Abstract
Herpes simplex virus (HSV) inhibits major histocompatibility complex (MHC) class I expression in infected cells and does so much more efficiently in human cells than in murine cells. Given this difference, if MHC class I-restricted T cells do not play an important role in protection of mice from HSV, an important role for these cells in humans would be unlikely. However, the contribution of MHC class I-restricted T cells to the control of HSV infection in mice remains unclear. Further, the mechanisms by which these cells may act to control infection, particularly in the nervous system, are not well understood, though a role for gamma interferon (IFN-γ) has been proposed. To address the roles of MHC class I and of IFN-γ, C57BL/6 mice deficient in MHC class I expression (β2 microglobulin knockout [β2KO] mice), in IFN-γ expression (IFN-γKO mice), or in both (IFN-γKO/β2KO mice) were infected with HSV by footpad inoculation. β2KO mice were markedly compromised in their ability to control infection, as indicated by increased lethality and higher concentrations of virus in the feet and spinal ganglia. In contrast, IFN-γ appeared to play at most a limited role in viral clearance. The results suggest that MHC class I-restricted T cells play an important role in protection of mice against neuroinvasive HSV infection and do so largely by mechanisms other than the production of IFN-γ.
Two gene products of herpes simplex virus (HSV) block presentation of viral proteins by class I major histocompatibility complex (MHC) molecules: the viral host shutoff protein (vhs), which is present in the viral particle, and the immediate-early protein ICP47 (1, 14, 41, 42). Through the sequential action of these proteins, antigen presentation by MHC class I is inhibited early in the viral replication cycle. ICP47 binds to human transporter associated with antigen-processing proteins (TAP), thereby inhibiting peptide loading on MHC class I and recognition by HSV-specific, MHC class I-restricted, CD8+ T cells (1, 14, 42, 43). This effect is greatest in nonhematopoietic cells in which the abundance of MHC class I and TAP are lower than in antigen-presenting cells (41). As a consequence, HSV is more likely to impair recognition of infected target cells in the tissues than to block the generation of antigen-specific CD8+ T cells. Consistent with this, recent studies indicate that HSV antigen-specific CD8+ cytotoxic-T-lymphocyte (CTL) precursors can be readily detected in the blood and cutaneous lesions of HSV-infected individuals (16, 31, 32). However, NK cells and HSV antigen-specific CD4+ T cells are detected earlier than antigen-specific CD8+ T cells in lesions of humans with recurrent HSV-2 disease (16). This finding has led to the proposal that gamma interferon (IFN-γ) produced by infiltrating NK and CD4+ T cells overrides the inhibitory effects of HSV on TAP function and MHC class I expression (22, 41), thereby allowing the eradication of virus by CD8+ T cells, whose numbers increase in lesions around the time of viral clearance (16, 31). In patients with AIDS, a lower frequency in the blood of HSV antigen-specific CD8+ CTL precursors is associated with more frequent and severe recurrences of genital disease (32). These correlative data suggest that CD8+ T cells may play an important role in the clearance of HSV in humans, at least from mucocutaneous lesions.
ICP47 inhibits murine TAP poorly (1, 42), which may explain the greater ease with which anti-HSV CD8+ CTLs have been detected in mice than in humans (3, 8, 28, 34, 35). Despite the weak interaction of ICP47 with murine TAP, results of a recent study (12) suggested that ICP47 impairs CD8+ T-cell-dependent viral clearance from the nervous system: CD8+ T cells protected susceptible BALB/c or A/J mice from lethal, nervous system infection with an HSV mutant lacking ICP47 but did not appear to protect against infection with wild-type HSV or to contribute to clearance of either virus from the eye. These findings are consistent with data suggesting that CD8+ T cells limit persistence of HSV in the spinal ganglia and decrease spread to the central nervous system (35, 36). However, other studies have concluded that CD4+ T cells but not CD8+ T cells play the critical role in viral clearance and protection from lethal primary infection with wild-type HSV (20, 23, 24) or that either CD4+ or CD8+ T cells are sufficient for protection (26, 37). Since the effects of ICP47 are likely to be greater in humans than in mice, if MHC class I-restricted CD8+ T cells do not play an important role in protection of mice from lethal, neuroinvasive infection due to wild-type HSV, an important role in humans would be unlikely.
The mechanisms by which T cells may limit the spread of infection in the nervous system are not clearly understood. Studies by Simmons and colleagues suggested that CD8+ T cells may lyse infected Schwann cells or satellite cells but that they probably do not lyse infected neurons (31, 32). They and others have proposed that CD8+ T cells protect neurons through the production of cytokines, in particular IFN-γ (35, 36). IFN-γ contributes to the clearance of HSV from mucocutaneous sites (4, 24, 25, 37, 44). However, the role of IFN-γ in protection from lethal, neuroinvasive infection is uncertain and may vary with the strain of mice, method used to inhibit IFN-γ function, and route of inoculation (4, 5, 24, 37, 44). IFN-γ is produced in the ganglia of mice with acute or latent HSV infection (5, 13, 19). Both CD4+ and CD8+ T cells (and NK cells) produce IFN-γ, but CD4+ T cells appear to be the predominant source of IFN-γ following intravaginal infection with HSV (24, 25). Thus, it is possible that the disparity in results regarding the relative importance of CD4+ and CD8+ T cells in protection from lethal, neuroinvasive HSV infection reflects their redundant roles in production of this cytokine or that IFN-γ and CD8+ T cells contribute independently to control of infection in the nervous system.
To address in parallel the contributions of MHC class I-restricted T cells and of IFN-γ to protection of mice from HSV, MHC class I and CD8+ T-cell-deficient β2 microglobulin knockout (β2KO) mice, IFN-γ knockout (IFN-γKO) mice, and mice deficient in both MHC class I and IFN-γ expression (IFN-γKO/β2KO) were studied. The results indicated that loss of MHC class I expression in β2KO mice substantially increased their susceptibility to HSV, whereas the loss of IFN-γ expression had a much more limited effect. These findings indicate that MHC class I-restricted T cells play an important role in protection against neuroinvasive HSV infection in mice and that they do so largely by mechanisms other than the production of IFN-γ. Though MHC class I expression is more severely impaired in β2KO mice than in human cells infected with wild-type HSV, these findings support the notion that inhibition of MHC class I expression is an important factor in the virulence of this virus.
MATERIALS AND METHODS
Mice.
All mice were of the H-2b haplotype. B6 congenic β2KO mice (17, 33) were obtained from Jackson Laboratories; wild-type B6 mice were used as controls. IFN-γKO mice on a mixed B6 × 129 background were obtained from Tim Stewart (Genentech, South San Francisco, Calif.). IFN-γKO mice and wild-type controls were used after the seventh-generation backcross to B6 mice. IFN-γKO/β2KO mice and mice heterozygous for one or both of these genes were derived from the second intercross of IFN-γKO mice and β2KO mice. The genotypes of the mice were determined by PCR and in some cases by Southern blot analysis or flow cytometry to detect the lack of CD8+ T cells in peripheral blood of β2KO mice. Animals were housed under specific pathogen-free conditions and were used at 8 to 18 weeks of age.
Virus.
The wild-type KOS strain of HSV-1 was a gift of Edward Wagner (University of California, Irvine). Virus stocks were produced and titrated in mycoplasma-free Vero cells. A lysate of uninfected (mock) Vero cells was prepared in parallel. Aliquots were stored at −80°C and thawed just before use.
Analysis of the course of HSV infection.
The footpad model of infection was used (3, 6, 7). Mice were anesthetized with ketamine-xylazine, and the hind footpads were injected intradermally with 100 μl of pyrogen-free 9% NaCl solution. Two to three hours later, both footpads were gently abraded and then inoculated topically with 10 μl of virus diluted to yield the desired inoculum. Thereafter, mice were evaluated daily for the presence of footpad lesions, hind limb paralysis, and gross motor ataxia. In preliminary experiments, it was found that >80% of mice which developed bilateral hind limb paralysis or gross motor ataxia died within 24 to 36 h. Thus, in subsequent experiments, mice which developed paralysis or ataxia were immediately euthanized. One outcome variable analyzed was the number of days mice survived without developing paralysis or gross ataxia. The other outcome variable analyzed was clearance of virus from the feet and nervous system. For determination of viral concentrations, both hind feet and the lumbosacral dorsal root ganglia and associated spinal cord were snap frozen and stored at −80°C. Tissues were subsequently homogenized in phosphate-buffered saline (PBS), diluted serially, and titrated by plaque assay on Vero cells. The viral density was corrected for the weight of the tissues and expressed as log10 PFU per gram of tissue.
Lymphocyte proliferation and cytokine production.
The draining popliteal lymph nodes were obtained from mice at the time of sacrifice. Lymph nodes from mice of the same genotype were pooled for analysis. Single-cell suspensions were obtained by pressing tissue through fine mesh sieves, and then cells were washed once and resuspended in HL-1 medium (Biowhittaker, Walkersville, Md.) at a concentration of 2.5 × 106 cells/ml. Cells were added to wells of microtiter trays to which medium alone (unstimulated), anti-CD3 monoclonal antibody (1452C11) at an optimal concentration (positive control), HSV antigen (UV-inactivated viral stock) in a concentration ranging from 1:2,500 to 1:10,000, or mock antigen (UV-inactivated, sham-infected Vero cells) was added. After incubation for 72 h at 37°C in an atmosphere of 5% CO2–95% air, [3H]thymidine (0.4 μCi) was added, and uptake was assessed 24 h later. Supernatants harvested after 72 h from additional microtiter wells were assayed for IFN-γ, interleukin 10 (IL-10), or IL-4 by enzyme-linked immunosorbent assay as described previously (15) with antibody pairs and recombinant standards obtained from Genzyme (Cambridge, Mass.).
Assessment of anti-HSV antibody production.
Preinfection sera and sera obtained at the time of sacrifice were collected and stored at −80°C. Isotype-specific antibodies to HSV glycoprotein B were assessed by enzyme-linked immunosorbent assay. Plates were coated overnight with 1 μg of recombinant glycoprotein B (a gift from Chiron Corp, Emeryville, Calif.) per ml in carbonate buffer (pH 9.6), blocked with PBS containing 3% bovine serum albumin and 0.05% Tween 20, washed, and incubated with serum samples that were diluted serially in 10% PBS, 0.3% Tween 20, and 0.01 M EDTA. Plates were then washed, incubated with isotype-specific, peroxidase-conjugated antisera and developed as previously described (15).
Statistics.
The significance of differences in levels of paralysis-free survival was determined by life-table analysis and log rank test, and differences in viral concentrations in tissues were determined by Student’s two-tailed t test on log-transformed viral titers with Statview software (Abacus Concepts, Berkeley, Calif.).
RESULTS
MHC class I-restricted T cells play a critical role in defense against HSV after footpad inoculation.
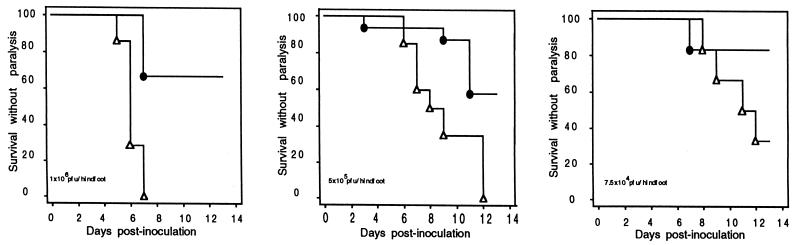
To explore the role of MHC class I-restricted T cells in resistance to primary infection with HSV, β2KO mice and heterozygous littermate controls were challenged by bilateral footpad inoculation. In this and similar models, virus is cleared from the skin and spinal ganglia between days 5 and 10 in a T-cell-dependent manner (3, 6, 7, 26, 35, 36). As previously reported for mice of the B6 background, controls were relatively resistant to HSV: in three independent experiments, 60 to 86% of wild-type B6 mice survived without neurological signs after bilateral footpad inoculation with 7.5 × 106 PFU, the highest inoculum tested. In contrast, β2KO mice were markedly compromised: more than 50% died or developed hind limb paralysis and/or marked ataxia after infection with doses as low as 7.5 × 104 PFU (Fig. 1). In the experiments whose results are shown, paralysis or death occurred by 13 days, but in other experiments occasional deaths occurred in β2KO mice (but not wild-type B6 or heterozygous littermate control mice) as late as the 19th day.
FIG. 1.
Outcome of HSV infection in β2KO (▵) and control (●) mice. The results show the fraction of mice surviving without neurological impairment (paralysis or gross motor ataxia) over time in days after bilateral footpad inoculation with 106 (left), 5 × 105 (middle), or 7.5 × 104 (right) PFU/footpad. The numbers of mice per group and P values by log rank test were 7 β2KO mice, 6 controls, and P of 0.004 for the group inoculated with 106 PFU; 20 β2KO mice, 16 controls, and a P of 0.002 for the group inoculated with 5 × 105 PFU; and 6 β2KO mice, 6 controls, and a P of 0.14 for the group inoculated with 7.5 × 104 PFU.
The role of IFN-γ compared to that of MHC class I-restricted T cells in resistance to acute HSV infection.
The mechanisms by which MHC class I-restricted T cells protect against HSV is not certain. In addition to cytolytic function, these cells are potent producers of IFN-γ and tumor necrosis factor (TNF), production of which has been proposed as a mechanism by which HSV may be cleared from neurons without their destruction (5, 12, 29, 35, 36). If production of IFN-γ is an important mechanism by which MHC class I-restricted T cells control HSV infection and these cells are an important source of IFN-γ, then (i) IFN-γKO mice should be more susceptible to HSV and (ii) the contribution of IFN-γ should be less evident in β2KO mice.
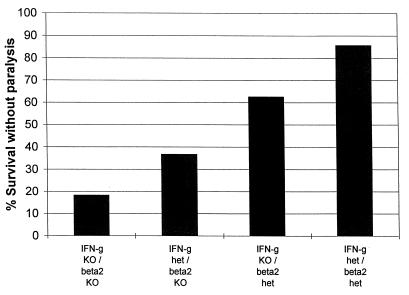
Following inoculation with 7.5 × 106 PFU, the survival of IFN-γKO mice (56%) did not differ from that of wild-type B6 mice (71%, n = 16, P = 0.23). Also, following inoculation with 5 × 105 to 5 × 106 PFU, the survival of IFN-γKO mice (13 of 17, 76%) was similar to that of heterozygous littermate controls (14 of 17, 82%). When β2KO mice were crossed with IFN-γKO mice and littermates of the four resultant genotypes were infected with >106 PFU, there was no difference in outcome between IFN-γ heterozygous/β2KO and IFN-γKO/β2KO mice: ≥80% died or developed hind limb paralysis between days 6 and 12, whereas >50% of IFN-γKO/β2 heterozygous and IFN-γ heterozygous/β2 heterozygous mice survived without neurological signs for up to 21 days (data not shown). However, when these groups of mice were infected with smaller amounts of virus, there appeared to be a modest contribution by IFN-γ to protection. In these experiments, one cohort of mice of each of the four genotypes was monitored to determine the fraction that developed paralysis or died by day 10, when the survivors were sacrificed and viral concentrations in the feet and lumbosacral ganglia and spinal cord were determined; viral concentrations in another cohort infected in parallel and sacrificed on day 7 or 8 were also evaluated. Following inoculation with 5 × 105 PFU, survival was lowest in the IFN-γKO/β2KO mice, greater in IFN-γ heterozygous/β2KO mice, even greater in the IFN-γKO/β2 heterozygous mice, and greatest in IFN-γ heterozygous/β2 heterozygous mice (Fig. 2). These results provided further evidence that MHC class I played a critical role in protection from lethal HSV infection and suggested an incremental, and partially independent, contribution of IFN-γ to protection.
FIG. 2.
Outcome of HSV infection in IFN-γKO and β2KO mice. The results show the fraction of mice surviving to day 10 (at which time surviving mice were sacrificed) without neurological impairment (paralysis or gross motor ataxia) over time in days after bilateral footpad inoculation with 5 × 105 PFU/footpad. The concentrations of virus in the footpads and spinal ganglia of the sacrificed mice are shown in Table 1, experiment 1. The numbers of mice evaluated for survival to day 10 were 11 for the IFN-γKO/β2KO mice, 8 for the IFN-γKO/β2 heterozygous (het) mice, 6 for the IFN-γ heterozygous/β2KO mice, and 7 for the IFN-γ heterozygous/β2 heterozygous mice. The overall levels of survival survival were different by log rank test (P = 0.005). Independent comparisons by Fisher’s exact test were as follows: IFN-γ heterozygous/β2 heterozygous versus IFN-γKO/β2KO mice (P = 0.01) and versus IFN-γ heterozygous/β2KO mice (P = 0.1), and IFN-γKO/β2KO versus IFN-γKO/β2 heterozygous mice (P = 0.07).
Consistent with this notion, by day 10 the amounts of virus present in the spinal ganglia and feet of IFN-γKO/β2KO mice were consistently greater than in the other three groups, even in the few mice of this genotype that survived to day 10 (Table 1, experiment 1). The amounts of virus were also increased in the IFN-γKO/β2 heterozygous and IFN-γ heterozygous/β2KO mice compared to amounts in control mice heterozygous for both genes. The difference between the groups became evident only during the period (5 to 10 days after inoculation) when viral clearance is mediated by T cells (3, 7, 26, 34–36). There was no difference among the four groups in the amounts of virus in the feet and spinal ganglia 3 days after inoculation (not shown). Differences were first evident at days 7 and 8, and differences were greatest at day 10 (Table 1). Since mice that died were shown in other experiments to have high concentrations of virus in the feet and spinal ganglia (not shown), the results shown in Table 1 may underestimate the magnitude of the differences in viral burdens between the IFN-γKO/β2KO and IFN-γ heterozygous/β2KO mice and the other two groups. A similar trend of greater virus concentrations was seen in mice inoculated with 106 PFU (Table 1, experiment 2) and when IFN-γ wild-type β2KO mice were compared to IFN-γ wild-type β2 heterozygous mice (not shown).
TABLE 1.
Viral clearance in IFN-γ KO, β2KO, and IFN-γKO/β2KO mice
| Expt (inoculum)a | Genotypeb | Mean log10 PFU/gram of tissue ± SD (no. of mice with detectable virus/total no. of mice)c in:
|
No. of mice surviving to day 10/total no. of mice | |||
|---|---|---|---|---|---|---|
| Feet (day 8) | Spinal ganglia | Feet (day 10) | Spinal ganglia | |||
| 1 (5 × 105 PFU) | IFN-γKO/β2KO | 7.7 ± 0.0 (2/2) | 5.4 ± 0.2 (2/2) | 6.2 ± 1.4 (2/2) | 3.9 ± 0.5 (2/2) | 2/11 |
| IFN-γKO/β2het | 4.8 ± 2.3 (4/4) | 2.6 ± 1.9 (2/4) | 4.1 ± 2.1 (4/5) | 2.4 ± 1.3 (3/5) | 5/8 | |
| IFN-γhet/β2KO | 6.0 ± 0.3 (2/2) | 4.1 ± 0.5 (2/2) | 5.7 ± 0.7 (2/2) | 2.7 ± 2.4 (1/2) | 2/6 | |
| IFN-γhet/β2het | 3.3 ± 2.8 (1/3) | 2.0 ± 2.0 (1/4) | <1 (0/5)d | <1 (0/5)e | 6/7 | |
| 2 (106 PFU) | IFN-γKO/β2KO | 7.9 ± 0.0 (2/2) | 6.3 ± 0.2 (2/2) | 7.5 (1/1) | 4.8 (1/1) | 1/4 |
| IFN-γKO/β2het | 7.0 ± 1.0 (3/3) | 5.1 ± 0.6 (3/3) | 6.1 ± 0.9 (2/2) | 3.5 ± 0.0 (2/2) | 2/4 | |
| IFN-γhet/β2KO | 6.4 ± 0.8 (5/5) | 5.7 ± 1.0 (5/5) | 6.7 ± 0.1 (2/2) | 2.7 ± 0.4 (2/2) | 2/4 | |
| IFN-γhet/β2het | 6.7 ± 0.7 (4/4) | 3.2 ± 2.6 (2/4) | 4.9 ± 2.0 (2/2) | 2.4 ± 1.0 (1/2) | 2/4 | |
Amount of virus inoculated into each footpad.
IFN-γhet, IFN-γ heterozygous; β2het, β2 heterozygous.
The limit of detectability was 1 log10; mice with undetectable virus were assigned a value of 1 log10/gram of tissue for purposes of analysis.
P < 0.001 with IFN-γ heterozygous/β2 heterozygous mice versus IFN-γKO/β2KO mice and IFN-γ heterozygous/β2KO mice. P = 0.01 with IFN-γ heterozygous/β2 heterozygous mice versus IFN-γKO/β2 heterozygous mice.
P < 0.001 with IFN-γ heterozygous/β2 heterozygous mice versus IFN-γKO/β2KO mice. P = 0.04 with IFN-γ heterozygous/β2 heterozygous mice versus IFN-γKO/β2 heterozygous mice.
Lymphocyte proliferation, cytokine production, and antibody production are not impaired in β2KO mice.
As expected, the numbers of CD8+ T cells were profoundly reduced in the draining lymph nodes of β2KO mice. With this exception, the total numbers of lymphocytes, NK cells, CD4+ T cells, and CD4+ CD44hi memory or effector T cells in the draining lymph nodes of β2KO and control mice were similar at 3 days and increased by similar extents by 7 to 8 days after infection (data not shown). NK1.1+ T cells were rare even in the nodes of control mice. Proliferation and cytokine production in response to HSV antigen by cells from the draining lymph nodes were detected 8 and 10 (but not 3) days after infection (Table 2). Results with cells from the four groups were similar, with the exception that IFN-γ was not detected in culture supernatants from IFN-γKO mice. Lymphocyte proliferation responses in the β2KO groups at day 10 were greater in the experiment whose results are shown, but this was not observed in a second experiment and was not observed in either experiment at days 7 to 8 (Table 2 and data not shown). The high [3H]thymidine uptake in unstimulated cultures at day 8 likely reflects the presence of cells proliferating in situ prior to isolation and may have masked, at least in part, HSV antigen-specific proliferation as assessed in vitro. IL-10 production by cells from IFN-γKO mice was not increased (Table 2), and IL-4 was not detected in culture supernatants from any of the groups (data not shown). Thus, the inability to produce IFN-γ did not cause a shift in the response towards the production of Th-2 cytokines. Production of antibody to HSV glycoprotein B antigen was also similar, with the exception that the ratio of immunoglobulin G1 (IgG1) to IgG2a antibody was reduced in the IFN-γKO and IFN-γKO/β2KO mice compared to those of the other groups (Table 3), as reported previously for IFN-γKO mice (27, 44). These results suggest that the functions of CD4+ T cells and B cells in β2KO mice were intact.
TABLE 2.
Lymphocyte proliferation and cytokine productiona
| Assay | Genotypeb | Day 8 result with:
|
Day 10 result with:
|
||||
|---|---|---|---|---|---|---|---|
| Unstimulated | Mock antigen | HSV antigen | Unstimulated | Mock antigen | HSV antigen | ||
| Proliferationc | IFN-γKO/β2KO | 26,574 | 24,533 | 52,059 | 2,343 | 2,174 | 25,257 |
| IFN-γKO/β2het | 30,029 | 30,720 | 52,286 | 2,298 | 3,238 | 6,977 | |
| IFN-γhet/β2KO | 21,981 | 27,533 | 59,642 | 5,362 | 5,331 | 31,080 | |
| IFN-γhet/β2het | 10,769 | 13,222 | 56,057 | 4,450 | 4,233 | 7,027 | |
| IFN-γ productiond | IFN-γKO/β2KO | <50 | <50 | <50 | <50 | <50 | <50 |
| IFN-γKO/β2het | <50 | <50 | <50 | <50 | <50 | <50 | |
| IFN-γhet/β2KO | 800 | 761 | 5,801 | 140 | <50 | >30,000 | |
| IFN-γhet/β2het | 204 | 313 | 6,513 | <50 | <50 | >30,000 | |
| IL-10 productiond | IFN-γKO/β2KO | 216 | 317 | 1,353 | NDe | ND | ND |
| IFN-γKO/β2het | 143 | 189 | 2,672 | ND | ND | ND | |
| IFN-γhet/β2KO | 140 | 219 | 2,545 | ND | ND | ND | |
| IFN-γhet/β2het | <30 | <30 | 2,379 | ND | ND | ND | |
Results are from one experiment in which cells from two to four mice of the same genotype (with the exception of the day 10 sample from a IFN-γKO/β2KO mouse [i.e., cells from one mouse]) were pooled and then stimulated or not stimulated as indicated.
IFN-γhet, IFN-γ heterozygous; β2het, β2 heterozygous.
Counts per minute.
Picograms per milliliter of triplicate samples.
ND, not determined.
TABLE 3.
Anti-glycoprotein B antibody titers
| Genotypea | Log10 titer ± SD
|
|||
|---|---|---|---|---|
| Day 8
|
Day 10
|
|||
| IgG1 | IgG2a | IgG1 | IgG2a | |
| IFN-γKO/β2KO | 2.5 ± 0.3 | <1 | 3.1 ± 0.0 | 2.2 ± 0.0 |
| IFN-γKO/β2het | 2.4 ± 0.8 | 1.3 ± 0.4 | 3.1 ± 0.4 | 1.8 ± 0.5 |
| IFN-γhet/β2KO | 1.5 ± 0.6 | 1.6 ± 0.4 | 1.6 ± 0.0 | 2.4 ± 0.2 |
| IFN-γhet/β2het | 1.4 ± 0.6 | 2.1 ± 0.4 | 1.9 ± 0.9 | 2.2 ± 0.8 |
IFN-γhet, IFN-γ heterozygous; β2het, β2 heterozygous.
DISCUSSION
These results indicate that MHC class I-restricted T cells play an important role and that IFN-γ plays at most a limited role in protection of B6 mice from lethal neuroinvasive HSV infection. B6 mice, like immunocompetent humans, are relatively resistant to lethal primary HSV infection (7, 20, 35, 37). The predominant defect in β2KO mice is a marked reduction in MHC class I expression and numbers of CD8+ T cells (17, 33), which results in the inability to generate CD8+ CTLs in response to HSV (28). Although β2KO mice have reduced NK cell function in the absence of infection and reduced numbers of NK1.1+ (natural) T cells (2), it is unlikely that these differences accounted for their poor outcome. β2KO mice have a normal NK response to infection with murine cytomegalovirus (40), and the differences in disease and virus concentrations in tissues in this study first became evident by days 7 to 8, during the period when antigen-specific mechanisms act to clear the infection (3, 7, 26, 34–36). Also, few NK1.1+ T cells were present in the draining lymph nodes of HSV-infected control mice.
These results extend those of Goldsmith and colleagues (12) by demonstrating an important role for CD8+ T cells in the control of infection with wild-type HSV and not just with HSV mutants lacking ICP47. The current results differ somewhat from those of Manickan and Rouse (20), who concluded that β2KO mice on a B6 background were as resistant as controls to lethal HSV infection following flank inoculation, whereas mice lacking CD4+ T cells were highly susceptible. In their studies, mice were challenged either with a very high dose (108) of HSV, which was lethal for 100% of wild-type mice, or a very low dose (104), which was lethal only for the CD4+ T-cell-deficient mice. In the present study, in which intermediate doses of virus were inoculated into the footpads, the 50% lethal dose for β2KO mice was at least 2 log10 lower than that for controls. The varying conclusions reached in other studies regarding an independent role for MHC class I-restricted T cells in protection against wild-type HSV in the mouse may be related to differences in the methods of infection, methods by which the contributions of different T-cell subsets were assessed, and strains of mice (12, 23, 24, 26, 34, 35, 37). Nonetheless, more profound defects in the control of primary HSV have been observed in CD4+ KO mice than in β2KO mice (20) and in mice depleted of CD4+ T cells than in mice depleted of CD8+ T cells by treatment with monoclonal antibodies (23, 24). This may reflect a role for CD4+ T cells in multiple aspects of antiviral defense, including a requirement for these cells in the generation and survival of effector CD8+ CTL and in the upregulation MHC class I expression on infected cells in the tissues (21, 24, 37, 38).
The mechanisms by which CD4+ or CD8+ T cells limit viral replication in the tissues and peripheral nervous system and spread to the central nervous system are not fully defined and are likely to be multiple (12, 20, 36). IFN-γ appears to contribute to the clearance of HSV outside of the nervous system in mice (4, 24, 37, 44). However, conclusions regarding the role of IFN-γ in protection from lethal nervous system infection with HSV have been highly varied (4, 5, 24, 37, 44). The present study indicates that IFN-γ contributes to viral clearance from the peripheral tissues and nervous system but that it plays a limited role compared to that of CD8+ T cells. These results are similar to those of Cantin et al. (5), who found a small but reproducible difference in levels of viral clearance and survival in mice in which the action of IFN-γ was blocked. The contribution of IFN-γ appeared to be at least partially independent of the contribution of MHC class I, suggesting that MHC class I-restricted T cells are not the major source of IFN-γ in this infection and that IFN-γ is not the major mechanism by which these cells contribute to protection. These conclusions also suggest that the role of IFN-γ in HSV infection is not limited to upregulation of MHC class I but that it may include induction of MHC class II, protection of neurons from apoptosis, and inhibition of HSV replication (5, 9, 10, 13, 18). Though TNF may contribute to noncytolytic inhibition of HSV replication and upregulation of MHC class I expression in the nervous system (9, 11), the outcome of HSV infection in type I TNF receptor KO mice on a B6 background (30) was similar to that in controls in preliminary experiments (unpublished observations). TNF and IFN-γ may play partially redundant roles in the control of HSV, but the greater severity of HSV disease in β2KO mice than in IFN-γKO or type I TNF receptor KO mice suggests that T-cell-mediated protection from HSV is not mediated solely by these cytokines. The present results do not exclude a role for these cytokines in CD8+ T-cell-mediated control of HSV infection but suggest that other mechanisms are more important. There is considerable evidence that other cells, including γδ T cells, NK cells, CD4+ T cells, and neurons themselves may produce IFN-γ and TNF in response to HSV in the nervous system, so this function of CD8+ T cells is likely to be redundant.
In summary, this study indicates that MHC class I-restricted T cells play an important role and that IFN-γ plays a limited role in protection of mice from lethal nervous system infection due to wild-type HSV. The importance of MHC class I expression is consistent with the presence of two viral genes that inhibit MHC class I-mediated antigen presentation and with the reduced neurovirulence of strains lacking ICP47 (12) and vhs (39), though in the latter case it is not certain that the lower neurovirulence results from evasion of host defenses. IFN-γ can override the effects of ICP47 on MHC class I expression (22, 41), suggesting that IFN-γ may play a more critical role in the control of HSV in humans than in mice. Nonetheless, since the effects of ICP47 are greater in human than in murine cells, the present findings strongly suggest that inhibition of MHC class I-mediated antigen presentation is an important strategy by which HSV evades the immune response.
ACKNOWLEDGMENTS
This work was supported in part by grants HD18184 (C.B.W.), T32 GM07270 (K.E.), AI34616 (D.M.K.), AI30731, and AI42528 (L.C.) from the National Institutes of Health.
We thank Phil Greenberg for helpful discussions and Heidi Jessup, Matthew L. Johnson, and Annete Peck for technical assistance.
REFERENCES
- 1.Ahn K, Meyer T H, Uebel S, Semp’e P, Djaballah H, Yang Y, Peterson P A, Fruh K, Tamp’e R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- 2.Bendelac A, Rivera M N, Park S H, Roark J H. Mouse CD1-specific NK1 T cells: development, specificity and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 3.Bonneau R H, Salvucci L A, Johnson D C, Tevethia S S. Epitope specificity of H-2Kb-restricted, HSV-1-, and HSV-2-cross-reactive cytotoxic T lymphocyte clones. Virology. 1993;195:62–70. doi: 10.1006/viro.1993.1346. [DOI] [PubMed] [Google Scholar]
- 4.Bouley D M, Kanangat S, Wire W, Rouse B T. Characterization of herpes simplex virus type-1 infection and herpetic stromal keratitis development in IFN-gamma knockout mice. J Immunol. 1995;155:3964–3971. [PubMed] [Google Scholar]
- 5.Cantin E M, Hinton D R, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook M L, Stevens J G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973;7:272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook M L, Stevens J G. Restricted replication of herpes simplex virus in spinal ganglia of resistant mice is accompanied by an early infiltration of immunoglobulin G-bearing cells. Infect Immun. 1983;40:752–758. doi: 10.1128/iai.40.2.752-758.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cose S C, Kelly J M, Carbone F R. Characterization of a diverse primary herpes simplex virus type 1 gB-specific cytotoxic T-cell response showing a preferential Vβ bias. J Virol. 1995;69:5849–5852. doi: 10.1128/jvi.69.9.5849-5852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feduchi E, Alonso M A, Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989;63:1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geiger K D, Gurushanthaiah D, Howes E L, Lewandowski G A, Reed J C, Bloom F E, Sarvetnick N E. Cytokine-mediated survival from lethal herpes simplex virus infection: role of programmed neuronal death. Proc Natl Acad Sci USA. 1995;92:3411–3415. doi: 10.1073/pnas.92.8.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gold R, Toyka K V, Hartung H P. Synergistic effect of IFN-gamma and TNF-alpha on expression of immune molecules and antigen presentation by Schwann cells. Cell Immunol. 1995;165:65–70. doi: 10.1006/cimm.1995.1187. [DOI] [PubMed] [Google Scholar]
- 12.Goldsmith K, Chen W, Johnson D C, Hendricks R L. Infected cell protein (ICP)47 enhances herpes simplex virus neurovirulence by blocking the CD8+ T cell response. J Exp Med. 1998;187:341–348. doi: 10.1084/jem.187.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halford W P, Gebhardt B M, Carr D J J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 14.Hill A, Jugovic P, York I, Russ G, Bennink J, Yewdell J, Ploegh H, Johnson D. Herpes simplex virus turns off the TAP to evade host immunity. Nature. 1995;375:411–415. doi: 10.1038/375411a0. [DOI] [PubMed] [Google Scholar]
- 15.Kay M A, Holterman A X, Meuse L, Gown A, Ochs H D, Linsley P S, Wilson C B. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat Genet. 1995;11:191–197. doi: 10.1038/ng1095-191. [DOI] [PubMed] [Google Scholar]
- 16.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koller B H, Marrack P, Kappler J W, Smithies O. Normal development of mice deficient in Beta2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 18.Lewandowski G A, Lo D, Bloom F E. Interference with major histocompatibility complex class II-restricted antigen presentation in the brain by herpes simplex virus type 1: a possible mechanism of evasion of the immune response. Proc Natl Acad Sci USA. 1993;90:2005–2009. doi: 10.1073/pnas.90.5.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Tang Q, Hendricks R L. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manickan E, Rouse B T. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse models. J Virol. 1995;69:8178–8179. doi: 10.1128/jvi.69.12.8178-8179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mercadal C M, Martin S, Rouse B T. Apparent requirement for CD4+ T cells in primary anti-herpes simplex virus cytotoxic T-lymphocyte induction can be overcome by optimal antigen presentation. Viral Immunol. 1991;4:177–186. doi: 10.1089/vim.1991.4.177. [DOI] [PubMed] [Google Scholar]
- 22.Mikloska Z, Kesson A M, Penfold M E T, Cunningham A L. Herpes simplex virus protein targets for CD4 and CD8 lymphocyte cytotoxicity in cultured epidermal keratinocytes treated with interferon-gamma. J Infect Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 23.Milligan G N, Bernstein D I. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following infection with herpes simplex virus type 2. Virology. 1995;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 24.Milligan G N, Bernstein D I. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 25.Milligan G N, Bernstein D I, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- 26.Nash A A, Jayasuriya A, Phelan J, Cobbold S P, Waldmann H, Prospero T. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J Gen Virol. 1987;68:825–833. doi: 10.1099/0022-1317-68-3-825. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen L, Knipe D M, Finberg R W. Mechanism of virus-induced Ig subclass shifts. J Immunol. 1994;152:478–484. [PubMed] [Google Scholar]
- 28.Niemialtowski M G, Godfrey V L, Rouse B T. Quantitative studies on CD4+ and CD8+ cytotoxic T lymphocyte responses against herpes simplex virus type 1 in normal and beta 2-m deficient mice. Immunobiology. 1994;190:183–194. doi: 10.1016/s0171-2985(11)80268-8. [DOI] [PubMed] [Google Scholar]
- 29.Paul W E, Seder R A. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 30.Peschon J, Dauphine D, Stocking K, Glaccum M, Otten C, Willis C, Charrier K, Morissey P, Ware C, Mohler K. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 31.Posavad C M, Koelle D M, Corey L. Tipping the scales of herpes simplex virus reactivation: the important responses are local. Nat Med. 1998;4:381–382. doi: 10.1038/nm0498-381. [DOI] [PubMed] [Google Scholar]
- 32.Posavad C M, Koelle D M, Shaughnessy M F, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci USA. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raulet D H. MHC class I-deficient mice. Adv Immunol. 1994;55:381–421. doi: 10.1016/s0065-2776(08)60514-3. [DOI] [PubMed] [Google Scholar]
- 34.Schmid D S, Rouse B T. The role of T cell immunity in control of herpes simplex virus. Curr Top Microbiol Immunol. 1992;179:57–74. doi: 10.1007/978-3-642-77247-4_4. [DOI] [PubMed] [Google Scholar]
- 35.Simmons A, Tscharke D, Speck P. The role of immune mechanisms in control of herpes simplex virus infection of the peripheral nervous system. Curr Top Microbiol Immunol. 1992;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- 36.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith P M, Wolcott R M, Chervenak R, Jennings S R. Control of acute cutaneous herpes simplex virus infection: T cell-mediated viral clearance is dependent upon interferon-gamma (IFN-gamma) Virology. 1994;202:76–88. doi: 10.1006/viro.1994.1324. [DOI] [PubMed] [Google Scholar]
- 38.Stohlman S A, Bergmann C C, Lin M T, Cua D J, Hinton D R. CTL effector function within the central nervous system requires CD4+ T cells. J Immunol. 1998;160:2896–2904. [PubMed] [Google Scholar]
- 39.Strelow L I, Leib D A. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J Virol. 1995;69:6779–6786. doi: 10.1128/jvi.69.11.6779-6786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tay C H, Welsh R M, Brutkiewicz R R. NK cell response to viral infections in beta 2-microglobulin-deficient mice. J Immunol. 1995;154:780–789. [PubMed] [Google Scholar]
- 41.Tigges M A, Leng S, Johnson D C, Burke R L. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J Immunol. 1996;156:3901–3910. [PubMed] [Google Scholar]
- 42.Tomazin R, van Schoot N E, Goldsmith K, Jugovic P, Semp’e P, Fruh K, Johnson D C. Herpes simplex virus type 2 ICP47 inhibits human TAP but not mouse TAP. J Virol. 1998;72:2560–2563. doi: 10.1128/jvi.72.3.2560-2563.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.York I A, Roop C, Andrews D W, Riddell S R, Graham F L, Johnson D C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 44.Yu Z, Manickan E, Rouse B T. Role of interferon-gamma in immunity to herpes simplex virus. J Leukoc Biol. 1996;60:528–532. doi: 10.1002/jlb.60.4.528. [DOI] [PubMed] [Google Scholar]