Abstract
Purpose
Serum lipids are modifiable, routinely collected blood test features associated with cardiovascular health. We examined the association of commonly collected serum lipid measures (total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], and triglycerides) with intraocular pressure (IOP).
Design
Cross-sectional study in the UK Biobank and European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohorts.
Participants
We included 94 323 participants from the UK Biobank (mean age, 57 years) and 6230 participants from the EPIC-Norfolk (mean age, 68 years) cohorts with data on TC, HDL-C, LDL-C, and triglycerides collected between 2006 and 2009.
Methods
Multivariate linear regression adjusting for demographic, lifestyle, anthropometric, medical, and ophthalmic covariables was used to examine the associations of serum lipids with corneal-compensated IOP (IOPcc).
Main Outcome Measures
Corneal-compensated IOP.
Results
Higher levels of TC, HDL-C, and LDL-C were associated independently with higher IOPcc in both cohorts after adjustment for key demographic, medical, and lifestyle factors. For each 1-standard deviation increase in TC, HDL-C, and LDL-C, IOPcc was higher by 0.09 mmHg (95% confidence interval [CI], 0.06–0.11 mmHg; P < 0.001), 0.11 mmHg (95% CI, 0.08–0.13 mmHg; P < 0.001), and 0.07 mmHg (95% CI, 0.05–0.09 mmHg; P < 0.001), respectively, in the UK Biobank cohort. In the EPIC-Norfolk cohort, each 1-standard deviation increase in TC, HDL-C, and LDL-C was associated with a higher IOPcc by 0.19 mmHg (95% CI, 0.07–0.31 mmHg; P = 0.001), 0.14 mmHg (95% CI, 0.03–0.25 mmHg; P = 0.016), and 0.17 mmHg (95% CI, 0.06–0.29 mmHg; P = 0.003). An inverse association between triglyceride levels and IOP in the UK Biobank (–0.05 mmHg; 95% CI, –0.08 to –0.03; P < 0.001) was not replicated in the EPIC-Norfolk cohort (P = 0.30).
Conclusions
Our findings suggest that serum TC, HDL-C, and LDL-C are associated positively with IOP in 2 United Kingdom cohorts and that triglyceride levels may be associated negatively. Future research is required to assess whether these associations are causal in nature.
Keywords: Cholesterol, Glaucoma, Intraocular pressure, Lipids
Elevated intraocular pressure (IOP) is one of the most important risk factors for glaucoma, and IOP reduction is the only proven treatment to reduce the development and progression of the disease.1 Identifying potential systemic associations with IOP may provide further insight into the pathophysiologic features of glaucoma.
Previous studies have demonstrated a potential association between elevated IOP and traditional cardiovascular risk factors such as age,2 body mass index (BMI),3 smoking,4 and hyperlipidemia.5 Serum lipids (including total cholesterol [TC] and triglycerides) and cholesterol components (including low-density lipoprotein cholesterol [LDL-C] and high-density lipoprotein cholesterol [HDL-C]) are collected routinely, are widely available, and are modifiable causal risk factors for cardiovascular disease.6,7 The association between serum lipid fractions and cholesterol components (specifically LDL-C, HDL-C, and triglycerides) and IOP has been examined previously with variable results. Higher LDL-C and triglyceride levels have been associated with higher IOP in several studies, whereas higher HDL-C has been associated with lower IOP in some observational studies.8,9 A recent meta-analysis observed that hyperlipidemia was associated with a modest but statistically significant higher IOP, and this association was maintained when LDL-C and triglycerides were assessed individually, but no association was found between IOP and HDL-C.5
The generalizability of the existing literature is limited because most studies were performed in Asian populations8, 9, 10, 11, 12 and did not adjust for important potential confounders of this association, including BMI,8,11 diabetes status,9 or use of cardiovascular medications, including statins and β-blockers,8,9,12 all of which have potential associations with IOP or corneal biomechanical properties that may influence IOP and with serum lipids. In this large observational study, we examined the association of individual serum lipids and cholesterol components (specifically TC, HDL-C, LDL-C, and triglycerides) with corneal-compensated IOP (IOPcc) in 2 independent cohorts, the UK Biobank and the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk.
Methods
UK Biobank
Study Population
The UK Biobank is a large community-based cohort of 502 506 United Kingdom residents registered with the National Health Service and between 40 and 69 years of age at enrollment. Baseline examinations were carried out between 2006 and 2010 at 22 study assessment centers. The North West Multi-center Research Ethics Committee approved the study in accordance with the principles of the Declaration of Helsinki. All participants gave written informed consent before enrollment in the study. The overall study protocol (https://www.ukbiobank.ac.uk/media/gnkeyh2q/study-rationale.pdf) and protocols for individual tests (http://biobank.ctsu.ox.ac.uk/crystal/docs.cgi) are available online. Participants answered detailed touch-screen questionnaires that cover a wide range of demographic, health, and lifestyle information.13 The choices for ethnicity included White (English/Irish or other White background), Asian or British Asian (Indian/Pakistani/Bangladeshi or other Asian background), Black or Black British (Caribbean, African, or other Black background), Chinese, mixed (White and Black Caribbean or African, White and Asian, or other mixed background), or other ethnic group (not defined). We subsequently combined ethnicity groups into White and non-White. The Townsend deprivation index was determined according to the participants’ postcode at recruitment and the corresponding output area from the preceding national census. The index was calculated based on the output area’s employment status, home and car ownership, and household condition; the higher and more positive the index, the more deprived the area. Smoking and alcohol intake status were determined by self-report and subsequently were combined into ever smoker or never smoker and into alcohol consumption or no alcohol consumption. Diabetes status was defined by self-report of diabetes mellitus or use of diabetes medications. Statin and oral β-blocker medication use were self-reported. Blood pressure was measured twice using the HEM-70151T digital blood pressure monitor (Omron), and the mean was used in the analysis. Weight was measured with the BV-418 MA body composition analyzer (Tanita). Height was measured using a Seca 202 height measure (Seca). Body mass index was calculated as weight (kg)/height (m2).
Assessment of Serum Lipid and Lipoprotein Concentrations
Nonfasting venous blood sampling was conducted, and the collection procedures were standardized.14 Serum lipid concentrations, including TC, HDL-C, LDL-C, and triglycerides, were measured by biochemical assays from the blood samples collected at baseline using the Beckman Coulter AU5800 Platform and were reported in millimoles per liter. Details of the blood measurements and processing are available on the UK Biobank online showcase and protocol.15
Ocular Measurements
Ocular assessment was introduced as an enhancement in 2009. Ophthalmic data were collected for 128 180 UK Biobank participants at 6 assessment centers across the United Kingdom.16 Refractive error was measured using an autorefractor (Tomey RC 5000), and spherical equivalent was calculated as sphere power plus half cylinder power.17 The IOP was measured once for each eye using the Ocular Response Analyzer (ORA) noncontact tonometer (Reichert Corp).18 Participants who had undergone eye surgery within the previous 4 weeks or those with an eye infection were excluded from having IOP measured. The ORA flattens the cornea with a jet of air, causing an initial inward applanation, followed by an outward applanation event as the cornea returns to its original shape. An electro-optical system measures the air pressures at the initial inward applanation and the outward applanation event and combines them linearly to derive an IOP that accounts for corneal biomechanical properties (IOPcc). The average of the 2 ORA pressure values has been calibrated to derive an IOP correlated with Goldmann applanation tonometry as well.19 Participants who had a history of glaucoma laser therapy or surgery were excluded because of the impact of glaucoma treatment on IOP. Based on the mean IOP reduction achieved by medication, we imputed pretreatment IOP for participants using IOP-lowering medication by dividing the measured IOP by 0.7, a method used previously in genome-wide association studies for IOP.20 Participant-level IOP and spherical equivalent values were calculated as the average of both eyes or as either right or left eye value if data were available for only 1 eye.
European Prospective Investigation into Cancer and Nutrition-Norfolk
Study Population
The EPIC is a collaborative study involving 10 countries that began participant recruitment in 1989.21 The EPIC-Norfolk, a United Kingdom branch of this study, comprises a population-based cohort of 25 639 participants between 40 and 79 years of age at enrollment recruited from 35 participating general practices in Norfolk, United Kingdom.21 Baseline examinations were carried out between 1993 and 1997. An ophthalmic examination was performed of 8623 of these participants as part of the third health examination, carried out between 2004 and 2011.22 The EPIC-Norfolk Eye Study was carried out following the principles of the Declaration of Helsinki and the Research Governance Framework for Health and Social Care and was approved by the Norfolk Local Research Ethics Committee (identifier: 05/Q0101/191) and the East Norfolk and Waveney National Health Service Research Governance Committee (identifier: 2005EC07L). All participants gave written informed consent. The study protocol is available online at https://www.epic-norfolk.org.uk/.
Participants provided demographic information, completed general health questionnaires, which collected information on lifestyle factors and diet, and attended a baseline health check, during which blood samples were obtained for future analysis. The Townsend deprivation index was determined in the same fashion as described for the UK Biobank cohort. Smoking and alcohol use were determined by self-report. Participants were asked to bring their medications and related medical documents to the health examinations, and these were recorded by a research nurse using an electronic form. Diabetes status was determined mainly from a lifestyle questionnaire sent to participants at the baseline assessment (and related questions were repeated in follow-up health and lifestyle questionnaires), and additional data from sources, including deaths, hospital admissions, questionnaires, and general practitioner registers up to 2006, were also used to identify patients with diabetes at the time of the EPIC Eye Study. Blood pressure and heart rate were measured with the participants seated and resting using an objective measurement device (Accutorr Plus; Datascope Patient Monitoring, Mindray United Kingdom, Ltd). Height and weight were measured with participants wearing light clothing and no shoes. Height was measured to 0.1 cm using a stadiometer. Weight was measured to the nearest 0.1 kg using digital scales (Tanita UK Ltd), and BMI was calculated as weight (kg)/height (m2).
Assessment of Serum Lipid and Lipoprotein Levels
Nonfasting blood was drawn from study participants. Total cholesterol, HDL-C, and triglycerides were determined using the RA1000 analyzer (Bayer Diagnostics). Low-density lipoprotein cholesterol measurements were derived using the Friedewald formula.23 All serum lipids were reported in millimoles per liter.
Assessment of Ocular Measurements
Refractive error was measured once in each eye using an autorefractor (Humphrey model 500; Humphrey Instruments), and spherical equivalent was calculated as sphere power plus half cylinder power. Intraocular pressure was measured with the ORA, and IOPcc and Goldmann applanation tonometry–correlated IOP were obtained as described previously. Three ORA readings were obtained per eye, and the best signal value for each eye was used (based on the best-quality pressure waveform as assessed by the ORA software). The mean IOPs of the right and left eyes of each participant were used for analyses. If data were available only for 1 eye, then the IOP of that eye was considered for the participant. Participants who had a history of glaucoma laser therapy or surgery were excluded, and we imputed pretreatment IOP for participants reporting topical glaucoma medication using the same method as described previously.
Covariables in UK Biobank and EPIC-Norfolk
Demographic characteristics in the analysis included age, sex, ethnicity (White or non-White), and Townsend deprivation index. Health and lifestyle factors included BMI, smoking status (never smoked vs. ever smoked), alcohol status (nondrinker vs. current drinker), diabetes status (yes vs. no), and statin and oral β-blocker use (nonuser vs. user). These covariables were decided a priori on the basis of previously published studies demonstrating potential associations with IOP, IOP-related corneal biomechanical properties, and serum lipid levels. For example, age,24,25 sex,26,27 and systolic blood pressure26,28 have been associated with lipid levels and IOP. We additionally included height, smoking status, and diabetes status, which may be associated with both lipid levels and corneal biomechanical properties that influence IOP measurements,4,18,26,29, 30, 31as well as BMI, given its potential upstream association with serum lipid levels32,33 and consistent association with IOP.26
Statistical Analysis
The baseline characteristics of UK Biobank and EPIC-Norfolk participants were determined and are presented as mean ± standard deviation for continuous variables and number (percentage) for categorical variables. Pearson correlation coefficients were calculated for associations between each of the individual serum lipids (Table S1, available at www.aaojournal.org), and because of high correlation, linear regression was used to examine the associations of each serum lipid and lipoprotein individually with IOPcc. Primary analyses were carried out for each individual serum lipid as continuous variables and were categorized into quartiles within each cohort. All associations were examined using univariate- and multivariate-adjusted models. Because the range and standard deviation of values for each serum lipid measurement differ, standardized β coefficients were calculated and reported for associations between TC, HDL-C, LDL-C, triglycerides, and IOPcc.
Multivariate models were adjusted for age, sex, ethnicity, Townsend deprivation index, BMI, systolic blood pressure, height, smoking, alcohol use, diabetes status, statin use, oral β-blocker use, and spherical equivalent. These data were plotted using the multivariate regression and marginsplot commands using the STATA software package version 16 (StataCorp LP). We conducted various secondary analyses: (1) sex stratification and interaction for each of the aforementioned analyses, (2) additional analyses of the association of IOPcc with apolipoprotein A and B and with lipid ratios commonly used in clinical practice (TC to HDL-C, LDL to HDL-C, and TG to HDL-C),6 (3) sensitivity analyses adjusting for multiple lipid components in a single model, (4) sensitivity analyses excluding statin users, (5) sensitivity analyses excluding BMI as a covariable in multivariate regression models (to determine if upstream factors were driving potential associations), (6) sensitivity analyses restricted to participants reporting White ethnicity, and (7) sensitivity analyses additionally adjusting for age squared (to model potential nonlinear associations more accurately).
Results
A total of 94 323 participants in the UK Biobank were included in this analysis after excluding 29 766 participants with missing data and 4091 participants who had undergone glaucoma laser therapy or surgery. A total of 6230 participants from the EPIC-Norfolk cohort were included in this analysis after excluding 1028 with missing data and 62 who had undergone glaucoma laser therapy or surgery (Fig 1). Table 1 presents the baseline characteristics of both EPIC-Norfolk and UK Biobank participants included in the study. Compared with UK Biobank participants, EPIC-Norfolk participants were on average 12 years older, more likely to be White, women, shorter, and live in a less deprived area, and less likely ever to have smoked or currently to consume alcohol. They were more likely to use statin medication or oral β-blockers and had modest but significantly lower BMI, systolic blood pressure, and TC, LDL-C, and triglyceride levels. The mean IOPcc was 16.1 ± 3.3 mmHg and 17.1 ± 3.9 mmHg in the UK Biobank and EPIC-Norfolk cohorts, respectively.
Figure 1.
Flowchart showing participants included in the UK Biobank and European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohorts. BMI = body mass index; IOP = intraocular pressure; SBP = systolic blood pressure.
Table 1.
Baseline Characteristics of Participants Included in the Study
| UK Biobank | European Prospective Investigation into Cancer and Nutrition-Norfolk | P Value | |
|---|---|---|---|
| Sample size | 94 323 | 6230 | |
| Age (yrs) | 56.8 ± 8.0 | 68.6 ± 7.8 | <0.001 |
| Sex | <0.001 | ||
| Men | 44 286 (46.9) | 2739 (44.0) | |
| Women | 50 037 (53.1) | 3491 (56.0) | |
| Ethnicity | <0.001 | ||
| White | 85 947 (91.1) | 6193 (99.4) | |
| Non-White | 8376 (8.9) | 37 (0.59) | |
| Townsend deprivation index | –1.1 ± 3.0 | –2.29 ± 2.1 | <0.001 |
| Body mass index (kg/m2) | 27.3 ± 4.3 | 26.7 ± 4.2 | <0.001 |
| Height (cm) | 168.8 ± 9.2 | 166.3 ± 9.1 | <0.001 |
| Smoking status | <0.001 | ||
| Never smoked | 52 673 (55.8) | 3120 (50.1) | |
| Ever smoked | 41 650 (44.2) | 3110 (49.9) | |
| Alcohol status | <0.001 | ||
| Nondrinker | 7736 (8.2) | 880 (14.1) | |
| Drinker | 86 587 (91.8) | 5350 (85.9) | |
| Diabetes status | 0.76 | ||
| No | 89 650 (95.0) | 5943 (95.4) | |
| Yes | 4673 (5.0) | 296 (4.6) | |
| Systolic blood pressure (mmHg) | 137.3 ± 17.6 | 135.9 ± 16.2 | <0.001 |
| Total cholesterol (mmol/L) | 5.7 ± 1.1 | 5.4 ± 1.1 | <0.001 |
| Men | 5.4 ± 1.1 | 5.1 ± 1.1 | |
| Women | 5.9 ± 1.1 | 5.7 ± 1.1 | |
| HDL-C (mmol/L) | 1.5 ± 0.4 | 1.5 ± 0.4 | 0.99 |
| Men | 1.3 ± 0.3 | 1.3 ± 0.3 | |
| Women | 1.6 ± 0.4 | 1.7 ± 0.4 | |
| LDL-C (mmol/L) | 3.5 ± 0.8 | 3.2 ± 1.0 | <0.001 |
| Men | 3.5 ± 0.8 | 2.9 ± 1.0 | |
| Women | 3.6 ± 0.8 | 3.3 ± 1.0 | |
| Triglycerides (mmol/L) | 1.7 ± 0.9 | 1.6 ± 0.8 | <0.001 |
| Men | 1.9 ± 1.0 | 1.7 ± 0.8 | |
| Women | 1.5 ± 0.8 | 1.5 ± 0.7 | |
| Statin use | <0.001 | ||
| Nonuser | 76 393 (81.0) | 4867 (78.1) | |
| User | 17 930 (19.0) | 1363 (21.9) | |
| Oral β-blocker use | <0.001 | ||
| Nonuser | 88 977 (94.3) | 5506 (88.4) | |
| User | 5346 (5.7) | 724 (11.6) | |
| IOPcc (mmHg) | 16.1 ± 3.3 | 17.1 ± 3.9 | <0.001 |
| IOPg (mmHg) | 15.9 ± 3.4 | 16.3 ± 3.9 | <0.001 |
| Spherical equivalent (D) | –0.33 ± 2.7 | 0.18 ± 2.2 | <0.001 |
D = diopter; HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; IOPcc = corneal-compensated intraocular pressure; IOPg = Goldmann applanation tonometry–correlated intraocular pressure.
Data are presented as no., no. (%), or mean ± standard deviation.
Univariate Associations
Univariate analyses found that higher levels of TC and LDL-C were associated with higher IOPcc in both the UK Biobank and EPIC-Norfolk cohorts and that higher triglyceride levels were associated with higher IOPcc only in the UK Biobank cohort (Table S2, available at www.aaojournal.org). High-density lipoprotein cholesterol was not associated with IOPcc in univariate analyses in either cohort, but the model became significantly positively associated after the addition of sex and age into multivariate models in both cohorts. As such, sex-stratified analyses were performed subsequently (Table S3, available at www.aaojournal.org) that demonstrated that the associations between IOPcc and serum lipids remained significant and that no change in the direction of the association had occurred. Significant interactions between sex and each lipid component were identified (P = 0.003, P < 0.001, P = 0.049, and P = 0.003 for interaction for each of TC, HDL-C, LDL-C, and triglycerides, respectively). A stronger association was found in men, particularly for the association with HDL-C.
Multivariate Associations
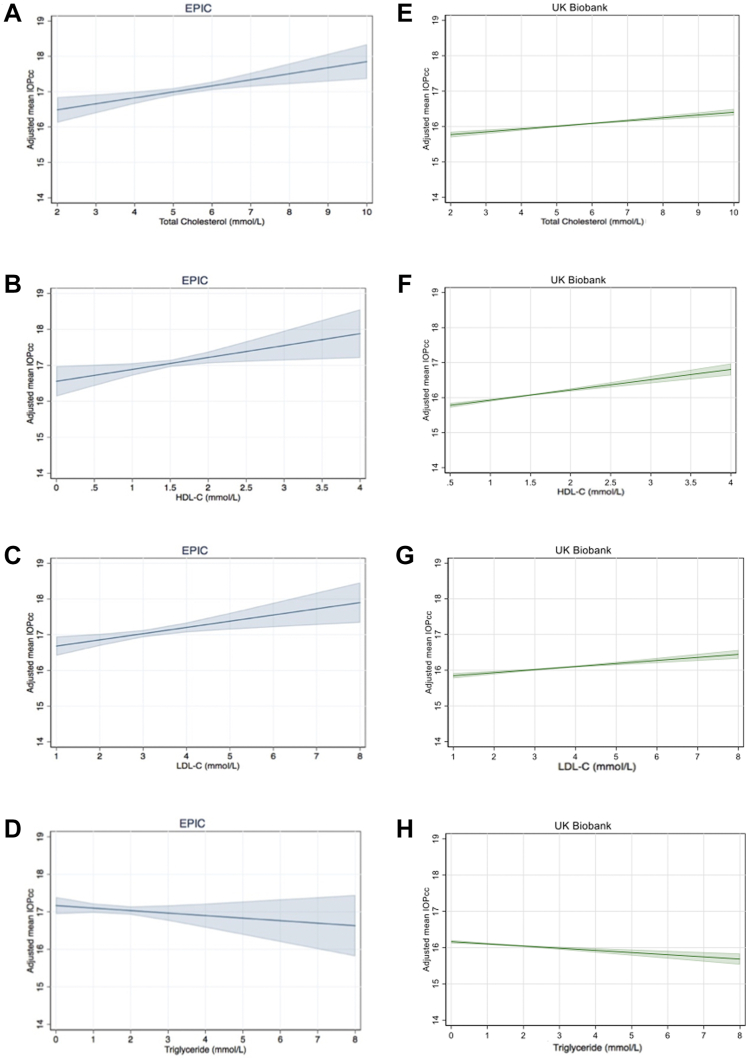
After adjusting for confounders including demographic, lifestyle, and cardiovascular risk factors and common cardiovascular medications, higher levels of TC, HDL-C, and LDL-C were associated with higher IOPcc in both cohorts, whereas higher triglyceride levels were associated with lower IOPcc in the UK Biobank only (Table 2). These associations are presented in Figure 2, which depict the IOP based on the lipid level and covariables from the multivariate regression analysis.
Table 2.
Multivariate Associations between Serum Lipid Levels and Corneal-Compensated Intraocular Pressure in the UK Biobank and European Prospective Investigation into Cancer and Nutrition-Norfolk Cohorts
| Serum Lipid Levels (mmol/L) | UK Biobank |
European Prospective Investigation into Cancer and Nutrition-Norfolk |
||||
|---|---|---|---|---|---|---|
| β Coefficient | 95% Confidence Interval | P Value | β Coefficient | 95% Confidence Interval | P Value | |
| Total cholesterol per 1-SD increase | 0.09 | 0.06–0.11 | <0.001 | 0.19 | 0.07–0.31 | 0.001 |
| Total cholesterol in quartiles | ||||||
| 1 | Reference | Reference | ||||
| 2 | 0.15 | 0.09–0.21 | <0.001 | 0.22 | –0.07 to 0.50 | 0.14 |
| 3 | 0.19 | 0.13–0.25 | <0.001 | 0.16 | –0.15 to 0.47 | 0.32 |
| 4 | 0.23 | 0.16–0.29 | <0.001 | 0.43 | 0.11–0.75 | 0.008 |
| P value for trend | <0.001 | 0.017 | ||||
| HDL-C per 1-SD increase | 0.11 | 0.08–0.13 | <0.001 | 0.14 | 0.03–0.25 | 0.016 |
| HDL-C in quartiles | ||||||
| 1 | Ref | Ref | ||||
| 2 | 0.13 | 0.07–0.19 | <0.001 | 0.12 | –0.15 to 0.39 | 0.39 |
| 3 | 0.21 | 0.15–0.27 | <0.001 | 0.28 | –0.01 to 0.57 | 0.06 |
| 4 | 0.30 | 0.23–0.37 | <0.001 | 0.27 | –0.04 to 0.58 | 0.09 |
| P for trend | <0.001 | 0.06 | ||||
| LDL-C per 1-SD increase | 0.07 | 0.05–0.09 | <0.001 | 0.17 | 0.06–0.29 | 0.003 |
| LDL-C in quartiles | ||||||
| 1 | Ref | Ref | ||||
| 2 | 0.08 | 0.02–0.14 | 0.009 | 0.09 | –0.19 to 0.37 | 0.53 |
| 3 | 0.11 | 0.05–0.18 | <0.001 | 0.13 | –0.17 to 0.43 | 0.40 |
| 4 | 0.18 | 0.12–0.24 | <0.001 | 0.48 | 0.15–0.81 | 0.004 |
| P value for trend | <0.001 | 0.007 | ||||
| Triglycerides per 1-SD increase | –0.05 | –0.08 to –0.03 | <0.001 | –0.05 | –0.15 to 0.05 | 0.30 |
| Triglycerides in quartiles | ||||||
| 1 | Ref | Ref | ||||
| 2 | 0.01 | –0.05 to 0.07 | 0.69 | 0.04 | –0.23 to 0.31 | 0.78 |
| 3 | –0.04 | –0.09 to 0.02 | 0.22 | 0.02 | –0.25 to 0.28 | 0.90 |
| 4 | –0.12 | –0.18 to –0.06 | <0.001 | –0.10 | –0.38 to 0.18 | 0.48 |
| P value for trend | <0.001 | 0.49 | ||||
| Apolipoproteins per 1-SD increase (g/dl) | ||||||
| A | 0.11 | 0.09–0.13 | <0.001 | — | — | — |
| B | 0.08 | 0.05–0.10 | <0.001 | — | — | — |
| Cholesterol components in same model per 1-SD increase | ||||||
| HDL-C∗ | 0.10 | 0.08–0.13 | <0.001 | 0.13 | 0.02–0.24 | 0.019 |
| LDL-C† | 0.07 | 0.05–0.09 | <0.001 | 0.17 | 0.05–0.28 | 0.004 |
| Serum lipids in same model | ||||||
| TC‡ | 0.11 | 0.08–0.13 | <0.001 | 0.22 | 0.10–0.34 | <0.001 |
| Triglycerides§ | –0.09 | –0.11 to –0.07 | <0.001 | –0.09 | –0.20 to 0.01 | 0.065 |
| Clinical serum lipid ratios | ||||||
| TC to HDL-C | –0.03 | –0.05 to –0.01 | 0.005 | –0.01 | –0.11 to 0.09 | 0.861 |
| LDL-C/HDL-C | –0.02 | –0.05 to –0.00 | 0.046 | 0.03 | –0.07 to 0.14 | 0.541 |
| Triglycerides to HDL-C | –0.08 | –0.10 to –0.06 | <0.001 | –0.10 | –0.20 to 0.01 | 0.055 |
HDL-C = high-density lipoprotein cholesterol; LDL-C = low-density lipoprotein cholesterol; SD = standard deviation; TC = total cholesterol.
All analyses are adjusted for age, sex, ethnicity, Townsend deprivation index, body mass index, systolic blood pressure, height, smoking, alcohol status, diabetes status, statin use, oral β-blocker use, and spherical equivalent. Quartiles in UK Biobank are defined as follows: (1) total cholesterol: quartile 1, ≤ 4.88; quartile 2, 4.89–5.62; quartile 3, 5.63–6.39; and quartile 4, ≥ 6.40; (2) HDL-C, quartile 1, ≤ 1.18; quartile 2, 1.19–1.41; quartile 3, 1.42–1.69; and quartile 4, ≥ 1.70; (3) LDL-C: quartile 1, ≤ 2.92; quartile 2, 2.93–3.48; quartile 3, 3.49–4.08; and quartile 4, ≥ 4.09; and (4) triglycerides: quartile 1, ≤ 1.02; quartile 2, 1.03–1.44; quartile 3, 1.45–2.07; and quartile 4, ≥ 2.08. Quartiles in EPIC-Norfolk are defined as follows: (1) TC: quartile 1, ≤ 4.67; quartile 2, 4.68–5.49; quartile 3, 5.50–6.19; and quartile 4, ≥ 6.20–10.20; (2) HDL-C: quartile 1, ≤ 1.21; quartile 2, 1.22–1.46; quartile 3, 1.47–1.77; and quartile 4, ≥ 1.78; (3) LDL-C: quartile 1, ≤ 2.46; quartile 2, 2.47–3.14; quartile 3, 3.15–3.83; and quartile 4, ≥ 3.84; and (4) triglycerides: quartile 1, ≤ 1.19; quartile 2, 1.20–1.57; quartile 3, 1.58–2.19; and quartile 4, ≥ 2.20.
Additionally adjusted for LDL-C.
Additionally adjusted for HDL-C.
Additionally adjusted for triglycerides.
Additionally adjusted for TC.
Figure 2.
A–H, Graphs showing multivariate linear regression of (A, E) total cholesterol, (B, F) high-density lipoprotein cholesterol (HDL-C), (C, G) low-density lipoprotein cholesterol (LDL-C), and (D, H) triglyceride levels with corneal-compensated (IOPcc) in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk and UK Biobank cohorts adjusted for age, sex, ethnicity, Townsend deprivation index, body mass index, height, systolic blood pressure, smoking, alcohol and diabetes status, statin use, oral β-blocker use, and spherical equivalent. These subfigures demonstrate the intraocular pressure based on the lipid level and covariables from the multivariate regression.
Similar multivariate associations were identified with Goldmann applanation tonometry–correlated IOP, although the association with triglyceride levels was not significant in either cohort (Table S4, Fig S1, available at www.aaojournal.org). In the UK Biobank, IOPcc was higher by 0.09 mmHg (95% confidence interval [CI], 0.06–0.11 mmHg; P < 0.001) for each 1-standard deviation increase in TC and was 0.11 mmHg (95% CI, 0.08–0.13 mmHg; P < 0.001) and 0.07 mmHg (95% CI, 0.05–0.09 mmHg; P < 0.001) higher for each 1-standard deviation increase in HDL-C and LDL-C, respectively. Intraocular pressure was 0.05 mmHg lower (95% CI, –0.08 to –0.03 mmHg; P < 0.001) for each additional 1-standard deviation increase in triglyceride levels in the UK Biobank. Results were of similar direction in the EPIC cohort but stronger; however, no significant association was identified with triglyceride levels (β = –0.05 mmHg; 95% CI, –0.15 to 0.05 mmHg; P = 0.30; Table 2). A positive trend across individual cohort-specific quartiles was identified for IOPcc with each of TC, HDL-C, and LDL-C in both cohorts (all P < 0.05, except for HDL-C in EPIC-Norfolk). In the UK Biobank, individuals in the highest quartile of TC, HDL-C, and LDL-C levels showed higher IOPcc (β = 0.23 mmHg, β = 0.30 mmHg, and β = 0.18 mmHg, respectively; P < 0.001 for all) than those in the lowest quartile of each individual serum lipid level. In the EPIC-Norfolk cohort, individuals in the highest quartile of TC and LDL-C showed higher IOPcc (β = 0.43 mmHg and β = 0.48 mmHg, respectively; P < 0.001 for all). The UK Biobank participants in the highest quartile of triglyceride levels showed lower IOPcc (β = –0.12 mmHg; P < 0.001) than those in the lowest quartile, and a significant linear trend was found overall (P < 0.001), but the association was not independently significant with quartiles 2 and 3, and these associations were not replicated in analyses of the EPIC-Norfolk cohort. The results of the quartile analyses for TC, HDL-C, LDL-C, and triglyceride levels (for UK Biobank only) indicated that the associations were consistent with linear relationships. The association with IOP was found to be linear across a spectrum of lipid levels (including both clinically normal and abnormal levels), and thus, higher HDL-C, LDL-C, and TC levels (even within the normal range) are associated with higher IOP.
Additional analyses of the UK Biobank cohort demonstrated that apolipoprotein A and B were associated positively with IOPcc; for each 1-standard deviation increase in apolipoprotein A, IOPcc was higher by 0.11 mmHg (95% CI, 0.09–0.13 mmHg; P < 0.001), and for each 1-standard deviation increase in apolipoprotein B, IOPcc was higher by 0.08 mmHg (95% CI, 0.05–0.10 mmHg; P < 0.001). Furthermore, associations between IOPcc and clinically relevant lipid ratios each were significant (Table 2): modestly lower IOPcc was identified for higher TC to HDL, LDL to HDL, and triglyceride to HDL-C ratios in the UK Biobank.
Sensitivity Analyses
Sensitivity analyses of the association of HDL-C with IOPcc, additionally adjusted for LDL-C, showed HDL-C to remain associated positively with IOPcc, although the association was very modestly attenuated (0.10 mmHg [95% CI, 0.08–0.13 mmHg; P < 0.001] in the UK Biobank; 0.13 mmHg [95% CI, 0.02–0.24 mmHg; P = 0.019] in EPIC-Norfolk; Table 2). Similarly, LDL-C was still associated with higher IOPcc in both cohorts in a multivariate model additionally adjusting for HDL-C (0.07 mmHg [95% CI, 0.05–0.09 mmHg; P < 0.001] in the UK Biobank; 0.17 mmHg [95% CI, 0.05–0.28 mmHg; P = 0.004] in EPIC-Norfolk; Table 2). Similar sensitivity analyses were performed assessing the association with TC and triglyceride levels (Table 2), demonstrating that TC remained positively associated with IOPcc in a multivariate model additionally adjusting for triglyceride levels (0.11 mmHg [95% 0.08–0.13 mmHg; P < 0.001] in the UK Biobank; 0.22 mmHg [95% CI, 0.10–0.34 mmHg; P < 0.001] in EPIC-Norfolk). Triglyceride levels remained associated with lower IOPcc in a model further adjusting for TC in the UK Biobank only (–0.09 mmHg [95% CI, –0.11 to –0.07 mmHg; P < 0.001]) and remained nonsignificant in EPIC-Norfolk. The magnitude and direction of all associations largely were unchanged in sensitivity analyses examining the association in statin users and nonstatin users separately (Table S5, available at www.aaojournal.org), and no interaction between statin use and IOP was identified. Our interpretation of the findings remained unchanged when we conducted sensitivity analyses including and excluding BMI as a covariable, when hypertension status (i.e., systolic blood pressure > 140 mmHg) was used in place of continuous measures of systolic blood pressure, when we restricted analyses to participants of self-reported White ethnicity, and when age squared was added to the multivariate models.
Discussion
After adjusting for key demographic, medical, and lifestyle covariables, higher levels of TC, HDL-C, and LDL-C were associated with higher IOPcc in both the UK Biobank and EPIC-Norfolk cohorts, and higher triglyceride levels were associated with lower IOPcc in the UK Biobank cohort only. When additional analyses of the UK Biobank were performed, apolipoprotein A and B were associated with higher IOPcc as well.
Significant gaps exist in the current literature on the association between individual lipid levels and IOP. Most existing studies are relatively small, and most were conducted in Asian populations, which complicates generalizability to other populations. Many studies did not comprehensively address important confounders of the association between lipid levels and IOP, including BMI,8,11 diabetes status,9 or relevant medication use such as statins,8,9,12 which may have associations with IOP.33,34 Substantial variability exists in the manner in which the exposures of interest were defined or ascertained in earlier studies, for example, using composite lipid measurements, such as hyperlipidemia, rather than examining individual lipid concentrations independently5 or evaluating lipids in the context of metabolic syndrome.8,10,35 Furthermore, no existing studies specifically have examined the association with IOPcc. By evaluating the association between serum lipid levels and IOPcc, our study reduced potential confounding by lipid associations with corneal biomechanical properties. For example, evidence suggests that patients with diabetes (a condition known to be associated with hyperlipidemia) have higher mechanical stiffness of the cornea, potentially associated with artifactually elevated IOP.18,31
Previous studies have reported positive associations between TC or LDL-C and IOP, and our study lends further support to these findings.8,9,12,35 Our study found a consistent positive association between HDL-C and IOP, clarifying inconsistent associations reported previously.8,9 Our findings showed opposing results to the previously reported positive associations with triglyceride levels8, 9, 10 and further support the previously demonstrated positive associations with apolipoprotein A and B.9
Cholesterol components and serum lipid levels are closely related physiologically, which poses challenges for determining meaningful associations with any one particular component. Notably, the associations with IOPcc were grossly unchanged or were attenuated only very modestly (and remained significant) when we conducted sensitivity analyses fitting cholesterol components (TC and triglyceride levels) and serum lipid fractions (HDL-C and LDL-C) into the same multivariate models.
Because lipid levels are known to differ by sex, sex-stratified analyses were performed (limited to the UK Biobank cohort because the sample size was large enough to afford sufficient statistical power in the subgroups), and these analyses demonstrated no change in direction or significance of the individual multivariate-adjusted associations. A significant interaction between sex and each lipid component was identified, and a stronger association was found in men, particularly for the association with HDL-C. Additional sensitivity analyses restricting analyses to White participants did not result in any significant changes, and no significant changes were found when we conducted the analyses excluding BMI from the model, further supporting the primary identified associations. In each of these analyses, actual lipid levels were used (regardless of statin use status) rather than imputed physiologic levels. This was carried out to examine whether the actual value of the prevalent lipid level in question (even if treated) in the study cohort was associated with IOP.
Genetic studies of IOP may offer a different perspective for understanding these complex relationships. Genetic associations have been identified between single nucleotide polymorphisms associated with IOP and single nucleotide polymorphisms associated with lipid metabolism. A recent meta-analysis of IOP genome-wide association studies identified a known IOP locus (rs9853115) to be located near the gene for diacylglycerol kinase gamma (DGKG),20 an enzyme that may contribute to both IOP regulation36 and lipid metabolism.37 Another locus known to be associated with IOP (rs247249) is located near the ATP binding cassette transporter A1 (ABCA1) gene, which codes for a transmembrane transporter that mediates cholesterol efflux to nascent high-density lipoprotein molecules.38 ABCA1 is expressed widely in ocular tissues, including trabecular meshwork and Schlemm’s canal,38 and an animal model study found that ABCA1 is upregulated in induced ocular hypertension.39 Furthermore, a single nucleotide polymorphism near ABCA1 (rs2487032) has been associated specifically with the high-tension primary open-angle glaucoma (POAG) endophenotype (defined as POAG with IOP > 22 mmHg).40 Associations with IOP also have been found with polymorphisms at the CAV1/2 gene loci (rs10281637) encoding caveolin 1 and caveolin 2, which may play a role in lipid metabolism.23 Caveolin 1 is a component of caveolae, which are involved in diverse cellular functions including transport, signal transduction, and the regulation of cholesterol metabolism.41 Interestingly, ABCA1 has been found to modulate caveolin 1 levels via pathways that also may play a role in IOP regulation.42
Although HDL-C and LDL-C do not share a common biochemical precursor and have functionally opposing roles along the atherogenesis pathway, similarities in their structural surface proteins (i.e., apolipoproteins) may help to explain both of their positive associations with IOP. Apolipoprotein B, for example, is expressed predominantly on LDL-C molecules and plays a key role in lipid transit. Previous studies have found that higher concentrations of apolipoprotein B are associated with higher IOP.9 Similarly, apolipoprotein A (the major protein component of HDL-C) also was associated previously with higher IOP in male patients.9 Our findings further support both of these associations with IOP, although further investigation is necessary to understand the underlying pathways driving these associations and to assess whether additional upstream factors may be at play.
The magnitude of these associations, although small, may have potentially meaningful clinical implications. For example, the effect of the association between IOP and the highest quartile of TC is 0.43 mmHg (95% CI, 0.11–0.75 mmHg; Table 2; Fig 2), which is similar to the effect size of the presence of TMC01 risk alleles on IOP.38,43 These are known to be strong genetic determinants of conversion to glaucoma.44 Furthermore, if the collective effects of individual lipid concentrations are operative, the effect of high lipid levels may exceed 1 mmHg (Fig 2), which may have important implications for conversion to glaucoma or progression of existing disease.45 Our results represent comparisons between participants with varying lipid levels rather than the difference in IOP that might be observed after a change in lipid level within an individual. It is likely that the magnitude of a within-individual effect would be more than the magnitude between individuals. A good example of this is the relatively small difference in IOP seen between systemic β-blocker users and nonusers (0.69 mmHg; 95% CI, 0.96–0.43 mmHg; P < 0.001)46 compared with the well-established profound IOP-lowering effect of systemic β-blockers within individuals,47 which prompted the development of topical β-blocker therapy for glaucoma. Although the predictive ability of these associations with IOP is relatively low (Tables S1 and S6, available at www.aaojournal.org), serum lipid levels are a readily available and routinely collected clinical measurement that may provide insight into potential pathophysiologic mechanisms for elevated IOP. Further research is warranted to identify whether these lipid components in fact help to risk-stratify patients in the general population and in clinical settings to identify individuals at high risk of glaucoma and of glaucomatous progression. Future studies may examine the association between lipids and risk of glaucoma, assessing for potentially causal associations using approaches such as Mendelian randomization.
Strengths of our study include its large sample size derived from 2 independent cohort studies, which provides substantial power and replicability to examine the association of various lipid fractions with IOP. In both cohorts, serum lipid level measurements were obtained using biochemistry assays in accordance with international standards for testing and calibration, and IOP was measured using the same method in both cohorts.14 Compared with previous studies, our study adjusted for a relatively comprehensive array of covariables, including demographic and lifestyle characteristics, medical history, medication use, and ocular factors.
Our study is limited in its use of cross-sectional analysis, preventing determination of potentially causal associations. Data collected using questionnaires are potentially subject to recall, social desirability, and misclassification biases. For example, participants may not recall accurately (or may underreport) the amount of alcohol they have consumed. Although our study is limited in its use of self-reported medications, self-reported statin and other lipid medication use specifically were validated in large epidemiologic studies with an accuracy of between 91% and 97%.48 One further limitation is that serum samples were obtained from nonfasting patients, which could be subject to recent meal ingestions. Serum samples from those who have not fasted generally would result in higher values than samples from those who have fasted, and this could mask real associations, although this likely would have reduced the statistical power of the analyses because of the reduced sample size, rather than affecting estimates of association. This potential bias additionally is offset by consistent findings across 2 independent cohorts. Furthermore, the identified associations could have been the result of unmeasured exposures that link serum lipids and IOP, and UK Biobank and EPIC-Norfolk participants may not necessarily be representative of the general population. Our study additionally is limited by missing data for 26% of the participants in the UK Biobank cohort and 15% of the participants in the EPIC-Norfolk cohorts for whom ophthalmic data were available. This is largely because of the exclusion of participants with missing data for variables included in the multivariate analyses. Excluded participants were more likely to be non-White and slightly shorter and were more likely to consume alcohol than included participants (Table S7, available at www.aaojournal.org). The small magnitude of such differences in characteristics between included and excluded participants likely is of low consequence because it is unlikely that the association between lipids and IOP would be different systematically between included and excluded participants, and the likely effect of excluding participants with missing data likely would bias associations toward the null because of reduced sample size.
In conclusion, data from 2 large, prospective United Kingdom cohorts suggest that higher concentrations of serum lipids (specifically TC, HDL-C, and LDL-C) are associated with higher IOP. Future research is required to assess whether this association may be causal in nature. The identification of an underlying causal association between lipids or cholesterol components and IOP would be clinically significant because lipid levels can be modified through diet, lifestyle, and medication. This potentially would allow for targeted dietary and lifestyle modification as a means of influencing IOP.
Manuscript no. OPHTHA-D-21-02462.
Footnotes
Supplemental material available atwww.aaojournal.org.
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The author(s) have made the following disclosure(s): L.R.P.: Consultant – Eyenovia, Twenty Twenty, Skye Biosciences
J.L.W.: Consultant – Allergan, Aerpio, Broadwing Bio, Editas, Maze, Regenxbio
Naveed Sattar: Consultant – Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Hanmi Pharmaceuticals, Merck Shark & Dohme, Novartis, Novo Nordisk, Pfizer, Sannofi, Roche Diagnostics
P.J.F.: Consultant – Alphasights, GLG, Google Health, Guidepoint, PwC, Santen
A.P.K.: Consultant – Aerie, Allergan, Google Health, Novartis, Reichert, Santen, Thea; Lecturer – AbbVie
Supported by UCL Overseas Research Scholarship (K.V.S.); Fight for Sight, London, United Kingdom (grant no.: 1956A [K.V.S.]); The Desmond Foundation (K.V.S.); the Wellcome Trust (grant no.: 220558/Z/20/Z [A.W.]); Alcon (P.J.F.); United Kingdom Research and Innovation Future Leaders Fellowship (A.P.K.); Moorfields Eye Charity (Springboard Award [R.N.L.] and Career Development Fellowship [A.P.K.]); the National Eye Institute, National Institutes of Health, Bethesda, Maryland (grant nos.: EY015473 [L.R.P.], EY032559 [L.R.P.], [J.L.W.]); Research to Prevent Blindness, Inc., New York, New York (Challenge Grant [L.R.P., J.L.W.]); The Glaucoma Foundation, New York, New York (L.R.P.); Astra Zeneca (N.S.); Boehringer Ingelheim (N.S.); Novartis (N.S.); Roche Diagnostics (N.S.); Association for Research in Vision and Ophthalmology Foundation (David Epstein Award [J.L.W.]); and UK Research and Innovation Future Leaders Fellowship (Medical Research Council grant no.: MR/T040912/1 [A.P.K.]). The authors acknowledge a proportion of their financial support from the United Kingdom Department of Health through an award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Biomedical Research Centre for Ophthalmology. This research used data from the UK Biobank Resource under data access request nos. 2112 and 36741. The UK Biobank Eye and Vision Consortium is supported by grants from Moorfields Eye Charity, The NIHR Biomedical Research Centre at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, the Alcon Research Institute, and the International Glaucoma Association (United Kingdom). The EPIC-Norfolk study was supported by the Medical Research Council, United Kingdom (grant nos.: SP2024/0201 and MR/N003284/1), and Cancer Research United Kingdom (grant nos.: G9502233 and C864/A8257). No funders had a direct role in the collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
HUMAN SUBJECTS: Human subjects were included in this study. The EPIC-Norfolk Eye Study was carried out following the principles of the Declaration of Helsinki and the Research Governance Framework for Health and Social Care and was approved by the Norfolk Local Research Ethics Committee (05/Q0101/191) and East Norfolk & Waveney NHS Research Governance Committee (2005EC07L). All participants gave written informed consent.
No animal subjects were included in this study.
Author Contributions:
Conception and design: Madjedi, Stuart, Chua, Luben, Warwick, Pasquale, Kang, Wiggs, Lentjes, Aschard, Sattar, Foster, Khawaja
Analysis and interpretation: Madjedi, Stuart, Chua, Luben, Warwick, Pasquale, Kang, Wiggs, Lentjes, Aschard, Sattar, Foster, Khawaja
Data collection: Madjedi, Stuart, Chua, Luben, Pasquale, Kang, Wiggs, Foster, Khawaja
Obtained funding: N/A; Study was performed as part of the authors' regular employment duties. No additional funding was provided.
Overall responsibility: Madjedi, Stuart, Chua, Luben, Warwick, Pasquale, Kang, Wiggs, Lentjes, Aschard, Sattar, Foster, Khawaja
Contributor Information
Kian M. Madjedi, Email: kian.madjedi1@ucalgary.ca.
Modifiable Risk Factors for Glaucoma Collaboration and the UK Biobank Eye and Vision Consortium:
Mark Chia, Ron Do, Alan Kastner, Jihye Kim, Giovanni Montesano, Denize Atan, Tariq Aslam, Sarah A. Barman, Jenny H. Barrett, Paul Bishop, Peter Blows, Catey Bunce, Roxana O. Carare, Usha Chakravarthy, Michelle Chan, Sharon Y.L. Chua, David P. Crabb, Philippa M. Cumberland, Alexander Day, Parul Desai, Bal Dhillon, Andrew D. Dick, Cathy Egan, Sarah Ennis, Paul Foster, Marcus Fruttiger, John E.J. Gallacher, David F. Garway-Heath, Jane Gibson, Dan Gore, Jeremy A. Guggenheim, Chris J. Hammond, Alison Hardcastle, Simon P. Harding, Ruth E. Hogg, Pirro Hysi, Pearse A. Keane, Sir Peng T. Khaw, Anthony P. Khawaja, Gerassimos Lascaratos, Andrew J. Lotery, Tom Macgillivray, Sarah Mackie, Keith Martin, Michelle McGaughey, Bernadette McGuinness, Gareth J. McKay, Martin McKibbin, Danny Mitry, Tony Moore, James E. Morgan, Zaynah A. Muthy, Eoin O’Sullivan, Chris G. Owen, Praveen Patel, Euan Paterson, Tunde Peto, Axel Petzold, Jugnoo S. Rahi, Alicja R. Rudnikca, Jay Self, Sobha Sivaprasad, David Steel, Irene Stratton, Nicholas Strouthidis, Cathie Sudlow, Dhanes Thomas, Emanuele Trucco, Adnan Tufail, Veronique Vitart, Stephen A. Vernon, Ananth C. Viswanathan, Cathy Williams, Katie Williams, Jayne V. Woodside, MaxM. Yates, Jennifer Yip, and Yalin Zheng
Supplementary Data
References
- 1.Stein J.D., Khawaja A.P., Weizer J.S. Glaucoma in adults—screening, diagnosis, and management: a review. JAMA. 2021;325(2):164–174. doi: 10.1001/jama.2020.21899. [DOI] [PubMed] [Google Scholar]
- 2.Klein B.E., Klein R. Intraocular pressure and cardiovascular risk variables. Arch Ophthalmol. 1981;99(5):837–839. doi: 10.1001/archopht.1981.03930010837009. [DOI] [PubMed] [Google Scholar]
- 3.Mori K., Ando F., Nomura H., et al. Relationship between intraocular pressure and obesity in Japan. Int J Epidemiol. 2000;29(4):661–666. doi: 10.1093/ije/29.4.661. [DOI] [PubMed] [Google Scholar]
- 4.Lee A., Rochtchina E., Wang J.J., et al. Does smoking affect intraocular pressure? Findings from the Blue Mountains Eye Study. J Glaucoma. 2003;12(3):209–212. doi: 10.1097/00061198-200306000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wang S., Bao X. Hyperlipidemia, blood lipid level, and the risk of glaucoma: a meta-analysis. Invest Ophthalmol Vis Sci. 2019;60(4):1028–1043. doi: 10.1167/iovs.18-25845. [DOI] [PubMed] [Google Scholar]
- 6.Arsenault B.J., Boekholdt S.M., Kastelein J.J.P. Lipid parameters for measuring risk of cardiovascular disease. Nat Rev Cardiol. 2011;8(4):197–206. doi: 10.1038/nrcardio.2010.223. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf P.S., Hawken S., Ôunpuu S., et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 8.Yokomichi H., Kashiwagi K., Kitamura K., et al. Evaluation of the associations between changes in intraocular pressure and metabolic syndrome parameters: a retrospective cohort study in Japan. BMJ Open. 2016;6(3) doi: 10.1136/bmjopen-2015-010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son J.H., Chung Y.K., Son J.S. Apolipoprotein B: novel indicator of elevated intraocular pressure. Eye. 2015;29(10):1315–1320. doi: 10.1038/eye.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C.P., Lin Y.S., Wu S.C., Ko Y.S. Age- and gender-specific association between intraocular pressure and metabolic variables in a Taiwanese population. Eur J Intern Med. 2012;23(1):76–82. doi: 10.1016/j.ejim.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Lee M.K., Cho S Il, Kim H., et al. Epidemiologic characteristics of intraocular pressure in the Korean and Mongolian populations: the healthy twin and the GENDISCAN study. Ophthalmology. 2012;119(3):450–457. doi: 10.1016/j.ophtha.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Kim M.J., Park K.H., Kim C.Y., et al. The distribution of intraocular pressure and associated systemic factors in a Korean population: the Korea National Health and Nutrition Examination Survey. Acta Ophthalmol. 2014;92(7):e507–e513. doi: 10.1111/aos.12327. [DOI] [PubMed] [Google Scholar]
- 13.Sudlow C., Gallacher J., Allen N., et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elliott P., Peakman T.C. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol. 2008;37(2):234–244. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 15.Fry D, Almond R, Moffat S, Gordon MSP. UK Biobank biomarker project companion document to accompany serum biomarker data. UK Biobank. Published 11/03/2019, Version 1.0. Available at: https://biobank.ndph.ox.ac.uk/showcase/showcase/docs/serum_biochemistry.pdf. Accessed online December 21, 2021.
- 16.Chua S.Y.L., Thomas D., Allen N., et al. Cohort profile: design and methods in the eye and vision consortium of UK Biobank. BMJ Open. 2019;9(2):25077. doi: 10.1136/bmjopen-2018-025077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cumberland P.M., Bao Y., Hysi P.G., et al. Frequency and distribution of refractive error in adult life: methodology and findings of the UK Biobank study. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan M.P.Y., Grossi C.M., Khawaja A.P., et al. Associations with intraocular pressure in a large cohort: results from the UK Biobank. Ophthalmology. 2016;123(4):771–782. doi: 10.1016/j.ophtha.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luce D. Methodology for corneal compensated IOP and corneal resistance factor for an Ocular Response Analyzer. Invest Ophthalmol Vis Sci. 2006;47:2266. [Google Scholar]
- 20.Khawaja A.P., Cooke Bailey J.N., Wareham N.J., et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat Genet. 2018;50(6):778–782. doi: 10.1038/s41588-018-0126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riboli E., Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(suppl 1):6–14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 22.Khawaja A.P., Chan M.P.Y., Hayat S., et al. The EPIC-Norfolk eye study: rationale, methods and a cross-sectional analysis of visual impairment in a population-based cohort. BMJ Open. 2013;3(3) doi: 10.1136/bmjopen-2013-002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 24.David R., Zangwill L., Stone D., Yassur Y. Epidemiology of intraocular pressure in a population screened for glaucoma. Br J Ophthalmol. 1987;71(10):766–771. doi: 10.1136/bjo.71.10.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paik J.K., Chae J.S., Kang R., et al. Effect of age on atherogenicity of LDL and inflammatory markers in healthy women. Nutr Metab Cardiovasc Dis. 2013;23(10):967–972. doi: 10.1016/j.numecd.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Khawaja A., Springelkamp H., Creuzot-Garcher C., et al. Associations with intraocular pressure across Europe: the European Eye Epidemiology (E3) Consortium. Eur J Epidemiol. 2016;31(11):1101–1111. doi: 10.1007/s10654-016-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs D.R., Mebane I.L., Bangdiwala S.I., et al. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the lipid research clinics prevalence study. Am J Epidemiol. 1990;131(1):32–47. doi: 10.1093/oxfordjournals.aje.a115483. [DOI] [PubMed] [Google Scholar]
- 28.Deedwania P.C., Pedersen T.R., DeMicco D.A., et al. Differing predictive relationships between baseline LDL-C, systolic blood pressure, and cardiovascular outcomes. Int J Cardiol. 2016;222:548–556. doi: 10.1016/j.ijcard.2016.07.201. [DOI] [PubMed] [Google Scholar]
- 29.Imamura H., Tanaka K., Hirae C., et al. Relationship of cigarette smoking to blood pressure and serum lipids and lipoproteins in men. Clin Exp Pharmacol Physiol. 1996;23(5):397–402. doi: 10.1111/j.1440-1681.1996.tb02748.x. [DOI] [PubMed] [Google Scholar]
- 30.Simonen P.P., Gylling H.K., Miettinen T.A. Diabetes contributes to cholesterol metabolism regardless of obesity. Diabetes Care. 2002;25(9):1511–1515. doi: 10.2337/diacare.25.9.1511. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., Xu G., Wang W., et al. Changes in corneal biomechanics in patients with diabetes mellitus: a systematic review and meta-analysis. Acta Diabetol. 2020;57(8):973–981. doi: 10.1007/s00592-020-01481-0. [DOI] [PubMed] [Google Scholar]
- 32.Gostynski M., Gutzwiller F., Kuulasmaa K., et al. Analysis of the relationship between total cholesterol, age, body mass index among males and females in the WHO MONICA Project. Int J Obes. 2004;28(8):1082–1090. doi: 10.1038/sj.ijo.0802714. [DOI] [PubMed] [Google Scholar]
- 33.Cohen E., Kramer M., Shochat T., et al. Relationship between body mass index and intraocular pressure in men and women: a population-based study. J Glaucoma. 2016;25(5):e509–e513. doi: 10.1097/IJG.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 34.Klein B.E.K., Klein R., Moss S.E. Intraocular pressure in diabetic persons. Ophthalmology. 1984;91(11):1356–1360. doi: 10.1016/s0161-6420(84)34142-2. [DOI] [PubMed] [Google Scholar]
- 35.Chang Y.C., Lin J.W., Wang L.C., et al. Association of intraocular pressure with the metabolic syndrome and novel cardiometabolic risk factors. Eye. 2010;24(6):1037–1043. doi: 10.1038/eye.2009.247. [DOI] [PubMed] [Google Scholar]
- 36.Choquet H., Thai K.K., Yin J., et al. A large multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. Nat Commun. 2017;8(1):1–9. doi: 10.1038/s41467-017-01913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiggs J.L., Pasquale L.R. Genetics of glaucoma. Hum Mol Genet. 2017;26(R1):R21–R27. doi: 10.1093/hmg/ddx184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hysi P.G., Cheng C.Y., Springelkamp H., et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat Genet. 2014;46(10):1126–1130. doi: 10.1038/ng.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L., Xu L., Chen W., et al. Reduced annexin A1 secretion by ABCA1 causes retinal inflammation and ganglion cell apoptosis in a murine glaucoma model. Front Cell Neurosci. 2018;12:347. doi: 10.3389/fncel.2018.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y., Lin Y., Vithana E.N., et al. Common variants near ABCA1 and in PMM2 are associated with primary open-angle glaucoma. Nat Genet. 2014;46(10):1115–1119. doi: 10.1038/ng.3078. [DOI] [PubMed] [Google Scholar]
- 41.Frank P.G., Cheung M.W.C., Pavlides S., et al. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am J Physiol Heart Circ Physiol. 2006;291(2):677–686. doi: 10.1152/ajpheart.01092.2005. [DOI] [PubMed] [Google Scholar]
- 42.Hu C., Niu L., Li L., et al. ABCA1 regulates IOP by modulating Cav1/eNOS/NO signaling pathway. Invest Ophthalmol Vis Sci. 2020;61(5):33. doi: 10.1167/iovs.61.5.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Koolwijk L.M.E., Ramdas W.D., Ikram M.K., et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012;8(5) doi: 10.1371/journal.pgen.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheetz T.E., Faga B., Ortega L., et al. Glaucoma risk alleles in the ocular hypertension treatment study. Ophthalmology. 2016;123(12):2527–2536. doi: 10.1016/j.ophtha.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leske M.C., Heijl A., Hussein M., et al. Factors for glaucoma progression and the effect of treatment: the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2003;121(1):48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 46.Khawaja A.P., Chan M.P.Y., Broadway D.C., et al. Systemic medication and intraocular pressure in a british population: the EPIC-Norfolk Eye Study. Ophthalmology. 2014;121(8):1501–1507. doi: 10.1016/j.ophtha.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips C.I., Howitt G., Rowlands D.J. Propranolol as ocular hypotensive agent. Br J Ophthalmol. 1967;51(4):222–226. doi: 10.1136/bjo.51.4.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhaskara S., Whitsel E.A., Ballantyne C.M., Folsom A.R. Validity of self-report of lipid medication use: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 2015;242(2):625–629. doi: 10.1016/j.atherosclerosis.2015.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.