Abstract
Introduction
Lead toxicity has been a major public health problem worldwide, yet no study has investigated the association between lead exposure and chronic pain.
Methods
We used data from three cycles of National Health and Nutrition Examination Survey (NHANES) with chronic pain status. We conducted univariate and multivariate logistic regression analyses to investigate the association between chronic pain and blood lead level (BLL). Subgroup analyses were performed to explore which confounding factor modified the association between chronic pain and BLL.
Results
A total of 13,485 participants were included in our final analysis, out of which 1950 (14.46%) had chronic pain. In the fully adjusted model, a 1 μg/dL increase of BLL was associated with 3% higher risk of chronic pain. The highest BLL quartile (BLL > 2.40 μg/dL) was associated with a 32% increase in the risk of chronic pain compared with the lowest BLL quartile (BLL < 0.90 μg/dL). In the subgroup analyses, hypertension (P for interaction = 0.018) and arthritis (P for interaction = 0.004) status modified the association between BLL and chronic pain. Higher quartiles of BLL were associated with a higher risk of chronic pain only in individuals with hypertension or arthritis but not those without these conditions.
Conclusion
A higher BLL was associated with a higher risk of chronic pain. Further research is warranted to investigate whether a causal relationship exists between the two, as well as potential underlying mechanisms.
Keywords: Chronic pain, Lead exposure, Blood lead level, Heavy metal
Key Summary Points
| Chronic pain has been a global health priority. |
| Lead is an environmental toxin that deleteriously affects multiple systems of human bodies. |
| The relationship between chronic pain and lead exposure has not been studied. |
| In the fully adjusted model, a 1 μg/dL increase of blood lead level (BLL) was associated with 3% higher risk of chronic pain. The highest BLL quartile (BLL > 2.40 μg/dL) was associated with a 32% increase in the risk of chronic pain compared with the lowest BLL quartile (BLL < 0.90 μg/dL). In the subgroup analyses, higher quartiles of BLL were associated with a higher risk of chronic pain only in individuals with hypertension or arthritis but not those without these conditions. |
| A higher BLL was associated with a higher risk of chronic pain. |
Introduction
Chronic pain, defined as pain that persists or recurs for longer than 3 months, has been a global health priority and is affecting an estimated 27.5% of people worldwide, though the prevalence of chronic pain ranges between 9.9% and 50.3% across countries [1]. Patients with chronic pain often suffer from restricted activities, physical or functional disability, anxiety, depression, and opioid addiction, which seriously affects people’s quality of life [2, 3]. Treatment of chronic pain includes medication therapy, surgical therapy, physical therapy, and lifestyle therapy [4]. However, chronic pain management remains a difficult problem. Considering its enormous global burden, scholars have argued for the recognition of chronic pain as a disease [4, 5].
Studies have found that many factors, such as genetics, socioeconomic status, lifestyles (smoking, alcohol intake, daily activity, and nutrition status) and occupational characteristics contribute to the development of chronic pain [6–8]. Studying the risk factors for chronic pain is necessary to further develop preventive and management strategies.
Lead is a known environmental toxin that deleteriously affects the nervous, cardiovascular, hematopoietic, skeletal, respiratory, and immune systems [9]. Although effort has been made to remove lead from paint, gasoline, drinking water, etc., lead exposure persists in our daily life [10]. In addition, lead toxicity has been a major public health problem worldwide [11]. Common sources of lead exposure are lead in drinking water, food, tobacco smoke, dust, soil, air, etc. [12]. Blood lead level (BLL) is the gold standard test for lead exposure [13]. Higher BLL was found to be associated with lower cognitive function [14], lower kidney function [15], higher blood pressure [16] and higher risk of cardiovascular disease, osteoarthritis [17], and cancer [18]. Although the US Centers for Disease Control and Prevention (CDC) recently updated the BLL reference value from 5 μg/dL to 3.5 μg/dL [19], there is evidence that lead exerts its detrimental effects even at lower levels, and there is no known safe BLL [20–23].
To the best of our knowledge, the relationship between chronic pain and lead exposure has not been studied. BLL measured in previous National Health and Nutrition Examination Survey (NHANES) programs has been the cornerstone of lead exposure surveillance in the USA. Accordingly, we sought to investigate the relationship between BLL and chronic pain using the 1999–2004 NHANES dataset.
Methods
Data Source and Study Population
Data from the NHANES were used for this study. The NHANES is a cross-sectional survey conducted by the National Center for Health Statistics (NCHS) to assess the health and nutritional status of the US population. The survey provides nationally representative data by conducting a series of interviews (demographic, socioeconomic, dietary, and health-related questions) and physical examinations (medical, dental, and physiological measurements, as well as laboratory tests) in 2-year cycles. Since the primary outcome (chronic pain) in our study is only available in three consecutive cycles (1999–2000, 2001–2002, and 2003–2004), we limited our analysis to adults aged 20 years or older from 1999 to 2004 datasets who completed the Miscellaneous Pain Questionnaire with the lead level in whole blood measured.
Ethical Statements
The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol, and the NHANES data are publicly available and can be downloaded on the NHANES website (https://wwwn.cdc.gov/nchs/nhanes/) by survey cycle.
Chronic Pain Assessment
In this study, chronic pain was assessed on the basis of the value of variable MPQ100 (had a problem with pain that lasted more than 24 h during the past month) and variable MPQ110 (the duration of the pain). According to the 11th version of the International Classification of Diseases (ICD-11), chronic pain was defined as persistent or recurrent pain lasting longer than 3 months[24]. On the basis of this criterion, participants who reported no pain problem during the past month (MPQ100 = 2) and those who had pain problems less than 3 months (MPQ100 = 1, MPQ110 = 1 or 2) were categorized as the no chronic pain group (control group). Participants with pain problems for 3 months or more (MPQ100 = 1, MPQ110 = 3 or 4) were classified as the chronic pain group.
Determination of Lead in Blood
Blood specimens were processed, stored, and shipped to the Division of Environmental Health Laboratory Sciences, National Center for Environmental Health, CDC for analysis. BLL was measured using atomic absorption spectroscopy in NHANES 1999–2002 and inductively coupled plasma mass spectrometry in NHANES 2003–2004. The NHANES quality assurance and quality control protocols met the 1988 Clinical Laboratory Improvement Act mandates. Detailed specimen collection and processing instructions were discussed in the NHANES Laboratory/Medical Technologists Procedures Manual (LPM).
Other Variables
On the basis of literature review and our clinical experience, we considered the following variables as potential confounders of the relationship between the BLL and chronic pain: gender (male and female), age, race (non-Hispanic white, non-Hispanic Black, Mexican American, or others), marital status (married or living with others, others), education level (less than high school, high school or equivalent, and college or above), poverty–income ratio (PIR: < 1, 1–3 and ≥ 3), body mass index (BMI), daily physical activity, smoking (had at least 100 cigarettes in a lifetime or not), alcohol consumption (in the past 12 months, on those days that participants drank alcoholic beverages, the amount of drinks they had on the average), and health conditions (hypertension, diabetes, coronary heart disease, arthritis, osteoporosis, and cancer or malignancy). Age, BMI, and alcohol consumption were adjusted as continuous variables. The self-reported daily physical activity contained four categories: mainly sit (sitting most of the day), walk around (walking around but no lifting or carrying), light load (lifting light loads and climbing stairs or hills), or heavy load (heavy work and carrying heavy loads).
Statistical Analysis
We described the baseline characteristics of the overall sample, the control group, and the chronic pain group. Continuous variables were presented as mean and standard deviation (SD), while categorical variables were presented as frequency and percentage. Differences between the control and chronic pain group were examined using the Student’s t-test for continuous variables and the chi-squared test for categorical variables. The univariate and multivariate logistic regression models were used to investigate the associations between BLL (both continuous and quartiles) and chronic pain (present or not). Subgroup analyses were performed to investigate whether the association between the BLL and chronic pain was modified by confounding factors. The P for interaction was further calculated using the log-likelihood ratio test comparing models with and without the interaction of confounders. The statistical analyses were conducted using R 3.6.1 software (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/) and EmpowerStats (X&Y Solutions, Boston, MA; http://www.empowerstats.com/). A two-sided P < 0.05 was considered statistically significant.
Results
Baseline Characteristics
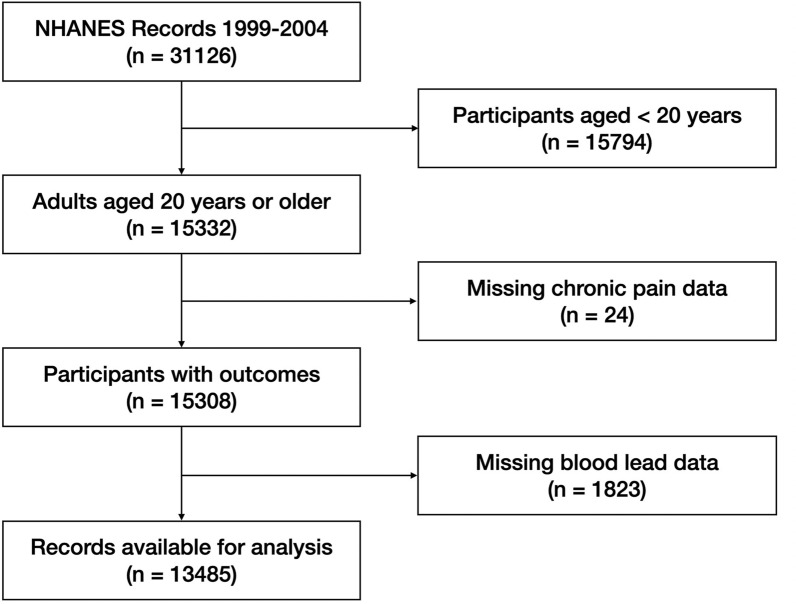
Of the 31,126 participants in NHANES 1999–2004 dataset, 13,485 participants who answered questions regarding chronic pain (MPQ100 and MPQ110) were included in our analyses (Fig. 1). Among included individuals, 1950 (14.46%) had chronic pain (Table 1). Participants with chronic pain are more likely to be older (P < 0.001), female (P < 0.001), white (P < 0.001), smokers (P < 0.001), more physically inactive (P < 0.001), have lower education level (P = 0.016), have a higher BMI (P < 0.001), have a lower PIR (P < 0.001), and have comorbidities including hypertension, diabetes, coronary heart disease, arthritis, osteoporosis, and cancer (P < 0.001 for all comorbidities).
Fig. 1.
Flow chart of participant selection
Table 1.
Participant characteristics in NHANES 1999–2004
| Characteristics | Total (N = 13,485) | Control (N = 11,535) | Chronic pain (N = 1950) | P-value |
|---|---|---|---|---|
| Age | 49.38 ± 19.34 | 52.45 ± 17.15 | < 0.001 | |
| Gender | < 0.001 | |||
| Male | 6401 | 5561 (48.2%) | 840 (43.1%) | |
| Female | 7084 | 5974 (51.8%) | 1110 (56.9%) | |
| Race | < 0.001 | |||
| Non-Hispanic white | 6867 | 5703 (49.4%) | 1164 (59.7%) | |
| Non-Hispanic Black | 2560 | 2201 (19.1%) | 359 (18.4%) | |
| Mexican American | 3060 | 2749 (23.8%) | 311 (15.9%) | |
| Others | 998 | 882 (7.6%) | 116 (5.9%) | |
| Marital status | 0.998 | |||
| Married or living with others | 8094 | 6920 (62.1%) | 1174 (62.1%) | |
| Others | 4930 | 4215 (37.9%) | 715 (37.9%) | |
| Education level | 0.016 | |||
| Less than high school | 4377 | 3738 (32.5%) | 639 (32.8%) | |
| High school or equivalent | 3189 | 2683 (23.3%) | 506 (26.0%) | |
| College or above | 5890 | 5086 (44.2%) | 804 (41.3%) | |
| PIR | < 0.001 | |||
| < 1 | 2283 | 1881 (17.9%) | 402 (22.2%) | |
| 1–3 | 5276 | 4501 (42.7%) | 775 (42.9%) | |
| > 3 | 4785 | 4154 (39.4%) | 631 (34.9%) | |
| BMI | 28.12 ± 6.06 | 29.59 ± 7.00 | < 0.001 | |
| Daily physical activity | < 0.001 | |||
| Mainly sit | 3453 | 2817 (24.4%) | 636 (32.7%) | |
| Walk around | 7090 | 6173 (53.6%) | 917 (47.1%) | |
| Light load | 2044 | 1765 (15.3%) | 279 (14.3%) | |
| Heavy load | 883 | 769 (6.7%) | 114 (5.9%) | |
| Smoking | < 0.001 | |||
| Less than 100 cigarettes in a lifetime | 6930 | 6138 (53.3%) | 792 (40.6%) | |
| At least 100 cigarettes in a lifetime | 6539 | 5382 (46.7%) | 1157 (59.4%) | |
| Alcoholic drinks/day in past 12 months | 2.83 ± 2.80 | 2.72 ± 2.56 | 0.205 | |
| Hypertension | < 0.001 | |||
| No | 9099 | 7962 (69.8%) | 1137 (58.6%) | |
| Yes | 4246 | 3442 (30.2%) | 804 (41.4%) | |
| Diabetes | < 0.001 | |||
| No | 11,967 | 10,323 (89.5%) | 1644 (84.4%) | |
| Yes | 1514 | 1209 (10.5%) | 305 (15.6%) | |
| Coronary heart disease | < 0.001 | |||
| No | 12,814 | 11,034 (96.2%) | 1780 (91.9%) | |
| Yes | 596 | 440 (3.8%) | 156 (8.1%) |
| Characteristics | Total (N = 13,485) | Control (N = 11,535) | Chronic pain (N = 1950) | P-value |
|---|---|---|---|---|
| Arthritis | < 0.001 | |||
| No | 10,018 | 9060 (78.6%) | 958 (49.4%) | |
| Yes | 3446 | 2463 (21.4%) | 983 (50.6%) | |
| Osteoporosis | < 0.001 | |||
| No | 12,712 | 10,988 (95.5%) | 1724 (88.9%) | |
| Yes | 736 | 521 (4.5%) | 215 (11.1%) | |
| Cancer or malignancy | < 0.001 | |||
| No | 12,324 | 10,614 (92.1%) | 1710 (88.0%) | |
| Yes | 1142 | 909 (7.9%) | 233 (12.0%) |
Data are presented as n (%) or mean ± SD. Bold text indicates a statistically significant difference with a P-value < 0.05
PIR poverty income ratio, BMI body mass index
BLL and Chronic Pain
The median value of BLL among all participants was 1.70 (0.70 [median of Q1] − 3.40 [median of Q4]) μg/dL. The prevalence of chronic pain in BLL quartiles was 12.23% (Q1), 15.11% (Q2), 14.51% (Q3), and 15.25% (Q4), respectively (Table 2).
Table 2.
Individuals with/without chronic pain by blood lead level (BLL) quartiles
| BLL quartiles (μg/dL) | Individuals, no. (%) | ||||
|---|---|---|---|---|---|
| Quartiles | Range | Median | Total sample | Control | Chronic pain |
| 1 | 0.20–0.90 | 0.70 | 2452 | 2152 (87.77%) | 300 (12.23%) |
| 2 | 1.00–1.40 | 1.20 | 2839 | 2410 (84.89%) | 429 (15.11%) |
| 3 | 1.50–2.30 | 1.80 | 3852 | 3293 (85.49%) | 559 (14.51%) |
| 4 | 2.40–54.00 | 3.40 | 4342 | 3680 (84.75%) | 662 (15.25%) |
| Total | 0.20–54.00 | 1.70 | 13,485 | 11,535 | 1950 |
BLL blood lead level
As a continuous variable, the BLL was positively associated with the risk of chronic pain in the unadjusted model (OR 1.02, 95% CI 1.00–1.04, P = 0.029) and three adjusted models (Model 1: OR 1.03, 95% CI 1.01—1.05, P = 0.006; Model 2: OR 1.03, 95% CI 1.00–1.05, P = 0.025; and Model 3: OR 1.03, 95% CI 1.00–1.07, P = 0.036).
To reduce the effect of extreme values on the analyses, we also calculated the OR for the risk of chronic pain in each BLL quartile using the lowest quartile (Q1) as the reference group. We found that, compared with individuals in the lowest BLL quartile, those in the highest quartile had 29% higher chronic pain risk in the unadjusted model (OR 1.29, 95% CI 1.12–1.49, P = 0.001). Such a difference remained significant in all the adjusted models (Model 1: OR 1.35, 95% CI 1.14–1.60, P = 0.001; Model 2: OR 1.44, 95% CI 1.20–1.74, P < 0.001; and Model 3: OR 1.32, 95% CI 1.01, 1.72, P = 0.043) (Table 3).
Table 3.
Relationship between blood lead level (BLL) and chronic pain in the unadjusted and adjusted logistic regression models
| Unadjusted modela OR (95% CI) P |
Adjusted model 1 OR (95% CI) P |
Adjusted model 2 OR (95% CI) P |
Adjusted model 3 OR (95% CI) P |
|
|---|---|---|---|---|
| BLL (continuous) | 1.02 (1.00, 1.04) 0.029 | 1.03 (1.01, 1.05) 0.006 | 1.03 (1.00, 1.05) 0.025 | 1.03 (1.00, 1.07) 0.036 |
| BLL (quartile) | ||||
| Q1(0.20–0.90) | Reference | Reference | Reference | Reference |
| Q2(1.00–1.40) | 1.28 (1.09, 1.50) 0.003 | 1.28 (1.09, 1.51) 0.003 | 1.36 (1.14, 1.62) 0.001 | 1.19 (0.94, 1.52) 0.149 |
| Q3(1.50–2.30) | 1.22 (1.05, 1.41) 0.010 | 1.23 (1.05, 1.45) 0.012 | 1.33 (1.11, 1.58) 0.002 | 1.18 (0.93, 1.50) 0.184 |
| Q4(2.40–54.00) | 1.29 (1.12, 1.49) 0.001 | 1.35 (1.14, 1.60) 0.001 | 1.44 (1.20, 1.74) < 0.001 | 1.32 (1.01, 1.72) 0.043 |
BLL blood lead level. Bold text indicates a statistically significant difference with a P-value < 0.05
Adjusted model 1: adjusted for age, gender, race
Adjusted model 2: adjusted for age, gender, race, education level, marital status, PIR, average level of physical activity each day, BMI
Adjusted model 3: adjusted for age, gender, race, education level, marital status, PIR, average level of physical activity each day, BMI, average alcoholic drinks per day in past 12 months, smoking, hypertension, diabetes, coronary heart disease, arthritis, osteoporosis, cancer or malignancy
aThe unadjusted model indicates no adjustment for other covariates
Subgroup Analyses
In the subgroup analyses, the association between the BLL and chronic pain risk was modified by hypertension status (P for interaction = 0.018) and arthritis status (P for interaction = 0.004). Among participants with hypertension, the BLL Q2, Q3, and Q4 were associated with higher risk of chronic pain compared with the BLL Q1 (Q2 versus Q1, OR 2.33, 95% CI 1.41–3.85, P < 0.001; Q3 versus Q1, OR 1.99, 95% CI 1.21–3.26, P = 0.007; Q4 versus Q1, OR 1.94, 95% CI 1.14–3.30, P = 0.014). Among participants with arthritis, the BLL Q3 and Q4 were associated with a higher risk of chronic pain compared with the BLL Q1 (Q3 versus Q1, OR 2.25, 95% CI 1.39–3.62, P = 0.001; Q4 versus Q1, OR 2.35, 95% CI 1.41–3.92, P = 0.001) (Table 4).
Table 4.
Relationship between blood lead level (BLL) and chronic pain in subgroups of potential effect modifiers
| Subgroups | BLL quartiles | P for interaction a | |||
|---|---|---|---|---|---|
| Q1 | Q2 OR (95% CI) P |
Q3 OR (95% CI) P |
Q4 OR (95% CI) P |
||
| Age, years | 0.679 | ||||
| < 55 | Reference | 1.11 (0.85, 1.45) 0.435 | 0.97 (0.73, 1.27) 0.799 | 1.10 (0.81, 1.48) 0.546 | |
| ≥ 55 | Reference | 1.09 (0.58, 2.04) 0.792 | 1.26 (0.71, 2.25) 0.426 | 1.30 (0.72, 2.33) 0.380 | |
| Gender | 0.668 | ||||
| Male | Reference | 1.33 (0.79, 2.24) 0.284 | 1.44 (0.88, 2.35) 0.152 | 1.68 (1.01, 2.77) 0.044 | |
| Female | Reference | 1.21 (0.91, 1.60) 0.186 | 1.12 (0.83, 1.52) 0.444 | 1.12 (0.78, 1.61) 0.535 | |
| Race | 0.121 | ||||
| Non-Hispanic white | Reference | 1.42 (1.05, 1.93) 0.023 | 1.31 (0.96, 1.80) 0.093 | 1.64 (1.16, 2.33) 0.005 | |
| Non-Hispanic Black | Reference | 1.76 (0.91, 3.40) 0.095 | 1.84 (0.95, 3.55) 0.069 | 2.25 (1.10, 4.62) 0.027 | |
| Mexican American | Reference | 0.54 (0.29, 0.98) 0.043 | 0.74 (0.43, 1.29) 0.291 | 0.61 (0.33, 1.12) 0.111 | |
| Others | Reference | 1.27 (0.32, 4.96) 0.732 | 1.69 (0.46, 6.20) 0.426 | 1.22 (0.29, 5.19) 0.784 | |
| Marital status | 0.739 | ||||
| Married or living with others | Reference | 1.11 (0.82, 1.50) 0.495 | 1.10 (0.81, 1.49) 0.530 | 1.31 (0.94, 1.82) 0.117 | |
| Others | Reference | 1.36 (0.90, 2.06) 0.147 | 1.31 (0.86, 1.97) 0.206 | 1.33 (0.84, 2.09) 0.223 | |
| Education level | 0.654 | ||||
| Less than high school | Reference | 1.28 (0.68, 2.38) 0.442 | 1.48 (0.81, 2.72) 0.203 | 1.79 (0.96, 3.33) 0.066 | |
| High school or equivalent | Reference | 1.18 (0.74, 1.89) 0.492 | 0.95 (0.59, 1.52) 0.826 | 0.95 (0.56, 1.60) 0.834 | |
| College or above | Reference | 1.12 (0.81, 1.55) 0.478 | 1.18 (0.85, 1.64) 0.332 | 1.32 (0.91, 1.92) 0.147 | |
| PIR | 0.324 | ||||
| < 1 | Reference | 2.16 (1.15, 4.06) 0.017 | 1.27 (0.66, 2.47) 0.472 | 1.83 (0.92, 3.63) 0.085 | |
| 1–3 | Reference | 1.02 (0.68, 1.51) 0.940 | 1.01 (0.68, 1.50) 0.965 | 1.10 (0.71, 1.70) 0.669 | |
| > 3 | Reference | 1.12 (0.78, 1.60) 0.538 | 1.31 (0.92, 1.87) 0.140 | 1.38 (0.92, 2.07) 0.114 | |
| BMI | 0.954 | ||||
| < 25 | Reference | 1.09 (0.70, 1.71) 0.698 | 1.06 (0.67, 1.68) 0.799 | 1.20 (0.72, 1.98) 0.484 | |
| ≥ 25 | Reference | 1.22 (0.92, 1.63) 0.173 | 1.22 (0.92, 1.63) 0.174 | 1.35 (0.98, 1.85) 0.063 | |
| Daily physical activity | 0.751 | ||||
| Mainly sit | Reference | 1.05 (0.66, 1.68) 0.837 | 1.08 (0.68, 1.72) 0.747 | 0.99 (0.58, 1.69) 0.963 | |
| Walk around | Reference | 1.35 (0.96, 1.90) 0.089 | 1.23 (0.87, 1.74) 0.236 | 1.55 (1.06, 2.26) 0.023 | |
| Light load | Reference | 0.92 (0.51, 1.65) 0.775 | 1.41 (0.78, 2.54) 0.260 | 1.25 (0.65, 2.39) 0.503 | |
| Subgroups | BLL quartiles | P for interactiona | |||
|---|---|---|---|---|---|
| Q1 | Q2 OR (95% CI) P |
Q3 OR (95% CI) P |
Q4 OR (95% CI) P |
||
| Daily physical activity | |||||
| Heavy load | Reference | 1.32 (0.44, 3.95) 0.622 | 0.98 (0.33, 2.90) 0.971 | 1.31 (0.43, 4.01) 0.635 | |
| Smoking | 0.189 | ||||
| Less than 100 cigarettes in a lifetime | Reference | 0.94 (0.67, 1.33) 0.740 | 1.15 (0.82, 1.63) 0.423 | 1.23 (0.82, 1.84) 0.323 | |
| At least 100 cigarettes in a lifetime | Reference | 1.49 (1.05, 2.12) 0.026 | 1.24 (0.87, 1.76) 0.234 | 1.46 (1.00, 2.11) 0.047 | |
| Alcoholic drinks per day in past 12 months | 0.510 | ||||
| < 9 | Reference | 1.19 (0.93, 1.52) 0.163 | 1.20 (0.94, 1.54) 0.143 | 1.34 (1.02, 1.75) 0.034 | |
| ≥ 9 | Reference | 1.35 (0.20, 9.07) 0.759 | 0.55 (0.09, 3.42) 0.519 | 0.86 (0.12, 6.24) 0.882 | |
| Hypertension | 0.018 | ||||
| No | Reference | 0.98 (0.74, 1.30) 0.888 | 1.00 (0.75, 1.33) 0.999 | 1.16 (0.85, 1.59) 0.352 | |
| Yes | Reference | 2.33 (1.41, 3.85) 0.001 | 1.99 (1.21, 3.26) 0.007 | 1.94 (1.14, 3.30) 0.014 | |
| Diabetes | 0.313 | ||||
| No | Reference | 1.17 (0.91, 1.50) 0.221 | 1.14 (0.88, 1.47) 0.328 | 1.33 (1.01, 1.76) 0.043 | |
| Yes | Reference | 1.74 (0.71, 4.29) 0.229 | 1.95 (0.81, 4.67) 0.137 | 1.32 (0.49, 3.51) 0.581 | |
| Coronary heart disease | 0.332 | ||||
| No | Reference | 1.19 (0.93, 1.52) 0.162 | 1.19 (0.93, 1.52) 0.171 | 1.35 (1.03, 1.78) 0.030 | |
| Yes | Reference | 1.09 (0.20, 5.95) 0.925 | 0.83 (0.17, 4.00) 0.812 | 0.51 (0.10, 2.46) 0.400 | |
| Arthritis | 0.004 | ||||
| No | Reference | 1.13 (0.86, 1.49) 0.388 | 0.86 (0.64, 1.16) 0.323 | 1.03 (0.74, 1.44) 0.840 | |
| Yes | Reference | 1.56 (0.95, 2.56) 0.081 | 2.25 (1.39, 3.62) 0.001 | 2.35 (1.41, 3.92) 0.001 | |
| Osteoporosis | 0.649 | ||||
| No | Reference | 1.22 (0.95, 1.56) 0.118 | 1.19 (0.92, 1.53) 0.185 | 1.29 (0.98, 1.70) 0.071 | |
| Yes | Reference | 0.95 (0.32, 2.82) 0.929 | 1.17 (0.41, 3.31) 0.771 | 1.86 (0.59, 5.85) 0.289 | |
| Cancer or malignancy | 0.708 | ||||
| No | Reference | 1.16 (0.90, 1.48) 0.250 | 1.14 (0.88, 1.46) 0.319 | 1.26 (0.95, 1.67) 0.103 | |
| Yes | Reference | 2.11 (0.71, 6.25) 0.177 | 1.90 (0.68, 5.32) 0.223 | 2.36 (0.81, 6.86) 0.116 | |
BLL blood lead level. Bold text indicates a statistically significant difference with a P-value < 0.05
aP for interaction was calculated using the likelihood ratio test comparing models with and without the interaction term
Discussion
This study found that BLL is positively correlated with the risk of chronic pain. A 1 μg/dL increase in the BLL is associated with 3% higher risk of chronic pain. Individuals in the highest BLL quartile had a 32% higher risk of chronic pain than those in the lowest. Furthermore, subgroup analyses showed that a stronger correlation between BLL and chronic pain was observed among those with hypertension or arthritis.
The CDC estimated that 20.4% of US adults had chronic pain in 2016 [3]. In this study, we found that 14.5% of the US population had self-reported chronic pain during 1999–2004, suggesting there might be an increase in the prevalence of chronic pain during the past two decades. Chronic pain affects people’s normal life and reduces quality of life [3]. Patients with chronic pain may require opioids, which exposes many people to misuse, abuse, and addiction to opioids and causes adverse events including respiratory suppression, constipation, and cognitive impairment, and even leads to increased morbidity and mortality [25, 26].
Chronic pain can be caused by nociceptive (from tissue injuries), neuropathic (from nerve injuries), or nociplastic (from a sensitized nervous system) stimuli. In addition, chronic pain can be triggered by one or more of the causes mentioned above [4]. Chronic exposure to heavy metals is a well-known cause of central and peripheral neuropathy. Lead toxicity primarily affects the nervous system compared with the other organ systems in the human body [27–29]. The traditional neuropathy associated with lead poisoning has mainly been the motor neuropathy with a demyelinating pattern [27, 28], and literature has indicated the possibility of mild sensory and autonomic fiber dysfunction [30–33]. Our study revealed a positive correlation between BLL and chronic pain, suggesting that lead exposure may exert an effect on chronic pain, possibly by acting directly on peripheral nerves [27, 34] or by causing other health conditions that consequently lead to chronic pain, such as various types of cancer (lung cancer [35], kidney cancer [36], etc.) and chronic kidney disease (CKD) [37].
In our subgroup analyses, we found that participants who suffered from hypertension might be more vulnerable to the negative effect of BLL on chronic pain. A possible explanation for this is that increased stimulation of baroreceptors in hypertension patients impaired the descending inhibitory pathways of pain, thus increasing pain sensitivity [38]. Pain sensitivity is also enhanced in patients with arthritis, possibly due to increased systemic inflammation [39–41]. Therefore, a moderate-to-high BLL (Q3–Q4) was associated with a higher risk of chronic pain compared with a very low BLL (Q1) in participants with arthritis but not those without arthritis.
Recently, CDC updated the BLL reference value from 5 μg/dL to 3.5 μg/dL [19]. However, we found that compared with the lowest BLL quartile (BLL < 0.90 μg/dL), the highest BLL quartile (BLL > 2.40 μg/dL) was associated with a 32% increase in the risk of chronic pain in the fully adjusted model, indicating that to prevent chronic pain, the lower BLL the better, even below the CDC’s BLL reference value.
The positive correlation between BLL and chronic pain implies the need to reduce lead exposure to ameliorate the impact of chronic pain on the economy and health. For those with hypertension or arthritis, BLL could be one of their health indicators. A high BLL should be taken into consideration by practitioners when providing health education or giving medical advice since they might be more vulnerable to the negative effect of lead exposure on chronic pain.
This is the first study to investigate the relationship between chronic pain and BLL. We used a large sample size of 13,485 participants in total. We conducted both unadjusted and adjusted regressions to confirm the robustness of the positive correlation between BLL and incidence of chronic pain. In addition, we performed subgroup analyses to explore populations more susceptible to the negative effect of lead exposure on chronic pain.
This study has several limitations. Firstly, self-reported chronic pain was not thoroughly evaluated in the NHANES. Secondly, this cross-sectional study could not determine a causal relationship between lead exposure and chronic pain. Thirdly, the NHANES discontinued asking about chronic pain in 2004. Therefore, a prospective study of a more recent population is warranted to confirm our findings.
Conclusion
A higher BLL was associated with a higher risk of chronic pain. Further research is warranted to investigate whether a causal relationship exists between the two as well as potential underlying mechanisms.
Acknowledgements
We thank the participants and investigators of the NHANES.
Funding
Sponsorship for this work and the Rapid Service Fee was funded by grants from the National Natural Science Foundation of China (grant 8217102207 to WA.Z.) and the Natural Science Foundation of Guangdong Province (grant 2021A1515220117 to WA.Z.).
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Wanyu Wang, and Qiang Li. The first draft of the manuscript was written by Wanyu Wang and Xiaoyun Lu, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
All authors declare that they have no competing interests.
Compliance with Ethics Guidelines
NHANES is conducted by the CDC and the NCHS. The NCHS Research Ethics Review Committee reviewed and approved the NHANES study protocol. The NHANES data used in this study is publicly available.
Data Availability
The datasets analyzed in the current study are publicly available on the official website of the NHANES (https://wwwn.cdc.gov/nchs/nhanes/).
Footnotes
Wanyu Wang and Xiaoyun Lu contributed equally.
Contributor Information
Dongtai Chen, Email: chendt@sysucc.org.cn.
Weian Zeng, Email: zengwa@mail.sysu.edu.cn.
References
- 1.Zimmer Z, Fraser K, Grol-Prokopczyk H, Zajacova A. A global study of pain prevalence across 52 countries: examining the role of country-level contextual factors. Pain. 2022;163(9):1740–1750. doi: 10.1097/j.pain.0000000000002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gignac MA, Cott C, Badley EM. Adaptation to chronic illness and disability and its relationship to perceptions of independence and dependence. J Gerontol B Psychol Sci Soc Sci. 2000;55(6):P362–P372. doi: 10.1093/geronb/55.6.P362. [DOI] [PubMed] [Google Scholar]
- 3.Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–2097. doi: 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 5.Raffaeli W, Arnaudo E. Pain as a disease: an overview. J Pain Res. 2017;10:2003–2008. doi: 10.2147/JPR.S138864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith BH, Macfarlane GJ, Torrance N. Epidemiology of chronic pain, from the laboratory to the bus stop: time to add understanding of biological mechanisms to the study of risk factors in population-based research? Pain. 2007;127(1–2):5–10. doi: 10.1016/j.pain.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 7.van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br J Anaesth. 2013;111(1):13–18. doi: 10.1093/bja/aet123. [DOI] [PubMed] [Google Scholar]
- 8.Bushnell MC, Case LK, Ceko M, et al. Effect of environment on the long-term consequences of chronic pain. Pain. 2015;156(Suppl 1(0 1)):S42–s9. [DOI] [PMC free article] [PubMed]
- 9.Mitra P, Sharma S, Purohit P, Sharma P. Clinical and molecular aspects of lead toxicity: an update. Crit Rev Clin Lab Sci. 2017;54(7–8):506–528. doi: 10.1080/10408363.2017.1408562. [DOI] [PubMed] [Google Scholar]
- 10.LeBrón AMW, Torres IR, Valencia E, et al. The state of public health lead policies: implications for urban health inequities and recommendations for health equity. Int J Environ Res Public Health. 2019;16(6). [DOI] [PMC free article] [PubMed]
- 11.Reuben A, Schaefer JD, Moffitt TE, et al. Association of childhood lead exposure with adult personality traits and lifelong mental health. JAMA Psychiat. 2019;76(4):418–425. doi: 10.1001/jamapsychiatry.2018.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gundacker C, Forsthuber M, Szigeti T, et al. Lead (Pb) and neurodevelopment: a review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int J Hyg Environ Health. 2021;238:113855. doi: 10.1016/j.ijheh.2021.113855. [DOI] [PubMed] [Google Scholar]
- 13.Anil L, Ma Z-Q, Nambiar A, Watkins SM. Blood lead level testing and retesting among newly arriving refugee children, Pennsylvania, 2015–2019. Am J Public Health. 2022;112(S7):S706–14. [DOI] [PMC free article] [PubMed]
- 14.Reuben A, Caspi A, Belsky DW, et al. Association of childhood blood lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA. 2017;317(12):1244–1251. doi: 10.1001/jama.2017.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muntner P, He J, Vupputuri S, Coresh J, Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63(3):1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 16.Lee KR, Ko KD, Hwang IC, Suh HS, Kim KK. Association between blood lead levels and blood pressures in a non-smoking healthy Korean population. Postgrad Med J. 2017;93(1103):513–518. doi: 10.1136/postgradmedj-2016-134208. [DOI] [PubMed] [Google Scholar]
- 17.Nelson AE, Shi XA, Schwartz TA, et al. Whole blood lead levels are associated with radiographic and symptomatic knee osteoarthritis: a cross-sectional analysis in the Johnston County Osteoarthritis Project. Arthritis Res Ther. 2011;13(2):R37. doi: 10.1186/ar3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schober SE, Mirel LB, Graubard BI, Brody DJ, Flegal KM. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ Health Perspect. 2006;114(10):1538–1541. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruckart PZ, Jones RL, Courtney JG, et al. Update of the blood lead reference value—United States, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(43):1509–1512. doi: 10.15585/mmwr.mm7043a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min MO, Singer LT, Kirchner HL, et al. Cognitive development and low-level lead exposure in poly-drug exposed children. Neurotoxicol Teratol. 2009;31(4):225–231. doi: 10.1016/j.ntt.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dórea JG. Environmental exposure to low-level lead (Pb) co-occurring with other neurotoxicants in early life and neurodevelopment of children. Environ Res. 2019;177:108641. doi: 10.1016/j.envres.2019.108641. [DOI] [PubMed] [Google Scholar]
- 22.He L, Chen Z, Dai B, Li G, Zhu G. Low-level lead exposure and cardiovascular disease: the roles of telomere shortening and lipid disturbance. J Toxicol Sci. 2018;43(11):623–630. doi: 10.2131/jts.43.623. [DOI] [PubMed] [Google Scholar]
- 23.Dinçkol Ö, Fuentes B, Tartaglione AM, Pino A, Calamandrei G, Ricceri L. Low-level lead exposure during development differentially affects neurobehavioral responses in male and female mouse offspring: a longitudinal study. Neurotoxicology. 2022;91:188–199. doi: 10.1016/j.neuro.2022.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Treede RD, Rief W, Barke A, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–1007. doi: 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voon P, Karamouzian M, Kerr T. Chronic pain and opioid misuse: a review of reviews. Subst Abuse Treat Prev Policy. 2017;12(1):36. doi: 10.1186/s13011-017-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA. 2016;315(15):1624–1645. doi: 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koszewicz M, Markowska K, Waliszewska-Prosol M, et al. The impact of chronic co-exposure to different heavy metals on small fibers of peripheral nerves. a study of metal industry workers. J Occup Med Toxicol. 2021;16(1):12. [DOI] [PMC free article] [PubMed]
- 28.Samuel C, Venkataraman S, VN, et al. Bioaccumulation of lead (Pb) and its effects on human: a review. J Hazard Mater Adv. 2022;7:100094.
- 29.Gilani SR, Zaidi SR, Batool M, Bhatti AA, Durrani AI, Mahmood Z. Report: central nervous system (CNS) toxicity caused by metal poisoning: brain as a target organ. Pak J Pharm Sci. 2015;28(4):1417–1423. [PubMed] [Google Scholar]
- 30.Sadeghniiat-Haghighi K, Saraie M, Ghasemi M, Izadi N, Chavoshi F, Khajehmehrizi A. Assessment of peripheral neuropathy in male hospitalized patients with lead toxicity in Iran. J Res Med Sci. 2013;18(1):6–9. [PMC free article] [PubMed] [Google Scholar]
- 31.Krieg EF, Jr, Chrislip DW, Brightwell WS. A meta-analysis of studies investigating the effects of lead exposure on nerve conduction. Arch Toxicol. 2008;82(8):531–542. doi: 10.1007/s00204-008-0292-z. [DOI] [PubMed] [Google Scholar]
- 32.Thomson RM, Parry GJ. Neuropathies associated with excessive exposure to lead. Muscle Nerve. 2006;33(6):732–741. doi: 10.1002/mus.20510. [DOI] [PubMed] [Google Scholar]
- 33.Rubens O, Logina I, Kravale I, Eglîte M, Donaghy M. Peripheral neuropathy in chronic occupational inorganic lead exposure: a clinical and electrophysiological study. J Neurol Neurosurg Psychiatry. 2001;71(2):200–204. doi: 10.1136/jnnp.71.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Shi F, Cui J, Pang S, Zheng G, Zhang Y. MiR-378a-3p/ SLC7A11 regulate ferroptosis in nerve injury induced by lead exposure. Ecotoxicol Environ Saf. 2022;239:113639. doi: 10.1016/j.ecoenv.2022.113639. [DOI] [PubMed] [Google Scholar]
- 35.Steenland K, Barry V, Anttila A, et al. A cohort mortality study of lead-exposed workers in the USA, Finland and the UK. Occup Environ Med. 2017;74(11):785–791. doi: 10.1136/oemed-2017-104311. [DOI] [PubMed] [Google Scholar]
- 36.Southard EB, Roff A, Fortugno T, et al. Lead, calcium uptake, and related genetic variants in association with renal cell carcinoma risk in a cohort of male Finnish smokers. Cancer Epidemiol Biomark Prev. 2012;21(1):191–201. doi: 10.1158/1055-9965.EPI-11-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moody EC, Coca SG, Sanders AP. Toxic metals and chronic kidney disease: a systematic review of recent literature. Curr Environ Health Rep. 2018;5(4):453–463. doi: 10.1007/s40572-018-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maixner W, Fillingim R, Kincaid S, Sigurdsson A, Harris MB. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med. 1997;59(5):503–511. doi: 10.1097/00006842-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Lee YC, Lu B, Bathon JM, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 2011;63(3):320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnstad ED, Iversen JM, Uglem M, et al. Pain sensitivity in young adults with juvenile idiopathic arthritis: a quantitative sensory testing study. Arthritis Res Ther. 2020;22(1):262. doi: 10.1186/s13075-020-02345-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vladimirova N, Jespersen A, Bartels EM, Christensen AW, Bliddal H, Danneskiold-Samsøe B. Pain sensitisation in women with active rheumatoid arthritis: a comparative cross-sectional study. Arthritis. 2015;2015:434109. doi: 10.1155/2015/434109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed in the current study are publicly available on the official website of the NHANES (https://wwwn.cdc.gov/nchs/nhanes/).