Abstract
Objective
To critically assess the effect and safety of platelet‐rich plasma (PRP) in chronic wounds and vitiligo.
Methods
A systematic literature searching was performed. Results were expressed as weight mean difference (WMD) or risk ratio (RR) with 95% confidence intervals (CIs). Pooled estimates were performed using a fixed‐effects model or random‐effects model, depending on the heterogeneity among studies.
Results
A total of 27 studies were included in this meta‐analysis. In patients with chronic diabetic ulcers, PRP significantly increased proportion of complete wound healing, percentage of wound area healed, and shortened the complete wound healing. In venous ulcers, PRP improved the epithelialized area and percentage of wound area healed. In vitiligo, PRP had better results in degree of improvement and mean repigmentation than controls. Regarding the safety profile, PRP did not increase the risk of infection in patients with chronic diabetic ulcers. Meta‐regression revealed that source of PRP and preparation method of PRP significantly affected the proportion of complete wound healing, whereas age, gender, country, duration of wound, and wound size had no impact on this outcome.
Conclusion
PRP is effective and safe, and can be used as a potential therapeutic adjunct or alternative treatment in chronic wounds of multiple etiologies and vitiligo.
Keywords: dermatology, platelet‐rich plasma, ulcers, vitiligo
1. INTRODUCTION
Chronic wounds are breaks in the skin that have failed to proceed through a normal, order, and timely produce anatomic and functional integrity; or wounds that proceed through the repair process without restoring anatomic and functional results. 1 When normal healing process is disrupted, a wound can become chronic in nature. Several risk factors can be attributed to poor wound healing, including local causes, systemic disease, and certain medications.
Platelet‐rich plasma (PRP) is an autologous product manufactured from patients’ venous blood, which avoids the risk of disease transmission. 2 PRP is a plasma fraction that contains a higher concentration of growth factors than whole blood, typically 3‐to 7‐fold the mean platelet concentration of whole blood. 3 , 4 , 5 Platelets play an essential role in hemostasis, and they mediate the anabolic effects of PRP by means of the releasing growth factors stored in their alpha granules. 6 These notable growth factors released from platelets interact with the local environment to promote cell differentiation, proliferation, and regeneration, including platelet‐derived growth factor (PDGF), transforming growth factor (TGF‐β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and insulin‐like growth factor (IGF‐1). 7 Leukocytes are central to the inflammatory response, host defense against infectious agents, and wound healing. 7 However, their proinflammatory and immunologic effects can also result in undesirable local cell and tissue damage, which reduce the intended effects of PRP therapy. 6
To date, it has yet to agree on how best to prepare PRP or the optimal concentrations of blood components to include in the product, since each PRP formulation has its unique biologic properties and effects, and this contributes to the different effects of PRP in the clinical trials. The production of PRP begins with the collection of 10–60 mL of whole blood on the day of treatment. Anticoagulants, such as acid citrate dextrose or sodium citrate, are added in the prevention of ex vivo coagulation and premature secretion of the alpha granules. 8 Then the cell types are separated from blood by centrifugation based on the specific gravity. Calcium or thrombin can be added before administration to induce release of a highly concentrated bolus of growth factor to the target tissue. Roh et al. 9 reported that the PRP activated with a low‐dose mixture of thrombin and calcium significantly increased growth factor release over 7 days when compared with nonactivated PRP. However, previous studies 10 , 11 showed inconsistent results regarding the PRP activation, which suggested that activated preparations were less effective in fibroblast differentiation and wound healing, but were equivalent bony regeneration compared with nonactivated preparations. 10 , 11
In the past decade, numerous therapeutic innovations with PRP have been applied into the field of dermatology. Recently, in other condition of dermatology, such as chronic wounds and vitiligo, PRP also has been investigated but received less attention. The present systematic review and meta‐analysis aim to critically assess the effects and safety of PRP in chronic wounds and vitiligo compared to standard treatment or any other alternative therapy.
2. MATERIALS AND METHODS
2.1. Literature search
This meta‐analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. 12
We searched several used electronic databases, including PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials, from their incept to May 15, 2020. The search terms were used as followings: (platelet‐rich plasma OR platelet releasate OR platelet gel OR platelet‐rich fibrin OR PRP) AND (dermatology OR skin OR cutaneous OR wound OR ulcer). There were no restrictions on publication status and language. Additionally, the references provided in the included studies or reviews were also manually searched to identify other reports that were not returned by the electronic searches.
2.2. Inclusion criteria
Clinical trials that focus on conditions of chronic wounds and vitiligo, and investigate the efficacy and safety of PRP are considered eligible for analysis. The predetermined study inclusion criteria were: (1) study design: randomized controlled trial (RCT), cohort study, case‐control study, or comparative trial; (2) population: adult patients aged 18 years or older with ulcers from many cause (such as arterial ulcers, venous ulcers, pressure ulcers, diabetic ulcers, traumatic wounds, and vitiligo); (3) intervention: autologous or allogeneic PRP (any method of collection, formulation and source); (4) control: standard care, placebo or alternative topical therapies; and (5) outcomes: proportion of complete wound healing, total epithelialized area, percentage of wound area healed, time to complete wound healing, wound complications, and adverse events.
2.3. Data extraction
On the basis of inclusion criteria, two independent investigators performed the titles and abstracts of studies. For the included studies, a standardized Excel file was used to extract data from each of the study: author, publication year, region, study design, source of finding, sample size in each study, patients’ characteristics, intervention details (source of PRP‐ autologous vs. allogeneic, methods of liberating growth factors from platelets‐platelet releasate vs. platelet lysate, methods of preparation for PRP‐home made, kits, or blood bank), comparators, and the main outcomes.
2.4. Quality assessment
Two independent investigators performed the assessment of methodological quality. For RCTs, the risk of bias was assessed using the method recommended by Cochrane Collaboration. 13 Methods used in each RCT were examined to test whether they were adequacy performed to generate the allocation sequence, allocation concealment and the level of blinding (clinician, participant or outcome assessor), or whether they presented the complete outcome data and selective reporting. 13 Each RCT was classified as being at high, unclear, or low risk of bias, according to the assessment details for risk of bias provided above.
For non‐RCTs, the methodological quality was evaluated by the modified Newcastle‐Ottawa Scale (NOS). 14 Each study was given scores according to the following items, including patient selection, comparability of the intervention/ control group, and outcome assessment. 14 The total score was 9 points, and higher scores indicated higher quality. A study scored more than 5 points was classified as high quality. 15
2.5. Statistical analysis
Meta‐analysis was performed using the STATA software version 12.0 (Stata Corporation, College Station, TX, USA). Statistical heterogeneity among the included studies was assess using Cochrane Q and I 2 statistic, 16 in which P < 0.1 or I 2 > 50% were considered to be significant. When significant heterogeneity was identified, a random‐effects model was used to pool the estimate, or a fixed‐effects model was applied. We performed a sensitivity analysis to investigate the influence of excluding any single trial on the overall estimate. In addition, we also conducted subgroup analysis based on study design and comparator to explore the potential sources of clinical heterogeneity. We also performed cumulative meta‐analysis to assess the evolution of evidence on PRP effectiveness over time. The studies were sorted out year‐wise and the effect estimates of the studies were added to the pooled estimates of the studies that have accrued till that date. A random‐effect model was used for the cumulative meta‐analysis.
For continuous variables, they were expressed as weight mean difference (WMD) with 95% confidence intervals (CIs); while dichotomous variables were pooled as risk ratio (RR) with 95% CIs. The assessment of publication bias was evaluated by using Egger 17 and Begger 18 test. A P‐value less than 0.05 was judged as statistically significant, except where otherwise specified.
2.6. Mete‐regression
We hypothesized that differences among included studies might be influenced by demographic (mean age, gender, country) and clinical (duration of wound, wound size, source of PRP, preparation methods of PRP) variables. Thus, we performed meta‐regression analysis to explore the possible impacts of these variables on the differences across included studies. In this regression model, proportion of complete wound healing was chosen as a dependent variable (y), and the above‐mentioned covariates were selected as independent variables (x).
3. RESULTS
3.1. Search results
The procedure of literature search and study selection is shown in Figure 1. The initial search identified 1016 publications, of which 394 were excluded because of duplicate records. The 622 articles were screened for title and abstract, and 580 of them were removed because of various reasons. Then 42 articles were left for full‐text information review, and 15 of them were excluded because of single‐arm trial (n = 3), without available data (n = 1), without outcomes of our interest (n = 3), and unrelated with our topics (n = 8). Finally, 27 studies 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 met the inclusion criteria and were included in this meta‐analysis.
FIGURE 1.
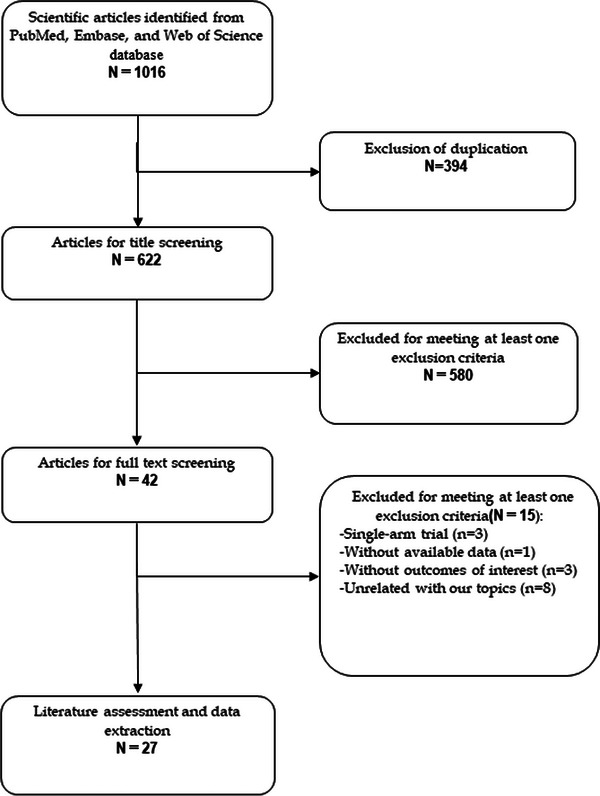
Eligibility of studies for inclusion in meta‐analysis.
3.2. Characteristics of included studies
The main characteristics of included studies are summarized in Table 1. These studies were published between 1992 and 2019. Of the included studies, 16 were RCTs 19 , 20 , 22 , 23 , 24 , 26 , 30 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 and 11 were cohort studies. 21 , 25 , 27 , 28 , 29 , 31 , 32 , 41 , 42 , 43 , 44 The majority were from Egypt, 19 , 23 , 27 , 28 , 31 , 41 , 44 USA, 33 , 34 , 35 , 39 Italy, 21 , 41 India, 25 , 38 and Spain, 30 , 37 with the remaining 10 studies from UK, 20 Korea, 22 Greece, 24 China, 26 Belgium, 29 Poland, 32 France, 36 Hungary, 42 Australia, 40 and Iran. 45 The sample size ranged greatly across the included studies, which ranged from 8 to 364. The mean age between PRP and control groups was comparably distributed, ranging from 24.9 to 82.5 years. Thirteen of the studies included diabetic foot ulcers, 19 , 20 , 21 , 22 , 24 , 26 , 31 , 33 , 34 , 39 , 41 , 43 , 45 six included venous leg ulcers, 27 , 32 , 35 , 37 , 40 five included vitiligo, 23 , 28 , 38 , 42 , 44 two included press ulcers, 25 , 30 and one included acute trauma wounds. 29 The mean wound size at baseline was 12.5 cm2, with 13.7 cm2 in PRP group and 11.2 cm2 in control group.
TABLE 1.
Baseline characteristics of patients in the trials included in the meta‐analysis.
| Study | Country | Study design | Treatment regimen | No. of patients | Male/female | Age (mean ± SD, y) | Wound etiology | NOS |
|---|---|---|---|---|---|---|---|---|
| Ahmed et al. 19 | Egypt | RCT | Autologous PRP | 28 | 20/8 | 43.2 ± 18.2 | Diabetic foot ulcers | NA |
| Antiseptic ointment dressing | 28 | 18/10 | 49.8 ± 15.4 | |||||
| Game et al. 20 | UK | RCT | Autologous PRP+ Standard care | 131 | 107/25 | 61.9 ± 11.4 | Diabetic foot ulcers | NA |
| Standard care | 134 | 110/24 | 62 ± 11.9 | |||||
| Saldalamacchia et al. 21 | Italy | Cohort | Autologous PRP + Standard care | 7 | 4/3 | 61.1 ± 9.4 | Diabetic foot ulcers | 5 |
| Standard care | 7 | 2/5 | 58.1 ± 7.8 | |||||
| Jeong et al. 22 | Korea | RCT | Allogenic PRP | 52 | 27/25 | 64.5 ± 8.1 | Diabetic foot ulcers | NA |
| Topicalfibrinogen and thrombin | 48 | 26/22 | 63.8 ± 6.4 | |||||
| Abdelghani et al. 23 | Egypt | RCT | Autologous PRP | 20 | 9/11 | 34.9 ± 15.39 | Non‐segmental vitiligo | NA |
| Laser | 20 | 6/14 | 29.6 ± 10.80 | |||||
| Kakagia et al. 24 | Greece | RCT | Autologous PRP | 17 | NR | 57 ± 12 | Diabetic foot ulcers | NA |
| Cellulose/collagen biomaterial | 17 | NR | 58 ± 10 | |||||
| Singh et al. 25 | India | Cohort | Autologous PRP | 25 | 19/6 | 36.84 ± 12.67 | Press ulcers | 5 |
| saline dressing | 25 | 19/6 | 36.84 ± 12.67 | |||||
| Li et al. 26 | China | RCT | Autologous PRP+ standard treatment | 59 | 37/22 | 61.4 ± 13.1 | Diabetic foot ulcers | NA |
| standard treatment | 58 | 38/20 | 64.1 ± 9.4 | |||||
| Moneib et al. 27 | Egypt | Cohort | Autologous PRP | 20 | 19/1 | 36.4 ± 10.2 | Chronic venous leg ulcers | 5 |
| conventional therapy | 20 | 20/0 | 32.5 ± 7.5 | |||||
| Ibrahim et al. 28 | Egypt | Cohort | Autologous PRP | 60 | NR | 28 ± 5.65 | Stable vitiligo | 6 |
| Narrowband—ultraviolet B | 60 | NR | 28 ± 5.65 | |||||
| Kazakos et al. 29 | Belgium | Cohort | Autologous PRP | 27 | 14/13 | 36 (20–56) | Acute trauma wounds | 6 |
| Conventional dressings | 32 | 20/12 | 38 (19–52) | |||||
| Ramos‐Torrecillas et al. 30 | Spain | RCT | Autologous PRP | 34 | NR | 82.5 | Press ulcers | NA |
| Standard care | 25 | NR | 82.5 | |||||
| Saad Setta et al. 31 | Egypt | Cohort | Autologous PRP | 12 | NR | 40–60 | Diabetic foot ulcers | 5 |
| Platelet‐poor plasma | 12 | NR | 40–60 | |||||
| Milek et al. 32 | Poland | Cohort | Autologous PRP | 50 | 16/34 | 53–89 | Venous leg ulcers | 6 |
| conventional hydrocolloid dressings | 50 | 11/39 | 54–79 | |||||
| Steed et al. 33 | USA | RCT | Allogenic PRP | 7 | 5/2 | 58.7 ± 12.4 | Diabetic foot ulcers | NA |
| Saline dressings | 6 | 4/2 | 54.2 ± 12.9 | |||||
| Steed et al. 34 | USA | RCT | Allogenic PRP | 11 | NR | NR | Diabetic foot ulcers | NA |
| Saline dressings | 5 | NR | NR | |||||
| Escamilla Cardeñosa et al. 35 | USA | RCT | Autologous PRP | 55 | 15/40 | 65 ± 13.72 | Venous leg ulcers | NA |
| Standard care | 47 | 16/31 | 69 ± 16.26 | |||||
| De Angelis et al. 41 | Italy | Cohort | Autologous PRP | 182 | NR | NR | Diabetic foot ulcers | 7 |
| Hyaluronic acid | 182 | NR | NR | |||||
| Senet et al. 36 | France | RCT | Autologous PRP | 7 | 4/3/ | 72.3 (45–88) | Venous leg ulcers | NA |
| Saline dressings | 6 | 3/3 | 72.3 (50–83) | |||||
| Burgos‐Alonso et al. 37 | Spain | RCT | Autologous PRP | 5 | 2/3 | 76.6 ± 8.7 | Venous leg ulcers | NA |
| Standard care | 3 | 3/0 | 69.7 ± 11.1 | |||||
| Kadry et al. 42 | Hungary | Cohort | Autologous PRP | 30 | NR | NR | Vitiligo | 6 |
| Laser | 30 | NR | NR | |||||
| Serra et al. 43 | Italy | Cohort | Autologous PRP | 26 | 19/7 | 67.5 (43–85) | Diabetic foot ulcers | 6 |
| Standard care | 32 | 27/5 | 63.5 (41–79) | |||||
| Khattab et al. 44 | Egypt | Cohort | Autologous PRP | 26 | 4/22 | 25.42 ± 7.6 | Vitiligo | 6 |
| Excimer laser | 26 | 6/20 | 24.9 ± 5.6 | |||||
| Parambath et al. 38 | India | RCT | Autologous PRP | 20 | NR | NR | Vitiligo | NA |
| Phosphate buffered saline | 20 | NR | NR | |||||
| Driver et al. 39 | USA | RCT | Autologous PRP | 40 | 32/8 | 56.4 ± 8.1 | Diabetic foot ulcers | NA |
| Saline dressings | 32 | 27/5 | 57.5 ± 9.1 | |||||
| Karimi et al. 45 | Iran | RCT | Autologous PRP | 25 | 20/5 | NR | Diabetic foot ulcers | NA |
| Saline dressings | 25 | 18/7 | NR | |||||
| Stacey et al. 40 | Australia | RCT | Autologous PRP | 42 | 15/27 | 72 (35–90) | Venous leg ulcers | NA |
| Placebo | 44 | 21/23 | 70 (26–92) |
Abbreviations: NR, not reported; PRP, platelet rich plasma; RCT, randomized control trial; SD, standard deviation.
The source of PRP varied across the included studies, with twenty‐four studies 19 , 20 , 21 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 35 used patients’ own blood to obtain a concentrate of platelets, and the remaining three studies used allogenic PRP. 22 , 33 , 34
3.3. Risk of bias
Figure 2 presented a summary of the risk of bias assessment in RCTs. Overall, seven studies 19 , 22 , 26 , 34 , 38 , 40 , 45 were classified as low risk of bias, six studies 20 , 24 , 30 , 33 , 35 , 36 regarded as unclear risk of bias, and three studies 23 , 37 , 39 were considered to be at high risk of bias. The most common reasons for the studies that were regarded as high risk of bias were that they did not blind to participants or personnel, or the outcome assessors; considering the nature of these studies, they might have a high risk of selection or performance bias or both. In other studies that were classified as unclear risk of bias, most of them did not adequately describe how they perform the random sequence generation or allocation concealment, or the methods for blinding.
FIGURE 2.
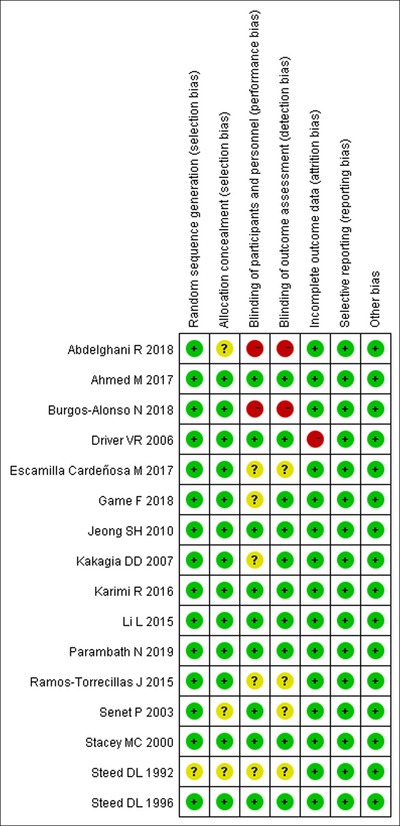
Risk of bias summary.
For non‐RCTs, the NOS score of each study was presented in Table 1. Overall, all the studies were given a score more than 5 points, which indicated that these studies were of high quality.
3.4. Chronic diabetic ulcers
3.4.1. Proportion of complete wound healing
Ten studies 19 , 20 , 21 , 22 , 26 , 33 , 34 , 39 , 43 , 45 with 11 sets of data reported the data of complete wound healing. Pooled estimate showed that, PRP significantly increased the proportion of complete wound healing as compared with control (RR = 1.53, 95% CI: 1.37, 1.72; P < 0.001) (Figure 3). There is no significant heterogeneity across the included studies (I 2 = 48.9%, P = 0.034).
FIGURE 3.
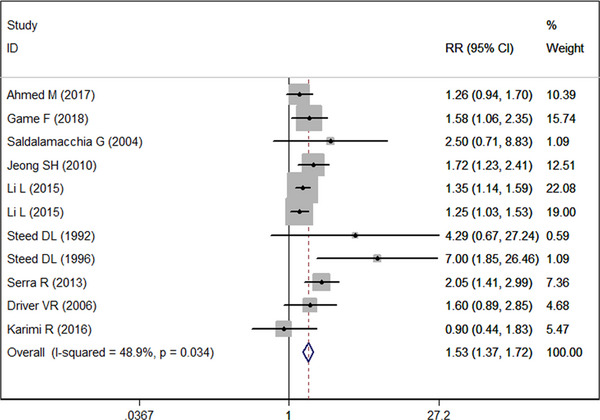
Forest plot showing the effect of PRP on wound healing rate in diabetic ulcers.
Subgroup analysis based on the comparator showed that, PRP was associated with a significantly higher proportion of complete wound healing than standard care (RR = 1.50, 95% CI: 1.31, 1.72; P < 0.001), topical fibrinogen and thrombin (RR = 1.72, 95% CI: 1.23, 2.41; P = 0.002), or saline dressings (RR = 1.78, 95% CI: 1.17, 2.72; P = 0.007). However, it had no superior effect than antiseptic ointment dressing (RR = 1.26, 95% CI: 0.94, 1.70; P = 0.122).
Subgroup analysis based on study design suggested that, the superior effect of PRP over control in terms of complete wound healing was observed in only RCTs (RR = 1.46, 95% CI: 1.23, 1.74; P < 0.001) but not in cohort studies (RR = 1.59, 95% CI: 0.99, 2.55; P = 0.056).
3.4.2. Percentage of wound area healed
Five studies 21 , 22 , 24 , 41 , 45 reported the data of percentage of wound area healed. PRP significantly improved the percentage of wound area healed than controls (WMD = 21.18%, 95% CI: 0.99%, 41.37%; P = 0.04) (Figure 4). There was significant heterogeneity across the included studies (I 2 = 98.9%, P < 0.001). Thus, we conducted sensitivity analysis. When we excluded the outlier, 21 the overall estimate changed a little (WMD = 11.25%, 95% CI: 10.64%, 11.87%; P = 0.02), but the heterogeneity was still present (I 2 = 99.2%, P < 0.001). When we further excluded any single study, the pooled WMD value ranged from 10.45% (95% CI: 9.83, 11.06; P < 0.001) to 11.47% (95% CI: 10.65, 11.87; P < 0.001). However, the evidence of heterogeneity was still present across the remaining studies.
FIGURE 4.
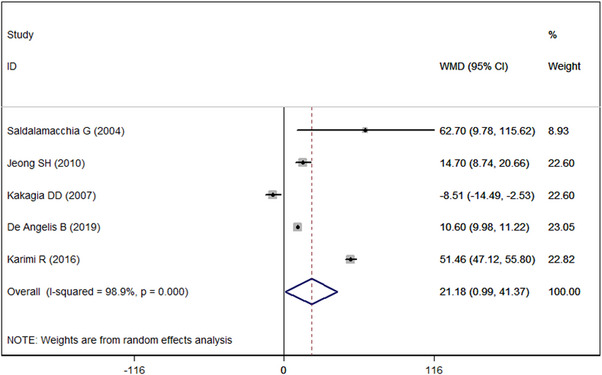
Forest plot showing the effect of PRP on percentage of wound area healed in diabetic ulcers.
Since there was paucity of available data on the percentage of wound area healed, we did not conduct subgroup analysis.
3.4.3. Total area epithelialized
Four studies 21 , 24 , 39 , 45 reported the data of total area epithelialized. PRP did not increase the epithelialized area compared to control (WMD = 0.26 cm2, 95% CI: −2.29, 2.82; P = 0.841). The test for heterogeneity was significant (I 2 = 90.1%, P < 0.001).
3.4.4. Time to complete wound healing
Four studies 20 , 22 , 31 , 39 reported the data of mean healing time. The mean time taken for wound healing was significantly less in the PRP group than in control group (WMD = −16.52 days, 95% CI: −25.17, −7.87; P < 0.001). Significant heterogeneity was identified among the included studies (I 2 = 86.7%, P < 0.001).
3.4.5. Wound area closure rate (cm2/week)
Three studies 19 , 33 , 39 reported the data of wound area closure rate. PRP did not significantly increase the wound area closure rate compared to control (WMD = −0.10 cm2/week, 95% CI: −0.28, 0.07; P = 0.234). The test for heterogeneity was significant (I 2 = 91.4%, P < 0.001).
3.4.6. Overall infection
Two studies 19 , 20 reported the data of infections. PRP did not increase the risk of infection compared to control (RR = 0.77, 95% CI: 0.58, 1.01; P = 0.438). There was no significant heterogeneity among the studies (I 2 = 0.0%, P = 0.438).
3.5. Venous ulcers
3.5.1. Proportion of complete wound healing
Two studies 36 , 40 reported the data of proportion of complete wound healing. The complete wound healing rate in PRP and control group was 69.4% and 70.0%, respectively. PRP had similar proportion of complete wound healing with control (RR = 1.01, 95% CI: 0.80, 1.27; P = 0.921). The test for heterogeneity was not significant (I 2 = 0.0%, P = 0.893).
3.5.2. Total area epithelialized
Four studies 27 , 32 , 35 , 37 reported the data of total area epithelialized. PRP significantly improved the epithelialized area in patients with venous ulcers when compared to control (WMD = 0.90 cm2, 95% CI: 0.73, 1.08; P < 0.001). There was no significant heterogeneity among the studies (I 2 = 0.0%, P = 0.521).
3.5.3. Percentage of wound area healed
Four studies 27 , 35 , 36 , 37 reported the data of percentage of wound area healed. A significantly greater area was healed in PRP group than in control group (WMD = 40.27%, 95% CI: 12.69%, 67.85%; P = 0.004). The test for heterogeneity was significant (I 2 = 84.0%, P < 0.001).
3.6. Pressure ulcers
3.6.1. Percentage of wound area healed
Two studies 25 , 30 reported the data of percentage of wound area healed. The percentage of wound area healed was significantly greater in PRP group than in control group (WMD = 52.21%, 95% CI: 35.87%, 68.55%; P < 0.001). The test for heterogeneity was significant (I 2 = 85.9%, P < 0.001).
3.7. Vitiligo
3.7.1. Degree of improvement – excellent and good response
Two studies 28 , 44 reported the data of degree of improvement. PRP was associated with a significantly greater degree of improvement than control (RR = 7.11, 95% CI: 3.88, 13.01; P < 0.001). The test for heterogeneity was significant (I 2 = 84.2%, P = 0.012).
3.7.2. Patient satisfaction
Two studies 23 , 38 reported the data of patient satisfaction. PRP had similar result in patient satisfaction with control (WMD = 1.43%, 95% CI: −18.14%, 21.0%; P = 0.886). The test for heterogeneity was significant (I 2 = 76.9%, P = 0.0038).
3.7.3. Mean repigmentation (%) by area
Two studies 38 , 42 reported the data of mean repigmentation by area. PRP resulted in significantly greater mean repigmentation than control (WMD = 13.73%, 95% CI: 3.28%, 24.18%; P = 0.010). There was no significant heterogeneity among the included studies (I 2 = 0.0%, P = 0.716).
3.8. Acute traumatic wounds
Since only one study 29 reported the use of PRP as aid in the management of acute trauma wounds, we did not perform meta‐analysis. Kazakos et al. carried out an open‐label RCT in 59 patients with acute wounds (open fracture, closed fractures with skin necrosis and friction burns) not requiring coverage with flap. 29 Compared with control, local application of PRP weekly for 3 weeks resulted in higher wound healing rate at week 1, 2 and 3 (P = 0.003, P < 0.001, and P < 0.001, respectively), and faster plastic reconstruction (21.26 ± 1.35 days vs. 40.6 ± 5.27 days, P < 0.001). 29
3.9. Meta‐regression
In order to further assess the impact of the clinical and demographic variables on the proportion of complete wound healing, meta‐regression analysis with a random‐effects model was performed. Results in Table 2 showed that source of PRP (standard error, SE = 0.92, t = 2.79, P = 0.021)and preparation method of PRP (SE = 0.43, t = −2.65, P = 0.027)affected the difference in proportion of complete wound healing between PRP and control groups; whereas the age (SE = 1.04, t = −1.07, P = 0.312), gender (SE = 1.07, t = 1.74, P = 0.116), country (SE = 0.29, t = 0.85, P = 0.416), duration of wound (SE = 0.92, t = 1.96, P = 0.082), and wound size (SE = 0.79, t = 0.99, P = 0.346) did not have significant influence on this outcome.
TABLE 2.
Results of meta‐regression analysis for the impact of clinical and demographic data in the proportion of complete wound healing.
| Variable | Coefficient | SE | t | P | 95% CI |
|---|---|---|---|---|---|
| Age | −1.115235 | 1.04 | −1.07 | 0.312 | −3.47, 1.24 |
| Gender | 1.866556 | 1.07 | 1.74 | 0.116 | −0.56, 4.30 |
| Country | 0.2462904 | 0.29 | 0.85 | 0.416 | −0.41, 0.90 |
| Duration of wound | 1.799416 | 0.92 | 1.96 | 0.082 | −0.28, 3.88 |
| Wound size | 0.7812874 | 0.79 | 0.99 | 0.346 | −1.00, 2.56 |
| Source of PRP | 2.549384 | 0.92 | 2.79 | 0.021 | 0.48, 4.62 |
| Preparation of PRP | −1.33735 | 0.43 | −2.65 | 0.027 | −2.10, −1.16 |
Abbreviations: 95% CI: 95% confidence interval; PRP, platelet‐rich plasma; SE, standard error.
3.10. Cumulative meta‐analysis
Year‐wise cumulative meta‐analysis was performed for the comparison between PRP and control in the proportion of complete wound healing. We observed that the evidence for the effect of PRP is available from the year 1992 and further studies just shortened the CI of overall effect size without change in the magnitude or direction of the estimates (Figure 5). From the year 2006, with the addition of estimate from new studies, the overall estimate remained relatively stable, which ranged from 1.49 to 2.65. This confirmed the reliable and significant effect of PRP in the proportion of complete wound healing.
FIGURE 5.
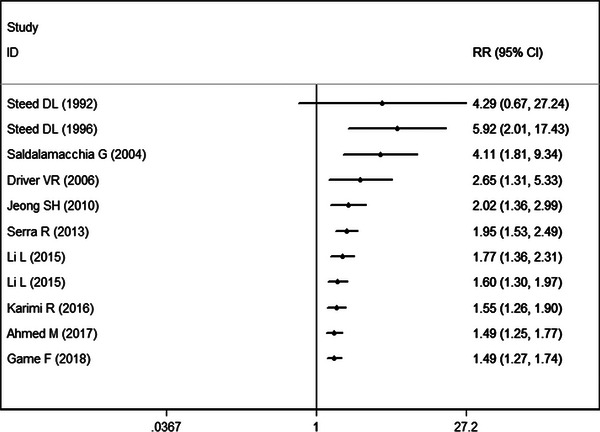
Forest plot showing the cumulative meta‐analysis by year‐wise for PRP with control group.
3.11. Publication bias
Egger's and Begg test were used to assess the publication bias, and results revealed that, there was no publication bias among the included studies (Egger's test: t = −1.32, P = 0.264; Begg test: Z = 0.58, P = 0.639).
4. DISCUSSION
This present meta‐analysis systematically and comprehensively investigated the utility of PRP in the treatment of ulcers of multiple etiologies across 27 studies. Our results demonstrated that PRP improved the complete wound healing rate, shorted the time to complete wound healing, while did not increase the risk of infection. Meta‐regression analysis revealed that, source of PRP and preparation method of PRP had a potential influence on the complete wound healing, but age, gender, country, duration of wound, wound size, and preparation method of PRP did not. Cumulative meta‐analysis suggested that the positive result was robust, which was unlikely to be overturned by further studies. Regarding the safety profile, PRP did not increase the risk of wound infection in patients with chronic diabetic ulcers.
This study has revealed the apparent effects of PRP applied in dermatological field in the management of ulcers of multiple etiologies. Despite there are increasing number of studies that have reported the benefit of PRP, no uniform standard has been used in their studies for the preparation of PRP. Different methods for PRP preparation might result in different therapeutic effects. And this has been confirmed in the present study. Meta‐regression suggested that preparation method of PRP had a significant impact on the wound healing rate (SE = 0.43, t = −2.65, P = 0.027). Two steps were followed in the preparation of PRP: preparing and activating PRP. The first step is responsible for the effect differences between different methods. Two‐step processing is widely applied in the first step. Sonnleitner, D 46 and Landesberg, R3 reported that, the recovery rate of platelets is higher when centrifugal force is low for the first time and high for the second time.
The centrifugation speeds used in each included studies varied greatly, which limited the ability to propose an optimal technique for collecting PRP. Previous research reported that, the highest platelet capture efficiency while preserving platelet function was noted with a first spin at 160 g for 10 min and a second spine at 250 g for 15 min. 47 However, the optimal PRP platelet concentration exists. When PRP contains 2–4 fold the peripheral platelet concentration, the fibroblastic proliferation and hyaluronic acid production would present the best effects, whereas angiogenesis decreases when platelet concentrations rise above 1.5 million platelets/μL. 48 , 49
For the chronic wound healing, PRP exhibits various advantages. First, it has the local microbicidal effects. 50 , 51 Second, PRP also plays an important role in the facilitation of ulcer healing, since it changes the matrix metalloproteinase and cytokine expression within 2 weeks after topical application. 52 The leukocyte inclusion in PRP might contribute to the inflammation because of induction of nuclear factor kappa β. 53 However, in the included studies, ulcers patients treated with leukocyte PRP achieved a higher percentage of complete healing than control. 19 , 20 , 27 , 31 This result contradicts to the concerns that high level of leukocyte in PRP might lead to deleterious inflammatory effects on the wound healing. PRP is widely regarded as safe without serious potential risk. However, a recent study reported that PRP was associated with vascular compromise and irreversible blindness for the periorbital rejuvenation. 54 This adverse event is seldom seen in the treatment of chronic ulcers and vitiligo since PRP is typically not used in the face, and is topical applied in most studies. Despite all this, dermatologists should proceed with caution duration injections of PRP.
In the present meta‐analysis, we found that patient's demographic and clinical characteristics, such as age, gender, wound size, and duration of size had no significant impact on the wound healing rate. Our result was in consistent with the finding of study reported by Oyibo et al. 55 In that study, the author carried out a 12‐month period research in diabetic patients with new foot ulcers to test the effects of ulcer size, patients’ age, sex and duration of diabetes on the outcome of diabetic foot ulcers. Cox regression analysis showed that, age [hazard ratio (HR) = 0.99, 95% CI: 0.97, 1.01; P = 0.31], sex (HR = 0.78, 95% CI: 0.51, 1.18; P = 0.26), ulcer size had no effect on the ulcer healing time; whereas ulcer size had an impact on the outcome (HR = 1.08, 95% CI: 1.01, 1.14; P = 0.04).
The source of PRP and preparation method of PRP in this study was found to have impact on the wound healing. Autologous PRP had higher proportion of complete wound healing than allogenic PRP. Ahmed et al. 19 reported that 96.15% of patients had healed ulcers by autologous PRP. Whereas Steed et al. 33 found that only 71.4% of patients in the allogenic PRP‐ treated group were healed. In addition to the advantaged effect, an autologous source would avoid any potential risk for transmissible diseases of graft‐versus‐host disease. 56
PRP releasate resulted in a higher proportion of complete wound healing than control, whereas PRP lysate had similar results with control. Our results were in agreement with the findings of the previously published studies. 47 , 48 , 49 Anitua et al. 47 performed a randomized open‐label control trial to assess the effectiveness of autologous preparation rich in growth factors (PRGF) in the treatment of chronic cutaneous ulcers. Their results showed that the mean percentage of surface healed in PRGF releasate group was higher (72.94% ± 22.25%) than standard care group (21.48% ± 33.56%) (P < 0.05). 47 Whereas in another randomized, double‐blind, placebo‐controlled trial, the authors found opposite results of PRP lysate for the treatment of chronic leg ulcerations. 49 In that study, 15 and 11 patients received autologous platelet lysate product mixed with collagen or platelet‐poor plasma mixed with collagen, respectively. 49 At the 28 weeks, there was no significant difference between the two groups in the rate of complete healing and area of wounds. 49
There were several other published meta‐analysis or systematic reviews that investigated the efficacy of PRP in wound care. Del Pino‐Sedeno et al. 57 and Li et al. 58 performed a meta‐analysis in diabetic foot ulcers and demonstrated the positive effects of PRP in wound healing. However, their results were limited by the low quality of included studies or potential confound factors. Martinez‐Zapata et al. performed a meta‐analysis focusing on autologous PRP for chronic wound in 2012 59 and an update meta‐analysis in 2016. 60 There was no definitive conclusion since the findings were based on low quality of small RCTs and had not sufficient statistical power. Hesseler et al. 8 reported the first systematic review of PRP applied in the conditions of medical dermatology. However, it was a narrative review but not a meta‐analysis. Whether PRP had better effects than control was not qualitatively and quantitatively assessed.
The present study expands on the previous systematic reviews or meta‐analysis to provide better evidence for PRP in the treatment of ulcers of multiple etiologies. First, this study included more studies and greater sample size than the previous analysis, which improved the statistical power. Second, there were substantial variations across the included studies, including patients’ demographic (age, gender, and country) and clinical characteristics (wound size, duration of wound, source of PRP, and preparation method of PRP). Considering that these variables might have an impact on the differences between treatments, we conducted meta‐regression analysis to detect the influence of these factors on the outcome. Furthermore, we also conducted cumulative meta‐analysis to assess the adequacy of evidence present till the date of analysis. Results showed that the addition of new clinical trials did not significantly change but narrowed the CI surrounding the pooled estimate. This confirms the robustness of our findings, and also indicates that there is no need of further clinical trials to focus on this outcome. However, these analyses were not performed in the previous reviews.
There is increasing interest in using PRP for the treatment of chronic wounds of multiple etiologies and vitiligo. This is because it contains growth factors which are thought to aid wound repair. Although PRP is extensively applied in the clinical practice, the evidence on its efficacy is still controversial since different results are obtained among the clinical studies. Meta‐analysis can systematically summarize current original studies on a specific topic, and provided some implications for future researches and decision making, especially controversial topics. Using meta‐analysis, our results support the conclusion that PRP can effectively improve symptoms as well as accelerate wound healing with acceptable safety. Thus, it could be considered as adjunctive therapy for the chronic wounds and vitiligo.
In addition to its effects on chronic wounds and vitiligo, PRP has also shown promising results in hair regrowth, female alopecia, and bone tissue defects. 61 , 62 , 63 , 64 A randomized placebo‐controlled trial by Gentile et al. 61 reported that three treatment cycles of PRP significantly improved the mean number of hairs, with a mean increase of 33.6 hairs in the target area and a mean increase in total hair density of 45.9 hairs per cm2 compared with baseline values. Similar findings were observed in a retrospective, blind, randomized trial, 62 where hair density measurements for patients treated with autologous activated PRP and non‐activated PRP significantly improved during a long‐term follow‐up of 58 weeks. Furthermore, in a systematic review (SR) assessing the safety and efficacy of PRP in female androgenetic alopecia (F‐AGA), five studies were included, and the results demonstrated a positive effect of PRP for F‐AGA treatment without major side effects. 63 Apart from PRP, stem cell therapy and biotechnology have also shown effectiveness in regenerative surgery for wound healing and soft tissue defects. Notable treatments include adipose‐derived mesenchymal stem cells (AD‐MSCs), stromal vascular fraction cells (SVFs), human follicle stem cells (HFSCs), and dermal substitutes. Clinical trials and SR have confirmed the efficacy of these treatment strategies in soft tissue defects and chronic wounds without major side effects. 65 , 66 , 67 For instance, a SR analyzing 72 articles on the use of AD‐MSCs, PRP, and biomaterials in chronic skin wounds and soft tissue defects showed their safety and efficacy. 66 Similarly, another SR comprising 18 articles describing outcomes in patients treated with AD‐MSCs, SVF, and F‐GRF reported the effectiveness and safety of these treatments in wound healing and scar treatment without major side effects. 65
There were several potential limitations in this study. First, there was significant heterogeneity across the included studies. However, one should not be surprising given the variability in inclusion criteria and exclusion criteria, wound size, duration of wound, preparation method of PRP, study design, and the comparators. These factors might account for the heterogeneity and have potential impact on our results. Second, some of the included studies had a relatively small sample size, which can limit the power to detect changes that might reach the threshold for a minimal clinically important difference in outcome measures. Third, the duration of observation period varied across the studies, which ranged from 2 to 12 weeks. For studies with shorter duration of follow‐up period, the ulcer healing and adverse events associated with long‐term follow‐up period are unable to be observed. Thus, our results might be biased by these incomplete data. Physicians should take into account this when interpreting results of present meta‐analysis.
Although the sources of heterogeneity across the included studies were difficult to identify, all of our results were confirmed in all subgroups and sensitivity analysis, and no publication bias was observed in the analysis. Moreover, in order to explore the effect of potential confounders on the different wound healing between PRP and control, we carried out meta‐regression, and all results were confirmed. None of these factors (age, gender, country, duration of wound, and wound size) was identified as a confounder responsible for the source of bias, which indicated the robustness our results. We also performed cumulative meta‐analysis, sequentially adding studies by year of publication into the pooled estimate, to explore the evolution of evidence on PRP effect over time. We observed that the CI of overall estimate just narrowed with the addition of studies, but the direction had not been changed. This confirmed the reliable and consistent benefit effect of PRP in the improvement of wound healing. And there is no need of further trials to identify this outcome since it is impossible to overturn this positive result.
5. CONCLUSION
Despite some limitations, this study provided an assessment of the effectiveness and safety of PRP for the treatment of chronic wounds of multiple etiologies and vitiligo, using meta‐regression and cumulative meta‐analysis. PRP presented benefit effects of more rapid or increased wound healing. Thus, it could be used as a promising therapeutic adjunct or alternative treatment. However, considering the limitations in this study, more large‐scale, high quality RCTs are needed to verify our findings.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Wang Z, Feng C, Chang G, Liu H, Li S. The use of platelet‐rich plasma in wound healing and vitiligo: A systematic review and meta‐analysis. Skin Res Technol. 2023;29:1–13. 10.1111/srt.13444
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489‐493. [PubMed] [Google Scholar]
- 2. Giordano S, Romeo M, Lankinen P. Platelet‐rich plasma for androgenetic alopecia: does it work? Evidence from meta analysis. J Cosmet Dermatol. 2017;16(3):374‐381. 10.1111/jocd.12331 [DOI] [PubMed] [Google Scholar]
- 3. Landesberg R, Roy M, Glickman RS. Quantification of growth factor levels using a simplified method of platelet‐rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58(3):297‐300; discussion 300‐291. 10.1016/s0278-2391(00)90058-2 [DOI] [PubMed] [Google Scholar]
- 4. Marx RE. Platelet‐rich plasma (PRP): what is PRP and what is not PRP? Implant Dent. 2001;10(4):225‐228. 10.1097/00008505-200110000-00002 [DOI] [PubMed] [Google Scholar]
- 5. Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet‐rich plasma produced by 2 different methods: curasan‐type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implants. 2002;17(2):184‐190. [PubMed] [Google Scholar]
- 6. Wu PI, Diaz R, Borg‐Stein J. Platelet‐rich plasma. Phys Med Rehabil Clin N Am. 2016;27(4):825‐853. 10.1016/j.pmr.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 7. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet‐rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429‐439. 10.1016/j.arthro.2011.10.018 [DOI] [PubMed] [Google Scholar]
- 8. Hesseler MJ, Shyam N. Platelet‐rich plasma and its utility in medical dermatology: a systematic review. J Am Acad Dermatol. 2019;81(3):834‐846. 10.1016/j.jaad.2019.04.037 [DOI] [PubMed] [Google Scholar]
- 9. Roh YH, Kim W, Park KU, Oh JH. Cytokine‐release kinetics of platelet‐rich plasma according to various activation protocols. Bone Joint Res. 2016;5(2):37‐45. 10.1302/2046-3758.52.2000540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeon YR, Jung BK, Roh TS, et al. Comparing the effect of nonactivated platelet‐rich plasma, activated platelet‐rich plasma, and bone morphogenetic protein‐2 on calvarial bone regeneration. J Craniofac Surg. 2016;27(2):317‐321. 10.1097/scs.0000000000002349 [DOI] [PubMed] [Google Scholar]
- 11. Scherer SS, Tobalem M, Vigato E, et al. Nonactivated versus thrombin‐activated platelets on wound healing and fibroblast‐to‐myofibroblast differentiation in vivo and in vitro. Plast Reconstr Surg. 2012;129(1):46e‐54e. 10.1097/PRS.0b013e3182362010 [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Higgins JP, Altman DG, Gotzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells G, Shea B, O'connell D, Peterson J, Welch V. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics. 2000:3‐5.
- 15. Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401‐406. 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 16. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327(7414):557‐560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088‐1101. [PubMed] [Google Scholar]
- 19. Ahmed M, Reffat SA, Hassan A, Eskander F. Platelet‐rich plasma for the treatment of clean diabetic foot ulcers. Ann Vasc Surg. 2017;38:206‐211. 10.1016/j.avsg.2016.04.023 [DOI] [PubMed] [Google Scholar]
- 20. Game F, Jeffcoate W, Tarnow L, et al. LeucoPatch system for the management of hard‐to‐heal diabetic foot ulcers in the UK, Denmark, and Sweden: an observer‐masked, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6(11):870‐878. 10.1016/s2213-8587(18)30240-7 [DOI] [PubMed] [Google Scholar]
- 21. Saldalamacchia G, Lapice E, Cuomo V, et al. A controlled study of the use of autologous platelet gel for the treatment of diabetic foot ulcers. Nutr Metab Cardiovasc Dis. 2004;14(6):395‐396. [DOI] [PubMed] [Google Scholar]
- 22. Jeong SH, Han SK, Kim WK. Treatment of diabetic foot ulcers using a blood bank platelet concentrate. Plast Reconstr Surg. 2010;125(3):944‐952. 10.1097/PRS.0b013e3181cb6589 [DOI] [PubMed] [Google Scholar]
- 23. Abdelghani R, Ahmed NA, Darwish HM. Combined treatment with fractional carbon dioxide laser, autologous platelet‐rich plasma, and narrow band ultraviolet B for vitiligo in different body sites: a prospective, randomized comparative trial. J Cosmet Dermatol. 2018;17(3):365‐372. 10.1111/jocd.12397 [DOI] [PubMed] [Google Scholar]
- 24. Kakagia DD, Kazakos KJ, Xarchas KC, et al. Synergistic action of protease‐modulating matrix and autologous growth factors in healing of diabetic foot ulcers. A prospective randomized trial. J Diabetes Complications. 2007;21(6):387‐391. 10.1016/j.jdiacomp.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 25. Singh R, Rohilla RK, Dhayal RK, Sen R, Sehgal PK. Role of local application of autologous platelet‐rich plasma in the management of pressure ulcers in spinal cord injury patients. Spinal Cord. 2014;52(11):809‐816. 10.1038/sc.2014.144 [DOI] [PubMed] [Google Scholar]
- 26. Li L, Chen D, Wang C, et al. Autologous platelet‐rich gel for treatment of diabetic chronic refractory cutaneous ulcers: a prospective, randomized clinical trial. Wound Repair Regen. 2015;23(4):495‐505. 10.1111/wrr.12294 [DOI] [PubMed] [Google Scholar]
- 27. Moneib HA, Youssef SS, Aly DG, Rizk MA, Abdelhakeem YI. Autologous platelet‐rich plasma versus conventional therapy for the treatment of chronic venous leg ulcers: a comparative study. J Cosmet Dermatol. 2018;17(3):495‐501. 10.1111/jocd.12401 [DOI] [PubMed] [Google Scholar]
- 28. Ibrahim ZA, El‐Ashmawy AA, El‐Tatawy RA, Sallam FA. The effect of platelet‐rich plasma on the outcome of short‐term narrowband‐ultraviolet B phototherapy in the treatment of vitiligo: a pilot study. J Cosmet Dermatol. 2016;15(2):108‐116. 10.1111/jocd.12194 [DOI] [PubMed] [Google Scholar]
- 29. Kazakos K, Lyras DN, Verettas D, Tilkeridis K, Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury. 2009;40(8):801‐805. 10.1016/j.injury.2008.05.002 [DOI] [PubMed] [Google Scholar]
- 30. Ramos‐Torrecillas J, Garcia‐Martinez O, De Luna‐Bertos E, Ocana‐Peinado FM, Ruiz C. Effectiveness of platelet‐rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol Res Nurs. 2015;17(2):152‐158. 10.1177/1099800414535840 [DOI] [PubMed] [Google Scholar]
- 31. Saad Setta H, Elshahat A, Elsherbiny K, Massoud K, Safe I. Platelet‐rich plasma versus platelet‐poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8(3):307‐312. 10.1111/j.1742-481X.2011.00797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Milek T, Nagraba L, Mitek T, et al. Autologous platelet‐rich plasma reduces healing time of chronic venous leg ulcers: a prospective observational study. Adv Exp Med Biol. 2019;1176:109‐117. 10.1007/5584_2019_388 [DOI] [PubMed] [Google Scholar]
- 33. Steed DL, Goslen JB, Holloway GA, Malone JM, Bunt TJ, Webster MW. Randomized prospective double‐blind trial in healing chronic diabetic foot ulcers. CT‐102 activated platelet supernatant, topical versus placebo. Diabetes Care. 1992;15(11):1598‐1604. 10.2337/diacare.15.11.1598 [DOI] [PubMed] [Google Scholar]
- 34. Steed DL, Edington HD, Webster MW. Recurrence rate of diabetic neurotrophic foot ulcers healed using topical application of growth factors released from platelets. Wound Repair Regen. 1996;4(2):230‐233. 10.1046/j.1524-475X.1996.40210.x [DOI] [PubMed] [Google Scholar]
- 35. Escamilla Cardenosa M, Dominguez‐Maldonado G, Cordoba‐Fernandez A. Efficacy and safety of the use of platelet‐rich plasma to manage venous ulcers. J Tissue Viability. 2017;26(2):138‐143. 10.1016/j.jtv.2016.11.003 [DOI] [PubMed] [Google Scholar]
- 36. Senet P, Bon FX, Benbunan M, et al. Randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers. J Vasc Surg. 2003;38(6):1342‐1348. 10.1016/s0741-5214(03)00908-x [DOI] [PubMed] [Google Scholar]
- 37. Burgos‐Alonso N, Lobato I, Hernandez I, et al. Autologous platelet‐rich plasma in the treatment of venous leg ulcers in primary care: a randomised controlled, pilot study. J Wound Care. 2018;27(Supp 6):S20‐S24. 10.12968/jowc.2018.27.Sup6.S20 [DOI] [PubMed] [Google Scholar]
- 38. Parambath N, Sharma VK, Parihar AS, Sahni K, Gupta S. Use of platelet‐rich plasma to suspend noncultured epidermal cell suspension improves repigmentation after autologous transplantation in stable vitiligo: a double‐blind randomized controlled trial. Int J Dermatol. 2019;58(4):472‐476. 10.1111/ijd.14286 [DOI] [PubMed] [Google Scholar]
- 39. Driver VR, Hanft J, Fylling CP, Beriou JM. A prospective, randomized, controlled trial of autologous platelet‐rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):68‐70, 72, 74 passim. [PubMed] [Google Scholar]
- 40. Stacey MC, Mata SD, Trengove NJ, Mather CA. Randomised double‐blind placebo controlled trial of topical autologous platelet lysate in venous ulcer healing. Eur J Vasc Endovasc Surg. 2000;20(3):296‐301. 10.1053/ejvs.2000.1134 [DOI] [PubMed] [Google Scholar]
- 41. De Angelis B, D'Autilio M, Orlandi F, et al. Wound healing: in vitro and in vivo evaluation of a bio‐functionalized scaffold based on hyaluronic acid and platelet‐rich plasma in chronic ulcers. J Clin Med. 2019;8(9):1486. 10.3390/jcm8091486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kadry M, Tawfik A, Abdallah N, Badawi A, Shokeir H. Platelet‐rich plasma versus combined fractional carbon dioxide laser with platelet‐rich plasma in the treatment of vitiligo: a comparative study. Clin Cosmet Investig Dermatol. 2018;11:551‐559. 10.2147/ccid.s178817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Serra R, Buffone G, Dominijanni A, Molinari V, Montemurro R, de Franciscis S. Application of platelet‐rich gel to enhance healing of transmetatarsal amputations in diabetic dysvascular patients. Int Wound J. 2013;10(5):612‐615. 10.1111/iwj.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Khattab FM, Abdelbary E, Fawzi M. Evaluation of combined excimer laser and platelet‐rich plasma for the treatment of nonsegmental vitiligo: a prospective comparative study. J Cosmet Dermatol. 2019:869‐877. 10.1111/jocd.13103 [DOI] [PubMed] [Google Scholar]
- 45. Karimi R, Afshar M, Salimian M, Sharif A, Hidariyan M. The effect of platelet rich plasma dressing on healing diabetic foot ulcers. Midwifery Stud. 2016;5:e30314. [Google Scholar]
- 46. Sonnleitner D, Huemer P, Sullivan DY. A simplified technique for producing platelet‐rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note. Int J Oral Maxillofac Implants. 2000;15(6):879‐882. [PubMed] [Google Scholar]
- 47. Yin W, Xu H, Sheng J, et al. Optimization of pure platelet‐rich plasma preparation: a comparative study of pure platelet‐rich plasma obtained using different centrifugal conditions in a single‐donor model. Exp Ther Med. 2017;14(3):2060‐2070. 10.3892/etm.2017.4726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anitua E, Sánchez M, Zalduendo MM, et al. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009;42(2):162‐170. 10.1111/j.1365-2184.2009.00583.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Giusti I, Rughetti A, D'Ascenzo S, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49(4):771‐778. 10.1111/j.1537-2995.2008.02033.x [DOI] [PubMed] [Google Scholar]
- 50. Moojen DJ, Everts PA, Schure RM, et al. Antimicrobial activity of platelet‐leukocyte gel against Staphylococcus aureus. J Orthop Res. 2008;26(3):404‐410. 10.1002/jor.20519 [DOI] [PubMed] [Google Scholar]
- 51. Chen L, Wang C, Liu H, Liu G, Ran X. Antibacterial effect of autologous platelet‐rich gel derived from subjects with diabetic dermal ulcers in vitro. J Diabetes Res. 2013;2013:1‐5. 10.1155/2013/269527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Li L, Chen D, Wang C, Liu G, Ran X. The effect of autologous platelet‐rich gel on the dynamic changes of the matrix metalloproteinase‐2 and tissue inhibitor of metalloproteinase‐2 expression in the diabetic chronic refractory cutaneous ulcers. J Diabetes Res. 2015;2015:1‐6. 10.1155/2015/954701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anitua E, Zalduendo M, Troya M, Padilla S, Orive G. Leukocyte inclusion within a platelet rich plasma‐derived fibrin scaffold stimulates a more pro‐inflammatory environment and alters fibrin properties. PLoS One. 2015;10(3):e0121713. 10.1371/journal.pone.0121713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kalyam K, Kavoussi SC, Ehrlich M, et al. Irreversible blindness following periocular autologous platelet‐rich plasma skin rejuvenation treatment. Ophthalmic Plast Reconstr Surg. 2017;33(3S Suppl 1):S12‐S16. 10.1097/iop.0000000000000680 [DOI] [PubMed] [Google Scholar]
- 55. Oyibo SO, Jude EB, Tarawneh I, et al. The effects of ulcer size and site, patient's age, sex and type and duration of diabetes on the outcome of diabetic foot ulcers. Diabet Med. 2001;18(2):133‐138. 10.1046/j.1464-5491.2001.00422.x [DOI] [PubMed] [Google Scholar]
- 56. Govindarajan B, Shah A, Cohen C, et al. Malignant transformation of human cells by constitutive expression of platelet‐derived growth factor‐BB. J Biol Chem. 2005;280(14):13936‐13943. 10.1074/jbc.M500411200 [DOI] [PubMed] [Google Scholar]
- 57. Del Pino‐Sedeno T, Trujillo‐Martin MM, Andia I, et al. Platelet‐rich plasma for the treatment of diabetic foot ulcers: a meta‐analysis. Wound Repair Regen. 2019;27(2):170‐182. 10.1111/wrr.12690 [DOI] [PubMed] [Google Scholar]
- 58. Li Y, Gao Y, Gao Y, et al. Autologous platelet‐rich gel treatment for diabetic chronic cutaneous ulcers: a meta‐analysis of randomized controlled trials. J Diabetes. 2019;11(5):359‐369. 10.1111/1753-0407.12850 [DOI] [PubMed] [Google Scholar]
- 59. Martinez‐Zapata MJ, Marti‐Carvajal AJ, Sola I, et al. Autologous platelet‐rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2012;10:Cd006899. 10.1002/14651858.CD006899.pub2 [DOI] [PubMed] [Google Scholar]
- 60. Martinez‐Zapata MJ, Marti‐Carvajal AJ, Sola I, et al. Autologous platelet‐rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016(5):Cd006899. 10.1002/14651858.CD006899.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, Cervelli V. The effect of platelet‐rich plasma in hair regrowth: a randomized placebo‐controlled trial. Stem Cells Transl Med. 2015;4(11):1317‐1323. 10.5966/sctm.2015-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gentile P, Garcovich S. Autologous activated platelet‐rich plasma (AA‐PRP) and non‐activated (A‐PRP) in hair growth: a retrospective, blinded, randomized evaluation in androgenetic alopecia. Expert Opin Biol Ther. 2020;20(3):327‐337. 10.1080/14712598.2020.1724951 [DOI] [PubMed] [Google Scholar]
- 63. Gentile P, Garcovich S. Systematic review: the platelet‐rich plasma use in female androgenetic alopecia as effective autologous treatment of regenerative plastic surgery. J Plast Reconstr Aesthet Surg. 2022;75(2):850‐859. 10.1016/j.bjps.2021.11.004 [DOI] [PubMed] [Google Scholar]
- 64. Gentile P, Scioli MG, Bielli A, et al. Platelet‐rich plasma and micrografts enriched with autologous human follicle mesenchymal stem cells improve hair re‐growth in androgenetic alopecia. Biomolecular pathway analysis and clinical evaluation. Biomedicines. 2019;7(2):27. 10.3390/biomedicines7020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gentile P, Sterodimas A, Calabrese C, Garcovich S. Systematic review: advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose‐derived mesenchymal stem cells in wound and scar treatment. Stem Cell Res Ther. 2021;12(1):318. 10.1186/s13287-021-02397-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gentile P, Garcovich S. Systematic review: adipose‐derived mesenchymal stem cells, platelet‐rich plasma and biomaterials as new regenerative strategies in chronic skin wounds and soft tissue defects. Int J Mol Sci. 2021;22(4):1538. 10.3390/ijms22041538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gentile P, Garcovich S. Concise review: adipose‐derived stem cells (ASCs) and adipocyte‐secreted exosomal microRNA (A‐SE‐miR) modulate cancer growth and proMote wound repair. J Clin Med. 2019;8(6):855. 10.3390/jcm8060855 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


