Abstract
The need for evidence-based data, to inform policy decisions on malaria vector control interventions in Nigeria, necessitated the establishment of mosquito surveillance sites in a few States in Nigeria. In order to make evidence-based-decisions, predictive studies using available data becomes imperative. We therefore predict the distribution of the major members of the Anopheles gambiae s.l. in Nigeria. Immature stages of Anopheles were collected from 72 study locations which span throughout the year 2020 resulted in the identification of over 60,000 Anopheline mosquitoes. Of these, 716 breeding sites were identified with the presence of one or more vector species from the An. gambiae complex and were subsequently used for modelling the potential geographical distribution of these important malaria vectors. Maximum Entropy (MaxEnt) distribution modeling was used to predict their potentially suitable vector habitats across Nigeria. A total of 23 environmental variables (19 bioclimatic and four topographic) were used in the model resulting in maps of the potential geographical distribution of three dominant vector species under current climatic conditions. Members of the An. gambiae complex dominated the collections (98%) with Anopheles stephensi, Anopheles coustani, Anopheles funestus, Anopheles moucheti, Anopheles nilli also present. An almost equal distribution of the two efficient vectors of malaria, An. gambiae and Anopheles coluzzii, were observed across the 12 states included in the survey. Anopheles gambiae and Anopheles coluzzii had almost equal, well distributed habitat suitability patterns with the latter having a slight range expansion. However, the central part of Nigeria (Abuja) and some highly elevated areas (Jos) in the savannah appear not suitable for the proliferation of these species. The most suitable habitat for Anopheles arabiensis was mainly in the South-west and North-east. The results of this study provide a baseline allowing decision makers to monitor the distribution of these species and establish a management plan for future national mosquito surveillance and control programs in Nigeria.
Subject terms: Ecological modelling, Entomology
Introduction
Anopheline mosquitoes remain successful in their ability to adapt to their changing natural environments, expanding opportunities for the propagation of their species. The majority of these adaptive processes comes at a cost to both the mosquitoes and their hosts as they serve as vectors of various human pathogens including malaria parasites, filarial worms and arboviruses (arthropod-borne viruses). Of specific importance are the members of the Anopheles gambiae complex (Anopheles coluzzii, Anopheles gambiae and Anopheles arabiensis) which have evolved over time, adapting and responding to ecological factors1,2, to become the primary and efficient vectors of malaria parasites in Africa3,4. Much of the efforts at combating malaria has been through massive investments in vector control targeted at these species, highlighting the importance of understanding vector distribution and dynamics5.
While the members of the An. gambiae complex commonly occupy similar ecological niches2, An. gambiae is widespread throughout Africa and is known to be ancestral6 whereas An. coluzzii is found mainly in West and Central Africa7 and An. arabiensis, which shares similar ecological areas with An. gambiae, prefers drier environments1. Both An. coluzzii and An. gambiae are highly anthropophilic and anthropophagic8,9 but An. arabiensis is more zoophilic10. These differences in habitat and feeding preferences may result in ecological variables differentially driving the dispersal patterns of these malaria vectors which potentially lead to a complex system influencing malaria parasite spread2. Knowledge of how geographical factors influence the dispersal of malaria vectors may therefore help in understanding vector distribution and adaptation and provide the baseline for evidence-based control interventions.
The recent WHO report5 clearly points out the need to provide greater evidence and invest more in malaria vector control in-country. Although Nigeria has invested a lot in vector control through mass distributions of long-lasting insecticidal nets (LLINs), its estimated malaria prevalence has not improved significantly and it still reports up to 54% of the total number of malaria cases in Africa5. The recent malaria indicator survey11 clearly points out the need to provide more data on both environmental and genetic factors that contribute to the survival and adaptation of the vectors in the face of ongoing insecticide-based vector interventions. The thrust of National Malaria Elimination Program’s (NMEP- Department of Public Health at the Federal Ministry of Health, Abuja) strategies for malaria control has been the scaling up of universal access to parasitological confirmation of malaria, treatment at all service delivery levels and provision of high impact vector control interventions12. This therefore led to a paradigm shift in the coordination of malaria control activities in Nigeria. To this end, the NMEP has been coordinating translational and operational research activities on vector dynamics, ecology and vectoral capacities of Anopheles mosquitoes vector species in different parts of Nigeria. The findings of such research activities are expected to inform decisions on appropriate strategies for sustainable vector control in the country.
Malaria vector control across Africa is becoming more problematic due to the development of resistance to the pyrethroid insecticides which are the only class of insecticides approved for use on bed nets for malaria control, in West and East Africa. In Nigeria, documented cases of resistance to pyrethroids and other commonly used insecticides have persisted13,14, severely limiting targeted malaria prevention and control efforts. Despite all evidence showing that insecticide resistance amongst malaria vector species is a major setback towards malaria control at the global level, the available information within Nigeria is not enough to inform an evidenced-based decision towards national malaria control. Therefore, advances in vector control that identify and target the most productive larval habitat and breeding sites are essential15. Owing to the limited movement of mosquito immature stages compared with that of free-flying adult mosquitoes, control of the immature stages can be more efficient16. In order to be able to design and implement control measures directed at the larval stages such as larval source reduction and larviciding, an understanding of the spatial and temporal distribution of malaria mosquito larvae in different malaria transmission settings is important17,18.
A lack of contemporary Anopheles distributional data limits the capacity for distributional modeling, hindering effective vector control which subsequently poses a serious challenge in combating malaria in Nigeria. Spatial modelling, such as Ecological Niche Modeling (ENM), can be used to bridge knowledge gaps in the distribution of organisms. When applied to mosquito vectors, it identifies areas of suitable habitat for each species where data is lacking which allows evidence-based estimates to be made on the risk of malaria transmission in areas not covered by the current interventions19,20. An ecological niche can be described as the set of natural conditions (biotic and abiotic variables) in which a species is able to preserve reasonable population sizes without migration21. Ecological Niche Modeling is a useful tool that extracts and identifies the fundamental boundaries of a species’ niche at each occurrence location in a selected dataset from a suite of relevant environmental covariates. Predictions of Ecological Niche Modeling can infill the voids resulting from surveillance data shortages22–27. This study therefore presents the spatial distribution of malaria vectors in 12 states in Nigeria and developed an ecological niche model that address information gaps and provide evidence-based predictions for public health decision makers to guide future national surveillance and control programs.
Materials and methods
Study area
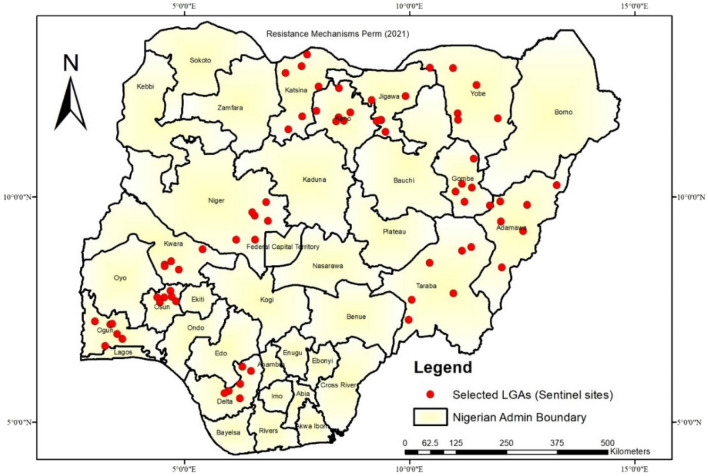
Nigeria (9.0820° N, 8.6753° E) has 36 states plus FCT with an estimated population of 215,266,57728. Routine mosquito surveillance (Larval collection) was conducted in twelve states (Table 1) which span all the ecological zones in the country (Fig. 1). Furthermore, mosquitoes were collected from 6 local government areas (LGAs) from each state (making a total of 72 LGAs). Generally, the climate in the twelve States is typically tropical with two distinct seasons, rainy (May to October) and dry (November to April). The mean annual weather conditions in the three states range from 24.0 °C to 30.20 °C (Temperature), 31.1–85% (Relative Humidity) and 314 mm–1871 mm (Rainfall). In terms of vegetation, the states in the north consist of Guinea, Sudan and Sahel Savannah while the southern part includes the mangrove and the forest in addition to the Guinea Savannah (Table 1). The main method of vector control in the study areas is insecticide-treated bed nets (ITNs). In the past 5 years, more ITNs have been deployed in the states through free mass distributions, resulting into high coverage (over 70%) in the study areas.
Table 1.
States, LGAs and Ecological zones of sites where mosquitoes were collected.
| S/N | States | Local government areas | Ecozone |
|---|---|---|---|
| 1 | Adamawa | Yola North, Numan, Shelleng, Mubi North, Song, Ganye | Guinea & Sudan Savannah |
| 2 | Delta | Ika North East, Ukwuani, Aniocha South, Ethiope East, Okpe, Isoko North | Rainforest & Mangroove |
| 3 | Gombe | Gombe, Balanga, Billiri, Funakaye, Akko, Yamaltu-Deba | Sudan & Sahel savannah |
| 4 | Jigawa | Dutse, Birnin-Kudu, Ringim, Taura, Kafin Hausa, Auyo | Sudan& Sahel Savannah |
| 5 | Katsina | Malumfashi, Funtua, Kusada, Bindawa, Kaita, Batagarawa | Sudan & Guinea Savannah |
| 6 | Kano | Kura, Warawa, Garun-Mallam, Gwarzo, Bunkure, Makoda | Sudan Savannah |
| 7 | Kwara | Moro, Asa, Ilorin South, Ilorin East, Ilorin West, Ifelodun | Guinea Savanah |
| 8 | Niger | Katchia, Chachanga, Lapai, Bosso, Shiroro, Paikoro | Guinea |
| 9 | Taraba | Jalingo, Ardokola, Gassol, Bali, Donga, Takum | Guinea & Sudan Savanah |
| 10 | Ogun | Odeda, Obafemi Owode, Shagamu, Ado Odo Ota, Yewa North, Abeokuta South | Rainforest |
| 11 | Osun | Obokun, Boripe, Ede, Oriade, Oshogbo, Egbedore | Rainforest |
| 12 | Yobe | Damaturu, Potiskum, Nguru, Nnengere, Bursalli, Bade | Sudan & Sahel savannah |
Figure 1.
Map of Nigeria showing the sentinel sites from 12 selected states for the surveillance project. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Available at https://www.R-project.org/. The Nigerian shapefile was obtained from World BankDataCatalog () an Open license standardized resource of boundaries (i.e., state, county) for every country in the world.
Mosquito collection and identification
The World Health Organization (WHO) and Center for Disease Control (CDC)’s entomological protocols for field and laboratory studies on malaria vectors were employed in carrying out the studies. Mosquitoes were uniformly collected on weekly basis during the raining season (between may and October) in the year 2020 across all the states. Surveys of larval breeding sites were carried out in the selected LGAs across the 12 States (Table 1). An. larvae were collected from identified breeding sites such as permanent water bodies, rice fields, small water pools, or impoundments, using a dipping method described by WHO29 and the location of each breeding site was georeferenced using a GPS device. Collected larvae were transported to the insectaries (for each state) for rearing where they were reared to adulthood under ambient laboratory conditions and the emerged adults were identified morphologically by two entomological technicians and finally validated by the Principal Investigator (for each state) using the keys of Gilles and Coetzee30 under × 1000 Dino-lite HD color CMOS sensor high speed digital microscope (model number, AD4113T-12 V).
Mosquitoes belonging to the An. gambiae complex were further identified using the An. gambiae species-specific PCR31. DNA from specimens identified as An. gambiae were subjected to PCR assays for identification of An. coluzzii and An. gambiae32.
Environmental data
Climatic variables such as temperature and precipitation influence species distributions at global and meso scales, topographic variables such as altitude and aspect have more influence at meso and topo-scales whereas land-cover variables such as percent canopy cover can influence distributions at the micro-scale33,34. Hence, climatic and topographic level variables were used here to predict the distribution of An. spp. in Nigeria.
A total of 716 positive breeding sites were discovered throughout the 12 states for the members of An. gambiae s.l. To determine the possible distribution of Anopheles spp. in Nigeria, a total of 421, 132 and 279 positive occurrences for An. coluzzii, An. arabiensis and An. gambiae respectively from the field surveys were used in our ENM (Fig. 2). The autocorrelation problems were addressed by eliminating redundant presences on the scale of the bioclimatic variables used in each 1 × 1 km grid35. In addition, records for spatial autocorrelation were screened in ArcGIS 10.7.1 using average nearest neighbor analyses to remove spatially correlated data points36,37. After this selection, a total of 329, 86 and 212 positive occurrence points for An. coluzzii, An. arabiensis and An. gambiae respectively were used in our prediction model. We considered 19 environmental and four topographical variables as potential predictors of the target species habitat distribution38,39. These variables were chosen based on their biological relevance to the target species distributions34,40–42. The nineteen bioclimatic variables with a 2.5 min spatial resolution (about. 1 km2) were downloaded from the WorldClim database (http://www.worldclim.org/)43. Elevation data 1 km2-resolution was obtained from the Shuttle Radar Topography Mission (SRTM). The elevation data was used to generate slope, aspect, and hillshade (all in degrees) using the Spatial Analyst tool/surface in using ArcGIS 10.4.1 software.
Figure 2.
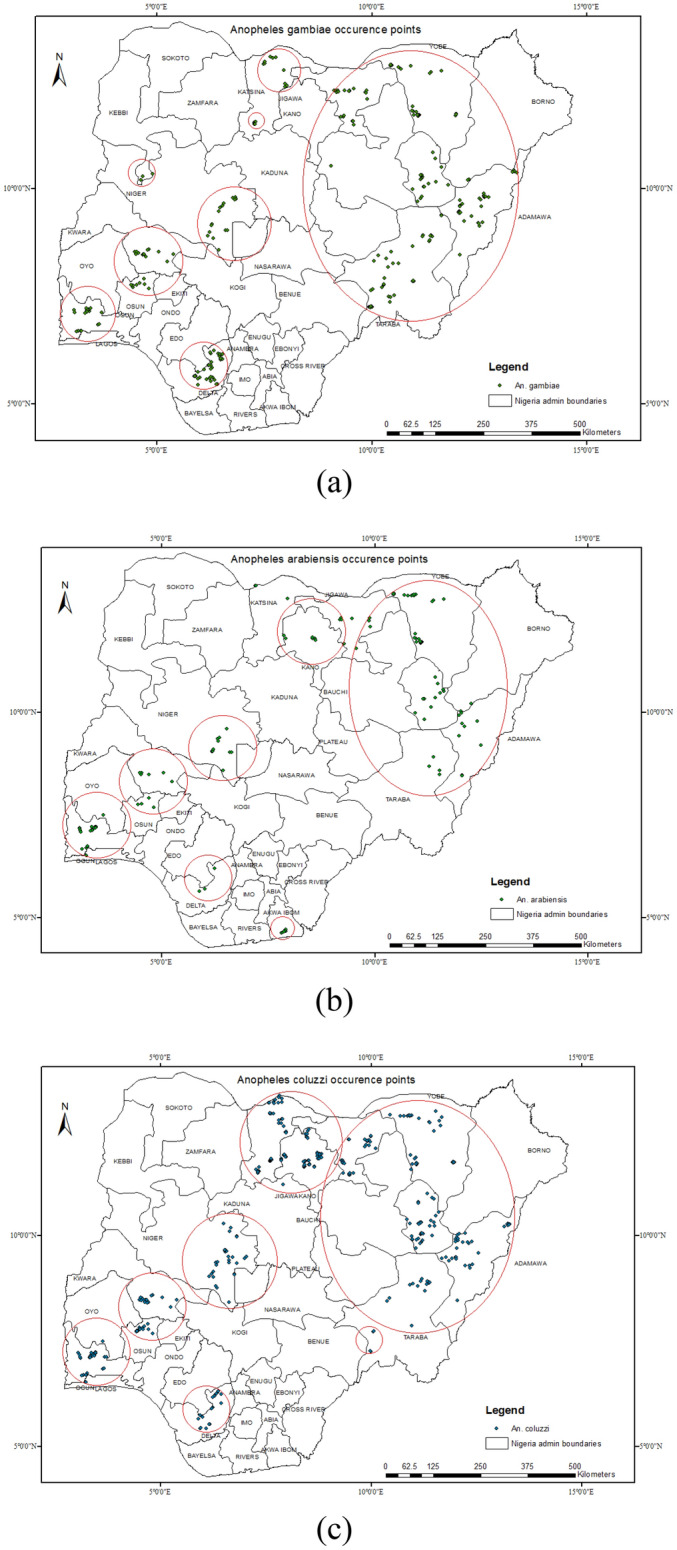
Map showing the positive occurrence records for each of the members of An. gambiae complex. (a) An. gambiae (b) An. arabiensis (c) An. coluzzii. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Available at https://www.R-project.org/. The Nigerian shapefile was obtained from World BankDataCatalog () an Open license standardized resource of boundaries (i.e., state, county) for every country in the world.
The coordinates for all occurrence data were taken in decimal degrees (to four decimal places) and plotted using Google Earth to check for annotation errors. After downloading the climatic files, the Nigeria layer was extracted by using a boundary mask. After that, extracted files were converted to ASCII format via using ArcGIS 10.7.1 software to be used later with Maxent software.
All combinations of the 23 environmental and topographic variables have been tested for multi-collinearity through the calculation of R-squared in linear regression analysis in R software ver. 4.1.2. In this study, because some of these bioclimatic variables were strongly correlated (R2 ≥ 0.7), only those variables that showed little correlation with other predictors were retained; following29,30. A total of 12 environmental and topographical variables were selected in this study (R2 < 0.7) (Table 2).
Table 2.
Environmental variables used for modeling the potential distribution of Anopheles spp. in the present study.
| No | Variable | Code/Unit | Source |
|---|---|---|---|
| 1 | Annual mean temperature | Bio1 (°C) | WorldClim |
| 2 | Mean Diurnal Range (Mean of monthly (max temp—min temp)) | Bio2 (°C) | WorldClim |
| 3 | Isothermality | Bio3 (°C) | WorldClim |
| 4 | Mean Temperature of Driest Quarter | Bio9 (°C) | WorldClim |
| 5 | Precipitation of the Wettest Month | Bio13 (mm) | WorldClim |
| 6 | Precipitation of the Driest Month | Bio14 (mm) | WorldClim |
| 7 | Precipitation of the Coldest Quarter | Bio19 (mm) | WorldClim |
| 8 | Elevation | – | SRTM |
| 9 | Slope | – | SRTM |
| 10 | Aspect | – | SRTM |
| 11 | Hillshade | – | SRTM |
Modelling
The modeling technique maximum entropy distribution or Maxent were used in this study; which has been found to be highly ranked among several different modeling methods27,38,44, and may continue to be effective even with small sample sizes39,41,45–47. For the study area, it only requires species presence data (not absence) and environmental variable (continuous or categorical) layers. We used the freely available Maxent software, version 3.3.3, which generates an estimate of the probability of the presence of the species that varies from 0 “unsuitable” to 0.99 “best habitat suitability”. ASCII files of the 8 selected environmental variables and a CSV file of species presence coordinates in decimal degrees were used to create the module. Maxent’s performance was assessed using a threshold independent Receiver-Operating Characteristic (ROC) analysis and Area Under Receiver- Operating Characteristic Curve (AUC) values (0.5 = random to 1 = perfect discrimination). The algorithm either runs 1000 iterations of these processes or continues until convergence is reached (threshold 0.00001).
For the model, the relative importance of each environmental predictor was evaluated using the percentage contribution of the Jackknife test, which is the best index for small sample sizes41. The default logistic output format was chosen, i.e. related to the probability of suitable conditions, ranging from 0 to 1. A total of 75% of the location point data were used for training, and the remaining 25% to test the predictive ability of the model (Model validation), in addition 10 replicates were considered. Average and Standard deviation values for training and test AUC for the 10 models were extracted from the Maxent text result output. The ASCII output map for the average model for the target species was loaded in ArcGIS 10.7.1 where the prediction models of habitat suitability were divided based on Choudhury et al.48 into 5 classes; very low (0–0.1), low (> 0.1–0.2), moderate (> 0.2–0.4), high (> 0.4–0.6) and very high (> 0.6) using natural breaks in the symbology tools to produce the habitat suitability model picture49.
Ethical approval
Ethical clearance for this study was obtained from the Ethics review committee, Federal Ministry of Health. All methods including mosquito larva collection and breeding, laboratory analysis and data management were performed in accordance with the 1964 Declarations of Helsinki.
Results
Spatial distribution of Anopheline species collected from the 12 states
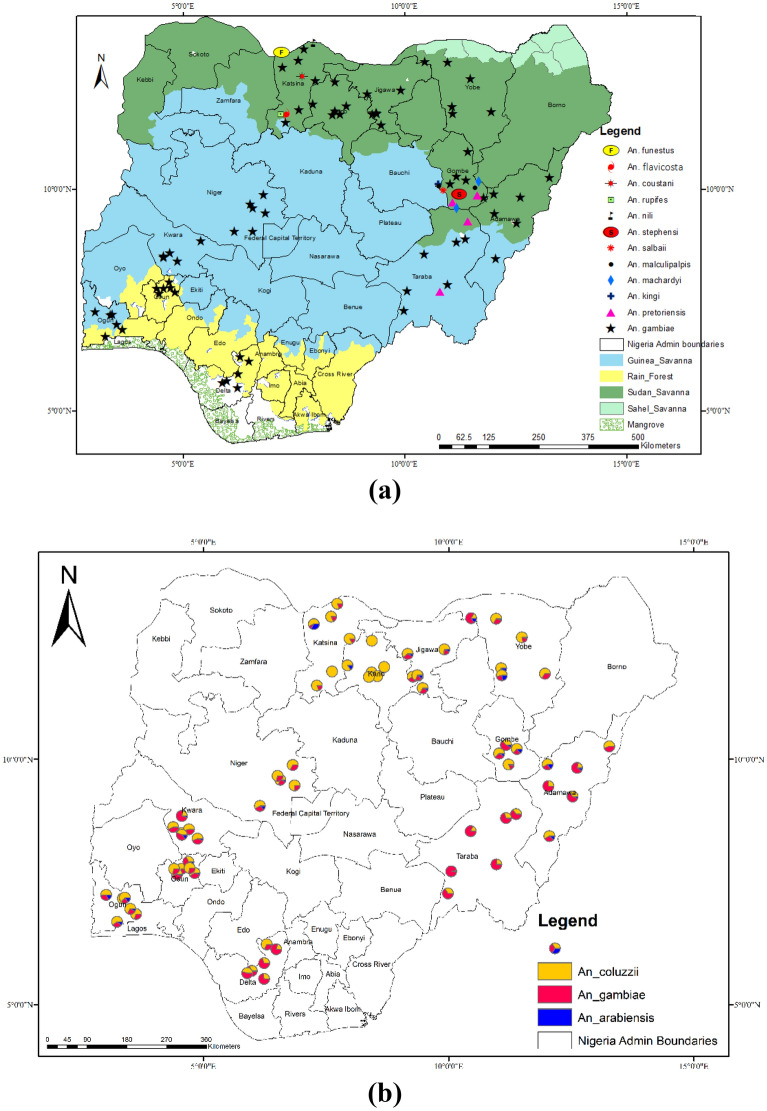
Members of the An. gambiae complex were the predominant species collected across all the LGAs from the 12 states, constituting up to 98% of total Anopheles collected. Furthermore, other Anophelines (less than 2% in each state, see Supplementary File 1) notably, An. salbaii, An. pretoriensis, An. funestus, An. flavicosta, An. coustani, An. machardyi etc., were encountered in three northern states in addition the An. gambiae complex. It is noteworthy, to state that a total of 14(0.3%) An. stephensi, an invasive species and efficient vector for urban malaria transmission was also encountered in Gombe State (though only at 2 sites in one LGA)50. Members of the An. gambiae complex identified were An. coluzzii, An. gambiae and An. arabiensis. These species were variably distributed in the 12 states with An. coluzzii dominating the majority of the collections in all areas, followed by An. gambiae and then An. arabiensis (Fig. 3).
Figure 3.
Spatial distribution of An. species collected from the 12 states. (a) Distribution of Anopheline mosquitoes in Nigeria. (b) Distribution of the members of the An. gambiae s.l. complex from the collection sites. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Available at https://www.R-project.org/. The Nigerian shapefile was obtained from World BankDataCatalog () an Open license standardized resource of boundaries (i.e., state, county) for every country in the world.
Potential habitat distribution for the members of An. gambiae complex in Nigeria
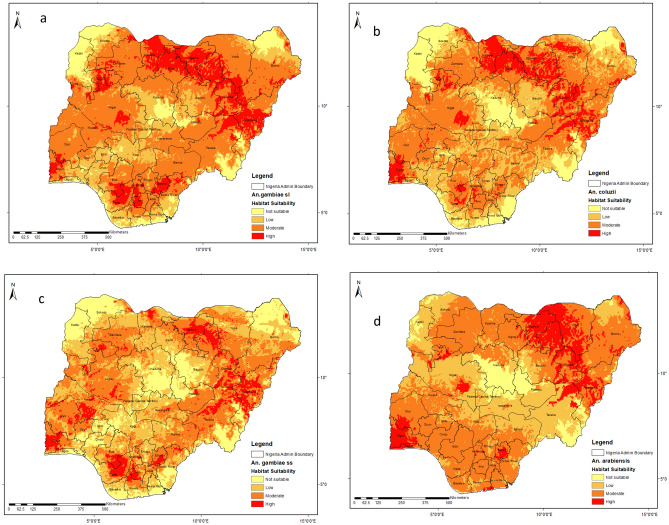
The potential habitat distribution of the members of An. gambiae complex., are presented in Fig. 4. The results showed that suitable larval habitats for An. gambiae complex are widely distributed across the country in varying proportions in (Fig. 4a). Habitat suitability for each of the members of the An. gambiae complex (An. coluzzii, An. gambiae and An. arabiensis) are highlighted below;
Figure 4.
Predictive maps of geographical spread of the members of An. gambiae s.l. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Available at https://www.R-project.org/. The Nigerian shapefile was obtained from World BankDataCatalog () an Open license standardized resource of boundaries (i.e., state, county) for every country in the world.
Our model predicts habitat suitability for An. coluzzii across eight states (Ogun, Lagos, Katsina, Kano, Jigawa, Bauchi, Gombe and Adamawa States). Conversely, it indicated only a few selected areas of Niger, Sokoto, Oyo, Yobe, Borno, Taraba, Kebbi, Delta, Anambra, Imo and Ebonyi States were highly suitable for An. coluzzii. Kebbi, Sokoto, Kaduna, Bauchi, Akwa-Ibom, Rivers and Bayelsa states were not predicted to be suitable for An. coluzzii. Oyo, Kwara, Osun, Niger, Zamfara, FCT, Nasarawa, Plateau, Yobe, Borno, Kogi, Edo, Imo, Ebonyi, Cross River, and Taraba States were moderately suitable for An. coluzzii (Fig. 4b).
For An. gambiae, larger part of Ogun, Delta, Kwara, Imo, Ebonyi, Adamawa and Gombe States were found to be highly suitable. Larger part of Oyo, Niger, Zamfara, Katsina, Kano, Yobe, Borno, Taraba, Benue, Nasarawa, and Plateau States were predicted to be moderately suitable for An. gambiae, while larger parts of Kebbi, Sokoto, Kaduna, Bauchi, Kogi, Ekiti, Ondo, Bayelsa, Akwa-Ibom, and FCT seems to be unsuitable for An. gambiae (Fig. 4c).
Larval habitats suitable for An. arabiensis were found in all parts of three states (Ogun, Lagos, Ebonyi) out of the 36 states. Also, larger parts of Jigawa, Yobe, Gombe, and Bauchi states were found to be highly suitable for An. arabiensis. Few parts of Oyo (especially in areas bordering Ogun), Niger, Taraba, and Borno states were highly suitable for An. arabiensis. Larger parts of Taraba, Plateau, Kaduna, Kebbi, FCT, Nasarawa, Niger, Sokoto and Benue states were predicted to be not suitable for An. arabiensis, while the remaining 20 states were moderately suitable for An. arabiensis (Fig. 4d).
Model performance and influencing factors
The average percent contribution (PC) and permutation importance (PI) of the 11variables used in the modeling of the members of An. gambiae s.l. distribution in this study were also assessed and highlighted below;
An. coluzzii
In this study, precipitation of the coldest quarter had the highest contribution with PC and PI of 25.2 and 29 respectively, followed by annual mean temperature PC of 22.1 and PI of 11 and mean temperature of driest quarter with PC of 13.7 and PI of 9.8. The results showed that these variables are strong predictors of An. coluzzii distribution in Nigeria, accounting for over 70% of the variations in distribution observed (Table 3).
Table 3.
Average percent contribution and permutation importance of the variables used in the modeling of An. coluzzii, An. gambiae and An. arabiensis distribution.
| Variable | Percent contribution | ||
|---|---|---|---|
| An. coluzzii | An. gambiae | An. Arabiensis | |
| Precipitation of the Coldest Quarter | 25.2a | 10.1f | 31.1b |
| Annual Mean Temperature | 22.1b | 16.6a | 13c |
| Mean Temperature of Driest Quarter | 13.7c | 3.9h | 36.3a |
| Isothermality (BIO/BIO7) (*100) | 11.9d | 14.6c | 4.9e |
| Precipitation of the Wettest Month | 8.4e | 12.7d | 0.3h |
| Mean Diurnal Range (Mean of monthly (max temp -min temp) | 5f | 16.1b | 1.4g |
| Aspect | 4.5g | 3.1i | 4f |
| Precipitation of the Driest Month | 3.3h | 11.3e | 0.3h |
| Elevation | 2.8i | 8g | 8.5d |
| Hillshade | 2.3j | 1.5k | 0.1k |
| Slope | 1k | 2j | 0.2j |
Superscripts (alphabets) are assigned to each value in a column in descending order of percentage contributions, such that value with superscript a connotes the environmental variable with the highest contribution and value with superscript k is the environmental variable with the lowest contribution of each of the sibling species.
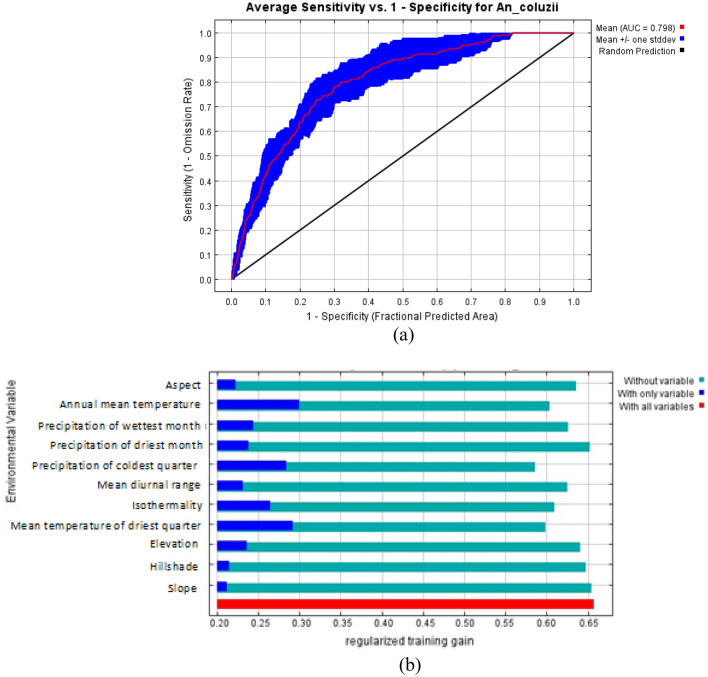
The ROC curve obtained as an average of the 10 replications runs is shown in Fig. 5a, and specificity was calculated. The average and standard deviation of the AUC for the 10 replicate runs was 0.798 ± 0.01. This value shows an excellent performance of the model as an AUC value of greater than 0.70 shows higher sensitivity and specificity for the presence of An. coluzzii.
Figure 5.
Estimation of model performance for An. coluzzii (a) Area under the curve (AUC) for An. coluzzii distribution. Red line indicates the mean value for 10 MaxEnt replicate runs. (b) Jackknife analysis for regularized training gain.
The relative importance of each variable to the distribution of An. coluzzii was also computed with the Jackknife test in Fig. 5b which gave a training gain of 0.67. The Jackknife test showed that mean temperature of the driest quarter and annual mean temperature are the two variables that will increase the gain the most when used alone. The Jackknife test also showed that mean temperature of the driest quarter and precipitation of the coldest quarter will decrease the gain the most when removed from the model.
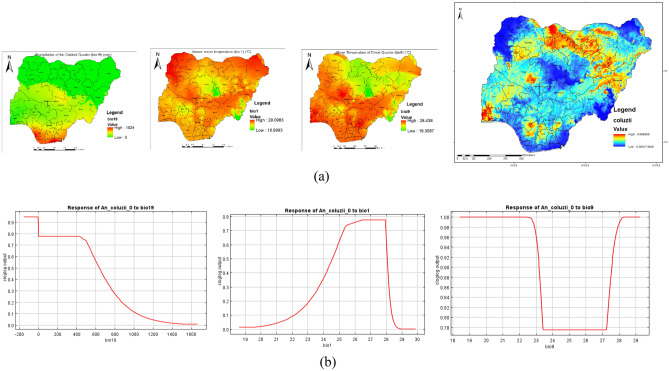
Figure 6 shows the main highest estimated environmental variables (contributions) that determines the distribution of An. coluzzii in Nigeria. Spatial distribution analysis was done to determine the geographical variability with regards to the selected environmental variables in the country. The response curves of three variables to An. coluzzii habitat suitability are shown in Fig. 6a. We found out that mean temperature of the driest quarter ranged from 19.3 to 28.4 °C, precipitation of the coldest quarter ranged from 0 to 1524 mm while annual mean temperature ranged from 18.9 to 29.1 °C. The response curves showed that between mean temperature of the driest quarter of 19 and 22 °C favors the potential distribution of An. coluzzii. Similarly, precipitation of the coldest quarter of − 200 to 400 mm significantly and potentially favoured the distribution of An. coluzzii while annual mean temperature ranging between 26 and 28 °C significantly favoured the distribution of An. coluzzii in Nigeria (Fig. 6b).
Figure 6.
Estimates of the highest contributing variables that determines the geographical distribution of An. coluzzii (a) The highest environmental variables that estimate to control the geographical distribution of An. coluzzii in Nigeria. Variable contributions (precipitation of coldest quarter, annual mean temperature and mean temperature of driest quarter), (b) Response curves of three environmental predictors used in MaxEnt model for An. coluzzii. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Available at https://www.R-project.org/. The Nigerian shapefile was obtained from World BankDataCatalog () an Open license standardized resource of boundaries (i.e., state, county) for every country in the world.
An. gambiae
In this study, annual mean temperature had the highest contribution with PC and PI of 16.6 and 7.2 respectively, followed by mean diurnal range PC of 16.1 and PI of 28.7 and isothermality with PC of 14.6 and PI of 25.2. The results showed that these variables are strong predictors of An. gambiae distribution in Nigeria, accounting for over 70% of the variations in distribution observed (Table 3).
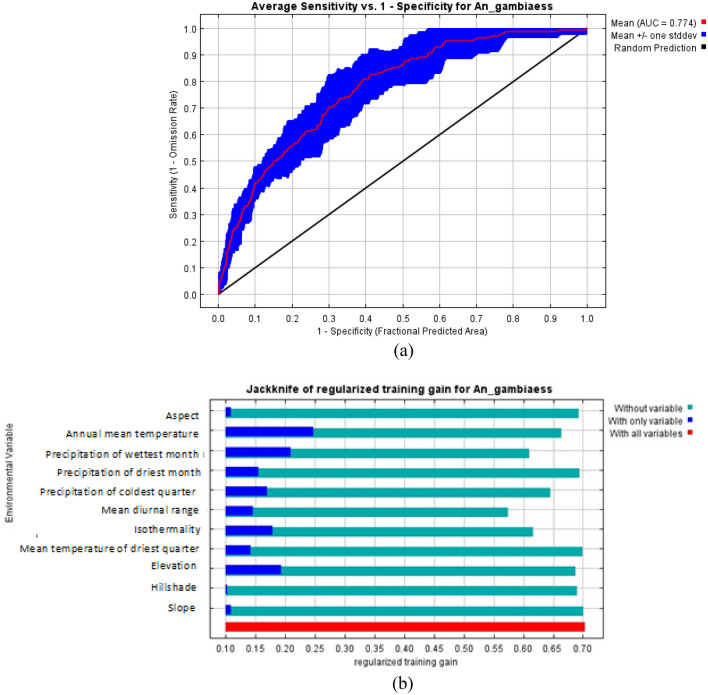
The ROC curve obtained as an average of the 10 replications runs is shown in Fig. 7a, and specificity was calculated. The average and standard deviation of the AUC for the 10 replicate runs was 0.774 ± 0.01. This value shows an excellent performance of the model as an AUC value of greater than 0.70 shows higher sensitivity and specificity for the presence of An. gambiae.
Figure 7.
Estimation of model performance for An. gambiae (a) Area under the curve (AUC) for An. gambiae distribution. Red line indicates the mean value for 10 MaxEnt replicate runs. (b) Jacknife analysis for regularized training gain.
The relative importance of each variable to the distribution of An. gambiae was also computed with the Jackknife test in Fig. 7b which gave a training gain of 0.71. The Jackknife test showed that precipitation of the wettest month and annual mean temperature are the two variables that will increase the gain the most when used alone. The Jackknife test also showed that mean diurnal range and precipitation of the wettest month will decrease the gain the most when removed from the model.
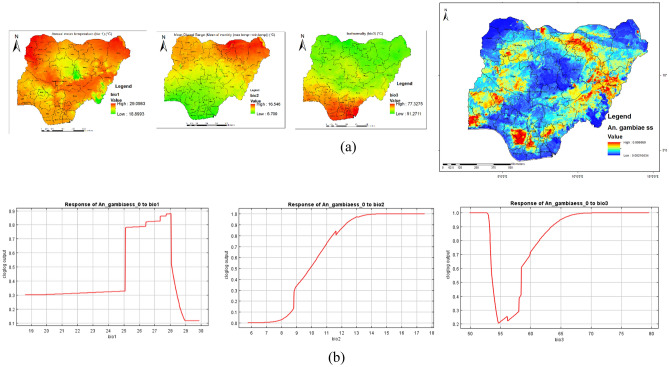
Figure 8a shows the main highest estimated environmental variables (contributions) that determines the distribution of An. gambiae in Nigeria. Spatial distribution analysis was done to determine the geographical variability with regards to the selected environmental variables in the country. The response curves of three variables to An. gambiae habitat suitability are shown in Fig. 10a. We found out that annual mean temperature ranged from 18.9 to 29.1 °C, mean diurnal range ranged from 6.7 to 16.6 °C while isothermality ranged from 51.3 to 77.3 °C. The response curves showed that between annual mean temperature of 25 and 28 °C favors the potential distribution of An. gambiae. Similarly, mean diurnal range of 11 to 18 °C significantly and potentially favoured the distribution of An. gambiae. while isothermality between 65 and 80 °C significantly favoured the distribution of An. gambiae in Nigeria (Fig. 8b).
Figure 8.
Estimates of the highest contributing variables that determines the geographical distribution of An. gambiae (a) The highest environmental variables that estimate to control the geographical distribution of An. gambiae in Nigeria. Variable contributions (annual mean temperature and mean diurnal range and isothermality), (b) Response curves of three environmental predictors used in MaxEnt model for An. gambiae. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Available at https://www.R-project.org/. The Nigerian shapefile was obtained from World BankDataCatalog () an Open license standardized resource of boundaries (i.e., state, county) for every country in the world.
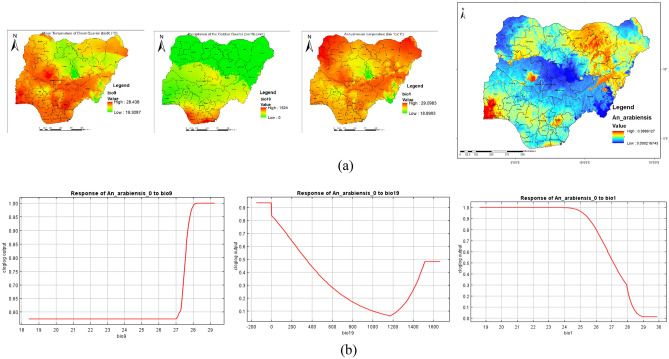
Figure 10.
Estimates of the highest contributing variables that determines the geographical distribution of An. arabiensis (a) The highest environmental variables that estimate to control the geographical distribution of An. arabiensis in Nigeria. Variable contributions (mean temperature of driest quarter, precipitation of coldest quarter and annual mean temperature), (b) Response curves of three environmental predictors used in MaxEnt model for An. arabiensis. This figure was created by the authors in R programming software (R version 4.1.2, Vienna, Austria).
Available at https://www.R-project.org/. The Nigerian shapefile was obtained from World BankDataCatalog () an Open license standardized resource of boundaries (i.e., state, county) for every country in the world.
An. arabiensis
In this study, mean temperature of the driest quarter had the highest contribution with PC and PI of 36.3 and 8.9 respectively, followed by precipitation of the coldest quarter with PC of 31.1 and PI of 30.5 and annual mean temperature with PC of 13 and PI of 16.8. The results showed that these variables are strong predictors of An. arabiensis distribution in Nigeria, accounting for over 80% of the variations in distribution observed (Table 3).
The ROC curve obtained as an average of the 10 replications runs is shown in Fig. 9a, and specificity was calculated. The average and standard deviation of the AUC for the 10 replicate runs was 0.753 ± 0.01. This value shows an excellent performance of the model as an AUC value of greater than 0.70 shows higher sensitivity and specificity for the presence of An. arabiensis.
Figure 9.
Estimation of model performance for An. arabiensis (a) Area under the curve (AUC) for An. arabiensis distribution. Red line indicates the mean value for 10 MaxEnt replicate runs. (b) Jackknife analysis for regularized training gain. The dark blue, light blue and red bars represent results of the model with each individual variable, all the remaining variables and all variables respectively.
The relative importance of each variable to the distribution of An. arabiensis was also computed with the Jackknife test in Fig. 9b which gave a training gain of 0.72. The Jackknife test showed that mean temperature of the driest quarter and annual mean temperature are the two variables that will increase the gain the most when used alone. The Jackknife test also showed that precipitation of the coldest quarter will decrease the gain the most when removed from the model.
Figure 10a shows the main highest estimated environmental variables (contributions) that determines the distribution of An. arabiensis in Nigeria. Spatial distribution analysis was done to determine the geographical variability with regards to the selected environmental variables in the country. The response curves of three variables to An. arabiensis habitat suitability are shown in Fig. 10b. We found out that mean temperature of the driest quarter ranged from 19.3 to 28.4° C, precipitation of the coldest quarter ranged from 0 to 1524 mm while annual mean temperature ranged from 18.9 to 29.1° C. The response curves showed that between mean temperature of the driest quarter of 28 and 29° C favors the potential distribution of An. arabiensis. Similarly, precipitation of the coldest quarter of 0 to 200 mm significantly and potentially favoured the distribution of An. arabiensis while annual mean temperature ranging between 19 and 25 °C significantly favoured the distribution of An. arabiensis in Nigeria (Fig. 10b).
Relationship between the strongest environmental predictors of the distribution of the three An. species
Annual mean temperature and precipitation of the coldest quarter were common predictors for the three species, while isothermality is peculiar to both An. gambiae and An. coluzzii. Furthermore, mean temperature of driest quarter is common to both An. coluzzii and An. arabiensis while elevation is a common predictor to both An. gambiae and An. arabiensis. However, An. gambiae is the only species to have three environmental variables; mean diurnal range, precipitation of the wettest month and precipitation of the driest month as predictors peculiar to it alone (Fig. 11).
Figure 11.
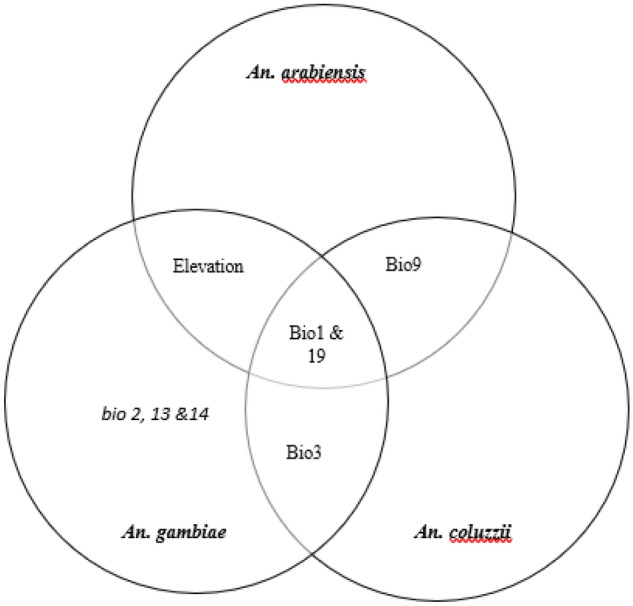
Relationship between the strongest environmental predictors of the distribution of the three Anopheles species.
Discussion
Vector surveillance is a very germane component of planning, implementation, monitoring and evaluation of vector control interventions. Having a holistic view of the distribution of major malaria vector populations for an entire country is a valuable asset for winning the battle against malaria transmission. Our work presents occurrence data for three malaria vectors in 12 states of Nigeria and a predicted distribution for the entire country using the maximum entropy algorithm approach.
Okorie et al.51 presented a comprehensive data base for the entire country, however, one major limitation to their study was that the methods of collection were not uniform as this may have its effect on predictive data especially in areas where samples were not collected. Second, the data takes into account a 100-year data from published manuscripts. As it is, the vector dynamics is changing and the current events may have overtaken this, over a decade manuscript. To the best our knowledge, our study is the first to report model-based estimates of dominant malaria vectors for the whole of Nigeria, using contemporary data.
Members of Anopheles gambiae complex constituted the majority of the populations of Anophelines collected during our study (occurring in all the 12 states) and may be responsible for malaria transmission within the country. This is not surprising as members of the complex have been implicated as the most important vectors of malaria in sub-Saharan. The complex has four species that occur in West Africa: An. coluzzii, An. gambiae, An. arabiensis and An. melas52–54.
An. coluzzii, An. gambiae, An. arabiensis were the members of the complex encountered in this study. This finding was somewhat similar to that of Okorie et al.51 who reported that An. gambiae complex, accounted for more than 60% of the overall composition of mosquitoes collected in their review. Some studies also buttressed the fact that the molecular S form (now referred to as An. gambiae) was predominant and had a wide distribution across Nigeria55,56. However, contrary to their findings, our work highlights the possibility of on-going species replacement from An. gambiae to An. coluzzii in the country. This could be a consequence of the recent efforts to increase LLIN distribution and use as well as possible changes in climatic conditions. These findings highlight the necessity for ongoing vector surveillance to provide data-based insight to control programmers and policy makers. Vector control strategies may need reshaping to target both of these major species. An. coluzzii has been reported to be an excellent vector for Plasmodium malariae, while both An. coluzzii and An. gambiae transmits both P. falciparium and P. ovale effectively57. Moreso, a study conducted in Benin suggested that An. coluzzii was more involved in malaria transmission in comparison with An. gambiae, which was previously believed to be the more efficient malaria vector58.
The importance of active vector surveillance cannot be overemphasized as it helps to understand how vector population thrives or changes over time, including the detection of new or invasive species. In this study, we also identified an invasive malaria vector; An. stephensi in one of the states in the northern part of the country50. An. stephensi is an excellent urban malaria vector and its presence in Nigeria is alarming owing to the behaviour of the species. Malaria in Africa has been essentially defined as a rural disease, but the establishment of An. stephensi population could result in a surge in cases of urban malaria59,60, putting at least 126 million people at risk61. Efforts to control malaria transmission in Nigeria have largely been concentrated on the members of the An. gambiae complex through deployment of LLINs due to the high predictability of their feeding behaviour (mostly anthropophilic) and ecology (strict breeding site preferences). It could be catastrophic if populations of An. stephensi have already established in the country, especially with the currently used control strategies in the country being targeted indoors.
Our model result suggests that the three sibling species; An. coluzzii, An. gambiae and An. arabiensis are widespread across all the ecological zones (from high to moderate), in a sympatric relationship, though the distribution of each of the species varied slightly. Of all the species, An. arabiensis seems to have the most restricted distribution, confined to two states in southern Nigeria and some portion of the northern parts of Nigeria.
The distribution of any disease vector including mosquito species are functions of the relationship that exists between factors such as climate, landforms, soil types and the human settlement patterns of different ecological zones61–63. However, this present study focused only on how climatic and topographic factors could affect the distribution pattern of major malaria vector in Nigeria. Mean temperature of the driest quarter, precipitation of the coldest quarter and annual mean temperature were strongly associated with the distribution of An. arabiensis. The ROC curve showed that warmer climates supported the breeding of An. arabiensis and are likely to be abundant in areas with lowest precipitation during the cooler part of the year. Other studies also suggested that An. arabiensis prefers warmer climates and thus limits their presence in cold swamps within the humid forest and high suitability in Sahel savanna localities64–66.
The high suitability observed for An. gambiae and An. coluzzii (more than two-thirds of the entire country is either highly or moderately suitable for both species) is also in keeping with the study of Akpan et al.66. It is important to note that areas with high suitability and low distribution density of both An. gambiae and An. coluzzii may have a serious likelihood of experiencing widespread prevalence alongside high distribution density, species migration and invasion67. Previous reports suggest that the range, relative abundance and ecological adaptability of An. gambiae complex are significantly influenced by seasonality, random temporal fluctuations and annual precipitation62,65,68,69. Therefore, their assertion agreed with our findings as all the aforementioned factors played a very crucial role in determining the distribution of the species in the study area. Our model suggests that higher temperatures and low precipitation influenced the presence of An. coluzzii, while the first three strongest environmental predictors for An. gambiae were related to temperature. The findings from our study indicate that certain environmental variables serve as common predictors for all three sibling species, while An. gambiae exhibits distinct environmental predictors not shared by the other species. This suggests that these variables play a unique role in influencing the distribution pattern of An. gambiae compared to the other mosquito species studied.
These findings highlight the importance of considering environmental factors, particularly climate and topography, in designing and implementing malaria control strategies in Nigeria. Understanding the distribution patterns and environmental preferences of mosquito species can help target when and where to deploy interventions, such as insecticide-treated bed nets, indoor residual spraying, and larval source management, especially to the area’s most at risk. For instance, larviciding in areas where precipitation is lower during the coolest part of the year may be highly impactful in controlling An. arabiensis. Additionally, monitoring and adapting control measures in response to changes in environmental conditions and vector behavior can enhance the effectiveness of malaria control efforts in the country.
One significant limitation of this study is the exclusion of parameters such as pH, dissolved oxygen, total dissolved solids, electrical conductivity, turbidity of the breeding sites, and demographic variables like the presence of cattle and human population density from our model. These variables are also crucial and may contribute to the explanation of mosquito species distribution. Further research should be conducted to incorporate these variables and evaluate their effects on the distribution of Anopheles mosquitoes in the country.
This study has, for the first time, predicted the potential distribution of the members of Anopheles gambiae complex across Nigeria using contemporary data involving standardized and uniform methods of mosquito collection. The Maximum entropy (MaxEnt) modeling used in this study is a general-purpose method for making predictions of inferences from incomplete information33. The model used in this study is a presence only modeling algorithm (i.e., absence data are not required). More so, the performance has been reported to be relatively better than other modeling methods27,34. The report that the model has been hardly influenced by small sample sizes with relatively robust prediction hence putting it among the top performing modeling tools27 made it the choice model for our study.
Conclusion
Our study provides comprehensive data on malaria vectors in Nigeria. Anopheles gambiae complex is the dominant vector, with species displacement observed. Active surveillance detects an invasive vector, An. stephensi, posing urban malaria risks. Varying environmental factors influence the distribution of different sibling species of the An. gambiae complex, requiring tailored control strategies. Active surveillance is very important in vector control. We have generated a model-based baseline species distribution of the major malaria vector population in the country using empirical data. Knowing that a country-wide mapping of vector distribution can be time consuming and expensive, hence the maps presented here could be used by the National Malaria Elimination Program to direct resources for vector control.
Supplementary Information
Acknowledgements
The authors extend their appreciation to the numerous entomological technicians and mosquito collectors for their efforts during the nation-wide mosquito breeding sites sampling.
Abbreviations
- MaxEnt
Maximum entropy
- LLIN
Long lasting insecticidal net
- IRS
Indoor residual spraying
- LSM
Larval source management
- WHO
World health organization
- NMEP
National malaria elimination program
- ENM
Ecological niche modeling
- FCT
Federal capital territory
- CDC
Center for diseases control
- PCR
Polymerize chain reaction
- LGA
Local government area
- ROC
Receiver operating characteristics
- AUC
Area under curve
- PC
Percentage contribution
- PI
Permutation importance
- SRTM
Shuttle radar topography mission
- GIS
Geographic information system
- GPS
Global positioning system
Author contributions
Conceptualization, A.A., A.S.B., O.O.O., P.U., C.A., B.S. and S.A.; methodology, A.A., A.S.B., T.O., A.O., O.A., R.T.I., A.O and O.O.; Data curation, A.A., A.S.B. and A.O; mapping, geospatial modeling and visualization, A.S.B.; investigation, A.A., O.O.O, M.A., C.O., D.A., A.D., J.M., O.S., O.A., P.U.I., L.S., A.O., M.D., P.K., S.M., R.S., C.A., M.A., M.B., O.A.I., A.A., I.O. and A.Y.; writing original draft, A.S.B. and A.A.; resources, A.A., A.S.B., O.O.O, M.B., M.E., M.O., P.U., S.A., M.S. and B.S.; review and editing, A.A., A.S.B., O.O.O., M.A., C.O., D.A., A.O, O.A.I., I.O., M.S., C.A., S.A. and B.S.; supervision, A.A., O.OO., M.O., P.U., S.A. and B.S.; project administration, A.A., O.O.O., M.A., C.O., D.A., A.D., J.M., O.S., O.A., P.U.I., L.S., A.O, M.D., P.K., S.M., R.S., C.A., M.A., M.B., O.A.I., A.A., I.O., A.S.B., S.A., B.S. and A.Y.
Funding
This work was supported by the Global Fund (Grant name: Global Fund supported grant NGA-M-NMEP, grant number: 1907).
Data availability
The data sets in this study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Adedapo Adeogun, Email: dapoadeogun@hotmail.com.
Ayodele Samuel Babalola, Email: ayodelebabalola2011@gmail.com.
Okefu Oyale Okoko, Email: oyalepp@yahoo.com.
Perpetua Uhomoibhi, Email: puhomoibhi@yahoo.com.
Samson Awolola, Email: awololas@hotmail.com.
Babatunde Salako, Email: tundesalako@hotmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-40929-5.
References
- 1.Sinka ME, et al. The dominant An. vectors of human malaria in Africa, Europe and the Middle East: Occurrence data, distribution maps and bionomic. Parasit. Vectors. 2010;3:117. doi: 10.1186/1756-3305-3-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hemming-Schroeder E, et al. Ecological drivers of genetic connectivity for African malaria vectors An. gambiae and An. arabiensis. Sci. Rep. 2020;10:19946. doi: 10.1038/s41598-020-76248-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coluzzii M. The clay feet of the malaria giant and its African roots: Hypotheses and inferences about origin, spread and control of Plasmodium falciparum. Parassitologia. 1999;41(1–3):277–283. [PubMed] [Google Scholar]
- 4.Coetzee M. Distribution of the African malaria vectors of the An. gambiae complex. Am. J. Trop. Med. Hyg. 2004;70(2):103–104. [PubMed] [Google Scholar]
- 5.WHO. World malaria report, 2022. WHO Geneva Switzerland. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwii3KPR5br9AhU0bvEDHekZDJsQFnoECBUQAQ&url=https%3A%2F%2Fwww.who.int%2Fteams%2Fglobal-malaria-programme%2Freports%2Fworld-malaria-report-2022&usg=AOvVaw3VYnKH7QBTFFiLnmC-0Vck. Accessed 15 February 2023 (2022)
- 6.Ayala FJ, Coluzzii M. Chromosome speciation: Humans, Drosophila, and mosquitoes. PNAS. 2005;102(Suppl 1):6535–6542. doi: 10.1073/pnas.0501847102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masendu HT, et al. The sympatric occurrence of two molecular forms of the malaria vector An. gambiae Giles sensu stricto in Kanyemba, in the Zambezi Valley, Zimbabwe. Trans. R. Soc. Trop. Med. Hyg. 2004;98(7):393–396. doi: 10.1016/j.trstmh.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Macdonald G. The Epidemiology and Control of Malaria. Oxford University Press; 1957. [Google Scholar]
- 9.Mala AO, et al. Plasmodium falciparum transmission and aridity: A Kenyan experience from the dry lands of Baringo and its implications for An. arabiensis control. Malar. J. 2011;10:121. doi: 10.1186/1475-2875-10-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillies, M. T. & De Mellion, B. The anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region), 2nd Publication no. 54 edn. South African Institute for Medical Research; Johannesburg. (1968).
- 11.NMIS: Nigeria Malaria Indicator Survey. https://dhsprogram.com/publications/publication-MIS41-MIS-Final-Reports.cfm. Accessed 14 January 2023 (2021).
- 12.NMSP: National Malaria Strategic Plan (NMSP 2014–2020), Federal Republic of Nigeria:https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjMrcS_5Lr9AhVEOHoKHbppBPwQFnoECAkQAQ&url=https%3A%2F%2Fwww.health.gov.ng%2Fdoc%2FNMEP-Strategic-Plan.pdf&usg=AOvVaw0VVqPBNHQ0wRsOAPBXF63p. Accessed 17 January, 2023 (2014)
- 13.Awolola TS, et al. Pyrethroids resistance intensity and resistance mechanisms in An. gambiae from malaria vector surveillance sites in Nigeria. PLoS One. 2018;13(12):e0205230. doi: 10.1371/journal.pone.0205230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The PMI VectorLink Project. The PMI VectorLink Nigeria Annual Entomology Report, October 2019–September 2020. Rockville, MD. VectorLink, Abt Associates Inc. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjQyYXc57r9AhXcgv0HHQBBDyYQFnoECAcQAQ&url=https%3A%2F%2Fd1u4sg1s9ptc4z.cloudfront.net%2Fuploads%2F2021%2F08%2FEntomological-Monitoring-Report-Nigeria-2019-2020.pdf&usg=AOvVaw1HW0v5FT_AwJZC_USxDHR8. Accessed 16 February, 2023 (2021)
- 15.Elimam AM, Elmalik KH, Ali FS. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling An. arabiensis and Culex quinquefasciatus mosquitoes. Saudi J. Biol. Sci. 2009;16:95–100. doi: 10.1016/j.sjbs.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaustein L, Kotler BP. Oviposition habitat selection by the mosquito, Culiseta longiareolata: Effects of conspecifics, food and green toad tadpoles. Ecol. Entomol. 1993;18(2):104–108. [Google Scholar]
- 17.Maheu-Giroux M, Castro MC. Impact of community-based larviciding on the prevalence of malaria infection in Dar es Salaam, Tanzania. PloS One. 2013;8(8):e71638. doi: 10.1371/journal.pone.0071638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maheu-Giroux M, Castro MC. Cost-effectiveness of larviciding for urban malaria control in Tanzania. Malar. J. 2014;13:477. doi: 10.1186/1475-2875-13-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson AT, Martinez-Campos C, Nakazawa Y, Martinez-Meyer E. Time-specific ecological niche modeling predicts spatial dynamics of vector insects and human dengue cases. Trans. R. Soc. Trop. Med. Hyg. 2005;99:647–655. doi: 10.1016/j.trstmh.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Escobar L, et al. Declining prevalence of disease vectors under climate change. Sci. Rep. 2016;6:39150. doi: 10.1038/srep39150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omar K, et al. Ecological niche modeling for predicting the potential geographical distribution of Aedes species (Diptera: Culicidae): A case study of Enugu State Nigeria. Parasite Epidemiol. Control. 2021;15:e00225. doi: 10.1016/j.parepi.2021.e00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiménez-Valverde A, et al. Use of niche models in invasive species risk assessments. Biol. Invasions. 2011;13:2785–2797. [Google Scholar]
- 23.Guisan A, Hofer U. Predicting reptile distributions at the mesoscale: Relation to climate and topography. J. Biogeogr. 2003;30:1233–1243. [Google Scholar]
- 24.Araújo MB, Pearson RG, Thuiller W, Erhard M. Validation of species-climate impact models under climate change. Glob. Change Biol. 2005;11:1504–1513. [Google Scholar]
- 25.Wintle BA, Elith J, Potts JM. Fauna habitat modelling and mapping: A review and case study in the Lower Hunter Central Coast region of NSW. Aust. Ecol. 2005;30:719–738. [Google Scholar]
- 26.Elith J, Leathwick JR. Species distribution models: Ecological explanation and prediction across space and time. Evol. Syst. 2009;40:677–697. [Google Scholar]
- 27.Elith J, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 28.United Nation. World Population Dashboard Nigeria. https://www.unfpa.org/data/world-population/NG.
- 29.WHO. Test procedures for insecticide resistance monitoringinmalaria vector mosquitoes. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjtzPvi8rr9AhUohv0HHcLaDdYQFnoECAsQAQ&url=https%3A%2F%2Fapps.who.int%2Firis%2Fbitstream%2Fhandle%2F10665%2F250677%2F9789241511575-eng.pdf&usg=AOvVaw2mfib4euOS-XutiHogtHS6. Accessed 14 February 2023 (2016).
- 30.Gillies MT, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara (Afro-Tropical region) South African Institute for Medical Research; 1987. [Google Scholar]
- 31.Scott J, Brogdon W, Collins F. Identification of single specimens of the An. gambiae complex by PCR. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 32.Favia G, et al. Polymorphisms detected by random PCR distinguishes between different chromosomal forms of An. gambiae. Proc. Natl. Acad. Sci. 1994;91:10315–10319. doi: 10.1073/pnas.91.22.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006;190:231–259. [Google Scholar]
- 34.Yaro CA, Kogi E, Luka SA. Spatial distribution and modeling of soil transmitted helminthes infection in Nigeria. Adv. Infect. Dis. 2018;8:82–107. [Google Scholar]
- 35.de Luis M, Bartolomé C, García Cardo Ó, Álvarez-Jiménez J. Gypsophila bermejoi: A possible case of speciation repressed by bioclimatic factors. PloS One. 2018;13(1):e0190536. doi: 10.1371/journal.pone.0190536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosso L, Di Febbraro M, Cristinzio G, Zoina A, Russo D. Shedding light on the effects of climate change on the potential distribution of Xylella fastidiosa in the Mediterranean basin. Biol. Invasions. 2016;18:1759–1768. [Google Scholar]
- 37.Smeraldo S, et al. Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: Lessons from bats. Biodivers. Conserv. 2018;27:2425–2441. [Google Scholar]
- 38.Khafagi O, Hatab EE, Omar K. Ecological niche modeling as a tool for conservation planning: Suitable habitat for Hypericum sinaicum in South Sinai, Egypt. Univers. J. Environ. Res. Technol. 2013;2(6):515–524. [Google Scholar]
- 39.Khafagi O, Hatab EE, Omar K. Predicting the potential geographical distribution of Nepeta septemcrenata in Saint Katherine Protectorate, South Sinai, Egypt using Maxent. Academ. Arena. 2011;3(7):45–50. [Google Scholar]
- 40.Guisan A, Graham CH, Elith J, Huettmann F, The NCEAS Species Distribution Modelling Group Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib. 2007;13:332–340. [Google Scholar]
- 41.Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007;34:102–117. [Google Scholar]
- 42.Olabimi IO, Ileke KD, Adu BW. Potential distribution of the primary malaria vector An. gambiae Giles [Diptera: Culicidae] in Southwest Nigeria under current and future climatic conditions. J. Basic Appl. Zool. 2021;82:63. [Google Scholar]
- 43.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005;25:1965–1978. [Google Scholar]
- 44.Ortega-Huerta MA, Peterson AT. Modeling ecological niches and predicting geographic distributions: A test of six presence-only methods. Rev. Mex. Biodivers. 2008;79:205–216. [Google Scholar]
- 45.Hernandez PA, Graham CH, Master LL, Albert DL. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography. 2006;29:773–785. [Google Scholar]
- 46.Papes M, Gaubert P. Modelling ecological niches from low numbers of occurrences: Assessment of the conservation status of poorly known viverrid (Mammalia, Carnivora) across two continents. Divers. Distrib. 2007;13:890–902. [Google Scholar]
- 47.Wisz MS, et al. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008;14:763–773. [Google Scholar]
- 48.Choudhury MR, Deb P, Singha H, Chakdar B, Medhi M. Predicting the probable distribution and threat of invasive Mimosa diplotricha Suavalle and Mikania micrantha Kunth in a protected tropical grassland. Ecol. Eng. 2016;97:23–31. [Google Scholar]
- 49.Sallam MF, Xue RD, Pereira RM, Koehler PG. Ecological niche modeling of mosquito vectors of West Nile virus in St. John’s County, Florida, USA. Parasit. Vectors. 2016;9(1):371. doi: 10.1186/s13071-016-1646-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adeogun, A. O. et al. First report of An. stephensi in West Africa: time to ramp up efforts towards implementation of more intense vector surveillance in Nigeria. https://www.ncbi.nlm.nih.gov/nuccore/ON856140. Accessed 2 March 2023 (2022).
- 51.Okorie PN, McKenzie FE, Ademowo OG, Bockarie M, Kelly-Hope L. Nigeria An. vector database: An overview of 100 years' research. PLoS One. 2011;6(12):e28347. doi: 10.1371/journal.pone.0028347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coluzzii M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the An. gambiae complex. Trans. R. Soc. Trop. Med. Hyg. 1979;73:483–497. doi: 10.1016/0035-9203(79)90036-1. [DOI] [PubMed] [Google Scholar]
- 53.Coluzzii M, Petrarca V, Di Deco MA. Chromosomal inversion intergradation an incipient speciation in An. gambiae. Boll. Zool. 1985;52:45–63. [Google Scholar]
- 54.Akogbeto M. Etude entomologique sur la transmission du paludisme cotier lagunaire: cas d'un village construit sur un lac d'eau saumâtre [Entomological study on the malaria transmission in coastal and lagoon areas: The case of a village built on a brackish lake] Soc. Belg. Med. Trop. 1995;75(3):219–227. [PubMed] [Google Scholar]
- 55.Awolola TS, Oyewole IO, Koekemoer LL, Coetzee M. Identification of three members of An. funestus (Diptera: Culicidae) group and their role in malaria transmission in two ecological zones in Nigeria. R. Soc. Trop. Med. Hyg. 2005;99:525–531. doi: 10.1016/j.trstmh.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Onyabe DY, et al. The distribution of M and S molecular forms of An. gambiae in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2003;97:605–608. doi: 10.1016/s0035-9203(03)80045-7. [DOI] [PubMed] [Google Scholar]
- 57.Zoh DD, et al. Role of An. gambiae s.s. and An. coluzzii (Diptera: Culicidae) in human malaria transmission in rural areas of Bouaké, in Côte d'Ivoire. J. Med. Entomol. 2020;57(4):1254–1261. doi: 10.1093/jme/tjaa001. [DOI] [PubMed] [Google Scholar]
- 58.Akogbéto MC, et al. Blood feeding behaviour comparison and contribution of An. coluzzii and An. gambiae, two sibling species living in sympatry, to malaria transmission in Alibori and Donga region, northern Benin, West Africa. Malar. J. 2018;17:307. doi: 10.1186/s12936-018-2452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takken W, Lindsay S. Increased threat of urban malaria from An. stephensi mosquitoes. Africa. Emerg. Infect. Dis. 2019;25:1431–1433. doi: 10.3201/eid2507.190301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sinka ME, et al. A new malaria vector in Africa: Predicting the expansion range of An. stephensi and identifying the urban populations at risk. Proc. Natl. Acad. Sci. U. S. A. 2020;117(40):24900–24908. doi: 10.1073/pnas.2003976117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tolulope O. Spatio-temporal clustering of malaria morbidity in Nigeria (2004–2008) J. Sci. Res. 2014;13:99–113. [Google Scholar]
- 62.Moffett A, Shackelford N, Sarkar S. Malaria in Africa: Vector species' niche models and relative risk maps. PLoS ONE. 2007;2(9):e824. doi: 10.1371/journal.pone.0000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Siteti MC, Injete SD, Wanyonyi WA. Malaria parasite species prevalence and transmission dynamics at selected sites in the Western highlands of Kenya. CHRISMED J. Health Res. 2016;3:45–50. [Google Scholar]
- 64.Oyewole IO, Ibidapo CA, Oduola AO, Obansa JB, Awolola TS. Anthropophilic mosquitoes and malaria transmission in a tropical rain forest area of Nigeria. Acta SATECH. 2005;2(1):6–10. [Google Scholar]
- 65.Kulkarni MA, Desrochers RE, Kerr JT. High resolution niche models of malaria vectors in Northern Tanzania: A new capacity to predict malaria risk? PLoS ONE. 2010;5(2):e9396. doi: 10.1371/journal.pone.0009396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akpan GE, Adepoju KA, Oladosu OR, Adelabu SA. Dominant malaria vector species in Nigeria: Modelling potential distribution of An gambiae sensu lato and its siblings with MaxEnt. PloS One. 2018;13(10):e0204233. doi: 10.1371/journal.pone.0204233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peterson AT. Shifting suitability for malaria vectors across Africa with warming climates. BMC Infect. Dis. 2009 doi: 10.1186/1471-2334-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lindsay SW, Parson L, Thomas CJ. Mapping the ranges and relative abundance of the two principal African malaria vectors, An. gambiae sensu stricto and An. arabiensis, using climate data. Proc. R. Soc. Lond. B. 1998;265:847–854. doi: 10.1098/rspb.1998.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Onyabe DY, Conn JE. The Distribution of Two Major Malaria Vectors, An. gambiae and An. arabiensis, in Nigeria. Mem. Inst. Oswaldo Cruz, Rio de Janeiro. 2001;96(8):1081–1084. doi: 10.1590/s0074-02762001000800009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets in this study are available from the corresponding author on reasonable request.