Abstract
Reproductive dysfunctions have been recently documented in male greater amberjack Seriola dumerili caught from the wild and reared in captivity. In the present study, we compared testis transcriptome in wild fish (WILD), hatchery-produced fish with apparently normal spermatogenesis (Normal Farmed; NormalF) and hatchery-produced fish with evident reproductive dysfunction (Dysfunctional Farmed; DysF). Gene expression analysis identified 2157, 1985 and 74 differentially expressed genes (DEGs) in DysF vs WILD, NormalF vs DysF and NormalF vs WILD comparisons, respectively. In DysF, a dysregulation of several interconnected biological processes, including cell assembly, steroidogenesis and apoptosis was found. Gene enrichment of progesterone-mediated oocyte maturation, oocyte meiosis and cell cycle pathways were identified in the DysF vs NormalF comparison. Most of the DEGs involved in the enriched pathways were downregulated in DysF. The comparison of NormalF vs WILD showed that most of the DEGs were downregulated in NormalF, including a gene that encodes for a regulatory protein with a protective role in apoptosis regulation (ptpn6), indicating that spermatogenesis was dysfunctional also in the apparently “normal” hatchery-produced fish. Hence, rearing of male greater amberjack in captivity, from eggs produced by captive breeders, did not prevent the appearance of reproductive dysfunctions, and these dysfunctions involved several biological processes and metabolic pathways.
Subject terms: Molecular biology, Bioinformatics, Gene expression analysis, Microscopy
Introduction
Fish reared in captivity are often affected by reproductive dysfunctions of variable severity1–3. In general, females show oogenesis alterations which may involve incapacity of oocytes to start secondary oocyte growth, block of vitellogenin uptake before completion vitellogenesis, or failure of oocytes to undergo maturation when vitellogenesis is accomplished. In males, spermatogenesis impairment results in reduction of sperm quantity and/or quality. These gametogenesis impairments are considered to be a consequence of confinement-induced stress4–7, lack of the natural spawning conditions8–10, and/or inadequate diet11,12.
The greater amberjack Seriola dumerili is a cosmopolitan species found throughout the temperate zone, including the Indo-West Pacific Ocean13, the Western Atlantic Ocean14,15, the Eastern Atlantic Ocean and the Mediterranean Sea16. The available total worldwide catches data of this species are outdated and indicate a global fishery production of ≈ 3300 tonnes in 200917. Due to the worldwide consumer’s appreciation and the high market quotations of this species, greater amberjack domestication represents an excellent opportunity for product diversification in aquaculture.
A few recent studies documented the occurrence of severe reproductive dysfunctions in greater amberjack of both sexes caught in the Mediterranean as juveniles and reared in captivity in marine cages for a few years until sexual maturity (see review by18). Compared with wild breeders, captive-reared greater amberjack males showed lower relative testicular mass (gonadosomatic index) and sex steroid plasma levels throughout the gonadal recrudescence, active gametogenesis and spawning phases of the reproductive cycle12. Moreover, captive-reared greater amberjack males exhibited smaller seminiferous lobules, early cessation of the active spermatogenesis phase, and high rate of germ cell apoptosis associated with abnormally high 17β-estradiol plasma concentrations during the gametogenesis recrudescence in spring. The observed reproductive anomalies finally resulted in the production of sperm of low quality, characterized by low percentage of motile spermatozoa, limited motility duration and velocity, and low ATP content19. Nevertheless, the documented spermatogenesis dysfunctions did not prevent the breeding of males and the production of fertilised eggs after the application of spawning induction therapies20–22, although the reported fertilization rate (30–45% in20 and 35–80% in21) and larval survival (5d larval survival, 5–30% in20 and 10–30% in21) were rather low and variable.
The present understanding of the endocrine mechanisms responsible for reproductive dysfunctions occurring in fish reared in captivity is limited. In many cases, the endocrine causes of gametogenesis impairment involve a reduced release (but not synthesis) of luteinizing hormone (Lh) from the pituitary2,23–25. In fact, the stimulation of Lh release from the pituitary through the administration of a gonadotropin releasing hormone agonist (GnRHa) has proven widely to be a useful tool to alleviate reproductive dysfunctions related to reduced sperm production and failure of oocyte maturation26–30, including greater amberjack20–22,31.
In the present study, we have undertaken a comparative analysis of testis transcriptome of hatchery-produced greater amberjack versus wild breeders sampled during the reproductive season, as part of a wider research aiming at describing the effects of captive rearing on reproductive function.
Methods
Ethics
For the present study, wild and farmed greater amberjack males were used. Wild fish were commercially caught from an authorized purse-seine fishing vessel during routine fishing operations. Immediately after death, male fish whose size was beyond that of first maturity32 were purchased and sampled on board. Farmed fish were produced from eggs obtained in Argosaronikos Fish Farm S.A. (Salamina Island, Greece) in 2017 and reared under routine farming condition. The use of the farmed fish used in the present study was approved by the Greek National Veterinary Services (AP 31337). All procedures involving animals were conducted in accordance to the “Guidelines for the treatment of animals in behavioral research and teaching”33, the Ethical justification for the use and treatment of fishes in research: an update34 and the “Directive 2010/63/EU of the European parliament and the council of 22 September 2010 on the protection of animals used for scientific purposes”35. The authors complied with the ARRIVE guidelines.
Sampling
Four wild and six farmed greater amberjack males were sampled on 31 May–01 June 2021 during the active gametogenesis period of the species in the Mediterranean Sea12. Wild fish were caught around the Pelagie Islands (Sicily, Italy) from a purse-seine fishing vessel and sampled on board immediately after death. Farmed fish used in the present study were produced from eggs obtained in Argosaronikos Fish Farm S.A. (Salamina Island, Greece) in 2017, after spawning induction of wild-caught breeders21,22. The hatchery-produced (first generation, F1) juveniles were stocked at the same farm and they were maintained following common aquaculture practices. A commercial broodstock diet (Skretting, Vitalis Prima) was administered 3 to 5 times a week until apparent satiation.
Before sampling, captive-reared fish were confined in a small cage area using a PVC curtain and then were tranquilized with about 0.01 ml l−1 clove oil (Roumpoulakis E.P.E., Greece) dissolved in ethanol at a 1:10 ratio. Then, they were gently directed into a PVC stretcher, brought on board of a service vessel, and anesthetized deeply with 0.03 ml l−1 clove oil. Then the fish were euthanized by decapitation, were placed in crushed ice and transferred to the farm facility for further collection of biometric data and tissue samples. The time interval between fish death and sampling ranged between 30 min and 2 h.
For each fish, biometric data (fork length, FL, nearest cm; body mass, BM, nearest hg; gonad mass, GM, nearest g) were recorded, and the gonado-somatic index was calculated as GSI = 100 GM BM−1 (Table 1). Testes were excised and preserved as below specified.
Table 1.
Greater amberjack sampling date, origin, biometric data, gonadosomatic index and reproductive state.
| Sampling date | Fish origin | Fork length (cm) | Body mass (kg) | Gonad mass (g) | GSI | Reproductive state* | Group** |
|---|---|---|---|---|---|---|---|
| 31/05/2021 | Wild | 95 | 9.3 | 50 | 0.5 | Advanced spermatogenesis | WILD |
| 31/05/2021 | Wild | 101 | 13.0 | 300 | 2.3 | Advanced spermatogenesis | WILD |
| 31/05/2021 | Wild | 92 | 9.2 | 100 | 1.1 | Advanced spermatogenesis | WILD |
| 31/05/2021 | Wild | 93 | 8.8 | 60 | 0.7 | Advanced spermatogenesis | WILD*** |
| 01/06/2021 | Farmed | 81 | 7.9 | 54 | 0.7 | Advanced spermatogenesis | NormalF |
| 01/06/2021 | Farmed | 75 | 6.1 | 86 | 1.4 | Advanced spermatogenesis | NormalF |
| 01/06/2021 | Farmed | 73 | 8.2 | 71 | 0.9 | Advanced spermatogenesis | NormalF |
| 01/06/2021 | Farmed | 80 | 7.7 | 69 | 0.9 | Advanced spermatogenesis | NormalF |
| 01/06/2021 | Farmed | 76 | 6.8 | 27 | 0.4 | Arrested spermatogenesis (spent) | DysF |
| 01/06/2021 | Farmed | 91 | 12.4 | 49 | 0.4 | Arrested spermatogenesis (spent) | DysF |
GSI, gonadosomatic index. *The reproductive state was assessed as described in the “Methods” section. **For comparative transcriptome analysis, fish were grouped according to their origin and reproductive state as described in the “Results” section. DysF, reproductively dysfunctional farmed fish; NornalF, non-dysfunctional farmed fish; WILD, wild fish with normal spermatogenic activity. ***This sample did not pass the RNA quality check and was excluded from RNA-seq analysis.
Histological analysis of greater amberjack testes and seminiferous tubule diameter
For the histological analysis of greater amberjack testes, 1-cm thick gonad slices were cut and fixed in Bouin’s solution, dehydrated in ethanol, clarified in xylene and embedded in paraffin wax. Five-μm thick sections were then stained with haematoxylin–eosin. For the assessment of the reproductive state, the type of spermatogenic cysts was recorded and the amount of spermatozoa in the lumen of seminiferous lobules was subjectively evaluated12.
At least 50 seminiferous tubules were selected randomly from one histological section and their diameter was measured from microphotographs taken with a digital camera (DFC 420; Leica, Cambridge, UK) connected to a light microscope (DIAPLAN; Leitz, Wetzlar, Germany). Measurements were performed using an image analysis software (Leica Application Suite, version 3.3.0, Cambridge, U.K.).
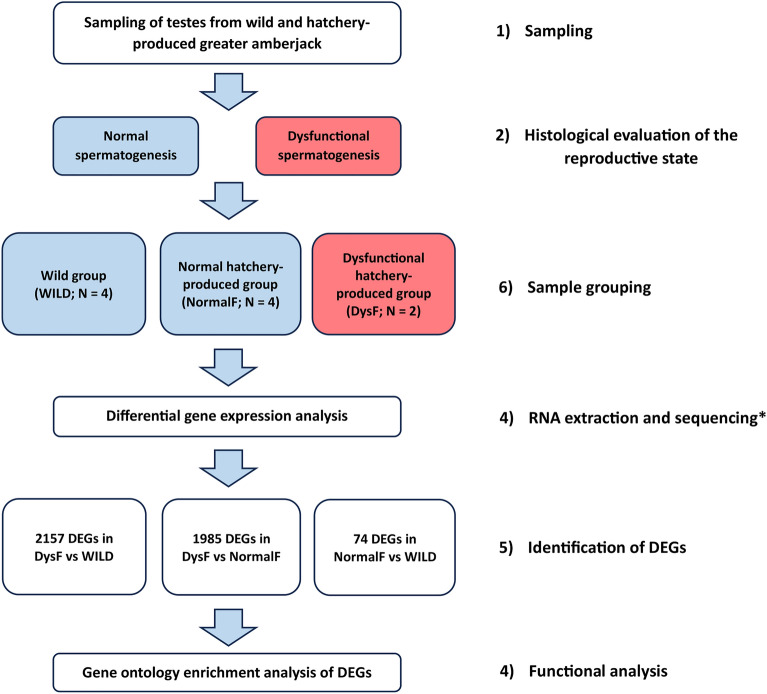
Based on the histological evaluation of the reproductive state, the fish were divided in three groups (see “Results” section) that underwent a comparative analysis of testis transcriptome (Fig. 1).
Figure 1.
Schematic representation of the experimental design. (1) Testis samples were taken from wild and hatchery-produced greater amberjack. (2) The reproductive state was assessed through the histological analysis of the testes. (3) Fish were then divided in three groups based on their origin and reproductive state: WILD (wild fish showing normal spermatogenesis), NormalF (hatchery-produced fish showing normal spermatogenesis); DysF (hatchery-produced fish showing altered spermatogenesis). (4) Testis RNA was extracted and sequenced. (5) Differentially expressed genes (DEGs) between groups were identified. (6) Functional analysis of DEGs was carried out. * After the evaluation of the RNA quality, one of the wild samples was excluded from further analyses.
RNA extraction and sequencing
For RNA-seq, small testis samples were stored in RNA later® (Thermo Fisher Scientific, Waltham, Massachusetts, U.S.), transported in the laboratory within one week and frozen at -80 °C. Total RNA extraction was performed on 2.5 mg testis samples, lysed and homogenised with TissueLyser II (Qiagen, Germany) setting 2 min and 20 Hz frequency, by RNeasy® Plus Micro kit (Qiagen, Germany) following the manufacturer's protocol. The quantity and quality of extracted Total RNA were checked for quantity and quality respectively by Nanodrop 1000 spectrophotometer (Thermo Scientific, Waltham, Massachusetts, U.S.) and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California, U.S.), respectively. After the evaluation of the RNA quality, one of the wild samples (Table 1, row 4) was excluded from library preparation and sequencing. All the other samples, showing high quality RNA (RIN range 7–8), were used to prepare the mRNA libraries by SureSelect Strand Specific RNA Library Preparation kit (Agilent Technologies, Santa Clara, California, U.S.). In particular, poly-A selection and directional mRNA libraries were carried out using 1 µg of total RNA. Finally, paired-end sequencing (2 × 75 bases) was performed on the Illumina NextSeq platform (Illumina Inc., San Diego, California, U.S.).
RNA-seq data analysis
Sequencing raw data in FASTQ format, were quality-checked using the FastQC program (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and adaptor sequences as well as low quality regions (phred cutoff of 25) were trimmed using fastp (version 0.20.0) (with parameters: --detect_adapter_for_pe -x -q 25 -n 1 -l 50 -y -w 8)36. Cleaned reads were aligned onto the Seriola dumerili reference genome (Sdu_1.0, assembly accession GCF_002260705, https://www.ncbi.nlm.nih.gov/assembly/GCF_002260705.1) using STAR (version 020201)37 with default parameters. Read counts per gene were performed by featureCounts (version 1.6.0)38 and differential gene expression analysis was carried out using DESeq239. Only genes with an adjusted P value ≤ 0.05, |log2(FC)|> 1.5 and |log2(FC)|< 1.5 were used for downstream analyses.
DAVID (Database for Annotation, Visualization, and Integrated Discovery database https://david.ncifcrf.gov/tools.jsp)40 and ShinyGO (http://bioinformatics.sdstate.edu/go)41 were used to perform the functional annotation of Differently Expressed Genes (DEGs) and the GO enrichment analysis. By using a False Discovery Rate (FDR < 0.05), these analyses were able to highlight specific categories (biological processes, molecular functions, cellular components and pathways), potentially involved in reproductive dysfunctions. A network based on protein–protein interaction (PPI) between DEGs associated to each comparison was built by STRING (https://string-db.org/). KEGG Mapper—Search (https://www.genome.jp/kegg/mapper/search.html) was used to explore DEGs specifically associated to apoptosis pathway42.
All queries launched on DAVID, ShinyGO and STRING were restricted to taxon ID 41447 (Seriola dumerili).
Statistical analysis
Differences in GSI and diameter of seminiferous tubules were evaluated by a two tailed Student’s t-test between the following groups that were identified based on testis histological analysis (see “Results” section): WILD vs NormalF; WILD vs DysF; NormalF vs DysF. The results are presented as means ± SD; the statistical probability significance was established at the P < 0.05 level.
Results
Evaluation of reproductive state and samples selection for RNA-seq
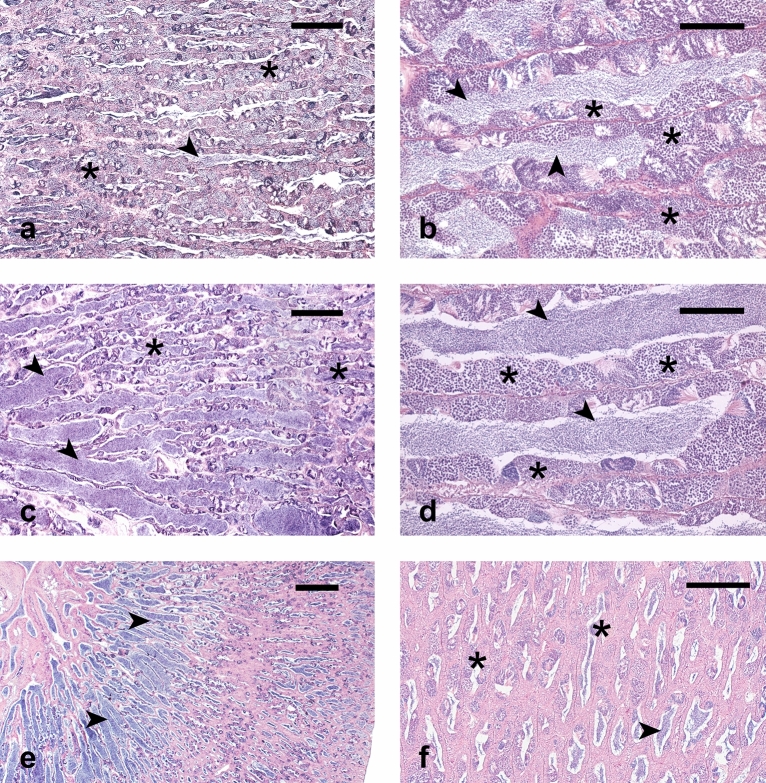
Wild greater amberjack had testes in active spermatogenesis, showing germ cell in all stages of gametogenesis, seminiferous tubules with large lumen and abundant luminal spermatozoa (Fig. 2a, b). Among farmed fish, two different sub-groups were identified: four individuals had testes in active spermatogenesis similar to wild fish, as evidenced from their histological appearance (Fig. 2c, d), GSI and seminiferous tubule diameter (Fig. 3); and two individuals that showed evident reproductive dysfunction characterized by reduced spermatogenic activity, seminiferous tubules with smaller lumen and limited amount of spermatozoa (Fig. 2e, f), lower GSI and smaller diameter of seminiferous tubules (Fig. 3).
Figure 2.
Micrographs of histological section from greater amberjack testes. (a, b) Wild specimen in active spermatogenesis phase (WILD group) showing large seminiferous tubules rich in spermatocysts. (c, d) Farmed specimen in active spermatogenesis with histological appearance similar to wild fish (NormalF group). (e, f) Farmed specimens showing arrested spermatogenesis (DysF group). Small seminiferous tubules with residual spermatocysts and luminal spermatozoa can be observed. Arrowheads indicate luminal spermatozoa; asterisks indicate spermatocysts. Hematoxylin–eosin staining. Bars = 100 μm in (b) and (d), 200 μm in (f), 300 μm in (a) and (c), 500 μm in (e).
Figure 3.
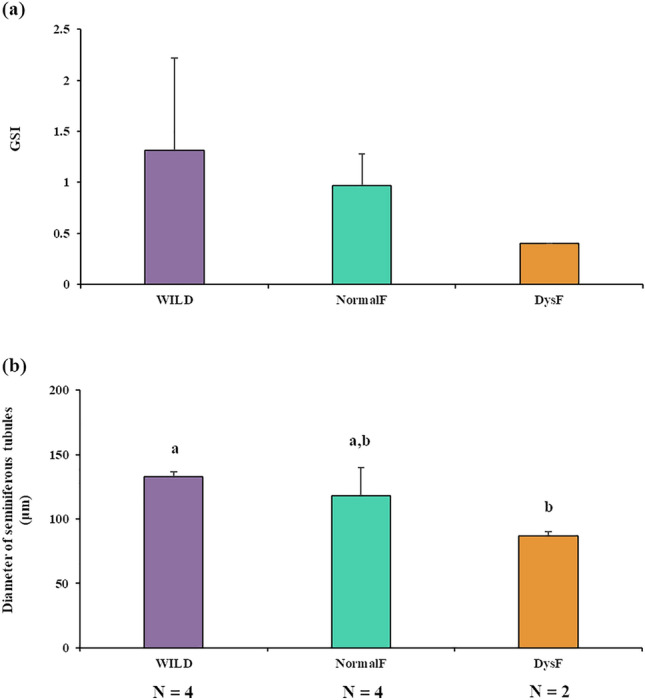
Gonadosomatic index (a) and seminiferous tubule diameter (b) of wild greater amberjack (WILD), non-dysfunctional farmed fish (NormalF) and dysfunctional farmed fish (DysF). Different letters indicate statistically significant differences (Student’s t-test; P < 0.05).
The comparative RNA-seq analysis was performed on the following groups of fish: wild fish (WILD; all of them with normal spermatogenic activity; N = 3); non-dysfunctional farmed fish showing apparently normal spermatogenic activity (NormalF; N = 4); reproductively dysfunctional farmed fish (DysF; N = 2).
Transcriptome analysis
The testis comparative transcriptome analysis among the three groups of fish in different reproductive conditions (WILD, NormalF and DysF) produced an average of 25 million paired-end reads per sample. After an appropriate cleaning procedure, high quality reads were aligned to the Seriola dumerili reference genome. About the 90% of cleaned reads were uniquely mapped to the reference genome.
The comparative transcriptome analysis showed that the majority of the expressed genes (20784) (with a normalized reads count of at least 10) were common to the three groups, while only 210, 183 and 218 genes were specifically expressed in WILD, NormalF and DysF respectively (Fig. 4).
Figure 4.
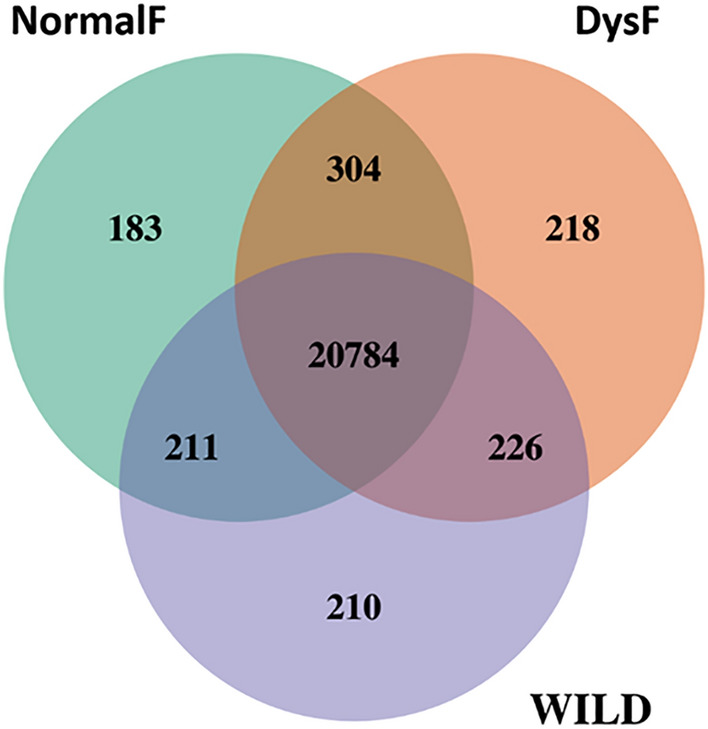
VENN diagram of shared and unique genes related to testes samples of wild (WILD) and hatchery-produced greater amberjack, dysfunctional (DysF) and non-dysfunctional (NormalF).
Differential gene expression analysis identified 2157, 1985 and 74 DEGs in DysF vs WILD, NormalF vs DysF and NormalF vs WILD comparisons, respectively. Among the DEGs, 24 were common to the comparisons DysF vs WILD and NormalF vs WILD, 15 were common to the comparisons DysF vs NormalF and NormalF vs WILD, whereas 1049 were common to the comparisons DysF vs NormalF and DysF vs WILD (Fig. 5; Supplementary Table S1).
Figure 5.
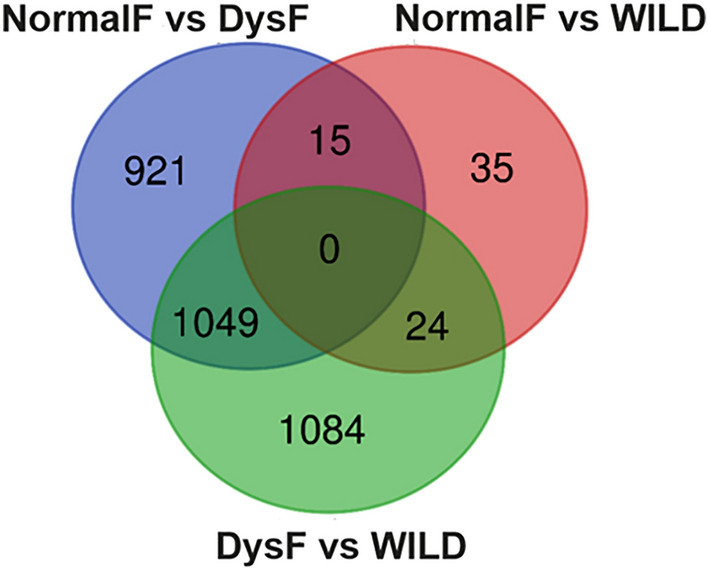
VENN diagram of DEGs shared and unique among DysF vs NormalF, DysF vs WILD and NormalF vs WILD comparisons. DysF, dysfunctional farmed; NormalF, non-dysfunctional Farmed; WILD, wild greater amberjack.
Biological categories related to gene ontology enrichment analysis performed on DEGs of each comparison are reported in Table 2.
Table 2.
Gene ontology enrichment analysis of DEGs from wild and hatchery-produced greater amberjack testes.
| Category | Term | Count | FDR | |
|---|---|---|---|---|
| DysF versus NormalF | UP_KW_BIOLOGICAL_PROCESS | KW-0970~Cilium biogenesis/degradation | 12 | 2.23E−05 |
| UP_KW_CELLULAR_COMPONENT | KW-0966~Cell projection | 39 | 1.19E−13 | |
| UP_KW_CELLULAR_COMPONENT | KW-0969~Cilium | 18 | 2.13E−10 | |
| UP_KW_CELLULAR_COMPONENT | KW-0206~Cytoskeleton | 36 | 4.76E−07 | |
| UP_KW_CELLULAR_COMPONENT | KW-0963~Cytoplasm | 114 | 2.79E−04 | |
| UP_KW_CELLULAR_COMPONENT | KW-0493~Microtubule | 18 | 4.38E−04 | |
| UP_KW_CELLULAR_COMPONENT | KW-0243~Dynein | 5 | 3.24E−02 | |
| UP_KW_CELLULAR_COMPONENT | KW-0282~Flagellum | 5 | 4.09E−02 | |
| UP_KW_MOLECULAR_FUNCTION | KW-0808~Transferase | 85 | 2.48E−02 | |
| KEGG_PATHWAY | sdu04914:ProgesteronE−mediated oocyte maturation | 21 | 6.69E−03 | |
| KEGG_PATHWAY | sdu04114:Oocyte meiosis | 24 | 6.69E−03 | |
| KEGG_PATHWAY | sdu04110:Cell cycle | 23 | 3.42E−02 | |
| DysF versus WILD | UP_KW_BIOLOGICAL_PROCESS | KW-0970~Cilium biogenesis/degradation | 10 | 4.39E−03 |
| UP_KW_CELLULAR_COMPONENT | KW-0966~Cell projection | 30 | 2.07E−06 | |
| UP_KW_CELLULAR_COMPONENT | KW-0969~Cilium | 14 | 9.60E−06 | |
| UP_KW_CELLULAR_COMPONENT | KW-0206~Cytoskeleton | 32 | 2.48E−04 | |
| UP_KW_CELLULAR_COMPONENT | KW-0493~Microtubule | 18 | 1.52E−03 | |
| NormalF versus WILD | KEGG_PATHWAY | sdu04145:Phagosome | 6 | 1.74E−02 |
Count indicates DEGs belonging to specific category. FDR (False Discovery Rate) are statistically significant (P < 0.05), corrected by the Benjamini–Hochberg procedure describing the level of significance of the enrichment. Term column corresponds to the denomination provided by the DAVID annotation tool.
DysF, dysfunctional farmed group; NormalF, normal farmed group; WILD, wild group.
In general, a statistically significant gene enrichment of biological processes and cellular components related to cilium was found in DysF vs NormalF as well as in DysF vs WILD comparisons. Enriched KEGG pathways, related to progesterone-mediated oocyte maturation, oocyte meiosis and cell cycle, were further identified in DysF vs NormalF comparison, while a unique enriched KEGG pathway associated to phagosome emerged when NormalF was compared to WILD. The connections between enriched categories are showed in Fig. 6.
Figure 6.
Relationship between enriched pathways in testis samples from the three analysed groups. Pathways (nodes) are connected if they share 20% (default) or more genes. Darker nodes are more significantly enriched gene sets. Bigger nodes represent larger gene sets. Thicker edges represent more overlapped genes.
In the three comparisons all the enriched categories were interconnected, except for the extracellular matrix structural constituent in NormalF vs WILD.
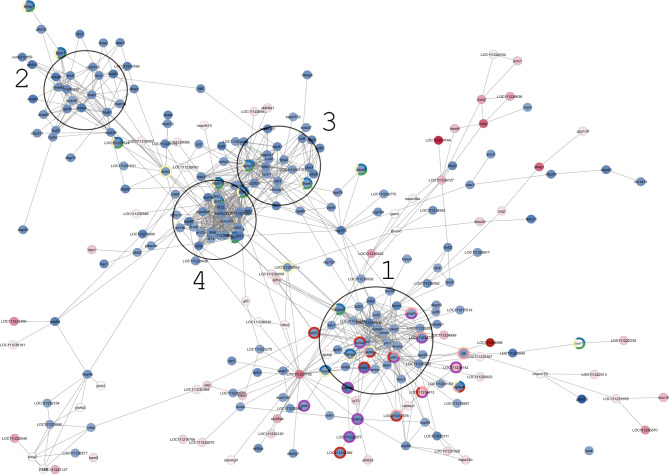
To evaluate the functional relations between DEGs for each comparison, a network based on Protein–Protein interaction (PPi) was generated (Figs. 7, 8 and 9; Supplementary Tables S2a, b and c). Four main protein-interaction groups emerged both in DysF vs NormalF and DysF vs WILD comparisons. Most of these proteins were associated to biological processes related to cilium organization, cilium assembly, plasma membrane bounded cell projection assembly, organelle assembly, cellular component organization and to cell cycle. Proteins related to male gamete generation, gamete generation, spermatogenesis (Spdya, Bbs4, Racgap1, Ift81, Ift20, Ift27, Hspb11, Nphp1, Plk1) as well as to the following KEGG pathways were also detected in both comparisons: progesterone-mediated oocyte maturation (Igf-1, Cdc), oocyte meiosis (AurkA, CycB) and cell cycle (CycA) (contour-coloured nodes in Figs. 7 and 8). Moreover, several proteins, involved in steroid synthesis (such as Cholesterol 25-hydroxylase like 3 and Cytochrome P450), as well as several heat shock proteins, were identified in addition to those included in PPi networks (Supplementary Table S2a, b).
Figure 7.
Protein–Protein interaction (PPi) network in DysF vs NormalF. The network was built using a confidence protein interaction (score = 0.7). Node background indicates gene upregulation (red, log2FC > 1.5) or downregulation (blue, log2FC < 1.5). Node contour indicates biological categories: male gamete generation (blue), gamete generation (yellow) spermatogenesis (green); KEGG: progesterone-mediated oocyte maturation (red), oocyte meiosis (pink), cell cycle (purple) pathways. Circles 1–4 are arbitrary representations of the main protein-protein interaction groups.
Figure 8.
Protein–Protein interaction (PPi) network in DysF vs WILD. The network was built using a confidence protein interaction (score = 0.7). Node background indicates gene upregulation (red, log2FC > 1.5) or downregulation (blue, log2FC < 1.5). Node contour indicates biological categories: male gamete generation (blue), gamete generation (yellow) spermatogenesis (green), KEGG: Progesterone-mediated oocyte maturation (red), KEGG: Oocyte meiosis (pink) KEGG: Cell cycle (purple). Circles 1–4 are arbitrary representations of the main protein-protein interaction groups.
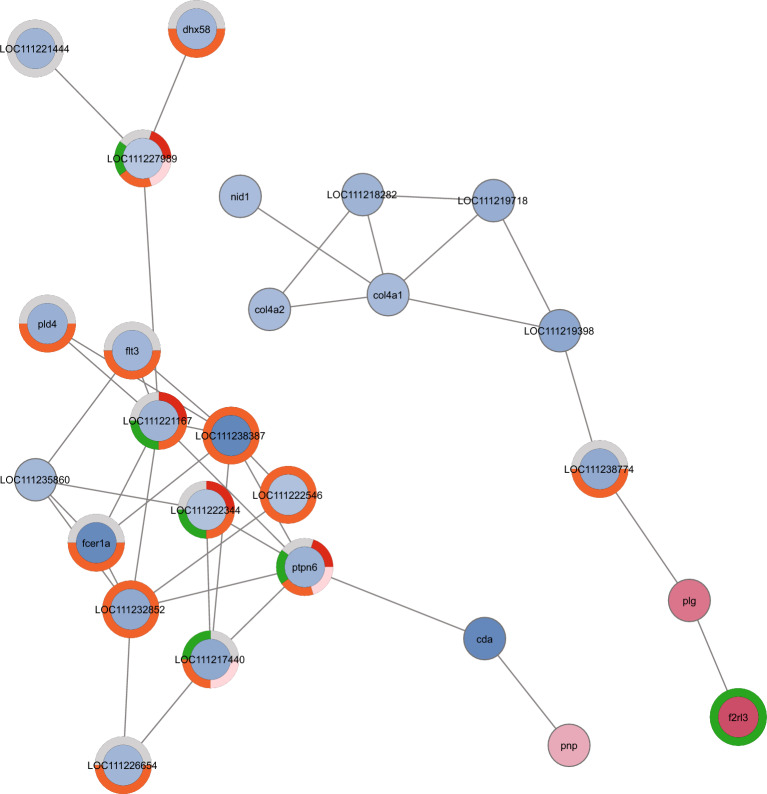
Figure 9.
Protein–Protein interaction (PPi) networks in NormalF vs WILD. Networks were built using a medium confidence protein interaction (score = 0.4). Nodes are coloured according to the following biological categories: regulation of tumor necrosis factor production (red); cell killing (pink); interspecies interaction between organisms (gray); sequestering of calcium ion (green); immune system process (orange).
Two main protein-interaction groups emerged from the comparison NormalF vs WILD. These proteins were associated to the regulation of tumor necrosis factor production and cell killing (Ptpn6), interspecies interaction between organisms (Fcer1a) immune system (Flt3) and regulation of sequestering and release of calcium ion (F2rl3) (Fig. 9; Supplementary Table S2c).
Gene expression levels related to genes detected in NormalF vs DysF and DysF vs WILD, as well as those observed in NormalF vs WILD were evaluated. Most of the DEGs involved in the biological categories male gamete generation and spermatogenesis, and KEGG pathways progesterone-mediated oocyte maturation (igf1, cdc), oocyte meiosis (aurka, cycb) and cell cycle (cyca) as well as the heat shock protein gene hsbp1 were downregulated in DysF compared with the two non-dysfunctional groups. On the contrary, DEGs belonging to the sterol desaturase family (e.g., LOC111231653: cholesterol 25-hydroxylase like 3) were upregulated in DysF. As shown in Fig. 9, most of the DEGs of the NormalF group were downregulated compared with the WILD group, except the gene f2rl3.
Since previous studies showed an increase of germ cell apoptosis in reproductively dysfunctional fish7,19,27, DEGs encoding for proteins involved in the apoptosis pathway were investigated in all comparisons and they were identified in DysF vs NormalF (N = 7) (Table 3; Supplementary Fig. S1), and DysF vs WILD (N = 17) (Table 3; Supplementary Fig. S2).
Table 3.
Dysregulated genes associated to apoptosis.
| Protein ID | Gene Entrez ID | KEGG identifier (K) product | DF vs NF | DF vs WILD | ||
|---|---|---|---|---|---|---|
| log2(FC)a | padjb | log2(FC) | padj | |||
| IAP/XIAP | 111220232 | K08731 baculoviral IAP repeat-containing protein 5-like | − 2.34 | 9.47E−04 | − 2.04 | 2.36E−03 |
| CytC | 111223891 | K08738 cytochrome c | − 2.94 | 9.47E−04 | − 2.77 | 1.15E−03 |
| α-tubulin | 111230200 | K07374 tubulin alpha-1C chain-like | − 2.26 | 3.69E−03 | − 1.62 | 4.03E−02 |
| Akt/PKB | 111235389 | K04456 RAC-gamma serine/threoninE−protein kinase | − 2.07 | 2.01E−02 | − 1.97 | 3.54E−02 |
| Cathepsin | 111231108 | K08568 cathepsin Z-like | − 2.18 | 1.04E−02 | – | – |
| Bcl-XL | 111234723 | K04570 bcl2l1; bcl-2-like protein 1 | − 1.58 | 2.50E−02 | – | – |
| IP3R | 111235419 | K04960 itpr3; inositol 1,4,5-trisphosphate receptor type 3 | 1.68 | 3.12E−02 | – | – |
| Lamin | 111220840 | K12641 lamin-A-like | – | – | − 2.75 | 4.08E−02 |
| Perforin | 111216537 | K07818 perforin-1-like | – | – | 2.12 | 2.05E−02 |
| α-tubulin | 111218500 | K07374 tubulin alpha chain-like | – | - | 1.88 | 2.77E−02 |
| Calpain | 111220564 | K01367 calpain-1 catalytic subunit-like isoform X1 | – | - | 1.87 | 3.16E−02 |
| Calpain | 111220638 | K03853 calpain-2 catalytic subunit-like | – | - | 3.81 | 5.02E−03 |
| Cathepsin | 111220923 | K01366 pro-cathepsin H-like | – | - | 1.92 | 9.42E−03 |
| Gaad45 | 111226142 | K04402 growth arrest and DNA damage-inducible protein GADD45 beta-like | – | – | 2.45 | 1.82E−03 |
| Cathepsin | 111230367 | K01379 cathepsin D-like | – | – | 1.76 | 8.16E−03 |
| Cathepsin | 111234098 | K01371 cathepsin L1-like | – | – | 4.81 | 1.19E−02 |
| Calpain | 111237555 | K03853 calpain-2 catalytic subunit-like | – | – | 2.37 | 1.82E−03 |
| Calpain | 111237566 | K01367 calpain-1 catalytic subunit-like | – | – | 1.61 | 1.36E−02 |
| IκBα | 111238330 | K04734 NF-kappa-B inhibitor alpha-like | – | – | 1.61 | 9.76E−03 |
| Perforin | 111239867 | K07818 perforin-1-like | – | – | 2.11 | 3.27E−02 |
alog2(FC)|> 1.5 upregulated gene, log2(FC)|< 1.5 downregulated gene; bBonferroni or Benjamini adjusted P value < 0.05.
In DysF vs NormalF, except for gene encoding for inositol 1,4,5-trisphosphate receptor type 3 (itpr3) all other DEGs were downregulated. In DysF vs WILD, 5 DEGs were downregulated while 12 were upregulated. Downregulated genes coding for the baculoviral IAP repeat-containing protein 5-like (IAP/XIAP), cytochrome c (CytC), tubulin alpha-1C chain-like (α tubulin) and RAC-gamma serine/threonine-protein kinase (Akt/PKB) were identified both in DysF vs NormalF and DysF vs WILD.
Discussion
Thanks to the integration of histological and RNA-seq data, this comparative study on wild vs hatchery-produced greater amberjack males provided new information on the molecular mechanisms underlying the spermatogenesis impairment observed in fish reared in captivity. Based on testicular development (GSI and histological appearance), hatchery-produced greater amberjack males in the present study were affected by a reproductive dysfunction similar to that displayed by individuals taken from the wild as juveniles and reared in captivity to reproductive maturity12,19. Although the number of dysfunctional fish was low due to the limited availability of farmed fish, which belonged to a small broodstock produced in captivity by hormonal induction of spawning, many significantly differentially expressed genes were identified in dysfunctional fish. Moreover, the comparative transcriptome analysis suggested that spermatogenesis was abnormal also in the histologically evaluated “normal” hatchery-produced fish, which might have been at an initial stage of a reproductive dysfunction.
Hereafter, for the sake of clarity, data interpretation and discussion will be referred to the comparison between DysF and WILD group if not further specified. The comparative analysis of RNA-seq data showed that 20784 genes were expressed in all the three groups and about 10% of these genes were differentially expressed between farmed fish showing clear reproductive dysfunction (DysF), and fish that did not show evident gametogenesis alteration (NormalF and WILD).
Among the mapped pathways, DEGs involved in “cell cycle”, “progesterone-mediated oocyte maturation” and “oocyte meiosis” were mostly downregulated in reproductively dysfunctional fish. The pathway “cell cycle” includes genes involved in cell proliferation. The pathways “progesterone-mediated oocyte maturation” and “oocyte meiosis”, despite the names they have been assigned, include genes related to both oogenesis and spermatogenesis processes; e.g., spyda encodes for a cell cycle regulator that plays a role in male germ cell meiotic maturation43; igf1 is associated with testicular activation by recombinant growth hormone in rats44 and with testicular germ cell proliferation and apoptosis in fish45; aurka is required for male germline maintenance and regulates sperm motility in mice46.
It is known that the reproductive dysfunctions occurring in fish reared in captivity result from a reduced pituitary release of gonadotropins, particularly Lh that is mainly involved in gamete maturation and ovulation/spermiation via 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P) synthesis1,2,23,47. Nevertheless, it is likely that the observed dysregulation of genes involved in steroid synthesis, cell cycle and meiosis may originate from the low levels of plasma Lh in hatchery-produced greater amberjack. Reduced sperm production associated with low levels of plasma Lh has been demonstrated in striped bass Morone saxatilis reared in captivity, in comparison with wild fish sampled in their spawning grounds23,24,48. Most of the identified DEGs encode for products associated with cellular components “cilium”, “cytoskeleton”, “microtubule”, “flagellum”, “microtubule”, “dynein”. The downregulations of these genes is likely related both to a decreased ability of spermatogonia to enter meiosis and the subsequent spermatid differentiation to spermatozoa. Moreover, several important members of the intraflagellar transport process (ift20, ift27, ift81, etc.) were downregulated. In mice Mus musculus, the absence of ift81is associated with abnormal flagellum formation and infertility49. These findings are coherent with the results of the histological analysis indicating that the dysfunctional fish had a reduced spermatogenic activity and with our previous study showing reduced meiosis in captive-reared greater amberjack19.
In agreement with50, who reported upregulation of glycolytic enzymes and downregulation of tricarboxylic acid cycle (TCA) and mitochondrial oxidative phosphorylation enzymes in gilthead seabream Sparus aurata ejaculated spermatozoa compared with diploid germ cells, we found downregulation of several genes belonging to these metabolic pathways. This finding is coherent with a reduced sperm production in dysfunctional fish. As expected, all of the enriched categories were interconnected, as they were all associated with the process of germ cell division and differentiation, except for the extracellular matrix structural constituent which appeared in the comparison between NormalF vs WILD. According to51, the testicular maturation of the rainbow trout Onchorhynchus mykiss is marked by changes of the expression pattern of genes encoding extracellular matrix proteins and this observation was supposed to be correlated with the reorganization of seminiferous tubules occurring during the testicular cycle42.
The PPi analysis confirmed that most of the proteins encoded by DEGs were associated to biological processes related to spermatogenesis, gamete maturation, meiosis, cell cycle and cell assembly. As expected, among DEGs, genes encoding for enzymes involved in steroid synthesis as well as several heat shock proteins were identified. In particular, the upregulation of gene encoding for cholesterol 25-hydroxylase like 3 is likely associated to stress-induced cortisol synthesis rather than sex steroid synthesis, since sex steroid secretion has been found to be compromised in greater amberjack confined in captivity12,19. This interpretation is supported by the upregulation of genes encoding for heat shock proteins, which are known biomarkers of fish exposure to stress52, as well as downregulation of genes encoding for CyP450, one of the main enzymes complexes involved in steroidogenesis, and insulin-like growth factors, known mediators of Fsh in the stimulation of spermatogenesis.
Further interesting information originated from the NormalF vs WILD PPi comparison that showed dysregulation of genes associated with tumor necrosis factor production, a cytokine produced by leukocytes and involved in inflammation, apoptosis signalling and cell killing. In particular, the ptpn6 gene, which encodes for a key regulatory protein involved in different pathways related to inflammation, apoptosis and necroptosis53, was differently expressed only in the NormalF vs WILD comparison. This gene has a protective role in the regulation of apoptosis54 and its downregulation in the NormalF group is coherent with the increased testicular apoptosis observed in captive-reared greater amberjack undergoing an apparently “normal” spermatogenic process19.
Some genes encoding regulatory factors involved in the apoptotic process (e.g. aculoviral IAP repeat-containing protein 5-like, cytochrome c, RAC-gamma serine/threonine-protein kinase) were downregulated in DysF vs NormalF, whereas other genes encoding for enzymes involved in the apoptotic process (e.g. cathepsin, calpain) were downregulated in DysF vs WILD, indicating an overall dysregulation of the apoptotic pathway in the two farmed groups. In the present study, several genes encoding for enzymes involved in the apoptotic process were downregulated in full-blown dysfunctional fish, indicating that when the spermatogenesis is arrested, the role of apoptosis in the removal of aberrant cells and assuring the correct germ cells/Sertoli cells ratio declines. In the DysF vs NormalF comparison, the only upregulated gene involved in the apoptotic process was itpr3, a pleiotropic gene which enables Ca2+ transfer from the endoplasmic reticulum to mitochondria, plays a role in metabolism and cell fate regulation and promotes either cell death or cell cycle progression and proliferation55. Hence, the upregulation of this gene in dysfunctional fish might be related to an increased germ cells apoptosis.
The downregulation of genes encoding for proteins involved in interspecies interaction between organisms and immune response in apparently non-dysfunctional fish may be interpreted as a further evidence of the inflammatory state induced by captivity-induced stress; furthermore, it may also suggest a reduced synthesis of factors involved in sperm-oocyte interaction.
In conclusion, 30% of the analysed hatchery-produced greater amberjack showed the same reproductive dysfunction previously observed in individuals caught from the wild and reared in captivity. Moreover, the presence of statistically significant differences in gene expression and disrupted pathways suggested that even apparently non-dysfunctional fish might have been experiencing an initial stage of reproductive impairment. The molecular mechanisms generating the observed spermatogenesis alteration involved dysregulation of many interconnected biological processes, such as steroidogenesis, cell cycle, meiosis, cell assembly, and apoptosis. Further studies are in progress on gene expression in pituitary and hypothalamus in order to provide a complete view of the alteration of the activity of the reproductive axis in greater amberjack reared under commercial farming conditions. The identification of the altered biological processes in fish reared in captivity will improve our understanding of the observed reproductive dysfunctions and will hopefully support the set-up of more effective broodstock management protocols in the aquaculture industry.
Supplementary Information
Acknowledgements
The authors are grateful to Mr Tasos Raftopoulos of Argosaronikos Fishfarming S.A. (Greece) for providing the hatchery-produced greater amberjack and for assistance during biological sampling. Thanks are also expressed to Mr Peppe, Giovanni and Vincenzo Billeci, and all the crew of the purse-seine fishing vessel ‘Graziella’ (Italy) for their friendly collaboration and hospitality on board during the sampling of wild greater amberjack. Thanks are due to Rezart Cuko for his technical support in the preparation of histological slides.
Author contributions
Project ideation: C.P., C.C.M., G.P. and A.C. Biological sampling: C.P., C.C.M., R.Z. and A.C. Molecular analyses: A.L., C.M. and C.DV. Elaboration of RNAseq data: A.L., C.P., L.M., S.N.C., C.LG and E.P. Histological analysis: C.P., R.Z. and G.V. Figure preparation: A.L. and R.Z. Funding: C.C.M., G.P. and A.C. Manuscript writing (first draft): A.L. and A.C. Manuscript review: all authors.
Funding
This work were funded by HORIZON EUROPE European Research Council (Grant no. 862658).
Data availability
Reads generated in this study are freely available through the SRA (Short Read Archive) database under the BioProject accession number PRJNA946197. All the other data produced and/or analyzed during the current study are included in this article and in Supplementary Figures and Supplementary Tables.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-40597-5.
References
- 1.Zohar Y, Mylonas CC. Endocrine manipulations of spawning in cultured fish: From hormones to genes. Aquaculture. 2001;197:99–136. [Google Scholar]
- 2.Mylonas CC, Fostier A, Zanuy S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010;165:516–534. doi: 10.1016/j.ygcen.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Mylonas CC, Duncan NJ, Asturiano JF. Hormonal manipulations for the enhancement of sperm production in cultured fish and evaluation of sperm quality. Aquaculture. 2017;472:21–44. [Google Scholar]
- 4.Sumpter, J. P., Pottinger, T. G., Rand-Weaver, M. & Campbell, P. M. The wide-ranging effects of stress in fish. In Perspectives in Comparative Endocrinology (eds. Davey, K. G. et al.) 535–538 (National Research Council of Canada, 1994).
- 5.Pankhurst, N. W. & Van der Kraak, G. Effects of stress on reproduction and growth of fish. In Fish Stress and Health in Aquaculture (eds. Iwama, G. K. et al.) 73–93 (Cambridge University Press, 1997).
- 6.Corriero A, et al. Evidence that severe acute stress and starvation induce rapid atresia of ovarian vitellogenic follicles in Atlantic bluefin tuna, Thunnus thynnus (L.) (Osteichthyes: Scombridae) J. Fish Dis. 2011;34:853–860. doi: 10.1111/j.1365-2761.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 7.Zupa R, et al. Comparative analysis of male germ cell proliferation and apoptosis in wild and captive Atlantic bluefin tuna (Thunnus thynnus L.) J. Appl. Ichthyol. 2013;29:71–81. [Google Scholar]
- 8.Zohar Y, et al. The bioactivity of gonadotropin-releasing hormones and its regulation in the gilthead seabream, Sparus aurata: In vivo and in vitro studies. Fish. Physiol. Biochem. 1989;7:59–67. doi: 10.1007/BF00004690. [DOI] [PubMed] [Google Scholar]
- 9.Zohar Y, Tosky M, Pagelson G, Finkelman Y. Induction of spawning in the gilthead seabream, Sparus aurata, using wD-Ala6-Pro9NEtx-LHRH: Comparison with the use of HCG. Israeli J. Aquacult. Bamidgeh. 1989;41:105–113. [Google Scholar]
- 10.Battaglene SC, Selosse PM. Hormone-induced ovulation and spawning of captive and wild broodfish of the catadromous Australian bass, Macquaria novemaculeata (Steindachner), (Percichthyidae) Aqua. Res. 1996;27:191–204. [Google Scholar]
- 11.Watanabe T, Vassallo-Agius R. Broodstock nutrition research on marine finfish in Japan. Aquaculture. 2003;227:35–61. [Google Scholar]
- 12.Zupa R, et al. Comparative study of reproductive development in wild and captive-reared greater amberjack Seriola dumerili (Risso, 1810) PLoS ONE. 2022;12(1):e0169645. doi: 10.1371/journal.pone.0169645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paxton, J. R., Hoese, D. F., Allen, G. R. & Hanley, J. E. Pisces. Petromyzontidae to Carangidae. Zoological Catalogue of Australia, Vol. 7 (Australian Government Publishing Service, 1989).
- 14.Cervigon, F. Los peces Marinos de Venezuela, vol. 2 (Caracas: Fundacion Cientıfica Los Roques, 1993).
- 15.Smith C. L. National Audubon Society field guide to tropical marine fishes of the Caribbean, the Gulf of Mexico, Florida, the Bahamas, and Bermuda (ed. Alfred, A. Knopf, Inc) 120 (New York, 1997).
- 16.Bauchot, M. L. Poisson osseux, Familie Carangidae. In Fiches FAO d'identification des espèces pour les besoins de la pêche (Révision 1). Méditerraneée et mar Noire. Zone de pêche 37. Volume II. Vertébrés (eds. Fischer, W. et al.) 1009–1030 (FAO, 1987).
- 17.FAO. Seriola dumerili. Cultured Aquatic Species Information Programme. In Fisheries and Aquaculture Division [online] (eds. Jerez Herrera, S. & Vassallo Agius, R.) (Rome, 2016). https://www.fao.org/fishery/en/culturedspecies/seriola_dumerili/en.
- 18.Corriero A, Wylie MJ, Nyuji M, Zupa R, Mylonas CC. Reproduction of greater amberjack (Seriola dumerili) and other members of the family Carangidae. Rev. Aquac. 2021;13:1781–1815. [Google Scholar]
- 19.Zupa R, et al. Rearing in captivity affects spermatogenesis and sperm quality in greater amberjack, Seriola dumerili (Risso, 1810) J. Anim. Sci. 2017;95:4085–4100. doi: 10.2527/jas2017.1708. [DOI] [PubMed] [Google Scholar]
- 20.Fakriadis I, Lisi F, Sigelaki I, Papadaki M, Mylonas CC. Spawning kinetics and egg/larval quality of greater amberjack (Seriola dumerili) in response to multiple GnRHa injections or implants. Gen. Comp. Endocrinol. 2019;279:78–87. doi: 10.1016/j.ygcen.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Fakriadis I, Miccoli A, Karapanagiotis S, Tsele N, Mylonas CC. Optimization of a GnRHa treatment for spawning commercially reared greater amberjack Seriola dumerili: Dose response and extent of the reproductive season. Aquaculture. 2020;521:735011. [Google Scholar]
- 22.Fakriadis I, et al. Control of reproduction of greater amberjack Seriola dumerili reared in aquaculture facilities. Aquaculture. 2020;519:734880. [Google Scholar]
- 23.Mylonas CC, Zohar Y. Endocrine regulation and artificial induction of oocyte maturation and spermiation in basses of the genus Morone. Aquaculture. 2001;202:205–220. [Google Scholar]
- 24.Mylonas CC, Scott AP, Zohar Y. Plasma gonadotropin II, sex steroids, and thyroid hormones in wild striped bass (Morone saxatilis) during spermiation and final oocyte maturation. Gen. Comp. Endocrinol. 1997;108:223–236. doi: 10.1006/gcen.1997.6967. [DOI] [PubMed] [Google Scholar]
- 25.Mylonas CC, Woods LC, III, Thomas P, Zohar Y. Endocrine profiles of female striped bass (Morone saxatilis) in captivity, during post-vitellogenesis and induction of final oocyte maturation via controlled-release GnRHa-delivery systems. Gen. Comp. Endocrinol. 1998;110:276–289. doi: 10.1006/gcen.1998.7073. [DOI] [PubMed] [Google Scholar]
- 26.Corriero A, et al. Histological study of the effects of treatment with gonadotropin-releasing hormone agonist (GnRHa) on the reproductive maturation of captive-reared Atlantic bluefin tuna (Thunnus thynnus L.) Aquaculture. 2007;272:675–686. [Google Scholar]
- 27.Corriero A, et al. Proliferation and apoptosis of male germ cells in captive Atlantic bluefin tuna (Thunnus thynnus L.) treated with gonadotropin-releasing hormone agonist (GnRHa) Anim. Reprod. Sci. 2009;116:346–357. doi: 10.1016/j.anireprosci.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Rosenfeld H, et al. GnRHa-mediated stimulation of the reproductive endocrine axis in captive Atlantic bluefin tuna, Thunnus thynnus. Gen. Comp. Endocrinol. 2012;175:55–64. doi: 10.1016/j.ygcen.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Mylonas CC, et al. Preparation and administration of gonadotropin-releasing hormone agonist (GnRHa) implants for the artificial control of reproductive maturation in captive-reared Atlantic bluefin tuna (Thunnus thynnus thynnus) Rev. Fish. Sci. 2007;15:183–210. [Google Scholar]
- 30.Mylonas CC, et al. Reproduction of hatchery-produced meagre Argyrosomus regius in captivity III. Comparison between GnRHa implants and injections on spawning kinetics and egg/larval performance parameters. Aquaculture. 2015;448:44–53. [Google Scholar]
- 31.Fakriadis I, Mylonas CC. Sperm quality of greater amberjack Seriola dumerili throughout the reproductive season and in response to GnRHa treatment with controlled release implants. Fish Physiol. Biochem. 2021;47:281–292. doi: 10.1007/s10695-020-00910-9. [DOI] [PubMed] [Google Scholar]
- 32.Kožul V, Skaramuca B, Kraljević M, Dulčić J, Glamuzina B. Age, growth and mortality of the Mediterranean amberjack Seriola dumerili (Risso 1810) from the south-eastern Adriatic Sea. J. Appl. Ichthyol. 2001;17:134–141. [Google Scholar]
- 33.Anonymous. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav.55, 251–257 (1998).
- 34.Metcalfe JD, Craig JF. Ethical justification for the use and treatment of fishes in research: An update. J. Fish Biol. 2011;78(2):393–394. doi: 10.1111/j.1095-8649.2010.02900.x. [DOI] [PubMed] [Google Scholar]
- 35.EU. "Directive 2010/63/EU of the European parliament and the council of 22 September 2010 on the protection of animals used for scientific purposes. In Official Journal of the European Union L 276/33(Animal use): Animal Protection (2010).
- 36.Chen S, Zhou Y, Chen Y, Gu J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dobin A, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao Y, Smyth GK, Shi W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 39.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman BT, et al. (2021) DAVID: A web server for functional enrichment analysis and functional annotation of gene lists. Nucleic Acids Res. 2022;50(W1):W216–W221. doi: 10.1093/nar/gkac194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge SX, Jung D, Yao R. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics. 2020;36:2628–2629. doi: 10.1093/bioinformatics/btz931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanehisa M, Sato Y. KEGG Mapper for inferring cellular functions from protein sequences. Protein Sci. 2020;29:28–35. doi: 10.1002/pro.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ray LB. Spermatogenesis lost in translation. Sci. Signal. 2009;2:146. [Google Scholar]
- 44.Xu Y, Han CY, Park MJ, Gye MC. Increased testicular insulin-like growth factor 1 is associated with gonadal activation by recombinant growth hormone in immature rats. Reprod. Biol. Endocrinol. 2022;20:72. doi: 10.1186/s12958-022-00944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreira DP, Melo RMC, Weber AA, Rizzo E. Insulin-like growth factors 1 and 2 are associated with testicular germ cell proliferation and apoptosis during fish reproduction. Reprod. Fertil. Dev. 2020;32:988–998. doi: 10.1071/RD20128. [DOI] [PubMed] [Google Scholar]
- 46.Lester WC, et al. Aurora A Kinase (AURKA) is required for male germline maintenance and regulates sperm motility in the mouse. Biol. Reprod. 2021;105:1603–1616. doi: 10.1093/biolre/ioab168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz RW, et al. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010;165:390–411. doi: 10.1016/j.ygcen.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 48.Mylonas CC, Zohar Y, Woods LC, Thomas P, Schulz RW. Hormone profiles of captive striped bass Morone saxatilis during spermiation, and long-term enhancement of milt production. J. World Aquac. Soc. 1998;29:379–392. [Google Scholar]
- 49.Qu W, et al. The essential role of intraflagellar transport protein IFT81 in male mice spermiogenesis and fertility. Am. J. Physiol. Cell Physiol. 2020;318(6):C1092–C1106. doi: 10.1152/ajpcell.00450.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castro-Arnau J, et al. Developmental RNA-Seq transcriptomics of haploid germ cells and spermatozoa uncovers novel pathways associated with teleost spermiogenesis. Sci. Rep. 2022;12(1):14162. doi: 10.1038/s41598-022-18422-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mazurais D, Montfort J, Delalande C, LeGac F. Transcriptional analysis of testis maturation using trout cDNA macroarrays. Gen. Comp. Endocrinol. 2005;142:143–154. doi: 10.1016/j.ygcen.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 52.da Silva HNP, et al. Stress response of Rhamdia quelen to the interaction stocking density—feeding regimen. Gen. Comp. Endocrinol. 2023;35:114228. doi: 10.1016/j.ygcen.2023.114228. [DOI] [PubMed] [Google Scholar]
- 53.Kiratikanon S, Chattipakorn SC, Chattipakorn N, Kumfu S. The regulatory effects of PTPN6 on inflammatory process: Reports from mice to men. Arch. Biochem. Biophys. 2022;721:109189. doi: 10.1016/j.abb.2022.109189. [DOI] [PubMed] [Google Scholar]
- 54.Speir M, et al. Ptpn6 inhibits caspase-8- and Ripk3/Mlkl-dependent inflammation. Nat. Immunol. 2020;21:54–64. doi: 10.1038/s41590-019-0550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosa N, Sneyers F, Parys JB, Bultynck G. Type 3 IP3 receptors: The chameleon in cancer. Int. Rev. Cel. Mol. Biol. 2020;351:101–148. doi: 10.1016/bs.ircmb.2020.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Reads generated in this study are freely available through the SRA (Short Read Archive) database under the BioProject accession number PRJNA946197. All the other data produced and/or analyzed during the current study are included in this article and in Supplementary Figures and Supplementary Tables.