Abstract
The importance of B cells in multiple sclerosis (MS) has been demonstrated through the advent of B-cell-depleting anti-CD20 antibody therapies. Ofatumumab is the first fully human anti-CD20 monoclonal antibody (mAb) developed and tested for subcutaneous (SC) self-administration at monthly doses of 20 mg, and has been approved in the US, UK, EU, and other regions and countries worldwide for the treatment of relapsing MS. The development goal of ofatumumab was to obtain a highly efficacious anti-CD20 therapy, with a safety and tolerability profile that allows for self-administration by MS patients at home and a positive benefit–risk balance for use in the broad relapsing MS population. This development goal was enabled by the unique binding site, higher affinity to B cells, and higher potency of ofatumumab compared to previous anti-CD20 mAbs; these properties of ofatumumab facilitate rapid B-cell depletion and maintenance with a low dose at a low injection volume (20 mg/0.4 ml). The high potency in turn enables the selective targeting of B cells that reside in the lymphatic system via subcutaneous (SC) administration. Through a comprehensive dose-finding program in two phase 2 studies (one intravenous and one SC) and model simulations, it was found that safety and tolerability can be further improved, and the risk of systemic injection-related reactions (IRRs) minimized, by avoiding doses ≥ 30 mg, and by reaching initial and rapid B-cell depletion via stepwise weekly administration of ofatumumab at Weeks 0, 1, and 2 (instead of a single high dose). Once near-complete B-cell depletion is reached, it can be maintained by monthly doses of 20 mg/0.4 ml. Indeed, in phase 3 trials (ASCLEPIOS I/II), rapid and sustained near-complete B-cell depletion (largely independent of body weight, race and other factors) was observed with this dosing regimen, which resulted in superior efficacy of ofatumumab versus teriflunomide on relapse rates, disability worsening, neuronal injury (serum neurofilament light chain), and imaging outcomes. Likely due to its fully human nature, ofatumumab has a low immunogenic risk profile—only 2 of 914 patients receiving ofatumumab in ASCLEPIOS I/II developed anti-drug antibodies—and this may also underlie the infrequent IRRs (20% with ofatumumab vs. 15% with the placebo injection in the teriflunomide arm) that were mostly (99.8%) mild to moderate in severity. The overall rates of infections and serious infections in patients treated with ofatumumab were similar to those in patients treated with teriflunomide (51.6% vs. 52.7% and 2.5% vs. 1.8%, respectively). The benefit–risk profile of ofatumumab was favorable compared to teriflunomide in the broad RMS population, and also in the predefined subgroups of both recently diagnosed and/or treatment-naïve patients, as well as previously disease-modifying therapy-treated patients. Interim data from the ongoing extension study (ALITHIOS) have shown that long-term treatment with ofatumumab up to 4 years is well-tolerated in RMS patients, with no new safety risks identified. In parallel to the phase 3 trials in which SC administration was carried out with a pre-filled syringe, an autoinjector pen for more convenient self-administration of the ofatumumab 20 mg dose was developed and is available for use in clinical practice.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-023-00518-0.
Keywords: ALITHIOS, Anti-CD20 monoclonal antibody, ASCLEPIOS I/II, Benefit-risk, Ofatumumab, Relapsing multiple sclerosis, Self-administration, Subcutaneous
Key Summary Points
| Ofatumumab is the first fully human anti-CD20 monoclonal antibody to be approved for the treatment of relapsing multiple sclerosis. |
| Rapid and sustained near-complete B-cell depletion can be achieved with ofatumumab treatment and maintained with monthly subcutaneous doses of 20 mg/0.4 ml. |
| The tolerability profile and at-home self-administration of ofatumumab enables patients to be compliant with and persistent on therapy over time. |
| The benefit–risk profile of ofatumumab supports its early initiation in the overall RMS population in order to maximize its long-term treatment benefits. |
Digital Features
This article is published with digital features, to facilitate understanding of the article. To view digital features for this article, go to 10.6084/m9.figshare.23508390.
The development of ofatumumab, a fully human anti-CD20 monoclonal antibody for practical use in relapsing multiple sclerosis treatment
Introduction
Multiple sclerosis (MS) is a chronic autoimmune and degenerative disorder affecting the central nervous system (CNS), and B and T cell interactions are thought to play a critical role in its pathogenesis [1]. The development of B-cell-depleting anti-CD20 monoclonal antibody (mAb) therapies has led to a better understanding of the role of B and T cells in this disease [2, 3], and, most importantly, has led to a new appreciation of the central role of B cells in MS pathophysiology [4, 5]. Preclinical studies have demonstrated that anti-CD20 mAbs reliably deplete B cells in the blood and cerebrospinal fluid, and less completely in lymphoid organs, with repletion of B cells in the majority of patients after treatment cessation [6]. Due to the potential maintenance of antibody production by plasma cells, which do not express CD20 and, hence, which are not targets for anti-CD20 mAbs, immunoglobulin levels can be maintained in patients on certain anti-CD20 therapies [7].
Rituximab is a murine–human chimeric anti-CD20 mAb, commonly used for off-label treatment of MS, and was the first anti-CD20 mAb to show efficacy on measures of inflammatory activity in a phase 2 clinical trial in patients with relapsing–remitting MS (RRMS) [2], as well as in observational settings [8]. This was followed by the development of ocrelizumab, a humanized anti-CD20 mAb [9, 10], which is approved for both relapsing multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS). Both molecules bind to the large extracellular loop of the CD20 receptor and are administered intravenously every 6 months at doses of 1000 mg for rituximab (in the clinical trial setting) and 600 mg for ocrelizumab [9, 11]. Ublituximab is a type I chimeric, intravenously administered high-dose anti-CD20 mAb, binding to a different epitope on the CD20 receptor compared to rituximab and ocrelizumab. Ublituximab recently completed phase 3 trials and has been approved at an initial dose of 150 mg, a second dose of 450 mg 2 weeks later, and subsequent doses of 450 mg every 24 weeks for the treatment of RMS patients [12]. Given that humanized and fully human mAbs have a reduced risk of inducing immune responses compared with chimeric mAbs [13], and humanized and chimeric mAbs consist of ~ 30% to ~ 40% mouse protein, the development of a fully human anti-CD20 mAb could potentially lead to increased tolerability with long-term use.
Ofatumumab is the first fully human anti-CD20 mAb approved for the treatment of RMS and it can be self-administered by the patient at home. Binding to a distinct CD20 epitope [14, 15], ofatumumab can achieve near complete B-cell depletion at a lower concentration than any of the aforementioned anti-CD20 mAbs due to its higher potency and affinity to B cells. Ofatumumab has been developed for subcutaneous (SC) administration at an initial dose of 20 mg (in 0.4 mL) at Weeks 0, 1 and 2 for a rapid and stepwise initial B-cell depletion; a maintenance regimen of subsequent 20 mg (in 0.4 mL) in monthly intervals starting from Week 4 was chosen to ensure a sustained near-complete depletion of B cells [14, 15]. The constant near-complete B-cell depletion seen with ofatumumab is believed to have advantages in terms of efficacy (disease control) and safety (less risk of severe systemic injection/infusion reactions).
Ofatumumab has been tested in the ASCLEPIOS I and II phase 3 trials (ASCLEPIOS I/II) in adult patients with RMS [16]. After the first month and appropriate training, patients had the option for self-administration at home, an option that was chosen by approximately 70% of the participants [16]. In support of this, and in parallel to ASCLEPIOS I/II, an autoinjector pen was developed and successfully tested in patients with RMS in the APLIOS study [17]. In ASCLEPIOS I/II, the SC route of administration was associated with high levels (98.8%) of compliance, and the autoinjector pen has offered patients a convenient method to easily administer treatment at home, which further facilitates treatment adherence [16, 17]. Other benefits associated the SC regimen include the elimination of the need for infusion center visits and the facilitation of a move towards the practical use of highly effective therapies early in the MS disease course, an approach shown to improve long-term outcomes in people with MS [18, 19].
In this article, we discuss the clinical development and properties of ofatumumab, and how they affect the efficacy, safety, tolerability, compliance, and ease of use of this subcutaneously administered anti-CD20 therapy for the treatment of patients with RMS. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Properties of Ofatumumab
Ofatumumab is primarily a complement-dependent cytotoxicity (CDC) activator, whose Fab domain binds to two noncontinuous regions on a unique conformational epitope of the CD20 receptor, a transmembrane phosphoprotein expressed on B lymphocytes, distinct from those of rituximab and ocrelizumab [14, 15, 20–22]. CDC is an immunological process in which target cells are killed by damaging their plasma membranes after triggering the complement cascade and forming a membrane attack complex (MAC), without the involvement of immune system cells [23]. Ofatumumab also mediates antibody-dependent cell-mediated cytotoxicity (ADCC) activity, but at lower levels than CDC [24]. ADCC is a lytic mechanism of cell-mediated immune defense through which Fc receptor-bearing effector cells of the immune system can actively recognize and eliminate an antibody-bound target cell [23].
Compared to other anti-CD20 antibodies [16], ofatumumab binds to the small exposed extracellular loop on CD20, which is closer to the cell membrane than other CD20 epitopes, and also shows signs of cell lysis induced by the MAC complex, all of which may account for its higher degree of CDC activity [25–27]. Ofatumumab, in comparison with ocrelizumab, was determined to induce greater complement-dependent B-cell lysis in vitro after exposure for 2 h and to maintain effectiveness in CDC induction when complement addition was delayed for 6 h [28]. The observed increased CDC potency of ofatumumab may account for its efficacy at a low dose (20 mg) compared with the higher doses required for rituximab, ocrelizumab, and ublituximab [24]. The unique binding regions of ofatumumab also yield a slower off-rate and greater binding affinity than other anti-CD20 mAbs, resulting in efficient B-cell lysis and consequent suppression of inflammatory activity [21, 22]. This slower off-rate, along with the monthly administration, is likely responsible for a more sustained B-cell depletion with ofatumumab compared to intravenous (IV) drugs that are administered at infrequent high doses with subsequent repletion in some patients between doses [29].
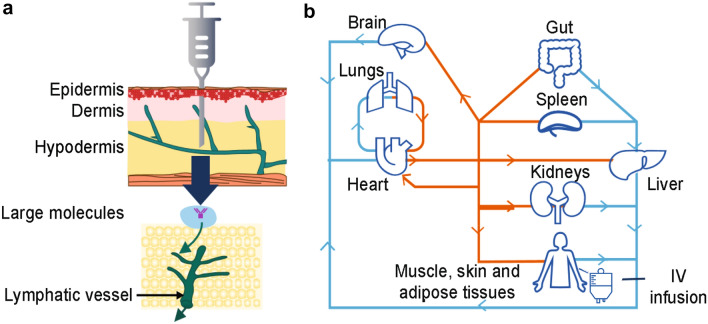
SC administration of ofatumumab may lead to more efficient and selective targeting of B cells residing in the lymphatic circulatory system compared with IV administration [30]; with SC administration, treatment is delivered into the hypodermis, while IV administered treatment directly enters the systemic circulation [31]. A summary of the potential mechanism by which SC versus IV administration achieves this selective targeting is outlined in Fig. 1.
Fig. 1.
Summary of anti-CD20 mAb access to lymphatic organs via SC and IV routes. Targeting of lymphatic circulatory system B cells by anti-CD20 mAbs via a SC administration, with direct access to lymphatic organs, and b IV administration, with access to lymphatic organs from the blood. IV intravenous, mAb monoclonal antibody, SC subcutaneous
In humanized-CD20 transgenic murine models, ofatumumab SC resulted in more direct access to lymph nodes than ofatumumab IV and ocrelizumab IV, and also demonstrated a 20-fold higher depleting potency on circulating B cells and equipotency on non-circulating B cells compared with ocrelizumab [30, 32, 33]. Additionally, single-photon emission computed tomography detected an increase in ofatumumab in the axillary and inguinal lymph nodes, where the B cells implicated in MS are located, of mice following SC versus IV administration [32]. Ofatumumab treatment also decreased the number of B and T cells in mice with focal delayed-type hypersensitivity lesions following SC and IV administration [32]. Furthermore, a separate study of 20-mg SC anti-mouse CD20 antibody treatment demonstrated that marginal zone progenitor cells, which are important for the rapid response to blood-borne pathogens and secretion of natural antibodies, were relatively spared with low-dose SC administration [6]. This was followed by significantly faster B-cell repletion in the lymph nodes, blood, and spleen of healthy mice compared with IV administration [6], speaking to the maintenance of IgG and IgM levels above the lower limit of normal (LLN) (5.7 g/L and 0.4 g/L, respectively) with the ofatumumab SC dose [7, 34].
The SC route of administration and at-home administration of ofatumumab have been tested in the well-controlled phase 3 ASCELPIOS I/II studies in a broad RMS population [16]. In this cohort, with a total of 1882 RMS patients, the SC dose yielded consistently low B-cell counts with mostly mild to moderate injection-related reactions (IRRs; 99.8%); after the first injection, the frequency and severity of IRRs was non-distinguishable from the IRRs with placebo injections (double-dummy design) in the teriflunomide control arm. One consequence of these phase 3 results is that the label for ofatumumab, to date unique among anti-CD20s approved for use in MS, specifically supports self-administration of monthly doses at home, and no premedication is required [14, 15]. This contrasts with intravenous MS DMTs, which typically require administration in infusion centers for ease of safety monitoring throughout treatment [8, 9, 35]. Rituximab, ocrelizumab, and ublituximab each pose a risk for infusion-related reactions, likely due to partial B-cell repletion between high but infrequent doses, and subsequent elimination of B cells after dosing, hence the need for pre-medications before each dose.
The favorable properties of ofatumumab do not come at a cost to efficacy, as shown by recent meta-analyses indicating that ofatumumab is superior to, or not statistically different from, all other disease-modifying therapies (DMTs) in MS, including high-efficacy mAbs, in terms of reducing relapse rate and disability worsening [36, 37]. Furthermore, an indirect comparative efficacy study showed a significant improvement in annualized relapse rate (ARR), and favorable results for no evidence of disease activity (NEDA)-3 and MRI outcomes for ofatumumab compared with ocrelizumab [38].
Clinical Development program of ofatumumab in MS and the 20 mg subcutaneous dosing regimen
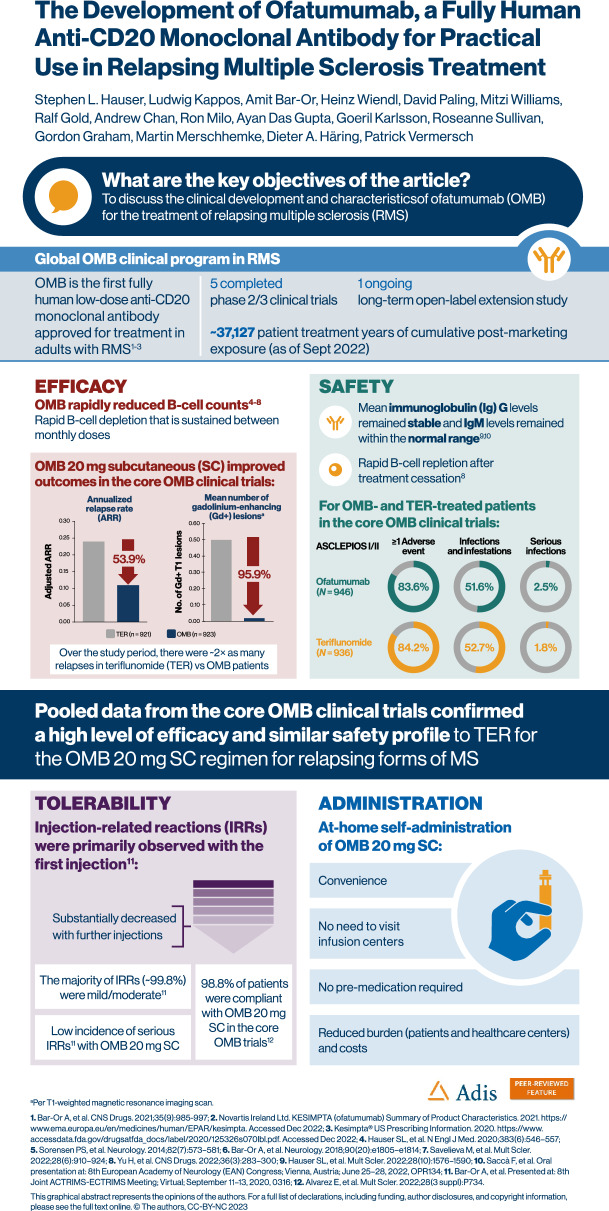
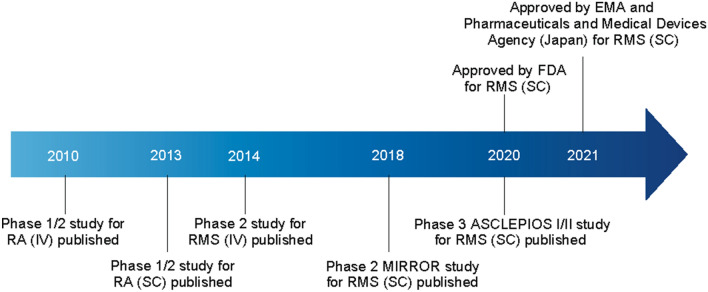
The global ofatumumab clinical program in RMS involved more than 3000 patients, and was the basis for the worldwide approval of ofatumumab 20-mg SC for the treatment of RMS, including in the US (in August 2020) and Europe (in March 2021) [14, 15]. A summary of the ofatumumab trials in MS is outlined in Table 1, while a timeline of the key publications and approvals of ofatumumab is presented in Fig. 2. As of 25 September 2022, the cumulative exposure in the overall safety pool was 6584.3 patient treatment years, while post-marketing exposure since the first launch of ofatumumab was ~ 37,127 patient treatment years.
Table 1.
Summary of studies in the ofatumumab clinical program in MS
| Clinical study | Clinical phase | Mode of administration; ofatumumab dosing; treatment duration | Number of participants | Primary outcome measure | Other outcome measures | Results |
|---|---|---|---|---|---|---|
| IV infusion study [39] | Phase 2 | IV; 2 infusions, 100 mg, 300 mg, or 700 mg 2 weeks apart or placebo (at Week 24, patients crossed over to opposite treatment arm); 48 weeks | 38 | Cumulative number of new Gd + lesions, new and/or enlarging T2 lesions, and T1 hypointense lesions measured on monthly MRI | Proportion of relapse-free patients; relapse rate and change in EDSS and MSFC scores from baseline to Week 24 and from Week 24 to Week 48; safety (AE assessments, MRI, and clinical laboratory tests) |
New brain MRI lesion activity was suppressed by > 99% in the first 24 weeks after ofatumumab administration across all doses, with statistically significant reductions (p < 0.001) favoring ofatumumab vs. placebo found in the number of new T1 Gd + lesions, total T1 Gd + lesions, and new and/or enlarging T2 lesions Ofatumumab did not provide significant benefit regarding T1 hypointense lesions Ofatumumab was associated with profound and selective reduction of B cells at each dose as measured by CD19 + expression No unexpected safety signals were identified for ofatumumab in the context of other indications, including rheumatoid arthritis |
| MIRROR [40] | Phase 2b | SC; 3, 30, or 60 mg q12w or 60 mg q4w or placebo; 24 weeks | 232 | Cumulative number of new Gd + lesions (per brain MRI) at Week 12 | Cumulative number of new Gd + lesions at Week 24; cumulative number and total volume of new and new plus persisting Gd + lesions; new and/or newly enlarging T2 lesions; T1-hypointense lesions at Weeks 12 and 24; safety |
The cumulative number of new lesions was reduced by 65% for all ofatumumab doses vs placebo (p < 0.001) Post-hoc analysis (excluding the Week 4 scan, consistent with the phase 2 approaches with other anti-CD20 products) revealed a clear dose–response and estimated a ≥ 90% lesion reduction vs. placebo (Week 12) for all cumulative ofatumumab doses ≥ 30 mg/12 weeks Dose-dependent CD19 B-cell depletion was observed SC ofatumumab demonstrated overall good tolerability and no new/unexpected safety findings No serious IRRs: IRRs were only seen at doses ≤ 30 mg/12 weeks |
| ASCLEPIOS I and II [16, 41] | Phase 3 | SC; 20 mg q4w after 20-mg loading doses at Days 1, 7, and 14 or teriflunomide; 30 months | 1882 | ARR | 3- or 6-month CDW; 6-month CDI; number of Gd + lesions per T1-weighted MRI scan; annualized rate of new or enlarging lesions on T2-weighted MRI scan; serum NfL chain levels at Month 3; change in brain volume; safety |
ARRs in the ofatumumab and teriflunomide groups were 0.1 and 0.2, respectively, in Trial 1 (difference, − 0.1; 95% CI, − 0.2 to − 0.1; p < 0.001) and 0.1 and 0.3 in Trial 2 (difference, − 0.2; 95% CI, − 0.2 to − 0.1; p < 0.001) 10.9% and 15.0% of patients on ofatumumab and teriflunomide, respectively, experienced 3-month CDW (HR, 0.7; p = 0.002) 8.1% and 12.0% of patients experienced 6-month CDW (HR, 0.7; p = 0.01) 11.0% and 8.1% of patients experienced 6-month CDI (HR, 1.4; p = 0.09) The mean number of Gd + lesions per T1-weighted MRI scan (1 lesion per 50 scans) was reduced by 97% and 94% with ofatumumab vs. teriflunomide (p < 0.001) in trial 1 and trial 2, respectively The annualized rate of lesions on T2-weighted MRI and serum NfL chain levels were reduced with ofatumumab vs. teriflunomide AEs that occurred in at least 10% of patients on ofatumumab were IRRs, nasopharyngitis, headache, injection-site reaction, upper respiratory tract infection, and urinary tract infection – AEs and AE rates were similar with ofatumumab and teriflunomide treatment; SAEs were reported in 9.1% of patients on ofatumumab and 7.9% of patients on teriflunomide In the ofatumumab group, two cases of basal-cell carcinoma, one case of malignant melanoma in situ, one case of recurrent non-Hodgkin’s lymphoma, and one case of invasive breast carcinoma were reported There was also one case of myocardial infarction in the ofatumumab group In the teriflunomide group, two cases of basal-cell carcinoma, one case of cervix carcinoma and one case of fibrosarcoma were reported Analysis of efficacy and safety of ofatumumab vs teriflunomide in the subgroup of RDTN patients (n = 615) from ASCLEPIOS I/II found ofatumumab to be an appropriate treatment option also in early patients |
| APLIOS (17) | Phase 2 | SC; 20 mg q4w after 20-mg loading doses at Days 1, 7, and 14; 12 weeks | 256 | Area under the plasma concentration–time curve over the dosing interval and Cmax after drug administration | Plasma concentration–time curve over the dosing interval and Cmax; proportion of patients with anti-ofatumumab Abs; MRI lesion activity; safety (AEs, including injection-related systemic reactions and laboratory abnormalities) |
Abdominal ofatumumab PK exposure was bioequivalent for autoinjector and PFS [geometric mean area under the plasma concentration–time curve over the dosing interval, 487.7 vs. 474.1 h × μg/mL (ratio 1.0); Cmax, 1.4 vs. 1.4 μg/mL (ratio 1.0)] B-cell counts (median cells/μL) depleted rapidly in all groups from 214.0 (baseline) to 2.0 (Day 14) The mean number of new/persisting Gd + T1 lesions decreased from 1.5 at baseline to 0.8, 0.3, and 0.1 by Weeks 4, 8, and 12, respectively; the proportion of patients free of Gd + T1 lesions increased over time in all ofatumumab groups Ofatumumab had favorable safety and tolerability profiles, in line with those reported in the ASCLEPIOS I/II trials |
| APOLITOS [42] | Phase 2 | SC; 20 mg q4w after 20-mg loading doses at Days 1, 7, and 14 or placebo; total duration 48 weeks (placebo-controlled initial 24 weeks) | 64 | Number of Gd + T1 lesions per scan over 24 weeks | Consistency of efficacy of ofatumumab on the cumulative number of Gd + T1 lesions on MRI scans at Weeks 12, 16, 20, and 24 across regions (Japan and Russia); the efficacy of ofatumumab vs. placebo on the annualized rate of new or enlarging T2 lesions; ARR; time to first relapse; safety |
Ofatumumab reduced Gd + T1 lesions vs. placebo by 93.6% (p < 0.001); results were consistent across regions Ofatumumab reduced annualized T2 lesion and relapse rate vs. placebo by Week 24 Both groups showed a benefit from ofatumumab in the APOLITOS extension-part (up to 48 weeks) Incidence of AEs was lower with ofatumumab vs. placebo (69.8% vs. 81.0%); IRRs were most common No deaths, opportunistic infections, or malignancies |
| ALITHIOS [43, 44] | Ongoing open-label extension of ASCLEPIOS I/II, APLIOS, APOLITOS | SC; 20 mg q4w for up to 5 years | 1367 | Number of patients that experience an AE or abnormal laboratory, vital and/or ECG results and positive suicidality outcomes | ARRs; confirmed 3- and 6- month disability worsening; confirmed 6-, 12- and 24-month disability improvement and improvement until end of study; safety |
86.2% (n = 1698) of patients experienced at least one AE in the ASCLEPIOS I/II + extension studies Overall rate of AEs and SAEs remained consistent with the rates observed during ASCLEPIOS I/II No new safety signals, compared to those reported in the ASCLEPIOS I and II trials, were identified In the between-group analysis, there was a 43.4% reduction in the number of confirmed relapses and near complete suppression of MRI lesions in the continuous ofatumumab group vs. the teriflunomide-ofatumumab switch group |
Ab antibody, AE adverse event, ARR annualized relapse rate, CDI confirmed disability improvement, CDW confirmed disability worsening, CI confidence interval, Cmax maximum plasma concentration, ECG electrocardiogram, EDSS Expanded Disability Status Scale, Gd+ gadolinium-enhancing, HR hazard ratio, IRR injection-related reaction, IV intravenous, MRI magnetic resonance ratio, MSFC Multiple Sclerosis Functional Composite, NfL neurofilament light, PFS pre-filled syringe, PK pharmacokinetic, q4w every 4 weeks, q12w every 12 weeks, RDTN recently diagnosed and treatment-naïve, SAE serious adverse event, SC subcutaneous
Fig. 2.
Timeline of ofatumumab key publications and approvals. EMA European Medicines Agency, FDA US Food and Drug Administration, IV intravenous, RA rheumatoid arthritis, RMS relapsing multiple sclerosis, SC subcutaneous
Prior to the demonstration of efficacy and safety in RMS, ofatumumab had been assessed in rheumatoid arthritis (RA). Indeed, support for the rationale of the eventual dosing regimen for ofatumumab in the RMS clinical trials was derived from the initial investigations carried out in patients with RA. In a phase 1/2 placebo-controlled study, ofatumumab IV at doses of 300, 700 and 1000 mg, at 2 infusions 2 weeks apart, were administered to patients with RA who did not respond to ≥ 1 disease-modifying antirheumatic therapy [45]. Infusions in doses up to 1000 mg were found to be clinically effective in these patients. Subsequently, an SC formulation of ofatumumab was tested in another placebo-controlled phase 1/2 study in rheumatoid arthritis patients who had taken methotrexate [46]. SC injections of up to 60 mg led to prolonged B-cell depletion in the blood and were well tolerated.
These studies ultimately led to the first clinical investigation of ofatumumab in MS, the first of which was a placebo-controlled phase 2 study, aiming to assess the efficacy and safety of IV doses in patients with RRMS [39]. Patients were randomized to receive ofatumumab (n = 26) or placebo (n = 12), with ofatumumab IV doses of 100 mg, 300 mg, or 700 mg (two doses at each dose strength, administered 2 weeks apart) over two 24-week treatment periods (Weeks 0–24 and Weeks 24–48), where patients received the alternate treatment at Week 24 in a crossover manner. For patients on ofatumumab, a > 99% reduction in gadolinium-enhancing T1 lesions was recorded, as well as profound B-cell depletion as measured by CD19 + expression, which was noted for all ofatumumab doses investigated. Infusions of up to 700 mg were not associated with unexpected or serious safety concerns, and were consistent with the known safety profile of ofatumumab based on previous indications, including rheumatoid arthritis [45]. The most common adverse event (AE), rash, occurred most frequently after the first infusion. These results indicated that, compared with placebo, ofatumumab decreased the number of new MRI lesions, and was well tolerated in RRMS patients [39].
Given the need for safety monitoring and on-site infusions with other anti-CD20 mAbs, the convenience and safety of self-administration at home was an important consideration for the development of ofatumumab in RMS. To examine the possibility that efficacy equivalent to an IV infusion could be attained with an SC regimen, ofatumumab SC was investigated in RRMS in the phase 2b placebo-controlled MIRROR study at 3-, 30-, or 60-mg doses every 12 weeks, or 60 mg every 4 weeks [40]. A dose-dependent reduction in B-cell counts was observed, and a post hoc analysis estimated a lesion reduction of ≥ 90% versus placebo at Week 12 for each ofatumumab dose ≥ 30 mg over 12 weeks. Furthermore, it was found that avoiding ≥ 30-mg doses for the initial injection significantly lowered the number of IRRs in RMS patients. Notably, IRRs were of mild to moderate severity in 97% of cases (n = 232) and were most commonly associated with the first dose (29–50%), reducing with subsequent dosing (1–18% at Week 12) [40].
Based on the results of the MIRROR trial, modeling studies were carried out to define a dose of ofatumumab that could reach and maintain a B-cell depletion target for efficacy based on related gadolinium-enhanced T1 lesion volumes and average B-cell counts. Once near complete B-cell depletion was achieved, it was estimated that B-cell depletion could be achieved and maintained based on monthly doses of 20 mg of ofatumumab. Repletion dynamics also supported the 20-mg dose. As discussed later in this article, a reduced repletion time has important implications for patients with RMS, especially regarding aspects such as vaccination, reproductive health, and any potential need for treatment switch.
Following the findings of the models, an ofatumumab regimen based on a 20-mg SC monthly dosing following an initial dose regimen of 20 mg at Weeks 0, 1, and 2 was selected for evaluation in phase 3. In line with the modeling studies, the phase 3 studies confirmed that the initial loading doses of ofatumumab achieved rapid target B-cell depletion, and the following maintenance regimen of 20-mg SC monthly was associated with a sustained B-cell depletion.
Efficacy and Safety of Ofatumumab SC in RMS
ASCLEPIOS I/II evaluated the efficacy and safety of SC ofatumumab versus oral teriflunomide in patients with RMS. Patients received either ofatumumab 20 mg SC every 4 weeks after initial 20 mg SC doses at Weeks 0, 1, and 2 (n = 946) or oral teriflunomide (14 mg daily; n = 936). Ofatumumab SC, compared with teriflunomide, was associated with significantly lower ARRs, and a reduced risk of 3- and 6-month confirmed disability worsening [risk reduction of 44% (p = 0.002) for 3-month confirmed disability worsening and 32% [p = 0.01] for 6-month confirmed disability worsening), close to complete abrogation of focal inflammation (1 gadolinium enhancing lesion per 50 scans) and a significant reduction in neuronal injury, reflected in a reduction in neurofilament light chain concentrations [16]. Pooled ARR and gadolinium-enhancing lesion data show rate reductions of 53.9% (p < 0.001) and 95.9% (p < 0.001), respectively, with ofatumumab versus teriflunomide treatment for ASCLEPIOS I/II (see Fig. S1). AEs and AE rates were similar with ofatumumab versus teriflunomide treatment, including infections overall and malignancies (see Table 2). One case of myocardial infarction was also noted in the ofatumumab group.
Table 2.
Adverse events from ASCLEPIOS I/II and long-term exposure in core and ALITHIOS extension trials as of first dose of ofatumumab (safety analysis set)
| Patients with at least one event, n (%) | ASCLEPIOS I/II ofatumumab (n = 946) | ASCLEPIOS I/II teriflunomide (n = 936) | Long-term (core + ALITHIOS extension) overall ofatumumab (n = 1969)a | |||
|---|---|---|---|---|---|---|
| n (%) | EAIR (95% CI) | n (%) | EAIR (95% CI) | n (%) | EAIR (95% CI) | |
| Patients with at least one AE | 791 (83.6) | 188.6 [175.9, 202.2] | 788 (84.2) | 188.9 [176.2, 202.6] | 1698 (86.2) | 135.1 [128.8, 141.7] |
| Patients with at least one SAE | 83 (8.8) | 5.6 [4.5, 6.9] | 73 (7.8) | 4.9 [3.9, 6.2] | 242 (12.3) | 5.0 [4.4, 5.6] |
| AEs leading to treatment discontinuation | 54 (5.7) | – | 49 (5.2) | – | 128b (6.5) | – |
| Infections | 488 (51.6) | 51.1 [46.8, 55.9] | 493 (52.7) | 52.6 [48.1, 57.4] | 1149 (58.4) | 41.0 [38.7, 43.4] |
| Serious infections | 24 (2.5) | 1.6 [1.0, 2.3] | 17 (1.8) | 1.1 [0.7, 1.8] | 78 (4.0) | 1.5 [1.2, 1.9] |
| Injection-related systemic reactions | 195 (20.6) | 15.5 [13.5, 17.8] | 143 (15.3) | 10.9 [9.3, 12.8] | 492 (25.0) | 12.4 [11.3, 13.5] |
| Injection site reactions | 103 (10.9) | 7.2 [5.9, 8.7] | 52 (5.6) | 3.5 [2.7, 4.7] | 233 (11.8) | 5.0 [4.4, 5.7] |
| Malignancies | 5 (0.5) | 0.3 [0.1, 0.8] | 4 (0.4) | 0.3 [0.1, 0.7] | 17 (0.9) | 0.3 [0.2, 0.5] |
| Deaths | 0 | 0 | 1 (0.1) | – | 6c (0.3) | – |
Only patients with SAEs that occurred until the last dosing date + 100 days are considered
AE adverse event, CI confidence interval, EAIR exposure-adjusted incidence rate, IgM immunoglobulin M, OMB ofatumumab, SAE serious adverse event
aAt least 1 dose of ofatumumab in core studies (ASCLEPIOS I/II, APLIOS, APOLITOS) or extension (ALITHIOS); cut-off date 25 September 2021bAEs related to reduced IgM levels were the most common reason for treatment discontinuation [71 (3.6%)]cPreferred term for these 6 cases includes sudden death (n = 1), completed suicide (n = 1), COVID-19 and COVID-19 pneumonia (n = 1), COVID-19 (n = 1), intestinal metastasis (n = 1), pneumonia and septic shock (n = 1)
The efficacy and safety of ofatumumab versus teriflunomide for first-line use in early RMS patients was also evaluated in ASCLEPIOS I/II on the basis of a protocol-defined cohort of 615 recently diagnosed and treatment-naïve RMS patients. Consistent with the overall ASCLEPIOS population, these early RMS patients achieved significantly higher levels of disease control (reductions in relapse rate, MRI activity, and risk of disability worsening) when treated with ofatumumab compared with teriflunomide. Safety findings were also consistent with those of the overall ASCLEPIOS population. The favorable disability outcomes and overall benefit–risk profile of ofatumumab versus teriflunomide in this protocol-defined cohort support the suitability of ofatumumab as an initial treatment in newly-diagnosed MS patients [41].
Running in parallel to the ASCLEPIOS trials were the phase 2 APLIOS and APOLITOS studies [17, 42]. The APLIOS trial demonstrated the bioequivalence of ofatumumab 20 mg administered by the pre-filled syringe (PFS, used during the ASCLEPIOS I/II trials described here) versus the autoinjector pen intended for commercial use. The autoinjector resulted in a lower treatment burden for patients by facilitating at-home self-administration. The efficacy of ofatumumab against MRI activity was similar regardless of the use of the PFS or autoinjector, and across both groups, the mean number of new or persisting gadolinium-enhancing T1 lesions decreased from 1.5 at baseline to 0.1 by Week 12, while the proportion of patients free of these lesions was 64.2% at baseline and 94.1% by Week 12 [17]. Concurrently, the APOLITOS study in RMS patients from Japan and Russia demonstrated the superior efficacy of ofatumumab versus placebo on new brain lesion activity at 24 weeks, with a 100% reduction in the number of gadolinium-enhancing T1 lesions from baseline by Week 48 with ofatumumab treatment [42]. These studies provided further evidence of the benefits of ofatumumab 20-mg SC and confirmed the consistency of effects across ethnic groups.
Long-term safety and benefits of ofatumumab 20-mg SC are being evaluated in the ongoing ALITHIOS extension study, which consists of patients who completed the ASCLEPIOS I/II, APLIOS or APOLITOS trials. Interim data have shown the long-term benefits and safety, and continued positive benefit–risk profile, of ofatumumab 20-mg SC treatment up to 4 years (core studies and extension) in patients with RMS [43]. The cohort originally randomized in ASCLEPIOS I/II showed a cumulative benefit (up to 4 years) of earlier initiation of ofatumumab 20-mg SC compared to those who were initiated on teriflunomide (active comparator) and then switched to ofatumumab in the extension study; these results may suggest that there is a benefit to using a high-efficacy anti-CD20 therapy before cycling through an oral RMS treatment. The results also indicate that extended treatment with ofatumumab up to 4 years is well tolerated in RMS patients with no new safety signals, high compliance on monthly injections, and no new safety risks identified [18, 43].
Confirming the Optimal Dose Regimen for Ofatumumab: Pharmacokinetic–Pharmacodynamic Model Simulations of the Ofatumumab SC Dose
In order to validate and confirm that the chosen 20-mg therapeutic dose of ofatumumab for RMS patients provided optimal characteristics as compared to other doses, a pharmacokinetic–pharmacodynamic (PKPD) model of B-cell counts versus ofatumumab concentration was developed using pooled data from phase 2 and phase 3 studies of ofatumumab in RMS [47]. The results of the simulations determined that both the monthly 20-mg and 40-mg SC doses were associated with a rapid reduction and near complete B-cell depletion in comparison to the lower simulated doses (1, 2, 5, or 10 mg), each administered at three loading doses given weekly, followed by monthly doses starting from Week 4 [47]. The 10-mg dose did not achieve a similar level of depletion compared with the 20-mg dose until after 6 months, and a higher proportion of patients did not achieve the same level of B-cell depletion across a range of B-cell depletion levels with the 10-mg dose compared with the 20-mg or higher doses. Importantly, the simulations also indicated that no dose adjustment was needed based on patient characteristics, i.e., age, body weight, baseline B-cell levels or injector device [47].
B-Cell Repletion Kinetics
According to the PKPD model simulations, depleted B-cell levels were consistent across patient weight, age, and baseline B-cell count, and the proportion of patients below one example B-cell level of 8 cells/μL was similar irrespective of body weight [47]. The simulated median time to return to B-cell count of 40 cells/μL (LLN) was estimated to be 23 weeks after dosing was interrupted at 2 years. Repletion to LLN (40 cells/µL) was estimated to range within 18–29 weeks after the last dose across all body weight categories [47]; this is in alignment with clinical results in which body weight did not have a significant effect on efficacy [48, 49]. It should be noted that repletion times could only be evaluated based on simulations, since most patients taking part in the ASCLEPIOS trial remained depleted due to ongoing treatment.
On treatment cessation, B-cell repletion has been reported to occur faster with ofatumumab than has been reported for IV-administered B-cell therapies in other studies [3, 50, 51]. In the PKPD model simulations, there was no difference in time to replete to the LLN after discontinuation of the 20-mg SC dosing regimen, regardless of each patient’s baseline B-cell count. [47]. Overall, this could have positive effects for patients scheduling vaccinations or for those who have concerns about infection, reproductive health, or any future need for treatment switch. Although no head-to-head comparisons are available, the median time to B-cell repletion to LLN post-treatment discontinuation with ofatumumab is 24.6 weeks, and the median time to B-cell repletion to baseline or LLN post-treatment discontinuation with ocrelizumab is 72 weeks, as per the respective product labels [10, 14].
PKPD modeling also indicated negligible B-cell repletion between doses of ofatumumab; PKPD model simulations of the B-cell profile for 20-mg SC every 4 weeks and 700-mg IV every 24 weeks demonstrated that the higher dose IV regimen resulted in repletion above the LLN (40 cells/µL) in 16% of patients between doses, while only 2% were estimated to be above the LLN between doses on 20-mg SC [47]. This maintenance of B-cell depletion with the 20-mg monthly SC dose is in line with the evidence (through these PKPD simulations and clinical data) that ofatumumab does not show a ‘wearing-off’ effect between monthly doses [16, 47].
Longer-Term Safety Profile of Ofatumumab SC and Immune Maintenance
Long-term safety data of ofatumumab to date (up to 4 years; cut-off date 25 September 2021) have not revealed new safety signals compared to the core ASCLEPIOS studies. According to recent results from the ALITHIOS study, the overall rates of AEs and serious adverse events (SAEs) across 4 years (core and extension studies) were consistent with the results from the ASCLEPIOS I/II trials (see Table 2). The most common serious infections were COVID-19 pneumonia/COVID-19 (1.2%), and appendicitis (0.1%), while a majority of serious infections resolved without discontinuing ofatumumab treatment [44]. Non-serious infections were the most common AEs, and the most frequent non-serious infections in the overall safety population (n = 1969) were nasopharyngitis (17.5%), upper respiratory tract infections (11.1%), and urinary tract infections (10.9%) [44]. Incidence rates of serious infections with ofatumumab remained stable and did not increase with long-term use up to 4 years [44].
Immune Maintenance with Ofatumumab SC
The favorable tolerability profile of ofatumumab has been supported by its prolonged low risk of immunogenicity. Mean serum immunoglobulin G (IgG) and immunoglobulin M (IgM) levels were measured up to 4 years of treatment in the core and long-term ALITHIOS extension studies. Over this time, IgG levels have remained stable and above the LLN (5.7 g/L), and, although there was a decrease in IgM, mean levels remained above the LLN (0.4 g/L) [7, 34]. At the individual patient level, the majority of patients on ofatumumab retained immunoglobulin levels above the LLN (98.4% in IgG and 73.4% in IgM) up to 4 years [34].
As per the core, and initially for the extension study, protocols, investigators were required to interrupt study treatment if IgM levels fell below 10% LLN or if IgG levels fell below 20% LLN, and treatment was not resumed until IgM or IgG levels returned to within normal limits. However, following an amendment to the protocol, the requirement to interrupt treatment due to low IgM or IgG levels was removed for the extension study and is currently left to the discretion of the investigator. Treatment interruption and discontinuation was reported in only 2 (0.1%) and 4 (0.2%) patients, respectively, due to low IgG levels, and in 193 (9.8%) and 71 (3.6%) patients, respectively, due to low IgM levels [34]. To date, no association has been observed between decreased immunoglobulin levels and the risk of serious infections with longer-term use of ofatumumab up to 4 years, indicating immune maintenance in RMS patients.
Immune Maintenance with Ofatumumab SC and COVID-19
In a report of COVID-19 cases and vaccination status in RMS patients treated with ofatumumab 20 -mg SC, the last assessed serum IgG and IgM levels before onset of COVID-19, and with respect to COVID-19 seriousness, were measured [52]. Of the 1703 patients enrolled in ALITHIOS receiving ofatumumab, 245 (14.4%) reported COVID-19; most cases were mild (44.1%) or moderate (46.5%) in severity. All patients had IgG levels above the LLN (5.7 g/l), while 23 had IgM levels below the LLN (0.4 g/l) before COVID-19 onset, 22 of whom had non-serious cases of mild-to-moderate severity. Most patients with COVID-19 (98.4%) recovered by the study cut-off date (25 September 2021), with few hospitalizations (9.4%) and two fatal outcomes (0.8%). Of the 90 confirmed COVID-19 post-marketing cases on ofatumumab treatment at the time of the report, 30 patients recovered or were recovering, 6 had no change in condition, and outcomes were not available for 54. No fatalities or life-threatening COVID-19 cases were reported [52].
These results suggest a favorable benefit–risk profile for the ofatumumab 20-mg SC dose in patients who contract COVID-19, and this may be associated with the evidence demonstrating that, although attenuated, patients receiving ofatumumab can mount an antibody response to the SARS-CoV-2 vaccine and may be capable of mounting a T-cell response [53]. Notably, it has also been reported that antibody levels for IV B-cell-depleting therapies (rituximab and ocrelizumab) were significantly lower than those for patients on SC B-cell-depleting therapies (ofatumumab; p < 0.001) [54].
Tolerability of IV Versus SC Ofatumumab
Ofatumumab has been generally well-tolerated by RMS patients, with many only experiencing mild headache across all injections [14, 55]. The IV and SC (and high dose vs. low dose) data from the RMS trials were pooled and used to assess the effects of dose-dependency and route of administration on the tolerability of ofatumumab [7, 16, 39, 40, 43, 56]. Infusion- or injection-related reactions occurring during the 24-h post-administration with ofatumumab IV versus SC were analyzed, and were characterized by overall incidence and association with the first infusion or injection (Fig. 3) [56]. The most commonly reported non-serious infusion-related reactions in the phase 2 ofatumumab IV dose trial included rash, erythema, upper respiratory tract infection, and viral infection [39]. Common injection-related systemic reactions across the phase 2b MIRROR and phase 3 ASCLEPIOS trials included fever, headache, chills, and influenza-like illness [16, 40].
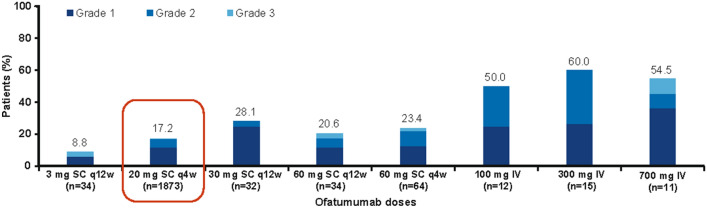
Fig. 3.
Proportion of patients with injection- or infusion-related reactions at the first injection/infusion across SC and IV studies*. *Cut-off date for analysis of pooled data: 30 November, 2019 (adapted from Bar-Or A, et al. Multiple Sclerosis Journal. 2020 [https://journals.sagepub.com/doi/pdf/10.1177/1352458520974937] [56]). IV intravenous, MS multiple sclerosis, n number of patients, q4w every 4 weeks, q12w every 12 weeks, SC subcutaneous
In the pooled MIRROR, ASCLEPIOS I/II, APLIOS, and ALITHIOS studies, the incidence of systemic and local-site IRRs was highest with the first injection in all treatment groups and decreased considerably for subsequent injections [56]. The incidence of severe IRRs with the 20-mg SC dose was low (0.2%) compared with the 3-mg and 60-mg SC doses, while the highest incidence of IRRs was reported with the ofatumumab 60-mg q4w dose (3.1%; Table 3). No other serious IRRs were reported during the study [56]. The incidence of discontinuations was highest with the IV route of administration and with higher doses of ofatumumab SC compared to the 20-mg SC dose. The infusion- and injection-related reactions observed here are consistent with the known safety profile of ofatumumab [7] and may be attributed to cytokine release after rapid B-cell depletion at first injection in patients with RMS.
Table 3.
Injection- or infusion-related reactions with ofatumumab across SC and IV studies
| Patients with at least one event, n (%) | SC doses | IV doses | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 mg q12wa (n = 34) | 20 mg q4w (n = 1873) | 30 mg q12wa (n = 32) | 60 mg q12wa (n = 34) | 60 mg q4wa (n = 64) | 100 mg (n = 12) | 300 mg (n = 15) | 700 mg (n = 11) | |
| Injection- or infusion-related reactions | 13 (38.2) | 435 (23.2) | 11 (34.4) | 14 (41.2) | 30 (46.9) | 8 (66.7) | 12 (80.0) | 10 (90.9) |
| Injection- or infusion-related reactions with first injection/infusion | 3 (8.8) | 322 (17.2) | 9 (28.1) | 7 (20.6) | 15 (23.4) | 6 (50.0) | 9 (60.0) | 6 (54.5) |
| Serious reaction | 0 | 2 (0.1) | 0 | 1 (2.9) | 2 (3.1) | 0 | 0 | 0 |
| Treatment discontinuation | 1 (2.9) | 1 (0.1) | 1 (3.1) | 0 | 1 (1.6) | 0 | 1 (6.7) | 0 |
| Treatment interrupted | 0 | 0 | 0 | 0 | 0 | 5 (41.7)b | 9 (60.0)b | 8 (72.7)b |
| Severe injection- or infusion-related reactions (Grade ≥ 3) | 1 (2.9) | 4 (0.2) | 0 | 1 (2.9) | 1 (1.6) | 0 | 0 | 2 (18.2) |
| Cytokine release syndrome | 0 | 0 | 0 | 1 (2.9) | 0 | 0 | 2 (13.3) | 0 |
AE adverse event, IV intravenous, n number of patients, q4w every 4 weeks, q12w every 12 weeks, SC subcutaneous
aUnlike the ASCLEPIOS I/II trials, no initial loading dose regimen was used in the MIRROR study
bInfusion paused and restarted
Treatment Initiation, Low Monitoring Burden and Treatment Preference
In addition to treatment efficacy, safety, and tolerability, decisions to treat with anti-CD20 agents rely on the onboarding processes and monitoring burdens of the therapy. As mentioned previously, infusible DMTs, such as ocrelizumab and rituximab, require premedication to mitigate their associated infusion-related reactions [8, 9]. Infusions also require access to infusion centers and healthcare professionals to administer treatment, which may increase the burden on patients, healthcare centers, and overall healthcare costs, as well as risks of exposure to community pathogens from travel outside the home. Premedication is not required with the ofatumumab 20-mg SC dose [14–16] and treatment may therefore be initiated more quickly, with good tolerability and with few IRRs, which can be managed with symptomatic treatment should they occur. The small volume of SC administration allows patients to self-administer the dose safely and conveniently [14], and this, combined with the good tolerability and lack of requirement for premedication, may foster greater treatment adherence versus traditional therapies [14].
Following the proportion of patients that were compliant with the 20-mg SC dose in the core part (98.8%), compliance in the ALITHIOS extension study has remained high in the overall ofatumumab, continuous ofatumumab, and newly-switched subgroups; as of 25 September 2021, 94.9% (1868/1969), 95.1% (1229/1292), and 94.4% (639/677) of patients in the overall, continuous and newly-switched subgroups remained on treatment [18].
Patient and nurse preferences regarding mode of administration have also been assessed, and MS patients have determined that the autoinjector pen is easy to use, while allowing treatment to be administered independently at home [17, 57]. Cost-effectiveness is another factor that often influences treatment preference among healthcare administrators, and SC administration is generally more cost-effective than infusion, as shown by the reduction in cost of SC rituximab in the real-world setting compared to the original IV rituximab dose, likely due to in-clinic visits being less frequent [58]. IV administration also requires the use of special resources and, hence, adds to the financial burden on healthcare systems [59]. In part, due to its independent administration, the National Institute for Health and Care Excellence (NICE) in the UK has recently regarded ofatumumab as a cost-effective therapy for the National Health Service [60].
Conclusion
The development target for the ofatumumab 20 mg SC dose was to tailor and achieve a favorable benefit-risk profile, and a convenient experience for patients with RMS. The efficacy of ofatumumab has been shown to be superior to teriflunomide in randomized double-blind phase 3 ASCLEPIOS I/II trials. In the ALITHIOS extension study, longer-term treatment up to 4 years has shown sustained benefits and good tolerability with no new safety signals. The safety profile, which importantly includes a reduced risk of IRRs with SC versus IV administration, has also been maintained over the long term. PKPD models further validated the use of the ofatumumab 20 mg dose for RMS patients by demonstrating a rapid reduction and near complete B-cell depletion with monthly administration. Simulations also indicated a shorter time to B-cell repletion after treatment cessation than other anti-CD20s, with attendant benefits for vaccination, reproductive health, and any potential need for treatment switch, and there is no significant effect on pharmacokinetics due to body weight. Based on network meta-analyses, ofatumumab is amongst the most highly efficacious treatments in MS (including ocrelizumab, natalizumab, alemtuzumab) [36, 37]; within this group, ofatumumab is the only treatment that has been specifically designed, tested and approved for self-administration by the patient at home. These unique benefit-risk and tolerability profiles enable patients to be compliant with, and persistent on, therapy over time. The at-home self-administration also meets patient preferences due to its convenience and was particularly beneficial during the COVID-19 pandemic by mitigating the risks of infection from in-clinic visits. The benefit-risk profile of ofatumumab supports its early initiation to maximize its long-term treatment benefits in the overall RMS population, as well as recently diagnosed and treatment-naïve RMS patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the participants of the studies referenced in this article.
Funding
Sponsorship for this study and Rapid Service Fee were funded by Novartis Pharma AG.
Medical Writing, Editorial, and Other Assistance
Writing assistance in the preparation of this article was provided by Brianna Fitzmaurice of Novartis Ireland Ltd. Support for this assistance was funded by Novartis Pharma AG. The authors would also like to thank Miriam King of Novartis Pharma AG for her contributions in organizing the production of this article.
Author Contributions
The conceptualization of this article was carried out by Goeril Karlsson, Roseanne Sullivan, Gordon Graham and Stephen Hauser. Roseanne Sullivan, Gordon Graham, and Dieter A. Häring assisted with the literature search. All authors contributed to the structuring and content of this article, as well as the critical revision and approval of the final draft.
Disclosures
Stephen L. Hauser has received personal compensation from Annexon, Alector, Accure, and Neurona; he has also received travel reimbursement from F. Hoffmann-La Roche Ltd and Novartis for CD20-related meetings and presentations. Ludwig Kappos has received no personal compensation. His institutions (University Hospital Basel/Stiftung Neuroimmunology and Neuroscience Basel) have received the following exclusively for research support: steering committee, advisory board and consultancy fees (Abbvie, Actelion, Auriga Vision AG, Bayer HealthCare, Biogen, Celgene, df-mp [Dörries Frank-Molnia & Pohlman], Eli Lilly, EMD Serono, Genentech, Genzyme, Glaxo Smith Kline, Janssen, Merck, Minoryx, Novartis, Roche, Sanofi, Santhera, Senda Biosciences, Shionogi and Wellmera AG); speaker fees (Bristol Myrers Squibb, Celgene, Janssen, Merck, Novartis, and Roche); support for educational activities (Biogen, Desitin, Novartis, Sanofi and Teva); license fees for Neurostatus products; and grants (European Union, Innosuisse, Novartis, Roche, Swiss MS Society and Swiss National Research Foundation). Amit Bar-Or has participated as a speaker in meetings sponsored by, and received consulting fees and/or grant support from, Accure, Atara Biotherapeutics, Biogen, BMS/Celgene/Receptos, GlaxoSmithKline, Gossamer, Janssen/Actelion, Medimmune, Merck/EMD Serono, Novartis, Roche/Genentech, Sanofi Genzyme. Heinz Wiendl has received honoraria for acting as a member of scientific advisory boards for Biogen, Evgen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Roche Pharma AG, and Sanofi-Aventis, as well as speaker honoraria and travel support from Alexion, Biogen, Cognomed, F. Hoffmann-La Roche Ltd., Gemeinnützige Hertie-Stiftung, Merck Serono, Novartis, Roche Pharma AG, Genzyme, Teva, and WebMD Global. Heinz Wiendl is acting as a paid consultant for AbbVie, Actelion, Biogen, IGES, Johnson & Johnson, Novartis, Roche, Sanofi-Aventis, and the Swiss Multiple Sclerosis Society. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Else Kröner Fresenius Foundation, Fresenius Foundation, the European Union, Hertie Foundation, NRW Ministry of Education and Research, Interdisciplinary Center for Clinical Studies (IZKF) Muenster and RE Children’s Foundation, Biogen, GlaxoSmithKline GmbH, Roche Pharma AG, and Sanofi-Genzyme. David Paling has participated as a speaker in meetings sponsored by, and received consulting fees from, Biogen, Celgene, Janssen, MedDay, Merck, Novartis, Sanofi Genzyme and Roche. Mitzi J. Williams has received consulting fees from EMD Serono, Horizon, Novartis, Alexion, Biogen, Sanofi, Genentech, Octave Biosciences, TG Therapeutics, Janssen, and Bristol Myers Squibb; and speaking fees from Genentech, Biogen, EMD Serono and TG Therapeutics. Ralf Gold has received compensation for serving as a consultant or speaker from Bayer HealthCare, Biogen Idec, Merck Serono, Novartis and Teva Neuroscience. He, or the institution he works for, has received research support from Bayer HealthCare, Biogen Idec, Merck Serono, Novartis and Teva Neuroscience. He has also received honoraria as a Journal Editor from SAGE and Thieme Verlag. Andrew Chan has received speakers’/board honoraria from Actelion (Janssen/J&J), Alexion, Almirall, Bayer, Biogen, Celgene (BMS), Merck KgaA, Novartis, Roche, Sanofi, and Teva, all for hospital research funds. He received research support from Biogen, Roche, Sanofi and UCB. Ron Milo has received research support from Bayer, Medison, Merck, Novartis and Teva; and honoraria or consulting fees from Actelion, Bayer, Biogen, Genzyme, Medison, Merck, Neopharm, Novartis, Roche, Sanofi, Teva and TG-Therapeutics. Patrick Vermersch has received honoraria and consulting fees from Biogen, Sanofi, Teva, Novartis, Merck, Imcyse, Roche and AB Science, and research support from Biogen, Sanofi and Merck. Ayan Das Gupta, Goeril Karlsson, Roseanne Sullivan, Gordon Graham, Martin Merschhemke and Dieter A. Häring are employees of Novartis.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
The original online version of this article was revised to correct the digital features text.
Change history
8/19/2023
The original online version of this article was revised to correct the digital features text.
References
- 1.van Langelaar J, Rijvers L, Smolders J, van Luijn MM. B and T cells driving multiple sclerosis: identity, mechanisms and potential triggers. Front Immunol. 2020;11:760. doi: 10.3389/fimmu.2020.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 4.Hauser SL, Cree BAC. Treatment of multiple sclerosis: A review. Am J Med. 2020;133(12):1380–90.e2. doi: 10.1016/j.amjmed.2020.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabatino JJ, Jr., Zamvil SS, Hauser SL. B-Cell Therapies in Multiple Sclerosis. Cold Spring Harb Perspect Med. 2019;9(2). [DOI] [PMC free article] [PubMed]
- 6.Huck C, Leppert D, Wegert V, Schmid C, Dunn R, Weckbecker G, et al. Low-dose subcutaneous anti-CD20 treatment depletes disease relevant B cell subsets and attenuates neuroinflammation. J Neuroimmune Pharmacol. 2019;14(4):709–719. doi: 10.1007/s11481-019-09872-z. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, Meuth SG, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28(10):1576–1590. doi: 10.1177/13524585221079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Genentech. Rituxan (rituximab) injection, for intravenous use: US prescribing information. 1997. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/103705s5464lbl.pdf. Accessed June 2023.
- 9.Genentech. Ocrevus (ocrelizumab) injection, for intravenous use: US prescribing information. 2017. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761053lbl.pdf. Accessed June 2023.
- 10.European Medicines Agency. Ocrevus (ocrelizumab). EU summary of product characteristics. 2018. Available at: https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf. Accessed June 2023.
- 11.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, et al. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 12.Steinman L, Fox E, Hartung HP, Alvarez E, Qian P, Wray S, et al. Ublituximab versus teriflunomide in relapsing multiple sclerosis. N Engl J Med. 2022;387(8):704–714. doi: 10.1056/NEJMoa2201904. [DOI] [PubMed] [Google Scholar]
- 13.Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. 2005;36(1):3–10. doi: 10.1016/j.ymeth.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency. Kesimpta (ofatumumab). EU summary of product characteristics. 2021. Available at: https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf. Accessed June 2023.
- 15.Novartis Pharmaceuticals Corporation. Kesimpta (ofatumumab) injection, for subcutaneous use: US prescribing information. 2020. Available at: https://www.hcp.novartis.com/products/kesimpta/rms. Accessed June 2023.
- 16.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 17.Bar-Or A, Wiendl H, Montalban X, Alvarez E, Davydovskaya M, Delgado SR, et al. Rapid and sustained B-cell depletion with subcutaneous ofatumumab in relapsing multiple sclerosis: APLIOS, a randomized phase-2 study. Mult Scler. 2022;28(6):910–924. doi: 10.1177/13524585211044479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarez E, Hersh CM, Robertson D, Das Gupta A, Hu X, Zielman R, et al. Compliance and persistence with ofatumumab treatment in patients with relapsing multiple sclerosis in clinical trials for up to 4 years. Mult Scler J. 2022;28(Suppl. 3):643–4.
- 19.He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. 2020;19(4):307–316. doi: 10.1016/S1474-4422(20)30067-3. [DOI] [PubMed] [Google Scholar]
- 20.Novartis Pharmaceuticals Australia Pty Limited. Kesimpta (ofatumumab) Australian product information. 2021.
- 21.Lin TS. Ofatumumab: a novel monoclonal anti-CD20 antibody. Pharmgenomics Pers Med. 2010;3:51–59. doi: 10.2147/pgpm.s6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klein C, Lammens A, Schafer W, Georges G, Schwaiger M, Mossner E, et al. Epitope interactions of monoclonal antibodies targeting CD20 and their relationship to functional properties. MAbs. 2013;5(1):22–33. doi: 10.4161/mabs.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Payandeh Z, Bahrami AA, Hoseinpoor R, Mortazavi Y, Rajabibazl M, Rahimpour A, et al. The applications of anti-CD20 antibodies to treat various B cells disorders. Biomed Pharmacother. 2019;109:2415–2426. doi: 10.1016/j.biopha.2018.11.121. [DOI] [PubMed] [Google Scholar]
- 24.Bar-Or A, O'Brien SM, Sweeney ML, Fox EJ, Cohen JA. Clinical perspectives on the molecular and pharmacological attributes of anti-CD20 therapies for multiple sclerosis. CNS Drugs. 2021;35(9):985–997. doi: 10.1007/s40263-021-00843-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teeling JL, Mackus WJ, Wiegman LJ, van den Brakel JH, Beers SA, French RR, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 26.Du J, Yang H, Guo Y, Ding J. Structure of the Fab fragment of therapeutic antibody Ofatumumab provides insights into the recognition mechanism with CD20. Mol Immunol. 2009;46(11–12):2419–2423. doi: 10.1016/j.molimm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Beum PV, Lindorfer MA, Beurskens F, Stukenberg PT, Lokhorst HM, Pawluczkowycz AW, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181(1):822–832. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 28.Touil I, Perrot C, Elain G, Weckbecker G. Ofatumumab and ocrelizumab differentially induce human primary B cell lysis by complement dependent cytotoxicity. Mult Scler J. 2019;25:157–165. [Google Scholar]
- 29.Jungquist R-MM, Douglas EA, Bouley AJ, Katz JD, Lathi ES, The Elliot Lewis Center for Multiple Sclerosis Care MA ACAPELLA: B-cell reconstitution in ocrelizumab-treated patients, 2021 update. Int J MS Care. 2021;23(Suppl. 2):33. [Google Scholar]
- 30.Eurpoean Medicines Agency. Kesimpta (ofatumumab). Assessment report. 2021. Available at: https://www.ema.europa.eu/en/documents/assessment-report/kesimpta-epar-public-assessment-report_en.pdf. Accessed June 2023.
- 31.Sanchez-Felix M, Burke M, Chen HH, Patterson C, Mittal S. Predicting bioavailability of monoclonal antibodies after subcutaneous administration: open innovation challenge. Adv Drug Deliv Rev. 2020;167:66–77. doi: 10.1016/j.addr.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Torres JB, Roodselaar J, Sealey M, Ziehn M, Bigaud M, Kneuer R, et al. Distribution and efficacy of ofatumumab and ocrelizumab in humanized CD20 mice following subcutaneous or intravenous administration. Front Immunol. 2022;28(13):814064. doi: 10.3389/fimmu.2022.814064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bigaud M, Anthony D, Lutzenburg P, Zipfel G, Uffelmann T, Helena V, et al. Comparative pharmacology of ofatumumab versus ocrelizumab in humanised-CD20 transgenic mice. Mult Scler J. 2022;28(Suppl. 3):335. [Google Scholar]
- 34.Saccà F, Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, et al. Longer-term safety of ofatumumab in patients with relapsing multiple sclerosis. Eur J Neurol. 2022;29(Suppl. 1):152. [Google Scholar]
- 35.TG Therapeutics. Briumvi (ublituximab) injection, for intravenous use: US prescribing information. 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761238s000lbl.pdf. Accessed June 2023.
- 36.Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Haring DA, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res. 2020;9(18):1255–1274. doi: 10.2217/cer-2020-0122. [DOI] [PubMed] [Google Scholar]
- 37.Samjoo IA, Worthington E, Drudge C, Zhao M, Cameron C, Haring DA, et al. Efficacy classification of modern therapies in multiple sclerosis. J Comp Eff Res. 2021;10(6):495–507. doi: 10.2217/cer-2020-0267. [DOI] [PubMed] [Google Scholar]
- 38.Samjoo IA, Klotz L, Giovannoni G, Drudge C, Haltner A, Worthington E, et al. Simulated treatment comparison of efficacy outcomes for ofatumumab in ASCLEPIOS I/II versus ocrelizumab in OPERA I/II for the treatment of patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2022;66:104031. doi: 10.1016/j.msard.2022.104031. [DOI] [PubMed] [Google Scholar]
- 39.Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82(7):573–581. doi: 10.1212/WNL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 40.Bar-Or A, Grove RA, Austin DJ, Tolson JM, VanMeter SA, Lewis EW, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR study. Neurology. 2018;90(20):e1805–e1814. doi: 10.1212/WNL.0000000000005516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gärtner J, Hauser SL, Bar-Or A, Montalban X, Cohen JA, Cross AH, et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: results from ASCLEPIOS I and II. Mult Scler. 2022;28(10):1562–1575. doi: 10.1177/13524585221078825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kira JI, Nakahara J, Sazonov DV, Kurosawa T, Tsumiyama I, Willi R, et al. Effect of ofatumumab versus placebo in relapsing multiple sclerosis patients from Japan and Russia: phase 2 APOLITOS study. Mult Scler. 2022;28(8):1229–1238. doi: 10.1177/13524585211055934. [DOI] [PubMed] [Google Scholar]
- 43.Hauser SL, Fox E, Aungst A, Su W, Zielman R, Das Gupta AXJ, et al. Long-term efficacy of ofatumumab in patients with relapsing multiple sclerosis. Neurology. 2022;98(Suppl. 18):2517. [Google Scholar]
- 44.Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, Meuth SG, et al. Long-term safety of ofatumumab in patients with relapsing multiple sclerosis. Neurology. 2022;98(Suppl. 18):2481. doi: 10.1177/13524585221079731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostergaard M, Baslund B, Rigby W, Rojkovich B, Jorgensen C, Dawes PT, et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying antirheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum. 2010;62(8):2227–2238. doi: 10.1002/art.27524. [DOI] [PubMed] [Google Scholar]
- 46.Kurrasch R, Brown JC, Chu M, Craigen J, Overend P, Patel B, et al. Subcutaneously administered ofatumumab in rheumatoid arthritis: a phase I/II study of safety, tolerability, pharmacokinetics, and pharmacodynamics. J Rheumatol. 2013;40(7):1089–1096. doi: 10.3899/jrheum.121118. [DOI] [PubMed] [Google Scholar]
- 47.Yu H, Graham G, David OJ, Kahn JM, Savelieva M, Pigeolet E, et al. Population pharmacokinetic-B cell modeling for ofatumumab in patients with relapsing multiple sclerosis. CNS Drugs. 2022;36(3):283–300. doi: 10.1007/s40263-021-00895-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, et al. B-cell depletion and efficacy outcomes with ofatumumab: subgroup analysis from the pooled phase 3 ASCLEPIOS I and II trials. Neurology. 2020;94(Suppl. 15):2356. [Google Scholar]
- 49.Wiendl H, Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, et al. Effect of ofatumumab on B-cell depletion and efficacy outcomes: subgroup analysis from the pooled phase 3 ASCLEPIOS I and II trials. Eur J Neurol. 2020;27(Suppl. 1):480. [Google Scholar]
- 50.Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: Benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020;44:102279. doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- 51.Thiel J, Rizzi M, Engesser M, Dufner AK, Troilo A, Lorenzetti R, et al. B cell repopulation kinetics after rituximab treatment in ANCA-associated vasculitides compared to rheumatoid arthritis, and connective tissue diseases: a longitudinal observational study on 120 patients. Arthritis Res Ther. 2017;19(1):101. doi: 10.1186/s13075-017-1306-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cross AH, Delgado S, Habek M, Davydovskaya M, Ward BJ, Cree BAC, et al. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol Ther. 2022;11(2):741–758. doi: 10.1007/s40120-022-00341-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ziemssen T, Groth M, Ettle B, Bopp T. Immune response to SARS-CoV-2 mRNA vaccines in an open-label multicenter study in participants with relapsing multiple sclerosis treated with ofatumumab. Vaccines (Basel). 2022;10(12):2167. doi: 10.3390/vaccines10122167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levit E, Longbrake EE, Stoll SS. Seroconversion after COVID-19 vaccination for multiple sclerosis patients on high efficacy disease modifying medications. Mult Scler Relat Disord. 2022;60:103719. doi: 10.1016/j.msard.2022.103719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Comi G, Hartung H-P, Bakshi R, Williams IM, Wiendl H. Benefit-risk profile of sphingosine-1-phosphate receptor modulators in relapsing and secondary progressive multiple sclerosis. Drugs. 2017;77(16):1755–1768. doi: 10.1007/s40265-017-0814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bar-Or A, Schubert-Tennigkeit AA, Mairon N, Kerloeguen C, Gufran M, Shaikh S, et al. Dose-dependent tolerability of intravenous and subcutaneous ofatumumab in clinical studies. Mult Scler J. 2020;26(Suppl. 3):118–659. [Google Scholar]
- 57.Ross AP, Besser C, Naval S, Stoneman D, Gaunt H, Barker N. Patient and nurse preference for Sensoready® autoinjector pen versus other autoinjectors in multiple sclerosis: results from a multicenter survey. 2022. (In development). [DOI] [PMC free article] [PubMed]
- 58.Delgado Sanchez O, Gutierrez A, do Pazo F, Gines J, Martorell C, Boyeras B, et al. Comparative Cost Analysis Of Intravenous And Subcutaneous Administration Of Rituximab In Lymphoma Patients. Clinicoecon Outcomes Res. 2019;11:695–701. [DOI] [PMC free article] [PubMed]
- 59.Baharnoori M, Bhan V, Clift F, Thomas K, Mouallif S, Adlard N, et al. Cost-effectiveness analysis of ofatumumab for the treatment of relapsing-remitting multiple sclerosis in Canada. Pharmacoecon Open. 2022;6(6):859–870. doi: 10.1007/s41669-022-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.National Institute for Health and Care Excellence. Ofatumumab for treating relapsing multiple sclerosis: technology appraisal guidance [TA699]. 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.