Abstract
Introduction
Based on real-world case data, this study intends to explore and analyze the impact of rescue conscious sedation (CS) on the clinical outcomes of patients with anterior circulation acute ischemic stroke (AIS) receiving mechanical thrombectomy (MT).
Methods
This retrospective study enrolled patients with anterior circulation AIS who received MT and were treated with either single local anesthesia (LA) or rescue CS during MT between January 2018 and October 2021. We used univariate and multivariate logistic regression methods to compare the impact of LA and CS on the clinical outcomes of patients with AIS who received MT, including the mRS at 90 days, the incidence of poststroke pneumonia (PSP), the incidence of symptomatic intracranial cerebral hemorrhage (sICH), and the mortality rate.
Results
We reviewed 314 patient cases with AIS who received MT. Of all patients, 164 met our search criteria. Eighty-nine patients received LA, and 75 patients received rescue CS. There was no significant difference between the two groups in the 90-day good prognosis (45.3% vs. 51.7%, p = 0.418) and mortality (17.3% vs. 22.5%, p = 0.414). Compared with the LA group, the incidence of postoperative pneumonia in the rescue CS group (44% vs. 25.8%, p = 0.015) was more significant. Multivariate stepwise logistic regression analysis revealed that intraoperative remedial CS was independently associated with PSP following MT. In a subgroup analysis, rescue CS was found to significantly increase the incidence of PSP in patients with dysphagia (OR = 7.307, 95% CI 2.144–24.906, p = 0.001). As the severity of the National Institutes of Health Stroke Scale (NIHSS) increased, intraoperative rescue CS was found to increase the risk of PSP (OR = 1.155, 95% CI 1.034–1.290, p = 0.011) by 5.1% compared to that of LA (OR = 1.104, 95% CI 1.013–1.204, p = 0.024).
Conclusion
Compared to LA, rescue CS during MT does not significantly improve the 90 days of good prognosis and reduce the incidence of sICH and mortality in patients with anterior circulation AIS. However, it has a significantly increased risk of poststroke pneumonia (PSP), particularly in patients with dysphagia.
Keywords: Rescue conscious sedation, Mechanical thrombectomy, Acute anterior circulation ischemic stroke, Poststroke pneumonia, Local anesthesia
Key Summary Points
| Why carry out this study? |
| The method of anesthesia may be a potential factor affecting the patient's prognosis. General anesthesia (GA), conscious sedation (CS), and local anesthesia (LA) are three alternative anesthetic methods, and there is no definitive conclusion as to which is optimal. |
| GA is not the anesthetic method of choice for operators in the real world because it is time-consuming. The simplicity and ease of operation of LA, the basic anesthetic method used for mechanical thrombectomy (MT), is faced with patient agitation intraoperatively. Notably, CS has a short operation time, practicality, and good sedation, which may be a good measure to control agitation during MT. |
| Unfortunately, there are few studies of MT anesthetic methods involving rescue CS during operation. The results of this study are based on a single-center experience developing a retrospective study of two anesthetic modalities, intraoperative rescue CS versus LA, to explore the impact of rescue CS on the clinical outcome of patients with anterior circulation acute ischemic stroke (AIS). |
| The hypothesis was that the rescue CS had an effect on clinical outcomes of patients who receive MT. |
| What was learned from the study? |
| Rescue CS was associated with the clinical outcomes of patients with anterior circulation AIS receiving MT.The rescue CS has a significantly increased risk of poststroke pneumonia (PSP), particularly in patients with dysphagia. |
| The implication of this study is that the rescue CS should be avoided if possible in patients with anterior circulation acute ischemic stroke with higher National Institutes of Health Stroke Scale (NIHSS) scores and dysphagia. |
Introduction
Mechanical thrombectomy (MT) is an endovascular treatment for an acute stroke of large vessel occlusion that is regarded as safe and effective [1–5]. Notably, as a measure of smooth functioning, the method of anesthesia may be a potential factor affecting the patient's prognosis. In 2017, a meta-analysis including three randomized controlled trials (RCTs) and 19 observational studies concluded that patients who received general anesthesia (GA) might have a worse prognosis compared with either rescue conscious sedation (CS) or local anesthesia (LA) [6]. Additionally, a 2018 meta-analysis of Hermes, including seven RCT studies from 2010 to 2017, came to a similar conclusion and concluded that patients who received GA had a worse prognosis at 3 months [7]. Observational studies based on ANGEL data have compared GA with non-GA (LA and CS) and still had similar outcomes, suggesting that patients receiving GA treatment had a poorer prognosis [8]. In 2016 and 2018, two single-center randomized clinical studies (SIESTA, GOLIATH) demonstrated that patients receiving GA had a better prognosis at 3 months than CS. However, GA is likely to result in an increased incidence of postoperative pneumonia (SIESTA trial 13.7% vs. 3.9%; p = 0.03) [9, 10]. In contrast, a single-center randomized clinical trial (AnStroke) in 2017 found no difference in the prognosis in patients with CS compared with GA over 3 months [11]. Furthermore, a 2020 review of the MR CLEAN database concluded that the LA had a better functional outcome than the GA and CS [12]. In a prospective multicenter registry study from France, LA was considered associated with a lower rate of good outcome (40.3% vs. 49.6%), lower rates of successful reperfusion (75.3% vs. 86.1%), and higher mortality (18.1% vs. 23.6%) compared with CS [13]. A meta-analysis of 18 non-randomized studies in 2021 found that there was no significant difference between general anesthesia and conscious sedation in terms of functional independence at 90 days, mortality at 90 days, symptomatic intracranial hemorrhage, and aspiration pneumonia [14]. Another meta-analysis of eight non-randomized studies in 2022 found that LA was not significantly different from CS and GA in terms of favorable functional outcome or mortality, but there was a trend towards achieving favorable functional outcome (mRS 1) [15]. Thus, GA, CS, and LA can be seen as three alternative anesthetic methods, and there is no definitive conclusion as to which is optimal.
In these studies, more attention is given to comparing the effects of GA, CS, and LA on stroke prognosis. GA is not the anesthetic method of choice for operators in the real world because it is time-consuming. The simplicity and ease of operation of LA, the basic anesthetic method used for MT are faced with patient agitation intraoperatively. Notably, CS has a short operation time, practicality, and good sedation, which may be a good measure to control agitation during MT.
Unfortunately, there are few studies of anesthetic methods involving rescue CS during MT. Therefore, based on a single-center experience, this study explores the effect of rescue CS on clinical outcomes in patients with acute ischemic stroke (AIS) in the anterior circulation receiving MT.
Methods
Patient selection
Patients included in this study were from a cohort (A New Parameter Derived from Digital Subtraction Angiography to Evaluate Cerebra Perfusion NCT03607565) of the Department of Neurology of the Xi’an No. 3 Hospital between January 2018 and October 2021. The inclusion criteria for this study were patients with (1) cerebral infarction caused by occlusion of the great arteries of the acute anterior circulation (ICA, MCA M1–M2 segment); (2) mechanical thrombectomy (thrombus aspiration, stent thrombectomy) performed; (3) inclusion up to 24 h after symptom onset; (4) age > 18 years old; (5) intraoperative remedial CS or LA accepted. The exclusion criteria were patients with (1) posterior circulation stroke (vertebral-basal artery infarction); (2) treatment with arterial thrombolytics, emergency stent implantation or balloon dilation therapy; (3) general anesthesia; (4) previous mRS > 2; (5) preoperative CT scan of the chest showing pneumonia; (6) swallowing function not evaluated preoperatively; (7) data missing. This was a retrospective study with prospectively collected data in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [16] and was approved by the local Institutional Review Board at the Xi’an No. 3 Hospital (no. SYXSLL-2018–010). Since the observational data we used came from the ongoing cohort, our study did not harm the patients without privacy exposure and only analyzed patient baseline data and clinical outcomes, so the informed consent form was waived. This study was conducted in accordance with the Declaration of Helsinki.
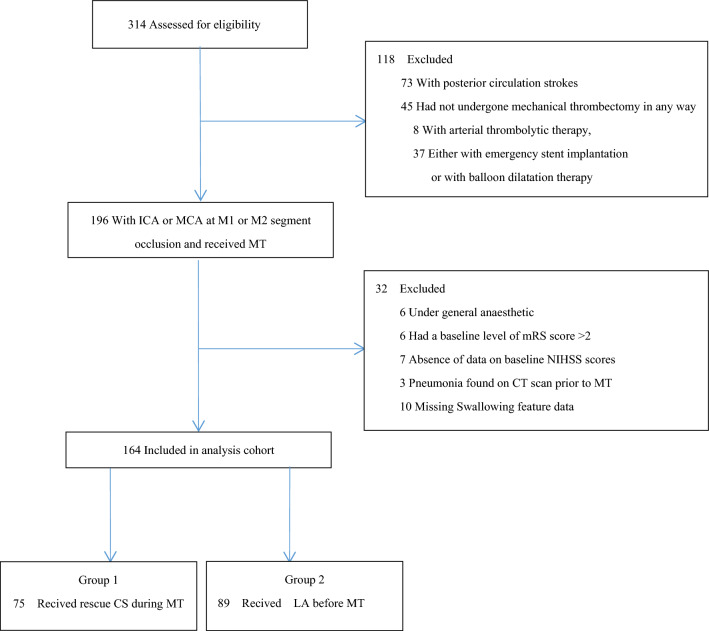
Of the 314 patients who agreed to endovascular therapy, 150 did not meet the inclusion or exclusion criteria. Additionally, the remaining 164 patients were divided into two groups: intraoperative rescue CS and LA according to anesthesia (Fig. 1). The rescue CS in this study was defined as continuous sedation with dexmedetomidine injection immediately when the patient became agitated during MT.
Fig. 1.
Flowchart of patient selection. ICA internal carotid artery; MCA middle cerebral artery; mRS modified Rankin Scale; NIHSS National Institutes of Health Stroke Scale; MT mechanical thrombectomy; CS conscious sedation; LA local anesthesia
LA was defined as local anesthesia at the puncture site, and no sedative medication was used during the procedure. Poststroke pneumonia after MT was defined as the following new manifestations within 7 days of MT (meeting at least one of the first and second criteria): (1) lung CT scan was indicative of inflammatory changes; (2) cough with purulent sputum, a microbial culture, or blood culture in the lower respiratory tract, leukocytosis, and a rise in C-reactive protein.
Variables and Follow-Up
The baseline characteristics of the patients that we used to build the database were: age, gender, previous stroke, hypertension, diabetes mellitus, atrial fibrillation, smoking status, alcohol consumption, site of occlusion (ICA, MCA, ICA + MCA), baseline stroke severity as assessed by the National Institutes of Health Stroke Scale (NIHSS), intravenous alteplase, endovascular therapy (thrombus aspiration, stent thrombus pulling, combined with both), time from onset to admission, time from admission to puncture, time from a puncture to recanalization, mTICI (2b or 3), dysphagia (prior to MT the patient appeared to be choking on water and coughing, or the swallowing irritation test was completed at least three times within 30 s), and ASTIN/SIR score (2 or 3). The primary outcome measure was the 90-day good functional outcome (mRS score of ≤ 2). The secondary outcome measures included the 90-day mRS, 90-day mortality, sICH (imaging evidence of cerebral hemorrhage defined according to the ECASS-II criteria) [17] and PSP (based on imaging, laboratory and clinical outcomes). At 3 months after mechanical thrombectomy, our follow-up physicians, who were blinded to the clinical details and anesthetic methods, completed the assessment of the clinical outcome by telephone follow-up. All data were reviewed anonymously and centrally by two independent physicians. Agreement was reached between physicians to resolve any disagreement; if no consensus was reached, a third physician blinded to the study participated in the review.
Statistical Analysis
The counting data are expressed in the form of n and %. If the measurement data obeyed the normal distribution, it was indicated by ; if it did not follow the normal distribution, it was indicated by M (IQR). The t-test, Mann-Whitney U test, -test, and Fisher exact method were used for univariate analysis. To compare the effects of two anesthesia methods on clinical outcomes, the odds ratios (ORs) of 95% confidence interval (CI) and the adjusted ORs of 95% CIs were calculated using univariate and multivariate logistic regression models. We adopted two kinds of multiple regression models to improve the reliability of the results. In model 1, we only adjusted age, sex, history of atrial fibrillation, and diabetes. In model 2, we additionally adjusted the variable of P < 0.05 level difference between the two groups. The data were analyzed using SPSS 26.0 (IBM, Armonk, NY, USA), and p < 0.05 was considered statistically significant.
Results
A total of 164 patients who met the study criteria were included in this study. Overall, the average age was 69 years, and males accounted for 53% of the population. The successful recanalization rate was 87.2%, 90 days good prognosis rate was 48.8%, mortality rate was 20.1%, sICH incidence rate was 13.4%, and PSP incidence rate was 34.1% (Tables 1 and 2). Eighty-nine patients received LA, and 75 patients received intraoperative rescue CS. There was no significant difference between the two groups in baseline data (Table 1). There was also no significant difference in 90-day good prognosis (45.3% vs. 51.7%, p = 0.418), incidence of sICH (10.1% vs. 17.3%, p = 0.176), and mortality (17.3% vs. 22.5%, p = 0.414) between the two groups. Notably, the incidence of postoperative pneumonia in the rescue CS group (44% vs. 25.8%, p = 0.015) was higher than that in LA (Table 2).
Table 1.
Analysis of the baseline characteristics between CS or LA groups for patients with anterior circulation ischemic stroke undergoing thrombectomy
| All patients(164) | Anesthetic mode | p | ||
|---|---|---|---|---|
| LA (89) | CS (75) | |||
| Age, mean (SD), years | 69 (12) | 69 (12) | 68 (14) | 0.832 |
| Sex (male), n (%) | 87 (53.0%) | 47 (52.8%) | 40 (53.3%) | 0.947 |
| Stroke history, n (%) | 26 (15.9%) | 15 (16.9%) | 11 (14.7%) | 0.702 |
| HBP, n (%) | 90 (54.9%) | 51 (57.3%) | 39 (52.0%) | 0.497 |
| AF, n (%) | 81 (49.4%) | 42 (47.2%) | 39 (52.0%) | 0.539 |
| DM, n (%) | 29 (17.7%) | 20 (22.5%) | 9 (12.0%) | 0.08 |
| Smoking, n (%) | 40 (24.4%) | 22 (24.7%) | 18 (24.0%) | 0.915 |
| Drinking, n (%) | 15 (9.1%) | 8 (9.0%) | 7 (9.3%) | 0.939 |
| NIHSS, median (IQR) | 15 (10, 19) | 14 (9, 19) | 16 (12, 19) | 0.163 |
| Dysphagia, n (%) | 45 (27.4%) | 23 (25.8%) | 22 (29.3%) | 0.618 |
| Occlusion site, n (%) | 0.118 | |||
| ICA | 44 (26.8%) | 20 (22.5%) | 24 (32.0%) | |
| MCA | 113 (68.9%) | 63 (70.8%) | 50 (66.7%) | |
| ICA and MCA | 7 (4.3%) | 6 (6.7%) | 1 (1.3%) | |
| Intravenous thrombolysis, n (%) | 62 (37.8%) | 32 (36.0%) | 30 (40.0%) | 0.595 |
| ASTIN/SIR = 2/3, n (%) | 63 (70.8%) | 63 (70.8%) | 55 (73.5%) | 0.718 |
| Thrombectomy method, n (%) | 0.872 | |||
| SWIM | 67 (40.9%) | 38 (42.7%) | 29 (38.7%) | |
| ADAPT | 59 (36.0%) | 31 (34.8%) | 28 (37.3%) | |
| SWIM and ADAPT | 38 (23.2%) | 20 (22.5%) | 18 (24.0%) | |
| Onset to admission, median (IQR),min | 152 (86, 295) | 139 (79, 289) | 188 (92, 303) | 0.642 |
| DPT, median (IQR), min | 138 (104, 187) | 139 (105, 193) | 135 (103, 177) | 0.501 |
| Puncture to recanalization, median (IQR), min | 72 (34, 109) | 79 (40, 118) | 60 (30, 95) | 0.059 |
| TICI = 2b/3, n (%) | 143 (87.2%) | 79 (88.8%) | 64 (85.3%) | 0.512 |
LA local anesthesia; CS conscious sedation; SD standard deviation; IQR interquartile range; HBP hypertension high blood pressure;DM diabetes mellitus; AF atrial fibrillation; NIHSS National Institutes of Health Stroke Scale; ICA internal carotid artery; MCA middle cerebral artery; ASTIN/SIR American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; SWIM Solitaire retriever stent combined with intracranial support catheter aspiration for mechanical thrombectomy; ADAPT a direct aspiration first-pass technology; DPT door to puncture time; TICI thrombolysis in cerebral infarction
Table 2.
Analysis of the clinical outcomes of patients undergoing CS or LA
| All patients (164) | LA (n = 89) | CS (n = 75) | p | |
|---|---|---|---|---|
| Primary outcome | ||||
| 90-day mRS 0–2, n (%) | 80 (48.8%) | 46 (51.7%) | 34 (45.3%) | 0.418 |
| Secondary outcomes | ||||
| 90-day mRS, median (IQR) | 3 (1, 5) | 2 (0, 5) | 3 (2, 5) | 0.13 |
| Mortality at 90 days, n (%) | 33 (20.1%) | 20 (22.5%) | 13 (17.3%) | 0.414 |
| Postoperative sICH, n (%) | 22 (13.4%) | 9 (10.1%) | 13 (17.3%) | 0.176 |
| PSP, n (%) | 56 (34.1%) | 23 (25.8%) | 33 (44.0%) | 0.015 |
LA local anesthesia; CS conscious sedation; mRS modified Rankin Scale; sICH symptomatic intracranial hemorrhage; PSP post-stroke pneumonia
After MT, there were significantly more patients with PSP than without PSP in terms of 90-day mRS (5 vs. 2, p = 0.010), mortality (32.1% vs. 13.9%, p = 0.006) at 90 days, and the incidence of sICH (21.4% vs. 9.3%, p = 0.030). The rate of 90-day good prognosis (33.9% vs. 56.5%, p = 0.006) was significantly lower than that of patients without PSP (Table 3).
Table 3.
Prognostic impact of PSP after MT
| No PSP (n = 108) | PSP (n = 56) | p | |
|---|---|---|---|
| 90-day mRS, median (IQR) | 2 (0, 5) | 5 (2, 6) | 0.010 |
| 90-day mRS 0–2, n (%) | 61 (56.5%) | 19 (33.9%) | 0.006 |
| Mortality at 90 days, n (%) | 15 (13.9%) | 18 (32.1%) | 0.006 |
| Postoperative sICH, n (%) | 10 (9.3%) | 12 (21.4%) | 0.030 |
PSP post-stroke pneumonia; MT mechanical thrombectomy; mRS modified Rankin Scale; sICH symptomatic intracranial hemorrhage
The univariate analysis of the occurrence of PSP after MT showed that intraoperative rescue CS, NIHSS, dysphagia, bridging thrombectomy, and ASTIN/SIR (2–3) were the risk factors for the occurrence of PSP after MT (Table 4). Consequently, we included several factors affecting the occurrence of PSP after MT, such as age, sex, AF, DM, dysphagia, and the severity of neurological deficit. Additionally, a stepwise multivariable logistic regression analysis for the independent variables with a univariate analysis of p < 0.1 demonstrated that rescue CS was an independent risk factor for the occurrence of PSP after MT (Table 5). In addition, there was also an independent relationship among the severity of neurological deficit, dysphagia, bridging thrombus ablation, and occurrence of PSP (Table 5).
Table 4.
Univariate analysis influencing the occurrence of pneumonia after thrombectomy in patients with anterior circulation acute ischemic stroke
| No PSP (108) | PSP (56) | p | |
|---|---|---|---|
| Age, mean (SD), years | 67 (13) | 71 (12) | 0.108 |
| Male, n (%) | 56 (51.9%) | 31 (55.4%) | 0.670 |
| Stroke history, n (%) | 14 (13%) | 12 (21.4%) | 0.159 |
| HBP, n (%) | 59 (54.6%) | 31 (55.4%) | 0.929 |
| AF, n (%) | 48 (44.4%) | 33 (58.9%) | 0.079 |
| DM, n (%) | 22 (20.4%) | 7 (12.5%) | 0.210 |
| Smoking, n (%) | 24 (22.2%) | 16 (28.6%) | 0.369 |
| NIHSS, median (IQR) | 13 (9, 18) | 18 (13, 21) | 0.000 |
| Dysphagia, n (%) | 22 (20.4%) | 23 (41.1%) | 0.005 |
| Occlusion site, n (%) | 0.138 | ||
| ICA | 24 (22.2%) | 20 (35.7%) | |
| MCA | 80 (74.1%) | 33 (58.9%) | |
| ICA and MCA | 4 (3.7%) | 3 (5.4%) | |
| Intravenous thrombolysis, n (%) | 33 (30.6%) | 29 (51.8%) | 0.008 |
| ASTIN/SIR = 2/3, n (%) | 84 (77.8%) | 34 (60.7%) | 0.021 |
| Thrombectomy technology, n (%) | 0.575 | ||
| SWIM | 41 (38.0%) | 26 (46.4%) | |
| ADPT | 41 (38.0%) | 18 (32.1%) | |
| SWIM and ADAPT | 26 (24.1%) | 12 (21.4%) | |
| Onset to admission, median (IQR),min | 143 (89, 307) | 180 (78, 290) | 0.690 |
| DPT, median (IQR),min | 137 (100, 187) | 141 (120, 193) | 0.265 |
| Puncture to recanalization, median (IQR),min | 72 (34, 100) | 72 (33, 115) | 0.881 |
| TICI = 2b/3, n (%) | 97 (89.8%) | 46 (82.1%) | 0.163 |
| CS, n (%) | 42 (38.9%) | 33 (58.9%) | 0.015 |
Table 5.
Effect of conscious sedation (CS) vs. local anesthesia (LA) on PSP, unadjusted (model A), adjusted for potential confounding variables (model B), and with additional adjustment for variables with p < 0.05 (model C)
| B | OR | 95% CI | p | |
|---|---|---|---|---|
| Model A | ||||
| CS | 0.718 | 2.050 | 1.052–3.993 | 0.035 |
| Model B | ||||
| CS | 0.692 | 1.997 | 1.006–3.962 | 0.048 |
| Male | − 0.347 | 0.707 | 0.344–1.452 | 0.345 |
| Age | 0.018 | 1.018 | 0.987–1.050 | 0.266 |
| AF | − 0.492 | 1.636 | 0.757–3.535 | 0.211 |
| DM | − 0.196 | 0.822 | 0.310–2.181 | 0.694 |
| Model C | ||||
| CS | 0.737 | 2.091 | 1.011–4.322 | 0.047 |
| NIHSS | 0.088 | 1.092 | 1.022–1.168 | 0.010 |
| Dysphagia | 0.768 | 2.155 | 1.359–7.099 | 0.048 |
| ASTIN/SIR 2–3 | − 0.570 | 0.588 | 0.256–1.252 | 0.160 |
| Intravenous thrombolysis | 0.744 | 2.104 | 1.011–4.379 | 0.047 |
OR odds ratio; CI confident interval; CS conscious sedation; HBP hypertension high blood pressure; DM diabetes mellitus; AF atrial fibrillation; NIHSS National Institutes of Health Stroke Scale; ASTIN/SIR American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology
PSP post-stroke pneumonia; SD standard deviation; IQR interquartile range; HBP hypertension high blood pressure; DM diabetes mellitus; AF atrial fibrillation; NIHSS National Institutes of Health Stroke Scale; ICA internal carotid artery; MCA middle cerebral artery; ASTIN/SIR American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology; SWIM Solitaire retriever stent combined with intracranial support catheter aspiration for mechanical thrombectomy; ADAPT a direct aspiration first-pass technology; DPT door to puncture time; TICI thrombolysis in cerebral infarction; CS conscious sedation
The subgroup analysis of anesthesia methods on the incidence of PSP showed that intraoperative rescue CS significantly increased the incidence of PSP in patients with dysphagia (OR = 7.307, 95% CI: 2.144–24.906, p = 0.001). With the increase of NIHSS severity, the incidence of PSP (OR = 1.155, 95% CI: 1.034–1.290, p = 0.011) increased by 5.1% compared with LA (OR = 1.104, 95% CI: 1.013–1.204, p = 0.024) (Table 6).
Table 6.
Subgroup analysis of the effect of anesthesia methods on the incidence of PSP
| B | OR | 95% CI | p | |
|---|---|---|---|---|
| CS | ||||
| NIHSS | 0.144 | 1.155 | 1.034–1.290 | 0.011 |
| Dysphagia | 1.989 | 7.307 | 2.144–24.906 | 0.001 |
| Intravenous thrombolysis | 0.934 | 2.544 | 0.814–07.950 | 0.108 |
| LA | ||||
| NIHSS | 0.099 | 1.104 | 1.013–1.204 | 0.024 |
| Dysphagia | -0.358 | 0.699 | 0.226–2.163 | 0.534 |
| Intravenous thrombolysis | 0.598 | 1.818 | 0.663–4.983 | 0.245 |
PSP post-stroke pneumonia; CS conscious sedation; LA local anesthesia; NIHSS National Institutes of Health Stroke Scale
Discussion
The anesthesia strategies of MT include GA, CS, and LA, among which CS and LA are often the first choices. Based on real-world case data, this study intends to explore and analyze the impact of rescue CS and LA on the clinical outcomes of patients who receive MT. Notably, we found that intraoperative rescue CS had no significant impact on 90-day good functional outcome, 90-day mortality, and incidence of sICH after MT. However, it was significantly related to the occurrence of PSP.
CS scheme refers to LA combined with sedatives such as dexmedetomidine [18, 19]. Additionally, there are two CS schemes in the real world. Initially, the patients were given sedatives actively before MT to prevent intraoperative agitation. Second, rescue CS was administered because of restlessness during MT. Much of the previous research on the prognostic effect of CS has focused on comparing the differences between CS and LA. Still, the timing of the use of sedatives in the CS scheme has not been extensively investigated. For example, in a French multicenter registry study in which the CS scheme group was given remifentanil preoperatively for MT, the results showed that LA was associated with reduced good outcomes, less successful reperfusion rates, and higher mortality compared with CS [13], suggesting that giving sedatives before MT may be beneficial to clinical outcomes. Meanwhile, in a study by the MR-CLEAN team, the CS scheme group was given sedatives during MT. They reported that this CS scheme led to a worse prognosis at 90 days than LA, and the incidence of PSP (20% vs. 11%, p < 0.01) was higher compared to LA [12], suggesting that giving sedatives during MT may be detrimental to clinical outcomes. In our study, we took the rescue conscious sedation of dexmedetomidine when patients were restless during MT, similar to MR-CLEAN [12], that is, sedative drugs during MT. The results showed that the 90-day good prognosis rate of patients with rescue CS during MT was slightly lower than for LA (44.2% vs. 51.7%, p = 0.418), and the incidence rate of PSP (44% vs. 25.8%, p = 0.015) was higher than for LA. However, there was no significant difference in 90-day mortality and postoperative sICH. Therefore, CS' start timing may affect EVT patients' prognosis differently. Compared with simple LA alone, rescue CS during MT has no significant advantage in improving the good prognosis rate at 90 days. However, it also can increase the incidence of PSP after MT, which may adversely affect patients' clinical outcome.
As a common complication of stroke [20], PSP can increase poor prognosis and mortality [18, 21]. Besides, it often burdens families and society because of increased hospital stays and medical expenses. Additionally, it will help reduce the incidence of PSP by controlling the risk factors [22]. The factors reported in the literature for the occurrence of PSP are not only related to the elderly, atrial fibrillation, male sex, diabetes, dysphagia, stroke severity, and so on [22–26] but also closely related to intravenous thrombolysis and/or mechanical thrombectomy [18]. Then, it is unclear whether CS significantly increases the risk of PSP. Compared with LA, some studies believe that CS increases the incidence of PSP [12], while others believe that CS has no significant impact on the incidence of pneumonia [27]. Notably, our study found that the severity of NIHSS, dysphagia, and bridging thrombectomy were closely related to the occurrence of PSP after incorporating age, sex, AF, DM, and other factors for univariate and multivariate regression analysis, which was similar to the literature reports that intraoperative rescue CS was significant. It was of vital importance that we found factors affecting the increase in the incidence of PSP, supporting the view that CS increased the incidence of PSP, as this was not thoroughly discussed in previous studies. Compared with uncontrollable factors such as old age, atrial fibrillation, male sex, diabetes, dysphagia, and stroke severity, intraoperative rescue CS is a controllable factor, which suggests that rational use of CS by clinicians can reduce the possibility of PSP.
CS and LA are the first choices of anesthesia for EVT compared with GA because of the convenient implementation and short time needed. However, there is no uniform standard for when LA is preferred or CS, as the operator primarily determines this according to the patient's specific situation. For example, the patient's restlessness may be the main reason for selecting CS administration [27]. The CS method in our study is to use remedial sedation based on the intraoperative restlessness of patients. Compared with LA in the subgroup analysis, this anesthesia significantly increased the incidence of PSP in patients with dysphagia (OR = 7.307). Additionally, with the increase of NIHSS severity, the incidence of PSP (OR = 1.155) in this anesthesia increased by 5.1% compared with LA (OR = 1.104). Unlike previous studies, our study has more reference significance for operators to choose anesthesia, suggesting that operators should fully consider the severity of patients’ baseline NIHSS, dysphagia, and other factors likely to induce PSP when choosing anesthesia and trying to avoid rescue CS during MT. For patients with severe symptoms and dysphagia, perhaps preoperative proactive CS is more conducive to good clinical outcomes. However, this requires further prospective large-sample RCT studies.
Our study has several limitations that need to be recognized. First, this retrospective study has a high possibility of selection bias, which may affect the research results. Second, this study only explores and analyzes LA and intraoperative passive CS. If we can compare preoperative active CS simultaneously, it may be more helpful to clarify the impact of CS application timing on patients. Third, this study has a relatively small sample size and is limited to the Chinese population. However, the implications of this study will prompt us to recruit more patients to expand the sample size in future studies, and appropriately designed prospective randomized controlled trials will be a good metric for exploring the relationship between rescue CS during MT and clinical outcomes of anterior circulation acute ischemic stroke in depth.
Conclusion
Our study revealed that rescue CS during MT significantly increased the risk of PSP in patients with anterior circulation acute ischemic stroke. Additionally, it was not superior to LA in improving the 90-day good prognosis of patients and in reducing the incidence of sICH and mortality, which was not conducive to the clinical outcome of patients, especially in patients with severe symptoms and dysphagia.
Acknowledgements
The authors thank all the participants in this study.
Author Contribution
Shilin Li, Gejuan Zhang, Nannan Han, Wenzhen Shi, and Mingze Chang contributed to the conception and design of the study. Shilin Li, Yu Zhang, Yizhuo Tian, Yixuan Xiao, Yinuo Niu, and Xiaojuan Ma collected and analyzed the original data. Shilin Li, Yu Zhang, Yizhuo Tian, Lin Qiao, and Mingze Chang revised the manuscript. Mingze Chang provided the technique and material support. All authors contributed to drafting the paper and were involved in the approval of the final version.
Funding
This study was supported by Xi’an Science and Technology Planning Project (21YXYJ0004, 21YXYJ0052 and 21YXYJ0035), Natural Science Basic Research Project of Shaanxi Province (2022JM-452). The journal’s Rapid Service fee was funded by the authors.
Data availability
The raw data required to reproduce the above findings cannot be shared at this time as the data also forms part of an ongoing study.The data will be shared on reasonable request to the corresponding author.
Editorial Assistance
The authors thank AiMi Academic Services (www.aimieditor.com) for English language editing and review services, which were funded by the authors.
Ethical Approval
This retrospective study involving human participants was reviewed and approved by The Institutional Review Board at the Xi’an No. 3 Hospital (no. SYXSLL-2018-010).Since the observational data we used came from the ongoing cohort, our study did not harm the patients without privacy exposure, and only analyzed baseline data and clinical outcomes, so the informed consent form was waived. This study was conducted in accordance with the Declaration of Helsinki.
Conflict of Interest
Shilin Li, Yu Zhang, Xiaobo Zhang, Gejuan Zhang, Nannan Han, Haojun Ma, Hanming Ge, Yong Zhao, Leshi Zhang, Yanfei Wang, Wenzhen Shi, Xiaojuan Ma, Yizhuo Tian, Yixuan Xiao, Yinuo Niu, Lin Qiao, and Mingze Chang declare that they have no competing interests.
References
- 1.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM, EXTEND-IA Investigators Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 2.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A, REVASCAT Trial Investigators Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 3.Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama-à-Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW, MR CLEAN Investigators A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 4.Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R, SWIFT PRIME Investigators Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD, ESCAPE Trial Investigators Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 6.Brinjikji W, Pasternak J, Murad MH, Cloft HJ, Welch TL, Kallmes DF, Rabinstein AA. Anesthesia-related outcomes for endovascular stroke revascularization: a systematic review and meta-analysis. Stroke. 2017;48(10):2784–2791. doi: 10.1161/STROKEAHA.117.017786. [DOI] [PubMed] [Google Scholar]
- 7.Campbell BCV, van Zwam WH, Goyal M, Menon BK, Dippel DWJ, Demchuk AM, Bracard S, White P, Dávalos A, Majoie CBLM, van der Lugt A, Ford GA, de la Ossa NP, Kelly M, Bourcier R, Donnan GA, Roos YBWEM, Bang OY, Nogueira RG, Devlin TG, van den Berg LA, Clarençon F, Burns P, Carpenter J, Berkhemer OA, Yavagal DR, Pereira VM, Ducrocq X, Dixit A, Quesada H, Epstein J, Davis SM, Jansen O, Rubiera M, Urra X, Micard E, Lingsma HF, Naggara O, Brown S, Guillemin F, Muir KW, van Oostenbrugge RJ, Saver JL, Jovin TG, Hill MD, Mitchell PJ, HERMES Collaborators Effect of general anaesthesia on functional outcome in patients with anterior circulation ischaemic stroke having endovascular thrombectomy versus standard care: a meta-analysis of individual patient data. Lancet Neurol. 2018;17(1):47–53. doi: 10.1016/S1474-4422(17)30407-6. [DOI] [PubMed] [Google Scholar]
- 8.Han H, Wang Y, Wang H, Sun H, Wang X, Gong J, Huo X, Zhu Q, Che F. General anesthesia vs local anesthesia during endovascular treatment for acute large vessel occlusion: a propensity score-matched analysis. Front Neurol. 2022;12:801024. doi: 10.3389/fneur.2021.801024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schönenberger S, Uhlmann L, Hacke W, Schieber S, Mundiyanapurath S, Purrucker JC, Nagel S, Klose C, Pfaff J, Bendszus M, Ringleb PA, Kieser M, Möhlenbruch MA, Bösel J. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA. 2016;316(19):1986–1996. doi: 10.1001/jama.2016.16623. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen CZ, Yoo AJ, Sørensen LH, Juul N, Johnsen SP, Andersen G, Rasmussen M. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. 2018;75(4):470–477. doi: 10.1001/jamaneurol.2017.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LöwhagenHendén P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, Dunker D, Schnabel K, Wikholm G, Hellström M, Ricksten SE. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the AnStroke Trial (Anesthesia During Stroke) Stroke. 2017;48(6):1601–1607. doi: 10.1161/STROKEAHA.117.016554. [DOI] [PubMed] [Google Scholar]
- 12.Goldhoorn RB, Bernsen MLE, Hofmeijer J, Martens JM, Lingsma HF, Dippel DWJ, van der Lugt A, Buhre WFFA, Roos YBWEM, Majoie CBLM, Vos JA, Boiten J, Emmer B, van Oostenbrugge RJ, van Zwam WH. Anesthetic management during endovascular treatment of acute ischemic stroke in the MR CLEAN Registry. Neurology. 2020;94(1):97–106. doi: 10.1212/WNL.0000000000008674. [DOI] [PubMed] [Google Scholar]
- 13.Benvegnù F, Richard S, Marnat G, Bourcier R, Labreuche J, Anadani M, Sibon I, Dargazanli C, Arquizan C, Anxionnat R, Audibert G, Zhu F, Mazighi M, Blanc R, Lapergue B, Consoli A, Gory B, ETIS Registry Investigators Local anesthesia without sedation during thrombectomy for anterior circulation stroke is associated with worse outcome. Stroke. 2020;51(10):2951–2959. doi: 10.1161/STROKEAHA.120.029194. [DOI] [PubMed] [Google Scholar]
- 14.Shen H, Ma X, Wu Z, Shao X, Cui J, Zhang B, Abdelrahim ME, Zhang J. Conscious sedation compared to general anesthesia for intracranial mechanical thrombectomy: a meta-analysis. Brain Behav. 2021;11(6):e02161. doi: 10.1002/brb3.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butt W, Dhillon PS, Podlasek A, Malik L, Nair S, Hewson D, England TJ, Lenthall R, McConachie N. Local anesthesia as a distinct comparator versus conscious sedation and general anesthesia in endovascular stroke treatment: a systematic review and meta-analysis. J Neurointerv Surg. 2022;14(3):221–226. doi: 10.1136/neurintsurg-2021-017360. [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M, STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, Larrue V, Bluhmki E, Davis S, Donnan G, Schneider D, Diez-Tejedor E, Trouillas P. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352(9136):1245–1251. doi: 10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 18.Schaller-Paule MA, Foerch C, Bohmann FO, Lapa S, Misselwitz B, Kohlhase K, Rosenow F, Strzelczyk A, Willems LM. Predicting poststroke pneumonia in patients with anterior large vessel occlusion: a prospective population-based stroke registry analysis. Front Neurol. 2022;13:8450. doi: 10.3389/fneur.2022.824450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou W, Zhang Y, Jiao Y, Yin W, Dong H, Xu S, Tang D, Jiang J, Shao J, Wang Z, Yu W. Dexmedetomidine maintains blood-brain barrier integrity by inhibiting Drp1-related endothelial mitochondrial dysfunction in ischemic stroke. Acta Biochim Biophys Sin (Shanghai) 2021;53(9):1177–1188. doi: 10.1093/abbs/gmab092. [DOI] [PubMed] [Google Scholar]
- 20.Katzan IL, Cebul RD, Husak SH, Dawson NV, Baker DW. The effect of pneumonia on mortality among patients hospitalized for acute stroke. Neurology. 2003;60(4):620–625. doi: 10.1212/01.WNL.0000046586.38284.60. [DOI] [PubMed] [Google Scholar]
- 21.van de Graaf RA, Samuels N, Chalos V, Lycklama A, Nijeholt GJ, van Beusekom H, Yoo AJ, van Zwam WH, Majoie CBLM, Roos YBWEM, van Doormaal PJ, Ben Hassen W, van der Lugt A, Dippel DWJ, Lingsma HF, van Es ACGM, Roozenbeek B, MR CLEAN Registry Investigators Predictors of poor outcome despite successful endovascular treatment for ischemic stroke: results from the MR CLEAN Registry. J Neurointerv Surg. 2022;14(7):660–665. doi: 10.1136/neurintsurg-2021-017726. [DOI] [PubMed] [Google Scholar]
- 22.Yuan MZ, Li F, Tian X, Wang W, Jia M, Wang XF, Liu GW. Risk factors for lung infection in stroke patients: a meta-analysis of observational studies. Expert Rev Anti Infect Ther. 2015;13(10):1289–1298. doi: 10.1586/14787210.2015.1085302. [DOI] [PubMed] [Google Scholar]
- 23.Souza JT, Ribeiro PW, de Paiva SAR, Tanni SE, Minicucci MF, Zornoff LAM, Polegato BF, Bazan SGZ, Modolo GP, Bazan R, Azevedo PS. Dysphagia and tube feeding after stroke are associated with poorer functional and mortality outcomes. Clin Nutr. 2020;39(9):2786–2792. doi: 10.1016/j.clnu.2019.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Zhang Y, Ma L, Niu X, Chang J. Risk of stroke-associated pneumonia during hospitalization: predictive ability of combined A2DS2 score and hyperglycemia. BMC Neurol. 2019;19(1):298. doi: 10.1186/s12883-019-1497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eltringham SA, Kilner K, Gee M, Sage K, Bray BD, Smith CJ, Pownall S. Factors associated with risk of stroke-associated pneumonia in patients with dysphagia: a systematic review. Dysphagia. 2020;35(5):735–744. doi: 10.1007/s00455-019-10061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Yu S, Wei L, Ye R, Lin M, Li X, Li G, Cai Y, Zhao M. The A2DS2 score as a predictor of pneumonia and in-hospital death after acute ischemic stroke in Chinese populations. PLoS ONE. 2016;11(3):e0150298. doi: 10.1371/journal.pone.0150298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels N, van de Graaf RA, van den Berg CAL, Nieboer D, Eralp I, Treurniet KM, Emmer BJ, Immink RV, Majoie CBLM, van Zwam WH, Bokkers RPH, Uyttenboogaart M, van Hasselt BAAM, Mühling J, Burke JF, Roozenbeek B, van der Lugt A, Dippel DWJ, Lingsma HF, van Es ACGM, MR CLEAN Registry Investigators Blood pressure during endovascular treatment under conscious sedation or local anesthesia. Neurology. 2021;96(2):e171–e181. doi: 10.1212/WNL.0000000000011006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data required to reproduce the above findings cannot be shared at this time as the data also forms part of an ongoing study.The data will be shared on reasonable request to the corresponding author.