Abstract
Cytoskeleton plays a significant role in the shape change, migration, movement, adhesion, cytokinesis, and phagocytosis of tumor cells. In clinical practice, some anti-cancer drugs achieve cytoskeletal therapeutic effects by acting on different cytoskeletal protein components. However, in the absence of cell-specific targeting, unnecessary cytoskeletal recombination in organisms would be disastrous, which would also bring about severe side effects during anticancer process. Nanomedicine have been proven to be superior to some small molecule drugs in cancer treatment due to better stability and targeting, and lower side effects. Therefore, this review summarized the recent developments of various nanomaterials disturbing cytoskeleton for enhanced cancer therapeutics, including carbon, noble metals, metal oxides, black phosphorus, calcium, silicon, polymers, peptides, and metal-organic frameworks, etc. A comprehensive analysis of the characteristics of cytoskeleton therapy as well as the future prospects and challenges towards clinical application were also discussed. We aim to drive on this emerging topic through refreshing perspectives based on our own work and what we have also learnt from others. This review will help researchers quickly understand relevant cytoskeletal therapeutic information to further advance the development of cancer nanomedicine.
Keywords: Nanomedicine, Disturbing cytoskeleton, Migration inhibition, Tumor therapy
Graphical abstract
Herein, a systematic summary of nanomaterials disturbing cytoskeleton for enhanced cancer therapeutics is presented. A comprehensive analysis of the characteristics of cytoskeleton therapy as well as the future prospects and challenges towards clinical application were also discussed.
Highlights
-
•
The importance of cytoskeleton for maintaining the functions of cells is illustrated.
-
•
The mechanisms of nanomaterials destroying tumor cytoskeleton are highlighted.
-
•
The future prospects and challenges towards clinical application were also discussed.
1. Introduction
1.1. The composition and function of the cytoskeleton
Cytoskeleton mainly refers to the protein fiber network framework system in eukaryotic cells composed of proteins such as microfilaments, microtubules, and intermediate fibers. Microfilaments are fibers with a diameter of about 7 nm, composed of two strands of actin filaments in the same direction in the form of a helix. Microfilaments are critical in cellular motility, signaling, and interactions with the extracellular environment and neighboring cells [1]. Microtubule, a polar cytoskeleton around 25 nm, is a hollow long tubular organelle structure composed of tubulin dimer, which is comprised of the combination of α and β tubulin subunits. Microtubules participate in maintaining cell shape and assisting transportation of intracellular materials, and generating various structures such as spindles, centrioles, flagella, cilia, neural tubes and other structures [2]. As the most stable and complex cytoskeleton component, intermediate fibers (about 10 nm in diameter) play an important role in cell structure. Unlike microtubules and microfilaments, intermediate fibers are non-polar and cannot drive the directional movement of molecules. Most of cells have one type of intermediate fiber, and a few cells express more than two [3]. The intermediate fibers in the cytoskeleton are related to cell differentiation, because the type and distribution of intermediate fibers are tissue-specific, and are specifically expressed with the differentiation of cells.
Cytoskeleton reorganization plays a significant role in the shape change, migration, movement, adhesion, cytokinesis, and phagocytosis of tumor cells. Cell shape determines cell behavior, so it is not surprising that cytoskeleton reorganization is related to extensive infiltration of cancer cells. The continuous movement of invasive and metastatic tumor cells is powered by tension fiber contraction and actin filament elongation, and Rho GTPases can power cell migration by regulating cytoskeleton reorganization [4]. Among them, Rho A, Rac1 and Cdc42 have been certified to be momentous for the progression and metastasis of different human tumors, containing melanoma, testicular breast, liver and ovarian cancers [5]. All three factors promote actin cytoskeleton reorganization but have different effects on cell shape and motility. Rho and its downstream Rho kinase activate the phosphorylation of LIMK, which eventually causes the phosphorylation of Coflin, thus losing the ability to depolymerize actin [6]. This results in increased levels of phosphorylated myosin light chain, stimulating cross-linking of actin filaments through myosin II and producing contractile force, which promotes membrane blistering, movement of cell bodies, and cell separation. In addition, Rho can also activate another important downstream effector mDia protein to incorporate actin monomers into the ends of actin filaments, inducing actin filaments to elongate and promote cell migration by preventing binding of capping protein [7]. The periphery of cells relies on Rac to form actin-rich lamellipodia. Cdc42 is involved in the formation of filopodia, which sense tropism signals and determine the direction of movement [8]. Therefore, for normal cells, the cytoskeleton can maintain its normal life activities, but when the cytoskeleton of a tumor is reorganized, it may significantly promote the process of tumor invasion and metastasis.
1.2. Mechanisms and challenges of anti-cancer agents in disturbing the cytoskeleton
In clinical practice, some drugs achieve therapeutic effects by acting on the cytoskeleton, and these drugs act on different cytoskeletal protein components. Antitumor drugs that act on intracellular microtubules are considered as some of the drugs with the highest clinical benefits. In recent years, various types of tubulin inhibitor drugs are newly developed, taking advantages from the dynamic properties of microtubules to regulate the cytoskeleton. The first kind drugs including colchicine and vinblastine compounds can inhibit the polymerization of microtubules and hinder the formation of the spindle, inhibiting the proliferation of tumors [9,10]. The second kind, as observed under action of drugs such as paclitaxel, evodiamine [11] and (Z)-1-Aryl-3-arylamino-2-propen-1-one (10) compound series [12], can promote microtubule polymerization, inhibit cell division, stop cell division in the mitotic phase, and finally cause tumor cell apoptosis. Paclitaxel has attracted much attention since the 1990s due to its unique microtubule polymerization-promoting effect. It has become an important chemotherapeutic drug for breast cancer, cervical cancer, and non-small cell lung cancer, and is widely used in clinical practice [13]. A third example, eribulin, a non-taxane microtubule kinetics inhibitor, promotes the suspension of microtubule growth and strongly inhibits the growth rate of microtubules, reducing microtubule dynamics overall [14]. As a promising compound, eribulin retains the unique properties of marine natural product halichondrin B and has excellent preclinical in vivo activity. In addition to preventing further tumor development by affecting microtubules, drugs can also achieve better antitumor effects by acting on microfilaments. Cytochalasin can prevent actin polymerization in tumor cells, resulting in inhibition of microfilament growth and destruction of microfilament mesh. As a result, the ability of tumor cells to migrate is greatly reduced [15]. Latrunculin is a macrolide compound isolated from the sponge of the Red Sea. The main mechanism of action is to combine with actin monomer (G-actin), thereby inhibiting the addition of G-actin to one end of F-actin and prevent further polymerization of microfilaments [16]. The different kinds of drugs listed above have extensive and effective applications in tumor treatment and we also summarized the cytoskeletal interference mechanism of other different drugs (Table 1). It is worth noting that although cytoskeletal antitumor drugs have achieved good efficacy, currently clinically applied drugs generally have problems such as drug resistance, toxic side effects, etc. Therefore, while developing and studying cytoskeletal target drugs with unique mechanisms of action, the main research direction is still the structural modification and transformation of the discovered lead compounds, in order to effectively reduce drug toxicity and improve its selectivity. Under a lack of cell-specific targeting, unwanted cytoskeletal reorganization in organisms would be catastrophic, and they would also bring about severe side effects in anticancer therapy.
Table 1.
Various anticancer drugs disturbing cytoskeleton for cancer therapy.
| Drugs | Mechanism | Effect | Position | Cell types | Disease types | Ref. |
|---|---|---|---|---|---|---|
| PTX-S-PTX, PTX–SS–PTX and PTX–SSS–PTX | Distinct redox-responsibility | More efficient drug release in tumor cells, significantly improved the antitumor activity of PTX. | Microtubule | 4T1, KB, B16–F10, L02 | Melanoma, BRCA | [17] |
| LMD | Occupy binding sites on a tubulin | Blocking mitosis | Microtubule | A549、MCF-7 | BRCA, NSCLC | [18] |
| MMAE | Occupy binding sites on a tubulin, TNTs' structural changes | Induce mitotic arrest | Microtubule | A549、MCF-7 | BRCA, NSCLC | [18] |
| Cucurbit [6] uril (CB [6]) | Interrupt the polymerization of tubulin | Microtubule depolymerization and significant tumor regression | Microtubule | A549, U87, MDA-MB231, B16F10 | Melanoma | [19] |
| Natural product thalicthuberine (TH) | Reduce tubulin polymer mass | Mitotic spindle defects | Microtubule | LNCaP, C4–2B, DuCaP, PC-3,BPH-1, WPMY-1, HeLa | Prostate, cervical cancer | [20] |
| SMART、SMART-OH、P-SMART | Cell cycle arrest | Cell apoptosis, DNA damage | Microtubule | A375, B16–F10 | Melanoma, BRCA, ovarian, colon, prostate cancer | [21] |
| 6α-Acetoxyanopterine | Reduce tubulin polymer mass | Inhibit cell proliferation, induce apoptosis | Microtubule | LNCaP, PC-3, HeLa, CCRF-CEM, | Metastatic castrate-resistant prostate cancer (mCRPC) | [22] |
| Cytochalasin | Inhibit the polymerization of microfilaments | Cell apoptosis, inhibit cell migration and angiogenesis | Microfilament | A549 | NSCLC | [23] |
| latrunculin | Bind to actin monomer, prevent actin from polymerizing | Induce depolymerization of tumor cytoskeleton | Microfilament | RMS | RMS | [24] |
| Staurosporine | Activate the actin depolymerization factor Cofilin | Cell accumulation in G2/M phase | Microfilament | A549 | NSCLC | [25] |
| Sinularin | Influence MAPK, EMT, and VEGF signaling pathways | Cell accumulation in G2/M phase | Microfilament | SK-HEP-1 | Liver cancer | [26] |
| VSM | Upstream Activa-Tor↓, PAx3-FoxO1↓ | Stabilize actin cytoskeleton | Microfilament | RAM, RH-30 | Rhabdomyosarcoma | [27] |
| Phalloidin | Stabilize F-actin, | Prevents fiber depolymerization | Microfilament | Viable cell | N/A | [28] |
| CCNY | Phosphorylate and activate LRP6 | Regulate the proliferation | Microfilament | HHCC | Lung cancer, liver cancer | [29] |
| PCA | P-JNK and P38↓ | Inhibit the adhesion, migration and invasion | Microfilament | HL60, U937 | Liver cancer, acute myeloid leukemia | [30] |
1.3. Anti-cancer nanomedicine acting on various organelles
Compared with conventional drugs, nanomedicines can increase the solubility and absorption of poorly soluble drugs with reduced adverse reactions, improved targeting, and more accurately controlled release. According to their own physical and chemical properties, nanomaterials can also treat tumors through photothermal, photodynamic, sonodynamic, radiosensitization, chemotherapy sensitization, inhibition of chronic inflammation to prevent tumor progression, and immunotherapy [31]. In addition, after nanomaterials enter cells, they can also produce different biological effects by targeting organelles (Table 2). The Golgi apparatus is the main suborganelle responsible for drug resistance. The translatable peptide C6RVRRF4KY can disrupt the Golgi apparatus membrane, leading to inhibition of cytokine secretion and decline drug resistance, eventually leading to cancer cell death [32]. Anodic alumina nanotubes combined with thapsigargin and autophagy inhibitor 3-methyladenine significantly reduced autophagy in breast cancer cells and induced ER stress-related cell death which stops the tumor from progressing any further [33]. After entering tumor cells, gold nanorods destroy lysosome membrane, resulting in increased permeability of lysosome membrane, which will cause lysosome enzyme leakage and destroy tumor cells. Moreover, gold nanorods can also cause mitochondrial dysfunction, which rapidly activates caspase-3, followed by explosive activation of apoptotic reaction [34]. Therefore, the strategy that nanomaterials disrupt the homeostasis of tumor cells by destroying organelles can significantly reduce the survival rate of tumor cells. Apart from cancer treatment, nanomaterials have shown promising results in treating other diseases by affecting organelles. Diabetic foot ulcer is a serious complication of diabetic patients. PBNPs@PLEL wound dressings reduce ROS production, protect mitochondria from oxidative stress-related damage, promote angiogenesis, and improve diabetic wound healing [35]. In conclusion, nanomaterials have been successfully applied to cancer by affecting a variety of organelles and have shown good therapeutic effects. It opens up new avenues for the treatment of many diseases that have failed conventional treatments.
Table 2.
Nanomaterials acting on various cell organelles for enhanced cancer theranostics.
| Materials | Targeted organelles | Mechanism | Detecting Methods | Cell | Ref. |
|---|---|---|---|---|---|
| GFNCs | Vascular endothelial gap junctions | VE-cadherin↓ | Laser Doppler imaging and MRI | HepG-2 | [36] |
| Ce6–C18-PEG/Cur | DNA damage | Destabilization | Confocal fluorescence micrographs of γ-H2AX-stained | 4T1 | [37] |
| SiNPs | ER | GRP78↑, CHOP↑, ERO1α↑ | Western blot analysis | RAW 264.7 | [38] |
| IONPs | ER | ER stress | RNA-seq and bioinformatics | RAW264.7 | [39] |
| AgNPs | ER, mitochondrion | GRP78↑, p-PERK↑, p-eIF2α↑, CHOP↑, XBP1↑, p-IRE↑ | Western blot analysis | SH-SY5Y | [40] |
| SiNPs | Lysosome | ROS/PARP/TRPM2 signaling-mediated lysosome impairment | LysoTracker Green DND-26 fluorescence | BEAS-2B | [41] |
| ZnONP | Lysosome | Lysosomal membrane destabilization | Acridine orange staining | Bronchial epithelial cells/THP1 cells | [42] |
| Ru-1@TPP-PEG-biotin SAN | Lysosome, ER | Lysosome degradation, GRP78↓, ER stress | Colocalization assay | MCF-7 and HepG2 | [43] |
| SeNPs | Mitochondrion | Target the mitochondria via TLR4/TRAF3/MFN1 pathway | Mitochondrial membrane potential, ROS, ATP content, | OVCAR-3 and EAC | [44] |
| Dual targeted MSN | Mitochondrion | TPP binds to mitochondria membrane | JC-1 assay | LNCaP | [45] |
| MSNAs-TPP | Mitochondrion | Effect TLR4/TRAF3/MFN1 | JC-1 assay | 4T1 | [46] |
| AuNCs/Cas9–gRNA | Nucleus | Release of Cas9–sgRNA plasmids into the cellular nucleus. | Cas9–sgRNA plasmid transport and release to achieve efficient genome editing | U2OS | [47] |
| Peroxisome-targeting peptide nanostructure | Peroxysome | SKL-COOH at the C-terminus of the PA, the nature of the secondary structure, and the morphology of the nanostructures | Confirmed by CLSM and 3D TEMT | HeLa | [48] |
| TiO2 | VE-cadherin | VE-cadherin is phosphorylated at intracellular residues (Y658 and Y731), and the interaction between VE-cadherin and p120 as well as b-catenin is lost. | Western blot analysis. | B16F10 | [49] |
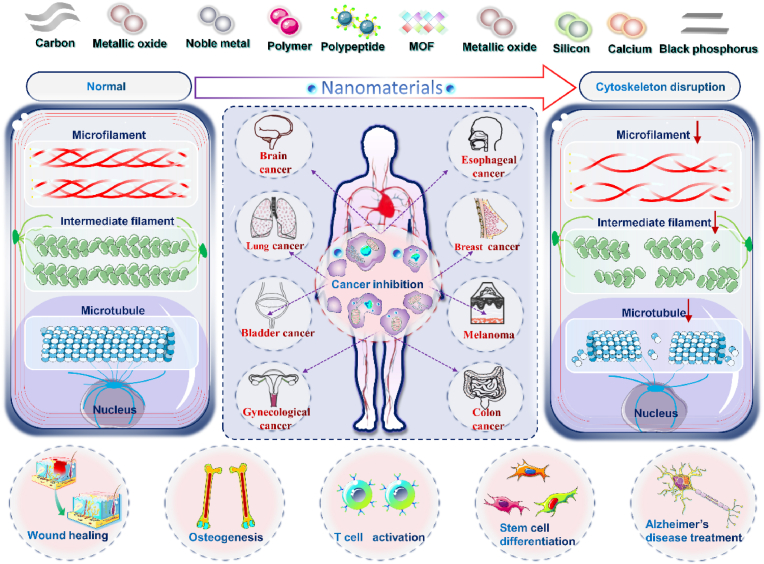
However, in the nanomedicine research domain, cytoskeleton as one of the organelles is rarely used in the research of disease treatment. Targeting the cytoskeleton has unique advantages over other therapeutic strategies. First, the destruction of the cytoskeleton is fatal to tumor growth and development. Therefore, targeting tumor cytoskeleton can inhibit proliferation by destroying tumor cell structure, regardless of tumor cell type and degree of differentiation. Secondly, the migration of tumor cells is a key step in the malignant invasion of tumors. Targeting the tumor cytoskeleton can significantly inhibit the formation of pseudopodia, which can prevent its metastasis to distant places. Therefore, the targeted cytoskeleton strategy not only inhibits the proliferation of tumor cells, but also prevents the metastasis of tumor cells, which shows an excellent strategy that the damage mechanism produces a dual therapeutic effect. Therefore, targeting the cytoskeleton with nanomedicine may be a promising disease treatment strategy. However, until now, there has been no systematic and comprehensive review. Thus, this review summarized the latest progress in the development of various types of nanomaterials affecting the cytoskeleton in the treatment of tumors and other diseases, including carbon, noble metals, metal oxides, black phosphorus, calcium, silicon, polymers, peptides, and MOFs based nanomaterials (Fig. 1), as well as the mechanisms, advantages, and limitations of them affecting the cytoskeleton.
Fig. 1.
Schematic illustration of various nanomedicine acting on cytoskeleton for cancer therapy.
2. Disturbing cytoskeleton by various nanomaterials for enhanced cancer therapeutics
As mentioned earlier, many drugs that affect the cytoskeleton have been used in clinical tumor therapy. However, drug resistance, toxicity and side effects are common in the drugs used at present, which seriously limit the process of tumor treatment. Compared with common drugs, nanomaterials have better biocompatibility and targeting, and have successfully treated cancer from the level of some organelles, which has great potential in cancer treatment. Therefore, it is a worthy research direction to explore the effect of nanomaterials on the cytoskeleton to treat cancer. At present, there are few reviews of nanomaterials for cancer treatment through affecting the cytoskeleton, and the discussions of these reviews are incomplete and systematic. In order to better understand the effect of nanomaterials on cytoskeleton and their influence on cancer and other diseases, this review summarizes the mechanism of the effect of nanomaterials such as carbon, precious metals, metal oxides, black phosphorus, calcium, silicon, polymers, peptides, MOFs on cytoskeleton treatment of cancer, and puts forward some thoughts on its application prospect and future development. We hope that the information from this review will lead to further development of this subfield of cancer nanomedicine.
2.1. Carbon based nanomaterials
According to the structural dimensions, carbon nanomaterials (CNMs) are divided into three categories, which are zero dimensional (0D), one-dimensional (1D) or two-dimensional (2D) materials. Due to their inherent hydrophobicity, they showed better biocompatibility and biosafety in CNMs compared with metal-based nanomaterials [50]. What's more, corresponding drugs can be carried by CNMs through hydrophobic interaction or π - π stacking, and then act as a highly efficient drug delivery platform for disease diagnosis and treatment [51]. Recently, graphene, fullerenes, carbon nanotubes, and carbon quantum dots have become increasingly popular as CNMs for cancer therapy and intracellular labeling, which can obtain better biocompatibility through covalent or noncovalent modification [52]. CNMs have been widely used in optics, medical implants, medical electronics, tissue scaffolds, sensors and other biomedical devices. Undoubtedly, they are also a promising tumor drug delivery system from a forward-looking perspective (Fig. 2A) [50].
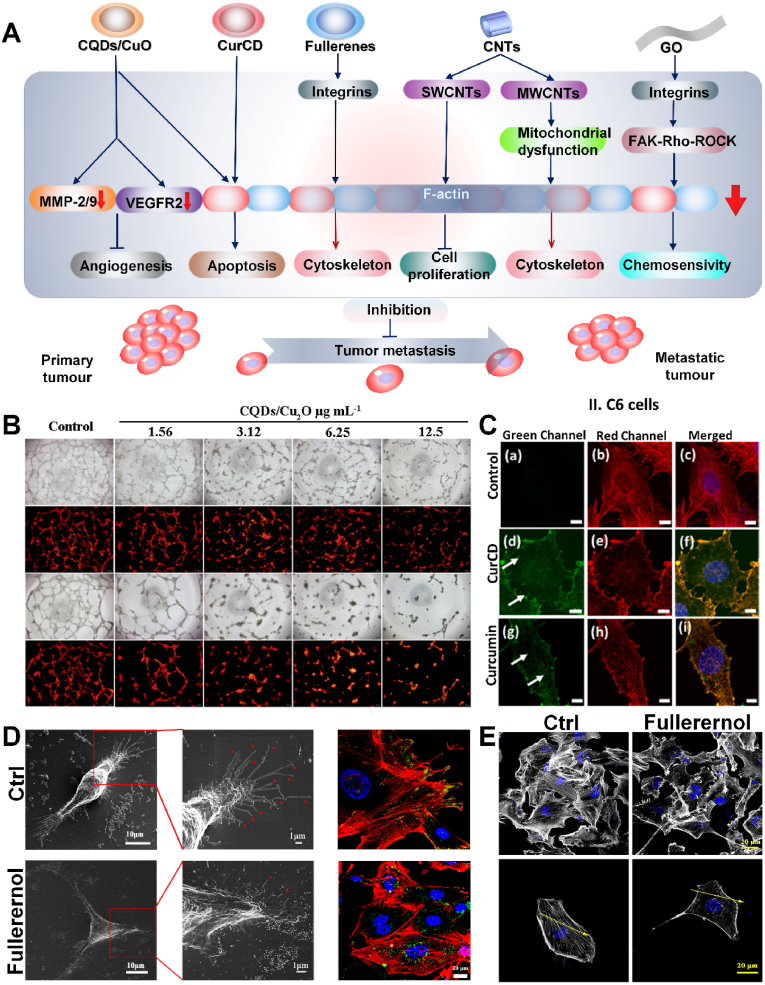
Fig. 2.
A) Carbon nanomaterials disturb F-actin, inhibit the expression of VEGFR2 and MMP-2/9 to cause tumor metastasis for enhanced cancer therapy. B) The CQDs/Cu2O complex inhibits angiogenesis [53]. Copyright 2021, Springer Nature. C) Actin staining of C6 cells after curcumin/CurCD treatment. The green channel is CurCD/curcumin attached to actin filaments, and the red channel is Phalloidin stained actin filaments [54]. Copyright 2021, Elsevier. D) Effect of fullerenol on filopodia formation and integrin distribution. Green = integrin β1, red = actin cytoskeleton, blue = nucleus. E) Effect of fullerenol nanoparticles on cell skeleton [55]. Copyright 2018, Springer Nature.
0D materials are defined as having size of 0.1–100 nm in each dimension (x, y, z), mainly including carbon quantum dots and fullerenes. Among many CNMs, owing to special 0D structure and excellent properties containing biocompatibility, low cytotoxicity, favorable water solubility and unique luminescent behaviors exist in carbon quantum dots (CQDs), having attracted much attention in the field of materials and biological applications [56]. It has been proved that CQDS and CQDS-based composites can be used in phototherapy and radiotherapy to selectively target tumor cells by regulating the expression of major angiogenic cytokines, including VEGF, FGF and VEGFR2 [53]. In addition, matrix metalloproteinases (MMPs) are involved in basement membrane remodeling and contribute to angiogenesis. Therefore, decreased MMPs expression or enzyme activity is considered to be a key factor that potentially inhibits migration and angiogenesis [57]. In 2017, Chen et al. synthesized CQDs/Cu2O with CuSO4 and C6H12O6, as the cytoskeleton filaments of SKOV3 cells treated with CQDs/Cu2O were broken, the expression level of F-actin decreased, and the destruction of cytoskeleton inhibited the migration of tumor cells [53]. In addition, tumor progression and metastasis depend on oxygen and nutrients from the abundant blood vessels within the tumor. Studies have shown that when CQDS/Cu2O significantly inhibits the expression of VEGFR2 and MMP-2/9, tumor metastasis and angiogenesis are also significantly limited (Fig. 2B). Additionally, the small size (<10 nm), better water solubility and photoluminescence properties of carbon dots endow them attractive in biosensors, biological imaging and drug delivery, improving the disadvantages of poor water solubility, low absorption, rapid metabolism and clearance of curcumin [58]. According to current understanding, curcumin is a polyphenol phytochemical, playing an important part in the treatment of cancer, arthritis and other chronic diseases thanks to its antioxidant and anti-inflammatory properties [59]. As an antiproliferative agent, curcumin has been shown to induce apoptosis in tumor cell lines, and following curcumin can inhibit the occurrence, development, invasion and metastasis of tumors [60]. For comparing with the property of curcumin, in 2021, Sharma et al. synthesized curcumin derived carbon nanodots (CurCD) through a one-step synthesis method to study the effect on glioblastoma [54]. With the unique structure of curcumin promoting its direct combining to the vicinity of cytochalasin sites in the cytoskeleton and leading to disturbance, CurCD showed the effect of promoting tumor cell apoptosis, as well as causing the destruction of microfilaments and microtubules of glioblastoma C6 cells by producing more ROS, which apparently inhibited the migration of tumor cells. Compared with curcumin, CurCD had better solubility, stability, and biocompatibility without affecting normal cells and has higher safety. Furthermore, the special capability of CurCD to selectively bind actin filaments enabled its application in assessing the state of the cytoskeleton and imaging (Fig. 2C). Both CQDS/Cu2O and CurCD were successful in destroying the cytoskeleton. These findings may open up a new pathway for CQDS/Cu2O and CurCD as potential therapies for cancer chemotherapy.
Fullerenes have a foothold in the field of electronics, optics, cosmetics, catalysts, environment, and nano medicine. However, fullerenes are poorly dissolved in water. To overcome this problem, molecular functionalization was used to produce water-soluble derivatives with different biocompatibility [61]. Presently, many studies of this materials are hydroxylated fullerenes, among which fullerenols have been proved with a broad range of biological activities [62]. From the point of view of chemical structure, hydroxylated fullerenol C60(OH)24 is a C60 cage with symmetrically arranged hydroxyl groups, about 1 nm in size. One of the characteristics of this structure is the formation of aggregates through agglomeration, which can range in size from a few nanometers to hundreds of nanometers, with an average size of 40∼60 nm [63]. There are very promising properties of fullerenol, including its antioxidant activity and protective effect, as well as its potential applications in biomedical field, such as nano drug delivery [64]. Integrins are transmembrane heterodimers composed of α and β subunits that connect the cell membrane and intracellular actin reticulum to the extracellular matrix (ECM), to coordinate cells to adapt to their extracellular microenvironment, but the disorder of integrin expression will damage the cell morphology, adhesion and migration functions [65]. Specifically, in 2018, Qin et al. found that the migration of MDA-MB-231 cells could be inhibited by small size fullerenol [55]. The results showed that fullerenol altered the distribution of integrins in cells and decreased the adhesion of cancer cells to ECM (Fig. 2D). In addition, the tumor cells treated with small fullerenol showed disturbance and reduction of actin, resulting in a significant decrease in the length and number of filopodia (Fig. 2E). However, this study is only preliminary and future studies should further evaluate the use of fullerenols in combating metastasis.
1D nanomaterials refer to only one of the three dimensions whose size is not between 0.1 and 100 nm. For example, the most typical carbon nanotubes (CNTs) have naturally become a rather attractive nanomaterial with their unique one-dimensional structure, the characteristics of external surface modification and special physical and chemical properties, including light weight, favorable flexibility, corrosion resistance, exceptional electrical properties and outstanding mechanical strength [66]. Purified and dispersed single-walled carbon nanotubes have been applied for drugs and proteins delivery without prominent cytotoxicity [67]. In 2020, the mechanism by which MWCNTs inhibit the migration and invasion of ovarian cancer cells was investigated by Zhang et al. [68]. These results clearly exhibited that MWCNTs caused a lower proportion of mitochondrial respiration by reducing the activity of mitochondrial electron transfer chain complex I–V, affected the assembly of actin cytoskeleton in a dose-dependent manner, thereby eventually inhibiting the migration and invasion of SKOV3 cells. In 2010, Holt et al. applied high-purity and dispersed SWCNTs to HeLa cells, showing that compared with untreated cells whose actin structure only existed in the cell cortex, in SWCNTs treated group, actin was distributed all over the inner part of the cells presenting spinous perinuclear projection with increased actin-related division defects, resulting in an obvious decrease in cell proliferation [69]. Although carbon nanotubes can inhibit tumors, carbon containing impurities (amorphous carbon, graphite materials, fullerenes) and metal catalyst particles in raw CNT samples remain toxic to other cells, such as adverse effects on local cell adhesion, cell shape, proliferation and differentiation [70]. However, in the long term, the therapeutic power and efficiency of carbon nanotubes are still open to exploration.
Lastly, 2D nanomaterials are defined as two of the three dimensions whose dimensions are not between 0.1 and 100 nm. Among them, the most common example of such materials is graphene (GO) and its derivatives. GO has high specific surface area and dispersity, and has broad application prospects in the biomedical field [71], including biological imaging, antibacterial activity, drug delivery, photothermal, and photoluminescence [72]. Further chemical modification and functionalization can provide a better compatibility and altered physical and chemical properties. In 2016, Tian et al. studied how GO inhibited lung cancer metastasis via regulating cytoskeleton [73]. After treating cells with GO nanosheets for 6 h, GO aggregation was found in the region of contracted actin filaments, the fluorescence intensity of actin filament had weakened, and the structure of F-actin contracted and became non-isotropic assembly. These results indicated that actin filaments were disturbed after GO nanosheets treatment. Molecular dynamics simulations found that GO caused tetramer separation by inserting the interchain gap of the actin tetramer, which ultimately resulted in actin filament rupture and significant inhibition of cell migration. When the cells cultured in vitro spread on the surface of the matrix, tight adhesive spots were often formed between specific areas of the plasma membrane and the matrix. Stress fibers are bundles of microfilaments arranged on the inner side of the plasma membrane attached to these spots, which are related to cell morphology, differentiation, and tissue construction. For regulating cytoskeletal remodeling, and maintaining cell morphology, motility, proliferation, and survival, the FAK−Rho-Rock pathway is known as essential. Moreover, Zhu et al. found that GO had the effect of chemotherapy sensitization by affecting the cytoskeleton [74]. They synthesized monolayers of GO with a thickness of about 1 nm and a width of about 100–300 nm through the modified Hummers' method. After GO treatment, the FAK−Rho−ROCK pathway in A549 cells was disordered, actin fibers disintegrated and stress fiber formation was reduced, and then cell membrane collapsed. At the same time, the destruction and loss of cytoskeleton also weaken the resistance of tumor cells to chemotherapy drugs, which make cancer cells sensitive to chemotherapy drugs. Some studies have also proved that GO can enter cells and induce the generation of intracellular ROS, therefore leading to secondary damage, such as cytoskeleton damage [75]. GO's ability to disrupt actin suggests that it can bind other target ligands and be used as a novel cytoskeletal binder in cancer therapy to overcome drug resistance and reduce side effects [74]. These findings provide new mechanisms by which GO nanotablets induce biophysical responses. Although GO has been proved to be a good candidate for disease diagnosis and treatment, the understanding of the biological effects and mechanisms of the interaction between graphene and biological systems is still limited. These uncertainties have greatly hindered the commercial application of graphene, especially in the biomedical field.
CNMs are also known for their excellent performance in a wide range of biomedical applications. However, their unique properties may also adversely affect the organism. For example, CNMs cause DNA damage, which may contribute to and accelerate cancer, affect fertility, or promote ecological genotoxicity [76]. Therefore, it is equally important to evaluate the carcinogenic or mutagenic potential of new substances when using CNMs. In general, we can take advantage of our knowledge of the genotoxicity of CNMs to reduce the potential adverse effects associated with them to protect human health and the environment. Studies have shown that some members of the carbon nanomaterials family, in addition to genotoxicity, have also been shown to induce oxidative damage, and inflammation, and activate different cell signaling pathways that lead to different cellular responses [77]. Additional in vitro studies, including biocompatibility analysis and the analysis of inflammation and genotoxicity of many healthy and transformed cell lines, will promote the fundamental work on CNM toxicity. Similarly, in vivo testing is beneficial for designing CNMs with desirable properties and high efficiency.
2.2. Noble metal based nanomaterials
Noble metal nanoparticles are unique nanomaterials which possess simple synthesis and strong optical. The physical and chemical properties of nanoparticles (NPs) are related to their composition, shape and size [78]. Many noble metal based nanomaterials, including gold (Au), Pd, Pt and silver (Ag), have been widely used as photosensitizers in the biomedical field. In particular, Au and Ag have become major materials due to their high biocompatibility, simple synthesis and surface functionalization, and adjustable optical and physical properties [79]. Some novel metal nanostructures show many unique features, for instance, strong light absorption and scattering as well as LSPR excited by local surface plasma, which enable them to be widely used in biomedical applications (Fig. 3A) [80].
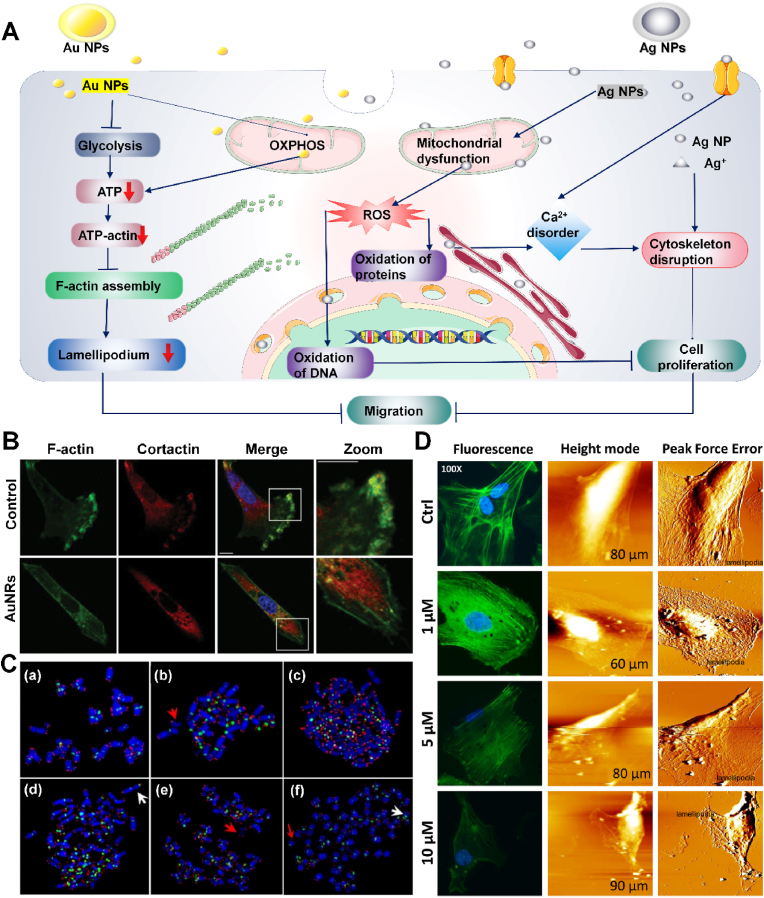
Fig. 3.
A) Noble metal nanomaterials inhibit the assembly of actin, increase ROS expression and cause Ca2+ disorder to inhibit tumor migration for enhanced cancer therapy. B) AuNRs damage F-actin skeleton assembly in cancer cells. F-actin = green, anti-corticosteroid antibody = red, DAPI = blue [81]. Copyright 2014, Wiley-VCH. C) Chromosome aberration occurred in AgNP treated cells [82]. Copyright 2009, Springer Nature. D) FAM image of cytoskeleton and topography structures of colon cancer cells treated with AgNP [83]. Copyright 2019, Elsevier.
Gold nanorods (AuNRs) have widespread applications in biological imaging, biosensing, drug delivery and photothermal therapy because of their unique physical, chemical and optical properties. [84] It has been reported that pseudopodia is a broad and flat lamellipodia assembled by actin at the front edge of migrating and moving cells and filopodia at the front end [85]. The extension of lamellipodia, the formation and stabilization of adhesion, the dissociation of adhesive plaques in cell tail and the rearrangement of cytoskeleton lead to the process of cell migration. In 2014, Zhou et al. studied the mechanism of AuNRs inhibiting tumor metastasis [81], synthesized CTAB modified AuNRs by using seed mediated growth method [86], and formed protein coated AuNRs with an average length of 54.4 ± 5.2 nm and a width of 18.2 ± 1.5 nm. The cytoskeletal assembly process of F-actin is known to consume intracellular ATP [87]. When AuNRs are internalized by cells and distributed in lysosomes and cytoplasm, the expression of energy production-related genes is down-regulated, leading to abnormal mitochondrial oxidative phosphorylation and glycolysis functions of MDA-MB-231 cells, decreased ATP synthesis, decreased ATP binding actin monomers, and decreased affinity. The decrease of ATP inhibits the assembly of actin, resulting in reduced lamellar and filamentous pseudopodia production AuNRs inhibit the formation of pseudopodia in tumor cells, thereby inhibiting tumor migration (Fig. 3B). In vivo, AuNRs injected mice showed significantly fewer metastatic nodules in lung slices compared with untreated cells. Thus, AuNRs disrupt the basis of cell migration in the first place by inhibiting ATP production and thus blocking the formation of cytoskeleton.
In different silver states, the elemental states of silver, especially silver nanoparticles (AgNPs), are widely used in life sciences, including medical imaging, drug carriers, antibacterial products and anti-tumor applications [88]. Glioblastoma multiforme (GBM) is the most malignant human brain tumor with a median survival of one year [89]. The current treatments including surgical resection, radiotherapy and chemotherapy, or their combination, cannot effectively prevent the progress of GBM. In 2009, Asharani et al. showed that AgNPs can destroy the cytoskeleton of glioblastoma [82] by affecting calcium homeostasis. Calcium ions can activate catabolic enzymes, damage mitochondrial membrane, release apoptotic factors, and cause cell damage [90]. AgNP binds to specific cell surface receptors on organelles, leading to direct Ca2+ transients or activation of other pathways. Cancer cells endocytose AgNP to form endosomes which gather around mitochondria, causing the change of mitochondrial permeability and the rise of ROS, resulting in DNA oxidation and chromosome aberration, and indirectly leading to Ca2+ transients (Fig. 3C) [91]. AgNP can also release silver ions through surface oxidation [92], acting at the same position as calcium ions, regulating the release of calcium ions from sarcoplasmic reticulum [93]. After AgNP treatment, the cytoskeleton was damaged and cells became more spherical with less diffusion. This was due to the disassembly of the membrane cytoskeleton caused by calcium ion disorder, and the phenomenon of cell plasma membrane blistering which is a typical feature of cell apoptosis and necrosis. In 2019, Xiao et al. examined the effect of AgNP on the cytoskeleton [83]. AgNP destroyed colon cancer HCT-116 cells in a dose-dependent manner, bringing about the damage of cell morphology, vagueness of cytoskeleton, contraction or disappearance of lamellar foot structure, disorder of nano structure of cell membrane, and significant reduction of cell adhesion and cell stiffness (Fig. 3D). Cell proliferation, migration, movement, and metastasis are closely related to the available AgNPs which can damage cytoskeleton integrity and alter morphology, thereby affecting the tumor cells' migration and transfer, providing supplementary scientific basis for further elucidation of AgNP treatment in cancer and experimental clues for clinical application. In conclusion, noble metal nanoparticles, such as AuNRs and AgNPs, can damage cytoskeleton by inhibiting ATP synthesis or disrupting calcium ion homeostasis, thus inhibiting tumor development. These two nanoparticles offer new approaches to cancer from different angles. However, their effect on normal cells remains to be studied.
2.3. Metallic oxide based nanomaterials
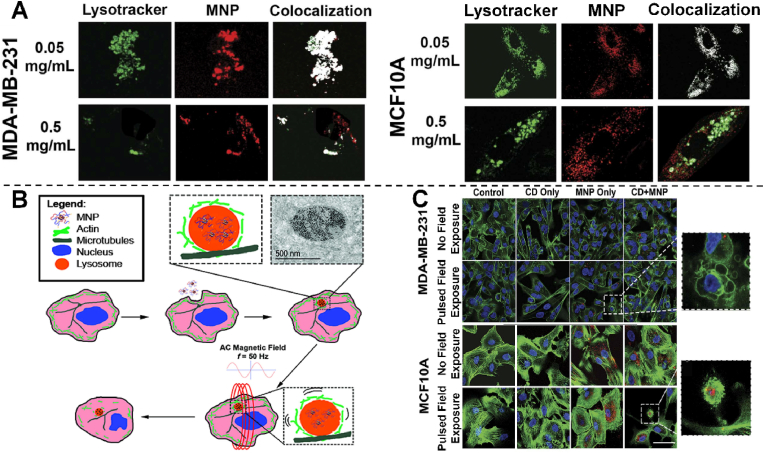
Due to its high specific surface area, thermal stability and mechanical strength, metal oxide nanomaterials exhibit outstanding physical and chemical properties, which make them have excellent optical, magnetic, and catalytic properties. In addition, as a new type of contrast agent, magnetic metal nanomaterials have been used in tumor therapy. Superparamagnetic iron oxide nanoparticles (SMNPs) can be remotely driven in an external magnetic field to kill cancer cells [95]. Magnetic hyperthermia takes advantage of the response of iron oxide particles to a relatively high frequency AC magnetic field. When particles are exposed to such a magnetic field, they generate heat through Néel, or Brownian relaxation to rise temperature to kill surrounding cells [96]. However, magnetic hyperthermia suffers from limitations such as difficulty in synthesizing nontoxic SMNP with sufficiently high specific absorption rate, difficulty in achieving sufficient intracellular SMNP concentration, and heat dissipation from tumors to adjacent healthy tissues. The hypothesis that magnetic nanoparticles cause cell damage through magneto-mechanical drive in AC magnetic field has also been put forward. The exposure to AC electric field will lead to mechanical movement of SMNP, resulting in stress and deformation of surrounding polymer coating and attached biomolecules [97]. In 2016, Master et al. synthesized superparamagnetic iron oxide SMNP complexed with PAA-b-P85-b-PAA pentablock copolymer, formed endosomes through endocytosis, vesicles fused, and nanoparticles accumulated in lysosomes (Fig. 4A), in which a single magnetite particle interacted with hydrophilic poly (ethylene oxide) chain and hydrophobic poly (propylene oxide) chain through expansion, and connected with lysosomal membrane to form aggregates [94]. Under the remote drive of AC magnetic field, PAA-P85-SMNPs can rotate inside the lysosome, thus generating torque and shear stress on the underlying cytoskeleton without lysosome leakage (Fig. 4B). The actin cytoskeleton in cancerous MDA-MB-231 cells exposed to PAA-P85-SMNPs and pulsed AC magnetic fields was significantly damaged, selectively acting on cancer cells while maintaining the integrity of normal cells (Fig. 4C). Notably, Cytochalasin (CD) damaged the normal cytoskeleton but had no effect on cancer cells. However, the pulsed AC magnetic field mechanism produced the same damage in cells in MCF10A after exposure to CD and SMNPs as was observed in cancer cells after exposure to SMNPs and pulsed AC magnetic fields. In conclusion, its high selectivity for cancer cells has great potential for cancer therapy and can be used as a technology platform for other biomedical applications.
Fig. 4.
A) Intracellular distributions of PAA-P85-SMNPs in MDA-MB-231 and MCF10A cells after 24 h of incubation with 0.05 or 0.5 mg/mL PAA-P85-SMNPs. B) Schematic diagram of cytoskeleton destruction caused by mechanical movement of lysosomal uptake SMNP. C) The picture shows representative confocal images of actin (green) of the MDA-MB-231 and MCF10A cells before and after exposure to a pulsed AC magnetic field with or without treatment with CD and/or P AA-P85-SMNP (red) [94]. Copyright 2016, Springer Nature.
In addition, in 2020, Yu et al. synthesized biocompatible tumor cytoskeleton-targeted multivalent supramolecular components [98]. The component consists of β-cyclodextrin-grafted hyaluronic acid (HACD) and iron oxide magnetic nanoparticles (MNPs) grafted by an actin-binding peptide (ABP) and adamantane (Ada)-modified polylysine. The experiment shows that MNP-ABP-Ada⊂HACD can realize the specific recognition and binding of tumor by targeting the HA receptor on the surface of tumor cells and actin cytoskeleton respectively with HACD and ABP. Under the effect of low frequency alternating magnetic field (200 Hz), MNP-ABP-Ada⊂HACD induces the serious actin destruction, leading to the obvious cell cycle arrest and cell death of tumor cells in vitro and in vivo, but has no obvious toxicity on normal cells. In addition, the component can form an extracellular network to prevent further metastasis of tumor cells. Therefore, the strategy that MNP-ABP-Ada treats tumor by targeting and damaging the cytoskeleton has a potential application prospect.
The current research results and hypotheses provide a theoretical basis for exploring the potential toxicity of metal nano oxides. However, the effect of metal oxide nanomaterials in humans remain unclear, the exposure dose, different genetic factors affect the degree of the damage exerted, and more importantly transport mechanism of nanometer materials can also be different. The application of metal oxides in disease treatment still needs more exploration.
2.4. Black phosphorus based nanomaterials
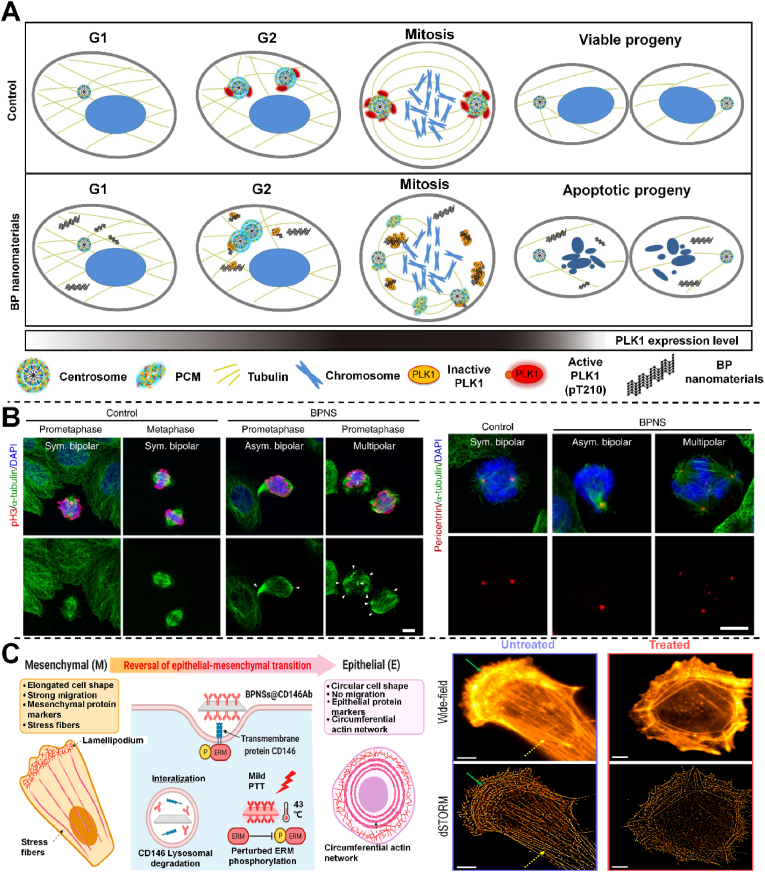
Black phosphorus (BP) is a naturally produced element in human body, 85% of which exists in bones and teeth, and has high biocompatibility and biodegradability [101]. A large amount of water or oxygen in the human body can easily degrade BP, thereby producing phosphate and other PxOy species. Despite its excellent biocompatibility and biodegradability, the use of this material is still a challenge due to its instability under environmental conditions, because even before implantation, lone pair electrons will cause partial degradation, which will greatly reduce its semiconductor properties and affect its performance [102]. However, the surface modification greatly improves the stability of BP, which makes it possible to be used in biomedical scaffolds and hydrogels. The whole cell cycle is known to be divided into four stages, in strict accordance with the sequence of G1, S, G2 and M. Obstruction of either stage does not allow mitosis to complete properly. Shao et al. found that the treatment of cells with low-concentration BP nanomaterials was found to cause cell division to specifically stop at the mitotic M stage of the cell cycle (Fig. 5A) [99]. Research shows that BP inhibits the transfer and activation of PLK1, a key kinase in cell division, to the centrosome, resulting in the inhibition of the normal separation of the centrosome and the fragmentation of the centrosome, leading to the formation of multipolar spindles, and ultimately inhibiting the completion of cell mitosis (Fig. 5B). Hence, the inhibition of cell cycle can be an effective anti-tumor strategy. In addition, as a new PLK1 inhibitor, BP nanomaterials were also seen to show excellent tumor inhibition effect in experimental animal models. In future research, BP nanomaterials have good potential to develop into a clinically available anti-tumor nanodrug. Cancer metastasis is known to lead to the decline and death of most cancer patients, and epithelial mesenchymal transformation (EMT) is the key mechanism for cancer cells with migration and invasion. Liu et al. used engineered black phosphorus nanosheets (BPNS) and mild photothermal therapy (PTT) in combination with the EMT inducer CD146 to reverse EMT in cancer cells [100]. CD146 targets BPNS and gentle PTT synergistically promotes EMT reversal by down-regulating membrane CD146 and disrupting its downstream EMT-associated signaling pathways, a method that converts highly metastatic mesenchymal breast cancer cells to an epithelial phenotype, thereby completely preventing cancer cell migration (Fig. 5C). Through the advanced nanomachinery and super-resolution imaging, the phenotypic transformation in cancer cells was verified, such as the change of actin tissue and cell morphology, the downregulation of mesenchymal protein markers and the up-regulation of epithelial protein markers. This method demonstrated the regulation of the phenotypic transformation in cancer cells can treat various cancer metastases. Therefore, BPNS successfully prevented the metastasis of cancer at the level of tumor cell differentiation. In conclusion, BP has successfully disrupted the further development of cancer by blocking mitosis or reversing the cancer cell phenotype, providing new insights into cancer treatment.
Fig. 5.
A) A Model to illustrate the molecular anticancer activity of BP. B) Co-immunostaining images of α-tubulin with pericentrin and pH3, respectively [99]. Copyright 2016, Springer Nature. C) Mechanism of EMT reversal with mild PTT and BPNSs@CD146 A b and Wide-field and dSTORM images of ventral actin organization in MDA-MB-231 cells [100]. Copyright 2022, American Chemical Society.
2.5. Calcium based nanomaterials
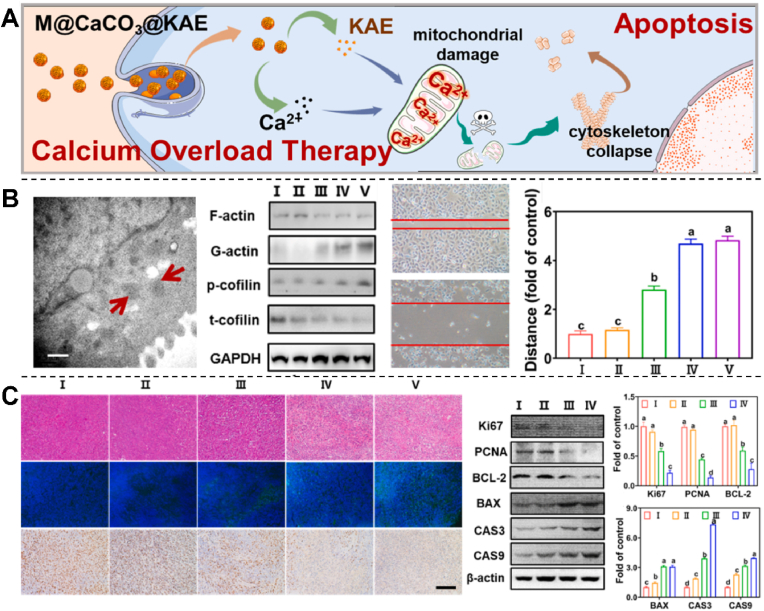
Calcium overload therapy is another treatment strategy that has received widespread attention in the field of oncology [104]. As a second messenger, calcium overload can lead to the loss of mitochondrial membrane potential and further stimulate apoptotic pathways [105]. However, due to the likely lack of calcium ions in the tumor sites, the efficiency of calcium entering the tumor is low, and its curative effect is limited, resulting in an unsatisfactory therapeutic effect. Kahenol-3-O-rutinside (KAE) is a biosafe flavonoid that can effectively disrupt calcium homeostasis and promote calcium influx and has an excellent anti-cancer ability [106]. Calcium carbonate (CaCO3) can decompose and generate calcium ions in an acidic environment. Li et al. synthesized A549 cell membrane coated M@CaCO3@KAE NPs to study its inhibitory effect on lung cancer cells (Fig. 6A) [103]. After reaching the tumor site, M@CaCO3@KAE NP entered cells through endocytosis and tumor microenvironment response, then was degraded into KAE and calcium ions. KAE regulated calcium signaling pathway in situ to break calcium homeostasis, increase calcium influx, and overload calcium. At the same time, the released calcium ions further exacerbated the KAE mediated calcium imbalance, triggered mitochondrial dysfunction, affected the energy supply during the formation of cytoskeleton, induced oxidative stress and cytoskeleton collapse [107], and F-actin degradation directly promoted the collapse of lamellar pseudopodia. At the same time, the filamentous pseudopodia became blurred, inhibited the ability of cell proliferation, migration, and invasion, eventually leading to cell apoptosis, achieving effective tumor treatment with reduced side effects (Fig. 6B). In addition, the animal experiment also obtained the same tumor inhibition effect as the cell experiment (Fig. 6C). M@CaCO3@KAE nanoparticles could be a candidate to successfully address the problem of low solubility and bioavailability of KAE, achieve the targeted delivery of KAE and calcium ions at tumor sites for highly synergistic and efficient calcium overload cancer therapy. This study designed a nanosystem that can act as a bionic calcium bomb to ensure targeted, collaborative, efficient, and biosafe therapy through calcium overload. However, solid CaCO3 NPs have low drug loading capacity and slow decomposition speed, which are difficult to reach the threshold of calcium ion to limit the treatment efficiency [108].
Fig. 6.
A) Diagram of M@CaCO3@KAE NP-mediated cell apoptosis. B) Transmission electron microscopy (TEM) image of mitochondria, cytoskeletal protein levels and cell migration in A549 cells treated with M@CaCO3@KAE NPs. I: Control; II: CaCO3 NPs; III: KAE; Ⅳ: CaCO3@KAE NPs; Ⅴ: M@CaCO3@KAE NPs. C) H&E staining, TUNEL and DAPI staining, IHC staining, proliferation and apoptosis protein expression levels in solid tumors. I: Control; II: CaCO3 NPs; III: KAE; Ⅳ: CaCO3@KAE NPs; V: M@CaCO3@KAE NPs [103]. Copyright 2021, Elsevier.
2.6. Silicon based nanomaterials
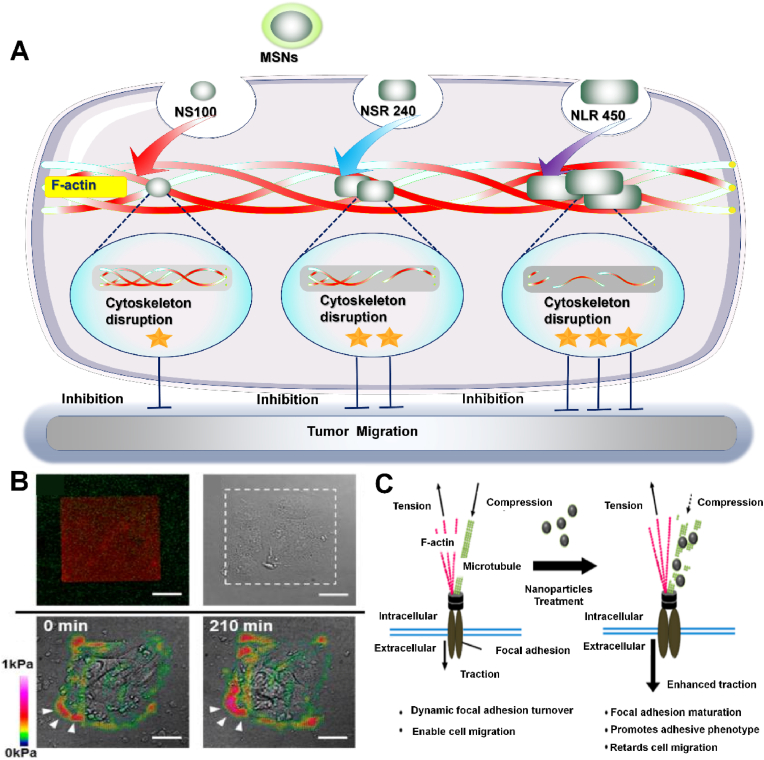
Mesoporous silica nanoparticles (MSNs) have high specific surface area, stable dispersion in water, good biocompatibility and biodegradability in vivo, which shows great potential in limiting cancer cell metastasis and drug delivery [110]. In one study, Huang et al. synthesized three kinds of MSNs with different aspect ratios (AR) to analyze the effect of particle shape on cell function in A375 cells (Fig. 7A) [111]. Because the plasma membrane is directly connected to the actin-based cytoskeleton and functionally integrated, endocytosis on the membrane will require actin rearrangement. Thus, the endocytosis of MSN leads to the destruction of cell membrane and the disintegration of cytoskeleton. When melanoma A375 cells endocytosed three different shapes of MSN, it was found that larger AR particles were endocytosed more and faster.In addition, the more large AR MSN is endocytosed, the greater the destruction of F-actin is, and the more obvious the migration inhibition of A375 cells. The research may provide useful information for the development of effective drug delivery systems and provide insights into nanotoxicology. In addition, Tay et al. explored the mechanism of Silicon dioxide (SiO2) NPs inhibiting the migration of oesophagal cancer TR146 cells [109]. The contractility of the cell is produced by actomyosin motor proteins sliding along the actin filaments, which are attached to the ECM by adhesion proteins. Microtubules can counteract contractility. In cells, actomyosin microtubules are in a strictly regulated state of homeostasis, which prevents disruption of cellular architecture and enables intracellular tension homeostasis [112]. Tay et al. demonstrated that SiO2 NPs can induce cells to form stable adhesion to the underlying matrix and thus limit cell migration (Fig. 7B). The reason for the limited cell migration is that SiO2 NPs interfere with the polymerization of microtubules by destroying tubulin, thus disrupting the balance regulation of intracellular tension and resulting in enhanced adhesion(Fig. 7C) [113]. These findings contribute to a better understanding of NPS-induced cellular responses and are expected to play an important role in exploring these effects.
Fig. 7.
A) Silicon based nanomaterials destroy F-actin to inhibit tumor migration for enhanced cancer therapy. B) Cell adhesion was enhanced after nanoparticle treatment. C) A hypothesized model of nanoparticle mediated changes in intracellular contractility and migration [109]. Copyright 2013, American Chemical Society.
2.7. Polymer based nanomaterials
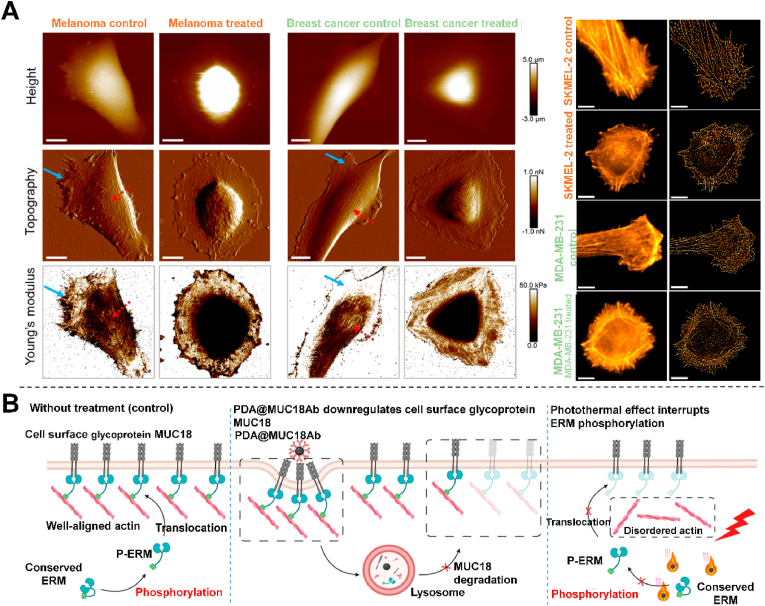
In the field of nanomedicine, polydopamine nanoparticles (PDA NPs) are considered a powerful substitute for gold nanoparticles because of their good biocompatibility and biodegradability, as well as their high photothermal conversion efficiency, and also an ideal light absorber for photothermal therapy [115]. MUC18 is a transmembrane glycoprotein whose expression promotes tumor progression and metastasis, leading to poor prognosis [116]. In 2021, Liu et al. investigated the effects of PDA NPs targeting transmembrane MUC18 combined with mild photothermal effect on actin cytoskeleton and migration of breast cancer cells [114]. Two unique actin cytoskeletal structures, plate-like processes and stress fibers, are closely related to cell migration [117]. The authors prepared a novel method to prepare MUC18 antibody on the surface of PDA NPs and co-incubated with breast cancer cells. dSTORM and AFM images showed that the untreated cells were flaky with the actins network in front of the cells in the same direction of motion and the ventral stress fibers were parallel to the long axis of the cells (Fig. 8A). In contrast, through PDA@Str@Muc18 antibody targeting and laser treatment, there was downregulated transmembrane MUC18 and release of membrane anchored actin filaments. At the same time, the mild photothermal effect interrupted the phosphorylation of ERM complex in the cytoplasm and further destroyed the actin cytoskeleton structure. It showed that the formation of actins network around the periphery of the whole cell and the loss of ventral stress fibers inhibited the migration of cancer cells (Fig. 8B). PDA can serve as a potential agent to address the difficulty in achieving PTT of cells due to insufficient load of light absorbers and insufficient laser penetration (i.e. insufficient temperature), which is a safer and more effective method. This method is not only a supplement to traditional photothermal therapy, but also has great potential in the treatment of chemotherapy-resistant tumor metastasis. As MUC18 is highly expressed in several other cancers, such as ovarian cancer, lung cancer and prostate cancer, which may be a potential treatment for other cancers [118].
Fig. 8.
A) dSTORM and AFM imaging of actin skeleton in a single cancer cell. B) Schematic of the mechanism by which PDA NPs targeting PsMUC18 and mild photothermal effects synergically disrupt the actin structure of cancer cells [114]. Copyright 2021, American Chemical Society.
Immunotherapy controls and eliminates tumors by restarting or enhancing the anti-tumor immune response in tumor patients. T cells can be divided into αβT cells and γδT cells according to the type of TCR. αβ T cells are widely distributed, and tumor-associated antigens, MHC molecules and co-stimulatory signals are involved in the activation of αβ T cells. Once they are absent, the anti-tumor response ability of αβ T cells will be reduced [119], and even lead to the loss of cell viability. γδ T cells are a group of innate immune cells with unique properties that mainly exist in the skin and mucous membranes. Various immune responses and regulatory processes are closely related to it. For example, γδ T cells can promote the progress of immune inflammatory responses, identify and kill specific tumor at the site [120]. More importantly, γδ T cells (specifically Vγ9Vδ2 subset) can recognize stress-induced phosphoantigens presented by cancer cells without the involvement of MHC, which makes Vγ9Vδ2 T cells have a unique advantage over αβ T cells in antitumor therapy. Chitosan (CTS) is a cationic polymer formed by deacetylation of chitin extracted from animal crustaceans, which is widely used because of its good biocompatibility, antibacterial activity and immunomodulatory effect [121,122]. In 2019, Lin et al. synthesized nano CSNPs using chitosan and sodium tripolyphosphate solution and found that CSNPs induced Vγ9Vδ2 T cells α- Tubulin to polarize [123]. Since the dynamic polarization of T cell microtubules is a necessary condition for the formation of immune synapses [124], the formation of immune synapses is a key step for T cells to recognize antigens, proliferate and activate, and an important part of cellular immune response [125]. The results indicated that CSNP could promote the formation of T cell immune synapse and may enhance its ability to kill cancer cells. CTS solves the problem of immunosuppression under the influence of tumor tissue by enhancing the effecting ability of T cells. Immunotherapy can make tumor removal more targeted, which makes it have great potential in the treatment of tumors.
2.8. Polypeptide based nanomaterials
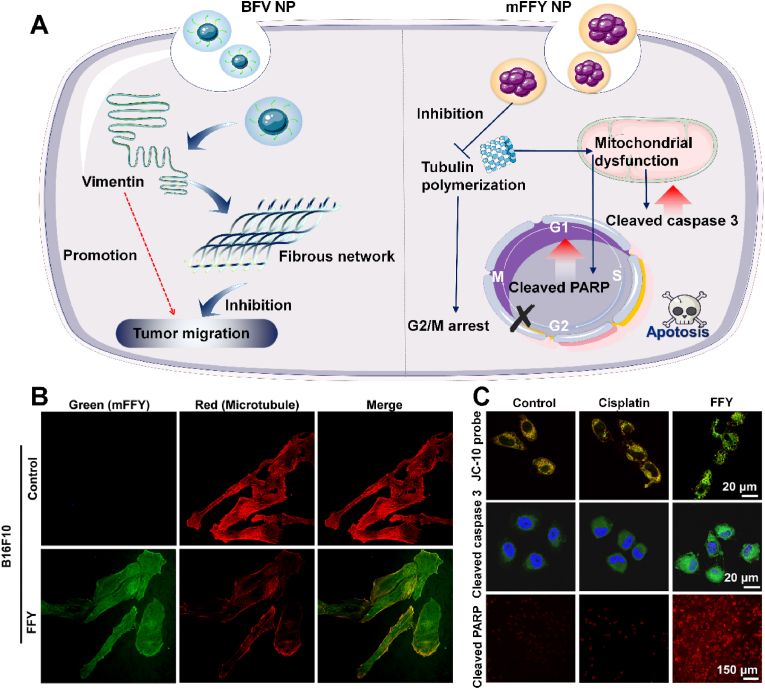
The unique structural properties make peptides very rich, and endow them with good biocompatibility and biorecognition ability. In addition, small peptides can translocate cell membranes without eliciting an immune response in the process. The unstable nature of free peptides lead to rapid degradation during in vivo circulation [127]. The Intermediate fibers vimentin was also identified to significantly enhance tumor cell invasion and metastasis. Vimentin overexpression and incorrect self-assembly lead to morphological changes and increased motility of tumor cells [128]. Fan et al. designed a binding-induced fibrillogenesis peptide, Bis-pyrene-KLVFF-VNTANST (BFV), which can form a fibrous network in situ, block the improper self-assembly of vimentin, and inhibit tumor metastasis (Fig. 9A) [129]. BFV NPs can bind to vimentin and subsequently undergo fibrillation, and the resulting polypeptide fiber network can prevent vimentin from participating in the formation of cytoskeleton, thereby inhibiting tumor cell migration and invasion. The experimental results showed that the tumor volume and the number of lung metastases in the mice were significantly reduced. In addition, the efficacy of BFV NPs is far superior to the small molecule anti-metastatic drug withaferin A. Sun et al. introduced tripeptide (FFY) into drug-resistant melanoma cells, and the tripeptide formed mFFY nanoparticles through enzymatic self-assembly in cells [126]. After “nondrug therapy” treatment, most tumor cells stagnate at the late stage of DNA synthesis or the interphase of division (i.e. G2/M) due to the inability of tubulin to polymerize into microtubules, thus inhibiting the proliferation of tumor cells (Fig. 9B). At the same time, mitochondrial dysfunction also induced the overexpression of apoptotic factors, such as cleaved caspase 3 and cleaved PARP to further promot the apoptosis of drug-resistant tumor cells and finally realized the reversal of drug resistance (Fig. 9C). In addition, Mohan et al. prepared peptide 5 (Pep5) encapsulated PLGA-PEI nanoparticles. These particles are further modified by TKD to target tumor cells, resulting in a dipeptide-functionalized multilayer nanodrug delivery system (Pep5-TPPN) [130]. Studies have reported that Pep5 is likely to promote the destruction of tumor cytoskeleton by binding to Plectin or CLIC1. The TKD targeting function is achieved by its specific binding to heat shock protein 70 on breast cancer cell membranes. The results showed that Pep5-TPPN displayed higher cell uptake than non-target groups. Moreover, after exposure to Pep5-TPPN, tumor cells showed significant morphological changes compared with control group. This result indicated that Pep5-TPPN caused significant loss of integrity of actin filaments in tumor cells. In addition, Pep5-TPPN can also induce lysosome rupture, activation of cleaved Caspase-3, and cleavage of PARP. These injuries can further lead to tumor cell death. In conclusion, in addition to inhibiting tumor development through cytoskeletal destruction, peptides can also destroy tumor cells in a variety of ways. More importantly, polypeptide nanomaterials often have better biocompatibility than other nanomaterials and have better application prospects.
Fig. 9.
A) Polypeptide nanomaterials block the improper self-assembly of vimentin and inhibit microtubule polymerization to inhibit tumor metastasis and cause tumor apoptosis for enhanced cancer therapy. B) Intracellular localization of green (mFFY) and red (microtubule) fluorescence signals after treatment. C) Analysis of mitochondrial dysfunction levels, cleaved caspase 3 and cleaved PARP levels [126]. Copyright 2022, American Chemical Society.
2.9. MOF based nanomaterials
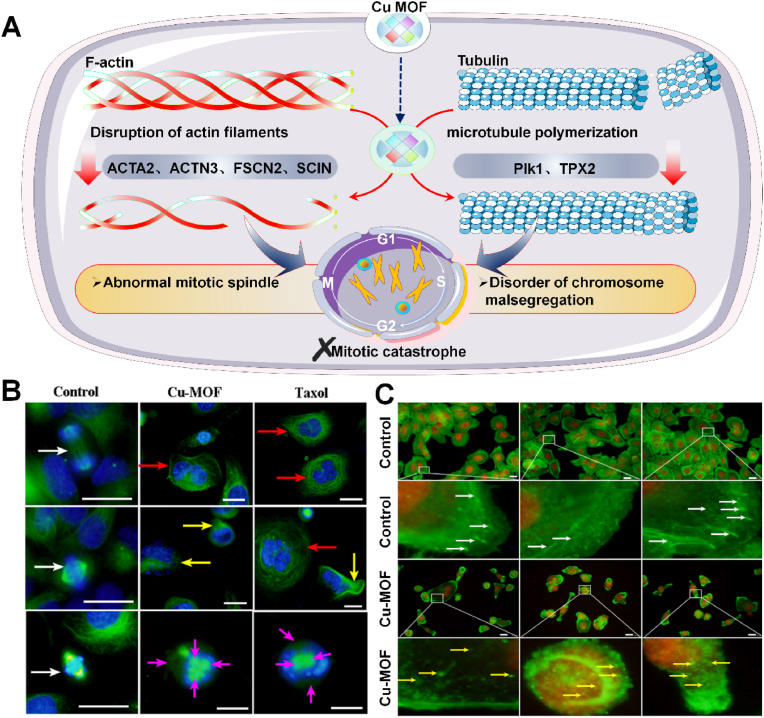
Metal-organic frameworks (MOFs) are composed of metal or cluster nodes linked by organic ligands [132]. MOFs have many excellent properties, such as controllable structure, tunable porosity, easy chemical modification, high biocompatibility, and good biodegradability, making MOF widely used in biomedical related fields [133,134]. In addition to inducing apoptosis of cancer cells, mitotic mutations provide new directions for anticancer therapy, and some of these strategies have been studied in preclinical and clinical phases [135]. Mitotic mutation is a specialized form of cell death caused by the premature or inappropriate entry of cells into mitosis, and mitotic mutation can be triggered by various factors that disrupt the mitotic process or DNA [136]. Mitotic mutations have detectable unique nuclear changes (giant cells with abnormal nuclei) for them to be distinguished from normal cells. In 2020, Chen et al. obtained 500–2500 nm Cu-MOF from copper nitrate and phenyltricarboxylic acid by hydrothermal method to treat SKOV3 ovarian cancer cells (Fig. 10A) [131]. It was found that Cu-MOF downregulated Plk1 and TPX2 of mitotic spindle regulatory genes, promoted tubulin polymerization and disrupted microtubule dynamics, resulting in abnormal mitotic spindle and chromosome segregation in SKOV3 cells, forming multinucleated giant cells (Fig. 10B). Furthermore, Cu-MOF surpressed actin regulatory genes ACTA2, ACTN3, FSCN2 and SCIN. In the Cu-MOF treatment group, many fluorescent spots were scattered in the cytoplasm, resulting in the significant cytoplasm shrinkage and cell morphology changing, indicating that microtubules and actin were damaged and destroyed by Cu-MOF (Fig. 10C). Albeit many methods have been developed for preparing nano MOF and its derivatives, manufacture MOF with controllable pore size, size and topology still faces many difficulties. These parameters determine the clinical antitumor efficiency of MOF-based antitumor drugs. Meanwhile, the biosafety of MOFs is another key factor limiting their application in cancer treatment. The metal ions and organic ligands released after MOF dissociation may potentially cause biosafety problems in organisms. Biodegradable and absorbable components might presumably be a solution. Clinical applications require low or non-toxic MOF composed of biocompatible ligands and metal ions [137].
Fig. 10.
A) MOF damage microtubules and F-actin to inhibit tumor proliferation for enhanced cancer therapy. B) Fluorescence image of abnormal mitosis of SKOV3 cells treated by Cu-MOF. C) Cu-MOF causes the damage of F-actin [131]. Copyright 2020, American Chemical Society.
3. Nanomedicine acting on cytoskeleton for other diseases treatment
3.1. Wound healing
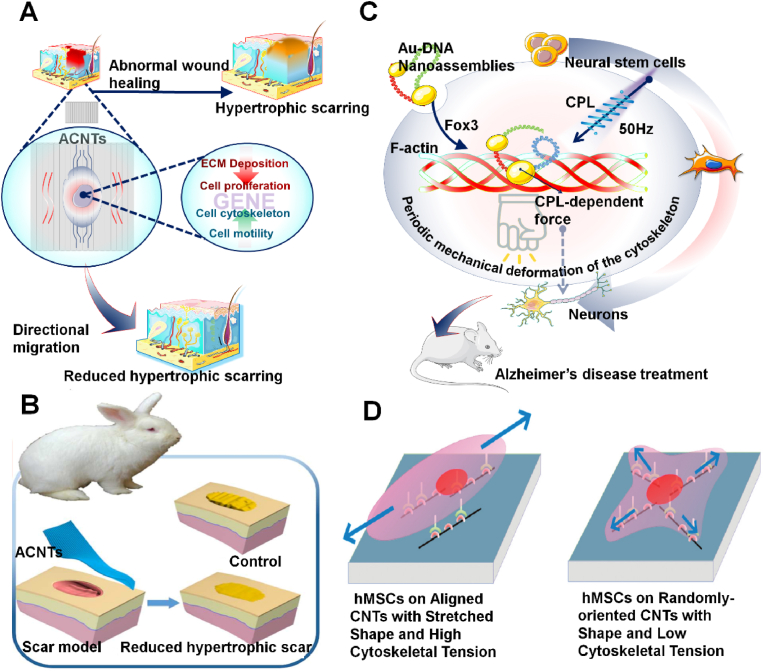
Hypertrophic scarring is the result of an abnormal wound healing process in which too much collagen is deposited in the wound area, causing the scar to rise above the surface of the skin. Second, when hypertrophic scarring appears above the joint, it often appears red and shiny, causing pain, itching, and sometimes even restricting movement, leading to severe morbidity. As a common complication after surgery, trauma, and burns, hypertrophic scarring is a notable challenge for surgeons [140]. Key factors to reduce scarring include adequate inhibition of fibroblast over-proliferation, excessive collagen deposition, and formation of well-structured fibrous tissue at the wound site [141]. However, the treatments available to reduce hypertrophic scarring, including surgery and drugs, do not live up to expectations [142,143]. In recent years, aligned structural materials with appropriate fiber diameters and spacing can induce cell directional growth, showing great potential in tissue engineering [144]. In 2018, Weng et al. synthesized ACNT arrays by chemical vapor deposition in a tubular furnace using iron as catalyst, alumina as a buffer layer, silicon (110) wafers as substrate, argon and hydrogen as the carrier gas, and ethylene as carbon source [138]. When human dermal fibroblast cells (HDF) and mouse embryonic fibroblast NIH3T3 cells were cultured on ACNT, the expression of fibroblast proliferation-related genes NFIB, CYP7B1, collagenous deposition-related genes COLA1A1 and COL3A1 [145,146], as well as the regulation of cytoskeleton genes TNS4 and KCNAB2 were altered [147,148]. Resultantly, the proliferation rate of fibroblast decreased significantly, and the type I collagen deposition was lower. F-actin and α-tubulin are rearranged in parallel to CNT, and the cells are elongated into a spindle shape with fewer filamentous pseudopods (Fig. 11A). This linear bundle of long, parallel filamentous structures is thought to induce directional migration (Fig. 11B) [149]. Studies have found that ACNTs can influence cell behavior and ECM composition by inhibiting cell proliferation, guiding its growth direction, and inhibiting collagen deposition. As a block material, ACNTs can inhibit the formation of hypertrophic scars in vivo. These findings may provide ACNTs’ applications in the field of bioengineering with an alternative strategy.
Fig. 11.
A) Nanomaterials acting on actin and tubulin to promote wound healing. B)ACNTs effectively reduced the formation of hypertrophic scars [138]. Copyright 2018, American Chemical Society. C) Nanomaterials acting on actin to promote stem cell differentiation. D) Plausible model to explain the hMSC responses to the aligned and the randomly oriented CNT networks [139]. Copyright 2011, American Chemical Society.
3.2. Stem cell differentiation
Mesenchymal stem cells (MSCs) are pluripotent stromal cells that can differentiate into mesenchymal tissue lineages such as osteoblasts, chondrocytes. Lately, nanostructured matrices have been investigated more and it has been found that nanomaterials can also control the growth and differentiation of human mesenchymal stem cells (hMSCs) without chemical additives [150]. In 2011, Namgung et al. thermally deposited 5 nm thick Ti layer and 10 nm Au layer on the covered glass, and spun CNTs in the suspension onto a gold substrate at a speed of 5000 rpm to prepare nanotubes assembled in a form of arrangement, and inoculated hMSCs on the substrate (Fig. 11C) [139]. The results showed that the actin filaments in hMSCs were also stretched along the alignment direction of individual CNTs (Fig. 11D). The alignment of actin filaments induced a stretched shape of hMSCs. Compared with randomly oriented CNT networks, hMSCs in aligned CNT networks have higher cytoskeletal tension and stronger proliferation and osteogenic differentiation ability. This study can provide a valuable example of employing designs of hybrid cell-nanostructure systems for regenerative tissue engineering and this research could be a boon for faster healing of broken bones.
Chiral inorganic nanoparticles can intensely interact with circularly polarized light (CPL) owing to their high polarizability, which may greatly enhance the biological response of chiral photons [151]. In 2021, Qu et al. synthesized the chiral assembly of DNA bridging gold nanoparticles and found that the chiral nanomaterial assembly could change its conformation after entering the cell [152]. The C30(D)S5–C20(L) nanomaterial was reconfigured to C30(D)-C20(L)S5 in the presence of Fox3 and then bound to the actins network. Subsequently, CPL applied a force required for polarization to the nanoassemblies as well as the nanofibers, and periodic mechanical deformation of the cytoskeleton under 50 Hz pulsed light stimulated neural stem cells (NSCs) to differentiate into a neuronal phenotype. When NSCs were implanted in the hippocampus of an AD bearing mouse l and irradiated according to a polarity optimization protocol, amyloid plaque formation was reduced by more than 70%. The results suggested that circularly polarized light have the potential in guiding cell development for biomedical applications.
3.3. Osteogenesis
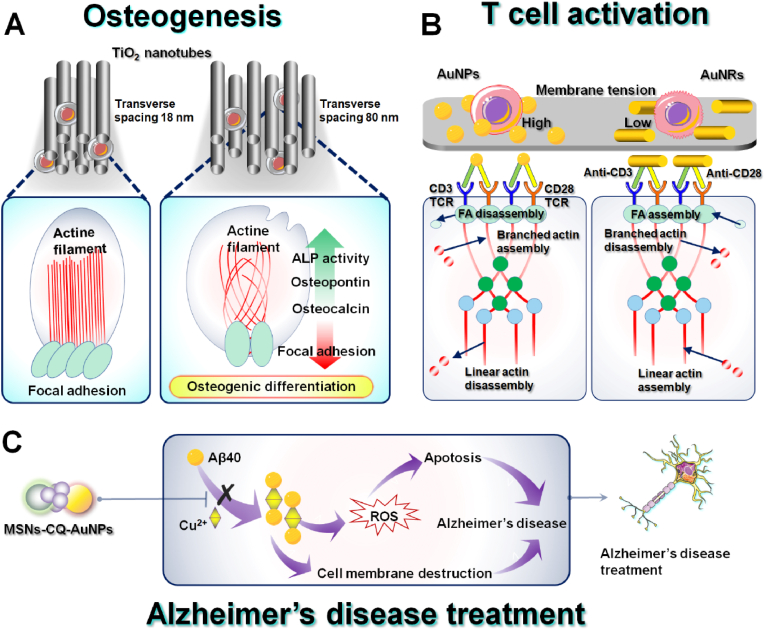
In human body, a layer of connective tissue forms between the implant metal and the underlying bone surface due to an immune response, which is affected by the thickness of the interface layer between the implant and bone. However, osseointegration usually occurs in the absence of a common layer of connective tissue [153]. Therefore, it is necessary to develop an implant with a bioactive surface layer that is both strong in binding and thin enough to minimize delamination. Modification of Ti surface with nanostructured TiO2 nanotubes can significantly enhance mineral formation, adhesion of osteoblasts in vitro [154] and strong adherent bone growth in vivo [155]. TiO2 nanotube surface system is generated directly from the underlying natural Ti composition with 300 nm thickness which is much thinner than previous coatings. This nanoscale length eliminates the tendency of layering that is common in thicker micron layers and improves osteogenesis [153]. In 2009, Brammer et al. prepared TiO2 nanotube surfaces on titanium plates by dual-electrode anodic oxidation process and inoculated MC3T3-E1 mouse osteoblasts on the surface of the materials [156]. Immunofluorescence images showed that compared with the cytoskeleton inoculated on the surface of titanium plates, actin images on the surface of TiO2 nanotubes elongated along with augmented nanotubes diameter, possibly because that osteoblast must further expand its filiform pseudopodia to a larger area, and forming a very slender shape to find the surface of a protein deposition. At the same time, the results also revealed that the bone formation marker ALP activity of osteoblasts in vitro increased proportional with the TiO2 nanotube diameter, indicating TiO2 nanotubes, especially large-sized (100 nm) nanotubes, have good biocompatibility and great potential as bone implant materials to improve the functional control of bone formation in advanced orthopedic implant technology. In 2019, Necula et al. studied the effect of transverse spacing of TiO2 nanotubes on osteoblasts and found that TiO2 nanotubes with large transverse spacing caused osteoblasts to show grid-like actin filaments branching and more filamentous pseudopods, while ALP activity increased [157]. The results showed that larger lateral spacing can also enhance osteogenic induction of pre-osteoblasts, and providing a new variable worth studying for the design and optimization of titania-based materials as bone regeneration and drug delivery platforms (Fig. 12A).
Fig. 12.
A) Nanomaterials acting on F-actin to promote osteogenesis. B) Nanomaterials acting on F-actin to activate T cells. C) Nanomaterials acting on tubulin to treat Alzheimer's disease.
3.4. T cell activation
T cells are important members of adaptive immunity that enhance or suppress the immune response by secreting cytokines or killing heterologous cells [158]. In T cells, microfilaments not only regulate cell morphology, but also participate in cell chemotactic migration, antigen recognition, immune synapse formation, activation, and proliferation [159]. It responds to T cell antigen receptor (TCR) mediated signal, promotes T cell polarization, and assists TCR to recruit and stabilize signal molecules. It also contributes to the movement and rearrangement of membrane molecules during signal transduction. Oh et al. examined the mechanism of extracellular ligand nano geometry in the activation of isolated T cells to activate immunotherapy [160]. Compared with low-aspect-ratio nanoparticles, anisotropic activating ligands using large-aspect-ratio AuNRs significantly enhanced T-cell proliferative ability, promoted activation-related morphological changes in T cells, such as linear actin cytoskeleton growth. Nanoscale anisotropic ligands promote the production of key cytokines by CD8+ T-cells, including IL-2, IFN-γ and TNF-α. Large aspect ratio ligand presentation also promotes the differentiation of mouse CD8+ T cells into the central memory phenotype. The results indicated the importance of manipulating the nano geometry of extracellular ligands in optimizing T cell behavior to enhance therapeutic efficacy. The dynamic regulation of T-cell microfilament skeleton plays an important role in maintaining cell homeostasis, activation, migration, and function. The assembly of TCR/CD3 signaling complex and cytoskeletal recombination induced by T-cell activation involves a series of protein phosphorylation, and the interactions between these protein molecules well-regulate the signal transduction after T-cell activation (Fig. 12B).
3.5. Alzheimer's disease treatment
One of the most characteristic pathological changes of AD is the accumulation of extracellular amyloid plaques (Aβ) [161]. Specifically, metal ions accelerate the aggregation of Aβ, which is deposited by selectively binding Zn2+ or Cu2+, and producing ROS, leading to the pathogenesis of AD [162]. In addition, microtubule structural defects affect axonal transport and contribute to various neurodegenerative diseases. MSNs are excellent drug carriers owing to their excellent thermal and pH resistance. Yang et al. developed a gold nanoparticle-terminated mesoporous silica-based H2O2-responsive controlled-release system for delivery of the metal chelator clioquinol (CQ) to inhibit Aβ self-assembly and metal-induced aggregation in AD (Fig. 12C) [163]. MSN-CQ-AuNPs inhibited Cu2+-induced Aβ40 aggregation and protected PC12 cells from Aβ40-Cu2+ complex-induced cell membrane disruption and ROS-mediated apoptosis. In the presence of MSN-CQ-AuNPs, PC12 cells had well-structured microtubules throughout the cell body and neurites. In addition, the AuNPs in the MSN-CQ-AuNPs system provided a method which could inhibit Aβ self-assembly. The high blood-brain barrier permeability, efficient cellular uptake, selective intracellular release of metal chelators, and good biocompatibility of MSN-CQ-AuNPs demonstrate their potential for future biomedical applications. In addition, Cheng et al. developed MSN as an advanced nanoplatform for simultaneous synergistic delivery of curcumin and RhoG to examine their therapeutic effects on neurodegenerative diseases (NDs) [164]. The researchers successfully encapsulated curcumin and adsorbed the plasmid RhoG-DsRed/TAT peptide complex, and constructed an MSN system (Cur@MSN-RhoG/TAT) co-loading drugs and genes. Plasmid RhoG-DsRed is involved in the formation of lamellipodia and filopodia to promote neurite outgrowth. The early release of curcumin plays a significant role in scavenging ROS to protect N2a cells and RhoG against ROS-mediated damage. This MSN-based platform combines actin cytoskeleton reorganization and pharmacology with anti-oxidative stress, providing a promising approach for NDs therapy.
4. Conclusion, discussion, and future perspectives
Nanotechnology provides many new research methods and ideas for tumor treatment, and has its unique advantages compared with other common treatment methods. Most nanomaterials focus on antitumor mechanisms is based on their own physicochemical properties or basic cell activity, ignoring how nano-drugs achieve the treatment process of tumors. Because the cytoskeleton plays a central role in cell structure, the influence of nanomaterials on cytoskeleton for the cancer therapy may be a promising strategy. Through continual research efforts, the development of nanomedicine has made great progress, but many nanotherapeutic strategies are still in their infancy, and some potential problems remain to be comprehensively assessed when designing nanomedicines to interfere with the cytoskeleton. The EPR effect of tumors is a preset window for nanomaterials to enter the tumor site, however the insufficiency of the EPR effect, as well as the numerous physicochemical barriers in the transport process, have been largely ignored. In particular, In particular, tumor in early stage have not obvious EPR effects, and the non-uniform TME around the tumor area also hinders the infiltration and intratumoral distribution of nanomaterials [165]. If nanodrugs cannot reach tumors in the first place, drug designs that targets cancer cell skeletal interference would be unable to perform their intended roles. In addition, some nanomaterials need to break cell junctions in order to enter tumor tissues, but may cause tumor cell metastasis when entering tumor sites due to the breakdown of cell junctions. In addition, the premise of biomedical applications of nanoparticles is biodegradability, which directly determines their prospects for clinical transformation. If nanomaterials are difficult to degrade, they can accumulate in the body for a long time and cause more complex long-term toxic effects. In the process of targeted therapy, the targeting of nanomaterials is usually relative, and can only improve the accumulation of tumor sites and reduce the biological distribution of normal organs to a certain extent. Therefore, it is also urgent to use safer and more efficient nanomaterials to achieve effective treatment of tumors and reduce the off-target effects.
At present, there are not many target ligands that effectively target tumor cytoskeleton, which limits the application of cytoskeletal therapy. Even if the nanomaterials successfully enter the tumor site, they have the potential to interact with other components of the tumor, such as immune cells, which can adversely affect immune response and reduce the body's ability to control infection. This concern is not groundless, as the actin-interacting protein WD repeat-containing protein 1 (WDR1) is known to promote cofilin-dependent actin filament turnover. It is identified that biallelic WDR1 mutation is a rare genetic disorder of the immune system, which are characterized by a dysfunctional immune system caused by a malfunction of lymphocytes' ability to rearrange actin. The ways to reduce the damage of nanomaterials to other beneficial cells in the tumor site is a question worthy of consideration. Although nanomedicine has great advantages in interfering with the cytoskeleton, the risk of long-term retention cannot be ignored. In addition, the strategy of targeting cytoskeleton for tumor therapy may be relatively slow compared to other therapeutic modalities. Because many nanomaterials disrupt the cytoskeleton depend primarily on downregulation of cytoskeleton protein gene expression and it may take certain time for the depletion of intracellular skeleton proteins. Although much research has been done on the cytoskeleton at the cellular level, strategies for observing cytoskeleton destruction in vivo are relatively lacking.
We provide a set of considerations on key issues for future innovation and potential shifts in nanomedicine strategies to maximize therapeutic outcomes. As a therapeutic concept which places great emphasis on grasping the optimal time window for administration, vascular normalization strategies appear to benefit greatly from the nanomaterials field's expertise in improving kinetics and effective doses for more predictable and stable delivery of useful drugs [127]. In the future, when designing more efficient and comprehensive nanomaterials to penetrate tumors, the complexity of different organs and different types of tumors should be fully considered. Presently, the bulk of nanomaterials may have rare chance of contacting with intended tumor cells, because any nanoparticle with a targeting moiety may only have one passing interaction with the target before subsequent systemic clearance. Therefore, one potential option is to increase the area of mutual contact between nanodrugs and tumor cells, thereby improving homotypic targeting between tumour cell types in vitro and in vivo. There could so be insufficient EPR effect of early-stage tumors, where inducing vascular leakage at early-stage tumor sites through nanomaterials, thereby increasing tumor penetration and cellular uptake of nanomedicines, and raising the contact probability of nanomedicines with the cytoskeleton, can be a promising direction.
Since tumor cells have the tendency to specifically metastasize and colonize specific organs, the encapsulation of metastatic cells could be achieved by combining some nanomaterials or nanomedicines on the cell surface, the colonization of specific organs by tumor cells could be reduced by decreasing competitive inhibition, therefore further reducing metastasis of tumors to specific organs. Hopefully, increasing strategies can emerge in the future to preferentially suppressfthe scaffold and inhibit the tumor metastatic cascade. Inspired by the fact that F-actin antibody can specifically recognize the cytoskeleton to realize fluorescence monitoring [166], in terms of cytoskeleton targeting, some nanomedicines coupled with F-actin antibody can be designed to achieve active cytoskeleton targeting in the future. In past studies on cancer treatment, the actual doses used have always far exceeded the expected dose levels [167]. After improving the scaffold targeting ability of nanomedicines, more cytosleleton-interfernce drugs are expected to enter the tumor, which will reduce the doses required for conventional medicines, thereby reducing the incidence of side effects on off-target healthy tissue.
Cells sense the external microenvironment and physical stimuli through their highly dynamic skeletal network, and at the same time regulate cell behavior. The cytoskeleton maintains cell shape and polarity. In addition to the design aimed at realizing the interference with the cytoskeleton, some researchers are still designing nano-drugs, using the self-assembled polymer formed by MDM2/MDMX dual-effect degradation peptide PMIV and gold ions to simulate the skeleton of the cell, and the red blood cell membranes as shells to mimic nanocells, to inhibit macrophage uptake and prolongs blood circulation time, resulting in better tumor enrichment. This idea can expand the application scope of cytoskeleton. In the future, when designing more efficient and comprehensive nanomaterials to penetrate tumors, the complexity of different organs and different types of tumors should be fully considered. The integrated design concept of smart nanomaterials that efficiently interfere with the cytoskeleton may represent a developmental breakthrough in cancer therapy. So far, toxicity testing studies of nano-cytoskeleton disruptors have mainly focused on short-term biocompatibility, and long-term follow-up studies of them in vivo effects remain to be further explored. Therefore, before the clinical application of nanomaterials, these risks must be clearly addressed through adequate demonstration to ensure the biosafety of cytoskeletal disruptors in vivo. Nanoparticles need to overcome barriers such as RES system, vascular structure, tumor matrix microenvironment, and tumor intercellular space before they enter tumor cells. Due to the complexity of the nanoparticle delivery process, taking advantage of the easier transport properties of small-molecule drugs or nanomaterials with smaller sizes, and inducing their self-assembly within cells to interfere with the cytoskeleton could also be a potential approach to treat tumors.
Ethics approval
Not applicable.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgements
We acknowledge the funding provided by the National Natural Science Foundation of China (22104073), the Natural Science Foundation of Shandong (ZR2021QA100, ZR2021QB119, 2022HWYQ-079), the Youth Innovation Science and Technology Program of Shandong Provincial Universities (2021KJ100).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Dawei Chen, Email: dave0505@yeah.net.
Xiao Sun, Email: sunxiao@sdfmu.edu.cn.
References
- 1.Svitkina T. The actin cytoskeleton and actin-based motility. Cold Spring Harbor Perspect. Biol. 2018;10:a018267. doi: 10.1101/cshperspect.a018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodson H.V., Jonasson E.M. Microtubules and microtubule-associated proteins. Cold Spring Harbor Perspect. Biol. 2018;10:a022608. doi: 10.1101/cshperspect.a022608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hohmann T., Dehghani F. The cytoskeleton-A complex interacting meshwork. Cells. 2019:8. doi: 10.3390/cells8040362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fife C.M., McCarroll J.A., Kavallaris M. Movers and shakers: cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2014;171:5507–5523. doi: 10.1111/bph.12704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haga R.B., Ridley A.J. Rho GTPases: regulation and roles in cancer cell biology. Small GTPases. 2016;7:207–221. doi: 10.1080/21541248.2016.1232583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bamburg J.R., Minamide L.S., Wiggan O., Tahtamouni L.H., Kuhn T.B. Cofilin and actin dynamics: multiple modes of regulation and their impacts in neuronal development and degeneration. Cells. 2021;10 doi: 10.3390/cells10102726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino D., Branch K.M., Weaver A.M. Signaling inputs to invadopodia and podosomes. J. Cell Sci. 2013;126:2979–2989. doi: 10.1242/jcs.079475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wennerberg K., Der C.J. Rho-family GTPases: it's not only Rac and Rho (and I like it) J. Cell Sci. 2004;117:1301–1312. doi: 10.1242/jcs.01118. [DOI] [PubMed] [Google Scholar]
- 9.Kingston D.G. Tubulin-interactive natural products as anticancer agents. J. Nat. Prod. 2009;72:507–515. doi: 10.1021/np800568j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L., Yang F., Yang X., Guan X., Hu C., Liu T., He Q., Yang B., Hu Y. Synthesis and biological evaluation of new 4beta-anilino-4'-O-demethyl-4-desoxypodophyllotoxin derivatives as potential antitumor agents. Eur. J. Med. Chem. 2011;46:285–296. doi: 10.1016/j.ejmech.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Liao C.H., Pan S.L., Guh J.H., Chang Y.L., Pai H.C., Lin C.H., Teng C.M. Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiae fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis. 2005;26:968–975. doi: 10.1093/carcin/bgi041. [DOI] [PubMed] [Google Scholar]
- 12.Reddy M.V., Akula B., Cosenza S.C., Lee C.M., Mallireddigari M.R., Pallela V.R., Subbaiah D.R., Udofa A., Reddy E.P. (Z)-1-aryl-3-arylamino-2-propen-1-ones, highly active stimulators of tubulin polymerization: synthesis, structure-activity relationship (SAR), tubulin polymerization, and cell growth inhibition studies. J. Med. Chem. 2012;55:5174–5187. doi: 10.1021/jm300176j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q., Xu S., Liu S., Wang Y., Liu G. Emerging nanomedicines of paclitaxel for cancer treatment. J. Contr. Release. 2022;342:280–294. doi: 10.1016/j.jconrel.2022.01.010. [DOI] [PubMed] [Google Scholar]
- 14.O'Shaughnessy J., Kaklamani V., Kalinsky K. Perspectives on the mechanism of action and clinical application of eribulin for metastatic breast cancer. Future Oncol. 2019;15:1641–1653. doi: 10.2217/fon-2018-0936. [DOI] [PubMed] [Google Scholar]
- 15.Low I., Dancker P. Effect of cytochalasin B on formation and properties of muscle F-actin. Biochim. Biophys. Acta. 1976;430:366–374. doi: 10.1016/0005-2728(76)90092-x. [DOI] [PubMed] [Google Scholar]
- 16.Coue M., Brenner S.L., Spector I., Korn E.D. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y., Zuo S., Zhang J., Liu T., Li X., Zhang H., Cheng M., Wang S., He Z., Sun B., Sun J. Prodrug nanoassemblies bridged by Mono-/Di-/Tri-sulfide bonds: exploration is for going further. Nano Today. 2022;44 doi: 10.1016/j.nantod.2022.101480. [DOI] [Google Scholar]
- 18.Kim J., Lee J., Lee J., Keum H., Kim Y., Kim Y., Yu B., Lee S.Y., Tanaka J., Jon S., Choi M.C. Tubulin-based nanotubes as delivery platform for microtubule-targeting agents. Adv. Mater. 2020;32 doi: 10.1002/adma.202002902. [DOI] [PubMed] [Google Scholar]
- 19.Barman S., Das G., Gupta V., Mondal P., Jana B., Bhunia D., Khan J., Mukherjee D., Ghosh S. Dual-arm nanocapsule targets neuropilin-1 receptor and microtubule: a potential nanomedicine platform. Mol. Pharm. 2019;16:2522–2531. doi: 10.1021/acs.molpharmaceut.9b00123. [DOI] [PubMed] [Google Scholar]
- 20.Levrier C., Rockstroh A., Gabrielli B., Kavallaris M., Lehman M., Davis R.A., Sadowski M.C., Nelson C.C. Discovery of thalicthuberine as a novel antimitotic agent from nature that disrupts microtubule dynamics and induces apoptosis in prostate cancer cells. Cell Cycle. 2018;17:652–668. doi: 10.1080/15384101.2017.1356512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang R., Mondal G., Ness R.A., Arnst K., Mundra V., Miller D.D., Li W., Mahato R.I. Polymer conjugate of a microtubule destabilizer inhibits lung metastatic melanoma. J. Contr. Release. 2017;249:32–41. doi: 10.1016/j.jconrel.2017.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levrier C., Sadowski M.C., Rockstroh A., Gabrielli B., Kavallaris M., Lehman M., Davis R.A., Nelson C.C., 6alpha-Acetoxyanopterine A novel structure class of mitotic inhibitor disrupting microtubule dynamics in prostate cancer cells. Mol. Cancer Therapeut. 2017;16:3–15. doi: 10.1158/1535-7163.MCT-16-0325. [DOI] [PubMed] [Google Scholar]
- 23.Xiu Z., Liu J., Wu X., Li X., Li S., Wu X., Lv X., Ye H., Tang X. Cytochalasin H isolated from mangrove-derived endophytic fungus inhibits epithelial-mesenchymal transition and cancer stemness via YAP/TAZ signaling pathway in non-small cell lung cancer cells. J. Cancer. 2021;12:1169–1178. doi: 10.7150/jca.50512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wurtemberger J., Tchessalova D., Regina C., Bauer C., Schneider M., Wagers A.J., Hettmer S. Growth inhibition associated with disruption of the actin cytoskeleton by Latrunculin A in rhabdomyosarcoma cells. PLoS One. 2020;15 doi: 10.1371/journal.pone.0238572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glassmann A., Carrillo Garcia C., Janzen V., Kraus D., Veit N., Winter J., Probstmeier R. Staurosporine induces the generation of polyploid giant cancer cells in non-small-cell lung carcinoma A549 cells. Anal. Cell Pathol. 2018 doi: 10.1155/2018/1754085. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko C.Y., Shih P.C., Huang P.W., Lee Y.H., Chen Y.F., Tai M.H., Liu C.H., Wen Z.H., Kuo H.M. Sinularin, an anti-cancer agent causing mitochondria-modulated apoptosis and cytoskeleton disruption in human hepatocellular carcinoma. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22083946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adamus A., Ali I., Vasileiadis V., Al-Hileh L., Lisec J., Frank M., Seitz G., Engel N. Vincetoxicum arnottianum modulates motility features and metastatic marker expression in pediatric rhabdomyosarcoma by stabilizing the actin cytoskeleton. BMC Complement Med. Ther. 2021;21:136. doi: 10.1186/s12906-021-03299-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith C.E.L., Lake A.V.R., Johnson C.A. Primary cilia, ciliogenesis and the actin cytoskeleton: a little less resorption, A little more actin please. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.622822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi K., Ru Q., Zhang C., Huang J. Cyclin Y modulates the proliferation, invasion, and metastasis of hepatocellular carcinoma cells. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:1642–1653. doi: 10.12659/msm.906075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye Q., Liao X., Fu P., Dou J., Chen K., Jiang H. Portulacerebroside A inhibits adhesion, migration, and invasion of human leukemia HL60 cells and U937 cells through the regulation of p38/JNK signaling pathway. OncoTargets Ther. 2016;9:6953–6963. doi: 10.2147/OTT.S117523. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Guan C., Zhu X., Feng C. DNA nanodevice-based drug delivery systems. Biomolecules. 2021;11 doi: 10.3390/biom11121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li R.S., Liu J., Shi H., Hu P.P., Wang Y., Gao P.F., Wang J., Jia M., Li H., Li Y.F., Mao C., Li N., Huang C.Z. Transformable helical self-assembly for cancerous Golgi apparatus disruption. Nano Lett. 2021;21:8455–8465. doi: 10.1021/acs.nanolett.1c03112. [DOI] [PubMed] [Google Scholar]
- 33.Wang Y., Kaur G., Chen Y., Santos A., Losic D., Evdokiou A. Bioinert anodic alumina nanotubes for targeting of endoplasmic reticulum stress and autophagic signaling: a combinatorial nanotube-based drug delivery system for enhancing cancer therapy. ACS Appl. Mater. Interfaces. 2015;7:27140–27151. doi: 10.1021/acsami.5b07557. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F., Zhu X., Gong J., Sun Y., Chen D., Wang J., Wang Y., Guo M., Li W. Lysosome-mitochondria-mediated apoptosis specifically evoked in cancer cells induced by gold nanorods. Nanomedicine (Lond) 2016;11:1993–2006. doi: 10.2217/nnm-2016-0139. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z., Liu Y., Ma R., Chen J., Qiu J., Du S., Li C., Wu Z., Yang X., Chen Z., Chen T. Thermosensitive hydrogel incorporating prussian blue nanoparticles promotes diabetic wound healing via ROS scavenging and mitochondrial function restoration. ACS Appl. Mater. Interfaces. 2022;14:14059–14071. doi: 10.1021/acsami.1c24569. [DOI] [PubMed] [Google Scholar]
- 36.Li X., Zhen M., Deng R., Yu T., Li J., Zhang Y., Zou T., Zhou Y., Lu Z., Guan M., Xu H., Shu C., Wang C. RF-assisted gadofullerene nanoparticles induces rapid tumor vascular disruption by down-expression of tumor vascular endothelial cadherin. Biomaterials. 2018;163:142–153. doi: 10.1016/j.biomaterials.2018.02.028. [DOI] [PubMed] [Google Scholar]
- 37.Qin Y., Shen M., Liu X., Gu J., Zhu M., Yi X. Photo-driven delivery of (125)I-labeled nanomicelles for nucleus-targeted internal conversion electron-based cancer therapy. ACS Appl. Mater. Interfaces. 2021;13:49671–49681. doi: 10.1021/acsami.1c13249. [DOI] [PubMed] [Google Scholar]
- 38.Chen F., Jin J., Hu J., Wang Y., Ma Z., Zhang J. Endoplasmic reticulum stress cooperates in silica nanoparticles-induced macrophage apoptosis via activation of CHOP-mediated apoptotic signaling pathway. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20235846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ying H., Ruan Y., Zeng Z., Bai Y., Xu J., Chen S. Iron oxide nanoparticles size-dependently activate mouse primary macrophages via oxidative stress and endoplasmic reticulum stress. Int. Immunopharm. 2022;105 doi: 10.1016/j.intimp.2022.108533. [DOI] [PubMed] [Google Scholar]
- 40.Li L., Cui J., Liu Z., Zhou X., Li Z., Yu Y., Jia Y., Zuo D., Wu Y. Silver nanoparticles induce SH-SY5Y cell apoptosis via endoplasmic reticulum- and mitochondrial pathways that lengthen endoplasmic reticulum-mitochondria contact sites and alter inositol-3-phosphate receptor function. Toxicol. Lett. 2018;285:156–167. doi: 10.1016/j.toxlet.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Wang M., Li J., Dong S., Cai X., Simaiti A., Yang X., Zhu X., Luo J., Jiang L.H., Du B., Yu P., Yang W. Silica nanoparticles induce lung inflammation in mice via ROS/PARP/TRPM2 signaling-mediated lysosome impairment and autophagy dysfunction, Part. Fibre Toxicol. 2020;17:23. doi: 10.1186/s12989-020-00353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho W.S., Duffin R., Howie S.E., Scotton C.J., Wallace W.A., Macnee W., Bradley M., Megson I.L., Donaldson K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes, Part. Fibre Toxicol. 2011;8:27. doi: 10.1186/1743-8977-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purushothaman B., Lee J., Hong S., Song J.M. Multifunctional TPP-PEG-biotin self-assembled nanoparticle drug delivery-based combination therapeutic approach for co-targeting of GRP78 and lysosome. J. Nanobiotechnol. 2020;18:102. doi: 10.1186/s12951-020-00661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu H.J., Qin Y., Zhao Z.H., Zhang Y., Yang J.H., Zhai D.H., Cui F., Luo C., Lu M.X., Liu P.P., Xu H.W., Li K., Sun B., Chen S., Zhou H.G., Yang C., Sun T. Lentinan-functionalized selenium nanoparticles target tumor cell mitochondria via TLR4/TRAF3/MFN1 pathway. Theranostics. 2020;10:9083–9099. doi: 10.7150/thno.46467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lopez V., Villegas M.R., Rodriguez V., Villaverde G., Lozano D., Baeza A., Vallet-Regi M. Janus mesoporous silica nanoparticles for dual targeting of tumor cells and mitochondria. ACS Appl. Mater. Interfaces. 2017;9:26697–26706. doi: 10.1021/acsami.7b06906. [DOI] [PubMed] [Google Scholar]
- 46.Xie C., Cen D., Wang H., Wang Y., Wu Y., Han G., Li X. Hierarchical nanoclusters with programmed disassembly for mitochondria-targeted tumor therapy with MR imaging. Biomater. Sci. 2021;9:8189–8201. doi: 10.1039/d1bm01423d. [DOI] [PubMed] [Google Scholar]
- 47.Tao Y., Yi K., Hu H., Shao D., Li M. Coassembly of nucleus-targeting gold nanoclusters with CRISPR/Cas9 for simultaneous bioimaging and therapeutic genome editing. J. Mater. Chem. B. 2021;9:94–100. doi: 10.1039/d0tb01925a. [DOI] [PubMed] [Google Scholar]
- 48.Kim I., Bang W.Y., Kim S., Jin S.M., Hyun J.Y., Han E.H., Lee E. Peroxisome-targeted supramolecular nanoprobes assembled with pyrene-labelled peptide amphiphiles, chem. Asian J. 2018;13:3485–3490. doi: 10.1002/asia.201800863. [DOI] [PubMed] [Google Scholar]
- 49.Setyawati M.I., Tay C.Y., Chia S.L., Goh S.L., Fang W., Neo M.J., Chong H.C., Tan S.M., Loo S.C., Ng K.W., Xie J.P., Ong C.N., Tan N.S., Leong D.T. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat. Commun. 2013;4:1673. doi: 10.1038/ncomms2655. [DOI] [PubMed] [Google Scholar]
- 50.Mohajeri M., Behnam B., Sahebkar A. Biomedical applications of carbon nanomaterials: drug and gene delivery potentials. J. Cell. Physiol. 2018;234:298–319. doi: 10.1002/jcp.26899. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang W.R., Wang Y., Cui P.F., Xing L., Lee J., Kim D., Jiang H.L., Oh Y.K. Applications of pi-pi stacking interactions in the design of drug-delivery systems. J. Contr. Release. 2019;294:311–326. doi: 10.1016/j.jconrel.2018.12.014. [DOI] [PubMed] [Google Scholar]
- 52.Gupta N., Rai D.B., Jangid A.K., Kulhari H. A review of theranostics applications and toxicities of carbon nanomaterials. Curr. Drug Metabol. 2019;20:506–532. doi: 10.2174/1389200219666180925094515. [DOI] [PubMed] [Google Scholar]
- 53.Chen D., Li B., Lei T., Na D., Nie M., Yang Y., Congjia Xie, He Z., Wang J. Selective mediation of ovarian cancer SKOV3 cells death by pristine carbon quantum dots/Cu(2)O composite through targeting matrix metalloproteinases, angiogenic cytokines and cytoskeleton. J. Nanobiotechnol. 2021;19:68. doi: 10.1186/s12951-021-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma A., Panwar V., Thomas J., Chopra V., Roy H.S., Ghosh D. Actin-binding carbon dots selectively target glioblastoma cells while sparing normal cells. Colloids Surf., B. 2021;200 doi: 10.1016/j.colsurfb.2021.111572. [DOI] [PubMed] [Google Scholar]
- 55.Qin Y., Chen K., Gu W., Dong X., Lei R., Chang Y., Bai X., Xia S., Zeng L., Zhang J., Ma S., Li J., Li S., Xing G. Small size fullerenol nanoparticles suppress lung metastasis of breast cancer cell by disrupting actin dynamics. J. Nanobiotechnol. 2018;16:54. doi: 10.1186/s12951-018-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Molaei M.J. A review on nanostructured carbon quantum dots and their applications in biotechnology, sensors, and chemiluminescence. Talanta. 2019;196:456–478. doi: 10.1016/j.talanta.2018.12.042. [DOI] [PubMed] [Google Scholar]
- 57.Fu L.Q., Du W.L., Cai M.H., Yao J.Y., Zhao Y.Y., Mou X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020;353 doi: 10.1016/j.cellimm.2020.104119. [DOI] [PubMed] [Google Scholar]
- 58.Yan H., Zhang B., Zhang Y., Su R., Li P., Su W. Fluorescent carbon dot-curcumin nanocomposites for remarkable antibacterial activity with synergistic photodynamic and photothermal abilities. ACS Appl. Bio Mater. 2021;4:6703–6718. doi: 10.1021/acsabm.1c00377. [DOI] [PubMed] [Google Scholar]
- 59.Feng T., Wei Y., Lee R.J., Zhao L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017;12:6027–6044. doi: 10.2147/IJN.S132434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mbese Z., Khwaza V., Aderibigbe B.A. Curcumin and its derivatives as potential therapeutic agents in prostate, colon and breast cancers. Molecules. 2019:24. doi: 10.3390/molecules24234386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Korzuch J., Rak M., Balin K., Zubko M., Glowacka O., Dulski M., Musiol R., Madeja Z., Serda M. Towards water-soluble [60]fullerenes for the delivery of siRNA in a prostate cancer model. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-89943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y., Shen X., Ma R., Hou Y., Qian Y., Fan C. Biological and biocompatible characteristics of fullerenols nanomaterials for tissue engineering. Histol. Histopathol. 2021;36:725–731. doi: 10.14670/HH-18-316. [DOI] [PubMed] [Google Scholar]
- 63.Grebowski J., Konopko A., Krokosz A., DiLabio G.A., Litwinienko G. Antioxidant activity of highly hydroxylated fullerene C(60) and its interactions with the analogue of alpha-tocopherol. Free Radical Biol. Med. 2020;160:734–744. doi: 10.1016/j.freeradbiomed.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 64.Bahuguna S., Kumar M., Sharma G., Kumar R., Singh B., Raza K. Fullerenol-based intracellular delivery of methotrexate: a water-soluble nanoconjugate for enhanced cytotoxicity and improved pharmacokinetics. AAPS PharmSciTech. 2017;19:1084–1092. doi: 10.1208/s12249-017-0920-0. [DOI] [PubMed] [Google Scholar]
- 65.Iwamoto D.V., Calderwood D.A. Regulation of integrin-mediated adhesions. Curr. Opin. Cell Biol. 2015;36:41–47. doi: 10.1016/j.ceb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park S., Srivastava D., Cho K. Generalized chemical reactivity of curved surfaces: carbon nanotubes. Nano Lett. 2003;3:1273–1277. doi: 10.1021/nl0342747. [DOI] [Google Scholar]
- 67.Lu Q., Moore J.M., Huang G., Mount A.S., Rao A.M., Larcom L.L., Ke P.C. RNA polymer translocation with single-walled carbon nanotubes. Nano Lett. 2004;4:2473–2477. doi: 10.1021/nl048326j. [DOI] [Google Scholar]
- 68.Zhang P., Teng J., Wang L. Multiwalled carbon nanotubes inhibit cell migration and invasion by destroying actin cytoskeleton via mitochondrial dysfunction in ovarian cancer cells. Aging (Albany NY) 2020;12:25294–25303. doi: 10.18632/aging.104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holt B.D., Short P.A., Rape A.D., Wang Y.L., Islam M.F., Dahl K.N. Carbon nanotubes reorganize actin structures in cells and ex vivo. ACS Nano. 2010;4:4872–4878. doi: 10.1021/nn101151x. [DOI] [PubMed] [Google Scholar]
- 70.Johnston D.E., Islam M.F., Yodh A.G., Johnson A.T. Electronic devices based on purified carbon nanotubes grown by high-pressure decomposition of carbon monoxide. Nat. Mater. 2005;4:589–592. doi: 10.1038/nmat1427. [DOI] [PubMed] [Google Scholar]
- 71.Patil R., Bahadur P., Tiwari S. Dispersed graphene materials of biomedical interest and their toxicological consequences. Adv. Colloid Interface Sci. 2020;275 doi: 10.1016/j.cis.2019.102051. [DOI] [PubMed] [Google Scholar]
- 72.Mousavi S.M., Low F.W., Hashemi S.A., Lai C.W., Ghasemi Y., Soroshnia S., Savardashtaki A., Babapoor A., Pynadathu Rumjit N., Goh S.M., Amin N., Tiong S.K. Development of graphene based nanocomposites towards medical and biological applications. Artif. Cells, Nanomed. Biotechnol. 2020;48:1189–1205. doi: 10.1080/21691401.2020.1817052. [DOI] [PubMed] [Google Scholar]
- 73.Tian X., Yang Z., Duan G., Wu A., Gu Z., Zhang L., Chen C., Chai Z., Ge C., Zhou R. Graphene oxide nanosheets retard cellular migration via disruption of actin cytoskeleton. Small. 2017;13 doi: 10.1002/smll.201602133. [DOI] [PubMed] [Google Scholar]
- 74.Zhu J., Xu M., Gao M., Zhang Z., Xu Y., Xia T., Liu S. Graphene oxide induced perturbation to plasma membrane and cytoskeletal meshwork sensitize cancer cells to chemotherapeutic agents. ACS Nano. 2017;11:2637–2651. doi: 10.1021/acsnano.6b07311. [DOI] [PubMed] [Google Scholar]
- 75.Mohammadinejad R., Moosavi M.A., Tavakol S., Vardar D.O., Hosseini A., Rahmati M., Dini L., Hussain S., Mandegary A., Klionsky D.J. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 2019;15:4–33. doi: 10.1080/15548627.2018.1509171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samadian H., Salami M.S., Jaymand M., Azarnezhad A., Najafi M., Barabadi H., Ahmadi A. Genotoxicity assessment of carbon-based nanomaterials; Have their unique physicochemical properties made them double-edged swords? Mutat. Res. Rev. Mutat. Res. 2020;783 doi: 10.1016/j.mrrev.2020.108296. [DOI] [PubMed] [Google Scholar]
- 77.Cui X., Wan B., Yang Y., Xin Y., Xie Y.C., Guo L.H., Mantell L.L. Carbon nanomaterials stimulate HMGB1 release from macrophages and induce cell migration and invasion. Toxicol. Sci. 2019;172:398–410. doi: 10.1093/toxsci/kfz190. [DOI] [PubMed] [Google Scholar]
- 78.Jeevanandam J., Barhoum A., Chan Y.S., Dufresne A., Danquah M.K. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arvizo R.R., Bhattacharyya S., Kudgus R.A., Giri K., Bhattacharya R., Mukherjee P. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future. Chem. Soc. Rev. 2012;41:2943–2970. doi: 10.1039/c2cs15355f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J.H., Cho H.Y., Choi H.K., Lee J.Y., Choi J.W. Application of gold nanoparticle to plasmonic biosensors. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19072021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou T., Yu M., Zhang B., Wang L., Wu X., Zhou H., Du Y., Hao J., Tu Y., Chen C., Wei T. Inhibition of cancer cell migration by gold nanorods: molecular mechanisms and implications for cancer therapy. Adv. Funct. Mater. 2014;24:6922–6932. doi: 10.1002/adfm.201401642. [DOI] [Google Scholar]
- 82.Asharani P.V., Hande M.P., Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65. doi: 10.1186/1471-2121-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao H., Chen Y., Alnaggar M. Silver nanoparticles induce cell death of colon cancer cells through impairing cytoskeleton and membrane nanostructure. Micron. 2019;126 doi: 10.1016/j.micron.2019.102750. [DOI] [PubMed] [Google Scholar]
- 84.Vigderman L., Khanal B.P., Zubarev E.R. Functional gold nanorods: synthesis, self-assembly, and sensing applications. Adv. Mater. 2012;24:4811–4841. doi: 10.1002/adma.201201690. 5014. [DOI] [PubMed] [Google Scholar]
- 85.Le Clainche C., Carlier M.F. Regulation of actin assembly associated with protrusion and adhesion in cell migration. Physiol. Rev. 2008;88:489–513. doi: 10.1152/physrev.00021.2007. [DOI] [PubMed] [Google Scholar]
- 86.Wang L., Liu Y., Li W., Jiang X., Ji Y., Wu X., Xu L., Qiu Y., Zhao K., Wei T., Li Y., Zhao Y., Chen C. Selective targeting of gold nanorods at the mitochondria of cancer cells: implications for cancer therapy. Nano Lett. 2011;11:772–780. doi: 10.1021/nl103992v. [DOI] [PubMed] [Google Scholar]
- 87.Carlier M.F., Pantaloni D. Control of actin assembly dynamics in cell motility. J. Biol. Chem. 2007;282:23005–23009. doi: 10.1074/jbc.R700020200. [DOI] [PubMed] [Google Scholar]
- 88.Lee S., Jun B.-H. Silver nanoparticles: synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.DeAngelis L.M. Global consequences of malignant CNS tumours: a call to action. Lancet Neurol. 2019;18:324–325. doi: 10.1016/s1474-4422(19)30083-3. [DOI] [PubMed] [Google Scholar]
- 90.Belizario J.E., Alves J., Occhiucci J.M., Garay-Malpartida M., Sesso A. A mechanistic view of mitochondrial death decision pores. Braz. J. Med. Biol. Res. 2007;40:1011–1024. doi: 10.1590/s0100-879x2006005000109. [DOI] [PubMed] [Google Scholar]
- 91.Chinopoulos C., Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 2006;273:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- 92.AshaRani P.V., Low Kah Mun G., Hande M.P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 93.Moutin M.J., Abramson J.J., Salama G., Dupont Y. Rapid Ag+-induced release of Ca2+ from sarcoplasmic reticulum vesicles of skeletal muscle: a rapid filtration study. Biochim. Biophys. Acta. 1989;984:289–292. doi: 10.1016/0005-2736(89)90295-2. [DOI] [PubMed] [Google Scholar]
- 94.Master A.M., Williams P.N., Pothayee N., Pothayee N., Zhang R., Vishwasrao H.M., Golovin Y.I., Riffle J.S., Sokolsky M., Kabanov A.V. Remote actuation of magnetic nanoparticles for cancer cell selective treatment through cytoskeletal disruption. Sci. Rep. 2016;6 doi: 10.1038/srep33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sun X., Zhang G., Du R., Xu R., Zhu D., Qian J., Bai G., Yang C., Zhang Z., Zhang X., Zou D., Wu Z. A biodegradable MnSiO(3)@Fe(3)O(4) nanoplatform for dual-mode magnetic resonance imaging guided combinatorial cancer therapy. Biomaterials. 2019;194:151–160. doi: 10.1016/j.biomaterials.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Ilg P., Kroger M. Dynamics of interacting magnetic nanoparticles: effective behavior from competition between Brownian and Neel relaxation. Phys. Chem. Chem. Phys. 2020;22:22244–22259. doi: 10.1039/d0cp04377j. [DOI] [PubMed] [Google Scholar]
- 97.Golovin Y.I., Gribanovsky S.L., Golovin D.Y., Klyachko N.L., Majouga A.G., Master capital A C., Sokolsky M., Kabanov A.V. Towards nanomedicines of the future: remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J. Contr. Release. 2015;219:43–60. doi: 10.1016/j.jconrel.2015.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu Q., Zhang B., Zhang Y.M., Liu Y.H., Liu Y. Actin cytoskeleton-disrupting and magnetic field-responsive multivalent supramolecular assemblies for efficient cancer therapy. ACS Appl. Mater. Interfaces. 2020;12:13709–13717. doi: 10.1021/acsami.0c01762. [DOI] [PubMed] [Google Scholar]
- 99.Shao X., Ding Z., Zhou W., Li Y., Li Z., Cui H., Lin X., Cao G., Cheng B., Sun H., Li M., Liu K., Lu D., Geng S., Shi W., Zhang G., Song Q., Chen L., Wang G., Su W., Cai L., Fang L., Leong D.T., Li Y., Yu X.F., Li H. Intrinsic bioactivity of black phosphorus nanomaterials on mitotic centrosome destabilization through suppression of PLK1 kinase. Nat. Nanotechnol. 2021;16:1150–1160. doi: 10.1038/s41565-021-00952-x. [DOI] [PubMed] [Google Scholar]
- 100.Liu J., Smith S., Wang C. Reversing the epithelial-mesenchymal transition in metastatic cancer cells using cd146-targeted black phosphorus nanosheets and a mild photothermal treatment. ACS Nano. 2022;16:3208–3220. doi: 10.1021/acsnano.1c11070. [DOI] [PubMed] [Google Scholar]
- 101.Lee H.U., Park S.Y., Lee S.C., Choi S., Seo S., Kim H., Won J., Choi K., Kang K.S., Park H.G., Kim H.S., An H.R., Jeong K.H., Lee Y.C., Lee J. Black phosphorus (BP) nanodots for potential biomedical applications. Small. 2016;12:214–219. doi: 10.1002/smll.201502756. [DOI] [PubMed] [Google Scholar]
- 102.Choi J.R., Yong K.W., Choi J.Y., Nilghaz A., Lin Y., Xu J., Lu X. Black phosphorus and its biomedical applications. Theranostics. 2018;8:1005–1026. doi: 10.7150/thno.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Y., Zhou S., Song H., Yu T., Zheng X., Chu Q. CaCO(3) nanoparticles incorporated with KAE to enable amplified calcium overload cancer therapy. Biomaterials. 2021;277 doi: 10.1016/j.biomaterials.2021.121080. [DOI] [PubMed] [Google Scholar]
- 104.Zhang M., Song R., Liu Y., Yi Z., Meng X., Zhang J., Tang Z., Yao Z., Liu Y., Liu X., Bu W. Calcium-overload-mediated tumor therapy by calcium peroxide nanoparticles. Chem. 2019;5:2171–2182. doi: 10.1016/j.chempr.2019.06.003. [DOI] [Google Scholar]
- 105.Yumnam S., Hong G.E., Raha S., Saralamma V.V., Lee H.J., Lee W.S., Kim E.H., Kim G.S. Mitochondrial dysfunction and Ca(2+) overload contributes to hesperidin induced paraptosis in hepatoblastoma cells, HepG2. J. Cell. Physiol. 2016;231:1261–1268. doi: 10.1002/jcp.25222. [DOI] [PubMed] [Google Scholar]
- 106.Li Y., Yu X., Wang Y., Zheng X., Chu Q. Kaempferol-3-O-rutinoside, a flavone derived from Tetrastigma hemsleyanum, suppresses lung adenocarcinoma via the calcium signaling pathway. Food Funct. 2021;12:8351–8365. doi: 10.1039/d1fo00581b. [DOI] [PubMed] [Google Scholar]
- 107.Yang C., Svitkina T.M. Ultrastructure and dynamics of the actin-myosin II cytoskeleton during mitochondrial fission. Nat. Cell Biol. 2019;21:603–613. doi: 10.1038/s41556-019-0313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park D.J., Min K.H., Lee H.J., Kim K., Kwon I.C., Jeong S.Y., Lee S.C. Photosensitizer-loaded bubble-generating mineralized nanoparticles for ultrasound imaging and photodynamic therapy. J. Mater. Chem. B. 2016;4:1219–1227. doi: 10.1039/c5tb02338f. [DOI] [PubMed] [Google Scholar]
- 109.Tay C.Y., Cai P., Setyawati M.I., Fang W., Tan L.P., Hong C.H., Chen X., Leong D.T. Nanoparticles strengthen intracellular tension and retard cellular migration. Nano Lett. 2014;14:83–88. doi: 10.1021/nl4032549. [DOI] [PubMed] [Google Scholar]
- 110.Tsai C.P., Hung Y., Chou Y.H., Huang D.M., Hsiao J.K., Chang C., Chen Y.C., Mou C.Y. High-contrast paramagnetic fluorescent mesoporous silica nanorods as a multifunctional cell-imaging probe. Small. 2008;4:186–191. doi: 10.1002/smll.200700457. [DOI] [PubMed] [Google Scholar]
- 111.Huang X., Teng X., Chen D., Tang F., He J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials. 2010;31:438–448. doi: 10.1016/j.biomaterials.2009.09.060. [DOI] [PubMed] [Google Scholar]
- 112.Romero S., Le Clainche C., Gautreau A.M. Actin polymerization downstream of integrins: signaling pathways and mechanotransduction. Biochem. J. 2020;477:1–21. doi: 10.1042/BCJ20170719. [DOI] [PubMed] [Google Scholar]
- 113.Tay C.Y., Cai P., Setyawati M.I., Fang W., Tan L.P., Hong C.H.L., Chen X., Leong D.T. Nanoparticles strengthen intracellular tension and retard cellular migration. Nano Lett. 2013;14:83–88. doi: 10.1021/nl4032549. [DOI] [PubMed] [Google Scholar]
- 114.Liu J., Kang L., Smith S., Wang C. Transmembrane MUC18 targeted polydopamine nanoparticles and a mild photothermal effect synergistically disrupt actin cytoskeleton and migration of cancer cells. Nano Lett. 2021;21:9609–9618. doi: 10.1021/acs.nanolett.1c03377. [DOI] [PubMed] [Google Scholar]
- 115.Cheng W., Zeng X., Chen H., Li Z., Zeng W., Mei L., Zhao Y. Versatile polydopamine platforms: synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano. 2019;13:8537–8565. doi: 10.1021/acsnano.9b04436. [DOI] [PubMed] [Google Scholar]
- 116.Feng R., Wang Y., Ramachandran V., Ma Q., May M.M., Li M., Zhou J.X., Xu X., Xu K., Fang S., Xia W., Sui D., Liu H., Gao X., Prieto V., Blacklow S.C., Lu M., Lee J.E. Characterization of novel neutralizing mouse monoclonal antibody JM1-24-3 developed against MUC18 in metastatic melanoma. J. Exp. Clin. Cancer Res. 2020;39:273. doi: 10.1186/s13046-020-01722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng B., Wan W., Huang G., Li Y., Genin G.M., Mofrad M.R.K., Lu T.J., Xu F., Lin M. Nanoscale integrin cluster dynamics controls cellular mechanosensing via FAKY397 phosphorylation. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aax1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zoni E., Astrologo L., Ng C.K.Y., Piscuoglio S., Melsen J., Grosjean J., Klima I., Chen L., Snaar-Jagalska E.B., Flanagan K., van der Pluijm G., Kloen P., Cecchini M.G., Kruithof-de Julio M., Thalmann G.N. Therapeutic targeting of CD146/MCAM reduces bone metastasis in prostate cancer. Mol. Cancer Res. 2019;17:1049–1062. doi: 10.1158/1541-7786.MCR-18-1220. [DOI] [PubMed] [Google Scholar]
- 119.Alnaggar M., Xu Y., Li J., He J., Chen J., Li M., Wu Q., Lin L., Liang Y., Wang X., Li J., Hu Y., Chen Y., Xu K., Wu Y., Yin Z. Allogenic Vgamma9Vdelta2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J. ImmunoTher. Cancer. 2019;7:36. doi: 10.1186/s40425-019-0501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carding S.R., Egan P.J. Gammadelta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 121.de Oliveira Pedro R., Hoffmann S., Pereira S., Goycoolea F.M., Schmitt C.C., Neumann M.G. Self-assembled amphiphilic chitosan nanoparticles for quercetin delivery to breast cancer cells. Eur. J. Pharm. Biopharm. 2018;131:203–210. doi: 10.1016/j.ejpb.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 122.Kean T., Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 123.Lin L., He J., Li J., Xu Y., Li J., Wu Y. Chitosan nanoparticles strengthen Vgamma9Vdelta2 T-cell cytotoxicity through upregulation of killing molecules and cytoskeleton polarization. Int. J. Nanomed. 2019;14:9325–9336. doi: 10.2147/IJN.S212898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Billadeau D.D., Nolz J.C., Gomez T.S. Regulation of T-cell activation by the cytoskeleton. Nat. Rev. Immunol. 2007;7:131–143. doi: 10.1038/nri2021. [DOI] [PubMed] [Google Scholar]
- 125.Neuberger J. Antibodies and primary biliary cirrhosis - piecing together the jigsaw. J. Hepatol. 2002;36:126–129. doi: 10.1016/s0168-8278(01)00303-8. [DOI] [PubMed] [Google Scholar]
- 126.Sun M., Wang C., Lv M., Fan Z., Du J. Intracellular self-assembly of peptides to induce apoptosis against drug-resistant melanoma. J. Am. Chem. Soc. 2022;144:7337–7345. doi: 10.1021/jacs.2c00697. [DOI] [PubMed] [Google Scholar]
- 127.Li Z., Di C., Li S., Yang X., Nie G. Smart nanotherapeutic targeting of tumor vasculature. Acc. Chem. Res. 2019;52:2703–2712. doi: 10.1021/acs.accounts.9b00283. [DOI] [PubMed] [Google Scholar]
- 128.Chen Z., Fang Z., Ma J. Regulatory mechanisms and clinical significance of vimentin in breast cancer. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111068. [DOI] [PubMed] [Google Scholar]
- 129.Fan J.Q., Li Y.J., Wei Z.J., Fan Y., Li X.D., Chen Z.M., Hou D.Y., Xiao W.Y., Ding M.R., Wang H., Wang L. Binding-induced fibrillogenesis peptides recognize and block intracellular vimentin skeletonization against breast cancer. Nano Lett. 2021;21:6202–6210. doi: 10.1021/acs.nanolett.1c01950. [DOI] [PubMed] [Google Scholar]
- 130.Mohan A.K., M M., Kumar T.R.S., Kumar G.S.V. Multi-layered PLGA-PEI nanoparticles functionalized with TKD peptide for targeted delivery of Pep5 to breast tumor cells and spheroids. Int. J. Nanomed. 2022;17:5581–5600. doi: 10.2147/IJN.S376358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen D., Li B., Jiang L., Li Y., Yang Y., Luo Z., Wang J. Pristine Cu-MOF induces mitotic catastrophe and alterations of gene expression and cytoskeleton in ovarian cancer cells. ACS Appl. Bio Mater. 2020;3:4081–4094. doi: 10.1021/acsabm.0c00175. [DOI] [PubMed] [Google Scholar]
- 132.Simon-Yarza T., Mielcarek A., Couvreur P., Serre C. Nanoparticles of metal-organic frameworks: on the road to in vivo efficacy in biomedicine. Adv. Mater. 2018;30 doi: 10.1002/adma.201707365. [DOI] [PubMed] [Google Scholar]
- 133.Lucena F.R., de Araujo L.C., Rodrigues Mdo D., da Silva T.G., Pereira V.R., Militao G.C., Fontes D.A., Rolim-Neto P.J., da Silva F.F., Nascimento S.C. Induction of cancer cell death by apoptosis and slow release of 5-fluoracil from metal-organic frameworks Cu-BTC. Biomed. Pharmacother. 2013;67:707–713. doi: 10.1016/j.biopha.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 134.He C., Lu K., Liu D., Lin W. Nanoscale metal-organic frameworks for the co-delivery of cisplatin and pooled siRNAs to enhance therapeutic efficacy in drug-resistant ovarian cancer cells. J. Am. Chem. Soc. 2014;136:5181–5184. doi: 10.1021/ja4098862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zielinska E., Zauszkiewicz-Pawlak A., Wojcik M., Inkielewicz-Stepniak I. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget. 2018;9:4675–4697. doi: 10.18632/oncotarget.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vakifahmetoglu H., Olsson M., Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 137.Gao P., Chen Y., Pan W., Li N., Liu Z., Tang B. Antitumor agents based on metal-organic frameworks. Angew. Chem., Int. Ed. Engl. 2021;60:16763–16776. doi: 10.1002/anie.202102574. [DOI] [PubMed] [Google Scholar]
- 138.Weng W., He S., Song H., Li X., Cao L., Hu Y., Cui J., Zhou Q., Peng H., Su J. Aligned carbon nanotubes reduce hypertrophic scar via regulating cell behavior. ACS Nano. 2018;12:7601–7612. doi: 10.1021/acsnano.7b07439. [DOI] [PubMed] [Google Scholar]
- 139.Namgung S., Baik K.Y., Park J., Hong S. Controlling the growth and differentiation of human mesenchymal stem cells by the arrangement of individual carbon nanotubes. ACS Nano. 2011;5:7383–7390. doi: 10.1021/nn2023057. [DOI] [PubMed] [Google Scholar]
- 140.Berman B., Maderal A., Raphael B. Keloids and hypertrophic scars: pathophysiology, classification, and treatment. Dermatol. Surg. 2017;43(Suppl 1) doi: 10.1097/DSS.0000000000000819. S3-S18. [DOI] [PubMed] [Google Scholar]
- 141.Landen N.X., Li D., Stahle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell. Mol. Life Sci. 2016;73:3861–3885. doi: 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tziotzios C., Profyris C., Sterling J. Cutaneous scarring: pathophysiology, molecular mechanisms, and scar reduction therapeutics Part II. Strategies to reduce scar formation after dermatologic procedures. J. Am. Acad. Dermatol. 2012;66:13–24. doi: 10.1016/j.jaad.2011.08.035. quiz 25-16. [DOI] [PubMed] [Google Scholar]
- 143.Lim A.F., Weintraub J., Kaplan E.N., Januszyk M., Cowley C., McLaughlin P., Beasley B., Gurtner G.C., Longaker M.T. The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast. Reconstr. Surg. 2014;133:398–405. doi: 10.1097/01.prs.0000436526.64046.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu T., Houle J.D., Xu J., Chan B.P., Chew S.Y. Nanofibrous collagen nerve conduits for spinal cord repair, Tissue. Eng. Part A. 2012;18:1057–1066. doi: 10.1089/ten.TEA.2011.0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Steele-Perkins G., Plachez C., Butz K.G., Yang G., Bachurski C.J., Kinsman S.L., Litwack E.D., Richards L.J., Gronostajski R.M. The transcription factor gene Nfib is essential for both lung maturation and brain development. Mol. Cell Biol. 2005;25:685–698. doi: 10.1128/MCB.25.2.685-698.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang K., Lu W., Jia J., Zhang J., Zhao M., Wang S., Jiang H., Xu L., Wang J. Noggin inhibits hypoxia-induced proliferation by targeting store-operated calcium entry and transient receptor potential cation channels. Am. J. Physiol. Cell Physiol. 2015;308:C869–C878. doi: 10.1152/ajpcell.00349.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yao F., Kausalya Jaya P., Sia Yee Y., Teo Audrey S.M., Lee Wah H., Ong Alicia G.M., Zhang Z., Tan Joanna H.J., Li G., Bertrand D., Liu X., Poh Huay M., Guan P., Zhu F., Pathiraja Thushangi N., Ariyaratne Pramila N., Rao J., Woo Xing Y., Cai S., Mulawadi Fabianus H., Poh Wan T., Veeravalli L., Chan Chee S., Lim Seong S., Leong See T., Neo Say C., Choi Poh Sum D., Chew Elaine G.Y., Nagarajan N., Jacques P.-É., So Jimmy B.Y., Ruan X., Yeoh Khay G., Tan P., Sung W.-K., Hunziker W., Ruan Y., Hillmer Axel M. Recurrent fusion genes in gastric cancer: CLDN18-ARHGAP26 induces loss of epithelial integrity. Cell Rep. 2015;12:272–285. doi: 10.1016/j.celrep.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 148.Krishnan H., Ochoa-Alvarez J.A., Shen Y., Nevel E., Lakshminarayanan M., Williams M.C., Ramirez M.I., Miller W.T., Goldberg G.S. Serines in the intracellular tail of podoplanin (PDPN) regulate cell motility. J. Biol. Chem. 2013;288:12215–12221. doi: 10.1074/jbc.C112.446823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park J., Kim D.H., Kim H.N., Wang C.J., Kwak M.K., Hur E., Suh K.Y., An S.S., Levchenko A. Directed migration of cancer cells guided by the graded texture of the underlying matrix. Nat. Mater. 2016;15:792–801. doi: 10.1038/nmat4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Oh S., Brammer K.S., Li Y.S., Teng D., Engler A.J., Chien S., Jin S. Stem cell fate dictated solely by altered nanotube dimension. Proc. Natl. Acad. Sci. U.S.A. 2009;106:2130–2135. doi: 10.1073/pnas.0813200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kim J.Y., Yeom J., Zhao G., Calcaterra H., Munn J., Zhang P., Kotov N. Assembly of gold nanoparticles into chiral superstructures driven by circularly polarized light. J. Am. Chem. Soc. 2019;141:11739–11744. doi: 10.1021/jacs.9b00700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qu A., Sun M., Kim J.Y., Xu L., Hao C., Ma W., Wu X., Liu X., Kuang H., Kotov N.A., Xu C. Stimulation of neural stem cell differentiation by circularly polarized light transduced by chiral nanoassemblies. Nat. Biomed. Eng. 2021;5:103–113. doi: 10.1038/s41551-020-00634-4. [DOI] [PubMed] [Google Scholar]
- 153.Cockerill I., Su Y., Lee J.H., Berman D., Young M.L., Zheng Y., Zhu D. Micro-/Nanotopography on bioresorbable zinc dictates cytocompatibility, bone cell differentiation, and macrophage polarization. Nano Lett. 2020;20:4594–4602. doi: 10.1021/acs.nanolett.0c01448. [DOI] [PubMed] [Google Scholar]
- 154.Oh S., Daraio C., Chen L.H., Pisanic T.R., Finones R.R., Jin S. Significantly accelerated osteoblast cell growth on aligned TiO2 nanotubes. J. Biomed. Mater. Res., Part A. 2006;78:97–103. doi: 10.1002/jbm.a.30722. [DOI] [PubMed] [Google Scholar]
- 155.Bjursten L.M., Rasmusson L., Oh S., Smith G.C., Brammer K.S., Jin S. Titanium dioxide nanotubes enhance bone bonding in vivo. J. Biomed. Mater. Res., Part A. 2010;92:1218–1224. doi: 10.1002/jbm.a.32463. [DOI] [PubMed] [Google Scholar]
- 156.Brammer K.S., Oh S., Cobb C.J., Bjursten L.M., van der Heyde H., Jin S. Improved bone-forming functionality on diameter-controlled TiO(2) nanotube surface. Acta Biomater. 2009;5:3215–3223. doi: 10.1016/j.actbio.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 157.Necula M.G., Mazare A., Ion R.N., Ozkan S., Park J., Schmuki P., Cimpean A. Lateral spacing of TiO(2) nanotubes modulates osteoblast behavior. Materials. 2019;12 doi: 10.3390/ma12182956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cavalli G., Dinarello C.A. Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 2018;281:179–190. doi: 10.1111/imr.12605. [DOI] [PubMed] [Google Scholar]
- 159.Colin-York H., Kumari S., Barbieri L., Cords L., Fritzsche M. Distinct actin cytoskeleton behaviour in primary and immortalised T-cells. J. Cell Sci. 2019;133 doi: 10.1242/jcs.232322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Oh J., Xia X., Wong W.K.R., Wong S.H.D., Yuan W., Wang H., Lai C.H.N., Tian Y., Ho Y.P., Zhang H., Zhang Y., Li G., Lin Y., Bian L. The effect of the nanoparticle shape on T cell activation. Small. 2022;18 doi: 10.1002/smll.202107373. [DOI] [PubMed] [Google Scholar]
- 161.Jakob-Roetne R., Jacobsen H. Alzheimer's disease: from pathology to therapeutic approaches. Angew. Chem., Int. Ed. Engl. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 162.Bush A.I. The metallobiology of Alzheimer's disease. Trends Neurosci. 2003;26:207–214. doi: 10.1016/S0166-2236(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 163.Yang L., Yin T., Liu Y., Sun J., Zhou Y., Liu J. Gold nanoparticle-capped mesoporous silica-based H(2)O(2)-responsive controlled release system for Alzheimer's disease treatment. Acta Biomater. 2016;46:177–190. doi: 10.1016/j.actbio.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 164.Cheng C.S., Liu T.P., Chien F.C., Mou C.Y., Wu S.H., Chen Y.P. Codelivery of plasmid and curcumin with mesoporous silica nanoparticles for promoting neurite outgrowth. ACS Appl. Mater. Interfaces. 2019;11:15322–15331. doi: 10.1021/acsami.9b02797. [DOI] [PubMed] [Google Scholar]
- 165.Tee J.K., Yip L.X., Tan E.S., Santitewagun S., Prasath A., Ke P.C., Ho H.K., Leong D.T. Nanoparticles' interactions with vasculature in diseases. Chem. Soc. Rev. 2019;48:5381–5407. doi: 10.1039/c9cs00309f. [DOI] [PubMed] [Google Scholar]
- 166.Sugizaki A., Sato K., Chiba K., Saito K., Kawagishi M., Tomabechi Y., Mehta S.B., Ishii H., Sakai N., Shirouzu M., Tani T., Terada S. POLArIS, a versatile probe for molecular orientation, revealed actin filaments associated with microtubule asters in early embryos. Proc. Natl. Acad. Sci. U.S.A. 2021;118 doi: 10.1073/pnas.2019071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mu Q., Yu J., Griffin J.I., Wu Y., Zhu L., McConnachie L.A., Ho R.J.Y. Novel drug combination nanoparticles exhibit enhanced plasma exposure and dose-responsive effects on eliminating breast cancer lung metastasis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0228557. [DOI] [PMC free article] [PubMed] [Google Scholar]