Abstract
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system, characterized by chronic, inflammatory, demyelinating, and neurodegenerative processes. MS management relies on disease-modifying drugs that suppress/modulate the immune system. Cladribine tablets (CladT) have been approved by different health authorities for patients with various forms of relapsing MS. The drug has been demonstrated to deplete CD4+ and CD8+ T-cells, with a higher effect described in the former, and to decrease total CD19+, CD20+, and naive B-cell counts. COVID-19 is expected to become endemic, suggesting its potential infection risk for immuno-compromised patients, including MS patients treated with disease-modifying drugs. We report here the available data on disease-modifying drug-treated-MS patients and COVID-19 infection and vaccination, with a focus on CladT. MS patients treated with CladT are not at higher risk of developing severe COVID-19. While anti-SARS-CoV-2 vaccination is recommended in all MS patients with guidelines addressing vaccination timing according to the different disease-modifying drugs, no vaccination timing restrictions seem to be necessary for cladribine, based on its mechanism of action and available evidence. Published data suggest that CladT treatment does not impact the production of anti-SARS-CoV-2 antibodies after COVID-19 vaccination, possibly due to its relative sparing effect on naïve B-cells and the rapid B-cell reconstitution following treatment. Slightly lower specific T-cell responses are likely not impacting the risk of breakthrough COVID-19. It could be stated that cladribine’s transient effect on innate immune cells likely contributes to maintaining an adequate first line of defense against the SARS-CoV-2 virus.
Keywords: Multiple sclerosis, Cladribine, COVID-19
Key Summary Points
| Patients with multiple sclerosis (MS) treated with cladribine tablets (CladT) are not at higher risk of developing severe COVID-19. |
| CladT treatment does not impact the production of anti-SARS-CoV-2 antibodies after COVID-19 vaccination. |
| CladT treatment does not increase the risk of breakthrough COVID-19 infection. |
| CladT’s transient effect on innate immune cells likely contributes to maintaining an adequate first line of defense against the SARS-CoV-2 virus. |
Introduction
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system (CNS), characterized by chronic, inflammatory, demyelinating, and neurodegenerative processes [1].
Cladribine [2-chlorodeoxyadenosine (2-CdA)] is a synthetic chlorinated deoxyadenosine analog which is biologically active in selected cell types and provides targeted and sustained reduction of circulating T- and B-lymphocytes implicated in the pathogenesis of MS. Cladribine tablets (CladT) have been approved by different health authorities for patients with various forms of relapsing MS [2]. A series of sequential phosphorylation steps, led by deoxycytidine kinase (dCK), generate the 2-CdA active triphosphate (2-CdATP) compound that interferes with DNA synthesis leading to cell death through apoptosis [3, 4]. 2-CdATP accumulation is prevented by 5′-deoxynucleotidases (5′-NT) enzymes. The preferential action of cladribine on lymphocytes compared to other cell types is related to their high intracellular dCK/5′-NT ratio, causing accumulation of 2-CdATP [4]. Activated T- and B-cells are susceptible to cladribine-induced apoptosis due to enhanced dCK expression and kinase activity [5]. Considering T-cells, cladribine treatment depletes both CD4+ and CD8+ T-cells, with a higher effect described in the former [6], and naïve CD4+cells and memory CD4+ T-cells, that are maximally depleted at 13–24 weeks from the first CladT administration [7].
An even more significant effect of drug treatment is observed on peripheral B-cells, which decrease rapidly upon treatment, with a nadir observed at week 13 from the beginning of the treatment [8, 9].
Thereafter, total CD19+, CD20+, and naive B-cell counts were subsequently reconstituted, but memory B-cells remained reduced by 93–87% over 12 months after the first course of CladT [10]. These cells are the main target of Epstein–Barr virus (EBV) infection and they are necessary for its replication [11]. EBV has been indicated to have a causal role in MS [12]. However, no studies have investigated the impact of CladT on EBV-infected memory B cells [13].
The decrease in plasmablasts was slower, reaching a nadir at month 3; similarly, short-lived CD38+ plasma cells reached their nadir at month 3. Immunoglobulin (Ig)G and IgM levels remained within the normal range over the 12-month study period, suggesting that long-lived, CD138+ plasma cells were not affected by CladT treatment, albeit this has not been proven [10]. These effects on IgG and IgM were also confirmed by Moser et al. who demonstrated that no reduction in antibody levels against measles, mumps, rubella, varicella-zoster (VZV), hepatitis B, diphtheria toxin, and tetanus toxin was associated with CladT treatment [14].
The pronounced effect on memory B-cells in the first 2 months after CladT treatment, the reduction in memory B-cells over the 12 months, and the moderate decrease of different T-cell subtypes may contribute to cladribine effects [15]. Depletion of other innate immune cells has been observed, although to a lesser extent. Indeed, treatment with CladT transiently decreases natural killer (NK) cells [8] and dampens the pro-inflammatory and phagocytic function of activated macrophages [16].
The effect of cladribine on immune cells is associated with a reduction of peripheral blood levels of pro-inflammatory cytokines and chemokines expression of adhesion molecules and reduced migration of mononuclear cells [6, 17].
Despite the overall effect of cladribine on the immune system, the risk of infections upon treatment is not increased, except for VZV infection [17]. The safety of cladribine has been confirmed even further during the COVID-19 pandemic [18].
Here, we discuss the evidence gathered since 2020 on the impact of cladribine treatment on COVID-19 risk and severity, and the efficacy of anti-COVID-19 vaccination in CladT-treated patients with MS.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Multiple Sclerosis and COVID-19 Infection
Immunological Response to COVID-19 Infection
The coronavirus disease 19 (COVID-19) is caused by the severe acute respiratory coronavirus-2 (SARS-CoV-2) infection. The virus is an RNA virus whose envelope Spike (S)-protein plays a key role in the inhibition and transmission of the COVID-19 infection, as it mediates the adhesion of the virus to the angiotensin-converting enzyme-related carboxypeptidase 2 expressed on type II pneumocytes of the human lung [19]. Infection of type II pneumocytes induces a local inflammatory response by releasing several cytokines and chemokines that foster the recruitment of circulating leukocytes [20, 21]. COVID-19 stimulates innate immune responses and cellular immunity, with specific cytotoxic T-cells against the virus and B-cells with the production of neutralizing antibodies [20–22]. The clinical course of COVID-19 is highly variable among affected unvaccinated subjects, with up to one-third having an asymptomatic course [23] and about one out of ten affected subjects developing severe, life-threatening bilateral pneumonia, a hyper-inflammatory state with possible sepsis, and disseminated intravascular coagulation with multi-organ failures [24, 25]. Higher risk for severe/fatal COVID-19 includes older age, obesity [26], and comorbidities such as diabetes, chronic kidney disease, and cardiovascular disease [27].
Immune response to SARS-CoV-2 involves both innate and adaptive immunity. Mechanisms of innate immune response include secretion of type I interferon (IFN) molecules by infected cells, activation of toll-like receptors on innate cells, and killing (through apoptosis) of infected cells by NK cells [28]. In the adaptive compartment, T- and B-cells mediate immunity against SARS-CoV-2. Among T-cells, both CD4+ and CD8+ cells respond to the Spike protein [29]; T-cell responses correlate with B-cell responses, as measured by the production of specific antibodies [30]. Neutralizing antibodies, especially the ones against the anti-receptor binding domain (anti-RBD), have been shown to have a role in the primary clearance of the virus [20]. Still, they are not strictly essential for the recovery from COVID-19 infection [21, 31]. The severe course of COVID-19 is associated with early suppression, delayed and prolonged production of type I IFN [32, 33] depression of NK cell function, and T- and B- cell lymphopenia [25, 34].
Evidence suggests that B-cell and antibody production have a role in preventing re-infection in both previously infected and vaccinated subjects [35, 36]. Patients experiencing mild-to-moderate COVID-19 have CD4+ and CD8+ cell activation and higher numbers of antibody-producing cells; such effects are even more pronounced in patients with severe COVID-19 [29, 37, 38]. The above data support the critical role of both T- and B-cells in the control and resolution of COVID-19.
COVID-19 Infection in MS Patients Treated with Disease-Modifying Drugs
It is well known that infections in general carry a higher risk of worse outcome in people with MS [39, 40]. In the first year of the SARS-CoV-2 pandemic, after the first case series were published (reviewed in [41]), two studies investigated the severity of COVID-19 in MS patients in the French and Italian populations, respectively [42, 43]. In the French cohort enrolling 347 MS patients, no disease-modifying therapy carried an increased risk of severe COVID-19. Only three patients were being treated with CladT, preventing any conclusions on the risk of such treatment on COVID-19 severity. The larger Italian cohort enrolled 844 MS patients with suspected/confirmed COVID-19, finding that patients treated with anti-CD20 monoclonal antibodies disease-modifying drugs (ocrelizumab/rituximab) had 2.4-fold increased risk for developing severe COVID-19 compared to untreated patients. None of the 11 patients treated with CladT in the Italian cohort developed severe disease, although the small sample size did not allow to obtain specific results in the multivariate analysis [43]. These results were also confirmed in another study where only 2 out of the 27 patients treated with CladT were hospitalized, but none of them were admitted to intensive care nor needed ventilation [44]. Detailed clinical outcome was available for 700 patients [44]; the severe and fatal cases mainly occurred in untreated patients but with previous cardiovascular disease and severe disability. Of the 27 patients treated with CladT, 19 had mild symptoms, and 6 were hospitalized but did not need ventilatory support; none experienced severe or fatal COVID-19 disease. A retrospective study that reviewed 873 published cases of MS patients with SARS-CoV-2 infection confirmed this result [45]. In a more recent case–control study from the Italian MS registry, 779 MS patients with confirmed COVID-19 were compared with matched 1558 MS patients without COVID-19 to investigate factors associated with the risk of getting COVID-19 [46]. The severity of COVID-19 clinical manifestation was associated to increased age, disability, and progressive MS. In this analysis [46], patients treated with CladT were 21 (2.7% of the total patients) COVID-19-positive and 25 (1.6% of the total patients) COVID-19-negative, no increased risk was reported in CladT treated patients as 19 of them showed mild symptoms, 2 showed moderate ones, and none had severe COVID-19.
Twice as much risk of developing severe COVID-19 was reported in MS patients compared to age- and sex-matched populations in a study aimed at investigating the COVID-19 outcomes in MS patients compared to non-MS patients in Italy [47]. However, in MS patients with Expanded Disability Status Scale score (EDSS) ≤ 3.0 and lacking co-morbidities, the risk of severe COVID-19 was similar to that of the matched population. Interestingly, in this latter group, only patients treated with anti-CD20 monoclonal antibodies had an increased risk of hospitalization.
The observation that CladT treatment does not lead to increased susceptibility to SARS-CoV-2 infection nor a more severe COVID-19 clinical course was confirmed by single-center case series, in which CladT-treated patients developed mild COVID-19 symptoms and mounted an adequate response in most cases [48–52].
Data extracted from the Merck Kga Global Patients Safety Database included 261 patients with confirmed (n = 160) and suspected (n = 101) COVID-19, and showed that the median time to COVID-19 occurrence was 162 days (i.e., about 5 months) from the most recent CladT treatment [53]. While 51% of these patients recovered, 7% did not recover (at the time of observation), 0.4% died, and 41% were not reported/missing/pending. In this cohort of patients, 15% experienced severe COVID-19 [53]. Such a rate is lower than the mean rate of severe COVID-19 (20.7%) in MS patients reported in the systematic review from Barzegar et al. [54].
A recent meta-analysis, focused on COVID-19 severity among MS patients, pooled data from 13 case series, including 5138 patients with COVID-19, of whom 107 (2.1%) were treated with CladT [55]. Pooled estimates of hospitalization and death were 9.36% and 0%, respectively, for patients treated with CladT, and 14.98% and 2.66% for MS patients under other treatments [55]. Table 1 summarizes the outcome of COVID-19 infection in CladT-treated MS patients.
Table 1.
Outcome of COVID-19 in cladribine-treated patients
| n° COVID-19 patients treated with Cladribine | No. of patients (%) with COVID-19 clinical manifestations severity | References |
|---|---|---|
| 3 | 3 (100%): not severe/fatal | [42] |
| 11 | 11 (100%): not hospitalized | [43] |
| 27 |
2 (7.4%): hospitalized; 0 (0%): ICU; 0 (0%): ventilation; 0 (0%): death |
[44] |
| 25 |
19 (76%): not hospitalized/mild symptoms; 6 (24%): hospitalized, not ventilated; 0 (0%): ventilated; 0 (0%): death |
[45] |
| 21 |
19 (90.5%): mild symptoms; 2 (9.5%): moderate, no severe/fatal |
[46] |
| 2 | 2 (100%): mild/self-limiting | [48] |
| 2 | 2 (100%): mild/asymptomatic | [50] |
ICU intensive care unit
Efficacy of Anti-COVID-19 Vaccination in Multiple Sclerosis
Vaccination against SARS-CoV-2 is a fundamental tool to control the COVID-19 pandemic [56], as confirmed by real-world evidence [57]. The safety of anti-COVID-19 vaccination in the MS population has been confirmed by different case series [58, 59].
Antibody Response
Different studies have evaluated antibody response to vaccination as a biomarker of efficacy. One of the first real-world data series on the ability of MS patients to mount a protective humoral response after the mRNA vaccine BNT62b2-COVID-19 was reported by Achiron et al. [60]. The authors evaluated SARS-CoV-2 antibodies in vaccinated healthy subjects (HS), untreated MS patients, and MS patients treated with disease-modifying drugs, and found that untreated MS patients, as well as MS patients treated with CladT (n = 23), developed an antibody response comparable to that of HS. In contrast, patients treated with fingolimod and anti-CD20 drugs produced lower levels of antibodies after vaccination [57]. Low numbers of circulating B lymphocytes is likely the cause of lower antibody response to COVID-19 in both ocrelizumab-treated [61] and fingolimod-treated patients [62].
The same data on the effect of disease-modifying drugs on the antibody responses emerged from other large cohorts. As for cladribine, in the Italian cohort, 25 treated patients responded to the vaccination by producing anti-SARS-CoV-2 antibodies [63]. Moreover, in a cohort from the United Kingdom, 16/20 (80%) subjects treated with CladT developed a serological response to SARS-CoV2 after anti-COVID-19 vaccination. The proportion of responders did not differ compared to untreated MS patients [64]. Serological response to two doses of anti-COVID-19 vaccine (BT162b2) in 67 MS patients, including untreated patients (n = 15), patients treated with IFN β-1a (n = 18), and patients treated with CladT (n = 34), was evaluated using two different assays [65]. All patients treated with CladT developed a serological response to the vaccine, similar to untreated and IFN-β-treated patients, and with comparable Ig levels in the three groups. In addition, no correlation was found between SARS-CoV-2 Ig levels and the total lymphocyte count in CladT-treated patients at vaccination, which occurred 12–120 weeks from the last drug dose. Normal antibody response to anti-COVID-19 vaccination was reported by several other studies [66, 67]. In an Italian study, all patients treated with CladT developed antibodies following vaccination, although anti-RBD titers were lower compared to healthy subjects [68]. Furthermore, 4/4 CladT-treated MS patients vaccinated with BNT162b2 Pfizer-BioNTech and 3/7 CladT-treated patients vaccinated with Beijing/Sinopharm BBIBP-CorV COVID-19 vaccines developed IgG antibodies upon vaccination [69]. Booster doses increased RBD-IgG titers in three CladT-treated MS patients [70].
Of note, neither timing of last treatment nor low lymphocyte counts at vaccination dampened the response to COVID-19 vaccination in 38 CladT-treated patients [71].
Data on antibody response to COVID-19 vaccination are summarized in Table 2.
Table 2.
Efficacy of COVID-19 vaccination on antibody responses, T-cell responses, and breakthrough infection in cladribine-treated MS patients
| N° patients treated with Cladribine | Antibody response after COVID-19 vaccination | T-cell response after COVID-19 vaccination | Breakthrough infection | References | ||||
|---|---|---|---|---|---|---|---|---|
| % of patients with IgG response | Magnitude of IgG levels | % of patients with T-cell response | Magnitude of T-cell response | |||||
| % of patients with positive SARS-CoV-2 Spike IgG Ab | % of patients with positive SARS-CoV-2RBD IgG Ab | SARS-CoV-2 Spike IgG Ab |
SARS-CoV-2 RBD IgG Ab | |||||
| 23 | 100% | ND | Not different to HC | ND | ND | ND | ND | [60] |
| 25 | ND | 100% | ND | Not different to untreated patients | ND | ND | ND | [63] |
| 20 | 80% | ND | ND | ND | ND | ND | ND | [64] |
| 34 | 100% | 100% | Not different to HC, untreated patients and IFN-β1a treated MS patients | Not different to HC | ND | ND | ND | [65] |
| 2 | ND | 100% | ND | Not different to untreated patients | ND | ND | ND | [66] |
| 3 | 100% | ND | ND | ND | ND | [82] | ||
| 38 | 100% | ND | Not impaired by the treatment | ND | ND | ND | ND | [71] |
| 20 | ND | 100% | ND | Lower than HC and IFN-β- treated patients | 70% | Lower than IFN-β- treated patients | ND | [68] |
| 570 | ND | ND | ND | ND | ND | 0.70% | [73] | |
| 15 | ND | 86.7% | ND | Not different from untreated patients | ND | ND | ND | [83] |
| 1 | 100% | ND | Not different from untreated patients | 100% | ND | [72] | ||
| 3 | ND | 100% | 66% | ND | [70] | |||
| 11 | 64% | ND | ND | ND | ND | [69] | ||
T-Cell Response
Specific T-cell responses induced by vaccinations likely contribute to their effectiveness, although data on breakthrough infections, discussed in the next paragraph, suggest that antibody responses are a better biomarker of vaccine efficacy. Data on treated MS patients show that T-cell responses to COVID-19 vaccination are mostly retained in ocrelizumab-treated patients [58], whereas they are largely impaired during treatment with fingolimod [69].
Regarding cladribine, 70% of 20 treated patients mounted T-cell responses after vaccination, a high, but significantly lower, response rate compared to 78/78 (100%) of healthy subjects [68]. In another cohort, one patient treated with CladT developed T-cell responses [72],
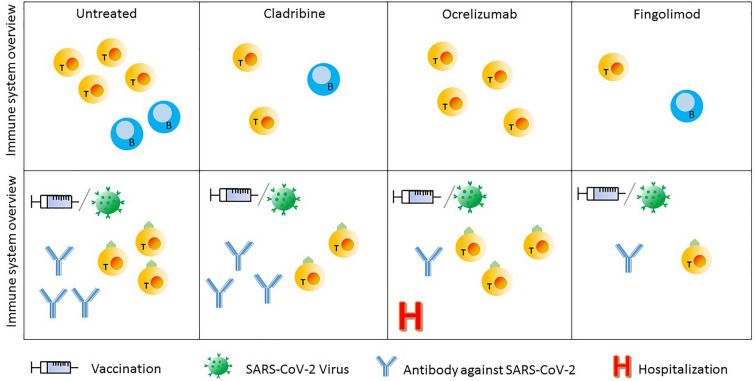
Data on T- cell responses after COVID-19 in CladT-treated patients are reported in Table 2. Figure 1 shows how the action of different disease-modifying therapies on B- and T-cell populations affects the immune response mounted after vaccination against the SARS-CoV-2 virus.
Fig. 1.
Overview of the immune system in SM patients. In the first row the representation of SM patient immune system when they are, respectively, untreated, treated with cladribine, ocrelizumab, or fingolimod. The second row shows how that immune system reacts to SARS-CoV-2 virus or vaccination
Risk of Breakthrough Infection
Different data are available on the effectiveness of vaccines in preventing COVID-19 infection in MS patients. The risk of a breakthrough infection was higher in MS patients treated with ocrelizumab and fingolimod [43], and this correlated with antibody levels [63], while no significant changes could be observed in patients treated with other DMDs, including CladT [73, 74]. Recently, Sormani et al. [74] reported a 4.5% cumulative incidence of COVID-19 breakthrough infection in an 8-month follow-up period in a cohort of 1705 MS patients receiving two mRNA vaccine doses. Of note, the rates of breakthrough infections in treated MS patients were associated with the level of humoral immunity to SARS-CoV-2. Data from a retrospective monocentric cohort of 254 MS patients in treatment with DMDs showed that the longer duration of anti-CD20 therapy and the decrease in CD20 cell counts were associated not only with blunted humoral response to vaccination but also with the breakthrough COVID-19 infection that was higher than in other/general population studies [75]. A summary of COVID-19 vaccination on antibody responses, T-cell responses, and breakthrough infection in CladT-treated MS patients is provided in Table 2.
Concluding Remarks
COVID-19 is expected to become endemic, suggesting its potential seasonal infection risk for immuno-compromised patients, including MS patients. MS patients treated with CladT are not at higher risk of developing severe COVID-19 [55]. The vaccination against COVID-19 is recommended for all MS patients. Advice for vaccination and guidelines addressing vaccination timing according to the different disease-modifying drugs taken by the patients have been published by National and International MS societies [76, 77]. COVID-19 vaccination should administered in all CladT-treated patients [78, 79], as it is safe and effective, likely due to the relative sparing of naïve B-cells by cladribine and by the rapid reconstitution of B-cells after drug treatment [80, 81].
As additional elements, the transient effect of cladribine on innate immune cells [7] likely contributes to maintaining an adequate first line of defense against SARS-CoV-2 [6, 16].
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Acknowledgements
Funding
Journal’s rapid service publication fee has been funded by Merck Serono S.p.A., Italy, an affiliate of Merck (CrossRef Funder ID: 10.13039/100009945). No funding was received for the writing of the review itself.
Medical Writing and/or Editorial Assistance
Salvatore Bianco, an independent medical writer on behalf of Fullcro S.r.l., provided medical writing assistance, funded by Merck Serono S.p.A., Italy, an affiliate of Merck (CrossRef Funder ID: 10.13039/100009945).
Author Contributions
Federico Carlini: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review and editing. Valeria Lusi: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review and editing. Caterina Rizzi: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review and editing. Francesco Assogna: Conceptualization; Writing – review and editing. Alice Laroni: Conceptualization; Methodology; Supervision; Validation; Writing – review and editing.
Disclosures
Federico Carlini and Valeria Lusi report no conflicts of interest. Alice Laroni received fees for consultation from Roche, Genzyme, Merck, Biogen, Novartis, Bristol-Myers Squibb. Caterina Rizzi and Francesco Assogna are employees of Merck Serono S.p.A., Italy, an affiliate of Merck KGaA.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Filippi M, Bar-Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 2.Rammohan K, Coyle PK, Sylvester E, et al. The development of cladribine tablets for the treatment of multiple sclerosis: a comprehensive review. Drugs. 2020;80(18):1901–1928. doi: 10.1007/s40265-020-01422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sasvari-Szekely M, Piroth Z, Kazimierczuk Z, Staub M. A novel effect of the new antileukemic drug, 2-chloro-2'-deoxyadenosine, in human lymphocytes. Biochem Biophys Res Commun. 1994;203(3):1378–1384. doi: 10.1006/bbrc.1994.2337. [DOI] [PubMed] [Google Scholar]
- 4.Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol. 2011;34(1):28–35. doi: 10.1097/WNF.0b013e318204cd90. [DOI] [PubMed] [Google Scholar]
- 5.Carlini F, Ivaldi F, Gualandi F, et al. Different susceptibility of T and B cells to cladribine depends on their levels of deoxycytidine kinase activity linked to activation status. J Neuroimmune Pharmacol. 2021 doi: 10.1007/s11481-021-09994-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stuve O, Soelberg Soerensen P, Leist T, et al. Effects of cladribine tablets on lymphocyte subsets in patients with multiple sclerosis: an extended analysis of surface markers. Ther Adv Neurol Disord. 2019;12:1756286419854986. doi: 10.1177/1756286419854986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sellner J, Rommer PS. Multiple sclerosis and SARS-CoV-2 vaccination: considerations for immune-depleting therapies. Vaccines (Basel) 2021;9(2):99. doi: 10.3390/vaccines9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser T, Schwenker K, Seiberl M, et al. Long-term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann Clin Transl Neurol. 2020;7(11):2199–2212. doi: 10.1002/acn3.51206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–174. doi: 10.1016/j.msard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 10.Wiendl H, Schmierer K, Hodgkinson S, et al. Specific patterns of immune cell dynamics may explain the early onset and prolonged efficacy of cladribine tablets: a MAGNIFY-MS substudy. Neurol Neuroimmunol Neuroinflamm. 2023 doi: 10.1212/NXI.0000000000200048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soldan SS, Lieberman PM. Epstein-Barr virus and multiple sclerosis. Nat Rev Microbiol. 2023;21(1):51–64. doi: 10.1038/s41579-022-00770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. doi: 10.1126/science.abj8222. [DOI] [PubMed] [Google Scholar]
- 13.Dyer Z, Tscharke D, Sutton I, Massey J. From bedside to bench: how existing therapies inform the relationship between Epstein-Barr virus and multiple sclerosis. Clin Transl Immunology. 2023;12(2):e1437. doi: 10.1002/cti2.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moser T, O'Sullivan C, Puttinger C, et al. Pre-existing humoral immunological memory is retained in patients with multiple sclerosis receiving cladribine therapy. Biomedicines. 2021 doi: 10.3390/biomedicines9111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiendl H, Schmierer K, Hodgkinson S, et al. Characterization of peripheral immune cell dynamics and repopulation patterns in the first 12 months of cladribine tablets treatment: MAGNIFY-MS study (2235) Neurology. 2021;96(15 Supplement):2235. [Google Scholar]
- 16.Mathiesen CBK, Rudjord-Levann AM, Gad M, Larsen J, Sellebjerg F, Pedersen AE. Cladribine inhibits secretion of pro-inflammatory cytokines and phagocytosis in human monocyte-derived M1 macrophages in-vitro. Int Immunopharmacol. 2021;91:107270. doi: 10.1016/j.intimp.2020.107270. [DOI] [PubMed] [Google Scholar]
- 17.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416–426. doi: 10.1056/NEJMoa0902533. [DOI] [PubMed] [Google Scholar]
- 18.Moser T, Ziemssen T, Sellner J. Real-world evidence for cladribine tablets in multiple sclerosis: further insights into efficacy and safety. Wien Med Wochenschr. 2022;172(15–16):365–372. doi: 10.1007/s10354-022-00931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golshani M, Hrdy J. Multiple sclerosis patients and disease modifying therapies: impact on immune responses against COVID-19 and SARS-CoV-2 vaccination. Vaccines (Basel) 2022 doi: 10.3390/vaccines10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19(7):409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz-Prado E, Simbana-Rivera K, Gomez-Barreno L, et al. Clinical, molecular, and epidemiological characterization of the SARS-CoV-2 virus and the coronavirus disease 2019 (COVID-19), a comprehensive literature review. Diagn Microbiol Infect Dis. 2020;98(1):115094. doi: 10.1016/j.diagmicrobio.2020.115094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koelle K, Martin MA, Antia R, Lopman B, Dean NE. The changing epidemiology of SARS-CoV-2. Science. 2022;375(6585):1116–1121. doi: 10.1126/science.abm4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siddiqi HK, Libby P, Ridker PM. COVID-19 - a vascular disease. Trends Cardiovasc Med. 2021;31(1):1–5. doi: 10.1016/j.tcm.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goumenou M, Sarigiannis D, Tsatsakis A, et al. COVID19 in northern Italy: an integrative overview of factors possibly influencing the sharp increase of the outbreak (Review) Mol Med Rep. 2020;22(1):20–32. doi: 10.3892/mmr.2020.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.(CDC) CfDCaP. Available online: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html. Accessed Oct 2020.
- 28.Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
- 29.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zohar T, Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat Rev Immunol. 2020;20(7):392–394. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuri-Cervantes L, Pampena MB, Meng W, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020 doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oberfeld B, Achanta A, Carpenter K, et al. SnapShot: COVID-19. Cell. 2020;181(4):954–e1. doi: 10.1016/j.cell.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park A, Iwasaki A. Type I and type III interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27(6):870–8. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu L, Yang P, Zhao Y, et al. Single-cell sequencing of peripheral mononuclear cells reveals distinct immune response landscapes of COVID-19 and influenza patients. Immunity. 2020;53(3):685–96.e3. doi: 10.1016/j.immuni.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21(2):e26–e35. doi: 10.1016/S1473-3099(20)30773-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pascual-Iglesias A, Canton J, Ortega-Prieto AM, Jimenez-Guardeno JM, Regla-Nava JA. An overview of vaccines against SARS-CoV-2 in the COVID-19 pandemic era. Pathogens. 2021 doi: 10.3390/pathogens10081030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiskopf D, Schmitz KS, Raadsen MP, et al. Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020 doi: 10.1126/sciimmunol.abd2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Zou Y, Wang H, Cui G, Yu Z, Ren Z. Immune response induced by novel coronavirus infection. Front Cell Infect Microbiol. 2022;12:988604. doi: 10.3389/fcimb.2022.988604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persson R, Lee S, Ulcickas Yood M, et al. Infections in patients diagnosed with multiple sclerosis: a multi-database study. Mult Scler Relat Disord. 2020;41:101982. doi: 10.1016/j.msard.2020.101982. [DOI] [PubMed] [Google Scholar]
- 40.Wijnands JM, Kingwell E, Zhu F, et al. Infection-related health care utilization among people with and without multiple sclerosis. Mult Scler. 2017;23(11):1506–16. doi: 10.1177/1352458516681198. [DOI] [PubMed] [Google Scholar]
- 41.Laroni A, Schiavetti I, Sormani MP, Uccelli A. COVID-19 in patients with multiple sclerosis undergoing disease-modifying treatments. Mult Scler. 2021;27(14):2126–36. doi: 10.1177/1352458520971817. [DOI] [PubMed] [Google Scholar]
- 42.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77(9):1079–88. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–9. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simpson-Yap S, De Brouwer E, Kalincik T, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870–e85. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohn N, Konen FF, Pul R, et al. Experience in multiple sclerosis patients with COVID-19 and disease-modifying therapies: a review of 873 published cases. J Clin Med. 2020 doi: 10.3390/jcm9124067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iaffaldano P, Lucisano G, Manni A, et al. Risk of getting COVID-19 in people with multiple sclerosis: a case-control study. Neurol Neuroimmunol Neuroinflamm. 2022 doi: 10.1212/NXI.0000000000001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sormani MP, Schiavetti I, Carmisciano L, et al. COVID-19 severity in multiple sclerosis: putting data into context. Neurol Neuroimmunol Neuroinflamm. 2022 doi: 10.1212/NXI.0000000000001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preziosa P, Rocca MA, Nozzolillo A, Moiola L, Filippi M. COVID-19 in cladribine-treated relapsing-remitting multiple sclerosis patients: a monocentric experience. J Neurol. 2021;268(8):2697–9. doi: 10.1007/s00415-020-10309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Celius EG. Normal antibody response after COVID-19 during treatment with cladribine. Mult Scler Relat Disord. 2020;46:102476. doi: 10.1016/j.msard.2020.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Angelis M, Petracca M, Lanzillo R, Brescia Morra V, Moccia M. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult Scler Relat Disord. 2020;45:102452. doi: 10.1016/j.msard.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haham N, Vaknin-Dembinsky A. COVID-19 in Cladribine-treated patient with multiple sclerosis. J Neuroimmunol. 2021;359:577690. doi: 10.1016/j.jneuroim.2021.577690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gelibter S, Orrico M, Filippi M, Moiola L. COVID-19 with no antibody response in a multiple sclerosis patient treated with cladribine: implication for vaccination program? Mult Scler Relat Disord. 2021;49:102775. doi: 10.1016/j.msard.2021.102775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jack D, Damian D, Nolting A, Galazka A. COVID-19 in patients with multiple sclerosis treated with cladribine tablets: an update. Mult Scler Relat Disord. 2021;51:102929. doi: 10.1016/j.msard.2021.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barzegar M, Mirmosayyeb O, Gajarzadeh M, et al. COVID-19 among patients with multiple sclerosis: a systematic review. Neurol Neuroimmunol Neuroinflamm. 2021 doi: 10.1212/NXI.0000000000001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albanese A, Sormani MP, Gattorno G, Schiavetti I. COVID-19 severity among patients with multiple sclerosis treated with cladribine: a systematic review and meta-analysis. Mult Scler Relat Disord. 2022;68:104156. doi: 10.1016/j.msard.2022.104156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dos Santos WG. Impact of virus genetic variability and host immunity for the success of COVID-19 vaccines. Biomed Pharmacother. 2021;136:111272. doi: 10.1016/j.biopha.2021.111272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27(8):1370–8. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler. 2021;27(6):864–70. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Di Filippo M, Cordioli C, Malucchi S, et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2022;93(4):448–50. doi: 10.1136/jnnp-2021-327200. [DOI] [PubMed] [Google Scholar]
- 60.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther Adv Neurol Disord. 2021;14:17562864211012835. doi: 10.1177/17562864211012835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021;27(11):1990–2001. doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiavetti I, Barcellini L, Lapucci C, et al. CD19+ B cell values predict the increase of anti-SARS CoV2 antibodies in fingolimod-treated and COVID-19-vaccinated patients with multiple sclerosis. Mult Scler Relat Disord. 2022;70:104494. doi: 10.1016/j.msard.2022.104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sormani MP, Inglese M, Schiavetti I, et al. Effect of SARS-CoV-2 mRNA vaccination in MS patients treated with disease modifying therapies. EBioMedicine. 2021;72:103581. doi: 10.1016/j.ebiom.2021.103581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tallantyre EC, Vickaryous N, Anderson V, et al. COVID-19 vaccine response in people with multiple sclerosis. Ann Neurol. 2022;91(1):89–100. doi: 10.1002/ana.26251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brill L, Rechtman A, Zveik O, et al. Effect of cladribine on COVID-19 serology responses following two doses of the BNT162b2 mRNA vaccine in patients with multiple sclerosis. Mult Scler Relat Disord. 2022;57:103343. doi: 10.1016/j.msard.2021.103343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Capone F, Lucchini M, Ferraro E, et al. Immunogenicity and safety of mRNA COVID-19 vaccines in people with multiple sclerosis treated with different disease-modifying therapies. Neurotherapeutics. 2021 doi: 10.1007/s13311-021-01165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bock H, Juretzek T, Handreka R, et al. Humoral and cellular immune responses to SARS CoV-2 vaccination in people with multiple sclerosis and NMOSD patients receiving immunomodulatory treatments. Mult Scler Relat Disord. 2022;59:103554. doi: 10.1016/j.msard.2022.103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tortorella C, Aiello A, Gasperini C, et al. Humoral- and T-cell-specific immune responses to SARS-CoV-2 mRNA vaccination in patients with MS using different disease-modifying therapies. Neurology. 2022;98(5):e541–e54. doi: 10.1212/WNL.0000000000013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drulovic J, Ivanovic J, Martinovic V, et al. Humoral response to SARS-CoV-2 COVID-19 vaccines in patients with multiple sclerosis treated with immune reconstitution therapies. Mult Scler Relat Disord. 2021;54:103150. doi: 10.1016/j.msard.2021.103150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aiello A, Coppola A, Ruggieri S, et al. Longitudinal characterisation of B and T-cell immune responses after the booster dose of COVID-19 mRNA-vaccine in people with multiple sclerosis using different disease-modifying therapies. J Neurol Neurosurg Psychiatry. 2022 doi: 10.1136/jnnp-2022-330175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grothe C, Steffen F, Bittner S. Humoral immune response and lymphocyte levels after complete vaccination against COVID-19 in a cohort of multiple sclerosis patients treated with cladribine tablets. J Cent Nerv Syst Dis. 2021;13:11795735211060118. doi: 10.1177/11795735211060118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Milo R, Staun-Ram E, Karussis D, et al. Humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis: an Israeli multi-center experience following 3 vaccine doses. Front Immunol. 2022;13:868915. doi: 10.3389/fimmu.2022.868915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schiavetti I, Cordioli C, Stromillo ML, et al. Breakthrough SARS-CoV-2 infections in MS patients on disease-modifying therapies. Mult Scler. 2022;28(13):2106–11. doi: 10.1177/13524585221102918. [DOI] [PubMed] [Google Scholar]
- 74.Sormani MP, Schiavetti I, Inglese M, et al. Breakthrough SARS-CoV-2 infections after COVID-19 mRNA vaccination in MS patients on disease modifying therapies during the Delta and the Omicron waves in Italy. EBioMedicine. 2022;80:104042. doi: 10.1016/j.ebiom.2022.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holroyd KB, Healy BC, Conway S, et al. Humoral response to COVID-19 vaccination in MS patients on disease modifying therapy: immune profiles and clinical outcomes. Mult Scler Relat Disord. 2022;67:104079. doi: 10.1016/j.msard.2022.104079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MS International Federation. MS tcavugahwmont-c-a-m-w-y-n-t-k. 2020. 10.1016/j.devcel.2019.05.023.
- 77.NMSC-VGfPLwMhwnoc-c--im-s-a-cc--v-g. 2021. 10.1039/c9sc00341j. Accessed Oct 05 2021.
- 78.Rieckmann P, Centonze D, Giovannoni G, et al. Expert opinion on COVID-19 vaccination and the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord. 2021;14:17562864211058298. doi: 10.1177/17562864211058298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moiola L, Barcella V, Benatti S, et al. The risk of infection in patients with multiple sclerosis treated with disease-modifying therapies: a Delphi consensus statement. Mult Scler. 2021;27(3):331–46. doi: 10.1177/1352458520952311. [DOI] [PubMed] [Google Scholar]
- 80.Baker D, Herrod SS, Alvarez-Gonzalez C, Zalewski L, Albor C, Schmierer K. Both cladribine and alemtuzumab may effect MS via B-cell depletion. Neurol Neuroimmunol Neuroinflamm. 2017;4(4):e360. doi: 10.1212/nxi.0000000000000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ceronie B, Jacobs BM, Baker D, et al. Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells. J Neurol. 2018;265(5):1199–209. doi: 10.1007/s00415-018-8830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mariottini A, Bertozzi A, Marchi L, et al. Effect of disease-modifying treatments on antibody-mediated response to anti-COVID19 vaccination in people with multiple sclerosis. J. Neurol. 2022;269(6):2840–2847. doi: 10.1007/s00415-022-11003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Disanto G, Sacco R, Bernasconi E, et al. Association of Disease-Modifying Treatment and Anti-CD20 Infusion Timing With Humoral Response to 2 SARS-CoV-2 Vaccines in Patients With Multiple Sclerosis. JAMA Neurol. 2021;78(12):1529–31. doi: 10.1001/jamaneurol.2021.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]