Abstract
Introduction
Patients with Parkinson’s disease (PD) exhibit alterations in eye movement control, primarily diverse oculomotor deficits which include hypometric saccade and impaired smooth pursuit with reduced pursuit-gain necessitating catch-up saccades. The effects of dopaminergic treatment of PD on eye movements are controversial. Previous studies suggest that smooth pursuit eye movements (SPEMs) are not directly influenced by the dopaminergic system. The nondopaminergic drug istradefylline, a selective adenosine A2A receptor antagonist, reduces the OFF time and improves somatomotor function in levodopa-treated PD. Here, we investigated whether istradefylline improves SPEMs in PD, and determined whether oculomotor performance is associated with somatomotor performance.
Methods
Using an infrared video eye tracking system, we quantified horizontal SPEMs in six patients with PD before and 4–8 weeks after initiation of istradefylline administration. A further five patients with PD were tested before and after a 4-week interval without istradefylline to control for practice effects. We evaluated smooth pursuit gain (eye velocity/target velocity), accuracy of smooth pursuit velocity, and saccade rate during pursuit before and after istradefylline administration during the ON state.
Results
Patients received istradefylline by single daily oral administration at 20 to 40 mg. Eye tracking data were obtained 4–8 weeks after initiation of istradefylline administration. Istradefylline increased smooth pursuit gain and the accuracy of smooth pursuit velocity, and tended to decrease saccade rates during pursuit.
Conclusions
Istradefylline ameliorated the oculomotor deficit in SPEM of patients with PD, although differences in somatomotor performance before and after istradefylline treatment were not significant during ON periods. The discrepancy observed between the oculomotor and somatomotor responses to istradefylline supports previous findings that SPEM is at least partially under nondopaminergic control.
Keywords: Eye tracking, Istradefylline, Parkinson’s disease, Smooth pursuit eye movement
Key Summary Points
| Treatment for Parkinson’s disease (PD)-associated oculomotor dysfunction has not been adequately studied, and a potential unmet clinical need remains. |
| Previous studies suggest that smooth pursuit eye movements (SPEMs) are not directly influenced by the dopaminergic system. |
| Our study investigated the effects of the nondopaminergic drug istradefylline on SPEM in patients with PD. |
| Istradefylline ameliorated SPEM in PD during the ON state, although differences in somatomotor performance before and after istradefylline treatment were not significant during the ON state. |
| The discrepancy observed between the oculomotor and somatomotor responses to istradefylline supports previous findings that SPEM is at least partially under nondopaminergic control. |
Introduction
Patients with Parkinson’s disease (PD) characteristically experience alterations in ocular movement. These oculomotor dysfunctions are diverse and include hypometric saccade, convergence insufficiency, and impaired smooth pursuit [1–4]. In particular, smooth pursuit eye movements (SPEMs) are generally impaired: when tracking a moving object on a stationary background, the gain of pursuit (i.e., eye velocity/target velocity) is decreased, requiring catch-up saccades and resulting in saccadic pursuit [1, 3, 5]. These aberrant movements are apparent during careful physical examination in routine clinical settings. To date, however, treatment for PD-associated oculomotor dysfunction has not been adequately studied, and a potential unmet clinical need remains.
The effect of dopaminergic treatment on SPEMs is controversial. Some studies show no improvement with levodopa [1, 2], while others suggest a beneficial effect [3, 6]. Despite its clear impact on somatomotor functions, dopaminergic treatment has minimal effects on SPEM [1, 7]. Previous studies suggest that SPEMs are not under the direct control of the dopaminergic system because of the lack of difference in smooth pursuit performance during the ON and OFF phases [8], indicating a cortical nondopaminergic pathomechanism for impaired SPEM in PD [7].
The adenosine A2A receptor antagonist istradefylline is a nondopaminergic drug which does not directly engage dopamine receptors or dopamine metabolism. Istradefylline reduces OFF time when used adjunctively with levodopa in PD [9]. To date, however, the effects of istradefylline on oculomotor movement have not been reported.
Here, we analyzed the effects of istradefylline on SPEM in patients with PD and determined whether oculomotor performance was associated with somatomotor performance.
Methods
Compliance with Ethics guidelines
This study was approved by the Ethics Committee of Fujita Neurological Hospital (approval H2801). This study was conducted in accordance with the Declaration of Helsinki and its later amendments. Informed consent was obtained from all participants in the study.
Subjects and Clinical Evaluation
We performed an open-label, non-randomized study. Subjects were recruited between July 2016 and January 2020 from among patients with a primary diagnosis of PD under outpatient treatment at Fujita Neurological Hospital. Inclusion criteria were (1) diagnosis of PD according to the UK Parkinson’s Disease Society Brain Bank Criteria; (2) having a daily OFF time; and (3) Hoehn and Yahr stages III–IV; istradefylline is covered by insurance in PD with wearing-off in Japan; moreover, PD with Hoehn and Yahr stage III or above can receive medical payment assistance from the government. Accordingly, more severe cases utilizing relatively expensive medication were more likely to be included in this study, resulting in the exclusion of individuals in Hoehn and Yahr stages I and II. Exclusion criteria were (1) visual impairment (best corrected visual acuity < 0.3); (2) severe dyskinesia; and (3) dementia (Hasegawa dementia scale-revised score < 21). We used an eye tracking system to investigate horizontal SPEMs in six patients with PD administered dopaminergic drugs before and 4–8 weeks after the initiation of istradefylline administration. To control for practice effects, a further five control patients with PD who met the inclusion criteria but were not treated with istradefylline were tested before and after a 4-week interval. All 11 patients experienced wearing-off motor fluctuation. Mean age in the istradefylline and control groups was 72.8 ± 7.4 years (mean ± SD) and 66.6 ± 9.3 years, and duration of illness was 6.0 ± 3.0 years and 5.6 ± 1.9 years, respectively. Patients’ Movement Disorder Society‐Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) III somatomotor scores (permission obtained to use the Japanese version of the MDS-UPDRS in paper format. http://www.movementdisorders.org/publications/rating_scales/) were evaluated during the ON state concurrently with SPEM assessment.
The positions of the right eyes of 11 patients were recorded using the ViewPoint EyeTracker (Arrington Research, Inc., http://www.ArringtonResearch.com) (sampling rate 60 Hz) to investigate horizontal SPEM. Visual stimuli were observed using a 31.5″ rear-illuminated liquid crystal monitor (Iiyama Co., ProLite X3291HS) positioned 45 cm in front of the subject’s eyes. Subjects sat with their head restrained by a chin and forehead rest in a semidarkened room. SPEM was elicited by simple ramp-pursuit (constant-velocity target). A 1-cm2 gray visual tracking target on a white background moved horizontally with a lateral constant velocity and a range of ± 15° of the visual field at 10°/s, 15°/s, or 30°/s. The target moved in a predictable direction. SPEM was tested five times at each velocity in each subject. Subjects were instructed to fixate on the target when it appeared and to follow the tracking target using only eye movements without turning their head.
We evaluated smooth pursuit gain (eye velocity/target velocity), accuracy of smooth pursuit velocity, and saccade rate (number of saccades per second) during pursuit before and after istradefylline administration in patients with PD during the ON state. Smooth pursuit gain was calculated by removing periods when the measured eye velocities were judged as saccades, blinks, or artifacts. This was achieved by excluding data when eye velocity exceeded 50°/s. Average eye velocities, except initial pursuit, were calculated for the remaining periods, and gain value was calculated by dividing the average eye velocity by the target velocity. The accuracy of smooth pursuit velocity was obtained by calculating the percentage of time during the entire movement range when eye movement velocity was within the target velocity boundaries with less than 20% absolute error from the target velocity. The eye movement data were evaluated by assessors blinded to the patients’ clinical data.
Statistical Analyses
The Wilcoxon signed-rank nonparametric test was used to compare MDS‐UPDRS III scores, smooth pursuit gain, accuracy, and saccade rate between pre- and post-treatment conditions. The non-parametric Mann–Whitney U test was used to compare smooth pursuit gain, accuracy, and saccade rate between the control group (before the 4-week interval) and the istradefylline group (pre-treatment). Statistical analyses were performed using R version 3.0.2 software (R Development Core Team). P values less than 0.05 were considered statistically significant.
Results
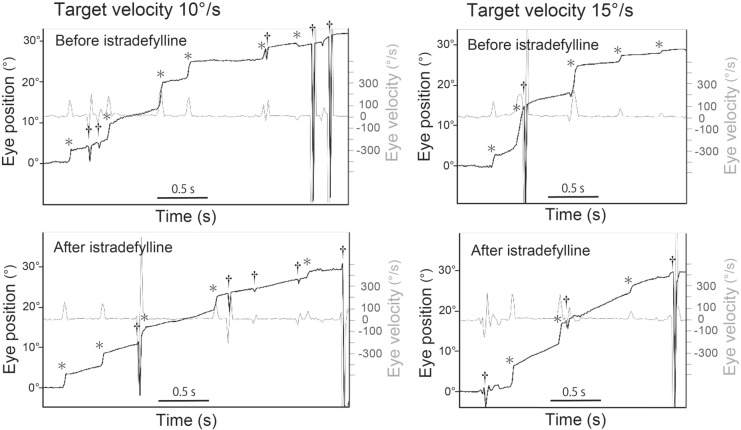
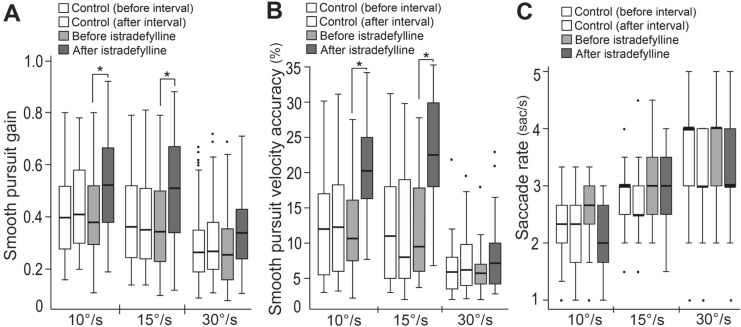
Baseline MDS-UPDRS III scores in the ON state were 58.0 ± 15.4 and 53.2 ± 14.2 in the istradefylline and control groups, respectively. Changes from baseline in scores during the ON state did not significantly differ between groups (− 1.3 ± 2.9 [mean ± SD] and 0.4 ± 1.7 points, respectively). The mean baseline MDS-UPDRS Part III scores in the OFF state were 64.8 ± 16.3 and 60.7 ± 9.8 in the istradefylline and control groups, respectively (there were missing values for one of six patients in the istradefylline group and two out of five patients in the control group). Four patients received a single daily oral dose of istradefylline at 20 mg for 4 weeks, while two patients were treated by subsequent administration of istradefylline at 40 mg for a further 4 weeks. The eye tracking data were obtained 4–8 weeks after the initiation of istradefylline administration (Fig. 1). Patients receiving istradefylline showed a significant increase in smooth pursuit gains compared with pre-administration at 10°/s and 15°/s (Fig. 2a). The accuracy of smooth pursuit velocity significantly increased with istradefylline compared with that before administration at 10°/s and 15°/s (Fig. 2b). Saccade rate during pursuit tended to decrease with istradefylline, although without statistical significance (Fig. 2c). In the five patients with PD not administered istradefylline, there were no significant changes in smooth pursuit gain, accuracy of velocity, or saccade rate before and after the 4-week interval. The parameters between groups in the control (before the 4-week interval) and istradefylline groups (pre-treatment) at baseline showed no significant changes in smooth pursuit gain, accuracy of velocity, or saccade rate.
Fig. 1.
Examples of eye position (black trace) and velocity (gray trace) traces during simple ramp-pursuit from a representative patient 10°/s and 15°/s before and after istradefylline administration. Note the increase in slope of the eye position trace excluding saccades, blinks, or artifacts after istradefylline treatment compared with before administration. *saccade. †blink or artifact
Fig. 2.
a Smooth pursuit gain for three target velocities. Istradefylline significantly increased smooth pursuit gains compared with before administration at 10°/s and 15°/s. b Smooth pursuit velocity accuracy for three target velocities. Istradefylline significantly increased smooth pursuit velocity accuracy compared with before administration at 10°/s and 15°/s. c Saccade rate during pursuit for three target velocities. The boxes represent interquartile ranges, and the horizontal line in each box represents the median. The whiskers show the minimum and maximum values or values up to 1.5 times the interquartile range below or above the first or third quartile if outliers are present (dots). *P < 0.05 versus before istradefylline administration
Discussion
Here, we show that istradefylline improved SPEM in patients with PD, while there was no significant improvement in somatomotor function during the ON state after istradefylline treatment. Decreased gain (eye velocity/target velocity) of smooth pursuit is a well-known characteristic of PD compared with age-matched controls without neurologic disorders [1–3], while normal aging results in low pursuit gain. While previous studies reported that dopaminergic agents such as levodopa, bromocriptine, and apomorphine improve SPEM in some patients with PD [3, 6], other studies reported conflicting findings, showing no improvement in SPEM following levodopa treatment in these patients [1, 2]. Furthermore, despite marked fluctuation in somatomotor symptoms between ON and OFF periods, there is no difference in smooth pursuit performance between these periods [8]. These findings [1, 2, 8] indicate differences in the mechanism of control between somatomotor and oculomotor movement, in turn suggesting that smooth eye movement does not primarily involve dopaminergic pathways.
In patients with PD, istradefylline has been suggested to counteract excessive striatopallidal activity through an indirect pathway by modulating GABAergic transmission in the striatum and external globus pallidus, which may lead to improvements in somatomotor function [10]. A meta-analysis of randomized controlled trials found that, relative to placebo, istradefylline at 20 mg/day and 40 mg/day as an adjunct to levodopa significantly decreases OFF time and increases ON time without troublesome dyskinesia. Further, the somatomotor score (UPDRS III) in the ON state demonstrates a trend toward improvement [9]. However, our present study is the first to investigate the effects of istradefylline on oculomotor movements.
Our present results show that istradefylline significantly improved smooth pursuit gain and pursuit velocity accuracies, and tended to reduce saccade rate during pursuit compared with those before administration. Regarding the mechanism of istradefylline’s effect on SPEM, our results are similar to findings that subthalamic nucleus deep brain stimulation (STN-DBS) alone following withdrawal of anti-PD medication improves smooth pursuit gain and accuracy in PD [11]. Likewise, a study of STN-DBS administered to patients who remained on anti-PD medication showed a trend towards improved smooth pursuit gain in DBS ON, although no significant differences in gain were found between DBS ON and OFF [7]. Although the therapeutic mechanism of DBS is debated, STN-DBS can achieve optimal regularization of subthalamic output, which is classically regarded as a component of the indirect pathway [12]. Moreover, STN-DBS activates nigrostriatal, pallidothalamic, cerebellothalamic, and pallidonigral tracts, which may contribute to its therapeutic effects [12]. We therefore assume that the mechanism of istradefylline in SPEM identified here may be at least partially analogous to the therapeutic mechanism of STN-DBS.
Attention contributes to smooth pursuit performance. For example, in a dual-task situation, the addition of attention-demanding secondary tasks during pursuit leads to decreased smooth pursuit gain in healthy subjects [13]. Cognitive assessment of a primate model treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine suggested that administration of istradefylline alleviated L-DOPA-induced deficits in attention and short-term memory [14]. These findings raise the possibility that istradefylline’s effect in alleviating cognitive function, including attention, may contribute to the improvement of SPEM observed here.
Our present results also showed that istradefylline was not associated with further improvement in MDS-UPDRS III somatomotor scores during the ON state, consistent with large-scale randomized controlled trials [9]. Accordingly, we found that istradefylline improved smooth pursuit oculomotor performance despite the lack of significant improvement in somatomotor performance during ON. Conversely, levodopa treatment showed no improvement in oculomotor deficits in smooth pursuit regardless of marked improvement in somatomotor performance [1, 2]. Together, these findings support the conclusion that SPEMs are not directly controlled by the dopaminergic system and that oculomotor control mechanisms may differ, at least in part, from those of the somatomotor system.
Improvements in SPEM may have clinical implications. SPEMs are essential to achieving clear vision of a moving object within our visual environment. An excessive increase in saccadic pursuit accompanied by decreased smooth pursuit velocity in PD may further increase saccadic suppression—defined as a temporal reduction of visibility around the time of the onset of saccade [15]—thereby potentially limiting visual cognitive performance. Thus, amelioration of SPEM may have a practical impact on the ability to perform tasks that depend on visual processing such as postural control, navigating obstacles, or operating a motor vehicle. This possibility warrants further investigation.
There are some limitations to the present study. First, the sample size was small, which limited the statistical power of the findings. We collected cases that met the inclusion criteria and had a clear ON and OFF status, which limited our study to the small number of cases that were collected at our institution. Second, we did not take account of potential oculomotor and cognitive fluctuations, or other changes in general condition which might have impacted oculomotor performance over time. Third, we did not account for the possibility of impaired attention among patients with PD. Allowing for these limitations, this study is the first to investigate the effect of istradefylline on oculomotor movement.
Conclusions
Although preliminary, these results indicate that istradefylline improved SPEM during the ON state. Moreover, the discrepancy observed between the oculomotor and somatomotor responses to istradefylline supports previous findings that SPEM is at least partially under nondopaminergic control.
Acknowledgements
The authors thank Yuzo Otani and Hiroshi Hashimoto of NAMOTO Company Limited for their technical assistance with the eye tracking device. We would also like to express our cordial gratitude to Dr. Kiyotaka Nakamagoe for helpful discussions on this work. We thank all the study participants for their participation and contribution.
Funding
No funding or sponsorship was received for this study or publication of this article. The journal’s fee was funded by the authors.
Medical Writing and/or Editorial Assistance
We received assistance from Drs Steve Tronick and Guy Harris of Dmed (Tokyo, Japan) for critical reading and editing of the manuscript. The authors provided the funding for this assistance.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Study concept and design: Youshi Fujita; Data collection and clinical assessment of subjects: Emi Kawaguchi, Takashi Toyomoto, Hirotaka Shirasaki, Youshi Fujita; Statistical analysis: Youshi Fujita; Drafting of the manuscript: Youshi Fujita.
Prior Presentation
This manuscript builds upon research previously shared at the World Congress of Neurology on September 17th, 2017 (Kyoto, Japan).
Disclosures
Youshi Fujita, Emi Kawaguchi, Takashi Toyomoto, and Hirotaka Shirasaki declare that they have no competing interests.
Compliance with Ethics Guidelines
This study was approved by the Ethics Committee of Fujita Neurological Hospital (approval H2801). This study was conducted in accordance with the Declaration of Helsinki and its later amendments. Informed consent was obtained from all participants in the study.
Data Availability
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
References
- 1.Rascol O, Clanet M, Montastruc JL, et al. Abnormal ocular movements in Parkinson's disease. Evidence for involvement of dopaminergic systems. Brain. 1989;112:1193–1214. doi: 10.1093/brain/112.5.1193. [DOI] [PubMed] [Google Scholar]
- 2.Waterston JA, Barnes GR, Grealy MA, Collins S. Abnormalities of smooth eye and head movement control in Parkinson's disease. Ann Neurol. 1996;39:749–760. doi: 10.1002/ana.410390611. [DOI] [PubMed] [Google Scholar]
- 3.Gibson JM, Pimlott R, Kennard C. Ocular motor and manual tracking in Parkinson's disease and the effect of treatment. J Neurol Neurosurg Psychiatry. 1987;50:853–860. doi: 10.1136/jnnp.50.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukushima K, Ito N, Barnes GR, et al. Impaired smooth-pursuit in Parkinson's disease: normal cue-information memory, but dysfunction of extra-retinal mechanisms for pursuit preparation and execution. Physiol Rep. 2015;3:e12361. 10.14814/phy2.12361. [DOI] [PMC free article] [PubMed]
- 5.Shibasaki H, Tsuji S, Kuroiwa Y. Oculomotor abnormalities in Parkinson's disease. Arch Neurol. 1979;36:360–364. doi: 10.1001/archneur.1979.00500420070009. [DOI] [PubMed] [Google Scholar]
- 6.Bares M, Brázdil M, Kanovský P, et al. The effect of apomorphine administration on smooth pursuit ocular movements in early Parkinsonian patients. Parkinsonism Relat Disord. 2003;9:139–144. doi: 10.1016/S1353-8020(02)00015-9. [DOI] [PubMed] [Google Scholar]
- 7.Pinkhardt EH, Jürgens R, Lulé D, et al. Eye movement impairments in Parkinson's disease: possible role of extradopaminergic mechanisms. BMC Neurol. 2012;29:5. doi: 10.1186/1471-2377-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpe JA, Fletcher WA, Lang AE, Zackon DH. Smooth pursuit during dose-related on-off fluctuations in Parkinson's disease. Neurology. 1987;37:1389–1392. doi: 10.1212/WNL.37.8.1389. [DOI] [PubMed] [Google Scholar]
- 9.Hauser RA, Hattori N, Fernandez H, et al. Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson's disease: a pooled analysis of 8 phase 2b/3 trials. J Parkinsons Dis. 2021;11:1663–1675. doi: 10.3233/JPD-212672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori A. How do adenosine A2A receptors regulate motor function? Parkinsonism Relat Disord. 2020;80:S13–S20. doi: 10.1016/j.parkreldis.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 11.Nilsson MH, Patel M, Rehncrona S, Magnusson M, Fransson PA. Subthalamic deep brain stimulation improves smooth pursuit and saccade performance in patients with Parkinson's disease. J Neuroeng Rehabil. 2013;3:33. doi: 10.1186/1743-0003-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163–171. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- 13.Hutton SB, Tegally D. The effects of dividing attention on smooth pursuit eye tracking. Exp Brain Res. 2005;163:306–313. doi: 10.1007/s00221-004-2171-z. [DOI] [PubMed] [Google Scholar]
- 14.Ko WKD, Camus SM, Li Q, et al. An evaluation of istradefylline treatment on Parkinsonian motor and cognitive deficits in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated macaque models. Neuropharmacology. 2016;110:48–58. doi: 10.1016/j.neuropharm.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Binda P, Morrone MC. Vision during saccadic eye movements. Annu Rev Vis Sci. 2018;15:193–213. doi: 10.1146/annurevvision-091517-034317. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of the present study are available from the corresponding author upon reasonable request.