Abstract
Introduction
Hereditary transthyretin (ATTRv; v for variant) amyloidosis, also known as hATTR amyloidosis, is a progressive and fatal disease associated with rapid deterioration of physical function and patients' quality of life (QOL). Vutrisiran, a subcutaneously administered RNA interference (RNAi) therapeutic that reduces hepatic production of transthyretin, was assessed in patients with ATTRv amyloidosis with polyneuropathy in the pivotal HELIOS-A study.
Methods
The phase 3 open-label HELIOS-A study investigated the efficacy and safety of vutrisiran in patients with ATTRv amyloidosis with polyneuropathy, compared with an external placebo group from the APOLLO study of the RNAi therapeutic patisiran. Measures of QOL and physical function were assessed.
Results
At month 18, vutrisiran improved Norfolk Quality of Life-Diabetic Neuropathy (Norfolk QOL-DN) total score (least squares mean difference [LSMD] in change from baseline [CFB]: –21.0; p = 1.84 × 10–10) and Norfolk QOL-DN domain scores, compared with external placebo. This benefit relative to external placebo was evident across all baseline polyneuropathy disability (PND) scores and most pronounced in patients with baseline PND scores I–II. Compared with external placebo, vutrisiran also demonstrated benefit in EuroQoL-Visual Analog Scale (EQ-VAS) score (LSMD in CFB: 13.7; nominal p = 2.21 × 10–7), 10-m walk test (LSMD in CFB: 0.239 m/s; p = 1.21 × 10–7), Rasch-built Overall Disability Score (LSMD in CFB: 8.4; p = 3.54 × 10–15), and modified body mass index (mBMI) (LSMD in CFB: 140.7; p = 4.16 × 10–15) at month 18. Overall, Norfolk QOL-DN, EQ-VAS, and mBMI improved from pretreatment baseline with vutrisiran, whereas all measures worsened from baseline in the external placebo group. At month 18, Karnofsky Performance Status was stable/improved from baseline in 58.2/13.1% with vutrisiran versus 34.7/8.1% with external placebo.
Conclusion
Vutrisiran treatment provided significant clinical benefits in multiple measures of QOL and physical function in patients with ATTRv amyloidosis with polyneuropathy. Benefits were most pronounced in patients with earlier-stage disease, highlighting the importance of early diagnosis and treatment.
Trial Registration Number
ClinicalTrials.gov: NCT03759379.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-023-00522-4.
Keywords: ATTRv amyloidosis, hATTR amyloidosis, Nutritional status, Physical function, Polyneuropathy, Quality of life, RNA interference, Vutrisiran
Key Summary Points
| Hereditary transthyretin (ATTRv; v for variant) amyloidosis, also known as hATTR amyloidosis, is a rare, rapidly progressive, and fatal disease, in which continued progression of neuropathy and cardiomyopathy is associated with debilitating symptoms, impaired physical function, and decline in patients' quality of life (QOL). |
| This analysis from the phase 3 HELIOS-A study assessed the impact of treatment with the RNA interference therapeutic vutrisiran, which is approved for the treatment of the polyneuropathy of ATTRv amyloidosis, on measures of QOL and physical function in patients with ATTRv amyloidosis with polyneuropathy over 18 months. |
| Vutrisiran treatment significantly improved multiple measures of QOL and also demonstrated significant benefit in measures of gait speed, disability, performance status, and nutritional status, compared with the external placebo arm of the APOLLO study, which included a similar patient population, over 18 months. |
| The worsening from baseline in multiple measures of QOL and physical function observed in the external placebo group, together with the observation that patients with lower baseline polyneuropathy disability scores at the start of treatment with vutrisiran retained a better Norfolk Quality of Life-Diabetic Neuropathy score after 18 months, highlights that early and effective treatment of ATTRv amyloidosis with polyneuropathy is critical to minimize the progressive negative impact of the disease on QOL and physical function. |
| The findings of this study support the clinical benefit of vutrisiran as an effective treatment that can improve the lives of patients with ATTRv amyloidosis with polyneuropathy and emphasize the importance of early and effective treatment. |
Introduction
Hereditary transthyretin (ATTRv; v for variant) amyloidosis, also known as hATTR amyloidosis, is a rare, inherited, rapidly progressive, debilitating, and fatal disease caused by variants in the transthyretin (TTR) gene [1–4]. The TTR protein typically circulates as a homotetramer, yet pathogenic TTR variants lead to tetramer destabilization and subsequent cleavage of the resulting monomers into amyloidogenic fragments, which misfold and accumulate as amyloid in multiple organs and tissues [5, 6]. Amyloid can deposit in nerves, heart, gastrointestinal (GI) tract, and musculoskeletal tissues [1, 2, 4, 7], resulting in a heterogeneous clinical presentation that typically includes sensory, motor, and autonomic neuropathy and cardiomyopathy [2, 8–10]. The majority of patients develop a mixed phenotype of both polyneuropathy and cardiomyopathy as a result of the disease [11, 12]. Prognosis is poor for untreated patients, with a median survival of 4.7 years following diagnosis, decreasing to 3.4 years for patients presenting with cardiomyopathy [13–16].
ATTRv amyloidosis has an aggressive course with rapid progression that is associated with debilitating symptoms and deterioration of physical function; the resulting decline in ambulatory ability [17, 18] and in the ability to perform activities of daily living [19] has a significant impact on a patient’s independence. The worsening in physical function, alongside continued progression of sensory neuropathy (leading to symptoms of numbness, burning sensation, and pain) [8] and autonomic neuropathy (GI involvement and deterioration of nutritional status) [20], has a detrimental impact on patient quality of life (QOL). Indeed, significant worsening of QOL is evident in natural history studies, and in the placebo arms of clinical studies of pharmacologic therapies in patients with ATTRv amyloidosis [19, 21–26]. In a small, prospective, multi-institutional, observational study, patients with ATTRv amyloidosis had severe impairment across a range of QOL outcomes, which deteriorated over time without treatment [25]. In another observational study, THAOS (Transthyretin Amyloidosis Outcomes Survey), GI symptoms increased with disease duration, negatively impacting nutritional status (modified body mass index; mBMI) [20]. These symptoms, which included vomiting, nausea, early satiety, constipation, diarrhea, and fecal incontinence, were each significantly associated with a negative impact on patients’ health-related QOL [20]. These observations are also replicated in pivotal clinical trials, in which substantial worsening over time across multiple measures of QOL (Norfolk Quality of Life-Diabetic Neuropathy [Norfolk QOL-DN] questionnaire, EuroQoL-5 dimensions-5 levels [EQ-5D-5L] questionnaire, EuroQoL-Visual Analog Scale [EQ-VAS], and Short-Form-36 questionnaire) and disability (Rasch-built Overall Disability Scale [R-ODS]) has been reported in placebo-treated patients [19, 26, 27].
The natural course of ATTRv amyloidosis highlights the need for early and effective treatment that can minimize the progressive negative impact of the disease on QOL and physical function. Treatment options include those that reduce levels of pathogenic TTR protein via silencing of the TTR gene, either by harnessing the natural process of RNA interference (RNAi) using a synthetic small interfering RNA (siRNA) or by antisense oligonucleotide-directed degradation of TTR mRNA, or those that stabilize the TTR tetramer [22, 23, 26, 28]. These different therapeutic approaches have shown various levels of clinical benefits in patients with ATTRv amyloidosis with polyneuropathy, including benefits on QOL measures compared with placebo [22, 23]. For example, the RNAi therapeutic patisiran, an approved treatment for patients with hATTR amyloidosis with polyneuropathy [29], demonstrated the potential to halt or reverse polyneuropathy progression and to improve multiple QOL and disability measures (Norfolk QOL-DN, EQ-5D-5L, EQ-VAS, and R-ODS) compared with placebo at 18 months in the pivotal phase 3 APOLLO study [19, 23]. Vutrisiran is also an RNAi therapeutic that, like patisiran, acts by reducing synthesis of both variant and wild-type TTR in the liver [30, 31], and has been approved for the treatment of the polyneuropathy of hATTR amyloidosis [32]. Vutrisiran is an siRNA conjugated to a triantennary N-acetyl galactosamine ligand that directs it to the liver, the primary site of TTR synthesis [33, 34]. This design, which utilizes next-generation enhanced stabilization chemistry, allows for increased potency and high metabolic stability, enabling vutrisiran to elicit a robust and sustained reduction in serum TTR with subcutaneous injection once every 3 months (Q3M) [30, 31]. In the phase 3 HELIOS-A study in patients with ATTRv amyloidosis with polyneuropathy, vutrisiran met the primary endpoint of change from baseline in neuropathy impairment (modified Neuropathy Impairment Score + 7 [mNIS + 7]), as well as all secondary efficacy endpoints, compared with an external placebo group from the APOLLO study, and demonstrated an acceptable safety profile [35].
Here, we report the impact of vutrisiran on QOL, measures of physical function (gait speed, disability score, and performance status), and nutritional status in patients with ATTRv amyloidosis with polyneuropathy during the 18-month treatment period of the HELIOS-A study.
Methods
Trial Design and Participants
The full methodology and study design details for HELIOS-A have been described previously [35]. In summary, HELIOS-A (NCT03759379) was a phase 3, global (57 sites in 22 countries), randomized, open-label study of vutrisiran in patients with ATTRv amyloidosis with polyneuropathy. The study protocol and amendments were approved by relevant Institutional Review Boards or Independent Ethics Committees. Written informed consent was obtained from each participant. The study was conducted in accordance with all applicable regulatory requirements, the current guidelines of Good Clinical Practice, and principles originating from the Declaration of Helsinki.
Eligible patients in HELIOS-A were aged 18–85 years with a documented TTR variant and diagnosis of ATTRv amyloidosis, neuropathy (baseline Neuropathy Impairment Score of 5–130), a polyneuropathy disability (PND) score of ≤ IIIb, adequate liver and renal function, and a Karnofsky Performance Status (KPS) score of ≥ 60%. Prior TTR stabilizer use was permitted, although patients were not allowed to use TTR stabilizers during their participation in the study. Enrolled patients were randomized 3:1 to 18 months of treatment with vutrisiran 25 mg subcutaneously Q3M, or patisiran 0.3 mg/kg intravenously (IV) once every 3 weeks, which served as a reference group. The placebo group of the APOLLO study [23], which had similar endpoints and eligibility criteria to HELIOS-A, was used as an external placebo control for the primary endpoint and most secondary and exploratory endpoints. In APOLLO, patients randomized to the placebo group received an IV administration of 0.9% normal saline with no lipid nanoparticles (LNPs) once every 3 weeks, in addition to the same precautionary premedication regimen (IV corticosteroid [dexamethasone 10 mg or equivalent], paracetamol 500 mg orally, and IV H1/H2 blockers) as the active treatment (patisiran) group to reduce the risk of infusion-related reactions (IRRs), before each saline infusion. Consequently, there was no potential for LNP-related adverse reactions and a low potential for IRRs more broadly in the APOLLO placebo arm to impact patients’ QOL or functional outcomes, with negligible resulting impact on comparisons with the HELIOS-A vutrisiran arm.
Assessments
Full details of the efficacy and safety endpoints of HELIOS-A have been described previously [35]. The primary endpoint was the change in neuropathy impairment from baseline as measured by mNIS + 7 score (range 0–304, with higher scores indicating greater neuropathy impairment) compared with the external placebo group of the APOLLO study at month 9. mNIS + 7 was also assessed at month 18 as a secondary endpoint. Measures of QOL assessed in HELIOS-A included change from baseline in Norfolk QOL-DN total score (range −4 to 136, higher score indicates worse QOL) at months 9 and 18 (secondary endpoints), individual domains of Norfolk QOL-DN (activities of daily living, physical functioning/large-fiber neuropathy, small-fiber neuropathy, autonomic neuropathy, and symptoms) at months 9 and 18 (post hoc analyses), and patient self-rated global health using EQ-VAS (range 0 [worst health]–100 [best health]) at months 9 and 18 (exploratory endpoints) [36]. Measures of physical function included change from baseline in gait speed assessed by 10-m walk test (10-MWT) at months 9 and 18 (secondary endpoints) and in disability (activity and social participation limitations) assessed by R-ODS (range 0–48, with lower scores indicating more disability) at months 9 (exploratory endpoint) and 18 (secondary endpoint), as well as the proportion of patients with worsened/stable/improved performance status assessed by KPS at month 18 (exploratory endpoint), where improvement was defined as an increase in KPS score from baseline. Nutritional status was also assessed by mBMI at months 9 (exploratory endpoint) and 18 (secondary endpoint). All analyses report the outcomes for patients receiving vutrisiran compared with the external placebo group and, where stated, with study baseline. KPS was only assessed at baseline in the APOLLO study; therefore, the KPS data for the external placebo group that are compared with the vutrisiran group month 18 data are derived from the patisiran Global open-label extension (OLE) study baseline. KPS was recorded at Global OLE baseline for all patients entering the Global OLE who were initially treated with placebo during APOLLO (APOLLO-placebo group). The mean treatment duration from APOLLO baseline to Global OLE baseline was 18.8 months.
Statistical Analyses
The primary population for efficacy analysis was the modified intent-to-treat population (defined as randomized patients who received any dose of study drug). In this analysis, estimates of treatment efficacy at months 9 and 18 for continuous endpoints were analyzed using a mixed-effects model for repeated measures (MMRM). For categorical outcomes, such as KPS and the presence or absence of symptoms as assessed by Norfolk symptom domain items, the number and percentage of patients in each category were calculated.
Primary and secondary endpoints in HELIOS-A were analyzed in a prespecified hierarchical order to control overall type I error, as reported previously [35]. Exploratory endpoints and post hoc comparisons were analyzed without a prespecified order.
Different statistical models were used to calculate the estimates and p values for endpoints at month 9 analysis (analysis of covariance; ANCOVA) versus month 18 analysis (MMRM). Hereby, estimates and p values are reported from month 18 data, unless otherwise specified.
Results
HELIOS-A Study Population
Details of the patient disposition, demographics, and baseline characteristics have been reported previously [35]. Among 164 patients randomized and treated in HELIOS-A, 122 received vutrisiran. A total of 117 (95.9%) vutrisiran-treated patients completed the 18-month treatment period. The external APOLLO placebo group included 77 patients. In the APOLLO study, 48 (62.3%) placebo-treated patients completed the 18-month treatment period.
Baseline demographics and disease characteristics were widely overlapping between the HELIOS-A vutrisiran group and the APOLLO placebo group; hence, the two populations were considered clinically comparable [35].
Baseline values for QOL, physical function, and nutritional status parameters in the HELIOS-A study are listed in Table 1. Baseline scores for all parameters were comparable between the HELIOS-A vutrisiran group and the external placebo group.
Table 1.
Baseline QOL and patient function parameters in the HELIOS-A study
| Assessment | Points range | External placebo (n = 77) | Vutrisiran (n = 122) |
|---|---|---|---|
| Norfolk QOL-DNa, mean (SD) | –4 to 136 | 55.5 (24.3) | 47.1 (26.3) |
| EQ-VASb, mean (SD) | 0–100 | 54.6 (18.0) | 64.5 (18.5) |
| R-ODSc, mean (SD) | 0–48 | 29.8 (10.8) | 34.1 (11.0) |
| 10-MWTd (m/s), mean (SD) | – | 0.790 (0.319) | 1.006 (0.393) |
| mBMIe (kg/m2 × g/L), mean (SD) | – | 989.9 (214.2) | 1057.4 (233.8) |
| KPSf, n (%) | |||
| 60% | – | 22 (28.6) | 17 (13.9) |
| 70−80% | – | 45 (58.4) | 73 (59.8) |
| 90−100% | – | 10 (13.0) | 32 (26.2) |
10-MWT 10-m walk test, BMI body mass index, EQ-VAS EuroQoL-Visual Analog Scale, KPS Karnofsky Performance Status, mBMI modified BMI, Norfolk QOL-DN Norfolk Quality of Life-Diabetic Neuropathy, QOL quality of life, R-ODS Rasch-built Overall Disability Scale, SD standard deviation
aThe Norfolk QOL-DN questionnaire [37] captures QOL components specifically relating to neuropathy, where higher score indicates worse QOL
bEQ-VAS records a respondent’s self-rated global health at the time of assessment, ranging from 0 (“the worst health you can imagine”) to 100 (“the best health you can imagine”) [36]
cLower R-ODS score indicates greater disability [44]
d10-MWT is a measure of gait speed that is calculated based on the mean time (seconds) taken to complete the 10-m walk across two assessments at each visit (imputed as 0 for patients unable to perform the walk), with lower gait speeds indicating worse ambulatory function
emBMI is calculated as BMI (in kg/m2) × serum albumin (in g/L), with lower scores indicating worse nutritional status
fRange 0–100%; where 100% indicates “normal no complaints,” 90% indicates “able to carry on normal activity,” 80% indicates “able to carry on normal activity with effort,” 70% indicates “unable to carry on normal activity/active work,” and 60% indicates “unable to work and requiring occasional assistance”
Measures of QOL
Norfolk QOL-DN
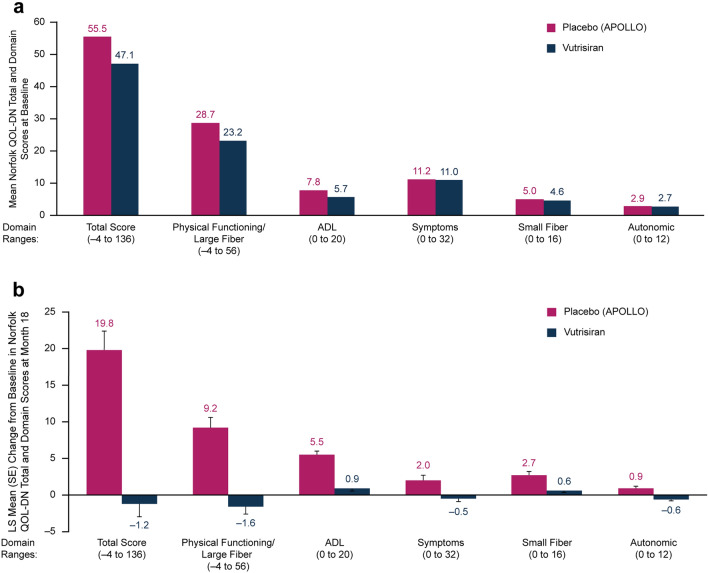
Baseline values for the Norfolk QOL-DN total and individual domain scores (Fig. 1a) reveal notable QOL impairment for patients in both the vutrisiran and external placebo groups. The Norfolk QOL-DN total score at baseline varied by disease stage in both groups, reflected by patients with baseline PND scores IIIa/IIIb reporting higher Norfolk QOL-DN total scores (indicating lower QOL) than those with lower baseline PND scores (Fig. 2). As reported previously, treatment with vutrisiran significantly improved Norfolk QOL-DN total score compared with the external placebo group at month 9 (least squares mean difference [LSMD] [95% confidence interval (CI)] in change from baseline: –16.2 [–21.7, –10.8]) and month 18 (LSMD [95% CI] in change from baseline: –21.0 [–27.1, –14.9]; p = 1.84 × 10–10) [35]. Compared with baseline, Norfolk QOL-DN total score improved at months 9 and 18 in the vutrisiran group and worsened in the external placebo group (LS mean ± standard error [SE] change in Norfolk QOL-DN total score from baseline: month 9, –3.3 [1.7] [vutrisiran] vs. +12.9 [2.2] [external placebo] points; month 18, –1.2 [1.8] vs. +19.8 [2.6] points, respectively). At month 18, 56.8% of the patients who received vutrisiran had an improvement (any decrease from baseline) in Norfolk QOL-DN total score compared with 10.4% of patients in the external placebo group, representing an odds ratio of 11.3 (95% CI: 5.0, 25.7; nominal p = 9.37 × 10–11) for improvement from baseline with vutrisiran versus external placebo.
Fig. 1.
Norfolk QOL-DN assessments in the HELIOS-A study. a Mean Norfolk QOL-DN total and domain scores at baseline in patients receiving vutrisiran or placebo (APOLLO). b LS mean change in Norfolk QOL-DN total score and in individual Norfolk QOL-DN domains from baseline to month 18 in patients receiving vutrisiran or placebo (APOLLO). Score ranges for each domain are shown on the x-axis; a higher score indicates worse quality of life. ADL activities of daily living, LS least squares, Norfolk QOL-DN Norfolk Quality of Life-Diabetic Neuropathy, SE standard error
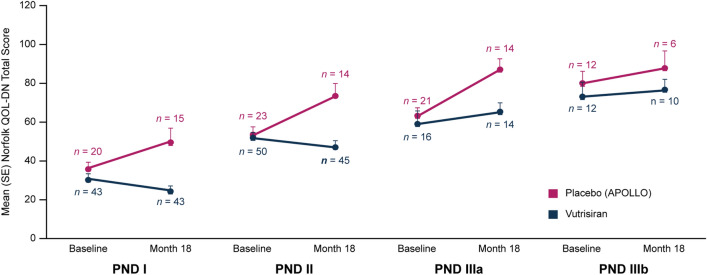
Fig. 2.
Mean Norfolk QOL-DN total score at baseline and at month 18 in HELIOS-A patients receiving vutrisiran or placebo (APOLLO), stratified according to baseline PND score. The Norfolk QOL-DN questionnaire captures QOL components specifically relating to neuropathy. The total score range is –4 to 136, where higher score indicates worse QOL. Norfolk QOL-DN Norfolk Quality of Life-Diabetic Neuropathy, PND polyneuropathy disability, QOL quality of life, SE standard error
The benefit in Norfolk QOL-DN observed in the vutrisiran group compared with the external placebo group was evident across all baseline PND score categories. However, patients with less advanced PND scores at baseline (i.e., I and II) exhibited greater benefit with vutrisiran over 18 months (Fig. 2). In contrast, worsening of QOL over 18 months was observed in the external placebo group across all baseline disease stages.
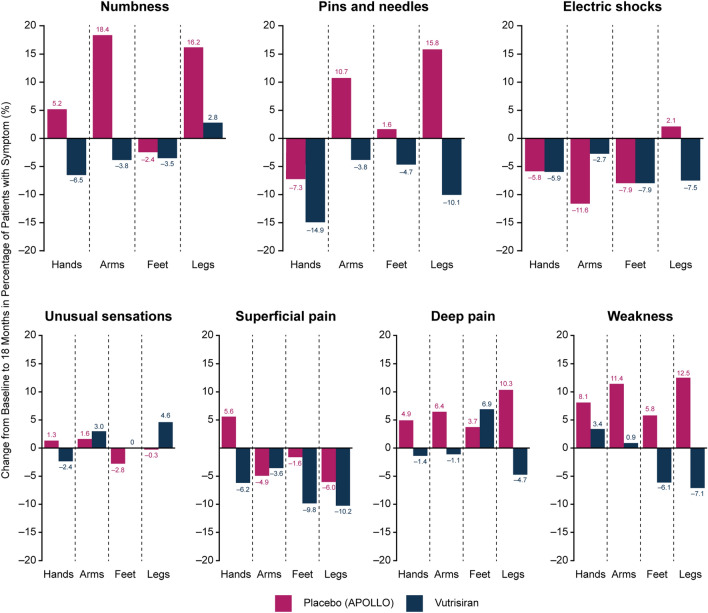
The treatment effect favoring vutrisiran over external placebo was consistent across all Norfolk QOL-DN domains at month 18 (Fig. 1b). More detailed analysis of specific components of the symptoms domain of the Norfolk QOL-DN (numbness, pins and needles, electric shocks, unusual sensations, superficial pain, deep pain, and weakness) also demonstrated a trend generally favoring vutrisiran treatment over external placebo, as measured by changes from baseline to month 18 in the percentage of patients experiencing these symptoms at specified extremity locations (i.e., hands, arms, feet, and legs) in each treatment group (Fig. 3).
Fig. 3.
Change from baseline at 18 months in percentage of patients reporting a specific symptom of the Norfolk QOL-DN symptoms domain at each of four specified extremity locations (hands, arms, feet, and legs). Norfolk QOL-DN Norfolk Quality of Life-Diabetic Neuropathy
EQ-VAS
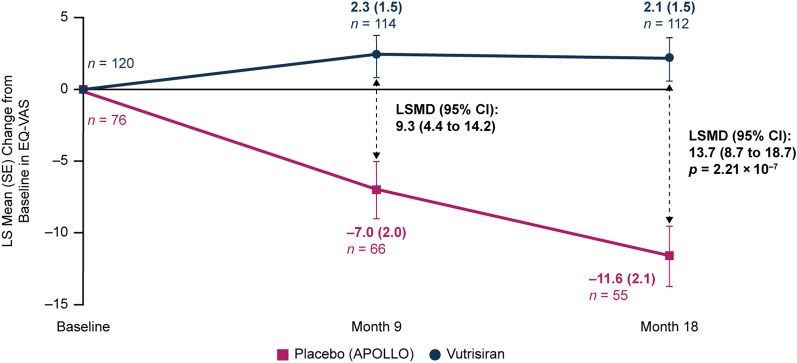
A significant benefit in patients’ self-rated global health, as measured by EQ-VAS, was observed with vutrisiran treatment compared with the external placebo group at month 9 (LSMD [95% CI] in change from baseline: 9.3 [4.4, 14.2]) and month 18 (LSMD [95% CI] in change from baseline: 13.7 [8.7, 18.7]; nominal p = 2.21 × 10–7) (Fig. 4). The initial improvement in EQ-VAS from baseline to month 9 was maintained over 18 months in the vutrisiran group (LS mean [± SE] change from baseline at months 9 and 18, +2.3 [1.5] and +2.1 [1.5] points, respectively), while patients in the external placebo group worsened from baseline at months 9 and 18 (–7.0 [2.0] and –11.6 [2.1] points, respectively) (Fig. 4).
Fig. 4.
LS mean change in EQ-VAS score (range 1–100) from baseline to month 18 in patients receiving vutrisiran or placebo (APOLLO). At baseline, the mean (± SD) EQ-VAS was 64.5 (18.5) in the vutrisiran group and 54.6 (18.0) in the external placebo group. mITT population. Value of n is the number of evaluable patients at each timepoint. Data plotted are MMRM data. EQ-VAS records a respondent’s self-rated health at the time of assessment (range 0–100), with minimum and maximum values ranging from “the worst health you can imagine” (0) to “the best health you can imagine” (100). CI confidence interval, EQ-VAS EuroQoL-Visual Analog Scale, LS least squares, LSMD LS mean difference, mITT modified intent-to-treat, MMRM mixed-effects model for repeated measures, SD standard deviation, SE standard error
Measures of Physical Function
Gait Speed: 10-MWT
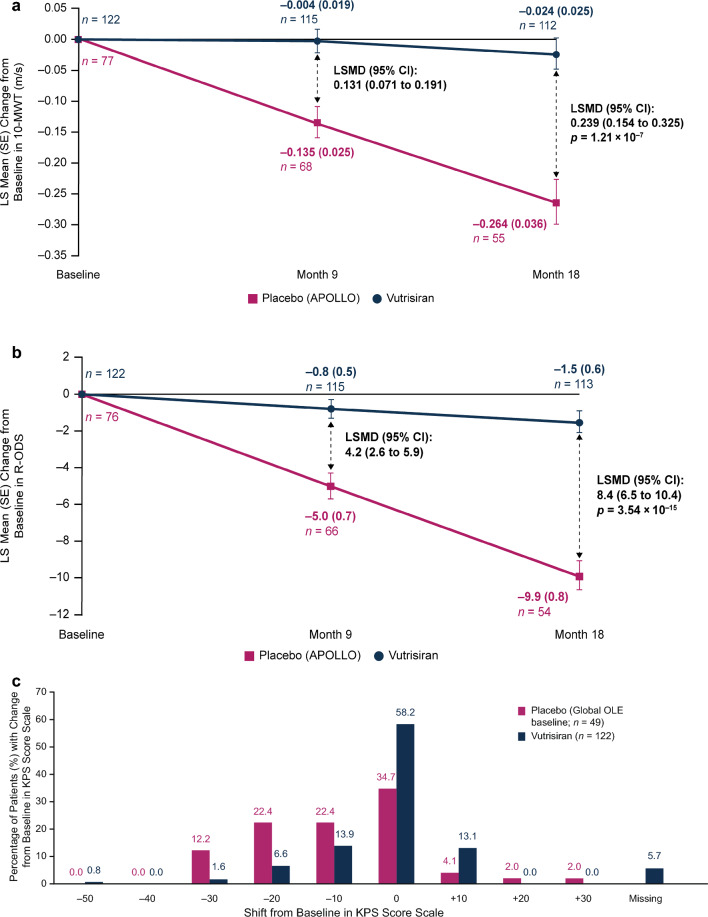
A significant benefit in gait speed, as measured by 10-MWT, was observed in patients in the vutrisiran group compared with the external placebo group at month 9 (LSMD [95% CI] in change from baseline: 0.131 m/s [0.071, 0.191]) and at month 18 (LSMD [95% CI] in change from baseline: 0.239 m/s [0.154, 0.325]; p = 1.21 × 10–7) (Fig. 5a). In the vutrisiran group, LS mean (± SE) change from baseline in gait speed on the 10-MWT showed a slight decrease of –0.004 (0.019) m/s and –0.024 (0.025) m/s, at months 9 and 18, respectively, while patients in the external placebo group showed a pronounced decline compared with baseline of –0.135 (0.025) m/s and –0.264 (0.036) m/s, respectively (Fig. 5a).
Fig. 5.
a LS mean change (MMRM analysis) in 10-MWT from baseline to month 18 in patients receiving vutrisiran or placebo (APOLLO). 10-MWT is a measure of gait speed that is calculated based on the mean time (seconds) taken to complete the 10-m walk across two assessments at each visit (imputed as 0 for patients unable to perform the walk), with lower gait speeds indicating worse ambulatory function. At baseline, the mean (± SD) gait speed on 10-MWT was 1.006 (0.393) m/s in the vutrisiran group and 0.790 (0.319) m/s in the external placebo group. b LS mean change (MMRM analysis) in overall R-ODS score from baseline to month 18 in patients receiving vutrisiran or placebo (APOLLO). R-ODS score range is 0–48, with lower scores indicating greater disability. At baseline, the mean (± SD) R-ODS score was 34.1 (11.0) in the vutrisiran group and 29.8 (10.8) in the external placebo group. c Distribution of patients according to level of shift in KPS score scale from HELIOS-A baseline to month 18 in the vutrisiran arm or from APOLLO baseline to Global OLE baseline in the placebo arm. Distribution of KPS scores at baseline in the vutrisiran arm of HELIOS-A and in the external placebo comparator arm is shown in Table 1. KPS is presented on an 11-point functional impairment scale (starting with 0% and increasing up to 100% in 10% increments) in which patients are classified along a range from normal functioning (100%) to dead (0%). Improvement is defined as an increase in KPS score from baseline. Lower scores indicate a lower ability to perform activities and a worse survival prognosis [45]. 10-MWT 10-m walk test, CI confidence interval, KPS Karnofsky Performance Status, LS least squares, LSMD LS mean difference, MMRM mixed-effects model for repeated measures, OLE open-label extension, R-ODS Rasch-built Overall Disability Scale, SD standard deviation, SE standard error
Disability (Activity and Social Participation Limitations): R-ODS
A significant benefit in R-ODS, which measures patients’ limitations in daily activities and social participation, was observed with vutrisiran treatment compared with the external placebo group at month 9 (LSMD [95% CI] in change from baseline: 4.2 [2.6, 5.9]) and month 18 (LSMD [95% CI] in change from baseline: 8.4 [6.5, 10.4]; p = 3.54 × 10–15). In the vutrisiran group, LS mean (± SE) change from baseline in R-ODS showed a decrease of –0.8 (0.5) points and –1.5 (0.6) points at months 9 and 18, respectively, while patients in the external placebo group showed a pronounced decline compared with baseline of –5.0 (0.7) points and –9.9 (0.8) points, respectively (Fig. 5b).
Performance Status: KPS
Baseline KPS data in the vutrisiran and external placebo groups are shown in Table 1; the majority of patients had a KPS score of 70–80% at baseline (unable to carry on normal activity/active work [70%] or able to carry on normal activity with effort [80%]). As demonstrated in Fig. 5c, a higher proportion of vutrisiran-treated patients than placebo-treated patients had stable (58.2% vs. 34.7%) or improved (13.1% vs. 8.1%) KPS at month 18 compared with baseline.
Measure of Nutritional Status
mBMI
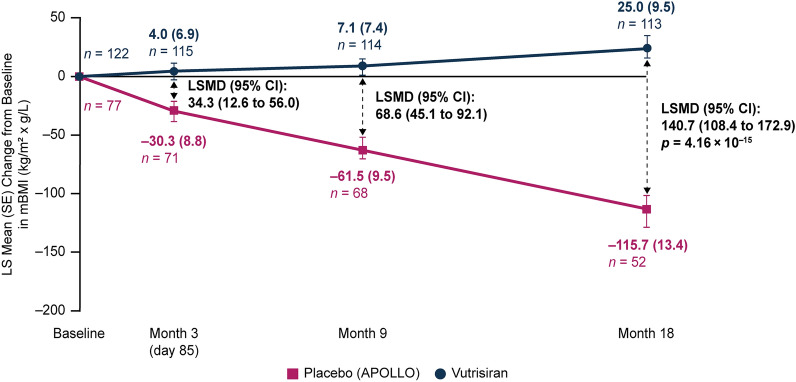
A significant benefit in nutritional status, as assessed by mBMI, was observed with vutrisiran treatment compared with the external placebo group at month 9 (LSMD [95% CI] in change from baseline: 68.6 kg/m2 × g/L [45.1, 92.1]) and month 18 (LSMD [95% CI] in change from baseline: 140.7 kg/m2 × g/L [108.4, 172.9]; p = 4.16 × 10–15) (Fig. 6). The favorable effect of vutrisiran on mBMI versus external placebo was observed as early as the first post-baseline assessment at month 3 (LSMD [95% CI] in change from baseline: 34.3 kg/m2 × g/L [12.6, 56.0]). At months 3, 9, and 18, patients in the vutrisiran group improved in their mBMI compared with baseline (LS mean [± SE] change from baseline, +4.0 [6.9], +7.1 [7.4], and +25.0 [9.5] kg/m2 × g/L, respectively), while patients in the external placebo group worsened at months 3, 9, and 18 compared with baseline (–30.3 [8.8], –61.5 [9.5], and –115.7 [13.4] kg/m2 × g/L, respectively) (Fig. 6).
Fig. 6.
LS mean change in mBMI from baseline to month 18 in patients receiving vutrisiran or placebo (APOLLO). At baseline, the mean (± SD) mBMI was 1057.4 (233.8) kg/m2 × g/L in the vutrisiran group and 989.9 (214.2) kg/m2 × g/L in the external placebo group. mITT population. Value of n is the number of evaluable patients at each timepoint. Data plotted are MMRM data. mBMI is calculated as BMI (in kg/m2) × serum albumin (in g/L), with lower scores indicating worse nutritional status. BMI body mass index, CI confidence interval, LS least squares, LSMD LS mean difference, mBMI modified BMI, mITT modified intent-to-treat, MMRM mixed-effects model for repeated measures, SD standard deviation, SE standard error
Discussion
Patients with ATTRv amyloidosis experience multisystem impairment as a result of peripheral and autonomic neuropathy, and/or cardiomyopathy, that collectively leads to a substantial and progressive impact on physical functioning and QOL [23, 37–39]. This is highlighted by the poor baseline scores across multiple QOL measures, consistently seen in clinical studies such as APOLLO [19] and HELIOS-A. Furthermore, observational studies indicate that patients with ATTRv amyloidosis may have a similar or even greater level of QOL impairment than patients with other chronic conditions such as Crohn's disease, cancer, and heart disease [40, 41], and that physical functioning and health-related QOL worsen over time with ATTRv amyloidosis disease progression [19, 25, 38].
In this analysis of patients with ATTRv amyloidosis with polyneuropathy in HELIOS-A, vutrisiran treatment over 18 months was associated with significant benefit compared with external placebo in measures of QOL (Norfolk QOL-DN, EQ-VAS), physical function (10-MWT, R-ODS, KPS), and nutritional status (mBMI). Indeed, within the Norfolk QOL-DN symptoms domain, results in terms of changes in symptom prevalence favored vutrisiran compared with external placebo for the majority of symptoms at most anatomical sites assessed over the course of treatment. The benefit of vutrisiran compared with external placebo was observed at month 9 or earlier for most measures assessed. Furthermore, vutrisiran-treated patients experienced improvement, stabilization, or minimal worsening in these measures at months 9 and 18 compared with their own pre-treatment baseline. While some measures, such as 10-MWT and R-ODS, did not improve from baseline, evidence of stabilization (or minimal worsening) over 18 months contrasts with the expected course of the disease, signaling a meaningful clinical benefit in a disease state where QOL and physical function would otherwise progressively worsen over time. Indeed, improvements from baseline or stabilization are particularly notable given the marked deteriorations seen in the external placebo group, as well as in the placebo groups of other clinical trials [19, 26–28] and in untreated patients in natural history studies [20, 25].
The favorable effects of vutrisiran treatment on QOL observed in HELIOS-A are consistent with the results of the APOLLO study of patisiran, an RNAi therapeutic with a similar mechanism of action, in patients with ATTRv amyloidosis with polyneuropathy. In the APOLLO study, patisiran yielded treatment benefits on scores for Norfolk QOL-DN (overall and across all domains), EQ-5D-5L, EQ-VAS, R-ODS, mBMI, and COMPASS-31 (a measure of autonomic neuropathy symptoms), compared with placebo over 18 months [19]. This consistency of results across studies of RNAi therapeutics supports the therapeutic strategy of TTR lowering with RNAi to improve or prevent the worsening of multiple disease-relevant endpoints in ATTRv amyloidosis.
The durability of the beneficial effects of RNAi therapeutics on QOL has been demonstrated in the patisiran Global OLE study, with the improvement from baseline in Norfolk QOL-DN scores observed in APOLLO being sustained over an additional 12 months of treatment in patients who received patisiran in APOLLO. Patients who were treated with placebo during APOLLO, and initiated patisiran in the Global OLE, also showed improvement in Norfolk QOL-DN relative to their Global OLE baseline [21]. However, the level of QOL attained at 12 months following a switch to patisiran did not reach that of patients who had received patisiran treatment from the start of APOLLO, likely due to irreversible disease progression while on placebo during APOLLO. Similarly, in this current analysis, patients with lower baseline PND scores (i.e., a less severe disease state) at vutrisiran initiation demonstrated a larger benefit and retained a higher level of QOL based on Norfolk QOL-DN score at 18 months of treatment. These findings further emphasize that effective treatment of ATTRv amyloidosis, early in an individual patient’s disease course, is critical to preserve QOL at as high a level as possible.
In addition to its acceptable safety profile [35] and favorable effects on QOL, the Q3M subcutaneous dosing regimen of vutrisiran has the potential to reduce the overall treatment burden on patients. This may better suit and improve not only the patient's life but also the lives of their caregivers and/or family members, who are frequently involved in the patient’s disease management. A study in the United States and Spain, and a separate cross-sectional online survey, both showed a substantial disease-related burden on the caregivers of patients with ATTR amyloidosis, who experienced poor mental well-being, characterized by depression and anxiety as well as reduced work productivity [42, 43].
This study has limitations, including its open-label design with the use of an external placebo control group, rather than a double-blind study design with a within-trial placebo group. HELIOS-A was designed to allow an efficient trial in which all patients could receive active treatment, given the availability of multiple effective therapies. Use of an external placebo comparator arm was supported by the well-defined natural history of the disease, with consistent and predictable disease progression observed in placebo arms and natural history cohorts across studies of patients with ATTRv amyloidosis with polyneuropathy. Furthermore, the results of patisiran in the APOLLO study supported that stabilization or improvement in measures of the disease could be expected by 9 months of vutrisiran treatment, based on the mechanism of action. The similarities between HELIOS-A and APOLLO with respect to inclusion and exclusion criteria, endpoints, and the timing of endpoints assessment also supported the use of the APOLLO placebo group as an external control in HELIOS-A.
A further potential limitation of this analysis is that measures of QOL specifically related to cardiac involvement were not assessed in HELIOS-A. While HELIOS-A recruited patients based on neuropathy impairment, approximately one-third of randomized patients also had evidence of cardiac involvement [35], and thus may have experienced benefit from vutrisiran treatment across cardiology-specific QOL domains that were not assessed here. The safety and efficacy of vutrisiran in patients with ATTR amyloidosis with cardiomyopathy are being assessed in the ongoing HELIOS-B study (NCT04153149), which includes the Kansas City Cardiomyopathy Questionnaire, a measure of patients’ perception of health status and QOL with a specific focus on impacts of cardiomyopathy, as a predefined secondary endpoint.
Conclusions
Findings from the HELIOS-A study demonstrate that vutrisiran treatment over 18 months provides significant clinical benefits compared with the external placebo group in multiple measures of QOL and physical function. Additionally, vutrisiran led to stabilization or improvement from baseline in several of those measures that would otherwise be expected to deteriorate rapidly and irreversibly based on the well-established natural course of ATTRv amyloidosis with polyneuropathy. These benefits of vutrisiran were seen in the overall study population and were most pronounced in patients with earlier-stage disease at baseline, highlighting the importance of initiating effective treatment for ATTRv amyloidosis with polyneuropathy early in the course of disease, in order to minimize the progressive negative impact on QOL and physical function. These data are consistent with the positive effect of vutrisiran on polyneuropathy, measured by mNIS + 7, observed in HELIOS-A compared with the external placebo group, and support the benefit of RNAi therapeutics for the treatment of patients with ATTRv amyloidosis with polyneuropathy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors would like to thank the members of the HELIOS-A Collaborators Study Group for their work on the study. A full list of the members of the HELIOS-A Collaborators Study Group is provided in the Supplementary Material. The authors would also like to thank the patients and their families for their participation in the HELIOS-A study.
Funding
This study was funded by Alnylam Pharmaceuticals Inc. The funder collaborated with authors during study design, data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and took final responsibility for the decision to submit the manuscript for publication. Alnylam Pharmaceuticals Inc. funded the journal’s rapid service publication fee.
Medical Writing/Editorial Assistance
Medical writing assistance was provided by Kristen Brown, PhD from Adelphi Communications Ltd (Macclesfield, UK) in accordance with GPP4 guidelines, and funded by Alnylam Pharmaceuticals Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Laura Obici, Senda Ajroud-Driss, Kon-Ping Lin, John L. Berk, Julian D. Gillmore, Parag Kale, Haruki Koike, David Danese, Emre Aldinc, and David Adams all contributed to data interpretation, drafting, and critical review of the manuscript. Chongshu Chen contributed to data analysis, data interpretation, drafting, and critical review of the manuscript. John Vest contributed to study design/methodology, data interpretation, drafting, and critical review of the manuscript.
Prior Presentation
Some data from this manuscript have been presented at the Peripheral Nerve Society Annual Meeting, Miami, FL, USA, May 14–17, 2022.
Disclosures
Senda Ajroud-Driss reports participating on Advisory Boards for Amylyx Pharmaceuticals, Biogen Inc., and Orphazyme. John L. Berk reports consultancy for Akcea Therapeutics, Corino Therapeutics, and Ionis Pharmaceuticals and research funding from Alnylam Pharmaceuticals, Eidos Therapeutics, and Ionis Pharmaceuticals. David Adams reports consultancy for Alnylam Pharmaceuticals, Eidos, and Pfizer Inc. Julian D. Gillmore reports consultancy for Alnylam Pharmaceuticals, AstraZeneca, ATTRalus, Intellia Therapeutics, Ionis Pharmaceuticals, and Pfizer Inc. Kon-Ping Lin has nothing to disclose. Parag Kale reports consultancy for Alnylam Pharmaceuticals. Haruki Koike reports being a member of the Editorial Board of Neurology and Therapy and reports consultancy for Alnylam Pharmaceuticals and Pfizer Inc. Dr. Koike’s current affiliation is Division of Neurology, Department of Internal Medicine, Saga University Faculty of Medicine, Saga, Japan. David Danese, Emre Aldinc, Chongshu Chen, and John Vest are employees of Alnylam Pharmaceuticals and also report ownership of equity in Alnylam Pharmaceuticals. Laura Obici reports speakers bureau fees from Akcea Therapeutics, Alnylam Pharmaceuticals, Pfizer Inc., and SOBI.
Compliance with Ethics Guidelines
The HELIOS-A study (NCT03759379) was conducted in accordance with the International Conference of Harmonisation guidelines for Good Clinical Practice, local regulatory requirements, and the principles of the Declaration of Helsinki. The study was approved by ethics committees or institutional review board for each study site (listed in the Supplementary Material), and all patients provided written informed consent.
Data Availability
Anonymized individual participant data that support these results would be made available in a secure-access environment 12 months after study completion and when the product and indication have been approved for no less than 12 months in the US and/or the EU. Access will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.
Contributor Information
Laura Obici, Email: LObici@smatteo.pv.it.
the HELIOS-A Collaborators Study Group:
Jonas Wixner, Rolf Backlund, Björn Pilebro, Intissar Anan, Fredrik Edbom, Anna Ekman, Sandra Arvidsson, Ulrika Englund, Karin Söderberg, Erik Nordh, Erica Uneus, Kristin Samuelsson, Anna Nilzen, Rayomand Press, Mirjam Bilecen, Teresa Coelho, Marta Novais, Patricia Rodrigues, Ana Martins da Silva, Inês Cardoso, Carla Rodrigues, Joana Ramalho, Helder Martins, Mónica Silva, Nádia Guimaraes, Javier Perez, Antonio Hipólito Reis, Julia Monte, Natalia Ferreira, Cristina Alves, Marcio Cardoso, Ricardo Teixeira, Isabel Conceição, Filipa Lamas, Miguel Oliveira Santos, Catarina Campos, Conceiçao de Azevedo Coutinho, José Castro, Isabel Castro, Daniela Silva, Susana Gonçalves, Eleonora Di Buduo, Claudia Sforzini, Roberta Mussinelli, Vittorio Rosti, Alessandro Lozza, Anna Racchi, Mario Sabatelli, Marco Luigetti, Giulia Bisogni, Angela Romano, Valeria Guglielmino, Andrea Di Paolantonio, Daniela Bernardo, Giuseppe Vita, Anna Mazzeo, Massimo Russo, Davide Pareyson, Daniela Calabrese, Silvia Fenu, Paola Saveri, Hans Nienhuis, Geert Bokhorst, Carlien Roos, Margriet Couperus, Greetje De Jong, Anne Brunger, Gea Drost, Fiete Lange, Adinda Colauto, Márcia Waddington-Cruz, Aline Abreu, Roberto Coury Pedrosa, Renata Gervais de Santa Rosa, Moisés Dias, Fetra Rakotondratafika, Andoni Echaniz-Laguna, Cecile Cauquil, Céline Labeyrie, Guillemette Beaudonnet, Yasmine Boubrit, Amina Gaouar, Halima Bourenane, Shahram Attarian, El Khansa Yahia, Annie Verschueren, Aude-Marie Grapperon, Emilien Delmont, Violaine Planté-Bordeneuve, Laetitia Vervoitte, Samar S. Ayache, Philippe Le Corvoisier, Raphaele Arrouasse, Thierry Gendre, Laure Abou Chakra, Cécile Focsénéanu, Caroline Barau, Guilhem Sole, Laurie Belin, Marie Helene Violleau, Fanny Duval-Bontemps, Rami Massie, Xin Dong, Francisco Muñoz-Beamud, Sandra García Garrido, Cristina Borrachero, Alvaro Gragera Martinez, Lucía Galán Dávila, Marta Palacios, Laura M. Vicente, Leopoldo Perez de Isla, Carlos Casasnovas, Carles Díez López, Elena Fabra, José González-Costello, Sonia Guerrero, Sergi Yun Viladomat, Yurema Martinez, Valentina Velez-Santamaria, Velina Nedkova-Hristova, Pablo Garcia Pavia, Ariadna Gonzalez Segovia, Fernando De Frutos, Esther Gonzalez-Lopez, Fernando Dominguez, Luis E. Escobar-López, Eva Cabrera-Romero, Paula Sánchez Gismera, María de la Iglesia, Fernando Martinez Valle, Gonzalo Mazuela Aguila, Karen Lorite, Núria Raguer, Pilar Suñé, Pablo Piera, Carlos Ortega, Carla Aguilar, Gisela Gili, Hartmut Schmidt, Christel Langenstroer, Anna Hüsing-Kabar, Iyad Kabar, Matthias Schilling, Frauke Friebel, Phil-Robin Tepasse, Frank Birklein, Monika Firros, Fabiola Escolano-Lozano, Caitlin Brueckner, Vanessa Bahnam, Michelle C. Kaku, K. H. Vincent Lau, Janice Wiesman, Martha Grogan, Susanna Miller, Janell Frantz, Diane C. Schmidt, Omar AbouEzzeddine, Wayne Miller, Grace Lin, Morie Gertz, Angela Dispenzieri, Thomas Brannagan, Raisy Fayerman, Elizabeth DuVerger, Jorge Cabrera, Mathew S. Maurer, Christina M. Ulane, Louis H. Weimer, Stephen Tsang, Jeffrey Shije, Nathan Carberry, Sai Si Thu, Dianna Quan, Brianna Blume, J. Scott Overcash, He Pu, Kia Lee, Hanh Chu, Karla Zepeda, Michael Waters, Thao Vuong, Derya Coskun, Kimberly Quillin, Allison Davis, Michael Polydefkis, Jing Ye, Xiaoling Li, Mohammad Khoshnoodi, Geno Vista, Tae Hwan Chung, Michele Watt, Dan Tsottles, Ahmad Masri, Dayna Carlson, Brian Drachman, Patricia Divito, Hansie Mathelier, Margaret Shanks, Karen Maslowski, Sami Khella, Janice Pieretti, Benjamin Joslin, Emma Schmidt, Miriam Freimer, Julie Agriesti, Fabio Barroso, Florencia Picone, Andrea Lautre, Lucas Orellana, Wenqin Du, Joost Felius, Alejandra González-Duarte, Karla Cardenas Soto, Rebecca Traub, Manisha Chopra, Chi-Chao Chao, Chia-Hua Hsu, Li-Kai Tsai, Ming-Jen Lee, Jen-Jen Su, Sung-Tsang Hsieh, Hsueh-Wen Hsueh, Hsi-Chieh Chou, Byoung-Joon Kim, Hyesun Kang, Ju-Hong Min, Eun-Seok Jeon, Yeon Hak Chung, Jae Hong Park, Jeeyoung Oh, Hyun Joo Jeong, Ivailo Tournev, Sashka Zhelyazkova, Yohei Misumi, Yumiko Sakamoto, Nami Hashimoto, Yoshimi Misumi, Aya Takahashi, Mitsuharu Ueda, Teruaki Masuda, Akihiko Ueda, Masahisa Katsuno, Kazuki Tajima, Momoko Sumi, Fujiko Hasegawa, Takahiro Okumura, Masahiro Iijima, Yuki Fukami, Daisuke Ito, Yoshiyuki Kishimoto, Tomoyuki Kazuta, Katsuhiko Kato, Naohiro Mouri, Soma Furukawa, Ryoji Nishi, Yoshiki Sekijima, Keiko Ito, Nagaaki Kato, Dai Kishida, Hideki Mochizuki, Kaori Okada, Kurumi Ohashi, Kensuke Ikenaka, Masayuki Nakamori, Makoto Kinoshita, Bella Ruth Mapalo, Steven Law, Liza Chacko, Helen Lachmann, Oliver Cohen, Yousuf Siu Kay Razvi, Sindhu Varughese, Ana Martinez-Naharro, Richard Orrell, Marianna Fontana, Lisa Rannigan, Sarah Louth, Eleni Zamba-Papanicolaou, Demetra Charalamnibous, Rana Abu Manneh, Kleopas Kleopa, Theodoros Christodoulides, Savvas Frangos, Michele Galganski-Cleanthous, Eftychia Gaglia, Irene Smoleski, Elena Kkolou, Andry Ploutarchou, Mariana Hanghiuc, Galini Chroidou, Olga Stylianou, Anastasia Krokou, Irene Zannetou, Efstathios Kastritis, Dimitra Papadopoulou, Ilias Spinasas, Panayiotis Bakalis, Nikolaos Kanellias, Despoina Fotiou, Ioanna Dialoupi, Magdalini Migko, Maria Gavriatopoulou, Soon-Chai Low, Mark Taylor, Graeme Stewart, Helen Knight, Steve Vucic, Antonia Carroll, Matthew Silsby, Dan Suan, Simon Gibbs, Carmela Corfield, Suzana Jakicic, Hayden Jina, Stephen Ting, Shi Qin Wong, Peter Mollee, Lynda McKinley, Emad Abro, Dariusz Korczyk, Gauthier Remiche, Nick Alaerts, Fabienne De Veylder, Kristl Claeys, Elisa Debien, Joyce Cremers, Ann D’hondt, and Bram De Wel
References
- 1.Hanna M. Novel drugs targeting transthyretin amyloidosis. Curr Heart Fail Rep. 2014;11(1):50–57. doi: 10.1007/s11897-013-0182-4. [DOI] [PubMed] [Google Scholar]
- 2.Mohty D, Damy T, Cosnay P, Echahidi N, Casset-Senon D, Virot P, et al. Cardiac amyloidosis: updates in diagnosis and management. Arch Cardiovasc Dis. 2013;106(10):528–540. doi: 10.1016/j.acvd.2013.06.051. [DOI] [PubMed] [Google Scholar]
- 3.Adams D, Coelho T, Obici L, Merlini G, Mincheva Z, Suanprasert N, et al. Rapid progression of familial amyloidotic polyneuropathy: a multinational natural history study. Neurology. 2015;85(8):675–682. doi: 10.1212/WNL.0000000000001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–638. doi: 10.3109/07853890.2015.1068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly JW. Amyloid fibril formation and protein misassembly: a structural quest for insights into amyloid and prion diseases. Structure. 1997;5(5):595–600. doi: 10.1016/S0969-2126(97)00215-3. [DOI] [PubMed] [Google Scholar]
- 6.Koike H, Katsuno M. Ultrastructure in transthyretin amyloidosis: from pathophysiology to therapeutic insights. Biomedicines. 2019;7(1):11. doi: 10.3390/biomedicines7010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damy T, Judge DP, Kristen AV, Berthet K, Li H, Aarts J. Cardiac findings and events observed in an open-label clinical trial of tafamidis in patients with non-Val30Met and non-Val122Ile hereditary transthyretin amyloidosis. J Cardiovasc Transl Res. 2015;8(2):117–127. doi: 10.1007/s12265-015-9613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin SC, Robinson-Papp J. Amyloid neuropathies. Mt Sinai J Med. 2012;79(6):733–748. doi: 10.1002/msj.21352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conceição I, Gonzalez-Duarte A, Obici L, Schmidt HH, Simoneau D, Ong ML, et al. "Red-flag" symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2016;21(1):5–9. doi: 10.1111/jns.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. 2019;15(7):387–404. doi: 10.1038/s41582-019-0210-4. [DOI] [PubMed] [Google Scholar]
- 11.Rapezzi C, Quarta CC, Obici L, Perfetto F, Longhi S, Salvi F, et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34(7):520–528. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- 12.Coelho T, Maurer MS, Suhr OB. THAOS—the Transthyretin Amyloidosis Outcomes Survey: initial report on clinical manifestations in patients with hereditary and wild-type transthyretin amyloidosis. Curr Med Res Opin. 2013;29(1):63–76. doi: 10.1185/03007995.2012.754348. [DOI] [PubMed] [Google Scholar]
- 13.Castaño A, Drachman BM, Judge D, Maurer MS. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015;20(2):163–178. doi: 10.1007/s10741-014-9462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swiecicki PL, Zhen DB, Mauermann ML, Kyle RA, Zeldenrust SR, Grogan M, et al. Hereditary ATTR amyloidosis: a single-institution experience with 266 patients. Amyloid. 2015;22(2):123–131. doi: 10.3109/13506129.2015.1019610. [DOI] [PubMed] [Google Scholar]
- 15.Sattianayagam PT, Hahn AF, Whelan CJ, Gibbs SD, Pinney JH, Stangou AJ, et al. Cardiac phenotype and clinical outcome of familial amyloid polyneuropathy associated with transthyretin alanine 60 variant. Eur Heart J. 2012;33(9):1120–1127. doi: 10.1093/eurheartj/ehr383. [DOI] [PubMed] [Google Scholar]
- 16.Gertz MA, Kyle RA, Thibodeau SN. Familial amyloidosis: a study of 52 North American-born patients examined during a 30-year period. Mayo Clin Proc. 1992;67(5):428–440. doi: 10.1016/S0025-6196(12)60388-7. [DOI] [PubMed] [Google Scholar]
- 17.Adams D. Recent advances in the treatment of familial amyloid polyneuropathy. Ther Adv Neurol Disord. 2013;6(2):129–139. doi: 10.1177/1756285612470192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mariani LL, Lozeron P, Theaudin M, Mincheva Z, Signate A, Ducot B, et al. Genotype-phenotype correlation and course of transthyretin familial amyloid polyneuropathies in France. Ann Neurol. 2015;78(6):901–916. doi: 10.1002/ana.24519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obici L, Berk JL, González-Duarte A, Coelho T, Gillmore J, Schmidt HH, et al. Quality of life outcomes in APOLLO, the phase 3 trial of the RNAi therapeutic patisiran in patients with hereditary transthyretin-mediated amyloidosis. Amyloid. 2020;27(3):153–162. doi: 10.1080/13506129.2020.1730790. [DOI] [PubMed] [Google Scholar]
- 20.Wixner J, Mundayat R, Karayal ON, Anan I, Karling P, Suhr OB, et al. THAOS: gastrointestinal manifestations of transthyretin amyloidosis - common complications of a rare disease. Orphanet J Rare Dis. 2014;9:61. doi: 10.1186/1750-1172-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams D, Polydefkis M, González-Duarte A, Wixner J, Kristen AV, Schmidt HH, et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. 2021;20(1):49–59. doi: 10.1016/S1474-4422(20)30368-9. [DOI] [PubMed] [Google Scholar]
- 22.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, et al. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PubMed] [Google Scholar]
- 23.Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379(1):11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 24.Yarlas A, Lovley A, McCausland K, Brown D, Vera-Llonch M, Conceicao I, et al. Early data on long-term impact of inotersen on quality-of-life in patients with hereditary transthyretin amyloidosis polyneuropathy: open-label extension of NEURO-TTR. Neurol Ther. 2021;10(2):865–886. doi: 10.1007/s40120-021-00268-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ines M, Coelho T, Conceicao I, Ferreira L, de Carvalho M, Costa J. Health-related quality of life in hereditary transthyretin amyloidosis polyneuropathy: a prospective, observational study. Orphanet J Rare Dis. 2020;15(1):67. doi: 10.1186/s13023-020-1340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berk JL, Suhr OB, Obici L, Sekijima Y, Zeldenrust SR, Yamashita T, et al. Repurposing diflunisal for familial amyloid polyneuropathy: a randomized clinical trial. JAMA. 2013;310(24):2658–2667. doi: 10.1001/jama.2013.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coelho T, Yarlas A, Waddington-Cruz M, White MK, Sikora Kessler A, Lovley A, et al. Inotersen preserves or improves quality of life in hereditary transthyretin amyloidosis. J Neurol. 2020;267(4):1070–1079. doi: 10.1007/s00415-019-09671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Plante-Bordeneuve V, Lozeron P, et al. Tafamidis for transthyretin familial amyloid polyneuropathy: a randomized, controlled trial. Neurology. 2012;79(8):785–792. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alnylam Pharmaceuticals Inc. US prescribing information: ONPATTRO (patisiran) lipid complex injection, for intravenous use. Food and Drug Administration. 2020. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210922s007lbl.pdf. Accessed 5 Jul 2023.
- 30.Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc. 2014;136(49):16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 31.Habtemariam BA, Karsten V, Attarwala H, Goel V, Melch M, Clausen VA, et al. Single-dose pharmacokinetics and pharmacodynamics of transthyretin targeting N-acetylgalactosamine-small interfering ribonucleic acid conjugate, vutrisiran, in healthy subjects. Clin Pharmacol Ther. 2021;109(2):372–382. doi: 10.1002/cpt.1974. [DOI] [PubMed] [Google Scholar]
- 32.Alnylam Pharmaceuticals Inc. US prescribing information: AMVUTTRA (vutrisiran) injection, for subcutaneous use. Food and Drug Administration. 2022. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/215515s000lbl.pdf. Accessed 5 July 2023.
- 33.Soprano DR, Herbert J, Soprano KJ, Schon EA, Goodman DS. Demonstration of transthyretin mRNA in the brain and other extrahepatic tissues in the rat. J Biol Chem. 1985;260(21):11793–11798. doi: 10.1016/S0021-9258(17)39100-7. [DOI] [PubMed] [Google Scholar]
- 34.Holmgren G, Steen L, Ekstedt J, Groth CG, Ericzon BG, Eriksson S, et al. Biochemical effect of liver transplantation in two Swedish patients with familial amyloidotic polyneuropathy (FAP-met30) Clin Genet. 1991;40(3):242–246. doi: 10.1111/j.1399-0004.1991.tb03085.x. [DOI] [PubMed] [Google Scholar]
- 35.Adams D, Tournev IL, Taylor MS, Coelho T, Plante-Bordeneuve V, Berk JL, et al. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: a randomized clinical trial. Amyloid. 2023;30:18–26. doi: 10.1080/13506129.2022.2091985. [DOI] [PubMed] [Google Scholar]
- 36.van Reenen M, Janssen B. EQ-5D-5L User Guide: Basic information on how to use the EQ-5D-5L instrument. 2015. Available from: https://euroqol.org/wp-content/uploads/2021/01/EQ-5D-5LUserguide-08-0421.pdf. Accessed 27 Jan 2023.
- 37.Vinik EJ, Vinik AI, Paulson JF, Merkies IS, Packman J, Grogan DR, et al. Norfolk QOL-DN: validation of a patient reported outcome measure in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. 2014;19(2):104–114. doi: 10.1111/jns5.12059. [DOI] [PubMed] [Google Scholar]
- 38.Coelho T, Vinik A, Vinik EJ, Tripp T, Packman J, Grogan DR. Clinical measures in transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2017;55(3):323–332. doi: 10.1002/mus.25257. [DOI] [PubMed] [Google Scholar]
- 39.Dyck PJ, Adams D, Coelho T, Polydefkis M, Gonzalez-Duarte A, Kristen A, et al. Neuropathy progression in patients with hATTR amyloidosis: analysis of the APOLLO placebo arm. Peripheral Nerve Society (PNS); July 22–25, 2018 Baltimore, MD, USA: Poster.
- 40.Mitchell PM, Al-Janabi H, Richardson J, Iezzi A, Coast J. The relative impacts of disease on health status and capability wellbeing: a multi-country study. PLoS ONE. 2015;10(12):e0143590. doi: 10.1371/journal.pone.0143590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yarlas A, Gertz MA, Dasgupta NR, Obici L, Pollock M, Ackermann EJ, et al. Burden of hereditary transthyretin amyloidosis on quality of life. Muscle Nerve. 2019;60(2):169–175. doi: 10.1002/mus.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart M, Shaffer S, Murphy B, Loftus J, Alvir J, Cicchetti M, et al. Characterizing the high disease burden of transthyretin amyloidosis for patients and caregivers. Neurol Ther. 2018;7(2):349–364. doi: 10.1007/s40120-018-0106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Acaster S, Lo SH, Nestler-Parr S. A survey exploring caregiver burden and health-related quality of life in hereditary transthyretin amyloidosis. Orphanet J Rare Dis. 2023;18(1):17. doi: 10.1186/s13023-022-02601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Nes SI, Vanhoutte EK, van Doorn PA, Hermans M, Bakkers M, Kuitwaard K, et al. Rasch-built Overall Disability Scale (R-ODS) for immune-mediated peripheral neuropathies. Neurology. 2011;76(4):337–345. doi: 10.1212/WNL.0b013e318208824b. [DOI] [PubMed] [Google Scholar]
- 45.Péus D, Newcomb N, Hofer S. Appraisal of the Karnofsky Performance Status and proposal of a simple algorithmic system for its evaluation. BMC Med Inform Decis Mak. 2013;13:72. doi: 10.1186/1472-6947-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized individual participant data that support these results would be made available in a secure-access environment 12 months after study completion and when the product and indication have been approved for no less than 12 months in the US and/or the EU. Access will be provided contingent upon the approval of a research proposal and the execution of a data sharing agreement. Requests for access to data can be submitted via the website www.vivli.org.