Abstract
Endometriosis is strongly associated with infertility. Several mechanisms have been reported in an attempt to elucidate the pathophysiological effects that lead to reduced fertility in women with endometriosis. However, the mechanisms by which endometriosis affects fertility have not been fully elucidated. Ferroptosis is a novel form of nonapoptotic cell death that is characterized by iron-dependent lipid peroxidation membrane damage. In past reports, elevated iron levels in ectopic lesions, peritoneal fluid and follicular fluid have been reported in patients with endometriosis. The high-iron environment is closely associated with ferroptosis, which appears to exhibit a double-edged effect on endometriosis. Ferroptosis can cause damage to ovarian granulosa cells, oocytes, and embryos, leading to endometriosis-related infertility. This article summarizes the main pathways and regulatory mechanisms of ferroptosis and explores the possible mechanisms of the formation of an iron-overloaded environment in endometriotic ectopic lesions, peritoneal fluid and follicular fluid. Finally, we reviewed recent studies on the main and potential mechanisms of ferroptosis in endometriosis and endometriosis-related infertility.
Subject terms: Cell death, Infertility
Facts
There is a high iron level in both peritoneal and follicular fluid in patients with endometriosis.
A high-iron environment may be key to triggering ferroptosis.
Ferroptosis may have a double-edged effect on the development of endometriosis.
Ferroptosis impairs the function of oocytes and granulosa cells in patients with endometriosis.
Open questions
How does endometriosis tolerate the high levels of iron in the peritoneal fluid?
Could ferroptosis inducers be the next potential treatment for endometriosis?
How do iron overload and ferroptosis affect endometriosis-related infertility?
How can ferroptosis be balanced to treat endometriosis and endometriosis-related infertility?
Introduction
Endometriosis refers to an oestrogen-dependent inflammatory disease characterized by the seeding and growth of endometrial tissue outside the uterine cavity [1]. These endometrial tissues can be seeded on the peritoneal cavity, ovaries, and fallopian tubes, as well as distant tissues and organs [2]. The simultaneous detection of endometrial stromal and glandular components in histological biopsies is necessary to ascertain endometriosis [3]. The common clinical symptoms of endometriosis include chronic pelvic pain and infertility, which severely affect the physical and mental health of patients [4]. A total of 25 to 50% of women with infertility are clinically treated for endometriosis, and 30 to 50% of women with endometriosis suffer from infertility [5, 6]. However, the exact link between endometriosis and infertility is unknown, and many factors may be involved in this link. For example, mechanical disruption by pelvic adhesions in women with advanced endometriosis affects oocyte release and transport, decreases sperm motility, and impairs zygote implantation, which leads to reduced fertility [7]. However, the causes of infertility in women with mild endometriosis remain unclear and are subject to numerous speculations, mainly relating to endocrine abnormalities, immune disorders, oxidative stress, and aberrant gene expression [8, 9].
Ferroptosis is a novel form of regulated cell death that is distinct from accidental cell death; it can be mediated by different molecular signalling pathways [10, 11]. Specifically, ferroptosis is defined as an iron-dependent regulated form of necrosis that is caused by massive lipid peroxidation-mediated membrane damage, and this regulated necrosis plays a crucial role in the development and disease of various organisms [12, 13]. Although many open questions remain in ferroptosis research, numerous reports have stated that ferroptosis is closely related to many diseases, such as cancer, ischaemic organ injury, and degenerative diseases [14]. In several recent reports, ferroptosis was detected in ectopic endometrial tissue in endometriosis characterized by periodic haemorrhage [15] and in the early embryo in iron-overloaded peritoneal fluid [16]. However, the specific role and mechanism of ferroptosis in endometriosis, as well as in endometriotic infertility, remain unclear. In this article, we explored the possible mechanisms of the formation of an iron-overloaded environment in endometriotic ectopic lesions, peritoneal fluid and follicular fluid. In addition, we summarized the main pathways and regulatory mechanisms of ferroptosis and discussed its involvement in endometriosis and endometriosis-related infertility to provide new insights into the discovery of novel therapeutic targets.
We propose the notion that a threshold exists for the occurrence of ferroptosis in ectopic endometrial tissue in endometriosis. Once beyond the threshold, iron overload and oxidative damage can lead to ferroptotic cell death. Multiple oxidative and antioxidant systems can be activated simultaneously and operate in parallel to adjust this threshold, which is implicated in the metabolic reprogramming of the affected cells [17]. On the one hand, ectopic endometrial tissues in patients with endometriosis present resistance to ferroptosis, probably because of the shared antioxidant system in macrophages and ectopic lesion cells in the peritoneal fluid. On the other hand, ectopic endometrial tissue is partially subjected to ferroptosis, which seems beneficial. However, this process is followed by the activation of a series of downstream signalling pathways and the release of cytokines that promote cell proliferation. Thus, ectopic endometrial tissue might shift the threshold at which ferroptosis occurs by metabolic reprogramming towards a proliferative advantage for itself, something that seems to be similar to that of cancer cells. However, the specific metabolic checkpoints of the altered thresholds need further exploration, which is a future research direction.
Ferroptosis
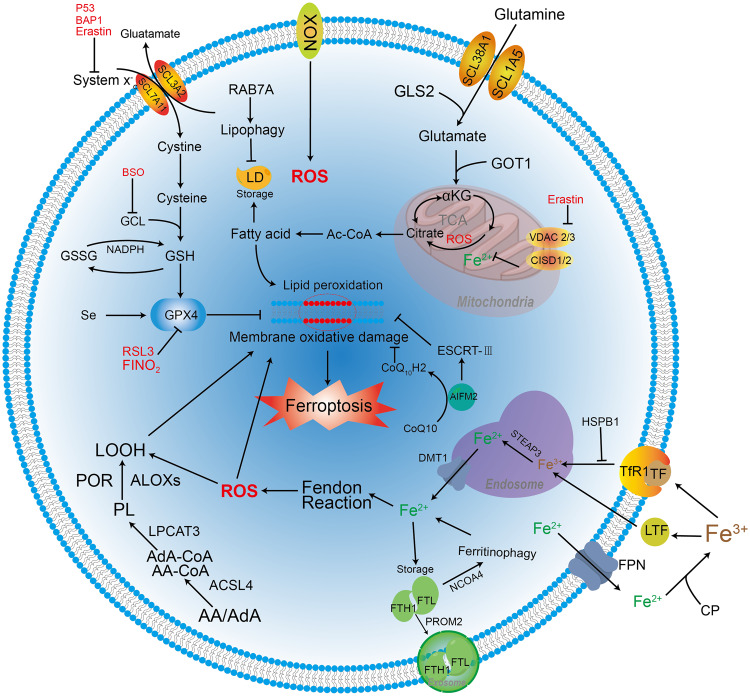
Dixon et al. first defined ferroptosis as a distinct iron-dependent form of non-apoptotic cell death in 2012 [11]. Ferroptosis is morphologically, biochemically, and genetically distinct from necrosis, apoptosis, and autophagy, and these differing features include abnormal mitochondrial membrane density, iron accumulation, lipid peroxidation, overexpression of ferroptosis biomarkers, and death of leucocyte subsets and the corresponding loss of immune function [11, 18–21]. Of note, ferroptosis that occurs within a cell can spread in a population of cells in a peroxidized lipid and iron-dependent manner [22]. Overall, the core molecular machinery of ferroptosis is regulated by various cellular signalling pathways and genes but is primarily mediated through two main pathways, namely, extrinsic or transporter-dependent pathways (e.g., reduced cysteine or glutamine uptake and increased iron uptake) and intrinsic or enzyme-regulated pathways (e.g., inhibition of glutathione (GSH) peroxidase 4 (GPX4) antioxidant system) (Fig. 1).
Fig. 1. Molecular machinery and regulation of ferroptosis.
The molecular machinery of ferroptosis involves cellular antioxidant and oxidative systems, and the regulation of ferroptosis includes iron metabolism and lipid peroxidation.
GPX4 pathway
GPX4 is a key factor in the antioxidant system that is regulated by multiple molecular mechanisms. In most cells, cysteine is obtained through the system xc- antiporter, which exchanges extracellular cystine with intracellular glutamate [23]. However, the deletion of the cystine/glutamate antiporter SLC7A11 in mice is well tolerated under unstressed environments [24], indicating that average cells have a low intake requirement for cystine. The activity of exogenously ingested cystine and glutamate–cysteine ligase (GCL) can regulate the synthesis of GSH, which is a major endogenous antioxidant [11, 25]. When GSH exerts an antioxidant effect, GSH can act as an electron donor and oxidize itself to the glutathione disulfide (GSSG) form. The GSH/GSSG ratio usually indicates the level of cellular oxidative stress, which accelerates the conversion of GSH to GSSG and decreases the GSH/GSSG ratio [26].
Based on its unique functions, GPX4 is considered a powerful antagonist of ferroptosis and plays a crucial role in regulating ferroptosis. As a key antioxidant system enzyme, GPX4 can catalyse the reduction in lipid peroxides in complex cellular membrane environments [27]. It can detoxify cellular lipid peroxidation by using the cofactor (GSH) by converting complex toxic lipid hydroperoxides, such as phospholipid hydroperoxides and cholesterol hydroperoxides, into their corresponding nontoxic lipid alcohols. The outcome is that GPX4 reduces the accumulation of ROS and acts against complex lipid peroxidation to reduce cell death [27, 28]. In addition, GPX4 is a kind of selenoprotein. Therefore, GPX4 synthesis is regulated by selenium (Se). Se protects neurons by activating the transcription factors TFAP2c and Sp1 coordinately. This, in turn, upregulates GPX4 and other genes to prevent fatal seizures [29]. Moreover, supplementation with Se could enhance the expression of GPX4 in follicular helper T cells and increase the number of helper T cells to improve the antibody responsiveness of immunized mice after vaccination [30], indicating that the regulation of Se on GPX4 plays an essential role in normal mammalian embryos. Selenocysteine (Sec) is the substitution of Se for sulfur from cysteine, which can enhance the resistance of GPX4 to irreversible peroxidation and prevent hydroperoxide-induced ferroptosis [13, 31].
Throughout the antioxidant system, the regulation of multiple inhibitors and ferroptosis inducers has been implicated. Inhibition of the GCL by buthionine sulfoximine (BSO) induces ferroptosis alone or enhances the sensitivity of cells to ferroptosis induced by other agents [32]. The activity of SLC7A11 is regulated by several factors, such as the transcription factor activating transcription factor 4 (ATF4) and/or nuclear factor erythroid 2-related 2 (NRF2) [33, 34], the epigenetic regulation-associated enzyme BAP1 [35], the tumour suppressor protein p53 [36], the autophagy mechanism component BECN1 [37], and the ferroptosis inducer erastin [38]. RSL3 can directly inhibit GPX4 activity but not its precursor GSH [27]. However, FINO2 indirectly inhibits GPX4 enzymatic function and directly induces ferrous (Fe2+) production [39].
Mitochondria-related pathways
Reactive oxygen species (ROS) are a byproduct of aerobic metabolism that are mainly derived from mitochondrial metabolism and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) on the cell membrane, and excessive ROS or the inappropriate location of ROS can damage cells [40]. ROS production in the mitochondria has been shown to be the signalling pathway that regulates the immune response and autophagy but is also important for the induction of ferroptosis [41, 42]. Mitochondria can promote the progression of cysteine-deprivation-induced ferroptosis but not inhibit GPX4-induced ferroptosis [18]. The metabolic network of ROS production can participate in ferroptosis. The transporter SLC38A1 and the amino acid transporter SLC1A5-mediated glutamine uptake and subsequent glutaminase 2 (GLS2)-mediated glutamate production are required for cysteine-deprivation-induced ferroptosis [43, 44]. Glutamate generates α-ketoglutarate (αKG) in mitochondria through transamination by the transaminase GOT1 [44]. αKG can generate acetyl-CoA, a metabolic precursor for lipid synthesis in the cytoplasm, and stimulate dihydrolipoamide dehydrogenase to produce mitochondrial ROS and increase local iron levels to promote ferroptosis [45]. In addition, the tricarboxylic acid cycle or electron transfer chain in the mitochondria can promote lipid ROS accumulation and is involved in cysteine-deprivation-induced ferroptosis [18]. However, ferristatin-1 can specifically prevent ferroptosis induced by erastin, but mitochondria are not involved in the function of ferristatin-1, suggesting that mitochondria may not be necessary for ferroptosis [46].
Notably, the voltage-dependent anion channel (VDAC) in the mitochondrial outer membrane, also known as the mitochondrial pore, acts as a gatekeeper for the entry and exit of mitochondrial metabolites and is a convergence point for its binding to various ligands and proteins to mediate various cell survival and cell death signals [47]. Erastin can directly bind to VDAC 2 and alter mitochondrial membrane permeability, thereby inducing nonapoptotic cell death [48]. Iron–sulfur cluster protein CDGSH iron sulfur domain (CISD) 1, a mitochondrial outer membrane protein, regulates VDAC in a redox-dependent manner in cells and closes mitochondrial pores to prevent iron accumulation in the mitochondria [49]. Nedd4 can be induced upon erastin treatment in melanoma cells, and Nedd4 leads to VDAC 2/3 ubiquitination and mitochondrial pore degradation [50]. These findings all illustrate that VDAC plays an important role in ferroptosis.
Regulation of ferroptosis
Iron-related pathways
Iron is an indispensable metal for the body and is essential for maintaining biological homoeostasis. Iron oxidation has two states, Fe2+ and ferric (Fe3+), which are mainly present intracellularly and extracellularly, respectively. The interconversion between Fe2+ and Fe3+ can either donate or accept electrons. This is a process that provides the premise for redox reactions and may affect the sensitivity of cells to ferroptosis. Interestingly, only iron, and not other metals, such as zinc, that also cause ROS generation via the Fenton reaction [51], can induce ferroptosis. Fe3+ can bind to transferrin (TF) in serum and is subsequently taken up by the TF receptor 1 (TfR1), which is encoded by TFRC on the cell membrane [52]. Similarly, lactotransferrin (LTF) on cancer cell membranes promotes ferroptosis by increasing intracellular iron levels [53]. Protein kinase C-mediated heat shock protein beta-1 (HSPB1) phosphorylation can stabilize the actin cytoskeleton, thereby inhibiting TfR1-mediated iron uptake and reducing lipid ROS production to limit ferroptotic cell death [54]. Subsequently, Fe3+ taken up into the cell is reduced to Fe2+ by STEAP3 metalloreductase in the endosome and is then released into the labile iron pool of the cytoplasm by divalent metal transporter 1 (DMT1) [55]. Fe2+ participates in various cellular metabolic and biochemical reactions and maintains cellular homoeostasis. The NFS1 cysteine desulfurase can promote iron–sulfur cluster biosynthesis. This results in increased Fe2+ availability to inhibit erastin-induced ferroptosis in lung tumour cells and attenuates dihydroartemisinin-induced ferroptosis in leukaemia cells [55, 56]. The CISD1 protein and CISD2 protein, which are present in mitochondria and the endoplasmic reticulum (ER), inhibit ferroptosis by reducing iron uptake from mitochondria and ROS production, respectively [57, 58]. The iron storage protein ferritin consists of ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1) and functions to store iron in cells [59]. This protein can create an iron-overloaded environment and lay the foundation for the occurrence of cellular ferroptosis. Interestingly, RSL3-induced ferroptosis could be inhibited by higher expression levels of mitochondrial ferritin under hypoxic conditions [60]. Moreover, the nuclear receptor coactivator 4 (NCOA4)-mediated selective autophagy pathway (ferritinophagy) increases cellular labile iron pool levels to promote the rapid intracellular accumulation of ROS, which is critical for ferroptosis [61].
Ferroportin1 (FPN1), the only identified mammalian nonhaem iron exporter, can transport Fe2+ from intracellular to extracellular spaces [62], and Fe2+ is subsequently oxidized to Fe3+ by the ferroxidase ceruloplasmin (CP) [63]. Erastin can decrease FPN1 expression and induce iron accumulation in ectopic endometrial stromal cells (ESCs) of women with endometriosis to promote ferroptosis [38]. Knockdown of FPN1 can promote ferroptosis in Alzheimer’s disease (AD) and induce AD-like hippocampal atrophy and memory deficits. Furthermore, differentially expressed genes of the ferroptosis-associated RNA-seq dataset are highly enriched in gene sets associated with AD [62]. Moreover, prominin-2, a member of the prominin family of pentaspan membrane glycoproteins, can mediate the release of ferritin into the extracellular space by exosomes in breast epithelial and breast cancer cells, thereby promoting cellular resistance to ferroptosis [64].
Lipid metabolism pathways
Lipids are not only important components of cell membranes but also precursors of various molecules that play important biological roles. However, the excessive accumulation of lipids has potentially toxic effects on individual cells, as well as on the whole body. Previous studies suggest that the peroxidation of polyunsaturated fatty acids in phospholipids by lipoxygenases (ALOX) is particularly important for ferroptosis [65, 66]. After lipid peroxidation occurs, the initiated generation of lipid hydroperoxides (LOOH) and subsequent generation of malondialdehyde (MDA) and 4-hydroxynonenal (4HNE) increase during ferroptosis, leading to a sustained oxidative stress response [67, 68]. Arachidonic acid (AA) and adrenic acid (AdA) are the main substrates of lipid peroxidation in ferroptosis [19], and the lipid peroxidation process involves three enzymes, namely, acyl-CoA synthetase long-chain family member 4 (ACSL4), lysophosphatidylcholine acyltransferase 3 (LPCAT3), and ALOX. ACSL4 binds to AA/AdA and catalyses the formation of AA/AdA-CoA; this is followed by the LPCAT3-mediated esterification of AA/AdA-CoA to phospholipids (PL). Finally, ALOX catalyses the generation of LOOH from PL to promote ferroptosis [69]. Cytochrome P450 (CYP450) oxidoreductase can promote lipid peroxidation by accelerating the cycling between Fe2+ and Fe3+ in the CYP450 haem fraction and is identified as the alternative source of ROS that induces ferroptosis-related lipid peroxidation [70]. Lipid droplets (LDs) generated from the ER can store lipids in cells and supply lipids for cellular metabolism. The LD cargo receptor RAB7A can mediate selective autophagy (lipophagy) to degrade LDs, which increases the production of free fatty acids and promotes lipid peroxidation. Thus, it ultimately leads to ferroptosis [71].
Summary
Under normal physiological conditions, iron plays an important role in metabolic processes. However, whenever the transporter is mutated or deleted, it will disrupt the iron balance and lead to excessive accumulation, triggering cellular oxidative damage and death [72, 73]. Similarly, ROS produced in normal physical processes play an important role in the maintenance of cell function, but excessive ROS may cause metabolic disorders, such as lipid peroxidation, and induce ferroptosis [74, 75]. GPX4 can inhibit ferroptosis by virtue of its special restorative function. Its depletion can lead to a decrease in the antioxidant capacity of cells and increase their sensitivity to ferroptosis [76]. In addition, as an NADPH-dependent coenzyme Q (CoQ) oxidoreductase, apoptosis-inducing factor mitochondria-associated 2 (AIFM2) can use NADPH to catalyse the regeneration of CoQ10 and act synergistically with GPX4 and GSH to inhibit phospholipid peroxidation and ferroptosis [77, 78]. GTP cyclohydrolase-1 (GCH1) can catalyse GTP to tetrahydrobiopterin to exert endogenous antioxidant effects and inhibit ferroptosis [79]. Dihydroorotate dehydrogenase (DHODH) is a flavin-dependent mitochondrial enzyme that can work in parallel with GPX4 to resist mitochondrial ferroptosis [80]. The upregulation of the tumour suppressor gene P53 leads to the accumulation of lipid hydroperoxides by inhibiting the expression of SLC7A11 and reducing the level of GSH, eventually triggering ferroptosis [81]. Ferroptosis is regulated by various factors and pathways.
In short, ferroptosis is an iron-dependent lipid peroxidation form of regulated cell death. Iron metabolism and lipid generation, storage, and degradation are all closely associated with ferroptosis. Excessive free iron levels and dysregulated lipid metabolism in cells trigger ferroptosis, but the oxidative and antioxidant systems are also involved in the regulation and maintenance of cellular processes. In addition, ferroptosis is regulated by many other factors. The complex mechanisms involved need to be elucidated further to help us better modulate the degree of ferroptosis caused by drugs or gene regulation for the treatment of diseases.
Iron-overloaded environment in endometriosis
Endometriosis can be divided into three phenotypes due to the diverse of underlying aetiologies: superficial peritoneal endometriosis, ovarian endometriosis, and deep infiltrating endometriosis [82]. Studies have shown that the levels of iron, ferritin, and haemoglobin are higher in the peritoneal fluid of women with endometriosis than in that of normal women [83]. Moreover, iron aggregates are present in endometriotic lesions of women with endometriosis and model mice [84, 85]. In addition, ovarian endometriomas contain high amounts of free iron, and the surrounding follicles nearby are also iron overloaded, which adversely affects oocyte development and quality [86]. However, the original cause of the iron-overloaded environment in ectopic lesions, peritoneal fluid, and follicular fluid of endometriosis is still unknown and may be related to the excessive degradation of red blood cells and increased influx caused by menstrual reflux and repeated bleeding of local lesions [87].
Retrograde menstruation and ectopic endometrial bleeding lesions can transport menstrual endometrial tissue and red blood cells to the peritoneal cavity. Some of these tissues and cells will be phagocytized, absorbed, and degraded by peritoneal macrophages and stored in the form of haemosiderin. Additionally, ferritin and haemoglobin are released into the peritoneal fluid [83]. The haem released by the hydrolytic digestion of haemoglobin is catabolized by haem oxygenase to generate active iron and forms iron-ferritin deposition. This overwhelms the iron homoeostasis and iron clearance system, finally leading to an iron-overloaded environment in peritoneal fluid and ectopic lesions of endometriosis [88]. In the environment of intraperitoneal iron overload, excess iron is transported by peripheral TF to cells within the ovary. This iron can bind to TfR1 on the surface of cells and trigger endocytosis [89]. In addition, menstrual reflux to the ovary and repeated bleeding in local lesions of the ovary may also lead to an iron-overloaded environment in follicular fluid. Excessive accumulation of intraperitoneal iron can lead to the overproduction of ROS and the enhanced activation of nuclear factor-kappaB (NF-κB). This enhances the migration ability of human endometriotic cells by promoting the expression of matrix metalloproteinases (MMPs), aggravating inflammation, angiogenesis, and cell adhesion to participate in the progression of endometriosis lesions [59]. Moreover, iron overload in follicular fluid can cause granulosa cell death and affect oocyte maturation and quality, ultimately increasing the risk of endometriosis-related infertility [89, 90].
Ferroptosis and endometriosis
The crosstalk between ferroptosis and inflammation
Endometriosis is a chronic inflammatory disease that is closely related to inflammation and the immune response. As a regulated form of cell death, ferroptosis can activate different downstream pathways and complex molecular effector mechanisms, leading to cell lysis in different forms and resulting in morphological changes and immune responses [10].
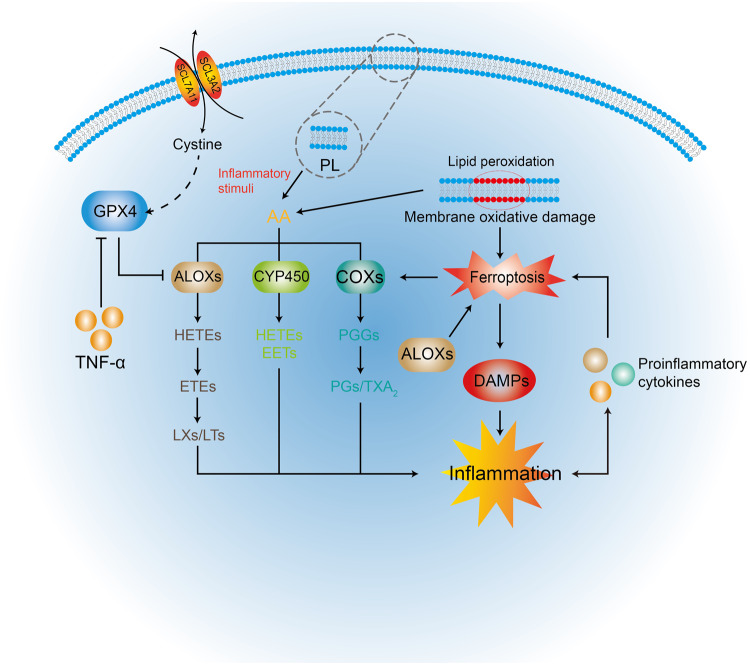
As the main substrate of lipid peroxidation released from PL in the cell membrane, AA is a precursor of proinflammatory mediators that can be metabolized by cyclooxygenases (COX), ALOX, and CYP450 monooxygenases to synthesize biologically active inflammatory mediators, such as prostaglandins (PGs) and leukotrienes [91]. Interestingly, ferroptosis induced by erastin or RSL3 can increase the expression of PTGS2 encoding COX2 [27]. Thus, ferroptosis can promote AA metabolism and inflammatory cytokine secretion via COX2 synthesis. The inactivation of the ferroptosis regulator GPX4, which can upregulate 12/15-ALOX and COX1 expression [92, 93], may accelerate AA metabolism and further promote inflammatory responses. Conversely, the release of inflammatory cytokines promotes the progression of ferroptotic cell death, such as the inhibition of GPX4 expression in tumour necrosis factor-α-treated cells (Fig. 2) [94]. Thus, there is crosstalk between ferroptosis and inflammation.
Fig. 2. The crosstalk between ferroptosis and inflammation.
AA is released from PL by inflammatory stimuli or by intercellular lipid peroxidation. The ALOX, COX, and CYP450 pathways promote further AA metabolization to inflammatory mediators. COX2 expression is increased by ferroptosis. ALOX can promote ferroptosis by catalysing the generation of LOOH. The large array of oxidized lipid mediators released by ferroptosis can contribute to the activity of COX and ALOX. GPX4 inhibits the activity of ALOX and COX directly by decreasing cellular redox states. Ferroptosis initiates inflammatory responses by releasing DAMPs that are immunogenic. Several proinflammatory cytokines play important roles in the crosstalk between ferroptosis and inflammation. For example, TNF can inhibit the activity of GPX4 to promote ferroptosis.
Similar to nonsilent immune forms of regulated necrosis, ferroptotic cell death can release damage-associated molecular patterns (DAMPs) that promote the development of multiple inflammatory diseases and trigger the innate immune system. These DAMPs can drive tissue inflammation and inflammation crosstalk with ferroptosis. This further promotes an autoamplification loop that exaggerates inflammation and cell death and leads to a more severe degree of cell death and a range of inflammation-related responses [95, 96]. For example, high mobility group box 1 (HMGB1), as a DAMP, is released in an autophagy-dependent manner by ferroptosis inducers and mediates inflammatory responses through the HMGB1-advanced glycation end-product-specific receptor (AGER) pathway, a pathway that activates the NF-κB pathway in innate immunity [94]. This promotes the expression of MMPs and aggravates inflammation, angiogenesis, and cell adhesion in endometriosis.
Double-edged roles of ferroptosis in endometriosis
Endometriosis is also an oestrogen-dependent gynaecological disease in which excessive oestrogen signalling transduction and altered oestrogen signalling pathways play an important role in its pathogenesis, resulting in oestrogen dominance and progesterone resistance [97, 98]. Oestrogen dependence may be due to the upregulation of the 17β-hydroxysteroid dehydrogenase-1 and aromatase genes, whereas progesterone resistance may result from the failure of progesterone receptor activation and transcription of progesterone target genes [99]. In normal endometrial tissue, oestrogen may inhibit autophagy in the endometrium by inhibiting the hypoxia-inducible factor-1/ROS/AMP-activated protein kinase signalling pathway and further activating mammalian target of rapamycin complex (mTOR) signalling during nonmenstrual periods [100]. However, the level of ROS is no longer suppressed by oestrogen in ectopic endometrial tissue cells. Thus, the level of ROS in ectopic endometrium is notably higher than that in normal eutopic endometrium. Perhaps this is because of the iron-overloaded environment in ectopic tissue cells [101].
The imbalance of iron metabolism plays an important role in the pathogenesis of endometriosis, and studies have confirmed that iron overload exists in the peritoneal fluid of patients with endometriosis [83, 102]. This phenomenon may be related to the increased degradation of red blood cells caused by menstrual reflux [87]. Overloaded iron generates a large amount of ROS by inducing the Fenton reaction, forming an imbalance between antioxidants and leading to oxidative stress reactions such as cellular oxidative damage [83]. Therefore, ectopic endometrial cell proliferation [103], the inflammatory response in the peritoneal cavity [104] and damage to the ovary and its cortex develop [105]. This iron-overloaded and peroxidative environment creates the conditions for the ferroptosis of ectopic endometrial tissue to occur in endometriosis. Li et al. found that a ferroptosis inducer could induce ferroptosis in ectopic endometrial stromal cells through ferroportin-mediated iron accumulation and then alleviate the ectopic lesions of endometriosis. However, the inducer had little effect in normal endometrial stromal cells [38]. The difference might be closely related to the special microenvironment of iron overload in ectopic endometrial stromal cells.
However, the role of ferroptosis in endometriosis appears to be bidirectional. On the one hand, ferroptosis inducers can promote ferroptosis in ectopic endometrial stromal cells, and thus, these inducers may become potential drugs for the treatment of endometriosis. On the other hand, ferroptotic endometrial stromal cells can release inflammatory cytokines and activate downstream regulatory pathways to promote proliferation and angiogenesis in surrounding tissues. Iron overload in ectopic endometrial stromal tissues from patients with ovarian endometriosis-induced ferroptosis, which promoted fibrosis and tissue adhesions, and the process was associated with endometrial stromal cell subpopulations [106]. In a recent study, Li et al. found that ferroptosis in ectopic endometrial stromal cells in patients with ovarian endometriosis could activate the p38 mitogen-activated protein kinase (p38 MAPK)/signal transducer and activator of transcription (STAT) 6 signalling pathway, thereby promoting local upregulation of vascular endothelial growth factor A (VEGFA) and interleukin-8 (IL-8) in ectopic lesions [15]. VEGFA and IL-8 could promote cell proliferation, adhesion, and angiogenesis of ectopic endometrial tissue, thereby promoting the development of endometriosis [107, 108]. In addition, ferroptosis, as a form of inflammatory cell death, is associated with the release of DAMPs, which can trigger the innate immune system and activate the NF-κB pathway through AGER [95, 109]. The excess of Fe2+ in ectopic ESCs generates ROS via the Fenton reaction, which contributes to the migration abilities of MMPs via the ROS-NF-κB pathway in ectopic endometrial cells [59]. The overproduction of ROS alters gene expression by regulating the redox-sensitive transcription factor NF-κB. NF-κB-mediated gene transcription in endometriotic cells promotes inflammation invasion, angiogenesis, and cell proliferation and inhibits the apoptosis of endometriotic cells. These effects favour the development and maintenance of endometriosis [110, 111].
Iron overload and ferroptosis do occur in endometriotic lesions, and the use of ferroptosis inducers may be a potential treatment for endometriosis. However, a series of downstream inflammatory pathways activated after ferroptosis cannot be ignored, and these pathways further promote angiogenesis and focal fibrosis (Fig. 3). Therefore, in the process of developing ferroptosis-related drugs with the potential to target endometriosis, a series of downstream reactions caused by ferroptosis in ectopic endometrial tissue should be considered, and these issues need to be further resolved in the future.
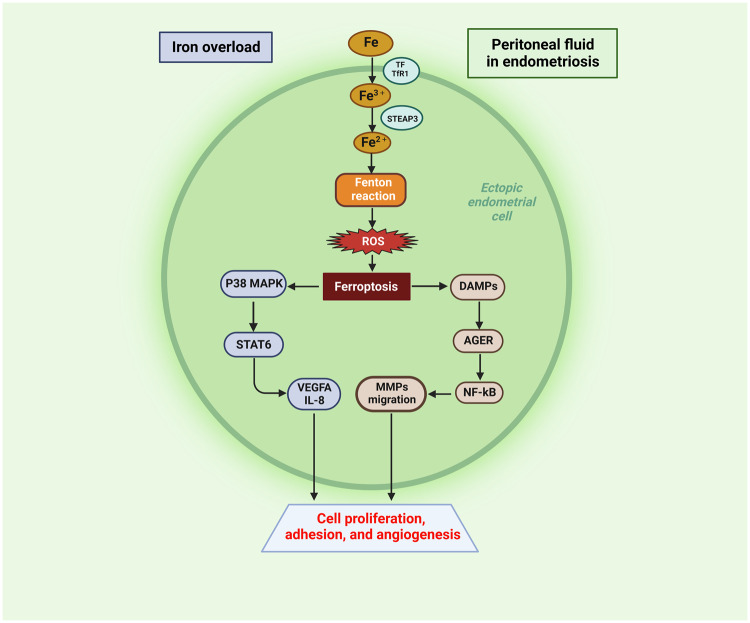
Fig. 3. Ectopic endometrial cells in iron-overloaded peritoneal fluid in endometriosis.
Iron-overloaded peritoneal fluid results in excess Fe2+ in ectopic endometrial cells. Excess Fe2+ generates ROS via the Fenton reaction, which contributes to ferroptosis in ectopic endometrial cells. Ectopic endometrial cells promote angiogenesis and cell proliferative adhesion through downstream DAMPs and the P38 MAPK/STAT6 pathways of ferroptosis. Created with BioRender.com.
Macrophage ferroptosis and endometriosis
Ferroptosis releases DAMPs and lipid oxidation products, which affect nonleukocytes and cause inflammatory cell death. However, it also mediates immune cell death that leads to losses of immune function, such as macrophage function. Macrophages phagocytose aged erythrocytes and process iron from erythrocytes to participate in iron metabolism. Excessive erythrophagocytosis leads to iron overload in macrophages and induces iron-dependent ferroptosis. Iron overload in bone marrow-derived macrophages can upregulate SLC7A11 expression via the ROS-NRF2-antioxidant response element (ARE) axis to reduce cellular sensitivity to ferroptosis [112]. In contrast, mice with GPX4-deficient bone marrow macrophages are susceptible to cell death caused by polymicrobial infection [113]. Furthermore, the release of DAMPs mediated by ferroptosis can affect macrophage polarization, and polarization imbalance can lead to various diseases or inflammatory conditions. For example, KrasG12D released from autophagy-dependent ferroptotic cancer cell death can limit the antitumour effects of macrophages by activating STAT3-mediated AGER-dependent M2 macrophage polarization [114]. Similarly, ferroptosis-mediated cell death that results in the release of 8-hydroxylamine (8-OHG) activates the STING1-dependent inflammatory pathway in surrounding macrophages and promotes M2 polarization [115]. Thus, ferroptosis directly impairs macrophages through the release of DAMPs.
Macrophages play an indispensable role in the chronic inflammatory disease mechanism of endometriosis. Previous studies have shown that macrophages allow the growth of ectopic endometrial tissue, promote angiogenesis, and recruit nerve fibres to contribute to chronic pain [101]. In the human peritoneal cavity, macrophages consist of 50% leucocytes [116]. Unlike other cells that acquire Fe2+ through TfRC and DMT1, the major source of iron for macrophages is through the disposal of haem-derived iron. Although macrophages have a remarkable ability to tolerate iron overload [117], the antioxidant capacity of macrophages is insufficient to cope with iron overload in this setting. This ultimately leads to the outcome of ferroptosis due to excessive phagocytosis of erythrocytes and ferritinophagy [118]. Activated M1 macrophages are more sensitive to ferroptosis than M2 macrophages, and this difference is associated with inducible nitric oxide synthase in M1 macrophages [119]. Therefore, iron overload in the peritoneal fluid may promote M2 macrophage polarization, inhibit the M1 macrophage phenotype and induce a subset of macrophage ferroptosis. Recent findings suggest that the M2 macrophage phenotypes with tissue repair effects predominate in the peritoneal fluid in women with endometriosis [120]. Therefore, the peritoneal environment possibly promotes ectopic endometrial tissue proliferation and growth by influencing macrophage M2 polarization via iron overload, which releases anti-inflammatory cytokines, growth factors, and other reparative components [121]. In summary, the intrinsic association between macrophages and endometriosis is much less well-studied than that for other diseases, such as cancer. The mechanisms by which macrophages resist ferroptosis help provide us with new insights into the mechanisms of ferroptosis in the endometriosis model.
Ferroptosis and endometriosis-related infertility
The iron-overloaded environment induced by retrograde menstruation is suspected to be an important factor in inducing the continued proliferation of ectopic endometrial tissue. In addition, ferroptosis promoted by an iron-overloaded environment appears to be detrimental to oocytes or embryos and is also closely related to endometriosis-related infertility. Peritoneal fluid and follicular fluid are the external microenvironments for oocyte maturation and blastocyst formation, and these abnormal microenvironments affected by iron overload may lead to impaired reproductive function.In recent years, studies on the role and mechanism of iron overload and ferroptosis in endometriosis-related infertility have been reported successively (Table 1).
Table 1.
Studies on the association of iron overload and ferroptosis with endometriosis-related infertility.
| Author, date (Ref.) | Model | Research content | Main results | Final outcomes |
|---|---|---|---|---|
| Chen et al., 2021 [104] |
In vivo: C57BL/6J female mice In vitro: mouse two-cell stage embryos |
Iron overload in endometriosis peritoneal fluid | Disrupted mitochondrial function, decreased ATP levels, increased ROS levels, hyperpolarized MMP, triggered apoptosis and ferroptosis | Compromised preimplantation mouse embryo development |
| Li et al., 2021 [16] |
In vivo: C57BL/6J female mice In vitro: mouse two-cell stage embryos |
Iron overload in endometriosis peritoneal fluid | Disrupted blastocyst formation, decreased GPX4 expression, disrupted mitochondrial function, decreased ATP levels, increased ROS levels and hyperpolarized MMP, upregulated HMOX1 | Embryotoxicity and early embryo ferroptosis |
| Ni et al., 2022 [110] |
In vivo: Kunming female mice In vitro: mouse granulosa cells and human granulosa cells |
Iron overload in endometriosis follicular fluid | Decreased GPX4 and GSH expression, increased NCOA4 expression, NCOA4-mediated ferritinophagy, released exosomes of granulosa cell containing abnormal miRNAs | Ferroptosis of granulosa cells and oocyte dysmaturity |
| Li et al., 2020 [109] | In vitro: mouse oocytes | Transferrin insufficiency and iron overload in endometriosis follicular fluid | Reduced concentration of transferrin with three analogues, increased concentration of ferricion, decreased maturation in vitro rate of mouse oocytes | Oocyte dysmaturity |
| Hu et al., 2021 [111] | In vitro: porcine oocytes | Iron overload-induced ferroptosis in porcine oocytes | Increased intracellular ROS generation, decreased intracellular free thiol levels, induced mitochondrial dysfunction, triggered autophagy, decreased embryonic developmental potential | Impaired oocyte meiosis, decreased oocyte quality and embryonic developmental competence |
| Ding et al., 2022 [112] | In vivo: C57BL/6J female mice | Iron overload in endometriosis ovarian function | Increased MDA levels, decreased GPX4 and GSH expression, decreased growing follicles numbers | Cellular ferroptosis, compromised ovarian function |
Iron overload in peritoneal fluid can affect embryonic development by leading to embryo toxicity and ferroptosis. Chen et al. showed that the pelvic iron-overloaded environment in patients with endometriosis impaired early embryonic development and caused embryo toxicity by triggering GPX4 downregulation-dependent ferroptosis in preimplantation mouse embryos. This leads to endometriosis-related infertility and adverse pregnancy outcomes [122]. During this process, excess iron could induce the excessive accumulation of ROS, which leads to oxidative stress and damages mitochondrial function in preimplantation mouse embryos. This triggers ATP generation impairment and decreases mitochondrial membrane potential (MMP) levels. Moreover, the expression of GPX4 in embryos was significantly decreased [122]. GPX4 is essential for embryonic development. GPX4 deficiency results in abnormal embryonic development compared to the deficiencies of all other GPX family members and ultimately produces lethal phenotypes in mice [123]. In addition to disrupting mitochondrial function, the iron-overload environment in the peritoneal fluid of endometriosis could also reduce the expression of GPX4 and induce lipid peroxidation. Thus, blastocyst formation is disrupted, and embryo toxicity and ferroptosis occur. The ferroptosis inhibitor Fer-1 could improve these adverse conditions [16]. In addition, haem oxygenase 1 (HMOX1) is upregulated in embryonic ferroptosis, and inhibition of HMOX1 can maintain normal mitochondrial function, thereby preventing ferroptosis from occurring [16]. Thus, HMOX1 may play an important role in mediating embryo ferroptosis. Its overexpression can play a pro-oxidative role and induce ferroptosis by increasing Fe accumulation and lipid peroxidation [124, 125].
The total iron levels and ferritin and TfR1 expression levels in endometrioma-proximal follicles are higher than those in endometrioma-distal follicles and healthy ovarian follicles. Moreover, the oocyte retrieval rates in endometrioma-proximal and -distal follicles are lower than those in healthy ovarian follicles [126]; this illustrates that excessive iron intake by follicles leads to cytotoxic accumulation that affects normal oocyte development.
In recent research, Li et al. studied specific proteins at different concentrations in the follicular fluid of patients with advanced endometriosis and found that the transferrin concentration of the three analogues of cDNA FLJ53691, cDNA FLJ54111, and TRF variant Fragment in the follicular fluid decreased. The iron ion concentration of these analogues increased. The environment of transferrin deficiency and iron overload could increase the level of ROS and lead to oxidative stress. Thus, the in vitro maturation rate of mouse oocytes could significantly decrease, which might be one of the causes of endometriosis-related infertility [89]. Ni et al. found that iron-overloaded follicular fluid could trigger ferroptosis in granulosa cells and immaturity of oocytes, thereby increasing the risk of endometriosis-related infertility [90]. The iron-overloaded environment of follicular fluid could not only inhibit the expression of GPX4 and its upstream regulatory target GSH but also cause the high expression of NCOA4 in granulosa cells. This would lead to NCOA4-dependent ferritinophagy, which increases lipid peroxidation in granulosa cells and promotes ferroptosis. Moreover, granulosa cells undergoing ferroptosis cannot exert nutritional and paracrine functions on oocytes and can release granulosa cell exosomes containing abnormal miRNAs. Therefore, oocyte maturation is inhibited, and endometriosis-related infertility can develop. The iron chelators deferoxamine mesylate and VITE could change these circumstances by increasing GPX4 expression and decreasing iron overload [90].
Furthermore, after in vitro ferroptosis inducer ferric ammonium citrate (FAC) intervention, mammalian oocytes experienced increases in ROS and autophagy-related protein LC3 and mitochondrial dysfunction. Additionally, there was significant accumulation of Fe2+ in the cytoplasm and decreases in the polar body (PB) expulsion rate and blastocyst formation rate. Thus, exogenous ferroptosis inducer-induced ferroptosis inhibits oocyte meiosis by increasing oxidative stress, inducing mitochondrial dysfunction, triggering autophagy splitting process and affecting oocyte quality [127]. Conversely, the inhibition of ferroptosis might not only inhibit the progression of endometriosis, but also improve the adverse effects of iron overload on ovarian function, thereby improving fertility and becoming a therapeutic approach for endometriosis-related infertility [128].
In summary, these findings suggest that iron overload and its induced ferroptosis in peritoneal fluid and follicular fluid in patients with endometriosis play an important role in the progression of endometriosis-related infertility (Fig. 4). Therefore, mitigating the impact of iron stress on the local microenvironment, such as the use of antioxidant agents or iron chelators, is expected to be an effective approach for the prevention and treatment of endometriosis-related infertility.
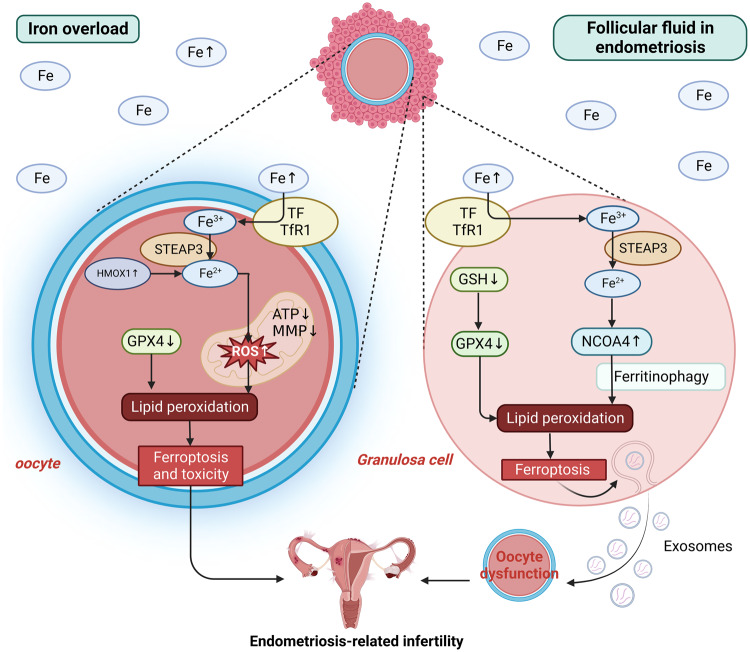
Fig. 4. Oocyte and granulosa cells in iron-overloaded follicular fluid in endometriosis.
Iron-overloaded follicular fluid in endometriosis plays an important role in the progression of endometriosis-related infertility. Iron overload in peritoneal fluid affects the mitochondrial function of oocytes and decreases GPX4 expression, thereby inducing ferroptosis and toxicity by promoting lipid peroxidation. Moreover, iron overload in follicular fluid not only decreases GPX4 and GSH expression, but also increases NCOA4 expression and mediates ferritinophagy. Thus, granulosa cell ferroptosis is induced by promoting lipid peroxidation. Granulosa cells undergoing ferroptosis cause oocyte dysmaturity by releasing exosomes containing abnormal miRNAs. These situations can contribute to endometriosis-related infertility. Created with BioRender.com.
Conclusion
In recent years, researchers have gradually appreciated and revealed the potential role of ferroptosis in endometriosis. These findings highlight the ability of ectopic endometrial tissue to resist iron overload-induced ferroptosis and promote ectopic lesion growth by mediating local cellular ferroptosis in peritoneal fluid in patients with endometriosis. However, oocytes from patients with endometriosis-related infertility are threatened by iron overload, and the development and maturation of oocytes are affected and prone to trigger cellular ferroptosis. This is possibly due to the immature antioxidant system and membrane repair mechanisms of the oocyte. Furthermore, although iron accumulation and lipid peroxidation are unique intermediate events in the onset of ferroptosis, they are not the ultimate executors. Lipid peroxidation can also occur in other cell death types, which depend on different ultimate effectors. Key regulators of ferroptosis can also regulate other types of cell death. For example, GPX4, a key factor in the antioxidant system, also inhibits apoptosis and necroptosis to protect cells from various insults [129, 130]. Therefore, unique markers of ferroptosis in ectopic endometrial tissue require further identification. Currently, the detailed regulatory mechanisms of ferroptosis in endometriosis have not been fully elucidated. In conclusion, ferroptosis and its role in endometriosis, as well as endometriosis-related infertility, require systematic and in-depth studies.
Author contributions
YL, YH, and WC contributed equally to the literature review in preparation for writing. YL, YH, and ZZ conducted the image production and manuscript editing. ZN and CY reviewed and supervised the manuscript. All authors have contributed to the manuscript and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 82074206], the Science and Technology Innovation Action Plan of Shanghai Science and Technology Commission [grant number 21Y21920500] and Changhai Hospital “Gu Hai” plan.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yangshuo Li, Yalun He, Wen Cheng.
These authors jointly supervised this work: Zhexin Ni, Chaoqin Yu.
Contributor Information
Zhexin Ni, Email: nizxzg@163.com.
Chaoqin Yu, Email: chqyu81@163.com.
References
- 1.Ni Z, Sun S, Bi Y, Ding J, Cheng W, Yu J, et al. Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am J Reprod Immunol. 2020;84:e13307. doi: 10.1111/aji.13307. [DOI] [PubMed] [Google Scholar]
- 2.Vercellini P, Vigano P, Somigliana E, Fedele L. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10:261–75. doi: 10.1038/nrendo.2013.255. [DOI] [PubMed] [Google Scholar]
- 3.Kiesel L, Sourouni M. Diagnosis of endometriosis in the 21st century. Climacteric. 2019;22:296–302. doi: 10.1080/13697137.2019.1578743. [DOI] [PubMed] [Google Scholar]
- 4.Alimi Y, Iwanaga J, Loukas M, Tubbs RS. The clinical anatomy of endometriosis: a review. Cureus. 2018;10:e3361. doi: 10.7759/cureus.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmood TA, Templeton A. Prevalence and genesis of endometriosis. Hum Reprod. 1991;6:544–9. doi: 10.1093/oxfordjournals.humrep.a137377. [DOI] [PubMed] [Google Scholar]
- 6.Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 7.de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376:730–8. doi: 10.1016/S0140-6736(10)60490-4. [DOI] [PubMed] [Google Scholar]
- 8.Máté G, Bernstein LR, Török AL. Endometriosis is a cause of infertility. does reactive oxygen damage to gametes and embryos play a key role in the pathogenesis of infertility caused by endometriosis? Front Endocrinol. 2018;9:725. doi: 10.3389/fendo.2018.00725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanbo T, Fedorcsak P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet Gynecol Scand. 2017;96:659–67. doi: 10.1111/aogs.13082. [DOI] [PubMed] [Google Scholar]
- 10.Tang D, Kang R, Berghe TV, Vandenabeele P, Kroemer G. The molecular machinery of regulated cell death. Cell Res. 2019;29:347–64. doi: 10.1038/s41422-019-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingold I, Berndt C, Schmitt S, Doll S, Poschmann G, Buday K, et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell. 2018;172:409–22.e21. doi: 10.1016/j.cell.2017.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Stockwell BR, Jiang X, Gu W. Emerging mechanisms and disease relevance of ferroptosis. Trends Cell Biol. 2020;30:478–90. doi: 10.1016/j.tcb.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Lin Y, Zhang Y, Gu N, Yang B, Shan S, et al. Endometrial stromal cell ferroptosis promotes angiogenesis in endometriosis. Cell Death Discov. 2022;8:29. doi: 10.1038/s41420-022-00821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li S, Zhou Y, Huang Q, Fu X, Zhang L, Gao F, et al. Iron overload in endometriosis peritoneal fluid induces early embryo ferroptosis mediated by HMOX1. Cell Death Discov. 2021;7:355. doi: 10.1038/s41420-021-00751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31:107–25. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao M, Yi J, Zhu J, Minikes AM, Monian P, Thompson CB, et al. Role of mitochondria in ferroptosis. Mol Cell. 2019;73:354–63.e3. doi: 10.1016/j.molcel.2018.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–68. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayır H, Anthonymuthu TS, Tyurina YY, Patel SJ, Amoscato AA, Lamade AM, et al. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem Biol. 2020;27:387–408. doi: 10.1016/j.chembiol.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riegman M, Sagie L, Galed C, Levin T, Steinberg N, Dixon SJ, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22:1042–8. doi: 10.1038/s41556-020-0565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, et al. Redox imbalance in cystine/glutamate transporter-deficient mice. J Biol Chem. 2005;280:37423–9. doi: 10.1074/jbc.M506439200. [DOI] [PubMed] [Google Scholar]
- 25.Kang YP, Mockabee-Macias A, Jiang C, Falzone A, Prieto-Farigua N, Stone E, et al. Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab. 2021;33:174–89.e7. doi: 10.1016/j.cmet.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitakata H, Endo J, Matsushima H, Yamamoto S, Ikura H, Hirai A, et al. MITOL/MARCH5 determines the susceptibility of cardiomyocytes to doxorubicin-induced ferroptosis by regulating GSH homeostasis. J Mol Cell Cardiol. 2021;161:116–29. doi: 10.1016/j.yjmcc.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–31. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu K, Yan M, Liu T, Wang Z, Duan Y, Xia Y, et al. Creatine kinase B suppresses ferroptosis by phosphorylating GPX4 through a moonlighting function. Nat Cell Biol. 2023;25:714–25. doi: 10.1038/s41556-023-01133-9. [DOI] [PubMed] [Google Scholar]
- 29.Alim I, Caulfield JT, Chen Y, Swarup V, Geschwind DH, Ivanova E, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177:1262–79.e25. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J, et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol. 2021;22:1127–39. doi: 10.1038/s41590-021-00996-0. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Roh JL. Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience. Cancer Lett. 2023;559:216119. doi: 10.1016/j.canlet.2023.216119. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–84. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Y, Mi Y, Zhang X, Ma Q, Song Y, Zhang L, et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J Exp Clin Cancer Res. 2019;38:402. doi: 10.1186/s13046-019-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodson M, Castro-Portuguez R, Zhang DD. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23:101107. doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20:1181–92. doi: 10.1038/s41556-018-0178-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang R, Kroemer G, Tang D. The tumor suppressor protein p53 and the ferroptosis network. Free Radic Biol Med. 2019;133:162–8. doi: 10.1016/j.freeradbiomed.2018.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song X, Zhu S, Chen P, Hou W, Wen Q, Liu J, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system X(c)(-) activity. Curr Biol. 2018;28:2388–99.e5. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Zeng X, Lu D, Yin M, Shan M, Gao Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum Reprod. 2021;36:951–64. doi: 10.1093/humrep/deaa363. [DOI] [PubMed] [Google Scholar]
- 39.Gaschler MM, Andia AA, Liu H, Csuka JM, Hurlocker B, Vaiana CA, et al. FINO(2) initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14:507–15. doi: 10.1038/s41589-018-0031-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–85. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lloberas J, Muñoz JP, Hernández-Álvarez MI, Cardona PJ, Zorzano A, Celada A. Macrophage mitochondrial MFN2 (mitofusin 2) links immune stress and immune response through reactive oxygen species (ROS) production. Autophagy. 2020;16:2307–9. doi: 10.1080/15548627.2020.1839191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schofield JH, Schafer ZT. Mitochondrial reactive oxygen species and mitophagy: a complex and nuanced relationship. Antioxid Redox Signal. 2021;34:517–30. doi: 10.1089/ars.2020.8058. [DOI] [PubMed] [Google Scholar]
- 43.Badgley MA, Kremer DM, DelGiorno KE, Firl CEM, Decker AR, Iuga A. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science. 2020;368:85–9. doi: 10.1126/science.aaw9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin D, Lee J, You JH, Kim D, Roh JL. Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol. 2020;30:101418. doi: 10.1016/j.redox.2019.101418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaschler MM, Hu F, Feng H, Linkermann A, Min W, Stockwell BR. Determination of the subcellular localization and mechanism of action of ferrostatins in suppressing ferroptosis. ACS Chem Biol. 2018;13:1013–20. doi: 10.1021/acschembio.8b00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoshan-Barmatz V, De Pinto V, Zweckstetter M, Raviv Z, Keinan N, Arbel N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Asp Med. 2010;31:227–85. doi: 10.1016/j.mam.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–8. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipper CH, Stofleth JT, Bai F, Sohn YS, Roy S, Mittler R, et al. Redox-dependent gating of VDAC by mitoNEET. Proc Natl Acad Sci USA. 2019;116:19924–9. doi: 10.1073/pnas.1908271116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang Y, Luo M, Zhang K, Zhang J, Gao T, Connell DO, et al. Nedd4 ubiquitylates VDAC2/3 to suppress erastin-induced ferroptosis in melanoma. Nat Commun. 2020;11:433. doi: 10.1038/s41467-020-14324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hübner C, Haase H. Interactions of zinc- and redox-signaling pathways. Redox Biol. 2021;41:101916. doi: 10.1016/j.redox.2021.101916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, Liu Y, Liu J, Kang R, Tang D. NEDD4L-mediated LTF protein degradation limits ferroptosis. Biochem Biophys Res Commun. 2020;531:581–7. doi: 10.1016/j.bbrc.2020.07.032. [DOI] [PubMed] [Google Scholar]
- 54.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, et al. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–25. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551:639–43. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356–69. doi: 10.1016/j.freeradbiomed.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 57.Yuan H, Li X, Zhang X, Kang R, Tang D. CISD1 inhibits ferroptosis by protection against mitochondrial lipid peroxidation. Biochem Biophys Res Commun. 2016;478:838–44. doi: 10.1016/j.bbrc.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 58.Li B, Wei S, Yang L, Peng X, Ma Y, Wu B, et al. CISD2 promotes resistance to sorafenib-induced ferroptosis by regulating autophagy in hepatocellular carcinoma. Front Oncol. 2021;11:657723. doi: 10.3389/fonc.2021.657723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woo JH, Choi YS, Choi JH. Iron-storage protein ferritin is upregulated in endometriosis and iron overload contributes to a migratory phenotype. Biomedicines. 2020;8:454. doi: 10.3390/biomedicines8110454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fuhrmann DC, Mondorf A, Beifuß J, Jung M, Brüne B. Hypoxia inhibits ferritinophagy, increases mitochondrial ferritin, and protects from ferroptosis. Redox Biol. 2020;36:101670. doi: 10.1016/j.redox.2020.101670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–32. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–62. doi: 10.1038/s41418-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Q, Liu W, Zhang S, Liu S. The cardinal roles of ferroportin and its partners in controlling cellular iron in and out. Life Sci. 2020;258:118135. doi: 10.1016/j.lfs.2020.118135. [DOI] [PubMed] [Google Scholar]
- 64.Brown CW, Amante JJ, Chhoy P, Elaimy AL, Liu H, Zhu LJ, et al. Prominin2 drives ferroptosis resistance by stimulating iron export. Dev Cell. 2019;51:575–86.e4. doi: 10.1016/j.devcel.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wenzel SE, Tyurina YY, Zhao J, St Croix CM, Dar HH, Mao G, et al. PEBP1 wardens ferroptosis by enabling lipoxygenase generation of lipid death signals. Cell. 2017;171:628–41.e26. doi: 10.1016/j.cell.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113:E4966–4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fritz KS, Petersen DR. An overview of the chemistry and biology of reactive aldehydes. Free Radic Biol Med. 2013;59:85–91. doi: 10.1016/j.freeradbiomed.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ayala A, Muñoz MF, Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17:2054–81. doi: 10.1080/15548627.2020.1810918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou Y, Li H, Graham ET, Deik AA, Eaton JK, Wang W, et al. Cytochrome P450 oxidoreductase contributes to phospholipid peroxidation in ferroptosis. Nat Chem Biol. 2020;16:302–9. doi: 10.1038/s41589-020-0472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu J, Kuang F, Kroemer G, Klionsky DJ, Kang R, Tang D. Autophagy-dependent ferroptosis: machinery and regulation. Cell Chem Biol. 2020;27:420–35. doi: 10.1016/j.chembiol.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song N, Wang J, Jiang H, Xie J. Ferroportin1 and hephaestin overexpression attenuate iron-induced oxidative stress in MES23.5 dopaminergic cells. J Cell Biochem. 2010;110:1063–72. doi: 10.1002/jcb.22617. [DOI] [PubMed] [Google Scholar]
- 73.Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharm Sci. 2018;22:3826–36. doi: 10.26355/eurrev_201806_15267. [DOI] [PubMed] [Google Scholar]
- 74.Ferreira CA, Ni D, Rosenkrans ZT, Cai W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018;11:4955–84. doi: 10.1007/s12274-018-2092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lundgren CAK, Sjöstrand D, Biner O, Bennett M, Rudling A, Johansson AL, et al. Scavenging of superoxide by a membrane-bound superoxide oxidase. Nat Chem Biol. 2018;14:788–93. doi: 10.1038/s41589-018-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woo JH, Shimoni Y, Yang WS, Subramaniam P, Iyer A, Nicoletti P, et al. Elucidating compound mechanism of action by network perturbation analysis. Cell. 2015;162:441–51. doi: 10.1016/j.cell.2015.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–8. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 78.Wei X, Yi X, Zhu XH, Jiang DS. Posttranslational modifications in ferroptosis. Oxid Med Cell Longev. 2020;2020:8832043. doi: 10.1155/2020/8832043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mao L, Zhao T, Song Y, Lin L, Fan X, Cui B, et al. The emerging role of ferroptosis in non-cancer liver diseases: hype or increasing hope? Cell Death Dis. 2020;11:518. doi: 10.1038/s41419-020-2732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gnanapradeepan K, Basu S, Barnoud T, Budina-Kolomets A, Kung CP, Murphy ME. The p53 tumor suppressor in the control of metabolism and ferroptosis. Front Endocrinol (Lausanne) 2018;9:124. doi: 10.3389/fendo.2018.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chapron C, Marcellin L, Borghese B, Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat Rev Endocrinol. 2019;15:666–82. doi: 10.1038/s41574-019-0245-z. [DOI] [PubMed] [Google Scholar]
- 83.Defrère S, Lousse JC, González-Ramos R, Colette S, Donnez J, Van Langendonckt A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008;14:377–85. doi: 10.1093/molehr/gan033. [DOI] [PubMed] [Google Scholar]
- 84.Van Langendonckt A, Casanas-Roux F, Donnez J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil Steril. 2002;78:712–8. doi: 10.1016/S0015-0282(02)03346-0. [DOI] [PubMed] [Google Scholar]
- 85.Van Langendonckt A, Casanas-Roux F, Eggermont J, Donnez J. Characterization of iron deposition in endometriotic lesions induced in the nude mouse model. Hum Reprod. 2004;19:1265–71. doi: 10.1093/humrep/deh182. [DOI] [PubMed] [Google Scholar]
- 86.Wu Y, Yang R, Lan J, Wu Y, Huang J, Fan Q, et al. Iron overload modulates follicular microenvironment via ROS/HIF-1α/FSHR signaling. Free Radic Biol Med. 2023;196:37–52. doi: 10.1016/j.freeradbiomed.2022.12.105. [DOI] [PubMed] [Google Scholar]
- 87.Lousse JC, Van Langendonckt A, Defrere S, Ramos RG, Colette S, Donnez J. Peritoneal endometriosis is an inflammatory disease. Front Biosci. 2012;4:23–40. doi: 10.2741/e358. [DOI] [PubMed] [Google Scholar]
- 88.Lousse JC, Defrère S, Van Langendonckt A, Gras J, González-Ramos R, Colette S, et al. Iron storage is significantly increased in peritoneal macrophages of endometriosis patients and correlates with iron overload in peritoneal fluid. Fertil Steril. 2009;91:1668–75. doi: 10.1016/j.fertnstert.2008.02.103. [DOI] [PubMed] [Google Scholar]
- 89.Li A, Ni Z, Zhang J, Cai Z, Kuang Y, Yu C. Transferrin insufficiency and iron overload in follicular fluid contribute to oocyte dysmaturity in infertile women with advanced endometriosis. Front Endocrinol. 2020;11:391. doi: 10.3389/fendo.2020.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ni Z, Li Y, Song D, Ding J, Mei S, Sun S, et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis. 2022;13:579. doi: 10.1038/s41419-022-05037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou Y, Khan H, Xiao J, Cheang WS. Effects of arachidonic acid metabolites on cardiovascular health and disease. Int J Mol Sci. 2021;22:12029. doi: 10.3390/ijms222112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Probst L, Dächert J, Schenk B, Fulda S. Lipoxygenase inhibitors protect acute lymphoblastic leukemia cells from ferroptotic cell death. Biochem Pharm. 2017;140:41–52. doi: 10.1016/j.bcp.2017.06.112. [DOI] [PubMed] [Google Scholar]
- 93.Araújo AC, Wheelock CE, Haeggström JZ. The eicosanoids, redox-regulated lipid mediators in immunometabolic disorders. Antioxid Redox Signal. 2018;29:275–96. doi: 10.1089/ars.2017.7332. [DOI] [PubMed] [Google Scholar]
- 94.Wen Q, Liu J, Kang R, Zhou B, Tang D. The release and activity of HMGB1 in ferroptosis. Biochem Biophys Res Commun. 2019;510:278–83. doi: 10.1016/j.bbrc.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 95.Linkermann A, Stockwell BR, Krautwald S, Anders HJ. Regulated cell death and inflammation: an auto-amplification loop causes organ failure. Nat Rev Immunol. 2014;14:759–67. doi: 10.1038/nri3743. [DOI] [PubMed] [Google Scholar]
- 96.Proneth B, Conrad M. Ferroptosis and necroinflammation, a yet poorly explored link. Cell Death Differ. 2019;26:14–24. doi: 10.1038/s41418-018-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bulun SE. Endometriosis. N. Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 98.Zhao Y, Gong P, Chen Y, Nwachukwu JC, Srinivasan S, Ko C, et al. Dual suppression of estrogenic and inflammatory activities for targeting of endometriosis. Sci Transl Med. 2015;7:271ra279. doi: 10.1126/scitranslmed.3010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: origins, consequences and interventions. Acta Obstet Gynecol Scand. 2017;96:623–32. doi: 10.1111/aogs.13156. [DOI] [PubMed] [Google Scholar]
- 100.Shen HH, Zhang T, Yang HL, Lai ZZ, Zhou WJ, Mei J, et al. Ovarian hormones-autophagy-immunity axis in menstruation and endometriosis. Theranostics. 2021;11:3512–26. doi: 10.7150/thno.55241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cacciottola L, Donnez J, Dolmans MM. Can endometriosis-related oxidative stress pave the way for new treatment targets? Int J Mol Sci. 2021;22:7138. doi: 10.3390/ijms22137138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolfler MM, Meinhold-Heerlein IM, Henkel C, Rath W, Neulen J, Maass N, et al. Reduced hemopexin levels in peritoneal fluid of patients with endometriosis. Fertil Steril. 2013;100:777–81. doi: 10.1016/j.fertnstert.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 103.Tosti C, Pinzauti S, Santulli P, Chapron C, Petraglia F. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod Sci. 2015;22:1053–9. doi: 10.1177/1933719115592713. [DOI] [PubMed] [Google Scholar]
- 104.Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, et al. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev. 2017;2017:7265238. doi: 10.1155/2017/7265238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goud PT, Goud AP, Joshi N, Puscheck E, Diamond MP, Abu-Soud HM. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil Steril. 2014;102:151–59.e5. doi: 10.1016/j.fertnstert.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y, Liu X, Deng M, Xu C, Zhang Y, Wu D, et al. Ferroptosis induced by iron overload promotes fibrosis in ovarian endometriosis and is related to subpopulations of endometrial stromal cells. Front Pharm. 2022;13:930614. doi: 10.3389/fphar.2022.930614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sikora J, Smycz-Kubańska M, Mielczarek-Palacz A, Kondera-Anasz Z. Abnormal peritoneal regulation of chemokine activation-the role of IL-8 in pathogenesis of endometriosis. Am J Reprod Immunol. 2017. 10.1111/aji.12622. [DOI] [PubMed]
- 108.Hsu CY, Hsieh TH, Tsai CF, Tsai HP, Chen HS, Chang Y, et al. miRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J Pathol. 2014;232:330–43. doi: 10.1002/path.4295. [DOI] [PubMed] [Google Scholar]
- 109.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 110.Defrère S, González-Ramos R, Lousse JC, Colette S, Donnez O, Donnez J, et al. Insights into iron and nuclear factor-kappa B (NF-kappaB) involvement in chronic inflammatory processes in peritoneal endometriosis. Histol Histopathol. 2011;26:1083–92. doi: 10.14670/HH-26.1083. [DOI] [PubMed] [Google Scholar]
- 111.González-Ramos R, Van Langendonckt A, Defrère S, Lousse JC, Colette S, Devoto L, et al. Involvement of the nuclear factor-κB pathway in the pathogenesis of endometriosis. Fertil Steril. 2010;94:1985–94. doi: 10.1016/j.fertnstert.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 112.Wang H, An P, Xie E, Wu Q, Fang X, Gao H, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449–65. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kang R, Zeng L, Zhu S, Xie Y, Liu J, Wen Q, et al. Lipid peroxidation drives gasdermin D-mediated pyroptosis in lethal polymicrobial sepsis. Cell Host Microbe. 2018;24:97–108.e4. doi: 10.1016/j.chom.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, et al. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–83. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dai E, Han L, Liu J, Xie Y, Zeh HJ, Kang R, et al. Ferroptotic damage promotes pancreatic tumorigenesis through a TMEM173/STING-dependent DNA sensor pathway. Nat Commun. 2020;11:6339. doi: 10.1038/s41467-020-20154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kubicka U, Olszewski WL, Tarnowski W, Bielecki K, Ziółkowska A, Wierzbicki Z. Normal human immune peritoneal cells: subpopulations and functional characteristics. Scand J Immunol. 1996;44:157–63. doi: 10.1046/j.1365-3083.1996.d01-297.x. [DOI] [PubMed] [Google Scholar]
- 117.Winn NC, Wolf EM, Cottam MA, Bhanot M, Hasty AH. Myeloid-specific deletion of ferroportin impairs macrophage bioenergetics but is disconnected from systemic insulin action in adult mice. Am J Physiol Endocrinol Metab. 2021;321:E376–91. doi: 10.1152/ajpendo.00116.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Recalcati S, Cairo G. Macrophages and iron: a special relationship. Biomedicines. 2021;9:1585. doi: 10.3390/biomedicines9111585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kapralov AA, Yang Q, Dar HH, Tyurina YY, Anthonymuthu TS, Kim R, et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat Chem Biol. 2020;16:278–90. doi: 10.1038/s41589-019-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gou Y, Li X, Li P, Zhang H, Xu T, Wang H, et al. Estrogen receptor β upregulates CCL2 via NF-κB signaling in endometriotic stromal cells and recruits macrophages to promote the pathogenesis of endometriosis. Hum Reprod. 2019;34:646–58. doi: 10.1093/humrep/dez019. [DOI] [PubMed] [Google Scholar]
- 121.Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharm Toxicol. 2011;51:267–88. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen X, Zhou Y, Wu D, Shu C, Wu R, Li S, et al. Iron overload compromises preimplantation mouse embryo development. Reprod Toxicol. 2021;105:156–65. doi: 10.1016/j.reprotox.2021.08.010. [DOI] [PubMed] [Google Scholar]
- 123.Ufer C, Wang CC. The roles of glutathione peroxidases during embryo development. Front Mol Neurosci. 2011;4:12. doi: 10.3389/fnmol.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2018;416:124–37. doi: 10.1016/j.canlet.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 125.Fernandez-Mendivil C, Luengo E, Trigo-Alonso P, Garcia-Magro N, Negredo P, Lopez MG. Protective role of microglial HO-1 blockade in aging: implication of iron metabolism. Redox Biol. 2021;38:101789. doi: 10.1016/j.redox.2020.101789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanchez AM, Papaleo E, Corti L, Santambrogio P, Levi S, Viganò P, et al. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum Reprod. 2014;29:577–83. doi: 10.1093/humrep/det466. [DOI] [PubMed] [Google Scholar]
- 127.Hu W, Zhang Y, Wang D, Yang T, Qi J, Zhang Y, et al. Iron overload-induced ferroptosis impairs porcine oocyte maturation and subsequent embryonic developmental competence in vitro. Front Cell Dev Biol. 2021;9:673291. doi: 10.3389/fcell.2021.673291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ding J, Zhao Q, Zhou Z, Cheng W, Sun S, Ni Z, et al. Huayu Jiedu Fang protects ovarian function in mouse with endometriosis iron overload by inhibiting ferroptosis. Evid Based Complement Altern Med. 2022;2022:1406820. doi: 10.1155/2022/1406820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ran Q, Liang H, Gu M, Qi W, Walter CA, Roberts LJ, 2nd, et al. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–46. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 130.Canli Ö, Alankuş YB, Grootjans S, Vegi N, Hültner L, Hoppe PS, et al. Glutathione peroxidase 4 prevents necroptosis in mouse erythroid precursors. Blood. 2016;127:139–48. doi: 10.1182/blood-2015-06-654194. [DOI] [PMC free article] [PubMed] [Google Scholar]