Abstract
Causative genes have been identified only in four types of lipid storage myopathies (LSMs): SLC22A5 for primary carnitine deficiency (PCD); ETFA, ETFB, and ETFDH for multiple acyl-coenzyme A dehydrogenation deficiency (MADD); PNPLA2 for neutral lipid storage disease with myopathy (NLSDM); and ABHD5 for neutral lipid storage disease with ichthyosis. However, the frequency of these LSMs has not been determined. We found mutations in only 9 of 37 LSM patients (24%): 3 in SLC22A5; 4 in MADD-associated genes; and 2 in PNPLA2. This low frequency suggests the existence of other causative genes. Muscle coenzyme Q10 levels were normal or only mildly reduced in two MADD patients, indicating that ETFDH mutations may not always be associated with CoQ10 deficiency. The 2 patients with PNPLA2 mutations had progressive, non-episodic muscle disease with rimmed vacuoles. This suggests there is a different pathomechanism from other LSMs.
Keywords: ABHD5, ETF, ETFDH, lipid storage myopathy, PNPLA2, SLC22A5
Defects in muscle lipid metabolism are due to a heterogeneous group of metabolic conditions. They are caused by problems in transport of fatty acids and carnitine, mitochondrial matrix β-oxidation enzymes, or endogenous triglyceride synthesis. The clinical spectrum of these disorders is variable. Patients often present with hypotonia, muscle weakness, recurrent rhabdomyolysis, and peripheral neuropathy.21 Lipid storage myopathies (LSMs), which are categorized under the broad category of disorders of lipid metabolism, are invariably characterized by accumulation of lipid droplets in muscle fibers. Among LSMs, genetic causes have been identified in only four disorders: primary carnitine deficiency (PCD); multiple acyl-coenzyme A (acyl-CoA) dehydrogenation deficiency (MADD); neutral lipid storage disease with myopathy (NLDSM); and neutral lipid storage disease with ichthyosis (NLDSI).4,10,21
PCD is an autosomal-recessive disorder caused by mutations of the SLC22A5 gene, which encodes an integral plasma membrane protein, organic cation transporter 2 (OCTN2). It functions to transport extracellular carnitine into cells.17,22 OCTN2 mutations lead to defective renal reabsorption and reduced tissue storage of carnitine and impairment of long fatty acid metabolism, as carnitine is necessary to incorporate long-chain fatty acids into the mitochondrial matrix for β-oxidation. Clinical features of PCD include severe hypoglycemia and dilated cardiomyopathy in addition to skeletal muscle involvement.21
MADD, also known as glutaric aciduria type II, is an autosomal-recessive disorder of fatty and amino acid metabolism6 caused by defects in electron transfer flavoprotein (ETF) or ETF dehydrogenase (ETFDH). ETF is a heterodimeric protein consisting of two subunits, α and β, that are encoded by different genes, ETFA and ETFB. ETF receives electrons from mitochondrial flavin-containing dehydrogenases to ETFDH in the inner mitochondrial membrane. ETDFH, in turn, transfers electrons to coenzyme Q. The MADD phenotype varies widely from a fatal neonatal-onset form19,20 to a much milder late-onset form, which is often associated with a lipid storage myopathy that manifests with muscle weakness and pain. Recently, patients with ETDFH mutations were shown to have secondary coenzyme Q10 (CoQ10) deficiency.7
Neutral lipid storage disease is characterized by systemic accumulation of triglycerides (TG) in the cytoplasm and includes two distinct diseases: NLSDM and NLSDI (also called Chanarin–Dorfman syndrome). NLSDM is caused by mutations in a gene that encodes adipose triglyceride lipase (ATGL), which is also referred to as patatin-like phospholipase domain-containing protein 2 (PNPLA2).4,9,23 This protein catalyzes the initial step in TG hydrolysis. On the other hand, NLSDI is due to defects in the gene that encodes the coactivator of ATGL, comparative gene identification-58 (CGI-58), which is also known as abhydrolase domain–containing 5 (ABHD5).10
Although the pathological characteristics of LSM are rather uniform, the phenotypic manifestations are remarkably heterogeneous, possibly due to different genetic backgrounds. Thus, genetic analysis has always posed a challenge. In this study, we analyzed all known causative genes for LSM (SLC22A5, ABHD5, PNPLA2, ETFA, ETFB, and ETFDH), as well as LIPE, which encodes hormone-sensitive lipase (HSL),8 among patients who had pathological confirmation of LSM. Our aim was to determine the actual frequency of identifiable mutations and to look for genotype–phenotype correlations that could be helpful for diagnosis.
METHODS
Patients.
We retrospectively recruited cases diagnosed with LSM at the National Center of Neurology and Psychiatry (NCNP) from a total of 9639 muscle biopsies obtained between 1978 and 2006. The diagnosis of LSM was made based on characteristic muscle pathology findings: small clear vacuoles on hematoxylin and eosin staining and intramyofiber accumulation of lipid droplets on oil-red-O staining. We excluded cases with obvious mitochondrial abnormalities such as ragged-red fibers, strongly succinate dehydrogenase (SDH)-reactive vessels, and cytochrome c oxidase deficiency. Detailed retrospective review of the clinical and pathological findings was performed. Informed consent was obtained from the patients using a form approved by the NCNP ethics board committee.
Mutation Analysis.
We sequenced all exons and their flanking regions of all the known causative genes for LSM: SLC22A5, ABHD5, PNPLA2, ETFA, ETFB, and ETFDH in genomic DNA of patients with LSM.
Genomic DNA was extracted from the muscle biopsies using a standard method.16 We sequenced all exons and their flanking regions of SLC22A5, ABHD5, PNPLA2, ETFA, ETFB, ETFDH, and LIPE. Primers were designed from the genomic sequences reported in GenBank (Gene IDs: 6584 for SLC22A5, 51099 for ABHD5, 57104 for PNPLA2, 2108 for ETFA, 2109 for ETFB, 2110 for ETFDH, and 3991 for LIPE). We performed direct sequencing of amplified fragments using an automated 3100 DNA sequencer (Applied Biosystems, Foster City, California) with the BigDye Terminator cycle sequencing system, and analyzed DNA sequences with the SeqScape program (Applied Biosystems).
We performed quantitative reverse transcript–polymerase chain reaction (RT-PCR) in RNA obtained from muscle using the QuantiTect SYBR-Green PCR Kit (Qiagen GmbH, Hilden, Germany) and iCycler iQ real-time PCR detection system (Bio-Rad, Hercules, California). We analyzed the amount of transcript for ETFDH relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA.
Biochemical Analyses.
We measured CoQ10 in frozen muscles from patients with ETF mutations using a high-performance liquid chromatography (HPLC) method described previously7 in 2 cases with enough sample size for analysis (patients 6 and 7). For muscle lipid analysis, total lipid was extracted from muscles according to the methods of Folch et al.5 Extracted lipids were adopted to TLC with petroleum ether/diethyl ether/acetic acid (60:40:1) as a developing solvent to separate TG, cholesterol (Cho), and free fatty acids (FFA) from phospholipid (PL). The lipids were visualized with 50% sulfuric acids/methanol vapor. Band intensities were measured with Quantity One software (Bio-Rad Laboratories). We measured the levels of TG, PL, and FFA relative to Cho amount (TG/Cho, PL/Cho, FFA/Cho). Muscle carnitine palmitoyltransferase type II (CPT II) activity was measured using a method described previously.2
RESULTS
Pathological and Clinical Features of LSM.
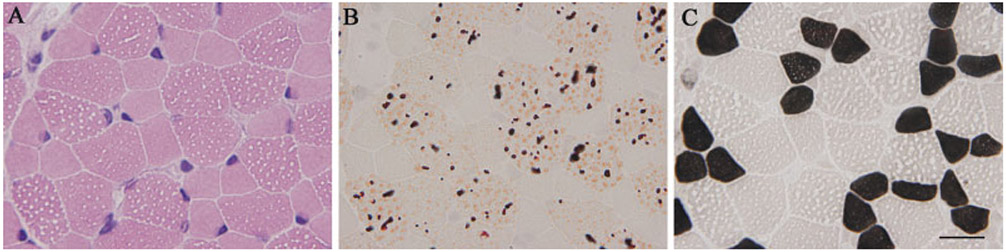
Of 9639 frozen muscle biopsies that we had examined pathologically, 47 (0.5%) had LSM. In all 47 patients, there were numerous small vacuoles that were filled with lipid droplets in scattered type 1 and 2 muscle fibers (Fig. 1A, B). Measurement of the width of these vacuoles, indirectly representing the amount of lipid, did not reveal any significant differences among patients (data not shown). In addition, these lipid droplets were found predominantly in type 1 fibers (Table 1 and Fig. 1C), except in patients 10 and 11, who exhibited lipid droplets predominantly in type 2 fibers (Fig. 2).
FIGURE 1.
Muscle pathology in patient 1 with the SLC22A5 mutation (PCD). Numerous small vacuoles seen on hematoxylin–eosin stain (A) are actually lipid droplets, as shown on oil-red-O (B). These vacuoles are seen predominantly in type 1 fibers (C). Bar = 20 μm.
Table 1.
Clinical summary of 47 patients with LSM
| Pt | Age | Sex | Clinical feature | Weakness | Hypotonia | Muscle pain and cramp |
Prodrome | Seizure | Coma | Respiratory failure |
Cardiac symptom |
Liver disease |
CK | Familial history |
Consanguinity | Lipid droplets distribution by fiber type |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mutations | ||||||||||||||||
| 1 | 8m | F | vomiting, diarrhea | NA | NA | NA | + | − | + | − | − | + | 243–1006 | − | − | type 1 > |
| 2 | 4y | M | gait disturbance | + | + | − | − | − | − | − | + | − | 150 | + | − | type 1 > |
| 3 | 5y | M | gait disturbance | + | + | − | − | − | − | − | + | − | 69 | + | − | type 1 > |
| 4 | 5m | F | + | + | NA | NA | − | − | − | − | + | 55 | − | − | type 1 > | |
| 5 | 6m | M | + | NA | NA | + | − | − | − | − | + | 2000–4000 | − | − | type 1 > | |
| 6 | 11m | M | diarrhea | + | + | NA | + | + | + | − | + | + | 128–618 | − | − | type 1 > |
| 7 | 13y4m | F | muscle weakness | + | + | − | + | − | − | − | − | + | 127 | − | − | type 1 > |
| 8 | 27y | M | muscle weakness | + | − | − | − | − | − | − | + | − | 757–1697 | − | − | type 1 > |
| 9 | 35y | F | gait disturbance | + | + | − | − | − | − | − | + | − | 654 | + | − | type 1 > |
| No Mutations | ||||||||||||||||
| 10 | 15y | M | muscle cramp | − | − | + | − | − | − | − | + | − | 587 | − | − | < type2 |
| 11 | 67y | M | gait disturbance | + | − | + | − | − | − | − | − | − | 4904 | − | − | < type2 |
| 12 | 37d | F | metabolic acidosis | + | + | NA | − | − | − | + | + | − | 44–200 | − | − | type1 = type2 |
| 13 | 4m | F | NA | NA | NA | NA | NA | NA | NA | + | NA | NA | NA | NA | type1 = type2 | |
| 14 | 4m | M | dyspnea | + | + | NA | − | − | − | + | + | − | 112 | + | − | type 1 > |
| 15 | 7m | M | developmental delay | + | + | NA | + | + | − | − | − | − | 67 | − | + | type 1 > |
| 16 | 1y | M | status epilepticus | − | + | NA | − | + | + | + | − | − | 1593 | − | − | type 1 > |
| 17 | 1y6m | F | metabolic acidosis | − | − | + | − | − | − | − | − | − | 330 | + | − | type 1 > |
| 18 | 1y1m | M | + | + | NA | + | − | − | + | + | − | 559 | − | − | type 1 > | |
| 19 | 1y2m | F | developmental delay | + | + | NA | + | + | + | + | − | − | NA | − | − | type 1 > |
| 20 | 1y6m | M | albinism | + | + | NA | − | − | − | − | − | − | 200–300 | − | − | type 1 > |
| 21 | 2y2m | M | diarrhea | NA | + | + | + | − | + | − | − | − | 163900 | − | − | type 1 > |
| 22 | 2y7m | M | developmental delay | − | + | − | − | − | − | − | − | − | 603 | − | − | type 1 > |
| 23 | 3y | M | status epilepticus | NA | NA | NA | + | + | + | − | − | − | 2034 | − | − | type 1 > |
| 24 | 3y | F | developmental delay | + | + | − | − | − | − | − | − | − | 63 | − | − | type 1 > |
| 25 | 3y | M | developmental delay | NA | + | NA | − | − | NA | + | − | − | NA | − | − | type 1 > |
| 26 | 4y | F | periodic paralysis | + | + | − | − | + | − | − | − | − | 162 | − | − | type 1 > |
| 27 | 5y7m | M | developmental delay | + | + | − | − | − | − | − | − | − | 47 | − | − | type 1 > |
| 28 | 6y | M | dyspnea, abdominal pain | + | + | − | + | − | + | + | − | − | 15 | + | + | type 1 > |
| 29 | 13y8m | F | muscle weakness | + | NA | − | − | − | − | − | − | − | normal | + | + | type 1 > |
| 30 | 30y | F | lumbago | − | − | + | − | − | − | − | − | − | NA | − | − | type1 = type2 |
| 31 | 40y | F | diplopia, muscle cramp | − | − | + | − | − | − | − | − | − | NA | − | − | type 1 > |
| 32 | 49y | F | hypokalemic myopathy | + | − | − | − | − | − | − | − | − | 3480 | − | − | type 1 > |
| 33 | 54y | F | weakness | + | − | − | − | − | − | − | − | − | 623 | − | − | type 1 > |
| 34 | 59y | F | dyspnea, weakness | + | − | − | − | − | − | + | − | − | 878 | − | − | type 1 > |
| 35 | 66y | F | gait disturbance | + | − | + | − | − | − | − | − | − | 49 | − | − | type 1 > |
| 36 | 69y | M | gait disturbance | − | − | + | − | − | − | − | − | − | 400 | − | − | type 1 > |
| 37 | 75y | F | gait disturbance | − | − | + | − | − | − | − | − | − | 5418 | − | − | type 1 > |
| No Available DNA | ||||||||||||||||
| 38 | 4m | F | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | type1 = type2 | |
| 39 | 7m | M | dyspnea | NA | NA | NA | NA | NA | NA | + | NA | NA | 125 | NA | NA | NC* |
| 40 | 9m | F | developmental delay | NA | + | NA | − | + | − | − | − | − | NA | − | − | type 1 > |
| 41 | 9m | M | ketoacidosis | − | − | − | + | − | − | − | − | + | 214 | − | − | type 1 > |
| 42 | 1y | M | vomiting, diarrhea | NA | NA | NA | + | + | + | − | − | − | 394 | − | − | type 1 > |
| 43 | 1y | F | dyspnea, weakness | + | − | − | − | + | NA | + | − | − | 12310 | − | − | type1 = type2 |
| 44 | 5y | M | fever, arthralgia | + | NA | + | − | − | − | − | − | − | 39 | + | − | type 1 > |
| 45 | 21y | F | vomiting, diarrhea | − | − | − | + | − | + | − | − | − | NA | − | − | type1 = type2 |
| 46 | 23y | F | muscle pain | − | − | + | − | − | − | − | − | − | 62180 | − | − | type 1 > |
| 47 | 24y | F | lumbago | − | − | + | − | − | − | − | − | − | 47–4797 | − | − | type 1 > |
NC = not counted
NA = not available
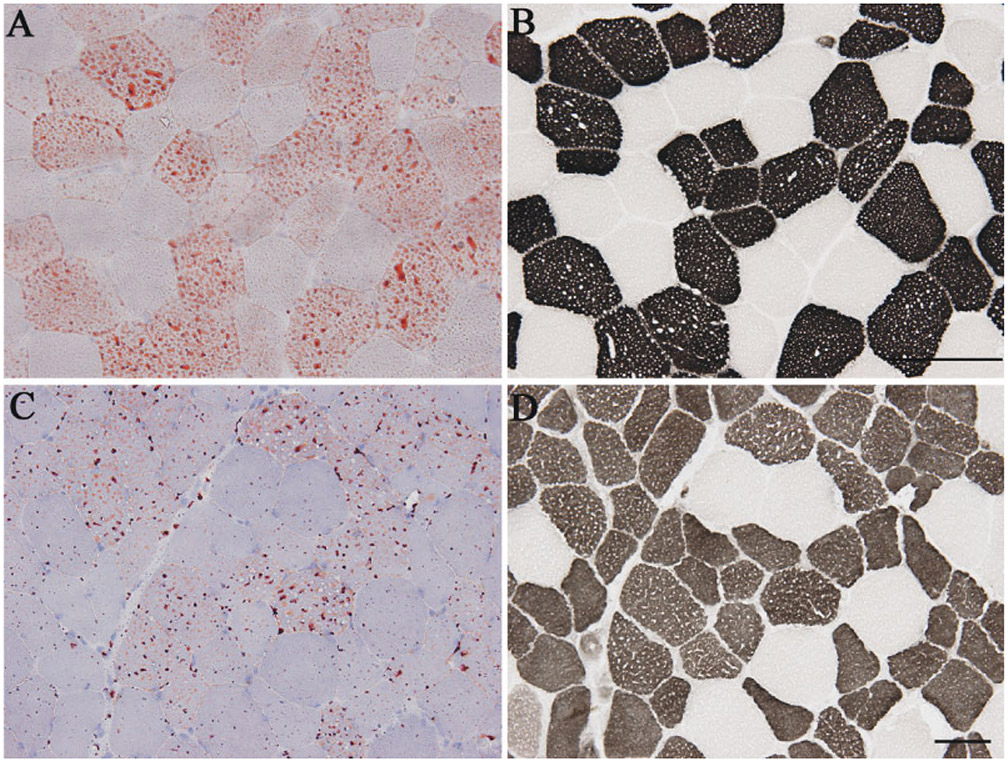
FIGURE 2.
Lipid accumulation in type 2 fibers of patients with no mutations in known genes associated with LSM: patient 10 (A, B) and patient 11 (C, D). Lipid droplets stained with oil-red-O (A, C) are only seen in type 2 fibers (routine adenosine triphosphatase stain) (B, D). Bar = 50 μm.
The clinical features of the 47 patients (23 males and 24 females) are summarized in Table 1. Age at onset varied from 37 days to 75 years. Eight patients had a positive family history. The majority of the patients (55%) had muscle weakness, and all except 1 had generalized or proximal dominant muscle weakness. No correlation was found between the clinical/pathological phenotype and genotype of patients (data not shown).
Genetic Analysis of LSM.
DNA was available for only 37 patients. We identified mutations in 9 (24%) patients: 3 in SLC22A5; 3 in ETFDH; 1 in ETFA; and 2 in PNPLA2 (Table 2). In patient 4, we identified a heterozygous c.1519T>G ETFDH mutation in genomic DNA; however, by RT-PCR, only the transcript with this mutation was detected, indicating absence of transcript from the other allele. All mutations were novel except in patients 2 and 3.11,12 We did not find similar mutations in 100 control chromosomes of Japanese individuals. In addition, we did not find any mutations in ABHD5 or LIPE.
Table 2.
Identified mutations.
| Patient | Age | Gender | Gene name | Nucleotide change | Amino acid change |
|---|---|---|---|---|---|
| 1 | 8 mo | F | SLC22A5 | c.396G>A* c.844C>T | p.W128X p.A282X |
| 211,12 | 4 y | M | SLC22A5 | −91_22del† | |
| 311,12 | 5 y | M | SLC22A5 | −91_22del† | |
| 4 | 5 mo | F | ETFDH | c.1519T>G* | p.Y507D |
| 5 | 6 mo | M | ETFDH | c.1208C>T† | p.A403V |
| 6 | 11 mo | M | ETFA | c.284T>G† | p.L95W |
| 7 | 13 y | F | ETFDH | c.524G>A* | p.R175H |
| c.1774T>G | p.C592R | ||||
| 8 | 27 y | M | PNPLA2 | c.477_478insCCTC* | Frameshift 178X |
| 9 | 35 y | F | PNPLA2 | c.477_478insCCTC* | Frameshift 178X |
Compound heterozygous.
Homozygous.
Biochemical Analysis.
CoQ10 levels were normal in patient 7 and mildly decreased in patient 6, who had ETFDH and ETFA mutations, respectively (Table 3). The size of the samples permitted lipid analysis in only 14 patients, including 2 patients with mutations: patient 1 with PCD, and patient 6 with MADD. The amount of TG was significantly elevated in all LSM patients (TG/Cho: 12.5 ± 2.26 [mean ± standard error of mean]) when compared with control individuals (5.95 ± 1.72). In contrast, FFA were not increased, and PL were not significantly different (data not shown). In all 10 patients tested, CPT II activity was normal.
Table 3.
Clinical summary of MADD patients.
| Patient | Age | Gender | Clinical feature | Serum CK (IU/L) |
CoQ10 level (μg/g muscle) |
Treatment | Clinical course |
|---|---|---|---|---|---|---|---|
| 4 | 5 mo | F | Muscle weakness, hepatomegaly | 55 | NA | NA | NA |
| 5 | 6 mo | M | Muscle weakness, hepatomegaly | 2000-4000 | NA | L-carnitine riboflavin | Normal development after treatment |
| 6 | 11 mo | M | Vomiting, hypertrophic cardiomyopathy | 128-618 | 24.1 | L-carnitine | Died at 2 years of age due to pulmonary alveolar bleeding |
| 7 | 13 y 4 mo | F | Progressive muscle weakness | 127 | 32.3 | L-carnitine riboflavin | No muscle weakness at present |
NA, not available. Normal range: CK, 57-197 IU/L; CoQ10, 32.1 ± 6.8 (mean ± standard deviation).
PCD Patients.
Patients 1, 2, and 3 harbored mutations in SLC22A5. Patient 1 exhibited normal early motor development and appeared healthy until age 8 months when she developed hepatomegaly, coma, hyperammonemia, and non-ketotic dicarboxylic aciduria. On liver biopsy, numerous lipid droplets were seen. Clinical improvement was seen with l-carnitine supplementation, but she eventually succumbed to heart failure when she had an infection. Patients 2 and 3, who are siblings, have been reported previously.2,12 Briefly, they had slowly progressive muscle weakness and hypertrophic cardiomyopathy, and their developmental milestones were normal until 3 years of age, when mild weakness in the lower limbs became evident. Laboratory examination showed transient high creatine kinase (CK) levels and hyperammonemia. Carnitine levels were decreased in skeletal muscles of these 3 patients (data not shown). Serum carnitine was likewise reduced in patients 2 and 3. Total and free carnitine levels (in μmol/L), respectively, were: 36.1 (normal: 67.6 ± 11.3) and 12.3 (normal: 52.2 ± 10.4) in patient 2; and 35.7 and 11.4 in patient 3. l-carnitine treatment in both cases resulted in marked clinical improvement.
On muscle pathology, both number and size of mitochondria were mildly increased (Fig. 4A). Lipid-containing vacuoles in skeletal muscle were predominantly observed in type 1 fibers (Fig. 4B). Patient 1 had type 2 fiber atrophy, whereas patients 2 and 3 showed type 2A fiber atrophy and type 2B fiber deficiency. On electron microscopy, there was an increase in number of lipid droplets and mitochondria. Incidentally, lipid droplets were often next to mitochondria (Fig. 5A, B).
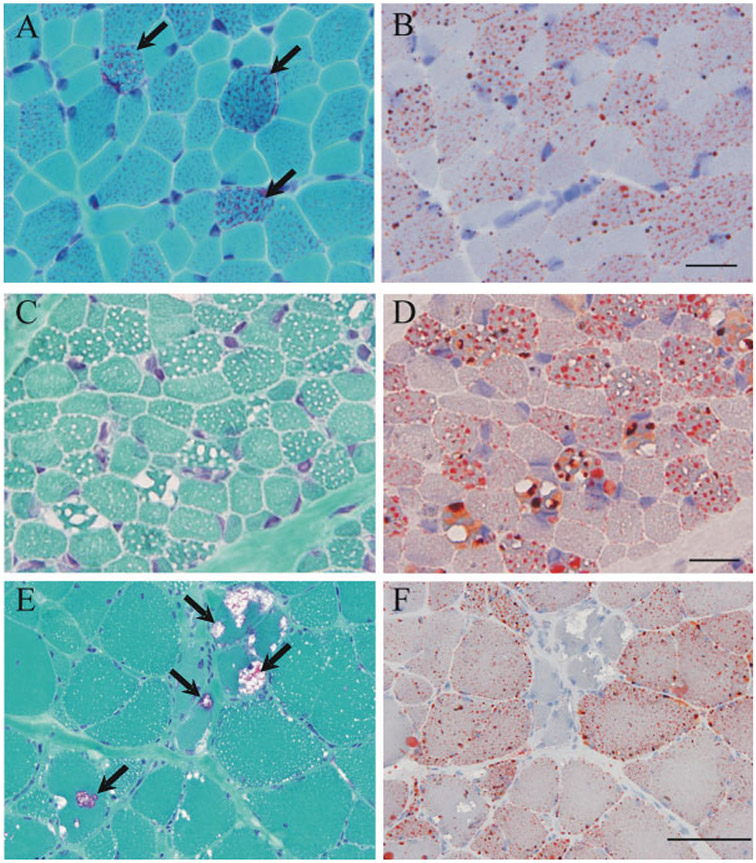
FIGURE 4.
Routine histochemical staining of patients with LSM. Ragged-red–like fibers are seen on modified Gomori trichrome staining (A) and numerous lipid droplets are seen with oil-red-O (B) in a PCD patient, but not in an MADD patient (C, D). Rimmed vacuoles are seen in myofibers of patients with NLSDM (E), in addition to the characteristic numerous lipid droplets predominantly in type 1 fibers (F). Bar = 20 μm.
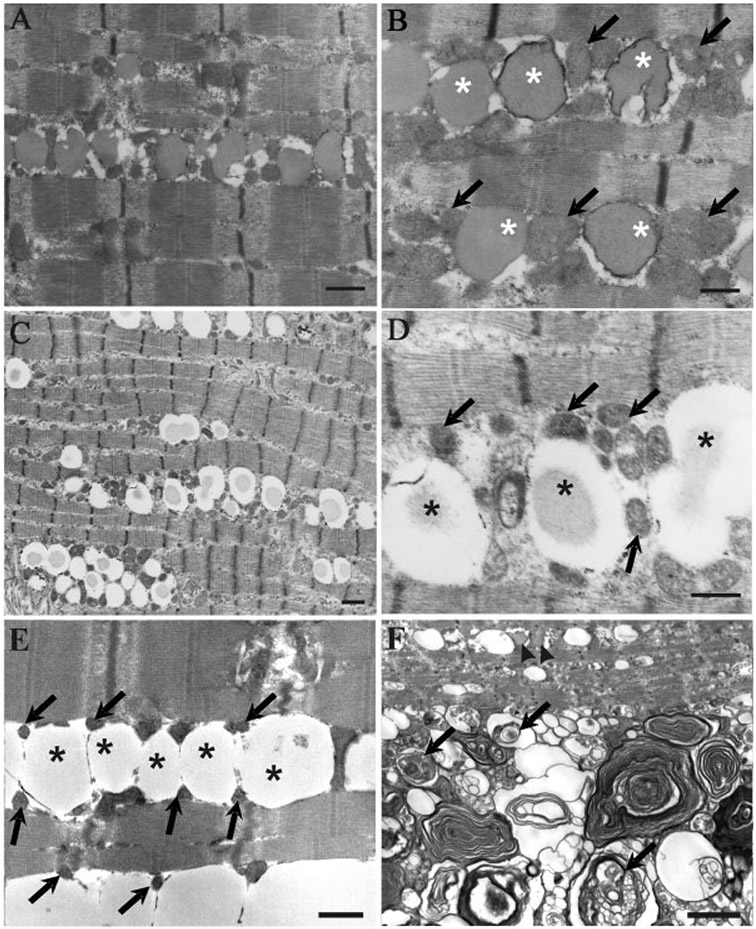
FIGURE 5.
Electron microscopy findings. In a patient with PCD, markedly increased lipid droplets and increased numbers of mitochondria are seen (A). On higher magnification, lipid droplets (asterisks) are seen next to mitochondria (arrows) (B). In a patient with MADD, note the increase in the number of lipid droplets (C) and the proximity of these droplets (asterisks) with mitochondria (arrows) (D), findings similar to those for PCD. In a patient with NLSDM, there is also a marked increase in the amount of lipid (asterisks) deposited within the myofibers; however, note that mitochondria appear pyknotic (arrows) (E). In this patient, numerous autophagic vacuoles (arrows) in close proximity to lipid deposition (arrowheads) are also observed (F). Bar = 1 μm in (A), (C), and (F). Bar = 0.5 μm in (B), (D), and (E).
MADD Patients.
The clinical features of the 4 patients genetically confirmed to have MADD are summarized in Table 3. The diagnosis of MADD in patients 6 and 7 was initially made based on the results of urinary organic acid analysis by gas chromatography/mass spectroscopy. All 4 patients had the infantile form. They all had generalized muscle weakness and hypotonia. Serum CK levels varied from normal to 4000 IU/L. Hepatomegaly was documented in patients 4 and 5. Patient 5, who received l-carnitine and riboflavin treatment, had normal growth and development, except for some mild metabolic episodes, and is now 20 years old. Patient 6 had hypertrophic cardiomyopathy. He was treated with l-carnitine, but he died of pulmonary alveolar bleeding at the age of 1 year and 11 months. Patient 7 was always a slow runner and poor athlete with easy fatigability since her preschool years. She developed nausea and vomiting at age 13 years and started experiencing difficulty climbing stairs. She had proximal dominant muscle weakness and atrophy on examination at age 13 years and 4 months. After treatment with l-carnitine and riboflavin, muscle weakness was ameliorated.
In skeletal muscle, lipids were observed predominantly in type 1 fibers. Mitochondria were not as prominent as in PCD (Fig. 4C, D). Type 2 fiber atrophy was seen in patient 5. Electron-microscopic findings were similar to those seen in PCD patients: intracytoplasmic lipid droplets were markedly increased both in number and size, and lipid droplets were often present next to mitochondria (Fig. 5C, D).
NLSDM Patients.
Patients 8 and 9 had mutations in PNPLA2. Patient 8 developed progressive weakness in the lower legs and fingers at age 20 years (article in submission); at age 27 years, echocardiogram revealed dilated cardiomyopathy with left ventricular enlargement. Serum CK was elevated from 757 to 1697 IU/L.14 Patient 9 was a slow runner since childhood.1 At age 33 years, she noticed weakness of all extremities and developed marked generalized muscle weakness at 35 years. Electrocardiogram (ECG) showed left ventricular hypertrophy, but echocardiogram was normal. Serum CK was elevated to 654 IU/L. In both patients, peripheral blood smear revealed lipid-containing vacuoles in leukocytes, namely Jordan’s anomaly. Both patients had numerous lipid droplets mainly in type 1 fibers in addition to variation in fiber size. Surprisingly, there were scattered rimmed vacuoles within the myofibers (Fig. 4E, F), which were demonstrated to be autophagic vacuoles on electron microscopy (Fig. 5F). Interestingly, increased lipid droplets were seen between myofibrils where mitochondria appeared pyknotic (Fig. 5E).
DISCUSSION
Among all LSM cases, we identified mutations in known causative genes in only 24% of the cases. This brings to our attention two possibilities: the existence of yet-unknown causative genes, and secondary increase of lipid in muscle under a variety of metabolic alterations without inheritance.
Analysis of muscle lipids demonstrated an increase in the amount of TG, but not FFA. The accumulated lipid droplets in the cytoplasm of skeletal myofibers are therefore likely to be mainly composed of TG. Although, theoretically, triglyceride accumulation should occur in NLSDM and NLSDI, in which genes encoding TG hydrolase or its activator are mutated, it is accumulated in virtually all patients analyzed regardless of the causative gene. Reduction of mitochondrial fatty acid metabolism may negatively regulate the hydrolysis of TG in cytosol.
We identified 3 PCD patients with mutations in SLC22A5. Their clinical characteristics were consistent with the typical PCD symptoms with severe hypoglycemia, dilated cardiomyopathy, and progressive muscle weakness, as reported elsewhere.11,12 A positive response to l-carnitine treatment was seen in all 3 patients, a feature that has been shown to be characteristic of PCD.11
Among the patients with MADD, 2 had a good response to riboflavin. Olsen et al. noted that riboflavin-responsive MADD may result from defects in ETFDH combined with general mitochondrial dysfunction.15 In support of this notion, both of our patients who responded to riboflavin had mutations in ETFDH. With regard to CoQ10 levels, however, our case contradicts the recent report.7 Although we measured CoQ10 levels in only 2 patients due to sample size limitation, the finding of a normal CoQ10 level in a patient with the ETDFH mutation is still relevant for clinicians, because it indicates that ETFDH mutations may not always be associated with CoQ10 deficiency. Further studies are necessary to determine whether there is a detailed relationship between the ETFDH mutation and CoQ10 deficiency.
The first step of the mitochondrial β-oxidation cycle is catalyzed by four fatty acyl-CoA dehydrogenases (very long, long, medium, and short chain), all of which are affected in MADD. We previously reported that very-long-chain acyl-CoA dehydrogenase (VLCAD) deficiency does not show increased lipid droplets in muscle.13 In contrast, MADD is characterized pathologically by lipid storage, raising the possibility that lipid droplets may not accumulate when one of the four acyl-CoA dehydrogenases, such as VLCAD, is defective.
Our patients with NLSDM presented with distal myopathy and cardiac symptoms, accompanied by lipid accumulation in muscle and peripheral leukocytes, suggesting multisystemic lipid accumulation. Notably, in the patient with NLSDM, mitochondria on electron microscopy were pyknotic, in stark contrast to those in PCD and MADD. This morphological difference is contrary to that expected from function of each causative gene, because PCD and MADD have defects in the mitochondrial β-oxidation cycle, whereas NLSDM is due to a defect in cytoplasmic TG hydrolysis. In addition, rimmed vacuoles were observed in the 2 NLSDM patients and not in the other LSM patients. Together with the fact that both patients had progressive, rather than episodic, muscle disease, these clinicopathological peculiarities should reflect a distinct pathomechanism that is yet to be elucidated. Clearly, further studies are necessary to fully understand the mechanism of the disease.
CPT II deficiency has been reported to show increased lipid droplets in muscle.3 However, in all patients whose samples were suitable for biochemical assays, normal enzymatic activity was seen. Furthermore, in our series, we had 7 patients with CPT II deficiency, but none showed lipid droplet accumulation on muscle pathology (data not shown). This suggests that lipid storage may not be a common pathological feature of CPT II deficiency, although analysis of a larger population of patients with CPT II deficiency would be needed to further support this contention.
In spite of an extensive genetic survey of known causative genes for LSM, we did not find mutations in 76% of the patients. One possible explanation for the absence of mutations in these patients is that they may have secondary LSM, as intramuscular lipid content is known to be secondarily increased under a variety of conditions, including diabetes, renal disease, iatrogenic conditions, gastrointestinal disturbance, elevated plasma fatty acid levels, and high dietary fat intake.18 The fact that 2 patients were taking antiepileptic drugs could be supportive of this notion. In the majority of patients without mutation, conditions associated with a secondary increase in muscle lipids were not seen. In addition, 2 patients had pathological features that differed from the rest of the patients in the form of lipid droplets almost exclusively in type 2 fibers, in contrast to the preference of lipid accumulation in type 1 fibers in all others, indicating the probability of a common pathomechanism, at least in these 2 cases.
Among the 28 patients without mutations in known causative genes, 5 had a positive family history and/or consanguinity (Table 1), suggesting that these individuals are likely to have primary genetic lipid disorders, rather than secondary LSM and the presence of additional yet-to-be-identified causative genes for LSM. Further analysis on biochemical analyses of accumulated metabolites and extended study of candidate genes involved in lipid metabolism will be helpful in the genetic diagnosis of LSM patients.
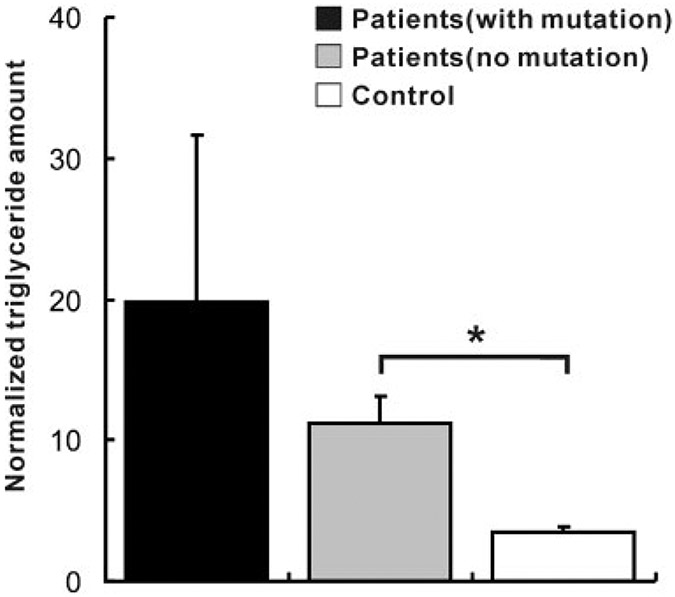
FIGURE 3.
TLC analysis of lipid composition of skeletal muscle with LSM. The bars represent the mean triglyceride (TG) amount which is normalized with cholesterol (Cho) content. Values are shown for patients with mutation (black bar; N = 2), patients with no mutation (gray bar; N = 12), and in controls (white bar; N = 4). Error bars represent standard error of means. Note the remarkable increase of TG in patients with mutations. *P < 0.05 (Student’s t-test).
Acknowledgments
This study was supported by Research on Psychiatric and Neurological Diseases and Mental Health, from Health and Labour Sciences Research Grants; Research on Health Sciences Focusing on Drug Innovation, from the Japanese Health Sciences Foundation; research grants for nervous and mental disorders (20B-12, 20B-13, 19A-4, and 19A-7), from the Ministry of Health, Labour and Welfare; and the Program for Promotion of Fundamental Studies in Health Sciences, National Institute of Biomedical Innovation (NIBIO).
Abbreviations:
- ABHD5
abhydrolase domain–containing 5
- ATGL
adipose triglyceride lipase
- CGI-58
comparative gene identification 58
- CPT II
carnitine palmitoyltransferase type II
- Cho
cholesterol
- CoA
coenzyme A
- CoQ10
coenzyme Q10
- ECG
electrocardiogram
- ETF
electron transfer flavoprotein
- ETFDH
electron transfer flavoprotein dehydrogenase
- FFA
free fatty acids
- HSL
hormone-sensitive lipase
- LSM
lipid storage myopathies
- MADD
multiple acyl-coenzyme A dehydrogenation deficiency
- NLSDI
neutral lipid storage with ichthyosis
- NLSDM
neutral lipid storage disease with myopathy
- OCTN2
organic cation transporter 2
- PCD
primary carnitine deficiency
- PL
phospholipids
- PNPLA2
patatin-like phospholipase domain–containing protein 2
- RT-PCR
reverse transcript–polymerase chain reaction
- SDH
succinate dehydrogenase
- TG
triglyceride
- VLCAD
very-long-chain acyl-CoA dehydrogenase
REFERENCES
- 1.Akiyama M, Sakai K, Ogawa M, McMillan JR, Sawamura D, Shimizu H. Novel duplication mutation in the patatin domain of adipose triglyceride lipase (PNPLA2) in neutral lipid storage disease with severe myopathy. Muscle Nerve 2007;36:856–859. [DOI] [PubMed] [Google Scholar]
- 2.Deufel T, Wieland OH. Sensitive assay of carnitine palmitoyl transferase activity in tissue homogenates with a modified spectrophotometric method for enzymatic carnitine determination. Clin Chim Acta 1983;135:247–251. [DOI] [PubMed] [Google Scholar]
- 3.Di Mauro S, Trevisan C, Hays A. Disorders of lipid metabolism in muscle. Muscle Nerve 1980;3:369–388. [DOI] [PubMed] [Google Scholar]
- 4.Fischer J, Lefv̀re C, Morava E, Mussini JM, Laforêt P, Negre-Salvayre A, et al. The gene encoding adipose triglyceride lipase (PNPLA2) is mutated in neutral lipid storage disease with myopathy. Nat Genet 2007;39:28–30. [DOI] [PubMed] [Google Scholar]
- 5.Folch JM, Lees M, Stanlex, GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 1957;226:497–509. [PubMed] [Google Scholar]
- 6.Freeman FE, Goodmann SI. Defects of electron transfer flavoprotein and electron transfer flavoprotein–ubiquinone oxidoreductase: glutaric aciduria type II. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001, p 2357–2365. [Google Scholar]
- 7.Gempel K, Topaloglu H, Talim B, Schneiderat P, Schoser BG, Hans VH, et al. The myopathic form of coenzyme Q10 deficiency is caused by mutations in the electron-transferring-flavoprotein dehydrogenase (ETFDH) gene. Brain 2007;130:2037–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haemmerle G, Zimmermann R, Strauss JG, Kratky D, Riederer M, Knipping G, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem 2002;277:4806–4815 [DOI] [PubMed] [Google Scholar]
- 9.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, et al. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin–Dorfman syndrome. Cell Metab 2006;3:309–319. [DOI] [PubMed] [Google Scholar]
- 10.Lefèvre C, Jobard F, Caux F, Bouadjar B, Karaduman A, Heilig R, et al. Mutations in CGI-58, the gene encoding a new protein of the esterase/lipase/thioesterase subfamily, in Chanarin–Dorfman syndrome. Am J Hum Genet 2001;69:1002–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsuishi T, Hirata K, Terasawa K, Kato H, Yoshino M, Ohtaki E, et al. Successful carnitine treatment in two siblings having lipid storage myopathy with hypertrophic cardiomyopathy. Neuropediatrics 1985;16:6–12. [DOI] [PubMed] [Google Scholar]
- 12.Nezu J, Tamai I, Oku A, Ohashi R, Yabuuchi H, Hashimoto N, et al. Primary systemic carnitine deficiency is caused by mutations in a gene encoding sodium ion-dependent carnitine transporter. Nat Genet 1999;21:91–94. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi Y, Hasegawa Y, Murayama K, Ogawa M, Hasegawa T, Kawai M, et al. A new diagnostic test for VLCAD deficiency using immunohistochemistry. Neurology 2004;22:62:2209–2213. [DOI] [PubMed] [Google Scholar]
- 14.Ohkuma A, Nonaka I, Malicdan MCV, Noguchi S, Nomura K, et al. Distal lipid storage myopathy due to PNLPA2 mutations. Neuromuscul Disord. 2008;18:671–674. [DOI] [PubMed] [Google Scholar]
- 15.Olsen RK, Olpin SE, Andresen BS, Miedzybrodzka ZH, Pourfarzam M, Merinero B, et al. ETFDH mutations as a major cause of riboflavin-responsive multiple acyl-CoA dehydrogenation deficiency. Brain 2007;130:2045–2054. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 17.Scaglia F, Longo N. Primary and secondary alterations of neonatal carnitine metabolism. Semin Perinatol 1999;23:152–161. [DOI] [PubMed] [Google Scholar]
- 18.Schrauwen-Hinderling VB, Hesselink MK, Schrauwen P, Kooi ME. Intramyocellular lipid content in human skeletal muscle. Obesity 2006;14:357–367. [DOI] [PubMed] [Google Scholar]
- 19.Stockler S, Radner H, Karpf EF, Hauer A, Ebner F. Symmetric hypoplasia of the temporal cerebral lobes in an infant with glutaric aciduria type II (multiple acyl-coenzyme A dehydrogenase deficiency). J Pediatr 1994;124:601–604. [DOI] [PubMed] [Google Scholar]
- 20.Takanashi J, Fujii K, Sugita K, Kohno Y. Neuroradiologic findings in glutaric aciduria type II. Pediatr Neurol 1999;20:142–145. [DOI] [PubMed] [Google Scholar]
- 21.Vockley J, Whiteman DA. Defects of mitochondrial beta-oxidation: growing group of disorders. Neuromuscul Disord 2002;12:235–246. [DOI] [PubMed] [Google Scholar]
- 22.Wu X, Prasad PD, Leibach FH, Ganapathy V. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem Biophys Res Commun 1998;246:589–595. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 2004;306:1383–1386. [DOI] [PubMed] [Google Scholar]