S., Boleslawski E., Artru F., Maggiotto F., Gantier E., Buob D., Leteurtre E., et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64:1949–1960. doi: 10.1136/GUTJNL-2014-308410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer B.J., Macmillan M.T., Brennan P.N., Forbes S.J. Cell therapy for advanced liver diseases: Repair or rebuild. J. Hepatol. 2021;74:185–199. doi: 10.1016/J.JHEP.2020.09.014. [DOI] [PubMed] [Google Scholar]
- Ellias S.D., Larson E.L., Taner T., Nyberg S.L. Cell-Mediated Therapies to Facilitate Operational Tolerance in Liver Transplantation. Int. J. Mol. Sci. 2021;22 doi: 10.3390/IJMS22084016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsegood C.L., Chan C.W., Degli-Esposti M.A., Wikstrom M.E., Domenichini A., Lazarus K., van Rooijen N., Ganss R., Olynyk J.K., Yeoh G.C.T. Kupffer cell-monocyte communication is essential for initiating murine liver progenitor cell-mediated liver regeneration. Hepatology. 2015;62:1272–1284. doi: 10.1002/HEP.27977. [DOI] [PubMed] [Google Scholar]
- Fisher R.A., Strom S.C. Human hepatocyte transplantation: worldwide results. Transplantation. 2006;82:441–449. doi: 10.1097/01.TP.0000231689.44266.AC. [DOI] [PubMed] [Google Scholar]
- Fisher R.A., Bu D., Thompson M., Tisnado J., Prasad U., Sterling R., Posner M., Strom S. Defining hepatocellular chimerism in a liver failure patient bridged with hepatocyte infusion. Transplantation. 2000;69:303–307. doi: 10.1097/00007890-200001270-00018. [DOI] [PubMed] [Google Scholar]
- Font-Burgada J., Shalapour S., Ramaswamy S., Hsueh B., Rossell D., Umemura A., Taniguchi K., Nakagawa H., Valasek M.A., Ye L., et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766–779. doi: 10.1016/J.CELL.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes S.J., Gupta S., Dhawan A. Cell therapy for liver disease: From liver transplantation to cell factory. J. Hepatol. 2015;62:S157–S169. doi: 10.1016/J.JHEP.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Fu G.B., Huang W.J., Zeng M., Zhou X., Wu H.P., Liu C.C., Wu H., Weng J., Zhang H.D., Cai Y.C., et al. Expansion and differentiation of human hepatocyte-derived liver progenitor-like cells and their use for the study of hepatotropic pathogens. Cell Res. 2019;29:8–22. doi: 10.1038/S41422-018-0103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadd V.L., Skoien R., Powell E.E., Fagan K.J., Winterford C., Horsfall L., Irvine K., Clouston A.D. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/HEP.26937. [DOI] [PubMed] [Google Scholar]
- Gao B., Tsukamoto H. Inflammation in Alcoholic and Nonalcoholic Fatty Liver Disease: Friend or Foe? Gastroenterology. 2016;150:1704–1709. doi: 10.1053/J.GASTRO.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti A., Monti M., Nevi L., Safarikia S., D’Ambrosio V., Brunelli R., Pajno C., Corno S., Di Donato V., Musella A., et al. Functions and the Emerging Role of the Foetal Liver into Regenerative Medicine. Cells. 2019;8:914. doi: 10.3390/CELLS8080914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliozzi A., Alpini G., Baroni G.S., Marucci L., Metalli V.D., Glaser S.S., Francis H., Mancino M.G., Ueno Y., Barbaro B., et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology. 2004;127:1198–1209. doi: 10.1053/J.GASTRO.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Gori M., Giannitelli S.M., Torre M., Mozetic P., Abbruzzese F., Trombetta M., Traversa E., Moroni L., Rainer A. Biofabrication of Hepatic Constructs by 3D Bioprinting of a Cell-Laden Thermogel: An Effective Tool to Assess Drug-Induced Hepatotoxic Response. Adv. Healthc. Mater. 2020;9:e2001163. doi: 10.1002/ADHM.202001163. [DOI] [PubMed] [Google Scholar]
- Gridelli B., Vizzini G., Pietrosi G., Luca A., Spada M., Gruttadauria S., Cintorino D., Amico G., Chinnici C., Miki T., et al. Efficient human fetal liver cell isolation protocol based on vascular perfusion for liver cell-based therapy and case report on cell transplantation. Liver Transpl. 2012;18:226–237. doi: 10.1002/LT.22322. [DOI] [PubMed] [Google Scholar]
- Guyette J.P., Gilpin S.E., Charest J.M., Tapias L.F., Ren X., Ott H.C. Perfusion decellularization of whole organs. Nat. Protoc. 2014;9:1451–1468. doi: 10.1038/NPROT.2014.097. [DOI] [PubMed] [Google Scholar]
- Habeeb M.A., Vishwakarma S.K., Bardia A., Khan A.A. Hepatic stem cells: A viable approach for the treatment of liver cirrhosis. World J Stem Cells. 2015;7:859–865. doi: 10.4252/WJSC.V7.I5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibullah C.M., Syed I.H., Qamar A., Taher-Uz Z. Human fetal hepatocyte transplantation in patients with fulminant hepatic failure. Transplantation. 1994;58:951–952. doi: 10.1097/00007890-199410270-00016. [DOI] [PubMed] [Google Scholar]
- Han Y., Glaser S., Meng F., Francis H., Marzioni M., McDaniel K., Alvaro D., Venter J., Carpino G., Onori P., et al. Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp. Biol. Med. 2013;238:549–565. doi: 10.1177/1535370213489926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Pu W., Liu X., Zhang Z., Han M., Li Y., Huang X., Han X., Li Y., Liu K., et al. Proliferation tracing reveals regional hepatocyte generation in liver homeostasis and repair. Science. 2021;371:eabc4346. doi: 10.1126/SCIENCE.ABC4346. [DOI] [PubMed] [Google Scholar]
- Hu H., Gehart H., Artegiani B., LÖpez-Iglesias C., Dekkers F., Basak O., van Es J., Chuva de Sousa Lopes S.M., Begthel H., Korving J., et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell. 2018;175:1591–1606.e19. doi: 10.1016/J.CELL.2018.11.013. [DOI] [PubMed] [Google Scholar]
- Huang P., Zhang L., Gao Y., He Z., Yao D., Wu Z., Cen J., Chen X., Liu C., Hu Y., et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370–384. doi: 10.1016/J.STEM.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S.W., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/NATURE11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Gehart H., van Boxtel R., Hamer K., Blokzijl F., Verstegen M.M.A., Ellis E., van Wenum M., Fuchs S.A., de Ligt J., et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015;160:299–312. doi: 10.1016/J.CELL.2014.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Lopez C., Obach M., Vallano A., Agustí A. Comparison of regulatory pathways for the approval of advanced therapies in the European Union and the United States. Cytotherapy. 2021;23:261–274. doi: 10.1016/j.jcyt.2020.11.008. [DOI] [PubMed] [Google Scholar]
- Joshi M., B Patil P., He Z., Holgersson J., Olausson M., Sumitran-Holgersson S. Fetal liver-derived mesenchymal stromal cells augment engraftment of transplanted hepatocytes. Cytotherapy. 2012;14:657–669. doi: 10.3109/14653249.2012.663526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Yamada T., Wada T., Kosugi S. Current status and legal/ethical problems in the research use of the tissues of aborted human fetuses in Japan. Congenit. Anom. 2020 Nov;60:166–174. doi: 10.1111/cga.12381. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Shaik M v, Parveen N., Rajendraprasad A., Aleem M.A., Habeeb M.A., Srinivas G., Raj T.A., Tiwari S.K., Kumaresan K., et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409–418. doi: 10.3727/096368910X498241. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Parveen N., Mahaboob V.S., Rajendraprasad A., Ravindraprakash H.R., Venkateswarlu J., Rao P., Pande G., Narusu M.L., Khaja M.N., et al. Treatment of Crigler-Najjar Syndrome type 1 by hepatic progenitor cell transplantation: a simple procedure for management of hyperbilirubinemia. Transplant. Proc. 2008;40:1148–1150. doi: 10.1016/J.TRANSPROCEED.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Parveen N., Mahaboob V.S., Rajendraprasad A., Ravindraprakash H.R., Venkateswarlu J., Rao P., Pande G., Narusu M.L., Khaja M.N., et al. Management of hyperbilirubinemia in biliary atresia by hepatic progenitor cell transplantation through hepatic artery: a case report. Transplant. Proc. 2008;40:1153–1155. doi: 10.1016/J.TRANSPROCEED.2008.03.110. [DOI] [PubMed] [Google Scholar]
- Khan A.A., Habeeb A., Parveen N., Naseem B., Babu R.P., Capoor A.K., Habibullah C.M. Peritoneal transplantation of human fetal hepatocytes for the treatment of acute fatty liver of pregnancy: a case report. Trop. Gastroenterol. 2004;25:141–143. [PubMed] [Google Scholar]
- Kholodenko I.v., Kholodenko R.v., Lupatov A.Y., Yarygin K.N. Cell Therapy as a Tool for Induction of Immunological Tolerance after Liver Transplantation. Bull. Exp. Biol. Med. 2018;165:554–563. doi: 10.1007/S10517-018-4213-8. [DOI] [PubMed] [Google Scholar]
- Kim Y., Kang K., Lee S.B., Seo D., Yoon S., Kim S.J., Jang K., Jung Y.K., Lee K.G., Factor V.M., et al. Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. J. Hepatol. 2019;70:97–107. doi: 10.1016/J.JHEP.2018.09.007. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Kim D.W., Choi J.Y., Kim D.G., Min W.S., Lee J.W., Kim C.C. Full donor chimerism using stem-cell transplantation for tolerance induction in the human leukocyte antigen-matched liver transplant setting. Transplantation. 2009;88:601–603. doi: 10.1097/TP.0B013E3181B164D5. [DOI] [PubMed] [Google Scholar]
- Ko I.K., Peng L., Peloso A., Smith C.J., Dhal A., Deegan D.B., Zimmerman C., Clouse C., Zhao W., Shupe T.D., et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature. Biomaterials. 2015;40:72–79. doi: 10.1016/J.BIOMATERIALS.2014.11.027. [DOI] [PubMed] [Google Scholar]
- Koike H., Iwasawa K., Ouchi R., Maezawa M., Giesbrecht K., Saiki N., Ferguson A., Kimura M., Thompson W.L., Wells J.M., et al. Modelling human hepato-biliary-pancreatic organogenesis from the foregut-midgut boundary. Nature. 2019;574:112–116. doi: 10.1038/S41586-019-1598-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanthier N., Lin-Marq N., Rubbia-Brandt L., Clément S., Goossens N., Spahr L. Autologous bone marrow-derived cell transplantation in decompensated alcoholic liver disease: what is the impact on liver histology and gene expression patterns? Stem Cell Res. Ther. 2017;8:88. doi: 10.1186/S13287-017-0541-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanthier N., Rubbia-Brandt L., Lin-Marq N., Clément S., Frossard J.L., Goossens N., Hadengue A., Spahr L. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J. Hepatol. 2015;63:609–621. doi: 10.1016/J.JHEP.2015.04.003. [DOI] [PubMed] [Google Scholar]
- Lanthier N., Spahr L. Resident liver progenitor cells: Proofs of their contribution to human liver regeneration. Clin. Res. Hepatol. Gastroenterol. 2019;43:646–648. doi: 10.1016/J.CLINRE.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Lanthier N. Haemopoietic stem cell therapy in cirrhosis: the end of the story? Lancet. Gastroenterol. Hepatol. 2018;3:3–5. doi: 10.1016/S2468-1253(17)30359-X. [DOI] [PubMed] [Google Scholar]
- Lanzoni G., Cardinale V., Carpino G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: A new reference frame for disease and regeneration. Hepatology. 2016;64:277–286. doi: 10.1002/HEP.28326. [DOI] [PubMed] [Google Scholar]
- Li Q., Uygun B.E., Geerts S., Ozer S., Scalf M., Gilpin S.E., Ott H.C., Yarmush M.L., Smith L.M., Welham N.V., Frey B.L. Proteomic analysis of naturally-sourced biological scaffolds. Biomaterials. 2016;75:37–46. doi: 10.1016/J.BIOMATERIALS.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S., Nascimento E.M., Gajera C.R., Chen L., Neuhöfer P., Garbuzov A., Wang S., Artandi S.E. Distributed hepatocytes expressing telomerase repopulate the liver in homeostasis and injury. Nature. 2018;556:244–248. doi: 10.1038/S41586-018-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B.L., Chen J.F., Qiu W.H., Wang K.W., Xie D.Y., Chen X.Y., Liu Q.L., Peng L., Li J.G., Mei Y.Y., et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209–219. doi: 10.1002/HEP.29189. [DOI] [PubMed] [Google Scholar]
- Lin W.R., Lim S.N., McDonald S.A.C., Graham T., Wright V.L., Peplow C.L., Humphries A., Kocher H.M., Wright N.A., Dhillon A.P., Alison M.R. The histogenesis of regenerative nodules in human liver cirrhosis. Hepatology. 2010;51:1017–1026. doi: 10.1002/HEP.23483. [DOI] [PubMed] [Google Scholar]
- Liu Y., Dong Y., Wu X., Xu X., Niu J. The assessment of mesenchymal stem cells therapy in acute on chronic liver failure and chronic liver disease: a systematic review and meta-analysis of randomized controlled clinical trials. Stem Cell Res. Ther. 2022;13:204. doi: 10.1186/s13287-022-02882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W.Y., Bird T.G., Boulter L., Tsuchiya A., Cole A.M., Hay T., Guest R.V., Wojtacha D., Man T.Y., Mackinnon A., et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat. Cell Biol. 2015;17:971–983. doi: 10.1038/NCB3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli N., Kamileri I., Keogh A., Malinka T., Sarris M.E., Talianidis I., Schaad O., Candinas D., Stroka D., Halazonetis T.D. R-spondin 1 and noggin facilitate expansion of resident stem cells from non-damaged gallbladders. EMBO Rep. 2016;17:769–779. doi: 10.15252/EMBR.201642169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghsoudlou P., Georgiades F., Smith H., Milan A., Shangaris P., Urbani L., Loukogeorgakis S.P., Lombardi B., Mazza G., Hagen C., et al. Optimization of Liver Decellularization Maintains Extracellular Matrix Micro-Architecture and Composition Predisposing to Effective Cell Seeding. PLoS One. 2016;11:e0155324. doi: 10.1371/JOURNAL.PONE.0155324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manco R., Clerbaux L.A., Verhulst S., Bou Nader M., Sempoux C., Ambroise J., Bearzatto B., Gala J.L., Horsmans Y., van Grunsven L., et al. Reactive cholangiocytes differentiate into proliferative hepatocytes with efficient DNA repair in mice with chronic liver injury. J. Hepatol. 2019;70:1180–1191. doi: 10.1016/J.JHEP.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Manzia T.M., Angelico R., Toti L., Angelico C., Quaranta C., Parente A., Blasi F., Iesari S., Sforza D., Baiocchi L., et al. Longterm Survival and Cost-Effectiveness of Immunosuppression Withdrawal After Liver Transplantation. Liver Transpl. 2018;24:1199–1208. doi: 10.1002/LT.25293. [DOI] [PubMed] [Google Scholar]
- Mayorca-Guiliani A.E., Madsen C.D., Cox T.R., Horton E.R., Venning F.A., Erler J.T. ISDoT: in situ decellularization of tissues for high-resolution imaging and proteomic analysis of native extracellular matrix. Nat. Med. 2017;23:890–898. doi: 10.1038/nm.4352. [DOI] [PubMed] [Google Scholar]
- Mazza G., Rombouts K., Rennie Hall A., Urbani L., Vinh Luong T., Al-Akkad W., Longato L., Brown D., Maghsoudlou P., Dhillon A.P., et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci. Rep. 2015;5:13079. doi: 10.1038/SREP13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J.M, Weissman I.L. The Ban on US Government Funding Research Using Human Fetal Tissues: How Does This Fit with the NIH Mission to Advance Medical Science for the Benefit of the Citizenry? 2019;13:777–786. doi: 10.1016/j.stemcr.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer C., Wiezer M.J., Diehl A.M., Schouten H.J., Schouten H.J., Meijer S., van Rooijen N., van Lambalgen A.A., Dijkstra C.D., van Leeuwen P.A. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66–77. doi: 10.1034/J.1600-0676.2000.020001066.X. [DOI] [PubMed] [Google Scholar]
- Meyburg J., Hoerster F., Schmidt J., Poeschl J., Hoffmann G.F., Schenk J.P. Monitoring of intraportal liver cell application in children. Cell Transplant. 2010;19:629–638. doi: 10.3727/096368909X485058. [DOI] [PubMed] [Google Scholar]
- Minnis-Lyons S.E., Ferreira-González S., Aleksieva N., Man T.Y., Gadd V.L., Williams M.J., Guest R.V., Lu W.Y., Dwyer B.J., Jamieson T., et al. Notch-IGF1 signaling during liver regeneration drives biliary epithelial cell expansion and inhibits hepatocyte differentiation. Sci. Signal. 2021;14 doi: 10.1126/SCISIGNAL.AAY9185. [DOI] [PubMed] [Google Scholar]
- Mohamadnejad M., Alimoghaddam K., Bagheri M., Ashrafi M., Abdollahzadeh L., Akhlaghpoor S., Bashtar M., Ghavamzadeh A., Malekzadeh R. Randomized placebo-controlled trial of mesenchymal stem cell transplantation in decompensated cirrhosis. Liver Int. 2013;33:1490–1496. doi: 10.1111/LIV.12228. [DOI] [PubMed] [Google Scholar]
- Moroni F., Dwyer B.J., Graham C., Pass C., Bailey L., Ritchie L., Mitchell D., Glover A., Laurie A., Doig S., et al. Safety profile of autologous macrophage therapy for liver cirrhosis. Nat. Med. 2019;25:1560–1565. doi: 10.1038/S41591-019-0599-8. [DOI] [PubMed] [Google Scholar]
- Nevens F., Gustot T., Laterre P.F., Lasser L.L., Haralampiev L.E., Vargas V., Lyubomirova D., Albillos A., Najimi M., Michel S., et al. A phase II study of human allogeneic liver-derived progenitor cell therapy for acute-on-chronic liver failure and acute decompensation. JHEP Rep. 2021;3:100291. doi: 10.1016/J.JHEPR.2021.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevi L., Cardinale V., Carpino G., Costantini D., di Matteo S., Cantafora A., Melandro F., Brunelli R., Bastianelli C., Aliberti C., et al. Cryopreservation protocol for human biliary tree stem/progenitors, hepatic and pancreatic precursors. Sci. Rep. 2017;7:6080. doi: 10.1038/S41598-017-05858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevi L., Carpino G., Costantini D., Cardinale V., Riccioni O., di Matteo S., Melandro F., Berloco P.B., Reid L., Gaudio E., Alvaro D. Hyaluronan coating improves liver engraftment of transplanted human biliary tree stem/progenitor cells. Stem Cell Res. Ther. 2017;8:68. doi: 10.1186/S13287-017-0492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevi L., Safarikia S., Di Matteo S., Biancaniello F., Chiappetta M.F., Cardinale V. Hyaluronan-Based Grafting Strategies for Liver Stem Cell Therapy and Tracking Methods. Stem Cells Int. 2019;2019:3620546. doi: 10.1155/2019/3620546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome P.N., Fox R., King A.L., Barton D., Than N.N., Moore J., Corbett C., Townsend S., Thomas J., Guo K., et al. Granulocyte colony-stimulating factor and autologous CD133-positive stem-cell therapy in liver cirrhosis (REALISTIC): an open-label, randomised, controlled phase 2 trial. Lancet. Gastroenterol. Hepatol. 2018;3:25–36. doi: 10.1016/S2468-1253(17)30326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen D.G., Funk J., Robbins J.B., Crogan-Grundy C., Presnell S.C., Singer T., Roth A.B. Bioprinted 3D Primary Liver Tissues Allow Assessment of Organ-Level Response to Clinical Drug Induced Toxicity In Vitro. PLoS One. 2016;11:e0158674. doi: 10.1371/JOURNAL.PONE.0158674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M.P., Jain V., Iansante V., Mitry R.R., Filippi C., Dhawan A. Clinical application of hepatocyte transplantation: current status, applicability, limitations, and future outlook. Expert Rev. Gastroenterol. Hepatol. 2020;14:185–196. doi: 10.1080/17474124.2020.1733975. [DOI] [PubMed] [Google Scholar]
- Ogasawara H., Inagaki A., Fathi I., Imura T., Yamana H., Saitoh Y., Matsumura M., Fukuoka K., Miyagi S., Nakamura Y., et al. Preferable Transplant Site for Hepatocyte Transplantation in a Rat Model. Cell Transplant. 2021;30 doi: 10.1177/09636897211040012. 9636897211040012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando G., Soker S., Wood K. Operational tolerance after liver transplantation. J. Hepatol. 2009;50:1247–1257. doi: 10.1016/J.JHEP.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Ott H.C., Matthiesen T.S., Goh S.K., Black L.D., Kren S.M., Netoff T.I., Taylor D.A. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/NM1684. [DOI] [PubMed] [Google Scholar]
- Overi D., Carpino G., Cardinale V., Franchitto A., Safarikia S., Onori P., Alvaro D., Gaudio E. Contribution of Resident Stem Cells to Liver and Biliary Tree Regeneration in Human Diseases. Int. J. Mol. Sci. 2018;19 doi: 10.3390/IJMS19102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overi D., Carpino G., Franchitto A., Onori P., Gaudio E. Hepatocyte Injury and Hepatic Stem Cell Niche in the Progression of Non-Alcoholic Steatohepatitis. Cells. 2020;9 doi: 10.3390/CELLS9030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlowski M., Ortmann D., Bertero A., Tavares J.M., Pedersen R.A., Vallier L., Kotter M.R.N. Inducible and Deterministic Forward Programming of Human Pluripotent Stem Cells into Neurons, Skeletal Myocytes, and Oligodendrocytes. Stem Cell Rep. 2017;8:803–812. doi: 10.1016/j.stemcr.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peloso A., Dhal A., Zambon J.P., Li P., Orlando G., Atala A., Soker S. Current achievements and future perspectives in whole-organ bioengineering. Stem Cell Res. Ther. 2015;6:107. doi: 10.1186/S13287-015-0089-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L., Xie D.y., Lin B.L., Liu J., Zhu H.p., Xie C., Zheng Y.b., Gao Z.l. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/HEP.24434. [DOI] [PubMed] [Google Scholar]
- Peng W.C., Kraaier L.J., Kluiver T.A. Hepatocyte organoids and cell transplantation: What the future holds. Exp. Mol. Med. 2021;53:1512–1528. doi: 10.1038/S12276-021-00579-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.C., Logan C.Y., Fish M., Anbarchian T., Aguisanda F., Álvarez-Varela A., Wu P., Jin Y., Zhu J., Li B., et al. Inflammatory Cytokine TNFα Promotes the Long-Term Expansion of Primary Hepatocytes in 3D Culture. Cell. 2018;175:1607–1619.e15. doi: 10.1016/J.CELL.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perruche S., Marandin A., Kleinclauss F., Angonin R., Fresnay S., Baron M.H., Tiberghien P., Saas P. Association of mixed hematopoietic chimerism with elevated circulating autoantibodies and chronic graft-versus-host disease occurrence. Transplantation. 2006;81:573–582. doi: 10.1097/01.TP.0000183878.53367.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinato G., Lehoux S., Ramanathan R., Salem M.M., He L.X., Muse O., Flaumenhaft R., Thompson M.T., Rouse E.A., Cummings R.D., et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019;9:8920. doi: 10.1038/S41598-019-45514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosi G., Vizzini G., Gerlach J., Chinnici C., Luca A., Amico G., D'Amato M., Conaldi P.G., Li Petri S., Spada M., et al. Phases I-II Matched Case-Control Study of Human Fetal Liver Cell Transplantation for Treatment of Chronic Liver Disease. Cell Transplant. 2015;24:1627–1638. doi: 10.3727/096368914X682422. [DOI] [PubMed] [Google Scholar]
- Podestà M.A., Remuzzi G., Casiraghi F. Mesenchymal Stromal Cells for Transplant Tolerance. Front. Immunol. 2019;10:1287. doi: 10.3389/FIMMU.2019.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X., Guo X., Su C. Clinical outcomes of the transplantation of stem cells from various human tissue sources in the management of liver cirrhosis: a systematic review and meta-analysis. Curr. Stem Cell Res. Ther. 2015;10:166–180. doi: 10.2174/1574888X09666141112114011. [DOI] [PubMed] [Google Scholar]
- Raffin C., Vo L.T., Bluestone J.A. T reg cell-based therapies: challenges and perspectives. Nat. Rev. Immunol. 2020;20:158–172. doi: 10.1038/S41577-019-0232-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran P., Matchett K.P., Dobie R., Wilson-Kanamori J.R., Henderson N.C. Single-cell technologies in hepatology: new insights into liver biology and disease pathogenesis. Nat. Rev. Gastroenterol. Hepatol. 2020;17:457–472. doi: 10.1038/S41575-020-0304-X. [DOI] [PubMed] [Google Scholar]
- Rezvani M., Grimm A.A., Willenbring H. Assessing the therapeutic potential of lab-made hepatocytes. Hepatology. 2016;64:287–294. doi: 10.1002/HEP.28569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio M., Carnevale G., Cardinale V., Gibellini L., De Biasi S., Pisciotta A., Carpino G., Gentile R., Berloco P.B., Brunelli R., et al. The Fas/Fas ligand apoptosis pathway underlies immunomodulatory properties of human biliary tree stem/progenitor cells. J. Hepatol. 2014;61:1097–1105. doi: 10.1016/j.jhep.2014.06.016. [DOI] [PubMed] [Google Scholar]
- Richter M.L., Deligiannis I.K., Yin K., Danese A., Lleshi E., Coupland P., Vallejos C.A., Matchett K.P., Henderson N.C., Colome-Tatche M., Martinez-Jimenez C.P. Single-nucleus RNA-seq2 reveals functional crosstalk between liver zonation and ploidy. Nat. Commun. 2021;12:4264. doi: 10.1038/S41467-021-24543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimland C.A., Tilson S.G., Morell C.M., Tomaz R.A., Lu W.Y., Adams S.E., Georgakopoulos N., Otaizo-Carrasquero F., Myers T.G., Ferdinand J.R., et al. Regional Differences in Human Biliary Tissues and Corresponding In Vitro-Derived Organoids. Hepatology. 2021;73:247–267. doi: 10.1002/HEP.31252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos F.J.M., van Tienderen G.S., Wu H., Bordeu I., Vinke D., Albarinos L.M., Monfils K., Niesten S., Smits R., Willemse J., et al. Human branching cholangiocyte organoids recapitulate functional bile duct formation. Cell Stem Cell. 2022;29:776–794.e13. doi: 10.1016/j.stem.2022.04.011. [DOI] [PubMed] [Google Scholar]
- Salama H., Zekri A.R.N., Medhat E., al Alim S.A., Ahmed O.S., Bahnassy A.A., Lotfy M.M., Ahmed R., Musa S. Peripheral vein infusion of autologous mesenchymal stem cells in Egyptian HCV-positive patients with end-stage liver disease. Stem Cell Res. Ther. 2014;5:70. doi: 10.1186/SCRT459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaziotis F., Justin A.W., Tysoe O.C., Sawiak S., Godfrey E.M., Upponi S.S., Gieseck R.L., 3rd, de Brito M.C., Berntsen N.L., Gómez-Vázquez M.J., et al. Reconstruction of the mouse extrahepatic biliary tree using primary human extrahepatic cholangiocyte organoids. Nat. Med. 2017;23:954–963. doi: 10.1038/NM.4360. [DOI] [PubMed] [Google Scholar]
- Sampaziotis F., de Brito M.C., Madrigal P., Bertero A., Saeb-Parsy K., Soares F.A.C., Schrumpf E., Melum E., Karlsen T.H., Bradley J.A., et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nat. Biotechnol. 2015;33:845–852. doi: 10.1038/nbt.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Fueyo A., Whitehouse G., Grageda N., Cramp M.E., Lim T.Y., Romano M., Thirkell S., Lowe K., Fry L., Heward J., et al. Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am. J. Transplant. 2020;20:1125–1136. doi: 10.1111/AJT.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E, Zhang L, Bruce A, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007 doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E., Zhang L., Bruce A., Wauthier E., Ludlow J., Yao H.L., Moss N., Melhem A., McClelland R., Turner W., et al. Human hepatic stem cells from fetal and postnatal donors. J. Exp. Med. 2007;204:1973–1987. doi: 10.1084/JEM.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneeberger K., Sánchez-Romero N., Ye S., van Steenbeek F.G., Oosterhoff L.A., Pla Palacin I., Chen C., van Wolferen M.E., van Tienderen G., Lieshout R., et al. Large-Scale Production of LGR5-Positive Bipotential Human Liver Stem Cells. Hepatology. 2020;72:257–270. doi: 10.1002/HEP.31037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Attaran M., Meier P.N., Strassburg C., Manns M.P., Ott M., Barthold M., Arseniev L., Becker T., Panning B. Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation. 2006;82:1115–1116. doi: 10.1097/01.TP.0000232451.93703.AB. [DOI] [PubMed] [Google Scholar]
- Semeraro R., Carpino G., Cardinale V., Onori P., Gentile R., Cantafora A., Franchitto A., Napoli C., Anceschi M., Brunelli R., et al. Multipotent stem/progenitor cells in the human foetal biliary tree. J. Hepatol. 2012;57:987–994. doi: 10.1016/J.JHEP.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Shaheen M.F., Joo D.J., Ross J.J., Anderson B.D., Chen H.S., Huebert R.C., Li Y., Amiot B., Young A., Zlochiver V., et al. Sustained perfusion of revascularized bioengineered livers heterotopically transplanted into immunosuppressed pigs. Nat. Biomed. Eng. 2020;4:437–445. doi: 10.1038/S41551-019-0460-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smets F., Dobbelaere D., McKiernan P., Dionisi-Vici C., Broué P., Jacquemin E., Lopes A.I., Gonçalves I., Mandel H., Pawlowska J., et al. Phase I/II Trial of Liver-derived Mesenchymal Stem Cells in Pediatric Liver-based Metabolic Disorders: A Prospective, Open Label, Multicenter, Partially Randomized, Safety Study of One Cycle of Heterologous Human Adult Liver-derived Progenitor Cells (HepaStem) in Urea Cycle Disorders and Crigler-Najjar Syndrome Patients. Transplantation. 2019;103:1903–1915. doi: 10.1097/TP.0000000000002605. [DOI] [PubMed] [Google Scholar]
- Spahr L., Chalandon Y., Terraz S., Kindler V., Rubbia-Brandt L., Frossard J.L., Breguet R., Lanthier N., Farina A., Passweg J., et al. Autologous bone marrow mononuclear cell transplantation in patients with decompensated alcoholic liver disease: a randomized controlled trial. PLoS One. 2013;8:e53719. doi: 10.1371/JOURNAL.PONE.0053719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stärkel P., Schnabl B., Leclercq S., Komuta M., Bataller R., Argemi J., Palma E., Chokshi S., Hellerbrand C., Maccioni L., et al. Deficient IL-6/Stat3 Signaling, High TLR7, and Type I Interferons in Early Human Alcoholic Liver Disease: A Triad for Liver Damage and Fibrosis. Hepatol. Commun. 2019;3:867–882. doi: 10.1002/HEP4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stéphenne X., Najimi M., Sibille C., Nassogne M.C., Smets F., Sokal E.M. Sustained engraftment and tissue enzyme activity after liver cell transplantation for argininosuccinate lyase deficiency. Gastroenterology. 2006;130:1317–1323. doi: 10.1053/J.GASTRO.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Sterling R.K., Fisher R.A. Liver transplantation. Living donor, hepatocyte, and xenotransplantation. Clin. Liver Dis. 2001;5:431–460. doi: 10.1016/S1089-3261(05)70173-2. vii. [DOI] [PubMed] [Google Scholar]
- Strom S.C., Chowdhury J.R., Fox I.J. Hepatocyte transplantation for the treatment of human disease. Semin. Liver Dis. 1999;19:39–48. doi: 10.1055/S-2007-1007096. [DOI] [PubMed] [Google Scholar]
- Struecker B., Raschzok N., Sauer I.M. Liver support strategies: cutting-edge technologies. Nat. Rev. Gastroenterol. Hepatol. 2014;11:166–176. doi: 10.1038/NRGASTRO.2013.204. [DOI] [PubMed] [Google Scholar]
- Sun T., Pikiolek M., Orsini V., Bergling S., Holwerda S., Morelli L., Hoppe P.S., Planas-Paz L., Yang Y., Ruffner H., et al. AXIN2 + Pericentral Hepatocytes Have Limited Contributions to Liver Homeostasis and Regeneration. Cell Stem Cell. 2020;26:97–107.e6. doi: 10.1016/J.STEM.2019.10.011. [DOI] [PubMed] [Google Scholar]
- Takebe T., Sekine K., Enomura M., Koike H., Kimura M., Ogaeri T., Zhang R.R., Ueno Y., Zheng Y.W., Koike N., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484. doi: 10.1038/nature12271. [DOI] [PubMed] [Google Scholar]
- Takeishi K., Collin de l’Hortet A., Wang Y., Handa K., Guzman-Lepe J., Matsubara K., Morita K., Jang S., Haep N., Florentino R.M., et al. Assembly and Function of a Bioengineered Human Liver for Transplantation Generated Solely from Induced Pluripotent Stem Cells. Cell Rep. 2020;31:107711. doi: 10.1016/J.CELREP.2020.107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A.W., Humar A., Lakkis F.G., Metes D.M. Regulatory dendritic cells for promotion of liver transplant operational tolerance: Rationale for a clinical trial and accompanying mechanistic studies. Hum. Immunol. 2018;79:314–321. doi: 10.1016/J.HUMIMM.2017.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuluvath P.J., Thuluvath A.J., Hanish S., Savva Y. Liver transplantation in patients with multiple organ failures: Feasibility and outcomes. J. Hepatol. 2018;69:1047–1056. doi: 10.1016/J.JHEP.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Todo S., Yamashita K., Goto R., Zaitsu M., Nagatsu A., Oura T., Watanabe M., Aoyagi T., Suzuki T., Shimamura T., et al. A pilot study of operational tolerance with a regulatory T-cell-based cell therapy in living donor liver transplantation. Hepatology. 2016;64:632–643. doi: 10.1002/HEP.28459. [DOI] [PubMed] [Google Scholar]
- Tomaz R.A., Zacharis E.D., Bachinger F., Wurmser A., Yamamoto D., Petrus-Reurer S., Morell C.M., Dziedzicka D., Wesley B.T., Geti I., et al. Generation of functional hepatocytes by forward programming with nuclear receptors. Elife. 2022;11 doi: 10.7554/eLife.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryphonopoulos P., Tzakis A.G., Weppler D., Garcia-Morales R., Kato T., Madariaga J.R., Levi D.M., Nishida S., Moon J., Selvaggi G., et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am. J. Transplant. 2005;5:608–613. doi: 10.1111/J.1600-6143.2004.00743.X. [DOI] [PubMed] [Google Scholar]
- Tysoe O.C., Justin A.W., Brevini T., Chen S.E., Mahbubani K.T., Frank A.K., Zedira H., Melum E., Saeb-Parsy K., Markaki A.E., et al. Isolation and propagation of primary human cholangiocyte organoids for the generation of bioengineered biliary tissue. Nat. Protoc. 2019;14:1884–1925. doi: 10.1038/S41596-019-0168-0. [DOI] [PubMed] [Google Scholar]
- Uygun B.E., Soto-Gutierrez A., Yagi H., Izamis M.L., Guzzardi M.A., Shulman C., Milwid J., Kobayashi N., Tilles A., Berthiaume F., et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 2010;16:814–820. doi: 10.1038/NM.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hul N., Lanthier N., Español Suñer R., Abarca Quinones J., van Rooijen N., Leclercq I. Kupffer cells influence parenchymal invasion and phenotypic orientation, but not the proliferation, of liver progenitor cells in a murine model of liver injury. Am. J. Pathol. 2011;179:1839–1850. doi: 10.1016/J.AJPATH.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hul N.K.M., Abarca-Quinones J., Sempoux C., Horsmans Y., Leclercq I.A. Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology. 2009;49:1625–1635. doi: 10.1002/HEP.22820. [DOI] [PubMed] [Google Scholar]
- Vyas D., Baptista P.M., Brovold M., Moran E., Gaston B., Booth C., Samuel M., Atala A., Soker S. Self-assembled liver organoids recapitulate hepatobiliary organogenesis in vitro. Hepatology. 2018;67:750–761. doi: 10.1002/HEP.29483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Zhao L., Fish M., Logan C.Y., Nusse R. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–185. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Chen X., Cao W., Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat. Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lanzoni G., Carpino G., Cui C.B., Dominguez-Bendala J., Wauthier E., Cardinale V., Oikawa T., Pileggi A., Gerber D., et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis. Stem Cell. 2013;31:1966–1979. doi: 10.1002/STEM.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Qin J., Wang S., Zhang W., Duan J., Zhang J., Wang X., Yan F., Chang M., Liu X., et al. Conversion of Human Gastric Epithelial Cells to Multipotent Endodermal Progenitors using Defined Small Molecules. Cell Stem Cell. 2016;19:449–461. doi: 10.1016/J.STEM.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Wei Y., Wang Y.G., Jia Y., Li L., Yoon J., Zhang S., Wang Z., Zhang Y., Zhu M., Sharma T., et al. Liver homeostasis is maintained by midlobular zone 2 hepatocytes. Science. 2021;371:eabb1625. doi: 10.1126/SCIENCE.ABB1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y., Lambrecht J., Ju C., Tacke F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 2021;18:45–56. doi: 10.1038/S41423-020-00558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendon J., European Association for the Study of the Liver Electronic address easloffice@easlofficeeu. Clinical practice guidelines panel. Panel members. Cordoba J., Dhawan A., Larsen F.S., Manns M., Samuel D., Simpson K.J., et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J. Hepatol. 2017;66:1047–1081. doi: 10.1016/J.JHEP.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Wiemann S.U., Satyanarayana A., Tsahuridu M., Tillmann H.L., Zender L., Klempnauer J., Flemming P., Franco S., Blasco M.A., Manns M.P., Rudolph K.L. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/FJ.01-0977COM. [DOI] [PubMed] [Google Scholar]
- Wu M.C., Meng Q.H. Current understanding of mesenchymal stem cells in liver diseases. World J. Stem Cells. 2021;13:1349–1359. doi: 10.4252/WJSC.V13.I9.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang C., Du Y., Meng G., Soon Yi L., Sun S., Song N., Zhang X., Xiao Y., Wang J., Yi Z., et al. Long-term functional maintenance of primary human hepatocytes in vitro. Science. 2019;364:399–402. doi: 10.1126/SCIENCE.AAU7307. [DOI] [PubMed] [Google Scholar]
- Xiang Y., Miller K., Guan J., Kiratitanaporn W., Tang M., Chen S. 3D bioprinting of complex tissues in vitro: state-of-the-art and future perspectives. Arch. Toxicol. 2022;96:691–710. doi: 10.1007/S00204-021-03212-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi H., Fukumitsu K., Fukuda K., Kitago M., Shinoda M., Obara H., Itano O., Kawachi S., Tanabe M., Coudriet G.M., et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach. Cell Transplant. 2013;22:231–242. doi: 10.3727/096368912X654939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Sun L., Pang Y., Hu D., Xu H., Mao S., Peng W., Wang Y., Xu Y., Zheng Y.C., et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut. 2021;70:567–574. doi: 10.1136/GUTJNL-2019-319960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S.M., Gerasimidou D., Kuwahara R., Hytiroglou P., Yoo J.E., Park Y.N., Theise N.D. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology. 2011;53:964–973. doi: 10.1002/HEP.24122. [DOI] [PubMed] [Google Scholar]
- Zajicek G., Oren R., Weinreb M., Jr. The streaming liver. Liver. 1985;5:293–300. doi: 10.1111/j.1600-0676.1985.tb00252.x. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhang L., Liu W., Ma X., Cen J., Sun Z., Wang C., Feng S., Zhang Z., Yue L., et al. In Vitro Expansion of Primary Human Hepatocytes with Efficient Liver Repopulation Capacity. Cell Stem Cell. 2018;23:806–819.e4. doi: 10.1016/J.STEM.2018.10.018. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Wang L., Jin Y., Shi S. Fas Ligand Regulates the Immunomodulatory Properties of Dental Pulp Stem Cells. J. Dent. Res. 2012;91:948–954. doi: 10.1177/0022034512458690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Shan J., Guo Y., Li S., Long D., Li Y., Feng L. Effects of Adoptive Transfer of Tolerogenic Dendritic Cells on Allograft Survival in Organ Transplantation Models: An Overview of Systematic Reviews. J. Immunol. Res. 2016;2016:5730674. doi: 10.1155/2016/5730674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.H., Zhang D.H., Zhu C.W., Xu J., Guo C.L., Wu X.G., Cao Q.L., Di G.H. Adult stem cell transplantation combined with conventional therapy for the treatment of end-stage liver disease: a systematic review and meta-analysis. Stem Cell Res. Ther. 2021;12:558. doi: 10.1186/S13287-021-02625-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Rezvani M., Harbell J., Mattis A.N., Wolfe A.R., Benet L.Z., Willenbring H., Ding S. Mouse liver repopulation with hepatocytes generated from human fibroblasts. Nature. 2014;508:93–97. doi: 10.1038/NATURE13020. [DOI] [PMC free article] [PubMed] [Google Scholar]