Abstract
The c-myb oncogene has been a target of retroviral insertional mutagenesis in murine monocytic leukemias. One mechanism by which c-myb can be activated is through the integration of a retroviral provirus into the central portion of the locus, causing premature termination of c-myb transcription and translation. We had previously shown that a leukemia-specific c-Myb protein, truncated at the site of proviral integration by 248 amino acids, had approximately a fourfold-increased half-life compared to the normal c-Myb protein, due to its ability to escape rapid degradation by the ubiquitin-26S proteasome pathway. Here we provide evidence for the existence of more than one instability determinant in the carboxy-terminal region of the wild-type protein, which appear to act independently of each other. The data were derived from examination of premature termination mutants and deletion mutants of the normal protein, as well as analysis of another carboxy-terminally truncated protein expressed in leukemia. Evidence is provided that one instability determinant is located in the terminal 87 amino acids of the protein and another is located in the vicinity of the internal region that has leucine zipper homology. In leukemias, different degrees of protein stability are attained following proviral integration depending upon how many determinants are removed. Interestingly, although PEST sequences (rich in proline, glutamine, serine, and threonine), often associated with degradation, are found in c-Myb, deletion of PEST-containing regions had no effect on protein turnover. This study provides further insight into how inappropriate expression of c-Myb may contribute to leukemogenesis. In addition, it will facilitate further studies aimed at characterizing the specific role of individual regions of the normal protein in targeting to the 26S proteasome.
The c-myb gene is a frequent target of insertional mutagenesis in promonocytic leukemias induced in mice by retroviruses (reviewed in references 8 and 26). Transformation of myeloid cells by c-myb is probably due, at least in part, to inappropriate expression of the c-Myb protein. One supporting observation is that leukemias with retroviral integrations in the 5′ end of c-myb undergo promoter insertion at this locus. The LTR promoter bypasses the endogenous promoter and a transcriptional pause site in the first intron (23, 26, 28). The ultimate effect is that the c-myb gene is constitutively expressed, thereby avoiding the response to differentiation-related signals that normally result in the down regulation of the gene. Another observation supporting the notion that transformation can be due to inappropriate expression of c-myb is that constitutive ectopic expression of the full-length or leukemia-derived truncated form of protein in myeloid cells in vitro causes them to resist growth arrest signals (3, 4, 22, 27).
Promoter insertion is not the only mechanism involved in the activation of c-myb by retroviruses. Amino-terminal truncations occur in every case when the retrovirus inserts its promoter at the 5′ end of the gene, and although the role of this truncation in murine myeloid leukemia is not clear and may be a consequence of bypassing the elongation block, removal of similar sequences at the N terminus of avian c-Myb causes it to be more oncogenic in its induction of B-cell lymphomas (12). Recently, we have been focusing on a set of leukemias that have carboxy-terminal truncations due to virus integration into the central part of the locus, in the absence of promoter insertion. An example is the retrovirus-induced myeloid leukemia RI-4-11, where the c-Myb protein is overexpressed and aberrant expression is due to provirus-induced structural alterations that result in slower protein turnover (5, 18). Unlike the normal c-Myb protein that undergoes rapid degradation by the 26S proteasome, this protein is inefficiently degraded. (5, 17). In RI-4-11, c-Myb protein is truncated by 248 amino acids (aa) as a consequence of premature termination at a stop codon in the proviral long terminal repeat (LTR) in exon 9. This C-terminally truncated protein may be transforming, at least in part, because of its longer half-life in the cell. Studies by Gonda and coworkers, using an in vitro transformation assay with fetal liver cells, support this notion (7). They demonstrated that when cells expressing a C-terminally truncated version of c-Myb were seeded at low density, they could produce a higher level of transformation than cells expressing wild-type c-Myb. Interestingly, truncated c-Myb protein has an increased potential for transactivation (10). Protein stabilization may represent a common mechanism of oncogenic activation, since it has also been observed for the viral versions of two other transcription factors, v-Jun and v-Fos. Transduction by retroviruses of the proto-oncogenes that encode these proteins results in truncations that remove sequences important for recognition and processing by the 26S proteasome (20, 25).
The present study was initiated to determine the mechanism of c-myb activation in a cell line derived from a leukemia induced by infection of mice with Friend murine leukemia virus (F-MuLV) (19). We have found that this leukemic cell line has a proviral integration in exon 13, leading to a truncated mRNA and protein. Interestingly, the c-Myb protein produced in this cell line, which is truncated by 96 aa, has a longer half-life than the wild-type protein, but it is not as stable as the protein truncated by 248 aa, as previously described (5). This prompted us to investigate the sequence requirements for c-Myb protein degradation, since this should ultimately lead to a better understanding of the mechanism involved in targeting the c-Myb protein to the 26S proteasome. This analysis has led to the identification of two regions, one around the region that has leucine zipper homology and the other one in the last 87 aa of the C-terminal region, that are involved in destabilization of the normal full-length protein.
MATERIALS AND METHODS
Cell lines.
The RI-4-11 and 45-16 cell lines were established from leukemias that had been serially transplanted at least once in the peritoneal cavities of pristane-treated DBA/2N mice as previously described (18). COS-7 cells (American Type Culture Collection, Manassas, Va.) were maintained in Dulbecco’s modified Eagle’s medium with 10% fetal calf serum.
Construction of premature termination and deletion mutants.
Each premature termination mutant was constructed by first amplifying a unique size fragment from the 3′ end of the full length c-myb cDNA and joining this to a previously cloned 5′ fragment in the pcDNA3.1(+) vector. A sense oligonucleotide primer overlapping an EcoRI site in c-myb (14) was utilized in the amplification for all the mutants (see Fig. 7A). The unique antisense oligonucleotides included different c-myb sequences, a translation termination codon (TGA), and the restriction site for XbaI. The amplified sequences were digested with EcoRI and XbaI and cloned directly into the expression vector pcDNA3.1(+) (In Vitrogen, Carlsbad, Calif.) in which the 5′ end of the c-myb gene up to the EcoRI site had been previously cloned. Amplification was carried out with low-error-rate Pwo DNA polymerase (Boehringer Mannheim, Indianapolis, Ind.). The oligonucleotide primers used for the amplifications are available upon request. The structures of these mutants and those described below were verified by sequencing.
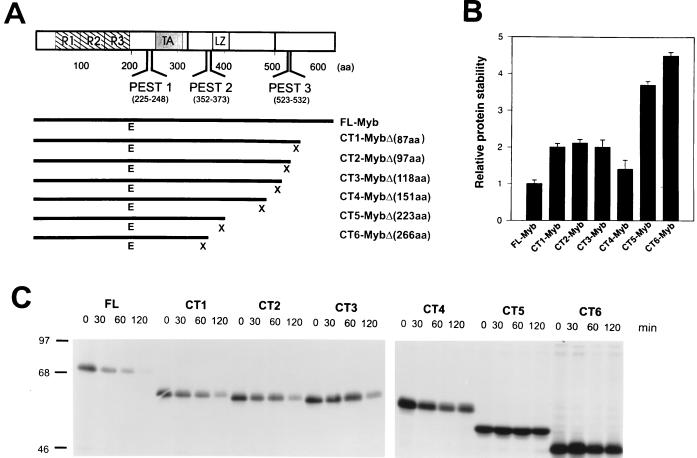
FIG. 7.
Stability of proteins with various C-terminal truncations. pcDNA3.1 plasmids expressing full-length or premature termination mutations were transiently expressed in COS-7 cells, and a pulse chase experiment was performed as in Fig. 4. (A) The structures of the mutants with the premature termination mutations are shown below the full-length c-Myb. E (EcoRI) and X (XbaI) are restriction endonuclease sites used in the preparation of the recombinants, as described in Materials and Methods. The number of amino acids removed in each mutant is shown to the right. (B) Bar graph showing the relative stabilities of the truncated proteins compared to the full-size protein. (C) Half-life calculations were performed as described in Materials and Methods and averaged for two experiments. A representative experiment is shown.
The mutants with internal deletion mutations, including those with the PEST sequences removed (see Fig. 5 and 7), were constructed by preparing two amplification products corresponding to sequences which flanked the deletion, one upstream of the deletion and the other downstream of the deletion. These two products were joined through a BamHI site which was introduced into the ends of the DNA during amplification (see Fig. 7A). The 5′ amplification product began upstream of a unique EcoRI site in c-myb and ended at different sites in c-myb depending on the particular deletion. The 3′ product began at different sites in c-myb depending on the particular deletion and ended at the terminus of the coding region of c-myb. An XbaI site was introduced at the terminus with the antisense oligonucleotide primer. The two amplification products were joined through the BamHI site by sequential cloning into Litmus 28 (New England Biolabs, Inc., Beverly, Mass.). EcoRI-XbaI fragments containing individual deletions were excised and ligated separately into pcDNA3.1(+), which already contained DNA encoding the amino terminus of c-Myb up to the EcoRI site.
FIG. 5.
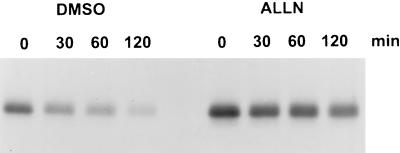
Turnover of c-Myb in COS-7 cells in the absence or presence of the 26S proteasome inhibitor, ALLN. COS-7 cells were transiently transfected with pcDNA3.1 expressing full-length c-Myb, and after 36 h the cells were pulse-labeled with [35S]methionine-[35S]cysteinine and chased for the indicated times. ALLN (N-acetyl-l-leucinyl-l-leucinyl-norleucinal) dissolved in dimethyl sulfoxide (DMSO) was added for the last 15 min of the pulse and kept at the same concentration during the chase. Cells treated with 0.5% dimethyl sulfoxide were used as a negative control for this experiment.
DNA transfection.
COS-7 cells were transfected with pcDNA3.1(+) plasmids containing normal c-myb or mutants by using the DOSPER liposomal transfection reagent (Boehringer Mannheim) as specified by the manufacturer.
RT-PCR and sequencing of c-myb mRNA.
Reverse transcription-PCR (RT-PCR) of mRNA from M1 and 45-16 cells was performed with the Titan RT-PCR one-tube system (Boehringer Mannheim). A sense oligonucleotide primer corresponding to a sequence from c-myb exon 6 (CAAGAACCACTGGAATTCCACC [bp 807 to 828]) (2) was used in conjunction with sense and antisense long terminal repeat (LTR) oligonucleotides (GAGTGATTGACTACCCGTCTC [bp 110 to 130] [14] and CTGCAGCTATCAGGCTAAGC [14], respectively) or an antisense c-myb oligonucleotide (CACTGAGGTAGCATCTTCAGG [bp 1700 to 1718] [2]).
To determine the sequence depicted in Fig. 1, one of the PCR products spanning the virus-myb junction in leukemic cell line 45-16 was cloned into the pCRII vector (InVitrogen) and partially sequenced by BioServe Biotechnologies, Laurel, Md.
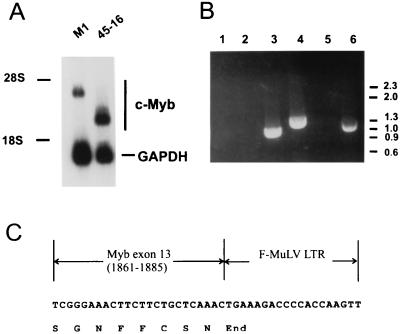
FIG. 1.
Integration of F-MuLV into exon 13 of c-myb in the leukemia cell line 45-16. (A) Northern analysis of c-myb mRNA in a cell line derived from 45-16. Total RNAs from the myeloblast cell line, M1, and from the 45-16 cell line were electrophoresed, blotted, and hybridized with c-myb cDNA and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probes. (B) RT-PCR analysis of 45-16. Total RNA from 45-16 was amplified following reverse transcription and electrophoresed on a 1% agarose gel containing 1 μg of ethidium bromide per ml. RNAs from M1 cells (lanes 1 to 3) or 45-16 cells (lanes 4 to 6) were reverse transcribed and amplified with an oligonucleotide primer overlapping the EcoRI site in exon 6 of c-myb in conjunction with an antisense F-MuLV LTR oligonucleotide (lanes 1 and 4), a sense LTR oligonucleotide (lanes 2 and 5), or an oligonucleotide with sequences from exons 11 and 12 (lanes 3 and 6). (C) Sequences at the c-myb exon 13–F-MuLV proviral junction in the mRNA from 45-16.
Southern analysis.
Genomic DNA was prepared from the 45-16 cell line with the Wizard genomic DNA purification kit (Promega, Madison, Wis.). Samples (10 μg) were digested to completion with restriction endonucleases, electrophoresed through horizontal agarose gels, and blotted onto nylon membranes. A Stratalinker 1800 (Stratagene, La Jolla, Calif.) was used to UV cross-link DNA to the membranes, which were then hybridized to either a probe from the myb locus, exons 12 through 13 (see Fig. 2B), or an F-MuLV LTR probe. The c-myb probe was labeled by random priming. The LTR probe was a HinfI-BglI fragment that was labeled by PCR amplification as described previously (15).
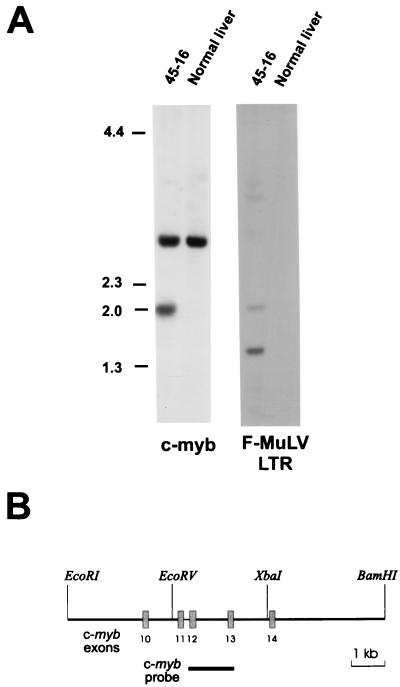
FIG. 2.
Southern analysis demonstrates a rearranged c-myb allele in the leukemia cell line 45-16. (A) Genomic DNA from the leukemia cell line 45-16 or normal DBA liver cells was digested with EcoRV and XbaI and hybridized with either a c-myb probe (B) or an F-MuLV LTR probe, as described in Materials and Methods. (B) Map of part of the c-myb locus showing the location of the probe used in panel A.
Immunoprecipitation.
For immunoprecipitation, 107 cells were labeled as previously described (5). Transiently transfected COS-7 cells were metabolically labeled 36 h after transfection. Briefly, the cells were starved for 15 min in methionine- and cysteine-free medium, metabolically labeled for 45 min with 400 μCi of Tran35S-label (ICN), washed, and then chased by adding prewarmed RPMI 1640 medium plus 10% horse serum (M1 cells) or Dulbecco’s modified Eagle’s medium with 10% fetal calf serum (45-16, 3A2-11, and RI-4-11 leukemic cell lines) for 0, 30, 60, and 120 min. At the indicated time points, the cells were then disrupted in cold lysis buffer, and immunoprecipitation was carried out with rabbit antiserum raised against the bacterial fusion protein glutathione S-transferase–cMyb(I) as described previously (3). Precipitates were electrophoresed under reducing conditions on an 8% polyacrylamide gel and visualized by fluorography. Quantitative analyses of dried gels were performed on a PhosphorImager 425 with ImageQuant software (Molecular Dynamics), and the half-lives of the proteins (t1/2) were calculated from the formula t1/2 = (0.693 × t)/ln (Nt/N0), as described previously (3).
RESULTS
Analysis of c-myb gene structure and protein products in 45-16 leukemia cells.
A promonocytic leukemia cell line, 45-16, was obtained by intravenous inoculation of F-MuLV into irradiated and pristane-treated DBA/2 mice (19). Interestingly, c-myb had not undergone activation by promoter insertional mutagenesis, which is the most common type of alteration observed for these leukemias (26). However, c-myb seemed to be involved in the development of the leukemia, because we observed that the c-myb mRNA from a cell line established from this leukemia cell line was smaller than that of full-length mRNA (Fig. 1A). To localize a potential deletion in one of the ends of the coding region, we attempted to amplify separately by RT-PCR sequences corresponding to either the 5′ or 3′ portion of the c-myb mRNA. Although a DNA product of the predicted size was amplified from the 5′ end of c-myb, no amplification was obtained from the 3′ end with a sense oligonucleotide primer homologous to the central portion of c-myb and an antisense primer from exon 15, the last exon (data not shown). A Southern blot analysis was performed to determine if there was an alteration in the c-myb DNA in this leukemia cell line that would indicate the presence of a provirus. As shown in Fig. 2A, a rearranged allele was detected in 46-16 when the DNA from this cell line was digested with EcoRV and XbaI and hybridized with a genomic c-myb probe spanning the region from exons 12 to 13 (Fig. 2B). A 2-kb leukemia-specific fragment, not detected in normal liver DNA, also hybridized with an LTR probe, indicating the presence of a provirus between the EcoRV and XbaI sites. To determine the position and orientation of the virus, which was presumably integrated in the central region of the gene, another RT-PCR analysis was performed with primers homologous to the LTR, in both the sense and antisense orientations, in conjunction with a primer from c-myb. The results, depicted in Fig. 1B, demonstrate that the provirus is positioned in the same orientation as the c-myb gene. This is evident from lane 4, which contains an RT-PCR product obtained with a c-myb sense primer homologous to sequences in exon 6 and the LTR antisense primer. An RT-PCR analysis performed on M1 cells, which did not have a provirus integrated in the c-myb locus, served as a control. A positive control for the RT-PCR assay was the amplification product in lanes 3 (M1) and 6 (45-16) of Fig. 1B, obtained with sense and antisense oligonucleotides from c-myb. The sequence of the partial cDNA covering the junction of the c-myb and the LTR in 45-16 cells (Fig. 1C) reveals that the provirus is integrated in exon 13 and that termination of translation occurs immediately at the beginning of viral LTR.
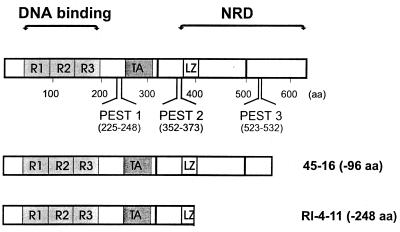
The predicted structure of the protein expressed in 45-16 cells is depicted in Fig. 3, where it is compared to the normal c-Myb and a previously described c-Myb protein from RI-4-11 leukemia cells, which is more severely truncated. The smaller protein from RI-4-11 (with 248 aa deleted) has all of the C terminus removed up to and including some of the leucine zipper-related region. Therefore, all of the negative regulatory domain, defined previously by investigators who showed that its removal resulted in increased transactivation (10), is missing. In contrast, the protein from 45-16 (with 96 aa deleted) had only a short C-terminal region of unknown function missing.
FIG. 3.
Structure of carboxy-terminally truncated c-Myb proteins expressed in two leukemia cell lines, RI-4-11 and 45-16. The top of the figure depicts the structure of the normal wild-type c-Myb protein, showing the DNA binding domain consisting of three imperfect direct repeats (R1, R2, and R3), the transactivation domain (TA), the negative regulatory domain (NRD), and the region with similarity to leucine zippers (LZ). PEST1, PEST2, and PEST3 were identified with PESTfind (21). Below are the structures of two leukemia-specific proteins that are truncated at the C terminus.
Turnover of c-Myb protein in leukemic cell line 45-16.
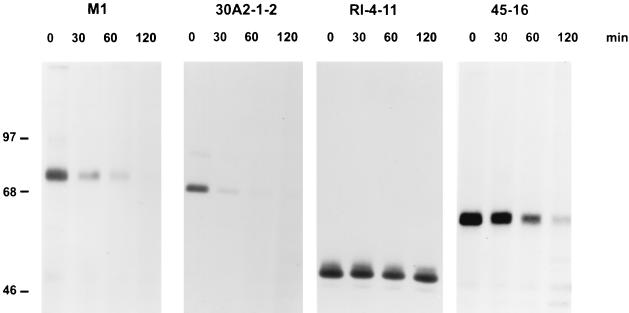
We previously showed by immunoprecipitation that the steady-state level of a truncated c-Myb with 248 aa deleted, which is expressed in an established leukemia cell line, is increased compared to that of endogenous full length c-Myb expressed in the M1 myeloblastic cell line (5). This increase in the protein level was shown to be a consequence of a longer half-life compared to the full-length protein. We therefore decided to examine the half-life of the c-Myb protein expressed in 45-16 cells. To compare the stability of the 45-16 c-Myb protein with that of wild-type c-Myb, we performed a pulse-chase experiment. Cells were labeled with radioactive methionine and cysteine for 45 min and chased for up to 120 min. The results are depicted in Fig. 4, which shows the proteins truncated by 96 aa in 45-16 cells and by 248 aa in RI-4-11 cells are more stable than the full-length protein in M1 cells or the amino-terminally truncated protein in another leukemic cell line, 30A2-1-2 (28). Quantitative analysis of results from several experiments showed that the t1/2 of the protein expressed in 45-16 cells was about twofold longer than that of the normal protein and that the t1/2 of the shorter RI-4-11 protein was at least fourfold longer. This data, therefore, suggests that more than one protein determinant is responsible for degradation of full-length c-Myb in these cells and led us to analyze the protein in more detail and to localize potential determinants.
FIG. 4.
Pulse-chase experiment demonstrating altered stabilities of C-terminally truncated proteins. The M1 myeloblast cell line, a promonocytic leukemia cell line with an amino-terminally truncated c-Myb, 30A2-1-2, and two cell lines with C-terminally truncated c-Myb, RI-4-11 and 45-16, were metabolically labeled with radioactive methionine and cysteine and chased in complete medium for the designated times. Proteins were immunoprecipitated with polyclonal rabbit anti-c-Myb serum (3) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The mobilities of molecular mass standards in kilodaltons are on the left.
Localization of sequences responsible for targeting c-Myb for degradation.
Since the c-Myb protein is recognized and degraded in M1 cells by the 26S proteasome (5) and the leukemia-specific truncated forms are stabilized to different extents depending upon the size of the C-terminal truncation, we became interested in localizing determinants, particularly in the C terminus, that might be responsible for recognition and targeting of the protein to the 26S proteasome. To analyze different c-myb deletion mutants, we decided to transfect COS-7 cells with plasmids containing the sequence modifications. First, however, we confirmed that degradation of the full-length protein occurred similarly in COS-7 cells to that in M1 cells and that the protein was degraded by the 26S proteasome. As shown in Fig. 5, the kinetics of proteolysis of full-length c-Myb were the same as those previously observed in M1 cells, and proteolysis could be blocked by a potent inhibit of the proteasome, N-acetyl-l-leucinyl-l-leucinyl-norleucinal (ALLN).
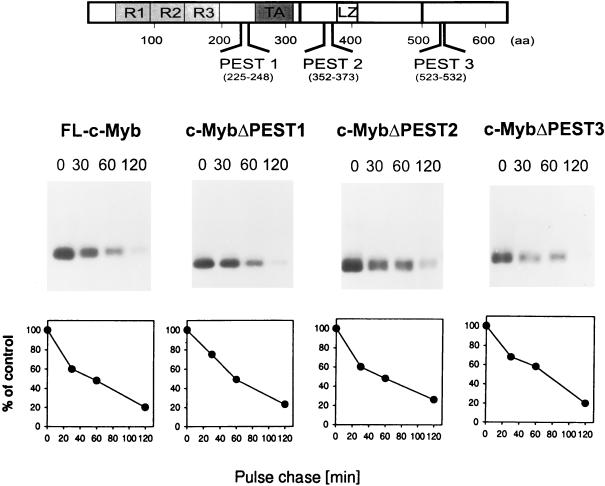
The first deletions prepared were those that removed the putative PEST sequences identified in the c-Myb protein by using PESTfind (Fig. 3) (5). PEST sequences are rich in proline (P), glutamine (E) and/or aspartic acid, serine (S), and threonine (T) and have been shown for some proteins, such as c-Fos and IκB, to be conditional proteolytic signals (21) involved in targeting of the protein to the ubiquitin-26S proteasome pathway. Although PEST1 represented a poor PEST sequence, PEST2 and PEST3 had high scores of 9.3 and 8.0, respectively. Both of these sites were interesting candidates for playing a role in degradation. PEST2 is upstream of the leucine zipper-like sequences and at the amino-terminal edge of the negative regulatory domain, and it has the highest score. On the other hand, PEST3 is highly conserved among c-Myb proteins of higher vertebrates and is removed in the c-Myb protein expressed in RI-4-11. Deletion mutants were prepared that removed PEST sequences, and these were inserted into the pcDNA3.1 vector. They were then transiently transfected into COS-7 cells. However, a pulse-chase analysis of the mutant proteins demonstrated that removal of PEST1, PEST2, or PEST3 had no detectable effect on the stability of the mutated proteins compared to full-length c-Myb (Fig. 6).
FIG. 6.
Turnover of proteins with mutations in the PEST regions. c-Myb with mutations in the PEST sequences was transiently expressed in COS-7 cells as described in the legend to Fig. 4. The decay of full-length c-Myb (FL-c-Myb) and proteins with mutations in PEST1, PEST2, or PEST3 is depicted in the graphs at the bottom.
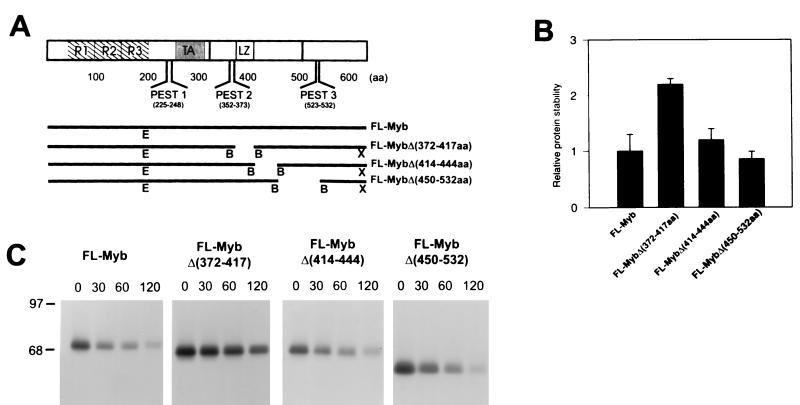
Since the PEST sequence mutants did not affect proteolysis, we began to search for other regions in the C terminus that might be involved in the proteolytic processing. For this purpose, a series of progressively greater C-terminal deletions were prepared from full length c-Myb, as demonstrated in Fig. 7A. These altered c-Myb proteins were than expressed from the pcDNA3.1 vector in COS-7 cells. After 36 h, the cells were metabolically labeled with [35S]methionine and chased for 0 to 120 min. The results for the electrophoresed proteins from one experiment are depicted in Fig. 7B. Half-life calculations were made and averaged for two experiments, and the relative stabilities of the truncated proteins, compared to that of the full-length protein, are plotted in Fig. 7C. The shortest deletion of 87 aa (producing the CT1 mutant) resulted in partial stabilization with an increase in the half-life of approximately twofold. No further stabilization was noted as additional sequences totaling 106 aa were deleted (CT4). Removal of 151 to 223 aa (CT5), however, resulted in a further stabilization, so that the protein was almost four times more stable than the wild type. Interestingly, this deletion was up to but not including the leucine zipper-like sequences. The leucine zipper domain has been proposed to function in protein-protein interactions, and although the sequence was intact in CT5, this alteration may have still interfered with its putative interaction with another protein that could shorten the half-life of c-Myb. Loss of the next 43 aa resulted in only a slight increase in stability (CT6), and removal of the entire C terminus, including the entire leucine zipper region, resulted in the same half-life as for CT6 (data not shown).
To confirm our interpretation of the results with the C-terminal deletions, internal deletions were prepared as depicted in Fig. 8A. The mutant proteins were tested for their resistance to degradation, as in the above experiment, after transfection of COS-7 cells. Three regions were deleted, one overlapping the leucine zipper and two others downstream from the leucine zipper region. As shown in Fig. 8B and C, the leucine zipper mutant with aa 371 to 417 deleted was the only protein that demonstrated a significant increase in stability. We therefore concluded from the analysis of both sets of mutants that two regions may be important for recognition and targeting of c-Myb to the proteasome, the last C-terminal 87 aa and a region in the vicinity of the leucine zipper-like sequence.
FIG. 8.
Stability of proteins with various internal C-terminal deletions. pcDNA3.1 plasmids expressing full-length or C-terminally deleted mutants were expressed in COS-7 cells, and a pulse-chase experiment was performed as described in the legend to Fig. 4. (A) The structures of the C-terminally deleted mutants are shown below that of the full-length c-Myb. B (BamHI), E (EcoRI), and X (XbaI) are restriction endonuclease sites used in the preparation of the recombinants, as described in Materials and Methods. (B) Bar graph showing the relative stabilities of the deleted proteins compared to the full-size protein. (C) Half-life calculations were performed as described in Materials and Methods and averaged for two experiments. A representative experiment is shown.
DISCUSSION
In the present study we have provided evidence for a function of the C terminus of c-Myb. An analysis of aberrant leukemia-specific proteins and proteins expressed from constructed mutants demonstrates the existence of separate determinants involved in the proteolysis of the normal c-Myb protein. One is in a region adjacent to or perhaps including the putative leucine zipper domain, and the other is in a region at the extreme C terminus, a region to which no function has previously been assigned. The exact locations of the determinants cannot be determined precisely at this time, because we may have produced conformational changes in portions of the molecule outside the deletions. In any case, more than one determinant was found that can function independently of each other. For example, the loss of the one determinant at the extreme C terminus in the leukemia cell line 45-16 can result in partial slowing of c-Myb protein turnover, whereas the combined loss of these, as in the leukemia cell line RI-4-11, has a maximum effect on stability. These truncations of c-Myb, caused by the integrated provirus, represent a basic mechanism which can contribute to inappropriate expression of c-Myb in monocytic leukemia. We cannot determine presently if this is the only mechanism involved in oncogenic activation. Other contributing factors may include increased mRNA stability (our own unpublished observations) and altered transcription at the locus due to the introduction of viral enhancers to the central portion of the gene.
PEST sequences, rich in proline, glutamate, serine, and threonine and interrupted by positively charged residues, are commonly found in proteins that are rapidly degraded and in many cases have been demonstrated to be required for proteolysis. Examples include cyclins, c-Fos, IκBα, and ornithine decarboxylase (6, 21). Previously, we used the algorithm called PESTfind to identify such sequences in c-Myb, and three regions depicted in Fig. 3, especially PEST2 and PEST3, had significant scores. For this reason, our first attempt to find sites that could be important in targeting c-Myb to the proteasome involved preparing mutants that would disrupt these sites and determining the half-life of these mutants in a common cell background, that of the COS-7 cells. However, none of these deletions had any effect on c-Myb stability. One conclusion from these results is that other sequences such as those described above play a role in the rapid destruction of c-Myb. This is not surprising, because other proteins contain alternative degradation motifs. For example c-Jun has a delta domain at its amino terminus that is required for its rapid degradation and some proteins such as cyclin B have short regions of sequence known as cyclin destruction boxes (9, 21).
The regions of c-Myb that we identified as important to its rapid degradation could potentially perform one or more functions relevant to the proteasome proteolytic pathway. Since our previous data suggest that polyubiquitination may be a required step in the processing of c-Myb for degradation (5), sequences at these sites may be involved in aspects of this modification. It is intriguing that one of the regions in c-Myb, determined from analysis of premature termination and deletion mutants to be important in the degradation process, overlaps the region with sequence similarity to leucine zippers (Fig. 7 and 8). Although this region has not been demonstrated to form an amphipathic α-helical structure, there is evidence that it can bind proteins. For example, p160 was identified and cloned from cells based on its ability to bind to the leucine zipper (1). Also, others have observed the binding of two cellular proteins, p26 and p28, to the avian v-Myb (24). The leucine zipper region is part of a large domain of c-Myb called the negative regulatory domain (Fig. 3). Removal of this region has been demonstrated to cause an increase in transactivation by c-Myb. In addition, mutation of the leucine zipper can cause a similar increase in its transactivating function and transforming capacities (13). The increased capacity of leucine zipper mutants to transform cells compared to wild-type c-Myb has been demonstrated by using retroviral vectors that expressed the proteins in murine fetal liver cells and then assaying these cells for colony formation in semisolid medium. Although we have never found deletions that specifically removed the leucine zipper in monocytic leukemia cells, a spontaneous internal deletion of this region has been observed in a bovine T-cell lymphoma (11). Based on our analysis here of an analogous mutant, it is interesting to speculate that these alterations in the protein may have affected the transactivation capacity of c-Myb and its ability to transform because of increased levels of protein following escape from degradation. The involvement of interacting proteins in recognition and targeting to the proteasome has been demonstrated for other transcription factors. For example, the stability of c-Fos is decreased upon interaction with phosphorylated c-Jun (20). Also, p53 can be induced to degrade rapidly by interaction with MDM2 (16). Further studies are required to determine if overexpression of leucine zipper binding proteins can affect the stability of c-Myb.
ACKNOWLEDGMENTS
We thank Douglas Lowy for critical reading of the manuscript and Richard Koller for excellent technical assistance.
This work was supported in part by grant 2/5052/98 from the Slovak Academy of Sciences.
REFERENCES
- 1.Bartunek P, Karafiat V, Dvorakova M, Zahorova V, Mandikova S, Zenke M, Dvorak M. The Myb leucine zipper is essential for leukemogenicity of the v-Myb protein. Oncogene. 1997;15:2939–2949. doi: 10.1038/sj.onc.1201457. [DOI] [PubMed] [Google Scholar]
- 2.Bender T P, Kuehl W M. Murine myb protooncogene mRNA: cDNA sequence and evidence for 5′ heterogeneity. Proc Natl Acad Sci USA. 1986;83:3204–3208. doi: 10.1073/pnas.83.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bies J, Hoffman B, Amanullah A, Giese T, Wolff L. B-Myb prevents growth arrest associated with terminal differentiation of monocytic cells. Oncogene. 1996;12:355–363. [PubMed] [Google Scholar]
- 4.Bies J, Mukhopadhyaya R, Pierce J, Wolff L. Only late, nonmitotic stages of granulocyte differentiation in 32Dcl3 cells are blocked by ectopic expression of murine c-myb and its truncated forms. Cell Growth Differ. 1995;6:59–68. [PubMed] [Google Scholar]
- 5.Bies J, Wolff L. Oncogenic activation of c-Myb by carboxyl-terminal truncation leads to decreased proteolysis by the ubiquitin-26S proteasome pathway. Oncogene. 1997;14:203–212. doi: 10.1038/sj.onc.1200828. [DOI] [PubMed] [Google Scholar]
- 6.Chevaillier P. PEST sequences in nuclear proteins. Int J Biochem. 1993;25:479–482. doi: 10.1016/0020-711x(93)90653-v. [DOI] [PubMed] [Google Scholar]
- 7.Ferrao P, MacMillan E M, Ashman L K, Gonda T J. Enforced expression of full length c-Myb leads to density-dependent transformation of murine haemopoietic cells. Oncogene. 1995;11:1631–1638. [PubMed] [Google Scholar]
- 8.Golay J, Basilico L, Loffarelli L, Songia S, Broccoli V, Introna M. Regulation of hematopoietic cell proliferation and differentiation by the myb oncogene family of transcription factors. Int J Clin Lab Res. 1996;26:24–32. doi: 10.1007/BF02644770. [DOI] [PubMed] [Google Scholar]
- 9.Hilt W, Wolf D H. Proteasomes: destruction as a programme. Trends Biochem Sci. 1996;21:96–102. [PubMed] [Google Scholar]
- 10.Hu Y L, Ramsay R G, Kanei-Ishii C, Ishii S, Gonda T J. Transformation by carboxyl-deleted Myb reflects increased transactivating capacity and disruption of a negative regulatory domain. Oncogene. 1991;6:1549–1553. [PubMed] [Google Scholar]
- 11.Ishiguro N, Ohzono T, Shinagawa T, Horiuchi M, Shinagawa M. A spontaneous internal deletion of the c-myb protooncogene enhances transcriptional activation in bovine T lymphoma cells. J Biol Chem. 1994;269:26822–26829. [PubMed] [Google Scholar]
- 12.Jiang W, Kanter M R, Dunkel I, Ramsay R G, Beemon K L, Hayward W S. Minimal truncation of the c-myb gene product in rapid-onset B-cell lymphoma. J Virol. 1997;71:6526–6533. doi: 10.1128/jvi.71.9.6526-6533.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanei-Ishii C, MacMillan E M, Nomura T, Sarai A, Ramsay R G, Aimoto S, Ishii S, Gonda T J. Transactivation and transformation by Myb are negatively regulated by a leucine-zipper structure. Proc Natl Acad Sci USA. 1992;89:3088–3092. doi: 10.1073/pnas.89.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koch W, Hunsmann G, Friedrich R. Nucleotide sequence of the envelope gene of Friend murine leukemia virus. J Virol. 1983;45:1–9. doi: 10.1128/jvi.45.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koller R, Krall M, Mock B, Bies J, Nazarov V, Wolff L. Mml1, a new common integration site in murine leukemia virus-induced promonocytic leukemias, maps to mouse chromosome 10. Virology. 1996;224:224–234. doi: 10.1006/viro.1996.0524. [DOI] [PubMed] [Google Scholar]
- 16.Kubbutat M H, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 17.Luscher B, Eisenman R N. c-myc and c-myb protein degradation: effect of metabolic inhibitors and heat shock. Mol Cell Biol. 1988;8:2504–2512. doi: 10.1128/mcb.8.6.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyaya R, Wolff L. New sites of proviral integration associated with murine promonocytic leukemias and evidence for alternate modes of c-myb activation. J Virol. 1992;66:6035–6044. doi: 10.1128/jvi.66.10.6035-6044.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nazarov V, Wolff L. Novel integration sites at the distal 3′ end of the c-myb locus in retrovirus-induced promonocytic leukemias. J Virol. 1995;69:3885–3888. doi: 10.1128/jvi.69.6.3885-3888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papavassiliou A G, Treier M, Chavrier C, Bohmann D. Targeted degradation of c-Fos, but not v-Fos, by a phosphorylation-dependent signal on c-Jun. Science. 1992;258:1941–1944. doi: 10.1126/science.1470918. [DOI] [PubMed] [Google Scholar]
- 21.Rechsteiner M, Rogers S W. PEST sequences and regulation by proteolysis. Trends Biochem Sci. 1996;21:267–271. [PubMed] [Google Scholar]
- 22.Selvakumaran M, Liebermann D A, Hoffman-Liebermann B. Deregulated c-myb disrupts interleukin-6 or leukemia inhibitory factor-induced myeloid differentiation prior to c-myc: role in leukemogenesis. Mol Cell Biol. 1992;12:2493–2500. doi: 10.1128/mcb.12.6.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen-Ong G L, Wolff L. Moloney murine leukemia virus-induced myeloid tumors in adult BALB/c mice: requirement of c-myb activation but lack of v-abl involvement. J Virol. 1987;61:3721–3725. doi: 10.1128/jvi.61.12.3721-3725.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tavner F J, Simpson R, Tashiro S, Favier D, Jenkins N A, Gilbert D J, Copeland N G, MacMillan E M, Lutwyche J, Keough R A, Ishii S, Gonda T J. Molecular cloning reveals that the p160 Myb-binding protein is a novel, predominantly nucleolar protein which may play a role in transactivation by Myb. Mol Cell Biol. 1998;18:989–1002. doi: 10.1128/mcb.18.2.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treier M, Staszewski L M, Bohmann D. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell. 1994;78:787–798. doi: 10.1016/s0092-8674(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 26.Wolff L. Myb-induced transformation. Crit Rev Oncog. 1996;7:245–260. doi: 10.1615/critrevoncog.v7.i3-4.60. [DOI] [PubMed] [Google Scholar]
- 27.Wolff L, Koller R, Bies J, Nazarov V, Hoffman B, Amanullah A, Krall M, Mock B. Retroviral insertional mutagenesis in murine promonocytic leukemias: c-myb and Mml1. Curr Top Microbiol Immunol. 1996;211:191–199. doi: 10.1007/978-3-642-85232-9_19. [DOI] [PubMed] [Google Scholar]
- 28.Wolff L, Koller R, Davidson W. Acute myeloid leukemia induction by amphotropic murine retrovirus (4070A): clonal integrations involve c-myb in some but not all leukemias. J Virol. 1991;65:3607–3616. doi: 10.1128/jvi.65.7.3607-3616.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]